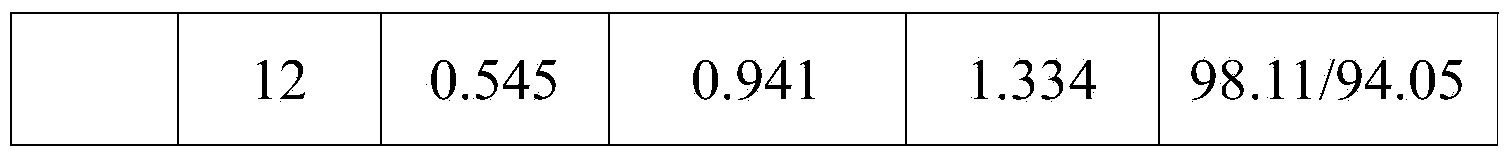Double-layer tablet containing ezetimibe and rosuvastatin and preparation method thereof
A technology of rosuvastatin and ezetimibe, which is applied in the field of compound double-layer tablets, can solve problems such as difficulty in in vitro dissolution, difficult release of ezetimibe, and poor dissolution of ezetimibe. The effect of reducing adverse drug reactions, increasing drug safety, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
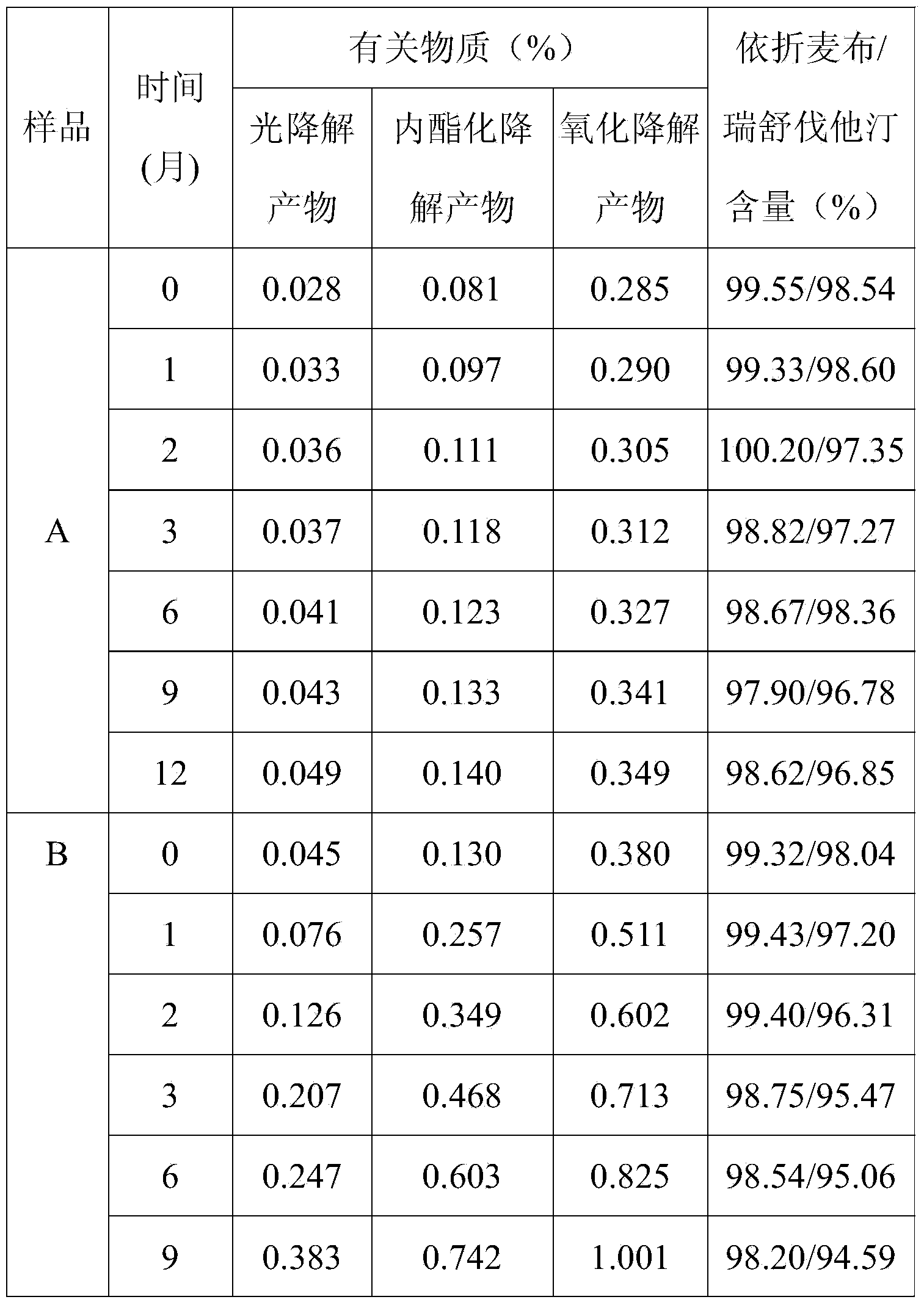
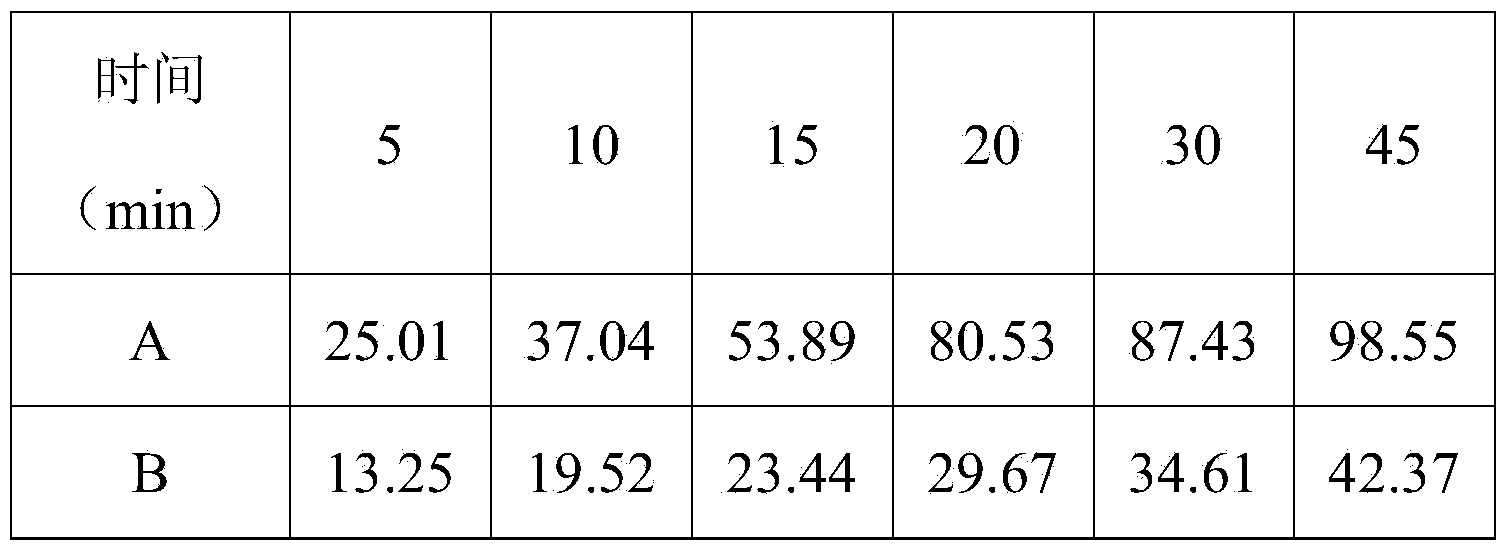
[0047] Example 1: A bilayer tablet containing ezetimibe and rosuvastatin and one of its preparations (prescription quantity: 1000 tablets)
[0048]
[0049] Preparation method: Ezetimibe is micronized in advance (see Example 6 for the specific implementation process), passed through a 200-mesh sieve, and the remaining raw and auxiliary materials are respectively passed through an 80-mesh sieve for later use. Ezetimibe layer: put ezetimibe, lactose, microcrystalline cellulose, croscarmellose sodium, and sodium lauryl sulfate into the wet granulator according to the composition ratio of the prescription and mix evenly, add 8 The solution of povidone K30 of % recipe is granulated, dried, granulated with a 20 mesh sieve, added with magnesium stearate and mixed evenly. Rosuvastatin layer: put rosuvastatin, lactose, microcrystalline cellulose, croscarmellose sodium, tert-butyl-4-hydroxyanisole, and meglumine into the wet process according to the composition ratio of the prescript...
Embodiment 2
[0050] Example 2: A bilayer tablet containing ezetimibe and rosuvastatin and its preparation 2 (prescription quantity: 1000 tablets)
[0051]
[0052] Preparation method: Ezetimibe is micronized in advance (see Example 6 for the specific implementation process), and passed through a 200-mesh sieve, and the remaining raw and auxiliary materials are respectively passed through an 80-mesh sieve for later use. Ezetimibe layer: Put ezetimibe, lactose, pregelatinized starch, carboxymethyl starch sodium, and Tween-80 into the wet granulator according to the composition ratio of the prescription and mix them evenly, and add 8% of the prescription amount of polysaccharide The vitamin K90 solution is granulated, dried, sized with a 20-mesh sieve, added with silicon dioxide and mixed evenly. Rosuvastatin layer: put rosuvastatin, lactose, pregelatinized starch, sodium carboxymethyl starch, propyl gallate, and disodium hydrogen phosphate into a wet granulator according to the compositio...
Embodiment 3
[0053] Example 3: A bilayer tablet containing ezetimibe and rosuvastatin and its preparation 3 (prescription quantity: 1000 tablets)
[0054]
[0055] Preparation method: Ezetimibe is micronized in advance (see Example 6 for the specific implementation process), and passed through a 200-mesh sieve, and the remaining raw and auxiliary materials are respectively passed through an 80-mesh sieve for later use. Ezetimibe layer: put ezetimibe, microcrystalline cellulose, pregelatinized starch, crospovidone, and Tween-80 into the wet granulator according to the composition ratio of the prescription and mix evenly, add 10% of the prescription A certain amount of ethyl cellulose solution is granulated, dried, granulated with a 20-mesh sieve, added with glyceryl behenate and mixed evenly to obtain the product. Rosuvastatin layer: granulate rosuvastatin, microcrystalline cellulose, pregelatinized starch, crospovidone, citric acid, sodium hydroxide, and 10% prescription ethylcellulose ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com