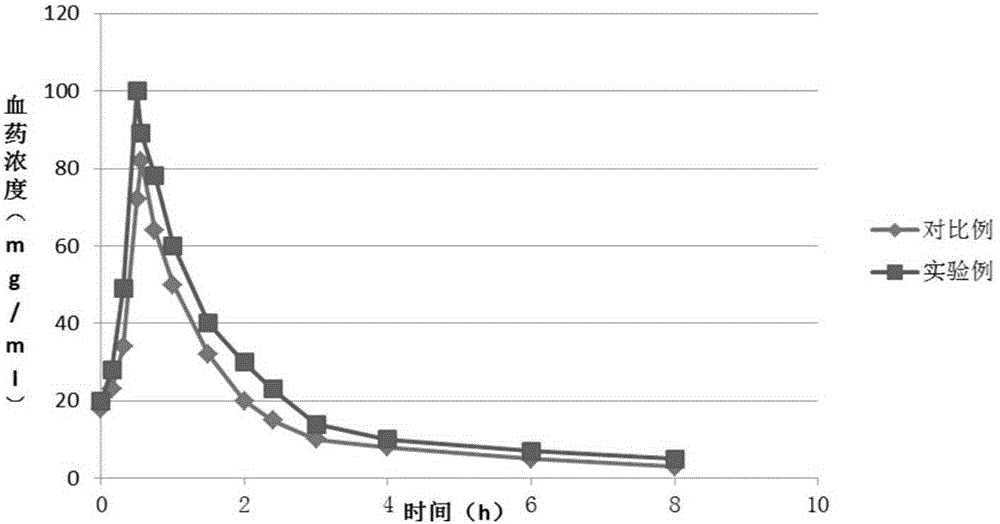
A kind of cefepime hydrochloride compound
A technology for cefepime hydrochloride and compounds, which is applied in the field of cefepime hydrochloride compounds, can solve the problems of biological activity that needs to be further tested, and achieve the effects of safe and reliable clinical application, easy separation and collection, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Prepare 2L of aqueous solution of crude cefepime hydrochloride 0.6g / ml;
[0034] 2. In a sound field with a frequency of 30KHz and an output power of 30W, under the condition of nitrogen filling, add tetrahydrofuran at 5°C while stirring. The volume of tetrahydrofuran added is 4L, and the addition speed is 20ml / min;
[0035] 3. After adding tetrahydrofuran, adjust the frequency of the sound field to 25KHz, and continue to add 5L of a mixed solution of isopropanol and cyclohexane at 3°C at a speed of 100ml / min; remove the sound field after adding the mixed solvent, and cool down to 0°C , the cooling rate is 0.25° C. / hour; the crystal is grown for 6 hours, washed and dried to obtain the cefepime hydrochloride compound.
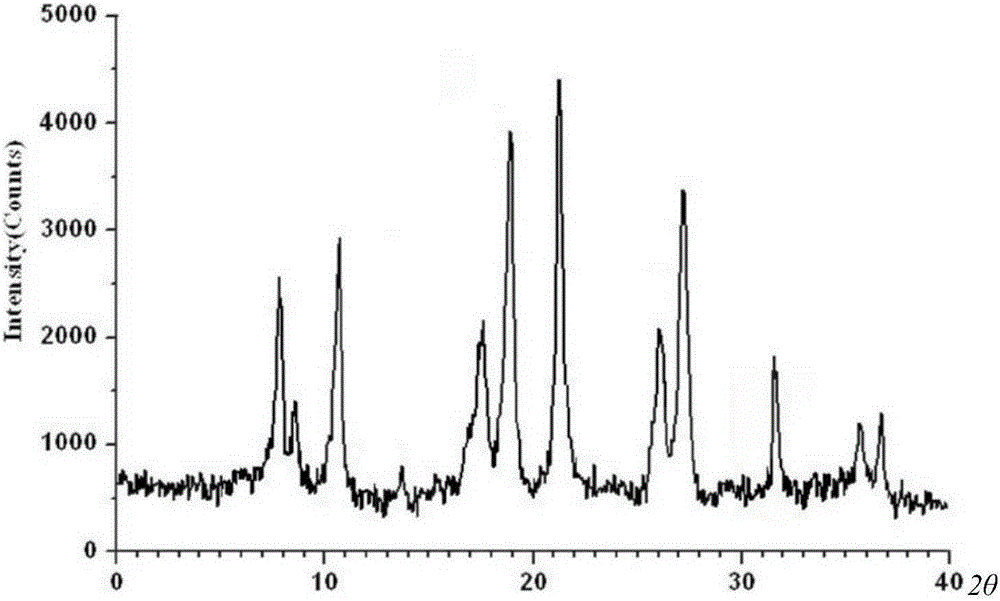
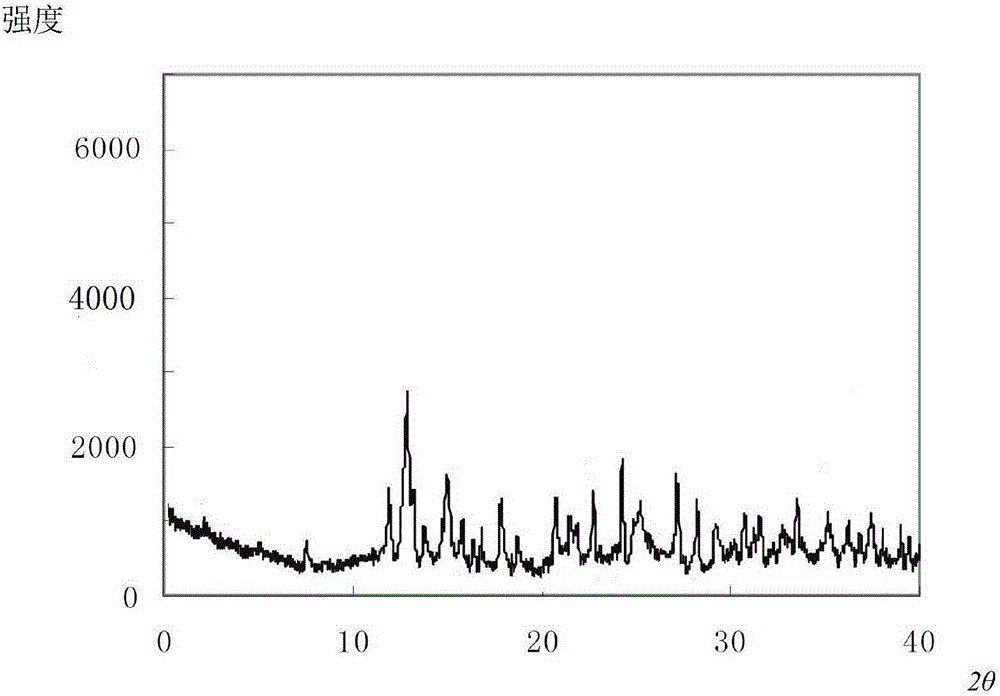
[0036] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 96.8%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 2 As shown; thro...
Embodiment 2
[0038] 1. Prepare 2 L of an aqueous solution of 0.5 g / ml crude product of cefepime hydrochloride;
[0039] 2. In a sound field with a frequency of 28KHz and an output power of 30W, under nitrogen filling conditions, add tetrahydrofuran at 8°C while stirring; the volume of tetrahydrofuran added is 3L; the addition speed is 20ml / min;
[0040]3. After adding tetrahydrofuran, adjust the frequency of the sound field to 22KHz, and continue to add 4L of a mixed solution of isopropanol and cyclohexane at 1°C at a speed of 40ml / min. After adding the mixed solvent, remove the sound field and cool down to 0°C , the cooling rate was 0.2° C. / hour, the crystal was grown for 4 hours, washed and dried to obtain the cefepime hydrochloride compound.
[0041] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 96.8%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 2 As shown; t...
Embodiment 3
[0043] 1. Prepare 2L of aqueous solution of crude cefepime hydrochloride 0.6g / ml;
[0044] 2. In a sound field with a frequency of 26KHz and an output power of 40W, under the condition of nitrogen filling, add tetrahydrofuran at 6°C while stirring. The volume of tetrahydrofuran added is 4L, and the addition speed is 20ml / min;
[0045] 3. After adding tetrahydrofuran, adjust the frequency of the sound field to 21KHz, and continue to add 5L of a mixed solution of isopropanol and cyclohexane at 1°C at a speed of 50ml / min; remove the sound field after adding the mixed solvent, and cool down to 0°C , the cooling rate is 0.25° C. / hour, the crystal is grown for 6 hours, washed and dried to obtain the cefepime hydrochloride compound.
[0046] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 96.8%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 2 As shown; through...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com