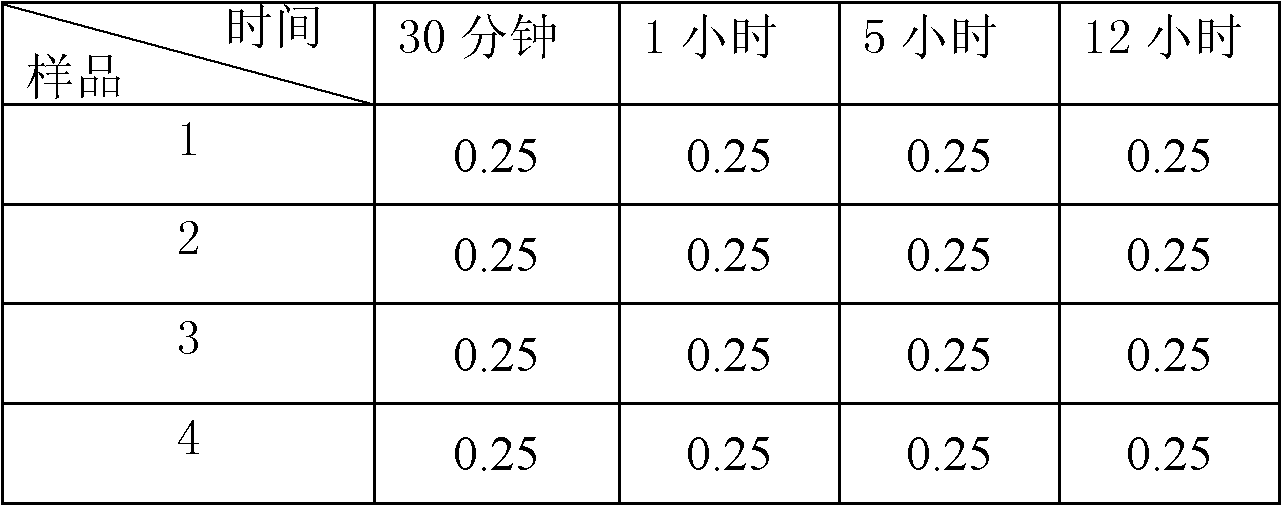
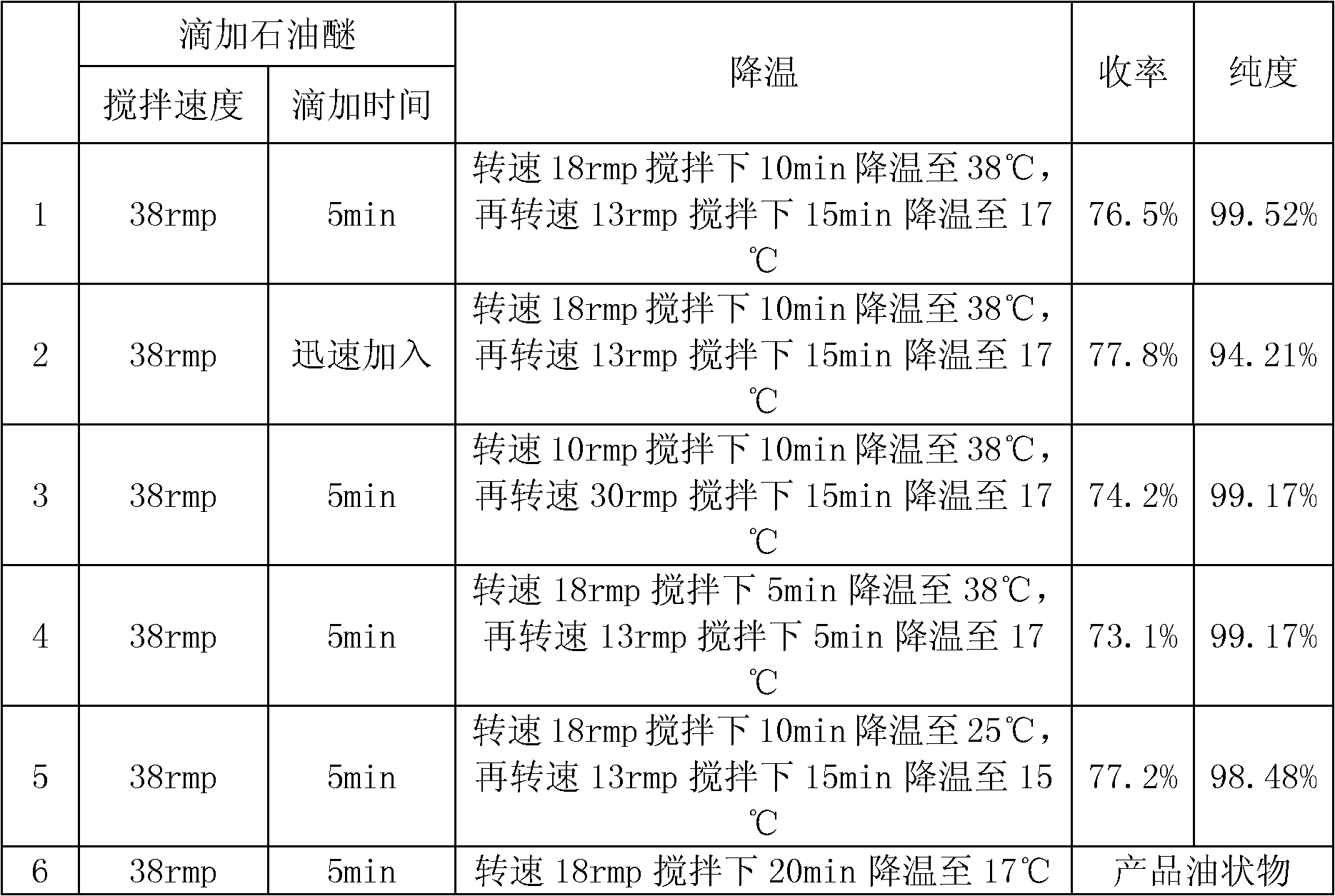
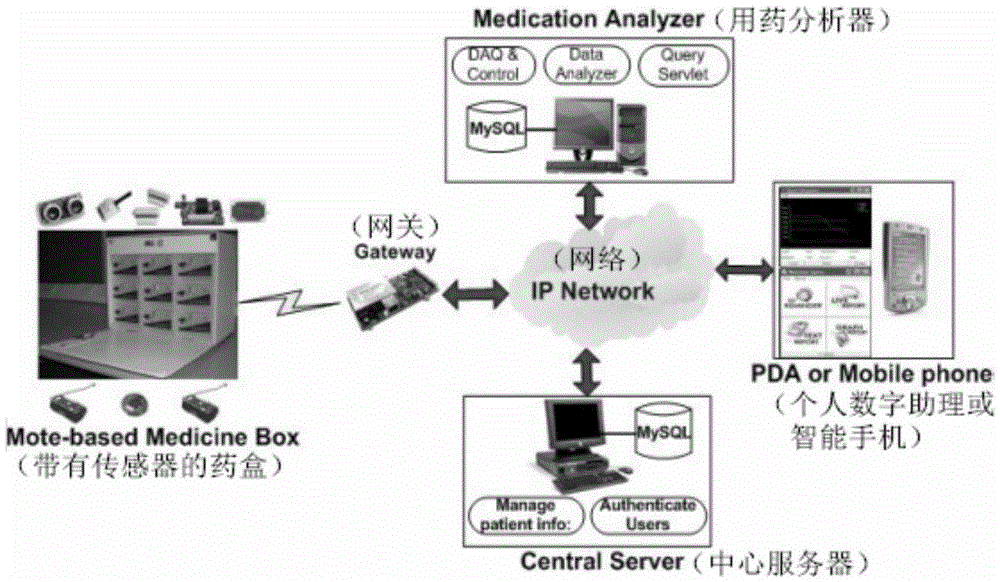
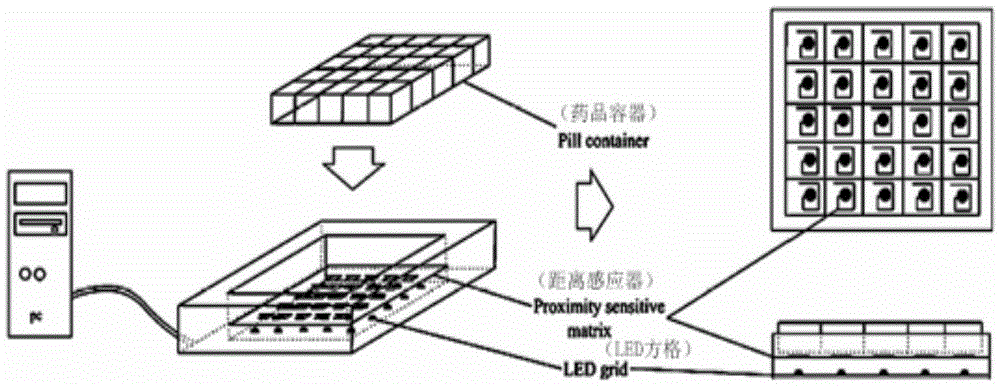
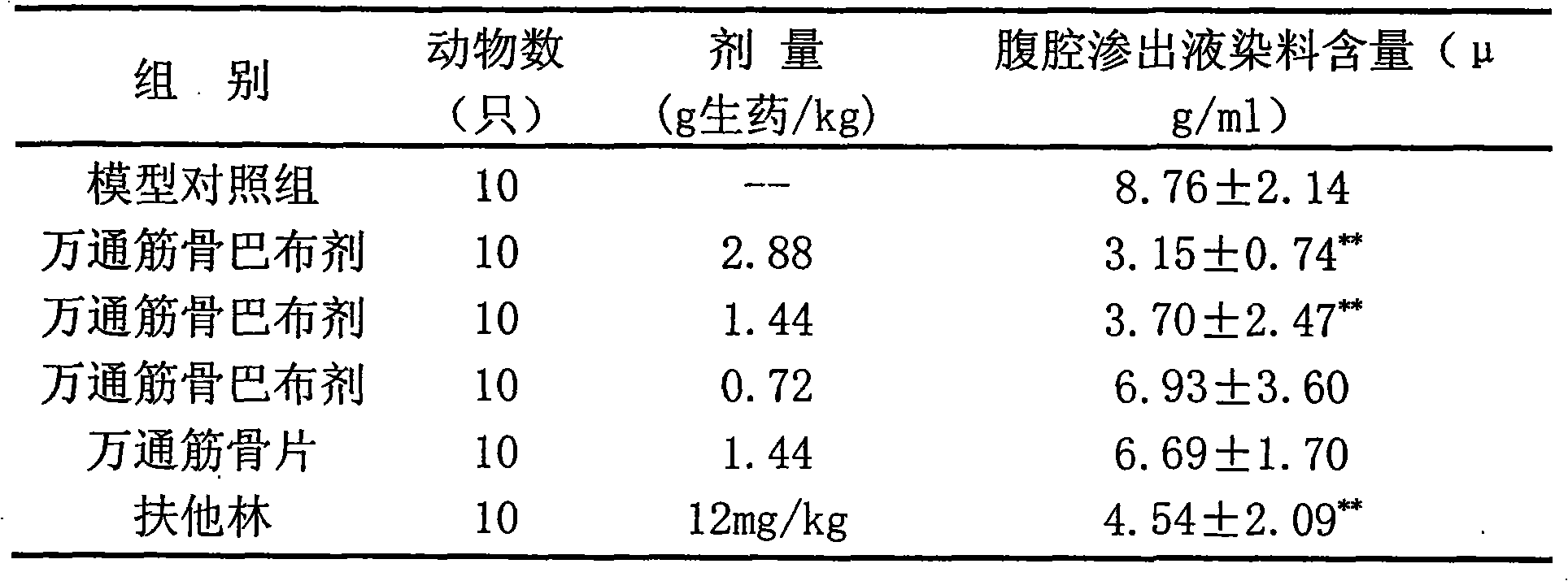
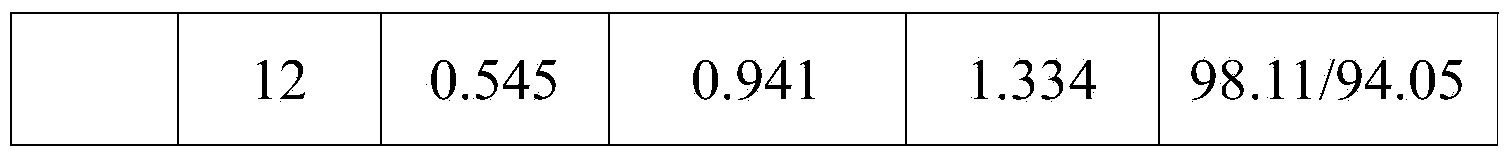Patents
Literature
792results about How to "Improve medication safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Soluble epoxide hydrolase inhibitor
ActiveCN102093320ARegulate blood pressureProtectiveOrganic active ingredientsSulfonic acid amide preparationHydrogenBlood pressure
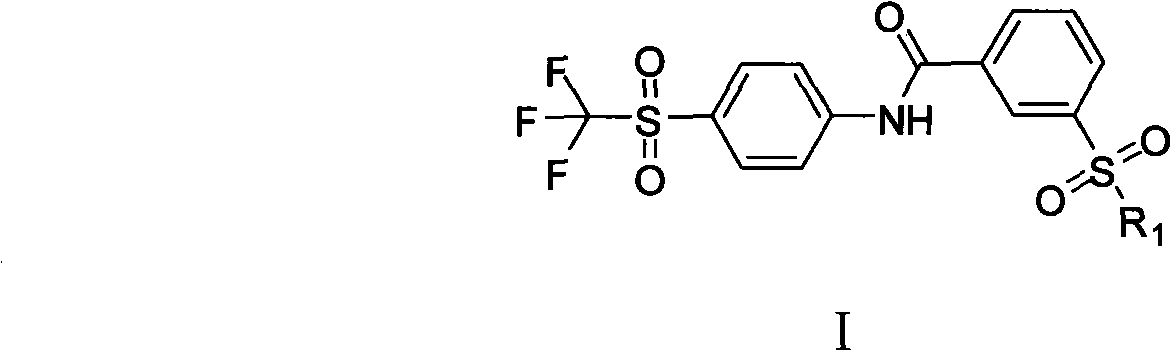
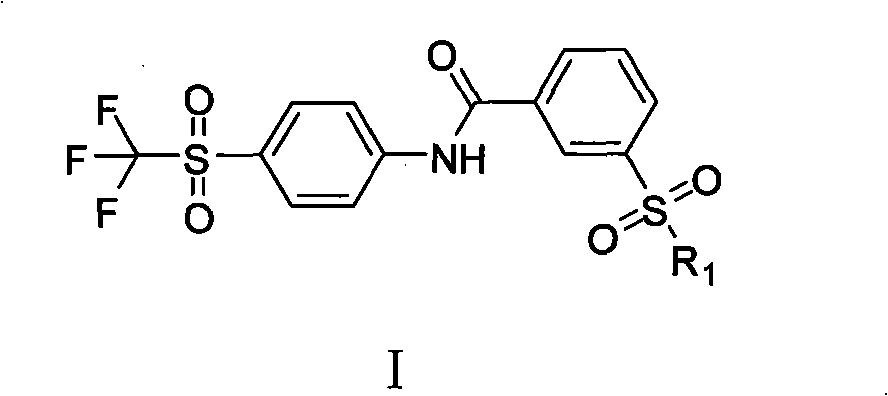
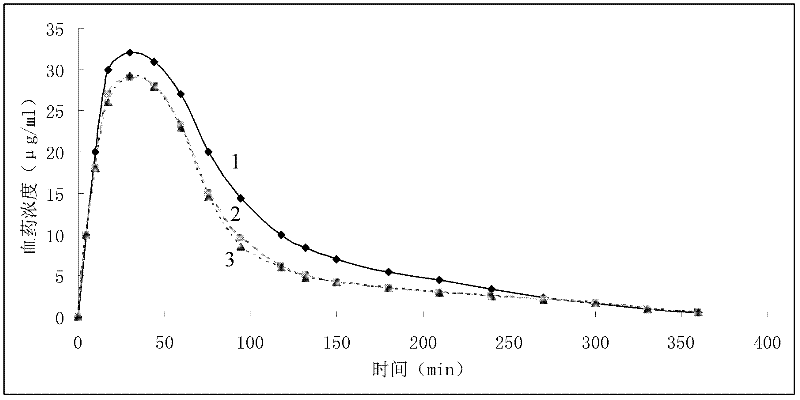
The invention provides a soluble epoxide hydrolase inhibitor, which has the following structural formula I, wherein R1 is N-R2R3; R2 and R3 are hydrogen, C1-C4 alkyl, aromatic ring, aromatic heterocyclic ring, or C1-C4 alkyl connected with the aromatic ring or the aromatic heterocyclic ring respectively; and the R2 can also be connected with the R3 to form a ring, then R1 is a quinary, hexahydricor heptabasic heterocyclic ring containing 1 nitrogen atom or 1 oxygen atom, and the nitrogen atom can be directly connected with the hydrogen and can also be connected with the C1-C4 alkyl. Rat experiment group results in an anesthesia state and a non-anesthesia state show that the inhibitor has certain blood pressure reducing effect, can be used for preparing medicaments for preventing and treating cardiovascular diseases, and has broad clinical application prospect. The invention provides a preparation method.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Freeze-dried powder injection of pantoprazole sodium and its preparation
ActiveCN1679563ALittle side effectsImprove stabilityOrganic active ingredientsPowder deliveryDisodium EdetateFreeze-drying
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
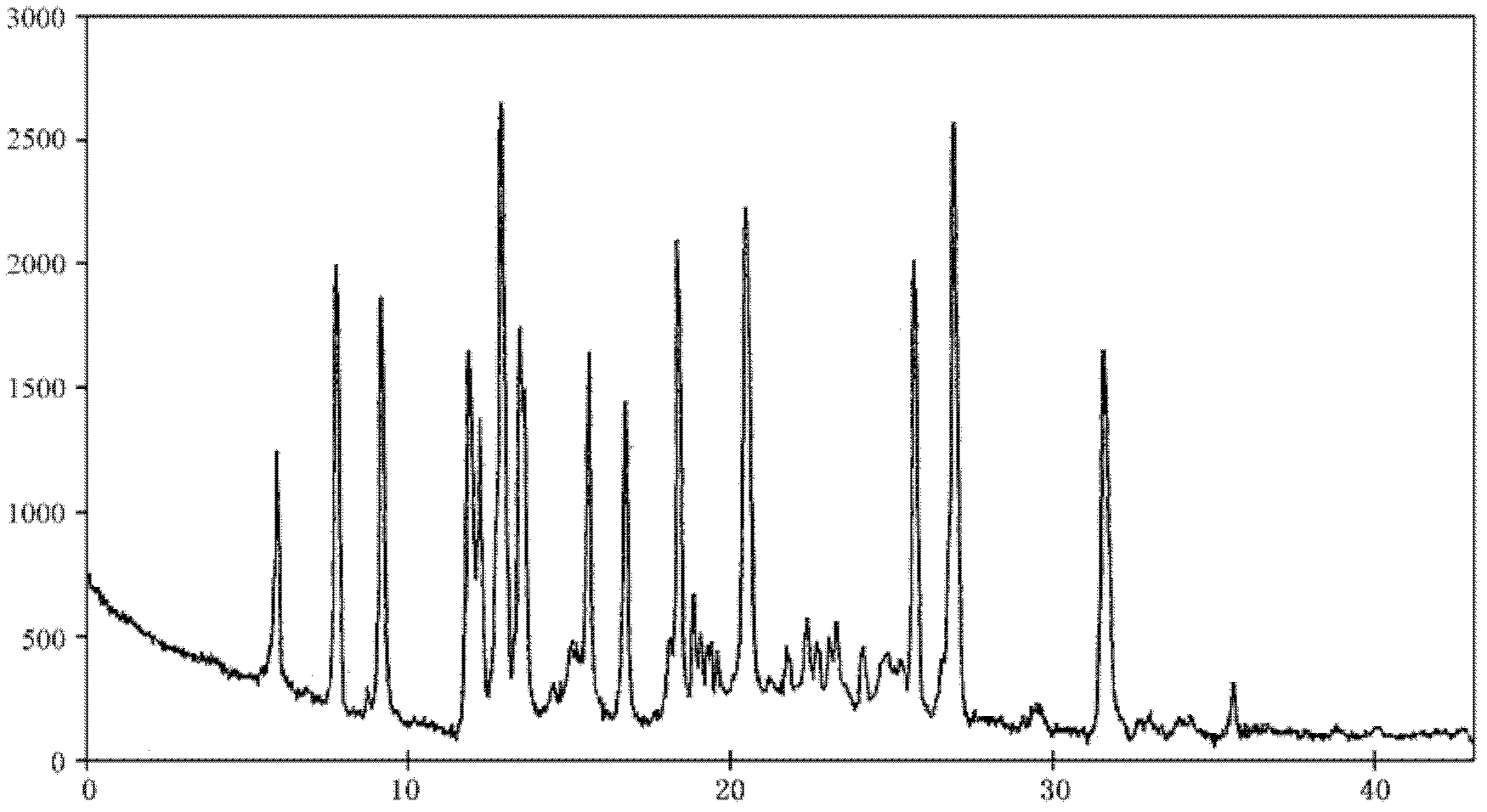
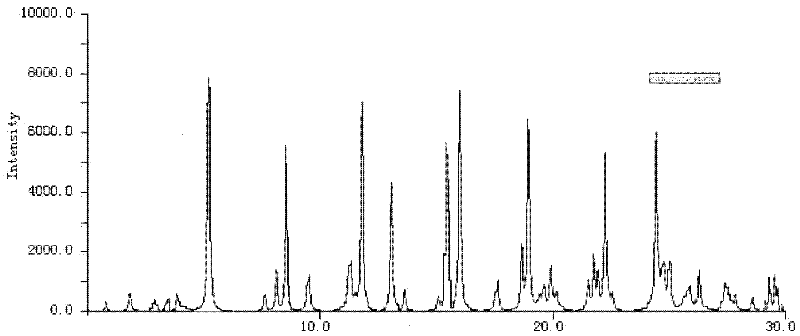
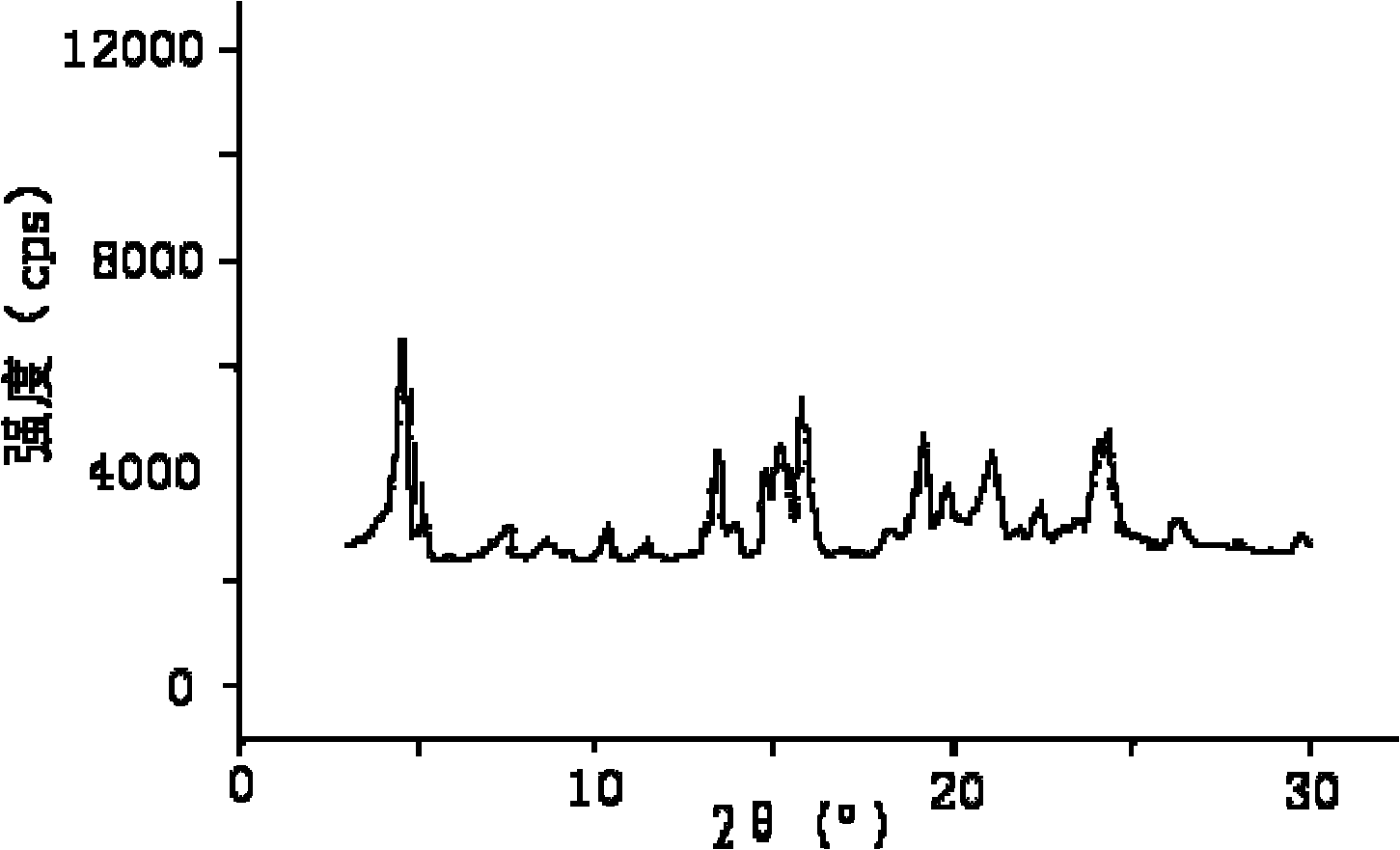
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
Fat-decreasing biscuit
InactiveCN101073332AReduce irreversible liver damageReduce fatty degeneration and necrosis of liver cellsDough treatmentMetabolism disorderDigestionLoss weight
The invention is concerned with the food combination and preparation method for digestion and reducing fat, the compositions and ratio is: red sage root, hawkthorn, fleece-flower root, notoginseng, chrysanthemum, hemlock parsley, medlar, purslane, which is better loss weight effect, and lower price.
Owner:FANGXIA ENTERPRISE INFORMATION CONSULTING WUJIANG
Oryzanol composition and preparation method thereof
InactiveCN101480405AStable in natureGood water solubilityOrganic active ingredientsNervous disorderSolubilityFreeze-drying
The invention discloses a pharmaceutical composition of oryzanol, which comprises the oryzanol, phospholipid, bile acid and / or salt of bile acid. The pharmaceutical composition effectively improves the water solubility and the stability of the oryzanol and has better grain diameter and stability than the prior product, and further, the invention provides injectio and freeze-dried powder of the pharmaceutical composition, and preparation methods thereof.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
Old people medication system based on Android platform
ActiveCN105380801ASimple and fast operationEasy to operateOral administration deviceOlder peopleMedication information
The invention provides an old people medication system based on an Android platform, relates to the technical field of old people medication assistant software development, and aims at solving the problems that old people cannot accurately take medicine on time and at the proper dose. The old people medication system based on the Android platform comprises a client side and a server which achieve medication information interaction, wherein the client side comprises a medicine barcode scanning and analyzing module, a voice reading module and a timing medicine taking reminding module which are directly operated by a user; the medicine barcode scanning and analyzing module is used for scanning a medicine barcode identification and analyzing acquired medicine barcode information; the client side is used for sending a query request, and the server is used for responding the query request of the client side, receiving the query request of the client side and a medicine barcode and generating a statement query medicine information database recognized by a medicine information database to query the medicine information database according to the submitted query request and the corresponding medicine barcode information; the voice reading module is used for presenting medication information to a user of the client side and helping the user accurately acquire medicine information to improve the medication safety; the timing medicine taking reminding module is used for helping the user to take the medicine on time and at the proper dose according to a set medicine taking time.
Owner:HARBIN INST OF TECH
Individual drug dosage adjustment method and device
InactiveCN105844112AAvoid the risk of after-the-fact adjustmentsImprove medication safetyMolecular designComputer-assisted medicine prescription/deliveryDosage adjustmentMedicine
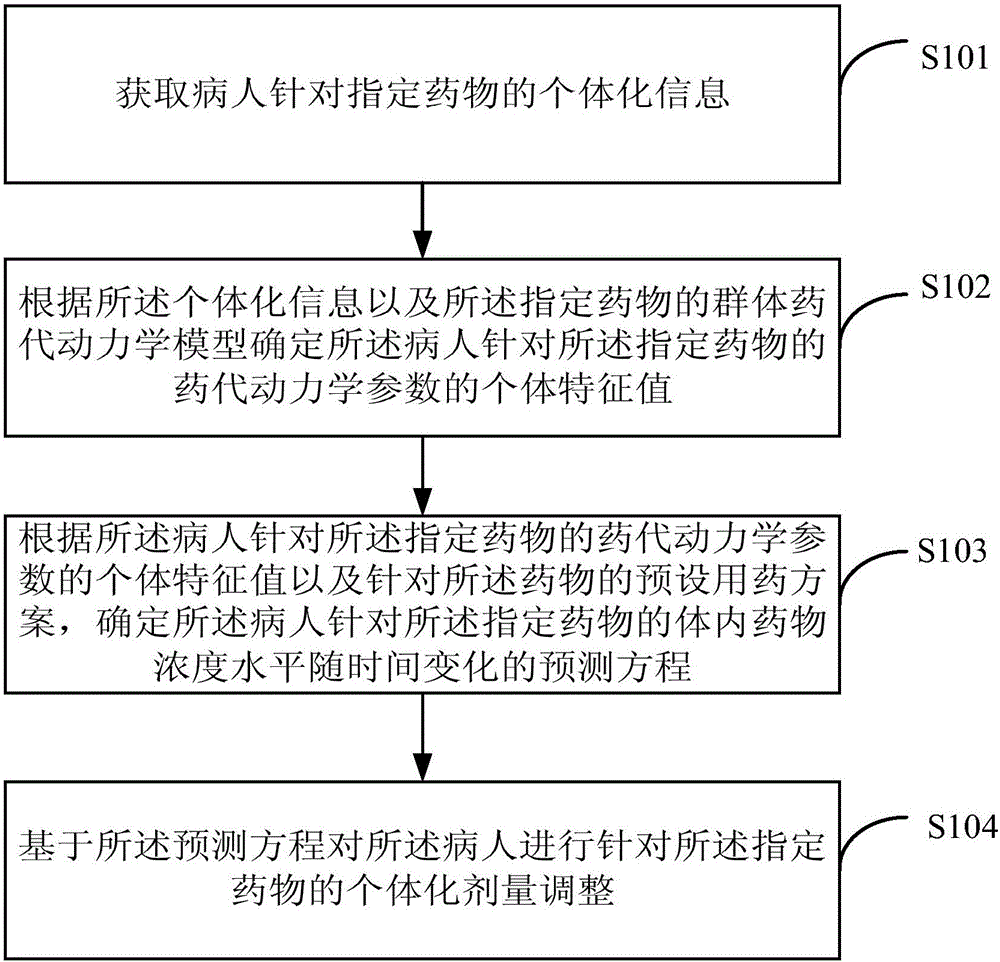
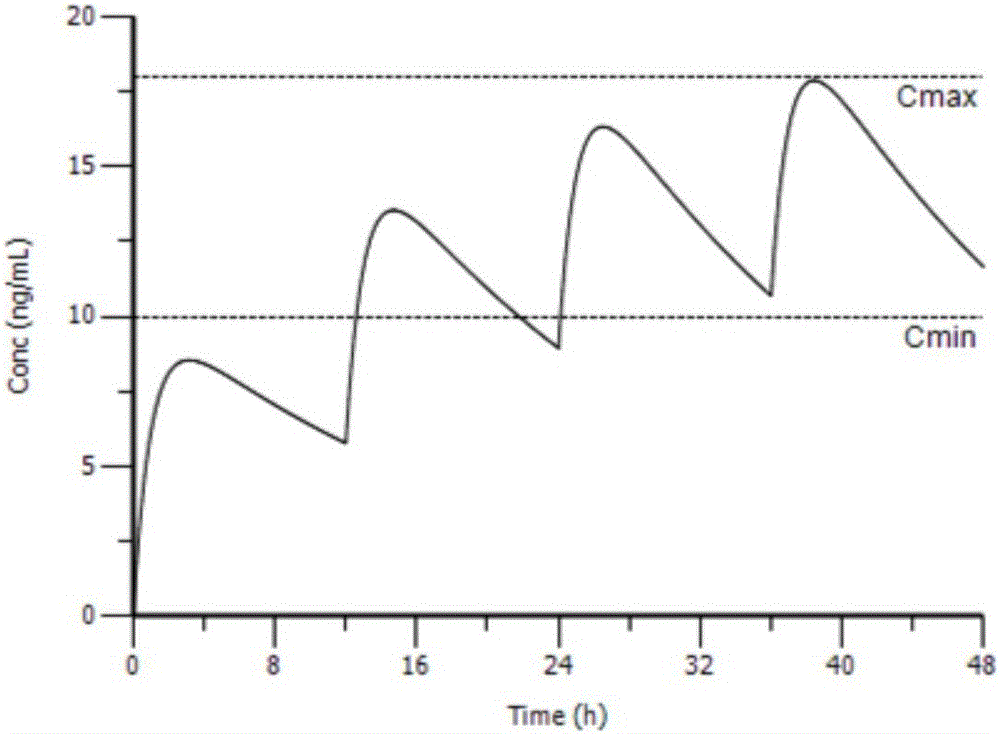
The invention provides an individual drug dosage adjustment method and device. The method comprises steps as follows: the individual feature value of a pharmacokinetic parameter of a designated drug for a patient is determined according to individual information and a group pharmacokinetic model of the designated drug; a time-variable prediction equation of the drug concentration level of the designated drug for the patient is determined according to the individual feature value of the pharmacokinetic parameter of the designated drug for the patient as well as a preset medication plan of the drug; individual dosage adjustment of the designated drug is performed on the patient on the basis of the prediction equation. The concentration of the drug in every patient corresponding to a preset medication plan is accurately predicted according to the individual information of the patient, accordingly, the preset medication plan is adjusted according to a prediction result, and risks caused by adjustment afterwards are avoided.
Owner:BEIJING DRYAS PHARMA TECH CO LTD
Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
ActiveCN102358744AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCefoxitin SodiumSodium benzoate
The invention relates to a cefoxitin sodium crystal compound. The method of powder X-ray diffractometry is utilized to determine the cefoxitin sodium crystal compound, and an X-ray powder diffraction pattern represented by the diffraction angle of 2theta+-0.2 degrees has characteristic diffraction peaks at 5.3 degrees, 8.6 degrees, 11.9 degrees, 13.2 degrees, 15.5 degrees, 16.1 degrees, 19.0 degrees, 22.3 degrees and 24.4 degrees. The invention also relates to a cefoxitin sodium composition powder injection containing the cefoxitin sodium crystal compound, and the cefoxitin sodium composition powder injection comprises 95 to 100 parts of the cefoxitin sodium crystal compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres, and preparation method thereof
InactiveCN102302458ASmall particle size distributionFlat surfaceAntibacterial agentsOrganic active ingredientsWound dressingMicrosphere
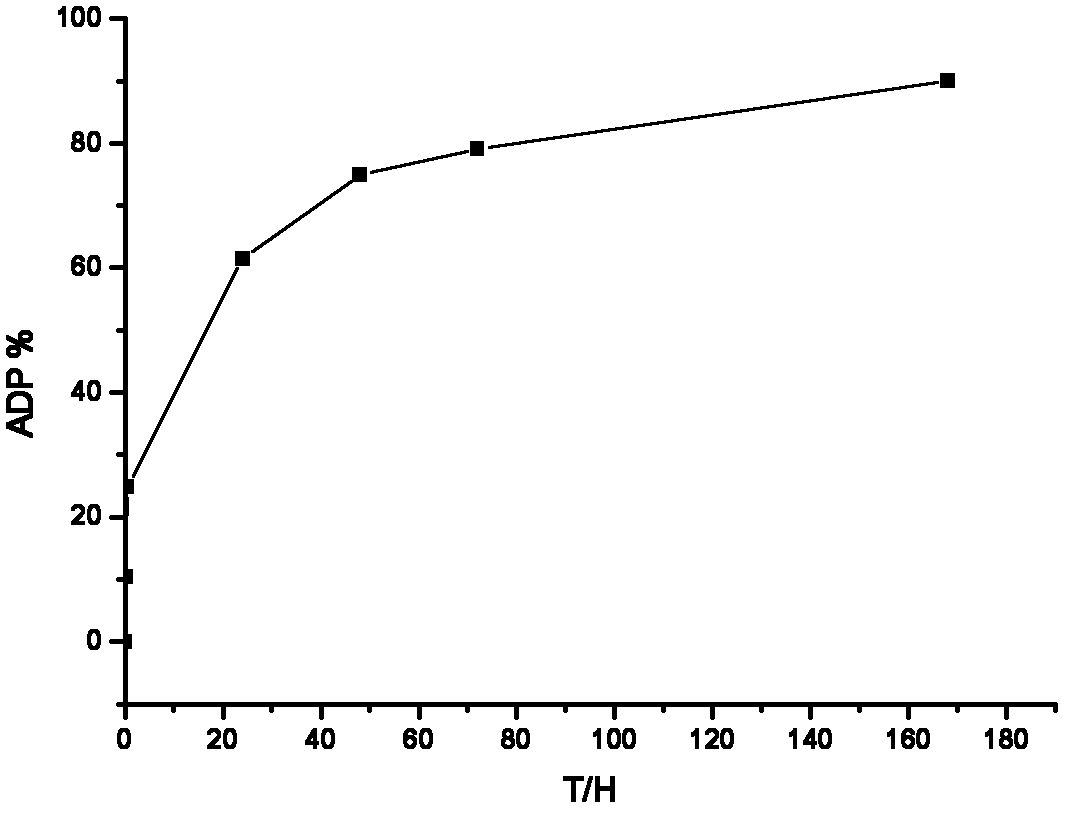
The invention discloses a long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres. According to the preparation method of the wound dressing, levofloxacin sustained-release microspheres with grain diameter between 10 and 20mu m are fixed in a composite medical non-woven fabric which mainly comprises chitosan fiber, alginate fiber, viscose fiber, and hydrophobic ethylene propylene fiber; and the long-acting sustained-release wound dressing is prepared by taking the levofloxacin sustained-release microspheres as a medicinal component. In a use process, the medicament quickly release levofloxacin after contacting the blood, tissues and surrounding skin of the wound, and can continuously release levofloxacin for a long time, and the effective release of levofloxacin accumulatively reaches 168h. The long-acting sustained-release wound dressing is applied to treatment of bacterium infection of burnt and scalded skin, and the microsphere state medicament can remarkably improve the bioavailability of effective components of the medicament; and the long-acting sustained-release wound dressing has excellent moisture penetrability, hygroscopicity, mechanical tensile property and biocompatibility, the frequency of dressing change can be reduced, and the curative effect and administration safety cam be enhanced.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
Pharmaceutical combination with repaglinide and metformin as active components and preparation method thereof
InactiveCN102218064AEasy to recycleAchieve separationOrganic active ingredientsOrganic chemistryAdhesiveActive component
The invention relates to a pharmaceutical combination with repaglinide and metformin as active components and a preparation method thereof. The pharmaceutical combination comprises 0.1-10 weight portions of repaglinide and 100-1500 weight portions of metformin as active components and pharmaceutic adjuvants, wherein, the pharmaceutic adjuvants comprise filler, disintegrating agent, adhesive, flavoring agent, lubricant and swelling accessory, and the repaglinide is repaglinide crystal. The invention adopts the repaglinide crystal with small particle size, metformin and pharmaceutic adjuvants to prepare the pharmaceutical combination, so as to realize the purpose of simultaneous release. Since the particle size of the repaglinide used in the invention is small, the dissolvability is improved, so that the dissolution is improved and the bioavailability is increased.
Owner:HAINAN JINRUI PHARMA CO LTD
Improved method for preparing amoxicillin by enzymic method
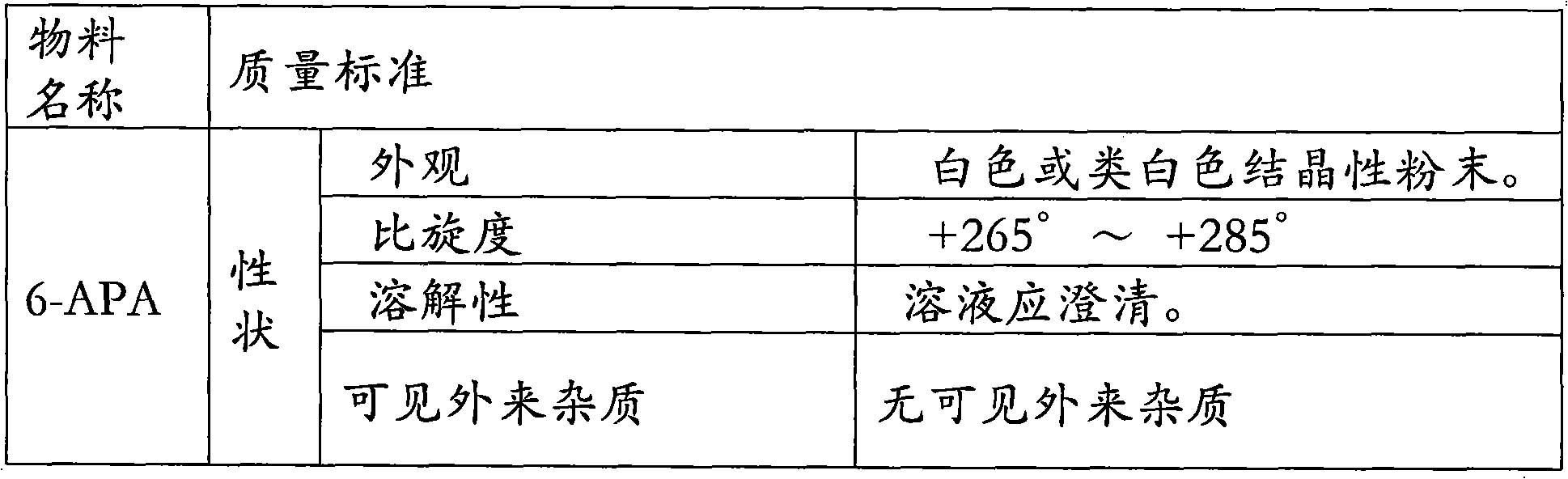
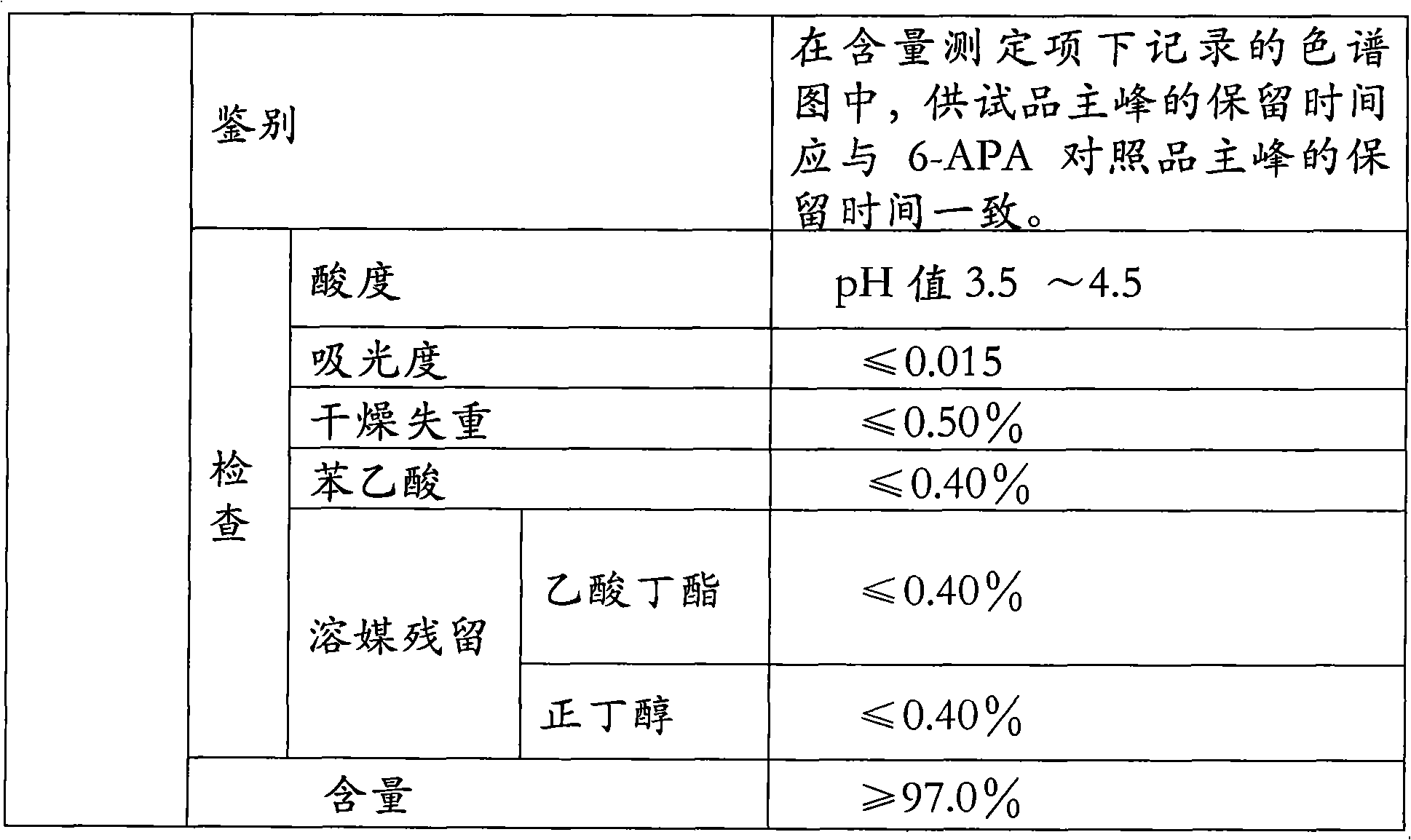
The invention relates to the field of pharmacy, and provides an improved method for preparing amoxicillin by an enzymic method, and a product obtained by the improved method for preparing amoxicillin by the enzymic method. The method comprises the following steps of: 1) dissolving 6-aminopenicillanic acid (6-APA) at the temperature of between 10 and 20 DEG C by using water or / and aqueous solution of ammonia which has the pH value of 7.0 to 8.0, and adding D-p-Hydroxyphenylglycine methyl ester hydrochlorid and penicillin G acyltransferase; 2) adjusting the pH value of a solution obtained in the step 1) to be 6.0 to 6.5, and reacting at the temperature of between 21 and 30 DEG C until the content of 6-APA is less than 5mg / ml to obtain a solution of an amoxicillin product; and 3) separating the penicillin G acyltransferase from the solution of the amoxicillin product, adjusting by using hydrochloric acid until the solution of the amoxicillin product is clarified, adding the aqueous solution of ammonia, adjusting the pH value to be 5.5 to 6.5, and crystallizing at the temperature of between 0 and 5 DEG C to obtain amoxicillin. By the improved method for preparing amoxicillin by the enzymic method, the quality of the amoxicillin product is greatly improved, and the medication safety of the amoxicillin product is further improved.
Owner:UNITED LAB INNER MONGOLIA CO LTD
A kind of cefminox sodium crystalline compound and its composition powder injection
ActiveCN102276630AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCEFMINOX SODIUMX-ray
The invention relates to a cefminox sodium crystalline compound. The cefminox sodium crystalline compound is determined by a powder X-ray diffraction determination method; and characteristic diffraction peaks are displayed at 5.1, 6.9, 8.5, 10.3, 12.1, 15.1, 15.9, 17.4, 19.5, 21.7 and 24.6 degrees in an X-ray powder diffraction pattern represented by a diffraction angle of 2 theta+ / -0.2 degree. The invention also relates to cefminox sodium composition powder injection containing the cefminox sodium crystalline compound. The composition powder injection comprises the following components: 95 to 100 parts of cefminox sodium crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Stavudine sustained release tablet and its preparing process
InactiveCN1634116AEnsure stabilityImprove medication safetyOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletAdjuvant
The invention relates to stavudine sustained release tablet and its preparing process, wherein the epicuticle comprises Stavudine and gel matrix core as medicinal adjuvant, the weight ratio of the Stavudine and gel matrix core is 1 : 0.68-2.32. The preparing process comprises the steps of making core and dressing.
Owner:NORTHEAST PHARMA GRP
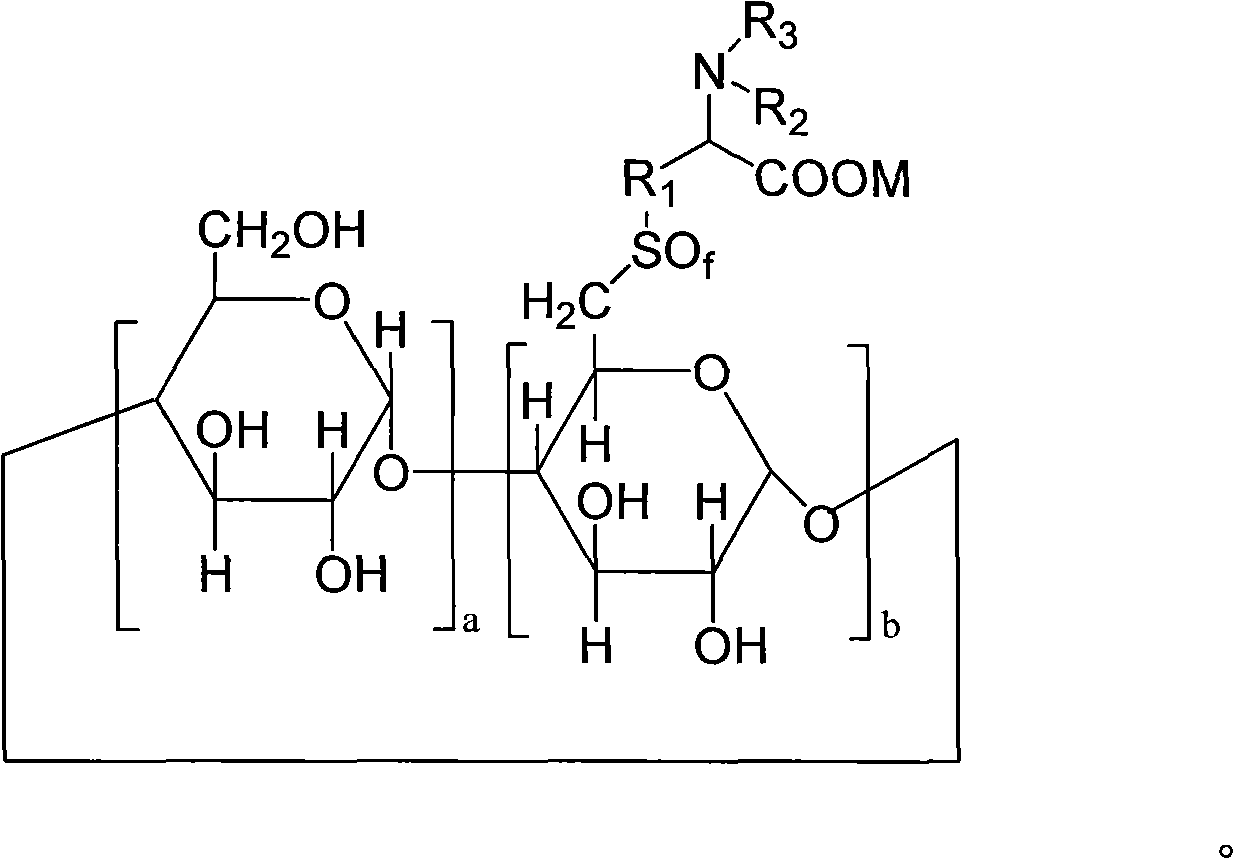
6-deoxy alpha-amino acid derivative cyclodextrin, preparation and application thereof
ActiveCN102060941AThe preparation process is stableHigh yieldOrganic active ingredientsMuscular disorderCyclodextrin derivativeThioether
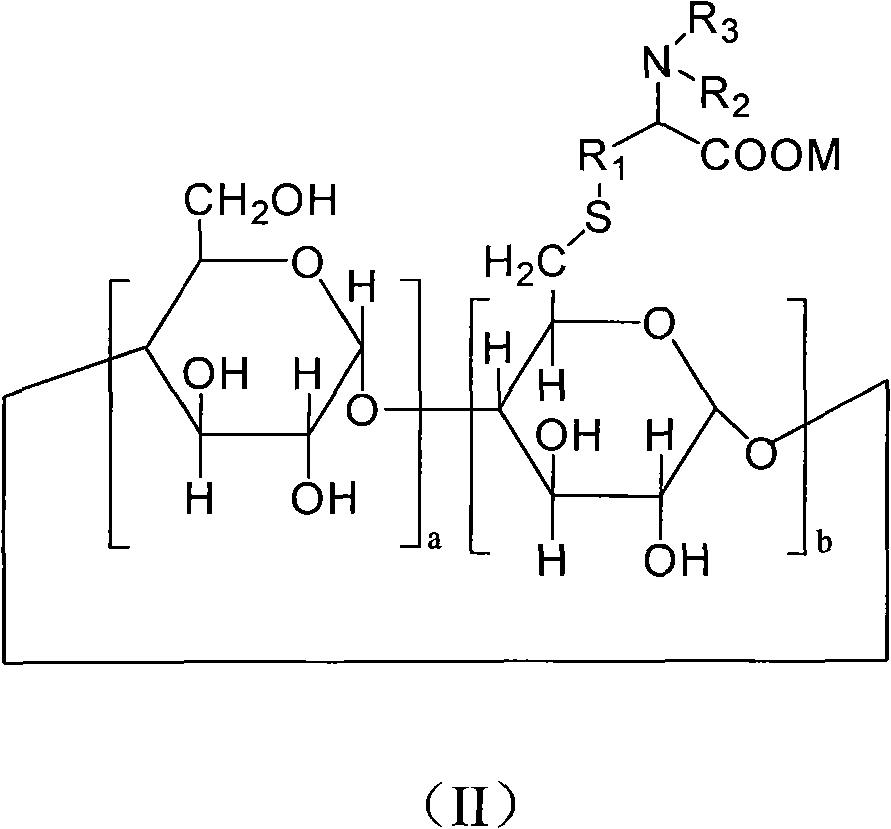
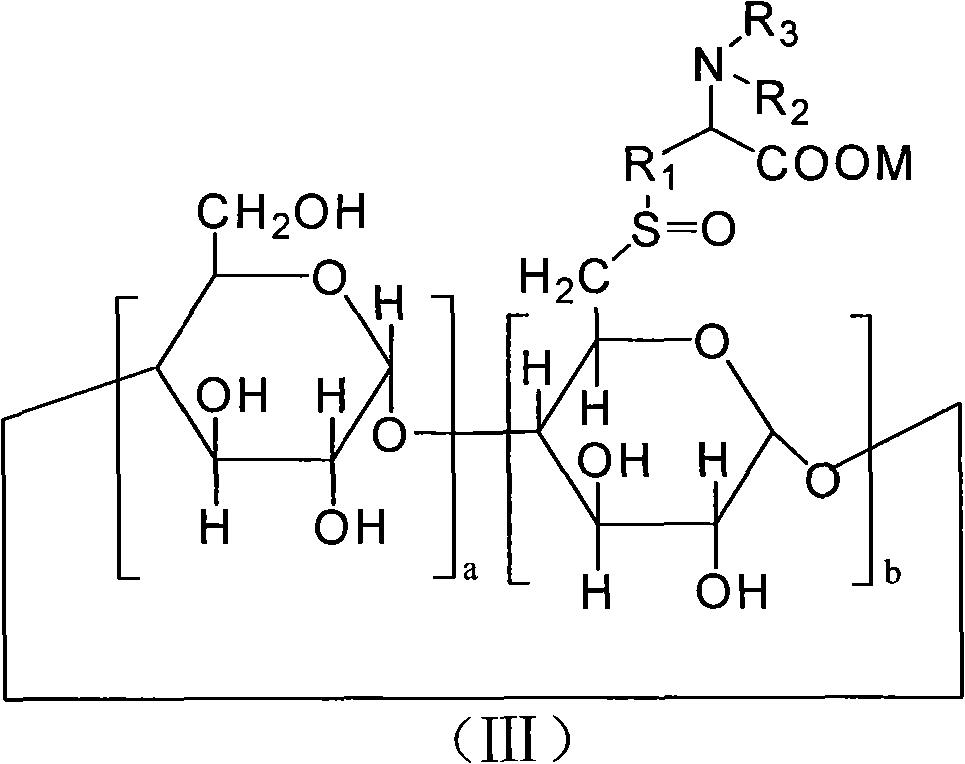
The invention provides 6-deoxy alpha-amino acid derivative cyclodextrin which is a target substance obtained by the condensation of an amino acid derivative and halogenated cyclodextrin in the presence of alkali. The 6-deoxy alpha-amino acid cyclodextrin derivatives comprise a 6-deoxy thioether alpha-amino acid derivative cyclodextrin, a 6-deoxy sulfoxide alpha-amino acid derivative cyclodextrin and a 6-deoxy sulfonyl alpha-amino acid derivative cyclodextrin. The compounds provided by the invention can be used for reversing the phenomenon of nerve muscle relaxation induced by human or animal muscle relaxants, have rapid reversing and antagonizing effects on muscle relaxation induced by the muscle relaxants, can be used for preparing medicaments with muscle relaxation antagonizing effect, have the characteristics of low production cost and wide muscle relaxation reversing and antagonizing range and have effectively increased clinical application range. More importantly, the safe dose of the compounds provided by the invention is increased by at least one time compared with the known compounds. The general formula (I) of the compounds is shown in the specifications.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
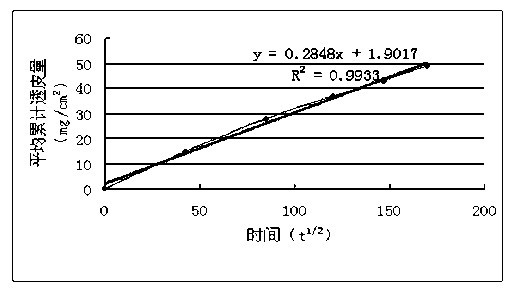
Microemulsion transdermal gel agent of butylphthalide or derivative thereof, and preparation method thereof
ActiveCN102178643APromote transdermal absorptionEasy to preparePharmaceutical delivery mechanismEster active ingredientsMedicineCentrifugation
The invention relates to a microemulsion transdermal gel agent of butylphthalide or a derivative thereof, and a preparation method thereof, and belongs to the technical field of medicaments. The technical scheme is that: the microemulsion transdermal gel agent is prepared mainly from the following ingredients in part by weight: 1 to 5 parts of oil phase, 1 to 5 parts of emulsifying agent, 0.1 to 1 part of auxiliary emulsifying agent, 1.5 to 15 parts of purified water and 0.003 to 0.5 part of transdermal accelerator. Compared with a comparison example, the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof almost does not influence the microemulsion appearance and granular size under the conditions of centrifugation and high temperature, and is a dynamic and thermodynamic stable system. An in vitro transdermal performance test proves that the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof has good transdermal absorbability. In conclusion, the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof has the simple preparation method, does not need the procedures of high-speed cutting or homogenizing and the like, has small product granular size, and contributes to industrialization.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Lyophilization process of glutathione for injection
InactiveCN1994461AQuality improvementHigh yieldPowder deliverySenses disorderFreeze-dryingEngineering
The invention relates to a freezing technique of injection reduction glutathione, wherein it comprises that: packing liquid reduction glutathione; freezing and drying. The invention can standardize the freezing technique of injection reduction glutathione, with stable product quality and high yield, to improve intake safety.
Owner:上海复旦复华药业有限公司
A kind of cefoperazone sodium tazobactam sodium pharmaceutical composition
ActiveCN102274233AReduce security risksFew kindsAntibacterial agentsHeterocyclic compound active ingredientsPowder diffractionSubstance content
The invention relates to a medicinal composition of cefoperazone sodium and tazobactam sodium. The medicinal composition comprises the following components in part by weight: 4 to 8 parts of cefoperazone sodium and 1 part of tazobactam sodium, wherein the tazobactam sodium is measured by a powder X-ray diffraction measuring method, and characteristic diffraction peaks are shown at the positions of 6.9 degrees, 10.5 degrees, 11.4 degrees, 16.6 degrees, 19.2 degrees, 22.7 degrees, 27.0 degrees, 29.7 degrees and 33.5 degrees in an X-ray powder diffraction map expressed by a diffraction angle of between 2 theta+ / -0.2 degree. The medicinal composition has the advantages of high stability, low relevant substance content, controllable quality and the like, and the administration safety of patients is improved. The invention also relates to a method for preparing the tazobactam sodium with the technical characteristics of the characteristic diffraction peaks.
Owner:江西璟瑞药业有限公司
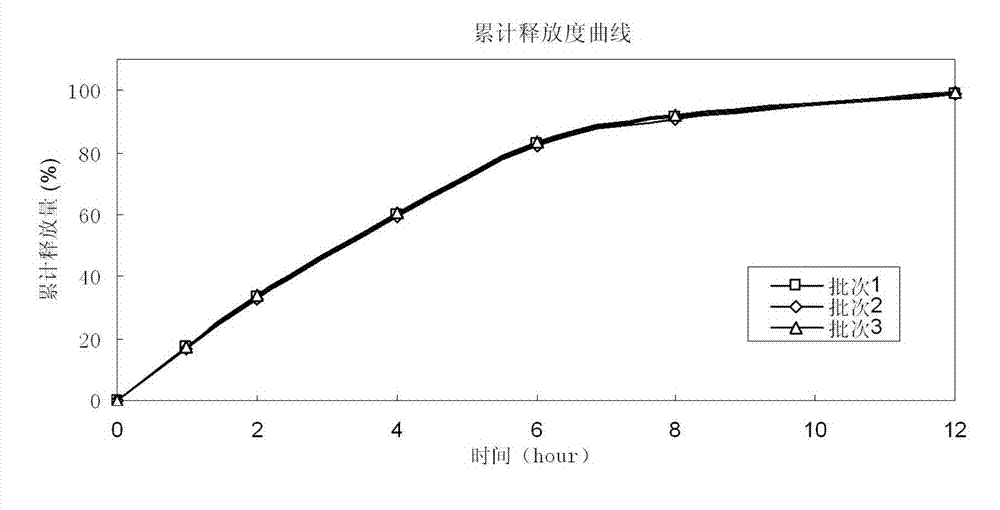
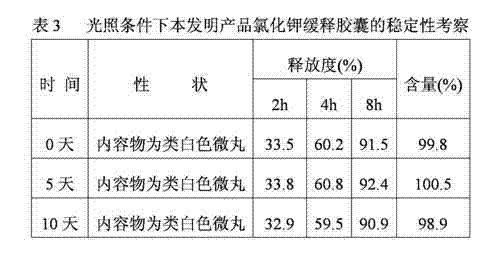
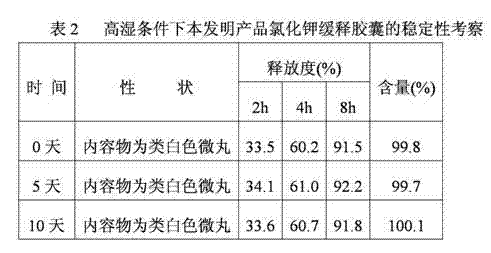
Potassium chloride slow release capsule
ActiveCN102961363ASimple manufacturing processSolve problems such as complianceMetabolism disorderGranular deliveryPlasticizerLong term treatments
The invention discloses a potassium chloride slow release capsule. A content of the potassium chloride slow release capsule is a slow release micro-pill; and the slow release micro-pill consists of 70-97 percent by weight of the potassium chloride used as a raw material, 0-10 percent by weight of forming materials, 2-20 percent of slow release materials, 0.5-5 percent of plasticizers and 0-10 percent of antisticking agents. The potassium chloride and the forming materials are uniformly mixed; a medicine carrier micro-pill is prepared into a dry type granulator; the slow release materials, the plasticizers and the antisticking agents are mixed to prepare slow release layer coating solution; the medicine carrier micro-pill is put into a fluidized bed; the prepared slow release layer coating solution is ejected into the fluidized bed; the medicine carrier micro-pill is coated according to the conventional method so as to prepare the slow release micro-pill; and the slow release micro-pill is filled into the capsule so as to obtain the potassium chloride slow release capsule. The prepared potassium chloride slow release capsule is low in toxin side effects, convenient to treat patients for a long time, improves the medicine safety and improves the compliance of the medicines.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
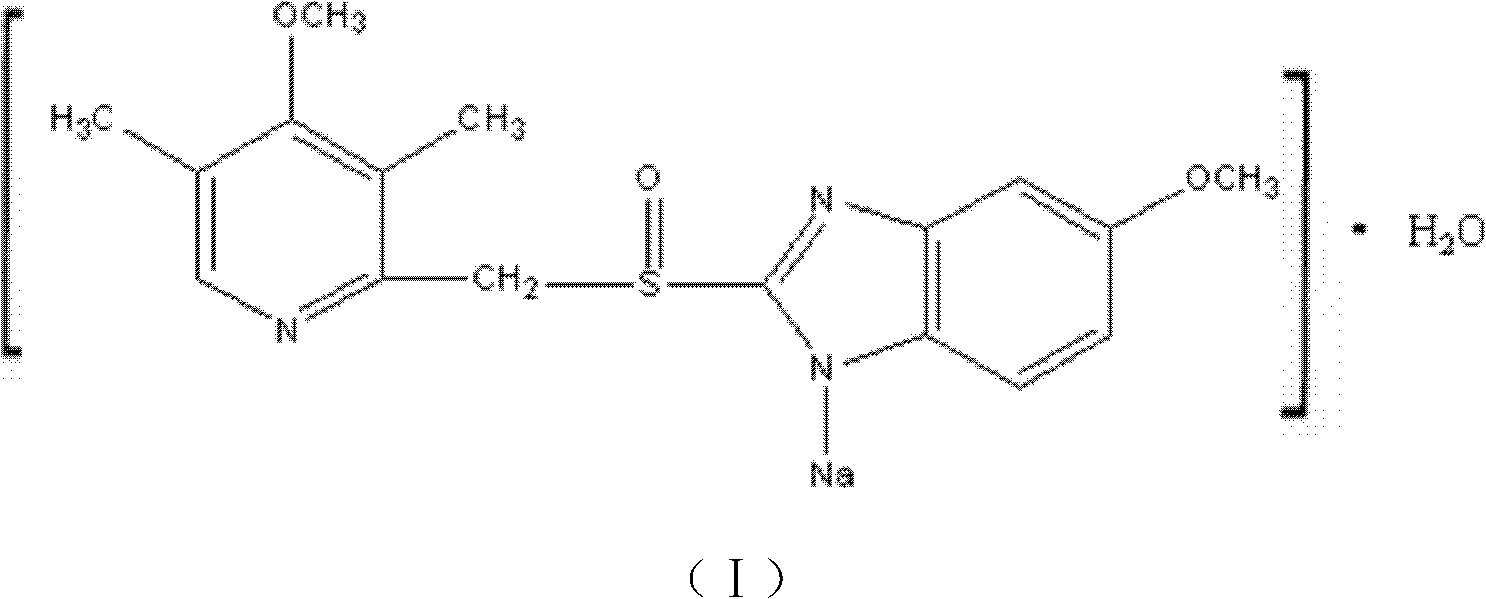
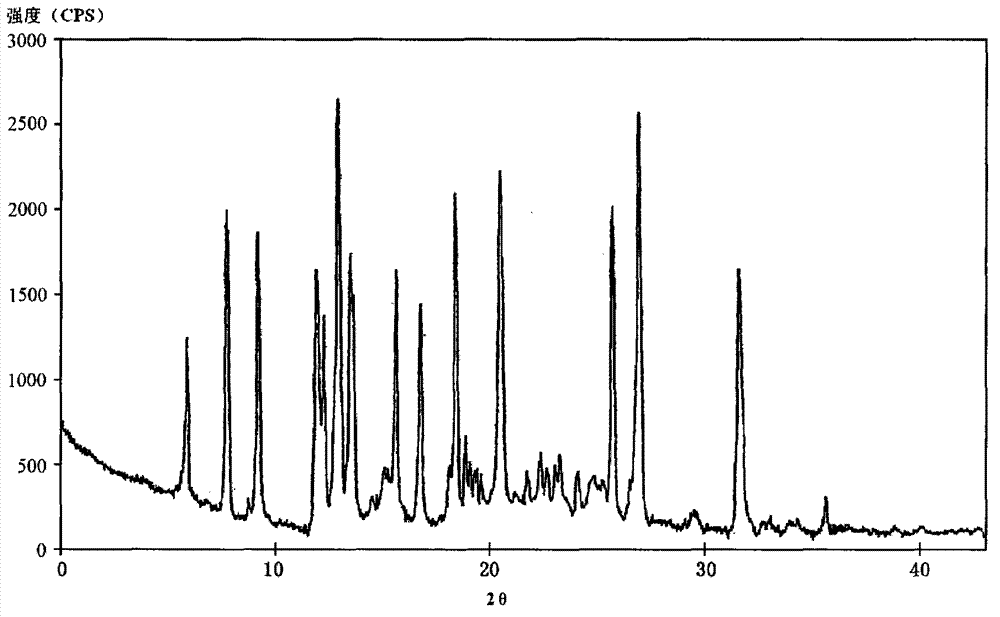
Novel omeprazole sodium compound and medicinal composition thereof
ActiveCN102351846AHigh thermodynamic stabilityHigh yieldOrganic active ingredientsPowder deliveryOmeprazole SodiumFreeze-drying
The invention discloses a novel omeprazole sodium compound. Characteristic peaks of the omeprazole sodium compound in an X-ray powder diffraction pattern obtained through Cu-K alpha-ray measurement are displayed when 2 theta is 3.2, 5.9, 7.6, 9.2, 10.5, 10.6, 12.3, 16.2, 18.2, 20.8, 23.4, 27.1, 30.4, 32.3 and 34.6. The omeprazole sodium compound has a new crystal form different from the prior art, has the thermodynamic stability higher than that with known crystal form, and has the advantage of not absorbing moisture basically. The invention also discloses a medicinal composition, which comprises the novel omeprazole sodium compound. Omeprazole sodium freeze-dried powder injection can be directly prepared under the condition of not adding lyoprotectants, and the omeprazole sodium freeze-dried powder injection has the advantages of high stability and high re-dissolubility.
Owner:周晓东
Yellow-peach can
The invention is concerned with the preparation method for can-combination, with the functions of dispelling wind and eliminating dampness, clearing away the heat-evil and expelling superficial evils, insect disinfestations and diminishing inflammation. The main compounding is yellow peach, especially for treat anemia, edema of woman climacteric period, and without side effect.
Owner:FANGXIA ENTERPRISE INFORMATION CONSULTING WUJIANG
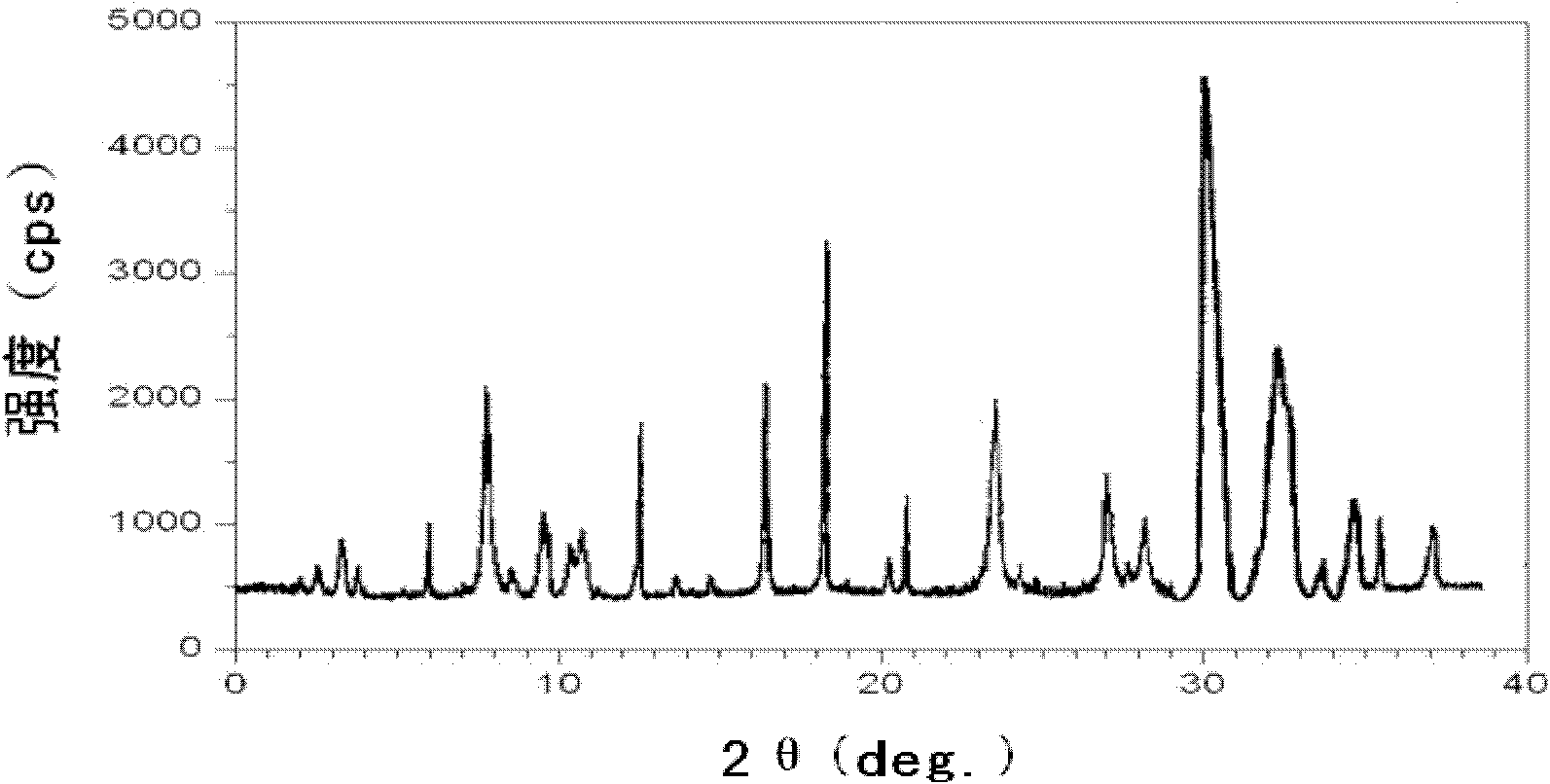
Ceftazidime crystal compound, preparation method of compound and pharmaceutical composition of compound in sterile mixed powder form
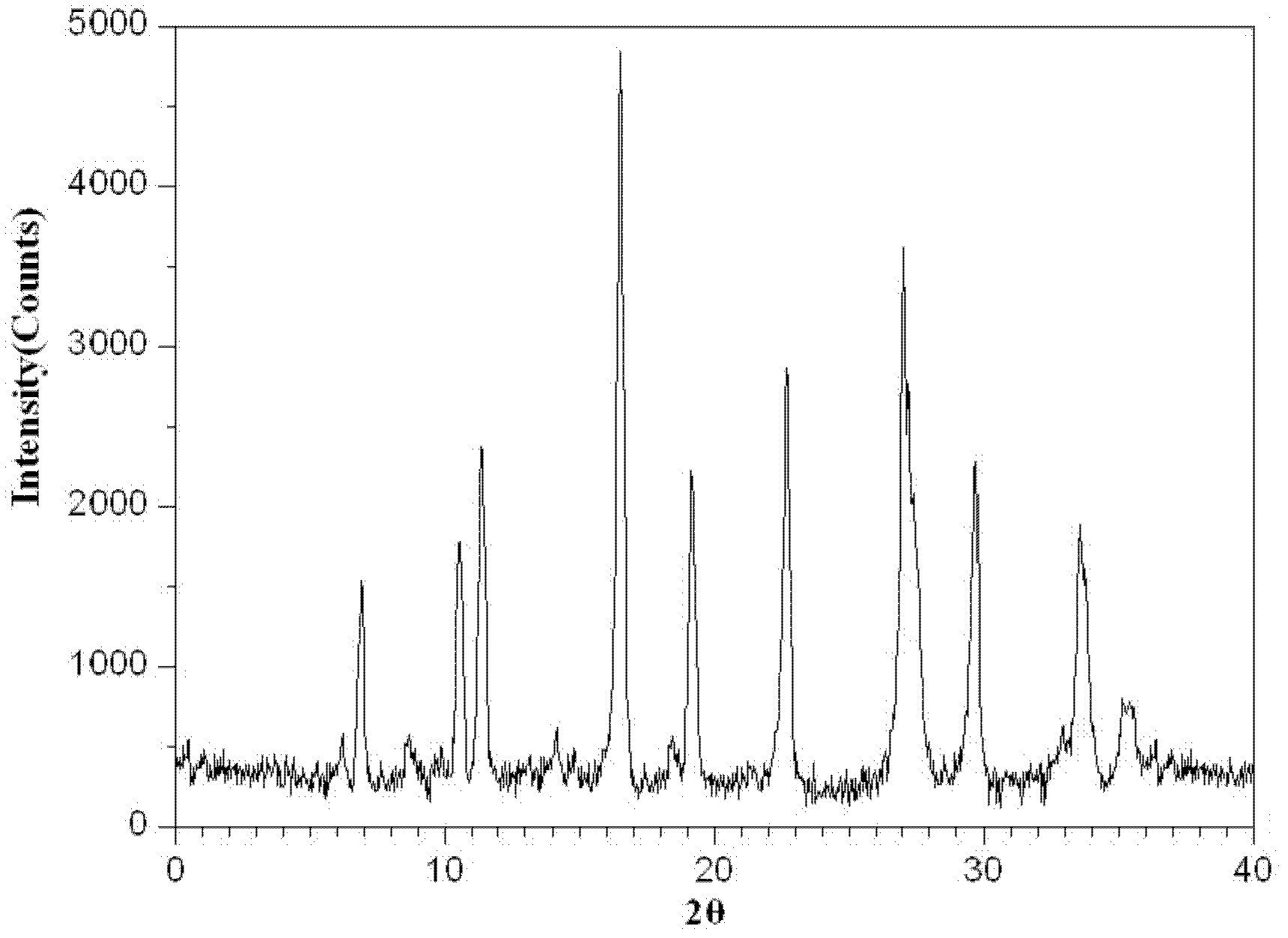
ActiveCN102924483AHigh purityGood thermal stabilityAntibacterial agentsOrganic active ingredientsCeftazidimePowder diffraction
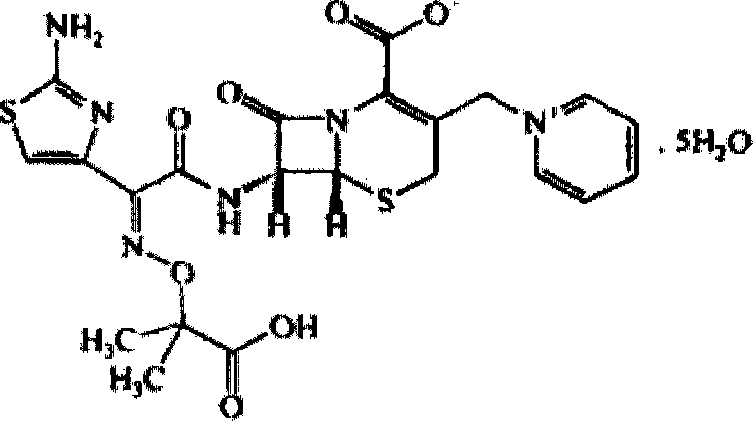
The invention relates to a ceftazidime crystal compound, a preparation method of the compound and a pharmaceutical composition of the compound in a sterile mixed powder form. The structural formula of the ceftazidime crystal compound is as shown in a formula (I); and the crystal compound is measured by using a powder X-ray diffraction measurement method, and an X-ray powder diffraction map expressed by a 2theta+ / -0.2-degree diffraction angle shows characteristic diffraction peaks at 5.7 degrees, 7.6 degrees, 9.1 degrees, 11.8 degrees, 12.2 degrees, 12.6 degrees, 13.3 degrees, 15.5 degrees, 16.7 degrees, 18.4 degrees, 20.5 degrees, 25.8 degrees, 26.3 degrees and 31.8 degrees. The crystal compound is high in purity and stability, and almost does not absorb moisture.
Owner:HAINAN HERUI PHARMA
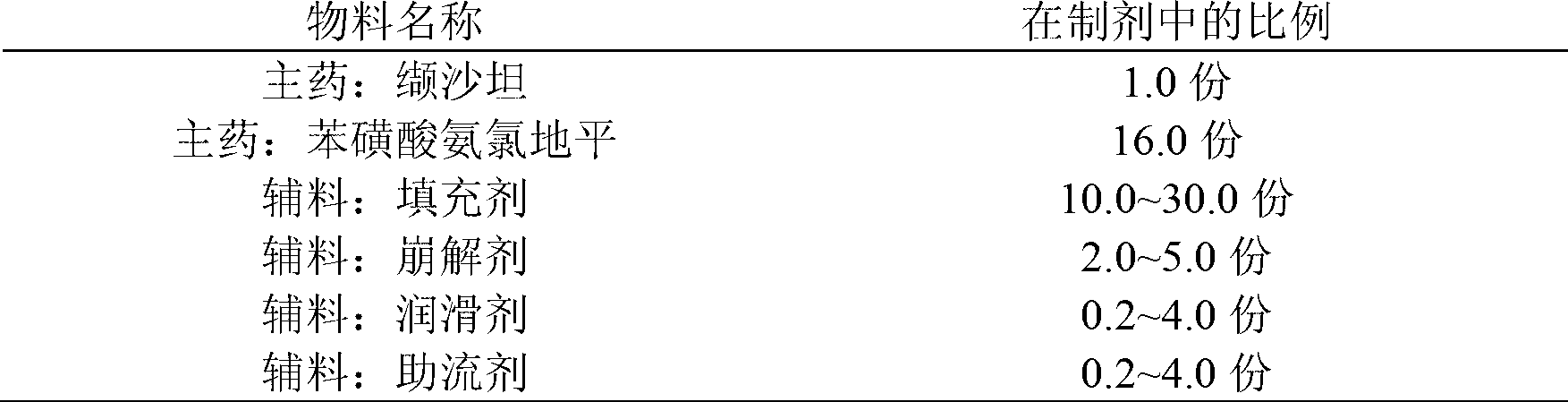
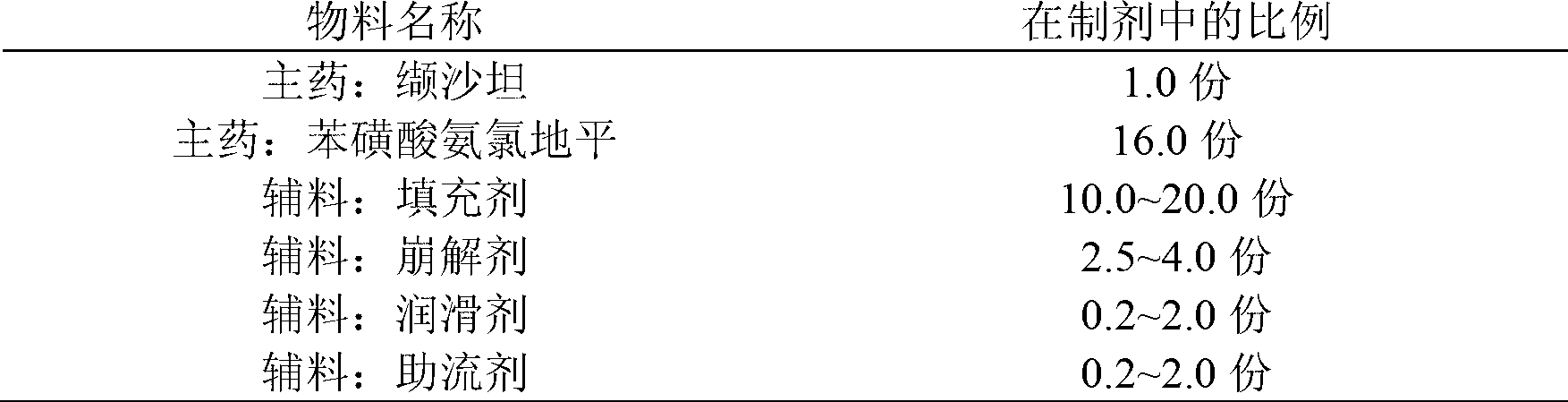
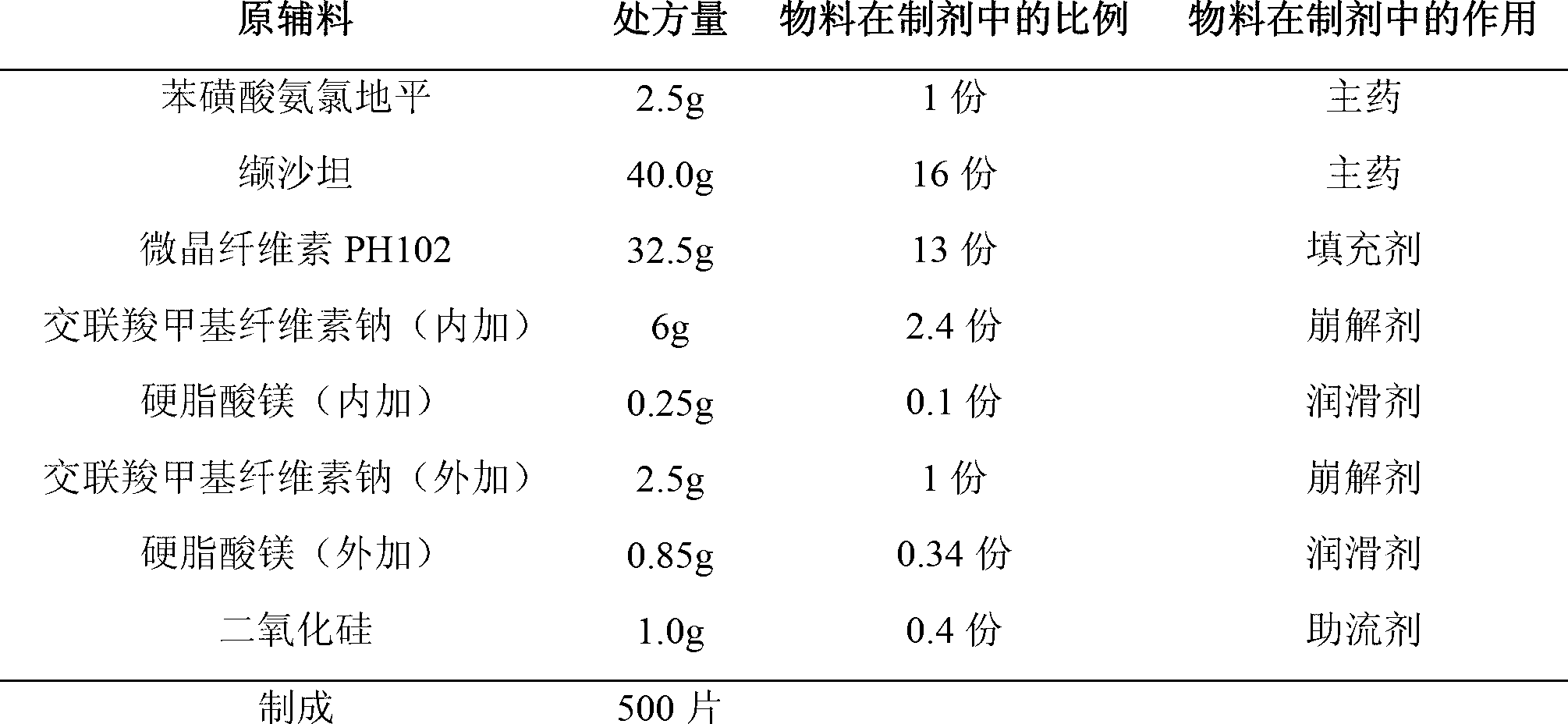
Compound preparation of valsartan amlodipine tablet (I) and preparation method thereof
ActiveCN103006649AReduce the introductionReduce lossesDrageesCardiovascular disorderValsartanAmlodipine besilate
The invention provides a compound preparation of valsartan amlodipine tablet (I) and a preparation method thereof. The compound preparation comprises a tablet, a film coating layer, the tablet comprises main drug and auxiliary materials, the main drug comprises valsartan, benzenesulfonic acid amlodipine, the auxiliary material comprises filler, a disintegrating agent, a lubricant, and glidant, the tablet comprises the valsartan, benzenesulfonic acid amlodipine, the filler, the disintegrating agent, the lubricant and the glidant, and the tablet is obtained by being coated with the film coating layer. According to the method, the defect of unsafety of medicine use caused by increase of relevant material due to wet granulation is overcome, and manufacture cost can be lowered and labor intensity can be relieved due to the dry granulation technology.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and preparation method thereof
InactiveCN101559128ANo allergiesAvoid stimulationAnthropod material medical ingredientsAntipyreticDiseaseIrritation
The invention provides an externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and a preparation method thereof. The medicine is prepared by 25 types of medicinal materials such as radix aconiti preparata, wild aconite root, nux vomica (processed), epimedium, achyranthes root, notopterygium root, cyrtomium fortunei, phellodendron, zaocys dhumnade, hairy antler, dipsacus root, dark plum, asarum, Chinese ephedra, cassia twig, safflower, acanthopanax, honeysuckle, earth worm, loranthus, licorice, drynaria (scald), anisetree bark, myrrh gum (processed) and red ginseng. The medicine links closely with pathogen and pathogenesis of a disease and a plurality of medicines are compatible reasonably, thus expelling wind and clearing away cold as well as activating meridians to stop pain. As an externally used cataplasm, the medicine is taken through skin, thus avoiding the irritation of an oral preparation to gastrointestinal tract; in addition, relatively slow percutaneous absorption process inevitably greatly mitigates drug toxicity to the whole body. Neither skin sensibility nor skin irritation occurs. Therefore, the transdermal drug delivery of new preparation improves the safety of medicine taking to a certain extent and provides new choices for safe clinical medicine use.
Owner:潘首德
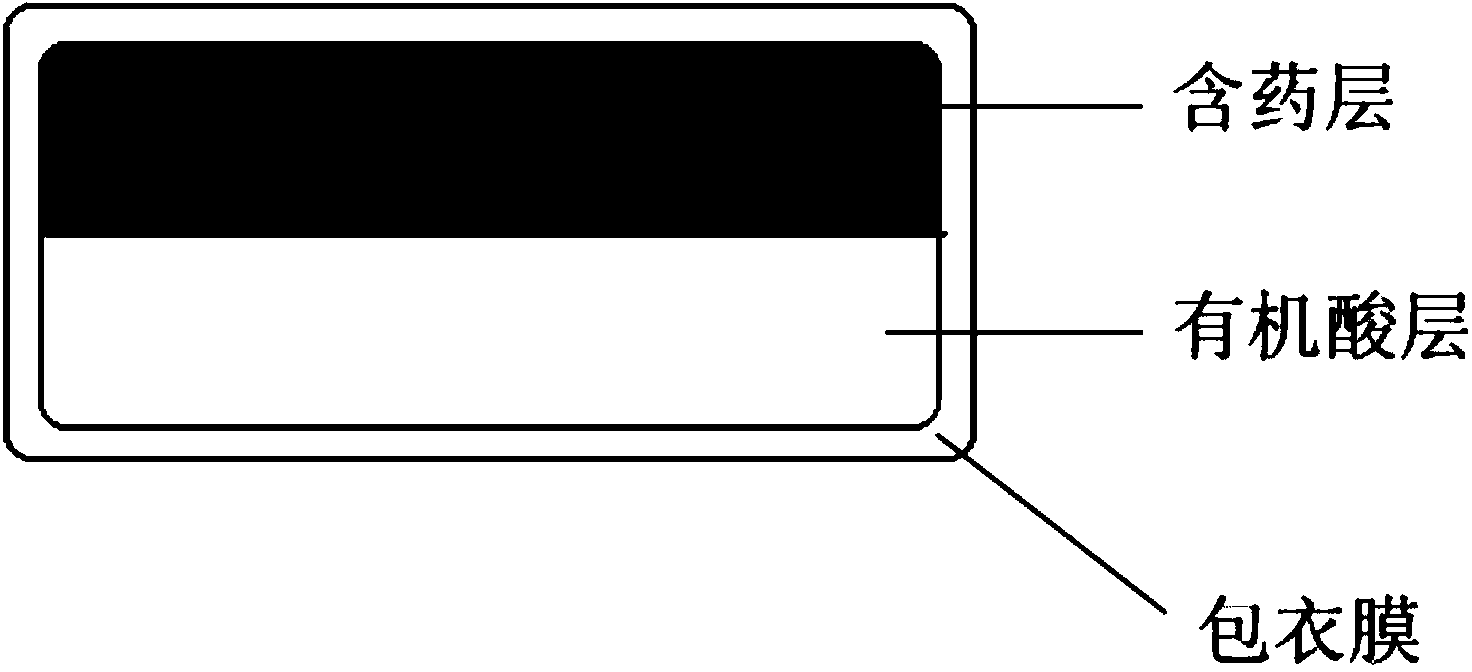
Multi-layer tablets containing dabigatran etexilate mesylate
ActiveCN104042588AIncrease absorption rateAddress disintegration risksOrganic active ingredientsPharmaceutical delivery mechanismSolubilityOrganic acid
The invention discloses a multi-layer tablet containing dabigatran etexilate mesylate. The multi-layer tablet comprises a tablet core and a coating film; the tablet core comprises at least one drug-containing layer containing dabigatran etexilate mesylate and at least one organic acid layer. The multi-layer tablet is capable of increasing the solubility in vivo and the absorption rate in vivo, and also capable of effectively controlling the release time in vivo, and further is low in batch difference, and therefore, the safety of drug use is improved. Meanwhile, the multi-layer tablet is used for solving the problem that other substances are generated due to crystal transformation in the production and storage processes, and the production quality is improved and the storage time is prolonged. In addition, the multi-layer tablet further has the characteristics of convenience for swallowing, simple production process and high reproducibility.
Owner:ZHEJIANG JINGXIN PHARMA
Multifunctional liquid medicine fertilizer and preparation method and application thereof
InactiveCN106831137AImprove blending effectGrowth inhibitionAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersPhosphateInsect pest
The invention discloses a multifunctional liquid medicine fertilizer and a preparation method and application thereof, and the multifunctional liquid medicine fertilizer comprises the following components by weight: 10-30 parts of urea, 1-20 parts of ammonium dihydrogen phosphate, 1-10 parts of potassium dihydrogen phosphate, 1-12 parts of potassium nitrate, 1-5 parts of potassium sulfate, 1-10 parts of potassium chloride, 1-5 parts of cytex, 1-5 parts of fulvic acid potassium, 1-5 parts of chitosan oligosaccharide, 0.1-5 parts of chlorantraniliprole, 1-30 parts of hymexazol, 1-10 parts of a surfactant, 1-5 parts of other additives and 60-90 parts of water. The multifunctional liquid medicine fertilizer is a multifunctional medicine fertilizer product prepared by compounding of fertilizers, the cytex, fulvic acid, the chitosan oligosaccharide and other biostimulant substances, and high-efficiency wide-spectrum low-toxicity pesticide chlorantraniliprole and fungicide hymexazol, can provide crops nutrition, also can prevent crop diseases and insect pests, and is convenient to use, time-saving and labor-saving; the cytex, the fulvic acid and the chitosan oligosaccharide can effectively improve crop resistance, can improve pesticide efficacy, reduces pesticide use amount, and improves agricultural production yield, quality and safety.
Owner:山东康德源农业科技有限公司
Pistachio galls pressure ulcer powder
InactiveCN101129578AImprove satisfaction rateSignificant effect on symptomsPowder deliveryHydroxy compound active ingredientsSide effectTraditional medicine
The invention relates to a medicinal and dietary composition for treating bedsore and its preparing process, wherein the composition is prepared mainly from frankincense, Chinese angelica root, dahurian angelica root, dried rehmannia root, goldthread root and boneol, the medicament can also be used as a conventional health dietary preparation having no side effects easy administering.
Owner:王军
Targeted, control-release and thin film coating material made of denatured starch for oral medicine to treat colonic diseases, its prepn. and use
ActiveCN1903368AIncrease release rateFast release ratePharmaceutical delivery mechanismPharmaceutical non-active ingredientsOral medicineControlled release
A release controllable film coating material targetting to colon is prepared from modified starch through adding the modified starch to coating solvent, and adding plasticizer. Its application is also disclosed.
Owner:SOUTH CHINA UNIV OF TECH
Coix seed wine
InactiveCN101085963ADilated blood vesselsLower blood sugarAlcoholic beverage preparationSide effectAcute Pharyngitis
The invention relates to a kind of medical wine that possesses functions of excreting dampness, eliminating arthralgia syndrome, apocenosis and invigorating the spleed and strengthening the middle-warmer energy. It mainly takes coix seed as raw material, and the effect for treating dropsy, asthma, lung abscess, nephritis, nephropyeltis, beriberi, dysuria, arthritis with muscle contracture, diarrhea of deficiency type, acute pharyngitis and flat wart is distinctive. The product can be used as normal health- care beverage, which is non side effect, and possesses good economic and social benefit.
Owner:FANGXIA ENTERPRISE INFORMATION CONSULTING WUJIANG
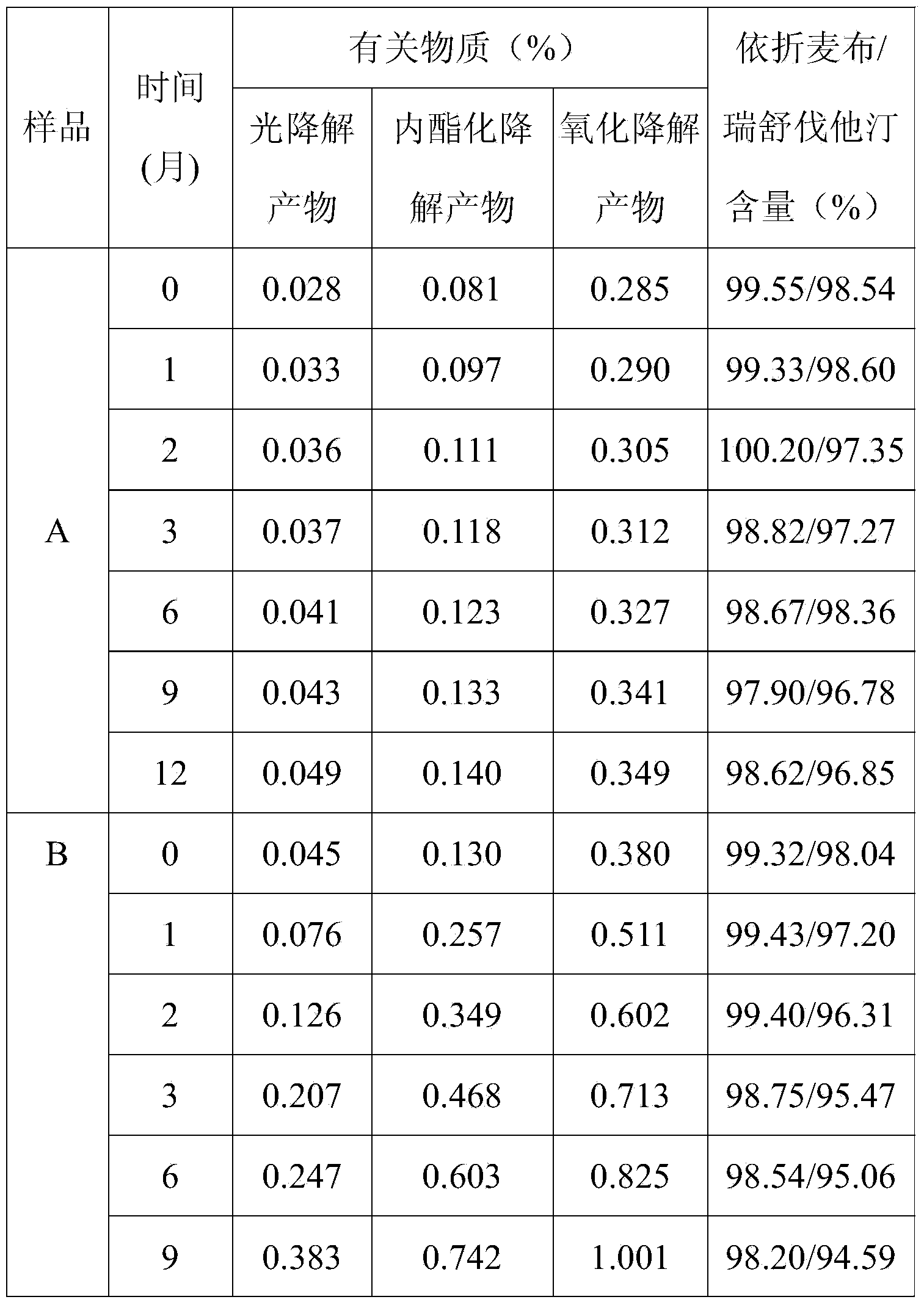
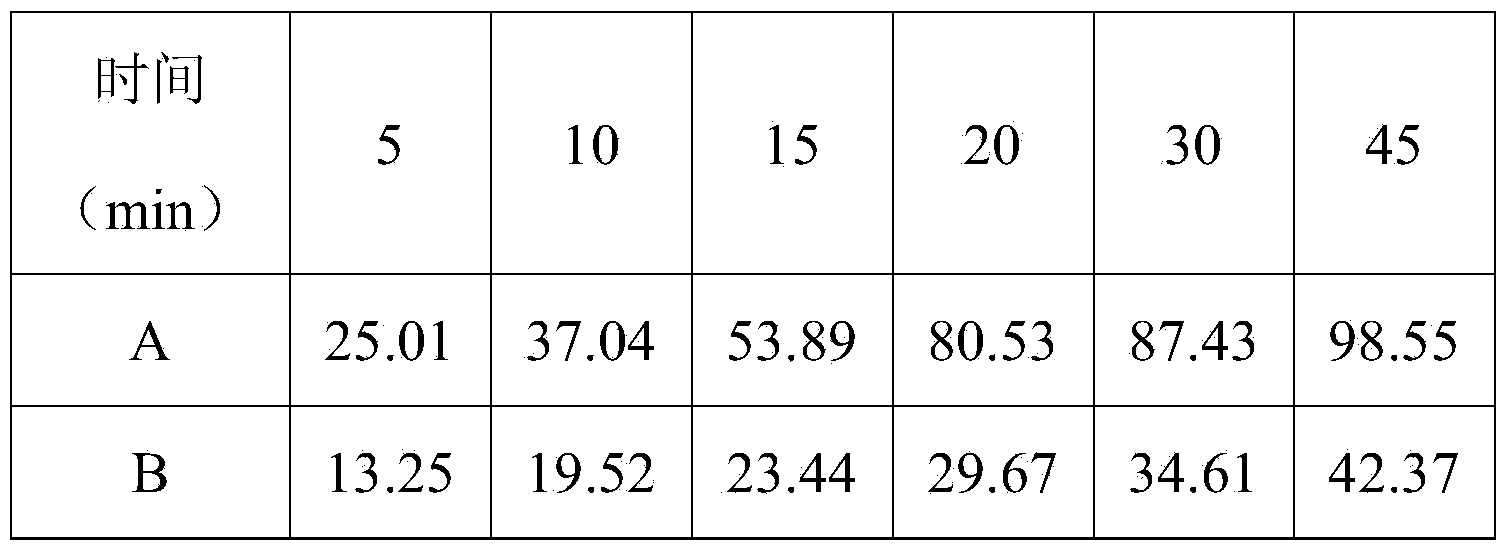
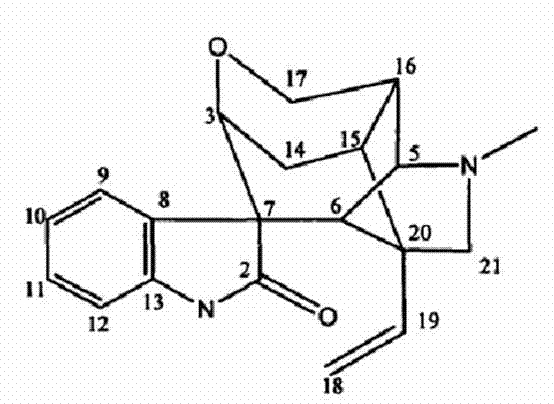
Double-layer tablet containing ezetimibe and rosuvastatin and preparation method thereof
ActiveCN103585157AImprove the quality of lifeReduce adverse reactionsOrganic active ingredientsMetabolism disorderSolubilityAdditive ingredient
The invention relates to a double-layer tablet which contains ezetimibe and rosuvastatin serving as effective components and has a lipid-lowering effect and a preparation method thereof. Aiming at the problems poor water solubility of ezetimibe, instability of rosuvastatin to acid and oxygen and the like, the method adopts a micronization technology to improve the dissolution rate of ezetimibe and adopts an anti-oxidizing agent and a double-layer tabletting technology to improve the stability of rosuvastatin in a human body, further, ingredients of a medicine is enabled to take effect sufficiently, and the best synergistic effect is achieved; and a compound preparation agent is mainly applied to treatment of diseases relative to hypercholesteremia.
Owner:WUHAN WUYAO SCI & TECH
Applications of gelsemine, koumine and 1-methoxyl gelsemine to preparation of medicine for treating anxiety
ActiveCN102240287AImprove medication safetyExpanding the Safe Therapeutic WindowOrganic active ingredientsNervous disorderSide effectPharmaceutical medicine
The invention discloses applications of gelsemine, koumine and 1-methoxyl gelsemine to the preparation of a medicine for treating the anxiety, especially an application of gelsemium alkaloid monomer gelsemine as the sole active ingredient or a pharmaceutically acceptable salt thereof to the preparation of the medicine for treating the anxiety, an application of gelsemium alkaloid monomer koumine as the sole active ingredient or the pharmaceutically acceptable salt thereof to the preparation of the medicine for treating the anxiety, and an application of gelsemium alkaloid monomer 1-methoxyl gelsemine as the sole active ingredient or the pharmaceutically acceptable salt thereof to the preparation of the medicine for treating the anxiety; and the gelsemine, the koumine and the 1-methoxyl gelsemine can play significant roles in treating the murine anxiety in far below the toxic dose so that the safe therapeutic window is remarkable expanded, have no tolerance, low addiction and side effect, and are more suitable for the preparation of the medicine for treating the anxiety in comparison with gelsemium total alkaloids.
Owner:FUJIAN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com