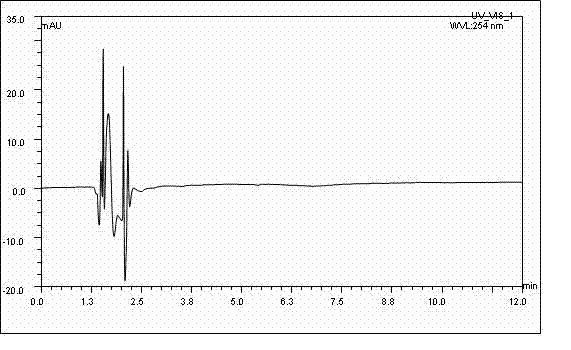
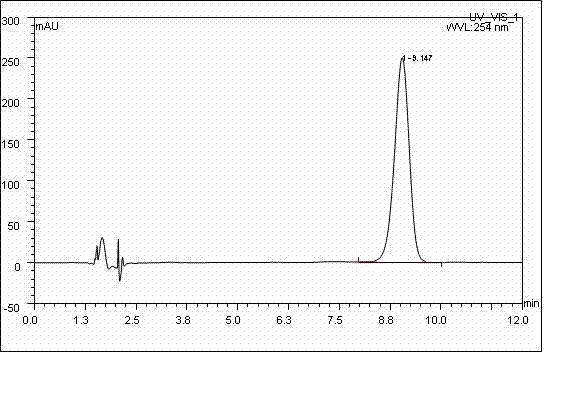
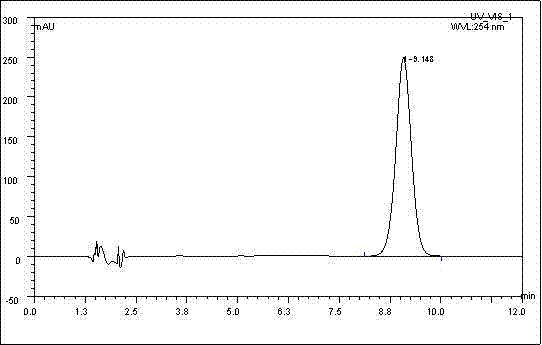
Long-acting ceftiofur hydrochloride injection and preparation method thereof
A technology of ceftiofur hydrochloride and injection, which is applied in the field of long-acting ceftiofur hydrochloride suspension injection and its preparation, can solve the problems of short action time and achieve the effects of improving fluidity, good fluidity, and eliminating half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 7.5g of ceftiofur hydrochloride to 40ml of glycerol triacetate, stir magnetically, and mix well;
[0028] (2) Add 0.3g of compound suspending agent (hydrogenated castor oil: lecithin 1:2 (g / g) mixture) into glycerol triacetate at 90°C to dissolve;
[0029] (3) Cool the solution in step (2) to room temperature and add it to the stirring suspension in step (1), and stir evenly;
[0030] (4) Add 0.5g bacteriostatic agent chlorobutanol to the suspension in step (3), adjust to 100ml with the remaining triacetin, and stir thoroughly;
[0031] (5) Stir and mix for 30 minutes, pass through a high-speed shear disperser for 30 minutes at a speed of 1200r / min, pass through a colloid mill for 4 hours, and finally sterilize the sample by irradiating 60Co-γ rays at 3KGY to obtain a milky white or light yellow suspension .
[0032] The properties of the suspension are milky white or light yellow, the particle size distribution is uniform, the average particle size is 8.38 μm...
Embodiment 2
[0034] (1) Add 7.5g of ceftiofur hydrochloride to 40ml of composite solvent (36ml of glycerol triacetate and 4ml of polyethylene glycol 200), stir magnetically, and mix well;
[0035] (2) Add 0.3g of compound suspending agent (hydrogenated castor oil: lecithin 1:2 (g / g) mixture) into glycerol triacetate at 90°C to dissolve;
[0036] (3) Cool the solution in step (2) to room temperature and add it to the stirring suspension in step (1), and stir evenly;
[0037] (4) Add 0.2g of bacteriostatic agent benzyl alcohol to the suspension in step (3), adjust to 100ml with the remaining triacetin, and stir thoroughly;
[0038] (5) Stir and mix for 30 minutes, pass through a high-speed shear disperser for 30 minutes at a speed of 1200r / min, pass through a colloid mill for 4 hours, and finally sterilize the sample by irradiating 60Co-γ rays at 3KGY to obtain a milky white or light yellow suspension .
[0039] The properties of the suspension are milky white or light yellow, the particle...
Embodiment 3
[0041] (1) Add 7.5g of ceftiofur hydrochloride to 40ml of compound solvent (36ml of glycerol triacetate and 4ml of polyethylene glycol 200: Span 80 at a ratio of 2:1 (v / v) mixture), stir magnetically, and mix well ;
[0042](2) Add 0.3g of compound suspending agent (hydrogenated castor oil: lecithin 1:2 (g / g) mixture) into glycerol triacetate at 90°C to dissolve;
[0043] (3) Cool the solution in step (2) to room temperature and add it to the stirring suspension in step (1), and stir evenly;
[0044] (4) Add 0.5g of bacteriostatic agent benzyl alcohol to the suspension in step (3), adjust to 100ml with the remaining triacetin, and stir thoroughly;
[0045] (5) Stir and mix for 30 minutes, pass through a high-speed shear disperser for 30 minutes at a speed of 1200r / min, pass through a colloid mill for 4 hours, and finally sterilize the sample by irradiating 60Co-γ rays at 3KGY to obtain a milky white or light yellow suspension .
[0046] The properties of the suspension are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com