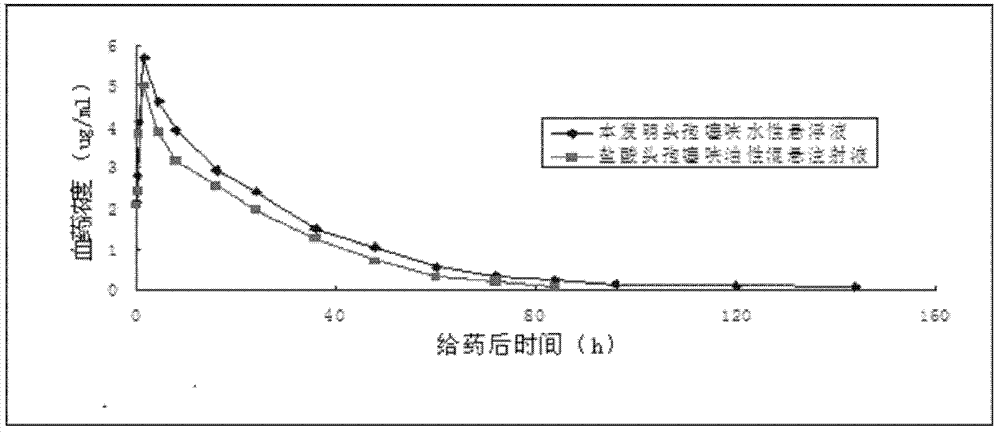
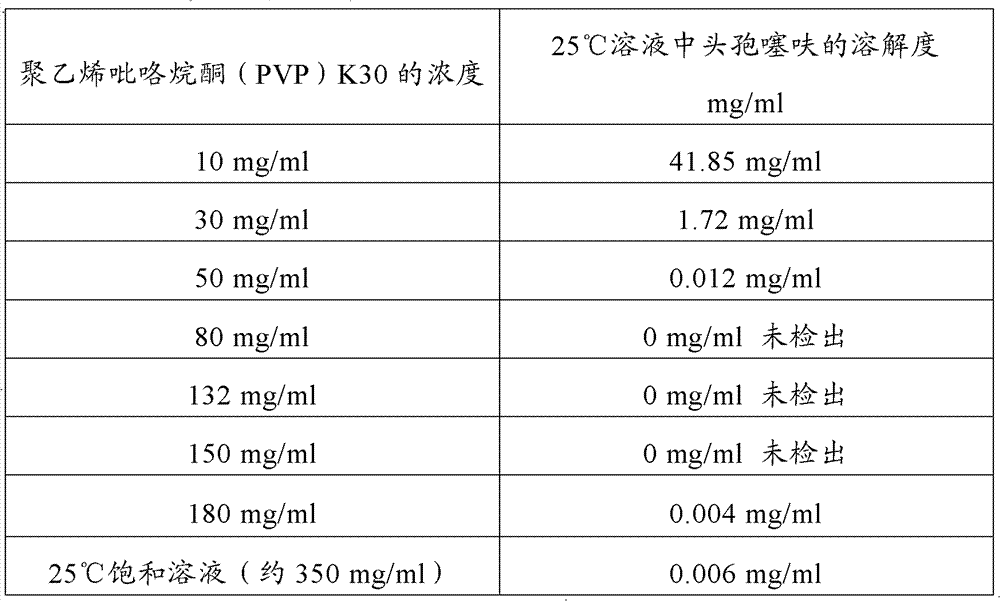
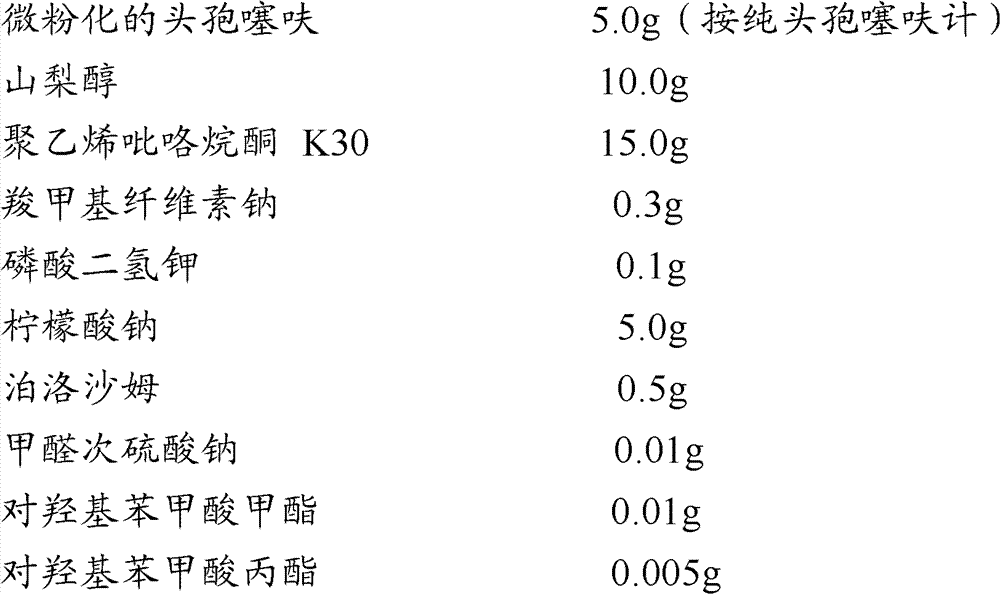
Aqueous suspension injection of ceftiofur, and preparation method thereof
A water-based suspension, ceftiofur technology, applied in medical preparations containing active ingredients, liquid delivery, pharmaceutical formulations, etc., can solve the problems of difficult extraction and injection, high product cost, inconvenient use, etc., and achieve drug release Slow, extended shelf life, less painful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The selection of the water-soluble excipient in the ceftiofur aqueous suspension of the present invention of embodiment 1
[0053] 1. Solubility of ceftiofur in ceftiofur aqueous suspension without excipients
[0054] Put ceftiofur (produced by Luoyang Huizhong Veterinary Medicine Co., Ltd., batch number: 20100801) in 100ml water for injection at 25±2°C, leave it at room temperature for 2 hours, and shake vigorously for 30 seconds every 5 minutes. Leave it for 2 hours and observe. Ceftiofur sinks to the bottom of the water for injection and cannot be suspended. The solution was filtered, and the filtrate was taken, and the content of ceftiofur in the solution was determined by HPLC according to the method under the content determination item of ceftiofur in "The Veterinary Pharmacopoeia of the People's Republic of China". Through determination, the content of ceftiofur in water for injection is 98.2mg / ml.
[0055] Therefore, the solubility of ceftiofur in aqueous sus...
Embodiment 2
[0075] The preparation of the aqueous suspension of embodiment 2 ceftiofur
[0076] The raw material used in the examples of the present invention, ceftiofur, is produced by Luoyang Huizhong Veterinary Medicine Co., Ltd., batch number: 20100801. Ceftiofur hydrochloride, produced by Binzhou Xinda Chemical Co., Ltd., batch number: 11CF1015H. Ceftiofur crystalline free acid is prepared according to the method of Patent Example 2 whose publication number is CN1119016A. Ceftiofur sodium, produced by Zhejiang Hisun Pharmaceutical Co., Ltd., batch number: 100702.
[0077] The QYF-2600 jet mill produced by Kunshan Miyou Machinery Manufacturing Co., Ltd. was used for ultrafine pulverization. Set the pressure of the air compressor to 0.4Mpa, the speed of the classifying wheel to 7200 rpm, the crushing pressure of the main air valve to 0.8MPa, and the pressure to the blowback valve to 0.4-0.6MPa. The crushing amount is 5-10g / sec. After crushing, micronized ceftiofur with suitable par...
preparation example 1
[0078] Preparation Example 1: 5% w / v Ceftiofur Aqueous Suspension Injection (100ml)
[0079]
[0080]
[0081] Ceftiofur particle size inspection: check 400 micropowder particles under a microscope, of which 93.5% have a diameter in the range of 2-20 μm, 5.5% have a particle size in the range of 20-30 μm, and 30-40 μm The average particle size of micronized ceftiofur was 12.2 μm.
[0082]Preparation method: Weigh 10.0 g of sorbitol, 15.0 g of polyvinylpyrrolidone K30, 5.0 g of sodium citrate, 0.1 g of potassium dihydrogen phosphate, 0.5 g of poloxamer, 0.01 g of sodium formaldehyde sulfoxylate, and methyl p-hydroxybenzoate 0.01g, 0.005g of methyl p-hydroxybenzoate, add to 50ml of water, stir to dissolve completely.
[0083] Weigh 0.3 g of sodium carboxymethyl cellulose, add it to 30 ml of water, slowly heat to about 60° C., stir while heating until the sodium carboxymethyl cellulose is completely dissolved, and prepare 30 ml of a 1.0% sodium carboxymethyl cellulose solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com