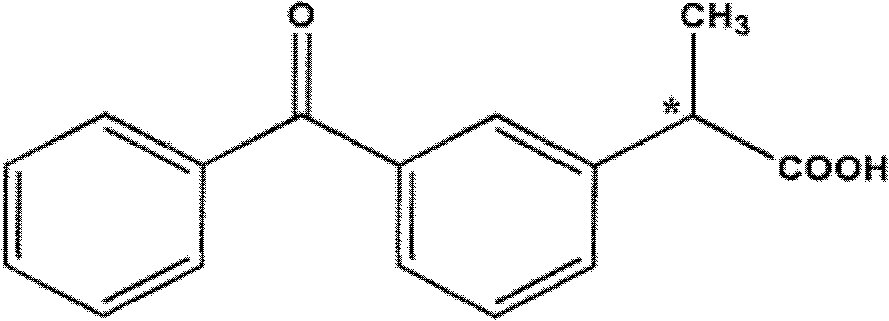
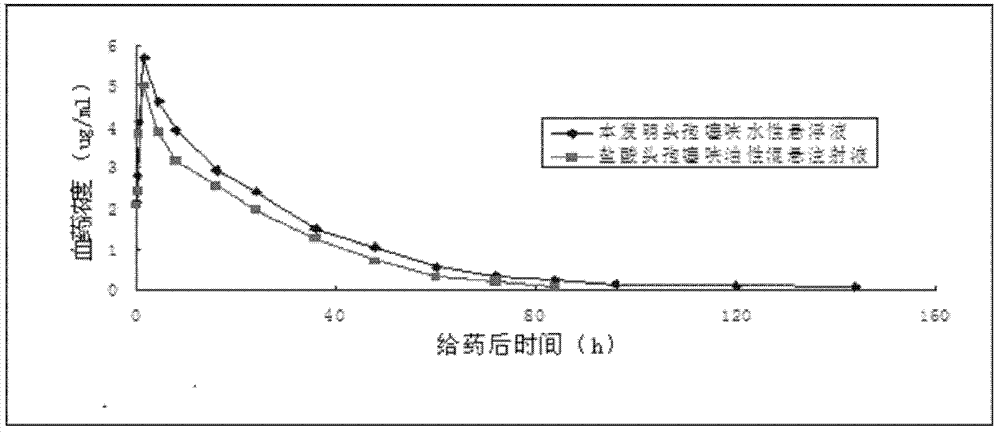
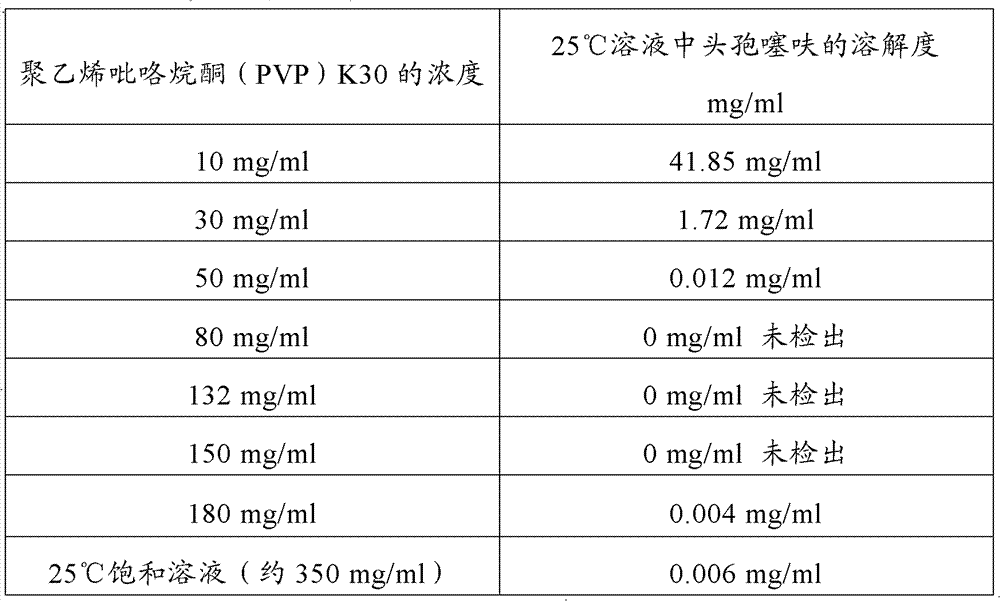
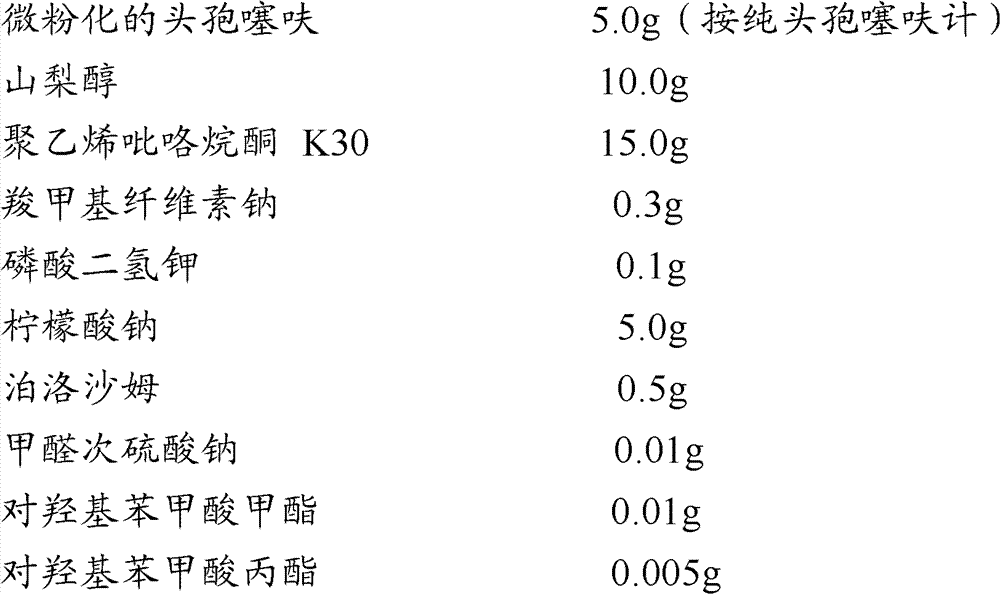
Patents
Literature
78 results about "Ceftiofur" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ceftiofur is an antibiotic of the cephalosporin type (third generation), licensed for use in veterinary medicine. It was first described in 1987. It is marketed by pharmaceutical company Zoetis as Excenel, Naxcel, and Excede and is also the active ingredient in that company's Spectramast LC (lactating cow formulation) and Spectramast DC (dry cow formulation) product.
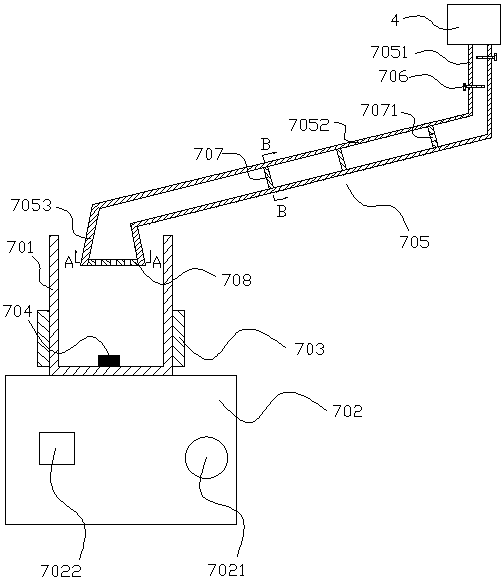
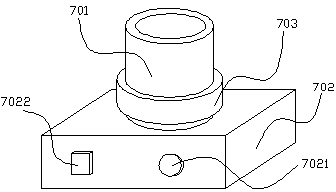
Preparation method of ceftiofur long-acting injector
InactiveCN101416968AReduce stress responseGood curative effectAntibacterial agentsOrganic active ingredientsHalf-lifeTherapeutic effect
A ceftiofur long acting injection consists of the following substances: 2.5g to 5.0g of ceftiofur crude drugs, 0.5g to 3.0g of span 80, 0.1g to 0.3g of Vitamin E, 0.2g to 0.5g of aluminium stearate, 0.25g to 0.5g of chlorobutanol and soybean oil. The preparation method of the ceftiofur long acting injection comprises the following steps: (1) smashing the ceftiofur crude drugs till the particle size of the ceftiofur crude drugs is 5 Mum; (2) adding the span 80 into a mortar for grinding till the span 80 becomes even; (3) then, adding the aluminium stearate, the Vitamin E and the chlorobutanol into the mortar together; and (4) moving the mixture into a measuring vessel and adding the soybean oil into the measuring vessel till the total volume of the substances in the measuring vessel reaches 100ml. The ceftiofur long acting injection has the advantages of obvious therapeutic effect, long half-life period, less adverse reaction, reducing medication times, saving manual labor and time and the like.
Owner:PU LIKE BIO ENG
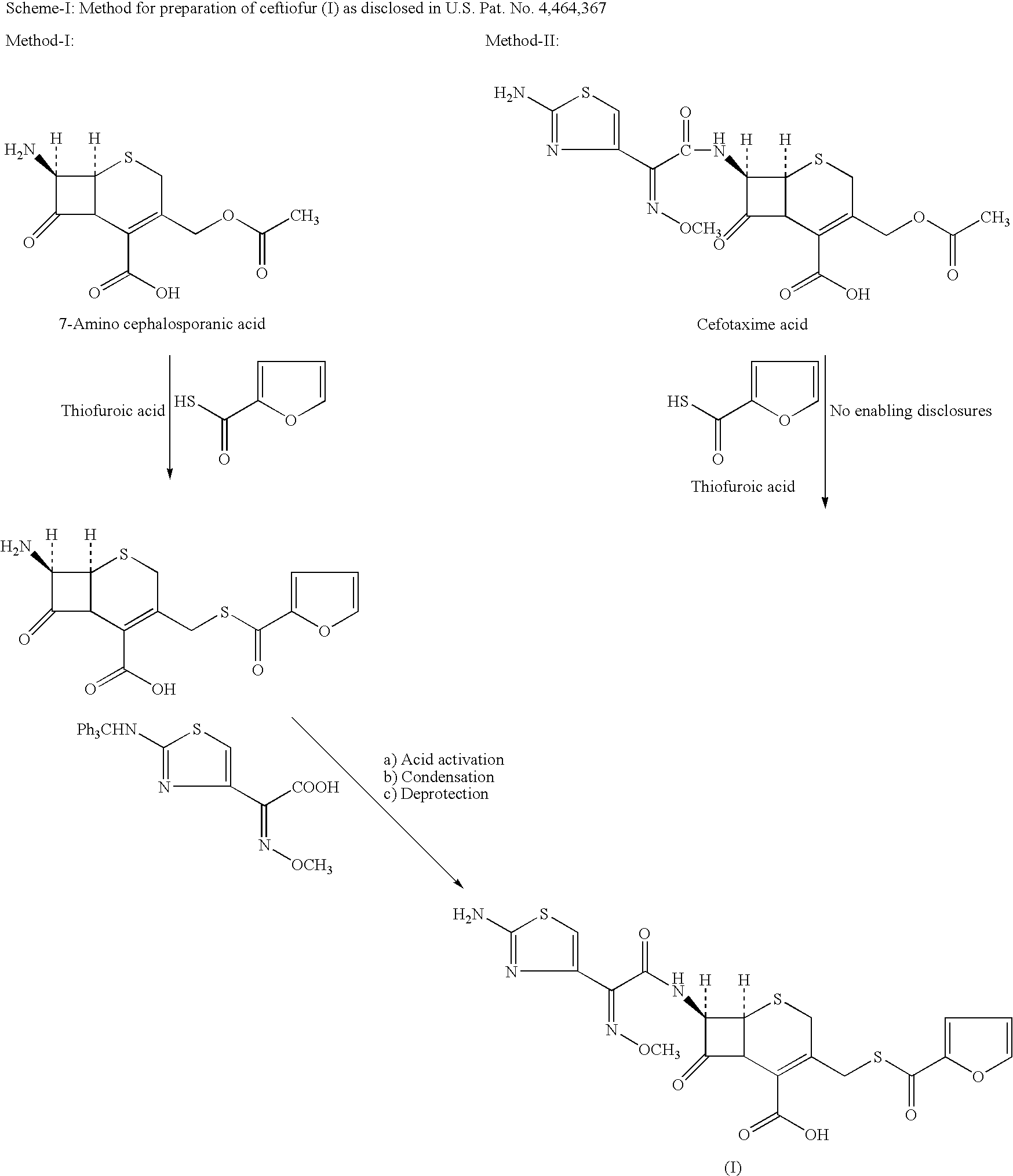
Method for manufacture of ceftiofur
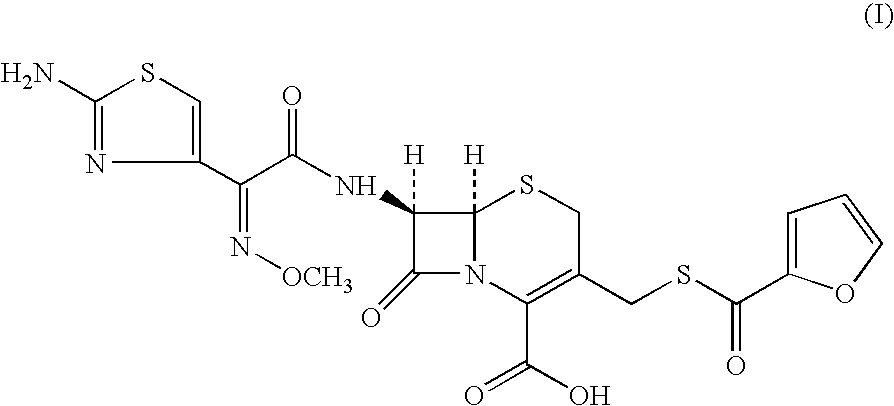
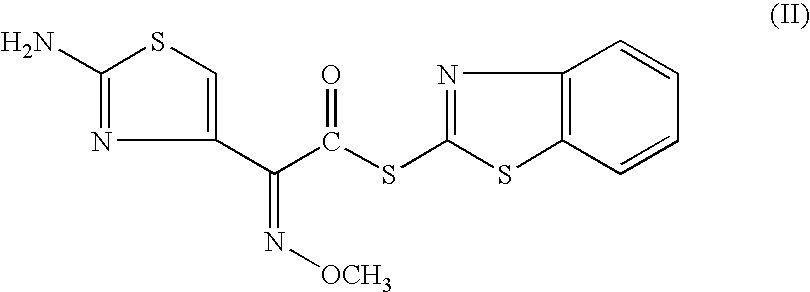
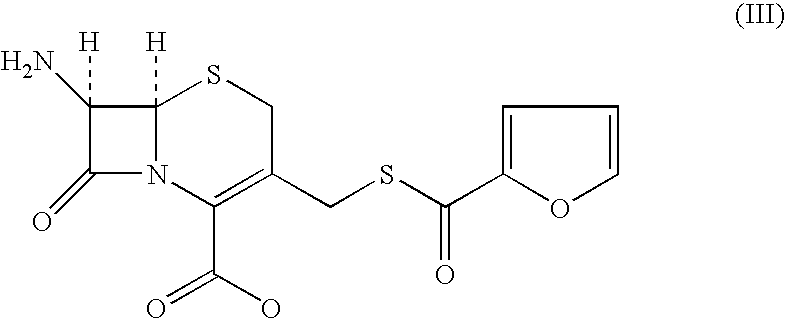
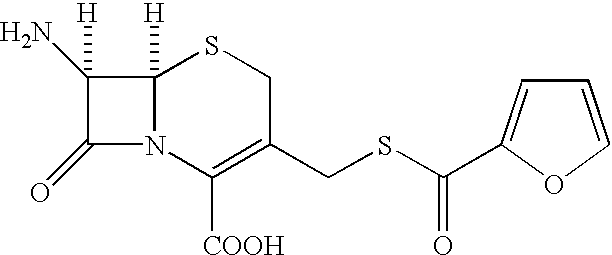
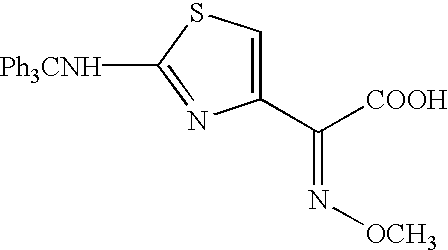
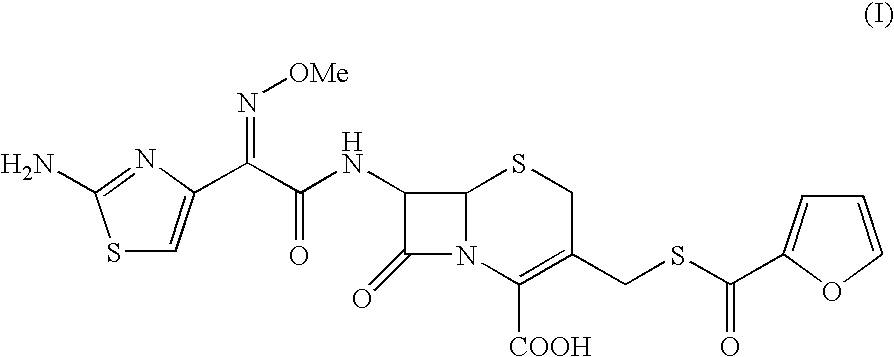
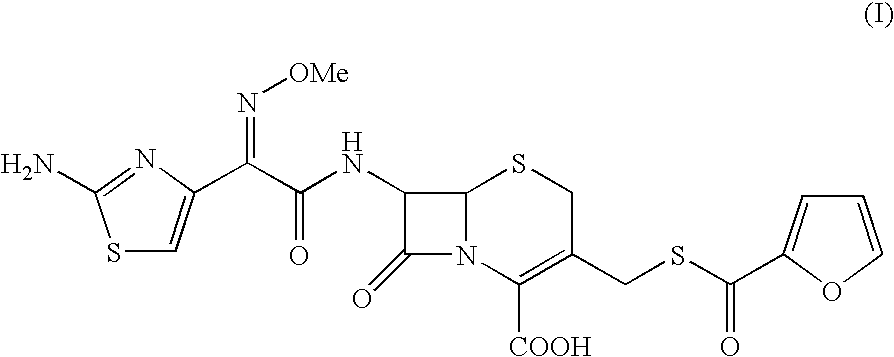
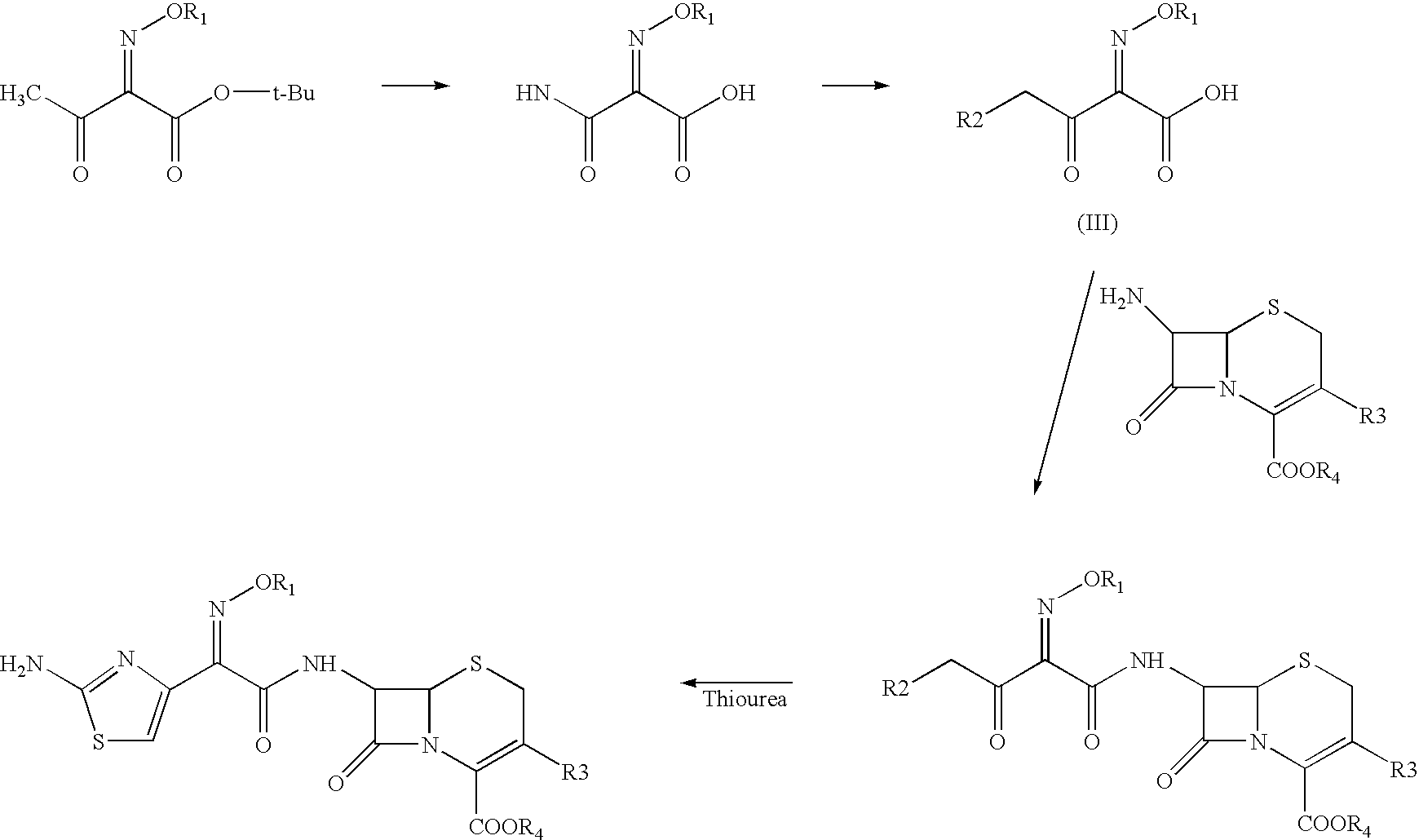
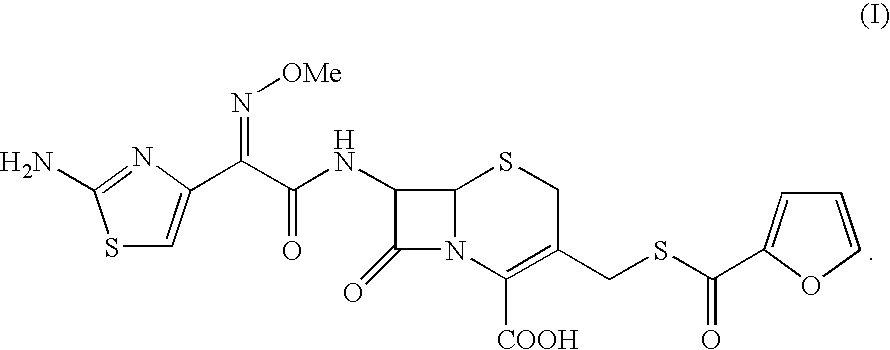
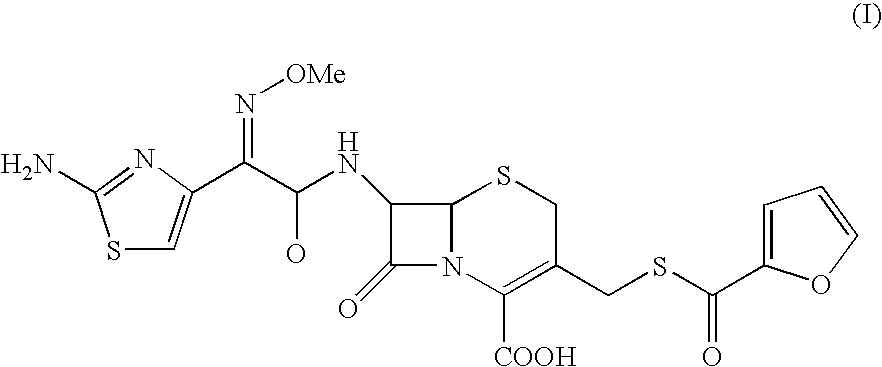
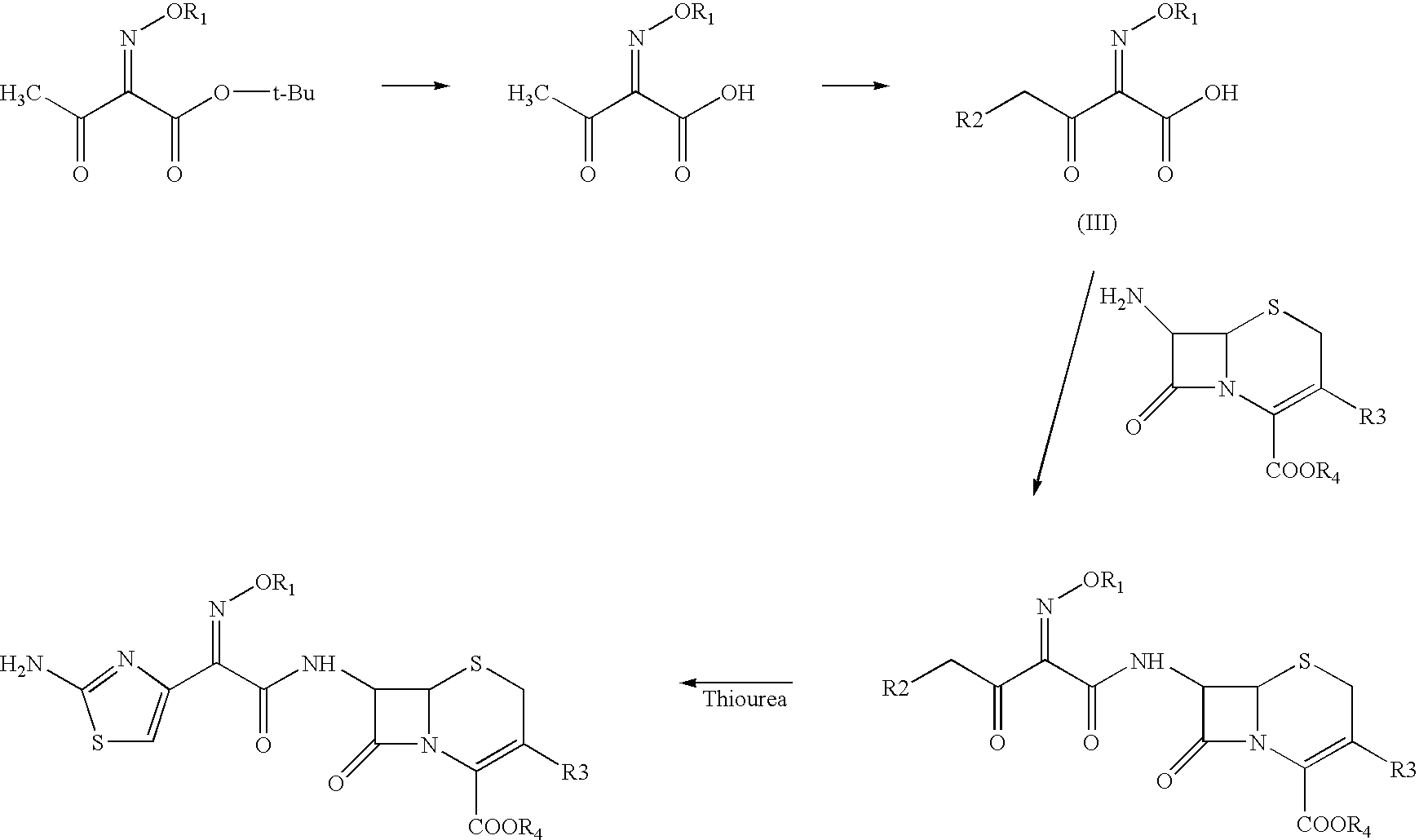
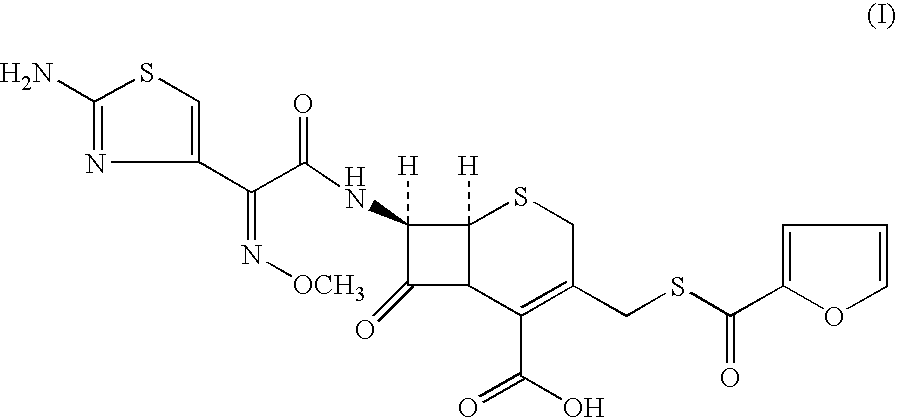
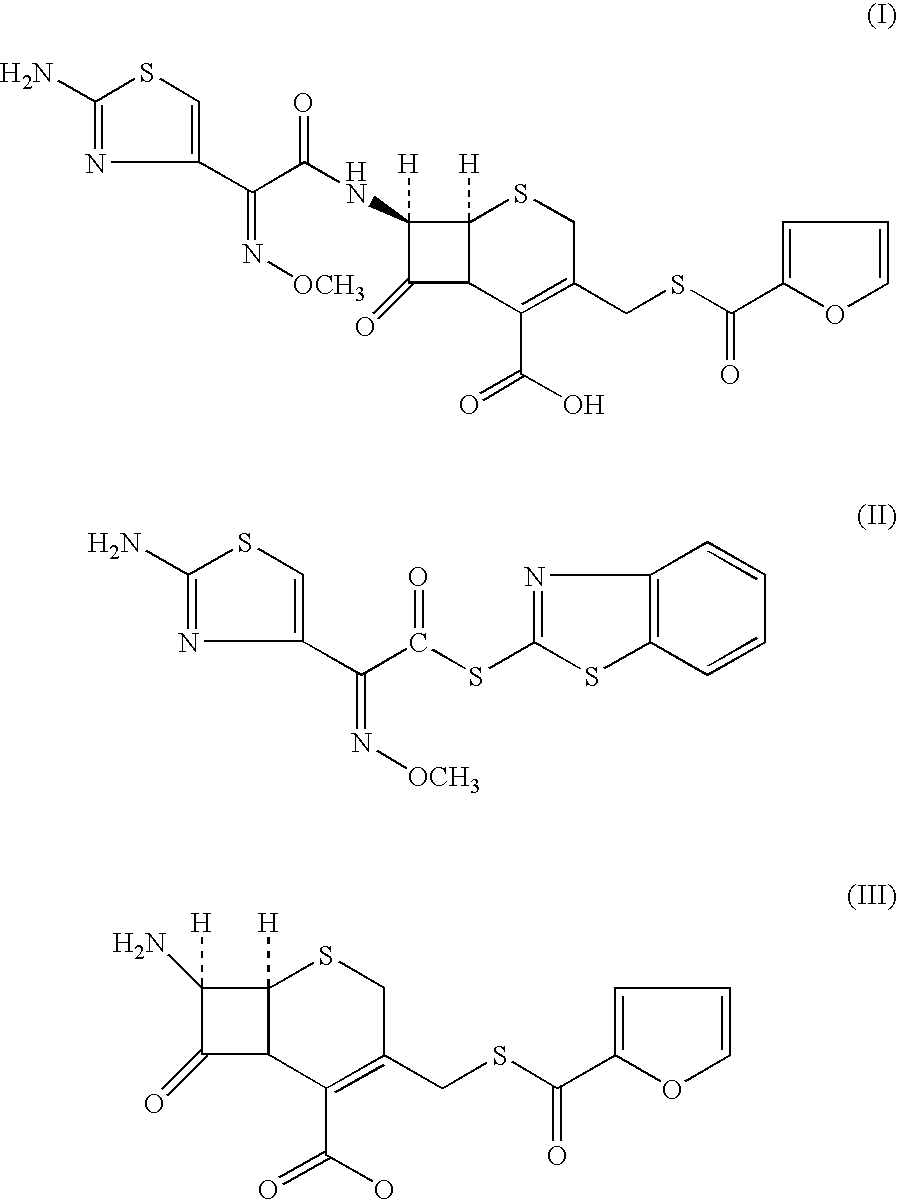
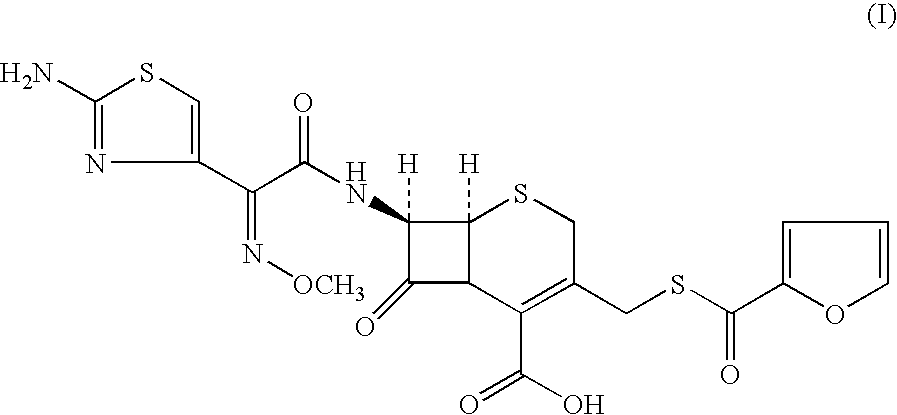
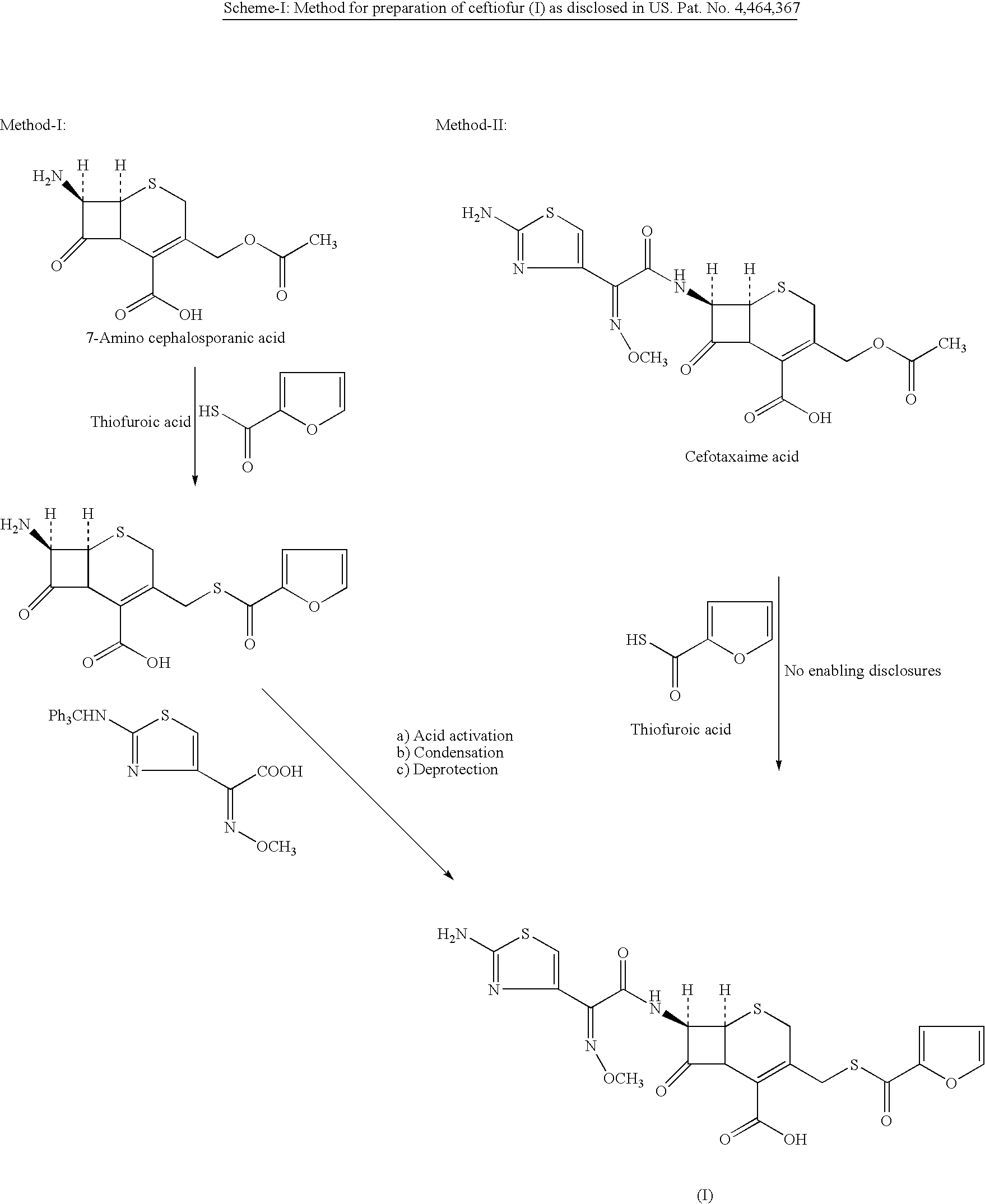
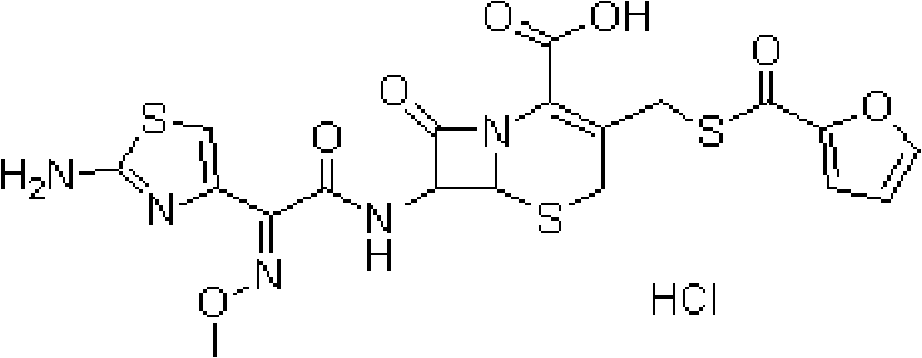
A process for preparation of ceftiofur of formula (I)having purity greater than 97% is disclosed. The process comprises reacting [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester of formula (II),with 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of formula (III)in the presence of a mixture of an water-immiscible inert organic solvent and water and in the presence of a organic base and isolating ceftiofur of formula (I) substantially free of impurities by,a) adding water to the reaction mixture and selectively partitioning the impurities in the organic phase and ceftiofur (I) in the form of a salt with the base in the aqueous phase,b) acidifying the aqueous phase containing ceftiofur (I) in the form of a salt with the base in the presence of a mixture containing a water-miscible and a water-immiscible organic solvent and in the presence of a saturated aqueous solution of an alkali or alkaline earth containing salt, to partition ceftiofur (I) in the organic phase, andc) isolating ceftiofur (I) of high purity and substantially free of impurities by evaporation of the organic solvent or precipitation by addition of a anti-solvent.
Owner:LUPIN LTD
Cephalosporin compound and a process for its preparation
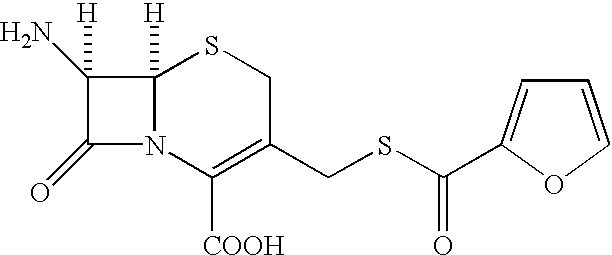
A process is disclosed for the preparation of 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of the formula This compound is useful as an intermediate for the preparation of ceftiofur cephalosporin antibiotic used for bovine respiratory infections.
Owner:AUROBINDO PHARMA LTD
Veterinary medicament for treating bacteriosis, virosis and mixed infection of bird and preparation method thereof
The invention relates to a veterinary medicine for treating poultry bacterial diseases, viral diseases and mixed infection and preparation method thereof, prepared by the following components by weight parts: 300-400 parts of honeysuckle, 300-400 parts of scutellaria, 700-800 parts of fructus forsythiae, 0.5-1.5 parts of ceftiofur, 0.5-1.0 parts of synergist, 20-30 parts of bacteriostatic factor, 5-15 parts of dissolution factor. The veterinary medicine is capable of clearing away the heat-evil and expelling superficial evils and dispelling wind and cooling blood with strong antibiotic, antivirus, antitoxin function and quickly activating immune organ and adjusting immunologic function and recovering all kinds of physiological functions and thoroughly killing grey pathogen, and being equipped with the synergist to enlarge antibacterial spectrum and bactericidal power therefore the curative effect is obviously reinforced.
Owner:ZHENGZHOU HOUYI PHARMA
Process for the preparation of cephalosporin antibiotic
InactiveUS20060058281A1Easy to condenseImpurity formation is highOrganic active ingredientsArsenic organic compoundsCephalosporin AntibioticOrganic chemistry
An improved one-pot process for the preparation of Ceftiofur of the formula (I) or its salt, without isolating intermediate compound.
Owner:ORCHID CHEM & PHARM LTD
Compound long-effect injection containing ceftiofur and meloxicam and preparation method thereof
InactiveCN109568255ASimple preparation processGood batch-to-batch repeatabilityAntibacterial agentsOrganic active ingredientsMeloxicamDisease
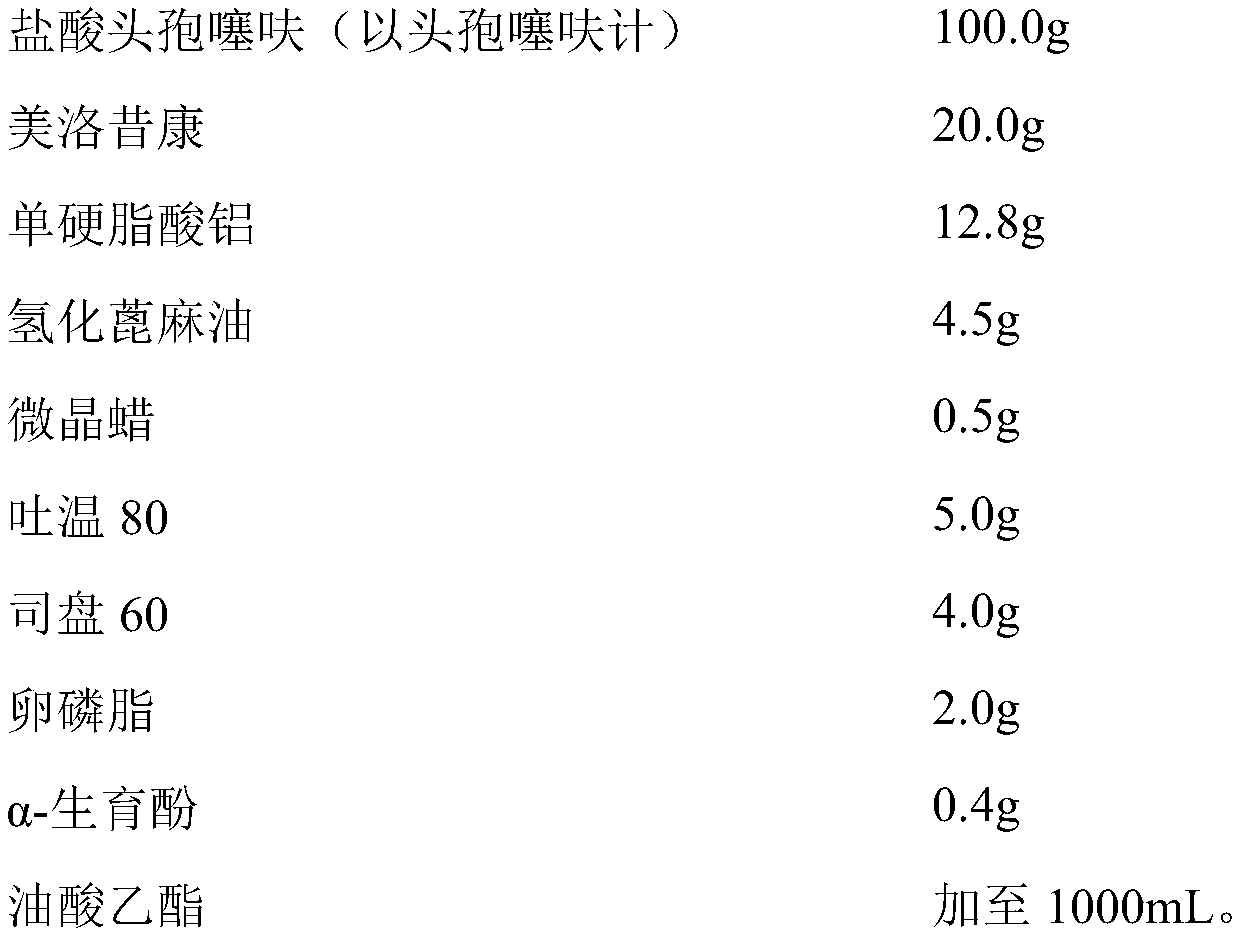
The invention discloses a compound long-effect injection containing ceftiofur and meloxicam. Every 1000 mL of injection contains 20 to 250 g of ceftiofur (based on ceftiofur), 4 to 80 g of meloxicam,1 to 50 g of macromolecular retardants, 1 to 50 g of wetting agents, 0.1 to 10 g of antioxidants and the balance of dispersion media. The granularity D90 of the ceftiofur is lower than or equal to 5 mum; the granularity D90 of meloxicam is lower than or equal to 5 mum. The injection has the advantages that the medicine microparticle mixing is uniform; the ceftiofur stability is good; just throughone injection, the injection can reach the treatment effect of one treatment coarse medication of 3 to 5 times of a conventional injection; both principal and secondary aspects of diseases can be treated; the cost is reduced; the time and the labor are saved; the animal stress is reduced, and the animal adaptability can be enhanced; the long-effect slow release effect is achieved; meanwhile, the medication safety is improved.
Owner:NANJING AGRICULTURAL UNIVERSITY
Lung-targeting ceftiofur microsphere and preparation method thereof
ActiveCN101756909AGood biocompatibilityPromote degradationOrganic active ingredientsAntiinfectivesSide effectTreatment effect
The invention provides a lung-targeting ceftiofur microsphere and a preparation method thereof, aiming at solving the problems that less medicine can reach the target part in the prior art, so that a great deal of medicine is wasted, the treatment effect is not remarkable, the toxic and side effect of the medicine is increased, and the probability of generating drug resistance is high. The technical scheme comprises: the microsphere takes ceftiofur as raw material and PLA as carrier material, and the weight ratio between the ceftiofur and the PLA is 1:0.5-10. The invention also provides the method for preparing the microsphere by emulsification. The microsphere prepared by the method has round appearance and shape, even grain fineness distribution, the average grain diameter of about 20mu m and higher medicine-loading rate. The microsphere can be used for treating lung infection of livestock and poultry with high effect, and leads the ceftiofur to be made into the lung-targeting microsphere so as to improve the concentration of the medicine at the lung tissues of animals and lead the medicine effect to be more remarkable, thus achieving the aims of slow release, long-term effect and target.
Owner:QINGDAO VLAND BIOTECH INC +2
Process for the preparation of cephalosporin antibiotic
InactiveUS7345169B2Easy to condenseOrganic active ingredientsArsenic organic compoundsCephalosporin AntibioticPhotochemistry
An improved one-pot process for the preparation of Ceftiofur of the formula (I) or its salt, without isolating intermediate compound
Owner:ORCHID CHEM & PHARM LTD
Nanometer compound ceftiofur suspension and preparation method thereof
InactiveCN103301147ASmall particle sizeUniform particle size distributionAntibacterial agentsOrganic active ingredientsAstragalus polysaccharideSuspending Agents
The invention discloses a nanometer compound ceftiofur suspension and a preparation method thereof, belonging to the technical field of antibiotic preparations for animals. The nanometer compound ceftiofur suspension comprises 1 to 30% of ceftiofur, 1 to 30% of astragalus polysaccharide, 0.1 to 40% of a surfactant, 0.1 to 30% of a wetting agent, 0.05 to 20% of a suspending agent, 0.1 to 10% of an anti-oxidant and 0.1 to 10% of an antiseptic, with the balance being soybean oil for injection. The nanometer compound ceftiofur suspension provided by the invention has the advantages of a scientific and reasonable formula, safety, high efficiency, a small particle size, uniform particle size distribution, simple preparation process, good stability and suitability for industrial mass production.
Owner:河南省针剂兽药工程技术研究中心
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cephalosporin antibiotics
ActiveCN104558187AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureAntibiotic YCephalosporin Antibiotic
The invention discloses a specific monoclonal antibody capable of resisting various cephalosporin antibiotics such as ceftiofur, ceftriaxone and the like. The invention further discloses an enzyme-linked immunosorbent assay method and kit for detecting the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like. According to the invention, the monoclonal antibody is secreted by a hybridoma cell 4D5 of which the preservation number is CCTCC No. C201341. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
Method for manufacture of ceftiofur
A process for preparation of ceftiofur of formula (I) of high purity and substantially free from impurities is disclosed. The process comprises reacting [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester of formula (II), with 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of formula (III) in the presence of a mixture of an water-immescible inert organic solvent and water and in the presence of a organic base and isolating ceftiofur of formula (1) substantially free of impurities by, d) adding water to the reaction mixture and selectively partitioning the impurities in the organic phase and ceftiofur (I) in the form of a salt with the base in the aqueous phase, e) acidifying the aqueous phase containing ceftiofur (I) in the form of a salt with the base in the presence of a mixture containing a water-miscible and a water-immiscible organic solvent and in the presence of a saturated aqueous solution of an alkali or alkaline earth containing salt, to partition ceftiofur (I) in the organic phase, and f) isolating ceftiofur (I) of high purity and substantially free of impurities by evaporation of the organic solvent or precipitation by addition of a co-solvent.
Owner:LUPIN LTD
Lung targeting ceftiofur microsphere and preparation method
ActiveCN101756910ANo toxicityEfficient infectionAntibacterial agentsOrganic active ingredientsSide effectMicrosphere
The invention provides lung targeting ceftiofur microsphere and a preparation method which can solve the problems the prior art that few positions reach a target, so that a lot of medicine is wasted, the therapical effect is insignificant, the toxin and side effect of the medicine are improved and the chance of leading to medicine resistance is great. The technical scheme is as follows: the microsphere takes ceftiofur as the raw material, PLGA as the carrier material, and the weight ratio of ceftiofur to PLGA is 1:1 to 1:50. The invention also provides a method for preparing the microsphere through an emulsion process. The microsphere can treat the lung infection of animals with high efficiency; and the ceftiofur is prepared into lung targeting microsphere, thereby improving the concentration of the medicine in the animal lung tissue, so that the therapical effect is more significant. The microsphere prepared through the method has the advantages of very good morphology and very strong controllable particle size, can achieve high lung targeting property by optimizing the particle size, so that the particle size of more than 90 percent of microsphere is 7 to 30mu m, the encapsulation rate of the microsphere is more than 65 percent, the drug loading can reach 8 to 24 percent, and the microsphere has better releasing effect.
Owner:QINGDAO VLAND BIOTECH INC +1
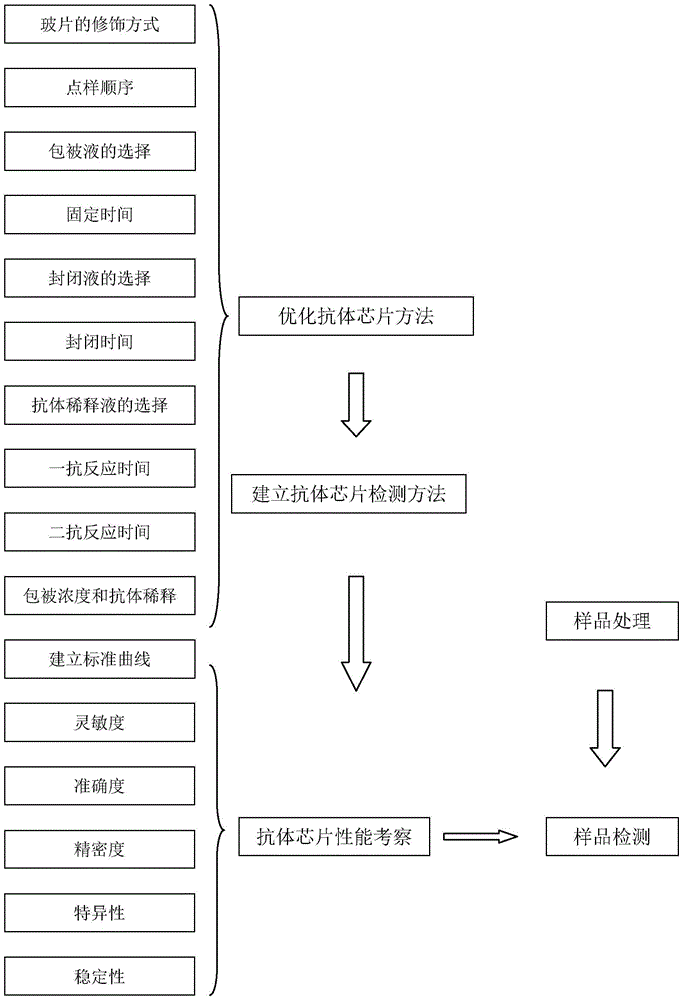
Antibody chip kit and method for detecting residual cephalosporin antibiotics in food
The invention discloses an antibody chip kit for detecting residual cephalosporin antibiotics in food, and belongs to the technical field of the drug residue detection. The kit comprises a chip, an antibody, a second antibody marked by Cy3, and an extraction reagent, the antibody is composed of a cefalexin monoclonal antibody and a ceftiofur monoclonal antibody, and the cefalexin monoclonal antibody is secreted by hybridoma 3A6 with a preservation number of CCTCC NO: C201340; the ceftiofur monoclonal antibody is secreted by hybridoma 4D5 with a preservation number of CCTCC NO: C201341, and the chip fixes cefalexin coating antigen and cefalexin coating antigen. The invention further discloses an antibody chip method for detecting residual cephalosporin antibiotics in food, the method can be used for simultaneously detecting 8 kinds of cephalosporins, and has the advantages of high accuracy, high precision, high efficiency, etc.
Owner:HUAZHONG AGRI UNIV
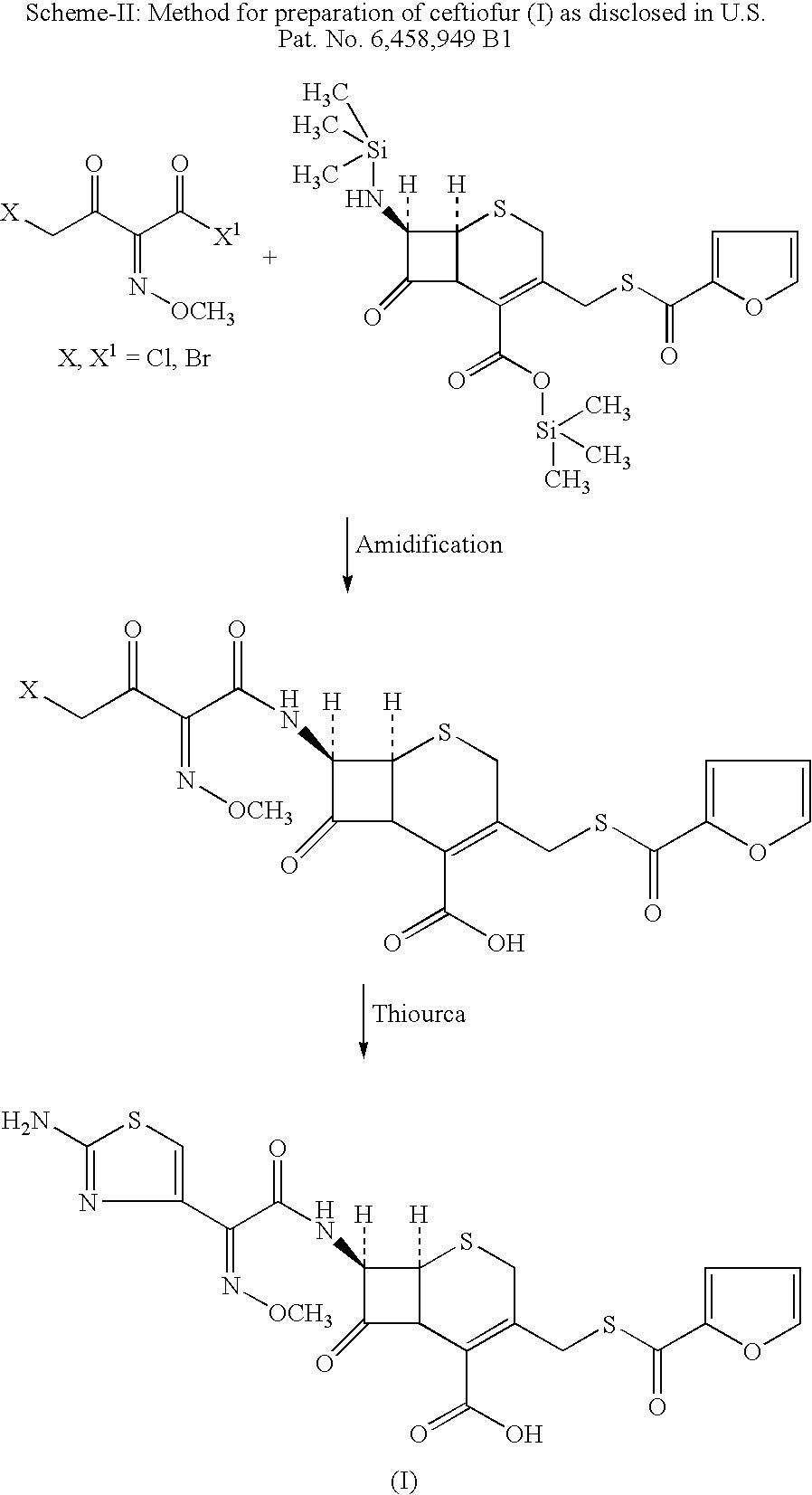
Method for manufacture of ceftiofur
InactiveUS20060149054A1High yieldNo impuritiesOrganic chemistryThioester synthesisAlkaline earth metal
A process for preparation of ceftiofur of formula (I) of high purity and substantially free from impurities is disclosed. The process comprises reacting [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester of formula (II), with 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of formula (III) in the presence of a mixture of an water-immescible inert organic solvent and water and in the presence of a organic base and isolating ceftiofur of formula (I) substantially free of impurities by, d) adding water to the reaction mixture and selectively partitioning the impurities in the organic phase and ceftiofur (I) in the form of a salt with the base in the aqueous phase, e) acidifying the aqueous phase containing ceftiofur (I) in the form of a salt with the base in the presence of a mixture containing a water-miscible and a water-immiscible organic solvent and in the presence of a saturated aqueous solution of an alkali or alkaline earth containing salt, to partition ceftiofur (I) in the organic phase, and f) isolating ceftiofur (I) of high purity and substantially free of impurities by evaporation of the organic solvent or precipitation by addition of a co-solvent.
Owner:LUPIN LTD
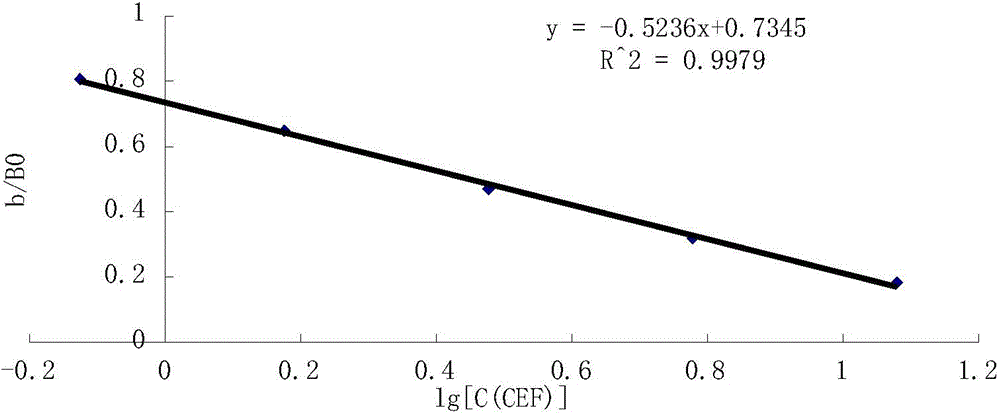
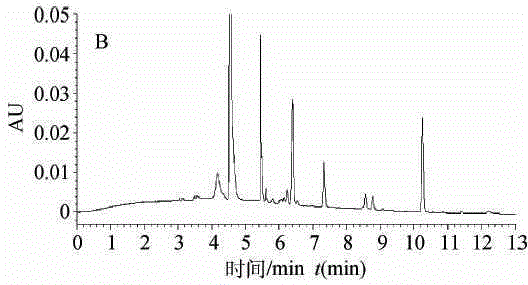
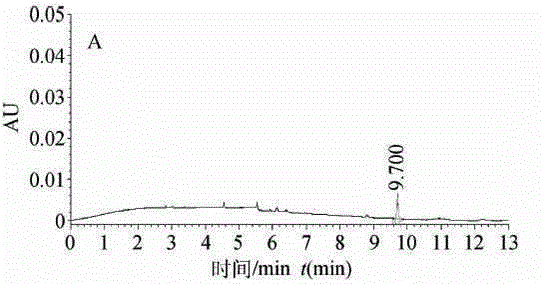
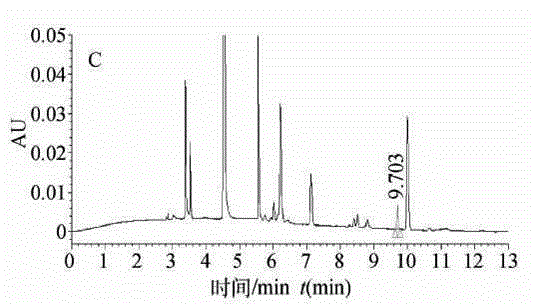
High performance liquid chromatography for detection of residual ceftiofur in pork tissues
InactiveCN105277631AHigh resolutionGood repeatabilityComponent separationChromatographic separationRelative standard deviation
The invention discloses a high performance liquid chromatography (HPLC) for detection of residual ceftiofur in pork tissues. The ceftiofur relevant residual amount in cattle and pig muscles and kidneys is determined by the high performance liquid chromatography. A sample is extracted by a dithioerythritol solution, is derived by amide, is purified by solid phase extraction columns (C18, SAX and SCX), is separated by 8 column chromatography, and is subjected to gradient elution with acetonitrile-water (with the volume ratio of 15 to 85) containing a 0.1% trifluoroacetic acid as a mobile phase, and is subjected to 6 nm ultraviolet detection. The ceftiofur residual amount shows good linearity with a peak area within 0.016-1.28 mg / L, and the correlation coefficient r is more than 0.9999. The recovery rate with the sample addition concentration of 200-8000 [mu]g / kg is 90%-91%, and the relative standard deviation is 118%-315%. The detection limit of the method (the signal-to-noise ratio S / N is 3) is 50 [mu]g / kg. The method comprises the following steps: 1, instruments and reagents; 2, solution preparation; 3, sample treatment; 4, standard substance treatment; 5, experiment implementation; and 6, data processing.
Owner:JIANGSU WISE SCI & TECH DEV
Method of preparing ceftiofur
InactiveCN101108855ASolve discolorationHigh antibacterial activityOrganic chemistryThio-Sodium hydrosulfide
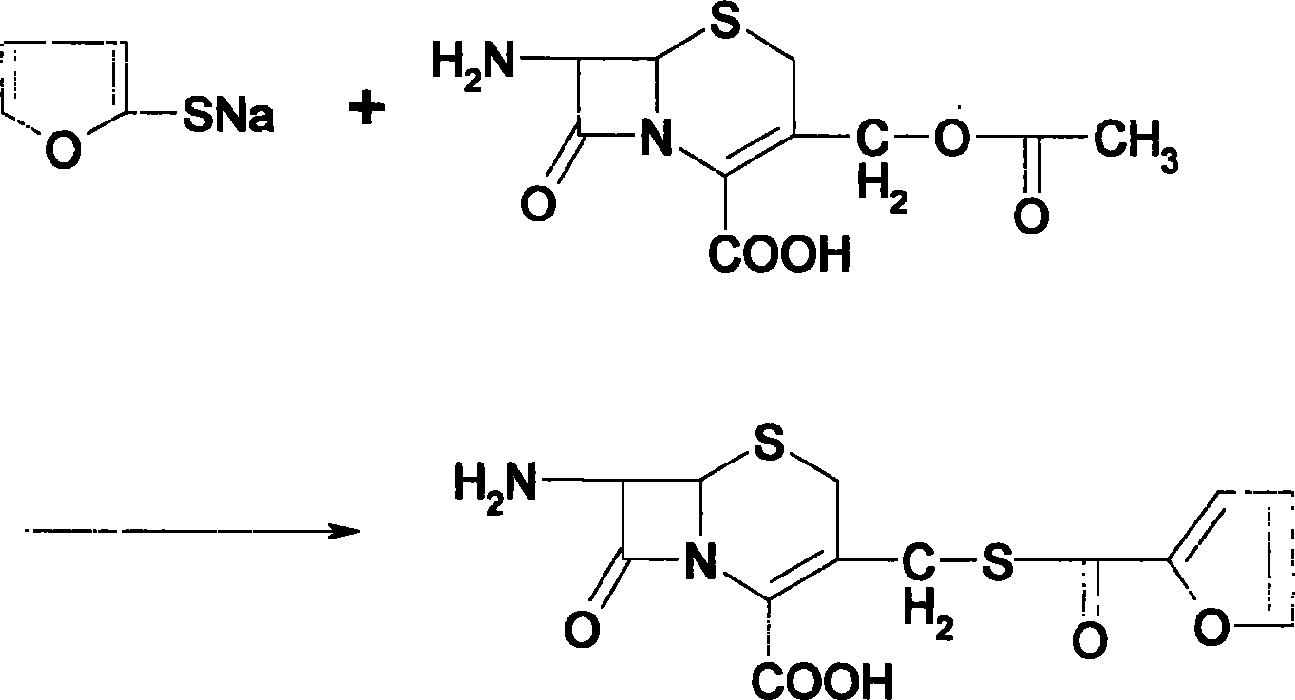
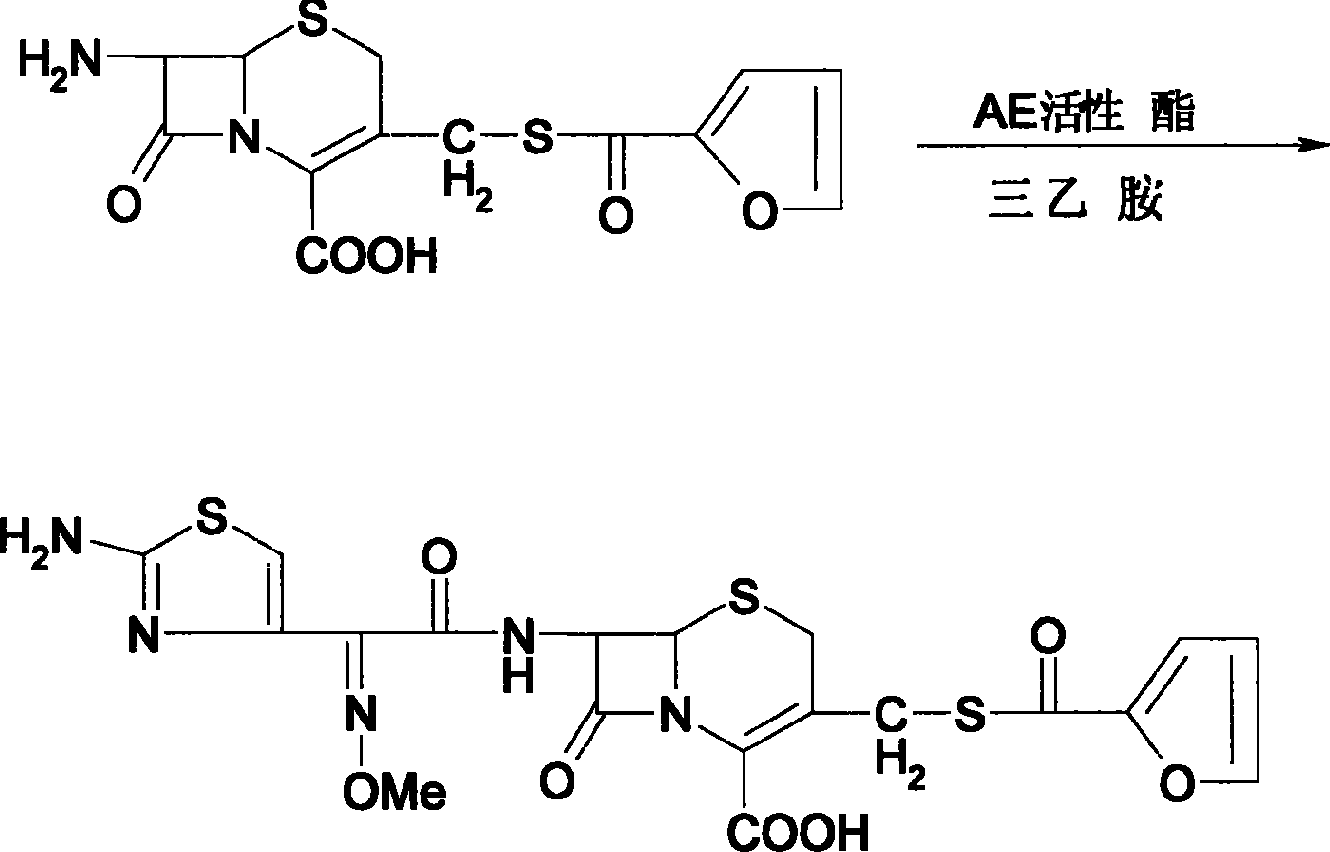
A preparation method of the ceftiofur is provided. The method is that the sodium hydrosulfide and the furoyl chlorine are adopted as the raw materials and react under the alkali condition to gain the thio furancarboxlic acid. The organic solvents such as ethyl formate, ethyl acetate, acetone, chloroform and methylene chloride are adopted to extract and react in the aqueous solution when the pH value is between 8 to 11 and the temperature between 25 DEG C. to 75 DEG C., and then condensed with 7-aminocephalosporanic acid (7-ACA) and dissolved by the organic solvents such as ethyl formate, ethyl acetate, acetone, chloroform and methylene chloride after reacting with the AE active ester, and is added with the active carbon or diatomite to stir and decolor under normal temperature and is filtered through a titanium bar filter, and is added to the purified water by drops and is stirred, filtered. The filter cake is added with sodium carbonate to dissolve in right amount, and is frozen and dried to gain the ceftiofur.
Owner:PU LIKE BIO ENG
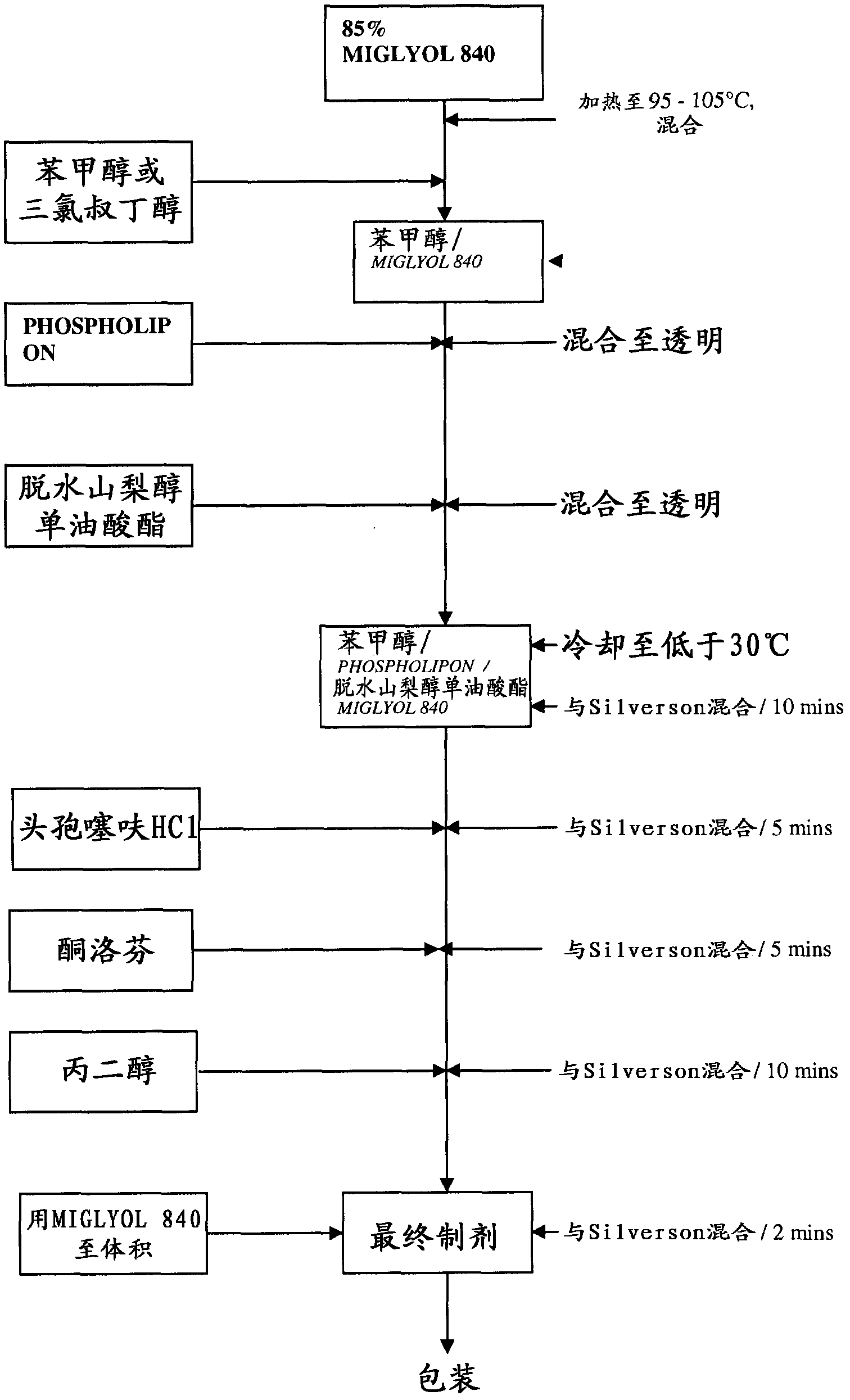
Formulations comprising ceftiofur and ketoprofen or ceftiofur and benzyl alcohol
ActiveCN102341125AAntibacterial agentsOrganic active ingredientsBovine respiratory diseaseKetoprofen
The present invention relates to veterinary or pharmaceutical formulations which may comprise ceftiofur, ketoprofen, benzyl alcohol, or effective combinations thereof. The formulations of the present invention may include a wetting or dispersing agent, a preservative, a flocculating agent or resuspendability enhancer, and / or a biocompatible oil vehicle. This invention also provides for, inter alia, formulations for the treating, controlling and preventing of respiratory disorders, particularly bovine respiratory disease (BRD), in warm-blooded animals, such as livestock,. This invention further provides for methods of increasing the resuspendability of an oily formulation which may comprise the addition.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Preparation method of ceftiofur microspheres
ActiveCN112353765AImprove stabilityReduce dispersionAntibacterial agentsOrganic active ingredientsMicrospherePolythylene glycol
The invention discloses a preparation method of ceftiofur microspheres, and relates to the field of veterinary drugs, and the preparation method comprises the following steps: 1, dissolving a polylactic acid-polyethylene glycol segmented copolymer as a carrier material in an organic solvent; 2, adding ceftiofur, and stirring and mixing to prepare a dispersion phase; 3, adding the dispersion phaseinto water, adding an emulsifier, and magnetically stirring and emulsifying to obtain an emulsion; 4, rapidly stirring the emulsion for a period of time T1, supplementing water, and continuously stirring for a period of time T2 until the organic solvent is completely volatilized; and 5, centrifuging, washing, collecting and drying in vacuum to obtain the ceftiofur microsphere. According to the ceftiofur sustained-release microsphere disclosed by the invention, a drug is more uniformly dispersed in a carrier material, the drug loading capacity is high, a dispersion system of drug particles is reduced by virtue of a nanoscale microsphere technology, and the ceftiofur sustained-release microsphere has better needle permeability, so that the drug has a stronger sustained-release effect and a longer sustained drug effect.
Owner:山东华辰制药有限公司
Ceftiofur lung targeting microsphere used for beasts and birds and preparation method thereof
ActiveCN101773477AHigh drug loadingHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsPulmonary infectionSide effect
The invention provides a ceftiofur lung targeting microsphere used for beasts and birds and a preparation method thereof, which can solve the problems of waste of a large amount of medicine, indistinctive curative effect, medicine toxic and side effect enhancement and high possibility of generating medicine resistance caused by few reached targeting parts in the prior art. The invention has the technical scheme that ceftiofur is used as raw materials, gelatin is used as carriers, and the weight ratio of the ceftiofur to the gelatin is 1 / 0.5 to 20. The invention also provides a preparation method of the lung targeting microsphere. Both the medicine carrying rate and the encapsulation rate of the microsphere are high, the encapsulation rate can reach more than 60 percent, and more than 94 percent of sorted microspheres have the grain diameter between 7 and 30 mum, so the requirement of the lung targeting microsphere on the grain diameter is met, the tissue selectivity of the medicine is improved, the effect of lung targeting is reached, the concentration of the medicine in other tissues is lowered, the toxic and side effect is reduced, and thus, pulmonary infection of animals can be treated at high efficiency.
Owner:QINGDAO VLAND BIOTECH INC +2
Aqueous suspension injection of ceftiofur, and preparation method thereof
ActiveCN103191057AReduce viscosityEasy extractionAntibacterial agentsOrganic active ingredientsOrganic solventSuspending Agents
The invention provides an aqueous suspension injection of ceftiofur. The aqueous suspension injection comprises 5-20% W / V of a ceftiofur micronized raw material, 8-15% W / V of a water-soluble excipient, 5-25% W / V of a suspending agent, 0.1-0.5% W / V of a suspending aid, and balance of injection water. Compared with traditional ceftiofur oily suspension liquids, no organic solvent is added into the ceftiofur aqueous suspension injection. The ceftiofur aqueous suspension injection is substantially a pure water solution. Therefore, problems of high viscosity and inconvenient application of current ceftiofur oily suspension liquids in market are solved.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Compound rifaximin uterus injectant as well as preparation method and application thereof
InactiveCN104586855AGood treatment effectSolve the problem of abandonment periodAntibacterial agentsOrganic active ingredientsALUMINUM STEARATESAntioxidant
The invention provides a compound rifaximin uterus injectant as well as a preparation method and application thereof. The compound rifaximin uterus injectant comprises the following components in percentage by weight: 0.8%-1.2% of rifaximin, 4.8%-5.2% of ceftiofur crystal free acid, 1.8%-2.5% of surface active agents, 0.2%-0.3% of antioxidants and the balance of oil phases, wherein the surface active agents are one or any mixture of two of glycerin monostearate, aluminum stearate and ethyl cellulose, the antioxidants are one or any mixture of two of BHA, VE oil, BHT and phenol, and the oil phases are one or any mixture of two of soybean oil, castor oil and cotton seed oil. The compound rifaximin uterus injectant provided by the invention is prepared through a mixing dissolution mode and achieves more outstanding treatment effect on bacterial endometritis.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
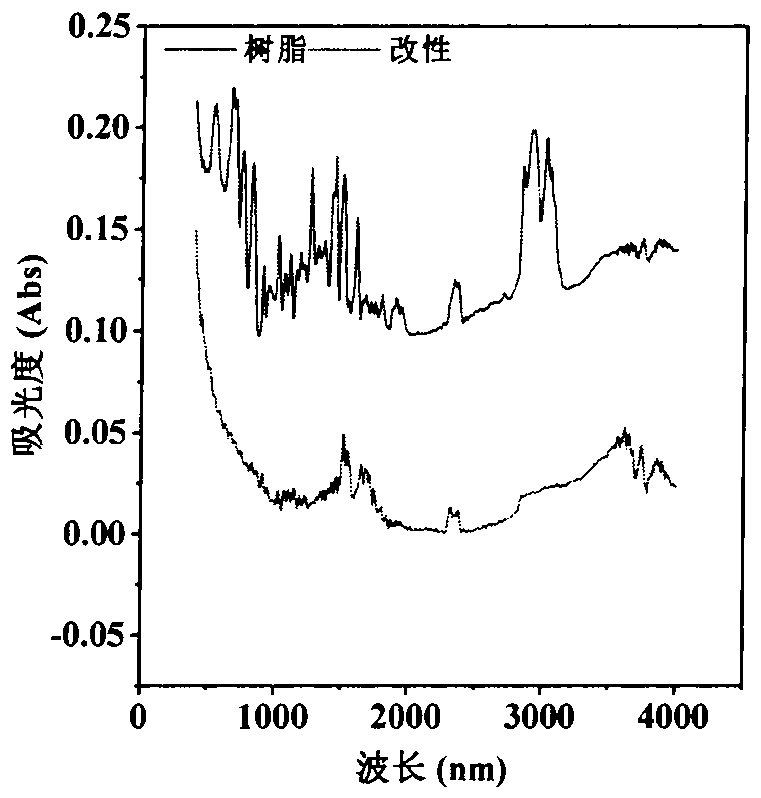
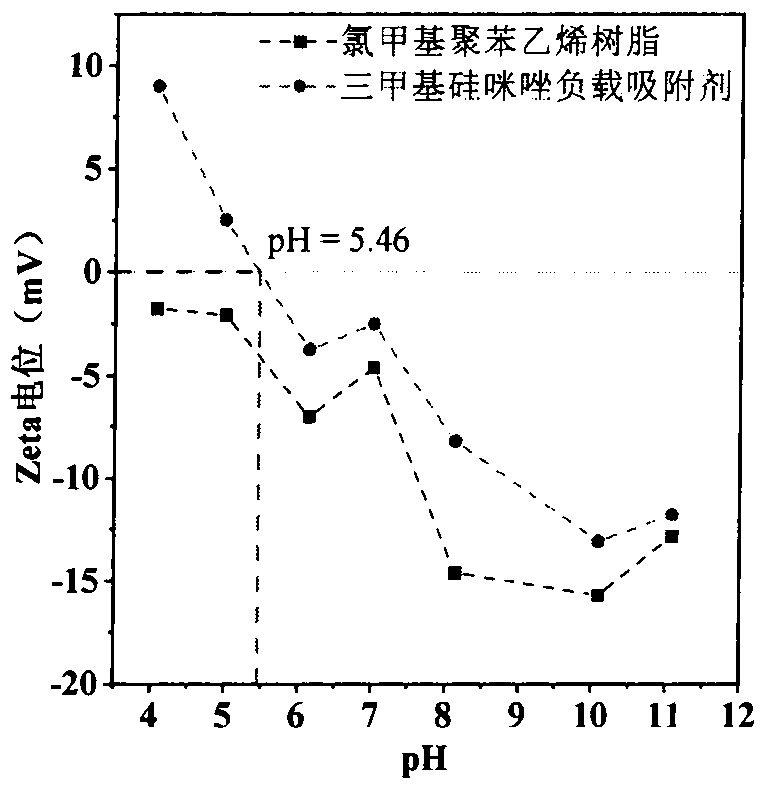
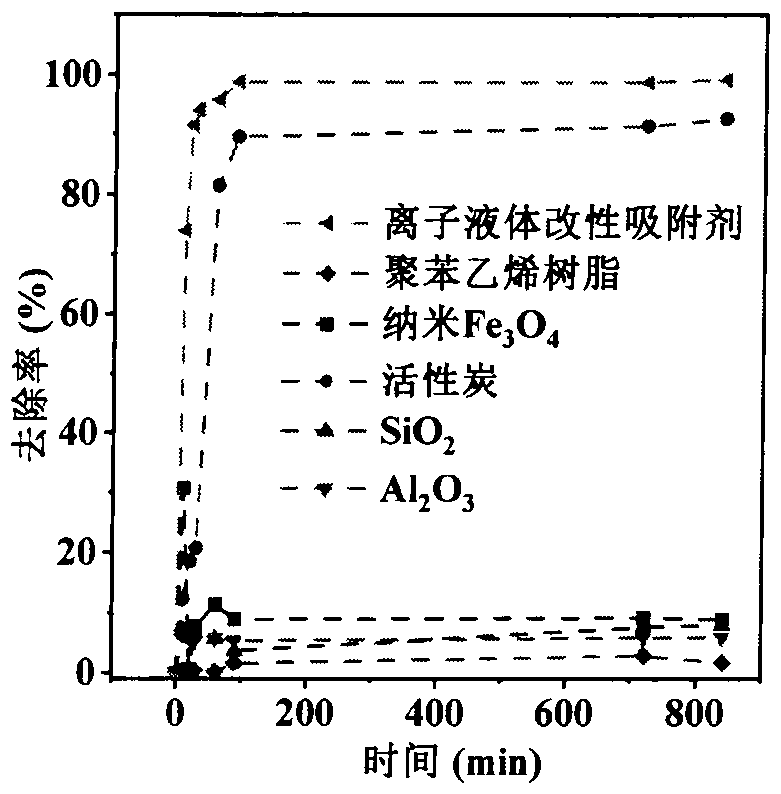
Preparation and application of trimethylsilyl imidazole adsorbentfor antibiotics
PendingCN113600151AImprove adsorption capacityFast reaction kineticsOther chemical processesWater contaminantsPolystyreneSulfanilamide
The invention discloses a preparation method of a 1-(trimethylsilyl) imidazole ionic liquid adsorbent for antibiotics in a water body and an applied synthesis method and application thereoft, and belongs to the technical field of water body organic micropollutant treatment. According to the invention, the 1-(trimethylsilyl) imidazole ionic liquid is grafted on the surface of the chloromethyl polystyrene resin through chemical bonding so that the product is prepared. The specific application effect of the synthesized 1-(trimethylsilyl) imidazole ionic liquid adsorbent in adsorption of four typical antibiotics including beta-lactam antibiotics (ceftiofur), sulfonamide antibiotics (sulfamethoxazole), fluoroquinolone antibiotics (ciprofloxacin) and tetracycline antibiotics (aureomycin) is released for the first time; the synthesis method is simple and convenient to operate and mild in reaction condition, and the product has the advantages of large adsorption quantity, fast reaction kinetics, high adsorption efficiency and the like on various antibiotics.
Owner:TIANJIN UNIV
Electrochemical receptor sensor for detecting beta-lactam antibiotics, and preparation method and application thereof
InactiveCN110243907AHigh sensitivityHigh precisionMaterial electrochemical variablesPenicillinAntibiotics beta lactam
Owner:HUAZHONG AGRI UNIV
Veterinary suspension containing ceftiofur and baicalein and preparing method thereof
ActiveCN105769874ALow incidence of drug resistanceDelay drug resistanceAntibacterial agentsOrganic active ingredientsEffective actionMaterial resources
The invention discloses a veterinary suspension containing ceftiofur and baicalein and a preparing method thereof.The veterinary suspension is formed in the mode that active constituents are dispersed into dispersing solvent, wherein the active constituents are ceftiofur and baicalein.Each 100 mL of suspension comprises 2.5-10 g of ceftiofur, 2.5-10 g of baicalein, and the balance dispersing solvent.According to the veterinary suspension containing ceftiofur and baicalein, oily solvent is adopted as the preparation dispersing solvent, in the oily suspension, the drug is released slowly, and for time-dependent antibiotics, T> MIC is prolonged, that is, the effective acting time is prolonged, feeding is conducted every 24 hours, and manpower and material resources are saved.The veterinary suspension containing ceftiofur and baicalein is obtained in the mode that ceftiofur and baicalein are dispersed into the dispersing solvent through a dispersing method, a suspending agent, a wetting agent and antioxygen are added, and a colloid mill is used for conducting grinding; the production technology is simple, cost is reduced, and industrialization is easily achieved.
Owner:CHINA AGRI UNIV
Ceftiofur hydroxypropyl-beta-cyclodextrin inclusion compound and preparation method thereof
ActiveCN103751196AFree from oxidationFree from hydrolysisAntibacterial agentsOrganic active ingredientsSide effectOxidation resistant
The invention discloses a preparation method of a solid inclusion compound of ceftiofur and hydroxypropyl-beta-cyclodextrin (CD). The preparation method comprises the following steps of taking ceftiofur and hydroxypropyl-beta-cyclodextrin in a molar ratio of 1: (1 to 3) as reaction raw materials; adding water which is 5 times the total weight of the reaction raw materials under a temperature of 20 to 50 DEG C; grinding for 2 to 5 hours; drying for 12 hours under vacuum; and filtering to obtain white powder which is the inclusion compound. The prepared inclusion compound has the effects of effectively preventing a chemical compound from oxidation reaction or hydrolysis reaction and other reactions, improving the water solubility of a medicine, enhancing the stability, reducing the toxic and side effects, controlling the medicine release, and improving the bioavailability. The prepared hydroxypropyl-beta-CD inclusion compound improves the solubility and bioavailability of ceftiofur serving as a raw medicine. The prepared inclusion compound shows remarkable capacity of improving oxidation resistance of the raw medicines under light, high temperature, humidity and other conditions, and brings effective means for developing a novel form of ceftiofur.
Owner:河南华牧生物科技有限公司
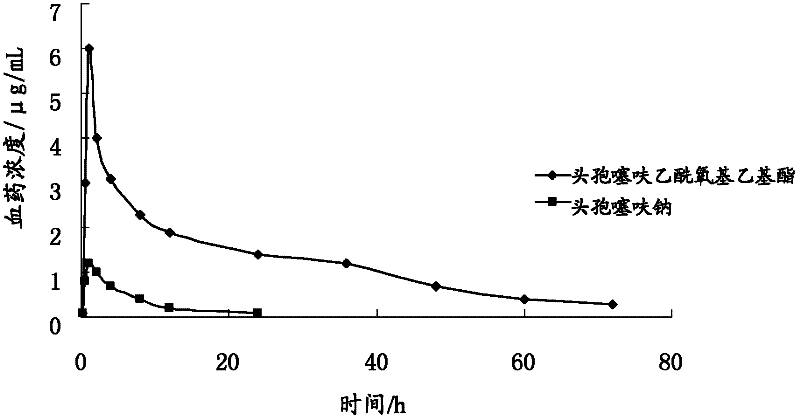
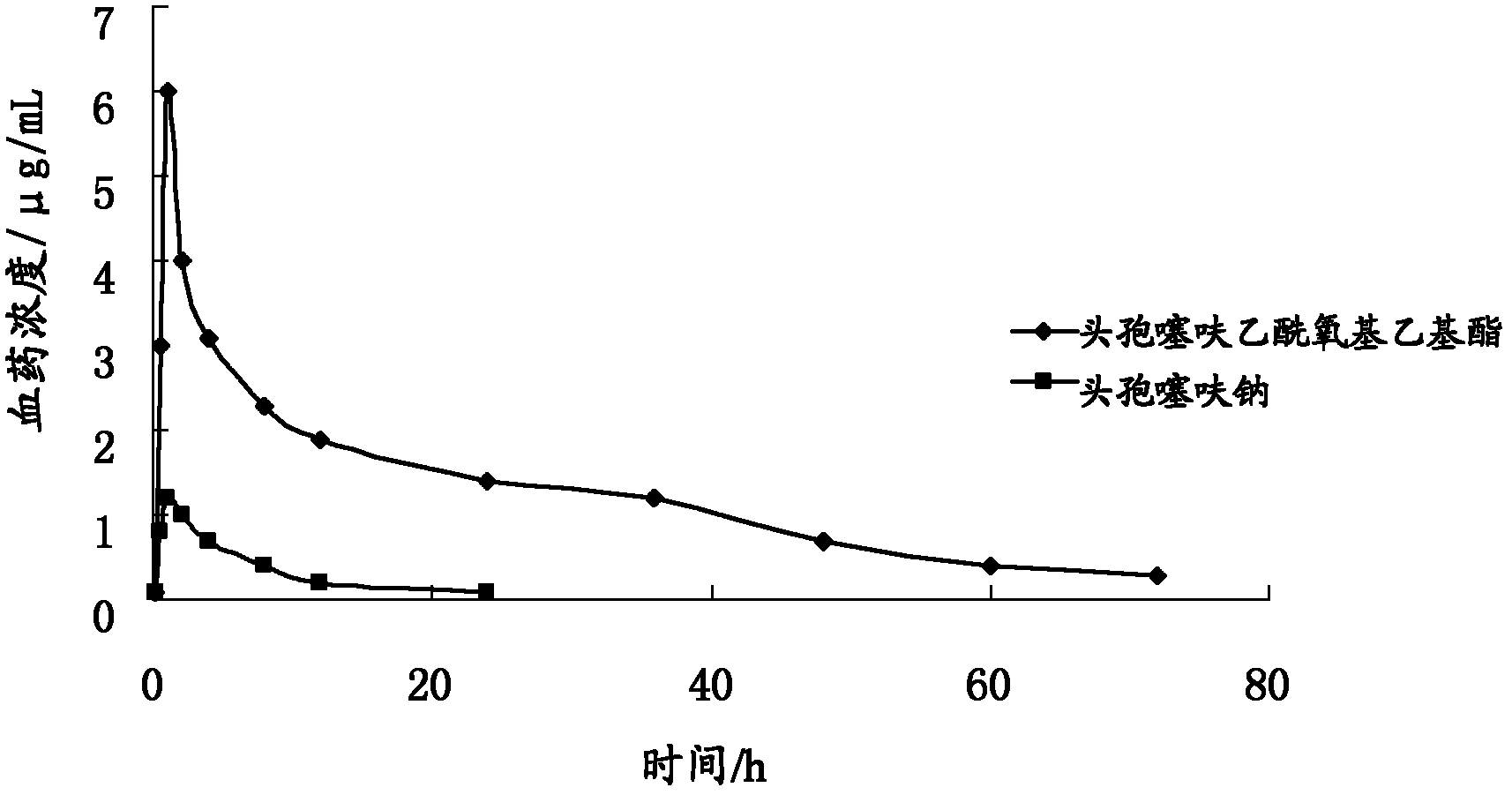
Ceftiofur acetoxyethyl ester and preparation method thereof
ActiveCN102268020APromote absorptionImprove bioavailabilityAntibacterial agentsOrganic active ingredientsEscherichia coliStaphylococcus aureus
The invention provides a ceftiofur acetoxy ethyl ester compound and a preparation method thereof. The ceftiofur acetoxy ethyl ether compound is prepared by a step of adding esterification reagent such as 1-bromoethyl acetate into organic solvent of ceftiofur or sodium salt of ceftiofur to obtain the ceftiofur acetoxy ethyl ester. The ceftiofur acetoxy ethyl ester compound can be better absorbed in gastrointestinal tract of animals than ceftiofur or sodium salt of ceftiofur, has a characteristic of high bioavailability, and can be used for treating or preventing infection of digestive tract, respiratory tract and urogenital tract of pigs, poultry, cattle, sheep, dogs and other animal with drug-resistant staphylococcus aureus as well as gram negative bacteria including Escherichia coli, salmonella, typhoid bacillus, dysentery bacillus, pasteurella and the like.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Specific anti-ceftiofur monoclonal antibody hybridoma cell strain 2E5 and application thereof
ActiveCN105505886AHigh detection sensitivityHigh affinityTissue cultureImmunoglobulinsAntibiotic YSpecific antibody
The invention discloses a specific anti-ceftiofur monoclonal antibody hybridoma cell strain 2E5 and the application thereof and belongs to the technical field of food safety immunodetection. The monoclonal cell strain 2E5 is preserved in the China General Microbiological Culture Collection Center, CGMCC for short, with the preservation number of CGMCC No.10873. The crude drug ceftiofur and BSA are coupled to prepare immunogen by means of the active ester method, and ceftiofur and OVA are coupled to prepare coating antigen. The specific anti-ceftiofur hybridoma cell strain is obtained after a mouse is immunized. The monoclonal antibody secreted by the cell strain only aims at ceftiofur specifically, the crossing-over rates between the monoclonal antibody and cefalexin and between the monoclonal antibody and cefquinome are 3.12% and 5.3% respectively, the crossing-over rates between the monoclonal antibody and other cephalosporin antibiotics are all smaller than 0.1%, and therefore specificity is high. The anti-ceftiofur monoclonal antibody hybridoma cell strain has high detection sensitivity and compatibility, the crossing-over rates with other cephalosporin antibiotics are low, and therefore specific detection of ceftiofur can be achieved. The invention also provides a new idea for preparing a specific antibody aiming at a specific cephalosporin so as to obtain a good specific monoclonal cell strain.
Owner:无锡迪腾敏生物科技有限公司
Heat-resistant insecticide composite material with high slow-release property
InactiveCN108208009AWell mixedGood sustained release effectBiocideAnimal repellantsMonopotassium phosphateBoron nitride
The invention discloses a heat-resistant insecticide composite material with a high slow-release property. The heat-resistant insecticide composite material with the high slow-release property is prepared from the raw materials: alkyl sodium sulfonate, 3-hydroxymethyl tetrahydrofuran, ceftiofur acid, a modified slow-release agent, a modified heat-resistant additive, sea-foam stone powder, calciumsulfate crystal whiskers, boron nitride nanopowder, nano aluminum hydroxide, polyadipic acid, hexadecyl trimethylamine, sodium polyphosphate, Na-montmorillonite, polyvinylpyrrolidone, n-butyl acetate,monopotassium phosphate, sodium tetraborate and a silane coupling agent KH-560. The heat-resistant insecticide composite material with the high slow-release property provided by the invention has excellent slow-release performance and heat resistance, is not easy to degrade through heating after being fed into soil, and is capable of efficiently releasing the insecticide raw materials so as to have a good insecticide effect, and can also effectively avoid nutrient depletion during an insecticide process.
Owner:定远县润泰化工有限责任公司
Ceftiofur acetoxy ethyl ester and preparation method thereof
ActiveCN102268020BAntibacterial agentsOrganic active ingredientsEscherichia coliStaphylococcus aureus
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
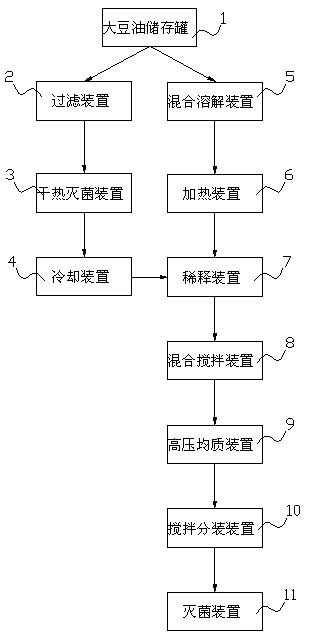
Tilmicosin and ceftiofur injection and preparing equipment and process
InactiveCN104958313ABroad spectrum antibacterialStrong therapeutic effectAntibacterial agentsOrganic active ingredientsSOYBEAN SEED OILTilmicosin
The invention relates to the field of veterinary medicine, in particular to a tilmicosin and ceftiofur injection and preparing equipment and process. The tilmicosin and ceftiofur injection contains tilmicosin, ceftiofur, aluminum stearate, vitamin C and soybean oil; the preparing equipment comprises a soybean oil storing tank, a filtering device, a mixing and dissolving device, a dry heat sterilizing device, a cooling device, a heating device, a diluting device, a mixing and stirring device, a high-pressure homogenizing device, a stirring and subpackaging device and sterilizing device; the preparing process includes the steps of preparation of sterilizing soybean oil, preparation of aluminum stearate factice of 10%, dilution, mixing and stirring, homogenizing and mixing, stirring and subpackaging and sterilizing. Through the specific preparing equipment and the excellent preparing process, tilmicosin and ceftiofur are prepared to be the oil suspension injection, so that ceftiofur and tilmicosin can be combined for administration to treat respiratory infectious diseases and systemic sepsis caused by mycoplasma secondary infection gram-positive bacteria and gram-negative bacteria infection.
Owner:侯强红
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com