Ceftiofur hydrochloride suspension injection and method for preparing ceftiofur hydrochloride suspension injection
A technology of ceftiofur hydrochloride and suspension injection, which is applied in the directions of liquid delivery, pharmaceutical formulation, emulsion delivery, etc., can solve problems such as the limitation of the application of ceftiofur hydrochloride in water-insoluble injection, and achieves increased biological safety, The effect of good adhesion, improved solubility and dissolution efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] A suspension injection of ceftiofur hydrochloride, containing in every 100mL injection: 4-6g of ceftiofur hydrochloride, 1-5g of aluminum stearate, 0.01-0.2g of BHT (dibutylhydroxytoluene), cecropin antibacterial Peptide 0.01-0.03g, the rest is injection grade soybean oil.
[0011] The above-mentioned preparation method of ceftiofur hydrochloride suspension injection comprises the following steps: adding aluminum stearate into soybean oil and heating to dissolve, dispersing the foam, controlling the oil temperature at about 120°C to form a light yellow viscous Add BHT after turning into a clear liquid, stir to dissolve, add ceftiofur hydrochloride after cooling, stir and mix, then add cecropin antimicrobial peptide, stir and mix, add injection-grade soybean oil to the full amount, stir and mix, homogeneous, pressure 30Mpa, Check that the semi-finished product is qualified, then pack it, cover it, label it, and pack it. During the entire production process, aseptic oper...
Embodiment 2
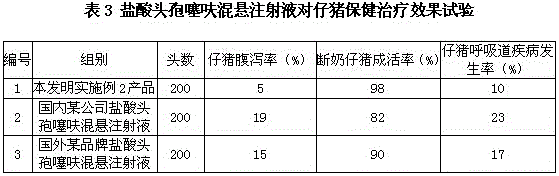
[0015] A suspension injection of ceftiofur hydrochloride, which contains 5 g of ceftiofur hydrochloride, 3 g of aluminum stearate, 0.1 g of BHT, 0.02 g of cecropin antimicrobial peptide, and the rest is injection-grade soybean oil per 100 mL of injection. All the other are with embodiment 1.
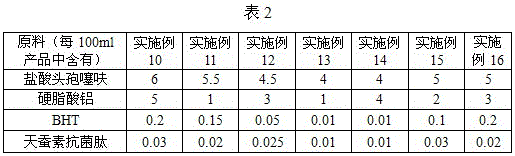
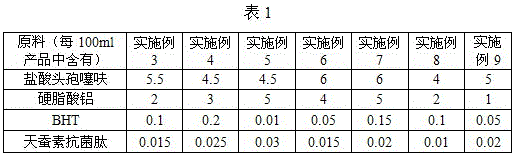
[0016] The formula of Ceftiofur Hydrochloride Suspension Injection in Example 3-16 of the present invention is shown in Table 1-2, and the others are the same as in Example 2.
[0017]
[0018]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com