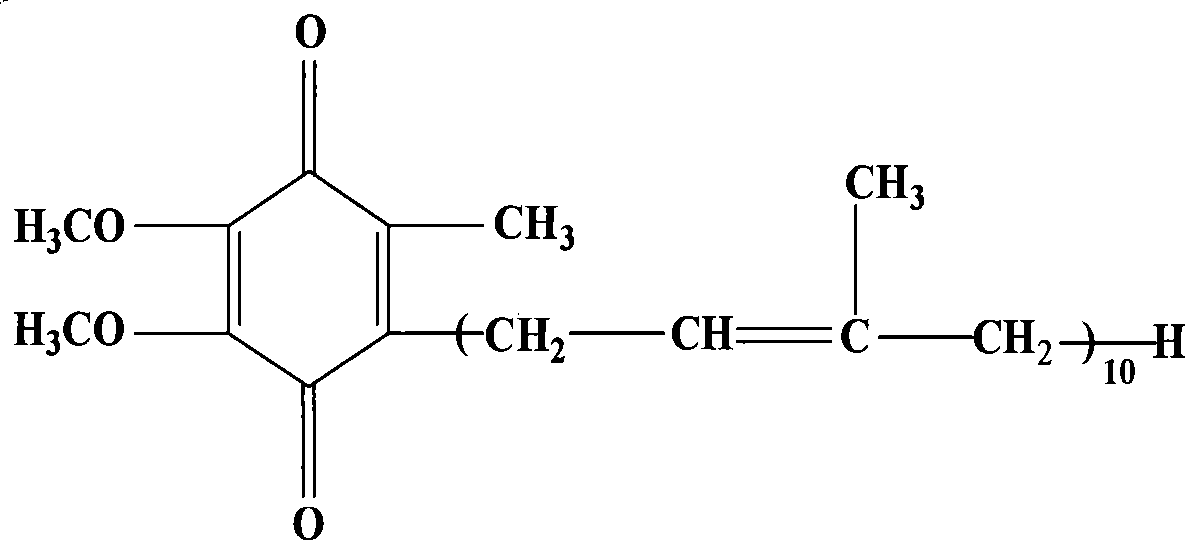
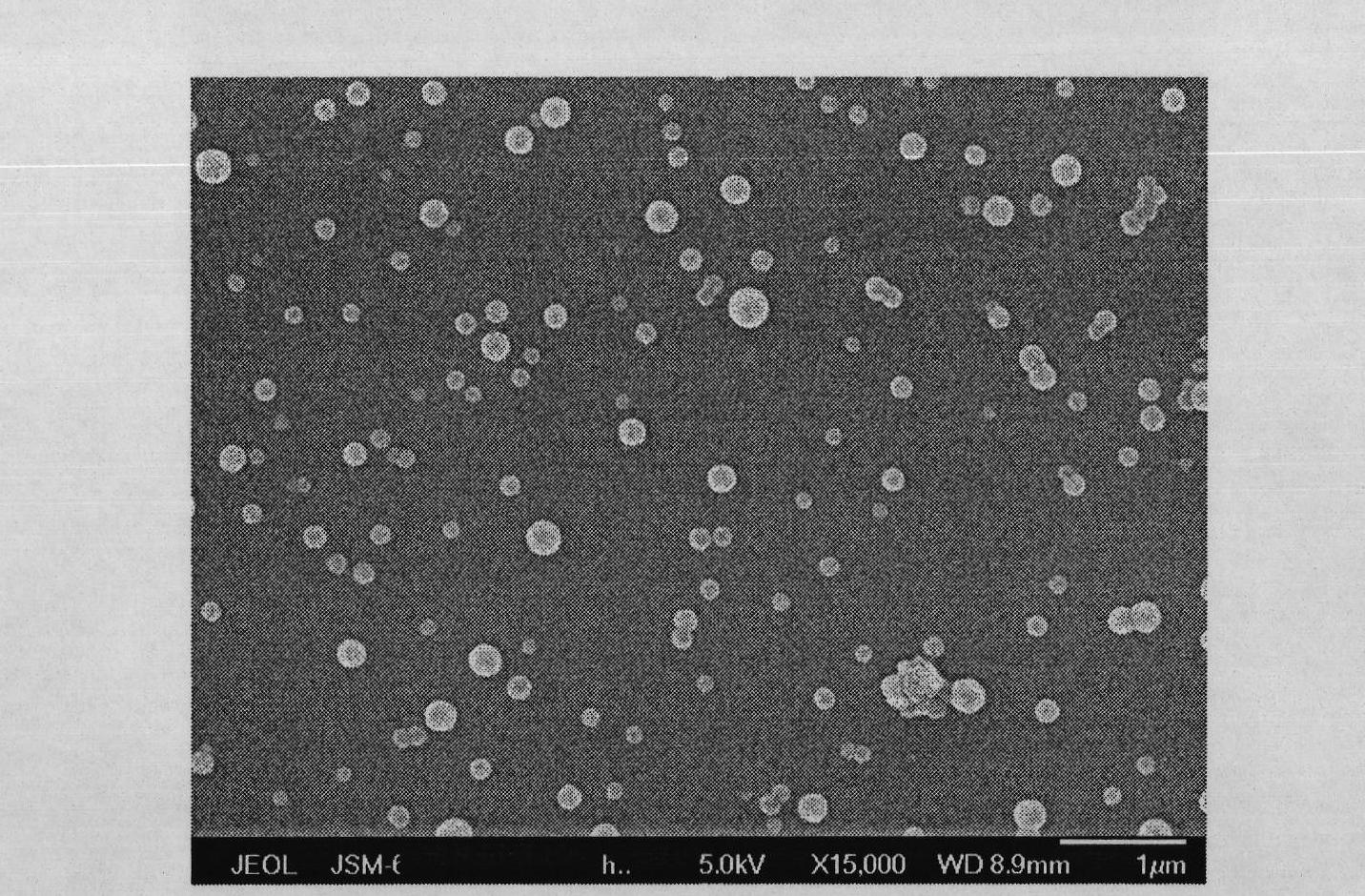
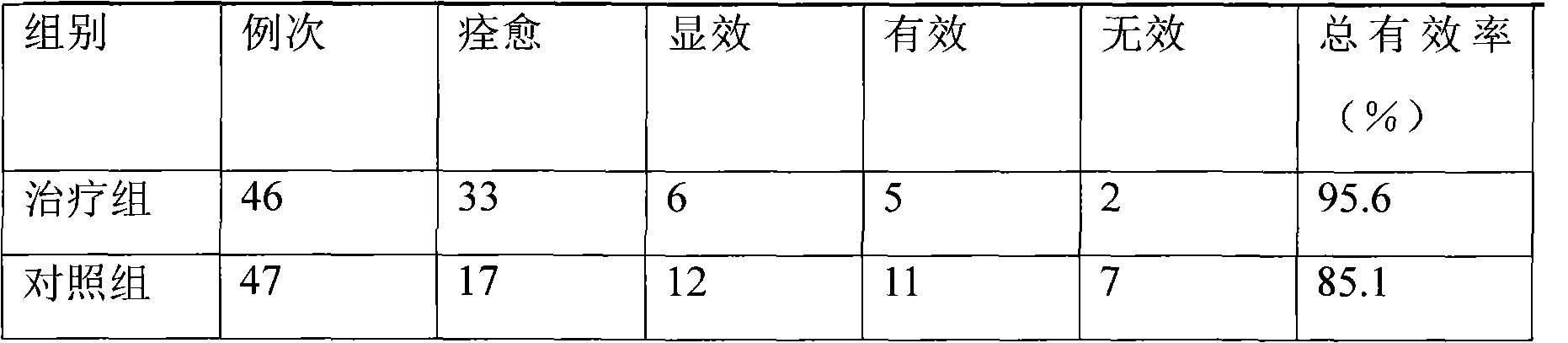
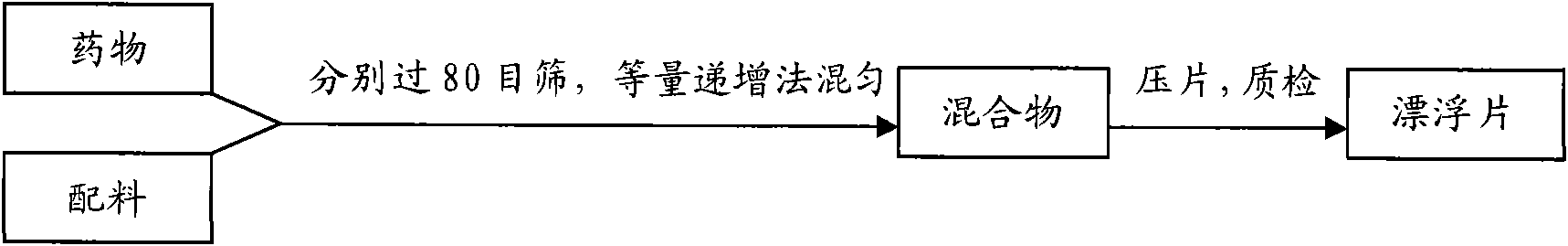
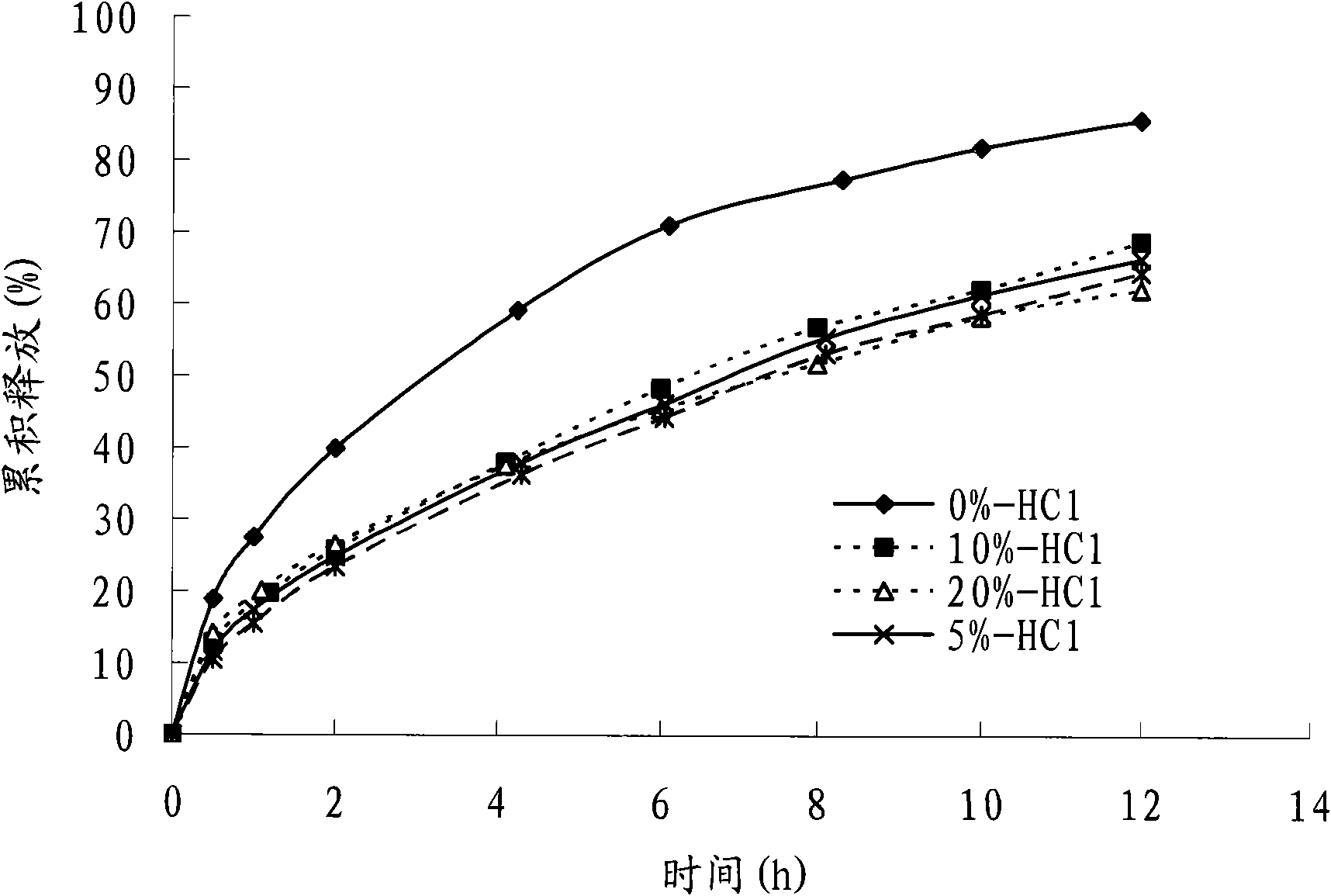
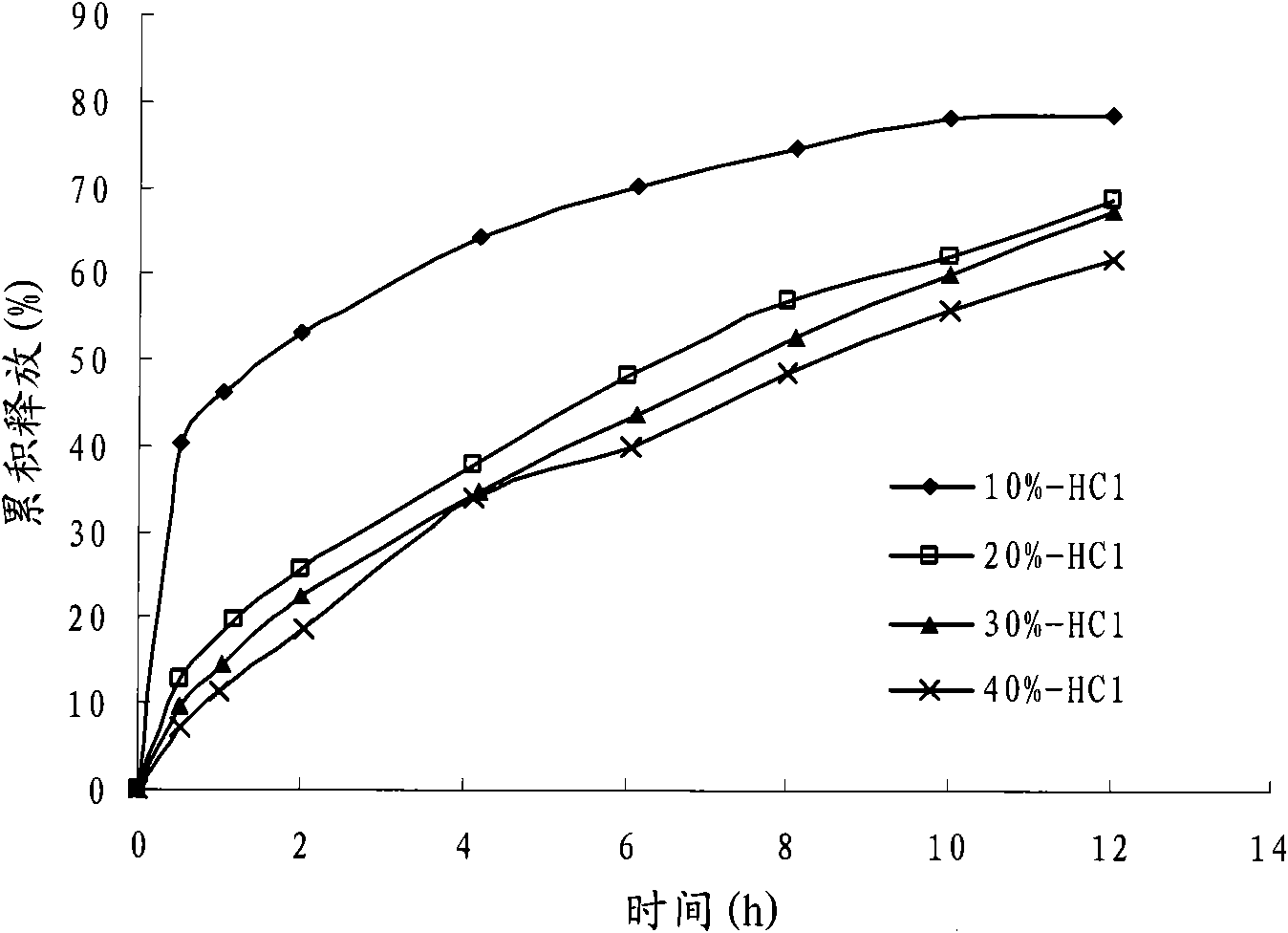
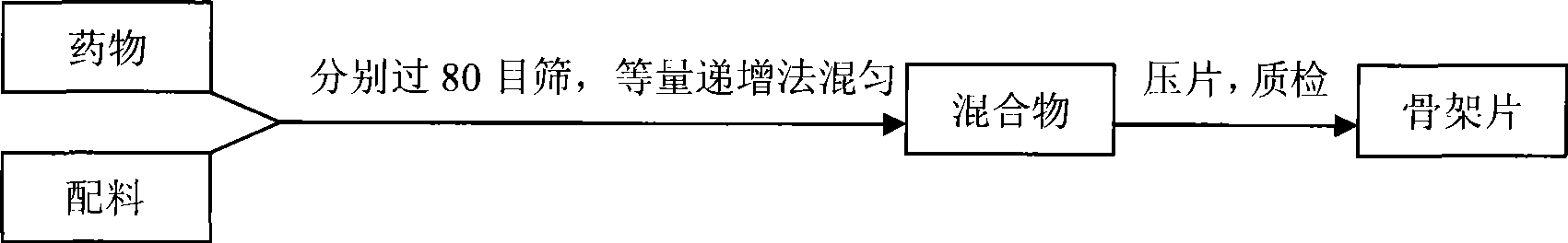
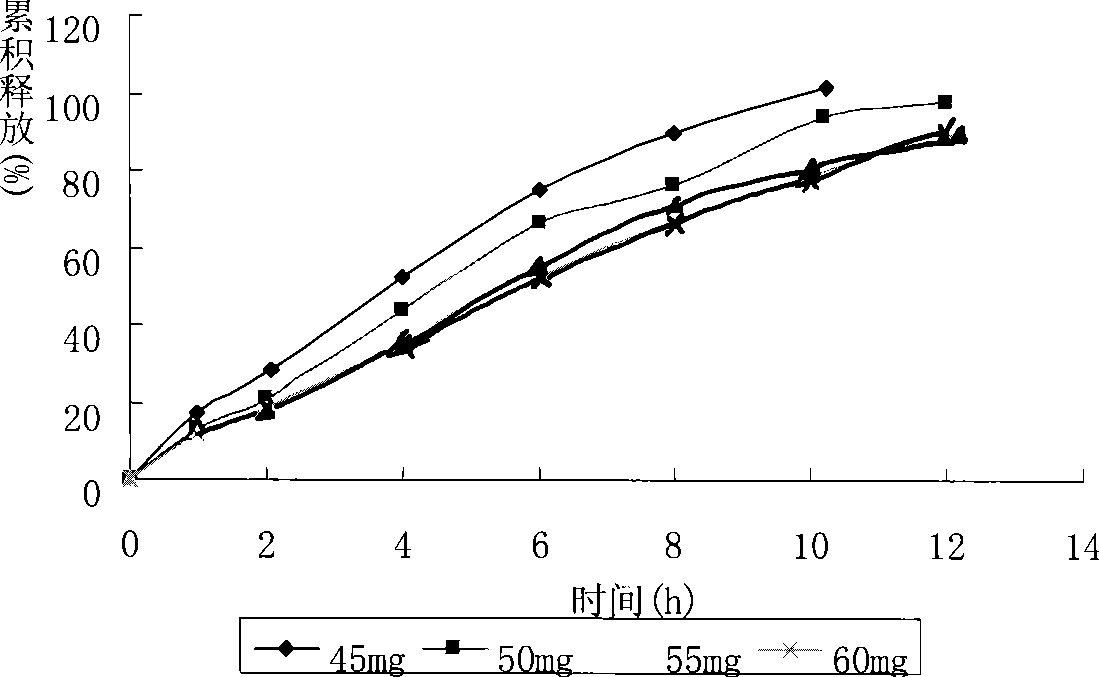
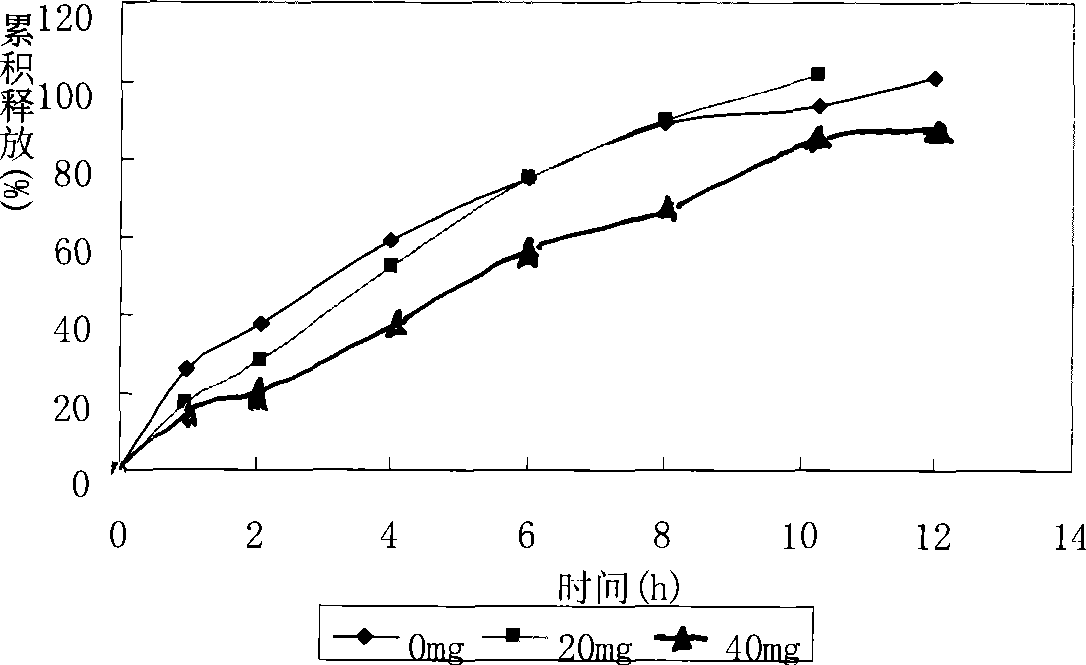
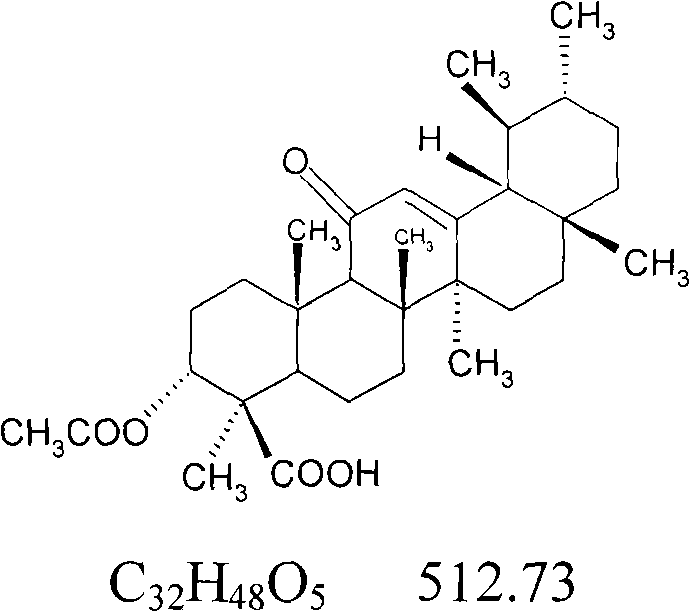
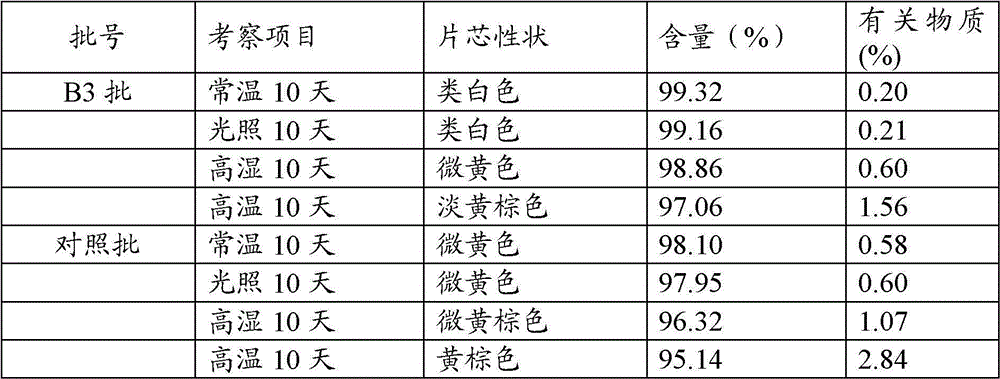
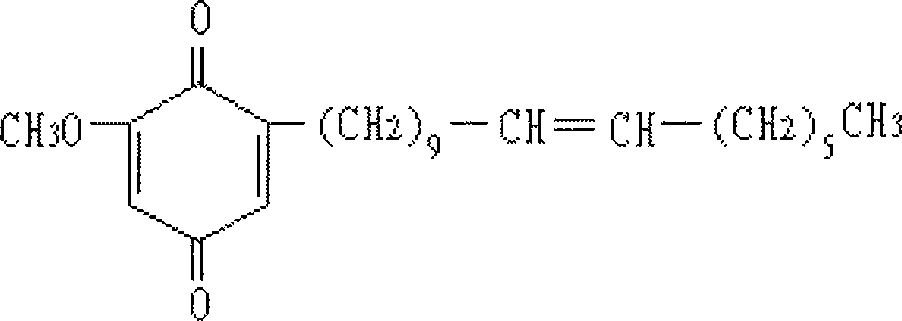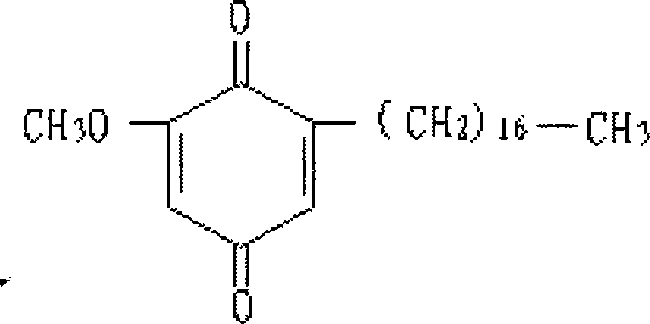Patents
Literature
702 results about "Pharmaceutic Adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacology, adjuvants are drugs that have few or no pharmacological effects by themselves, but may increase the efficacy or potency of other drugs when given at the same time. For instance, caffeine has minimal analgesic effect on its own, but may have an adjuvant effect when given with paracetamol (acetaminophen).
Composite type emulsifier, and emulsion prepared by using the emulsifier, and preparation method
InactiveCN101091890AGood chemical stabilityLess irritatingCosmetic preparationsToilet preparationsPolymer scienceOfficinal
The invention belongs to the medicine area, which involves a multi-emulsifier and a emulsion which be used by the multi-emulsifier and can resist high temperature and low temperature. This multi-emulsifier can combine by two or more then three emulsifiers. When combining, we can use two or more then three emulsifiers at will. The multi-emulsifier includes pharmaceutic adjuvant, oil and water. The emulsion includes drugs, oil phase, multi-emulsifier, the water and so on. The emulsion can be made after combining the oil and the water by isotropic device. The invention has some advantages: 1, the emulsion can resist high temperature and frost thawing. 2, easy to produce the average granularity smaller than the 150nm. 3, obtains the aseptic preparation without the high temperature. 4, produces the emulsion under the low pH value, so expand the application area. 5, increases the chemical stability of the medicine. 6, reduces the irritating quality.
Owner:SHENYANG PHARMA UNIVERSITY +1
Oral solid preparation of canagliflozin and preparation method thereof
The invention relates to an oral solid medicine composition of canagliflozin and a preparation method thereof. The composition comprises canagliflozin and pharmaceutic adjuvants, wherein the canagliflozin is in an amorphous form, and the average grain size of particles is 2.5-30 microns. The composition can be used for effectively solving the technical problems that the crystal transformation and the compressibility of the canagliflozin in the amorphous form are poor in the preparation process of the solid preparation.
Owner:CHONGQING PHARMA RES INST
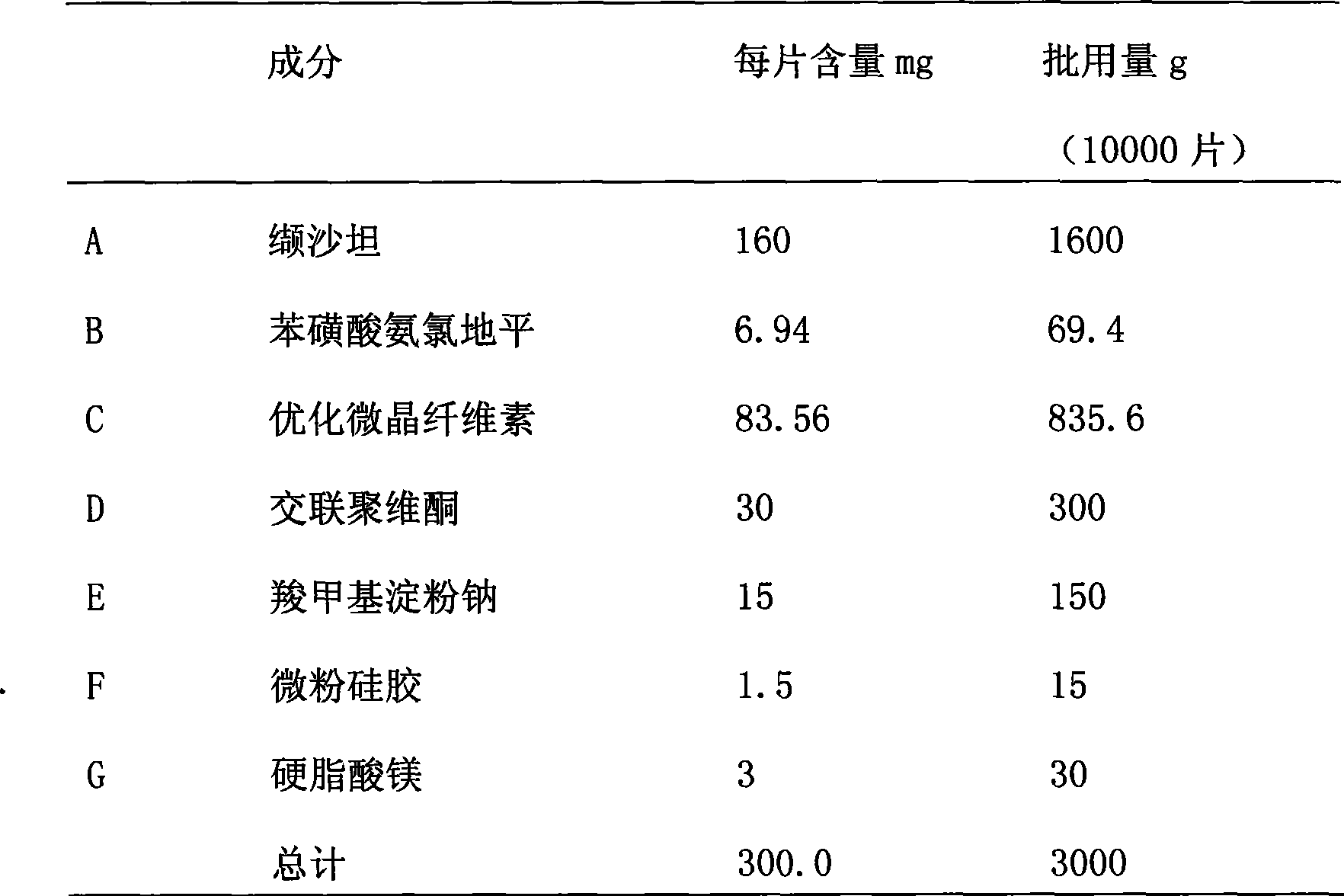
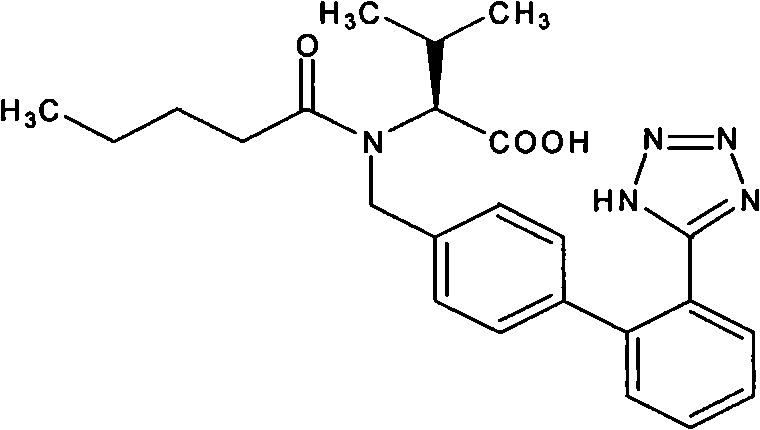
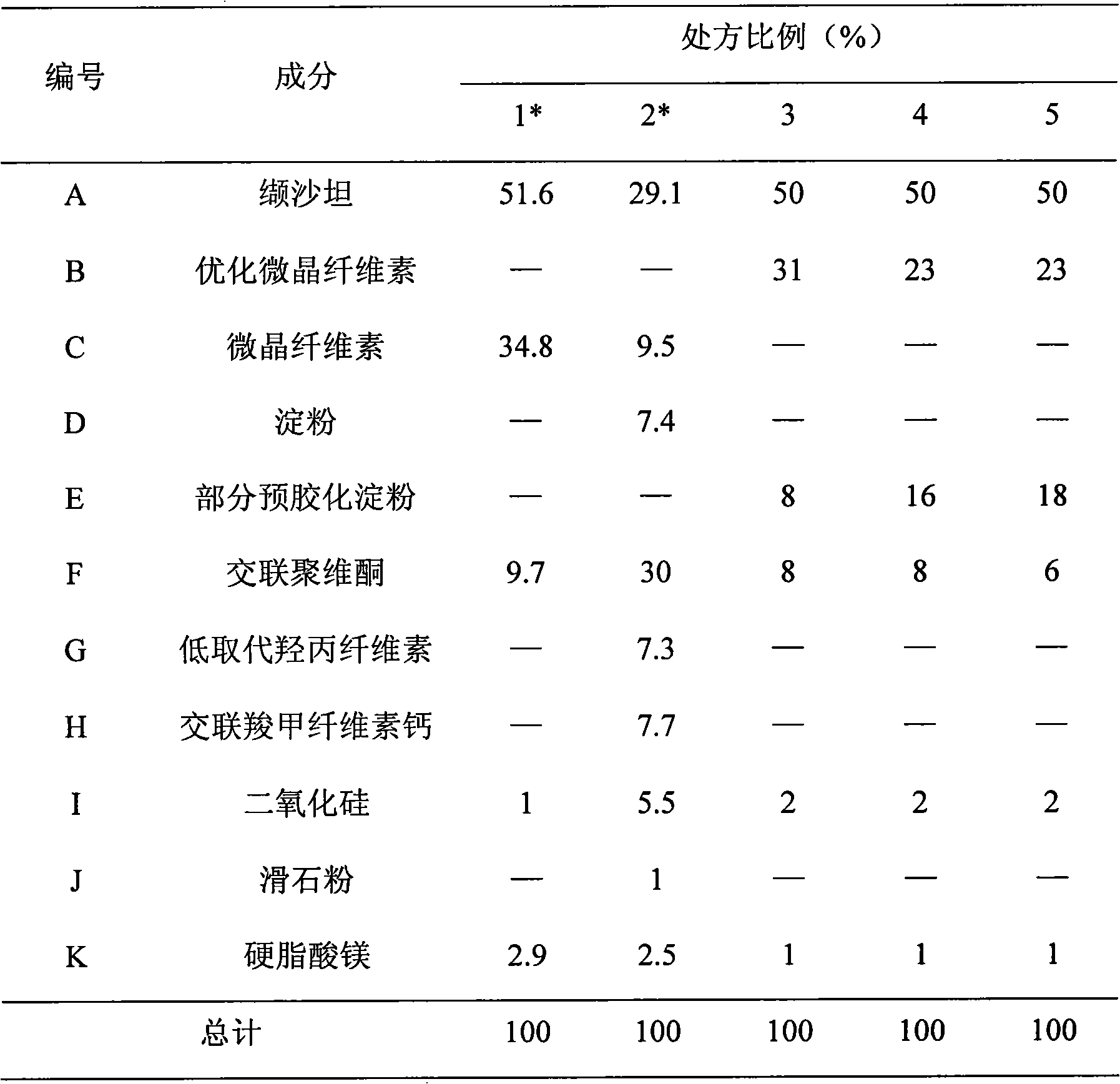
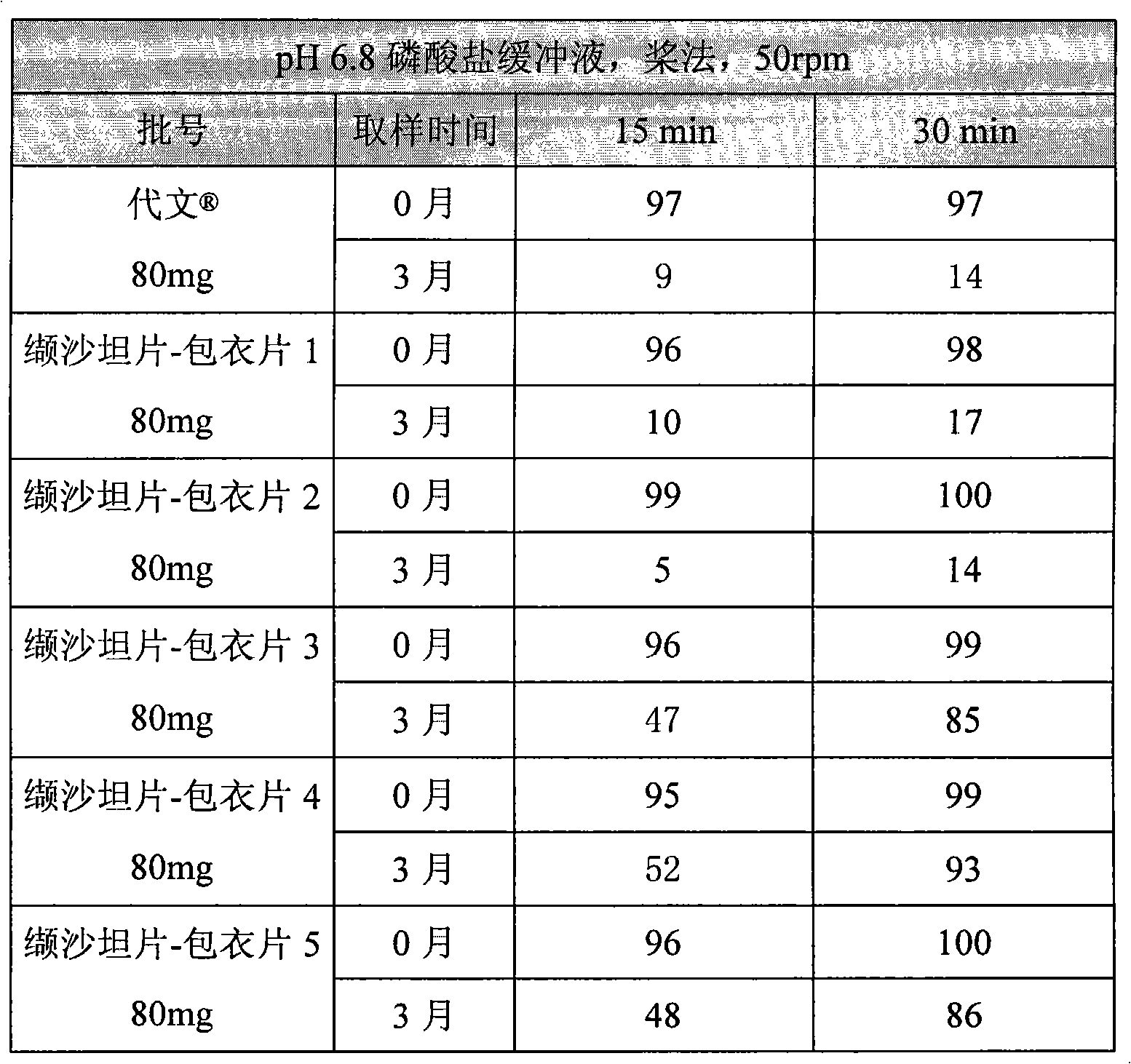
Diovan compound preparation and preparation method thereof
ActiveCN101485657AImprove liquidityNormal production processPharmaceutical product form changeCapsule deliveryValsartanBULK ACTIVE INGREDIENT
The invention discloses a valsartan compound preparation and a method for preparing the same. In the method, the valsartan or pharmacologically accepted salts of the valsartan and amlodipine or pharmacologically accepted salts of the amlodipine are used as active ingredients, and the active ingredients are pressed to prepare a compact by a rolling method; the compact is screened to prepare granules; and the granules are mixed with pharmaceutic adjuvants to prepare a tablet or a capsule. The method pretreats the active ingredients, so that materials have good fluidity; and the valsartan compound preparation and the method have the characteristics of simple process, low cost and suitability for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
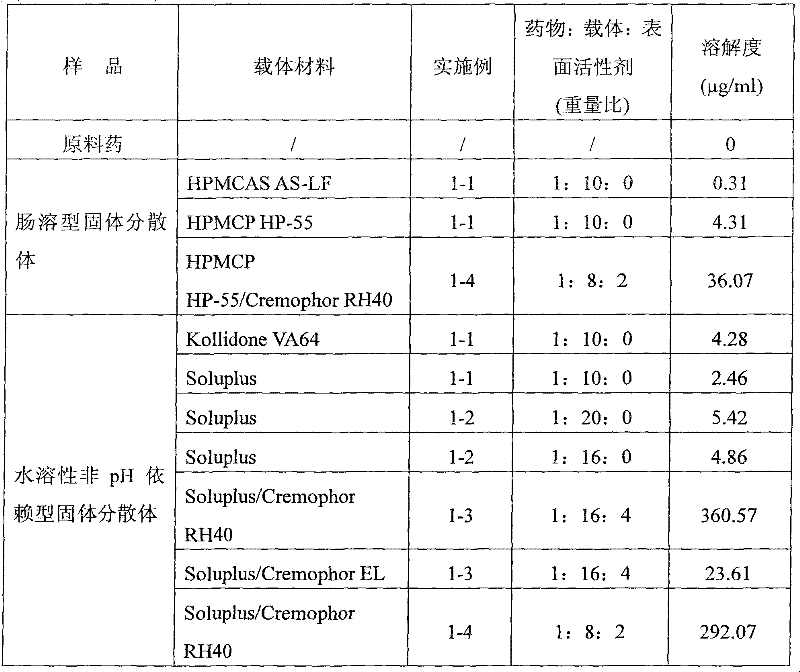
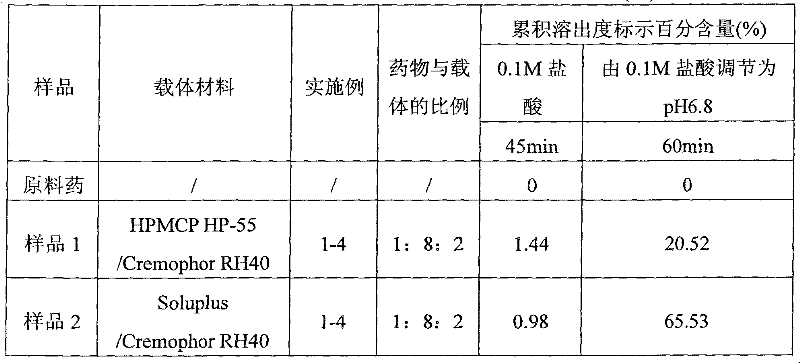
Enteric solid preparation containing lycopene, resveratrol or melatonin and preparation method of enteric solid preparation
The invention relates to the field of medical preparations, in particular to an enteric solid preparation containing lycopene, resveratrol or melatonin and a preparation method of the enteric solid preparation. The enteric solid preparation comprises one or more of lycopene, resveratrol and melatonin as an active ingredient, water-soluble and / or enteric carrier adjuvants or other pharmaceutic adjuvants. The water-soluble carrier adjuvants can be used as water-soluble solid dispersoid carriers; and the enteric carrier adjuvants are enteric polymers and can be used as enteric solid dispersoid carriers or enteric coating film materials. The lycopene, the resveratrol and the melatonin of the enteric solid preparation have favorable dissolubility in the intestinal tract, so that the medicament, namely, the enteric solid preparation, can be rapidly dissolved and released in the intestinal tract, and thus absorption and bioavailability of the lycopene, the resveratrol and the melatonin are increased. The enteric solid preparation containing the lycopene, the resveratrol and the melatonin can be suitable for application and industrial production of oral preparations, such as tablets, particles, pellets, capsules, enteric capsules, enteric coating tablets, enteric coating pellets, enteric coating particles and the like.
Owner:SINOTHERAPEUTICS
Preparation method of paraffin for pharmaceutic adjuvants
InactiveCN102533333AAchieve decolorizationAchieve deodorizationPetroleum wax refiningPetroleum wax recoveryChemical industryParaffin wax
The invention discloses a method for refining and deoiling paraffin for pharmaceutic adjuvants, and belongs to the field of medical materials. The method disclosed by the invention comprises the following steps of: refining hydrogenant paraffin which is used as a raw material by adopting an active substance so that the paraffin is effectively deodorized, de-colored and deodorized and the paraffin is odorless and tasteless; 'sweating' the paraffin by using a deoiling box so that the paraffin is deoiled and the oil content of the paraffin is reduced, wherein the active substance is active carbon, diatomite and / or active floridin; and the deoiling box is a device which enable the paraffin to be sweated according to a principle that heating is performed by using different melting points of the paraffin so that oil is separated to be infiltrated to the surface of the paraffin, and the deoiling effect of the deoiling box is that the oil content is reduced from 0.5 percent to 0.3 percent, so that the oil content is effectively reduced and the paraffin is difficult to oxidize. The invention aims to widen the application field of the paraffin, and widen the application field of the paraffin from food industry and daily chemical industry to pharmaceutic adjuvant industry; and the paraffin is mainly used as an ointment base hard-enhancing component and a sustained-release material in medicaments.
Owner:JIANGXI MASHAN CHEM
Oral solid composition of abiraterone and preparation method thereof
The invention relates to an oral solid composition of abiraterone and a preparation method thereof. The composition contains abiraterone acetate with a particle size of 1-30 micrometers and pharmaceutic adjuvants.
Owner:CHONGQING PHARMA RES INST
Method for preparing hydroxypropyl-beta-cyclodextrin
ActiveCN102040675AWill not cause decompositionLow impurity contentMacromolecular non-active ingredientsNanofiltrationPharmaceutic Adjuvant
The invention discloses a method for preparing hydroxypropyl-beta-cyclodextrin, which comprises the steps of etherification, neutralization, decoloring, nanofiltration, resin purification, and spray drying. The method of the invention is small in pollution, and the yield in weight is more than 80 percent; meanwhile, the product prepared by using the method of the invention is narrow in substituted ratio range and low in impurity content, and can be used as a pharmaceutic adjuvant.
Owner:石药集团中诺药业(石家庄)有限公司
Parenteral preparation containing active entecavir and preparation method thereof
The invention relates to a parenteral preparation containing active entecavir and a preparation method thereof. The invention is characterized in that the parenteral preparation comprises the active entecavir, xylitol, water-soluble filler, pH regulator, water for injection and osmo-regulator, wherein the active entecavir is 0.001 percent to 30 percent by weight, and the balance is pharmaceutic adjuvant or water. The parenteral preparation has the advantages of good stability and high bioavailability, thus solving the defects of the oral administration of entecavir in treating the acute exacerbation of viruses. The parenteral preparation is particularly suitable for treatment on the acute exacerbation of hepatitis of a diabetic.
Owner:TIANJIN PACIFIC PHARMA
Application of silicon dioxide aerogel in pharmacy
ActiveCN102961750AAchieve scaleShorten the development processOrganic active ingredientsPowder deliveryPharmacyAdjuvant
The invention discloses application of silicon dioxide aerogel in pharmacy, relates to application of the silicon dioxide aerogel, and in particular relates to application of silicon dioxide aerogel used as a nanoparticle medicine carrying system in the pharmacy. A new pharmaceutic adjuvant is searched in the field of pharmacy, and is not a nanoparticle material or nano-powder which is fashionably used in the present day, so that a medicine carrying hollow new structure is truly realized, the physical medicine carrying scale below 100 nanometers that any one of the adjuvants in the modern pharmaceutic adjuvant cannot realize is achieved, and the blank of the nanoscale pharmaceutic adjuvant in the international countries or the domestic countries is made up.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Cefdinir dispersible tablet and preparation method thereof
InactiveCN101352424AImprove solubilityLarge distribution areaAntibacterial agentsOrganic active ingredientsCefdinirPharmaceutic Adjuvant
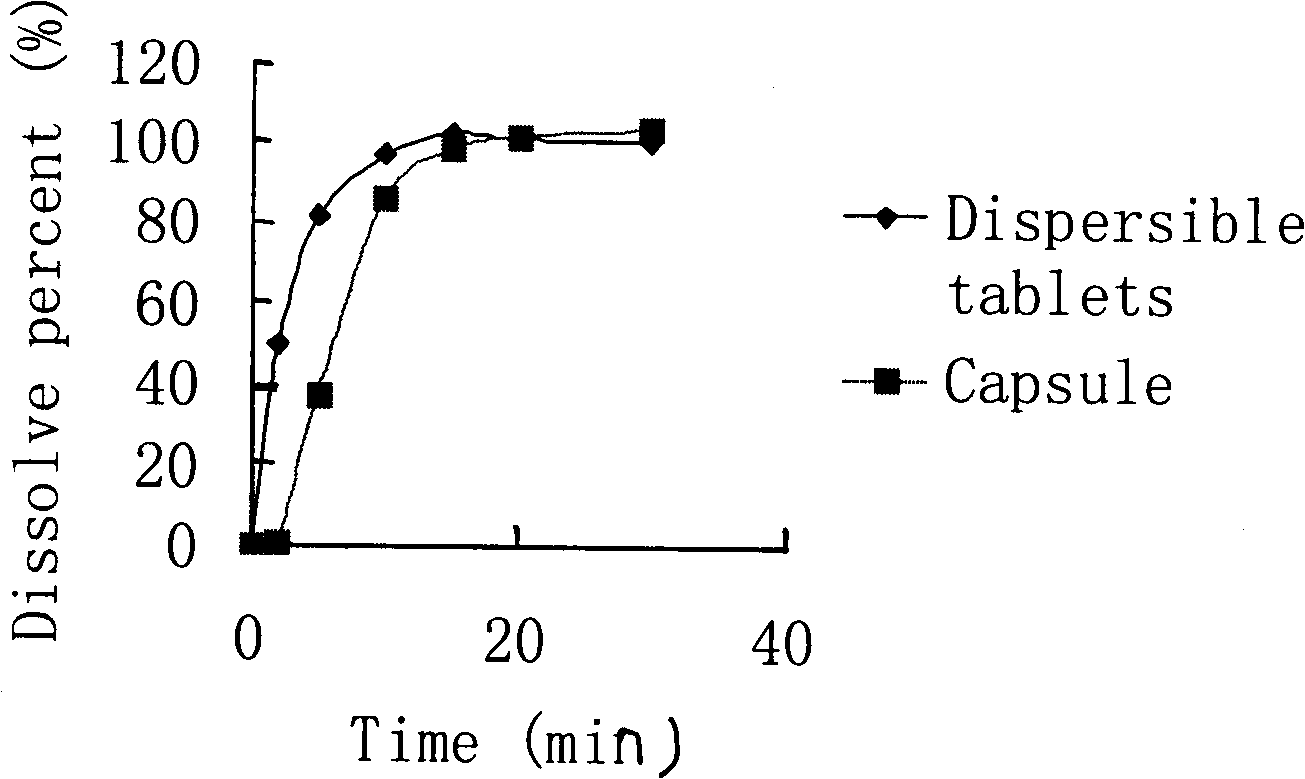
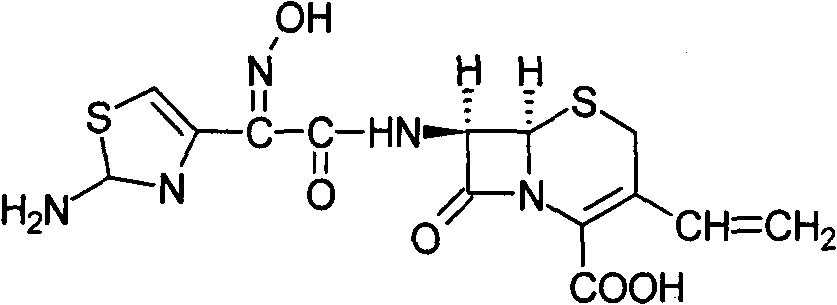
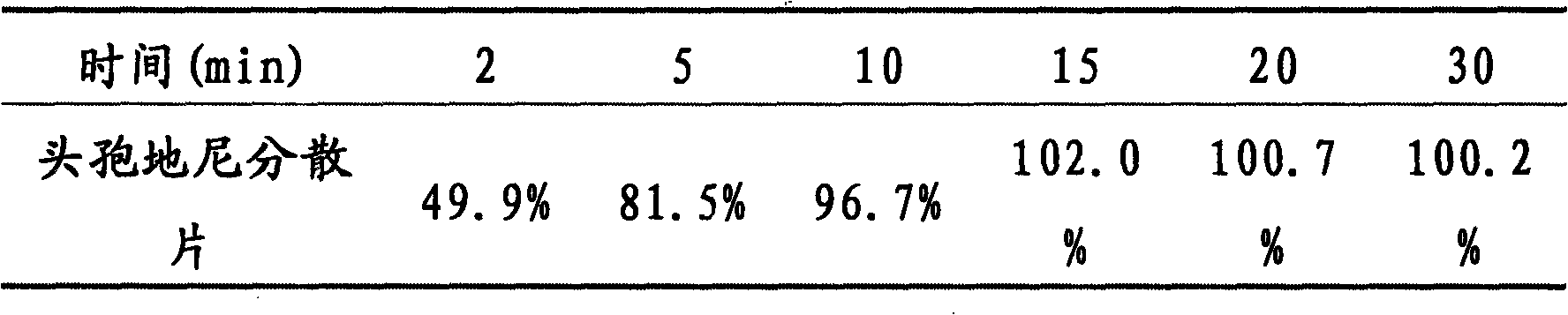
The invention provides a cefdinir dispersible tablet and a preparation method thereof, and the cefdinir dispersible tablet contains cefdinir with effective dose and pharmaceutic adjuvant which includes disintegrant and disintegrant-promoting aerosol; in every 100 parts of cefdinir, the dosage of the disintegrant is 2-60 parts, and the dosage of the disintegrant-promoting aerosol is 0.1-45 parts; after being taken orally, the cefdinir dispersible tablet of the invention can quickly disintegrate, and the cefdinir which is the active ingredient of the dispersible tablet has the accumulating dissolution rate of over 102.0% within 15 minutes. Compared with other medicines, the accumulating dissolution rate of cefdinir capsule is 98.3%. The cefdinir dispersible tablet are evenly dispersed into fine particles, so as to have remarkable disintegration and leachability capacity compared with the compressed tablets, realize the rapid absorption of medicine, the function of rapid onset, and improve the bioavailability of human body.
Owner:TIANJIN CENT PHARM CO LTD
Valsartan-containing solid preparation and preparation method thereof
ActiveCN101829111AIncrease humidityImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryValsartanMedicine
The invention relates to a valsartan-containing medicine preparation which at least comprises valsartan or pharmaceutically acceptable salt thereof that is taken as active ingredient, and at least two disintegrating agents, wherein the proportion of the two disintegrating agents is within the range of 1:2-2:1. The invention also relates to a preparation method of the valsartan-containing medicine preparation, which comprises the steps of: first, pelletizing the valsartan or the pharmaceutically acceptable salt thereof by a roller press way, and then mixing with the at least two disintegrating agents and other pharmaceutic adjuvant; and finally, tabletting or filling into capsules. Compared with the prior art, the valsartan-containing medicine preparation has better stability. The method has simple technique and high production efficiency, and is suitable for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
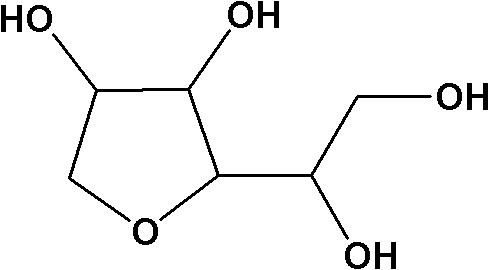
Preparation method of high-purity 1,4-sorbitan
Owner:NANJING WELL BIOCHEM
Preparation method of high-purity lecithin
InactiveCN102924506AHigh yieldHigh recovery ratePhosphatide foodstuff compositionsChromatographic separationNatural product
The invention belongs to the technical field of separation and extraction of natural products, and particularly relates to a preparation method of high-purity lecithin. The method is applied to the preparation of pharmaceutical high-purity lecithin. The invention provides a large-scale preparation and production method of high-purity lecithin for pharmaceutic adjuvant injection, adopts a simulated moving bed continuous chromatographic separation technology to ensure that lecithin is effectively separated from other constituents in material lipid, so as to obtain high-purity lecithin products, can also realize the recovery of residual products and the recycle of solvent, avoids the unreasonable consumption and waste of raw material and auxiliary material, completely eradicates the producing of wastewater and waste residue, ensures that the product yield and the product purity are more than 98 percent, ensures that the quality of the lecithin products meets the technical requirements of pharmaceutic adjuvant, greatly reduces the production cost, realizes the cleanability and no pollution of the lecithin production, and has a wide application prospect.
Owner:JIANGNAN UNIV +1
Paclitaxel freeze drying microemulsion for injection and method of producing the same
InactiveCN101428002ASimple manufacturing processStructure remains intactOrganic active ingredientsPowder deliverySide effectFreeze-drying
The invention provides taxol freeze dried micro emulsion used for injection and a preparation method thereof. The freeze dried micro emulsion comprises taxol in effective dosage as the drug and pharmaceutic adjuvant such as oil phase, an emulsifier, an assistant emulsifier, a pH regulator, isotonic regulator and a freeze dried protective agent. The preparation method comprises the following steps: weighing the emulsifier in the amount according to the prescription, after evenly stirring the assistant emulsifier and oil for injection, adding the taxol into the mixed liquor and stirring for complete dissolution, then adding water for injection, completely stirring, obtaining taxol micro emulsion after regulating the pH value, further adding the freeze dried protective agent, and obtaining the freeze dried micro emulsion after vacuum freeze drying. Micro emulsion can be rapidly recovered by using physiologically compatible solution such as normal saline, glucose solution or Ringer's solution for dilution before use. The freeze dried micro emulsion and the preparation method are characterized in that through the further freeze drying technique to the taxol micro emulsion, the stability and the efficacy of the taxol are obviously enhanced, and the toxic and side effects are greatly reduced; especially, the freeze-drying method realizes very good protection function to the micro emulsion and avoids effusion of drug, thereby breaking through a new prospect for the establishment of stable dose pattern of a taxol micro emulsion drug loaded system and long-term preservation thereof. The preparation method for the freeze dried micro emulsion has the advantages of simple preparation technique, no organic solvent and low cost, therefore, the invention is suitable for industrialized mass production.
Owner:李淑斌
Preparation process for ultrafine glibenclamide particles
InactiveCN102397257ASimple processEasy to operateMetabolism disorderSulfonylurea active ingredientsSlurryGlibenclamide
The invention discloses a preparation process for ultrafine glibenclamide particles, which belongs to the field of micronization of drugs. The process comprises the following steps: preparing an organic solution of glibenclamide; dissolving pharmaceutic adjuvants in water to form an aqueous solution and controlling the temperature of the aqueous solution to be 2 to 50 DEG C; mixing the above mentioned two solutions to prepare medicinal slurry, carrying out spray drying on the medicinal slurry, and controlling inlet temperature to be 100 to 170 DEG C, outlet temperature to be 60 to 95 DEG C, a feeding speed to be 5 to 40 ml / min and compressed air pressure to be 0.4 to 0.8 MPa so as to obtain ultrafine glibenclamide powder. The ultrafine powder provided in the invention has good stability, and more than 85% of the powder can be dissolved within 2.5 minutes; the process is simple and is easy to operate.
Owner:BEIJING UNIV OF CHEM TECH
Chinese medicine composition for treating neurasthenia
ActiveCN102526474AEasy to manufactureSignificantly nourishes yin and promotes body fluidNervous disorderPlant ingredientsCelluloseSide effect
The invention relates to a Chinese medicine composition for treating neurasthenia and preparation process of the Chinese medicine composition. The Chinese medicine composition is prepared by the following raw materials: astragalus mongholicus, fructus lycii, poria cocos, cocklebur fruit, herba epimedii, polygala tenuifolia, angelica sinensis, Chinese date, schisandra chinensis, bighead atractylodes rhizome, spina date seed, radix ophiopogonis, Chinese yam, elecampane, cortex albiziae, liquorice, prepared rehmannia root, ginseng, cortex moutan and pinellia ternate. The raw materials are prepared into forms of drugs including capsules, tablets, particles and the like after pharmaceutic adjuvant drugs including starch edible cellulose are added into the raw materials according to a normal preparation method. The Chinese medicine composition for treating the neurasthenia can treat both principal and secondary aspects of the neurasthenia, is free of toxic, harmful and side effect, low in cost and simple and convenient to manufacture, and has wide application prospect.
Owner:南通康威尔生物化工有限公司
Colchicines gastric floating sustained-release tablet and method for preparing same
InactiveCN101536990ASchematic diagram of the preparation processOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a colchicines gastric floating sustained-release tablet, which comprises active components of colchicines and pharmaceutic adjuvant according to the weight ratio of 1:24-1,999, wherein the pharmaceutic adjuvant comprises a hydrophilic gel framework material, a effervescing agent, a floating assistant material, a filler, a pH value regulator and a lubricant. The colchicines gastric floating sustained-release tablet can swell quickly in gastric juice or a similar gastric juice medium and can float on the gastric juice for at least 4 hours. The invention also relates to a method for preparing the colchicines gastric floating sustained-release tablet. The colchicines gastric floating sustained-release tablet can reach the effective blood-drug concentration quickly after being taken and then release drugs slowly, and can maintain the balanced blood-drug concentration so as to reduce the dose times, relieve the toxic side effect and improve the bioavailability.
Owner:普尔药物科技开发(深圳)有限公司
Colchicine framework controlled release tablets or capsules
InactiveCN101485637AStable release rateHigh Cumulative Release RateOrganic active ingredientsSkeletal disorderHydrophilic polymersSide effect
The invention discloses a colchicine framework controlled-release tablet or capsule, which comprises colchicines and framework materials having controlled-release function, wherein the framework materials contain hydrophilic polymers of which the weight against the weight of the tablet is at least 20 percent and other pharmaceutic adjuvants; and the tablet or capsule comprises the following components in percentage by weight: 0.01 to 1.0 percent of colchicine, 20 to 85 percent of hydrophilic polymer, 1 to 30 percent of retarder, 20 to 60 percent of bulking agent, and 0.1 to 2 percent of lubricant. Release degree test results show that more than 90 percent of drugs are continuously released in vitro in 12 hours; and compared with the common tablet, the tablet or capsule has the advantages of obviously reducing daily taking frequency, lowering toxic and side effects of the drugs and improving the compliance and curative effect of patients.
Owner:普尔药物科技开发(深圳)有限公司
Freeze dried powder injection of hirudin and its preparation method
InactiveCN1569226AHigh purityGood curative effectPowder deliveryPeptide/protein ingredientsPurification methodsFreeze-drying
The invention discloses a freeze dry injection of hirudin and its preparation process. It is characterized in that hirudin is extracted from fresh leech, pharmaceutic adjuvant can be added or not added after purification to prepare freeze dry injection formulation of hirudin, the character also lies in not adopting any organic solvent in the preparation method, but adopting purification methods of ultra filtration, ion exchange column chromatography and reverse osmosis condensation to get hirudin with comparative highly purity. The pharmacody experiment result indicates that the freeze dry injection has a favorable pharmacological action.
Owner:北京干露春科技有限公司
Medicine composition containing salvianolic acid A, preparation method and application thereof as well as freeze-dried powder injection and water injection containing composition
InactiveCN101596182AGood water solubilityImprove stabilityPowder deliveryOrganic active ingredientsDiseaseDistillation
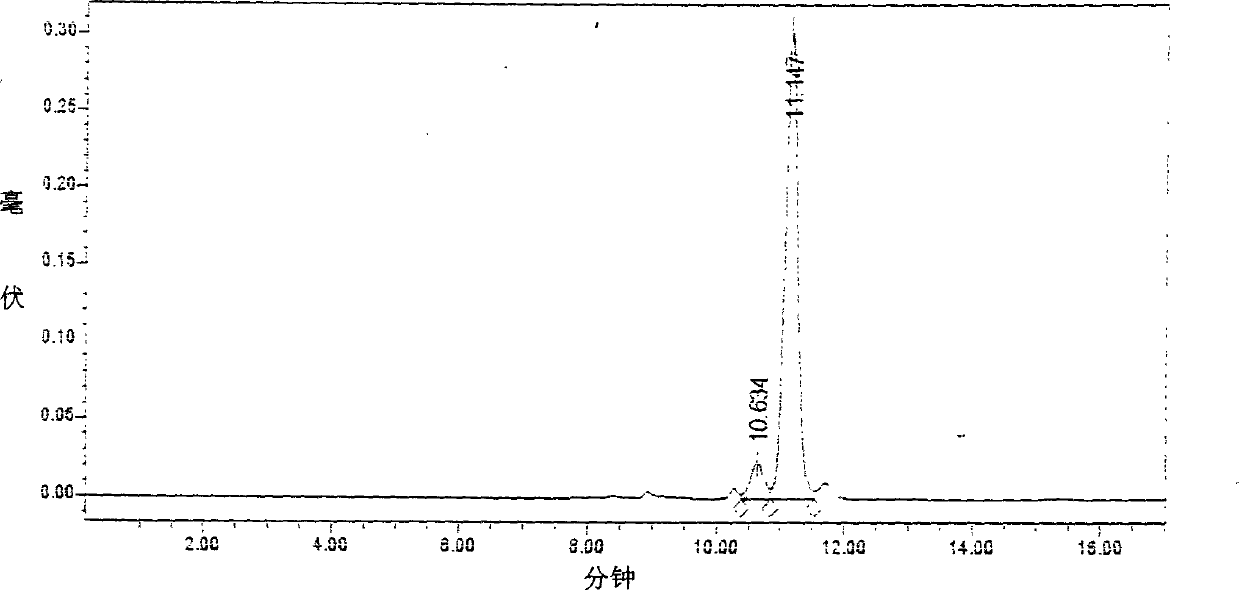
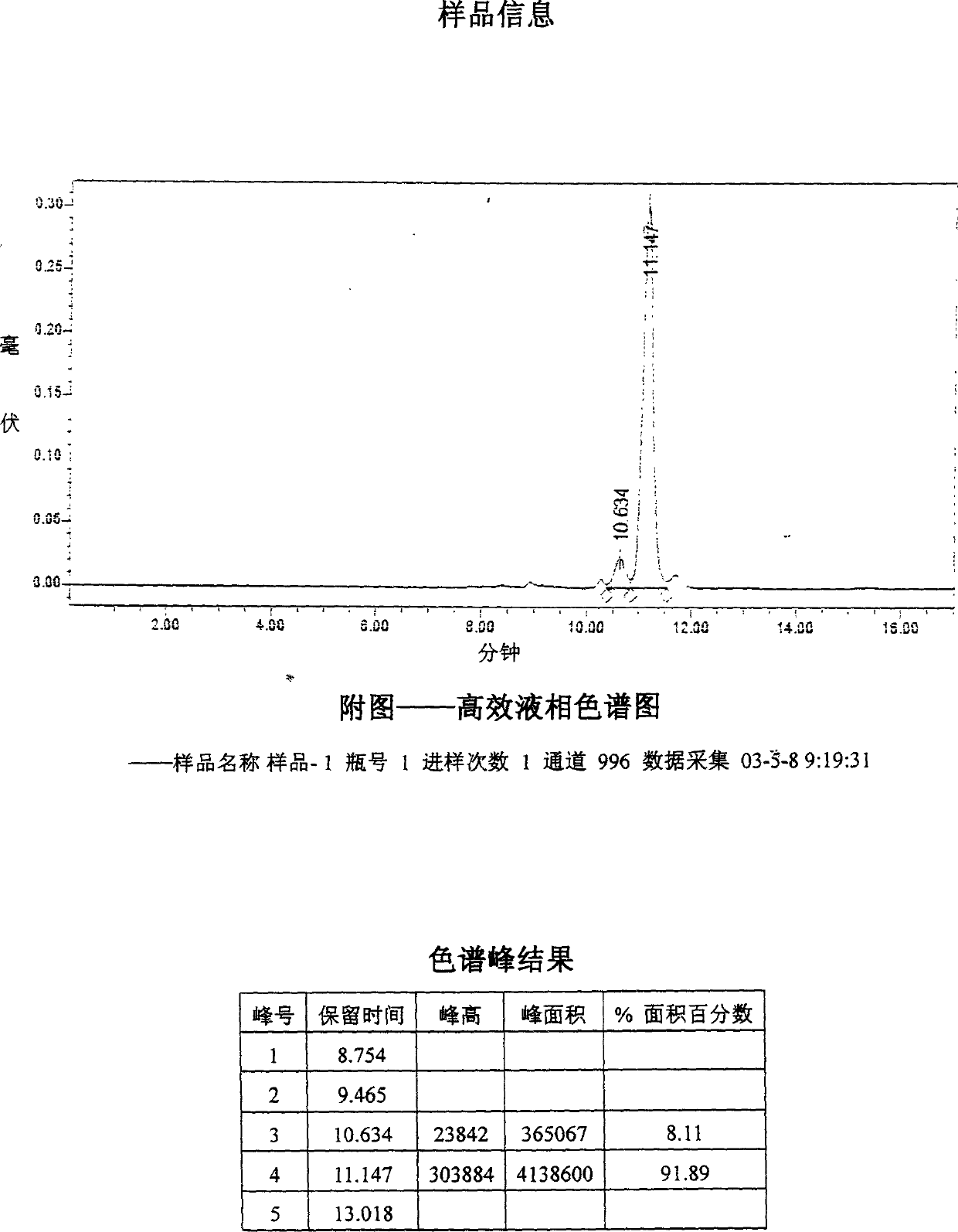
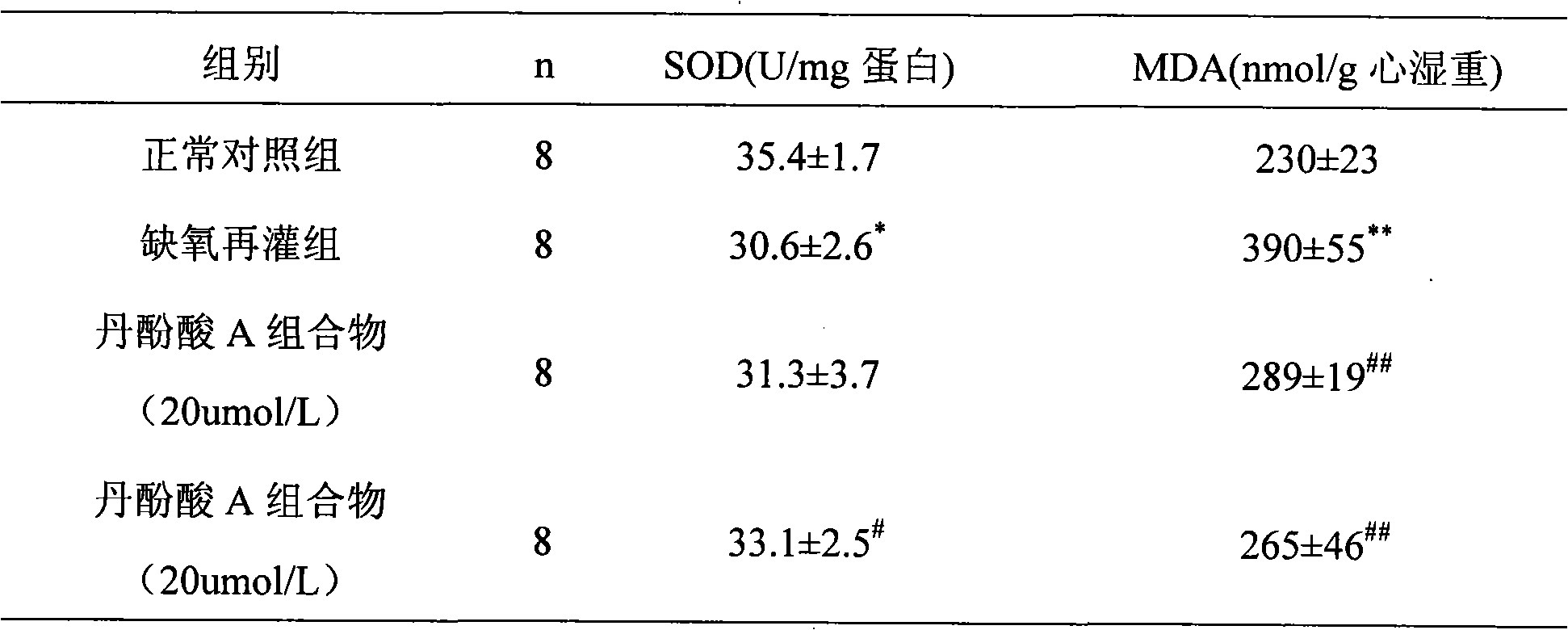
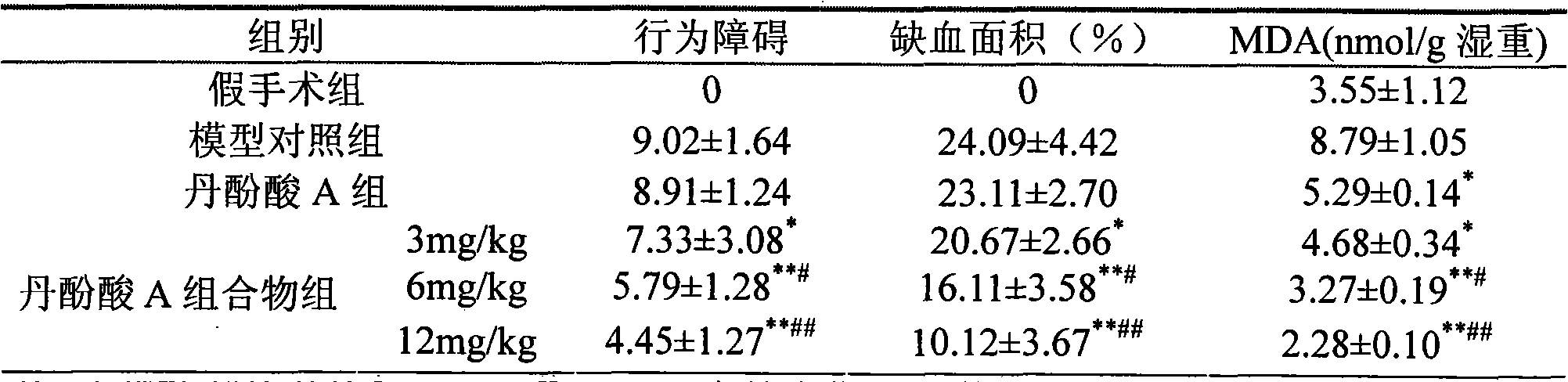
The invention relates to a medicine composition containing salvianolic acid A, the application and a preparation method thereof as well as freeze-dried powder injection and water injection containing the composition. The preparation method of the medicine composition comprises the following steps: taking salvianolic acid A to dissolve or disperse into water or ethanol, taking one or a plurality of alkaline sodium salt and alkaline kali salt to dissolve into water, mixing and fully stirring two solutions to be settled completely, and generally freeze-drying or decompression-drying by distillation to obtain a finished product, or directly adding water solution into pharmaceutic adjuvant to make the freeze-dried powder injection or water injection. The salvianolic acid A composition provided by the invention has strong stability, high dissolubility and uneasy oxidation in the water solution; the preparation method has good manufacturability, simple operation and low cost and can be used for preparing medicines for curing ischemic cardiovascular and cerebrovascular diseases; and the freeze-dried powder injection and water injection containing the salvianolic acid A have good stability and high uniformity and are accordant with requirements of medicines.
Owner:YANTAI TARGET DRUG RES
Preparation and application of pharmaceutic preparation of 11-carbonyl-betal- acetyl mastic acid and derivatives thereof extracted from frankincense
The invention discloses a preparation and an application of a pharmaceutic preparation of 11-carbonyl-betal- acetyl mastic acid and derivatives thereof extracted from frankincense, and the preparation is characterized by comprising the following steps: extracting, separating and purifying to obtain the 11-carbonyl-betal-acetyl mastic acid with the purity of 99% from the frankincense; and mixing the compound or the derivatives thereof with pharmaceutic adjuvants to prepare the pharmaceutically acceptable solid preparation and injection preparation. The pharmacological test result shows that the pharmaceutic preparation of the 11-carbonyl-betal-acetyl mastic acid and the pharmaceutic preparation of the derivatives thereof have obvious functions of analgesia, anti-inflammation, immunity regulation and the like, and can be applicable for treating patients suffering from rheumatic and rheumatoid arthritis.
Owner:凌沛学
Nasal gel or ointment preparation for preventing and/or treating aspiration allergy
InactiveCN102764230AAvoid accessEliminate or relieve allergy symptomsAerosol deliveryOintment deliveryNasal cavityExtensibility
The invention relates to a nasal preparation for preventing and / or treating aspiration allergy. With white vaseline as a matrix, the nasal gel or ointment preparation provided by the invention contains coating-extensibility pharmaceutic adjuvants required for adjustment of the gel or ointment preparation. When the nasal gel or ointment is smeared on the inner wall of nasal cavity, a layer of protective film, like an invisible mouth mask, is formed in the nasal cavity, thus effectively blocking inhalant allergens (such as pollen in the air and the like) from entering human body through the nasal cavity. Thereby, allergy symptoms are eliminated or alleviated, and the nasal gel or ointment containing drug components further can simultaneously play the role of drug treatment as well.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Health care product for relieving alcoholic liver
The invention discloses a health care product for relieving alcoholic liver, which comprises L-arabinose and mixed bacteria, and also comprises a food additive and / or pharmaceutic adjuvant. The problem of poor effect of relieving the alcoholic liver in the health care product for sobering up and protecting liver in the prior art is solved, therefore, the health care product for relieving the alcoholic liver, with remarkable effect for relieving the alcoholic liver, is provided.
Owner:SHENGQUAN HEALTANG
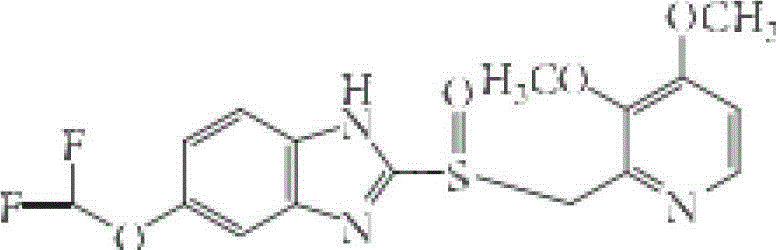
Enteric-coated tablet of S-pantoprazole or salt of S-pantoprazole, and preparation method thereof
ActiveCN102871980AAccurate doseEasy to carryOrganic active ingredientsDigestive systemChemistryPharmaceutic Adjuvant
The invention relates to an enteric-coated tablet of S-pantoprazole or salt of the S-pantoprazole, and a preparation method thereof, wherein the enteric-coated tablet comprises the components: a) a tablet core formed by the S-pantoprazole or the salt of the S-pantoprazole and pharmaceutic adjuvant; b) an isolating layer; and c) an enteric layer. The tablet core of the S-pantoprazole or the salt of the S-pantoprazole is formed by directly tabletting full powder, so that the damage of high temperature to the S-pantoprazole or the salt of the S-pantoprazole in the process of drying water and granules during wet granulation can be effectively avoided, and the stability of the preparation is improved. Furthermore, the isolating layer and the enteric layer have less composition, so that the preparation is simple and convenient. According to the invention, the preparation technology is simplified and the production cost is reduced; and the prepared S-pantoprazole enteric-coated tablet is better in dissolution rate and good in stability.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method for pharmaceutic adjuvant--magnesium stearate
InactiveCN103193614AImprove quality indicatorsThe production process conditions are easy to controlCarboxylic acid salt preparationSodium stearateMagnesium stearate
The invention provides a preparation method for a pharmaceutic adjuvant--magnesium stearate. The preparation method comprises the following two steps: step 1, subjecting stearic acid and sodium hydroxide to a saponification reaction; and step 2, reacting sodium stearate produced by stearic acid and sodium hydroxide with magnesium sulfate so as to produce the magnesium stearate. The pharmaceutic adjuvant magnesium stearate produced by using the method provided by the invention has stable quality indexes, and in particular, the content of MgO accords with requirements prescribed in Chinese Pharmacopoeia (2010 edition); production process conditions are easily controllable, and the produced magnesium stearate has a good color.
Owner:郑桂富
Pharmaceutical combination with repaglinide and metformin as active components and preparation method thereof
InactiveCN102218064AEasy to recycleAchieve separationOrganic active ingredientsOrganic chemistryAdhesiveActive component
The invention relates to a pharmaceutical combination with repaglinide and metformin as active components and a preparation method thereof. The pharmaceutical combination comprises 0.1-10 weight portions of repaglinide and 100-1500 weight portions of metformin as active components and pharmaceutic adjuvants, wherein, the pharmaceutic adjuvants comprise filler, disintegrating agent, adhesive, flavoring agent, lubricant and swelling accessory, and the repaglinide is repaglinide crystal. The invention adopts the repaglinide crystal with small particle size, metformin and pharmaceutic adjuvants to prepare the pharmaceutical combination, so as to realize the purpose of simultaneous release. Since the particle size of the repaglinide used in the invention is small, the dissolvability is improved, so that the dissolution is improved and the bioavailability is increased.
Owner:HAINAN JINRUI PHARMA CO LTD
Medicinal composition for resisting thrombotic diseases, preparation method thereof and application thereof
Owner:BEIJING SIHUAN PHARMA
Preparation method of decitabine freeze-dried powder injection
InactiveCN101843592ALow content of related substancesSafe and non-flammableOrganic active ingredientsPowder deliveryAdjuvantOrganic solvent
The invention discloses a preparation method of a decitabine freeze-dried powder injection, which is characterized by comprising the following steps: adding a pharmaceutic adjuvant into water and dissolving; adjusting the pH value to be 6.0-8.0 to obtain adjuvant solution; refrigerating the adjuvant solution to 0-10DEG C for later use; adding decitabine into the adjuvant solution and dissolving; keeping the liquid medicine at 0-10DEG C; adjusting the pH value to be 6.0-8.0; and adding active carbon, filtering, filling and freeze-drying to obtain the decitabine freeze-dried powder injection. In the process method, an organic solvent is not added and is replaced by water; and by controlling the pH value and the temperature, the contents of relative substances of the obtained preparation are low.
Owner:连云港杰瑞药业有限公司
Mulberry leaf chewing tablet with sugar-lowering function
The invention discloses a mulberry leaf chewing tablet with sugar-lowering function. The chewing tablet is characterized by being made from the following raw materials by weight: 1-10 parts of a fine powder extract, 10-80 parts of a pharmaceutic adjuvant and 1-30 parts of xylitol, wherein, the fine powder extract is the mulberry leaf fine powder extract containing not less than 20% of total flavonoid and is obtained by the following steps: decocting dried and cleaned mulberry leaves in water; condensing and centrifuging the obtained decoction at a high speed; adsorbing the obtained centrifugate with resin; eluting with 85% ethanol and performing alcohol precipitation; drying in vacuum after precipitation and dehydration; smashing, sieving and drying. The mulberry leaf chewing tablet can effectively control blood sugar level increase without being metallization by insulin, and provides a good clinical drug for diabetics.
Owner:TIANJIN JINGUIGU XYLITOL
Anti-cancer medicine composition containing antimetabolite
InactiveCN1634584AAntineoplastic agentsHeterocyclic compound active ingredientsWhole bodyTherapeutic effect
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and anti-metabolism medicine is disclosed. Wherein, the anti-metabolism medicine can effectively destroy DNA and / or protein synthesis and repairing function inside the tumor cell so as to inhibit the tumor cell growth, while the pharmaceutic adjuvant can mainly be biological compatible, degradable and absorbable macromolecule polymer, which can make the anti-metabolism drug to release slowly in the local tumor region in the degradation and absorption process, therefore it can both decrease considerably the whole body toxic reaction and sustain the local tumor effective drug level.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com