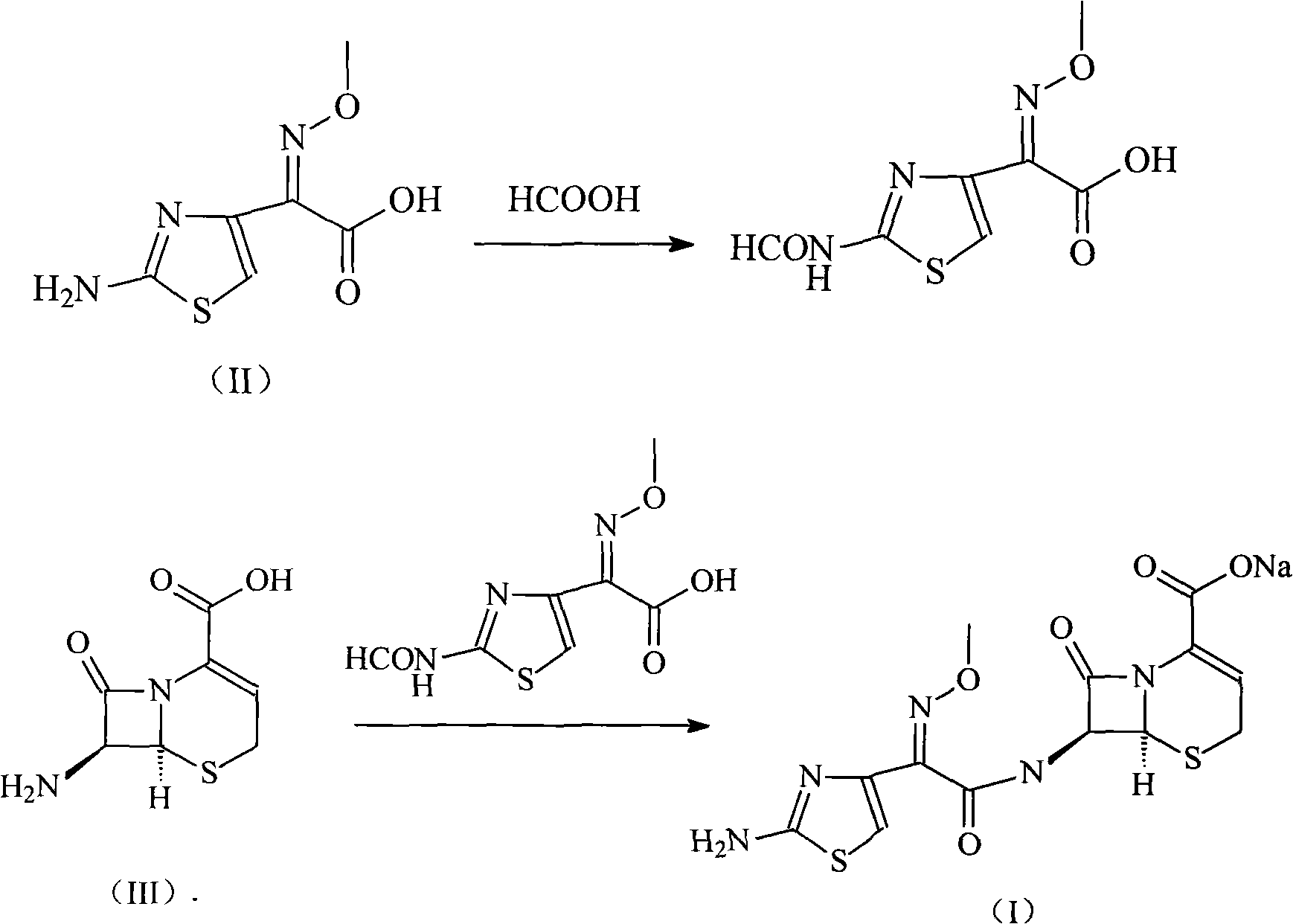
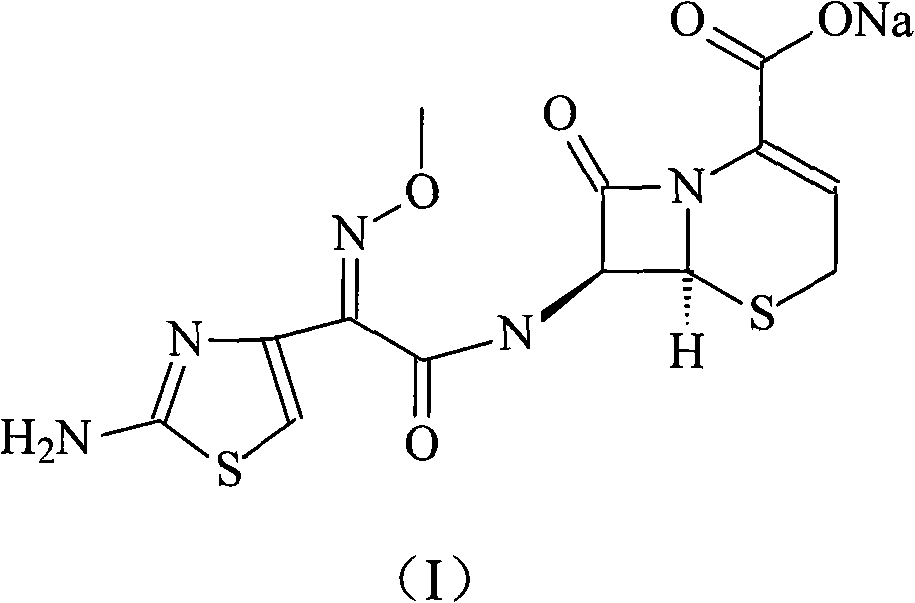
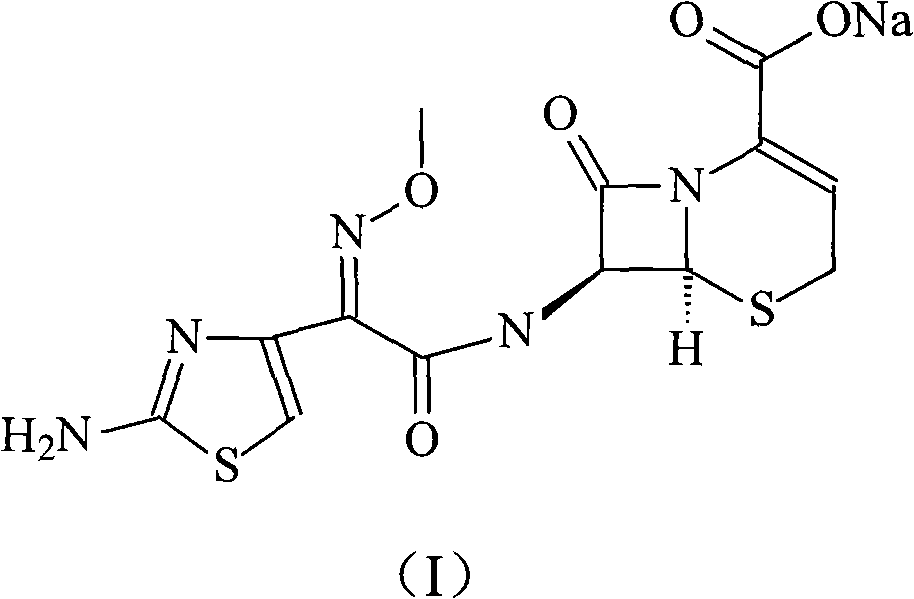
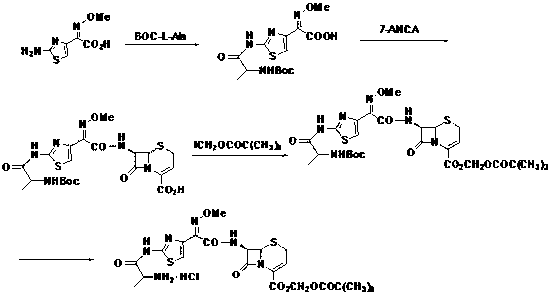
Patents
Literature
141 results about "Cephem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cephems are a sub-group of β-lactam antibiotics including cephalosporins and cephamycins. It is one of the most common 4-membered ring heterocycle.
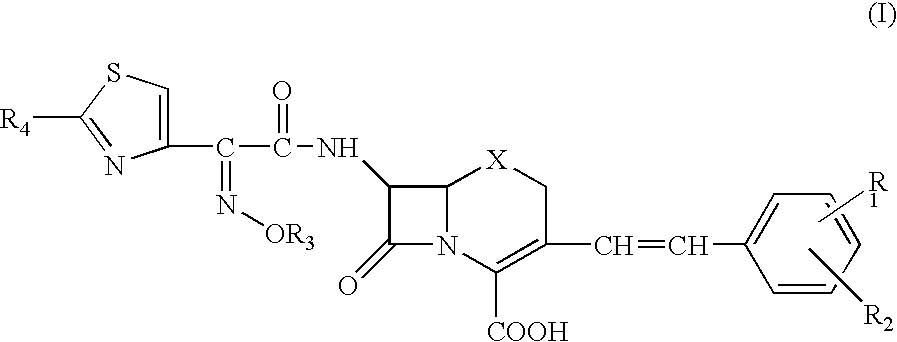
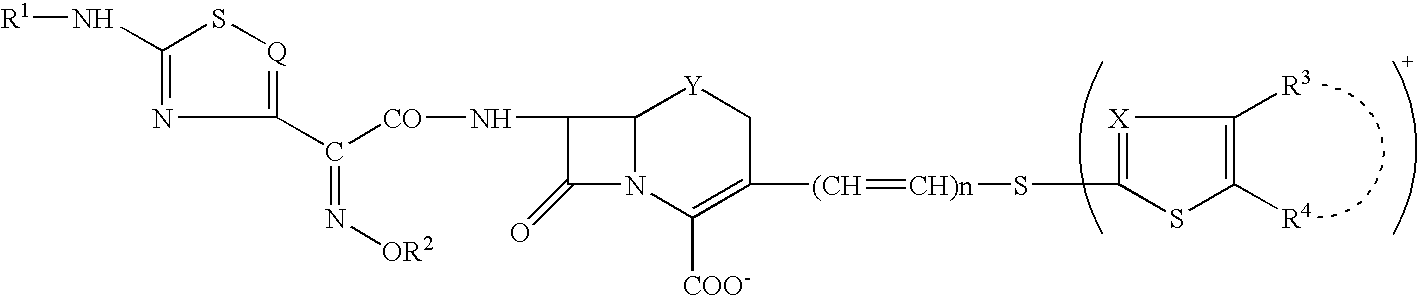
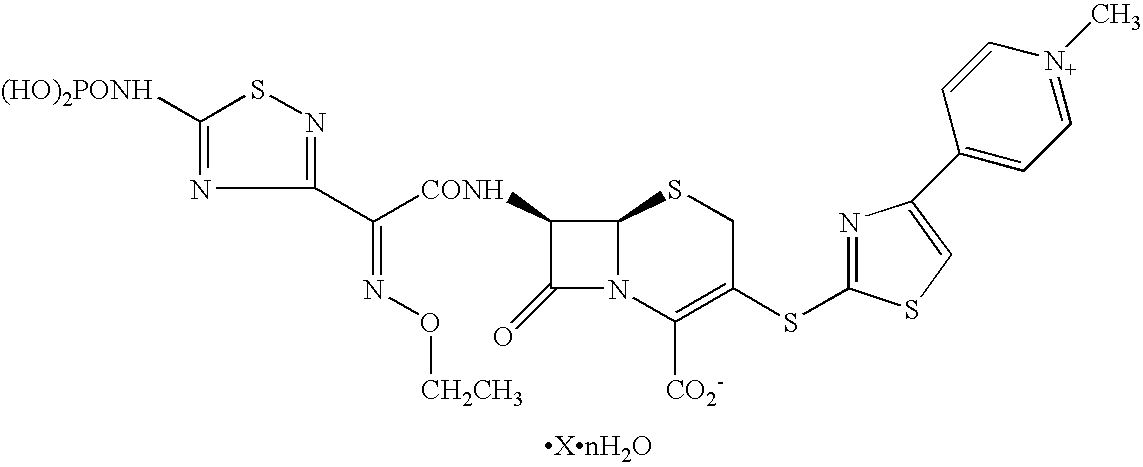
Phosphonocephem derivatives, process for the preparation of the same, and use thereof
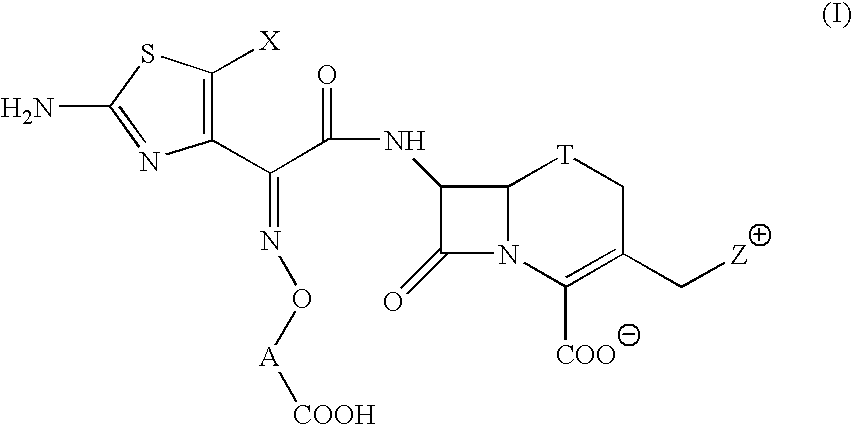
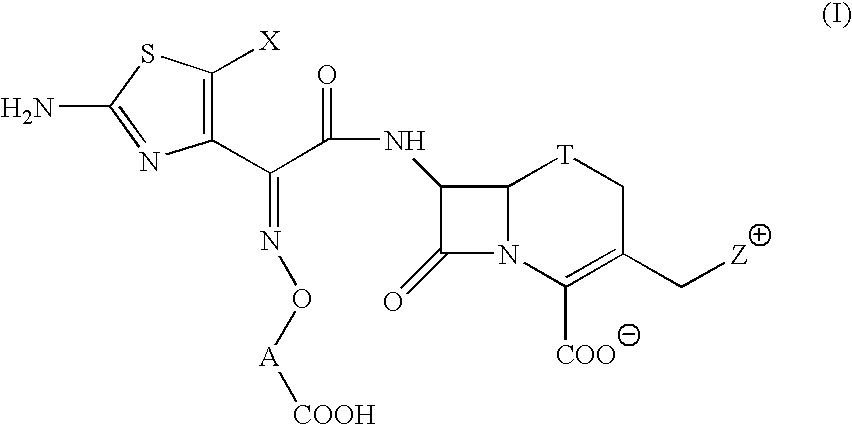
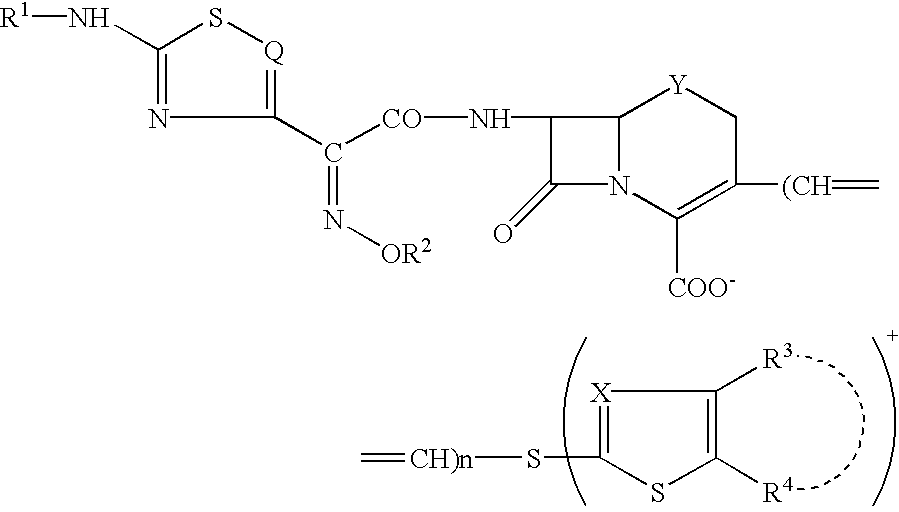
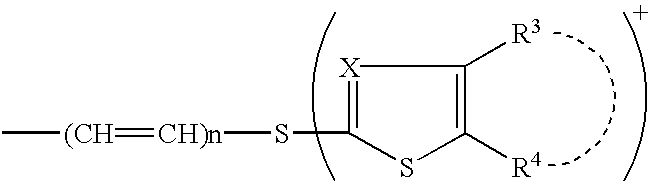
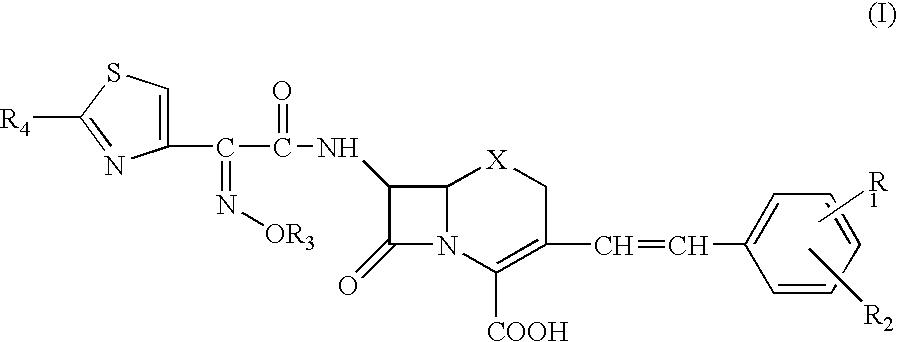
A novel cephem compound of the formula:wherein R1 is a phosphono group or a group convertible to a phosphono group; R2 is a hydrogen atom or a group having a linkage through a carbon atom; each of Q and X is a nitrogen atom or CH; Y is S, O or CH2; n is 0 or 1; one of R3 and R4 is a pyridinium group which may be substituted and the other is a hydrogen atom or hydrocarbon group which may be substituted, or R3 and R4 taken together may form a quaternalized nitrogen-containing heterocyclic ring which may be substituted, or its ester or its salt, which has a superior anti-bacterial activity, stability, absorbability, etc., a production thereof and a pharmaceutical composition containing it, is provided.
Owner:TAKEDA PHARMA CO LTD
Preparation of new intermediates and their use in manufacturing of cephalosporin compounds
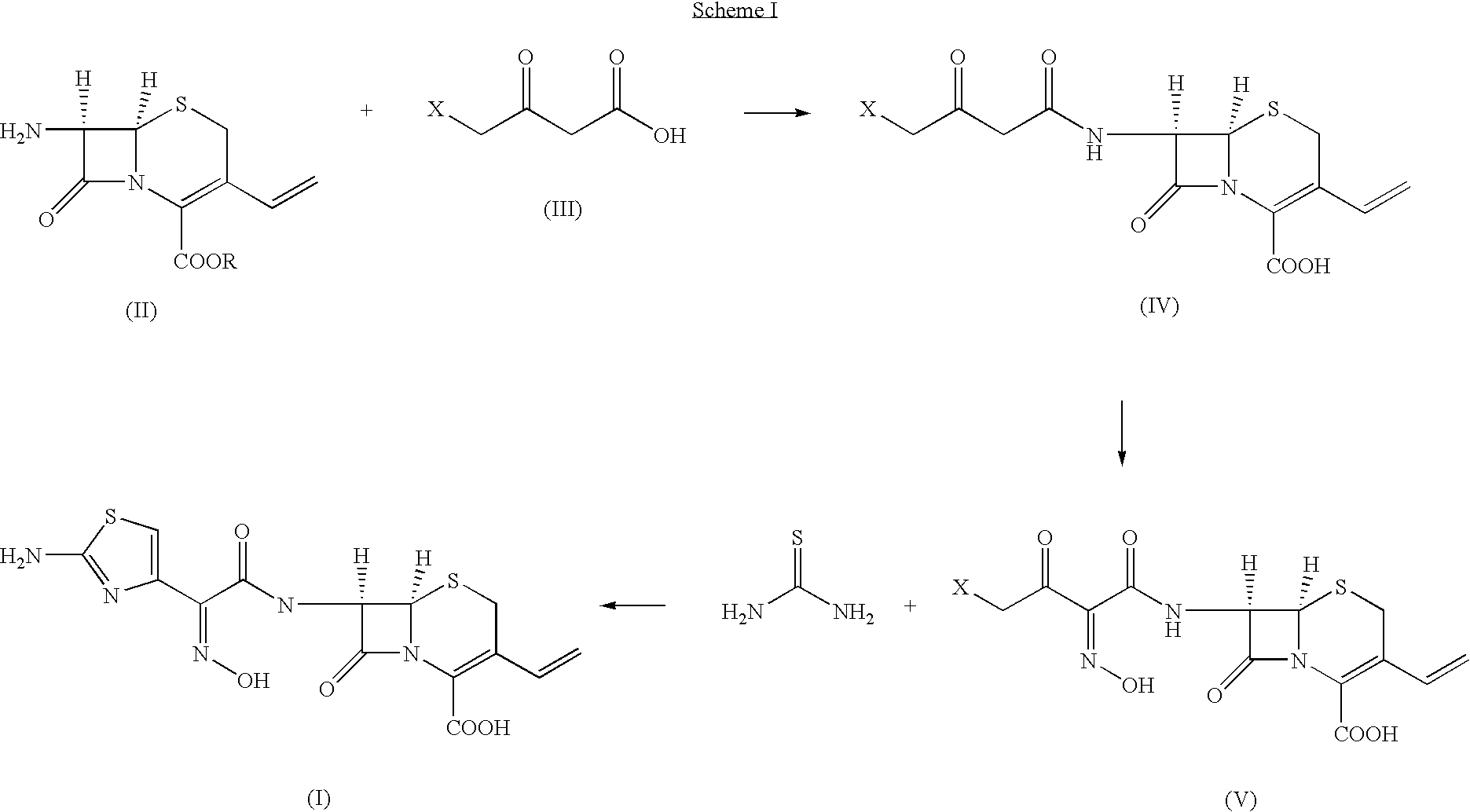
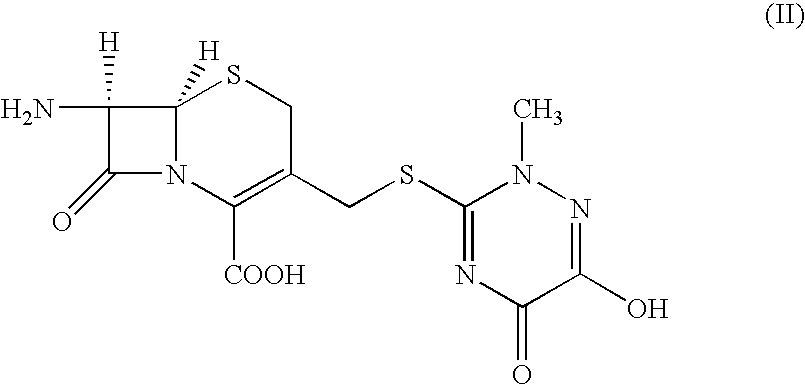
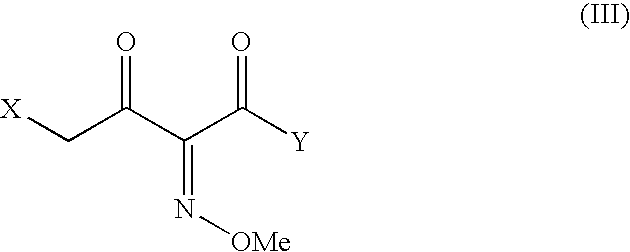
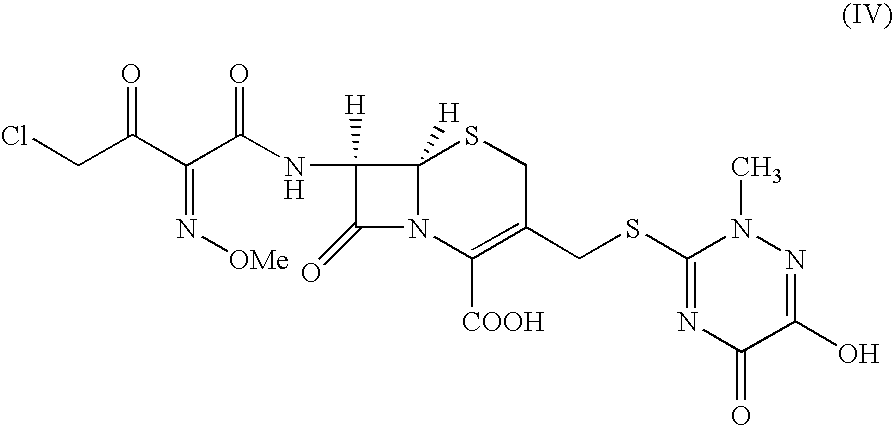
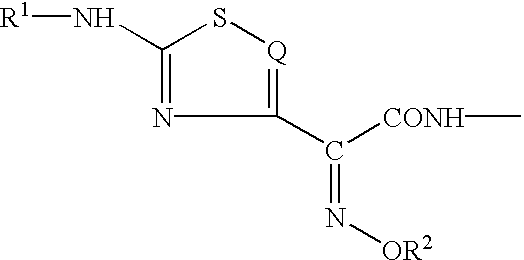
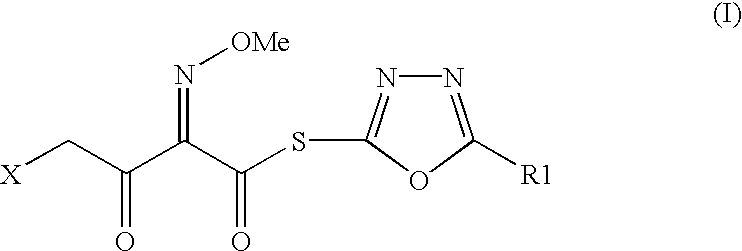
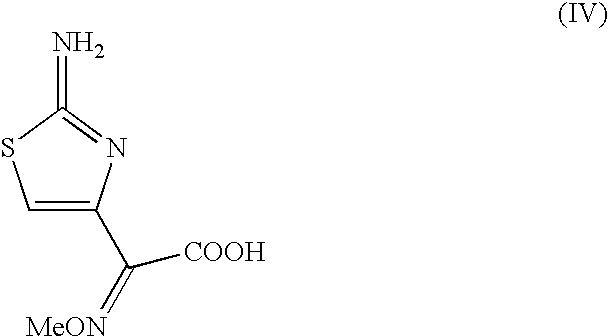
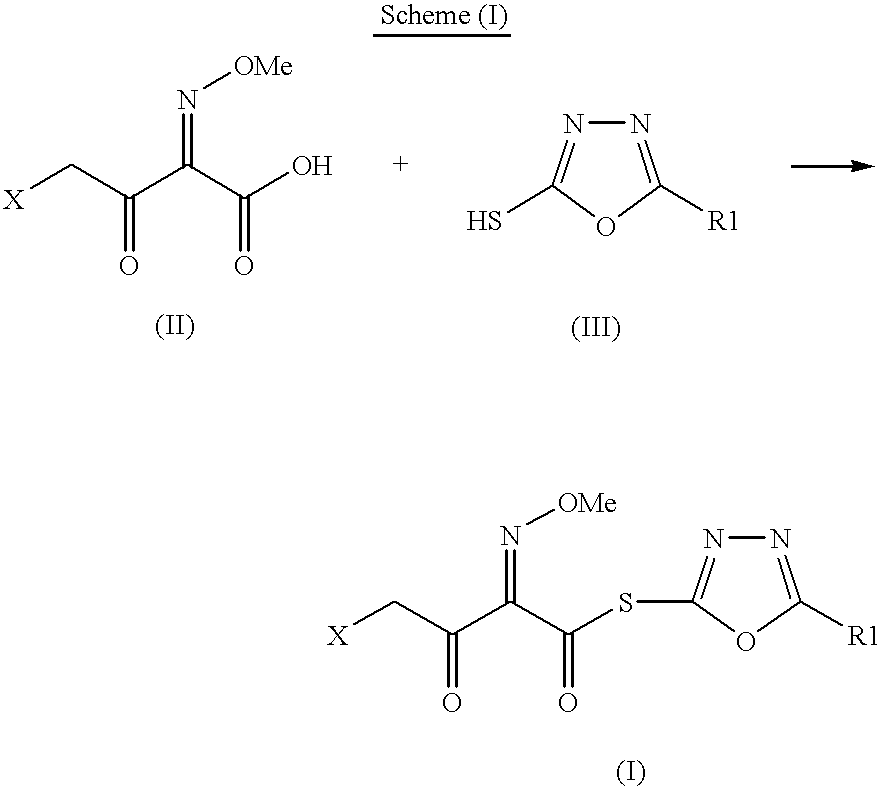
The present invention provides new thioester derivatives of 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (I), also, the invention provides a method by which the said thioester derivatives can be prepared by reacting 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (II) with 2-mercapto-5-substituted-1,3,4-oxadiazoles of the general formula (III) in a solvent, in the presence of DMF / POCl3 and in presence of an organic base and if desired the so obtained thioester derivatives so obtained are reacted with 7-amino-cephem carboxylic acids of the general formula (V) to produce condensed products which are insitu reacted with thiourea to get cephalosporin antibiotic compounds having the general formula (VI).
Owner:ORCHID CHEM &PHARMA LIMITED INDIA
Cephalosporin having catechol group
ActiveUS20110190254A1Potent antimicrobial spectrumHigh antibacterial activityAntibacterial agentsOrganic active ingredientsSide chainPharmaceutical medicine
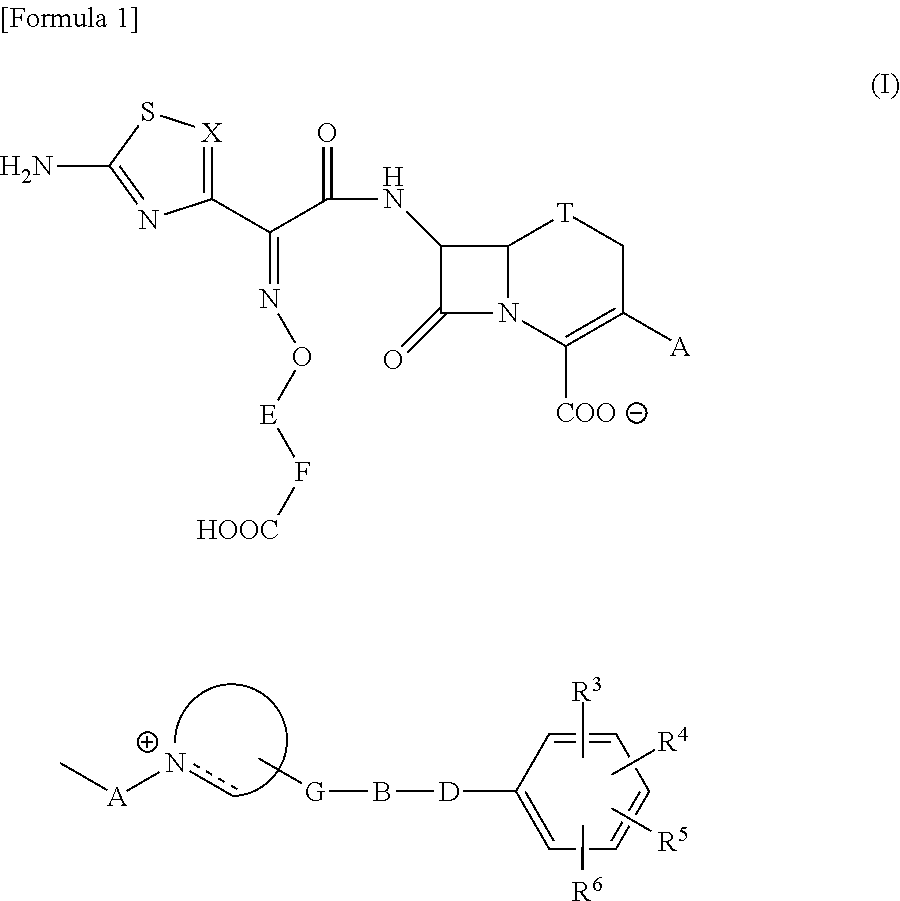
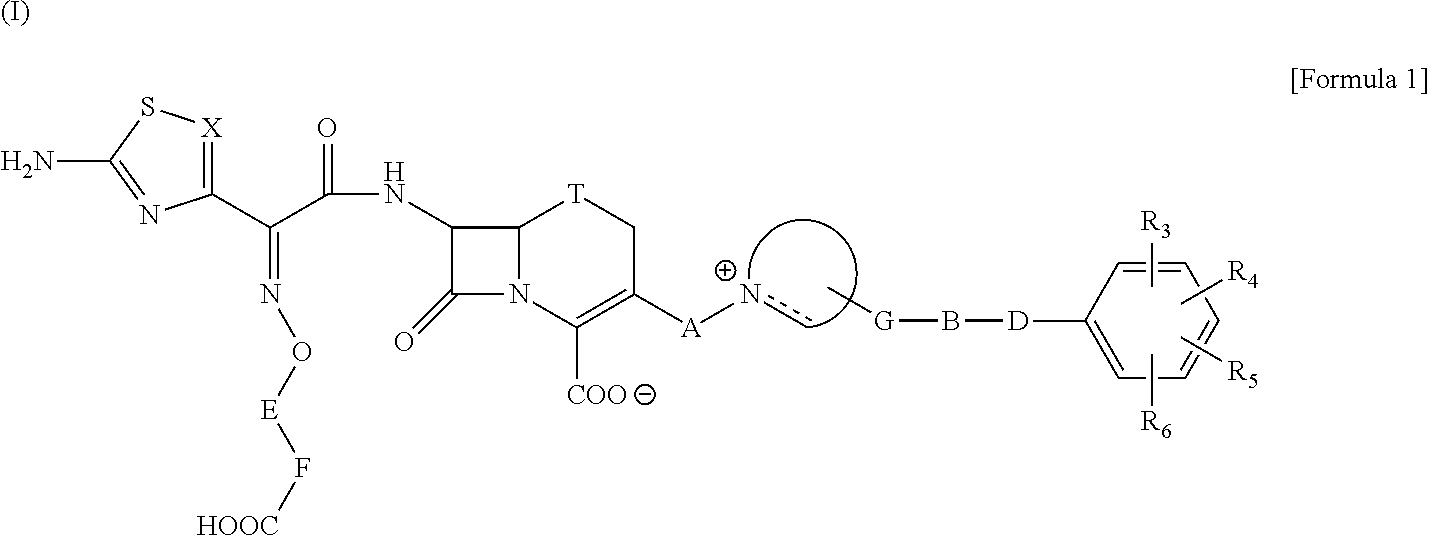
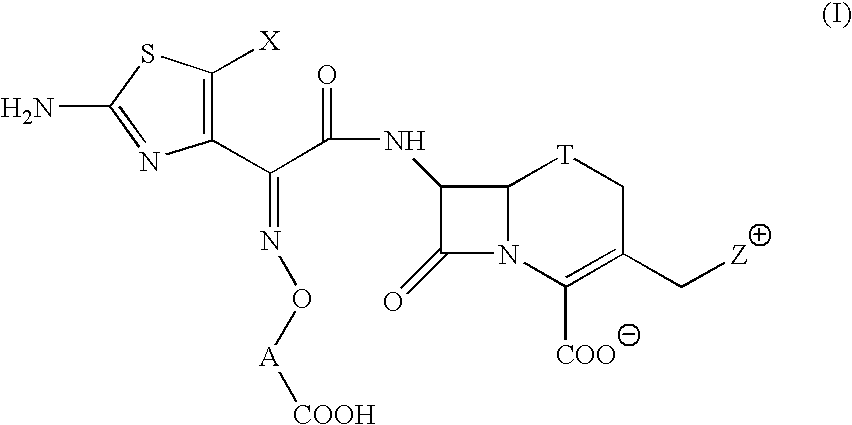
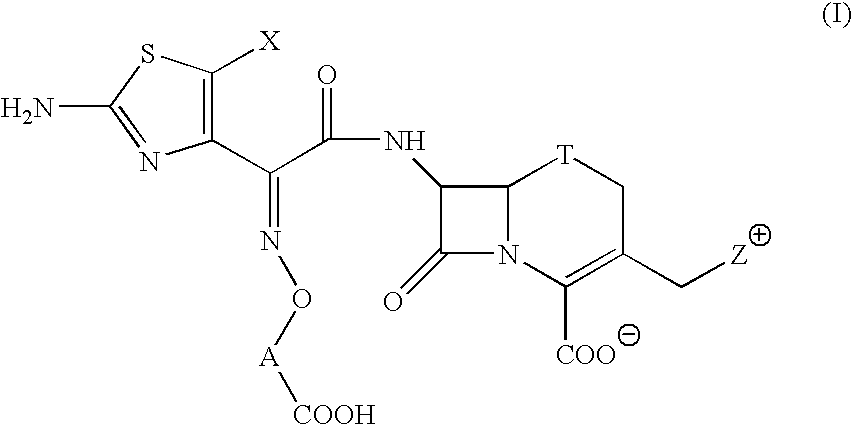
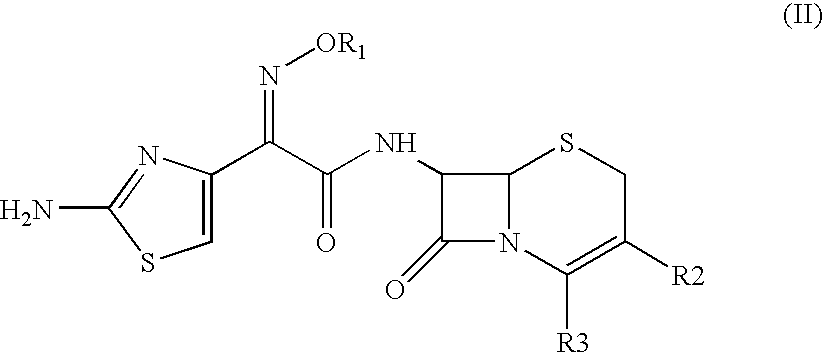
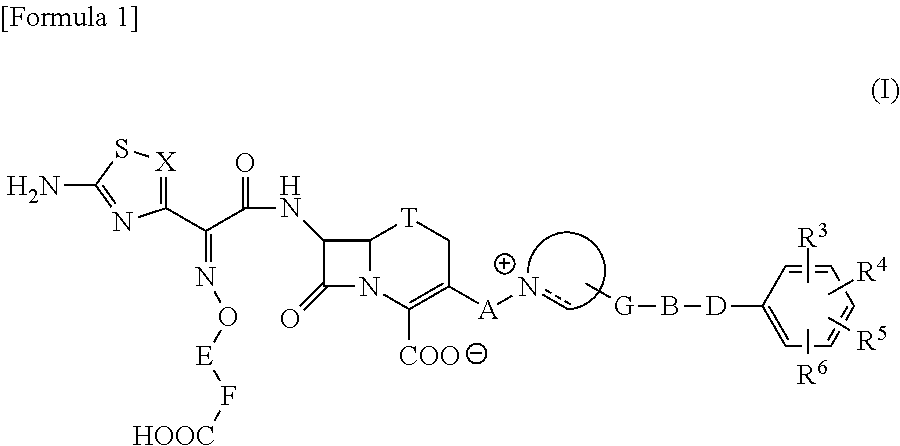
The present invention provides Cephem compounds which have a wide antimicrobial spectrum and have potent antimicrobial activity against beta-lactamase producing Gram negative bacteria as follows:A compound of the formula:wherein,X is N, CH or C—Cl;T is S or the like;A and G are lower alkylene or the like;B is a single bond or the like;D is a single bond, —NR7—, —CO—, —CO—NR7—, —NR7—CO—, —NR7—CO—NR7—, or the like;E is optionally substituted lower alkylene;F is a single bond or optionally substituted phenylene;R3, R4, R5 and R6 each is independently hydrogen, halogene, nitrile, or the like;or an ester, a compound protected at the amino on the ring in the 7-side chain, a pharmaceutically acceptable salt, or a solvate thereof.
Owner:SHIONOGI & CO LTD
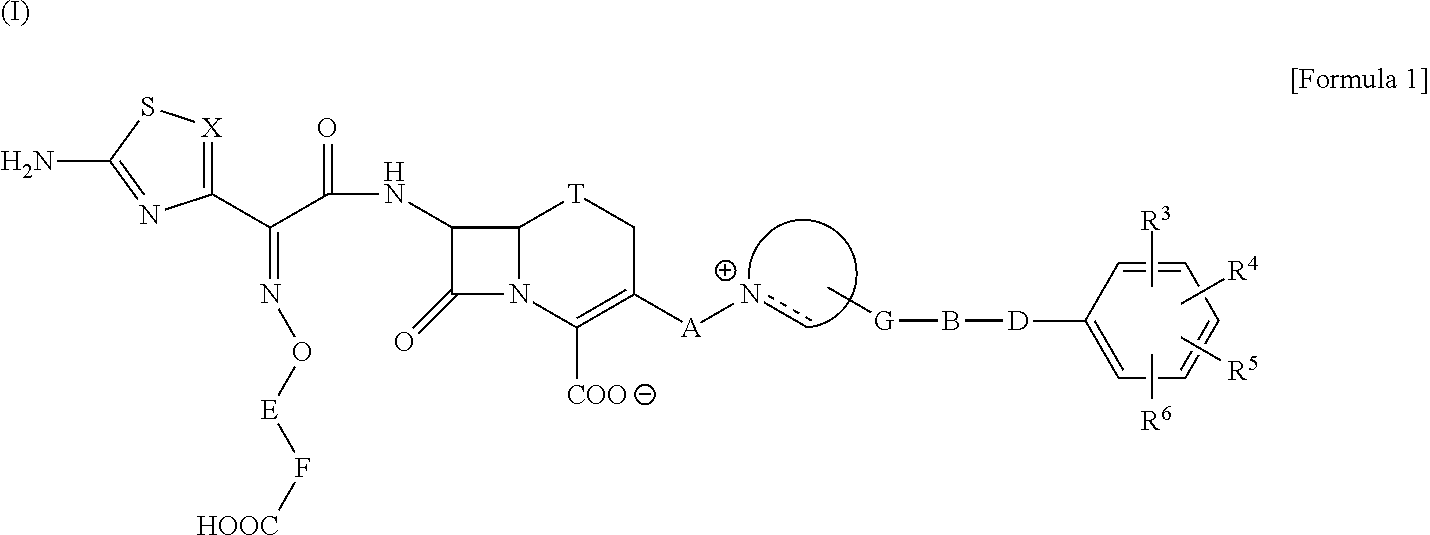
Broad spectrum cephem compounds
InactiveUS7384928B2Pronounced antibacterial activityAntibacterial agentsOrganic active ingredientsCompound aThiazole
A compound of the formula:(wherein,T is S, SO or O;X is halogen, CN, carbamoyl optionally substituted with lower alkyl, lower alkyl, lower alkoxy, or lower alkylthio;A is substituted lower alkylene (wherein the substituent is optionally substituted mono lower alkyl, optionally substituted lower alkylidene, or optionally substituted lower alkylene);Z+ is an optionally substituted, a cation and an N atom-containing heterocyclic group), ester, amino-protected compound wherein the amino bonds to a thiazole ring at the 7-position, or pharmaceutically acceptable salt or solvate thereof.
Owner:SHIONOGI & CO LTD
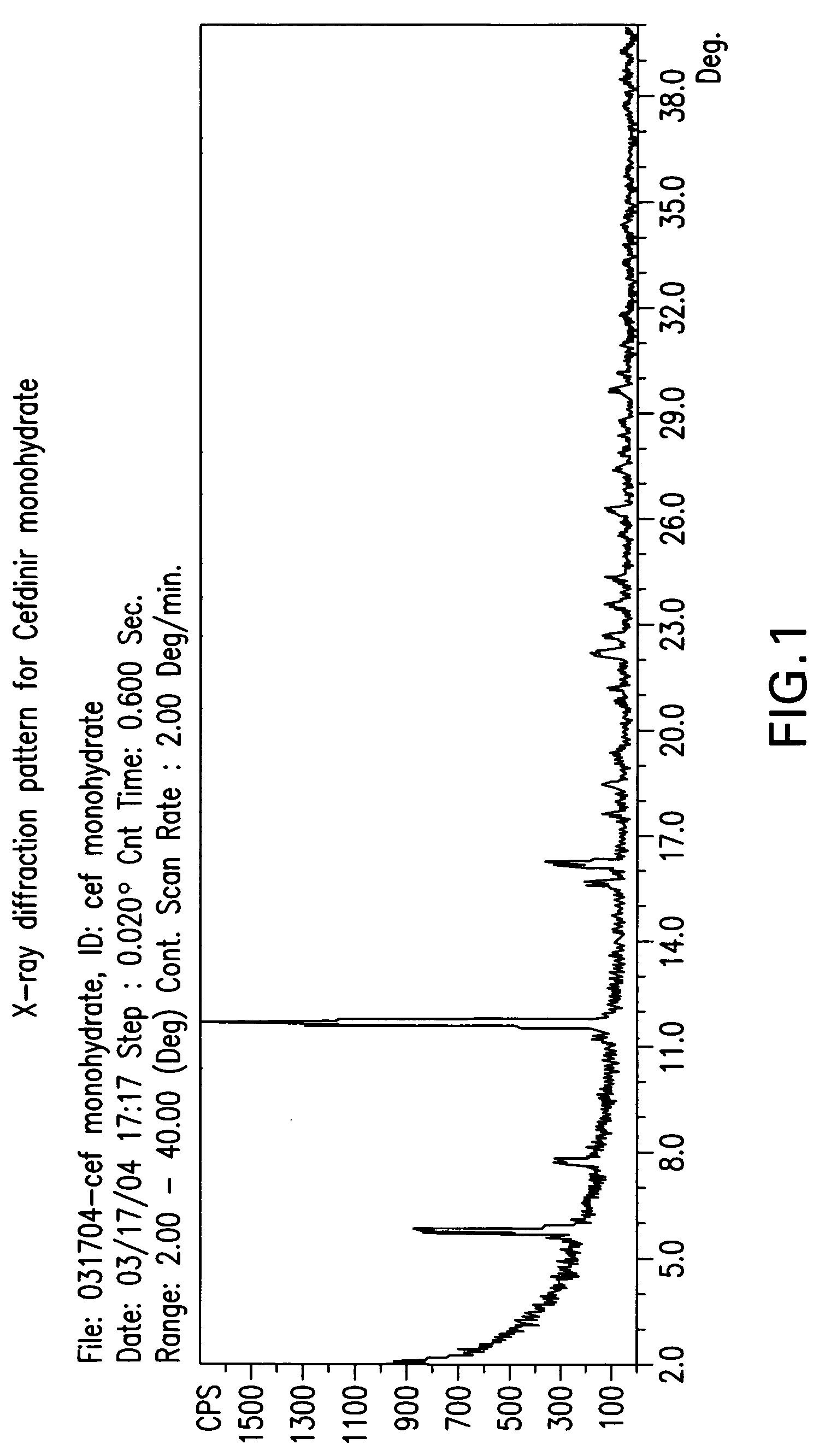
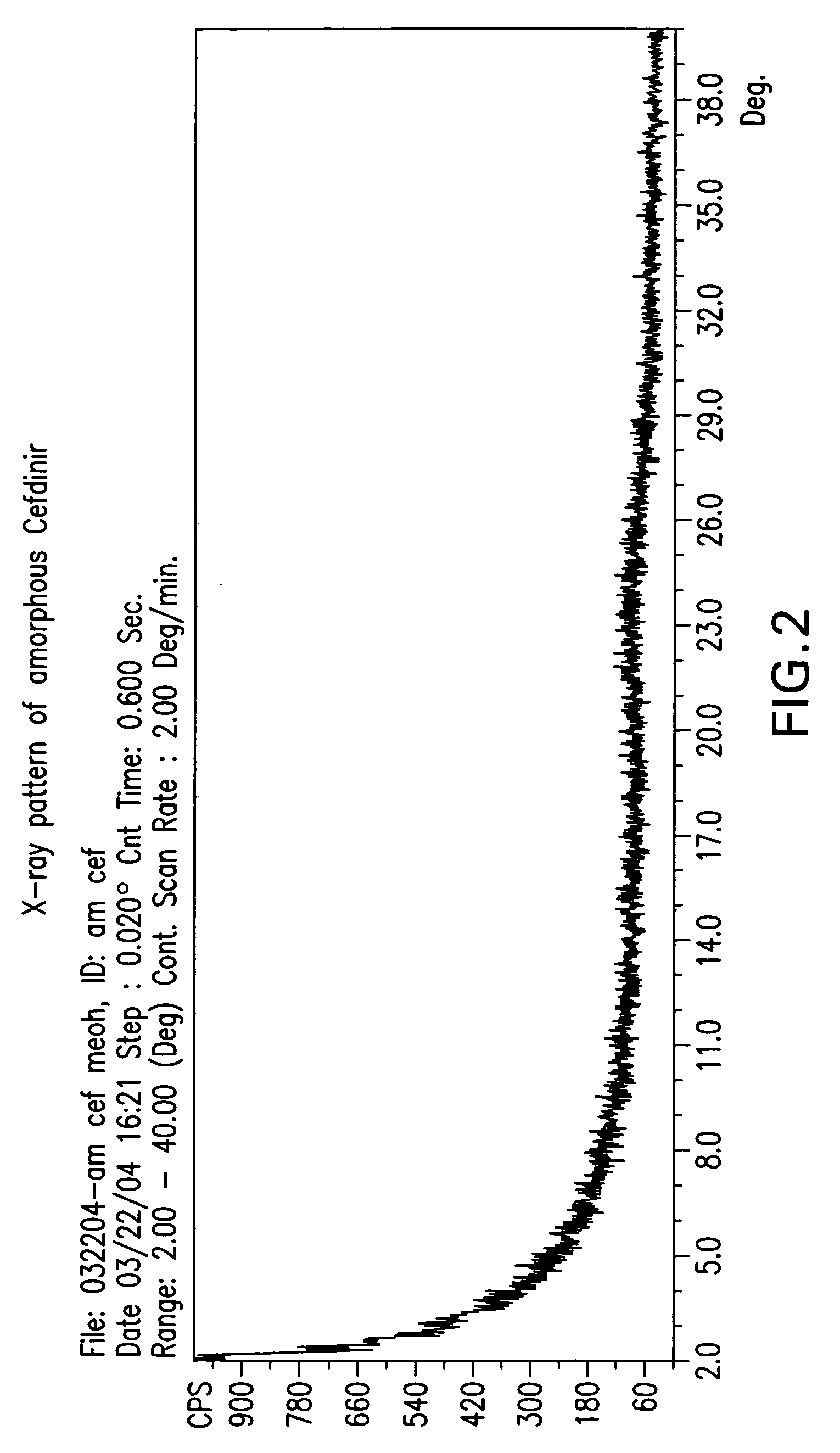
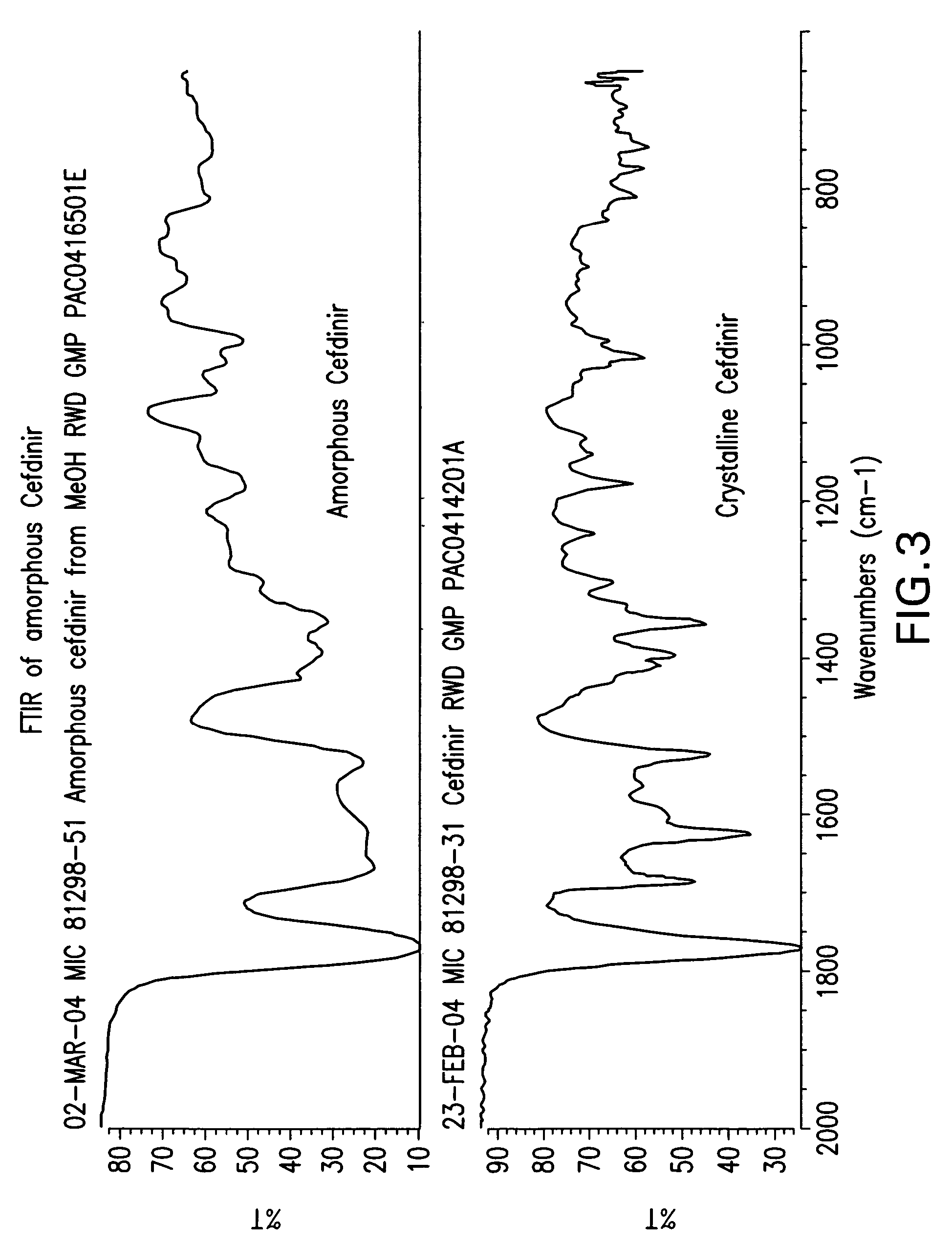
Novel amorphous hydrate of a cephalosporin antibiotic
InactiveUS20060094703A1Improve bioavailabilityUseful for developmentOrganic active ingredientsOrganic chemistryOrganic solventCarboxylic acid
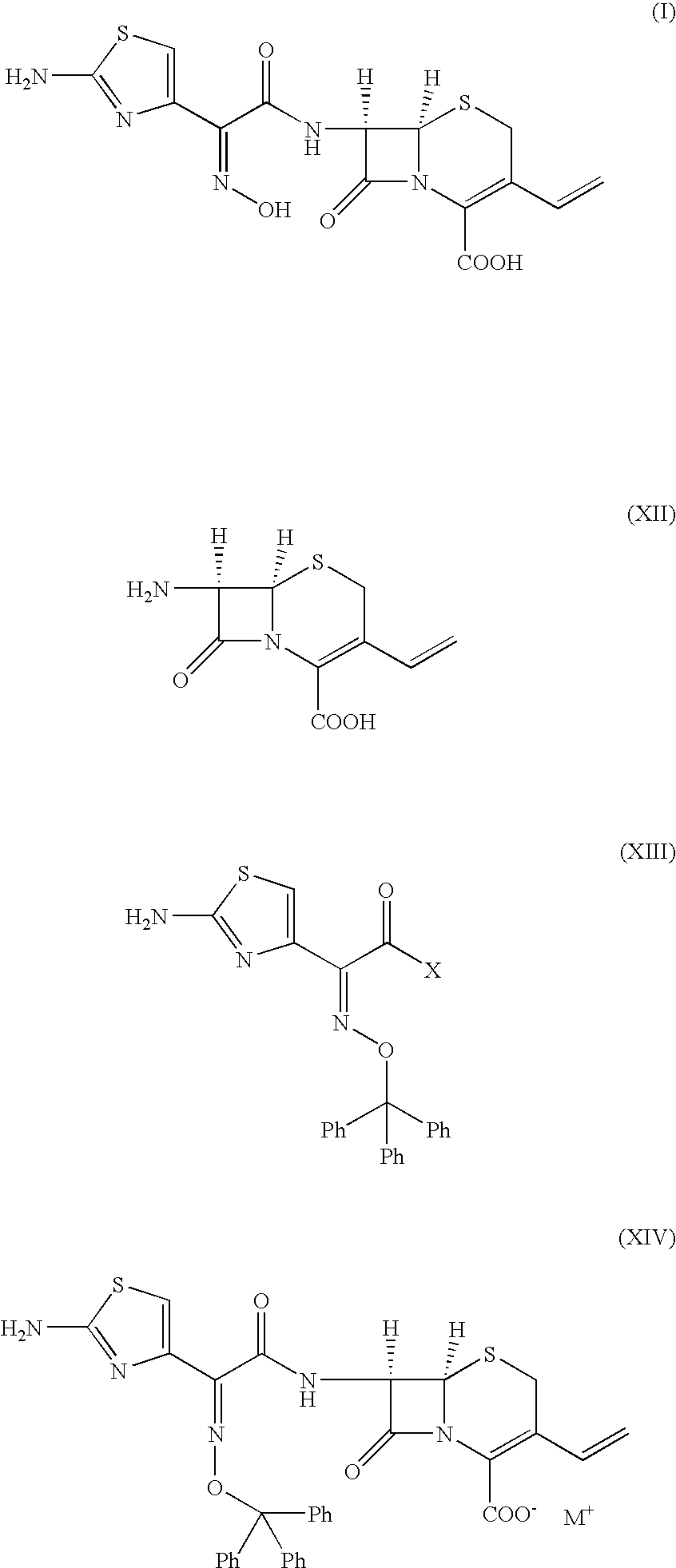
A process for the preparation of cefdinir of the formula (I) the said process comprising the steps of: i) condensing 7-amino-3-cephem-4-carboxylic acid of the formula (XII) wherein R1 is as defined above with compound of the formula (XIII) in the presence of a tertiary amine and an organic solvent, followed by treatment with a base to produce a salt of compound formula (XIV), wherein M+ is a counter ion and ii) hydrolyzing the compound of the formula (XIV) using an acid in the presence of a solvent to produce cefdinir of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Synthetic methods of ceftazidime intermediate and ceftazidime
The invention relates to a synthetic method of ceftazidime intermediate; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the ceftazidimeintermediate (6R, 7R)-7-amino-3-pyridine methyl-ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing ceftazidime by using the obtained intermediate halogen acid salt. The ceftazidime intermediate and ceftazidime prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
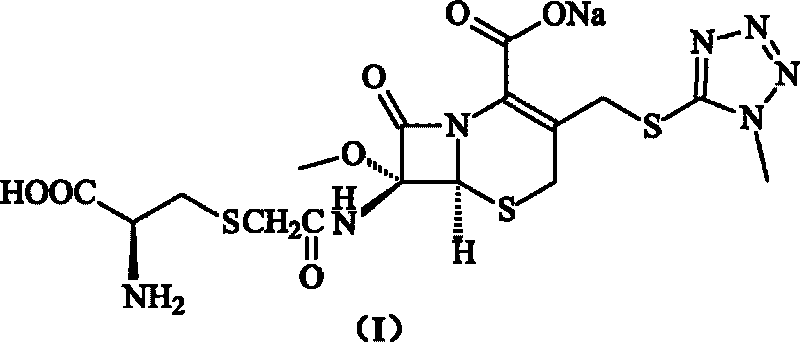
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
Stable amorphous cefdinir
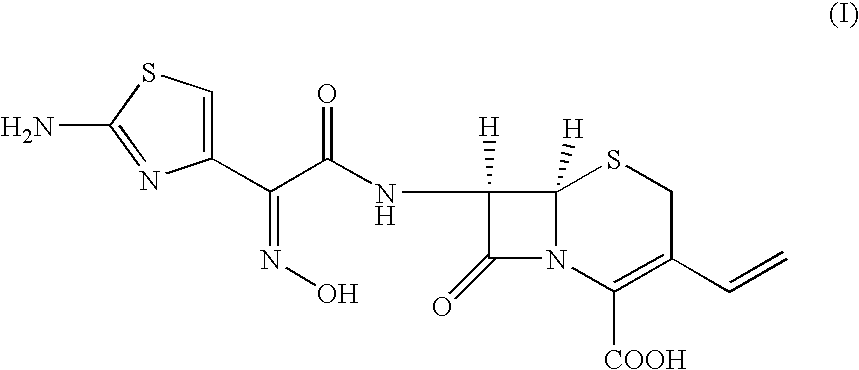
The present invention relates to stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer), methods for its preparation, and pharmaceutical compositions comprising stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer).
Owner:ABBOTT LAB INC
Cephem compounds and ESBL-detecting reagents containing the same
InactiveUS6897304B2Quick checkSure easyOrganic chemistryMaterial analysis by observing effect on chemical indicatorHydrogen atomReagent
A cephem compound or pharmaceutically acceptable salt thereof represented by the formula I: wherein R1 and R2 may be the same or different and each represent hydrogen atom, nitro or cyano; R3 represents C1-C6 alkyl which may be substituted with carboxyl; R4 represents hydrogen atom or amino; X represents —S— or —SO—, there being no case where both of R1 and R2 are simultaneously hydrogen atom.
Owner:ZENYAKU KOGYO KK
Process for producing 1-oxacephalosporin-7alpha-methoxy-3-chloromethyl derivative
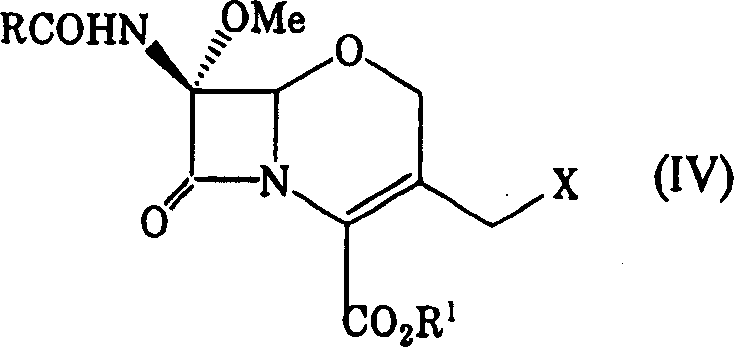
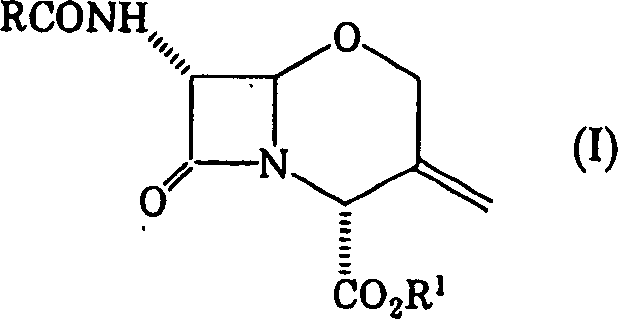
To provide a novel production method of oxacephem compound. [Solution] A method of producing Compound (IV) shown by the formula: (wherein R represents an acyl residue; R 1 represents carboxy protecting group; Me represents methyl and X represents halogen), the method comprising the steps of: (First step) letting Compound (I) shown by Formula: (wherein, R represents an acyl residue; R 1 represents carboxy protecting group) react with a halogenating agent in the presence of a base; (Second step) adding MOMe (M represents alkaline metal; Me represents methyl) in the presence of a halogenating agent after completion of the first step; and (Third step) adding a reducing agent after completion of the second step.
Owner:SHIONOGI & CO LTD
Reagent composition for detecting beta-lactamase, detection kit and detection method
InactiveCN1729299AMicrobiological testing/measurementBiological material analysisCarboxylic acidCephem
It is intended to provide a reagent composition for detecting beta-lactamase which contains 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido)-3-cephem-4-carboxylic acid or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxyimino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid as a substrate for detecting beta-lactamase together with at least one beta-lactamase inhibitor selected from among clavulanic acid, aztreonam, ethylenediaminetetraacetic acid and cloxacillin and by which beta-lactamase can be quickly and easily identified at a high sensitivity. Moreover, a detection kit containing the above detection reagent composition and a method of detecting beta-lactamase comprising contacting a liquid specimen containing the subject to be analyzed with the above composition are provided.
Owner:昭和药品化工股份有限公司 +1
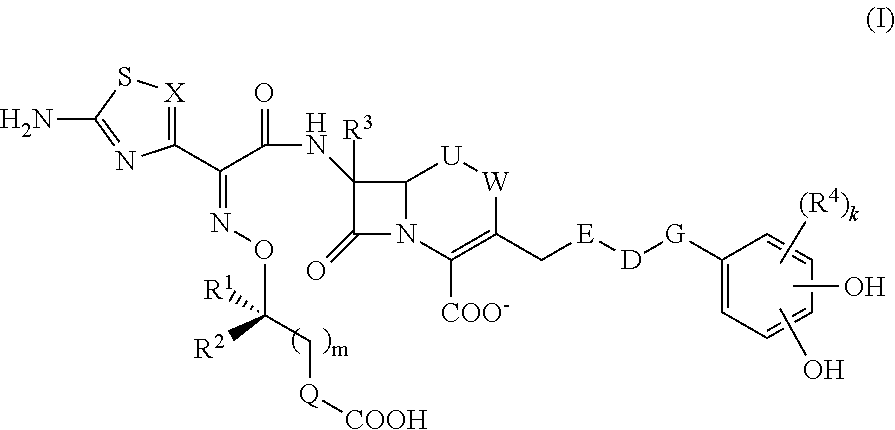
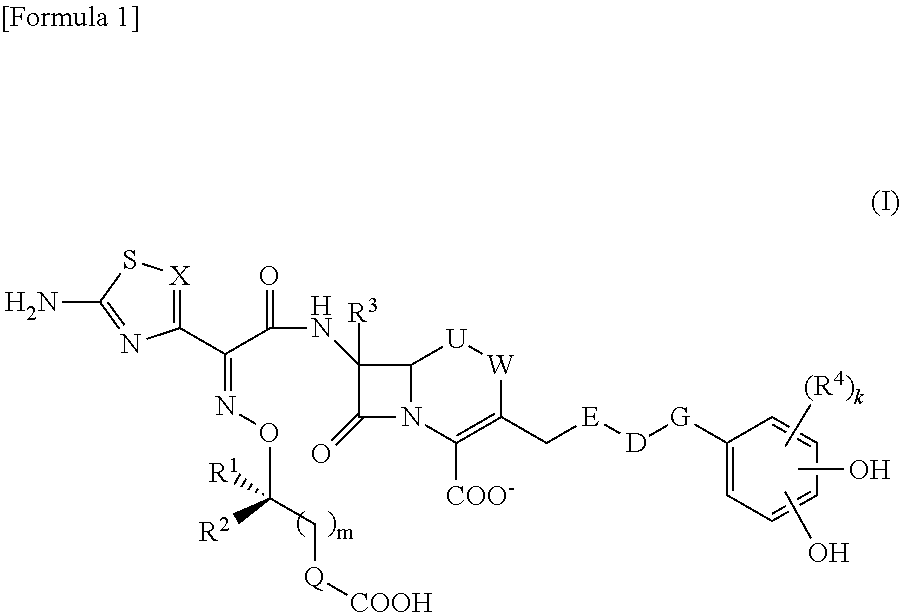
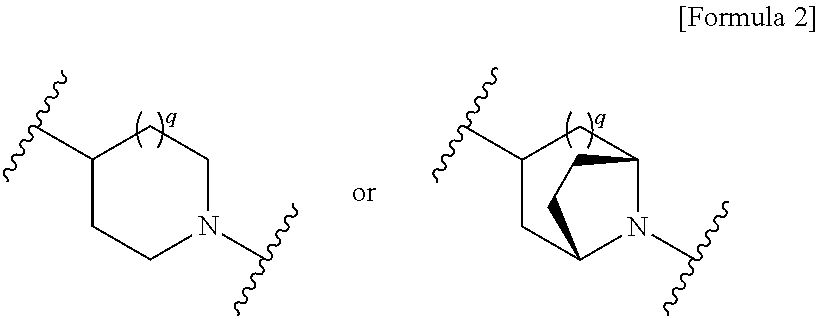
Novel cephem derivative
InactiveUS20130096299A1Potent antimicrobial spectrumHigh antibacterial activityAntibacterial agentsOrganic chemistryThiadiazolesPharmaceutical Substances
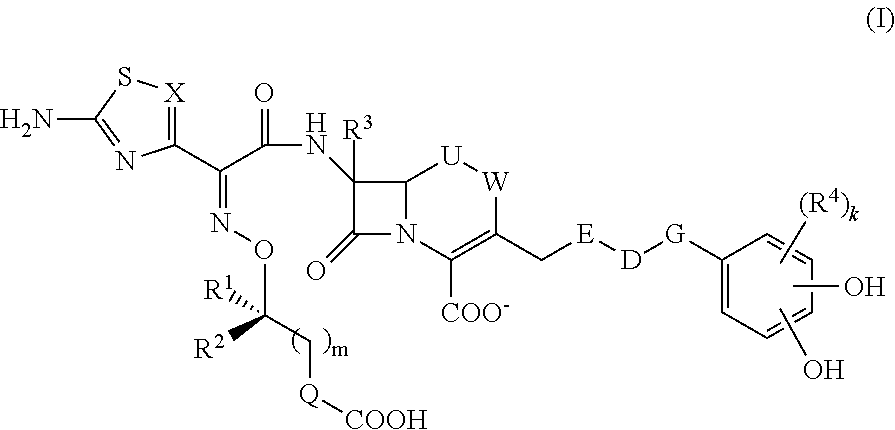
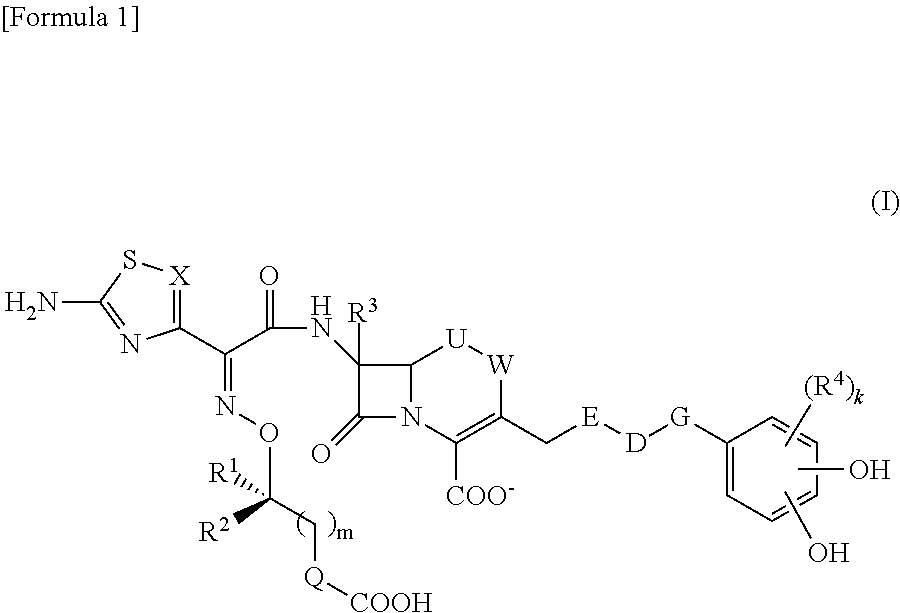
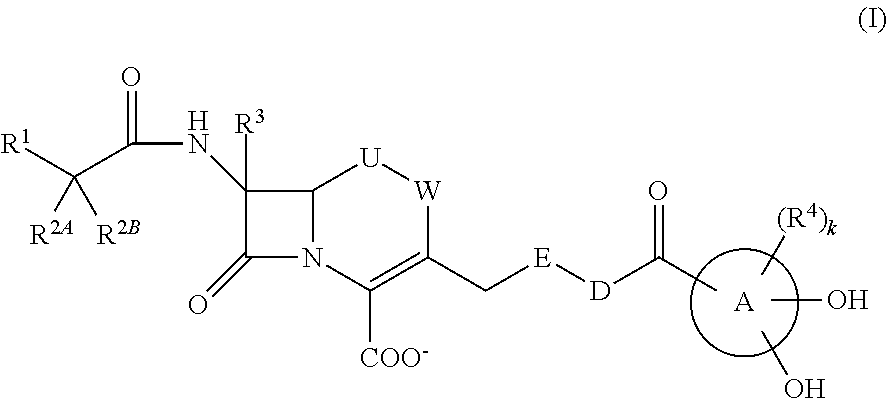
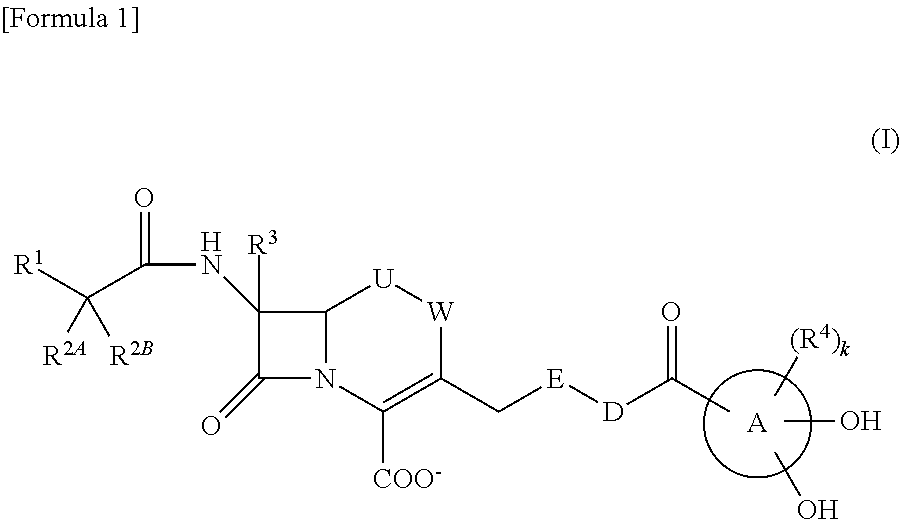
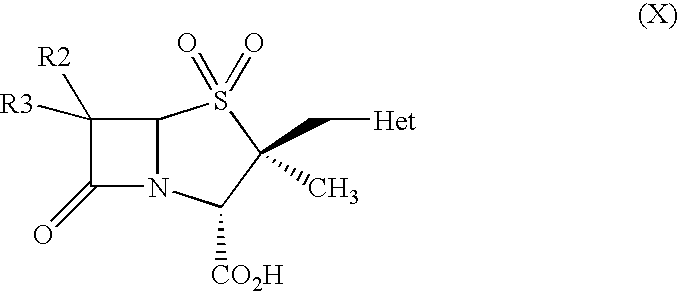
Provided is a cephem compound which has a wide antimicrobial spectrum, and in particular exhibit potent antimicrobial activity against beta-lactamase producing Gram negative bacteria, and pharmaceutical composition comprising the same.A Compound of the formula (I):whereinW and U are as defined in the specification;R1 is as defined in the specification;R2A and R2B are as defined in the specification, provided that R2A and R2B are not taken together to form an optionally substituted oxime group when R1 is aminothiazole or aminothiadiazole optionally protected at the amino group;ring A is a benzene ring or a 6-membered aromatic heterocyclic group having 1-3 nitrogen atoms;R3 is a hydrogen atom, —OCH3 or —NH—CH(═O);k is an integer from 0 to 2;R4 is as defined in the specification; andD and E are as defined in accordance with a) or b) described in the specification.
Owner:SHIONOGI & CO LTD
Cefminox sodium compound of new route
The invention provides a cefminox sodium compound of a new route, in particular a method for producing the cefminox sodium compound. The method comprises a step of generating cefminox sodium through the reaction between 7beta-bromoacetamido-7a-methoxyl-3-(1-methyl-1H-tetrazole-5-S-methyl)-3-cephem-4-carboxylic acid and D-cysteine hydrochloride, and is characterized in that: the reaction condition of the step is that the reaction is performed in aqueous solution, sodium iodide is used as a catalyst, the pH value of the reaction system is between 7.5 and 8.0, and the reaction temperature is kept at 30+ / -5 DEG C. Compared with the method for producing the cefminox sodium compound in the prior art, the method of the invention for producing the cefminox sodium compound greatly improves the product yield and the purity, and solves the problem that a cefminox sodium bulk drug always has a low purity in the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Process for the preparation of cephalosporin intermediate and its use for the manufacture of cephalosporin compounds
InactiveUS6919449B2High yieldHigh purityAntibacterial agentsUrea derivatives preparationThioureaBiological activation
The present invention relates to a method for the preparation of cephalosporin antibiotic of the formula (II), which comprises hydrolyzing and halogenating the ester of formula (III) by photochemical irradiation in one pot using a halogenating agent in the absence or presence of a solvent, to produce compound of formula (I), activating the 4-halogeno-2-substitutedimino-3-oxo-butyric acid of formula (I) using conventional activation agents gives compound of formula (IV), condensing the activated compound of the formula (IV) with 7-amino cephem derivative of the formula (V) to produce a compound of formula (VI), and cyclizing the compound of formula (VI) with thiourea to give cephalosporin compounds of the formula (II).
Owner:ORCHID CHEM & PHARM LTD
A kind of synthetic method of ceftazidime
The invention relates to a method for preparing ceftazidime. The synthesis method comprises the following steps: by taking 7-aminocephalosporanic acid (7-ACA) as a starting raw material, introducing a pyridine ion to 3-position methylene of 7-ACA so as to obtain 7-amino-3-(1-picolyl)-cephem acid (7-APCA) hydrochloride; then introducing a side chain with a thiazole ring on 7-position amino of the 7-APCA hydrochloride through acylation reaction; and carrying out hydrolyzing reaction, refining reaction and the like so as to obtain ceftazidime. The synthesis method for preparing the ceftazidime inthe invention is simple to operate and high in yield, and is suitable for industrial production.
Owner:哈药集团股份有限公司 +1
Process for preparation of penam derivatives from cepham derivatives
InactiveUS6936711B2High yieldHigh purityOrganic chemistry methodsBulk chemical productionHalogenHydrogen
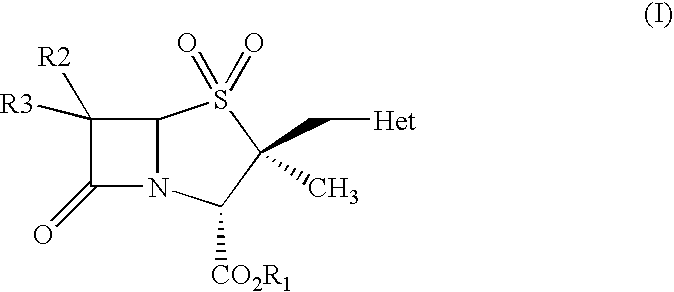
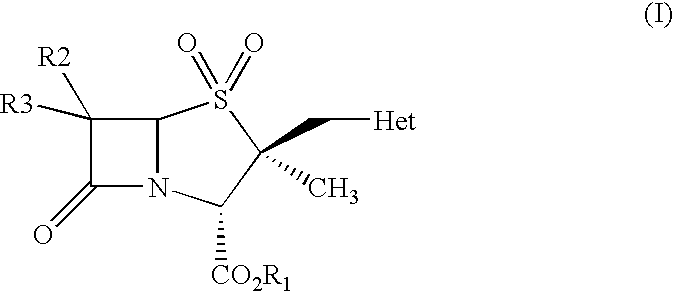
The present invention relates to a process for preparing 2α-methyl-2β-substituted methyl penam derivatives from cepham derivatives, more particularly the present invention provides a novel process for preparing 2β-heterocyclyl methyl penam derivatives of the formula (I) by reacting a cepham compound with a heterocylic amine to form an intermediate compound, and oxidizing the intermediate compound to produce the 2β-heterocyclyl methyl penam derivatives of the formula (I), wherein R1 represents carboxylic acid protecting group; R2 and R3 may be same or different and independently represent hydrogen, halogen, NH2, acylamino, phthalimido with a proviso that both R2 and R3 are not NH2, acylamino, phthalimido; Het represents a 5 or 6 membered nitrogen containing heterocycle ring system having one or more heteroatoms selected from O, S, or N.
Owner:ORCHID CHEM & PHARM LTD
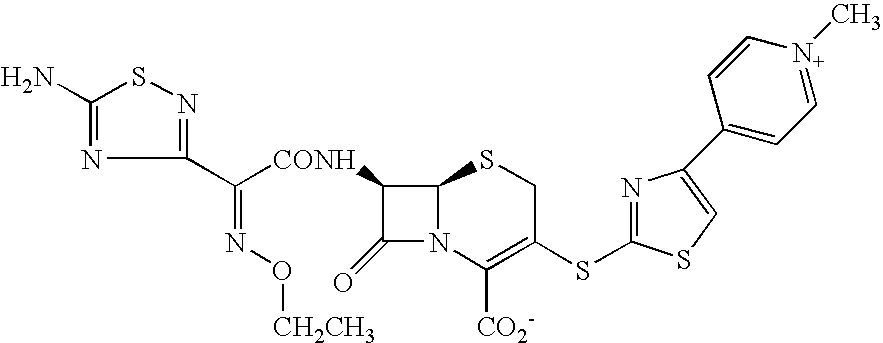
Cephem compound having pseudo-catechol group
InactiveUS20130079319A1Potent antimicrobial spectrumHigh antibacterial activityAntibacterial agentsOrganic active ingredientsHydrogenHalogen
A compound of the formula:whereinX is —N═, —CH═, or the like;W is —CH2— or the like;U is —S— or the like;R1 and R2 are each independently hydrogen, halogen, optionally substituted lower alkyl, or the like;Q is a single bond or the like;R3 is hydrogen or the like;Ring A is a 6-membered aromatic heterocyclic group having 1-3 nitrogen atoms;each R4 is independently hydrogen, halogen, or the like;m is an integer from 0 to 2;G is —C(═O)— or the like;D is a single bond, —NH—, or the like; andE is a cyclic quaternary ammonium group,or an ester, a protected compound at the amino on the ring in the 7-side chain, a pharmaceutically acceptable salt, or a solvate thereof.
Owner:SHIONOGI & CO LTD
Cephalosporin having catechol group
ActiveUS9238657B2Potent antimicrobial spectrumAntibacterial agentsOrganic active ingredientsSide chainPharmaceutical medicine
Owner:SHIONOGI & CO LTD
Soluble dosage forms containing cephem derivatives suitable for parenteral administration
The present invention relates to new dosage forms of cephem compounds, useful for the treatment of bacterial infections. The dosage forms are stable, exhibit enhanced solubility, and are particularly well suited for, e.g., parenteral administration.
Owner:FOREST LAB HLDG LTD
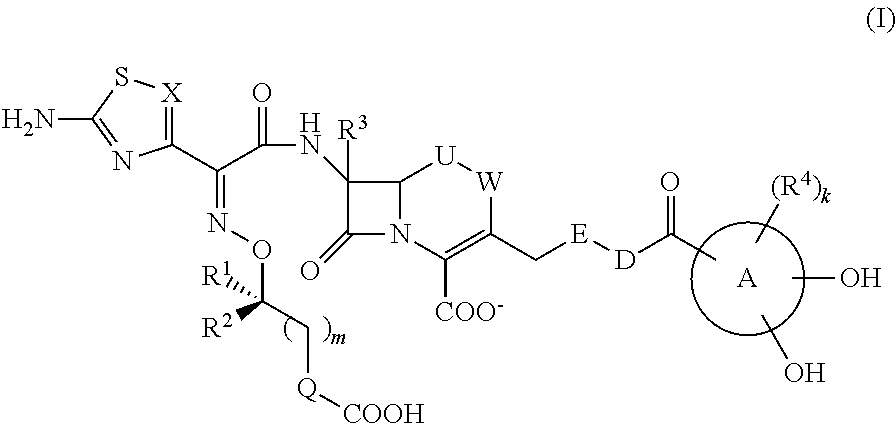
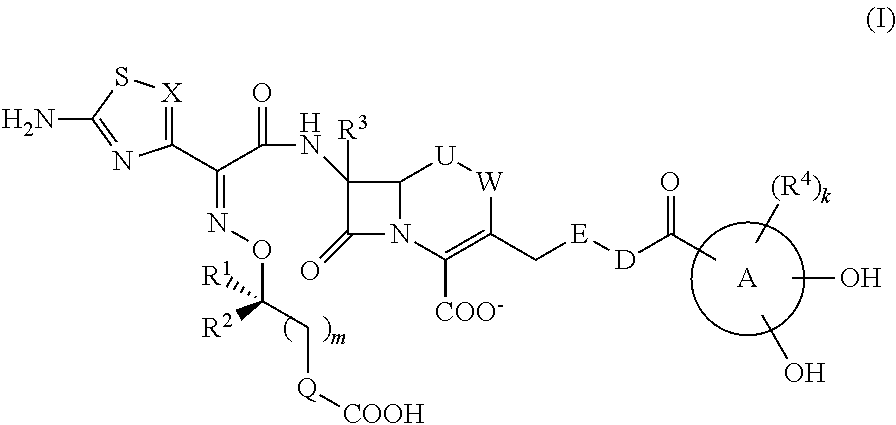
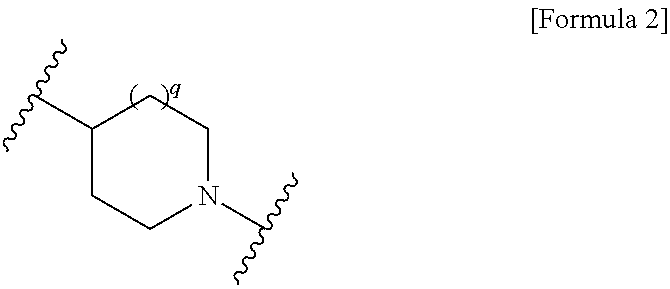
Cephem compound having catechol group
InactiveUS9145425B2Broad antimicrobial spectrumAntibacterial agentsOrganic active ingredientsSide chainPharmaceutical medicine
This invention provides Cephem compounds having the formula:or an ester, a protected compound at the amino on the ring in the 7-side chain, a pharmaceutically acceptable salt, or a solvate thereof, a pharmaceutical composition thereof, and a method for treating a bacterial infectious disease with the compound, the ester, the protected compound, the salt, or the solvate thereof, wherein the symbols in the formula are defined in the specification. The compounds exhibit potent antimicrobial spectrum against a variety of bacteria including Gram negative bacteria and / or Gram positive bacteria, preferably beta-lactamase producing Gram negative bacteria, more preferably, multi-drug resistant microbials, in particular, Class B type metallo-beta-lactamase producing Gram negative bacteria, and still preferably extended-spectrum beta-lactamase (ESBL) producing bacteria. The compounds most preferably do not exhibit cross-resistance against known Cephem drugs or Carbapenem drugs.
Owner:SHIONOGI & CO LTD
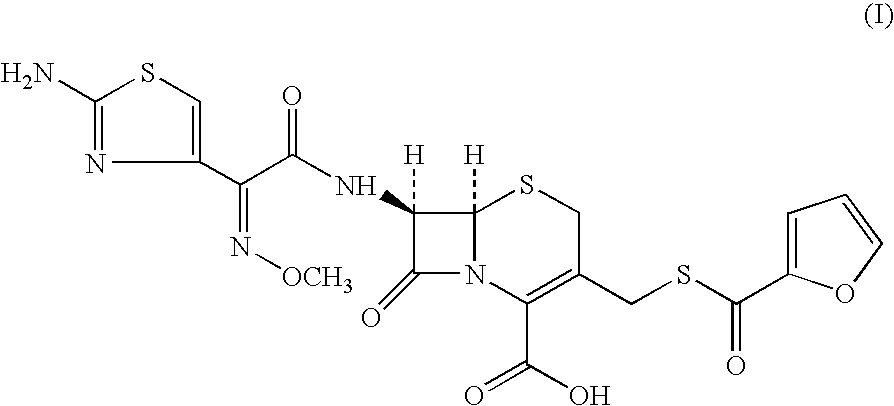
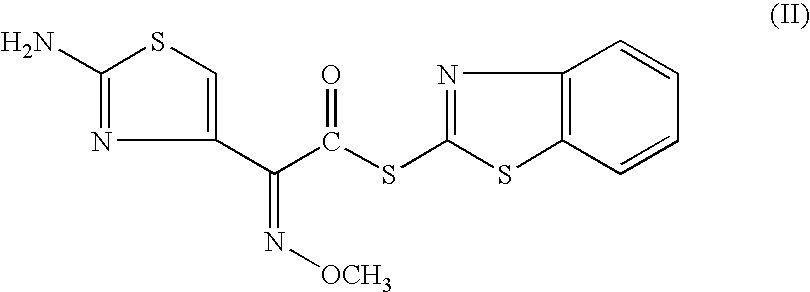
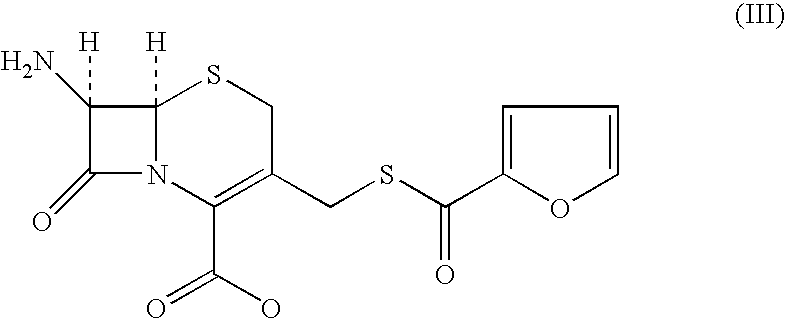
Method for manufacture of ceftiofur
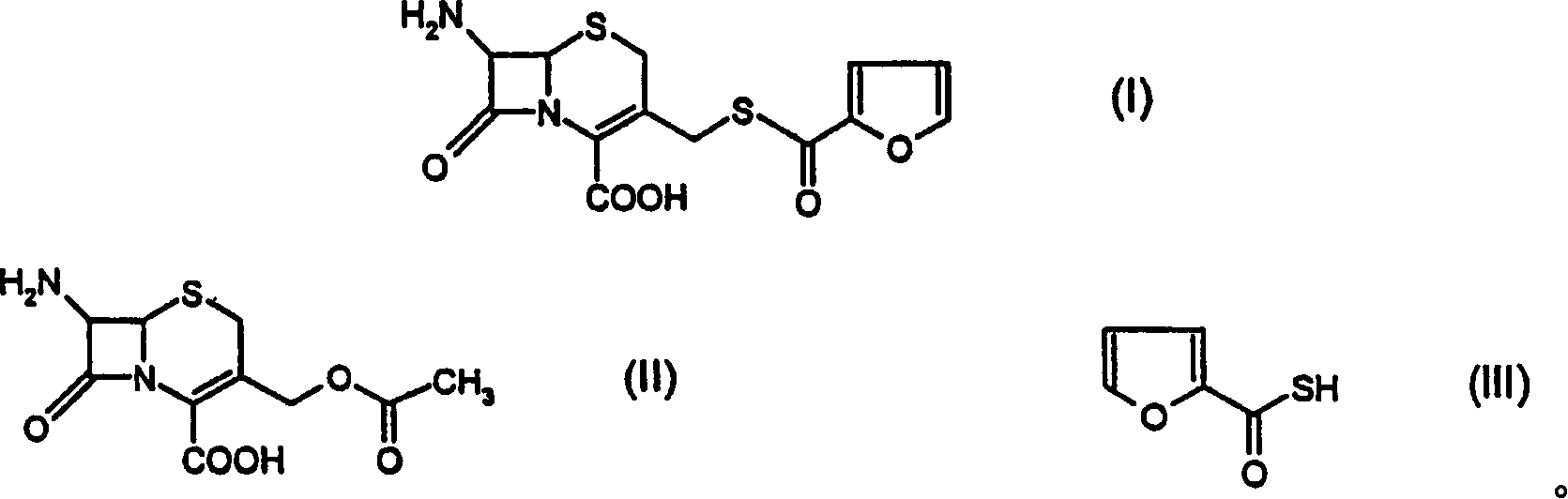
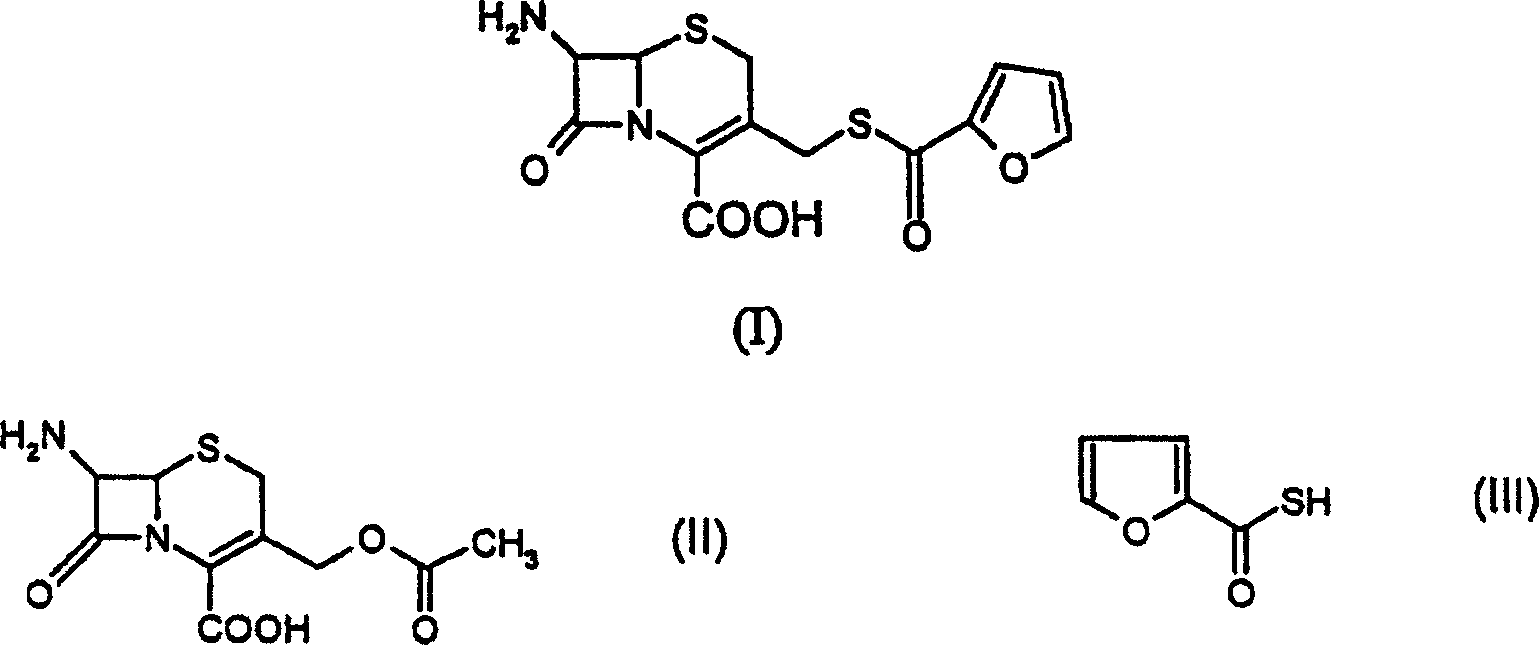
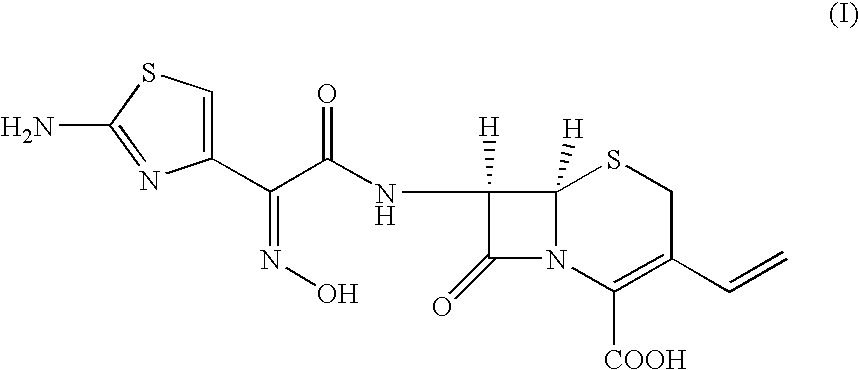
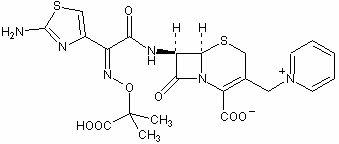
A process for preparation of ceftiofur of formula (I)having purity greater than 97% is disclosed. The process comprises reacting [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester of formula (II),with 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of formula (III)in the presence of a mixture of an water-immiscible inert organic solvent and water and in the presence of a organic base and isolating ceftiofur of formula (I) substantially free of impurities by,a) adding water to the reaction mixture and selectively partitioning the impurities in the organic phase and ceftiofur (I) in the form of a salt with the base in the aqueous phase,b) acidifying the aqueous phase containing ceftiofur (I) in the form of a salt with the base in the presence of a mixture containing a water-miscible and a water-immiscible organic solvent and in the presence of a saturated aqueous solution of an alkali or alkaline earth containing salt, to partition ceftiofur (I) in the organic phase, andc) isolating ceftiofur (I) of high purity and substantially free of impurities by evaporation of the organic solvent or precipitation by addition of a anti-solvent.
Owner:LUPIN LTD
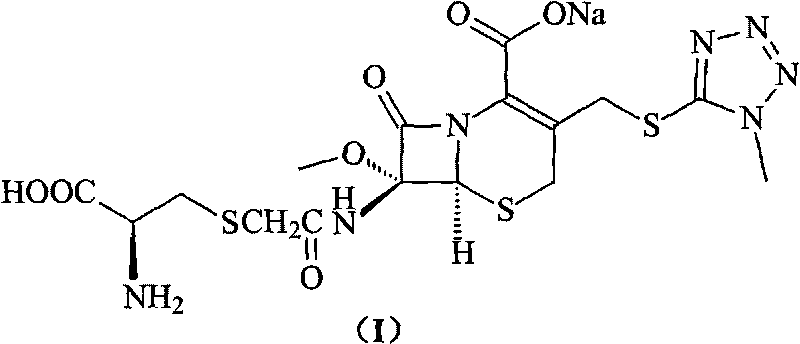
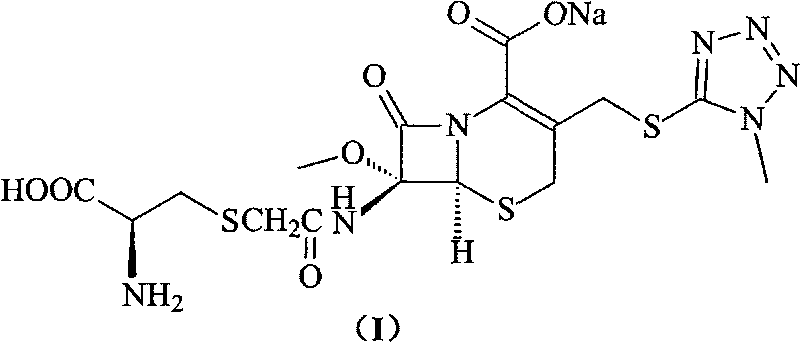
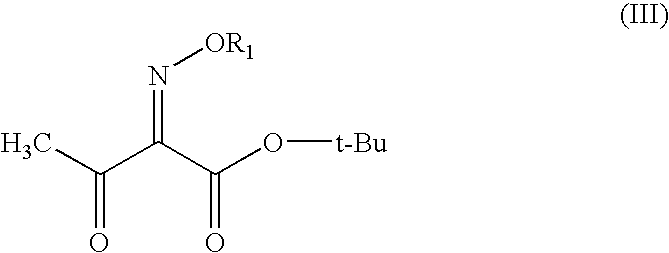
Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid
InactiveUS20060149096A1High purityHigh yieldGroup 4/14 element organic compoundsOrganic compound preparationCarboxylic acidHydrolysis
A process for preparation of 7-[D-α-amino-α-(4-hydroxyphenyl)acetamido]-3-(1-propen-1-yl)-3-cepham-4-carboxylic acid viz. Cefprozil of formula (I) igh purity, substantially free of impurities, which comprises preparation of mixed acid anhydride by selecting the sequence and temperature of addition of the reagents and its subsequent condensation with a protected 7-APCA; followed by hydrolysis, isolation and purification to give Cefprozil of formula (I) in the form of a monohydrate.
Owner:LUPIN LTD
A kind of preparation method of cefminox sodium
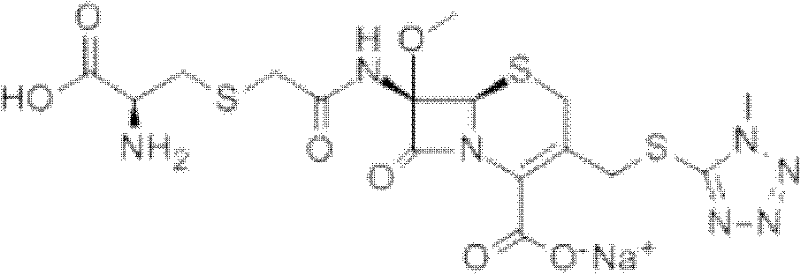
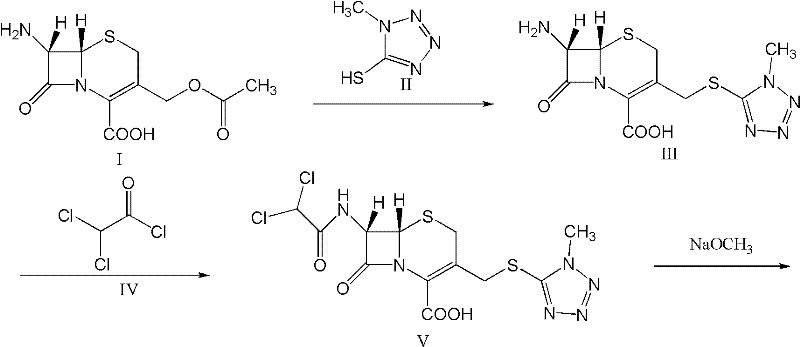
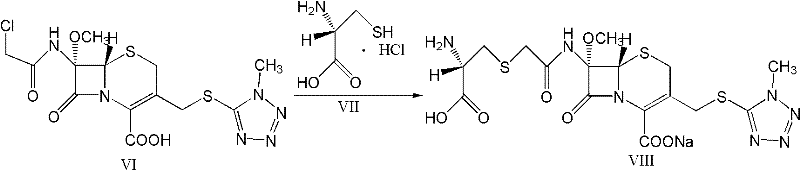
The present invention relates to a kind of preparation of medicinal compound, particularly a kind of preparation method of cefminox sodium. The present invention adopts a synthesis route using 7-ACA as a starting material, and the cost of raw materials is lower than that of a synthesis route using 7-MAC as a starting material. The new synthetic route uses dichloroacetyl chloride instead of bromoacetyl bromide, which reduces the toxicity and cost of raw materials. The new synthetic route increases the intermediate treatment process, reduces the amount of impurities brought by the intermediate into the next reaction, and improves the quality and stability of the finished product. The present invention overcomes the shortcomings of the synthetic route using 7-MAC as the starting material through the above improvements.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Amorphous hydrate of a cephalosporin antibiotic
InactiveUS7244842B2Improve bioavailabilityUseful for developmentOrganic active ingredientsOrganic chemistryOrganic solventCarboxylic acid
A process for the preparation of cefdinir of the formula (I) the said process comprising the steps of: i) condensing 7-amino-3-cephem-4-carboxylic acid of the formula (XII) wherein R1 is as defined above with compound of the formula (XIII) in the presence of a tertiary amine and an organic solvent, followed by treatment with a base to produce a salt of compound formula (XIV), wherein M+ is a counter ion and ii) hydrolyzing the compound of the formula (XIV) using an acid in the presence of a solvent to produce cefdinir of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Cephem compound having catechol group
InactiveUS20130102583A1Broad antimicrobial spectrumAntibacterial agentsOrganic active ingredientsSide chainPharmaceutical medicine
A compound of the formula:whereinX is —N═, —CH═, or the like;W is —CH2— or the like;U is —S— or the like;R1 and R2 are each independently hydrogen, halogen, optionally substituted lower alkyl, or the like;R3 is hydrogen or the like;each R4 is independently hydrogen, halogen, or the like;m is an integer from 0 to 2;Q is a single bond, or the like;G is —C(═O)—, or the like;D is a single bond, —NH—, or the like; andE is a cyclic quaternary ammonium group,or an ester, a protected compound at the amino on the ring in the 7-side chain, a pharmaceutically acceptable salt, or a solvate thereof.
Owner:SHIONOGI & CO LTD
An improved synthesis of ceftiofur intermediate
The present invention relates to a process for preparation of 7-amino-3-[2-(furylcarbonyl)thiomethyl]-3-cephem-4-carboxylic acid (I) by the condensation of 7-aminocephalosporanic acid (II) with furyl-2-carbonylthiol (III) in the presence of borontrifluoride or its complex, in an organic solvent or mixture of solvents at 0-50° C.
Owner:ORCHID CHEM & PHARM LTD
Ceftizoxime sosium compound of new way
InactiveCN101671348AHigh purityHigh yieldAntibacterial agentsOrganic chemistryAcetic acidTriphenylphosphine oxide
The invention relates to a ceftizoxime sosium compound of a new way. Ceftizoxime sosium is prepared by firstly reacting a cefotaxime acid with a methanoic acid to generate 2-(2-formyl aminothiazole-4-radical)-2-methoxyl imine acetic acid, then adding 7-amino-3-nor-3-cephem-4-carboxylic acid, taking triphenylphosphine oxide and triphosgene as catalysts, stirring and reacting, and regulating an acid-base pH value.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing ceftizoxime alapivoxil hydrochloride
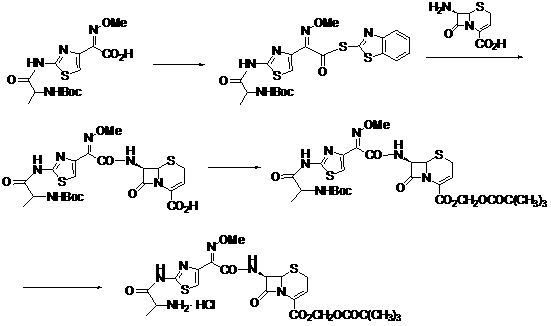
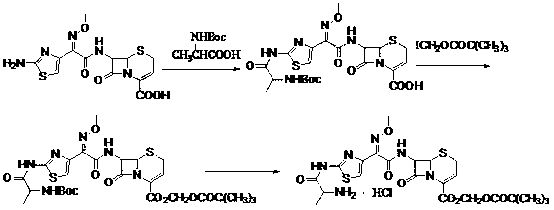
ActiveCN103059047AFew reaction stepsLow costOrganic chemistryBulk chemical productionBiotechnologyTert-Butyloxycarbonyl protecting group
The invention discloses a new method for preparing ceftizoxime alapivoxil hydrochloride and belongs to the technical field of medicine synthesis. The method comprises the following steps: step (1), performing Boc protection on 7-amino of raw material 7-amino-3-cephem-4-carboxylic acid (7-ANCA) and then making the raw material have reaction with iodomethyl pivalate, then removing Boc protection group to obtain a midbody 7-amino-3-cephem-4-carboxylic pivaloyl oxymethyl ester (7-ANCA-POM); step (2), making N-t-butyloxycarboryl-L-alanine (Boc-L-Ala) and methoxyiminoacetic acid (ATMA) have condensation reaction to obtain a midbody 2-(2-N-t-butyloxycarboryl-amino-(S)-triacylamino-thiazole-4-yl)-2-(Z)-methoxyimino-acetic acid (Boc-L-Ala-ATMA); step (3), activating the midbody Boc-L-Ala-ATMA and making the midbody Boc-L-Ala-ATMA have condensation reaction with the midbody 7-ANCA-POM, and then removing the Boc protection group to prepare a target compound ceftizoxime alapivoxil hydrochloride (CZX-AP-HC1,I). The method adopts a collection type synthesis route, is simple and convenient to operate and mild in technical conditions, has high product quality and low cost, and avoids the problem about benzothiazole residue caused by the use of AE active ester in the present technique, so the collection type synthesis route is a good synthesis route suitable for industrial production.
Owner:江苏慈星药业有限公司
Broad -spectrum cephem compounds
InactiveUS20050153950A1High antibacterial activityPronounced antibacterial activityAntibacterial agentsOrganic active ingredientsCompound aThiazole
A compound of the formula: (wherein, T is S, SO or O; X is halogen, CN, carbamoyl optionally substituted with lower alkyl, lower alkyl, lower alkoxy, or lower alkylthio; A is substituted lower alkylene (wherein the substituent is optionally substituted mono lower alkyl, optionally substituted lower alkylidene, or optionally substituted lower alkylene); [0001]Z+ is an optionally substituted, a cation and an N atom-containing heterocyclic group), ester, amino-protected compound wherein the amino bonds to a thiazole ring at the 7-position, or pharmaceutically acceptable salt or solvate thereof.
Owner:SHIONOGI & CO LTD
Process for the preparation of a cephalosporin antibiotic
An improved process for the preparation of ceftriaxone sodium comprising the steps of: i) reacting the 3-cephem derivative of formula (II) with halo acid derivative of formula (III) wherein X represents halogen and Y represent halogen in the presence of silylating agent and methylene chloride at −25 to 10° C., to produce (IV), ii) quenching the reaction by pouring the reaction mixture into water or in a aqueous solution of sodium carbonate, iii) preparing sodium salt solution of (IV) by adding sodium carbonate and separating the organic layer, iv) cyclizing the sodium salt of (IV) in the aqueous solution with thiourea at a temperature in the range of 0 to 30° C., v) adjusting the pH to 1.5 to 2.5 to precipitate the ceftriaxone free acid, vi) converting the ceftriaxone free acid to sodium salt using sodium-2-ethyl hexanoate in water and vii) precipitating and isolating the ceftriaxone sodium.
Owner:ORCHID CHEM & PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00001.png)
![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00002.png)
![Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid Process for preparation of 7-[a-amino (4-hydroxyphenyl) acetamido]-3-substituted-3-cephem-4-carboxylic acid](https://images-eureka.patsnap.com/patent_img/4c95adda-5677-4886-a0e7-3e1580e61e75/US20060149096A1-20060706-C00003.png)