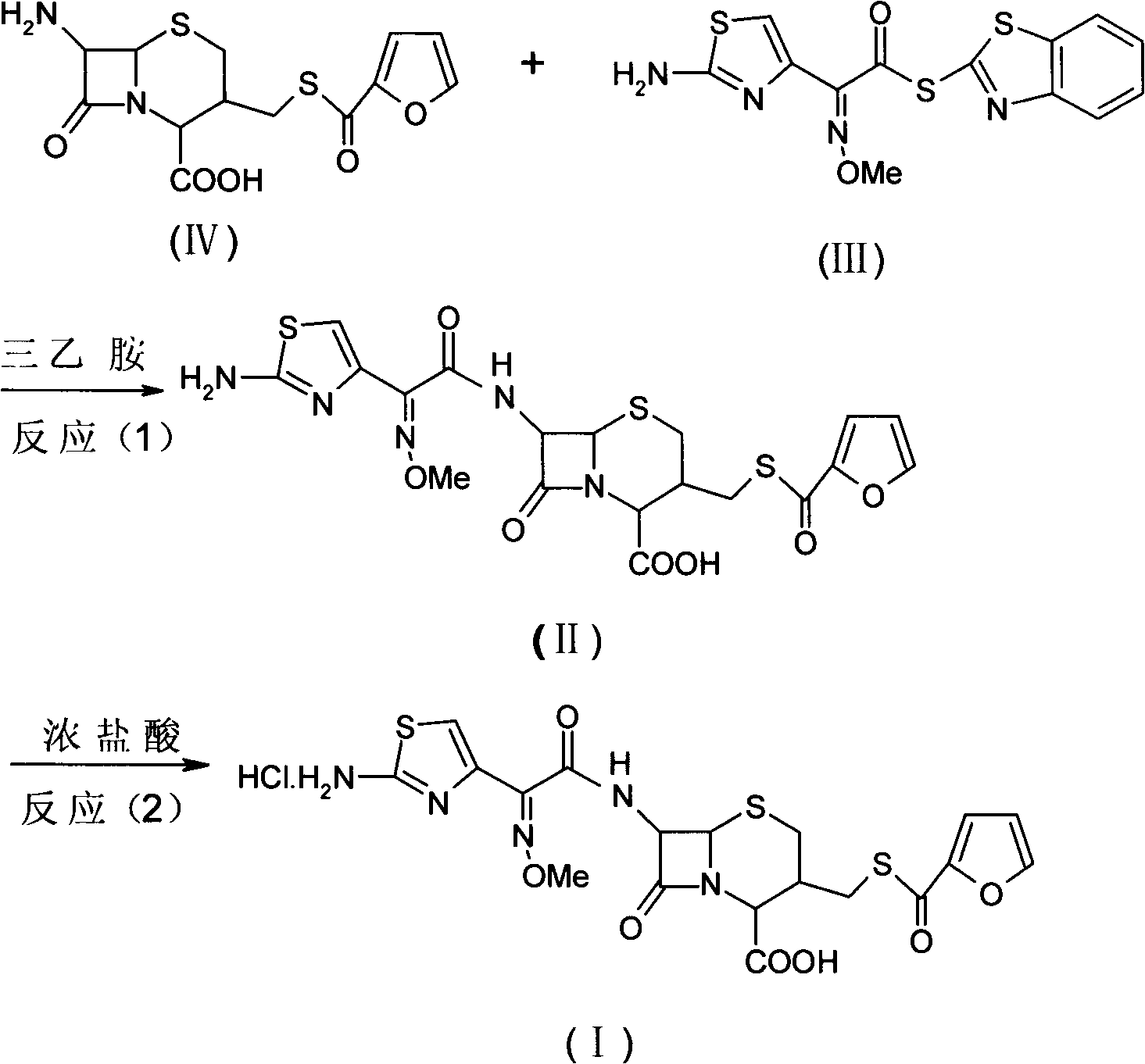
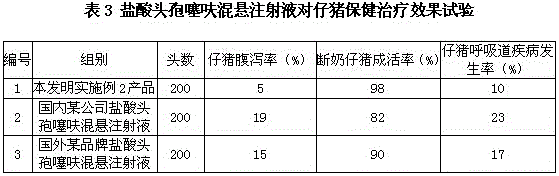
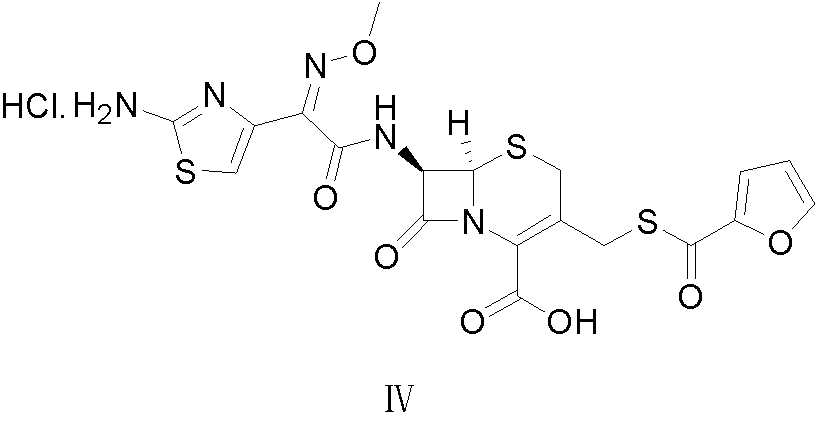
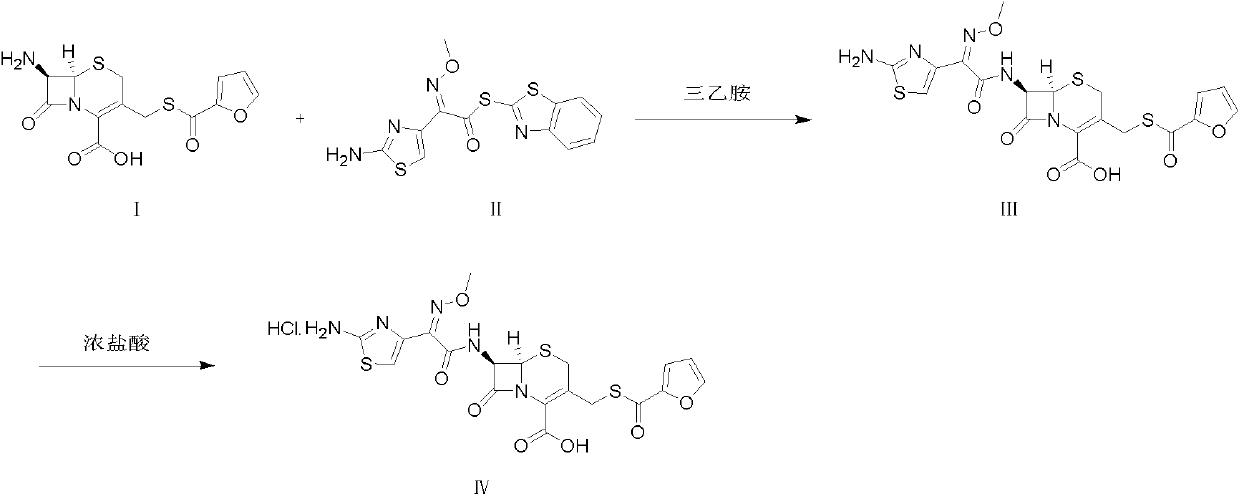
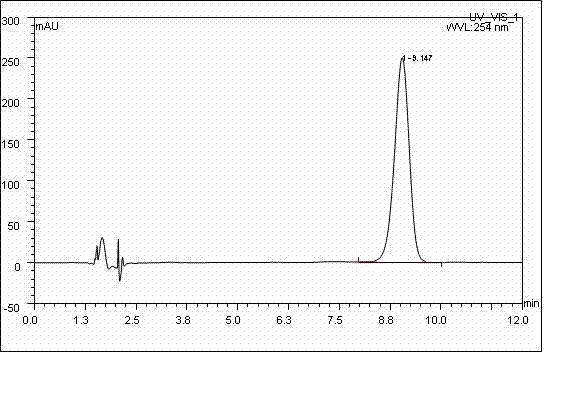
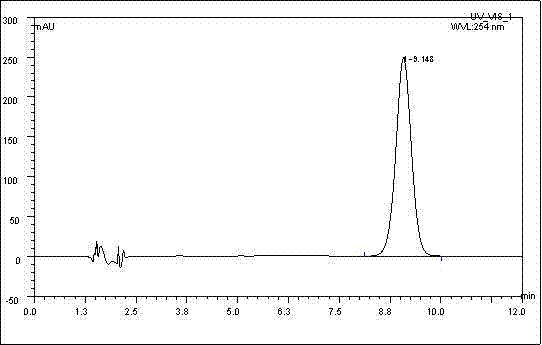
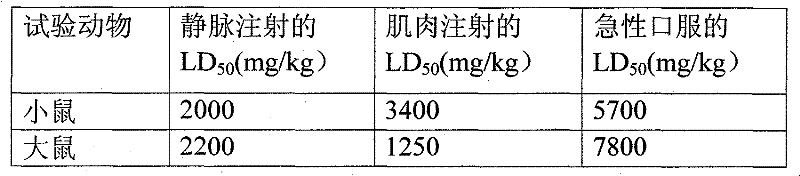
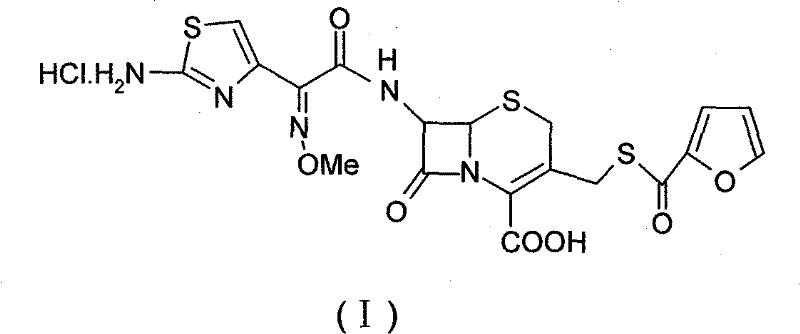
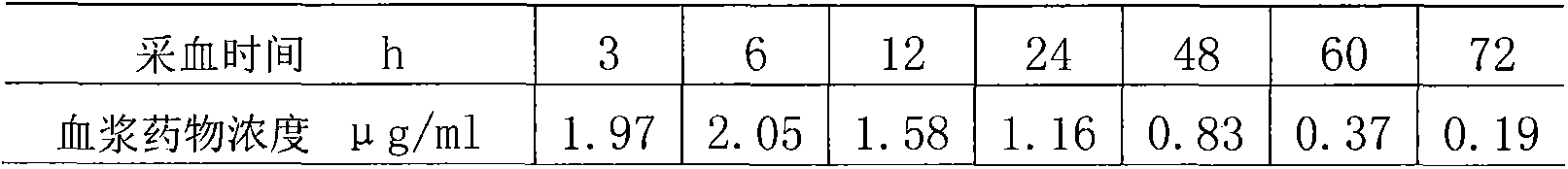Patents
Literature
59 results about "Ceftiofur Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
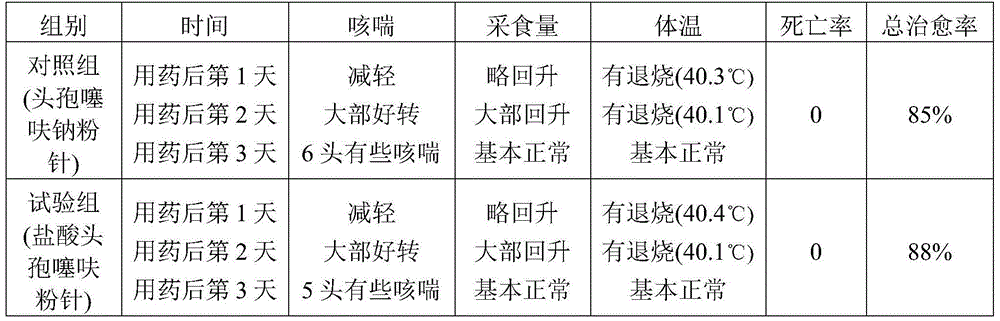
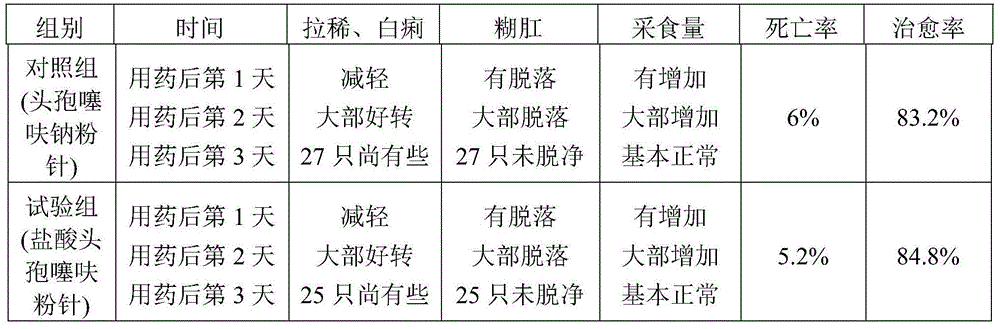
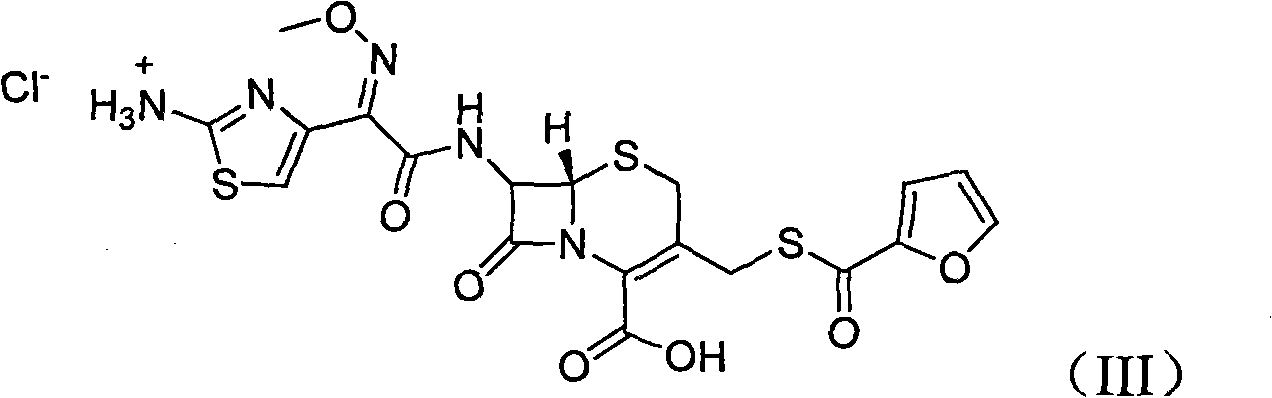
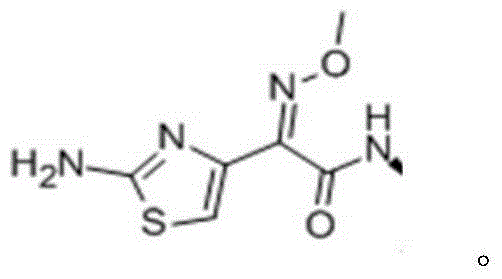
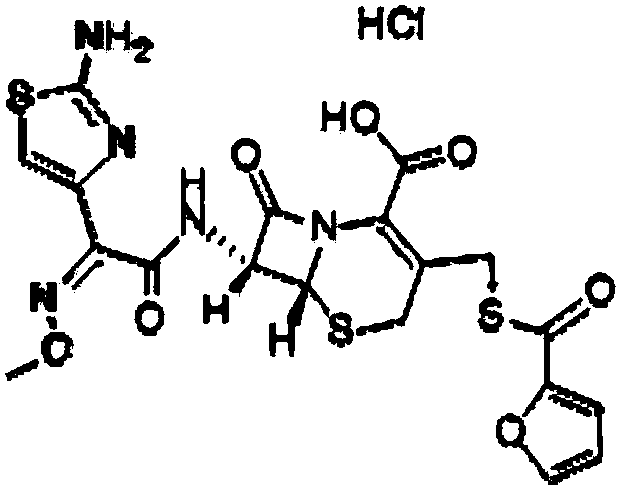
The hydrochloride salt form of ceftiofur, a semisynthetic, beta-lactamase-stable, broad-spectrum, third-generation cephalosporin with antibacterial activity. Ceftiofur binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis.
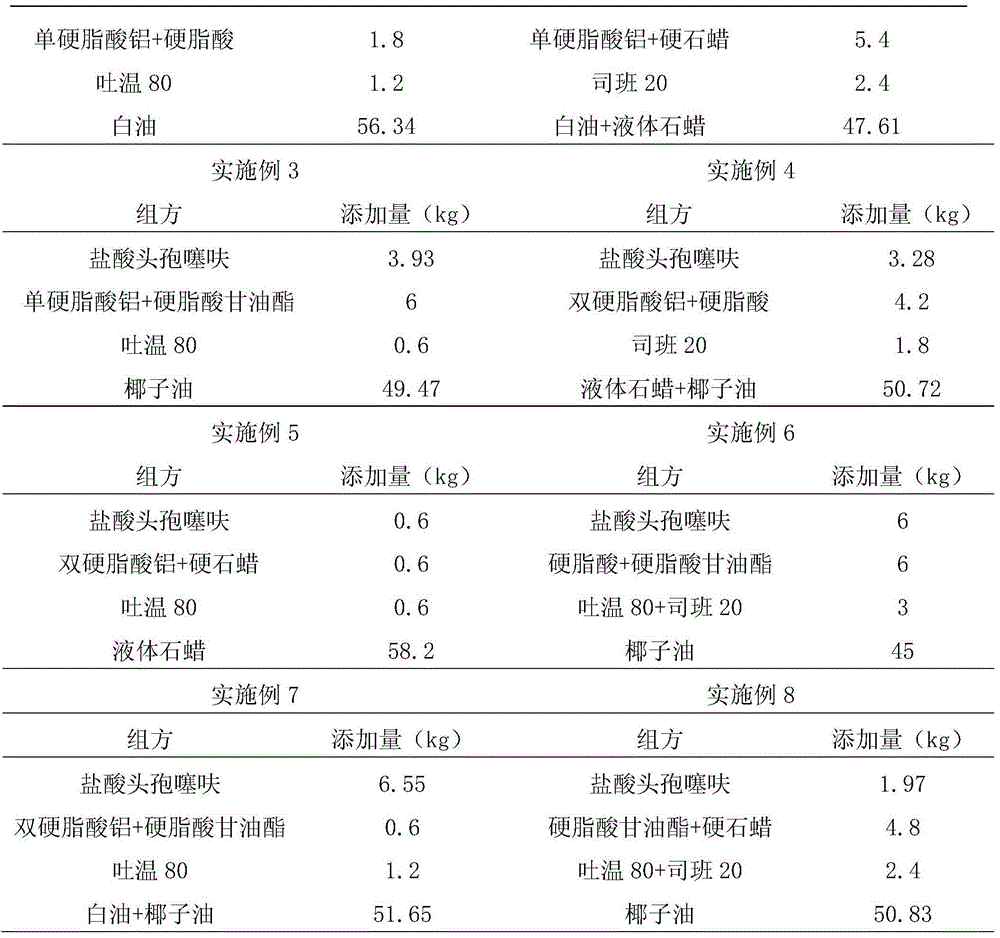
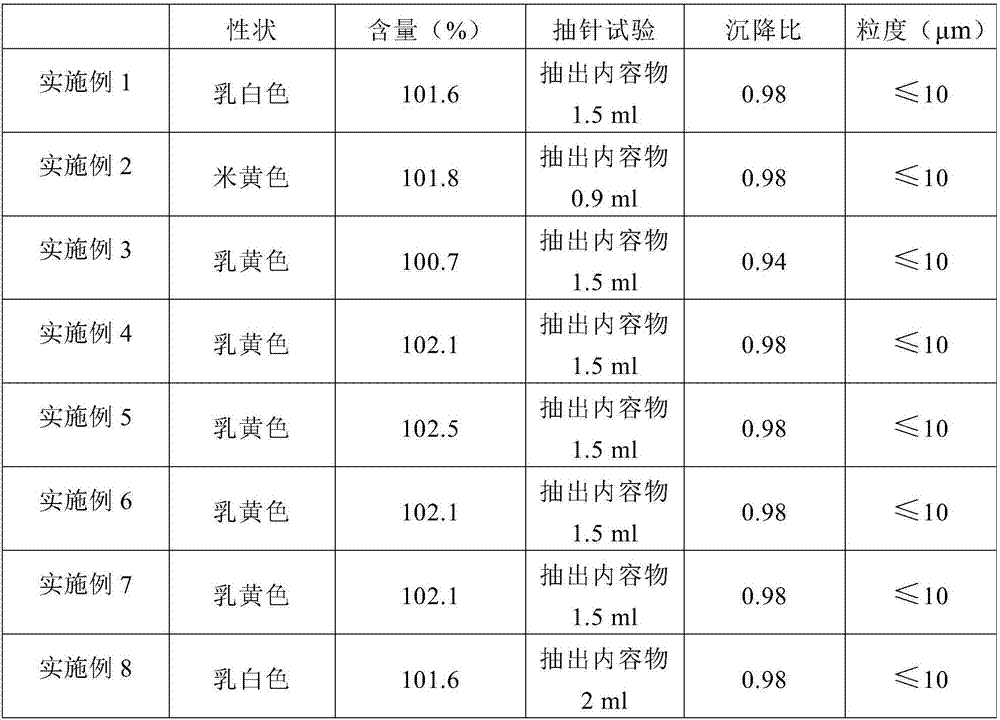
Technique for preparing compound ceftiofur oil suspension injection
InactiveCN101406447AAntibacterial agentsPharmaceutical delivery mechanismGraduated cylinderALUMINUM STEARATES
The invention discloses a preparation process for compound recipe ceftiofur oil suspensoid injection. The preparation process comprises the following steps: (1) taking neutral soybean oil, filtrating, heating and sterilizing and cooling down the soybean oil, adding aluminum stearate into the soybean oil to prepare gel, diluting the gel into factice of 2+-0.2 percent through the filtrated and sterilized soybean oil, and filtrating the factice through a No.3 sintered glass funnel for standby; and (2) taking hydrochloric acid ceftiofur / sulbactam according to a recipe, mixing the hydrochloric acid ceftiofur / sulbactam with the factice, grinding the mixture, adding lecithin into the mixture till the mixture is ground evenly, and then obtaining the compound recipe ceftiofur oil suspensoid injection through shifting the ground hydrochloric acid ceftiofur / sulbactam into a graduated cylinder and so on. As the invention adopts the preparation method, compared with a single recipe ceftiofur preparation, the compound recipe ceftiofur oil suspensoid injection has the advantage that the compound recipe ceftiofur oil suspensoid injection has antibacterial activity against bacteria capable of resisting the single recipe ceftiofur, that is, the compound recipe ceftiofur oil suspensoid injection can effectively treat bacterial infection generating ESBLs.
Owner:HENAN AGRICULTURAL UNIVERSITY
Pulmonary targeting microsphere of veterinary ceftiofur hydrochloride and preparation method thereof
ActiveCN101773478AEffective treatmentIncrease concentrationAntibacterial agentsOrganic active ingredientsSide effectTreatment effect
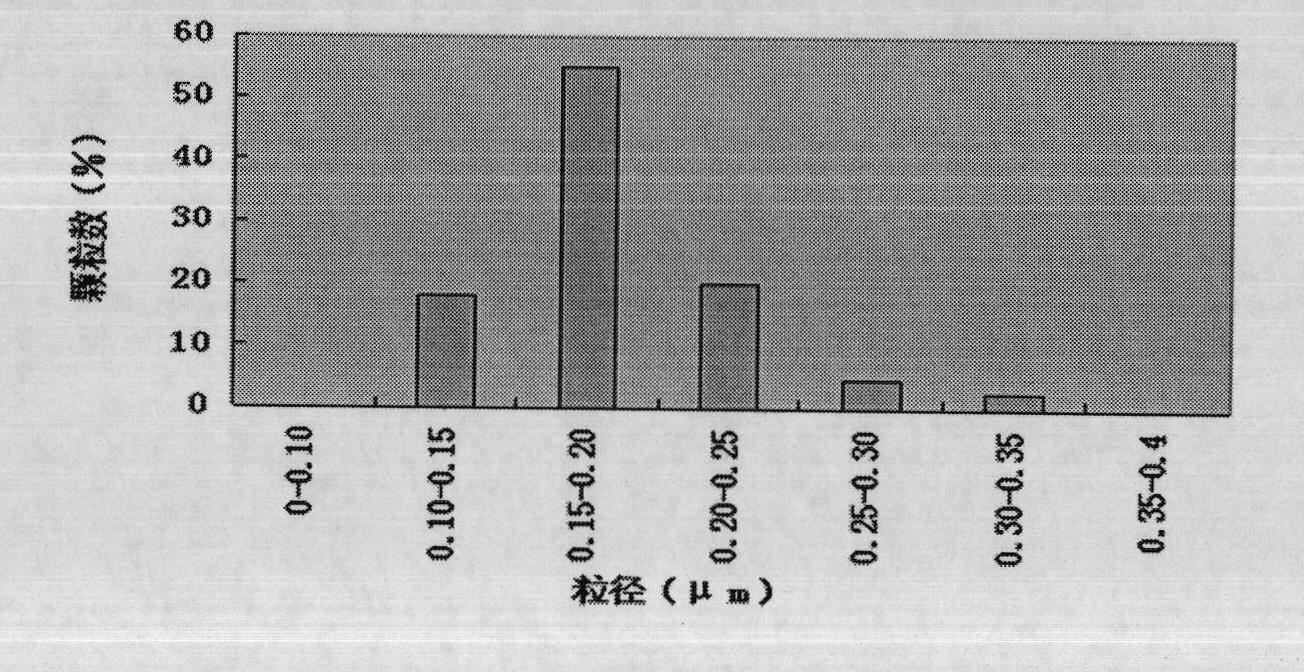
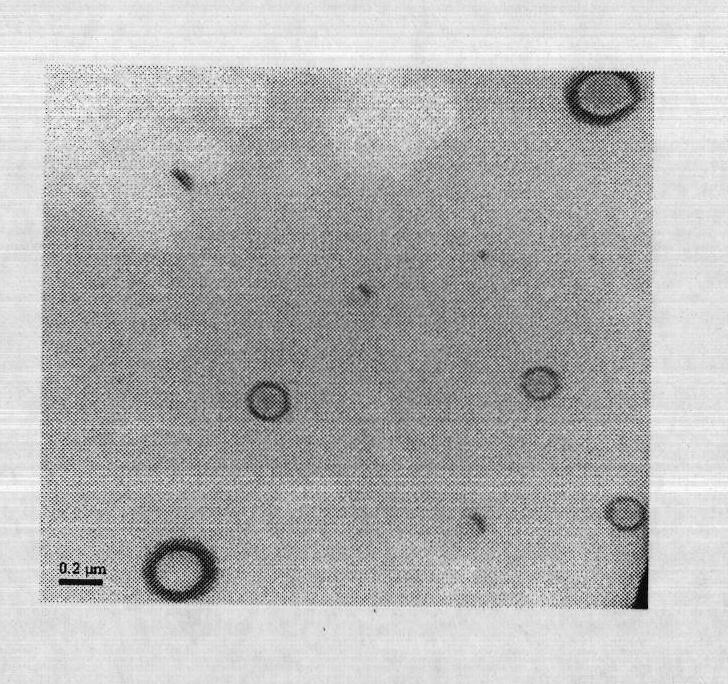
The invention provides a pulmonary targeting microsphere of veterinary ceftiofur hydrochloride and a preparation method thereof, which solves the problems of the prior art that the quantity of microspheres reaching the target site is small, thereby causing a large amount of drug waste, the therapeutic effect is no significant, the side effects of drugs are increased, and the possibility for generating drug resistance is high. In the technical scheme of the pulmonary targeting microsphere of veterinary ceftiofur hydrochloride, ceftiofur hydrochloride is used as a main component, poly-lactic-co-glycolic acid is used as a carrier material, and the mass ratio of the ceftiofur hydrochloride and the poly-lactic-co-glycolic acid is 1:1-1:50. The preparation method adopts the spray drying method. The particle sizes of the microspheres prepared in the method are relatively uniform, the large-scale preparation can be realized, the drug loading capacity and the encapsulation efficiency are both high, more than 89 percent of the particle sizes of the microspheres are all between 7mum and 30mum, thereby meeting the requirements of the pulmonary targeting microspheres for the particle sizes, and the microspheres can be concentrated in the lung, thereby enhancing the therapeutic effect.
Owner:QINGDAO VLAND BIOTECH INC +2
Ceftiofur hydrochloride cream and preparation method thereof
ActiveCN104042619AImprove immunityGood treatment effectAntibacterial agentsOrganic active ingredientsMonoglycerideEthylic acid
The invention relates to ceftiofur hydrochloride cream and a preparation method thereof. The ceftiofur hydrochloride cream comprises ceftiofur hydrochloride, prednisolone acetate, vitamin E, monoglyceride stearate, albolene, lanolin, liquid paraffin, span 80, polysorbate 80, ethylparaben and purified water. The preparation method comprises the following steps of preparing an oil phase and a water phase, adding ceftiofur hydrochloride and prednisolone acetate into the oil phase and the water phase, and carrying out mixing to obtain a uniform mixture. The ceftiofur hydrochloride cream can be used for treating cow mastitis.
Owner:LINYI UNIVERSITY
Long-acting ceftiofur hydrochloride injection and preparation method thereof
InactiveCN101874773AImprove stabilityEasy to prepareAntibacterial agentsOrganic active ingredientsCeftiofur HydrochlorideBlood drug concentration
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT +1
Long-acting compound ceftiofur suspension injection and its preparation method
InactiveCN102397282AFacilitated releaseHigh speedAntibacterial agentsOrganic active ingredientsSuspending AgentsAntibacterial activity
The invention discloses a long-acting compound ceftiofur suspension injection and its preparation technology. According to the invention, the compound ceftiofur suspension injection is prepared by using ceftiofur hydrochloride and tylosin tartrate as main drugs with assistance of appropriate auxiliary drugs. The suspension contains 5-20% (W / V) of ceftiofur hydrochloride, 5-20% (W / V) of tylosin tartrate, 0.5-2% (W / V) of a suspending agent, 0.01-0.5% (W / V) of an anti-oxidant, 0.1-3% (W / V) of a dispersant, and injection oil which is added to 100%. In comparison with a ceftiofur single preparation, the long-acting compound ceftiofur suspension injection has a strong antibacterial activity for Gram-negative bacteria and simultaneously has a strong effect of inhibiting Gram positive bacteria and mycoplasma. In addition, the preparation method provided by the invention provides a feasible approach for extended reproduction, and can be used to minimize times of drug administration and save manpower and material resources.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method for preparing ceftiofur hydrochloride parenteral solution
InactiveCN101601645ASolve the difficulty of injectionSolve for uniformityAntibacterial agentsOrganic active ingredientsCeftiofur HydrochlorideParenteral solutions
The invention relates to a method for preparing ceftiofur hydrochloride parenteral solution; the hydrochloride parenteral solution has the characteristics of even distribution and clear and transparent solution. The method comprises the following steps: (1) adding propylene glycol and ceftiofur hydrochloride with a volume equal to 60-70% of the total volume of ceftiofur hydrochloride parenteral solution to be prepared in a thick mixing tank; adding 2-10g ceftiofur hydrochloride for every 100ml of total volume of the ceftiofur hydrochloride parenteral solution; heating to 40-50 DEG C and stirring for rapid dissolving; (2) adding propylene glycol to reach the total volume of the ceftiofur hydrochloride parenteral solution to be prepared and cooling to 20-30 DEG C; (3) adding active carbon, stirring for 15-20 minutes and filtering; and (4) filling and sealing, sterilizing, light check and packing. The invention solves the problems of difficult and uneven injection of the ceftiofur hydrochloride parenteral solution of the existing technology and makes up the defects of easy-layer and inconvenient use of the ceftiofur hydrochloride suspension injection of the existing technology.
Owner:上海恒丰强生物技术有限公司
Compound ceftiofur suspension emulsion injection and preparation method thereof
InactiveCN101953889AReduce viscosityReduce usageAntibacterial agentsOrganic active ingredientsEmulsionAntioxidant
The invention discloses a compound ceftiofur suspension emulsion injection, which comprises the following components in percentage by mass: 1.5 to 15 percent of ceftiofur hydrochloride, 1.5 to 15 percent of magnolia flower oil, 1 to 10 percent of surfactant, 3 to 30 percent of oil for injection, 0.1 to 1 percent of antioxidant, 0.01 to 0.1 percent of thickening agent, and the balance of water for injection, wherein the sum of the mass percents of the components is 100 percent. The compound ceftiofur suspension emulsion injection has simple operation for the preparation method, strong antibacterial property and lasting pharmacological effect, and avoids stresses caused by multi-time administration of conventional formulations to animals; furthermore, the preparation cost is much lower than that of an oil suspension, and the compound ceftiofur suspension emulsion injection has a very high popularization and application value.
Owner:NORTHWEST A & F UNIV
Preparation method of hydrochloric acid ceftiofur
The invention discloses a preparation method of hydrochloric acid ceftiofur, which relates to the field of the chemical synthesis of bulk pharmaceutical chemicals for livestock and comprises the following steps: adding reaction organic solvents in a reaction bottle; taking AE-active ester; adding ceftiofur and an antioxidant in the reaction bottle; uniformly stirring to obtain a reaction solution;dripping an organic amine solution in the obtained reaction solution; keeping the temperature and reacting; adding the organic solvents for diluting; decoloring active carbon; filtering out the active carbon to obtain a reaction solution; adding the solvents in the reaction solution for diluting; adding water and concentrated hydrochloric acid; regulating pH value to be 1 to 2; stirring for 1 hour; gradually separating out crystals; putting and maintaining the crystals over a night at room temperature; filtering; rinsing; drying and eliminating the solvents to obtain the crystals of the hydrochloric acid ceftiofur. The ceftiofur is used as raw material; the condensation reaction process for preparing free acid of the ceftiofur and the reaction for generating hydrochloride of the free acidof the ceftiofur are combined; the preparation method is simple and an obtained target product has high purity and high-purity hydrochloric acid ceftiofur and can be industrially produced in large batch.
Owner:PU LIKE BIO ENG
Vitamin-D3-contained filling agent for preventing dairy cow mastitis and preparation method of filling agent
InactiveCN103006672AEasy to solveGood treatment effectAntibacterial agentsOrganic active ingredientsEscherichia coliAntioxidant
The invention provides a vitamin-D3-contained filling agent for preventing dairy cow mastitis and a preparation method of the filling agent. Every 10g of filling agent is prepared from the following materials by weight: 20mg-50mg of vitamin D3, 50mg-250mg of ceftiofur hydrochloride, 0.65g-2g of thickener, 50mg-100mg of antioxidant, and the balance of soybean oil or liquid paraffin. The preparation method of the vitamin-D3-contained filling agent comprises the following steps of: preparing the materials for future use; mixing the vitamin D3 and the ceftiofur hydrochloride, dispersing the obtained mixture by the soybean oil or the liquid paraffin, and ultrasonically stirring the mixture with the soybean oil or the liquid paraffin to uniformly disperse the mixture to obtain active ingredient mixed liquor; heating up residual solvent, and adding the thickener and the antioxidant into the mixture to obtain accessory mixed liquor; pouring the active ingredient mixed liquor into the accessory mixed liquor, uniformly stirring the mixture to obtain the vitamin-D3-contained filling agent for preventing the dairy cow mastitis. The vitamin-D3-contained filling agent is used for preventing and curing the dairy cow mastitis caused by pathogenic bacteria such as staphylococcus aureus, streptococcus, escherichia coli and salmonella.
Owner:QILU ANIMAL HEALTH PROD
Ceftiofur hydrochloride powder injection as well as preparation method and application thereof
ActiveCN104586777AProlong the action time of concentrationWell mixedAntibacterial agentsPowder deliveryCefepime hydrochlorideFreeze-drying
The invention belongs to the technical field of veterinary medicines, and particularly relates to a ceftiofur hydrochloride powder injection as well as a preparation method and an application thereof. The ceftiofur hydrochloride powder injection is prepared from the following effective components ceftiofur hydrochloride and a cosolvent at the weight ratio of (48-52) to (28-34). Ceftiofur sodium is replaced with the ceftiofur hydrochloride; veterinary cefepime hydrochloride powder injection is prepared by weighing the effective components and the cosolvent at the weight ratio of (48-52) to (28-34), mixing evenly, and sub-packaging; and the veterinary cefepime hydrochloride powder injection can be produced and stored at a room temperature. According to the ceftiofur hydrochloride powder injection, the problems that an existing veterinary cefepime hydrochloride powder injection needs to be produced by a freeze-drying method, the raw materials and the preparation need to be stored at a low temperature of 4-10 DEG C, the placement time in a room-temperature or high-temperature season is overlong, and the medication effect is poor due to degradation are solved; the prepared powder injection has the same antibacterial effect as a ceftiofur sodium freeze-dried powder injection; and the ceftiofur hydrochloride powder injection is low in cost and wide in application prospect.
Owner:广东省天宝生物制药有限公司
Oil injection containing antibacterial agents/polyethylene glycol drug-loading particles
InactiveCN103721263APromote absorptionRetain water swellingAntibacterial agentsPharmaceutical delivery mechanismCelluloseDoxycycline hydrochloride
The invention discloses an oil injection containing antibacterial agents / solid polyethylene glycol drug-loading particles. The oil injection is prepared by consisting of the drug-loading particles through antibacterial agents and solid polyethylene glycol and suspending the drug-loading particles in an oil medium. The antibacterial agents comprise enrofloxacin, danofloxacin mesylate, Marbofloxacin, mequindox, tilmicosin, tylosin, oxytetracycline hydrochloride, ceftiofur hydrochloride, cefquinome sulfate, lincomycin hydrochloride, florfenicol, erythrocin and doxycycline hydrochloride; preferentially the polyethylene glycol with the molecular weight of more than 6000 is used for preparing the preparation; more preferentially, any one of isopropyl myristate, soybean oil for injection, corn oil and tea-seed oil is used for preparing the preparation. Hydroxypropyl methyl cellulose or high-substituted hydroxy propyl cellulose can be added into the preparation.
Owner:王玉万
Pharmaceutical composition used for preventing and treating colibacillosis in livestock and poultry
InactiveCN102462686AEasy to prepareStable traitsAntibacterial agentsPeptide/protein ingredientsESCHERICHIA COLI ANTIGENBeta-Cyclodextrins
The invention relates to a pharmaceutical composition used for preventing and treating colibacillosis in livestock and poultry. Every 100 g of the pharmaceutical composition comprises 2 to 10 g of ceftiofur hydrochloride, 0.25 to 10 g of tazobactam sodium, 5 to 10 g of probenecid sodium, 0.1 to 0.5 g of dexamethasone sodium phosphate and 2 to 15 g of beta-cyclodextrin, with the balance being pharmaceutically acceptable accessories. The pharmaceutical composition has the advantages of reasonable composition, low cost, a remarkable curative effect on clinical pathogenic escherichia coli, especially on drug-resistant intractable escherichia coli, a simple preparation method, stable properties and convenience in large scale intensive production.
Owner:TIANJIN RINGPU BIO TECH
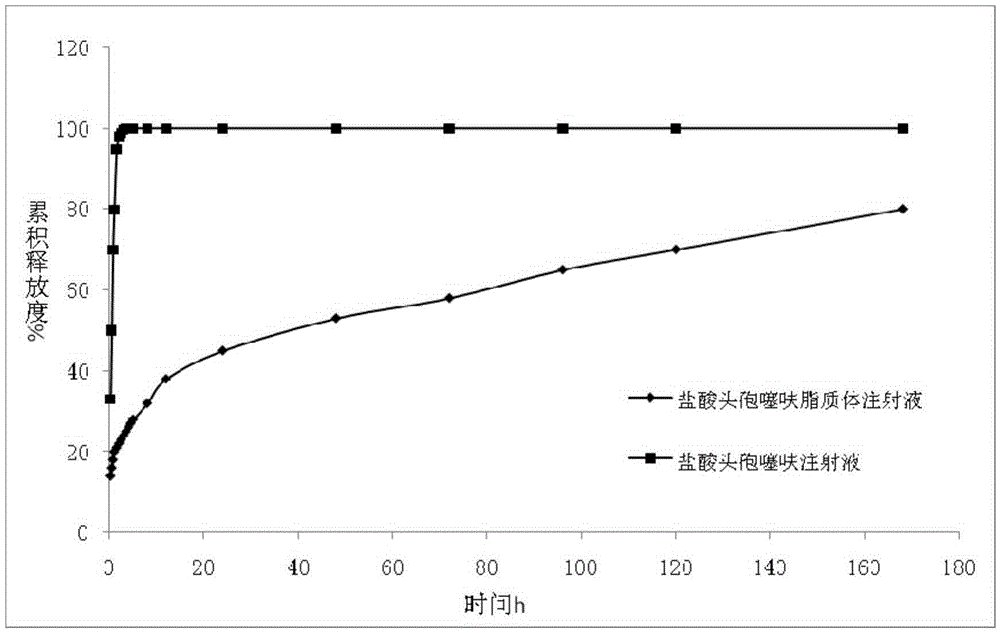
Ceftiofur hydrochloride lipidosome injection and preparation method thereof
InactiveCN106580878AUniform particle sizeHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsAntioxidantCeftiofur Hydrochloride
The invention relates to the field of pharmaceutical preparations, in particular to a ceftiofur hydrochloride lipidosome injection and a preparation method thereof. The ceftiofur hydrochloride lipidosome injection contains ceftiofur hydrochloride, a buffer salt system, a lipidosome matrix, an additive and an antioxidant, and ceftiofur hydrochloride lipidosome is prepared through a high-pressure homogenization method. The lipidosome obtained through the method has the high encapsulation efficiency on ceftiofur hydrochloride and can achieve a slow releasing effect on medicine.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
New preparation method of ceftiofur sodium
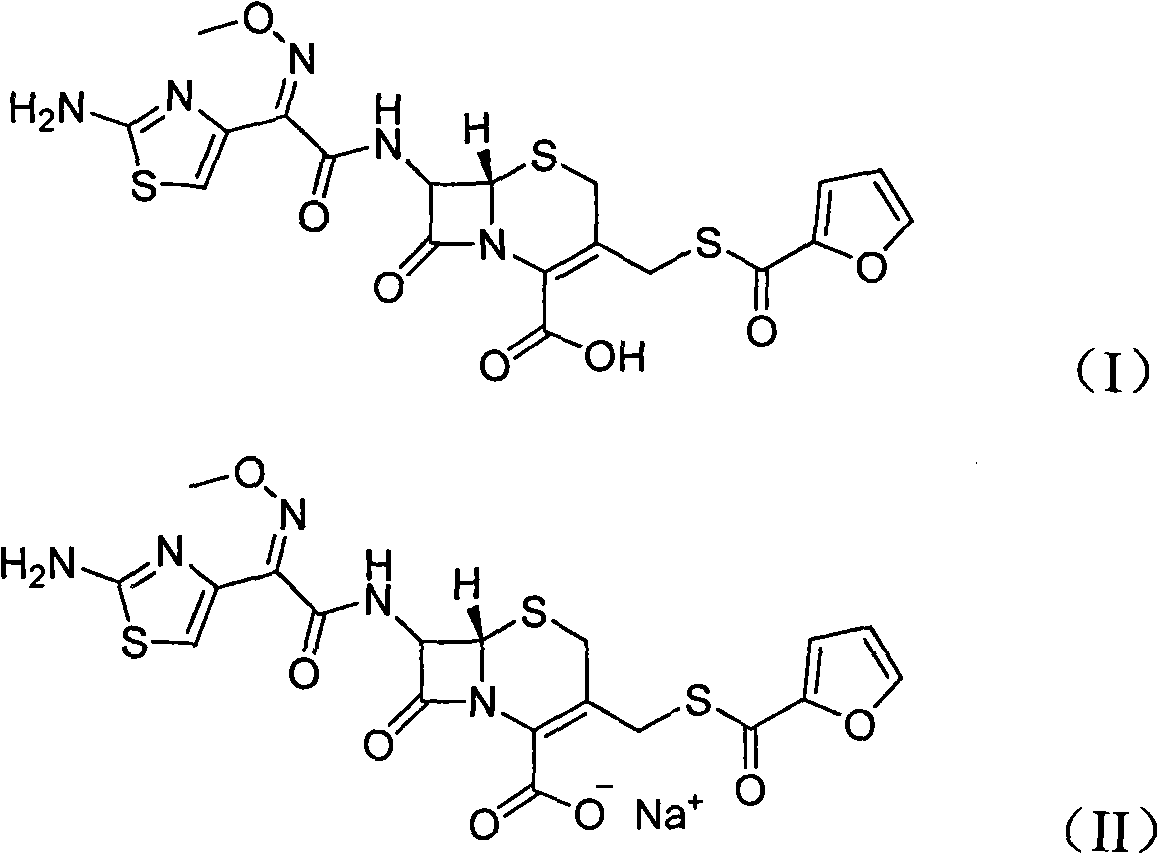
The invention provides a new method for preparing sodium salt from ceftiofur hydrochloride. The method comprises the following step of reacting inorganic weak base such as sodium bicarbonate and the like or organic weak acid with the ceftiofur hydrochloride by using a proper solvent to obtain the sodium salt with high yield. The quality of products reaches quality standard of the Ministry of agriculture. Compared with the conventional process route, the process route has the characteristics of cheap and readily-available selected raw materials and reagents, low process cost, environmental friendliness, high product yield (about 90 percent) and the like and is suitable for industrialized production.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
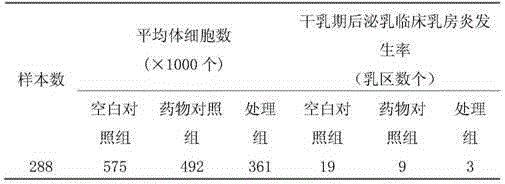
Ceftiofur hydrochloride breast injectant during dry period
ActiveCN105726461AGuaranteed uniformityGuaranteed softnessAntibacterial agentsOrganic active ingredientsMedicineCeftiofur Hydrochloride
The invention relates to a formula of a ceftiofur hydrochloride breast injectant during a dry period, and a preparation method of the injectant. The injectant comprises a suspending agent, a thickening agent, a surfactant, and a solid dispersion carrier, and is manufactured into a white or white-like ointment through special process. The injectant is simple to prepare, stable in quality and content, easy to inject, and soft in matrix. The injectant is an ointment, and compared with an injection, the injectant has advantages, such as high bioavailability, slow release, and long action time, can be sealed at nipple holes after being filled into milk chambers through milk ducts, and can prevent bacteria from intruding into the milk chambers by virtue of retrograde motion through the nipples.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Ceftiofur hydrochloride injection for dairy cattle and preparation method thereof
ActiveCN107049943AImprove dispersion stabilityGood acupunctureAntibacterial agentsOrganic active ingredientsDispersion stabilityAntioxidant
The invention provides ceftiofur hydrochloride injection for dairy cattle, comprising, by weight, 5-10 parts of ceftiofur hydrochloride, 0.5-3 parts of a suspending agent, 0.05-5 parts of a flocculant, 0.01-2 parts of an emulsifier, 0.1-2 parts of an antioxidant, and 55-100 parts of a lipid solvent. The ceftiofur hydrochloride injection has good dispersion stability and good needle passage, milk withdrawal period of dairy cattle injected with the ceftiofur hydrochloride injection is short, and the ceftiofur hydrochloride injection has a promising application prospect. A preparation method of the ceftiofur hydrochloride injection for dairy cattle is also provided; the preparation method is simple to perform and applicable to industrial application.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Method for preparing ceftiofur hydrochloride suspension injection
ActiveCN102813623AImprove performanceSimple preparation processAntibacterial agentsOrganic active ingredientsVegetable oilProcess engineering
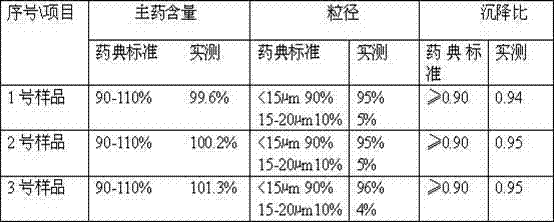
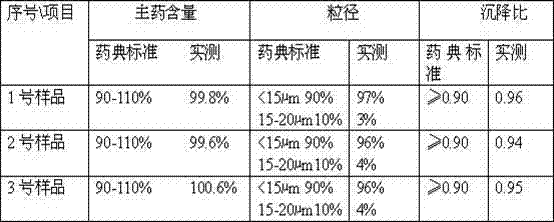
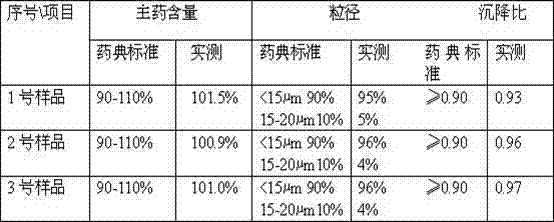
The invention discloses a method for preparing a ceftiofur hydrochloride suspension injection, which comprises the following steps: a) pulverizing ceftiofur hydrochloride in a pulverizer until the particle size of 90% particles is less than or equal to 20 mu m, and mixing; b) heating vegetable oil to 140-150 DEG C, keeping the temperature for 50-60 minutes for sterilization, and cooling to room temperature; c) adding the vegetable oil obtained in the step b) into the ceftiofur hydrochloride powder obtained in the step a), stirring uniformly, adding dehydrated sorbitol fatty acid ester, and continuing stirring uniformly until all the components are completely dispersed into a suspension; and d) refining the suspension obtained in the step c) with a homogenizer to obtain the ceftiofur hydrochloride suspension injection. Compared with the prior art, the method disclosed by the invention has the advantages of simple preparation technique, cheap and accessible raw and auxiliary materials, stable injection performance and obvious curative effect; compared with like products, the ceftiofur hydrochloride suspension injection has higher cost performance, and the product quality is superior to Relevant Standard for Pharmacopoeia, Edition 2010; and the invention is suitable for industrialized large-scale production, and therefore, has very wide application prospects.
Owner:SHANGHAI TONGREN PHARM CO LTD
Ceftiofur hydrochloride liposome lyophilized agent and preparation method thereof
ActiveCN104983695AHigh encapsulation efficiencyImprove stabilityAntibacterial agentsPowder deliveryClinical efficacyPenicillin
The invention discloses a ceftiofur hydrochloride liposome lyophilized agent and a preparation method thereof. The ceftiofur hydrochloride liposome lyophilized agent comprises, by weight, 3-10 parts of ceftiofur hydrochloride, 15-100 parts of lecithin, 15-100 parts of cholesterols, and 4.5-13.5 parts of polyvinyl alcohol 2000. The preparation method comprises the following steps: dissolving ceftiofur hydrochloride, lecithin and cholesterols, mixing, carrying out vacuum drying, and adding a phosphate buffer solution of polyethylene glycol 2000 to prepare a liposome suspension; and adding a lyophilizing protection agent to the suspension, uniformly mixing, packaging the obtained solution to a penicillin bottle, putting the penicillin bottle provided with the solution in a lyophilizer, and drying to obtain the ceftiofur hydrochloride liposome-carried lyophilized agent. The liposome entrapment rate is good, and a result of long-term stabilization investigation of the liposome lyophilized agent shows that the stability of the liposome lyophilized agent is good; and the lyophilized agent re-dissolved in a liquid medium has the characteristics of good dispersion and stable properties. Clinic curative effect tests prove that compared with suspensions, the liposome lyophilized agent has the advantages of prolonged circulation time in an administrated body, and definite therapeutic effect on porcine respiratory diseases induced by bacterial infection.
Owner:JIANGXI BOLAI PHARMACY CO LTD
Preparation method of long-acting hydrochloric ceftiofur injection
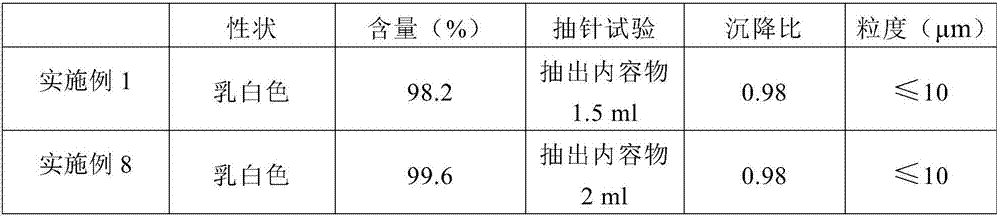
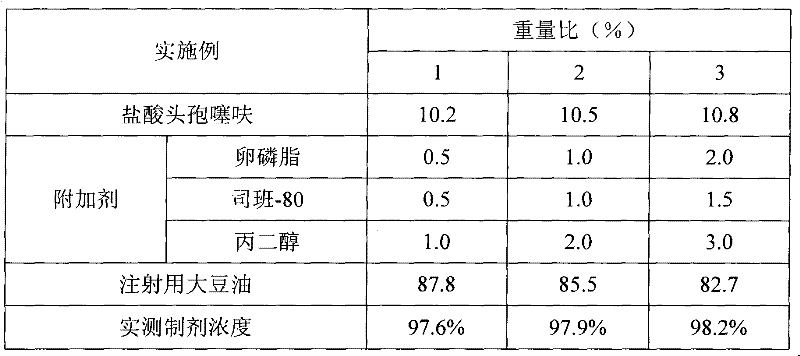
The invention discloses a preparation method of a long-acting hydrochloric ceftiofur injection. The preparation method is characterized by including: using soybean oil as a solvent, heating the soybean oil to 140-150 DEG C, holding the temperature for 60min for sterilizing, and cooling to room temperature; dissolving or dispersing 0.5-2.0wt% of and 0.5-1.5wt% of according to prescription amounts in 70-80% of the sterilized solvent; guiding into a colloid mill for grinding, adding 10.2-10.8wt% of hydrochloric ceftiofur according to a prescription amount after grinding is finished, adopting a mode of alternating circulating grinding and non-circulating grinding for grinding, checking whether particle fineness reaches 2.5-5.0um or not, stopping grinding after requirements are met, and adding the sterilized solvent to the prescription amount to obtain an intermediate product; testing ceftiofur content and product character of the intermediate product, after passing needle drawing experiment, filling, externally packing, warehousing, and obtaining the injection after passing tests of content, sterility, granularity and related matter. Results show that when consumption of and is 1%, production process and product quality of the injection are stable.
Owner:上海公谊药业有限公司
Ceftiofur hydrochloride suspension injection and method for preparing ceftiofur hydrochloride suspension injection
ActiveCN106176598AHigh drug loadingHigh biosecurityAntibacterial agentsOrganic active ingredientsSolubilityALUMINUM STEARATES
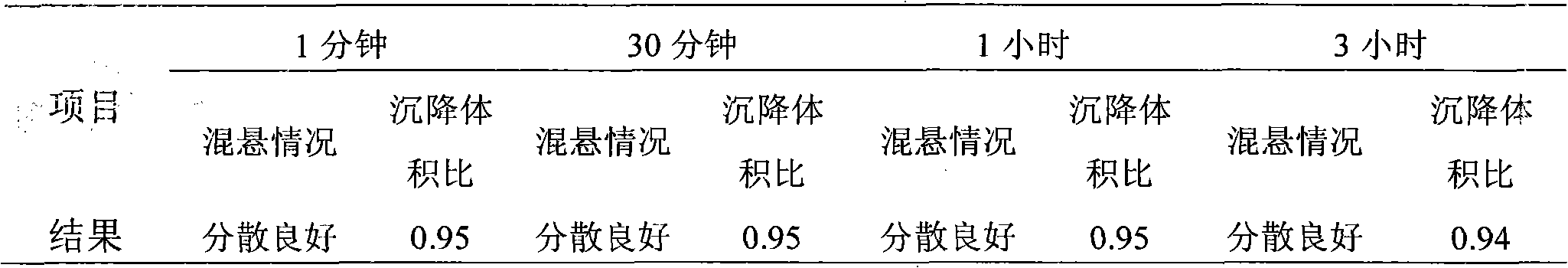
The invention discloses a ceftiofur hydrochloride suspension injection, which is prepared, in every 100 mL of the injection, from 4-6 grams of ceftiofur hydrochloride, 1 to 5 grams of aluminum stearate, 0.01-0.2 gram of BHT (butylated hydroxytoluene), 0.01 to 0.03 gram of cecropin and the balance of injection-grade soybean oil. The ceftiofur hydrochloride suspension injection provided by the invention improves the solubility and the dissolution efficiency of a medicine, is low in sedimentation rate and good in dispersivity, improves the stability of a medicinal preparation, is high in drug loading capacity, decreases the dosing volume, increases biological safety, has quite good adhesivity with mucosal tissue, and realizes targeted drug delivery; moreover, after the cecropin is added, a drug effect of the ceftiofur hydrochloride suspension injection can be obviously enhanced.
Owner:LINZHOU SINAGRI YINGTAI BIOLOGICAL PEPTIDES CO LTD
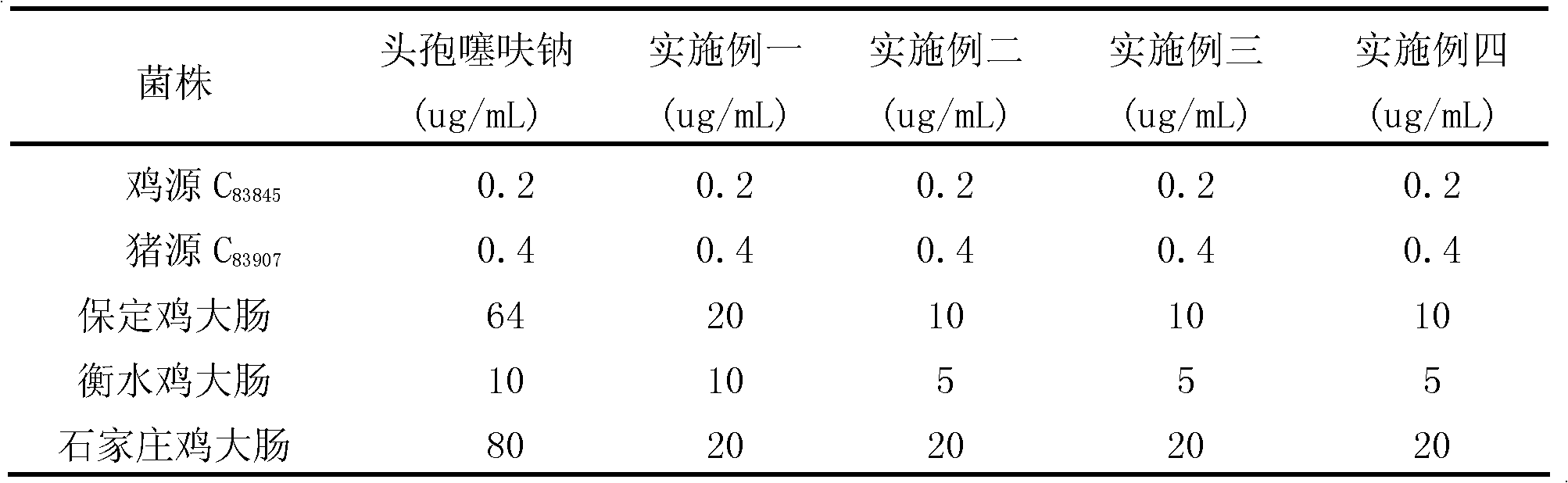
Preparation method of ceftiofur hydrochloride
The invention relates to preparation of a veterinary use chemical raw medicine and specifically relates to a preparation method of ceftiofur hydrochloride. Ceftiofur acid and AE active ester are used to synthetize ceftiofur in an organic solvent under the action of organic amine, a reaction liquid is extracted by water, hydrochloric acid is added after an aqueous phase extract liquid is decolorized to adjust potential of hydrogen (pH) to be 1-2, solid is separated out and filtered, a wet product is dissolved by an appropriate amount of acetone, water is slowly and dropwise added to elute crystals, filtering is performed, and the solid is dried to obtain the ceftiofur hydrochloride. According to the preparation method of the ceftiofur hydrochloride, the water is used as a crystallization solvent so that the organic solvent is reduced, reducing of production costs is facilitated, and environment pollution is reduced.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Long-acting ceftiofur hydrochloride injection and preparation method thereof
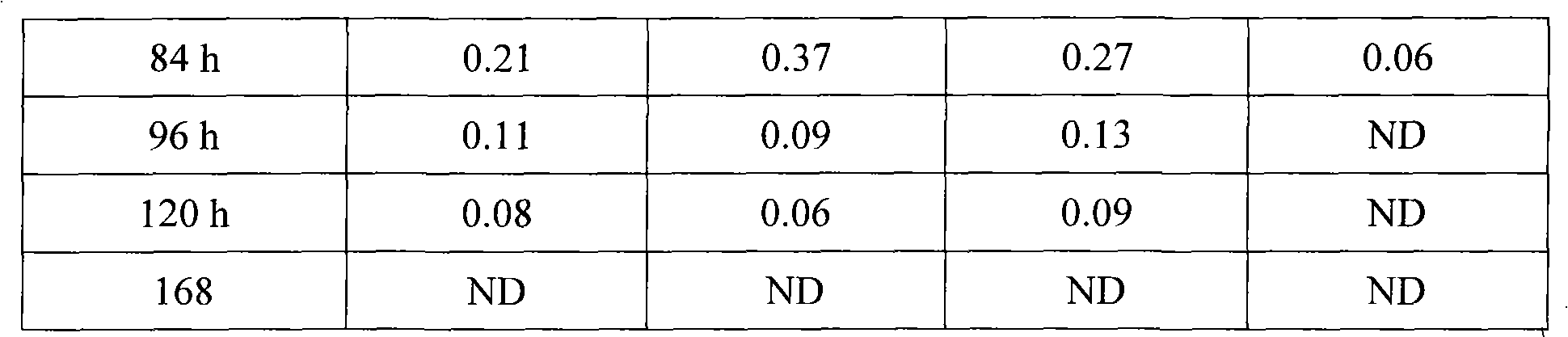
InactiveCN101874773BImprove stabilityEasy to prepareAntibacterial agentsOrganic active ingredientsCeftiofur HydrochlorideBlood drug concentration
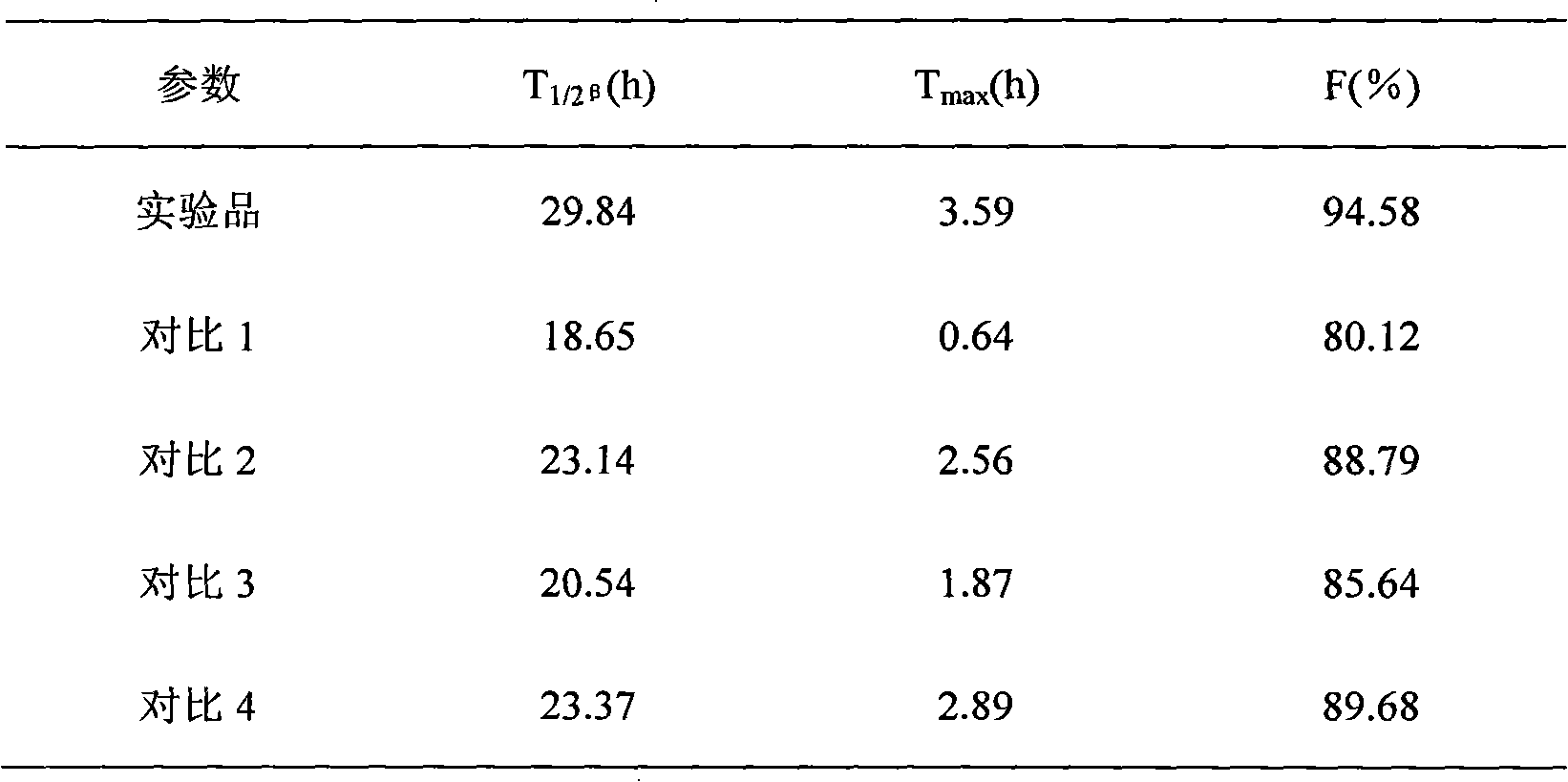
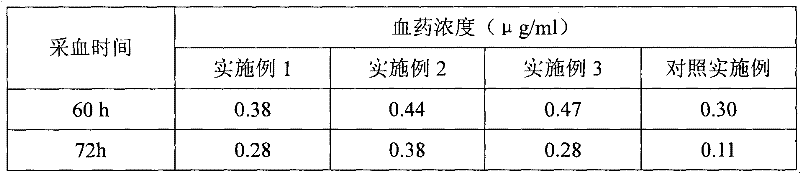
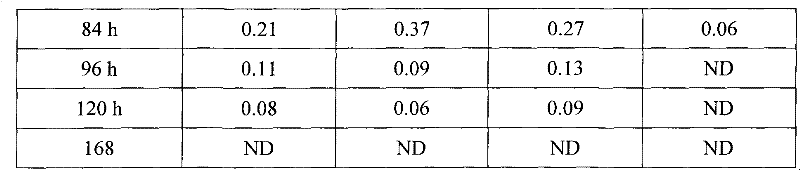
The invention discloses a long-acting ceftiofur hydrochloride injection and a preparation method thereof. The long-acting ceftiofur hydrochloride injection of the invention is prepared from the following raw materials in percentage by weight: 10.2%-10.8% of ceftiofur hydrochloride, 2.0%-6.5% of additive and balance of soybean oil for injection. The long-acting ceftiofur hydrochloride injection ofthe invention can slowly release drug effect; the duration time of the blood drug concentration of the long-acting ceftiofur hydrochloride injection is 72-84 hours, which is greatly longer than the duration time of the blood drug concentration of the current ceftiofur hydrochloride injection; and compared with the conventional injection which needs to be injected once per day, the long-acting ceftiofur hydrochloride injection only needs to be injected once every 3 days and has good stability.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT +1
Long-acting ceftiofur hydrochloride injection and preparation method thereof
ActiveCN102018669BExtended half-lifeElimination half-lifeAntibacterial agentsOrganic active ingredientsPhysical chemistryEngineering
Owner:武汉回盛生物科技股份有限公司
Lung targeting microsphere of veterinary ceftiofur hydrochloride and preparation method
ActiveCN101829063AHigh drug loadingHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsPulmonary infectionSide effect
The invention provides a lung targeting microsphere of veterinary ceftiofur hydrochloride and a preparation method and can solve the problems of harmful toxic and side effects on a tissue due to the increased concentrations of other tissues caused by the low tissue selectivity of medicaments and poor therapeutic effect of the medicaments in the prior art. The lung targeting microsphere adopts the technical scheme of taking ceftiofur hydrochloride as a raw material and glutin as a carrier, wherein the weight ratio of the ceftiofur hydrochloride to the glutin is 1:3 to 30. The invention further provides the preparation method of the lung targeting microsphere. The lung targeting microsphere has the advantages of higher medicament loading rate and encapsulation rate of over 60 percent; after separation, the grain diameter of over 92 percent of the microsphere is between 7 and 30 mu m, so that the lung targeting microsphere meets the grain diameter requirement, improves the tissue selectivity, targets to the lung, reduces the concentrations of the medicaments in other tissues and toxic and side effects, and efficiently treats the pulmonary infection of animals.
Owner:江西派尼生物药业有限公司
Preparation method of hydrochloric acid ceftiofur
The invention discloses a preparation method of hydrochloric acid ceftiofur, which relates to the field of the chemical synthesis of bulk pharmaceutical chemicals for livestock and comprises the following steps: adding reaction organic solvents in a reaction bottle; taking AE-active ester; adding ceftiofur and an antioxidant in the reaction bottle; uniformly stirring to obtain a reaction solution; dripping an organic amine solution in the obtained reaction solution; keeping the temperature and reacting; adding the organic solvents for diluting; decoloring active carbon; filtering out the active carbon to obtain a reaction solution; adding the solvents in the reaction solution for diluting; adding water and concentrated hydrochloric acid; regulating pH value to be 1 to 2; stirring for 1 hour; gradually separating out crystals; putting and maintaining the crystals over a night at room temperature; filtering; rinsing; drying and eliminating the solvents to obtain the crystals of the hydrochloric acid ceftiofur. The ceftiofur is used as raw material; the condensation reaction process for preparing free acid of the ceftiofur and the reaction for generating hydrochloride of the free acid of the ceftiofur are combined; the preparation method is simple and an obtained target product has high purity and high-purity hydrochloric acid ceftiofur and can be industrially produced in large batch.
Owner:PU LIKE BIO ENG
Ceftiofur long-acting injection and preparation method thereof
InactiveCN101401787BAccording to the law of pharmacokineticsDecreased peak plasma concentrationAntibacterial agentsOrganic active ingredientsVegetable oilVeterinary Drugs
The invention discloses a ceftiofur long-acting injection and a method for preparing the same. The ceftiofur long-acting injection is a suspension injection containing ceftiofur hydrochloride, and the main compositions of the solvent system of the drug are vegetable oil for injection and grease. The preparation process of the ceftiofur long-acting injection has simple operation, and the prepared suspension injection meets the requirements and has obvious long-acting effect after intramuscular injection, so that the therapeutic effect of the drug is improved and the disadvantages that a respiratory disease has a long course and needs multiple administrations in treatment are overcome, and the waste of human resources in a farm caused by frequent administrations and the animal stress reaction caused by multiple administrations are reduced, thus the ceftiofur long-acting injection is a novel veterinary drug preparation with long-acting effect on respiratory diseases of livestock and poultry, and has very high popularization and application value.
Owner:北京中农大动物保健品技术研究院
Ceftiofur-containing powder injection for beast
ActiveCN100534433CReduce manufacturing costQuick effectAntibacterial agentsPowder deliveryDiseaseCeftiofur sodium
The invention relates to a veterinary powder injection containing ceftiofur, which belongs to the technical field of drugs for preventing and treating animal microbial infectious diseases. It is characterized in that it contains 20-100% by weight of ceftiofur free acid or ceftiofur hydrochloride aseptic powder, and the rest is medical auxiliary agent. The above-mentioned veterinary powder injection containing ceftiofur has good quick-acting and sustained-release effects, and adopts ceftiofur free acid or ceftiofur hydrochloride to prepare aseptic powder, which is lower than that of preparing powder injection with ceftiofur sodium. More than 20% of the production cost is conducive to its popularization and application. The injection is suitable for the prevention and treatment of animal microbial infectious diseases. When in use, the sterile powder is mixed with a liquid dispersant, prepared into a solution, solution suspension, suspension or emulsion, and injected subcutaneously or intramuscularly.
Owner:浙江拜克生物科技有限公司 +1
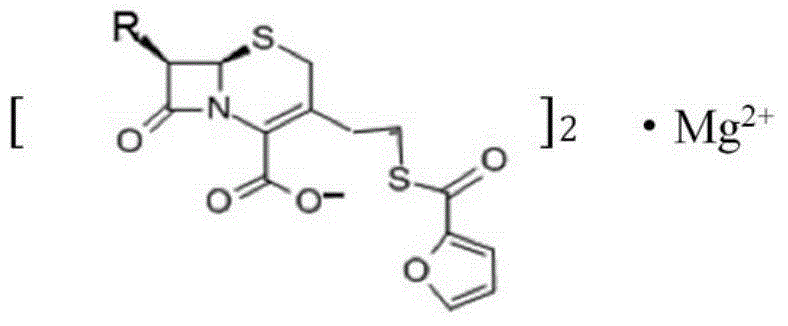
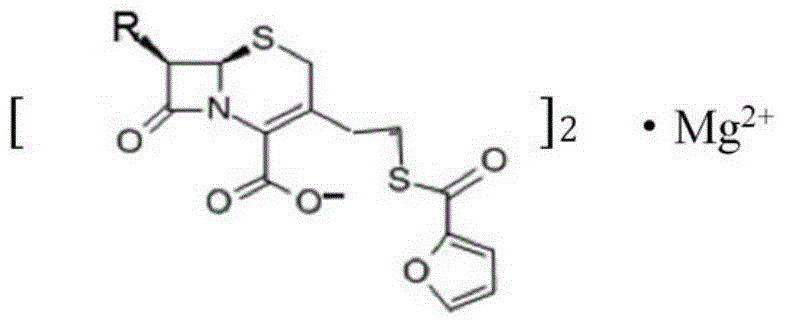
Magnesium ceftiofur as well as preparation method and using method thereof
ActiveCN104610281AHave antibiotic propertiesProlong the action timeAntibacterial agentsOrganic active ingredientsCeftiofur HydrochlorideAntibiotic Y
The invention relates to magnesium ceftiofur, and belongs to the field of special curative activity of a compound or a pharmaceutical preparation. The magnesium ceftiofur is prepared by virtue of ceftiofur hydrochloride and medicinal oxidase, and the structural formula of the magnesium ceftiofur is provided. Meanwhile, the invention provides a preparation method and a using method of the magnesium ceftiofur; the magnesium ceftiofur disclosed by the invention has a same antibacterial activity as the ceftiofur hydrochloride and the magnesium ceftiofur has the properties of antibiotics; the action time of drugs can be prolonged through magnesium ions; meanwhile, the magnesium ceftiofur can supplement magnesium ions to a human body, so that the medicinal value of the magnesium ceftiofur is improved; and the magnesium ceftiofur disclosed by the invention is simple in preparation process, low in cost and applicable to industrial production.
Owner:SIVEA QINGDAO BIOPHARM CO LTD
Applications of ceftiofur hydrochloride in prevention of mycobacterium tuberculosis infection
InactiveCN108125957AEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsMethionine aminopeptidaseMicrobiology
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Compound ceftiofur cream and preparation method thereof
InactiveCN106581033ATargetedEasy to useAntibacterial agentsOrganic active ingredientsOil phaseCeftiofur Hydrochloride
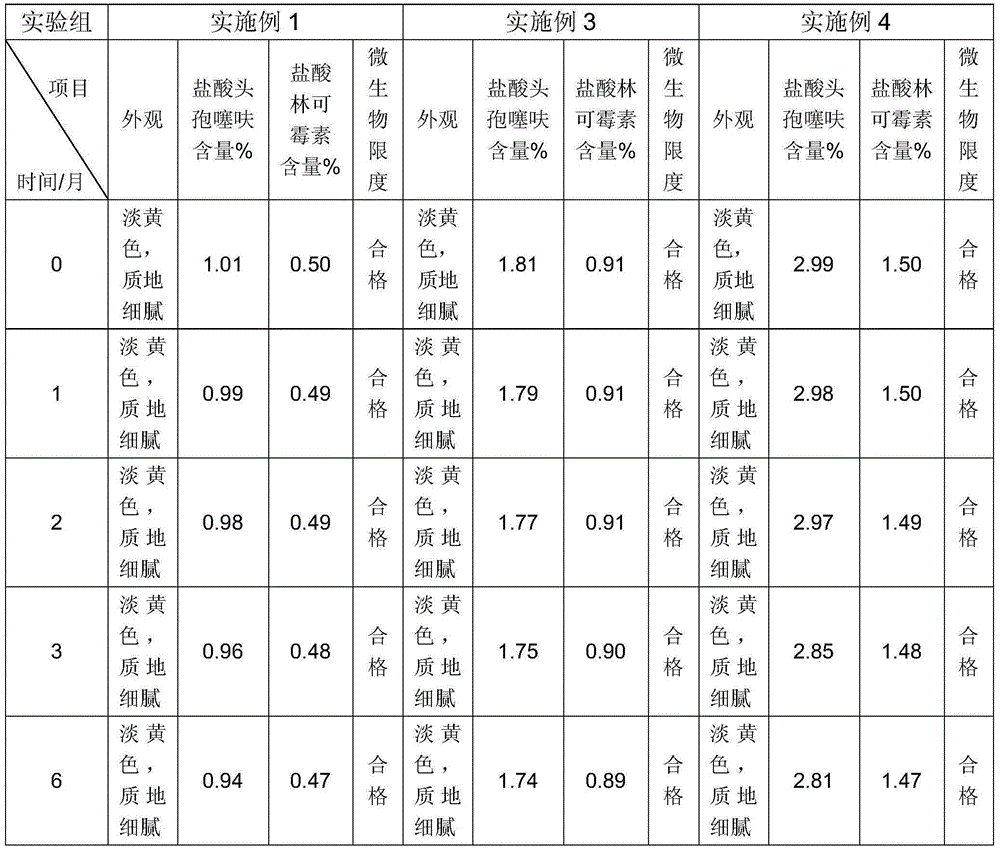
The present invention relates to a ceftiofur hydrochloride and lincomycin hydrochloride compound cream and a preparation method thereof, wherein the cream comprises ceftiofur hydrochloride, lincomycin hydrochloride and other suitable materials, and during the preparation, a water phase and an oil phase are prepared, then the water phase is added to the oil phase to prepare a cream, and sterilization is performed.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com