Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
A kind of technology of lansoprazole and crude product of lansoprazole, which is applied in the field of medicine, can solve problems such as poor stability of lansoprazole, and achieve the effects of improving drug safety, good dissolution stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
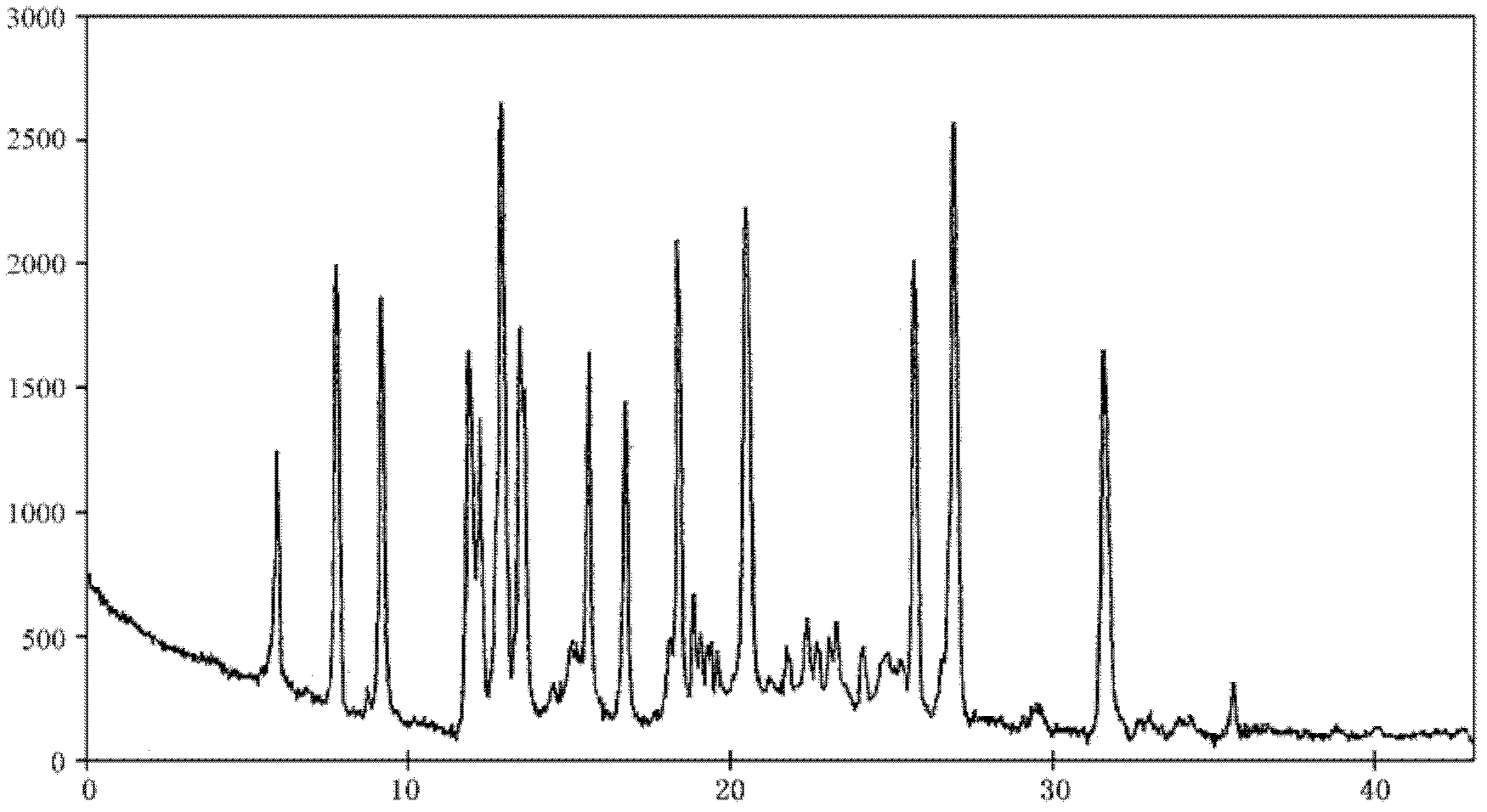
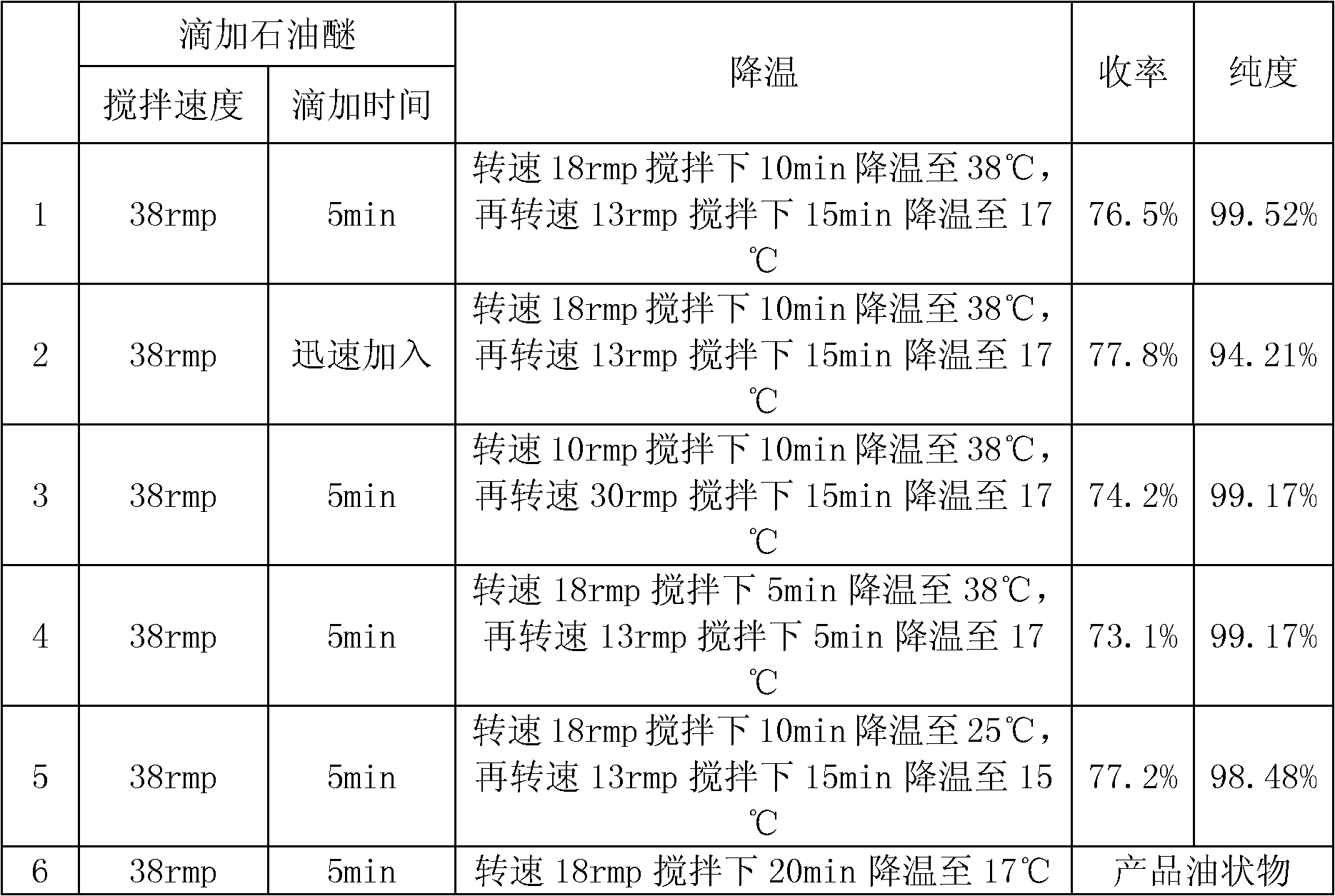
[0041] Get the crude product of lansoprazole, add volume and be that the volume ratio of 13 times of the crude product of lansoprazole is the acetone of 6: 3: 1: ethyl acetate: methanol solution, be heated to reflux; After the crude product of lansoprazole dissolves clear, Adding weight is that lansoprazole crude product weight 0.5 times active carbon decolorization 30min, microporous membrane filtration, under stirring, to filtrate drips the sherwood oil that volume is lansoprazole crude product weight 1.3 times, described stirring is 38rmp, described Dropping is to control the dropping time for 5 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 38°C for 10 minutes under stirring at a rotating speed of 18rmp, and then cool down to 17°C for 15min under stirring at a rotating speed of 13rmp, and stand for 18-20 minutes. hour, filter, with 6: 4 methanol: sherwood oil solution washing 2 times, each methanol: sher...
Embodiment 2
[0044] Get the crude product of lansoprazole, add volume and be that the volume ratio of 13 times of the crude product of lansoprazole is the acetone of 6: 3: 1: ethyl acetate: methanol solution, be heated to reflux; After the crude product of lansoprazole dissolves clear, Adding weight is that lansoprazole crude product weight 0.4 times active carbon decolorization 20min, microporous membrane filtration, under stirring, to filtrate drips the sherwood oil that volume is lansoprazole crude product weight 1.3 times, described stirring is 35rmp, described Dropping is to control the dropping time for 4 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 38°C for 40 minutes under stirring at a rotating speed of 20rmp, and then cool down to 18°C for 15min under stirring at a rotating speed of 14rmp, and stand for 18-20 minutes. hour, filter, with 6: 4 methanol: sherwood oil solution washing 2 times, each methanol: sh...
Embodiment 3
[0047] Get the crude product of lansoprazole, add volume and be that the volume ratio of 15 times of the crude product of lansoprazole is the acetone of 6: 3: 1: ethyl acetate: methanol solution, be heated to reflux; After the crude product of lansoprazole dissolves clear, Adding weight is that lansoprazole crude product weight 0.5 times active carbon decolorization 30min, microporous membrane filtration, under stirring, to filtrate drips the sherwood oil that volume is lansoprazole crude product weight 1.2 times, described stirring is 38rmp, described Dropping is to control the dropping time for 5 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 38°C for 10 minutes under stirring at a rotating speed of 18rmp, and then cool down to 17°C for 15min under stirring at a rotating speed of 13rmp, and stand for 18-20 minutes. hour, filter, with 6: 4 methanol: sherwood oil solution washing 2 times, each methanol: sher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com