Ceftiofur acetoxy ethyl ester and preparation method thereof
A technology for ceftiofur acetoxyethyl ester and cephalosporin, which is applied in the field of ceftiofur acetoxyethyl ester and its preparation, can solve the problem of low bioavailability, instability, poor absorption of ceftiofur and its sodium salt and other problems, to achieve the effect of high bioavailability, reduced side effects, and reduced usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 10.0g (0.018mol) of ceftiofur sodium (Zhejiang Haizheng batch number 20080501), add it into a 250mL three-necked bottle, add 100mL of N,N-dimethylacetamide, stir to dissolve, cool down to 0°C, 5.1 g (0.058 mol) of 1-bromoethyl acetate (Quanxi Chemical Factory, Huixian City) was put in, and the reactants were stirred and reacted at 1° C. for 90 minutes. Add 0.25g (0.0018mol) of potassium carbonate, and stir at 1-3°C for 2h. Add the reactant to a mixture composed of 200mL ethyl acetate and 50mL 3% W / V sodium bicarbonate, stir for 1h, let stand to separate layers, take the organic phase, and wash with 50mL 1mol / L hydrochloric acid and 15mL 20% sodium chloride solution respectively (containing 2% w / v sodium bicarbonate) wash. The three aqueous phases were all washed with 50 mL of ethyl acetate, the organic phases were combined, and 1 g of activated carbon was added and stirred for 30 min. The filtrate was rotary evaporated at 40°C to 30mL, and 100mL of isopropyl eth...
Embodiment 2
[0046] Weigh 10.0g (0.018mol) ceftiofur (Luoyang Huizhong Veterinary Medicine Co., Ltd., batch number 20090101), add it to a 250mL three-necked bottle, add 100mL tetrahydrofuran and stir to dissolve, it is in a solution state, cool down to 0°C, and weigh 5.1g (0.058mol) 1-bromoethyl acetate, and the reactant was stirred at 1°C for 90min. Add 0.25g (0.0018mol) of potassium carbonate, and stir at 1-3°C for 2h. Quickly add the reactant to the mixed solution consisting of 200mL ethyl acetate and 50mL 3% sodium bicarbonate, stir for 1h, let stand to separate layers, take the organic phase, and use 50mL1mol / L hydrochloric acid and 15mL20%W / V sodium chloride solution respectively (containing 2% w / v sodium bicarbonate) wash. The three aqueous phases were all washed with 50 mL of ethyl acetate, the organic phases were combined, and 1 g of activated carbon was added and stirred for 30 min. The filtrate was rotary evaporated at 40°C to 30mL, added 50mL of isopropanol, evaporated at 40°...
Embodiment 3
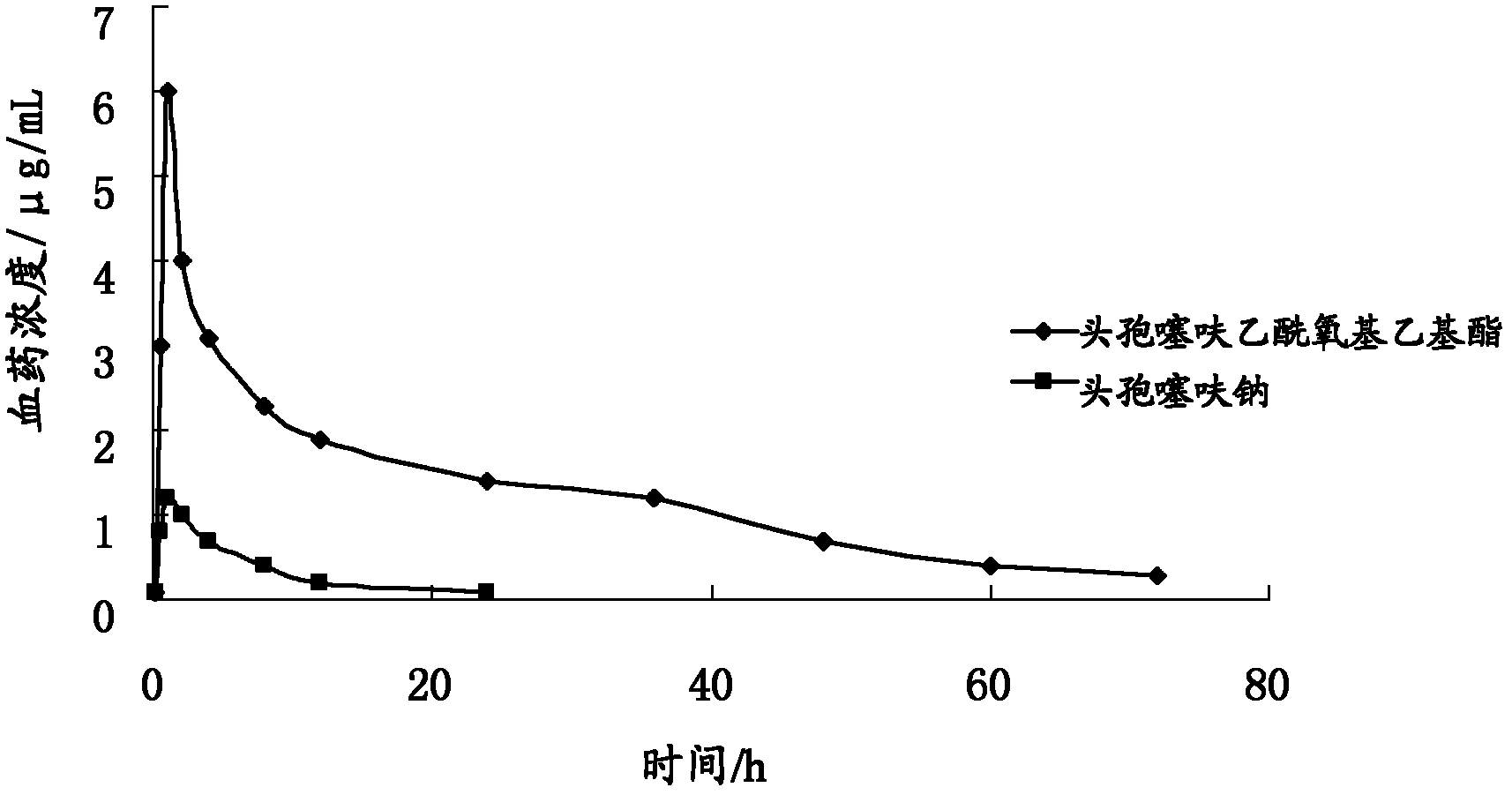
[0051] Ceftiofur sodium and ceftiofur acetoxyethyl ester were respectively added with appropriate amount of Baifumei powder (Henan Zhongyuan Biotechnology Co., Ltd.) to make a 10% premix (the content was calculated as the active ingredient), and the SPF experimental animal room was administered orally. Drug two groups of Du Dalong pigs, 20mg / kg (calculated as ceftiofur), 10 pigs in each group, 5mL of blood was collected at 15, 30min, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72h , Determination of blood concentration. The blood sample was added with dithioerythritol, derivatized with iodoacetamide, extracted and purified by solid-phase extraction, dried with nitrogen, added an appropriate amount of mobile phase, and separated on a C18 chromatographic column, using acetonitrile-trifluoroacetic acid-water (volume ratio acetonitrile: three Fluoroacetic acid: water is 300: 1: 700) as mobile phase, 266nm ultraviolet detects, fits and calculates pharmacokinetic parameter with pharmacokinetic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com