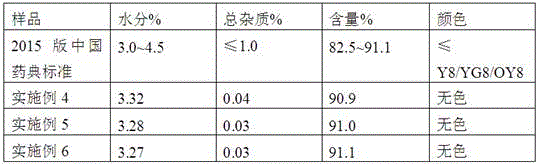
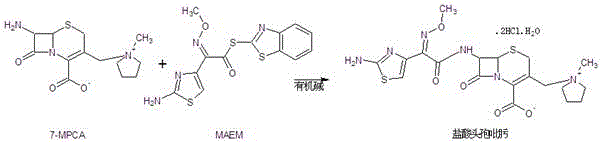
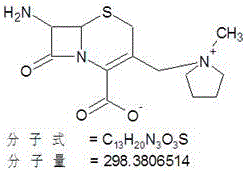
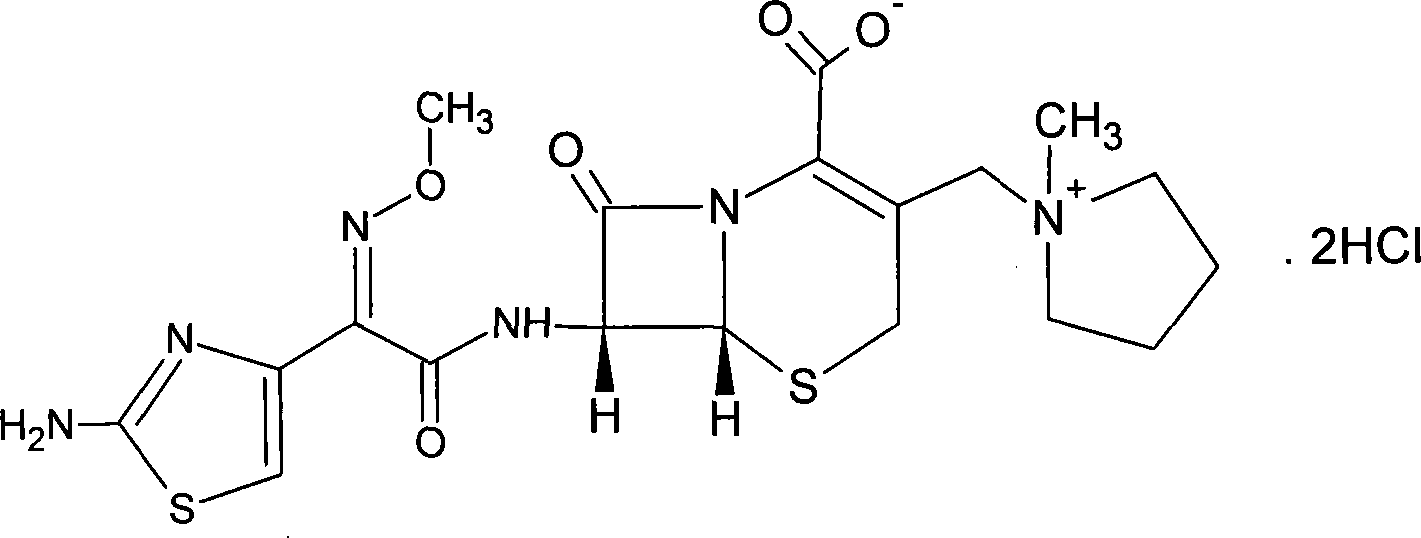
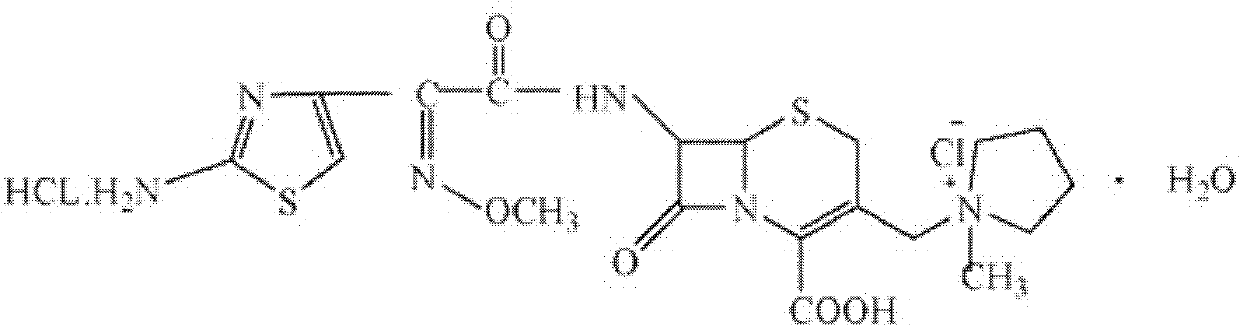
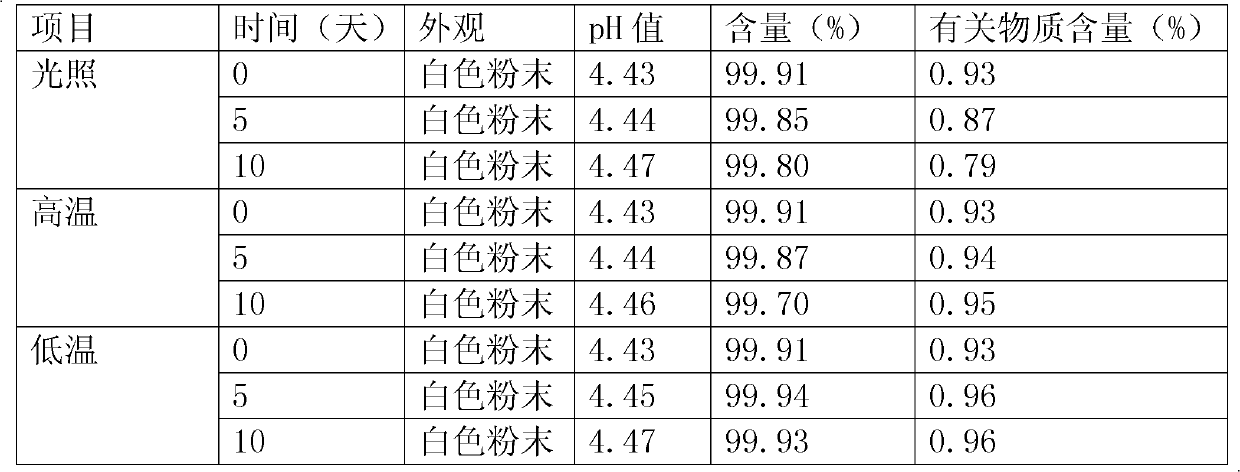
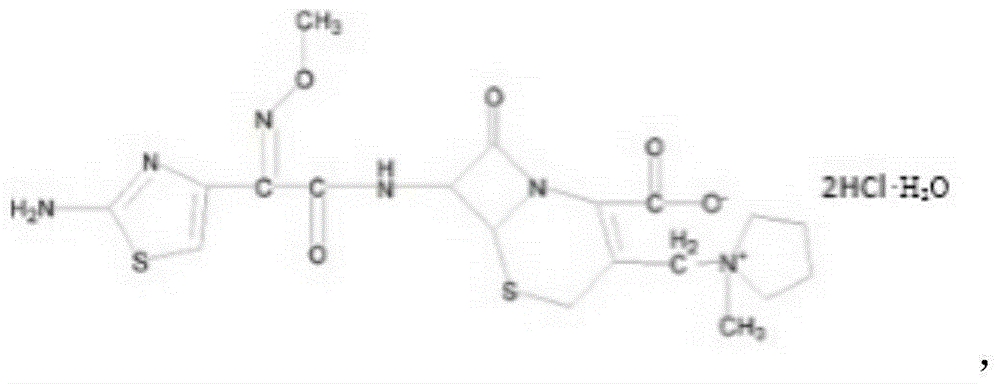
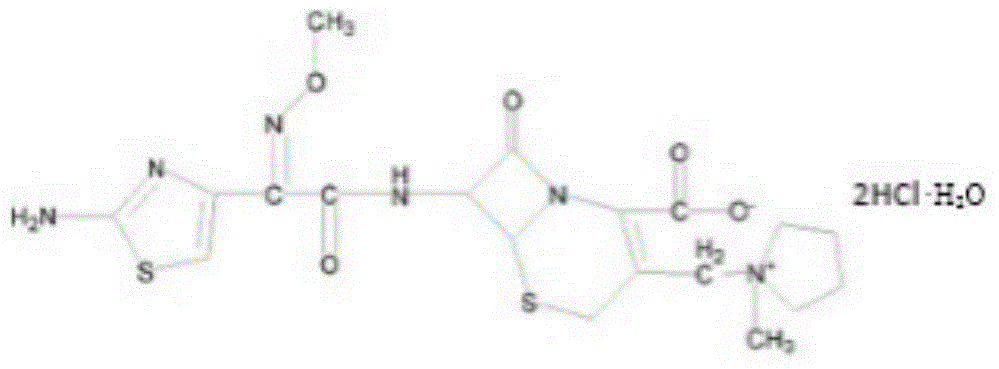
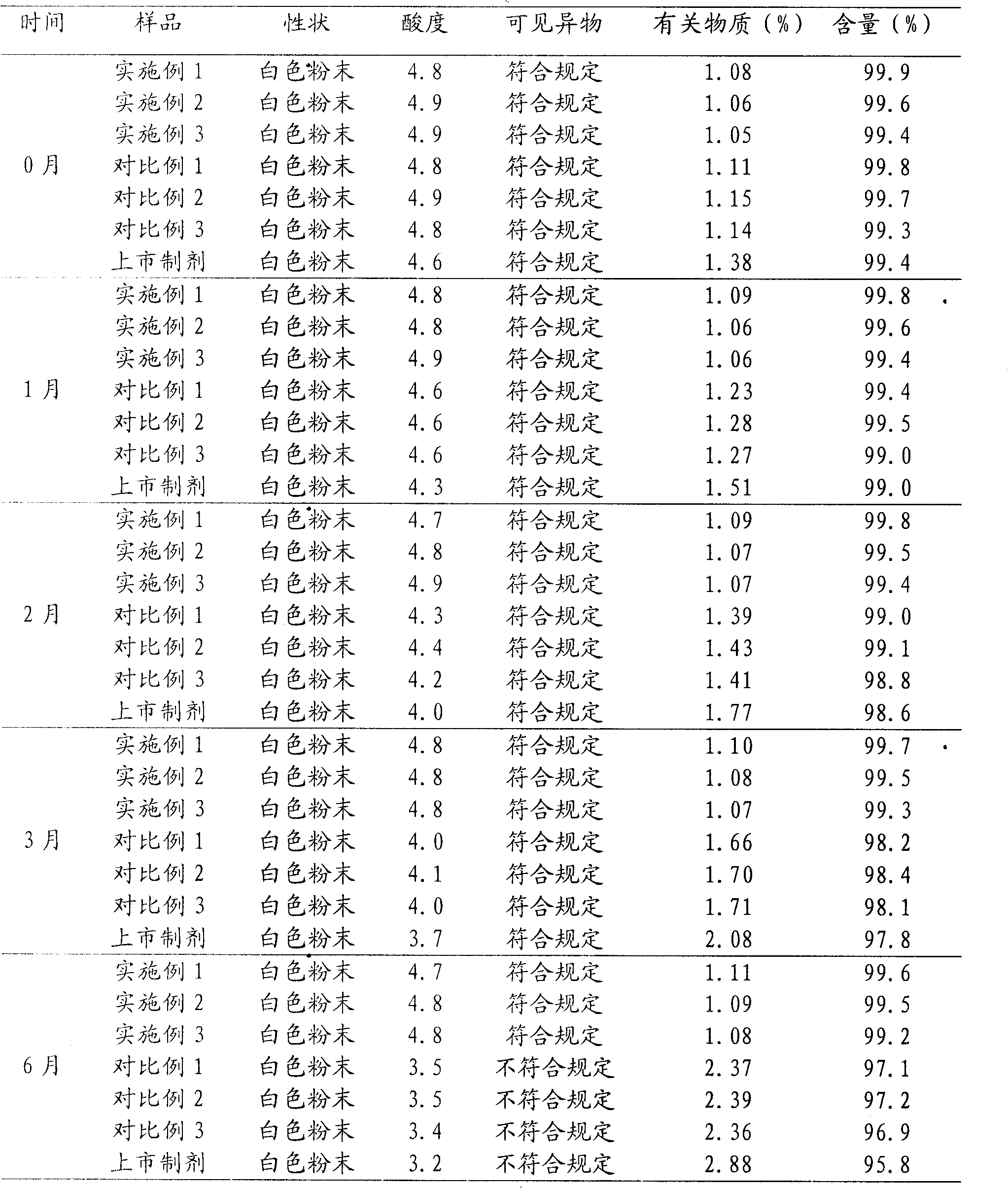Patents
Literature
52 results about "Cefepime hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
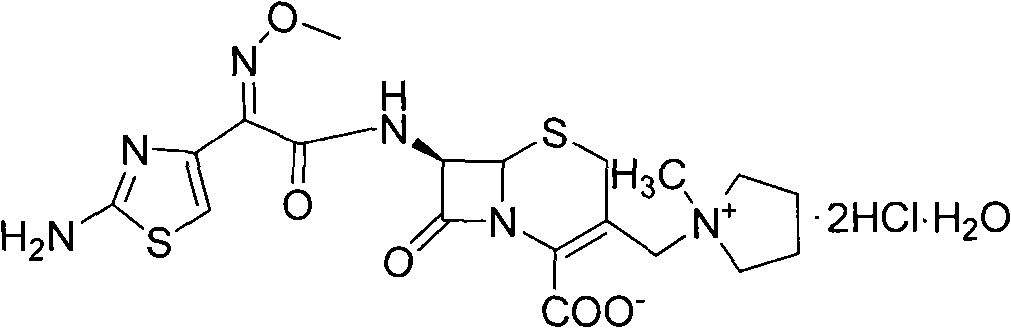
The hydrochoride salt of a semi-synthetic, beta-lactamase-resistant, fourth-generation cephalosporin antibiotic derived from an Acremonium fungal species with broad-spectrum bactericidal activity. Administered parenterally, cefipime inhibits bacterial cell wall synthesis by binding to and inactivating penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity, resulting in a reduction of bacterial cell wall stability and cell lysis. This agent is more active against a variety of Gram-positive pathogens compared to third-generation cephalosporins. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=38350&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=38350&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov:80/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C1041 NCI Thesaurus)
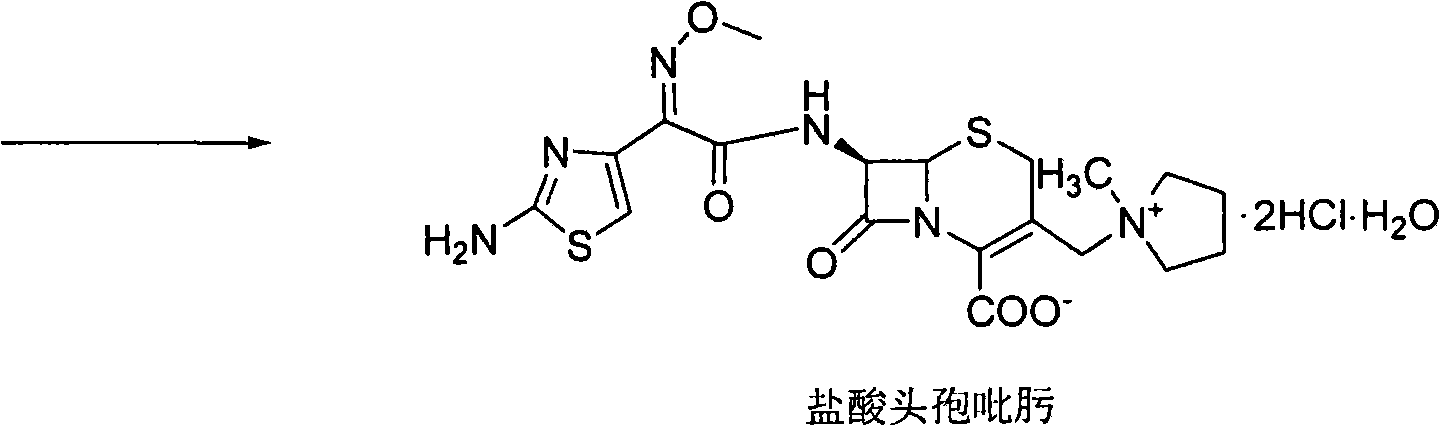
Synthesis method of cefepime hydrochloride
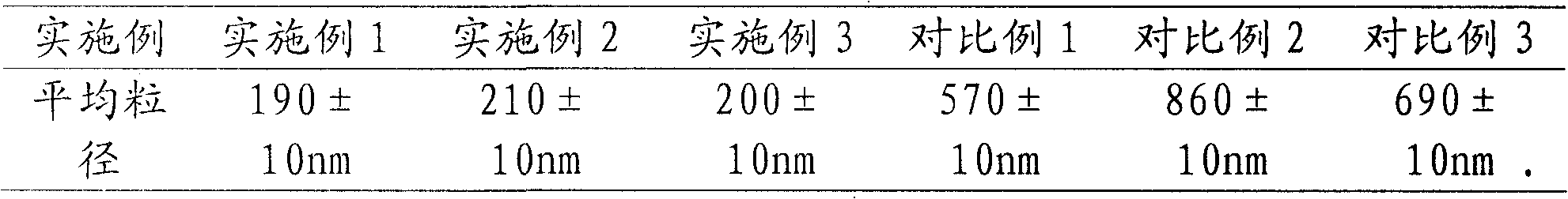
InactiveCN102408440AReduce the impactReduce degradationOrganic chemistryCefepime hydrochlorideSynthesis methods
The invention belongs to the field of medical intermediate and particularly relates to a synthesis method of cefepime hydrochloride. The method comprises the following steps: adding (6R,7R)-7-amino-3-[(1-methyl-1-pyrrolidine)methyl]cef-3-ene-4-carboxylic acid hydrochloride and AE active ester in a mixed solvent of water and a water-soluble organic solvent; adjusting the pH value to 5.5-7.5, and performing an acylation reaction while keeping the temperature; extracting after the reaction, and recycling the organic phase through reduced pressure distillation; and adjusting the pH value of the water phase with hydrochloric acid, and crystallizing to prepare cefepime hydrochloride. The reaction is milder and adopts an acidic or nearly neutral environment, the influence of the reaction on cefepime is lower, the reaction is easier to control, the degradation probability and ring opening probability can be reduced, the purity of the finished product can be increased, the yield of cefepime can be increased, the quality of cefepime can be increased and the purity is above 99.5% through high performance liquid chromatography (HPLC) detection.
Owner:YIYUAN XINQUAN CHEM
Method for synthesizing cefepime hydrochloride
InactiveCN101735251AHigh densityHigh reactivityAntibacterial agentsOrganic chemistryCefepime hydrochlorideVolumetric Mass Density
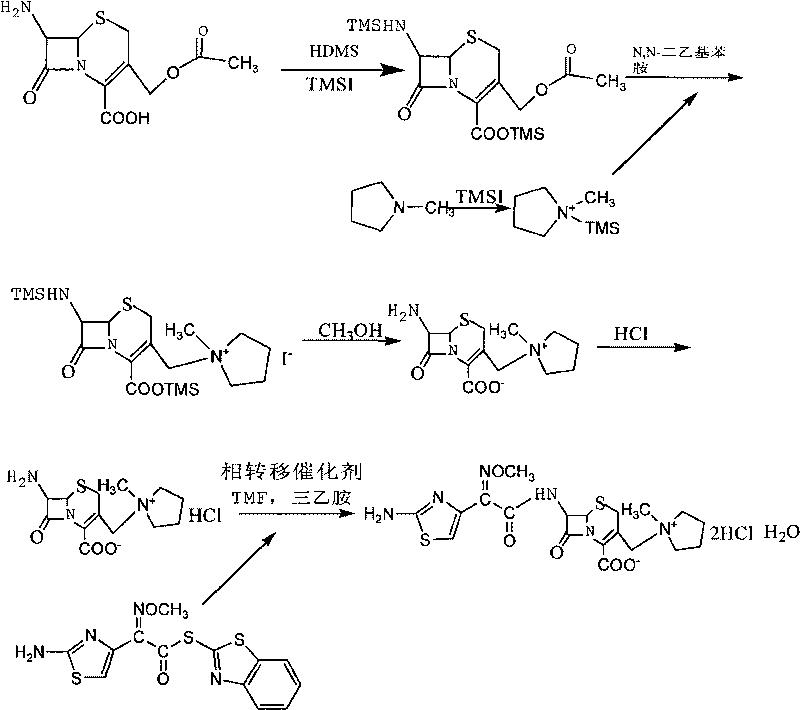
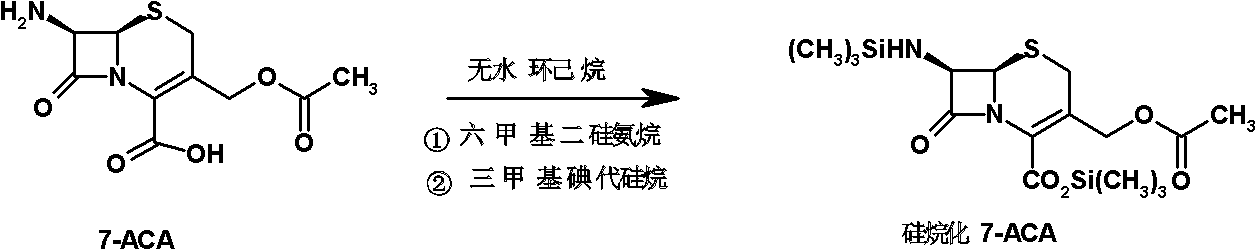
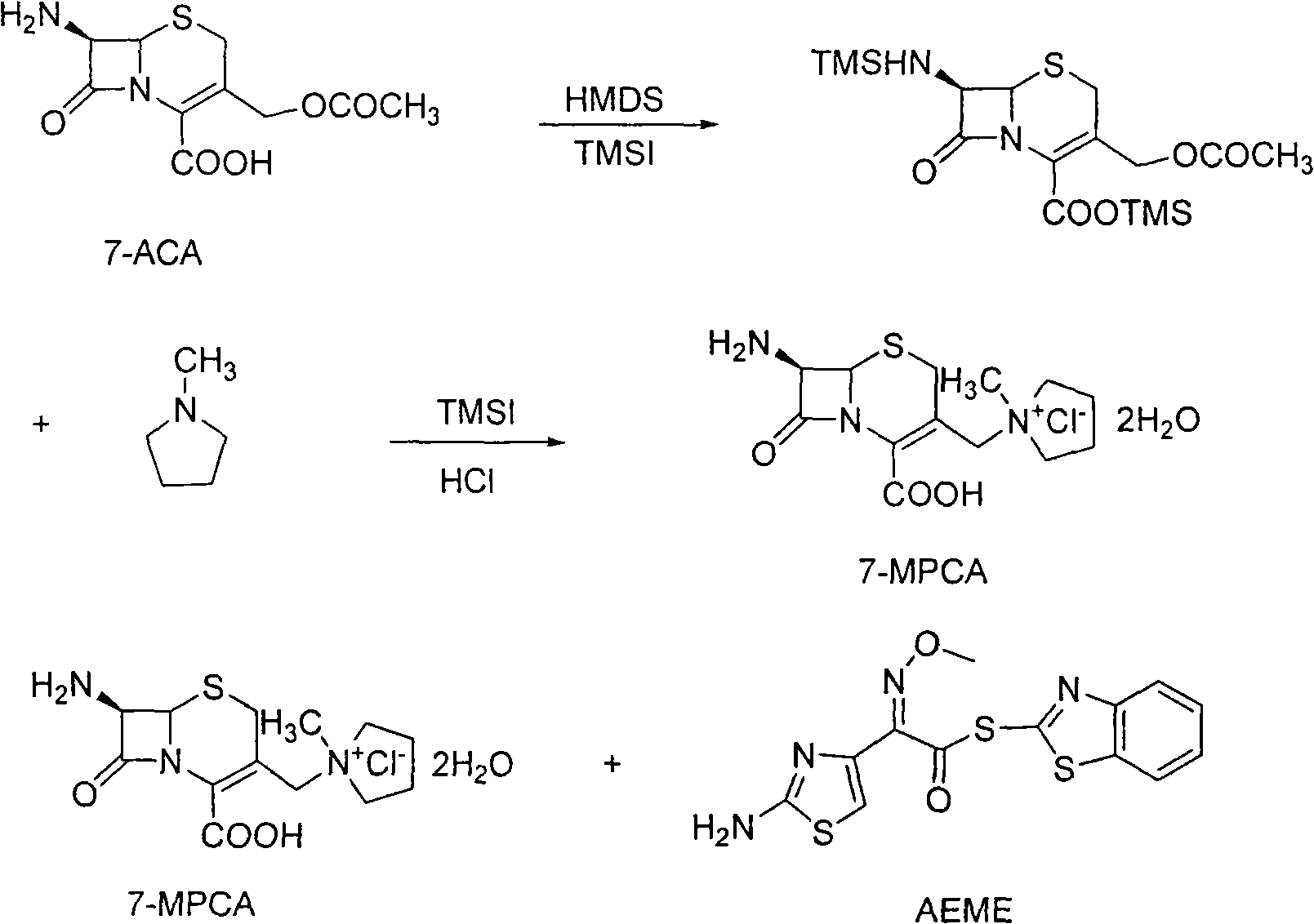
The invention relates to a method for synthesizing cefepime hydrochloride. The method comprises the following steps: taking 7-aminoce-phalosporanic acid (7-ACA) and N-methylpyrrolidine as raw materials, firstly, carrying out carboxylic and amino protection on the 7-ACA by HMDS, then preparing the N-methylpyrrolidine and iodotrimethylsilane into a quaternary ammonium salt intermediate, finally, adding the intermediate into the protected 7-ACA solution and reacting to prepare 7-MPCA; taking the 7-MPCA and AE-active ester, adding a phase transfer catalyst into an organic phase for carrying out an N-acidylating reaction, salifying and reacting to obtain the cefepime hydrochloride. The invention has the main characteristics that the quaternary ammonium salt intermediate is prepared in the step (1), the defects of high electron cloud density, strong reactivity and many side reactions of the N atom of N-methyl pyrrole are overcome, the yield is enhanced by 7%, and the product purity is enhanced. During the N-acidylating reaction in the organic phase in the step (2), the phase transfer catalyst is added, so that the conversion rate of the reaction is enhanced by 5%, and the product yield is enhanced.
Owner:YIYUAN XINQUAN CHEM
Cefepime hydrochloride powder injection and preparing method thereof
ActiveCN101229128AReasonable compositionThe preparation process is feasibleAntibacterial agentsPowder deliveryCefepime hydrochlorideMetallurgy
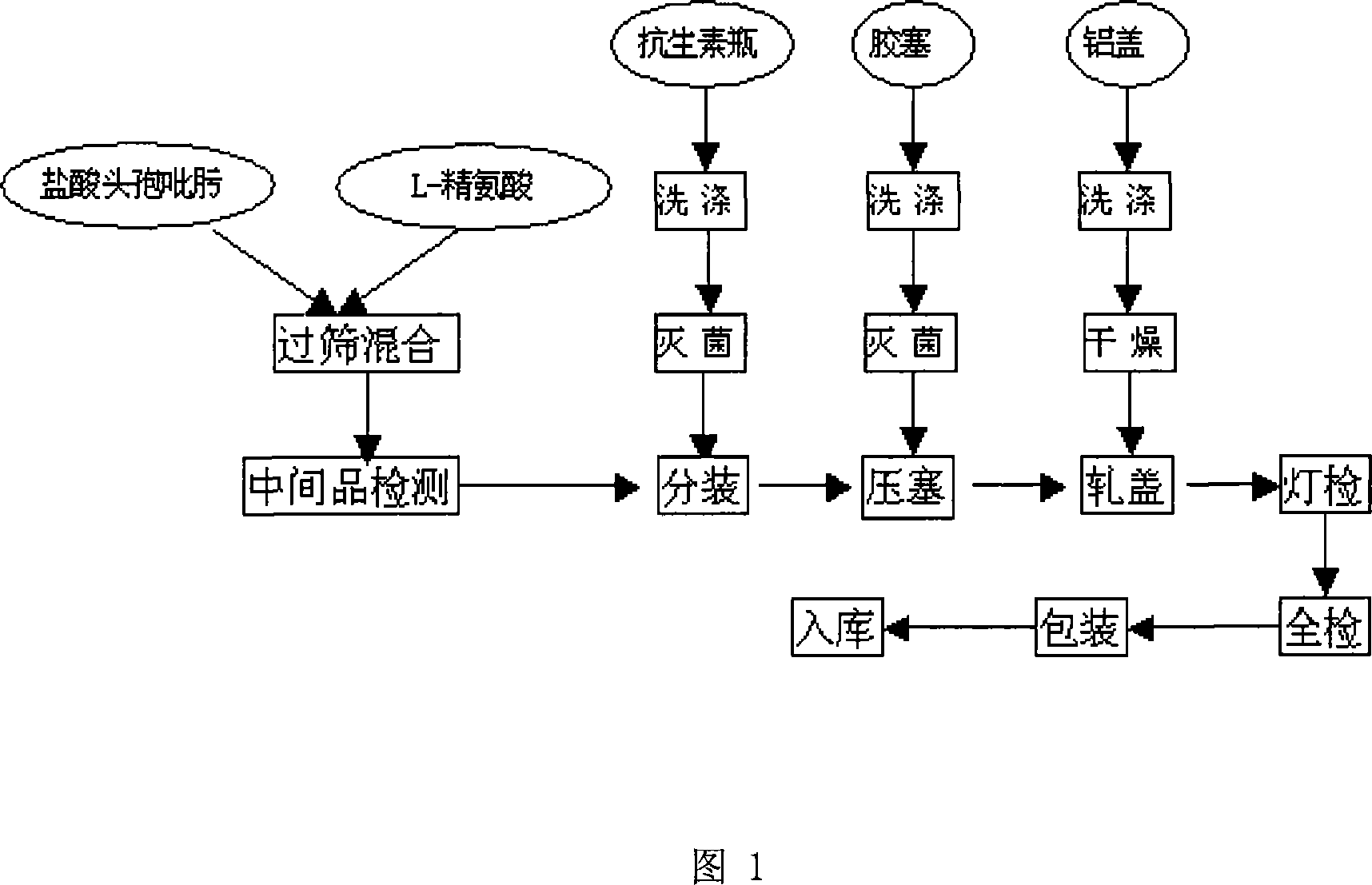
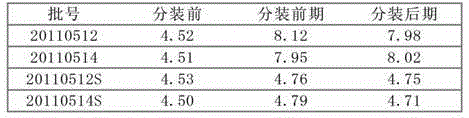
The invention which discloses a hydrochloric acid cefepime powder injection consists of the raw material hydrochloric acid cefepime and supplemental material L-arginine and is characterized in that in the hydrochloric acid cefepime powder injection for injection, the content of the supplemental material L-arginine is 83.5 percent of the raw material hydrochloric acid cefepime; the hydrochloric acid cefepime powder injection provided by the invention is provided with a pH value about 4.5 and is good in stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
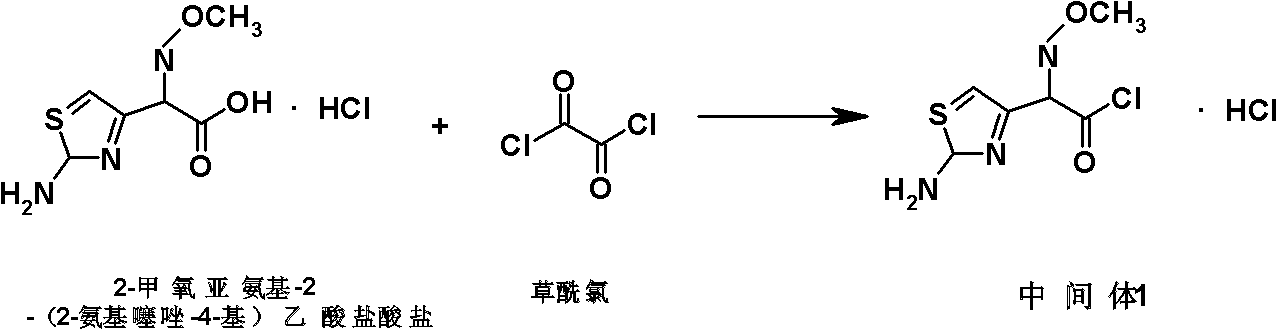
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Method for synthesizing antibiotic cefepime hydrochloride
ActiveCN101337971ASimple process conditionsEasy to operateAntibacterial agentsOrganic chemistryCefepime hydrochlorideHexamethyldisilane
The invention relates to a synthesis method of cefepime dihydrochloride that is a bacteriophage. 7-amin cethalosporanic acid (7-ACA) is used as starting material and reacts with hexamethyldisilane amine (HMDS) and iodotrimethylsilane (TMSI) first to obtain 7-ACA for protecting amino and carboxyl; then 7-ACA, amino and carboxyl of which are protected, reacts with iodotrimethylsilane and N-methylpyrrolidine to synthesize (6R, 7R)-7-amino-3-((1-methyl-1-pyrrolidine) methyl) cephalosporin-3-alkene-4-carboxylic acid hydrochloride (7-MPCA) through a one-pot method; 7-MPCA reacts with AE active ester to obtain a product of cefepime dihydrochloride through acidylation reaction and salifying reaction. Compared with the existing technical route, the synthesis method has the advantages that the process conditions are simple, the operation is convenient, the product yield is high, the product quality is stable, the method is suitable for the large-scale industrialized production, etc.
Owner:国药集团致君(苏州)制药有限公司
Cefepime hydrochloride composition sterile powder for injection
ActiveCN101849912AImprove the uniformity of dispensingWell mixedAntibacterial agentsPowder deliveryCefepime hydrochlorideArginine
The invention relates to cefepime hydrochloride composition sterile powder for injection. The cefepime hydrochloride composition sterile powder for injection consists of cefepime hydrochloride serving as a raw material and L-arginine serving as an auxiliary material, wherein the content of the L-arginine serving as the auxiliary material is 83.5 percent of that of the cefepime hydrochloride serving as the raw material and the amount of the cefepime hydrochloride is calculated by cefepime; and the cefepime hydrochloride is cefepime hydrochloride crystal with the bulk density of between 0.3 to 0.5 g / cm<3>. The bulk density of the cefepime hydrochloride crystal in the medical composition is close to that of the L-arginine and fits for the L-arginine, so mixing uniformity of the cefepime hydrochloride raw powder and the L-arginine is greatly improved. Thus, the packaging uniformity of cefepime hydrochloride powder injection is improved.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Synthesis method of cefepime hydrochloride
ActiveCN107201391AReduce usageLow costOrganic chemistryFermentationCefepime hydrochlorideSynthesis methods
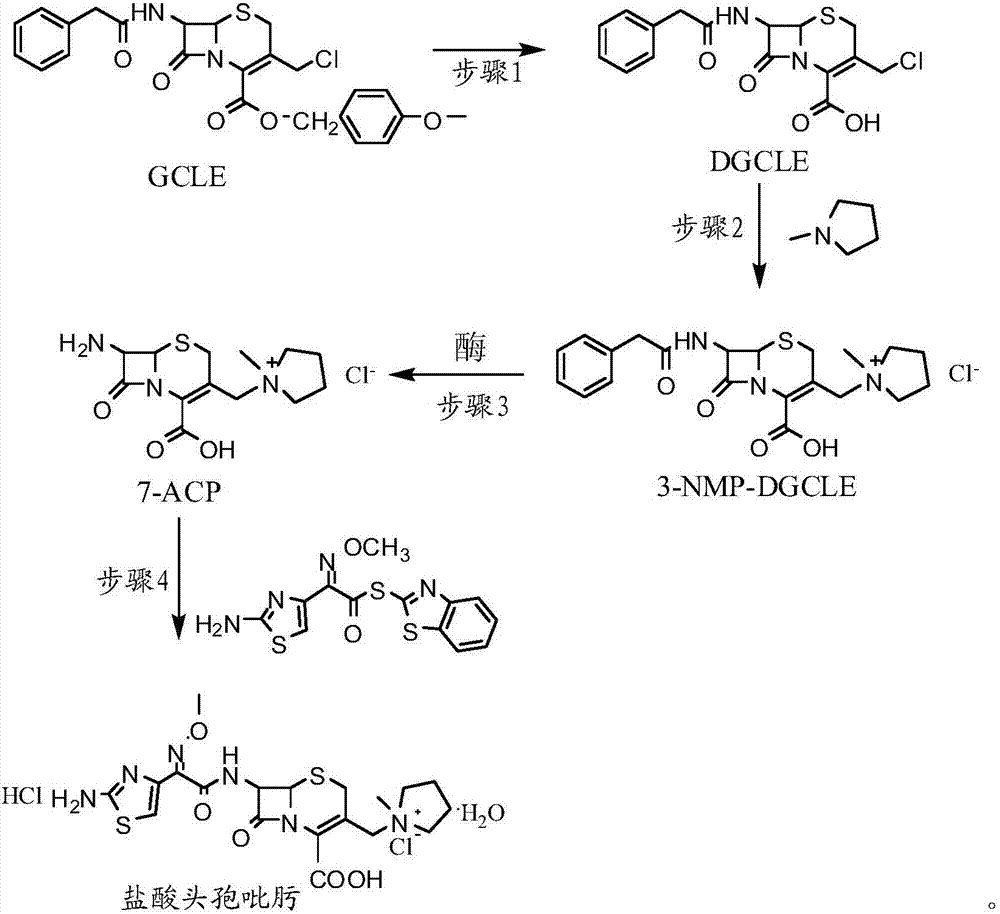
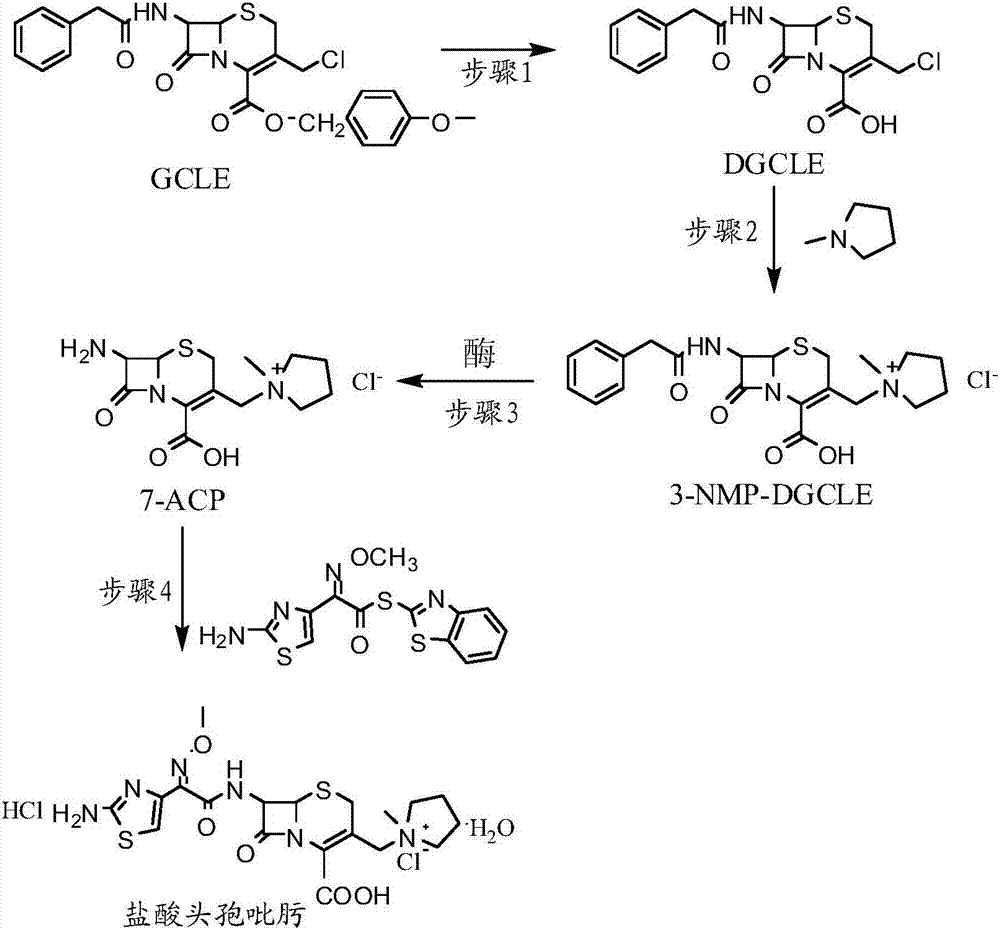
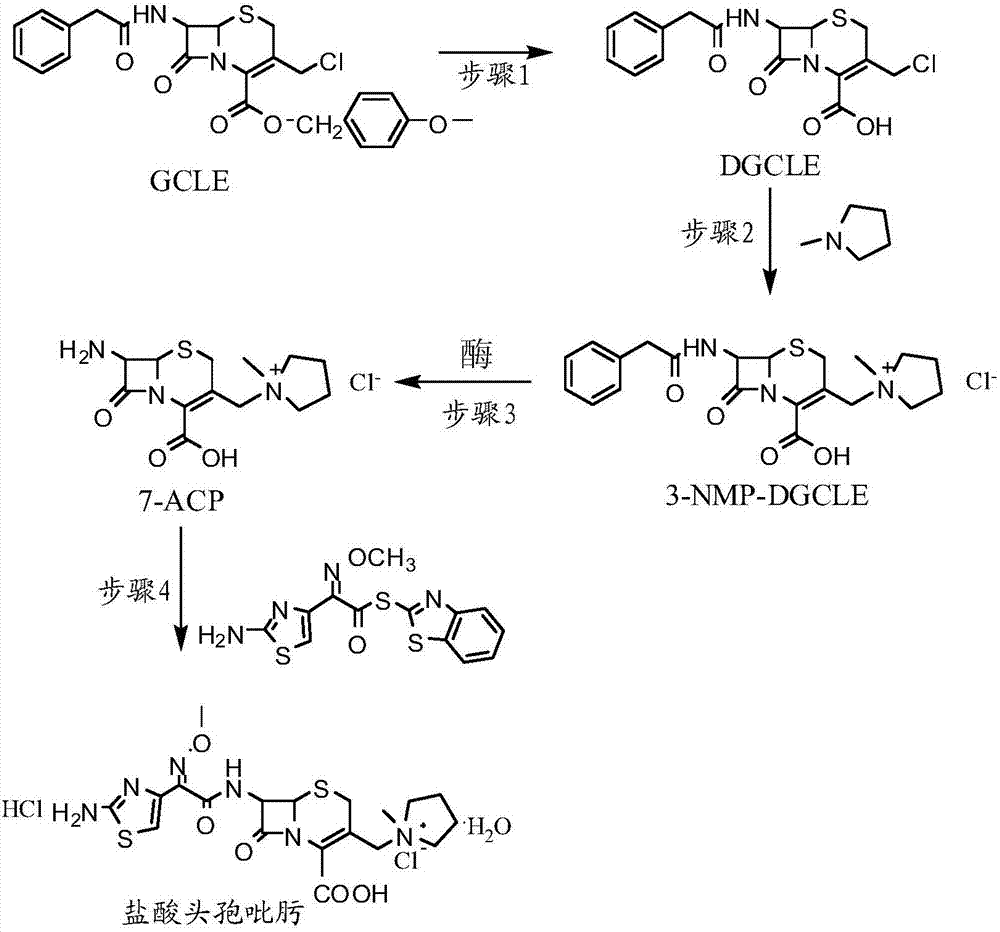
The invention provides a synthesis method of cefepime hydrochloride. The synthesis method comprises the following steps: taking GCLE (7-phenylacetamide-3-chloromethyl-3-cepham-4-carboxylic acid p-methyl-oxybenzyl ester) as a raw material; cutting a 4-site protecting group (p-methoxybenzyl) and enabling the 4-site protecting group to react with N-methylpyrrolidine (NMP); then cutting a 7-site protecting group through an enzyme method to obtain an immediate 7-amino-3-(1-methylpyrrolidine)methyl)-3-cepham-4-carboxylic acid hydrochloride (7-ACP); taking cheap and available methoxyiminoacetic acid mercaptobenzothiazole active ester (AE-active ester) and the 7-ACP to be subjected to 7-site acylation reaction, so as to finally prepare the cefepime hydrochloride. A route provided by the invention can be used for obtaining the high-yield and high-quality cefepime hydrochloride without a delta2 isomer. The synthesis method provided by the invention has the advantages of simple process, no harsh reaction conditions and the like and is very suitable for industrial production.
Owner:吉林省爱诺德生物工程有限公司
A kind of preparation method of cefepime hydrochloride
InactiveCN102276631AEffective protectionImprove industrial hygiene environmentOrganic chemistryCefepime hydrochlorideActivated carbon
The invention relates to a preparation of a pharmaceutical compound, in particular to a preparation method of cefepime hydrochloride. The present invention is an improvement after overcoming the shortcomings of the commonly used industrial production process and laboratory process. The improvement is that: 7-ACA adopts wet feeding when feeding; when 7-ACA is protecting, the protection used BSA is used as the agent; in the crystallization process of 7-MPCA, the process of adding crystallization solvent and growing crystals at room temperature is added; the amount of activated carbon used in the decolorization of cefepime hydrochloride is reduced.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Cefepime dihydrochloride preparation method suitable for industrial production
ActiveCN105859747AQuality improvementAvoid corrosionOrganic chemistryCefepime hydrochlorideStrong acids
The invention relates to the technical field of chemical medicine synthesizing, in particular to a cefepime dihydrochloride preparation method suitable for industrial production. The preparation method is characterized in that crude cefepime dihydrochloride is obtained by the acylation reaction of a compound I (7-MPCA) and a compound II (MAEM) and acidizing crystallization, and refining is performed to obtain the high-purify cefepime dihydrochloride. The preparation method has the advantages that a single solvent is used, and recycling and reusing are facilitated; the refining process is performed in an anhydrous system, product degradation under the strong acid condition during the refining process is reduced, and corrosion to production equipment is avoided; the acylation reaction and the refining are simple to operate, extremely high product conversion rate is achieved, production cost is low, and the method is suitable for industrial production of high-quality cefepime dihydrochloride.
Owner:QILU ANTIBIOTICS PHARMA
Cefepime hydrochloride combined medicament
ActiveCN101822679APain reliefReduced pyrogenic reactionAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSuperficial phlebitis
The invention discloses a cefepime hydrochloride combined medicament, which removes medicament heat and anaphylactic reaction of cefepime hydrochloride and adverse reactions thereof on liver damage, injection pain, phlebitis produced by injection and the like in the prior art. The cefepime hydrochloride combined medicament has the advantages of safe use, stable quality and reliable curative effect. A preparation process for the medicament is energy-saving, environmentally-friendly and pollution-free.
Owner:邓学峰
Method for preparing cefepime dihydrochloride and L-arginine mixed powder
The invention relates to a method for preparing a mixture powder of cefepime hydrochloride and L-arginine. The cefepime hydrochloride is dissolved in the water used for injection and then added with the L-arginine. The pH value of the solution is adjusted within a range from 4.0 to 6.0 and then the solution is filtered through a 0.22 micron filter membrane. The product, namely the powder mixed with cefepime hydrochloride and L-arginine, can be obtained after freeze drying. Such powder prepared through the method of freeze drying is good in homogeneity, quick in dissolution velocity and stable in content of product, water content, pH value and other major quality indexes.
Owner:QILU ANTIBIOTICS PHARMA
Cephalo olefine onium salt compound and its preparing method, and method for synthesizing cephalo pyoxime with said compound
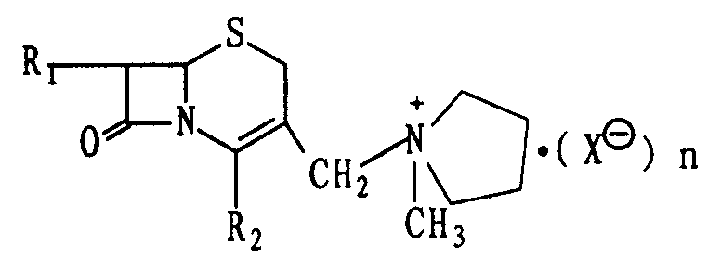
InactiveCN1392149AMild reaction conditionsReduce manufacturing costOrganic chemistryCefepime hydrochlorideMedicinal chemistry
The present invention relates to 7beta-alkylamido-3-(1-methyl-1-pyrrolidyl onium) methyl-3-cephalo olefine-4-carboxylate and its preparation process and the synthesizing process of cephalo pyroxime hydrochloride with the said intermediate. The present invention has mild reaction condition, no need of expensive reagent and benzyl penicillin acylase, low cost, high product purity, and simple and practical reaction path.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for preparing cefepime dihydrochloride
The invention provides a method for preparing cefepime dihydrochloride. The method employs the raw materials of 7-ACA, HMDS, TMSI, NMP and CH3OH, and a catalyst is hydrochloric acid having 98% of concentration. According to the invention, the method employs a one kettle way, a cefepime dihydrochloride crude product is prepared, then the cefepime dihydrochloride crude product is purified, and finally cefepime dihydrochloride is obtained. The purity of the cefepime dihydrochloride is 97%, yield can reach 31.3%, compared with the current synthesis technology, the yield is increased by 3.3-10% (by metering with 7-ACA), and the purity is increased by 4%. The preparation method has the advantages that the industrial production requirements of short reaction period, easily available raw materials and low cost can be satisfied, and has industrial application.
Owner:CHANGCHUN UNIV OF TECH
Cefepime hydrochloride/arginine pharmaceutical composition suspension injection
InactiveCN101773507AImprove stabilityEnsure product quality is qualifiedAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideArginine
The invention discloses a cefepime hydrochloride / arginine pharmaceutical composition suspension injection and a preparation method thereof. The suspension injection is characterized in that the injection is prepared by mixing cefepime hydrochloride suspension particles with arginine, wherein the weigh ratio of cefepime hydrochloride suspension particles (calculated by cefepime hydrochloride) to arginine is 1:0.62-0.75. Specifically, the cefepime hydrochloride suspension particles are prepared from the following components in parts by weight: 1 part of cefepime hydrochloride, 3.4-5.8 parts of surfactant, 0.05-0.8 part of antioxidant and 4.5-7.7 parts of support agent. More specifically, surfactant is composed of sodium deoxycholate and poloxamer 188 in a weight ratio of 4:1. The method of the invention adopts emulsification technology to prepare the cefepime hydrochloride suspension particles, and then the particles are mixed with arginine, and the mixture is subpackaged to obtain the cefepime hydrochloride / arginine pharmaceutical composition suspension injection, thus solving the problems that cefepime hydrochloride has poor stability and short expiration date and is easy to modify under the actions of light or heat, and obtaining satisfactory technical effects.
Owner:HAINAN LINGKANG PHARMA CO LTD
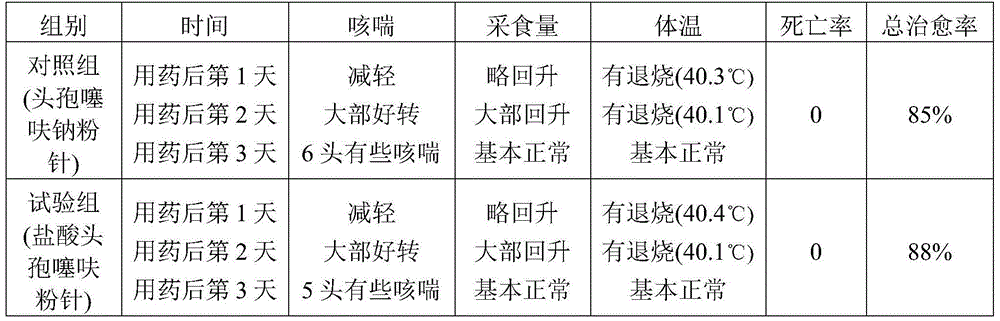
Ceftiofur hydrochloride powder injection as well as preparation method and application thereof
ActiveCN104586777AProlong the action time of concentrationWell mixedAntibacterial agentsPowder deliveryCefepime hydrochlorideFreeze-drying
The invention belongs to the technical field of veterinary medicines, and particularly relates to a ceftiofur hydrochloride powder injection as well as a preparation method and an application thereof. The ceftiofur hydrochloride powder injection is prepared from the following effective components ceftiofur hydrochloride and a cosolvent at the weight ratio of (48-52) to (28-34). Ceftiofur sodium is replaced with the ceftiofur hydrochloride; veterinary cefepime hydrochloride powder injection is prepared by weighing the effective components and the cosolvent at the weight ratio of (48-52) to (28-34), mixing evenly, and sub-packaging; and the veterinary cefepime hydrochloride powder injection can be produced and stored at a room temperature. According to the ceftiofur hydrochloride powder injection, the problems that an existing veterinary cefepime hydrochloride powder injection needs to be produced by a freeze-drying method, the raw materials and the preparation need to be stored at a low temperature of 4-10 DEG C, the placement time in a room-temperature or high-temperature season is overlong, and the medication effect is poor due to degradation are solved; the prepared powder injection has the same antibacterial effect as a ceftiofur sodium freeze-dried powder injection; and the ceftiofur hydrochloride powder injection is low in cost and wide in application prospect.
Owner:广东省天宝生物制药有限公司
Method for preparing cefepime hydrochloride
InactiveCN102675345AHigh crystal purityImprove liquidityOrganic chemistryCefepime hydrochlorideReflux
The invention discloses a method for preparing cefepime hydrochloride. The method comprises the following steps of: adding hexamethyldisilazane and trimethylsilyl iodide into 7-aminocephalosporanic acid (ACA), performing vacuum reflux at the temperature of 55 DEG C for 12h, reducing the temperature, diluting, adding N-methylpyrrolidone and trimethylsilyl iodide, reacting at the temperature of 40 DEG C for 23h, after-treating and recrystallizing to obtain 7-ACMP.HCl, dissolving the 7-ACMP.HCl into a solvent at the temperature of 0 to 5 DEG C, adding a small amount of AE-active ester and triethylamine for multiple times, and treating to obtain the cefepime hydrochloride after the reaction is finished. The method for preparing the cefepime hydrochloride is high in reaction speed, low in production cost, and high in product purity and flowability.
Owner:苏州盛达药业有限公司
Purification method of cefepime dihydrochloride
ActiveCN109776572AIncrease contentLess impuritiesOrganic chemistryCefepime hydrochloridePurification methods
The invention discloses a purification method of cefepime dihydrochloride, and belongs to the field of chemical pharmacy. The method comprises the following steps: cefepime dihydrochloride arginine istaken as a raw material, a complexing agent is added in the dissolving solution of the cefepime dihydrochloride arginine for decoloration; and a dispersant and a crystallization agent are added intothe decolorated solution for crystallization, so as to finally obtain the cefepime dihydrochloride. The purification method can enable the cefepime dihydrochloride arginine which does not meet the quality standard to be fully recycled, therefore the prepared cefepime dihydrochloride has the advantages of high content, less impurities and high stability; and the preparation method is simple, energy-saving and environmental-friendly, thereby being suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefepime dihydrochloride compound as well as preparation method and medicine composition thereof
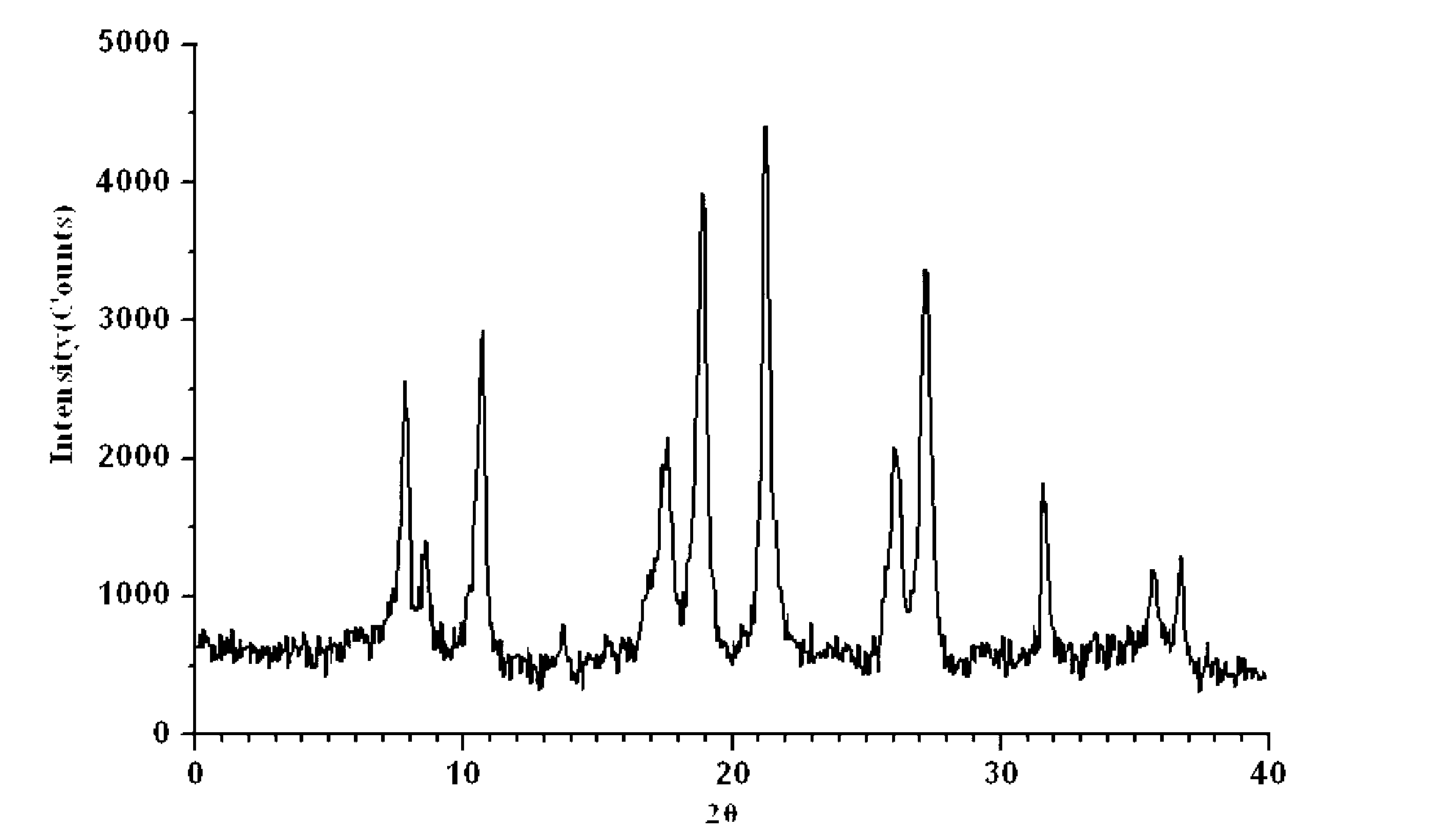
InactiveCN103304580ALow impurity contentGood storage stabilityAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideX-ray
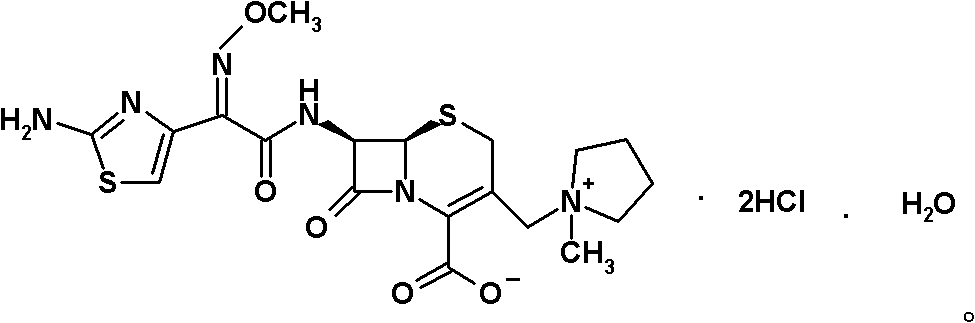
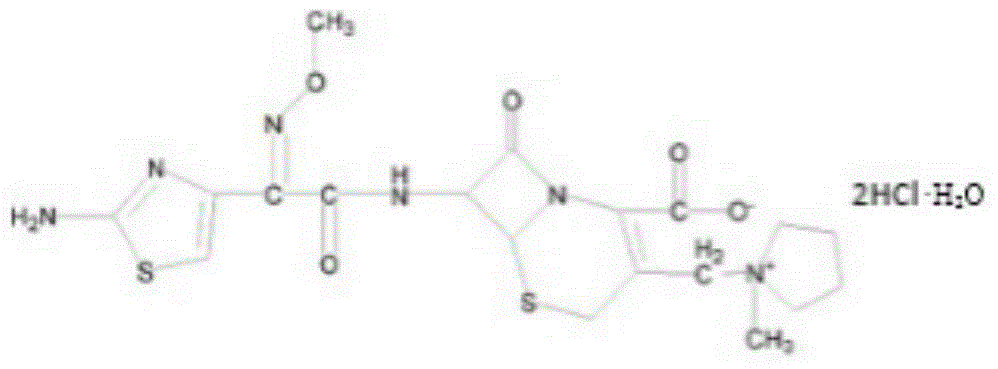
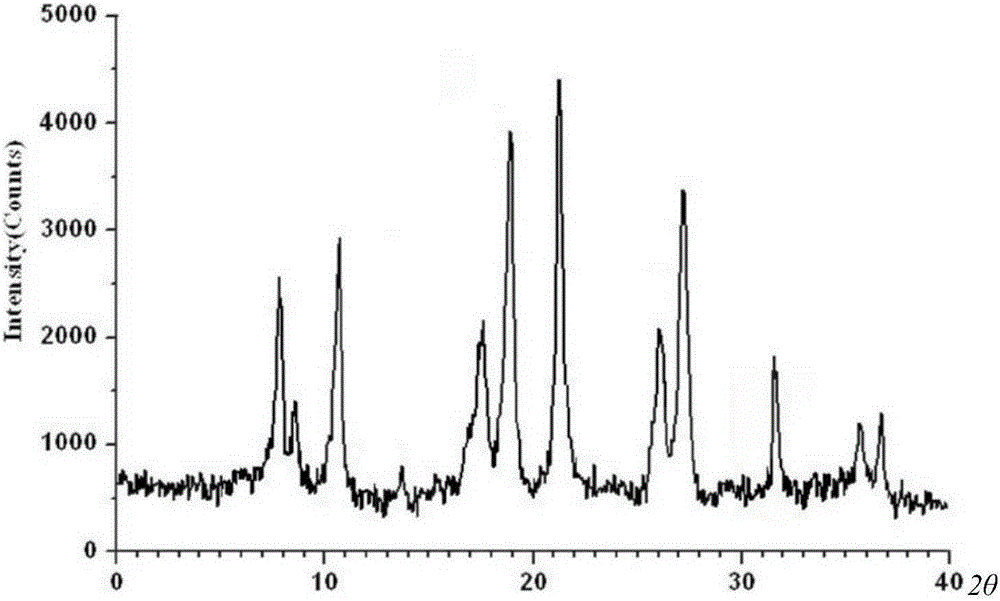
The invention belongs to the technical field of medicines, and in particular relates to a cefepime dihydrochloride compound. The structural formula of the cefepime dihydrochloride compound is as shown in the specification; and the X-ray powder diffraction spectrogram obtained through measurement by using Cu-Kalpha rays, of the cefepime dihydrochloride compound is as shown in figure 1. The invention further provides a preparation method of the cefepime dihydrochloride compound, a medicine composition containing the cefepime dihydrochloride compound and the preparation method of the medicine composition. The cefepime dihydrochloride compound is prepared into the dosage form of sterility powder-injection. Compared with the prior art, the cefepime dihydrochloride compound and the medicine composition thereof have better storage stability, and the medication safety of patients is greatly improved.
Owner:四川省惠达药业有限公司
Cefepime hydrochloride compound prepared by new synthetic method
InactiveCN101638412AHigh purityAntibacterial agentsOrganic chemistryCefepime hydrochlorideAcetic acid
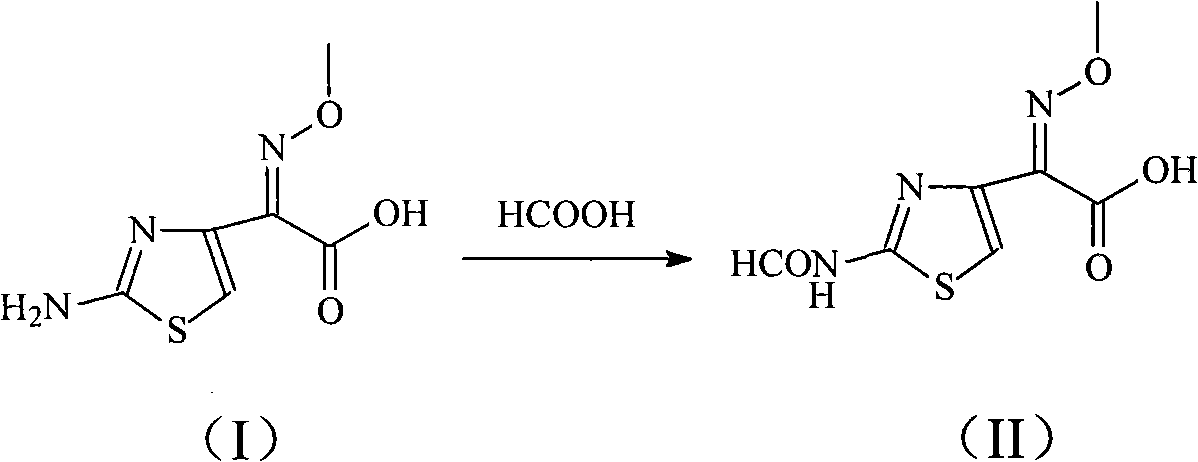
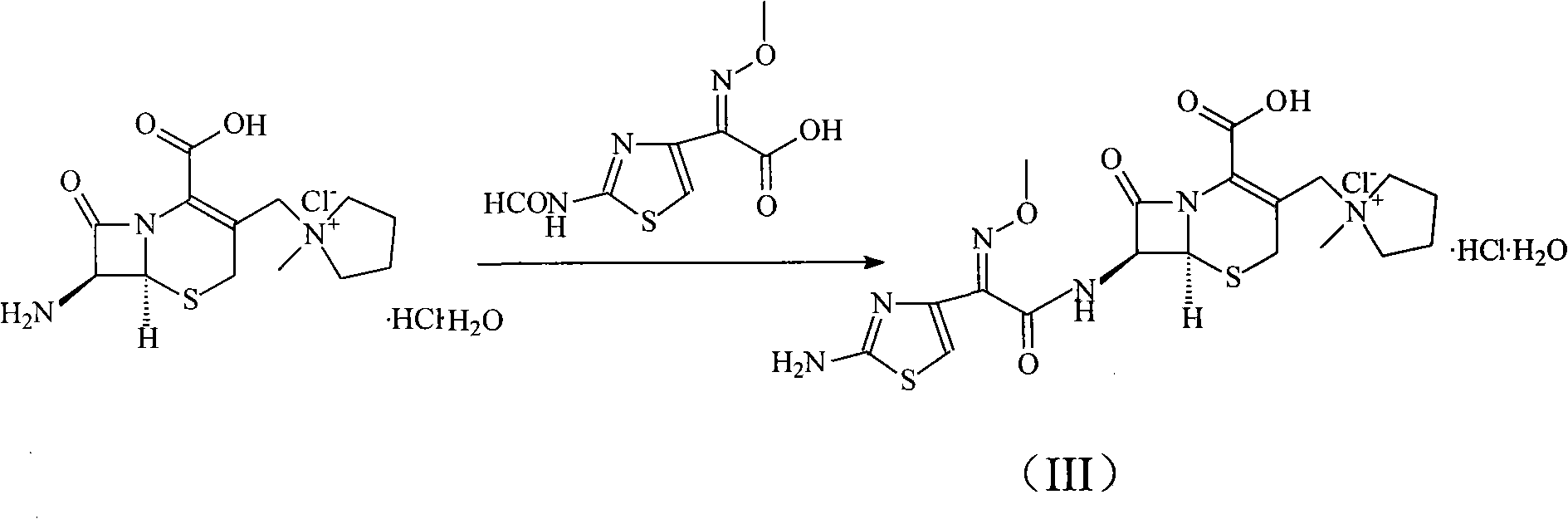
The invention relates to a cefepime hydrochloride compound prepared by a new synthetic method. The method comprises the following steps: first enabling aminothiazoly loximate to react with formic acidto generate 2-(2-formammidotiezol-4-group)-2-methoxyimino acetic acid, then adding 7-MPYCA and triethylamine, taking N,N-diisopropylethylamine and N,N-dimethylformamide as solvents and p-toluenesulfonyl chloride as a catalyst, stirring the above substances to react to prepare the cefepime hydrochloride.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefepime hydrochloride composition for injection and its preparation method
ActiveCN102824304AReasonable compositionSimple preparation processAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideArginine
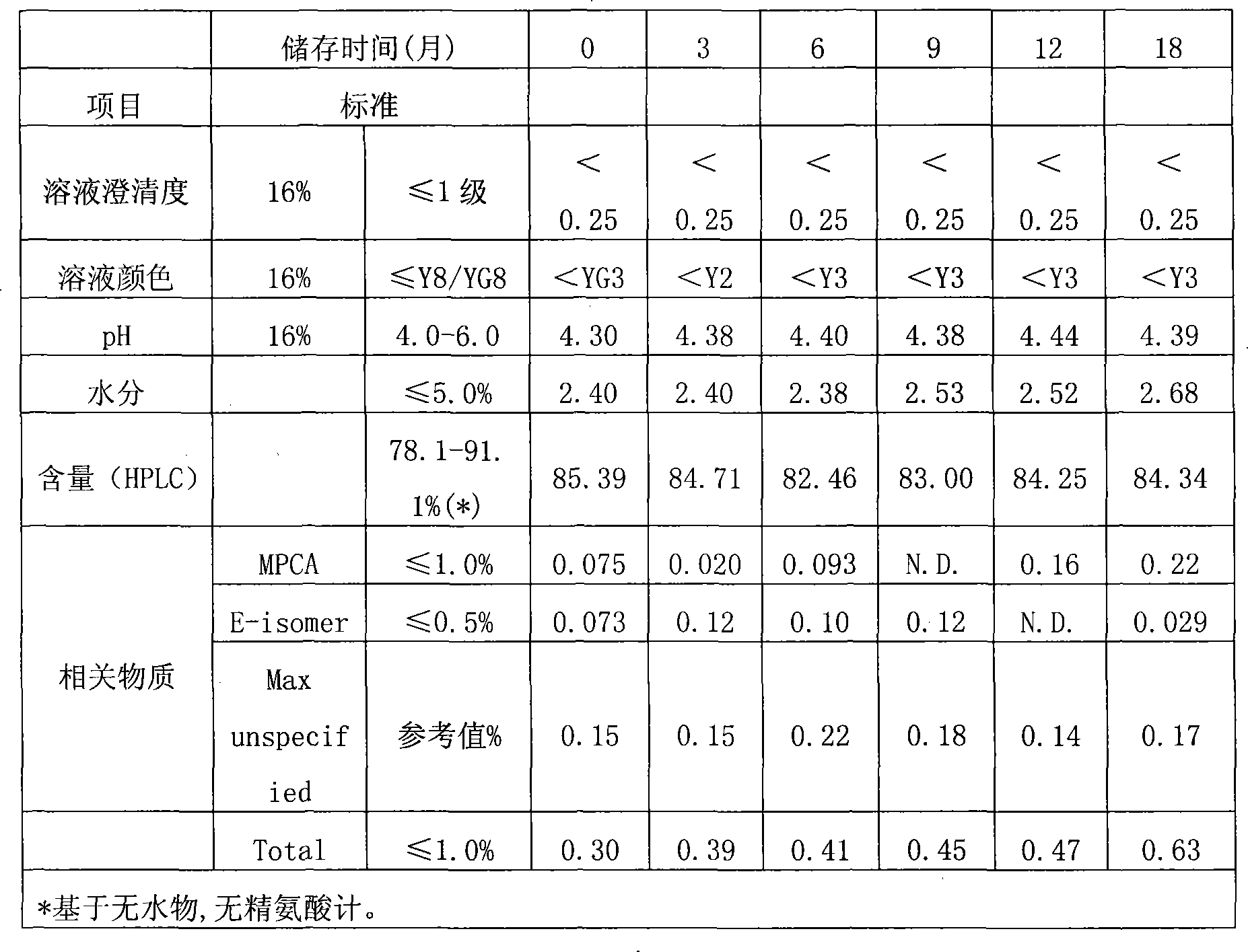
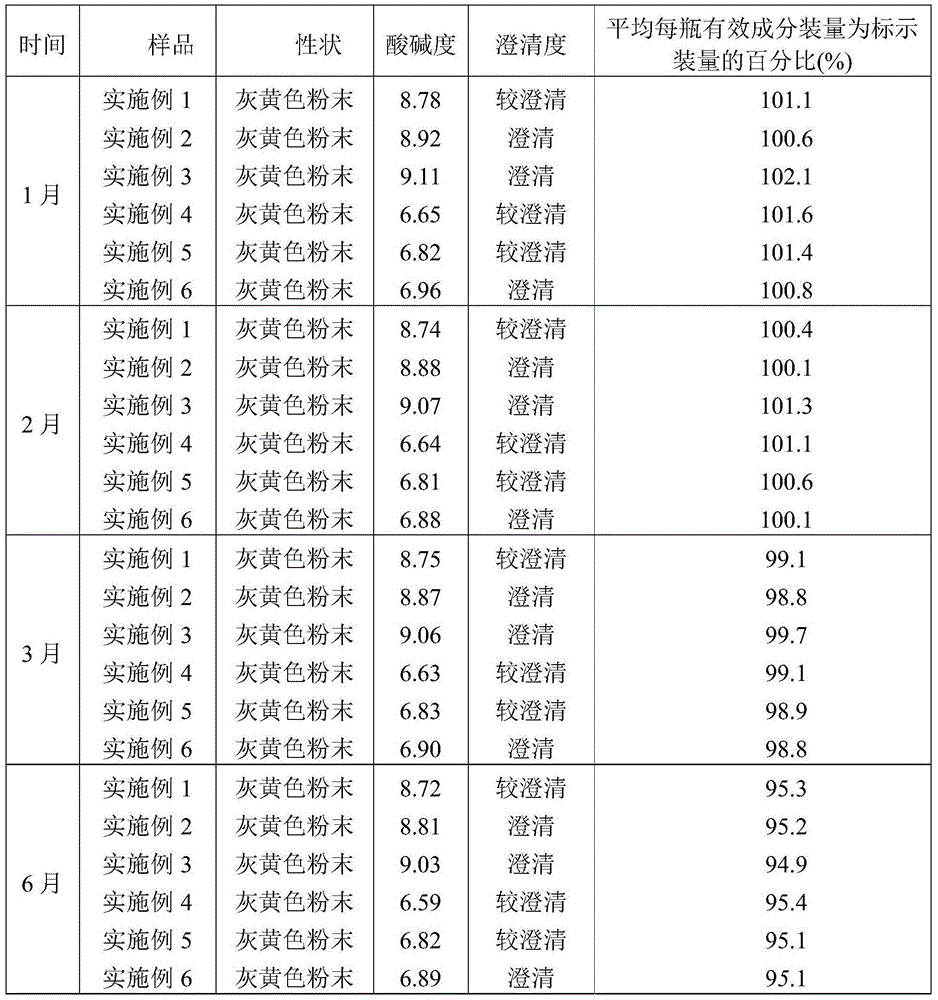
The invention discloses a cefepime hydrochloride composition for injection. The composition comprises a raw material and auxiliary materials, wherein the raw material is 1000 parts by weight of cefepime hydrochloride and the auxiliary materials contain 710 parts by weight of L-arginine and 10-20 parts by weight of L-lysine. The pH value of the cefepime hydrochloride composition can maintain between 4.0 and 6.0 for a long time. After 12 months of an accelerated test and 24 months of long-term sample storage for observation, contents of related substances are all less than 1%. The invention also aims to provide a preparation method of the cefepime hydrochloride composition for injection. According to the preparation method, the raw materials of cefepime hydrochloride, L-arginine and L-lysine are directly mixed. The preparation technology is simple, and the quality is stable and reliable.
Owner:上海欣峰制药有限公司
Preparation method of cefepime hydrochloride having content of genotoxic impurity 2-mercaptobenzothiazole reduced
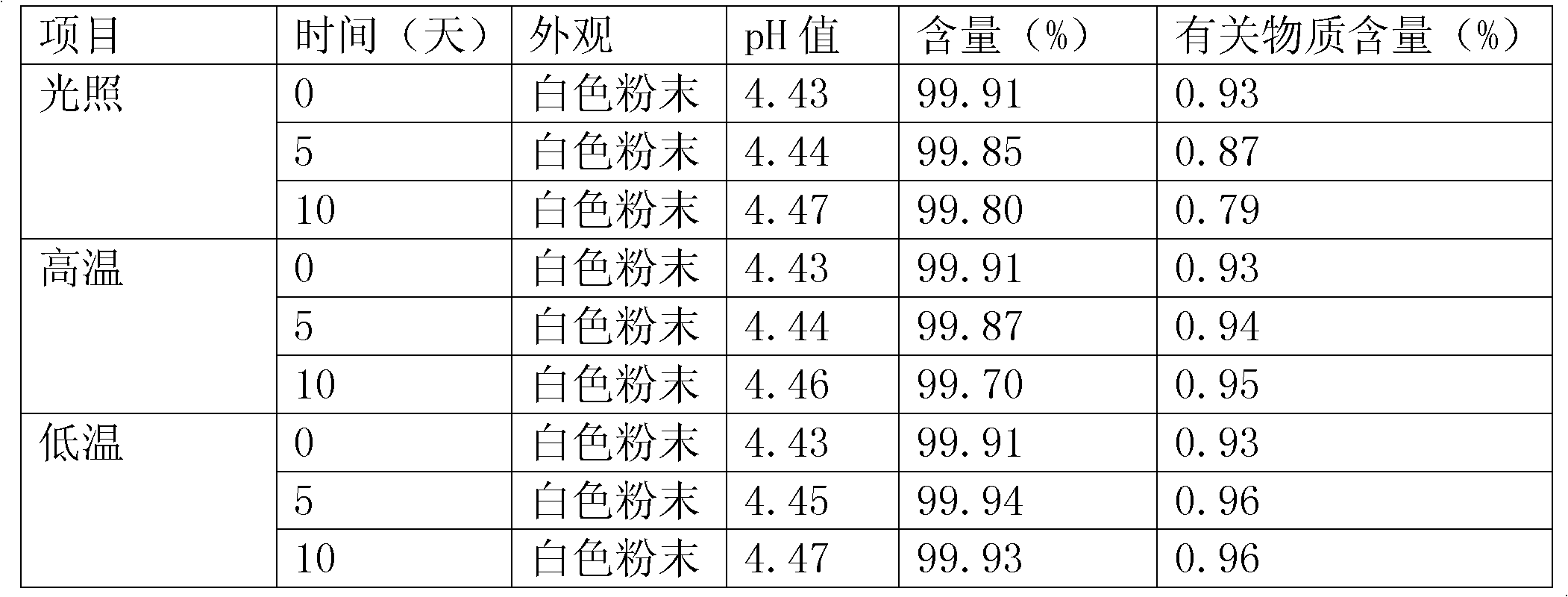
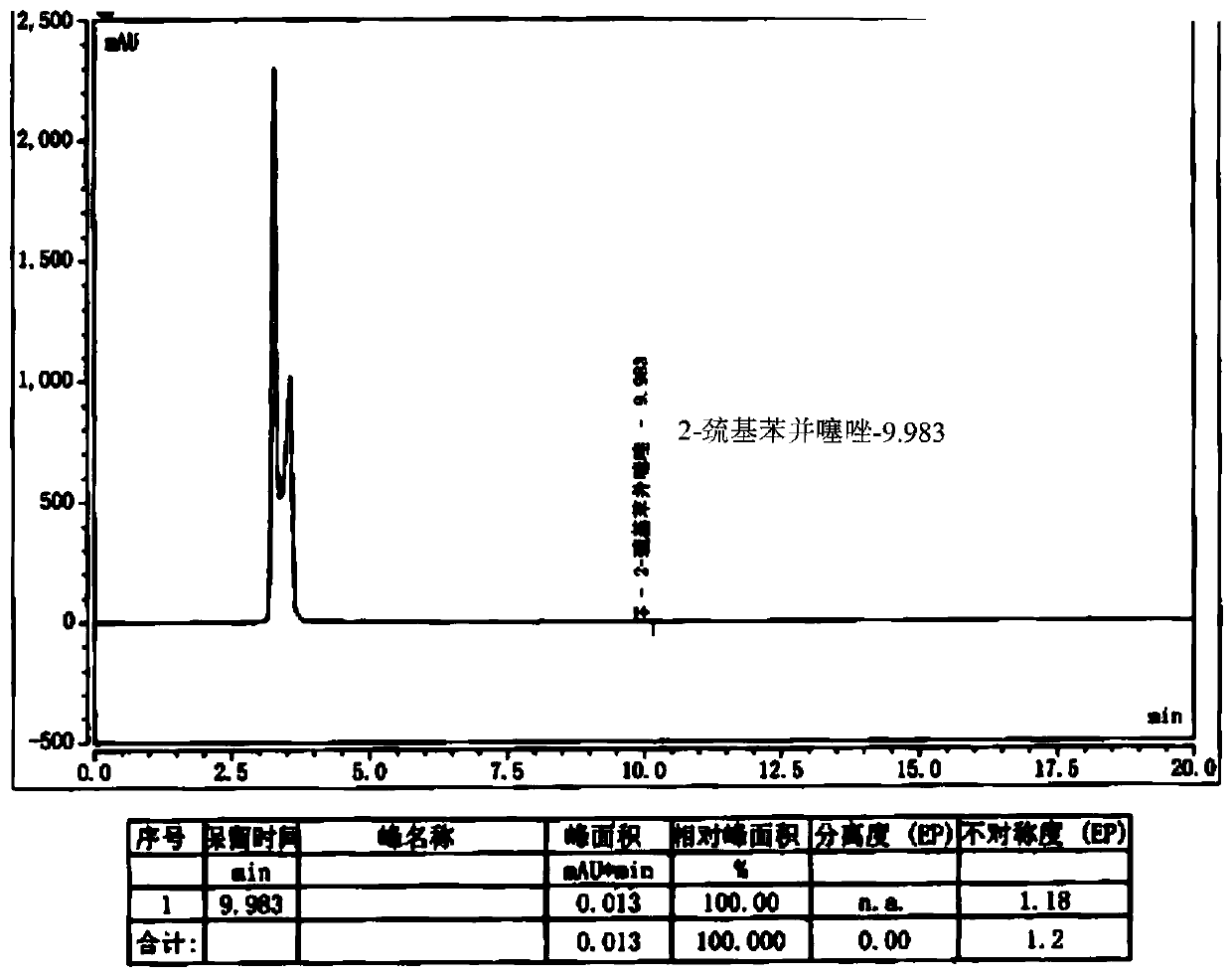
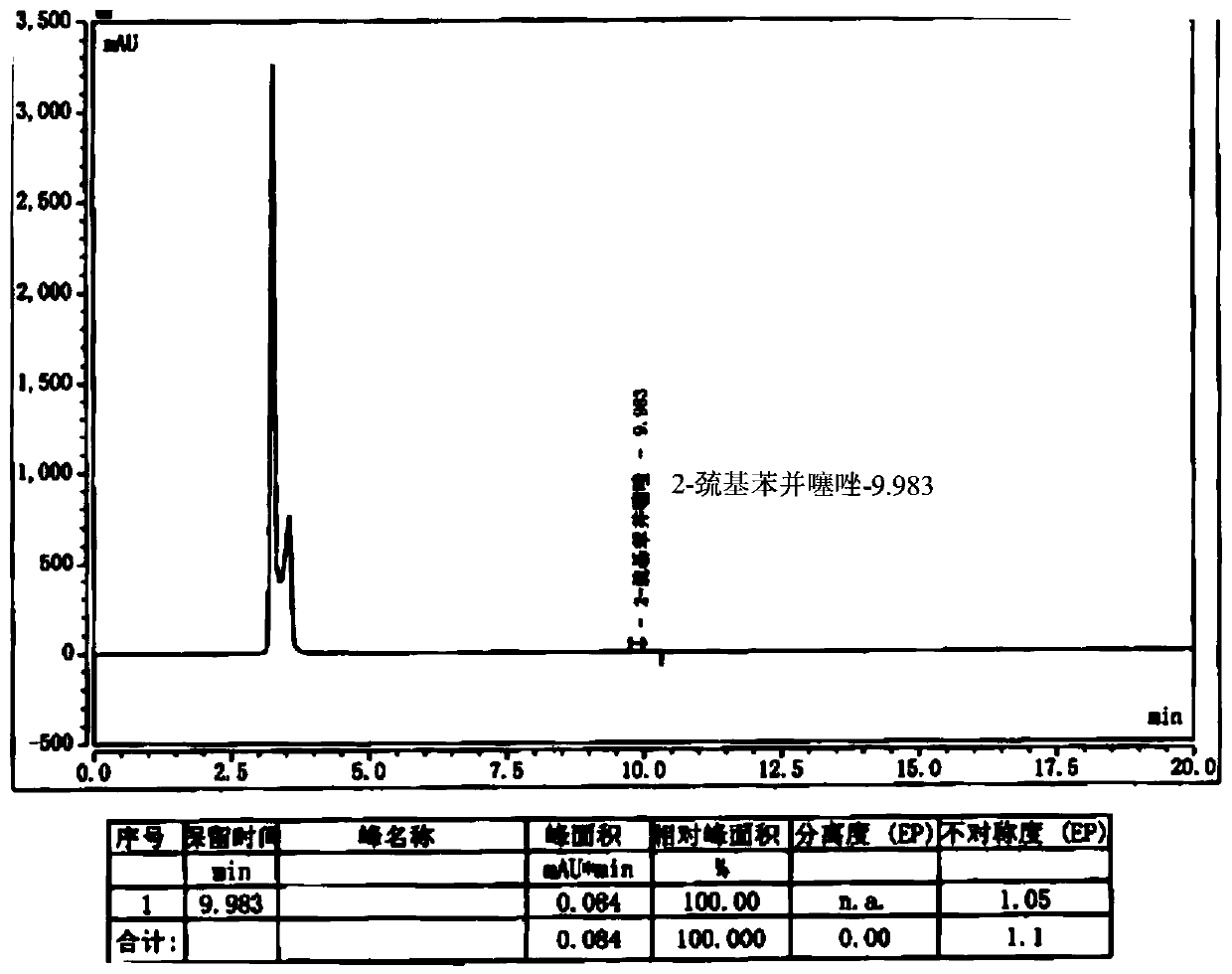
ActiveCN110655528AThe purpose of reducing residueImprove securityOrganic chemistryCefepime hydrochlorideSide chain
The invention belongs to the field of medicinal chemistry, and relates to a preparation method of cefepime hydrochloride. The preparation method comprises the steps: in a dichloromethane solvent, in the presence of triethylamine, carrying out acylation reaction on a cefepime side chain compound and AE active ester; after the reaction, adding water into the reaction liquid, and extracting to obtaina water phase; adding hydrochloric acid into the water phase to adjust the pH value of the water phase to 2.0-2.5, then adding a water-insoluble organic solvent, extracting, standing for layering, and separating out the water phase; adjusting the pH value of the water phase to 1.0-1.5 with hydrochloric acid after activated carbon decoloration, and adding a crystallization solvent to obtain cefepime hydrochloride. According to the cefepime hydrochloride prepared by the preparation method disclosed by the invention, the content of a genotoxic impurity 2-mercaptobenzothiazole in the cefepime hydrochloride is reduced to 1-5 mmp, even 1-3 mmp, so that the safety of a cefepime hydrochloride medicine is improved.
Owner:GUANGZHOU HC PHARM CO LTD
Cefepime hydrochloride proliposome preparation
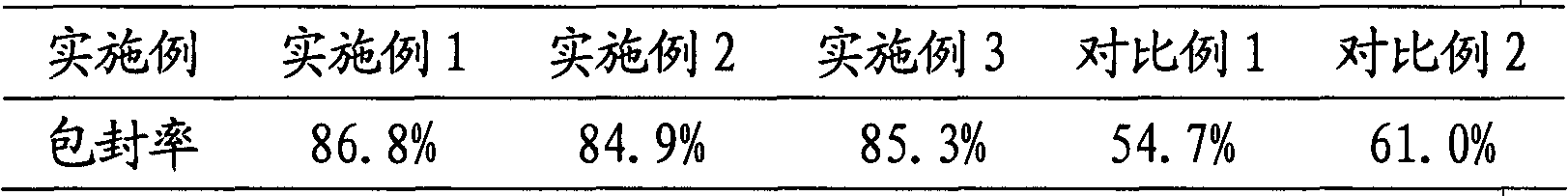
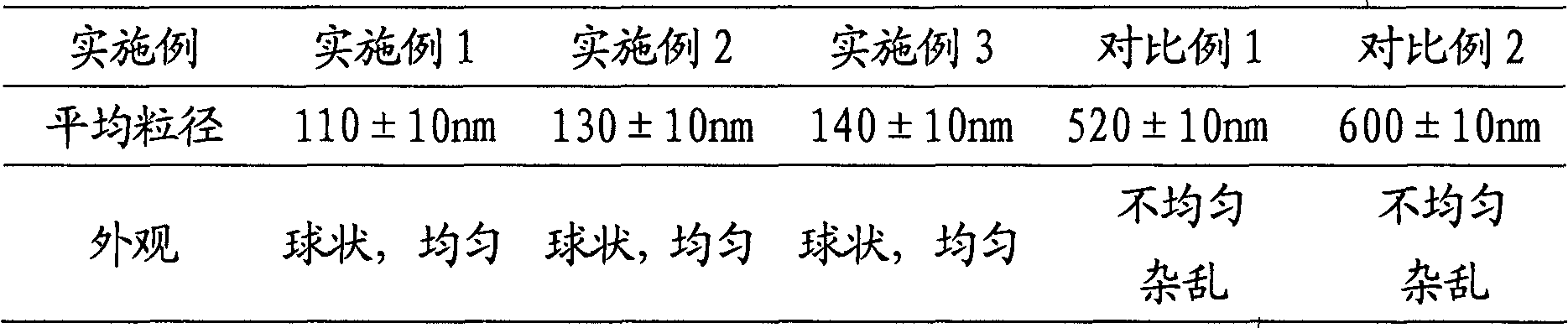
InactiveCN101623260AWon't breakEncapsulation efficiency will not decreaseAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideProcess equipment
The invention provides cefepime hydrochloride proliposome which comprises the following components by weight part: 3-20 parts of cefepime hydrochloride, 5-40 parts of dipalmitoyl phosphatidyl glycerol, 1-30 parts of cholesterol and 3-50 parts of proppant which consists of sodium chloride and mannitol with a weight ratio of 1:4. The cefepime hydrochloride proliposome greatly improves the stability, has in-vivo degradation of drug carrier liposome, no toxicity and no immunogenicity and can improve the medical therapeutic index and reduce drug toxicity and side effects. The cefepime hydrochloride proliposome can be prepared by conventional processing equipment and industrially and efficiently produced and has low production cost.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefepime hydrochloride medicine composition, powder-injection thereof and preparation method thereof
InactiveCN102743390AGood pH stabilityUniform product qualityAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSide effect
The invention relates to a cefepime hydrochloride medicine composition, a powder-injection thereof and a preparation method thereof. The medicine composition comprises the following ingredients: cefepime hydrochloride, L-arginine and hydroxypropyl-beta-cyclodextrin, wherein the weight of the L-arginine is 40% of that of the cefepime hydrochloride, and the weight of the hydroxypropyl-beta-cyclodextrin is 10% of that of the cefepime hydrochloride. The medicine composition is good in mixing uniformity, has small pH value change in subpackaging and transportation processes, is small in gastrointestinal tract side effect, and greatly improves the stability and safety of the medicine.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Cefepime hydrochloride medicine composition, powder-injection thereof and preparation method thereof
InactiveCN102743390BImproves pH stabilityUniform product qualityAntibacterial agentsPowder deliveryCefepime hydrochlorideSide effect
The invention relates to a cefepime hydrochloride medicine composition, a powder-injection thereof and a preparation method thereof. The medicine composition comprises the following ingredients: cefepime hydrochloride, L-arginine and hydroxypropyl-beta-cyclodextrin, wherein the weight of the L-arginine is 40% of that of the cefepime hydrochloride, and the weight of the hydroxypropyl-beta-cyclodextrin is 10% of that of the cefepime hydrochloride. The medicine composition is good in mixing uniformity, has small pH value change in subpackaging and transportation processes, is small in gastrointestinal tract side effect, and greatly improves the stability and safety of the medicine.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
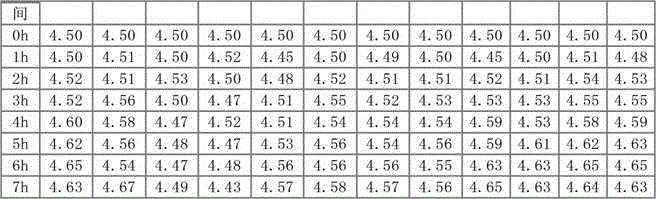
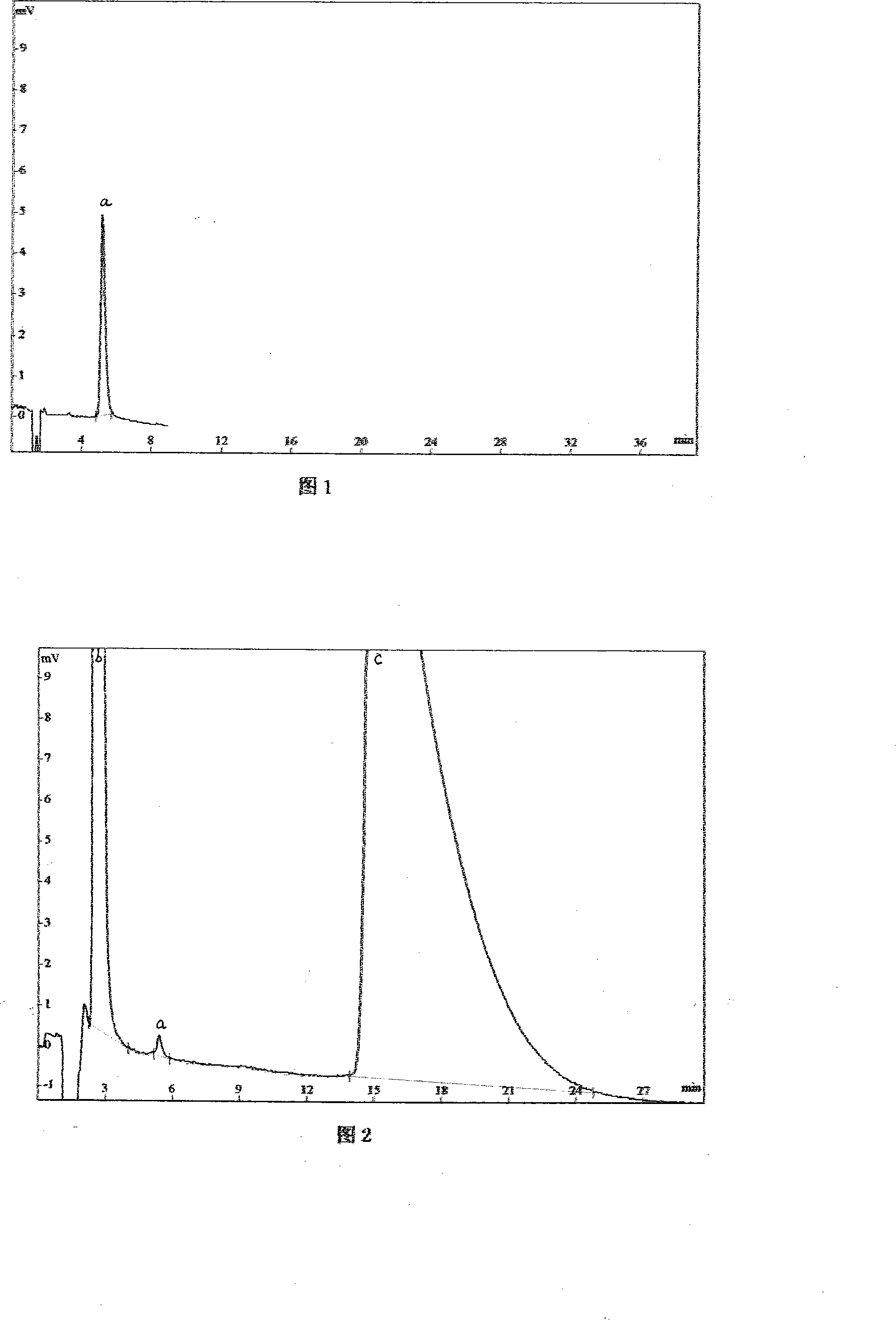
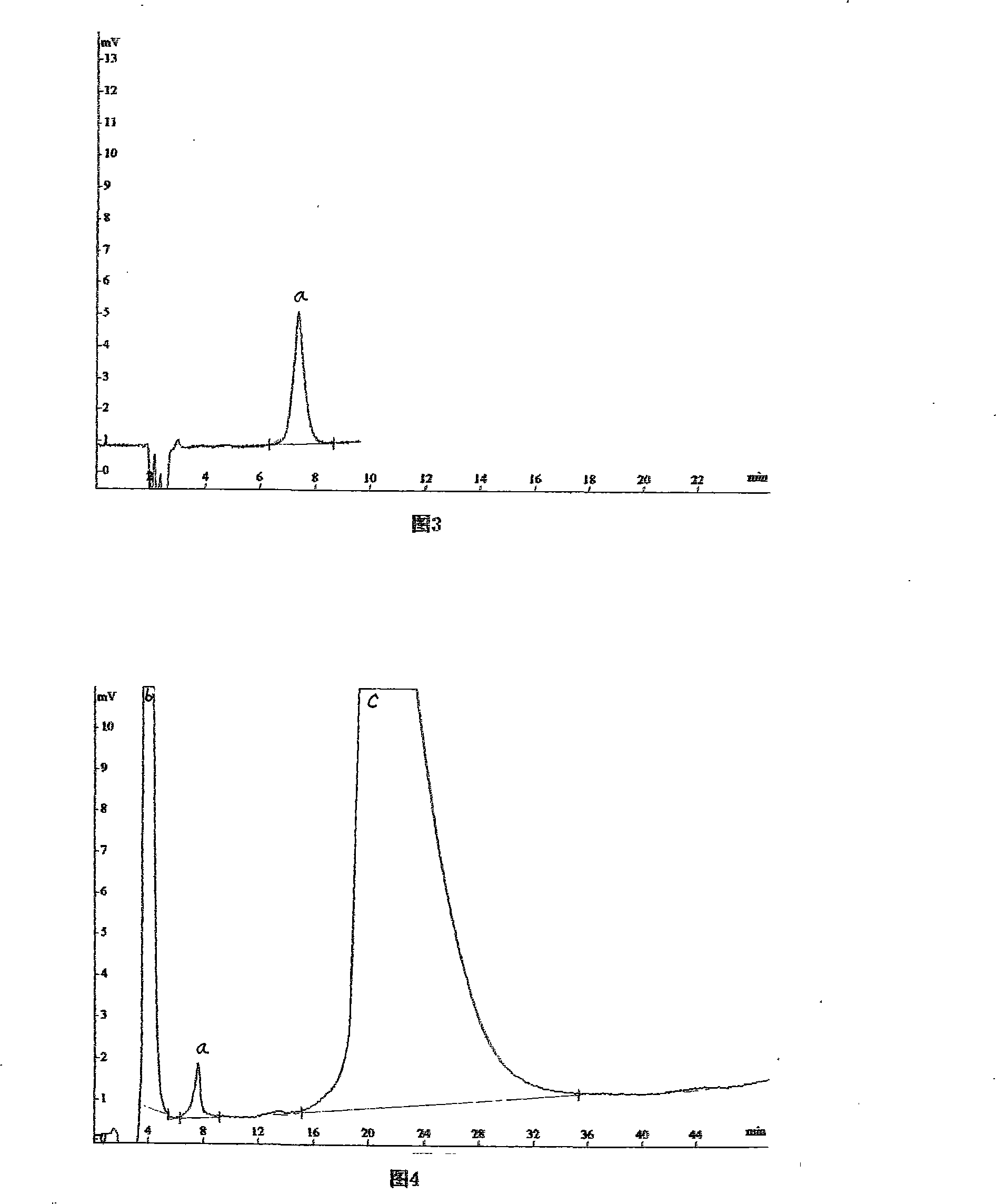
Hydrochloric acid cefepime raw material and method for measuring content of N-methyl pyrrolidine in preparation thereof
ActiveCN101226174AImprove balanceShort measurement timeComponent separationCefepime hydrochlorideRetention time
The invention relates to a measurement method of N-methylpyrrolidine content of cefepime dihydrochloride material and relative agent, for evaluating the quality of cefepime dihydrochloride material and relative agent. The invention discloses a liquid chromatograph which comprises using carboxylic cationic column as analysis column, uses a conductive detector to check, preparing flow phase, preparing sample solution and reference substance solution, using test method to respectively inject the sample solution and reference substance solution into a liquid chromatograph, recording high pressure liquid chromatographs, and calculating the N-methylpyrrolidine content of the sample. The inventive chromatograph system has easy balance, short sample test time, better repeatability in preserved time, stable baseline, durable column, high quality of chromatograph peak of N-methylpyrrolidine, better repeatability, accurate, simple and reliable process.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Cefepime hydrochloride composition for injection and its preparation method
ActiveCN102824304BReasonable compositionSimple preparation processAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideArginine
The invention discloses a cefepime hydrochloride composition for injection. The composition comprises a raw material and auxiliary materials, wherein the raw material is 1000 parts by weight of cefepime hydrochloride and the auxiliary materials contain 710 parts by weight of L-arginine and 10-20 parts by weight of L-lysine. The pH value of the cefepime hydrochloride composition can maintain between 4.0 and 6.0 for a long time. After 12 months of an accelerated test and 24 months of long-term sample storage for observation, contents of related substances are all less than 1%. The invention also aims to provide a preparation method of the cefepime hydrochloride composition for injection. According to the preparation method, the raw materials of cefepime hydrochloride, L-arginine and L-lysine are directly mixed. The preparation technology is simple, and the quality is stable and reliable.
Owner:上海欣峰制药有限公司
Cefepime dihydrochloride compound and pharmaceutical composition thereof
ActiveCN104610283AHigh purityIncrease contentAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSide effect
The invention discloses a cefepime dihydrochloride compound. The cefepime dihydrochloride compound is prepared by a following method comprising the following steps: (1) dissolving a cefepime dihydrochloride crude product into water and performing membrane separation and impurity removal by selecting a separating membrane with the molecular weight cut off of 500-3000; (2) adding the impurity-removed solution into an organic solvent, then adding active carbon into the obtained solution, stirring, filtering the solution to remove charcoal, and collecting the filter solution; (3) separating and purifying the filter solution with a chromatographic column, wherein a mobile phase is a mixed solvent of isopropanol and acetonitrile, and a stationary phase filler is silica gel or aluminum oxide; and (4) concentrating the separated and purified filter solution, and performing spray-drying to obtain the cefepime dihydrochloride compound. The invention also discloses a pharmaceutical composition containing the cefepime dihydrochloride compound, wherein the pharmaceutical composition is sterile powder for injection. The cefepime dihydrochloride compound and the pharmaceutical composition disclosed by the invention have low impurity content and few toxic and side effects, so that the medication safety of the patient is greatly improved.
Owner:YOUCARE PHARMA GROUP
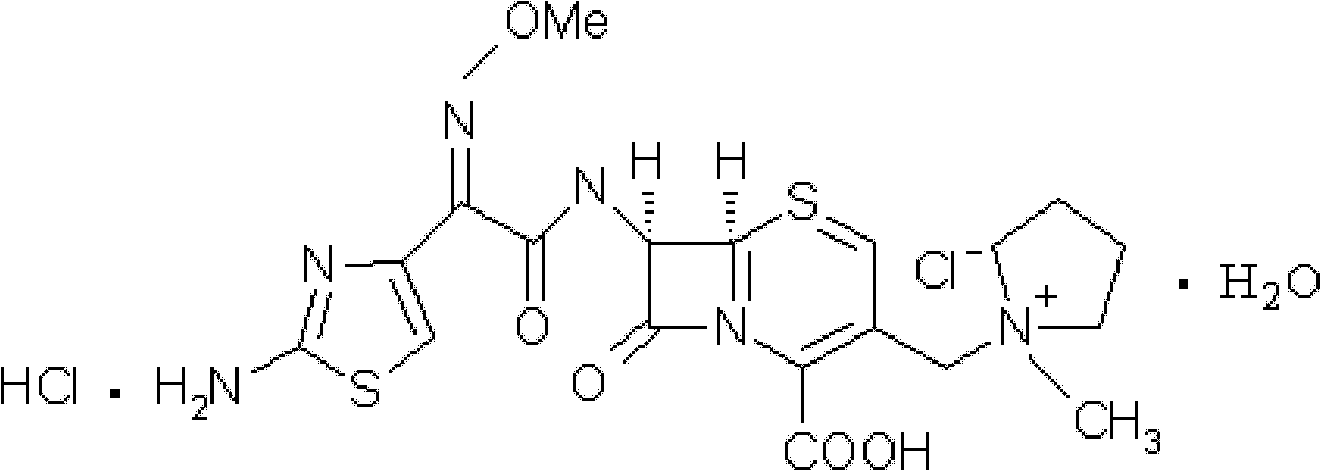
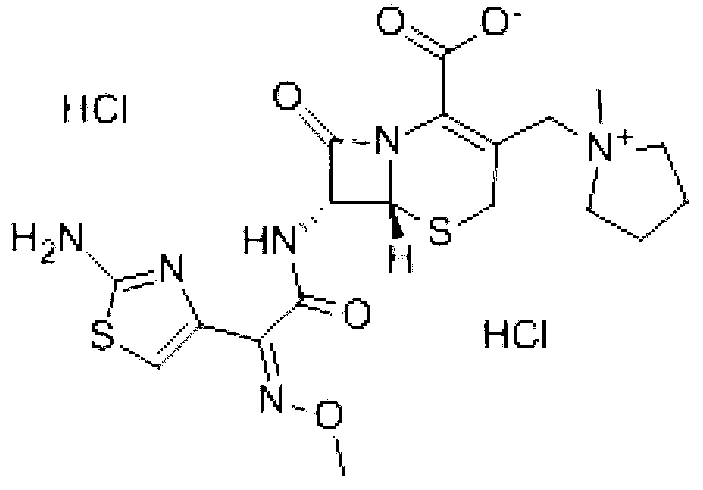
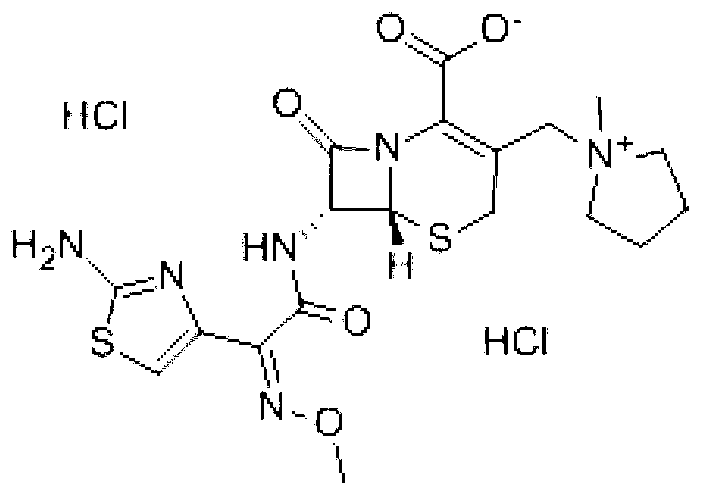
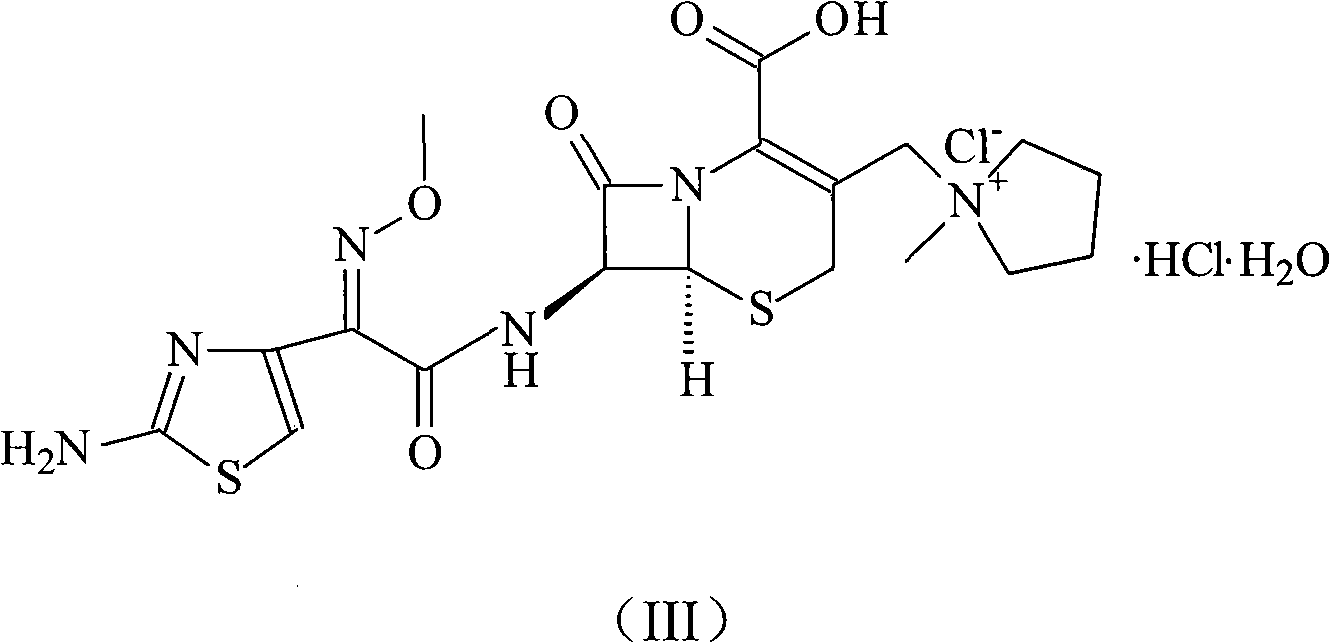
A kind of cefepime hydrochloride compound
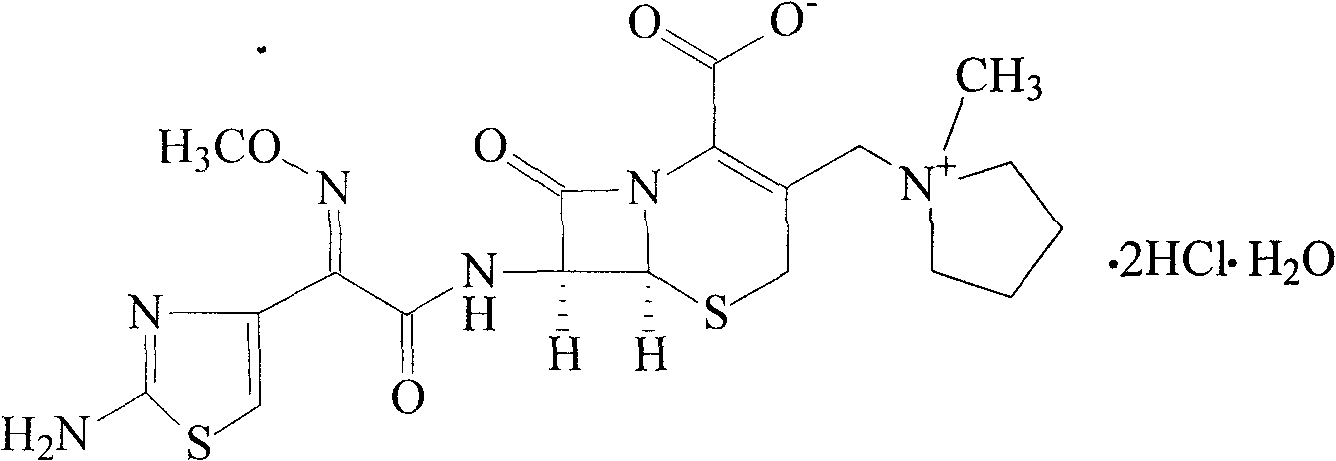
ActiveCN103804396BImprove bioavailabilityEasy to separateOrganic chemistryCefepime hydrochlorideElemental analysis
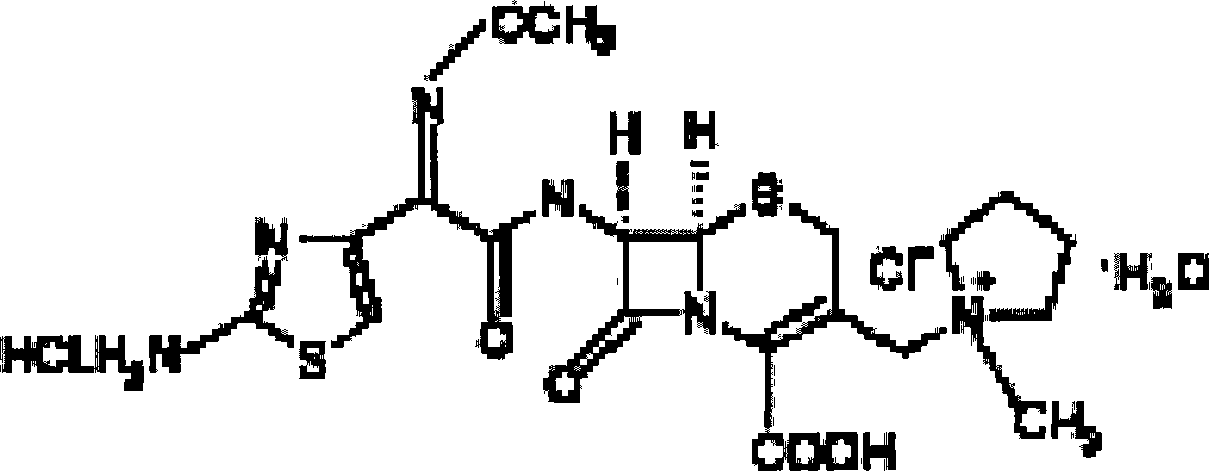
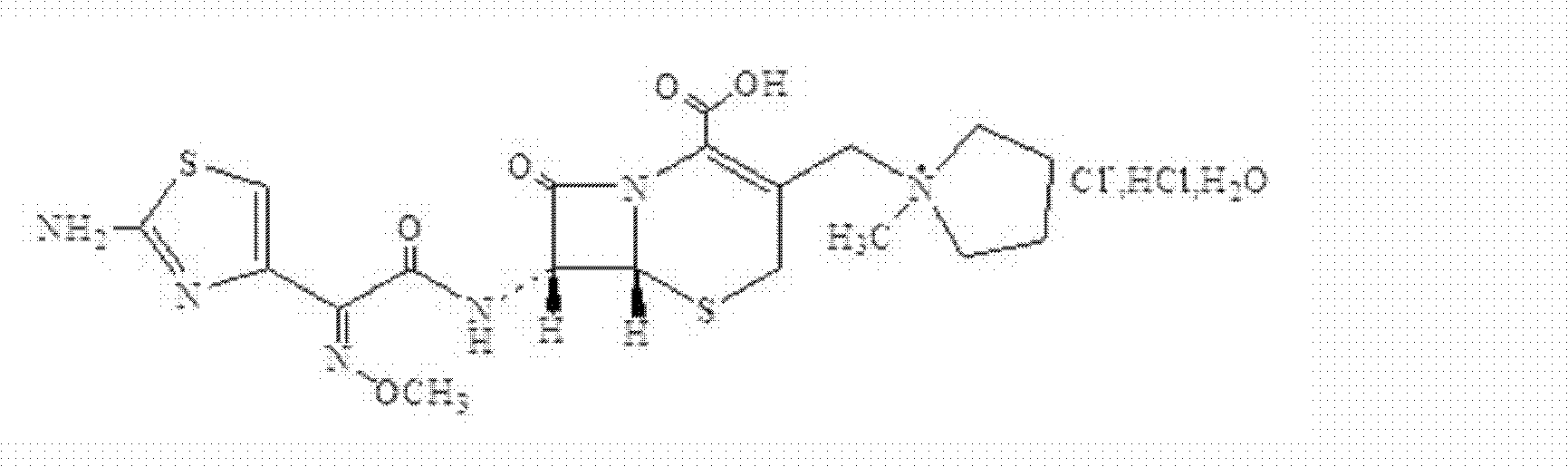
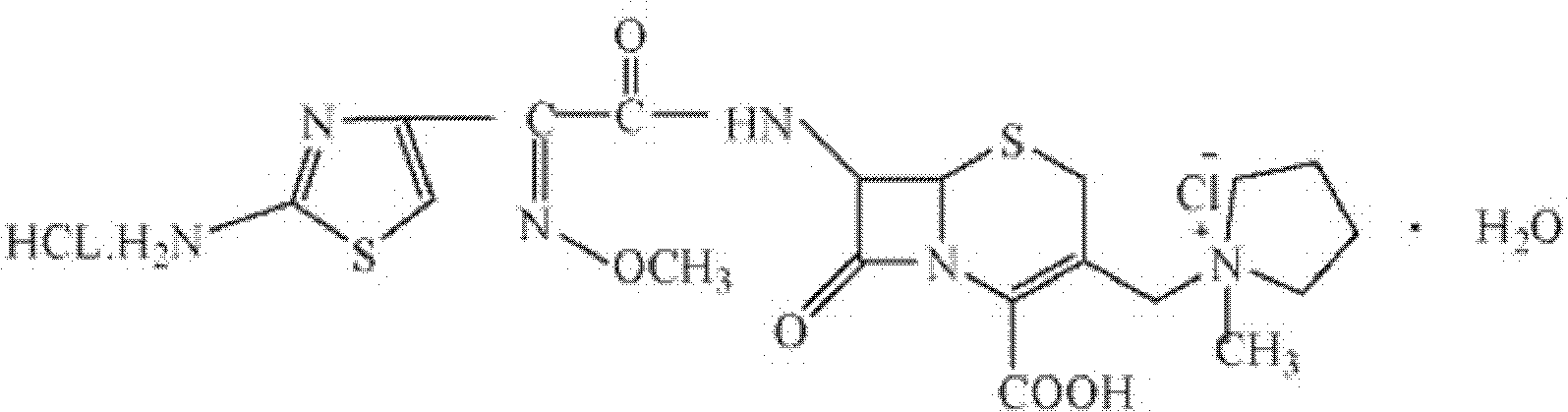
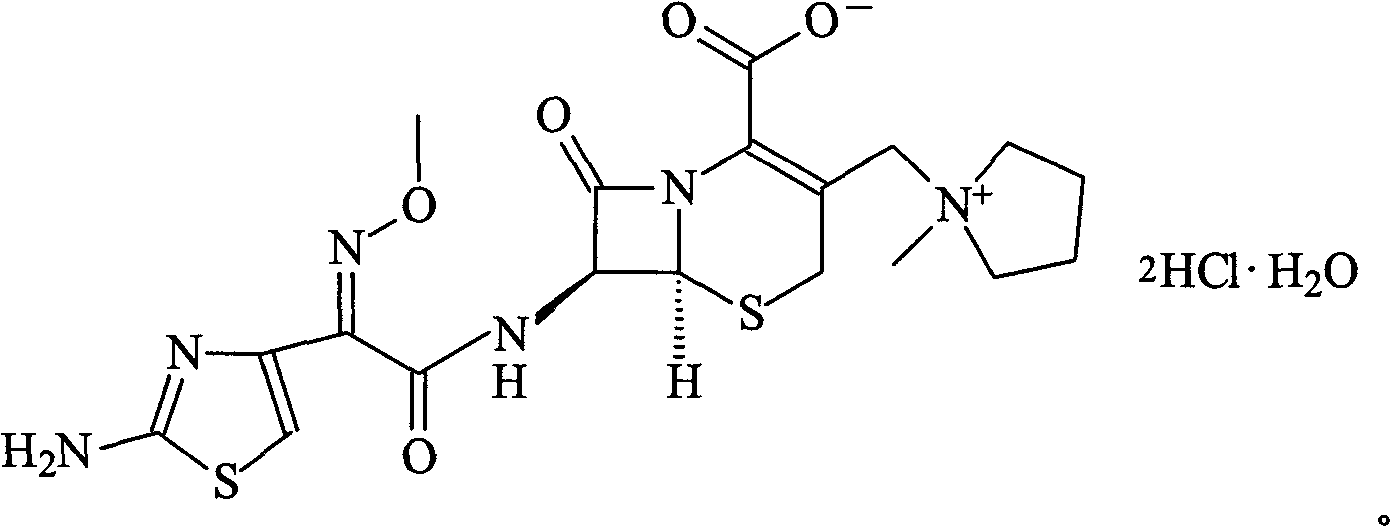
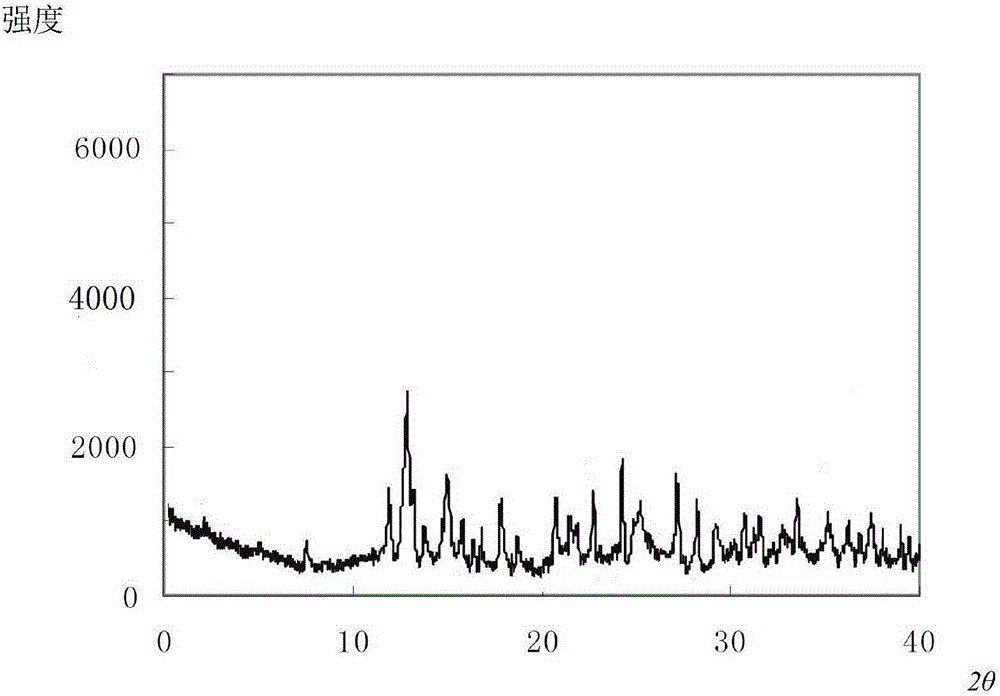
The invention relates to the field of pharmacy, and particularly relates to a cefepime dihydrochloride compound. The cefepime dihydrochloride is crystal, and an X-ray powder diffraction diagram measured by using a Cu-K alpha ray is shown in a figure 2. The purity of the cefepime dihydrochloride compound disclosed by the invention can be up to 99.95-99.98%; the molecular formula of the cefepime dihydrochloride obtained by elemental analysis and differential thermal analysis is C19H25ClN6O5S2.2HCl.H2O. The solvent used in the crystallization method disclosed by the invention is low in content, andthe cefepime dihydrochloride compound is safe and reliable in clinical application. A stability experiment proves that the cefepime dihydrochloride crystal compound provided by the invention is good in stability, and the prepared cefepime dihydrochloride compound is lower than that in the prior art in hygroscopicity, better than that in the prior art in bioavailability, and more applicable to clinical application.
Owner:YOUCARE PHARMA GROUP +1
Hydrochloric acid cefepime raw material and method for measuring content of N-methyl pyrrolidine in preparation thereof
ActiveCN101226174BImprove balanceShort measurement timeComponent separationCefepime hydrochlorideTested time
The invention relates to a measurement method of N-methylpyrrolidine content of cefepime dihydrochloride material and relative agent, for evaluating the quality of cefepime dihydrochloride material and relative agent. The invention discloses a liquid chromatograph which comprises using carboxylic cationic column as analysis column, uses a conductive detector to check, preparing flow phase, preparing sample solution and reference substance solution, using test method to respectively inject the sample solution and reference substance solution into a liquid chromatograph, recording high pressureliquid chromatographs, and calculating the N-methylpyrrolidine content of the sample. The inventive chromatograph system has easy balance, short sample test time, better repeatability in preserved time, stable baseline, durable column, high quality of chromatograph peak of N-methylpyrrolidine, better repeatability, accurate, simple and reliable process.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Synthesis method of cefepime hydrochloride
InactiveCN113024581ALow costEasy to handleOrganic chemistryBulk chemical productionCefepime hydrochlorideCarboxylic acid
The invention relates to a synthesis method of cefepime hydrochloride, which comprises the following steps: by taking 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid diphenylmethyl ester hydrochloride as a raw material, protecting amino by using di-tert-butyl dicarbonate ester, reacting with N-methylpyrrolidine, and removing 4-site and 7-site protecting groups by using acid to obtain an intermediate 7-amino-3-(1-methyltetrahydropyrrole)methyl)-3-cephem-4-carboxylic acid hydrochloride (7-ACP); and then carrying out an acylation reaction with aminothiazide sulfhydryl benzothiazole active ester (AE-active ester) to obtain the product. The route has the advantages of simple reaction treatment, harsh reaction conditions, less isomer generation, high yield, high purity and simple process, and the method is suitable for industrial production.
Owner:刀鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com