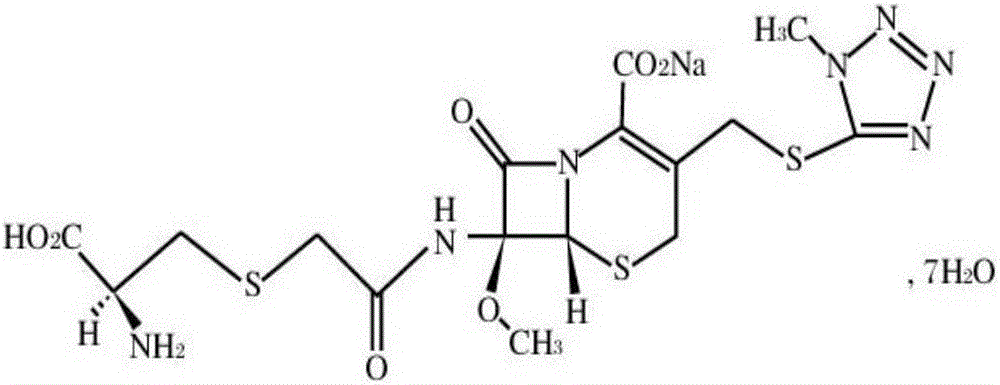
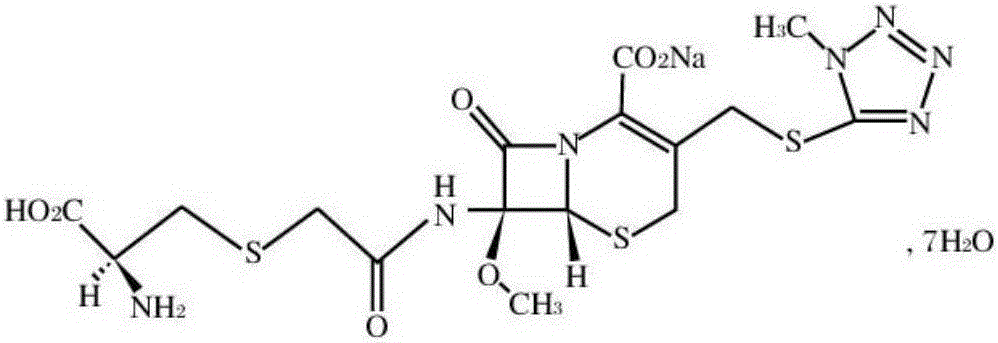
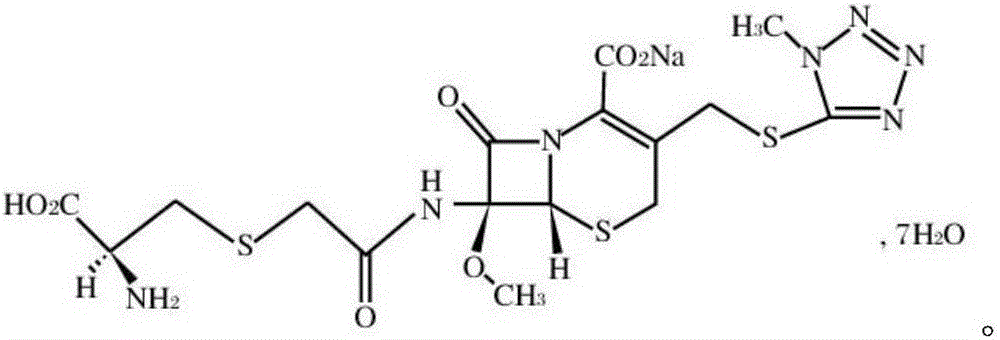
Cefminox sodium compound reducing adverse reactions and preparation thereof
A cefminox sodium and adverse reaction technology, applied in the field of antibiotics, can solve the problems of unsecured safety, complicated synthesis operation process, low yield of final product, etc. Simple and effective in reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) 358g 7β-amino-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 94.5g chloroacetic acid were dissolved In 3L of dichloromethane, add 30g of 3A molecular sieve and 200g of magnesium sulfate, react at 40°C, TLC detects that the reaction is complete, filter, wash with water, and distill off the solvent under reduced pressure to obtain 433g of 7β-chloroacetamido-7a-methoxy- 3-(1-Methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid;
[0029] (2) 433g 7β-chloroacetamido-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 157gD-semi Add cystine hydrochloride to 2L water, then add 20g EDTA and 309g sodium thiosulfate, react at 30°C, TLC detects that the reaction is complete, cool to room temperature, add 2L acetone, precipitate crystals, filter, and dry to obtain 528g cefamidox Nuo sodium, the yield is 97.5%, and the purity is determined by HPLC, and the purity is 99.2%.
Embodiment 2
[0031] (1) 358g 7β-amino-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 99.2g chloroacetic acid were dissolved In 3L of chloroform, add 40g of 3A molecular sieve and 220g of sodium sulfate, react at 50°C, TLC detects that the reaction is complete, filter, wash with water, and distill off the solvent under reduced pressure to obtain 432.2g of 7β-chloroacetamido-7a-methoxy-3 -(1-Methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid;
[0032](2) 432.2g 7β-chloroacetamido-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 164.5gD - Add cysteine hydrochloride to 2L of water, then add 25g of EDTA and 370g of sodium thiosulfate, react at 40°C, TLC detects that the reaction is complete, cool to room temperature, add 2L of ethanol, precipitate crystals, filter, and dry to obtain 525g Cefminox sodium, the yield is 97%, and the purity measured by HPLC is 99.0%.
Embodiment 3
[0034] (1) 358g 7β-amino-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 104g chloroacetic acid were dissolved in 2.5L ethyl acetate, add 20g 3A molecular sieve and 250g calcium chloride, react at 60°C, TLC detects that the reaction is complete, filter, wash with water, and distill off the solvent under reduced pressure to obtain 433.1g 7β-chloroacetamido-7a-methoxy Base-3-(1-methyl-1H-tetrazol-5-thiomethyl)-3-cephem-4-carboxylic acid;
[0035] (2) 433.1g 7β-chloroacetamido-7a-methoxy-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid and 173gD- Add cysteine hydrochloride to 2L water, then add 30g EDTA and 412g sodium thiosulfate, react at 50°C, TLC detects that the reaction is complete, cool to room temperature, add a mixed solution of 1500ml ethanol and 500ml acetone, and crystallize. Filter and dry to obtain 530g cefminox sodium, the yield is 97.9%, and the purity measured by HPLC is 99.5%. Comparative example 1:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com