Patents
Literature
4022 results about "Magnesium stearate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Magnesium stearate is the chemical compound with the formula Mg(C₁₈H₃₅O₂)₂. It is a soap, consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg²⁺). Magnesium stearate is a white, water-insoluble powder. Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics.
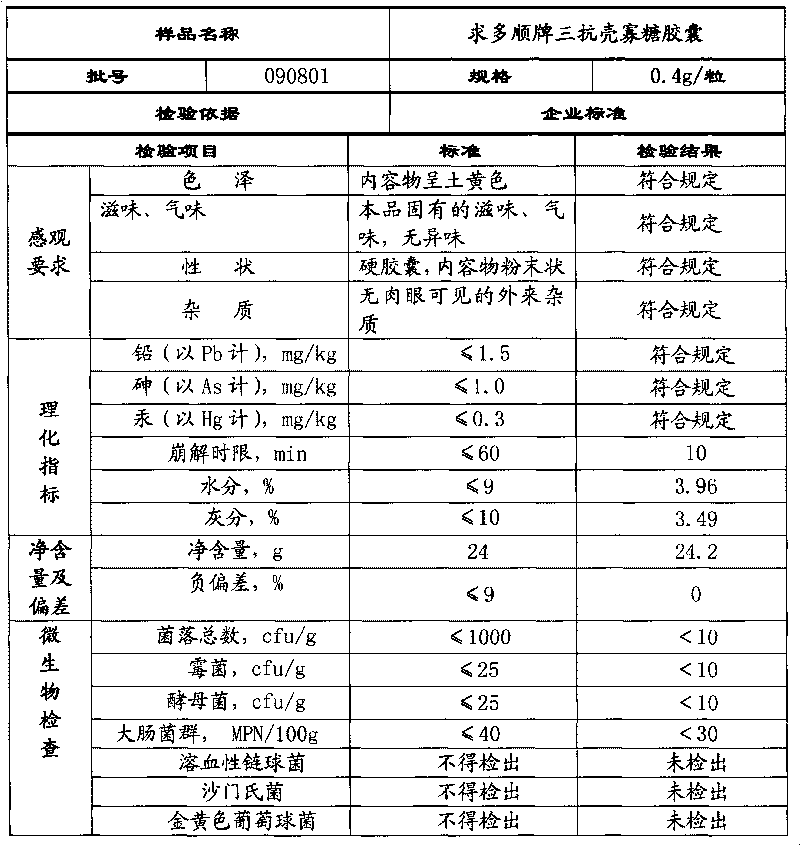
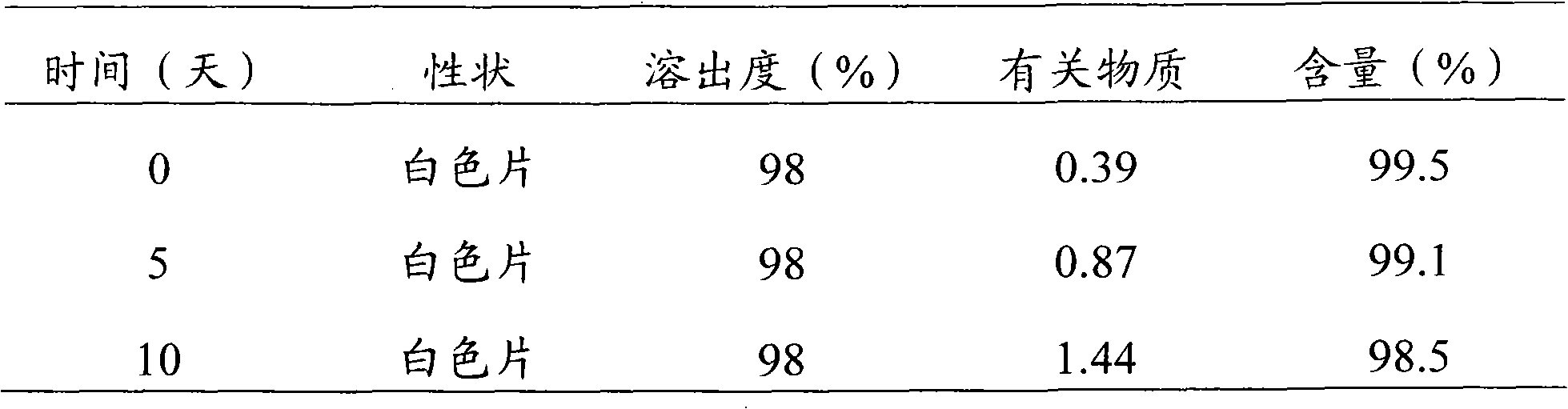
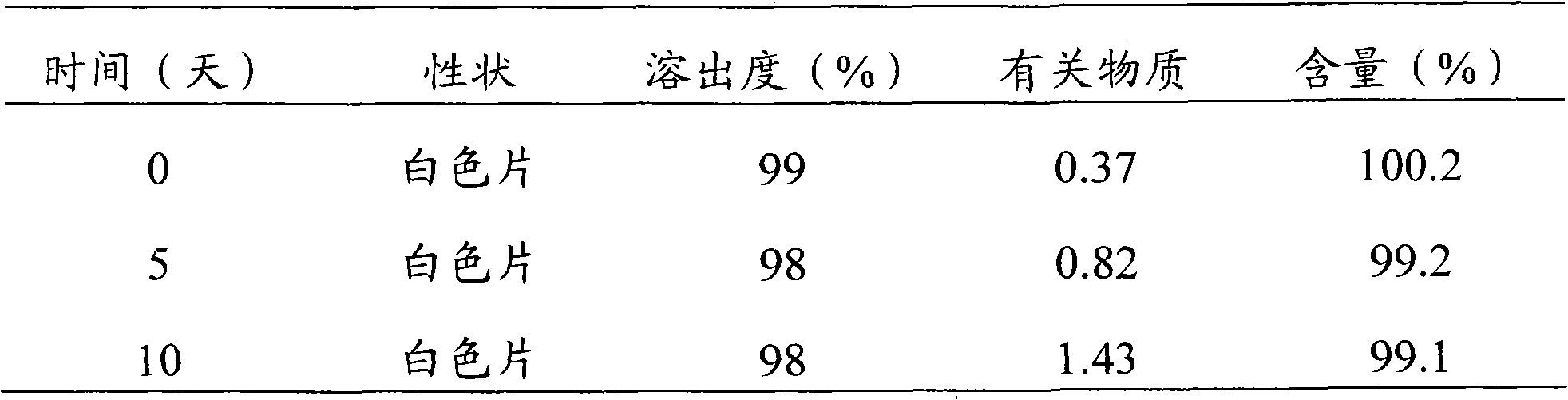
Rizatriptan benzoate capsule and preparation method thereof
ActiveCN101984960AImprove bioavailabilityKeep it workingOrganic active ingredientsNervous disorderMedicineMagnesium stearate
The invention discloses a medicinal preparation rizatriptan benzoate capsule for treating migraine and a preparation method thereof. Every 10000 capsules are prepared from the following formulation constituents: 29-250g of rizatriptan benzoate, 20-400g of microcrystalline cellulose, 220-1100g of starch, 100-400ml of 10-20% polyvinyl pyrrolidone ethanol solution and 4-30g of magnesium stearate. The invention also provides a preparation method of the rizatriptan benzoate capsule. The capsule of the invention covers the foreign taste of the main medicine; release and absorption of the medicine are accelerated; medical particles in the capsule are not punched or molten, thereby overcoming some defects of tablets; after the capsule is taken orally, the medicine is directly dispersed in the gastrointestinal fluid and is moistened by the gastrointestinal fluid, thus the effective surface area is large; and the medicine has higher dissolving rate in the gastrointestinal fluid, can be better absorbed and has high bioavailability.
Owner:四川梓橦宫药业股份有限公司
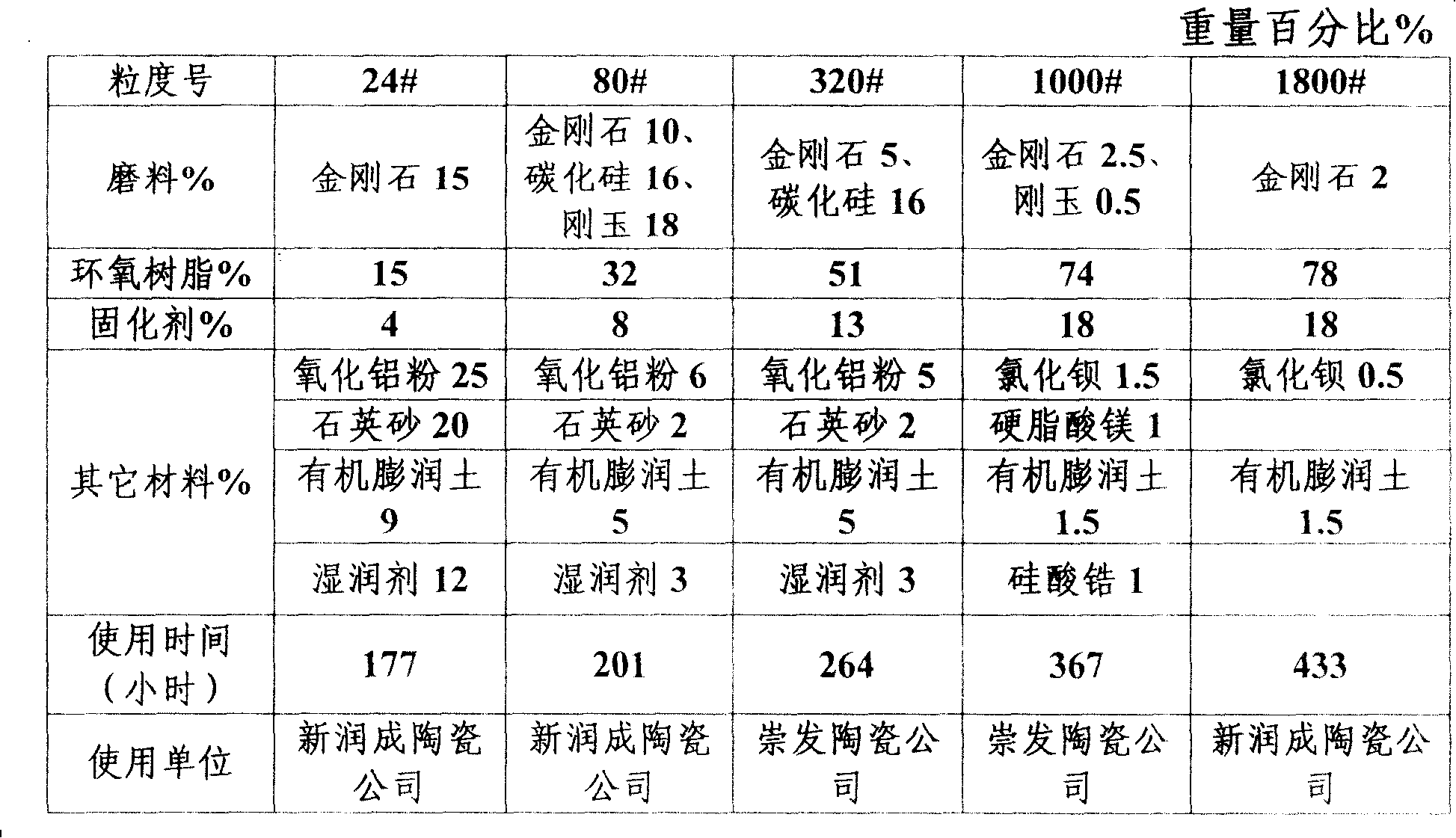
Abrasive tool of epoxy resin combined with compounded abrasive
Disclosed is an abrasive tool made of epoxy resin-bonded compound abrasive material, which comprises epoxy resin, abrasive material and curing agent. The weight percentages are as follow: the epoxy resin is 15 to 78 percent, the abrasive material is 2 to 44 percent and the curing agent is 4 to 18 percent. Wherein, the abrasive material comprises one of diamond, silicon carbide or corundum or the combination of more than one material. The curing agent comprises one of 4.4`-methylenedianil, solid diaminodiphenyl methane or liquid modified diaminodiphenyl methane or the combination of more than one material. The component of the abrasive tool also comprises the other auxiliary material, which comprises one of alumina, barium chloride, sodium chloride, quartz sand, organobentonite, wetting agent, zirconium silicate, calcium carbonate, graphite powder, garnet, magnesium sulfate, phenolic resin, zinc stearate and magnesium stearate, or the combination of more than one material. The product can be made into any shape the same with the present product, which can be applied in various grinding and polishing equipment with wide application scope.
Owner:广东奔朗新材料股份有限公司
Xanthophyll micro-capsule and its preparation method
InactiveCN101288662AHydroxy compound active ingredientsAntinoxious agentsAntioxidantMagnesium stearate
The invention relates to a xanthophylls microcapsule and a preparation method thereof. The core material of the microcapsule is xanthophylls and the wall material is natural macromolecule chitosan or glutin, gum acacia and antioxidant. The technique has the following steps: the xanthophylls is dissolved in an organic solvent, which is added into a gum acacia solution containing emulsifier and antioxidant for uniform emulsion; the well emulsified gum acacia is added into the chitosan or glutin solution for being condensed into capsules, and the capsules are processed with magnesium stearate after solidification; the microcapsules with excellent dispersivity are obtained after drying. The microcapsule prepared by the technique has the grain diameter of 1-200 Mum. The preparation technique has simple process, mild preparation condition and easy popularization; the microcapsule prepared has good dispersivity and high stability and is suitable for industries such as food, health care products, feed, etc.
Owner:UNIV OF JINAN
Dry powder for inhalation
InactiveUS7186401B2Reduce sensitivityMinimize impactBiocidePowder deliveryInhalable particlesInhalation
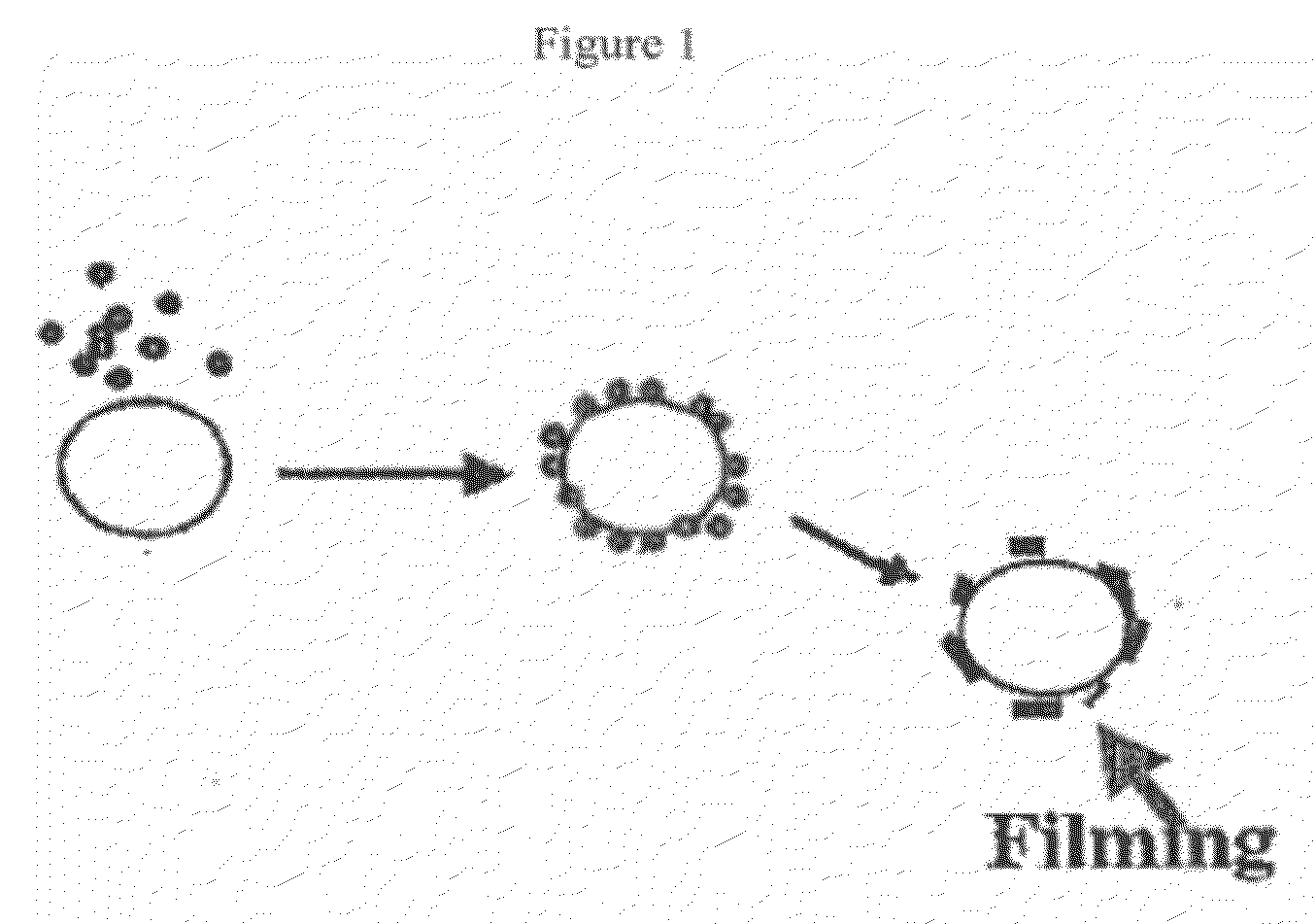
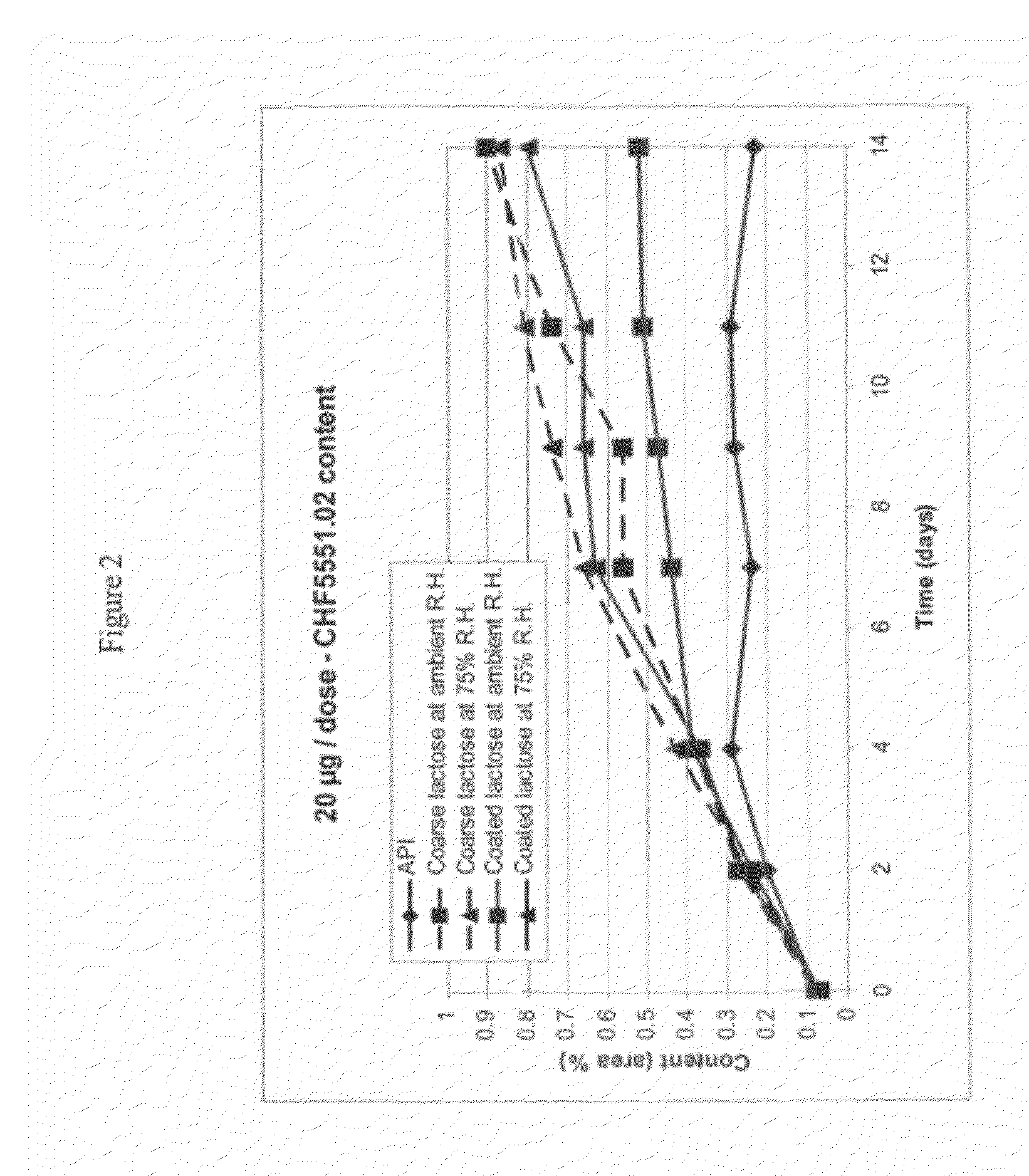
The aim of the invention is to improve the moisture resistance of dry powder formulations for inhalation which contain a pharmaceutically ineffective carrier of not-inhalable particle size and a finely divided pharmaceutically active compound of inhalable particle size and to also improve the storage stability of said formulations. To this end, magnesium stearate is used in said formulations. One of the features of the inventive dry powder is that a high fine particle dosage or fine particle fraction can be maintained also under relatively extreme temperature and humidity conditions.
Owner:JAGOTEC AG
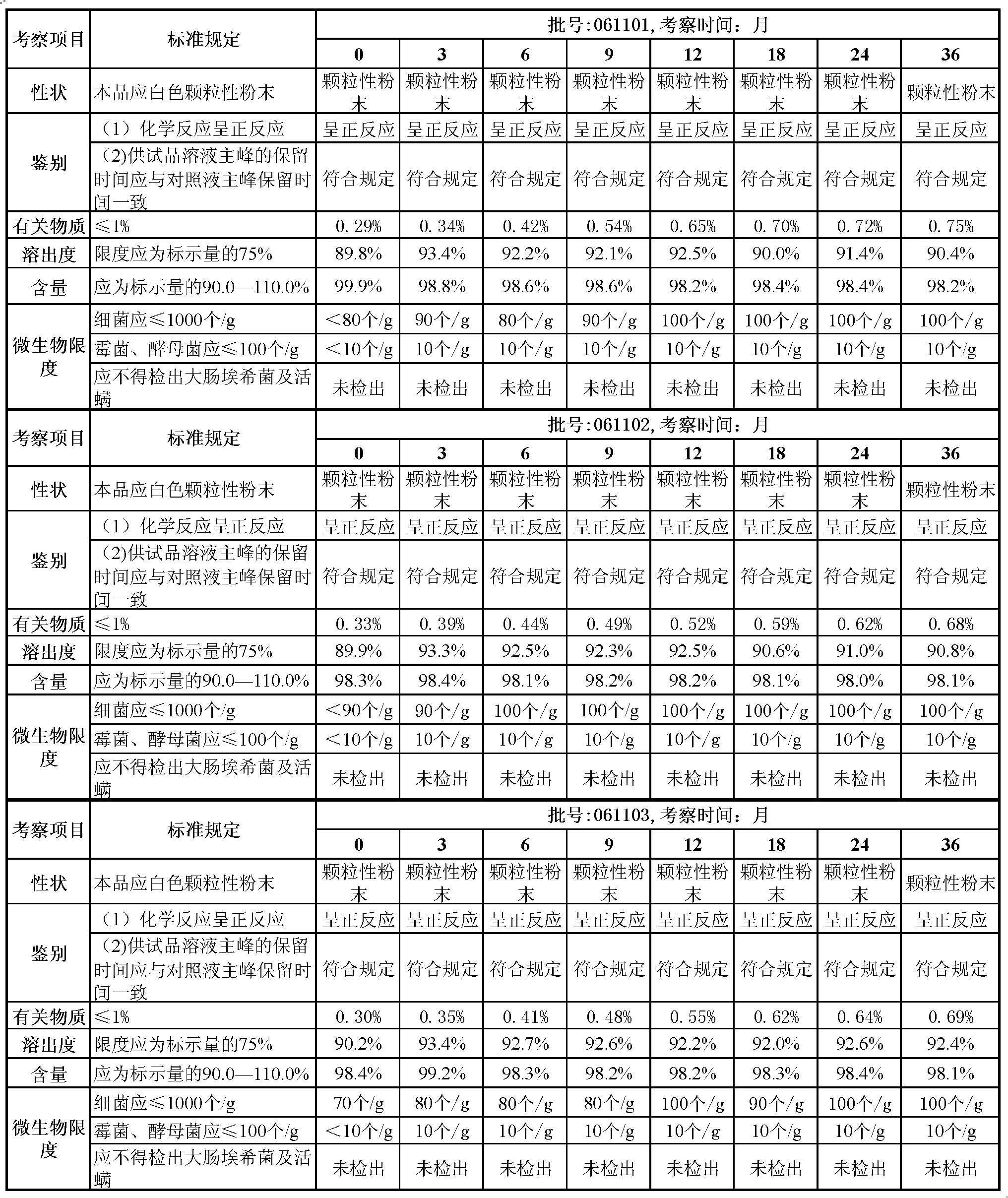
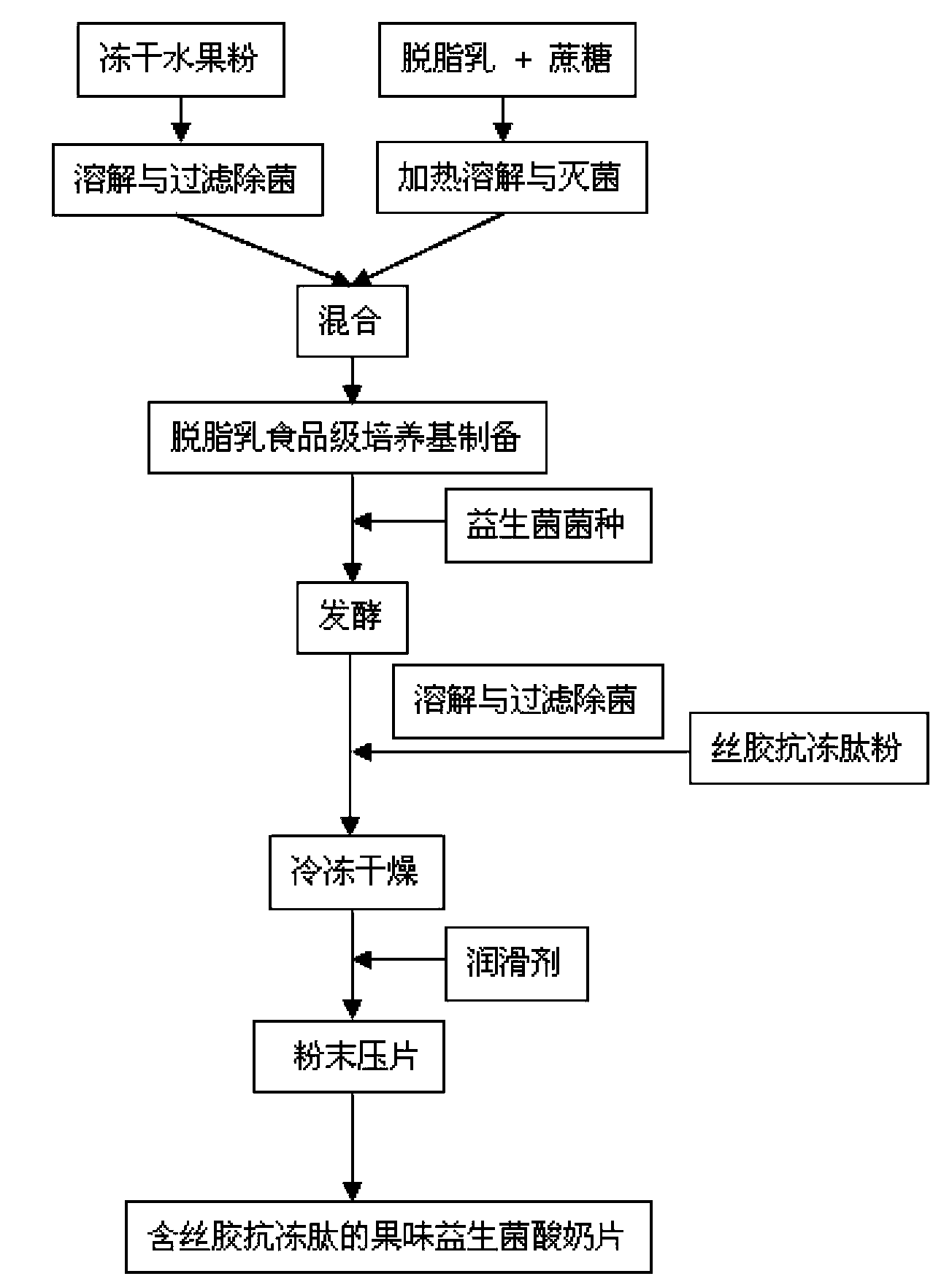
Fruity probiotic yogurt slice containing antifreeze sericin peptide and method for preparing same
ActiveCN103109930BRich in physiological functionsRich in antioxidantMilk preparationProbiotic yogurtBiotechnology
The invention discloses a fruity probiotic yogurt slice containing antifreeze sericin peptide. The raw materials for preparing the fruity probiotic yogurt slice comprise 10-14 parts of skimmed milk powder, 2-5 parts of sucrose, 1-3 parts of sericin peptide, 1-4 parts of fruit material, 0.1-0.6 part of lyophilized active probiotics and 0.1-0.4 part of magnesium stearate. The invention further discloses a method for preparing the product which is the fruity probiotic yogurt slice. The method mainly comprises the following steps of: preparing a skimmed-milk food-grade culture medium, fermenting the probiotics, adding the antifreeze sericin peptide, freezing and drying fermented milk in vacuum, and pressing powder into slices. As the fruity probiotic yogurt slice contains the antifreeze sericin peptide and the lyophilized fruit powder which is rich in the vitamin B group, the number of the probiotics in the fruity probiotic yogurt slice can be increased, the retention time of the activity of the probiotics can be prolonged, and the taste, flavor and nutritional and healthcare values of the fruity probiotic yogurt slice can be improved. The fruity probiotic yogurt slice can serve as a functional food for supplementing the vitamin B group, improving the immunity and promoting the intestinal health.
Owner:SHANGHAI JIAOTONG UNIV
Metal surface oil and rust removal agent
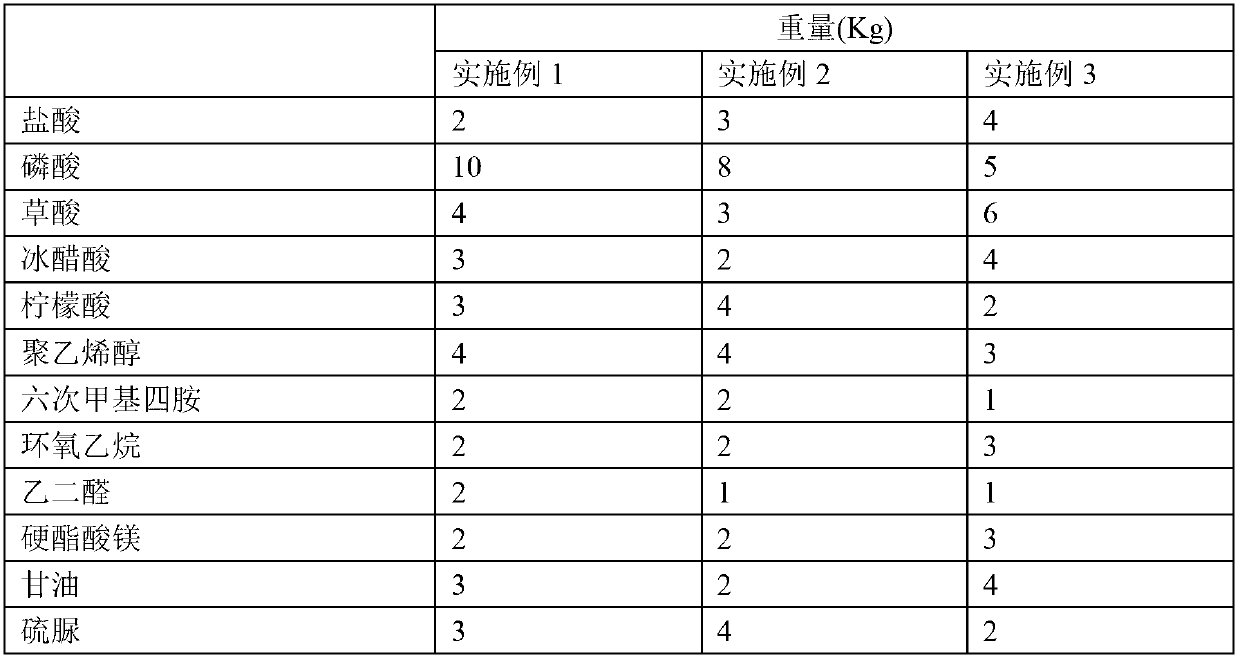
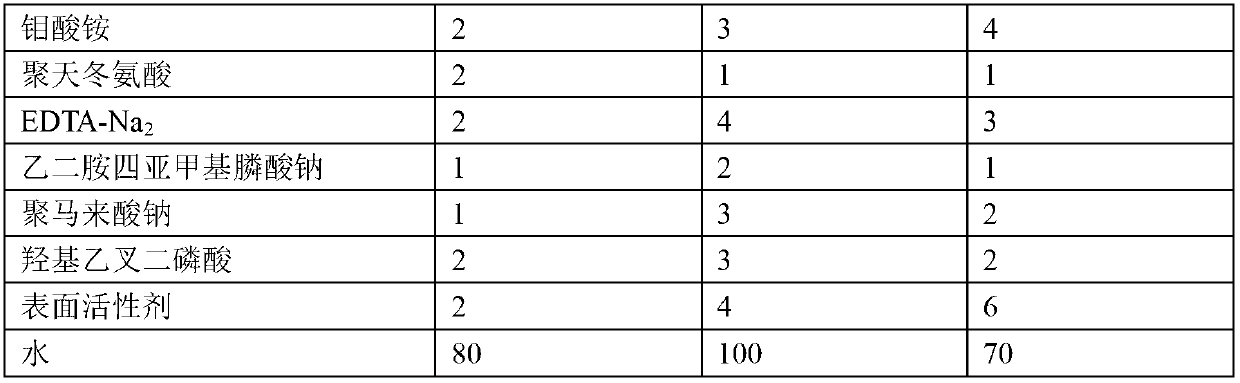
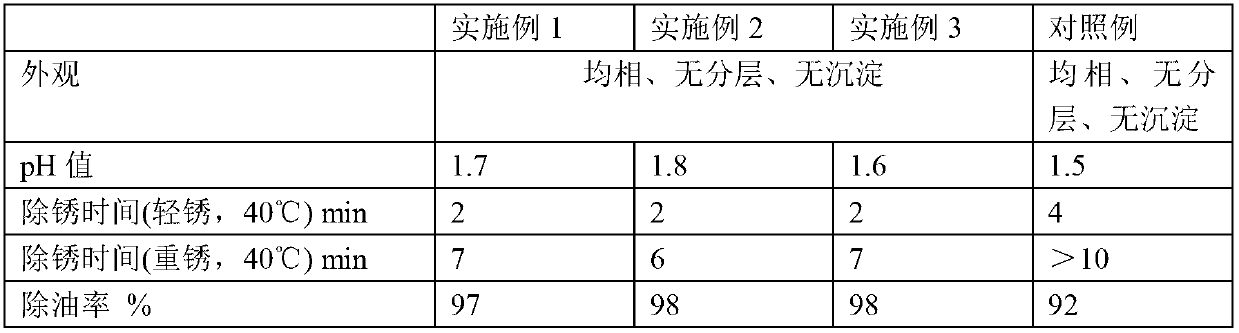
The invention provides a metal surface oil and rust removal agent, and belongs to the technical field of cleaning agents. The metal surface oil and rust removal agent comprises the following components by weight: 2 to 4 parts of hydrochloric acid, 5 to 10 parts of phosphoric acid, 3 to 6 parts of oxalic acid, 3 to 5 parts of glacial acetic acid, 2 to 4 parts of citric acid, 3 to 4 parts of polyvinyl alcohol, 1 to 2 parts of hexamethylene tetramine, 2 to 3 parts of ethylene oxide, 1 to 2 parts of glyoxal, 2 to 3 parts of magnesium stearate, 2 to 4 parts of glycerol, 2 to 4 parts of thiourea, 2 to 4 parts of ammonium molybdate, 1 to 2 parts of polyaspartic acid, 2 to 4 parts of EDTA-Na2, 1 to 2 parts of ethylene diamine tetra sodium, 1 to 3 parts of poly maleic acid sodium, 2 to 3 parts of HEDPA, 2 to 6 parts of surface active agent and 70 to 100 parts of water. The metal surface oil and rust removal agent is convenient to use, and removes light rust for about 2 minutes and heavy rust for 6 to 7 minutes, and the oil removal rate can reach more than 97%.
Owner:盐城创咏新能源投资有限公司
High-efficiency composite food cellulose and preparation method thereof
InactiveCN101317666AAchieve perfect unitySolve the rough tasteOrganic active ingredientsMetabolism disorderDiseaseSodium bicarbonate
The invention discloses a preparation method for highly effective compound dietary cellulose. The method of the invention is characterized in that: the highly effective compound dietary cellulose is prepared with the following materials, insoluble dietary fiber, water-soluble dietary fiber, vitamin C and lactic acid bacteria powder, in definite proportions. The product of the invention is the highly effective compound dietary cellulose which is provided with dual characteristics of insoluble dietary fiber and water-soluble dietary fiber and the dosage form of the product can be capsules or granules. The long-term taking of the product of the invention can ensure obvious curative effects on high blood pressure, high blood fat, high cholesterol, diabetes, obesity, constipation, gastro-intestinal diseases, etc. If being added with different materials such as microcrystalline celluloses, magnesium stearate, maltodextrin, citric acid, sodium bicarbonate and PEG6000, according to different preparation forms, the product can also be prepared into tablets, chewable tablets and effervescent tablets. The highly effective compound dietary cellulose of the invention has simple, scientific and reasonable prescription, simple technology and stable quality, and is easy to be produced in large scale, thus being a safe and green health-care food product.
Owner:罗文礼
Pharmaceutical formulation
The instant invention provides a pharmaceutical composition comprised of a cholesterol absorption inhibitor and an HMG-CoA reductase inhibitor, one or more anti-oxidants, microcrystalline cellulose, hydroxypropyl methylcellulose, magnesium stearate and lactose. The composition need not contain ascorbic acid in order to obtain desirable stability.
Owner:MERCK SHARP & DOHME LTD +2
Composition of composite potassium peroxymonosulfate disinfection tablet and preparation method thereof
ActiveCN101828550AEasy to useDisintegrates quicklyBiocideDisinfectantsSodium bicarbonateFiller Excipient
The invention discloses acomposite potassium peroxymonosulfate disinfection tablet which comprises 10.0-70.0 parts by weight of potassium monopersulfate, 1.0-65.0 parts by weight of fillers, 1.0-20.0 parts by weight of adhesives and 0.01-10.0 parts by weight of lubricant, wherein the fillers is formed by one or more then one of sodium sulfate, sodium chloride, potassium chloride, sodium carbonate, sodium bicarbonate, phosphate, inorganic acid, organic acid, starch, dextrin, glucose, solid emulsifier, xylitol and a metal ion complexing agent, the adhesives comprise cellulose, polyvinylpyrrolidone and gelatin; the lubricant is formed by one or more than one of magnesium stearate, talcum powder, micro-powder silica gel, sodium benzoate and polyethylene glycol; in addition, sodium percarbonate, calcium percarbonate, sodium perborate or calcium peroxide 1-2 are added. The invention is convenient to use, has the advantages of quick disintegration and dissolution, can significantly increase the effect of the disinfection tablet and can adjust pH value.
Owner:镇江威特药业有限责任公司 +1
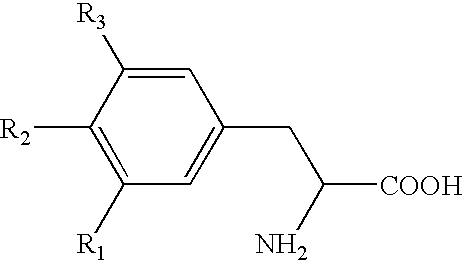
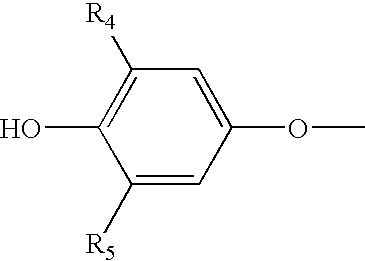
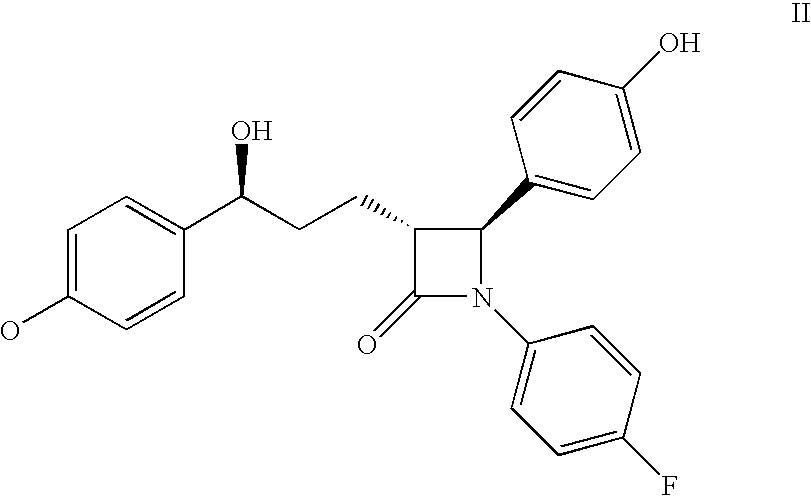
Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation
The subject invention provides a pharmaceutical tablet formulation comprising per unit dosage form e.g. per tablet the following ingredients:(a) a compound as an active ingredient, wherein the compound has a formula:wherein R is substituted adamantyl and n is an integer from 0 to 3 or a pharmaceutically acceptable salt thereof;(b) a hydroxypropyl methylcellulose with an apparent viscosity of 80,000 cP to 120,000 cP (nominal value 100,000 cP) when present in a 1% solution;(c) a microcrystalline cellulose; and(d) a magnesium stearate
Owner:KOWALSKI JAMES +3
Gingko leaf slow-releasing table and preparation process thereof
InactiveCN1371744AReduce the number of dosesStable blood concentrationUnknown materialsPill deliveryDiseaseAnhydrous ethanol
The preparation method of ginkgo leaf slow-released tablet mainly for curing cerebrovascular disease includes the following steps: 1. preparing gingko leaf extract; and 2. preparing slow-released tablet: using gingko leaf extract 120 portions, hydroxypropyl methyl cellulose 80-100 portions, lactose 50-80 portions and starch 30-80 portions, mixing them uniformly, adding anhydrous ethanol 0.8-1.2 portions to make granulation, then adding magnesium stearate 2-5 portions, uniformly mixing them and tabletting to obtain said invented product.
Owner:YABAO PHARMA GRP CO LTD
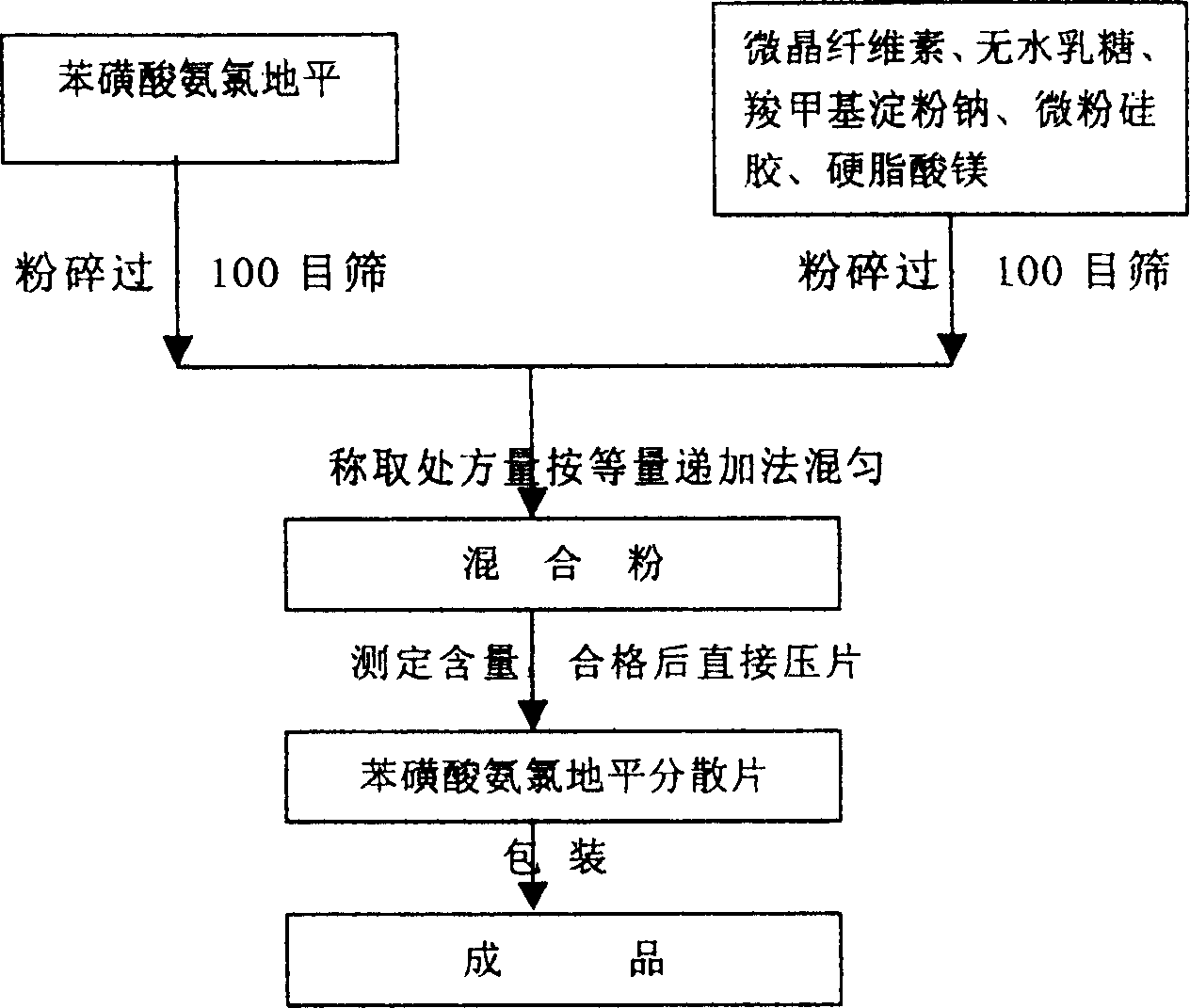
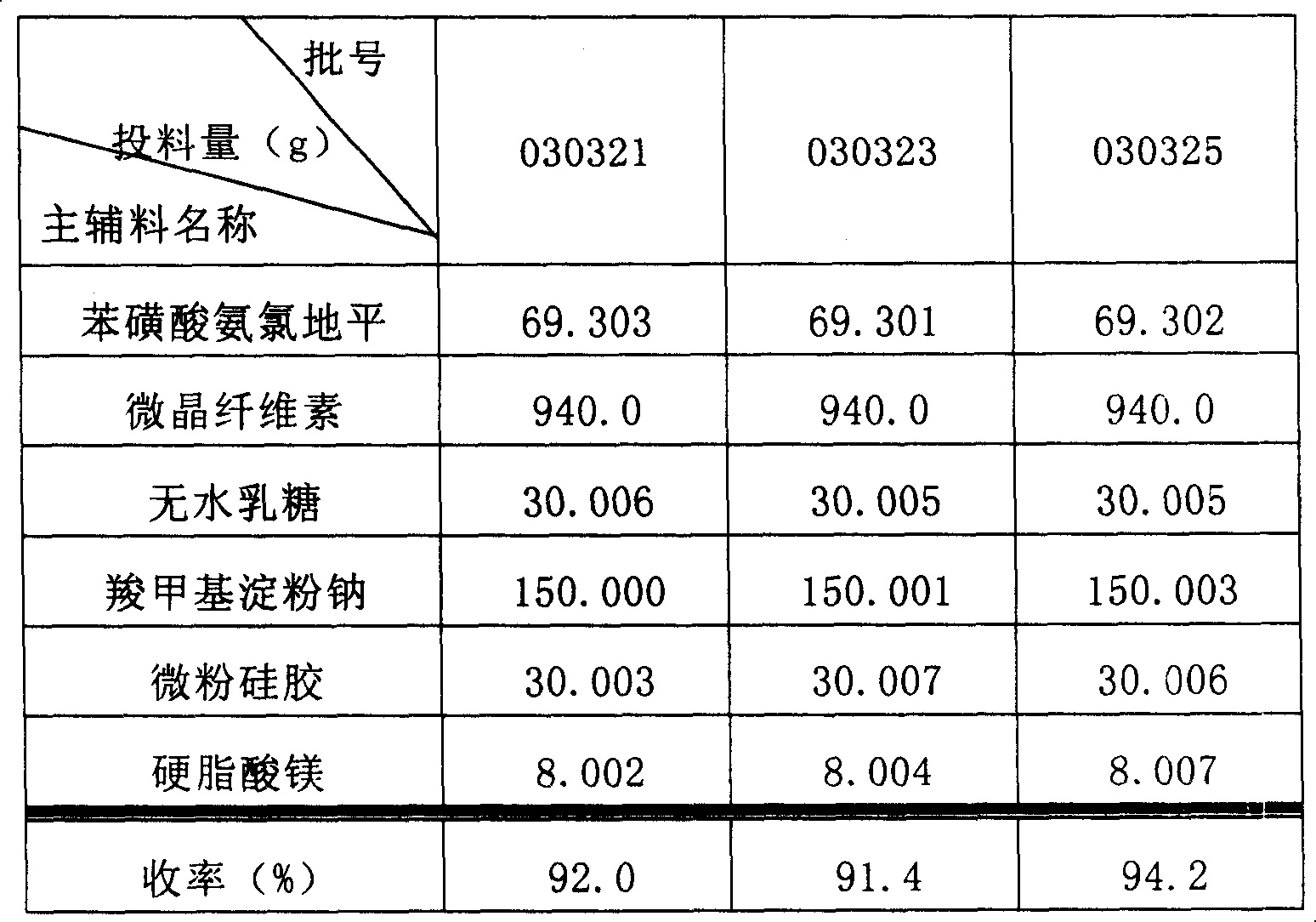
Phenylsulfonic acid amido chloro diping dispersion tablet and its preparation method
InactiveCN1686121AEasy to takeTake fastOrganic active ingredientsPill deliveryCarboxymethyl starchAmlodipine besilate
A dispersing table of amlodipine benzosulfonate for treating hypertension is proportionally prepared from amlodipine benzosulfonate, microcrystalline cellulose, anhydrous lactose, carboxymethyl starch sodium, micropowdered silica gel, and magnesium stearate.
Owner:YUNNAN BAIYAO GRP HEALTH PROD CO LTD
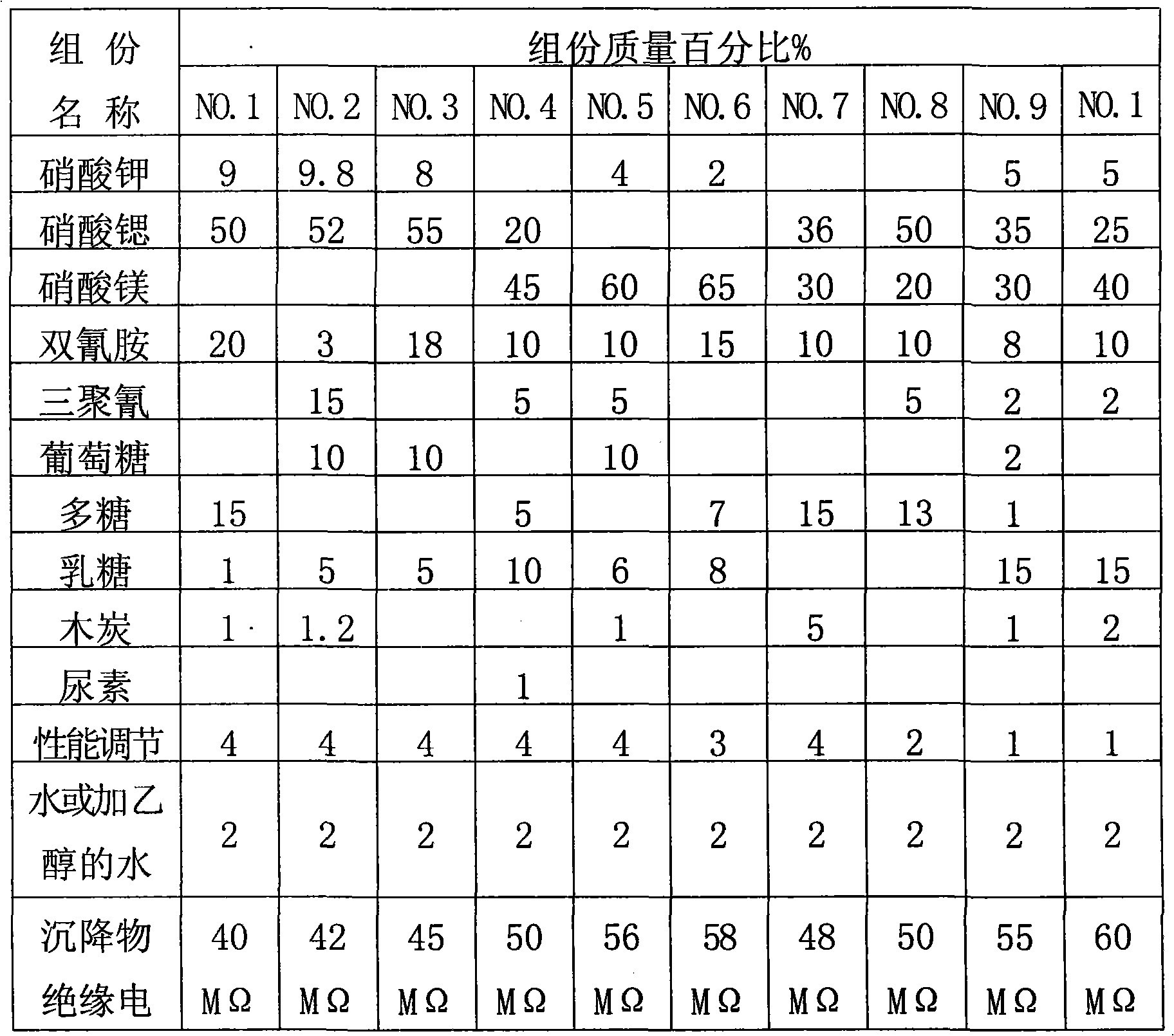
Compound type aerosol extinguishing agent
InactiveCN101862517ALow fire extinguishing concentrationImprove corrosion resistanceFire extinguisherSucroseNuclear chemistry
The invention discloses a compound type aerosol extinguishing agent comprising the following components in percentage by weight: 30-75 percent of oxidizing agent, 20-50 percent of reducing agent, 2-15 percent of performance regulating agent and no more than 2 percent of binding agent. The oxidizing agent is prepared by matching any two or three matched components selected from potassium nitrate, strontium nitrate and magnesium nitrate; the reducing agent is prepared by matching two or more components selected from polyhexose, glucose, starch, sorbitol, xylitol, lactose, dicyandiamide, melamine, carbamide and sucrose; the performance regulating agent is prepared by matching one or more component selected from carbon powder, light magnesium carbonate, magnesium stearate, aluminium nitrate, hexamethylenetetramine, aluminium oxide, magnesium oxide and light metal oxide; and the binding agent is prepared by matching water or 20-30 percent of ethanol added into the water. In the invention, production raw materials have a wide source and low cost, can be constantly obtained and are safe and reliable, and burnt residues have no toxicity and good environmental protection performance.
Owner:湖南省金鼎消防器材有限公司
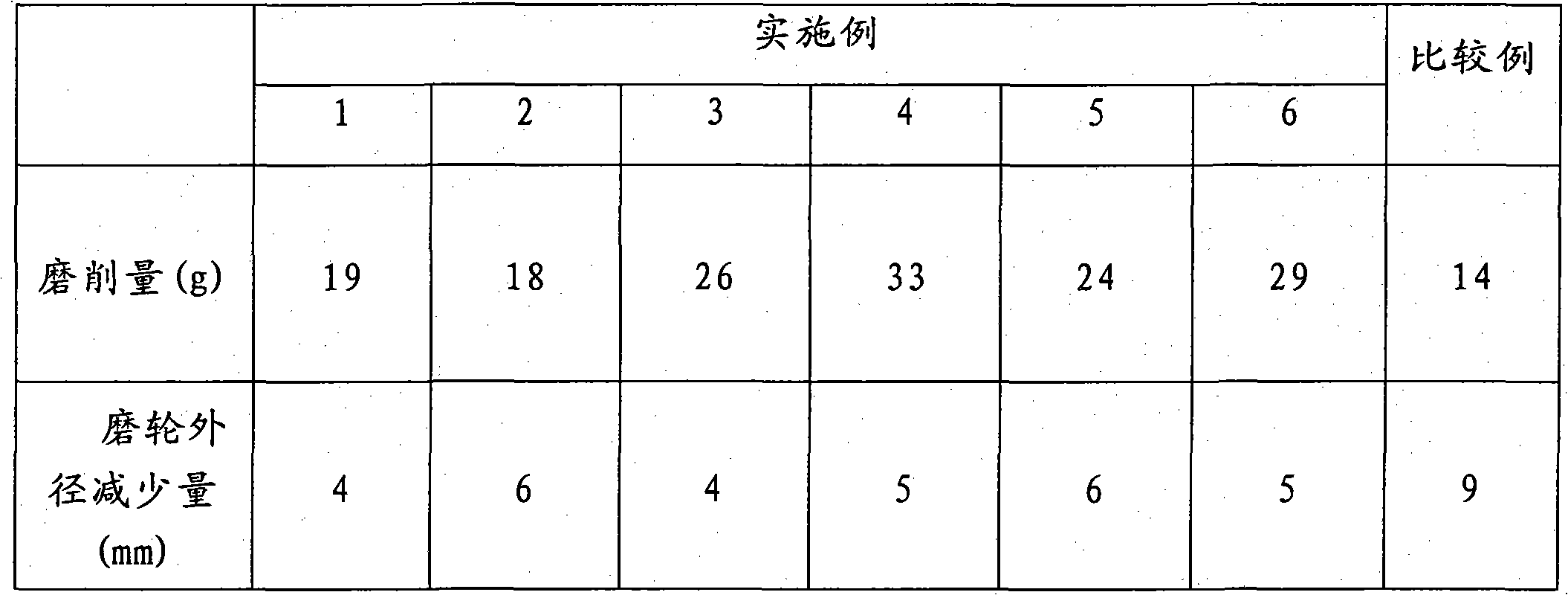
Method for preparing non-woven fabric polishing abrasive tool with high abrasion resistance
InactiveCN101913121AHigh viscosityImprove adhesionFibre typesGrinding devicesSilanesMagnesium stearate
The invention relates to a method for preparing a non-woven fabric polishing abrasive tool with high abrasion resistance. The method comprises the following steps of: taking 20 to 40 mass parts of epoxy resin, adding 4 to 25 mass parts of curing agent, 20 to 45 mass parts of abrasive, 8 to 15 mass parts of diluent, 2 to 8 mass parts of toughening agent, 1 to 3 mass parts of silane coupling agent and 3 to 8 mass parts of auxiliary materials into the epoxy resin, mixing the materials uniformly, then spraying the mixture onto a non-woven fabric, curing the mixture at a high temperature, and then performing punch forming, wherein the toughening agent comprises reactive epoxy toughening agent, terminal carboxyl liquid nitrile rubber and / or terminal carboxyl polybutadiene liquid rubber; the silane coupling agent comprises gamma-aminopropyltriethoxysilane, gamma-glycidyl ether oxypropyl trimethoxy silane and / or gamma-(methacryloyloxy) propyltrimethoxysilane; and the other auxiliary materials comprise 2,4,6-tri(dimethylaminomethyl) phenol DMP-30 accelerator, wax powder, graphite, pigment, magnesium stearate and / or zinc stearate. The non-woven fabric polishing abrasive tool is mainly used for polishing treatment of metal surfaces.
Owner:SOUTH CHINA UNIV OF TECH +1
Use of magnesium stearate in dry powder formulations for inhalation
InactiveUS20120082727A1Chemically stableInhibit and reduce chemical degradationPowder deliveryBiocideInhalationMagnesium stearate
Addition of magnesium stearate to a powder formulation for inhalation comprising carrier particles and an active ingredient bearing a group susceptible to hydrolysis is useful for inhibiting or reducing the chemical degradation of the active ingredient.
Owner:CHIESI FARM SPA
Palbociclib gastric-floating tablet and preparation method thereof
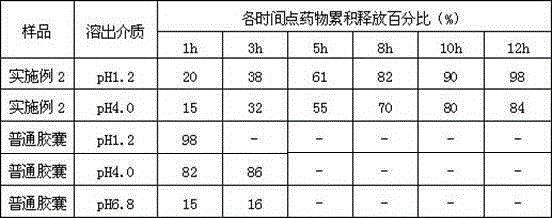
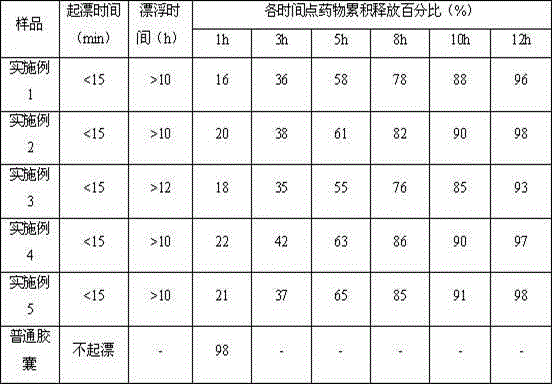
ActiveCN104887641ALow drift timeReduce dosageOrganic active ingredientsPill deliveryUse medicationPharmaceutical drug
The invention belongs to the technical field of medicine, and relates to a palbociclib gastric-floating tablet and a preparation method thereof. The palbociclib gastric-floating tablet comprises, by mass, 10%-30% of palbociclib, 20%-50% of hydroxypropyl methylcellulose, 20%-40% of bleaching auxiliaries, 2%-10% of foaming agents, 0%-25% of microcrystalline cellulose and 0.5%-3% of magnesium stearate. A dry granulating technology or a wet granulating technology can be used as the preparation technology. The palbociclib gastric-floating tablet is high in bioavailability, has a slow release tendency, and effectively lowers the total dosage. The palbociclib gastric-floating tablet and the preparation method thereof have the unique advantages that two different mechanisms are used for preparing the gastric-floating tablet, and accordingly the prepared tablet can keep floating in gastric juice by more than 10 hours and continuously release drugs in the hydrochloric acid solution with the pH being 1.2; the problem that the bioavailability is low due to the fact that drugs are extremely difficult to dissolve after the pH is higher than four is effectively solved; the medicine taking frequency is reduced; toxic and side effects are lightened; and the complaisance of a patient is effectively improved.
Owner:上海润泰医药科技有限公司
Chitosan oligosaccharide capsule
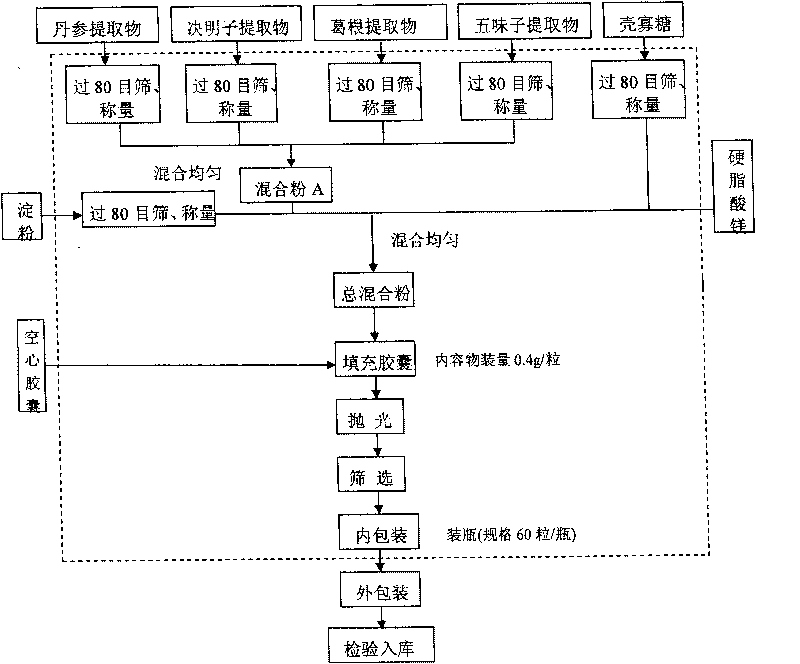
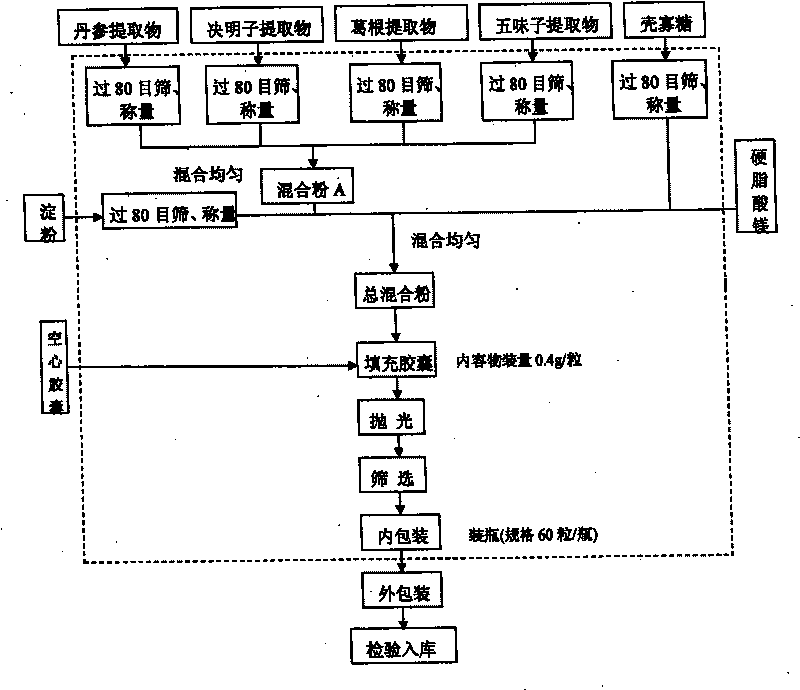
ActiveCN101744164AGood development valueHuge market potentialFood preparationAdditive ingredientPuerariae Radix extract
The invention discloses a chitosan oligosaccharide capsule; chitosan oligosaccharide with 2000-10000 of molecular weight, radix salviae miltiorrhizae extract, pueraria root extract, obtuseleaf senna seed extract and schisandra extract are used as main materials; starch and magnesium stearate are taken as auxiliary raw materials; the weight components of the formula of the chitosan oligosaccharide capsule include the chitosan oligosaccharide, the radix salviae miltiorrhizae extract, the pueraria root extract, obtuseleaf senna seed extract, schisandra extract, the starch and the magnesium stearate; the production process of the capsule is that: sieving, weighting, mixing, capsule filling, polishing, screening, inside and outside package, warehouse-in inspection are carried out to prepare the health-care product. The chitosan oligosaccharide capsule has the advantages that the main composition of the capsule comprises the chitosan oligosaccharide, general flavone and total anthraquinone; the capsule is taken orally two times one day, 3 granules each time, and the capsule has auxiliary protection function to the chemical liver damage.
Owner:江西省三抗保健品有限公司
Dendrobium candidum polysaccharide buccal tablet and preparing method thereof
ActiveCN101658639AImprove liquidityAvoid lossAntinoxious agentsPill deliveryAdditive ingredientMagnesium stearate
The invention provides a Dendrobium candidum polysaccharide buccal tablet comprising the following components by weight portions: 30-100 portions of Dendrobium candidum polysaccharide, 380-420 portions of xylithol as a carrier, 300-340 portions of aspartame, 0.5-1.5 portions of menthol, 0.5-1.5 portions of aerosol and 4-6 portions of magnesium stearate; and the invention also provides a preparingmethod of the Dendrobium candidum polysaccharide buccal tablet, comprising the following steps: taking the Dendrobium candidum polysaccharide as raw material, adding auxiliary material, uniformly mixing according a certain proportion, and tabletting and shaping to obtain the product. The Dendrobium candidum polysaccharide is easy to be taken, and fresh and cool in mouthfeel, can be directly absorbed through oral mucosa, avoids loss generated when nutrients are absorbed by organs such as human gastrointestine, liver and the like, improves the absorption utilization ratio of nutrients in Dendrobium candidum polysaccharide, and has important significance to clinical treatment and the prevention and development of health care products.
Owner:浙江森宇有限公司
Benzene sulfonic acid levo-amlodipine pill and preparation method thereof
InactiveCN101559043AHigh dissolution rateReasonable designOrganic active ingredientsPill deliveryBenzeneMagnesium stearate
The invention discloses benzene sulfonic acid levo-amlodipine pills and a preparation method thereof. On the basis of 1000 pills, the benzene sulfonic acid levo-amlodipine pill comprises 1-10g of benzene sulfonic acid levo-amlodipine, preferably 2.5g; 50-100g of lactose, preferably 67-87g and most preferably 80g; 5-55g of low-substituted hydroxy propyl cellulose, preferably 20-40g and particularly preferred 30g; 2-20g of crosslinked polyethylene ketopyrrolidine, preferably 5g; and 0.5-2.5g of magnesium stearate, preferably 1.5g. The benzene sulfonic acid levo-amlodipine pills have more than 95 percent of dissolution rate and good production stability.
Owner:NANCHANG HELIOEAST PHARMA
Melatonin two-layer release-controlled tablet and preparing process thereof
InactiveCN1488346AEffective plasma concentrationEffective hypnosisOrganic active ingredientsNervous disorderVitamin b6Polyethylene glycol
The invention is a melatonin double-layer controlled release tablet and making technique, using melatonin as main raw material, and composed of quick-release layer and sustained-release layer. The quick-release layerí»s components: melatonin 1-5%, Vitamin B6 3-15%, lactose 10-70%, pre-gel amylum 10-70%, hypromellose 0.03-0.3%, magnesium stearate 0.3-2% and SiO2 1.0-3.0%; the sustained layerí»s components: melatonin 2-6%, hypromellose 15-60%, stearic acid 15-65%, polyethylene glycol 10000 10-30%, and magnesium stearate 1.0-5.0%. It has efficacies of quickly hypnotizing and prolonging effective time of sleeping.
Owner:CHONGQING TAIJI MEDICAL RES INST CO LTD
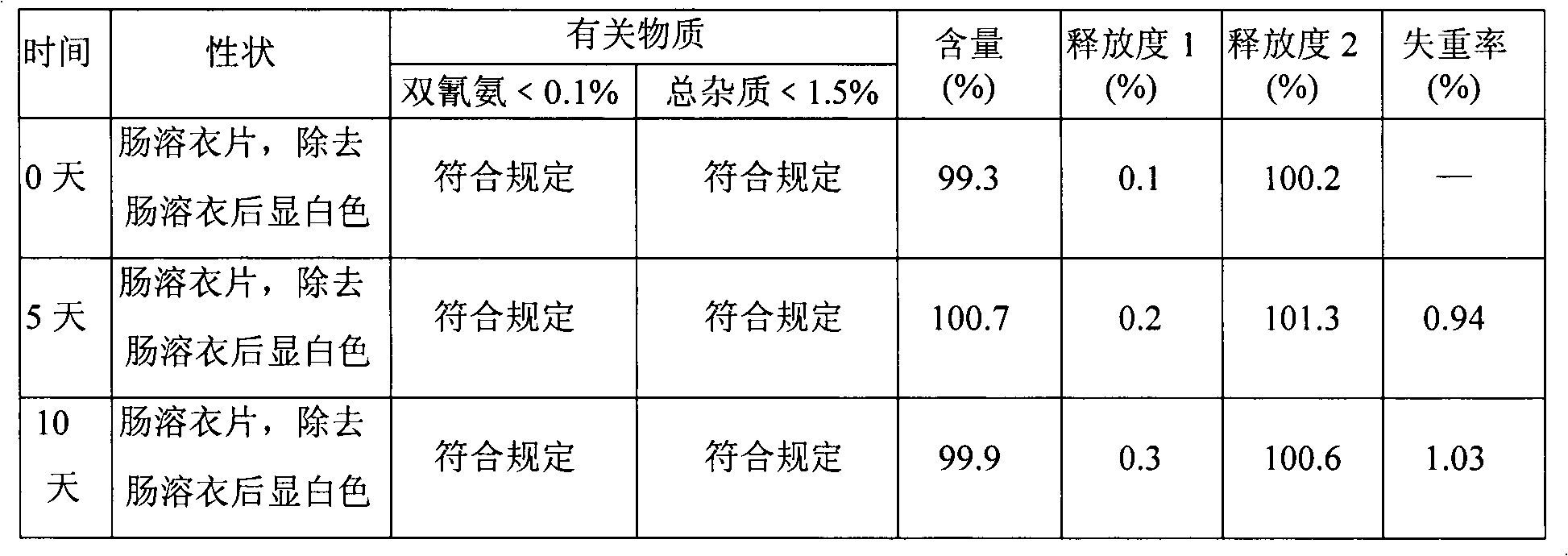
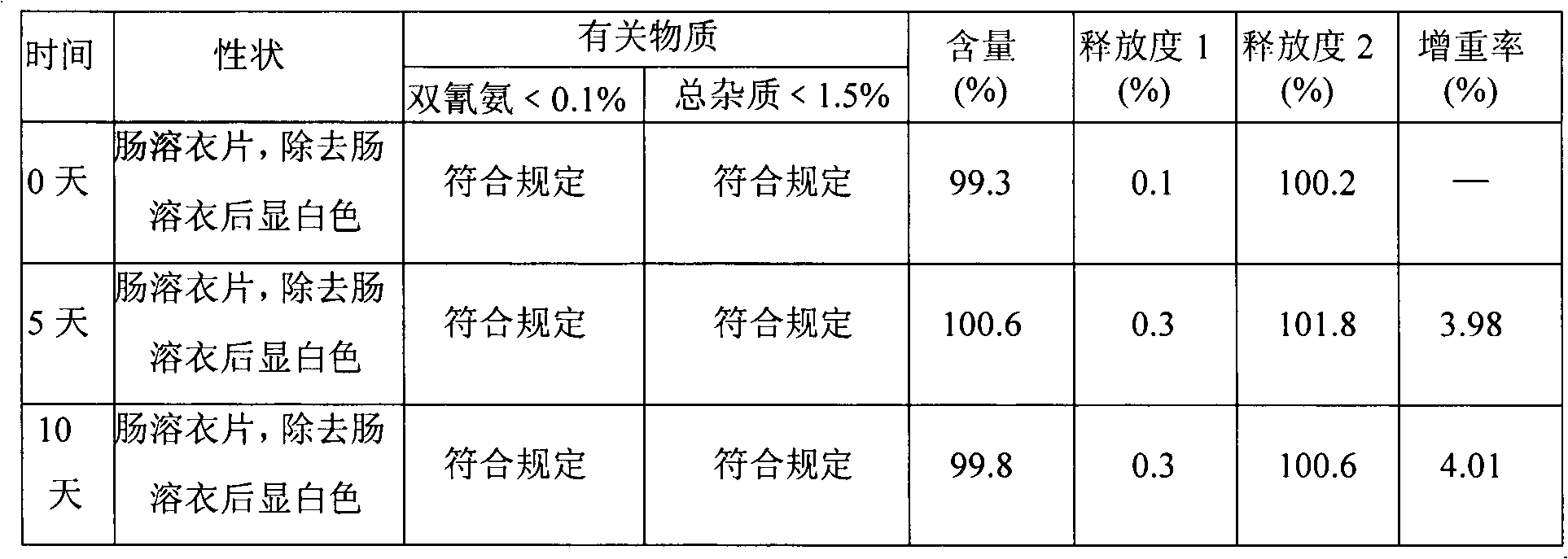
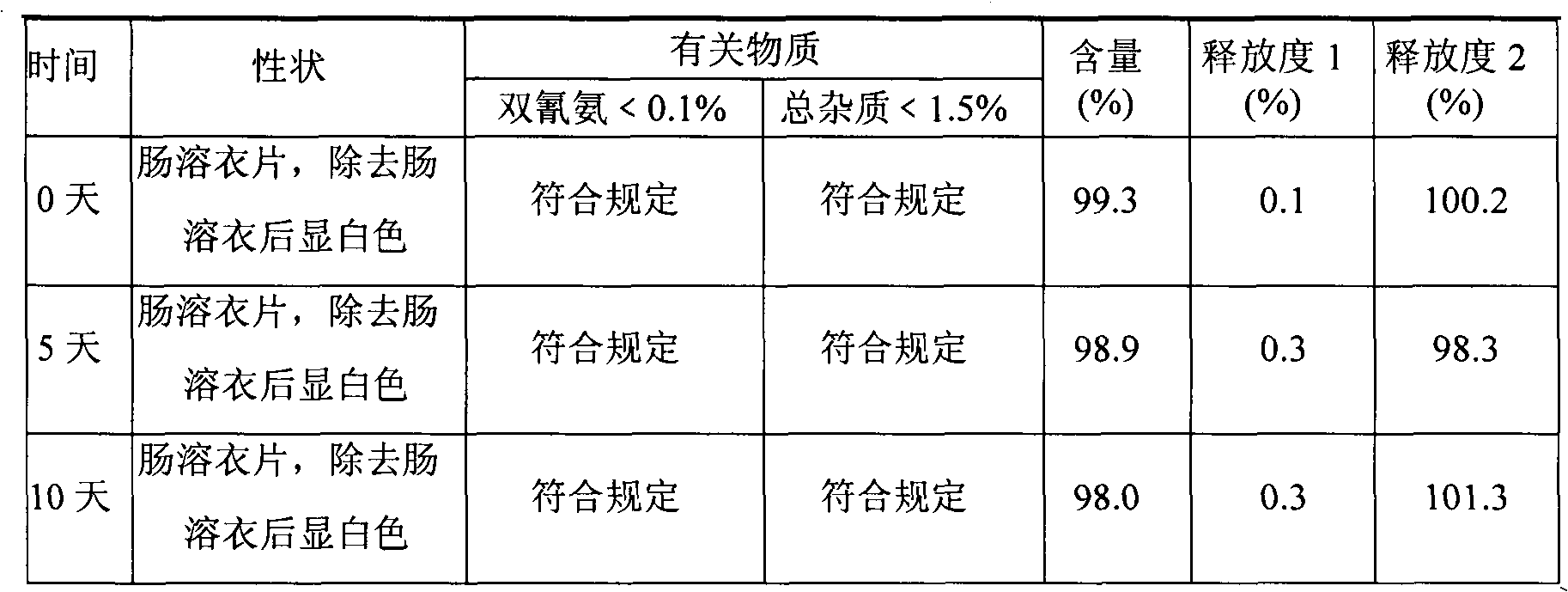
Metformin hydrochloride enteric-coated tablets and preparation method thereof
The invention belongs to the technical field of medicinal preparations, in particular relates to metformin hydrochloride enteric-coated tablets and a preparation method thereof, and provides stable metformin hydrochloride enteric-coated tablets. Each metformin hydrochloride enteric-coated tablet comprises a tablet core, an insulation coating and an enteric coating, wherein the tablet core is prepared from metformin hydrochloride, dextrin, hyprolose, magnesium stearate and talcpowder by adopting the ethanol aqueous solution of hypromellose as an adhesive; the insulation coating is prepared from a gastric soluble film coating premixed suspension agent and purified water; and the enteric coating is prepared from an enteric film coating premixed suspension agent and the purified water.
Owner:BEIJING JINGFENG PHARMA GRP
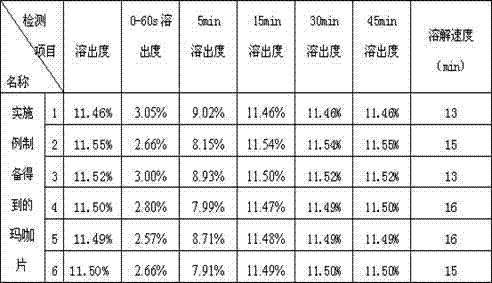
Maca tablet and preparation method thereof
InactiveCN102823798AImprove bioavailabilityRapid dissolutionFood preparationMagnesium stearateStearic acid
The invention provides a maca tablet and a preparation method thereof and relates to the technical field of health care products. The maca tablet is prepared by the following raw materials by weight: 581.84 g of maca powder, 56.48 g of microcrystalline cellulose, 30.4 g of lactose, 78.56 g of povidone, 15.12 g of magnesium stearate and 37.6 g of starch; and the average relative molecular weight of the povidone is 2000. The preparation method of the maca tablet comprises the following steps of: uniformly mixing all the raw materials, spraying 90 percent of ethanol solution to moisten, palletizing, drying, carrying out intermediate inspection, granulating and tabletting; and spraying 456.5 g of ethanol solution on every 800 g of raw materials. The maca tablet has the advantages that the effective components are quickly dissolved out, the biological availability is high, and the medical effects of effective component maca can be better realized, and the cost for drug consumption is reduced. The maca tablet has the effects of relieving the physical fatigue, improving the sexual function, improving the fertility, adjusting the internal secretion and the like, and particularly relieving the physical fatigue and the improving the sexual function.
Owner:SHANDONG YIBAO BIOLOGICS
Levamlodipine besylate tablet, preparation process thereof and control method for relevant materials
ActiveCN102028662AGood treatment effectStable buckOrganic active ingredientsPill deliveryCross-linkSolubility
The invention relates to a levamlodipine besylate tablet, a preparation process thereof and a control method for relevant materials. Each 1000 levamlodipine besylate tablets provided by the invention comprise the following compositions: 2.5g of levamlodipine besylate (measured in besylate), 30 to 50g of lactose (as a filler), 20 to 40g of beta-cyclodextrin (as an inclusion agent), 25 to 45g of microcrystalline cellulose (as a disintegrating agent), 5 to 15g of cross-linked polyvinylpyrrolidone (as a disintegrating agent), 1 to 2g of magnesium stearate (as lubricant), and 50 to 80g of 2.5% HPMC (hydroxypropyl methylcellulose) and 50% ethanol (as an adhesive). The levamlodipine besylate tablet provided by the invention has the advantages of making multi-item improvements on the properties of the levamlodipine besylate, increasing the solubility and apparent dissolution rate of the tablet, improving the stability of the tablet, reducing the excitability of the tablet, significantly reducing the limit of the relevant material, and having better clinical treatment effect, so that the blood pressure lowering of the patient with hypertension is more stable.
Owner:JIANGXI SHIMEI PHARM CO LTD
Dendrobium buccal tablets and preparation method for same
ActiveCN103285242ASimple processEasy to operatePill deliveryRespiratory disorderLoss rateSlice thickness
The invention belongs to the field of traditional Chinese medicines or healthcare foods, relates to a dendrobium preparation, and in particular relates to dendrobium buccal tablets. The formula of the dendrobium buccal tablets is composed of dendrobium ultra-fine powder, mannitol, white granulated sugar powder, magnesium stearate, aerosol, citric acid, borneol / menthol, mint oil and mint essence, wherein under the formula aforementioned, wet granulation is performed, and then tabletting is performed to prepare the buccal tablets; and the dendrobium buccal tablets prepared by the formula are high in bioavailability and good in taste. Additionally, the invention further discloses a preparation process for dendrobium ultra-fine powder. The preparation process specifically comprises the following steps of: peeling the cleaned fresh dendrobium bars; drying and slicing to achieve a thickness of not greater than 1 mm; and performing drying treatment at 50-60 DEG C to cause the water content to be 2-3%, and crushing for 10-30 minutes by an ultra-fine crusher, so as to obtain the dendrobium ultra-fine powder. According to the process, the loss rate of the precious medicine of dendrobium is further reduced by optimizing the slice thickness and omitting the step of processing crude powder.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
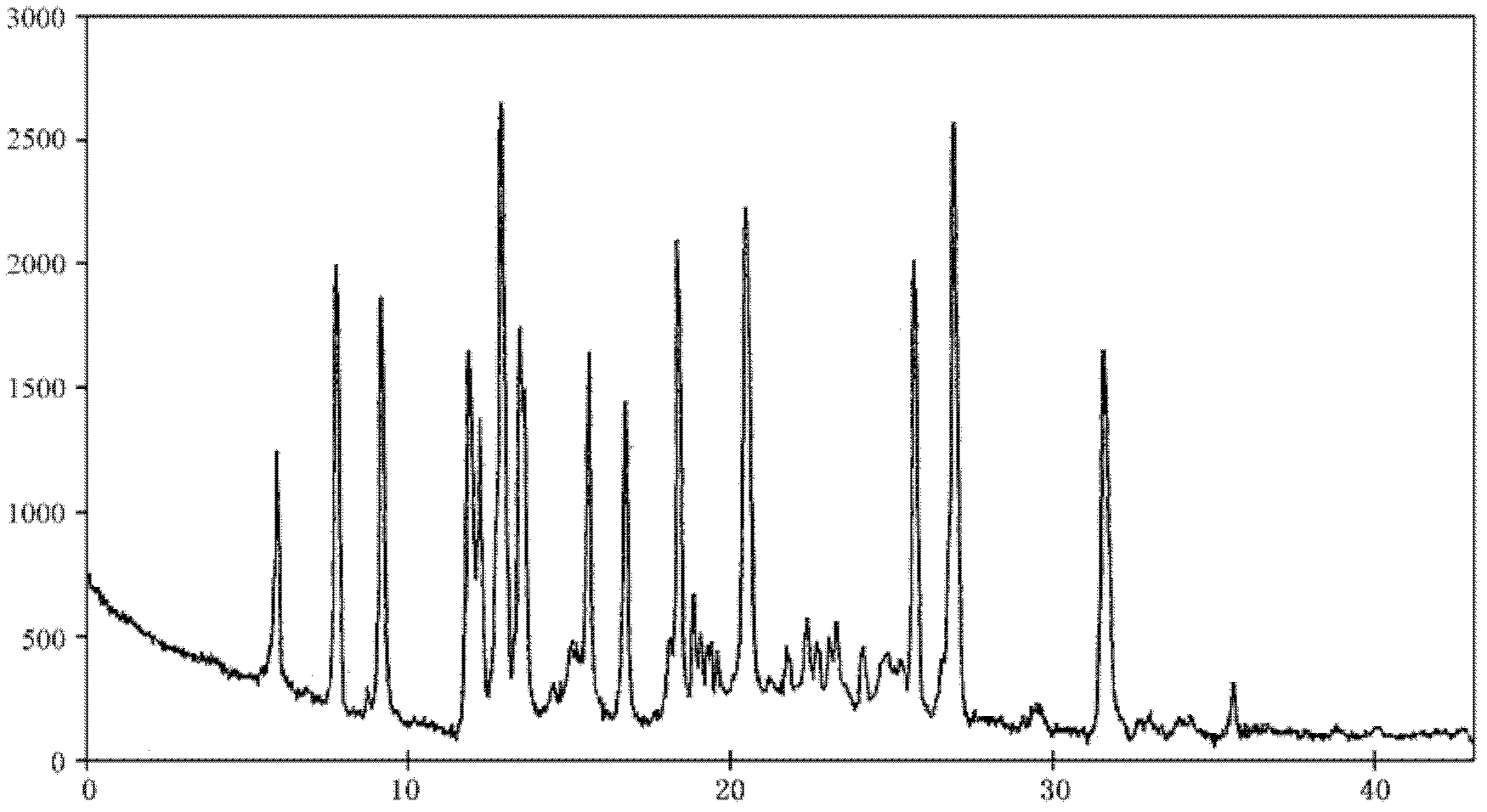
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
Composition and method for enhancing neuromuscular facilitation and cognitive functions
InactiveUS20070248696A1Function increaseIncrease in the neurotransmitter acetylcholineBiocideCarbohydrate active ingredientsGriffonia simplicifoliaDietary supplement
A composition and a method for improving neuromuscular facilitation, also known as “muscle memory,” and enhancing cognitive functions, such as memory and mental focus. The dietary supplement, and the method for the administration thereof, increases acetylcholine levels, which improves neuromuscular facilitation. In various embodiments, factors other than increasing acetylcholine levels within the body further contribute to the overall effect of the dietary supplement composition. In a preferred embodiment, the invention is a composition, and a method for the administration thereof, comprising effective amounts of choline, dimethylaminoethanol, cytidine 5′-diphosphocholine, turmeric extract, decaffeinated green tea extract, vitamin B1, vitamin B5, vitamin B6, vitamin B12, folic acid, dimethylglycine, huperzine A, Griffonia simplicifolia extract, and 1-phenylalanine, magnesium stearate, and silicon dioxide.
Owner:MIND SPORTS NUTRITION
Dry powder for inhalation
InactiveUS20070212422A1Reduce sensitivityMinimize impactPowder deliveryOrganic active ingredientsMagnesium stearateExtreme temperature
The aim of the invention is to improve the moisture resistance of dry powder formulations for inhalation which contain a pharmaceutically ineffective carrier of not-inhalable particle size and a finely divided pharmaceutically active compound of inhalable particle size and to also improve the storage stability of said formulations. To this end, magnesium stearate is used in said formulations. On of the features of the inventive dry powder is that a high fine particle dosage or fine particle fraction can be maintained also under relatively extreme temperature and humidity conditions.
Owner:KELLER MANFRED +1
Ultrafine moringa oleifera powder compressed buccal tablet, and preparation method thereof
The invention provides an ultrafine moringa oleifera powder compressed buccal tablet, and a preparation method thereof. The ultrafine moringa oleifera powder compressed buccal tablet is characterized in that the ultrafine moringa oleifera powder compressed buccal tablet includes the following components by weight percent: 40 to 60 percent of moringa oleifera, 10 to 40 percent of ultrafine maca powders, 5 to 20 percent of maltodextrin, 5 to 50 percent of fructose, and 0.5 to 1 percent of magnesium stearate. The preparation method for the ultrafine moringa oleifera powder compressed buccal tablet is characterized in that the preparation method includes the following steps: (1) freeze drying: fresh moringa oleifera leaves are placed in a freeze drying machine for freeze drying and moisture removal, so that the water content is kept between 3 percent and 6 percent; (2) ultrafine grinding: the moringa oleifera leaves after freeze drying undergo ultrafine grinding in an ultrafine grinder at the normal temperature; (3) compounding: the moringa oleifera, the ultrafine maca powders, the maltodextrin, the fructose and the magnesium stearate are poured into a mixing machine to be sufficiently and uniformly mixed; (4) tabletting: the mixed materials are tabletted through a tablet press. The buccal tablet provided by the invention can supplement daily values for calcium, protein, vitamins, potassium, iron and other elements for human bodies.
Owner:HEBEI COLLAGEN BIOTECH
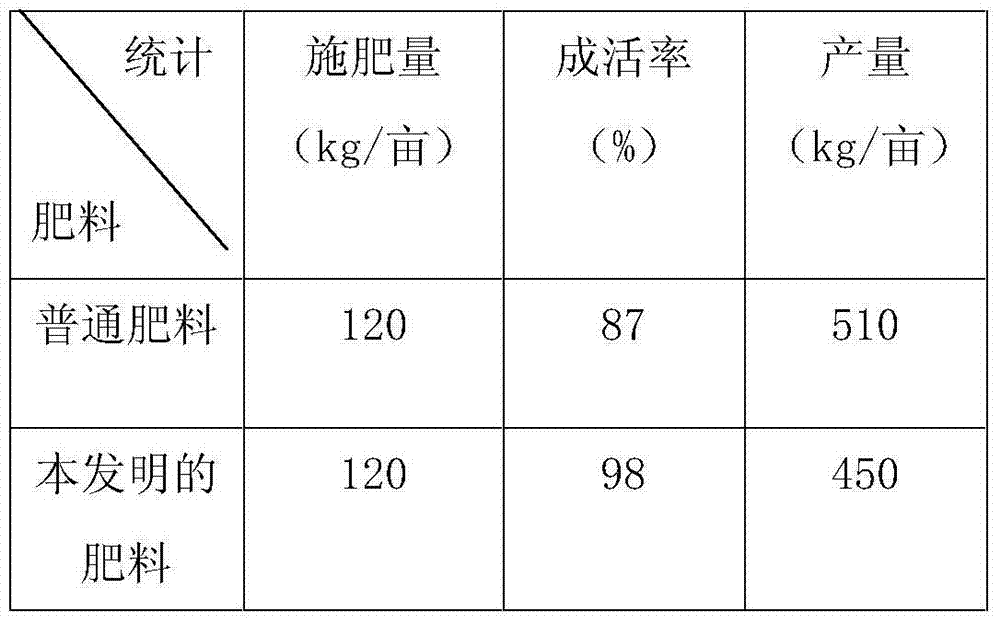
Ecological organic compound fertilizer and preparation method thereof
InactiveCN103755450AReduce the amount of applicationSupplementary trace elementsFertilizer mixturesEffective actionAdditive ingredient
The invention discloses an ecological organic compound fertilizer which is characterized by being prepared from the following raw materials in parts by weight: 40-45 parts of straw powder, 12-14 parts of ramulus mori powder, 20-23 parts of silkworm excrement, 12-14 parts of peanut shells, 5-7 parts of bock greenbrier rhizome, 34-38 parts of urea, 20-24 parts of calcium superphosphate, 18-20 parts of dipotassium phosphate, 14-17 parts of ammonium nitrate, 12-16 parts of humic acid, 2-3 parts of lime, 2-3 parts of hydroxypropyl methyl cellulose, 1-2 parts of magnesium stearate, 3-4 parts of an EM (Effective Microorganisms) fungicide, 4-6 parts of a developing agent and a proper amount of water. The fertilizer disclosed by the invention contains more and reasonable nutritional ingredients and can satisfy all fertilizers required by plants in the growing period, the fertilizer is excellent in slow release performance, has a long-term effective effect, supplements trace elements of soil, improves the yield and quality of the plants, is beneficial to human health and further improves the soil fertility at the same time. Meanwhile, the efficacy of the fertilizer can be further exerted to the maximum extent, and the application amount of the fertilizer is reduced, resources are saved, and the environment is protected. The fertilizer has economical and environmental benefits.
Owner:DANGTU COUNTY KEHUI TRADING
Storage stable thyroxine active drug formulations and methods for their production
InactiveUS6936274B2Improve stabilityPrevents decrease in effective dosagePowder deliveryBiocideSucroseMagnesium stearate
This invention provides a storage-stable dosage form of a thyroxine active drug composition which exhibits an improved stability. The formulation contains a thyroxine active drug substance, an alditol, and a saccharide, and, optionally, additional pharmaceutically accepted excipients. Levothyroxine sodium is the preferred active drug substance, mannitol is the preferred alditol, and sucrose is the preferred saccharide. Additional preferred excipients include, for example, microcrystalline cellulose, crospovidone, magnesium stearate, colloidal silicon dioxide, and sodium lauryl sulfate.
Owner:MYLAN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation](https://images-eureka.patsnap.com/patent_img/a0afd315-9f4c-4f27-b3eb-5346c6a36a85/US20100021539A1-20100128-D00000.png)
![Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation](https://images-eureka.patsnap.com/patent_img/a0afd315-9f4c-4f27-b3eb-5346c6a36a85/US20100021539A1-20100128-D00001.png)
![Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation Modified Release 1-[(3-Hydroxy-Adamant-1-Ylamino)-Acetyl]-Pyrrolidine-2(S)-Carbonitrile Formulation](https://images-eureka.patsnap.com/patent_img/a0afd315-9f4c-4f27-b3eb-5346c6a36a85/US20100021539A1-20100128-D00002.png)