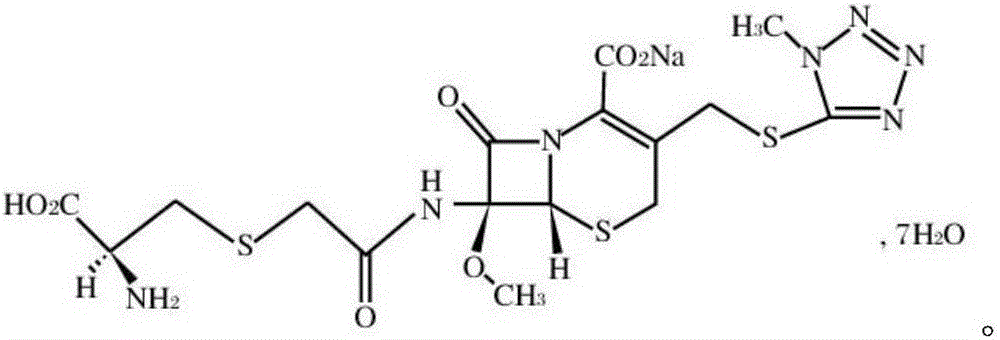
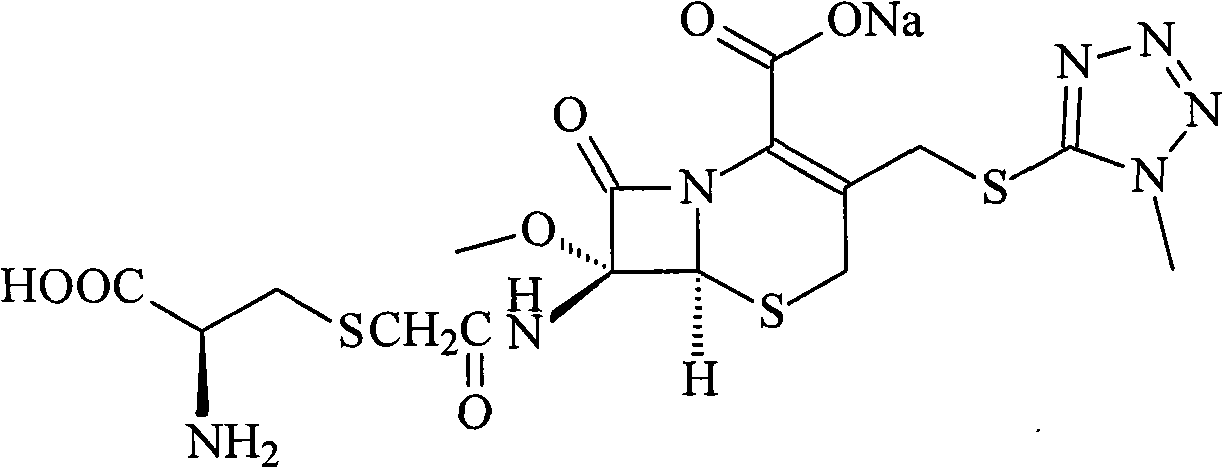
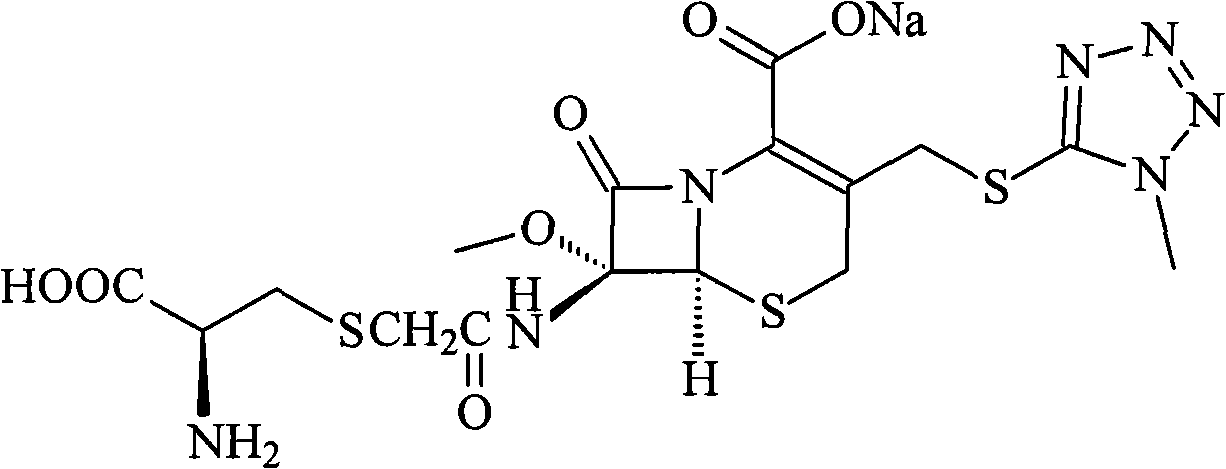
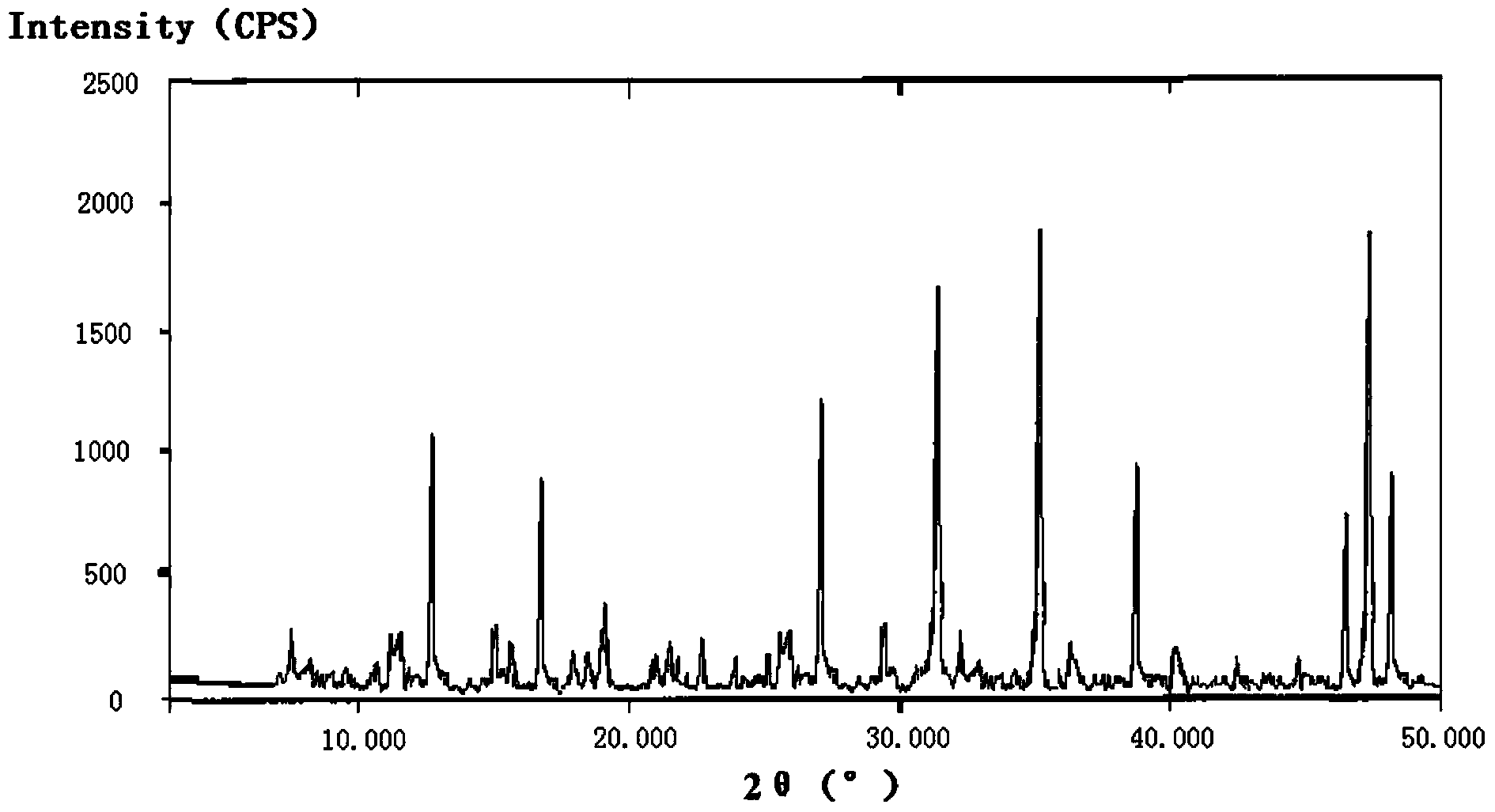
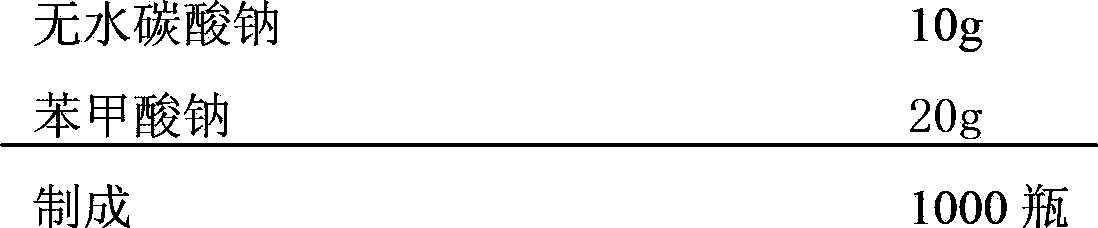Patents
Literature
50 results about "CEFMINOX SODIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
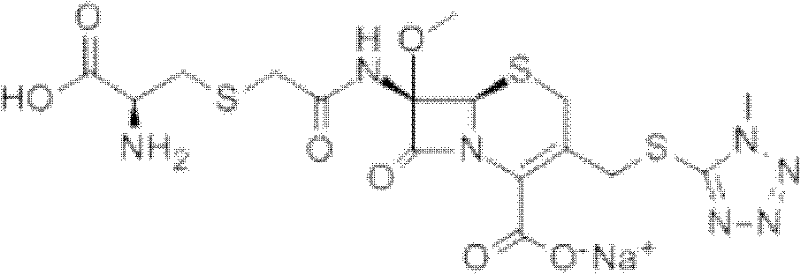
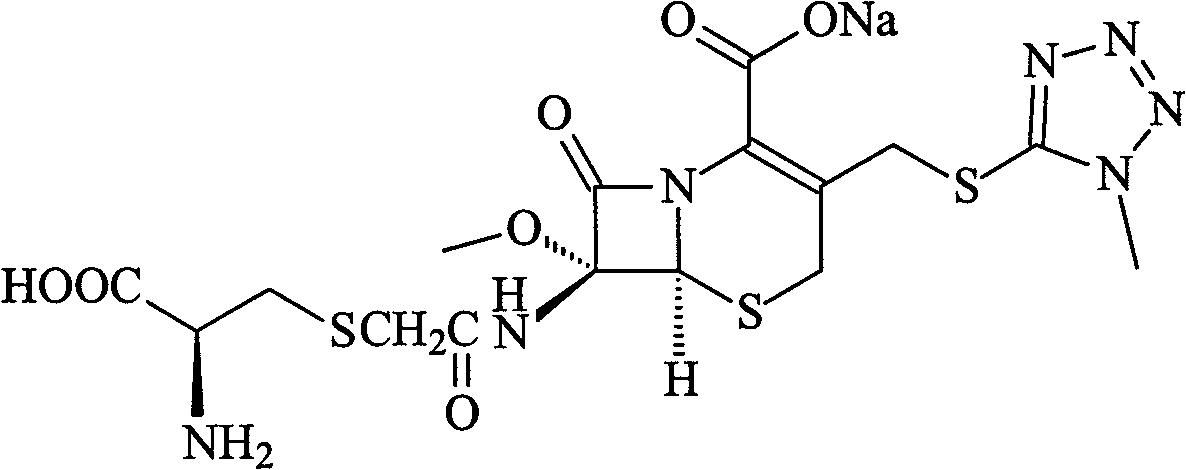
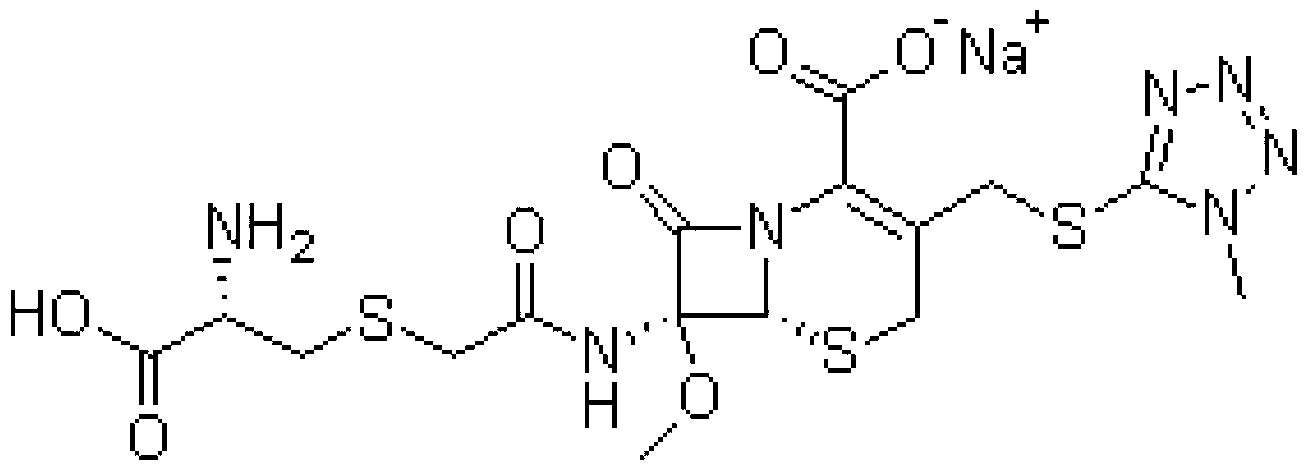
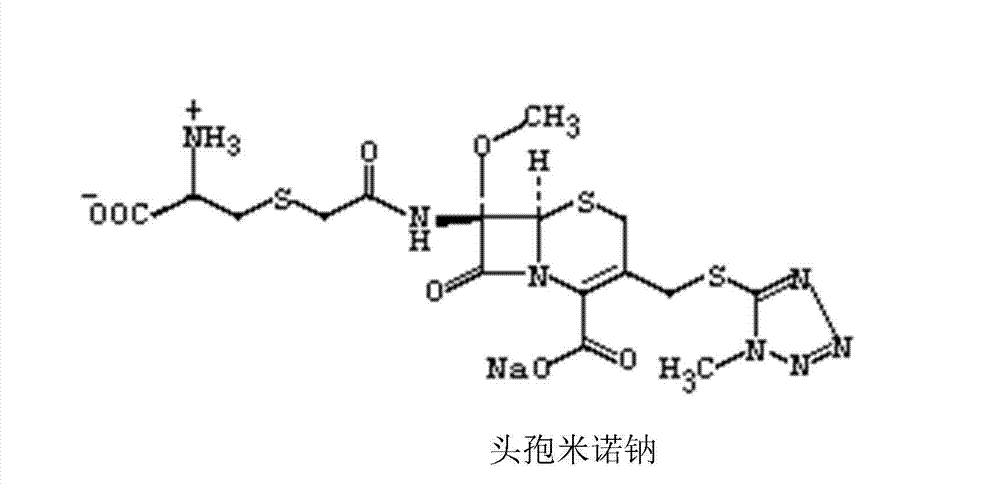
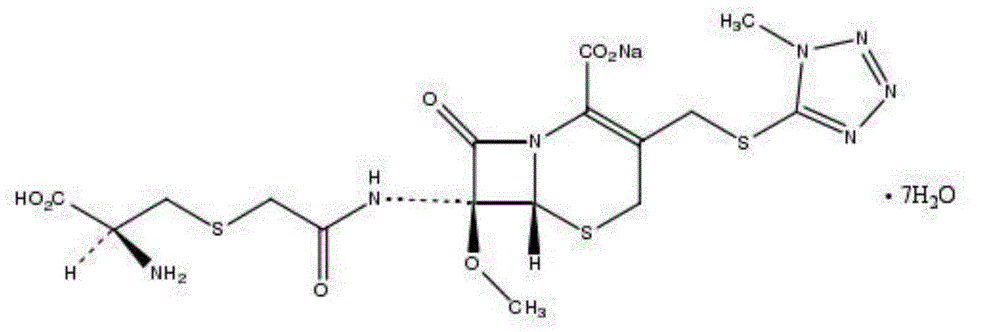
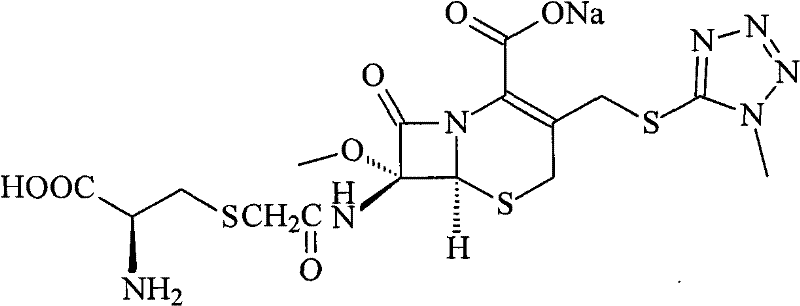
Cefminox Sodium is the sodium salt form of cefminox, a semi-synthetic, second-generation, beta-lactamase-stable cephalosporin with antibacterial activity. from NCIt MeSH Pharmacological Classification
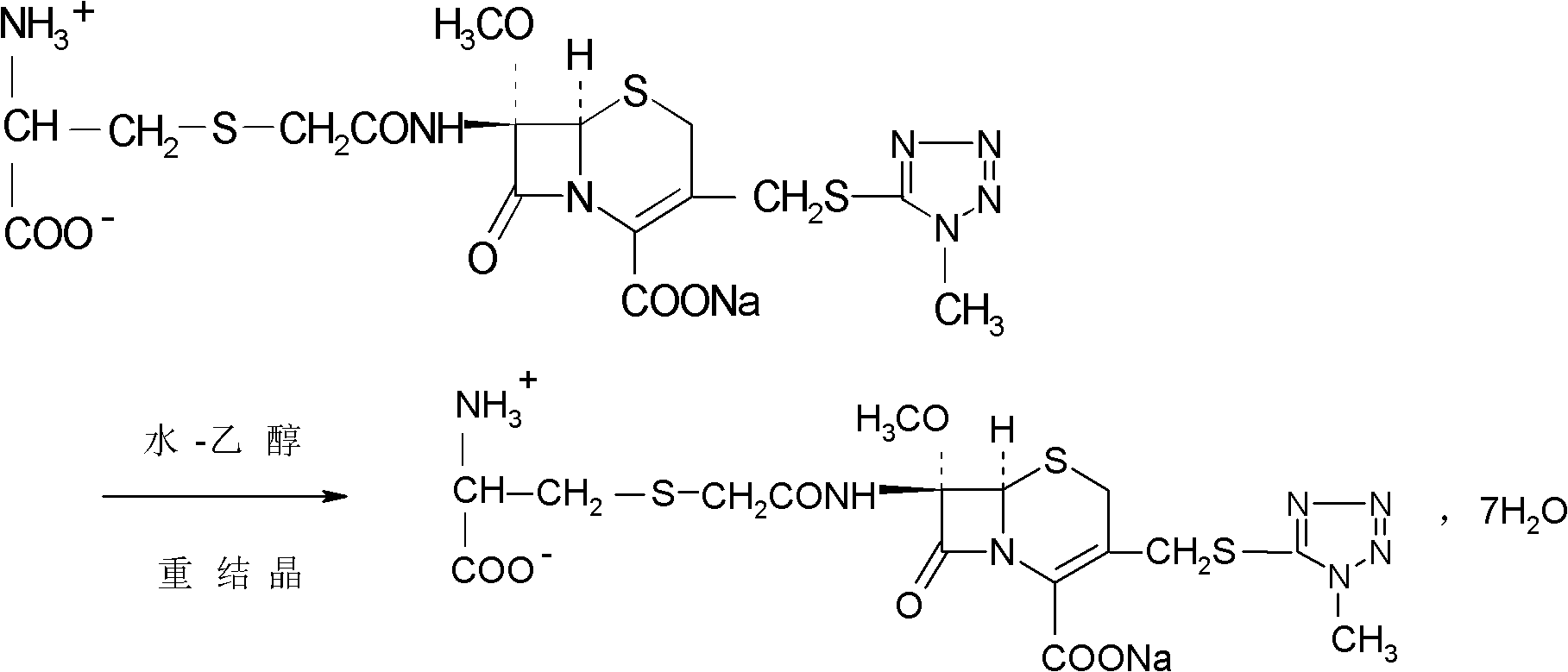
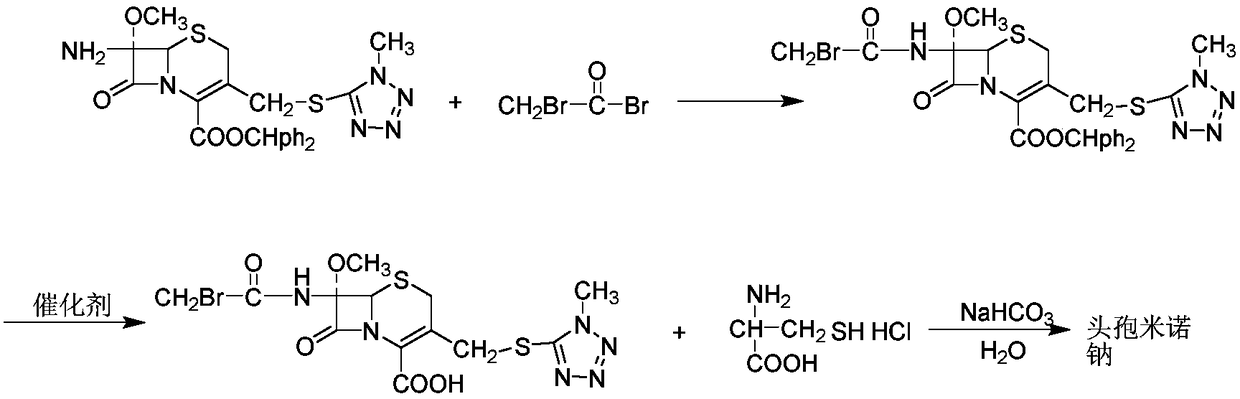
Cefminox sodium compound of new route
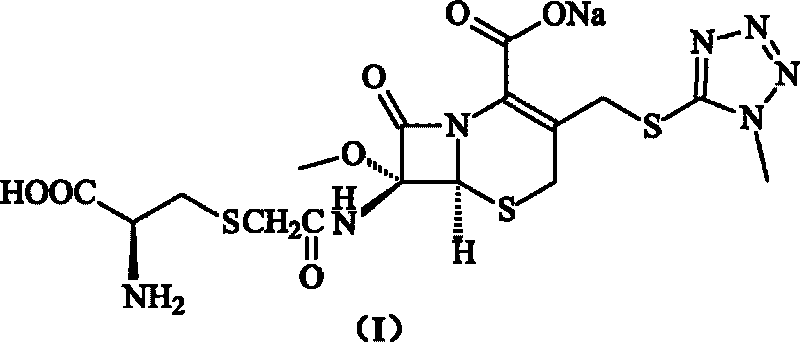
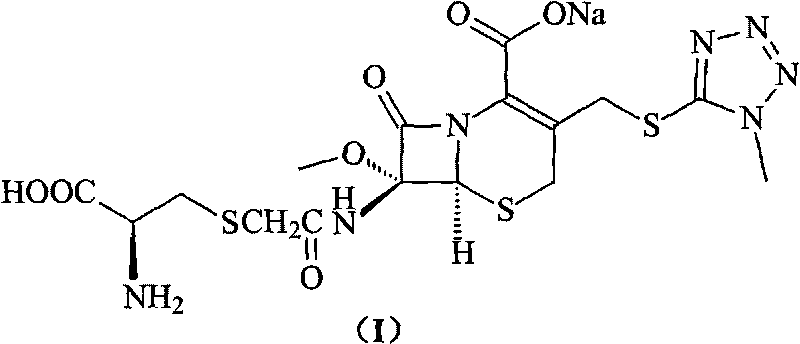
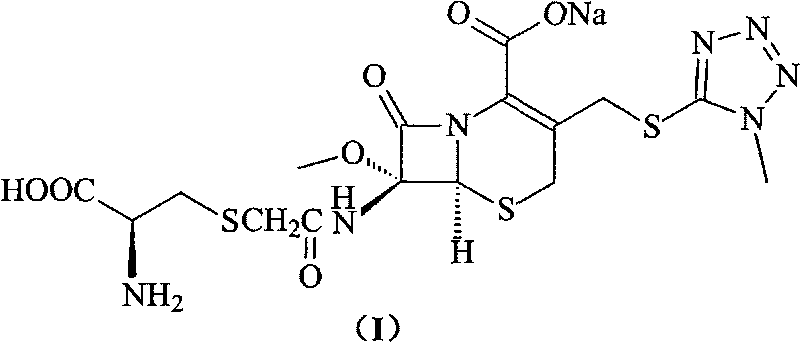
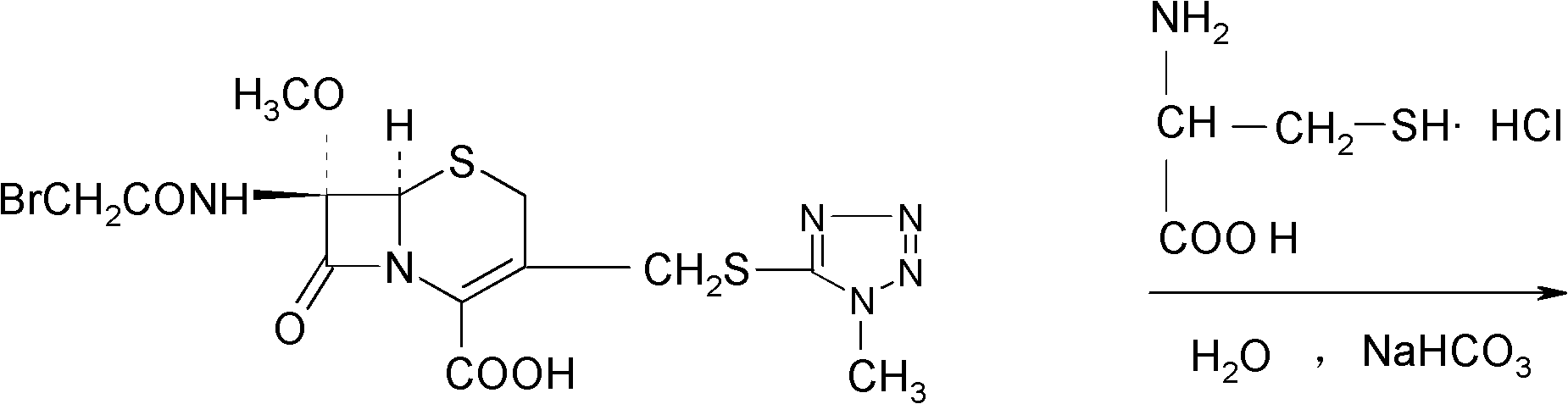
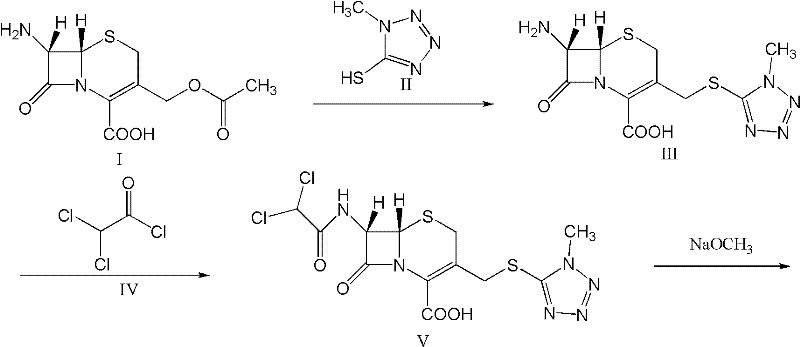
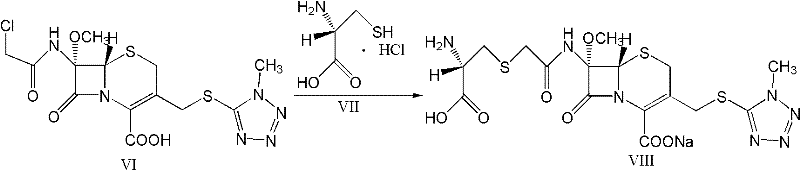
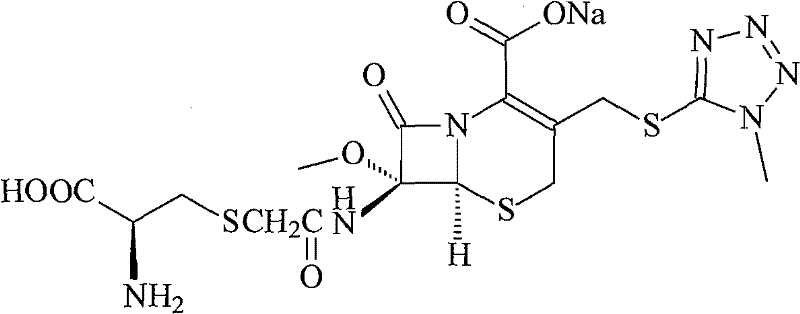
The invention provides a cefminox sodium compound of a new route, in particular a method for producing the cefminox sodium compound. The method comprises a step of generating cefminox sodium through the reaction between 7beta-bromoacetamido-7a-methoxyl-3-(1-methyl-1H-tetrazole-5-S-methyl)-3-cephem-4-carboxylic acid and D-cysteine hydrochloride, and is characterized in that: the reaction condition of the step is that the reaction is performed in aqueous solution, sodium iodide is used as a catalyst, the pH value of the reaction system is between 7.5 and 8.0, and the reaction temperature is kept at 30+ / -5 DEG C. Compared with the method for producing the cefminox sodium compound in the prior art, the method of the invention for producing the cefminox sodium compound greatly improves the product yield and the purity, and solves the problem that a cefminox sodium bulk drug always has a low purity in the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
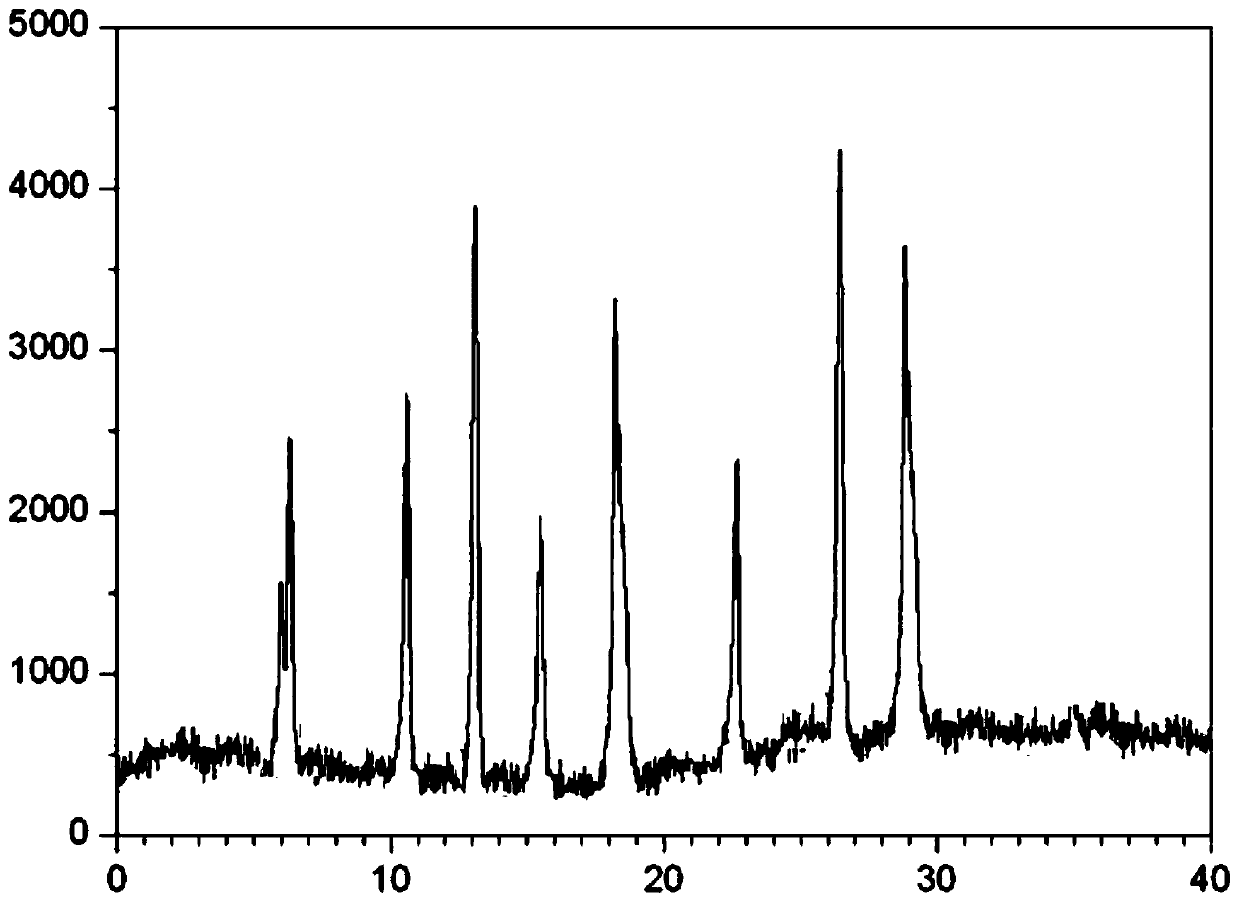
A kind of cefminox sodium crystalline compound and its composition powder injection
ActiveCN102276630AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCEFMINOX SODIUMX-ray
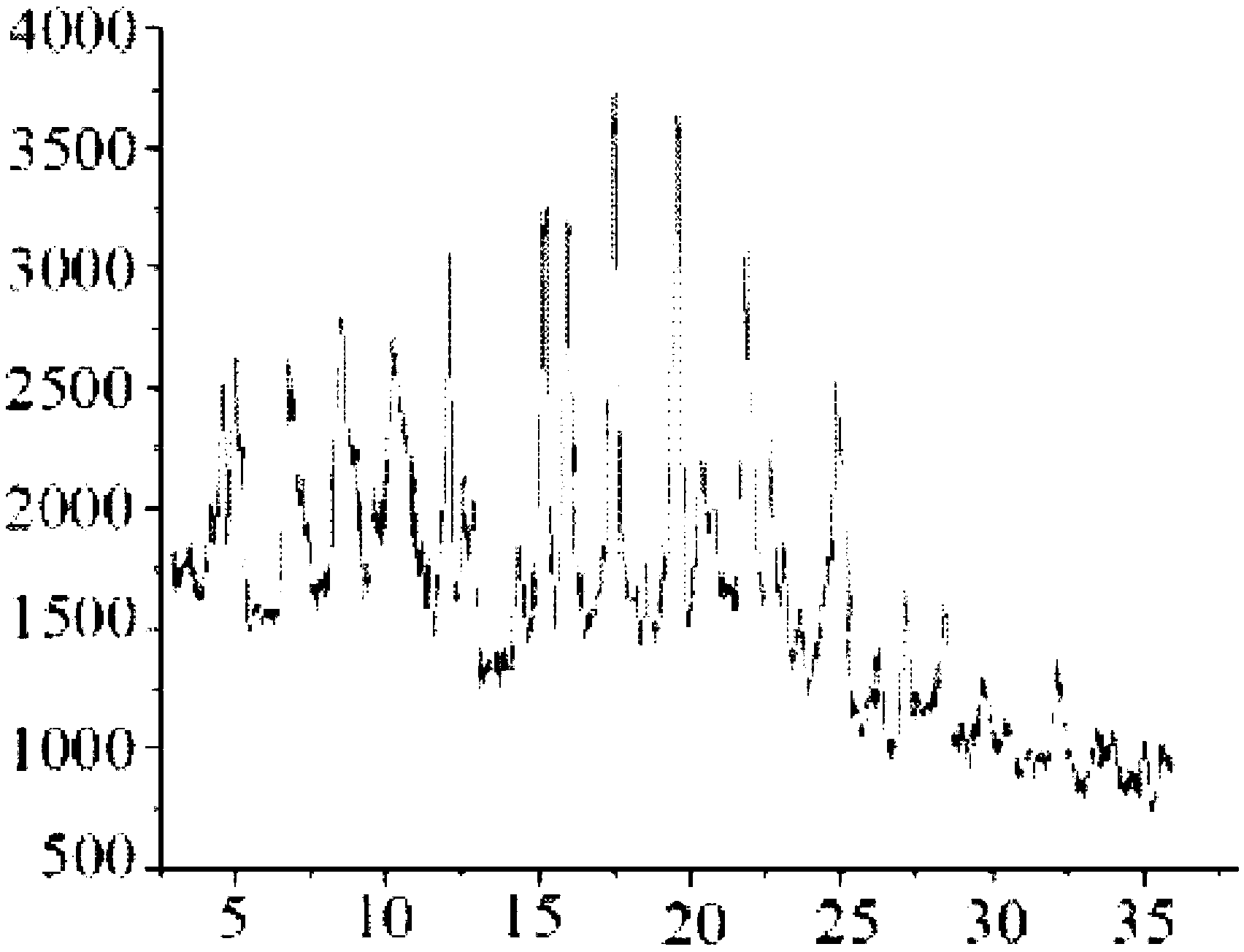
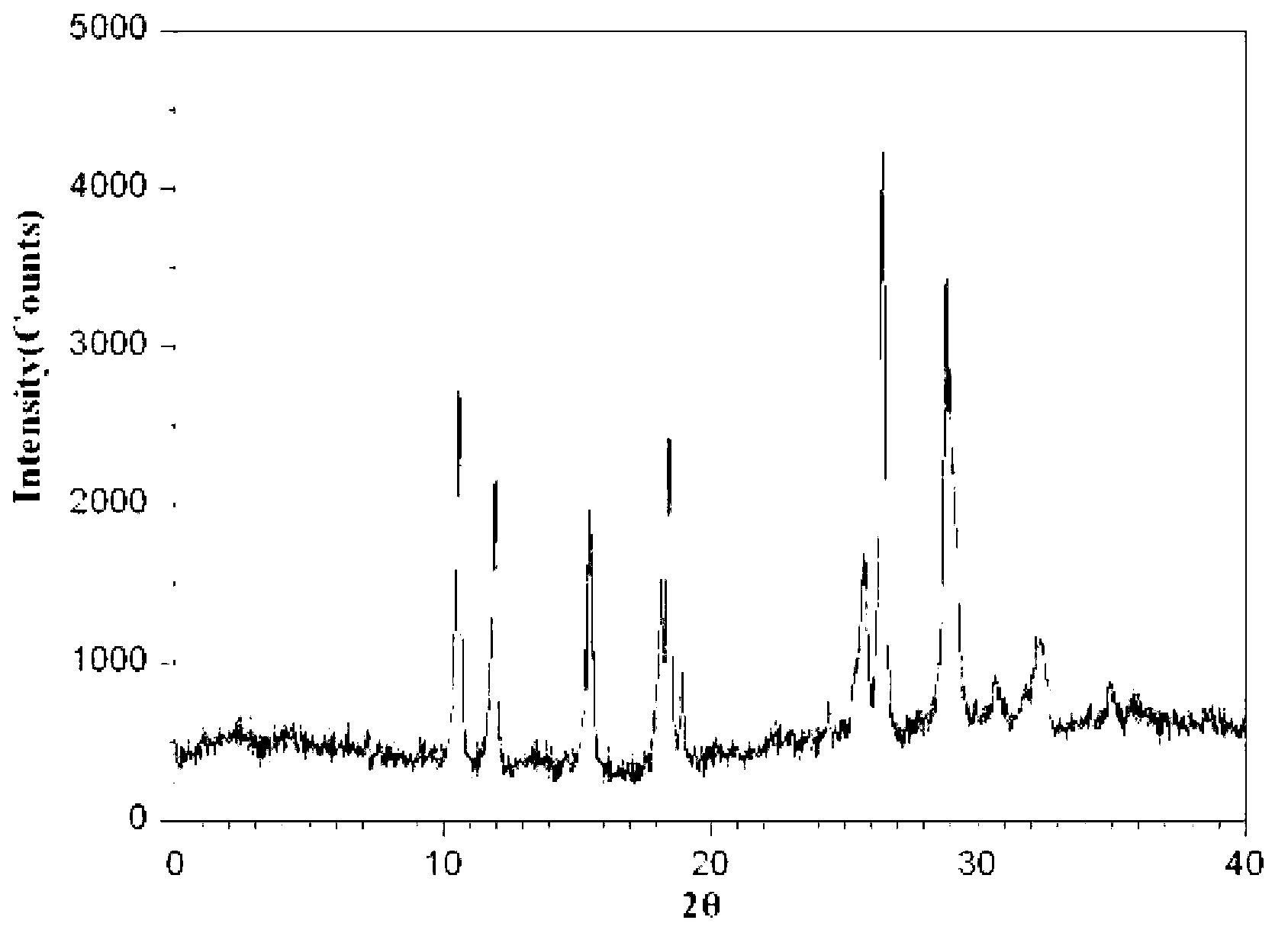
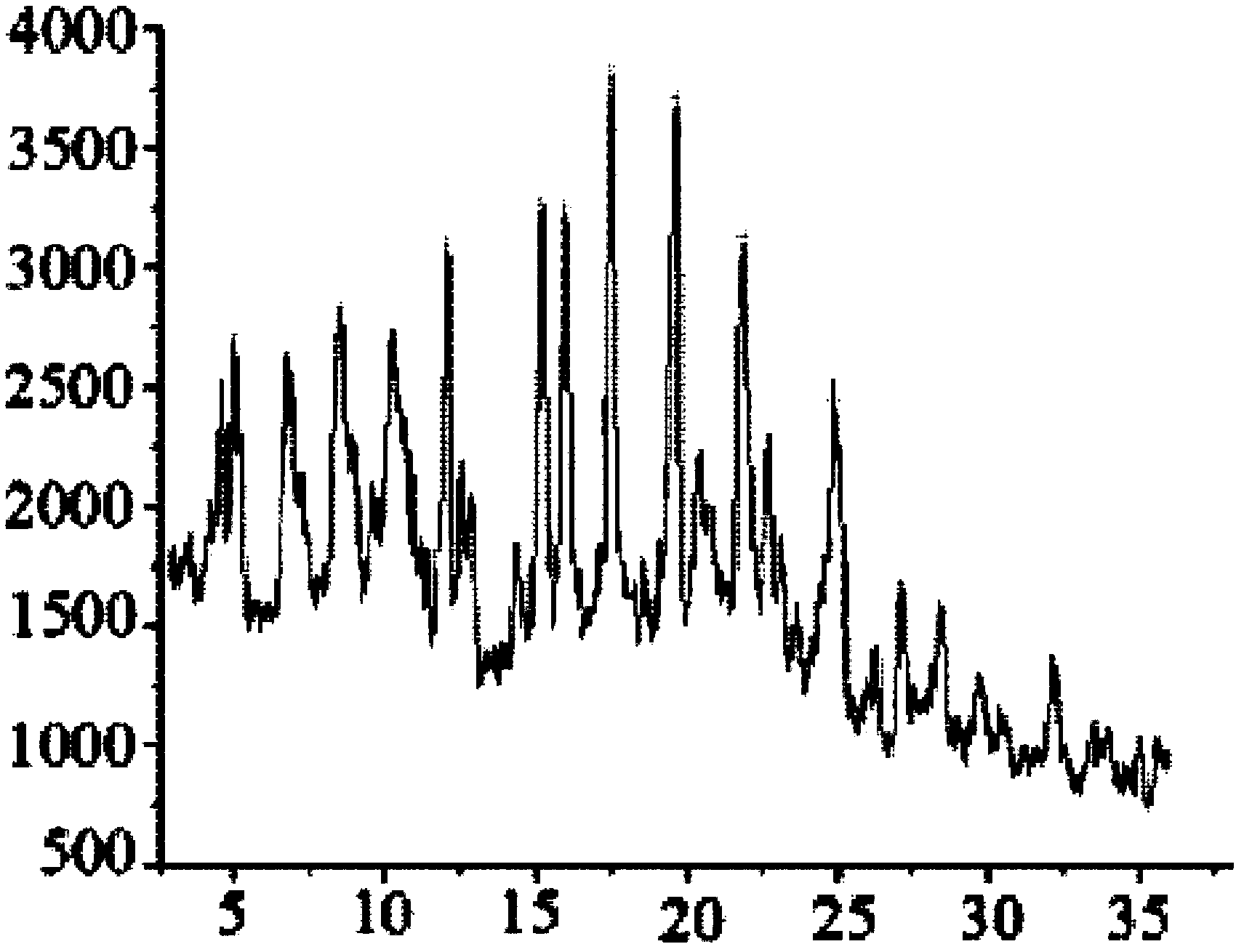
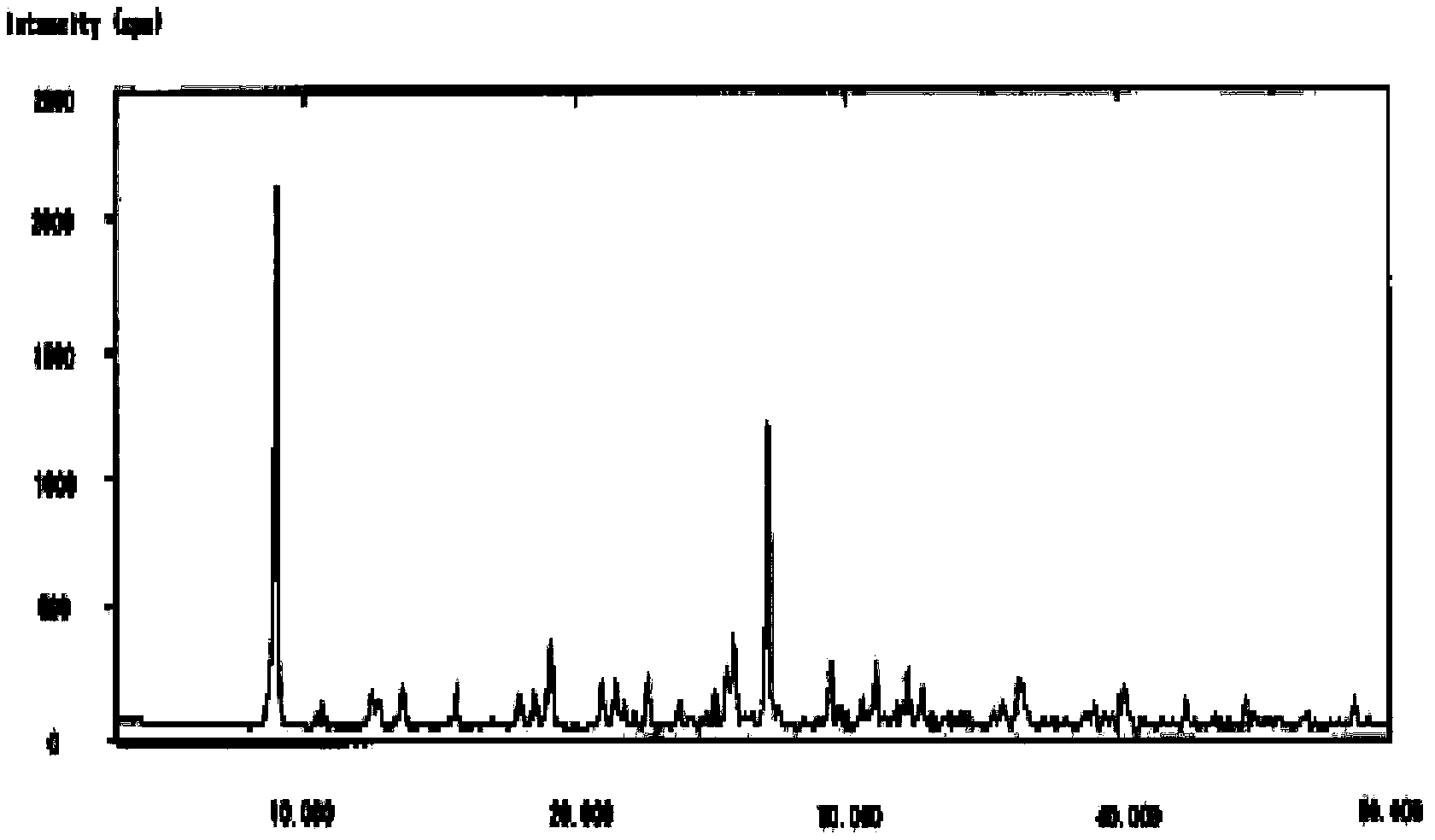
The invention relates to a cefminox sodium crystalline compound. The cefminox sodium crystalline compound is determined by a powder X-ray diffraction determination method; and characteristic diffraction peaks are displayed at 5.1, 6.9, 8.5, 10.3, 12.1, 15.1, 15.9, 17.4, 19.5, 21.7 and 24.6 degrees in an X-ray powder diffraction pattern represented by a diffraction angle of 2 theta+ / -0.2 degree. The invention also relates to cefminox sodium composition powder injection containing the cefminox sodium crystalline compound. The composition powder injection comprises the following components: 95 to 100 parts of cefminox sodium crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Preparation method of cefminox sodium
The invention discloses a preparation method of cefminox sodium, which comprises the following steps: 7 beta-bromoacetamide-7 alpha-methoxy-3-(1-methyl-1H-5-tetrazyl)sulfur methyl-3- cephem-4-carboxylic acid and D-cysteine hydrochloride are dissolved in water, the pH value is regulated to 6.0-7.0 by sodium bicarbonate, condensation reaction is carried out, and reaction products are post-treated to obtain the cefminox sodium. In the method, cefminox sodium raw material can be prepared through low-temperature reaction, a nonpolar macroporous resin X5 chromatography column is used for purification, ethanol-aqueous solution or anhydrous alcohol recrystallization and other simple operations are adopted to obtain target products, the yield and the purity of the target products are high, the products have uniform crystal forms and good fluidity, no special equipment is needed for the production, and the method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Cefminox sodium compound crystal, preparation method of cefminox sodium compound crystal and sterile powder injection containing cefminox sodium compound crystal
ActiveCN102838623AReduce contentImprove stabilityAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMX-ray
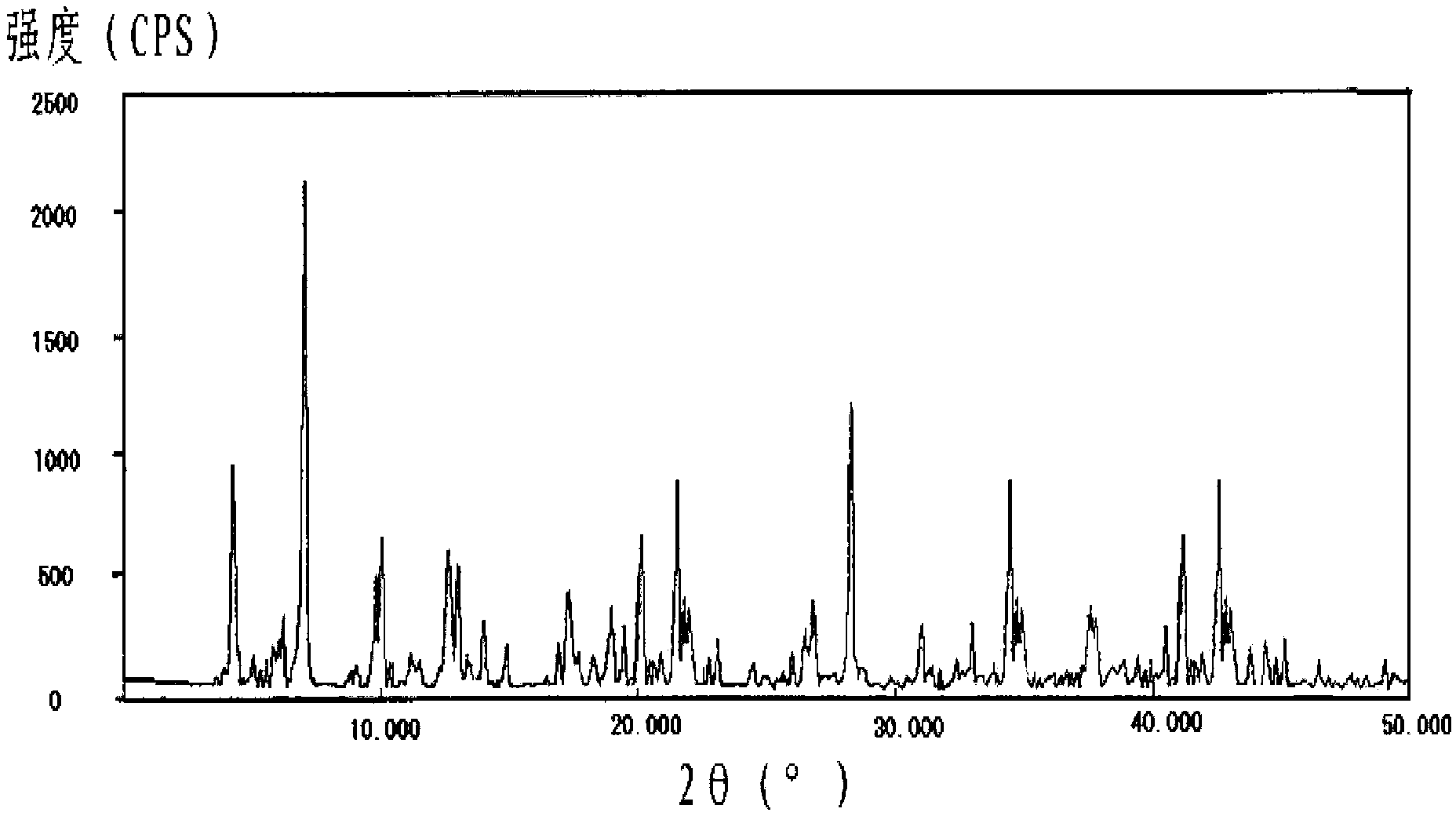
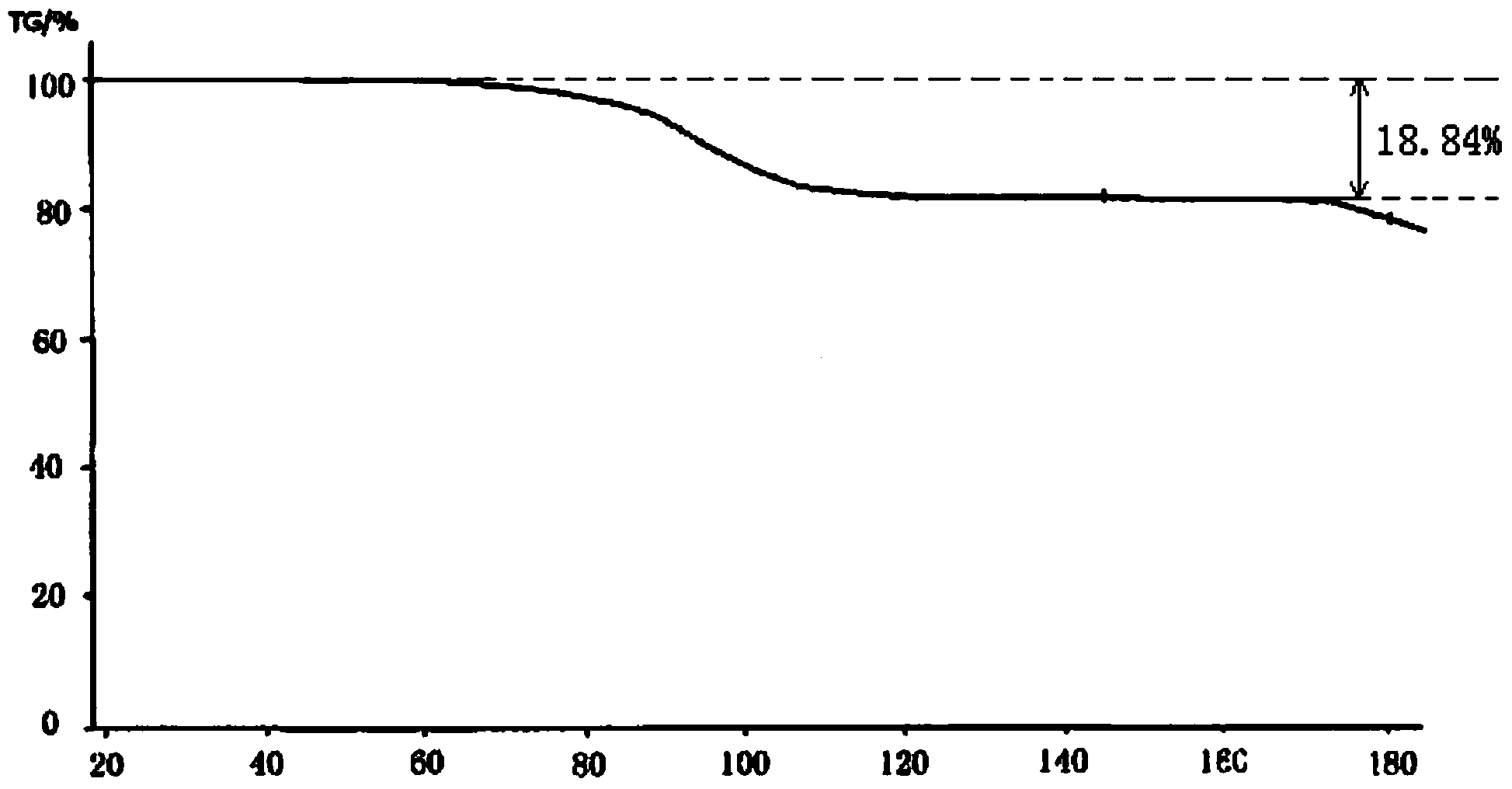
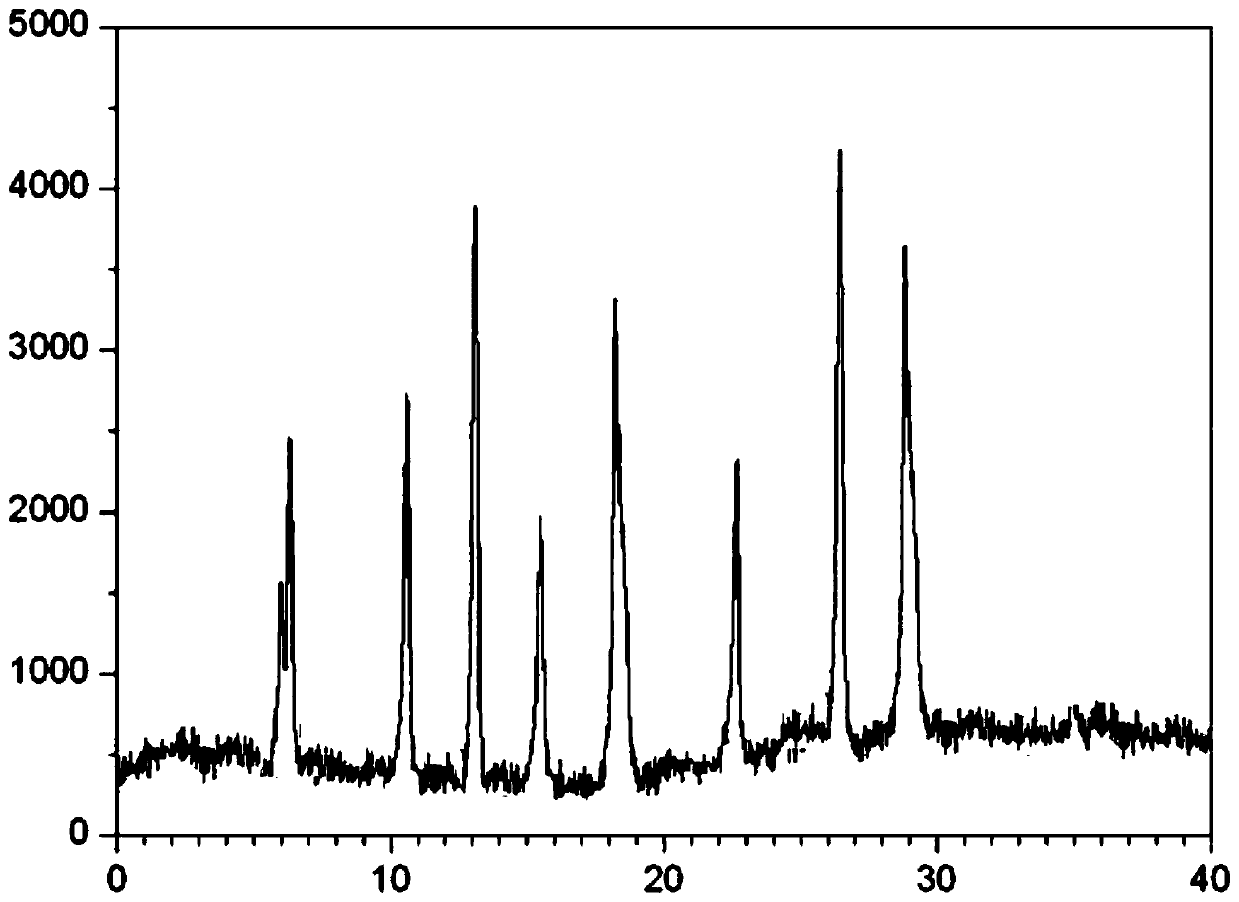
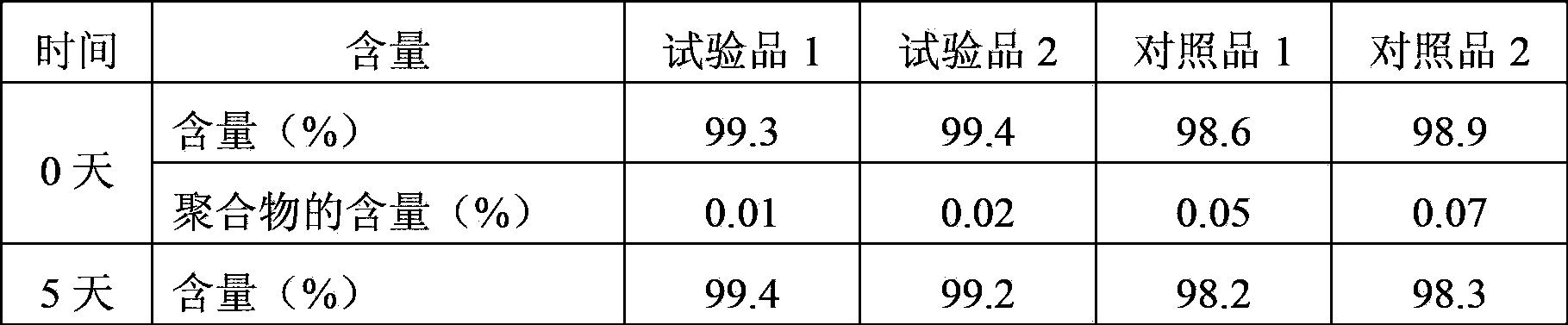
The invention relates to a cefminox sodium compound crystal, a preparation method of the cefminox sodium compound crystal and a sterile powder injection containing the cefminox sodium compound crystal. The cefminox sodium compound crystal is provided with an X-ray diffraction pattern as shown in a picture 3. The cefminox sodium compound crystal is a novel crystal form different from cefminox sodium heptahydrate reported in literatures, and tests prove that the cefminox sodium compound crystal in the crystal form has low content of a high-molecular polymer and excellent stability; in addition, the content of the high-molecular polymer slightly increases as the storage time prolongs.
Owner:HAINAN HERUI PHARMA
A kind of preparation method of cefminox sodium
The present invention relates to a kind of preparation of medicinal compound, particularly a kind of preparation method of cefminox sodium. The present invention adopts a synthesis route using 7-ACA as a starting material, and the cost of raw materials is lower than that of a synthesis route using 7-MAC as a starting material. The new synthetic route uses dichloroacetyl chloride instead of bromoacetyl bromide, which reduces the toxicity and cost of raw materials. The new synthetic route increases the intermediate treatment process, reduces the amount of impurities brought by the intermediate into the next reaction, and improves the quality and stability of the finished product. The present invention overcomes the shortcomings of the synthetic route using 7-MAC as the starting material through the above improvements.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Cefminox sodium liposome preparation
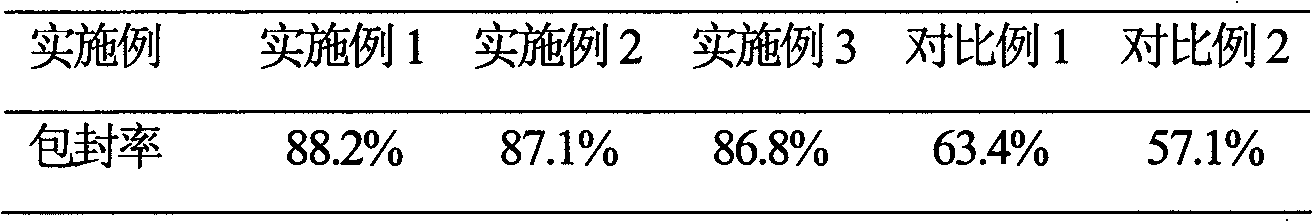
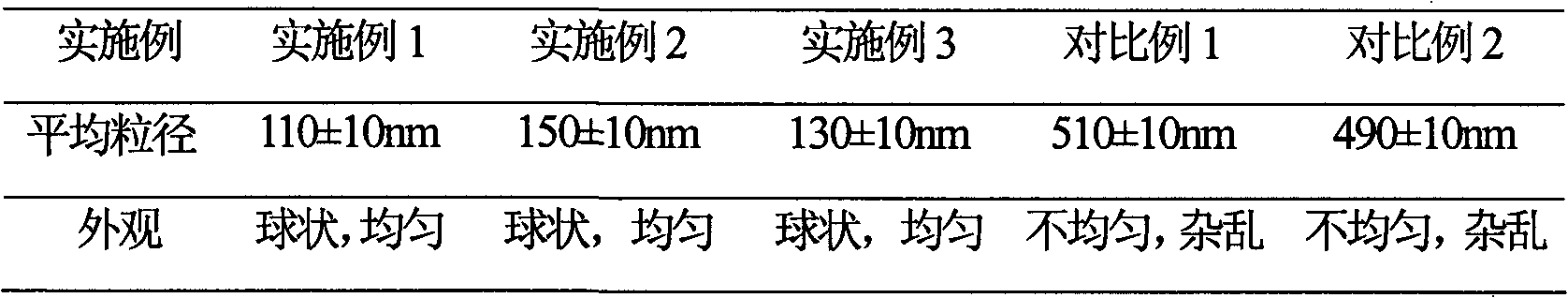
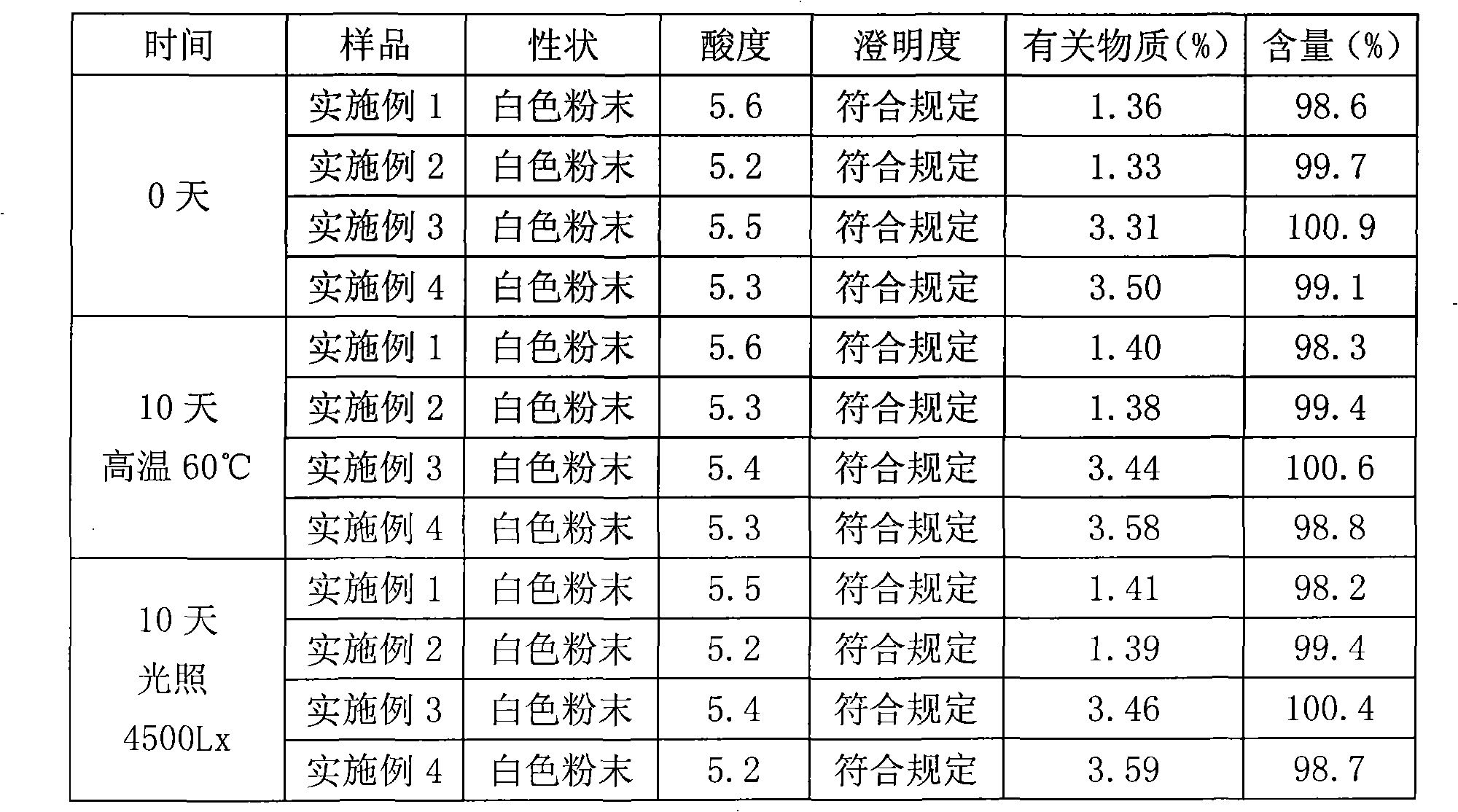
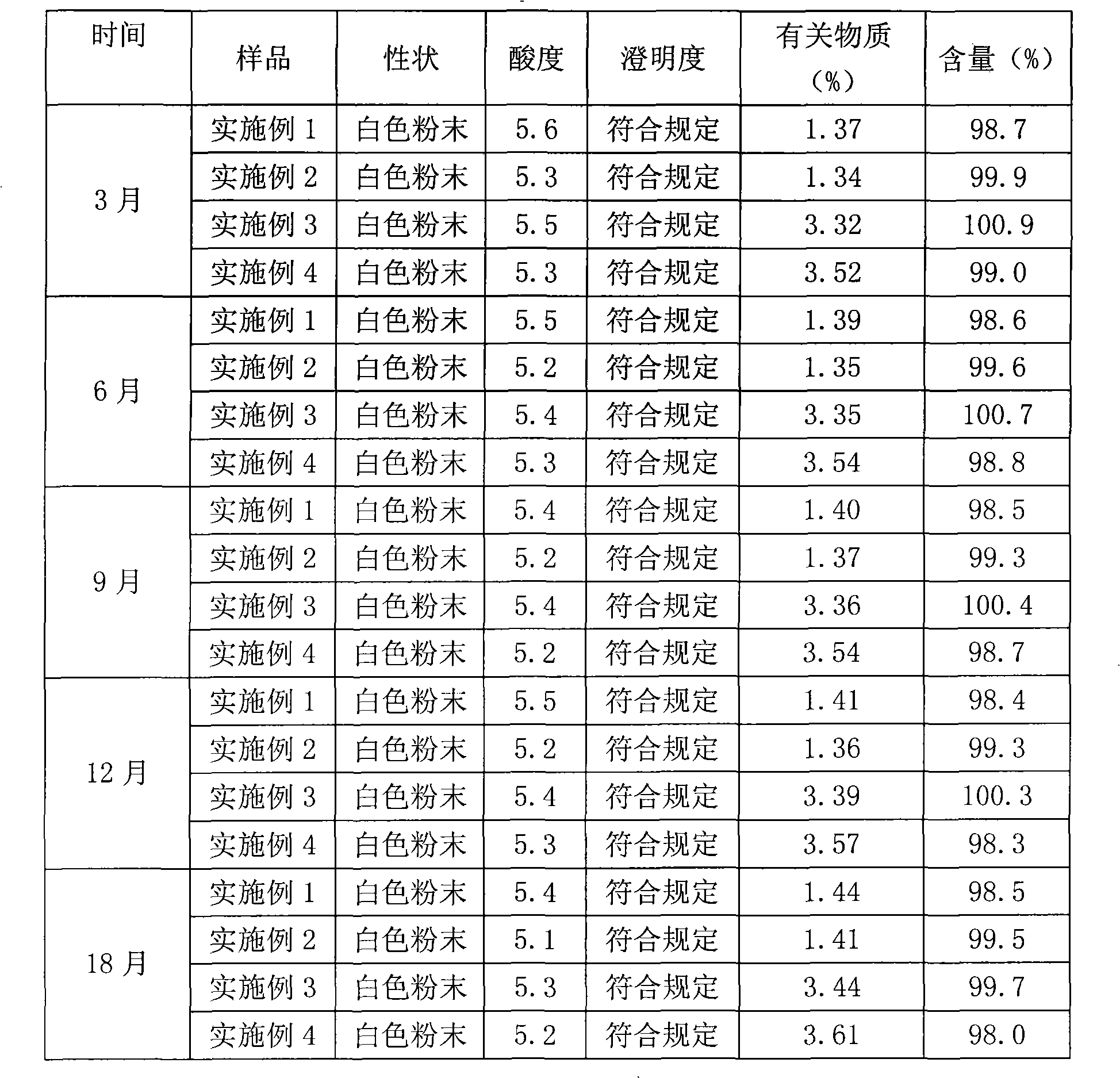
InactiveCN101623263AWon't breakHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsCholesterolAntioxidant
The invention provides a cefminox sodium liposome preparation which is a powder injection. Cefminox sodium is wrapped in liposome in the form of the liposome, and the cefminox sodium liposome preparation comprises the following components by weight part: 0.1-5 parts of cefminox sodium, 1-50 parts of soya bean lecithin, 0.1-40 parts of cholesterol, 0.1-20 parts of antioxidant and 1-60 parts of proppant. The cefminox sodium liposome preparation has good preparation stability and cannot crack because of dewatering, fusion, ice crystal generation, and the like in a freeze-drying process; and after hydrated re-dissolution, the cefminox sodium liposome preparation still can maintain good entrapment rate.
Owner:HAINAN LINGKANG PHARMA CO LTD
Separation and purification method of cefminox sodium and preparation of cefminox sodium freeze-dried powder injection
InactiveCN101279980AAvoid lostAvoid chemical denaturationAntibacterial agentsOrganic active ingredientsStationary phasePurification methods
The invention discloses a method to separate and purify cefminox sodium. Solvent is prepared from trichloromethane, ethyl acetate, carbinol and water, with the upper phase being stationary and the lower phase being mobile. The whole column of a high-speed countercurrent chromatograph is filled with stationary phase solvent and then the mobile phase solvent is pumped into the column; the raw material of cefminox sodium is dissolved in the solvent at the lower phase and the material is fed by an injection valve; above 98% of the product is collected according to the map of the detector and then the solvent is removed to obtain refined cefminox sodium. The method is good in effect and the product is of high purity. The cefminox sodium can be further froze and dried to prepare freeze-dried powder injection. The method is good in separation effect and the product is of high purity.
Owner:HAINAN LINGKANG PHARMA CO LTD
New crystal form composition of cefminox sodium and preparation method thereof
ActiveCN102942576AHigh entropyImprove solubilityAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMBioavailability
The invention provides a new crystal form composition of cefminox sodium and a preparation method thereof, relating to the technical field of medicines and preparation method of medicines. The new crystal form composition of cefminox sodium is measured by powder X-ray diffraction, and the powder X-ray diffraction spectrum represented by a diffraction angle of 2 theta + / - 0.2 degrees has no specific diffractive peaks. The new crystal form is amorphous, and is fused at 171-173 DEG C. According to the invention, the medicine of the crystal form has the advantages of high entropy, good dissolvability, high bioavailability, and good dissolving stability; by using novel freeze drying technology, the operation is simple, the method is suitable for industrial production; and the disadvantages of slow dissolving speed, bad stability, and solid precipitates after long time storage of cefminox sodium crystals prepared by the prior art can be solved.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
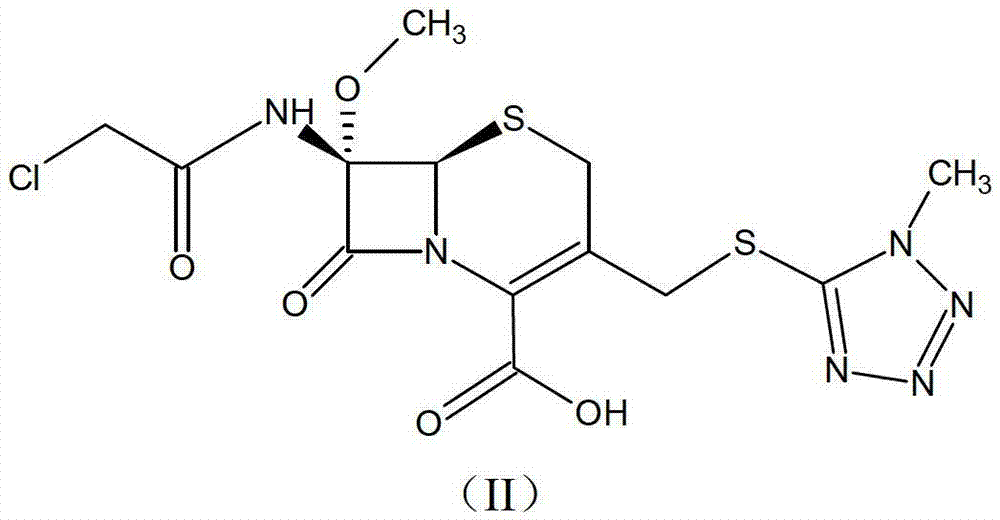
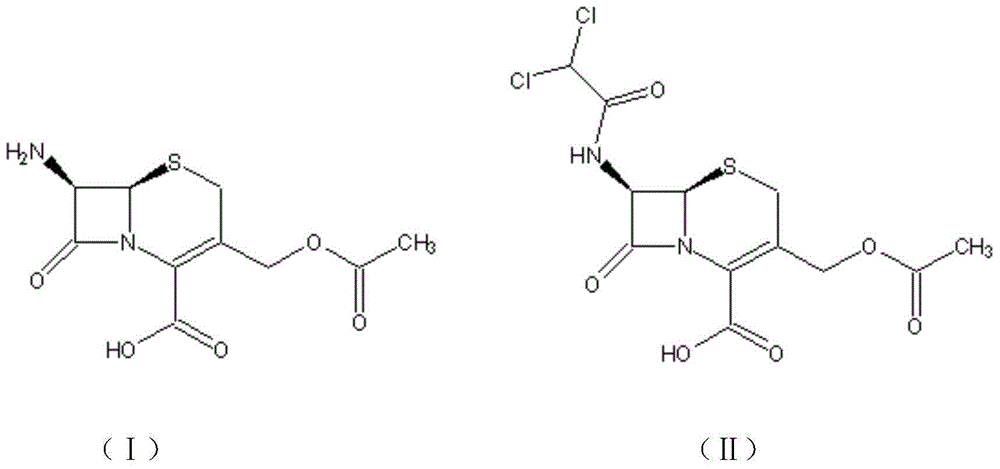
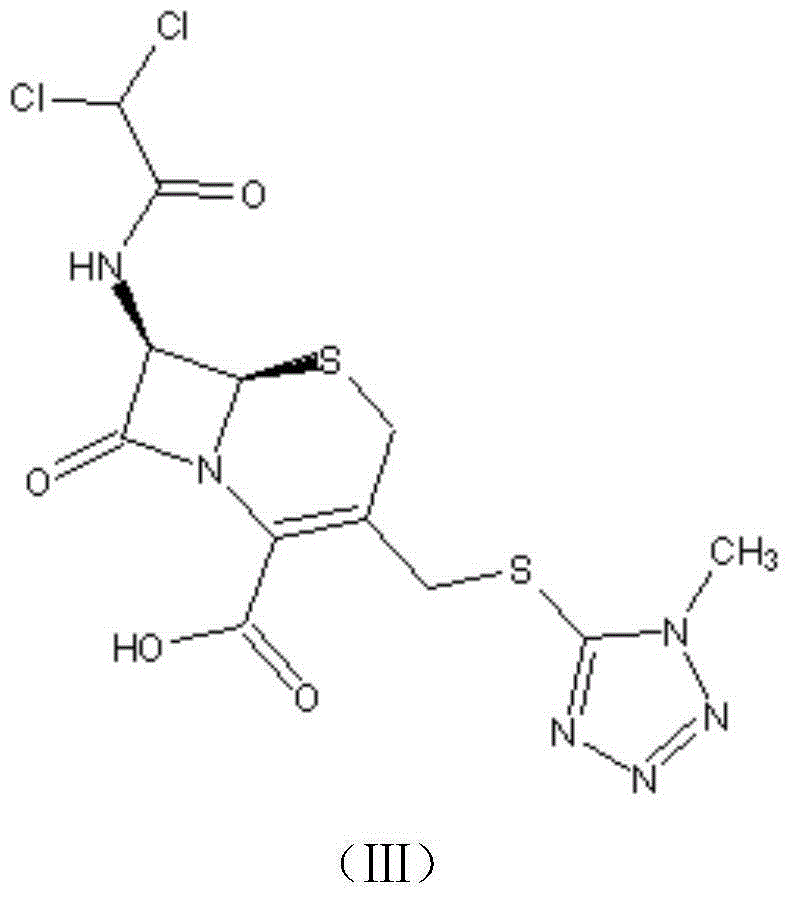
Cephamycin intermediate compound and preparation method thereof
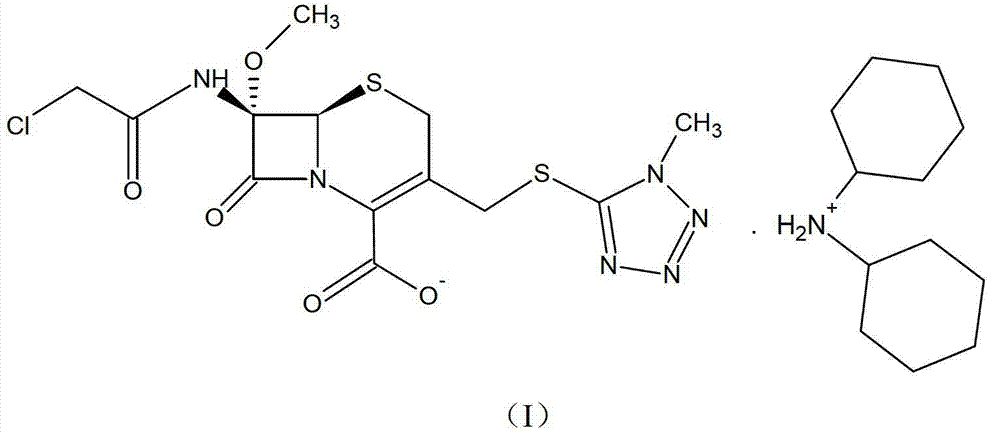
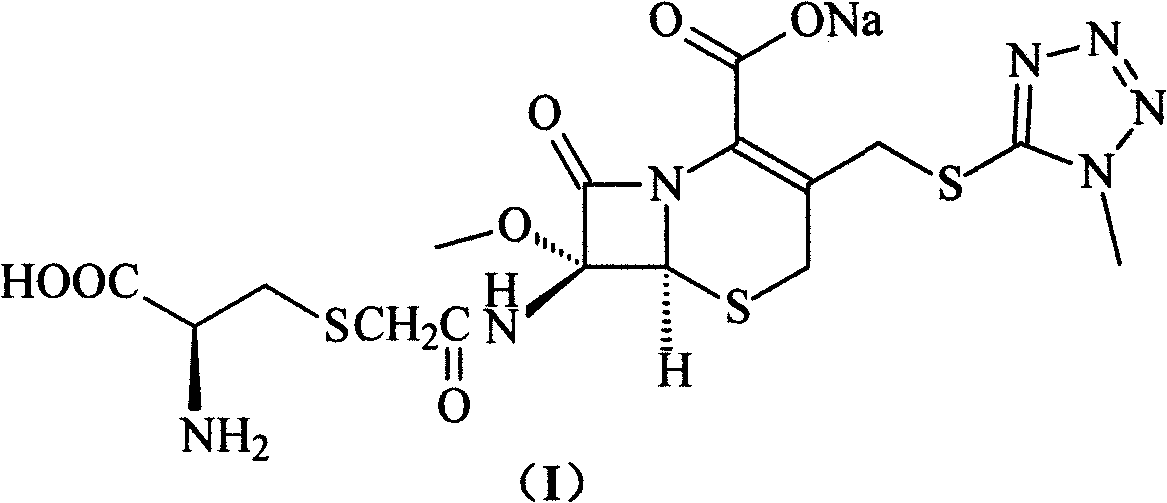
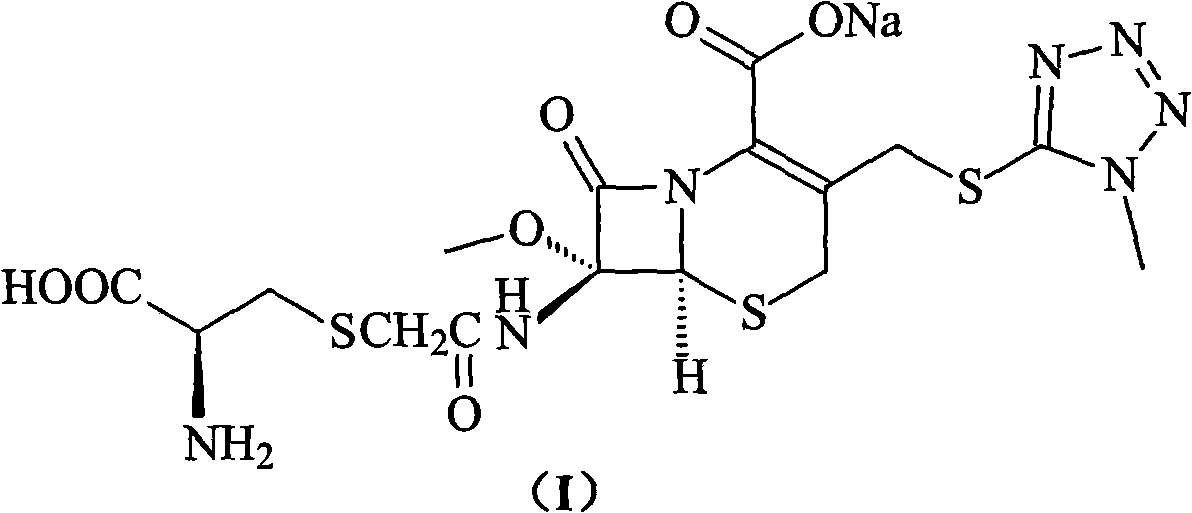
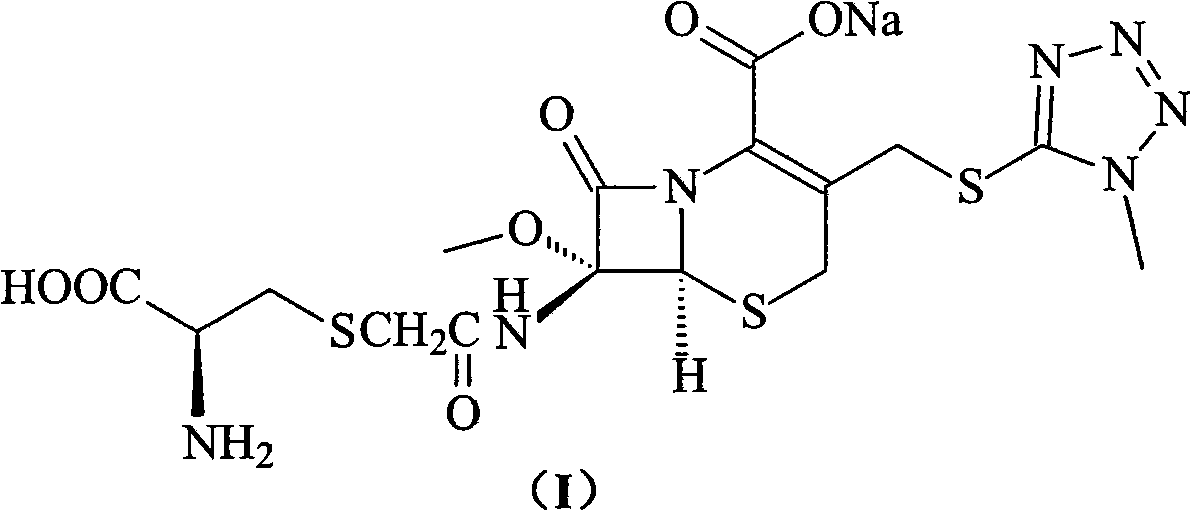
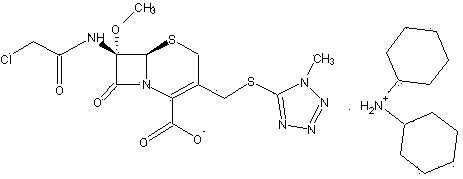
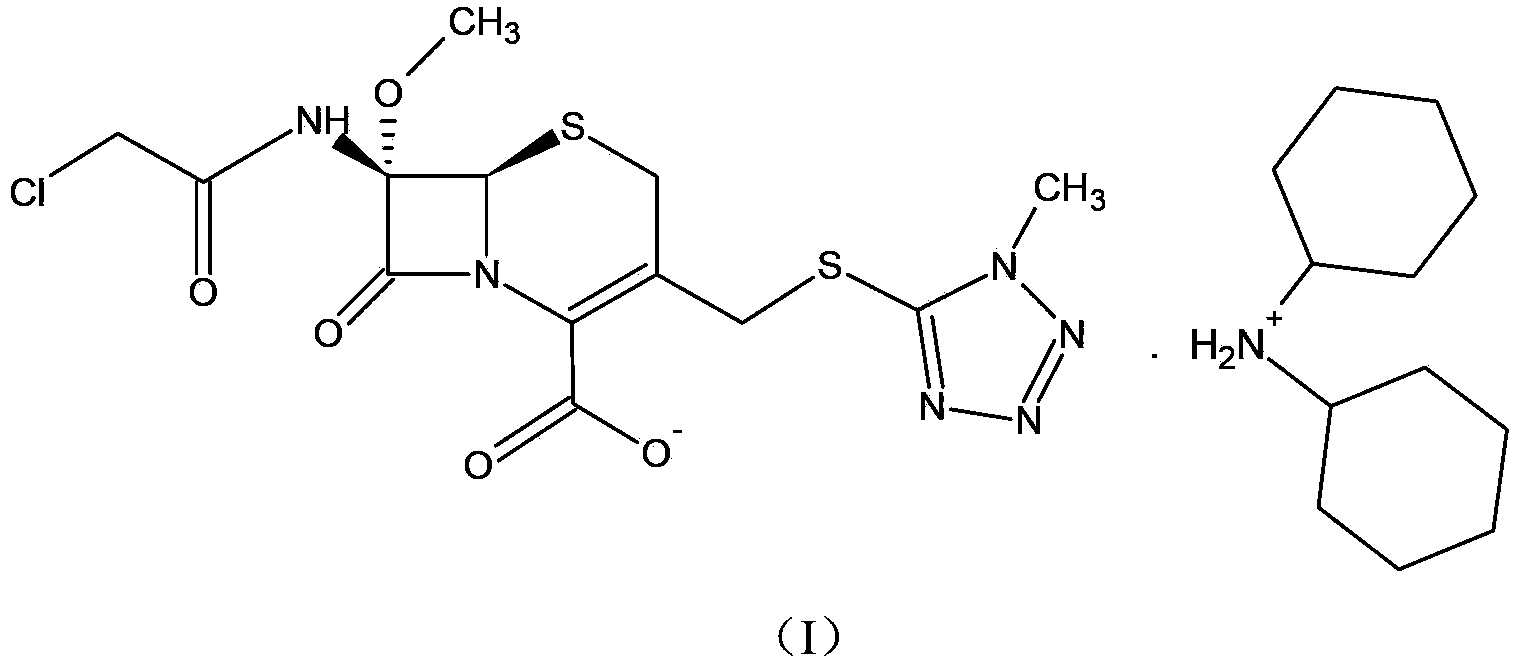
ActiveCN103193796ARaise the level of purityHigh purityOrganic compound preparationAmino compound preparationCEFMINOX SODIUMCarboxylic acid
The invention relates to a cephamycin intermediate compound and a preparation method thereof. The cephamycin intermediate compound has a structure of formula (I), and is prepared by directly reacting 7beta-chloroacetamide-7alpha-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid and dicyclohexylamine in a specific solvent to form a salt and crystallizing. The cephamycin intermediate compound has the characteristics of high purity, high stability and the like, is conductive to improving the purity of cephamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
Cefminox sodium compound reducing adverse reactions and preparation thereof
ActiveCN105924456AReduce generationReduce adverse reactionsAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMAntibiotic drug
The invention relates to an antibiotic drug, in particular to a preparation process of cefminox sodium compound reducing adverse reactions and a preparation of the cefminox sodium compound. The preparation process has the advantages such as high yield and high purity and is suitable for industrial production, and an obtained product has significant improvements in terms of product stability, preparations to reduce adverse reactions, and clinical application.
Owner:福安药业集团庆余堂制药有限公司 +2
Cefminox sodium compound and new preparation method thereof
The invention aims at providing a cefminox sodium compound and a new preparation method thereof. The refinement and the purification of the cefminox sodium compound can be achieved by acid-base reaction, activated carbon adsorption, electrodialyzer treatment and adsorption, separation and purification of a chromatographic column. The purity of final products is higher than that of the traditional product, the quality of preparation products is improved, the toxic and side effects are reduced, and the safety of clinical medicaments is ensured.
Owner:HAINAN LINGKANG PHARMA CO LTD
Synthesis method for cefminox sodium
The invention discloses a synthesis method for cefminox sodium. The method comprises the following steps: by taking cefminox mother nucleus 7-AMCA as a raw material, carrying out acylation reaction and decarboxylate protection reaction to obtain a mixed liquor; processing and condensing the mixed liquid into cefminox acid, and salifying without separation of an intermediate to synthesize cefminox sodium in one step. In the manner, by adopting the synthesis method for cefminox sodium disclosed by the invention, a plurality of reaction steps can be finished one time without separation, a sterile cefminox sodium product can be obtained by a continuous operation one time, the total yield can be up to 90% by taking 7-AMCA as an initial raw material, the process route is short, and the adopted reaction agent is low in price and low in cost.
Owner:苏州盛达药业有限公司
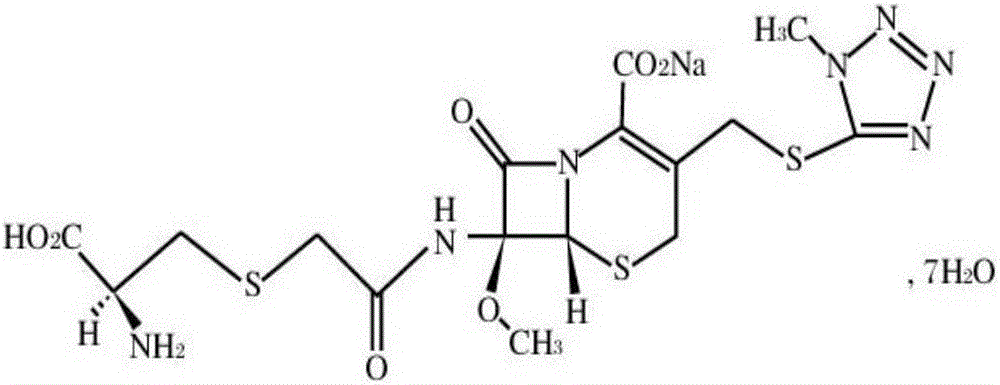
Cefminox sodium crystal form compound
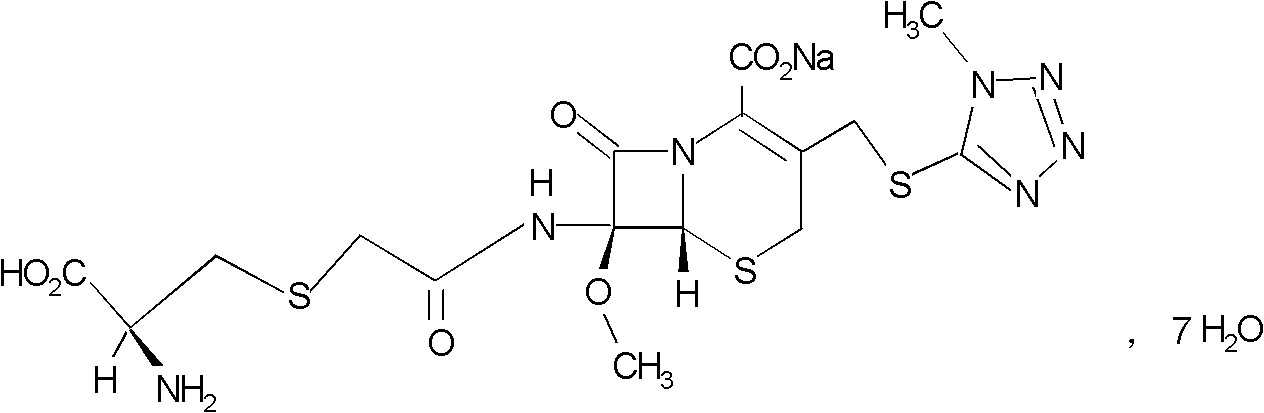
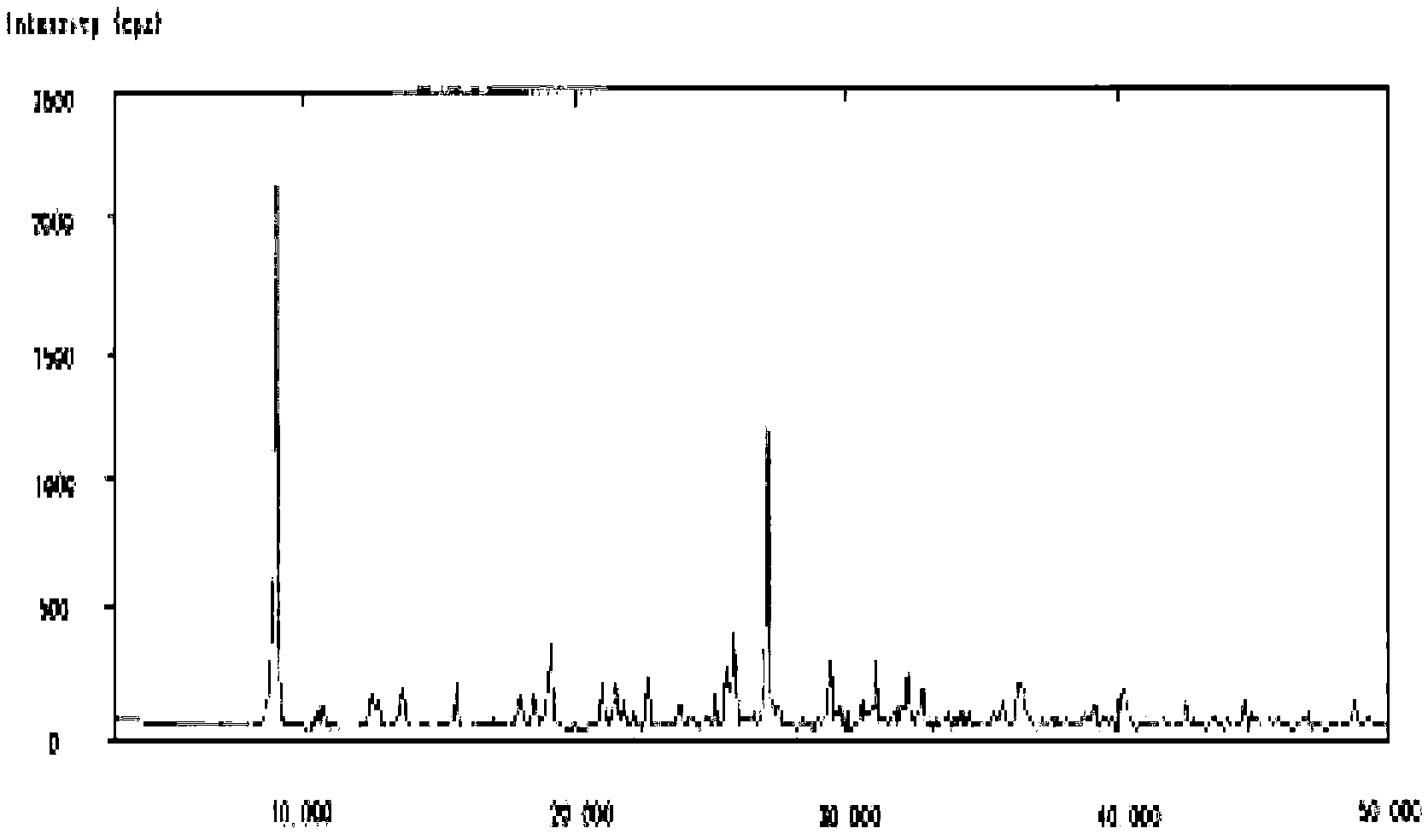
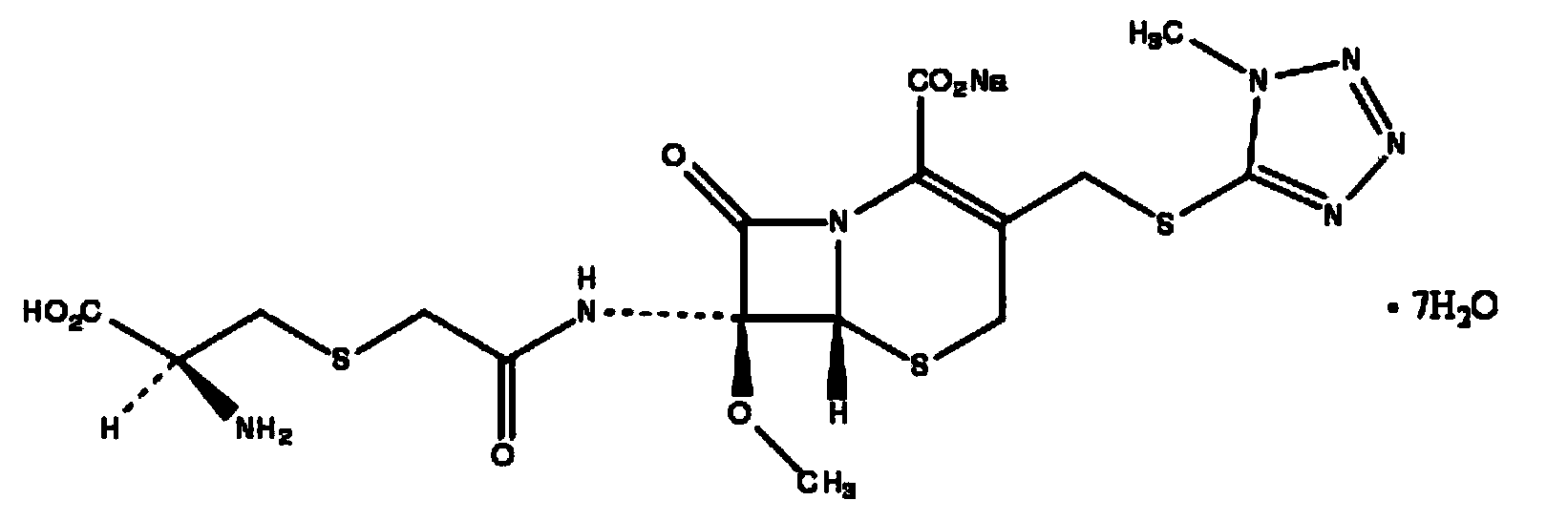
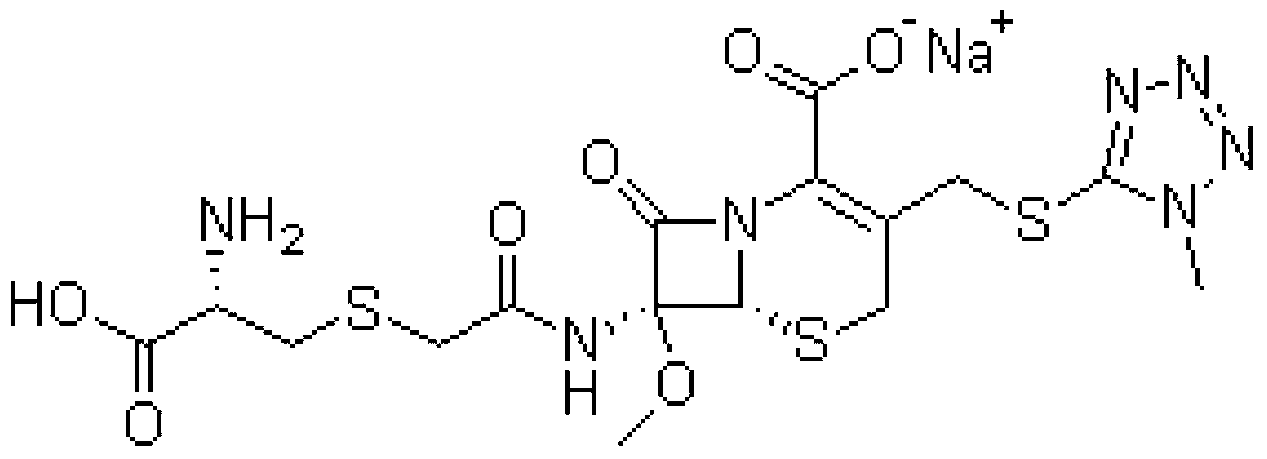
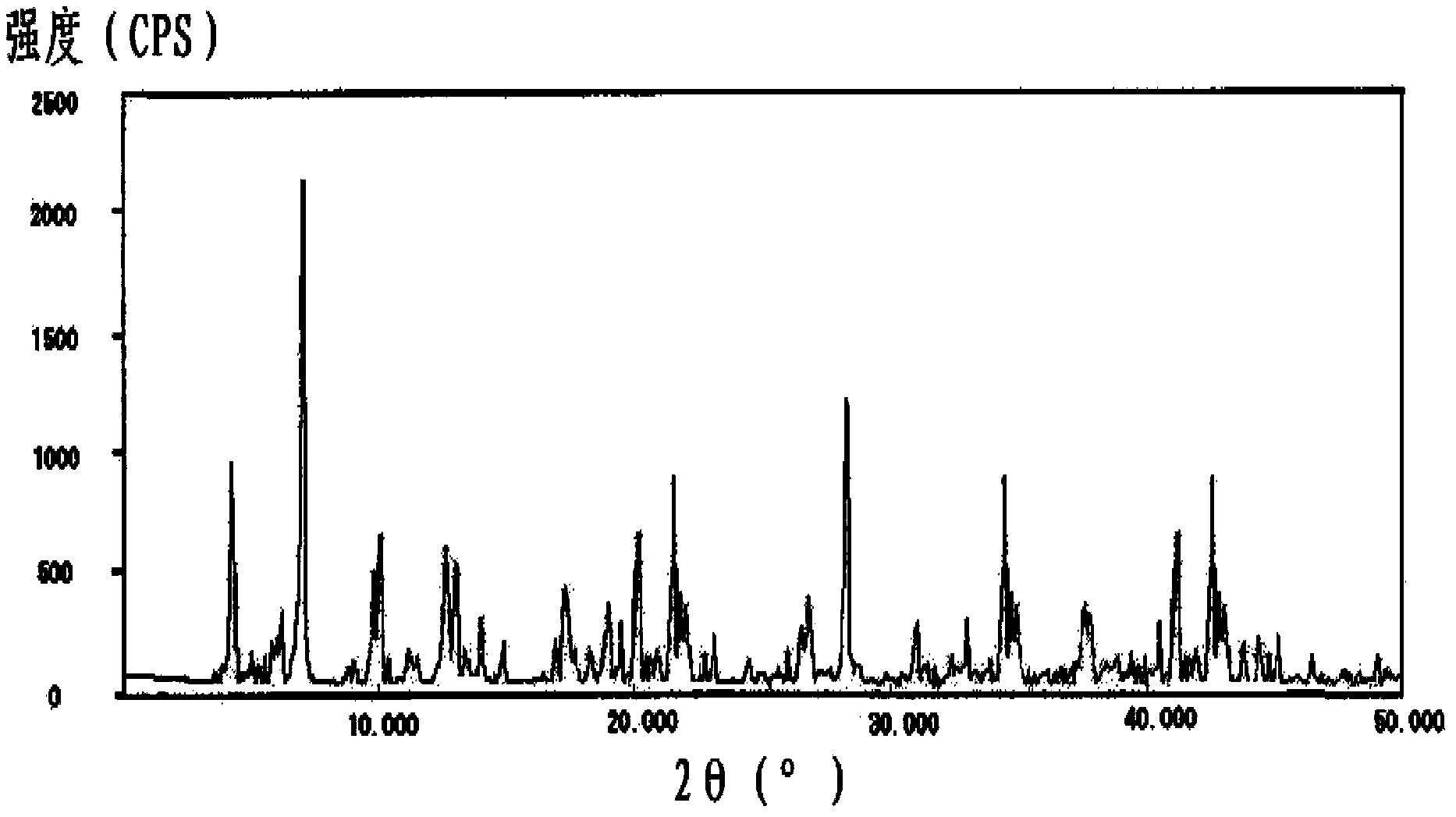
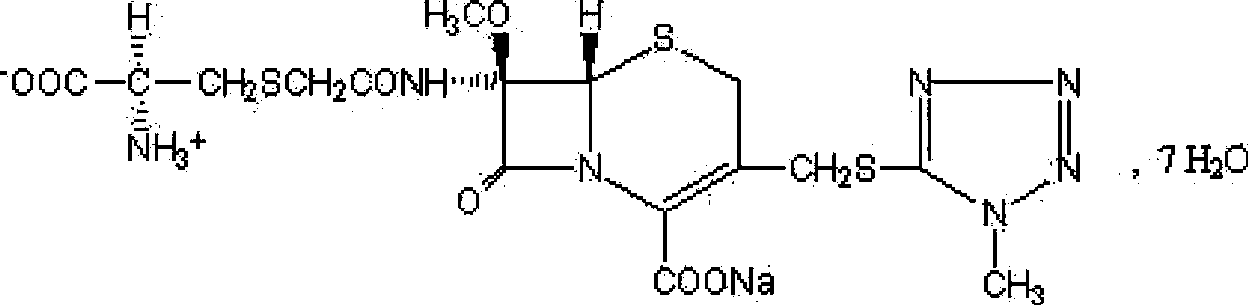
The invention belongs to the technical field of medicine, and particularly relates to a cefminox sodium crystal form compound. The structure formula of the cefminox sodium crystal form compound is shown as follows. The X-ray powder diffraction spectrum obtained by using Cu-K[alpha] ray of the cefminox sodium crystal form compound is shown as the figure 1. The cefminox sodium crystal form compound is a novel crystal form compound different from compounds in the prior art, and has better dissolution rate. Insoluble particles precipitated after the cefminox sodium crystal form compound is put for a long time are less than that of the compounds in the prior art. A cefminox sodium aseptic powder injection prepared from the cefminox sodium crystal form compound has good clinical effects for respiratory tract bacterial infection and bacteria curative effects, and has low occurrence rate of untoward effects.
Owner:YOUCARE PHARMA GROUP +1
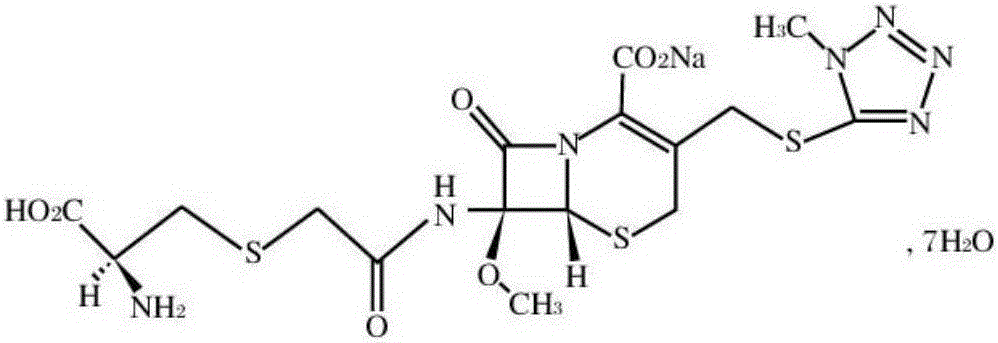
Cefminox sodium compound as well as preparation method and pharmaceutical composition of cefminox sodium compound
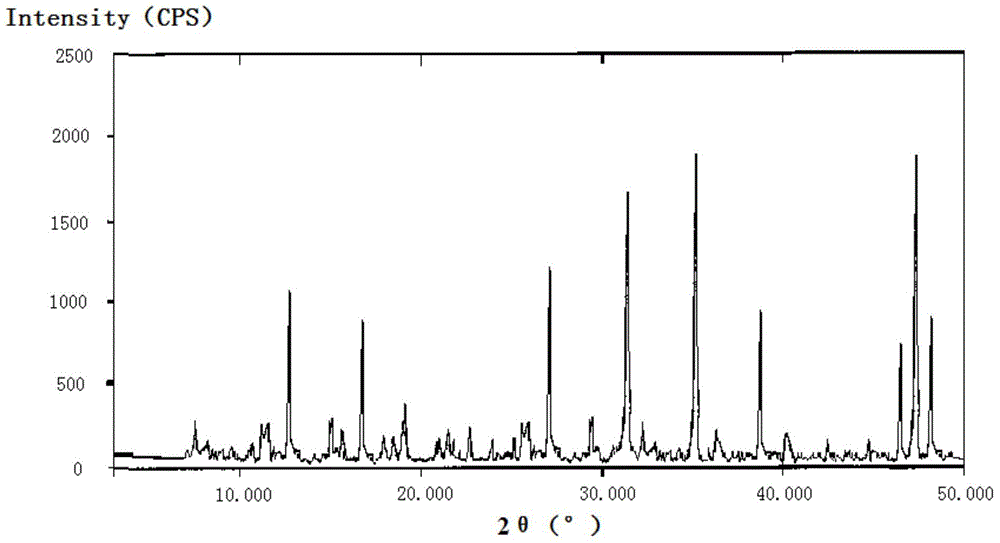
InactiveCN103304581AHigh lattice energyImprove stabilityAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMX-ray
The invention belongs to the technical field of medicines, and in particular relates to a cefminox sodium compound. The structural formula of the cefminox sodium compound is shown in the specification and an X-ray powder diffraction spectrum of the cefminox sodium compound, which is measured through Cu-K(alpha) ray, is shown in Figure 1. Furthermore, the invention provides a preparation method of the cefminox sodium compound, a pharmaceutical composition containing the cefminox sodium compound, and a preparation method of the pharmaceutical composition. The dosage form of cefminox sodium medicine is sterile powder injection. The cefminox sodium compound and the pharmaceutical composition thereof provided by the invention, in comparison with the prior art, have better storage stability and liquidity and are easier to sub-package and mix; and the cefminox sodium compound and the pharmaceutical composition thereof greatly enhance the medication safety of patients.
Owner:四川省惠达药业有限公司
Anti-infective pharmaceutical composition containing cefminox
InactiveCN101780087AAct as a diureticPromote secretionAntibacterial agentsOrganic active ingredientsDiseaseArginine
The invention belongs to the technical field of medicines, discloses an anti-infective pharmaceutical composition containing cefminox, and relates to a preparation method of the pharmaceutical composition and an application of the medicine in preparing the medicines for treating infectious diseases. By the combination of the cefminox, Na2HPO4 / NaH2PO4 and arginine, the invention solves the problem of the prior art that the cefminox is easy to hydrolyze and is unstable after storage and clinical dosage. The pharmaceutical composition has stable property, convenient storage and improved clinical medication safety.
Owner:深圳四环医药有限公司 +1
Synthesis method of cefminox sodium
The invention aims at providing a synthesis method of cefminox sodium. The synthesis method specifically comprises the following steps: firstly, mixing 7beta-amino-7alpha-methoxy-3-[(1-methyl-1H-5-tetrazolyl)-mercapto methyl]-3-cephem-4-methyl diphenyl carboxylate and dichloromethane (7-MAC), and bromoacetamide bromine under a certain condition to obtain 7beta-bromoacetyl amino-7alpha-methoxy-3-[(1-methyl-1H-5-tetrazolyl)-mercapto methyl]-3-cephem-4-methyl diphenyl carboxylate; then removing a carboxylic acid protection group to obtain 7beta-bromoacetyl amino-7alpha-methoxy-3-[(1-methyl-1H-5-tetrazolyl)-mercapto methyl]-3-cephem-4-carboxylic acid; then enabling the 7beta-bromoacetyl amino-7alpha-methoxy-3-[(1-methyl-1H-5-tetrazolyl)-mercapto methyl]-3-cephem-4-carboxylic acid to react withD-cysteine hydrochloride to obtain the cefminox sodium. According to the synthesis method provided by the invention, the product yield and purity of the synthesized cefminox sodium are high; phosphoric acid loaded activated carbon is used as a solid catalyst and is easy to separate from a reaction system, and the formation of acid waste liquid is avoided.
Owner:GUANGDONG LIGUO PHARMACY
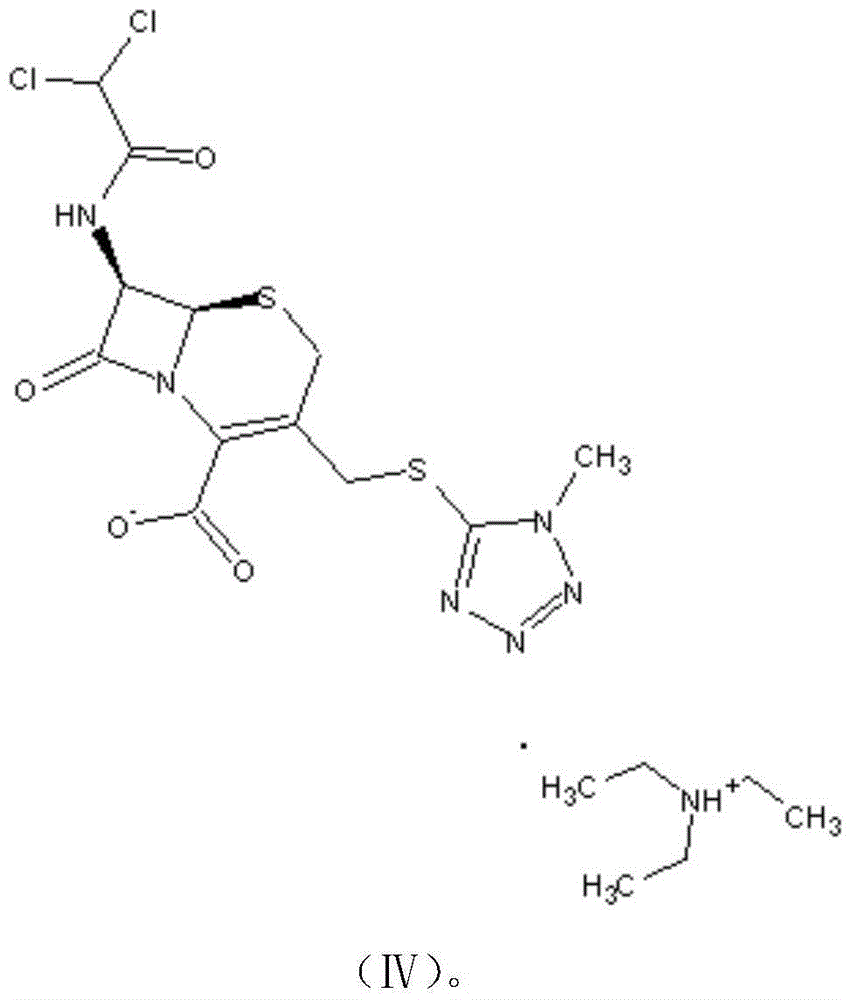
Preparation method for cephamycin intermediate
The invention relates to a preparation method for a cephamycin intermediate. A compound of the cephamycin intermediate can be seen in the formula (IV). The cephamycin intermediate is obtained by making 7-ACA react with dichloroacetyl chloride in specific solvent, making the obtained product react with MMT under catalysis of a sulfoacid catalyst and conducting crystallization. The cephamycin intermediate has the advantages of being high in purity and stability and the like, is beneficial for improving the purity of final cephamycin products such as cefminox sodium, cefmetazole and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
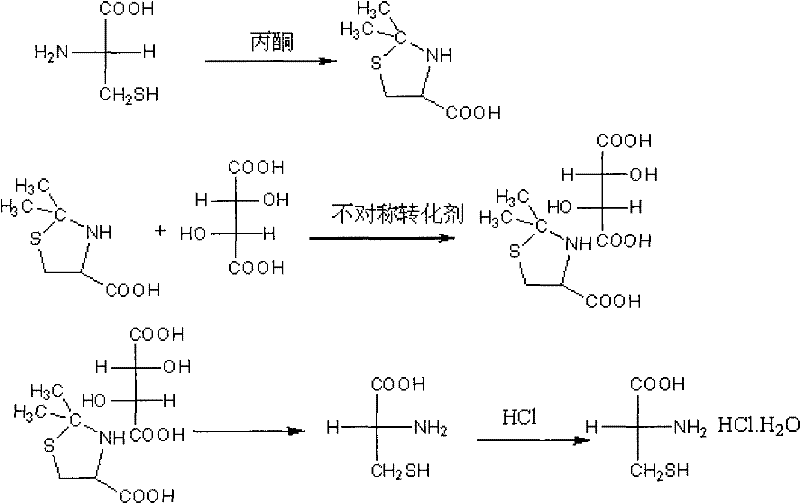
D-cysteine hydrochloride monohydrate preparation method
InactiveCN102234244AHigh chemical purityShorten drying timeThiol preparationPropanoic acidSalicylaldehyde
The invention discloses a preparation method of an intermediate D-cysteine hydrochloride monohydrate which is used for synthetizing third generation cephalosporin antibiotic cefminox sodium. The preparation method comprises the following steps: taking L-cysteine, acetone and L-tartaric acid as raw materials which are subjected to a reaction with acetone and acetate (or propionic acid) mediums, utilizing an asymmetric transforming agent salicylic aldehyde to carry out asymmetric transformation to form D-2,2-dimethyl thiazolidine-4-carboxylic acid-L-tartrate; carrying out hydrolyzation, crystallization, filteration and drying treatments on double salt to obtain D-cysteine; generating hydrochloride after a reaction between the D-cysteine and hydrochloric acid with a content of 16-31%, and carrying out dissolving, decolouring, distillation and recrystallization treatments to obtain the D-cysteine hydrochloride-hydrate product. The preparation method in the invention has the advantages of simple process, high product yield, high chemical purity and high optical purity. Recycle and reuse of input solvent and unreacted raw material facilitate industrialization production.
Owner:四平市精细化学品有限公司
Cefminox sodium preparation for injection and preparation and use method thereof
PendingCN110420184AMaintain morphological consistencyNarrow particle size distributionAntibacterial agentsOrganic active ingredientsSodium bicarbonateCEFMINOX SODIUM
The invention discloses a cefminox sodium preparation. The cefminox sodium preparation is prepared from the raw materials: cefminox sodium used as medicine, N-(beta-D-glucopyranose) octanamide and a film-forming lipid material; and the cefminox sodium preparation is further prepared from the raw materials: tartaric acid and solid sodium bicarbonate particles. In addition, the invention further discloses a preparation method and a use method of precursor liposomes. A precursor liposome pharmaceutical preparation is prepared by using solid dispersion, liposome suspension liquid is prepared by combining with an effervescence technology, the stability of the long-term storage of the precursor liposomes can be achieved, morphological consistency of the liposomes can further be maintained better, the particle size distribution is narrow, and the entrapment efficiency is not significantly reduced.
Owner:上海欣峰制药有限公司
Preparation method of cefminox sodium powder-needle preparation for injection
InactiveCN106562932AHigh puritySimple processAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMReaction temperature
The invention discloses a preparation method of a cefminox sodium powder-needle preparation for injection, and belongs to the technical field of biomedical synthesis. The method comprises: mixing the prepared raw materials, respectively carrying out an acylation reaction and a carboxyl protection removing reaction, treating the solution obtained after the reaction to obtain cefminox, adding sodium carbonate, and crystallizing to obtain cefminox sodium. According to the present invention, the steps are simple, the ladder temperature control mode is used in the reaction temperature control, the complete reaction is ensured through the material feeding time limiting while the production efficiency is improved, the quality of the prepared product is good, and the raw material utilization rate is high.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefminox sodium raw material as well as preparation method and preparation thereof
PendingCN110003240ASimple preparation processSuitable for industrial productionAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMSodium Chloride Injection
The invention belongs to the field of biomedical technology, and particularly relates to a cefminox sodium raw material as well as a preparation method and preparation thereof. After many years of creative experiments, applicants obtain the novel cefminox sodium crystal form provided by the invention, the crystal form drug and auxiliary material sodium citrate are combined to prepare the novel powder injection, after the powder injection and a sodium chloride injection or a glucose injection are mixed, the long-term stability is maintained, and insoluble microparticles and the like are hardlyincreased, so that the results further show that the preparation provided by the invention has better stability.
Owner:HARBIN GLORIA PHARMA +1
Synthesis method of cefminox sodium
The invention discloses a synthesis method of cefminox sodium, which comprises the following steps: completely dissolving methoxycephalosporin (7-MAC) and dichloromethane in a reaction flask (1), and adding pyridine and carbon tetrachloride; after complete reaction, adding a saturated aqueous solution of sodium chloride for extracting and layering, and performing vacuum concentration on a bottom-phase organic phase; adding dichloromethane and ethanol into a reaction flask (2), and introducing hydrogen chloride gas; after complete reaction, adding the concentrated liquid in the reaction flask (1) into the reaction flask (2); after complete reaction, adding a mixed solvent for extracting and layering; performing liquid separation on the organic phase by use of the saturated aqueous solution of sodium chloride, and keeping the bottom-phase organic phase; adding D-cysteine into the organic phase, adjusting the pH value of the solution to 6.0-6.5, and keeping the temperature at 20-25 DEG C; after sufficient reaction, feeding ethanol, and performing sufficient crystal precipitation and vacuum drying to obtain cefminox sodium. According to the method, the reaction process causes low toxicity, the operation is safe, the quality and yield of the product are increased, and the crystal form of the product is good and easy to dry, thus the method is suitable for popularization and application in mass production.
Owner:GUANGDONG LIGUO PHARMACY
Cefminox sodium compound of new route
InactiveCN101696214BAntibacterial agentsPhysical/chemical process catalystsSodium iodideCEFMINOX SODIUM
The invention provides a cefminox sodium compound of a new route, in particular a method for producing the cefminox sodium compound. The method comprises a step of generating cefminox sodium through the reaction between 7beta-bromoacetamido-7a-methoxyl-3-(1-methyl-1H-tetrazole-5-S-methyl)-3-cephem-4-carboxylic acid and D-cysteine hydrochloride, and is characterized in that: the reaction conditionof the step is that the reaction is performed in aqueous solution, sodium iodide is used as a catalyst, the pH value of the reaction system is between 7.5 and 8.0, and the reaction temperature is kept at 30+ / -5 DEG C. Compared with the method for producing the cefminox sodium compound in the prior art, the method of the invention for producing the cefminox sodium compound greatly improves the product yield and the purity, and solves the problem that a cefminox sodium bulk drug always has a low purity in the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
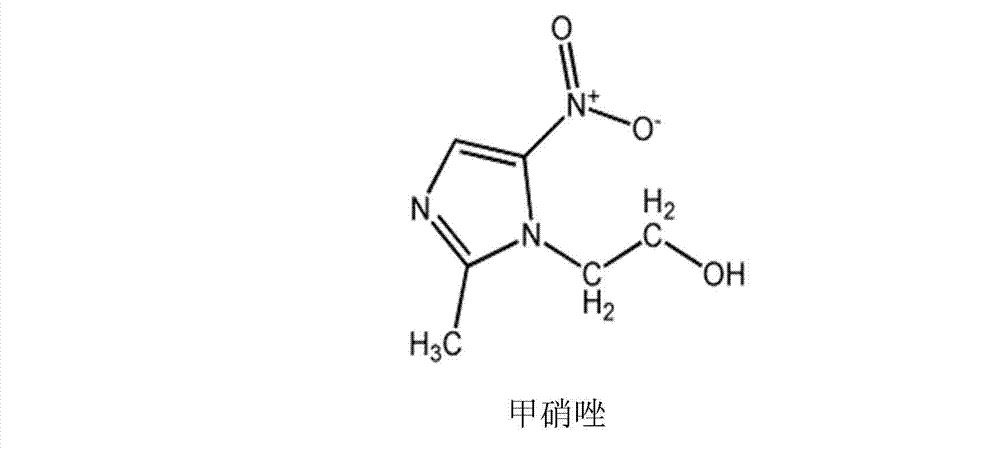
Cefminox sodium drug composition and preparation method thereof
InactiveCN103110645AReduce dosageReduce or avoid side effectsAntibacterial agentsOrganic active ingredientsSide effectMicrosphere
The invention provides a cefminox sodium drug composition and a preparation method thereof, relating to the fields of pharmaceutical preparations and preparation methods and mainly solving the problems that single preparations of cefminox sodium and metronidazole in the prior art have great dosages, poor treatment effects after being combined and big side effects. The drug composition comprises cefminox sodium and metronidazole lipid microspheres in a weight ratio of (100:3)-(100:10), wherein the weight of the metronidazole lipid microspheres is based on metronidazole. The drug composition is prepared into powder injection. The pH value of water solution of the drug composition is 6.5-7.0. The powder injection of the drug composition provided by the invention not only has high curative effect but also can reduce the dosage of metronidazole by over 80%, thus greatly reducing or avoiding the toxic and side effects, especially gastrointestinal reactions including nausea, emesis, anorexia, abdominal cramp and neurotoxicity, of metronidazole and further reducing the occurrence rate of adverse reactions.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Cefminox sodium preparation method
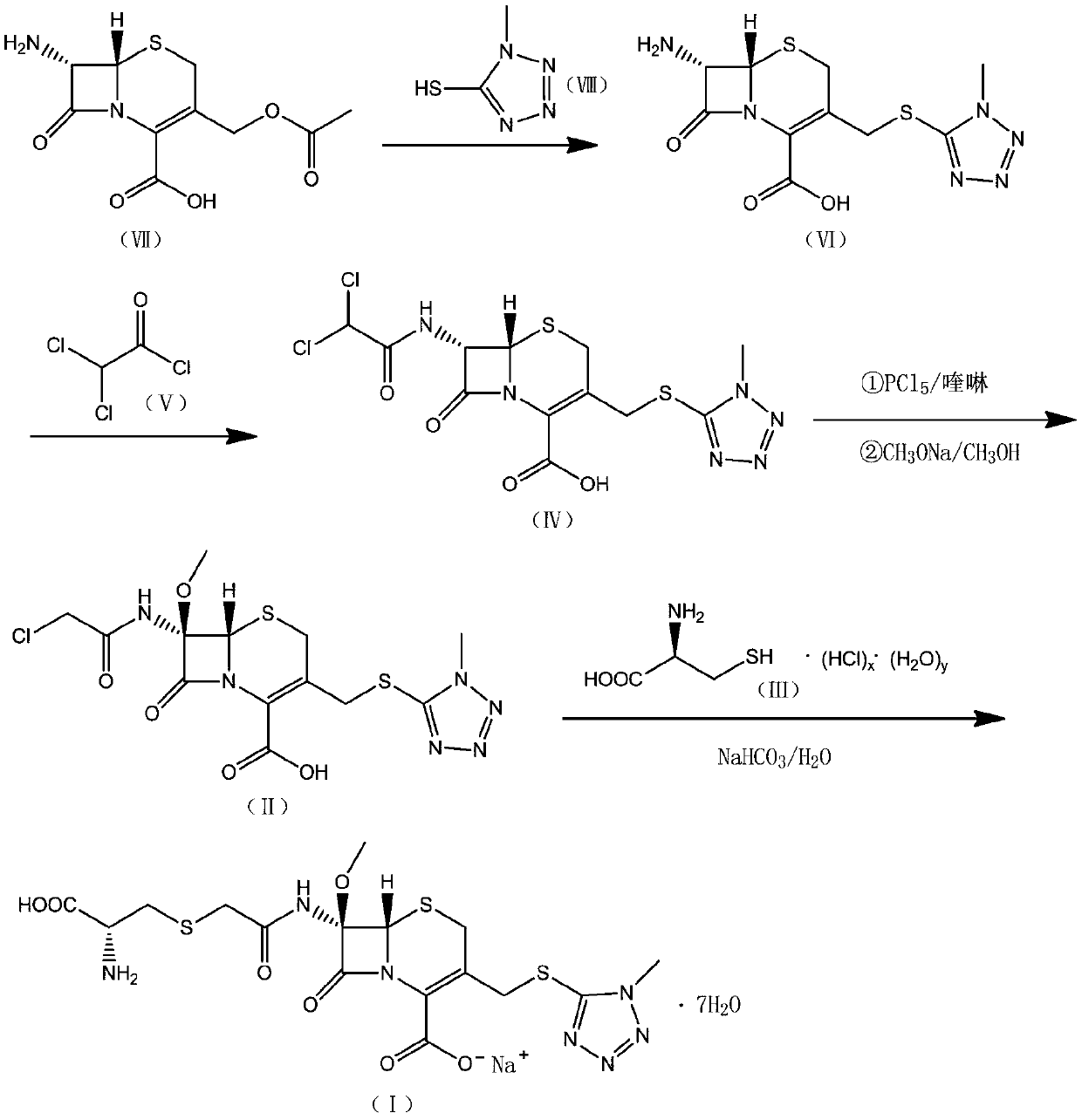
InactiveCN110590812AHigh selectivityHigh reactivityOrganic chemistryTemperature controlOrganic solvent
The invention provides a cefminox sodium preparation method, which comprises: (1) dispersing a compound having a structure represented by a formula (VII) in an organic solvent A to prepare a solutionor a suspension, adding a compound having a structure represented by a formula (VII), and carrying out vacuum drying to obtain a compound having a structure represented by a formula (VI); (2) dispersing the compound with the structure represented by the formula (VI) into an organic solvent C, adding a compound having a structure represented by a formula (V) in a dropwise manner, and drying to obtain a compound having a structure represented by a formula (IV); (3) dispersing the compound having the structure represented by the formula (IV) in an organic solvent, and removing the solvent by rotary evaporation under a pressure reducing condition to obtain an organic phase containing a compound having a structure represented by a formula (II); and (4) adding purified water into the solution containing the compound having the structure represented by the formula (II), heating, adjusting the pH value, adding a compound having a structure represented by a formula (III), carrying out temperature control stirring, transferring the water phase into a crystallizing tank, crystallizing, and carrying out pressure reducing drying to obtain the cefminox sodium heptahydrate. According to the invention, the synthesis process is shortened, the cost is reduced, and the product yield and the purity are high.
Owner:重庆天地药业有限责任公司
Cefminox sodium compound crystal, preparation method of cefminox sodium compound crystal and sterile powder injection containing cefminox sodium compound crystal
ActiveCN102838623BReduce contentImprove stabilityAntibacterial agentsPowder deliveryCEFMINOX SODIUMPhysical chemistry
Owner:HAINAN HERUI PHARMA
A kind of cefminox sodium crystal form compound
The invention belongs to the technical field of medicine, and particularly relates to a cefminox sodium crystal form compound. The structure formula of the cefminox sodium crystal form compound is shown as follows. The X-ray powder diffraction spectrum obtained by using Cu-K[alpha] ray of the cefminox sodium crystal form compound is shown as the figure 1. The cefminox sodium crystal form compound is a novel crystal form compound different from compounds in the prior art, and has better dissolution rate. Insoluble particles precipitated after the cefminox sodium crystal form compound is put for a long time are less than that of the compounds in the prior art. A cefminox sodium aseptic powder injection prepared from the cefminox sodium crystal form compound has good clinical effects for respiratory tract bacterial infection and bacteria curative effects, and has low occurrence rate of untoward effects.
Owner:YOUCARE PHARMA GROUP +1
Cephamycin intermediate compound and preparation method thereof
ActiveCN103193796BRaise the level of purityHigh purityOrganic compound preparationAmino compound preparationTetrazoleCEFMINOX SODIUM
The invention relates to a cephamycin intermediate compound and a preparation method thereof. The cephamycin intermediate compound has a structure of formula (I), and is prepared by directly reacting 7beta-chloroacetamide-7alpha-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid and dicyclohexylamine in a specific solvent to form a salt and crystallizing. The cephamycin intermediate compound has the characteristics of high purity, high stability and the like, is conductive to improving the purity of cephamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
Cefminox sodium compound and new preparation method thereof
The invention aims at providing a cefminox sodium compound and a new preparation method thereof. The refinement and the purification of the cefminox sodium compound can be achieved by acid-base reaction, activated carbon adsorption, electrodialyzer treatment and adsorption, separation and purification of a chromatographic column. The purity of final products is higher than that of the traditionalproduct, the quality of preparation products is improved, the toxic and side effects are reduced, and the safety of clinical medicaments is ensured.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pharmaceutical composition with cefminox sodium sterile mixed powder form
ActiveCN102973569BFast dissolutionImprove stabilityAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMHeat stability
The invention relates to a pharmaceutical composition with a cefminox sodium sterile mixed powder form, and the pharmaceutical composition comprises cefminox sodium, anhydrous sodium carbonate and sodium benzoate, wherein the mass ratio of the cefminox sodium, the anhydrous sodium carbonate and the sodium benzoate is 100g:(0.1-0.5)g:(0.1-1.0)g. Due to the combined application of the cefminox sodium, the anhydrous sodium carbonate and the sodium benzoate, the heat stability of the cefminox sodium can be greatly improved, and the stability of the cefminox sodium in a solution state can be improved, so that the clinical use safety is improved. Furthermore, the invention also relates to a preparation method of the pharmaceutical composition, and the preparation method is simple and practicable in technology, thus being suitable for large-scale production.
Owner:HAINAN HERUI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com