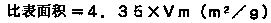Patents
Literature
254 results about "Differential thermal analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
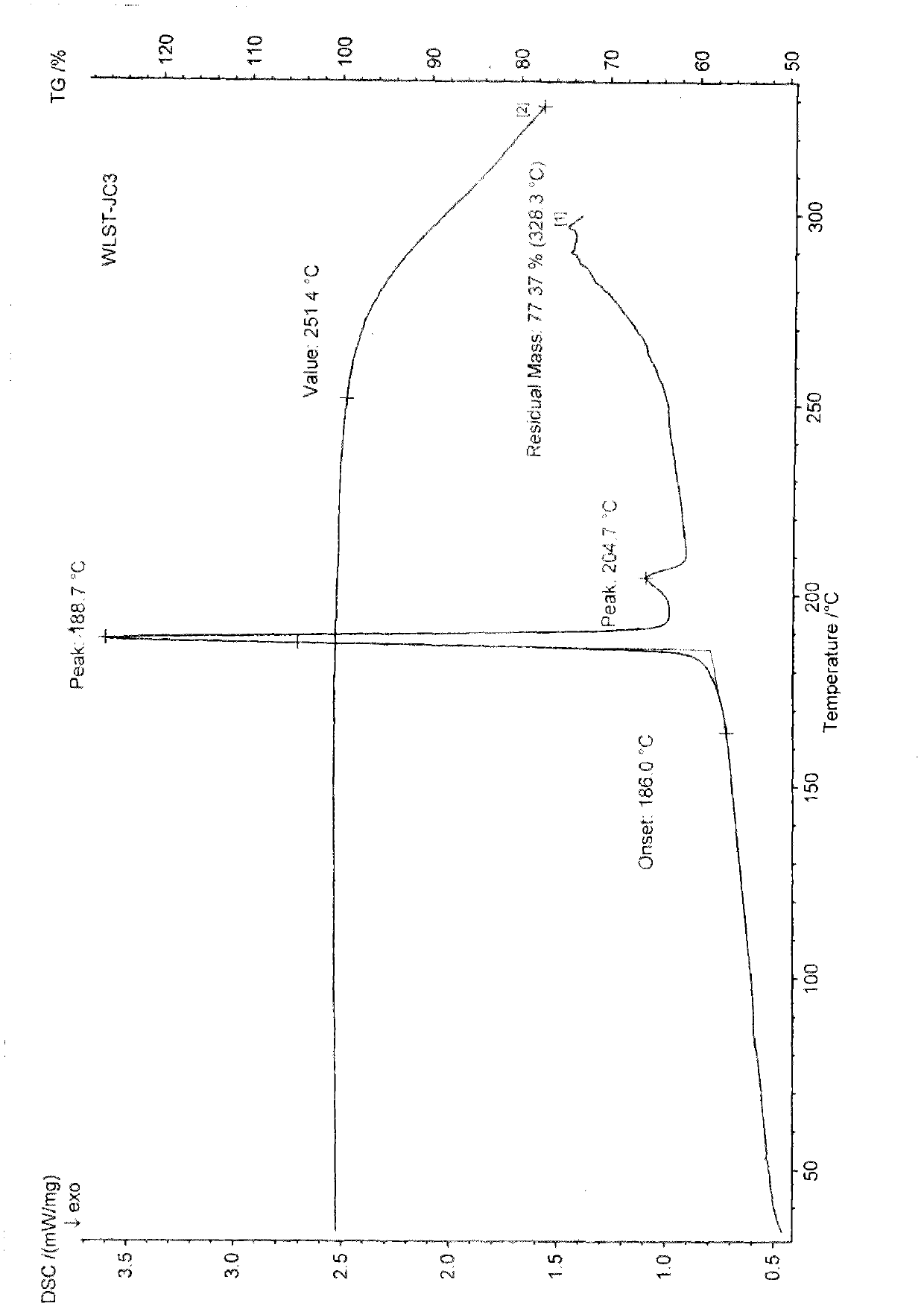
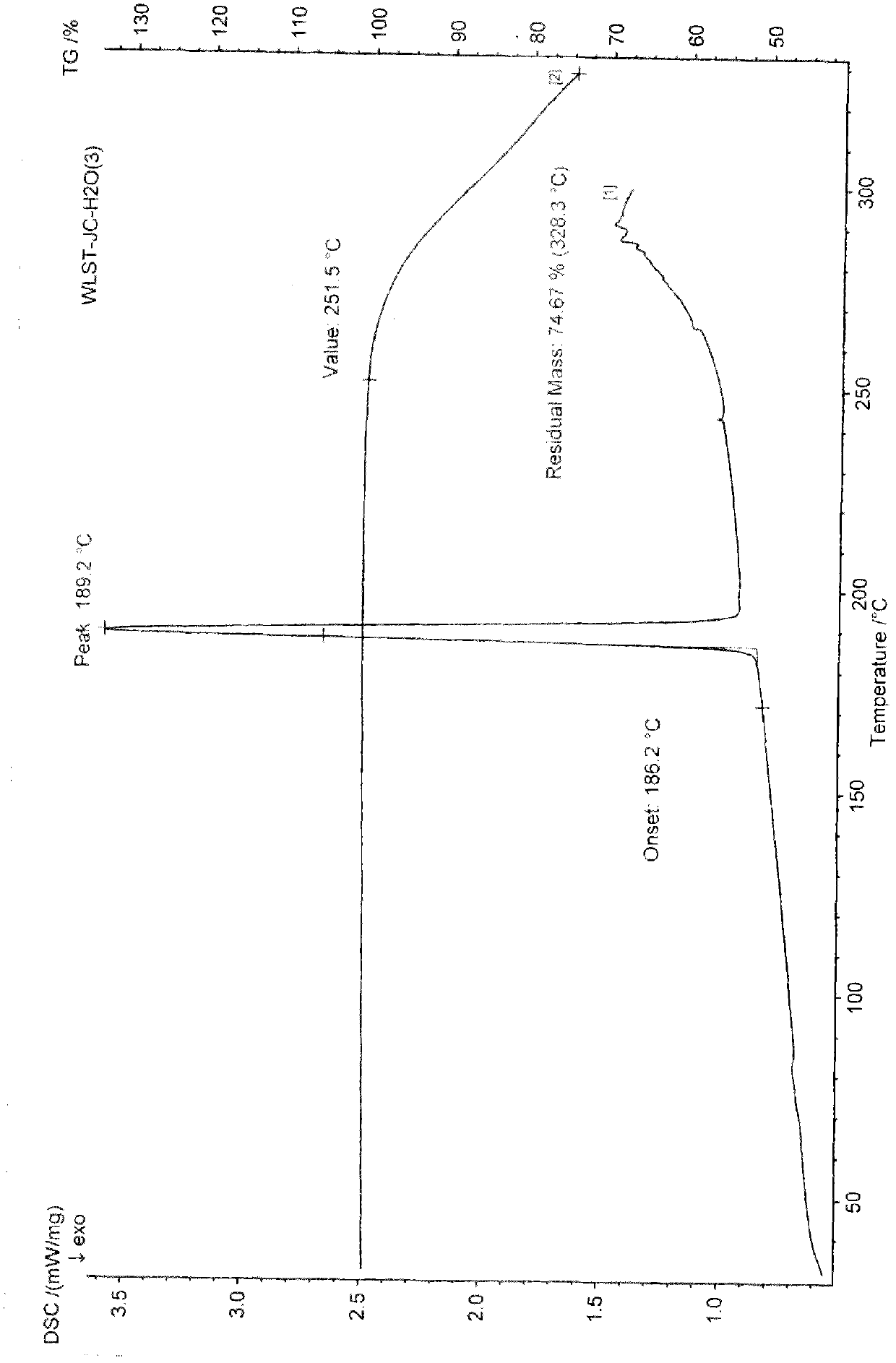
Differential thermal analysis (or DTA) is a thermoanalytic technique that is similar to differential scanning calorimetry. In DTA, the material under study and an inert reference are made to undergo identical thermal cycles, (i.e., same cooling or heating programme) while recording any temperature difference between sample and reference. This differential temperature is then plotted against time, or against temperature (DTA curve, or thermogram). Changes in the sample, either exothermic or endothermic, can be detected relative to the inert reference. Thus, a DTA curve provides data on the transformations that have occurred, such as glass transitions, crystallization, melting and sublimation. The area under a DTA peak is the enthalpy change and it's not affected by the heat capacity of the sample.
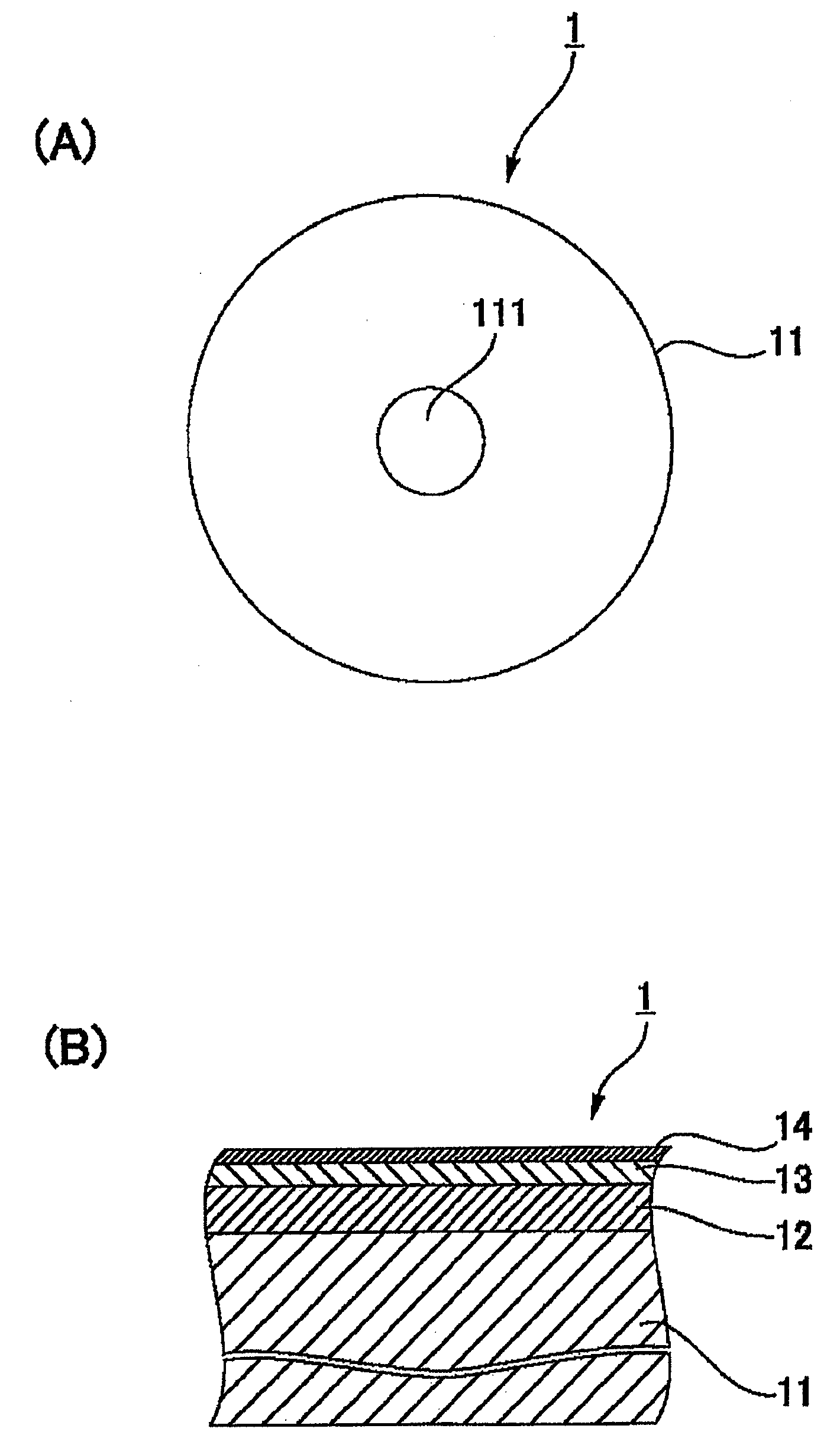
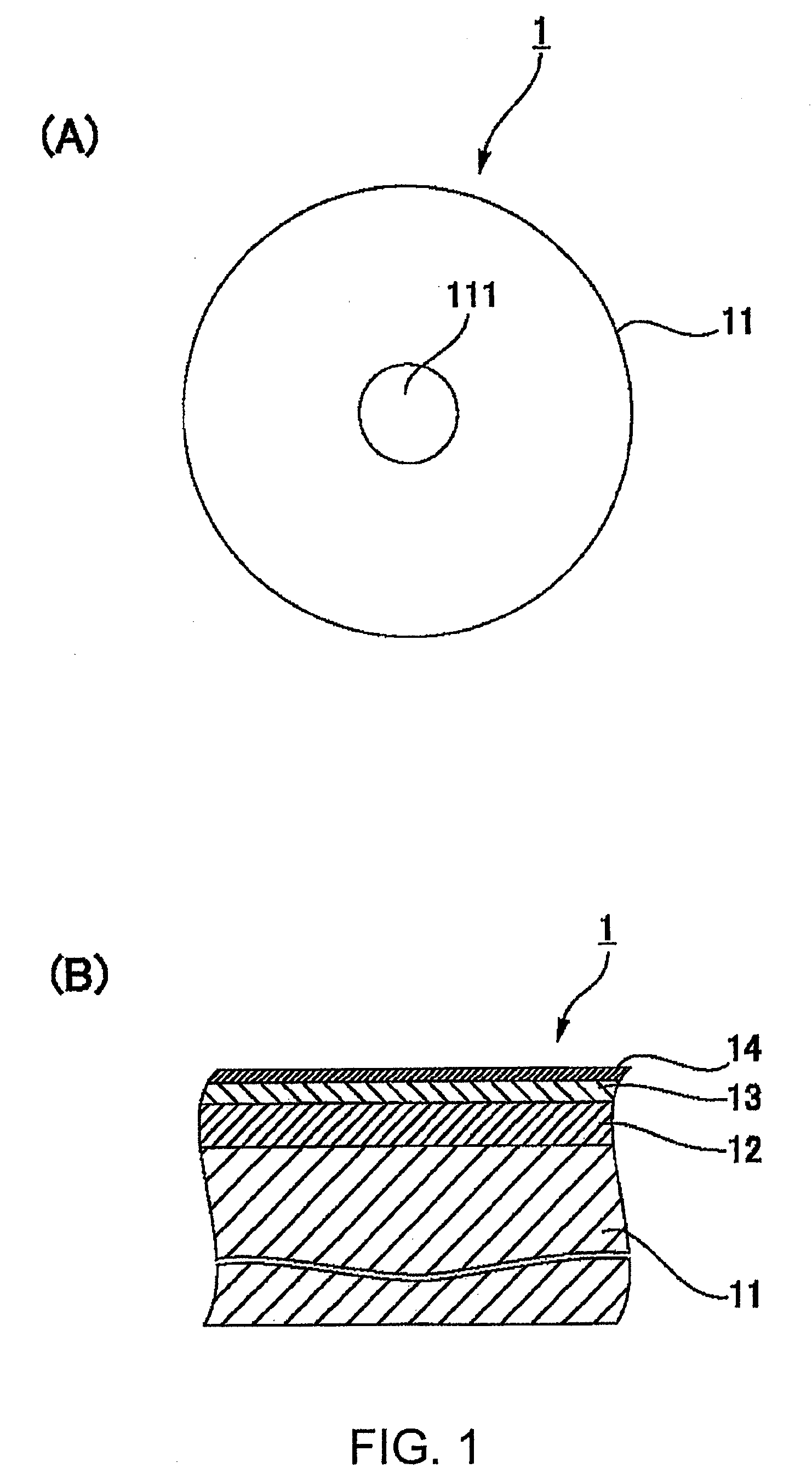
Magnetic recording disk and method for manufacture thereof
InactiveUS20090136784A1Improve reliabilityAvoid relative motionMagnetic materials for record carriersRecord information storageHeat resistanceMetallurgy
In a magnetic recording disk 1 including a magnetic layer 12, a protective layer 13, and a lubricating layer 14, the lubricating layer 14 is made from a lubricant prepared from a composition. The weight change of the lubricant ranges from −20% to −50% at 300° C. in the case where the lubricant is subjected to thermogravimetric analysis in such a manner that the lubricant is heated from 40° C. to 500° C. at a heating rate of 10° C. / min. The lubricant has a maximum peak at about 300° C. in the case where the lubricant is subjected to differential thermal analysis in the same manner as described above. The lubricant can be quantitatively measured for heat resistance. Therefore, a magnetic recording disk exhibiting stable performance at high temperatures and a method for manufacturing such a magnetic recording disk can be obtained.
Owner:WD MEDIA SINGAPORE PTE
Optical recording disk
InactiveUS6232036B1Small mark lengthSmall groove widthPhotosensitive materialsRadiation applicationsHigh densityEngineering
A high-density optical disk comprises a transparent substrate having a guide groove having a depth of 100 to 200 nm, width of 0.2 to 0.4 mum arranged with a track pitch of 0.7 to 1.0 mum. A recording layer is made of an organic pigment exhibiting a specific main weight reduction in a thermogravimetric analysis and a specific exothermic peak in a differential thermal analysis.
Owner:VERBATIM CORPORATION
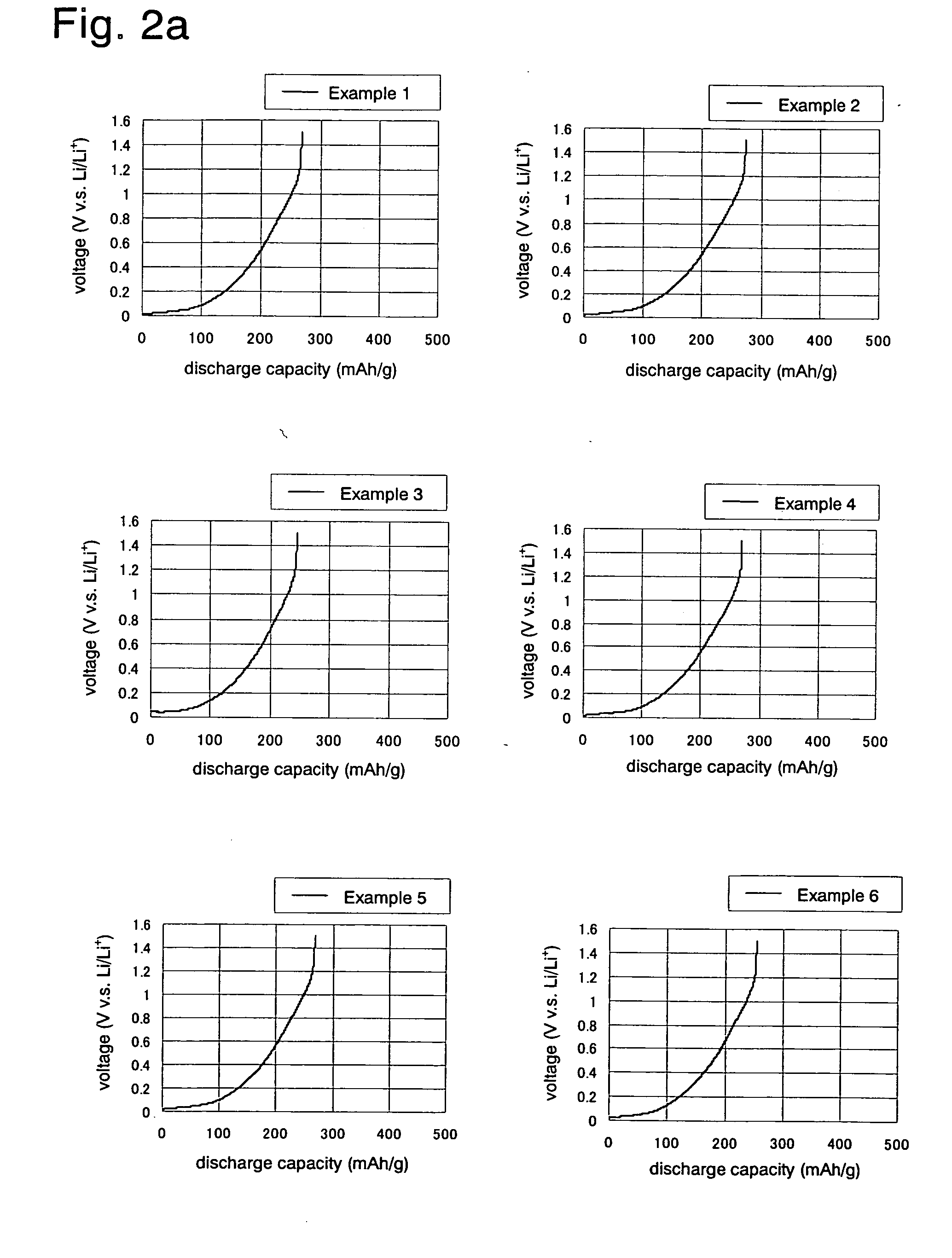
Negative Electrode Material for Non-Aqueous Electrolyte Secondary Battery, Process for Producing the Same, Negative Electrode, and Non-Aqueous Electrolyte Secondary Battery
ActiveUS20090297953A1Non-aqueous electrolyte accumulatorsFinal product manufactureElectric vehicleOxygen content
A negative electrode material for non-aqueous electrolyte secondary batteries, which is best suited for large current I / O non-aqueous electrolyte secondary batteries represented by those for hybrid electric vehicles (HEVs), which are unlikely to be influenced by the deterioration of battery characteristics due to water, and a production process thereof are provided.The negative electrode material having at least one exothermic peak in the range of not lower than 650° C. and lower than 700° C., and at least one exothermic peak in the range of not lower than 700° C. and lower than 760° C., in differential thermal analysis measured under an air flow. The production process of the negative electrode material for non-aqueous electrolyte secondary batteries is characterized by carbonizing a negative electrode material precursor having an oxygen content of not less than 5% by weight and less than 10% by weight, under an inert gas flow at a rate of not more than 120 ml / g·h, under a pressure of normal pressure to 10 kPa, at a temperature higher than 1100° C. and lower than 1500° C.
Owner:KURARAY CO LTD
Additive for plastic and plastic
InactiveUS20060188428A1Small amountCalcium/strontium/barium carbonatesMagnesium carbonatesCALCIUM CARBONATE/MAGNESIUM CARBONATEMagnesium carbonate / Magnesium Oxide
Disclosed is an additive for a plastic, comprising fine particles obtained by calcination and slaking of a dolomite which exhibits two endothermic peaks in the differential thermal analysis, said fine particles containing calcium carbonate, magnesium carbonate, magnesium oxide, calcium hydroxide and magnesium hydroxide as main chemical components and also containing an ignition loss component in an amount of 10 to 40% by weight based on the weight of said fine particles. A plastic hating hydrogen chloride scavenging properties and antimicrobial properties imparted by incorporating the additive for a plastic is also disclosed.
Owner:OSAKA MUNICIPAL TECHN RES INST +2
Process for producing regenerated hydrotreating catalyst and process for producing petrochemical product
InactiveUS8722558B2Easy to produceSolve the lack of activityCoke ovensTreatment with hydrotreatment processesPetrochemicalElectromotive force
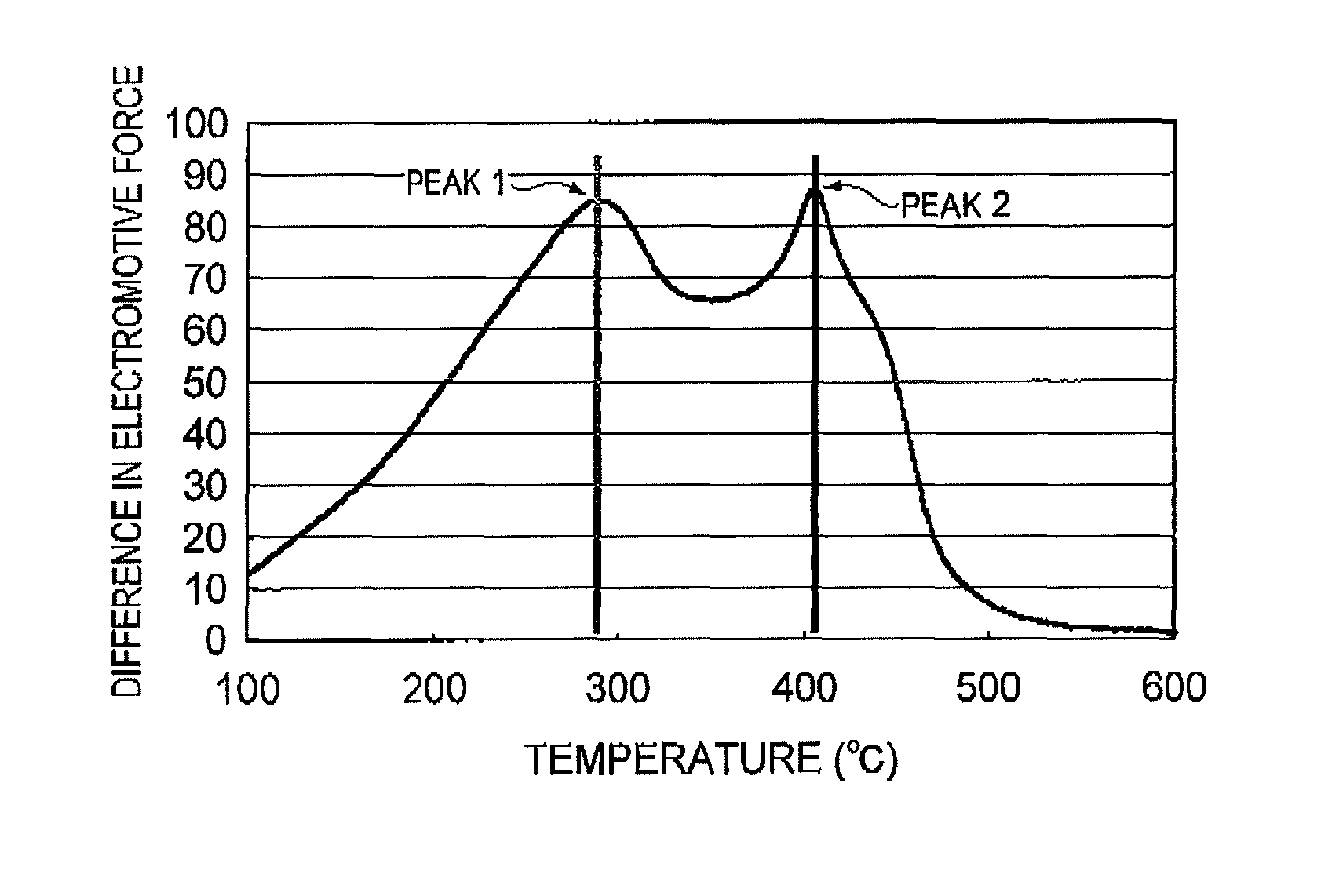
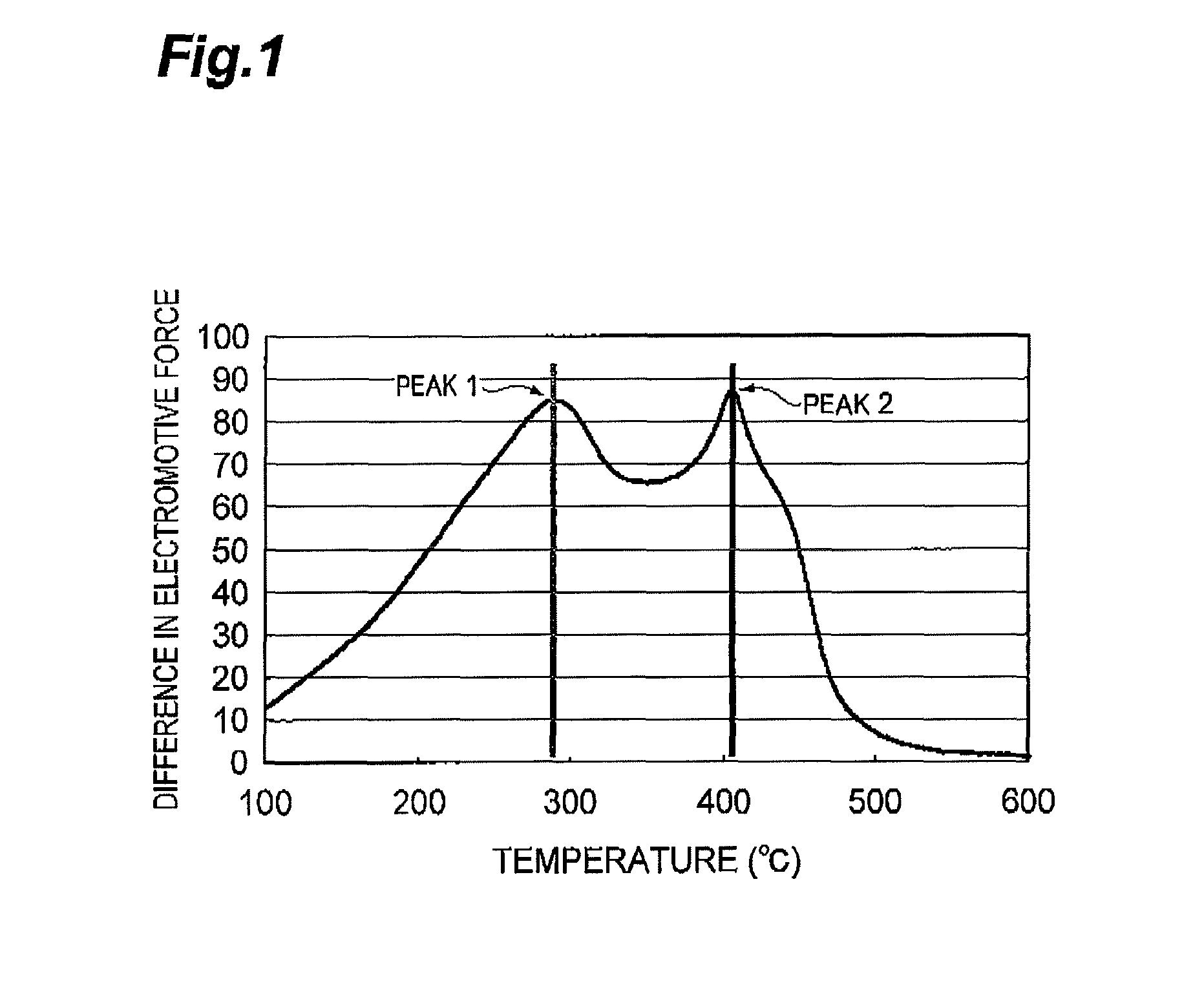
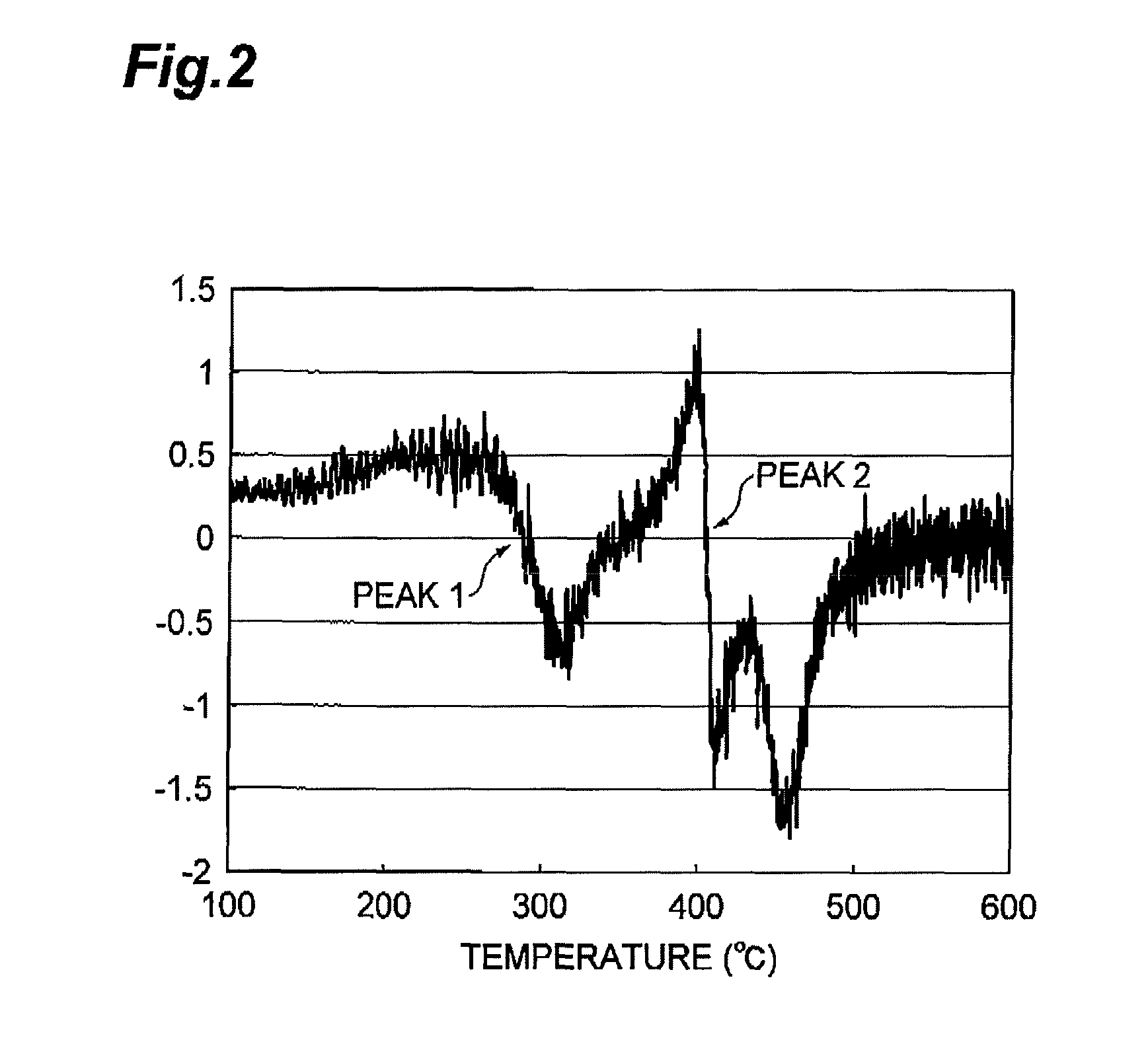
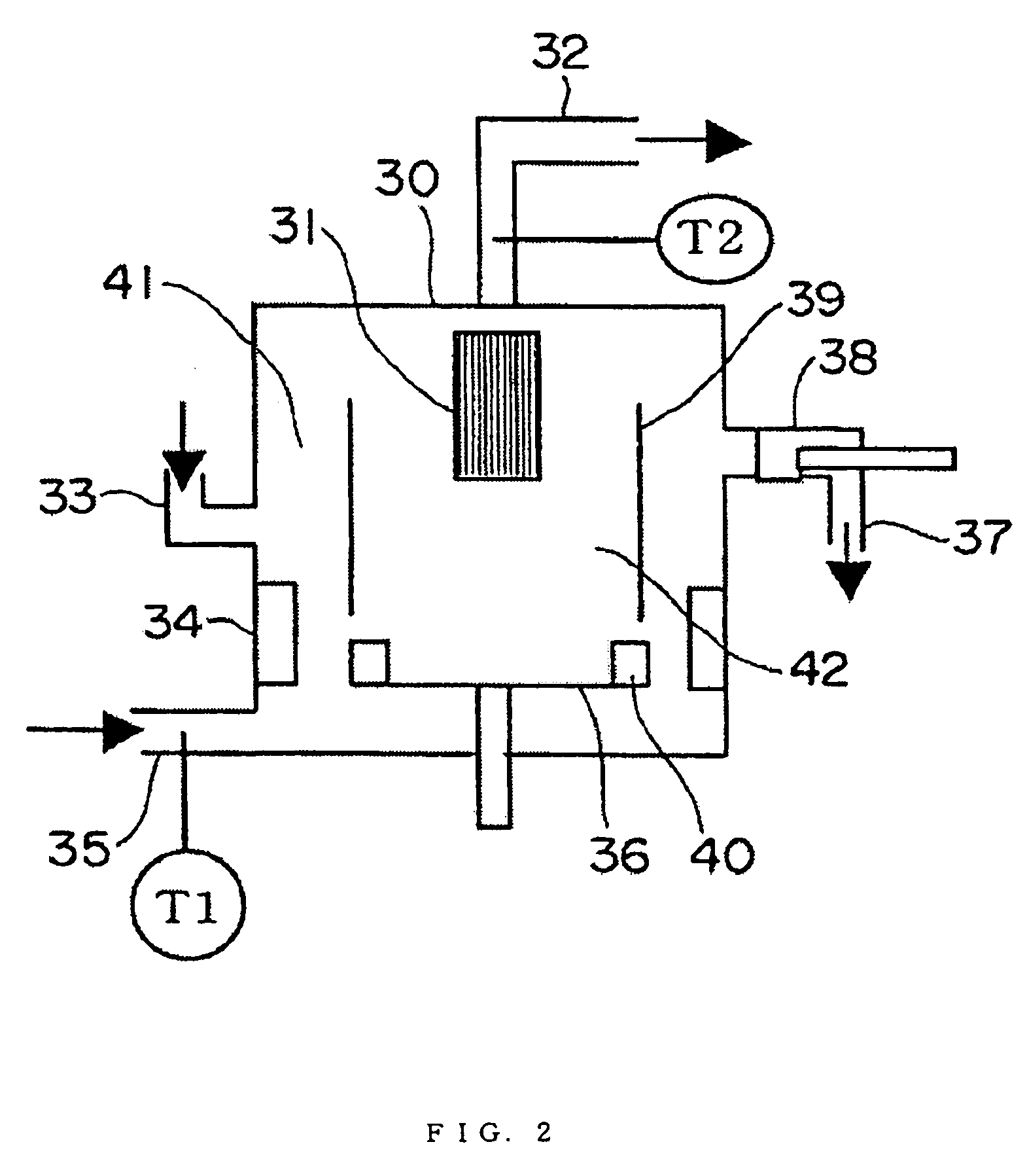
Provided is a process for producing a regenerated hydrotreating catalyst by regenerating a spent hydrotreating catalyst in a prescribed temperature range, wherein the prescribed temperature range is a temperature range of T1−30° C. or more and T2+30° C. or less, as determined by subjecting the spent hydrotreating catalyst to a differential thermal analysis, converting a differential heat in a measuring temperature range of 100° C. or more and 600° C. or less to a difference in electromotive force, differentiating the converted value twice by temperature to provide a smallest extreme value and a second smallest extreme value, and representing a temperature corresponding to the extreme value on the lower-temperature side as T1 and a temperature corresponding to the extreme value on the higher-temperature side as T2.
Owner:JX NIPPON OIL & ENERGY CORP
Color toner
ActiveUS7112395B2Sufficient fixable rangeGreat can be contaminationMobile jacksDevelopersPolyesterEngineering
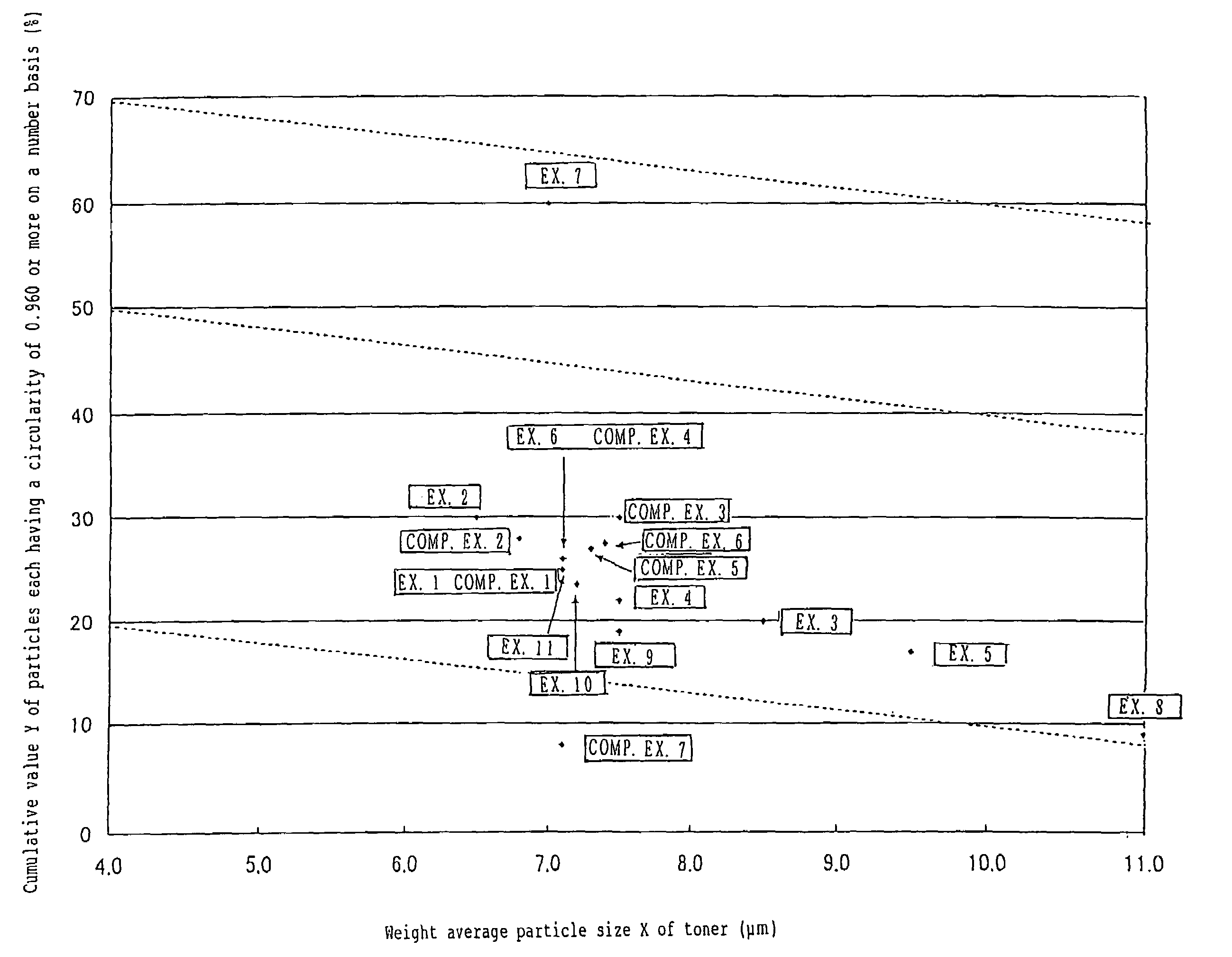
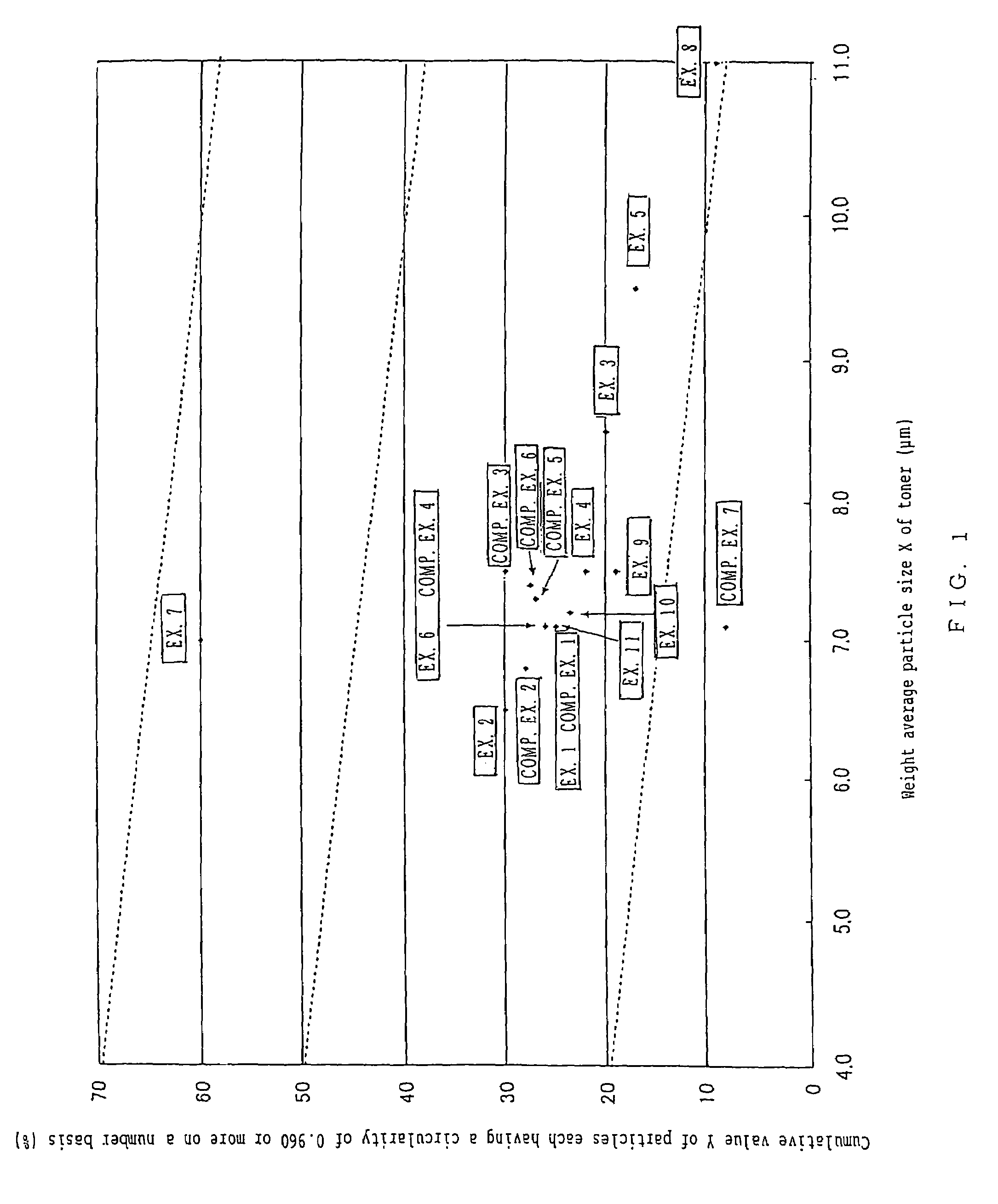
To provide a color toner which is effective in mitigating contamination of a charging member, which is good at low temperature fixing in high-speed copying, and which is excellent in blocking resistance and electrification stability in continuous copying. Provided is a color toner containing at least a binder resin, a colorant, and a releasing agent, in which: (i) the binder resin contains at least a polyester unit; (ii) a weight average particle diameter of the color toner is greater than 6.5 μm and equal to or less than 11 μm; (iii) an average circularity A of particles in the color toner each having a circle-equivalent diameter of 3 μm or more satisfies the relationship of 0.915≦A≦0.960; (iv) a permeability B (%) of the color toner in a 45 vol % aqueous solution of methanol satisfies the relationship of 10≦B≦70; and (v) an endothermic curve obtained through differential thermal analysis (DSC) measurement of the color toner has one or multiple endothermic peaks in the temperature range of 30 to 200° C., and a temperature Tsc of the highest endothermic peak of the one or multiple endothermic peaks satisfies the relationship of 65° C.<Tsc<105° C.
Owner:CANON KK
Crystallized glass, and method for producing crystallized glass
InactiveUS20070281849A1Stable productionImprove efficiencySemiconductor/solid-state device manufacturingOriginals for photomechanical treatmentLarge sizeMaterials science
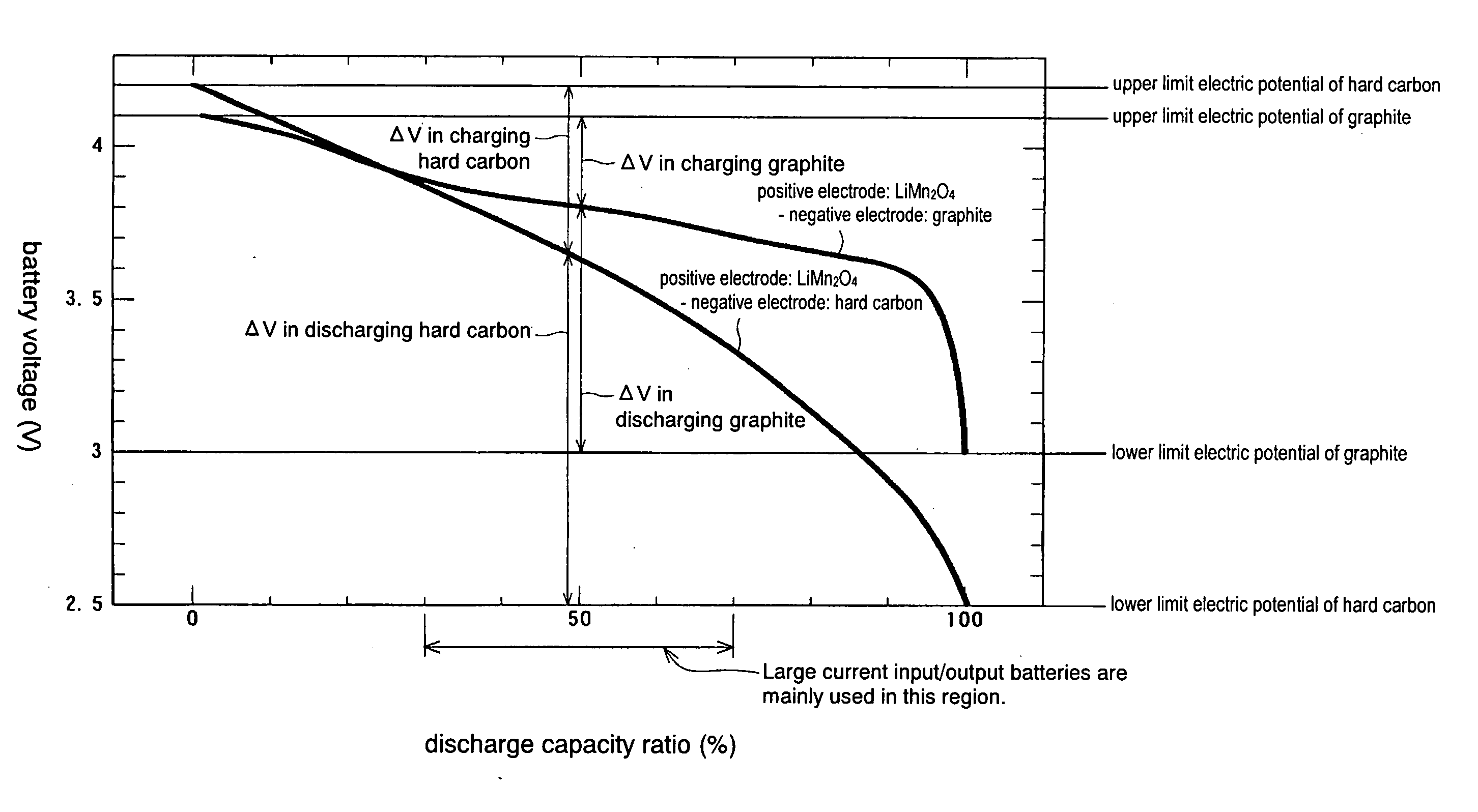
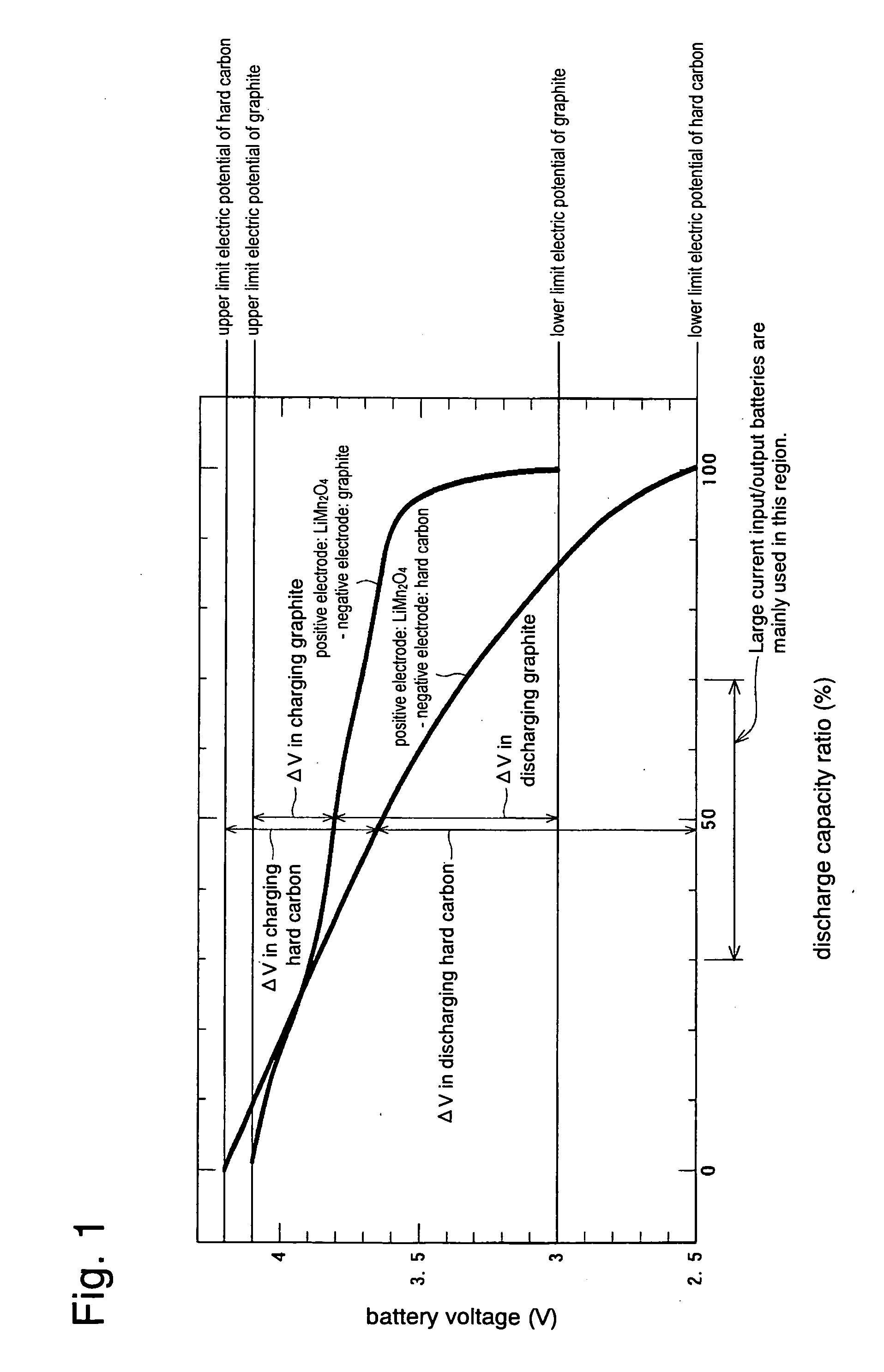
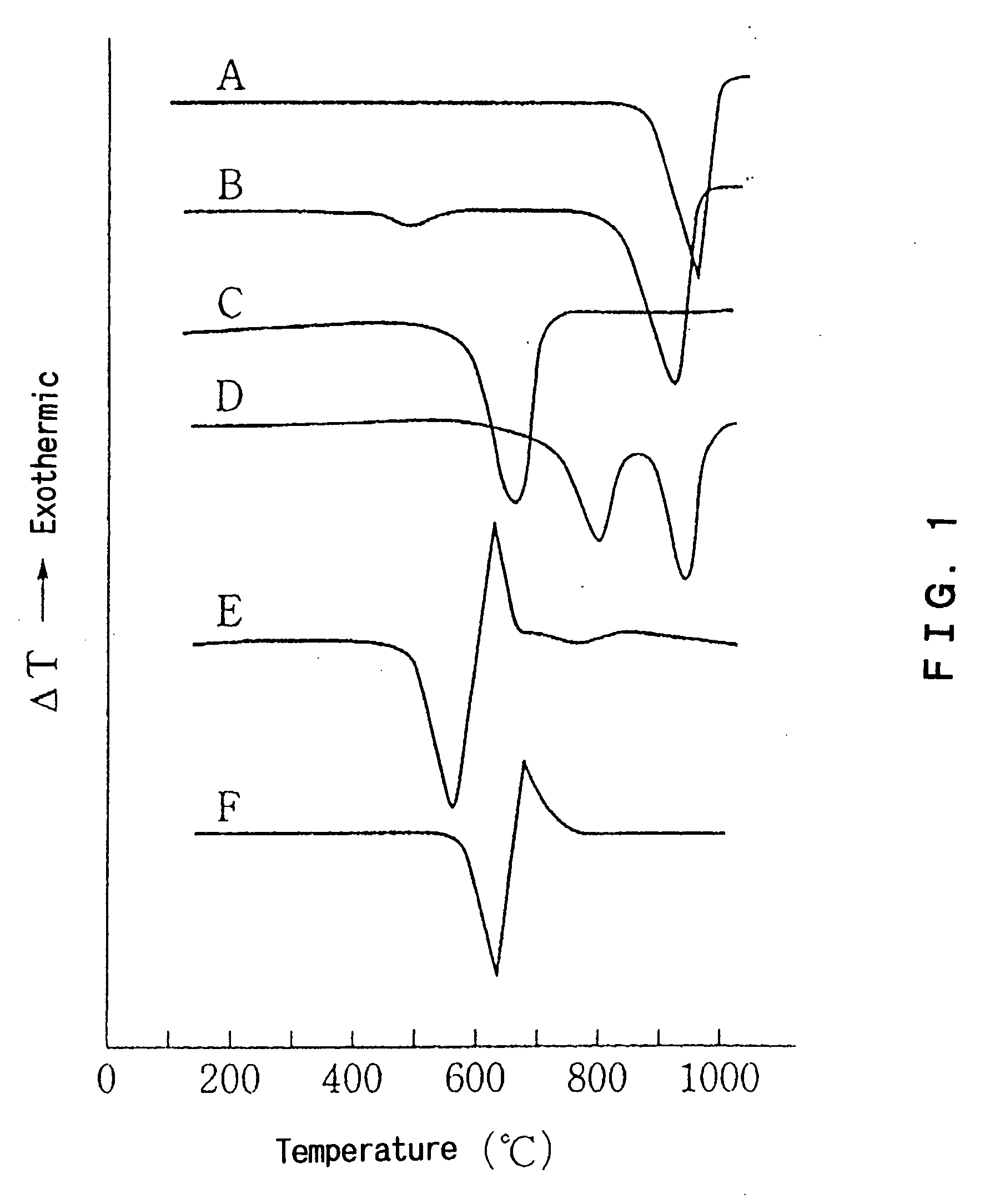
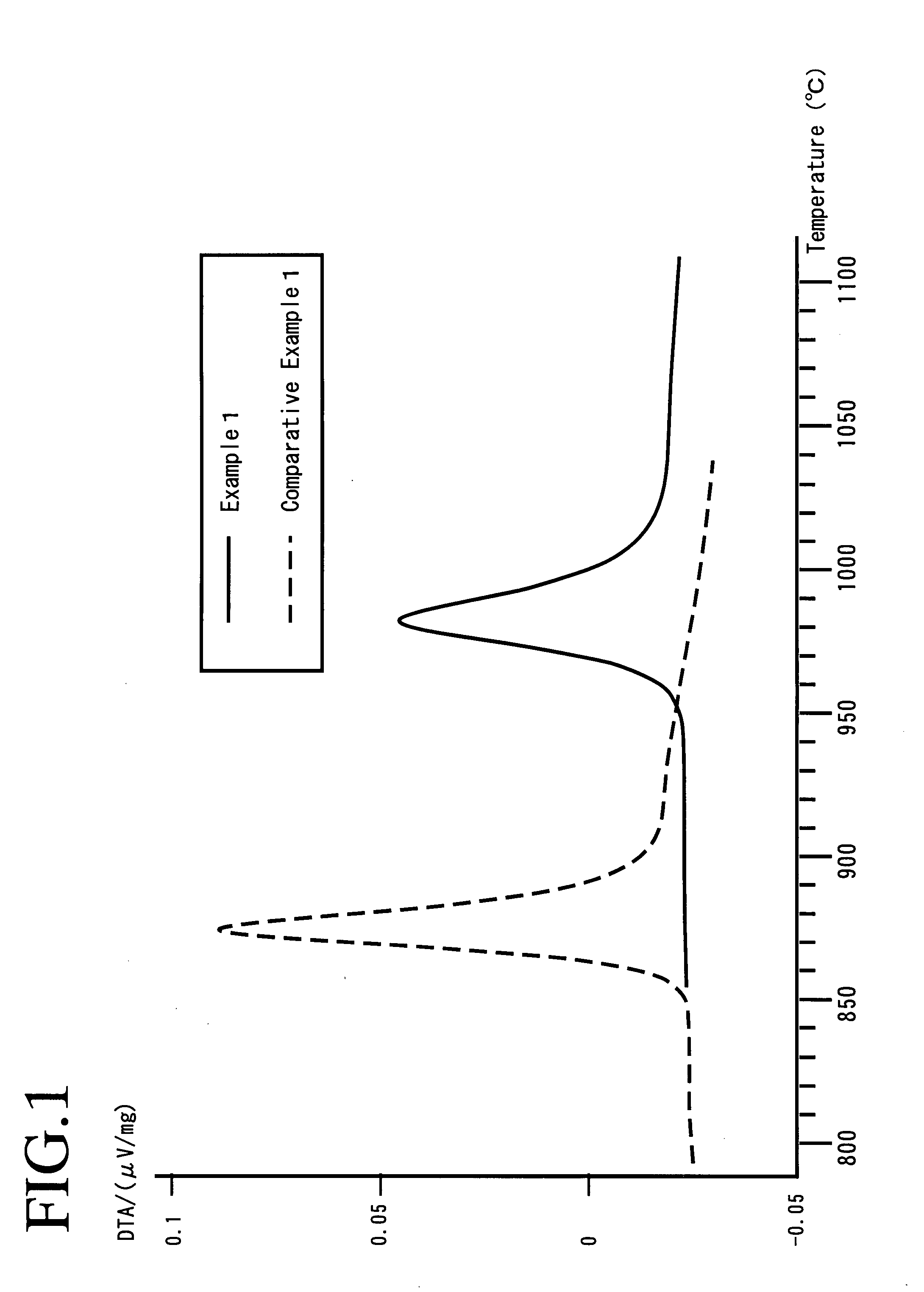
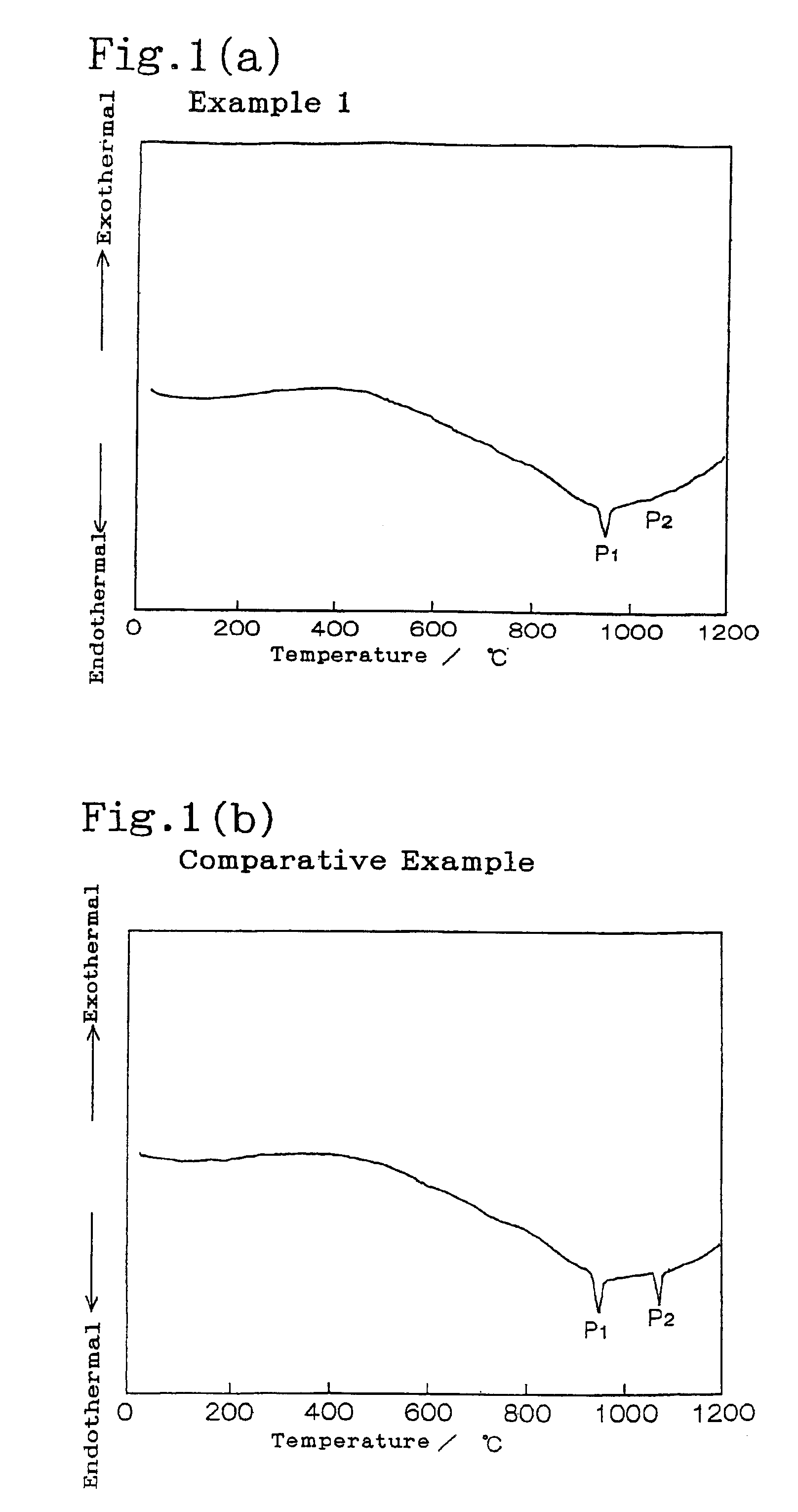
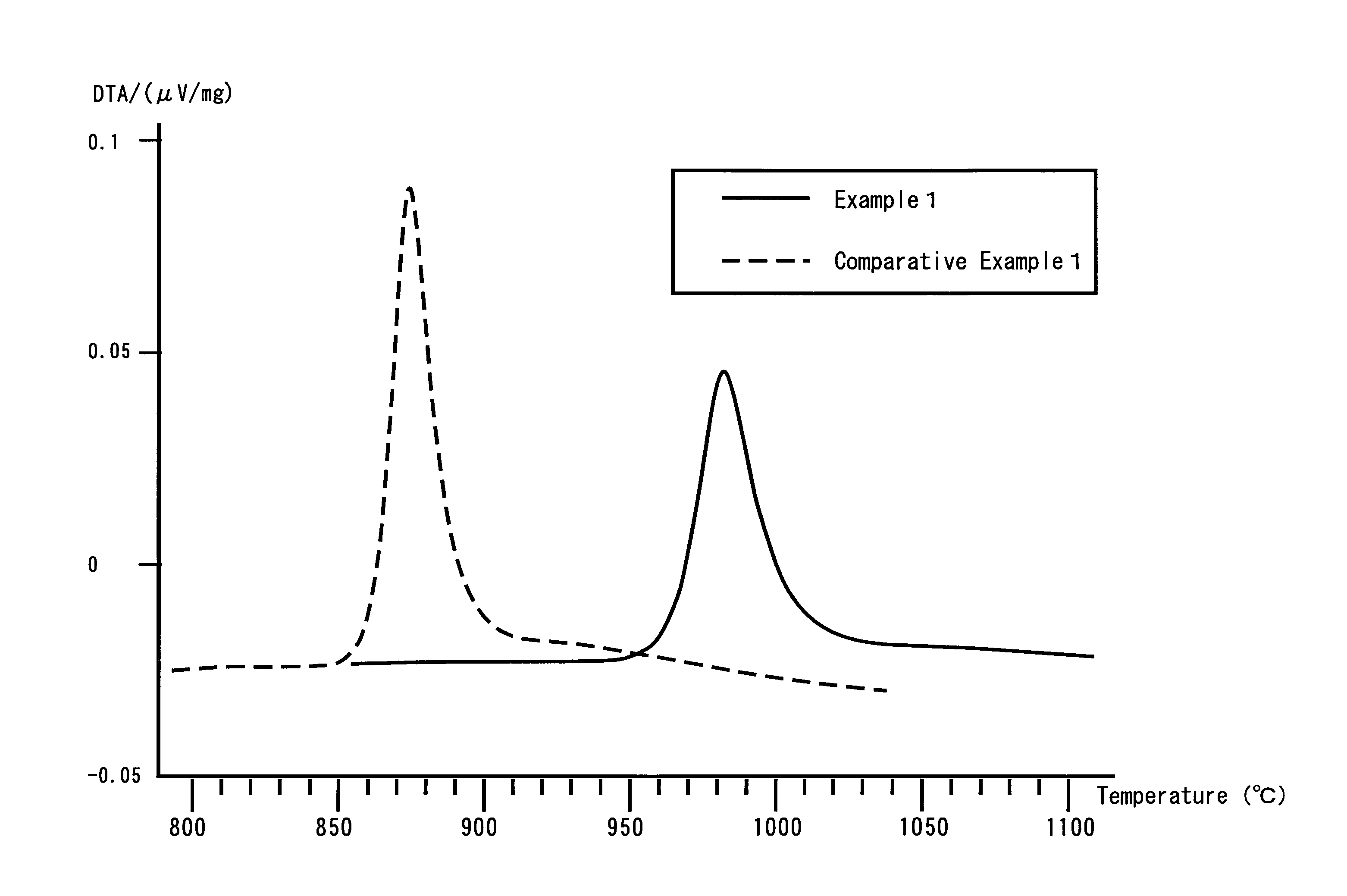
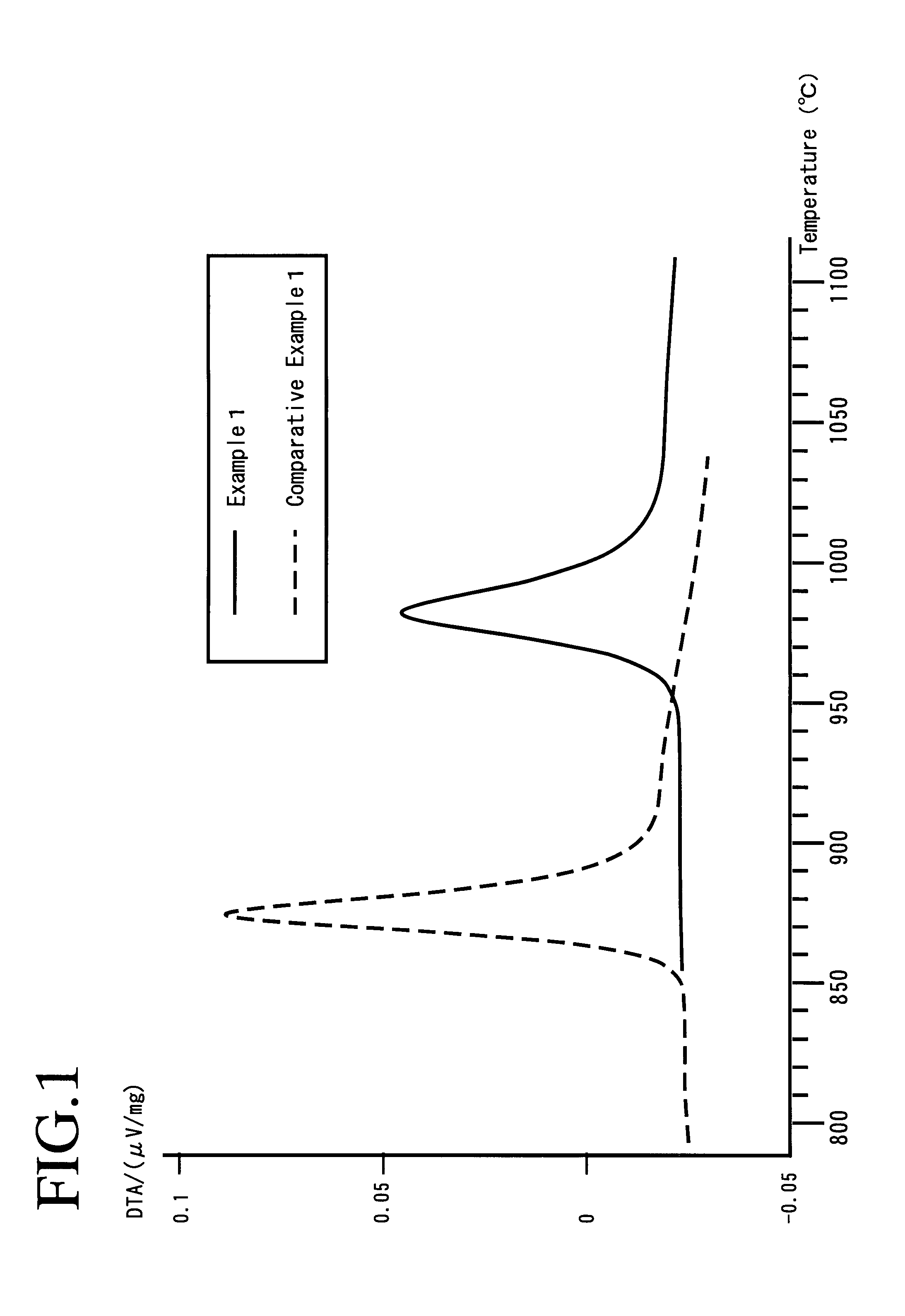
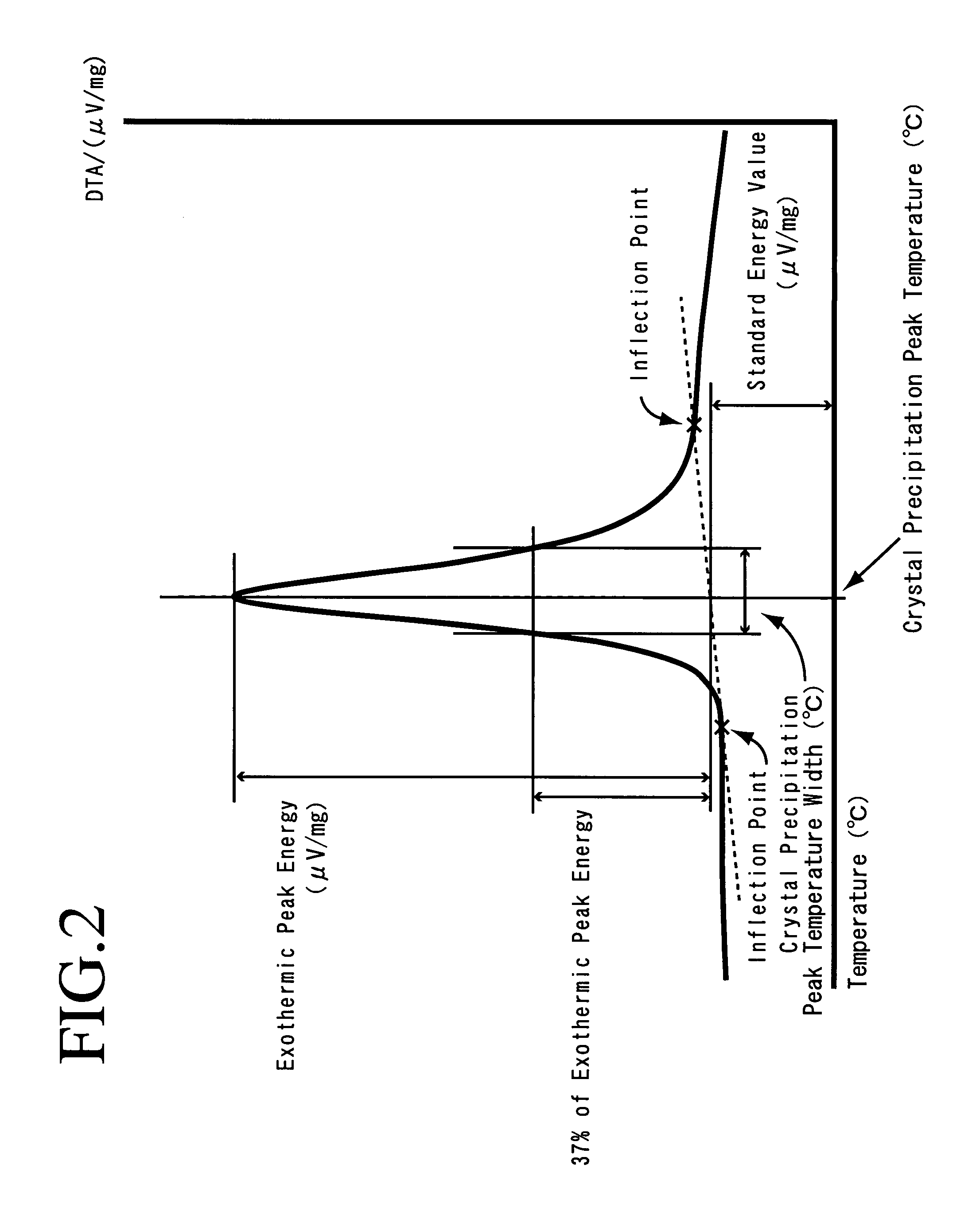
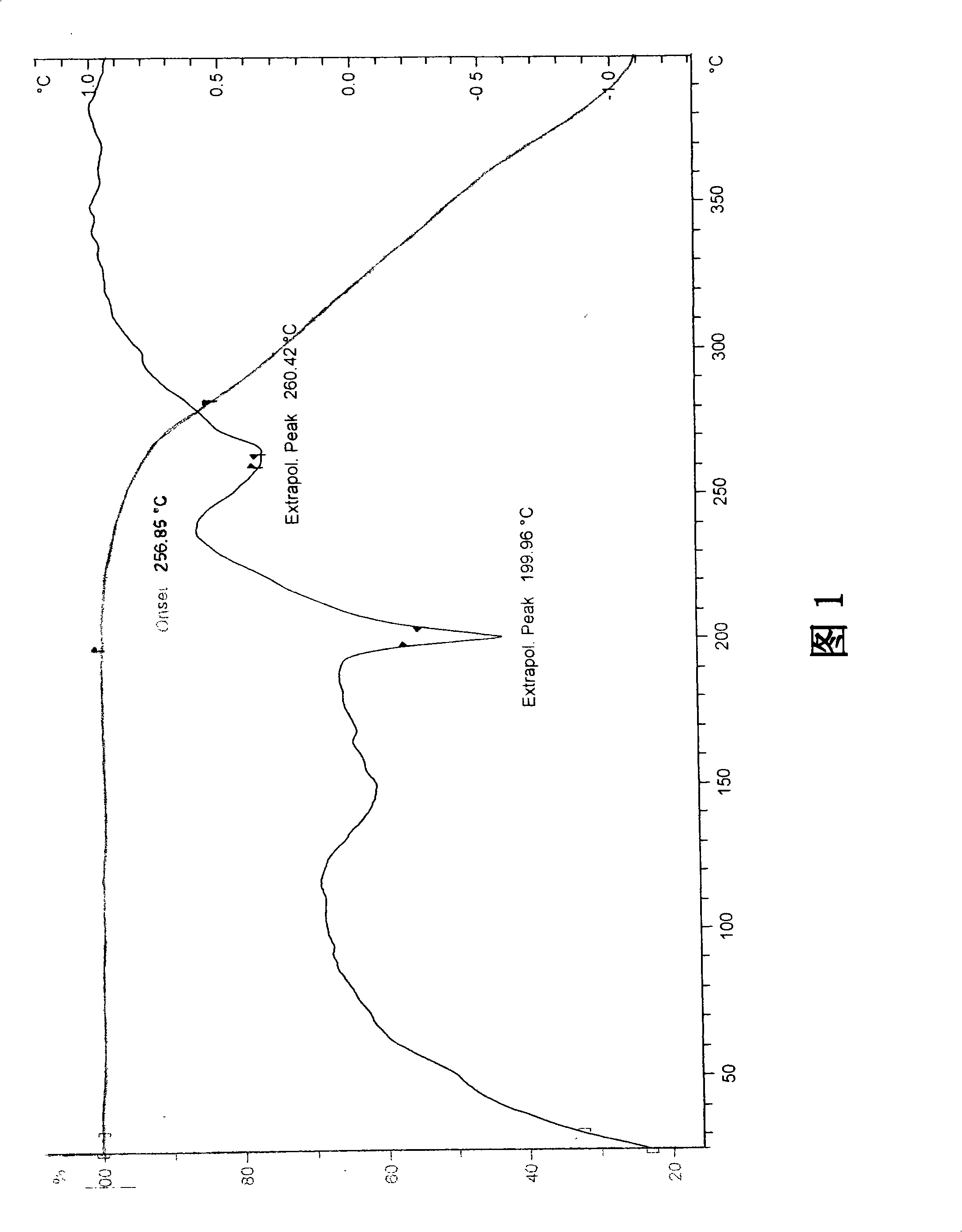
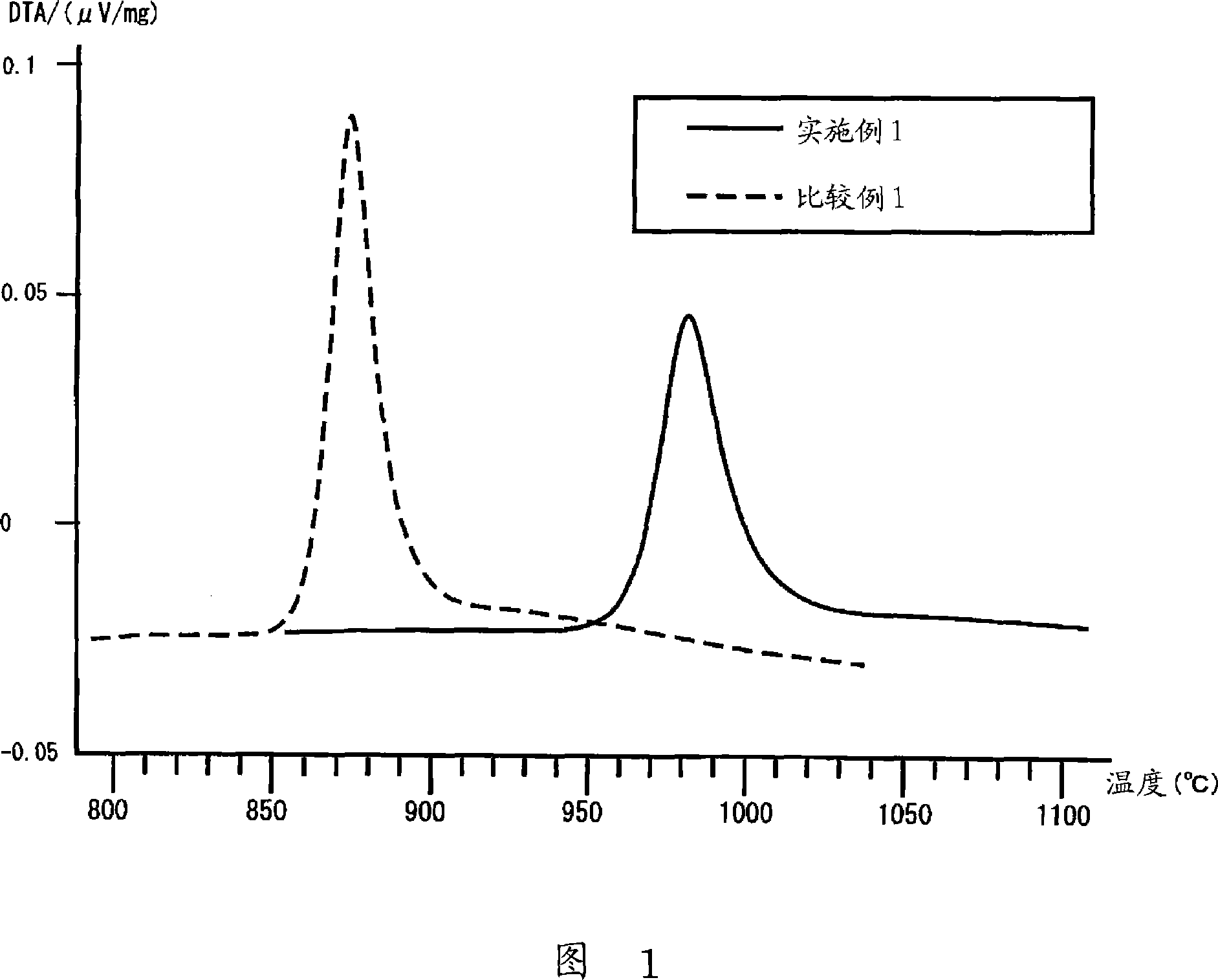
Provided are SiO2—Al2O3-based or Li2O—Al2O3—SiO2-based crystallized glass that solves the cause of cracking and fracture in forming it into large-size shaped articles, that has homogeneous inner quality and that may be produced stably at high efficiency; and a method for producing it. The crystallized glass contains components of SiO2 and Al2O3, wherein the crystal precipitation peak temperature width obtained in differential thermal analysis of amorphous glass, a precursor thereof, is at least 22° C. Preferably, the total amount of the TiO2 component and the ZrO2 component in the crystallized glass is within a range of from 3.0 to less than 4.3%. FIG. 1 is referred to.
Owner:OHARA
Lithium secondary battery
InactiveUS6964830B2Stable crystal structureImprove cycle performanceActive material electrodesNon-aqueous electrolyte accumulator electrodesSpinelLithium-ion battery
A lithium secondary battery using lithium manganese oxide as a positive active material and having excellent charge and discharge cycle properties.As a positive active material of a lithium secondary battery, lithium manganese oxide having a cubic spinel structure, in which the strength ratio (P2 / P1 strength ratio) of the primary endothermal peak (P1) appearing around 950° C. and the secondary endothermal peak (P2) appearing around 1100°0 C. in differential thermal analysis is under 1, is used.
Owner:NGK INSULATORS LTD
Crystallized glass, and method for producing crystallized glass
InactiveUS7645714B2Semiconductor/solid-state device manufacturingOriginals for photomechanical treatmentLarge sizeMaterials science
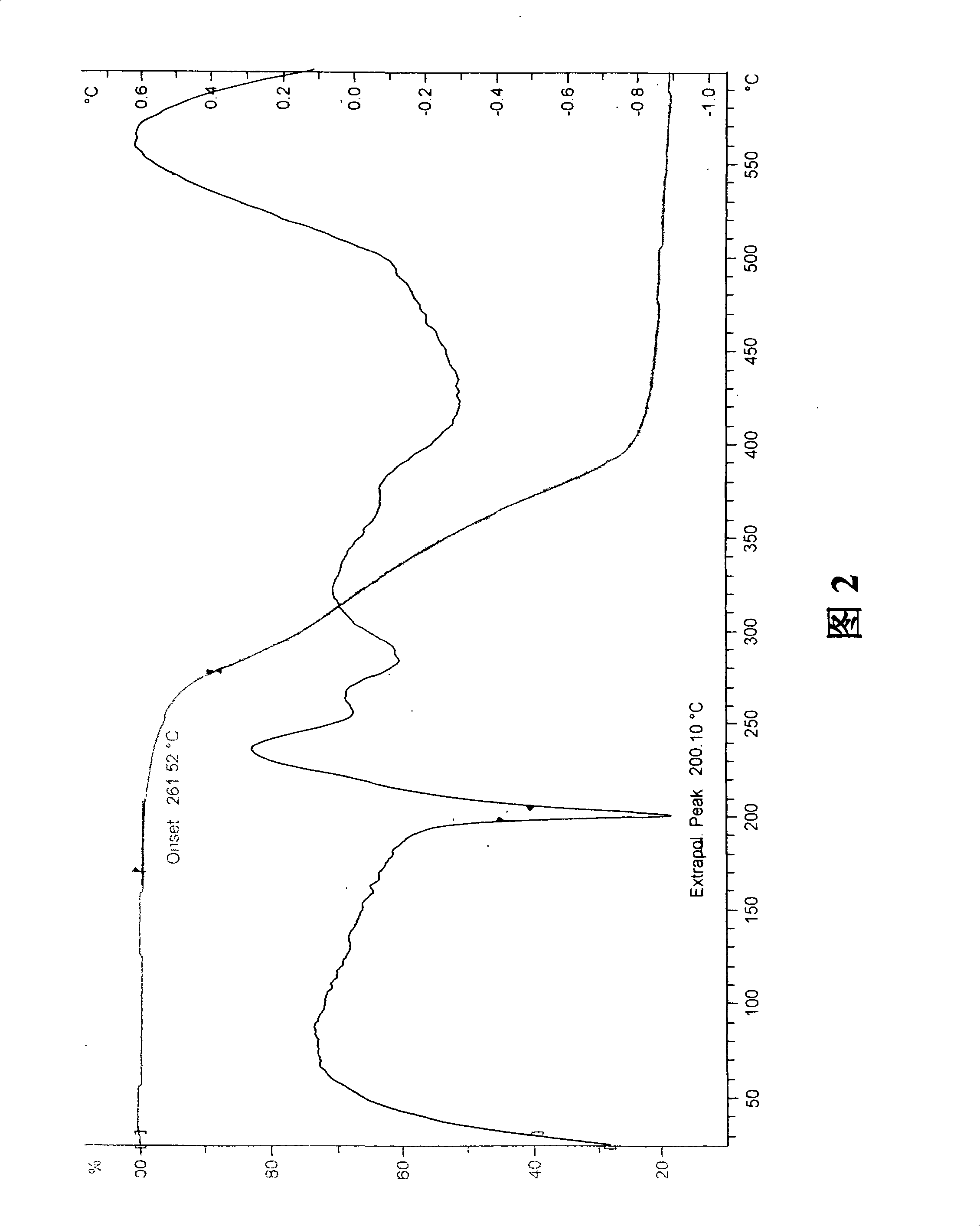
Provided are SiO2—Al2O3-based or Li2O—Al2O3—SiO2-based crystallized glass that solves the cause of cracking and fracture in forming it into large-size shaped articles, that has homogeneous inner quality and that may be produced stably at high efficiency; and a method for producing it. The crystallized glass contains components of SiO2 and Al2O3, wherein the crystal precipitation peak temperature width obtained in differential thermal analysis of amorphous glass, a precursor thereof, is at least 22° C. Preferably, the total amount of the TiO2 component and the ZrO2 component in the crystallized glass is within a range of from 3.0 to less than 4.3%. FIG. 1 is referred to.
Owner:OHARA
Multi-Color Coloring Laser Marking-Use Chromatic Color Colorant, Multi-Color Coloring Laser Marking-Use Composition And Molding Containing It, Multi-Color Making-Carrying Molding And Laser Marking Method
InactiveUS20080139707A1Conveniently and rapidly formedEasy to readDecorative surface effectsOrganic dyesDark colorBlack substance
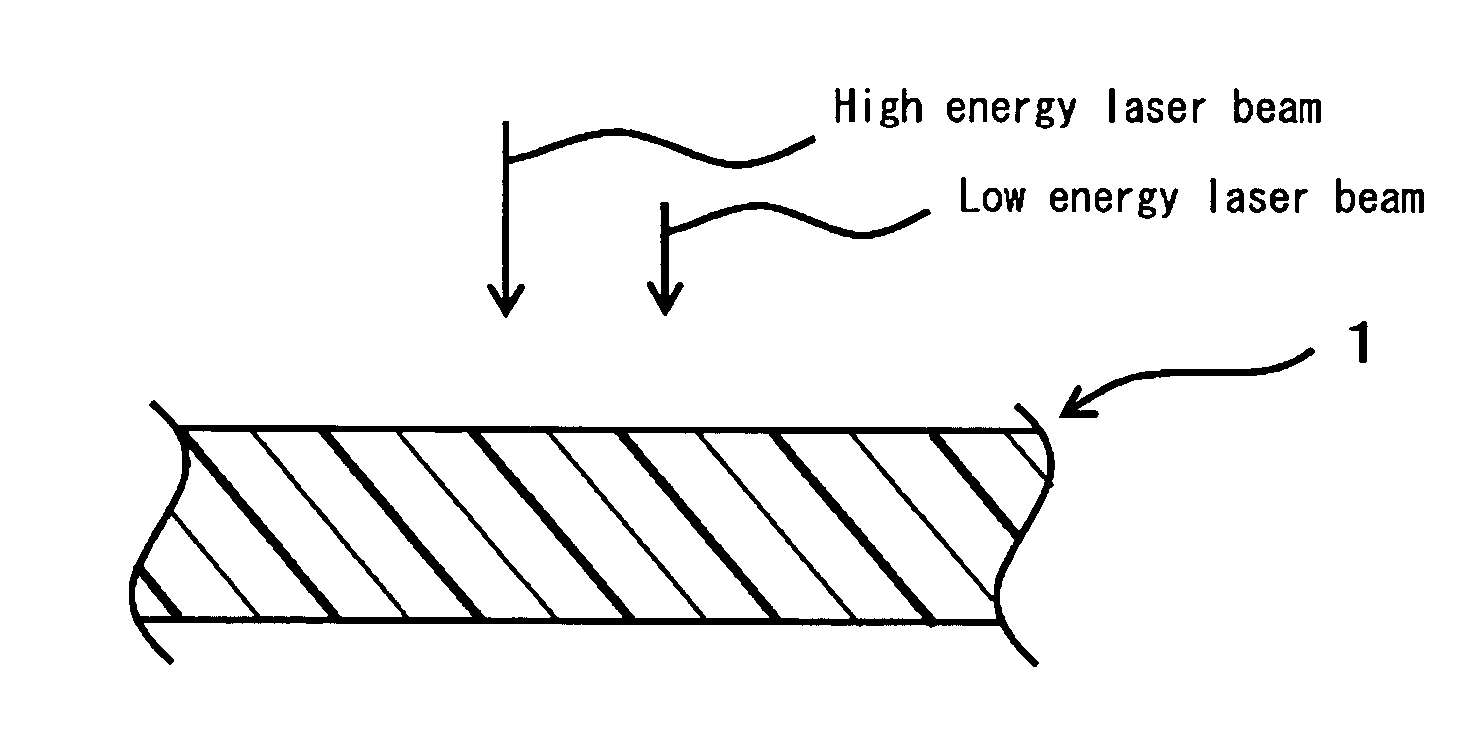
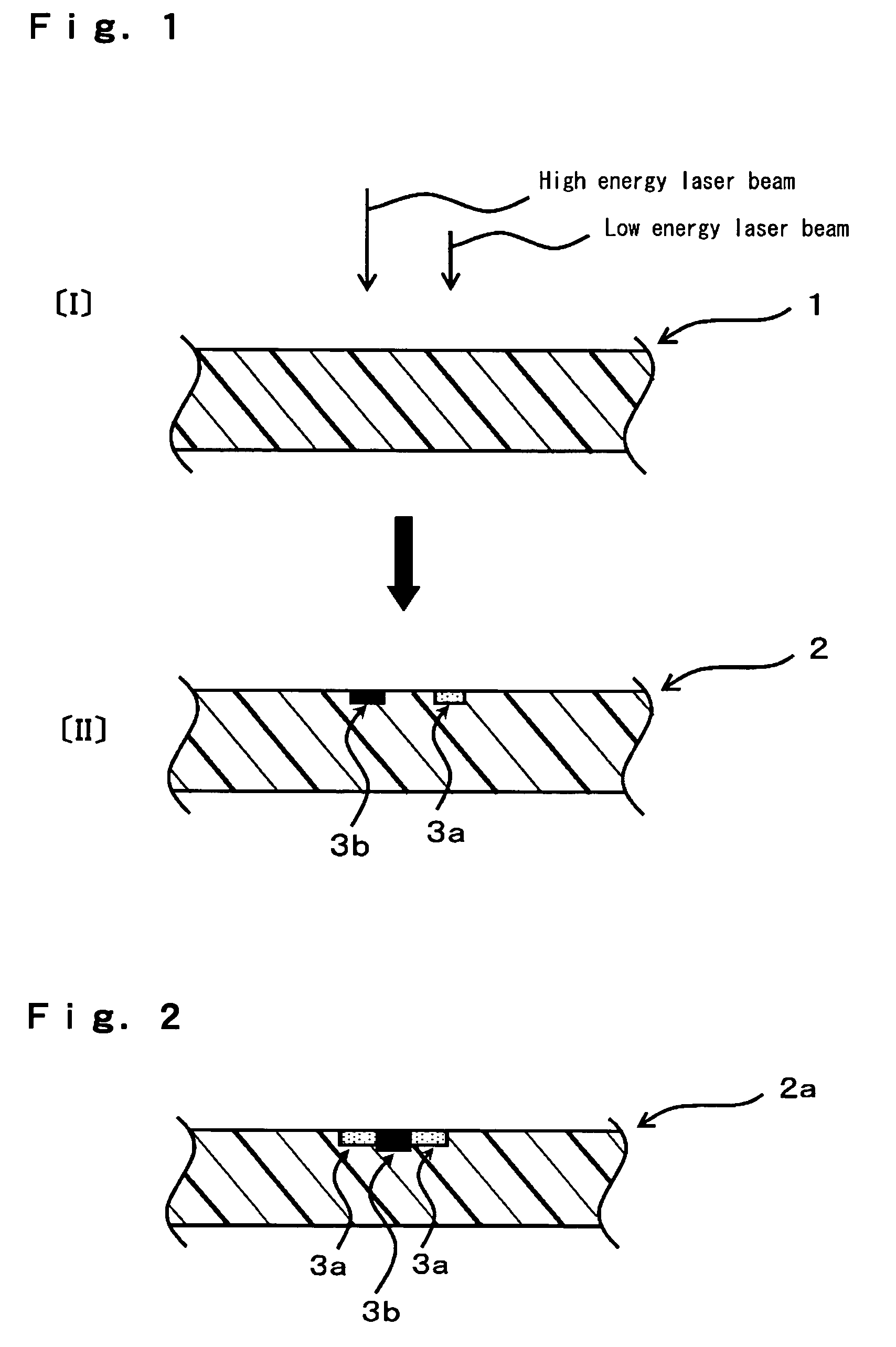
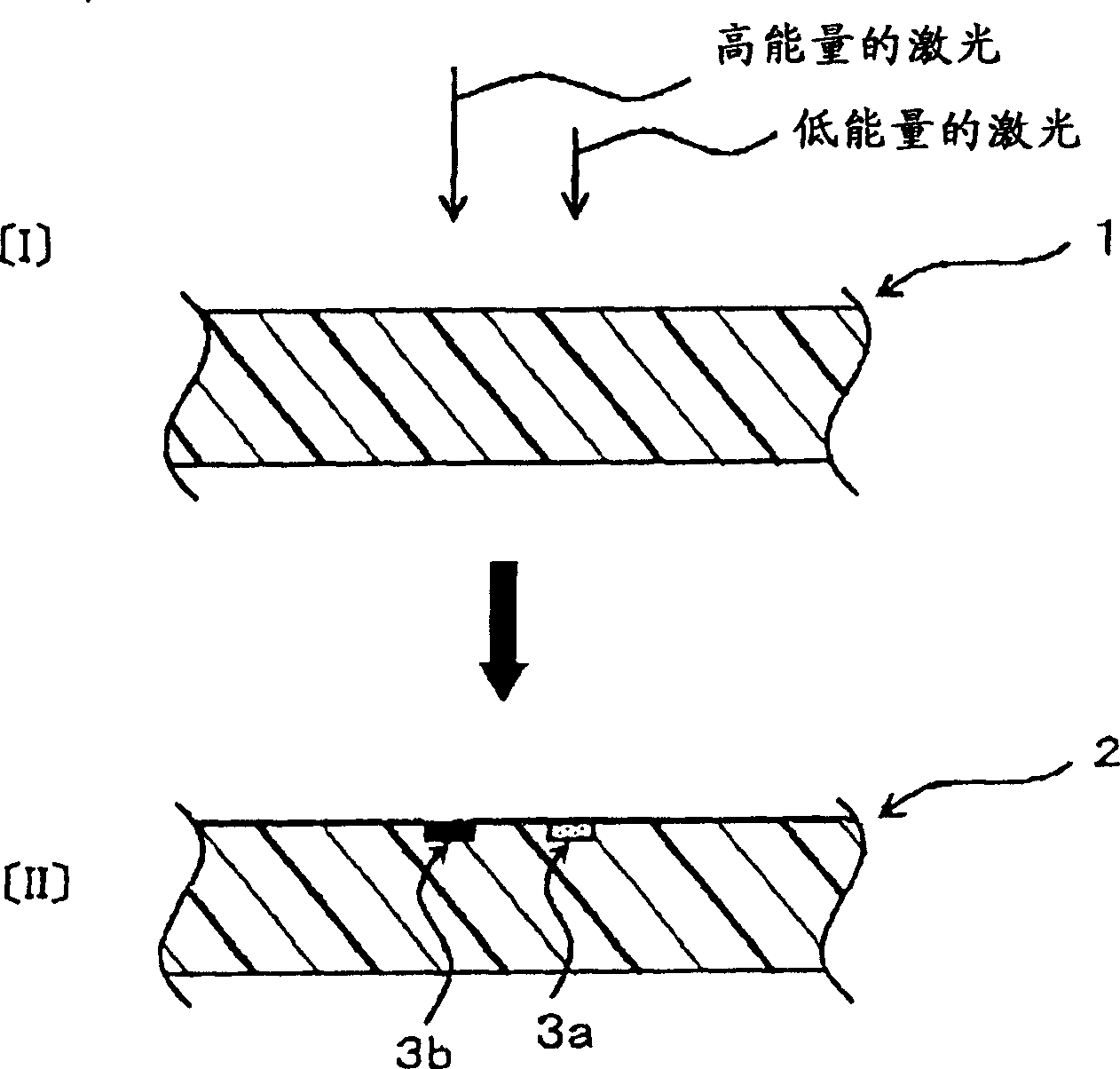
The objective of the present invention is to provide a chromatic coloring agent for multicolor laser marking, capable of forming clear markings having two or more different color tones when two or more laser beams having different energy levels are irradiated onto different places of a molded article, a composition for multicolor laser marking, for example, capable of forming a chromatic marking derived from the chromatic coloring agent and a white marking on the surface of a molded article whose base color is black or dark-color based color, a laser marking method, a multicolor-marked molded article and the like. The present chromatic coloring agent has an exothermic peak in the range of 360° C. or higher and 590° C. or lower, as measured by differential thermal analysis. The present laser marking composition comprises a chromatic coloring agent, a black substance (carbon black or the like) which is itself depleted or discolored by receiving a laser beam, and a polymer, and the contents of the chromatic coloring agent and the black substance are respectively 0.001 to 3 parts by mass and 0.01 to 2 parts by mass with respect to 100 parts by mass of the polymer.
Owner:TECHNO POLYMER CO LTD
Multi-color coloring laser marking-use chromatic color colorant, multi-color coloring laser marking-use composition and molding containing it, multi-color marking-carrying molding and laser marking me
InactiveCN1910238AAvoid deformationLess prone to wearOrganic dyesThermographyColored whiteBlack substance
Owner:TECHNO POLYMER CO LTD
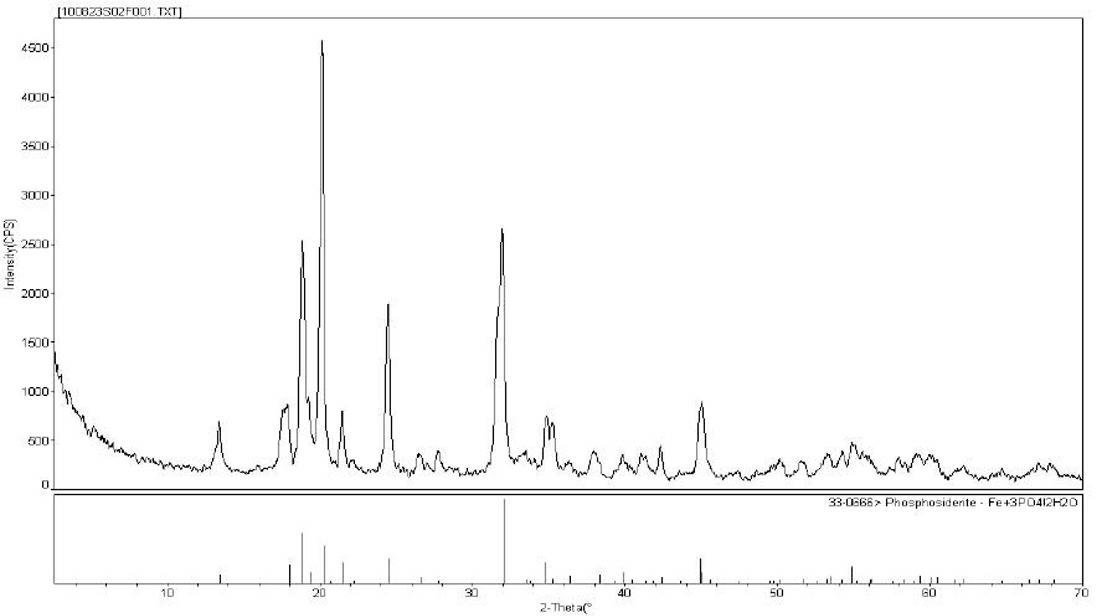
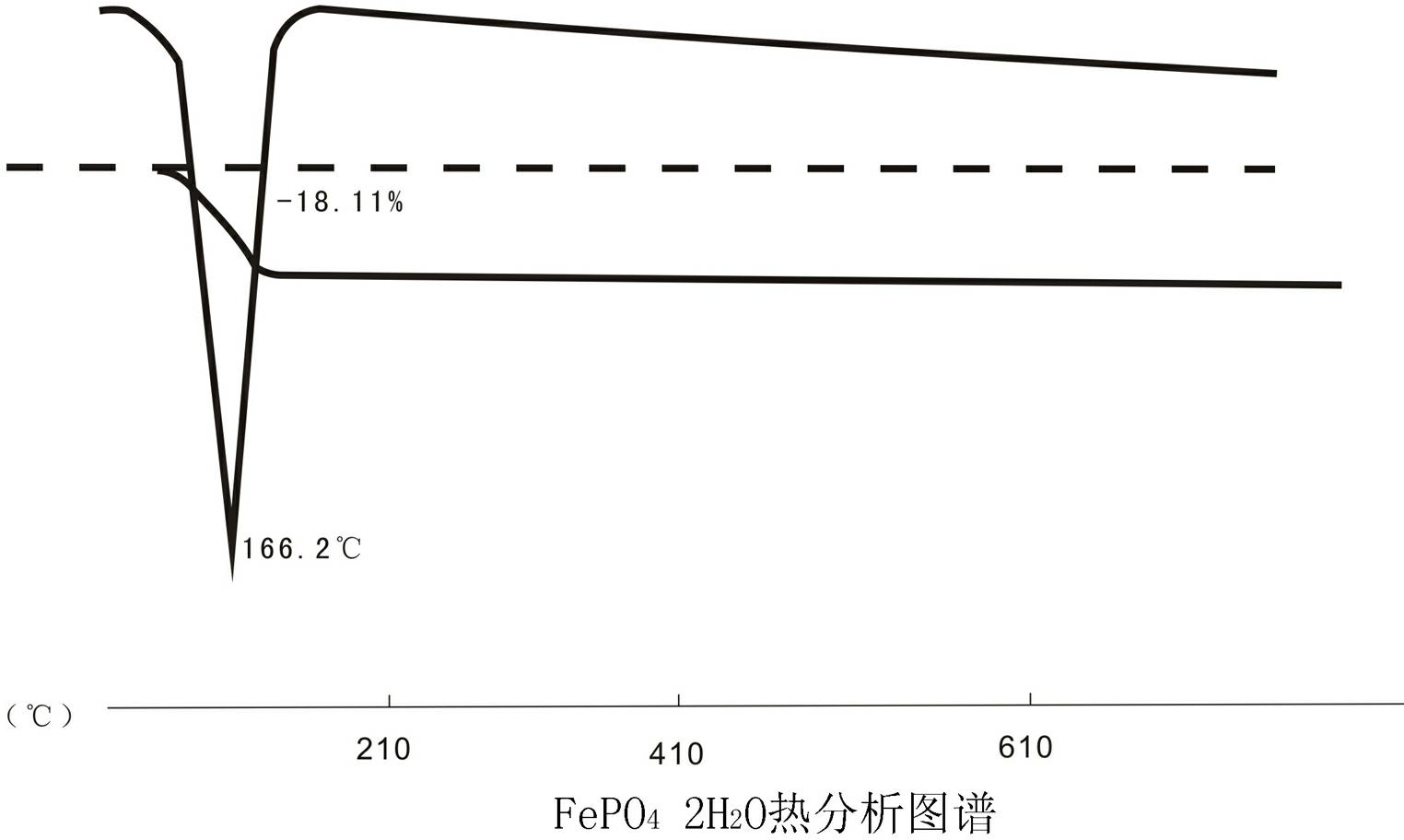
Method for preparing spherical iron phosphate for lithium iron phosphate cell material
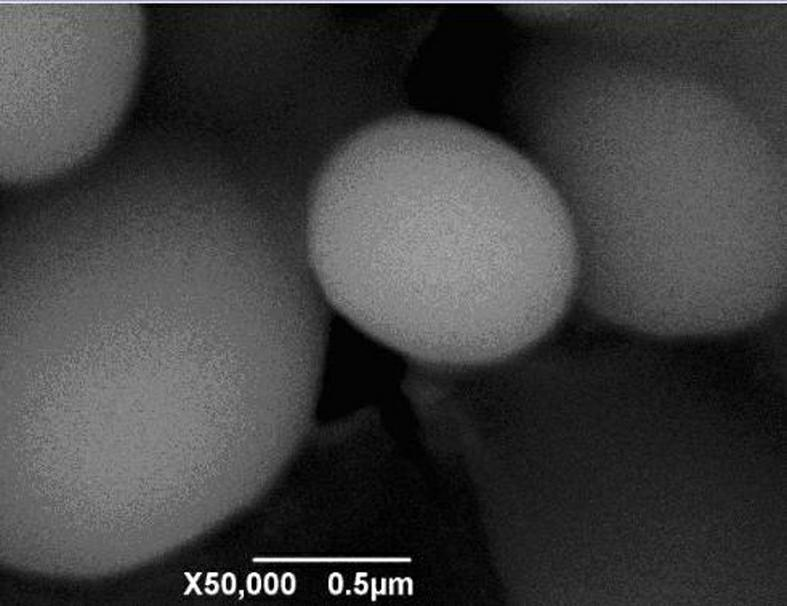
The invention relates to a method for preparing spherical iron phosphate for lithium iron phosphate cell material. In the method, sheet iron, scrap iron, inorganic ferric salt, oxide of ferrum or organic iron is used for preparing ultra-pure high-density spherical iron phosphate for lithium iron phosphate cell material. Iron phosphate products produced by using the method have high main content of over 99.5%, extremely low impurity content, high dispersibility and high flowability, and oscillation ratios are all higher than 0.95; size distribution is in a narrow range, wherein D50 is stabilized at about 3 mum; the shapes of the products are shown to be sphere-like under an SEM (Scanning Electron Microscope), XRD (X-Ray Diffraction) also shows that the obtained iron phosphate products are pure phases, namely iron phosphate products with dehydrate structures. TG-DTA (Thermal Gravimetric-Differential Thermal Analysis) proves that iron phosphate produced by using the method disclosed by the invention is standard iron phosphate with two crystal waters.
Owner:HUBEI HAOYUAN MATERIAL TECH
Reactive hot-melt composition and molded article using the same
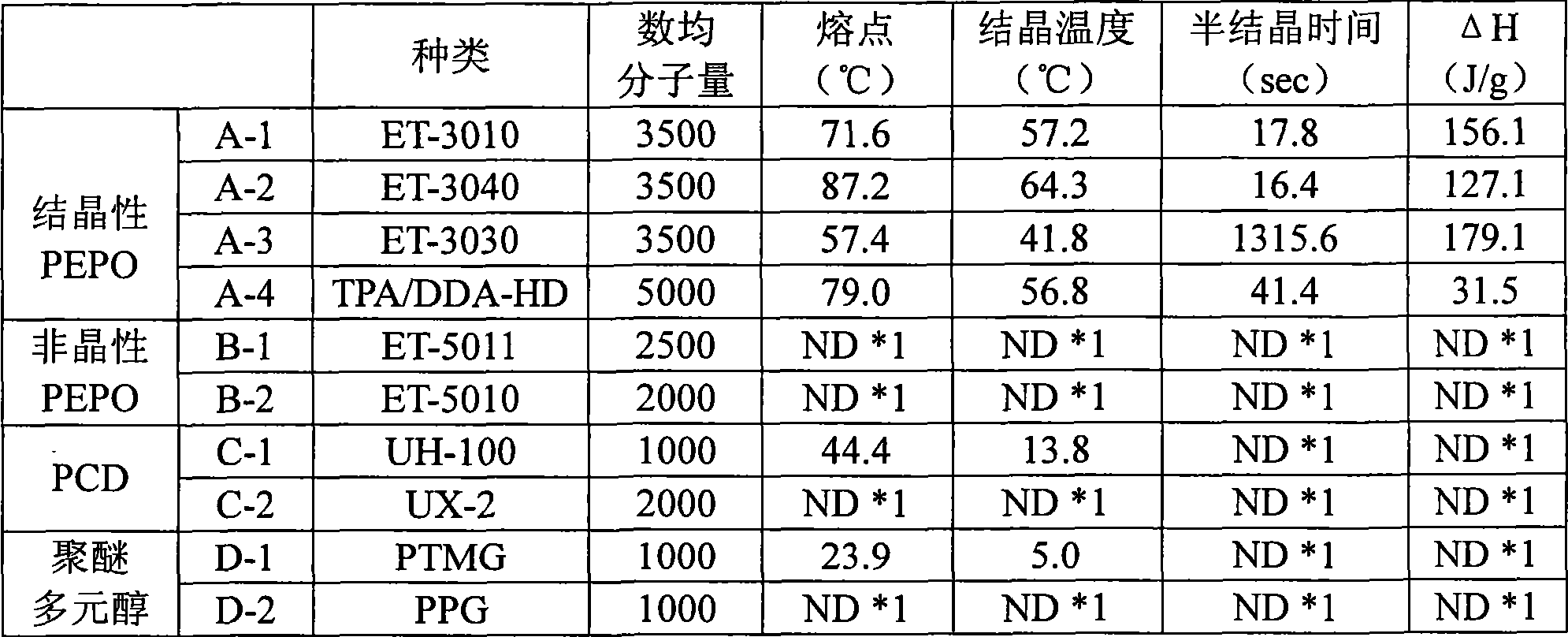
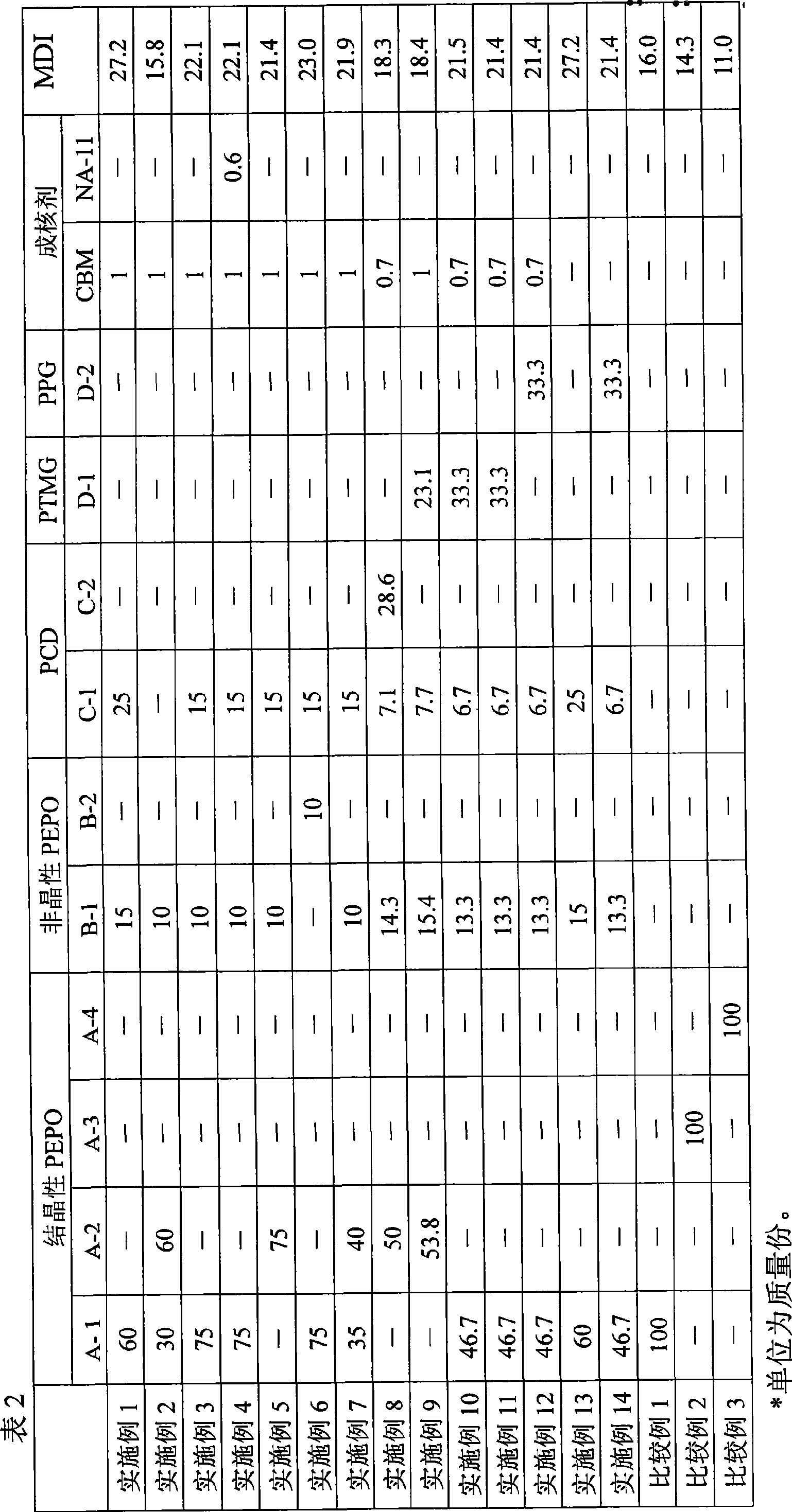
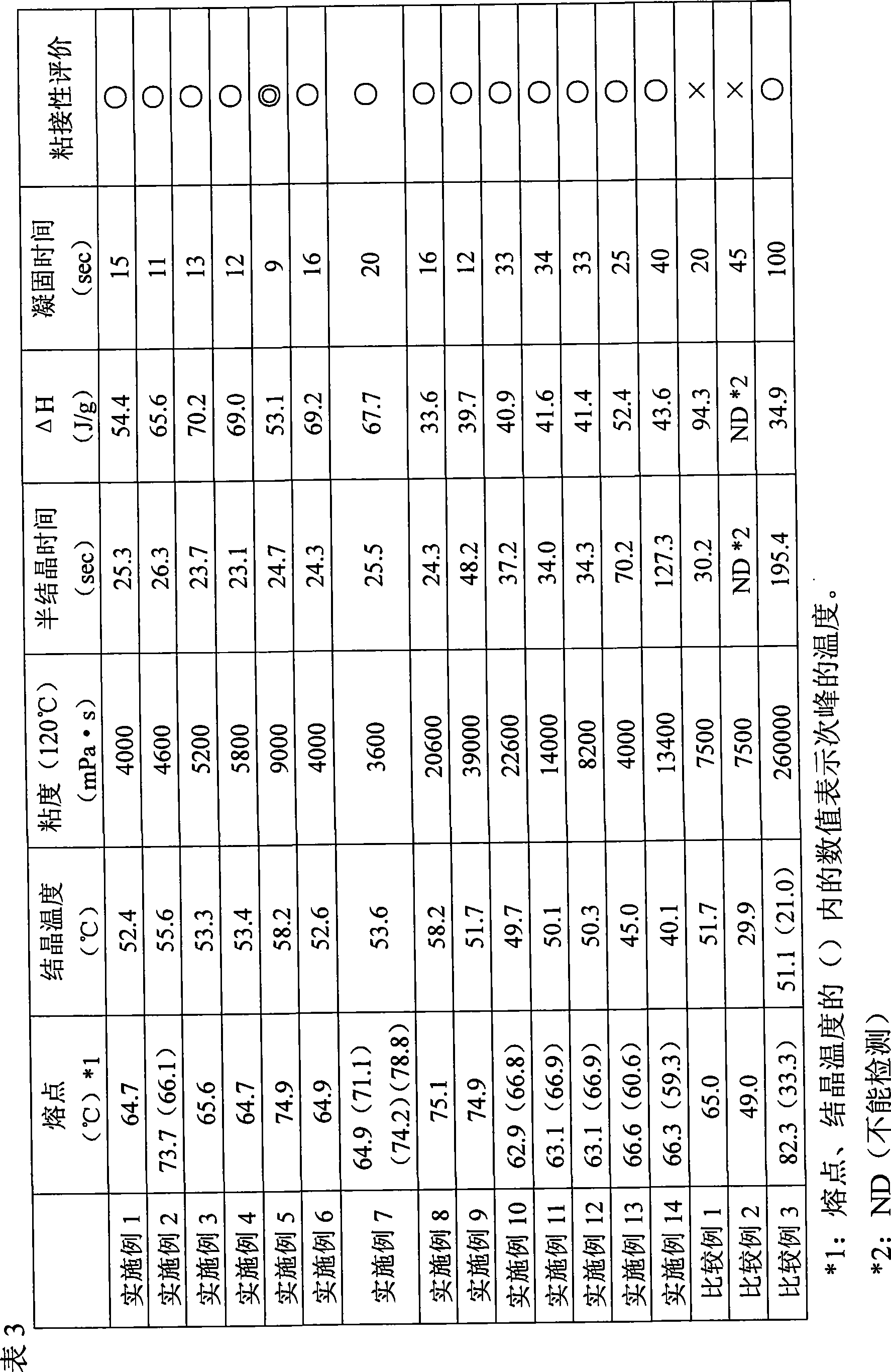
Disclosed is (1) a reactive hot-melt composition which enables the molding cycle to be curtailed and has excellent adhesion to a metal such as aluminum or the like. Specifically disclosed are: (1) a reactive hot-melt composition which can be produced by reacting a composition comprising a crystalline polyester polyol and a non-crystalline polyester polyol with a polyisocyanate, and which has a semicrystallization time of 150 seconds or shorter as measured by a differential thermal analysis method and a heat of crystallization of 30 to 90 J / g; (2) a reactive hot-melt composition which can be produced by reacting a composition comprising 100 parts by mass of a polyol comprising 30 to 95% by mass of a crystalline polyester polyol and 5 to 70% by mass of a non-crystalline polyester polyol and 0.05 to 5 parts by mass of a nucleating agent with a polyisocyanate; and (3) a molded article produced by molding any one of the above-mentioned compositions.
Owner:UBE IND LTD
Method for evaluating flame retardant efficiency of asphalt
InactiveCN103293079AAccurate evaluationFast testWeighing by removing componentMaterial heat developmentBituminous materialsEngineering
The invention discloses a method for evaluating the flame retardant efficiency of asphalt, belonging to the technical field of an asphalt pavement and solving the problem that a method for evaluating the flame retardant efficiency of the asphalt is difficultly accurately quantified by using an existing flame retardant. According to the method, based on the combination of a thermogravimetry-differential thermal analysis synchronous test and a thermal analysis kinetics theoretical equation, the flame retardant efficiency of a flame retardant to the asphalt is quantificationally evaluated. The method comprises the steps of: respectively testing the asphalt and prepared flame-retardant asphalt by adopting a thermogravimetry-differential thermal analyzer to obtain test data such as TGs (Thermal Gravity), DTGs (differential thermogravimetry), DTAs (Differential Thermal Analysis) and char yields; secondly, drawing curves of 1n[g(alpha) / T2] to 1 / T according to the thermal analysis kinetics theoretical equation, and determining reaction mechanism functions g(alpha) in the thermolysis process of the asphalt and the prepared flame-retardant asphalt through linear fitting of a least square method; thirdly, drawing a straight line of the 1n[g(alpha) / T2] to the 1 / T, solving kinetics parameter activation energies E and frequency factors A through a slope and an intercept; finally, comparing the E and the A of the asphalt with the E and the A of the prepared flame-retardant asphalt so as to completely and accurately evaluate the flame-retardant efficiency of the flame retardants with different types and doping quantities to the asphalt.
Owner:NANJING FORESTRY UNIV
Aluminum alloy board
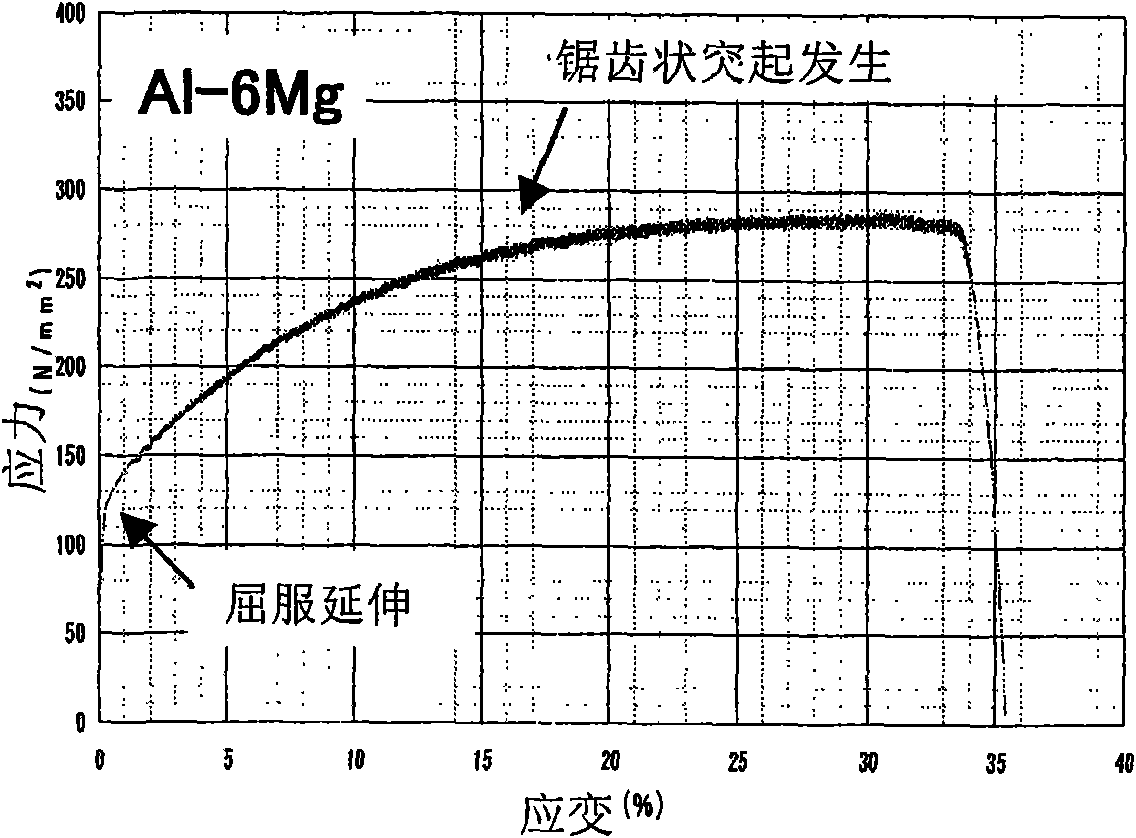
InactiveCN101684531AIncrease the critical strainIncrease the amount of formationSuperstructuresTensile strainThermal variation
Owner:KOBE STEEL LTD
Heat resistant adhesive film and electromagnetic steel sheet with said heat resistant adhesive film, iron core using said electromagnetic steel sheet, and process for manufacturing the same
ActiveCN101040022AAdhesive processesNon-macromolecular adhesive additivesSheet steelRoom temperature
This invention provides an electromagnetic steel sheet, which, after lamination, can be bonded by pressing and heating, can realize strain relieving annealing, and has a surface covered with a heat resistant adhesive insulating film, an iron core using the electromagnetic steel sheet, and a process for manufacturing the same. The electromagnetic steel sheet is covered with a heat resistant adhesive insulating film comprising a resin, which softens at room temperature or above and 300 DEG C or below, and a low-melting inorganic component having a softening temperature of 1000 DEG C or below asmeasured by a differential thermal analysis method. A strain relieving annealable bonded fixed iron core is provided by stacking this electromagnetic steel sheet and conducting press fixation.
Owner:NIPPON STEEL CORP
Dexrabeprazole sodium monohydrate crystal form and preparation method thereof
ActiveCN102924434AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistrySODIUM MONOHYDRATEPhysical chemistry
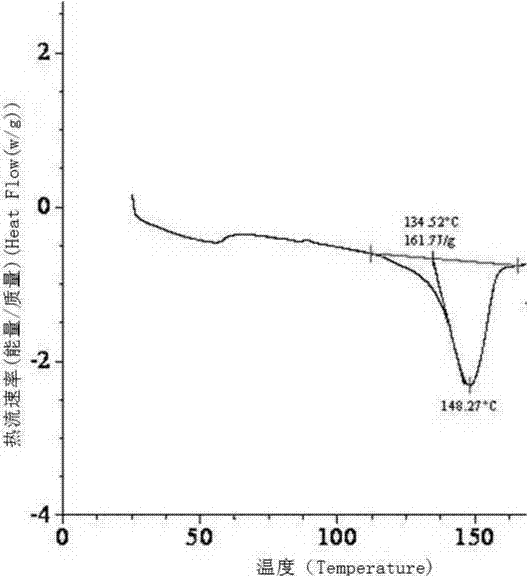
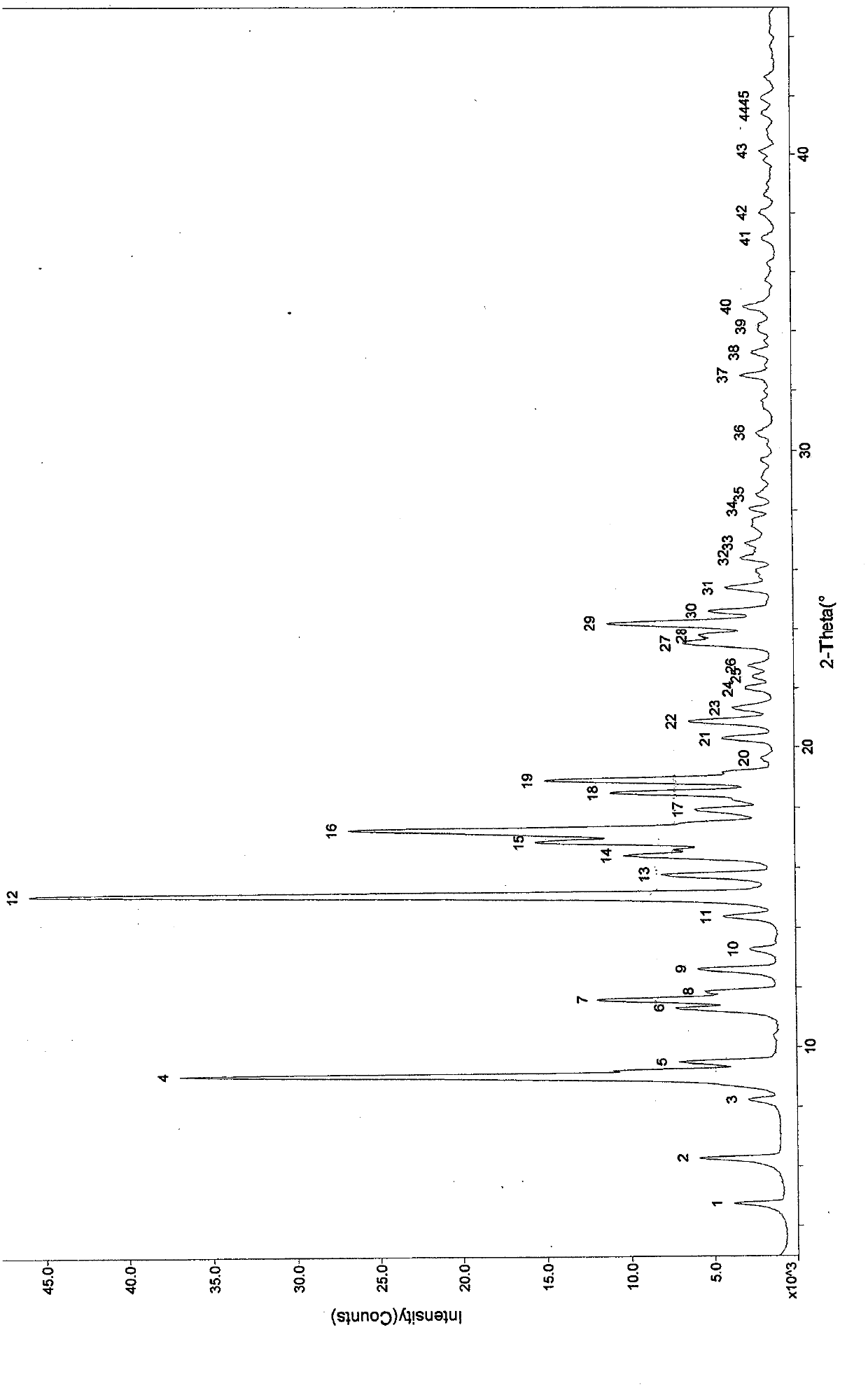
The invention provides a dexrabeprazole sodium monohydrate crystal form. The invention is characterized in that the dexrabeprazole sodium monohydrate crystal form has characteristic diffraction peaks at 10.5, 13.4, 17.1, 18.0, 18.5, 18.9, 19.5, 23.0, 23.3, 27.1 and 31.6 degrees in an X-ray powder diffraction spectrum represented by Cu-Kalpha radiation and 2theta; and an absorption peak exists at about 148.27 DEG C in a differential thermal analysis atlas of the dexrabeprazole sodium monohydrate crystal form. The crystal form has relatively high stability, solubility and dissolution, and relatively low hygroscopicity. The invention also provides a preparation method for the dexrabeprazole sodium monohydrate crystal form. The preparation method is simple, high in yield and low in preparation cost, and the quality of products is high.
Owner:JIANGSU CHENGXIN PHARMA
Method and device for investigation of phase transformations in metals and alloys
ActiveUS7473028B1Reliable and fast and inexpensive monitoringImprove bindingInvestigating phase/state changeAlloyMaterials science
A device and method for investigating phase transformation properties and structural changes of materials. In one form, the device simulates actual thermal processing conditions, while the method can be used in both simulations as well as in actual processing conditions. An analysis using at least one of the device and method is referred to as a single sensor differential thermal analysis, as it compares the temperature recorded in a measured specimen against a reference thermal history without requiring the derivation of the reference thermal history from measured reference temperatures.
Owner:THE OHIO STATES UNIV
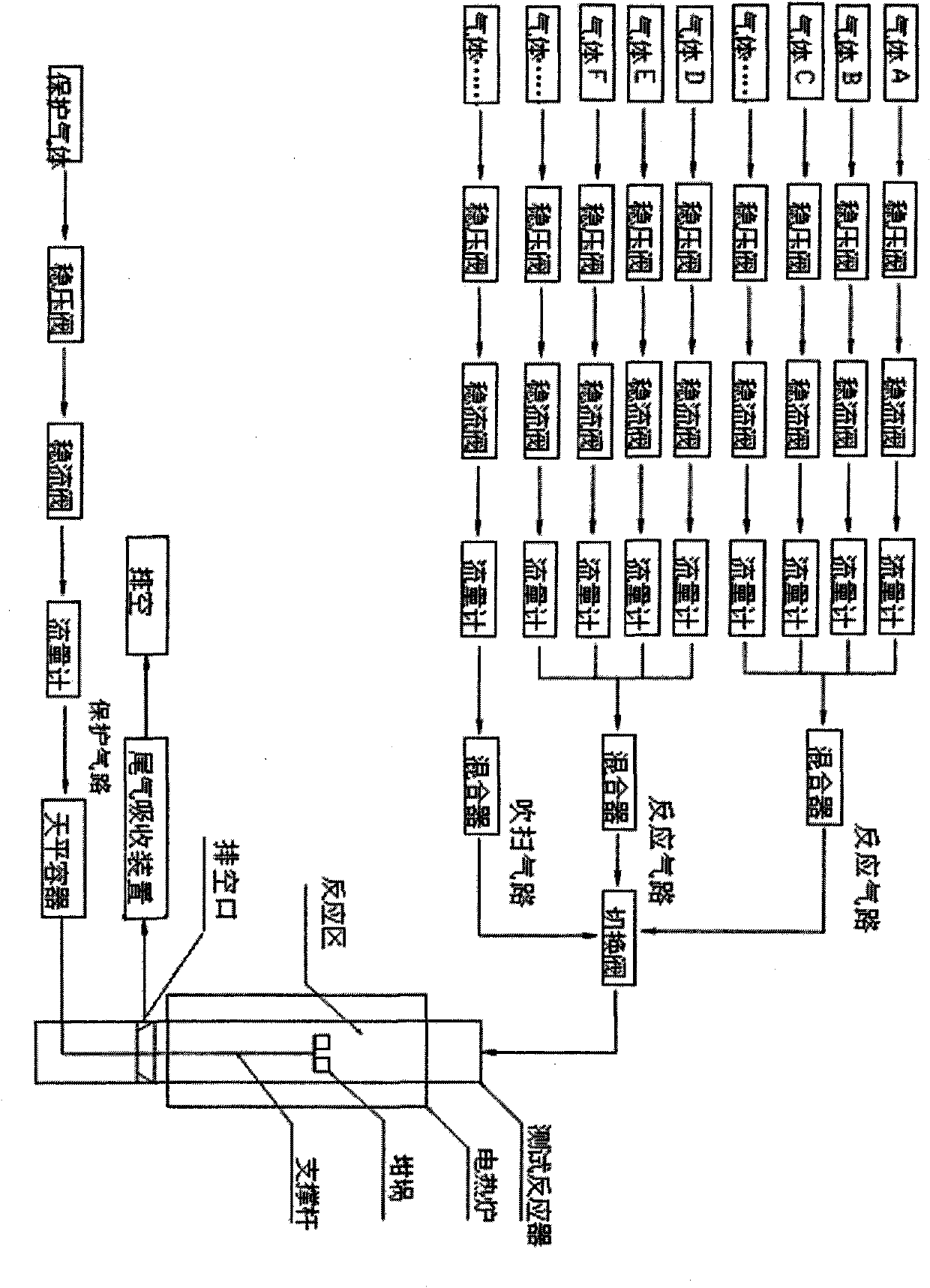
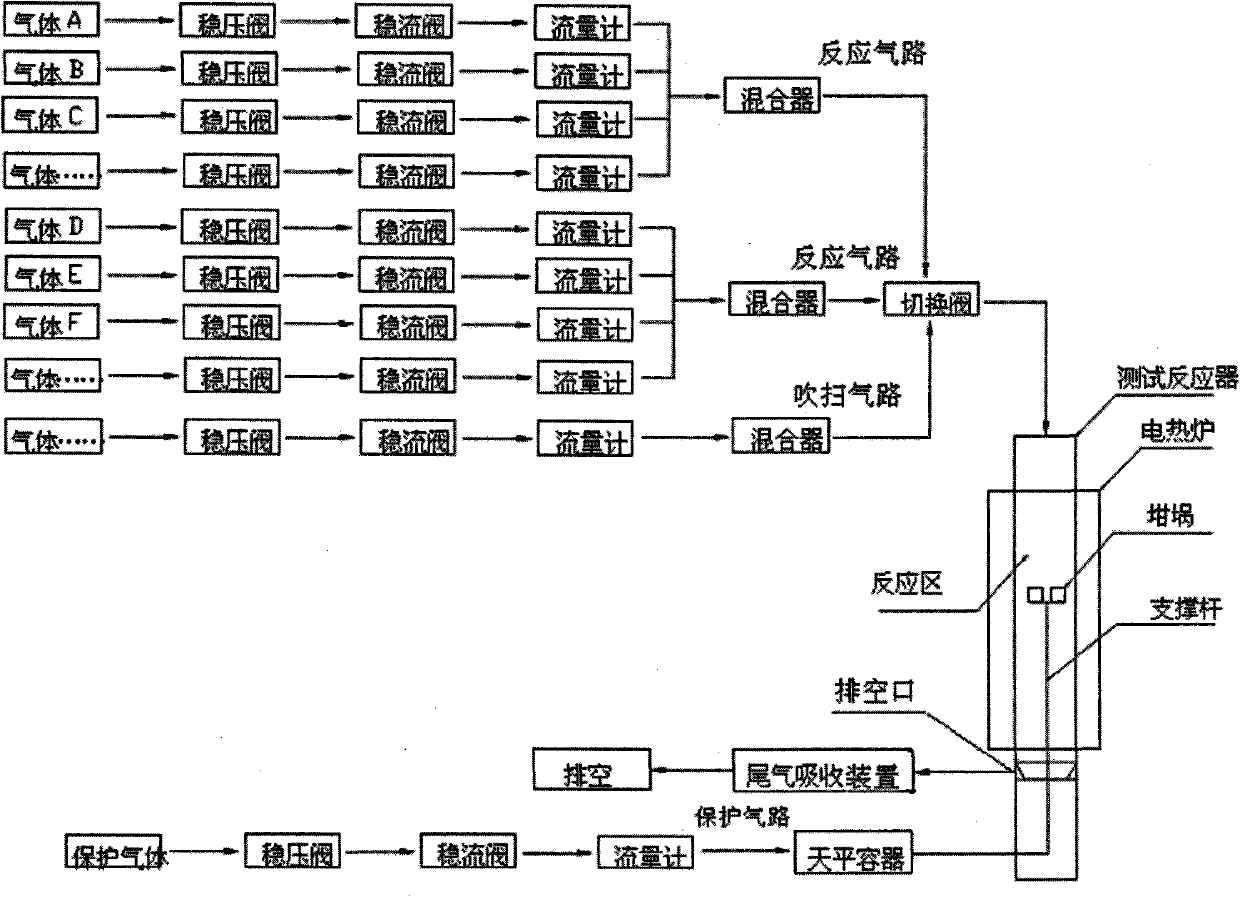
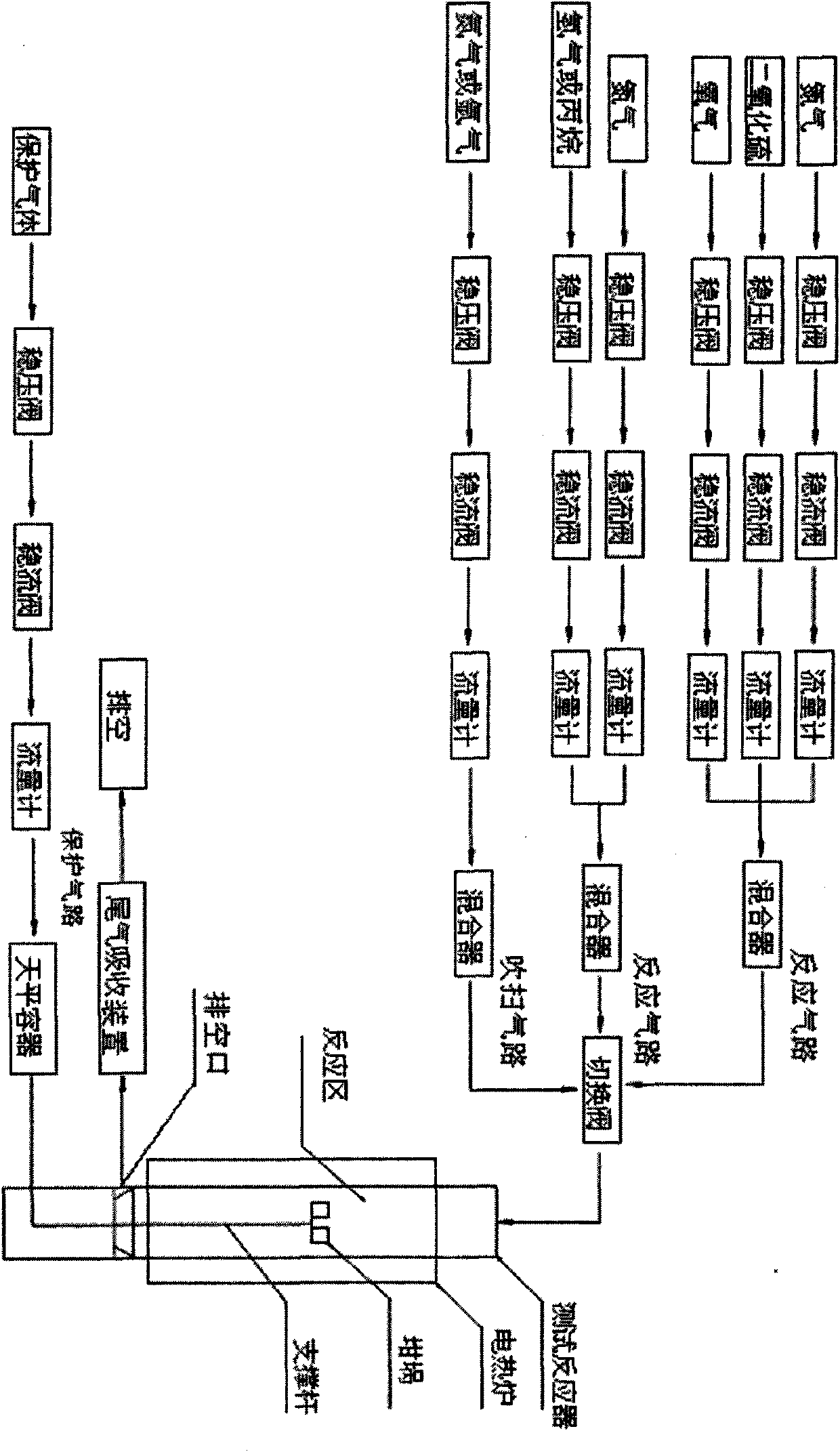
Multi-atmosphere dynamic thermogravimetric-differential thermal analyzer and application thereof in sulfur transfer performance simulation and evaluation of flue gas
InactiveCN101907591ARealize dynamic in-situ testingQuick evaluationMaterial heat developmentWeighing by absorbing componentPositive pressureHydrogen atmosphere
The invention discloses a multi-atmosphere dynamic thermogravimetric-differential thermal analyzer which belongs to the field of petroleum refining industry and researches application thereof in sulfur transfer performance simulation and evaluation of flue gas. The analyzer comprises a protection gas circuit, a multigroup reaction gas circuit, a purging gas circuit, a switching valve, a test reactor, a drain port, a tail gas absorption device and an electrothermal furnace. The analyzer can blend and provide different required multicomponent mixed gas through one or a plurality of groups of gas regulating and mixing systems, switch the atmospheres in the test reactor according to requirements, simulate reaction atmospheres with complicated changes, realize dynamic in-situ testing, more particularly simulate a sulfur dioxide atmosphere and a hydrogen atmosphere (or a hydrocarbon atmosphere) under the micro positive pressure and carry out safe switching between the sulfur dioxide atmosphere and the hydrogen atmosphere (or the hydrocarbon atmosphere), record changes of the weight and the temperature of a sample in thermal treatment process at the real time under the condition of programmed temperature controlling, carry out thermogravimetric analysis and differential thermal analysis and is used for simulating and evaluating the performance of a sulfur transfer agent of catalytically-cracked flue gas.
Owner:BEIJING UNIV OF CHEM TECH
Stable Ivabradine crystal and preparation thereof
The invention discloses a stable ivabradine hydrochloride crystal (formula I), which is verified by a DCS differential thermal analysis chart, a melting point and an X-ray powder diffraction diagram. By adopting the method, the ivabradine hydrochloride crystal (formula I) prepared by the method is very stable to temperature, illumination and humidity, thus being favorable for long-term storage. The solvent used in the crystal is safe, and is easy to be removed; therefore, the stable ivabradine hydrochloride crystal is applicable to industrialized production.
Owner:UTOPHARM SHANGHAI +1
Anode material for lithium ion secondary battery, anode for lithium ion secondary battery, and lithium ion secondary battery
ActiveUS20130143127A1Solve the small densityImprove featuresSecondary cellsNon-aqueous electrolyte accumulator electrodesLithiumX-ray
An anode material for a lithium ion secondary battery that includes a carbon material having an average interlayer spacing d002 as determined by X-ray diffraction of from 0.335 nm to 0.340 nm, a volume average particle diameter (50% D) of from 1 μm to 40 μm, a maximum particle diameter Dmax of 74 μm or less, and at least two exothermic peaks within a temperature range of from 300° C. to 1000° C. in a differential thermal analysis in an air stream.
Owner:RESONAC CORP
Oxide semiconductor layer and production method therefor, oxide semiconductor precursor, oxide semiconductor layer, semiconductor element, and electronic device
ActiveUS20160181098A1Reliably reducedReduce crackingTransistorGallium/indium/thallium compoundsDecompositionPeak value
The invention provides an oxide semiconductor layer that has less cracks and is excellent in electrical property and stability, as well as a semiconductor element and an electronic device each including the oxide semiconductor layer. The invention provides an exemplary method of producing an oxide semiconductor layer, and the method includes the precursor layer forming step of forming, on or above a substrate, a layered oxide semiconductor precursor including a compound of metal to be oxidized into an oxide semiconductor dispersed in a solution including a binder made of aliphatic polycarbonate, and the annealing step of heating the precursor layer at a first temperature achieving decomposition of 90 wt % or more of the binder, and then annealing the precursor layer at a temperature equal to or higher than a second temperature (denoted by X) that is higher than the first temperature, achieves bonding between the metal and oxygen, and has an exothermic peak value in differential thermal analysis (DTA).
Owner:JAPAN ADVANCED INST OF SCI & TECH +1
Heat treatment process of nickel base single crystal superalloy
InactiveCN104746145AImprove durabilityRaise the initial melting temperaturePolycrystalline material growthAfter-treatment detailsSingle crystal superalloySingle crystal
The invention discloses a heat treatment process of nickel base single crystal superalloy. A differential thermal analysis method and metallographic test method are used to determine the incipient melting temperature of the alloy at 1280 DEG C; and an optical metalloscope is used for observation of the microstructure of alloy after different solid solution treatments, and the stress rupture properties of the alloy are tested. The results show that optimum heat treatment process of the alloy is as below: 1245 DEG C / 2h, AC+1275 DEG C / 4h, AC+1100 DEG C / 2h, AC+850 DEG C / 24h, AC. The single crystal superalloy treated by the process has excellent durability, and creep rupture life reaching 159.35h under 980 DEG C and 235MPa.
Owner:QINGDAO YUGUANG PRECISION CASTING FACTORY
Carbonaceous material for non-aqueous electrolyte secondary battery negative electrode
InactiveCN103415948AImprove charge and discharge efficiencyReduce thicknessCell electrodesCarbon preparation/purificationAir atmosphereMicrometer
The purpose of the present invention is to provide a carbonaceous material for a non-aqueous electrolyte secondary battery negative electrode, which has large charge-discharge capacity, high charge-discharge efficiency and excellent charge-discharge cycle properties. The purpose is achieved by a carbonaceous material for a non-aqueous electrolyte secondary battery negative electrode, characterized in that the average interlayer spacing on face (002) is 0.365-0.40 nm as measured by an X-ray diffraction method, no exothermic peak appears in a temperature region of 620 DEG C or lower as measured by a differential thermal analysis under an air atmosphere, the BET specific surface area is 1-7 m2 / g, the average particle diameter (D50) is 5-25 micrometer, and the (D90-D10) / D50 ratio is 1.05 or less.
Owner:KUREHA KAGAKU KOGYO KK
Crystallized glass and method for producing crystallized glass
InactiveCN101085698AAvoid crackingIncrease productivitySemiconductor/solid-state device manufacturingOriginals for photomechanical treatmentMetallurgyLarge size
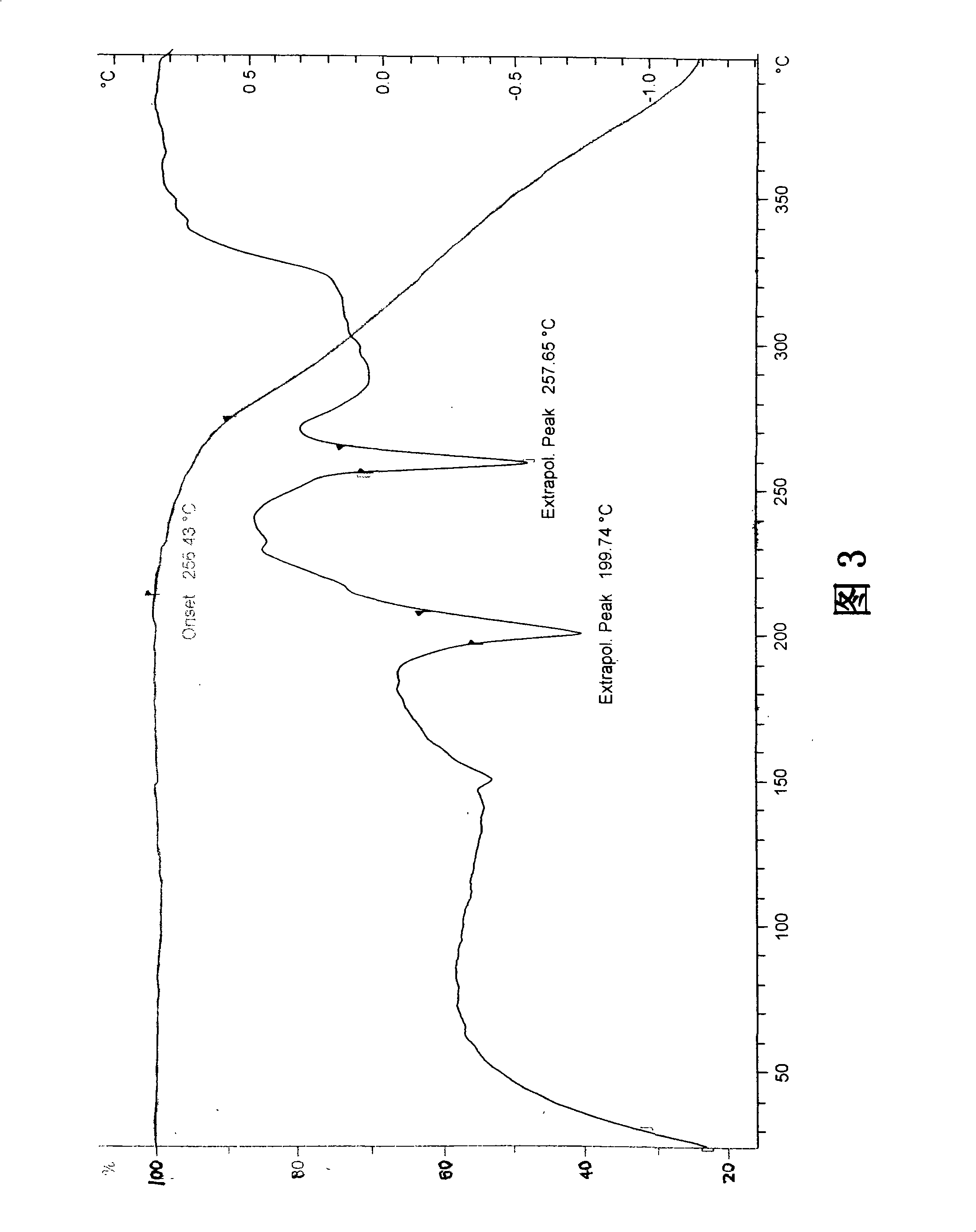
Provided are SiO 2 -Al 2 O 3 -based or Li 2 O-Al 2 O 3 -SiO 2 -based crystallized glass that solves the cause of cracking and fracture in forming it into large-size shaped articles, that has homogeneous inner quality and that may be produced stably at highefficiency; andamethodforproducingit. Thecrystallized glass contains components of SiO 2 and Al 2 O 3 , wherein the crystal precipitation peak temperature width obtained in differential thermal analysis of amorphous glass, a precursor thereof, is at least 22°C. Preferably, the total amount of the TiO 2 component and the ZrO 2 component in the crystallized glass is within a range of from 3.0 to less than 4.3 %.
Owner:OHARA
Polycrystal forms of ulipristal acetate and preparation method thereof
ActiveCN102675395AAvoid problems such as new impuritiesShorten the timeOrganic active ingredientsSteroidsInfraredX-ray
The invention discloses a polycrystal form A1 and a polycrystal form A2 of ulipristal acetate (formula I) free of solvate and a preparation method, a pharmaceutical composition and medical application of the polycrystal forms. By verification of melting point, X-ray diffraction powder spectrum (XRD), infrared spectrum (IR), differential thermal analysis spectrum (DSC) and thermogravimetric spectrum (TG), the polycrystal form A1 and polycrystal form A2 of ulipristal acetate prepared by the method disclosed by the invention are extremely stable to temperature, illumination and humidity, and favorable to long-term storage; the used crystallizing solvent is safe and easy to remove; the obtained polycrystal form A1 and the polycrystal form A2 can be directly used for preparation processing; and the preparation method is simple in operation and suitable for industrial production. The formula I is shown in the description.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Thermal analysis method for measuring contents of polydimethylsiloxane (PDMS), SiO2 and aluminum hydroxide (ATH) in silicone rubber composite insulator
ActiveCN104237299AMaterial heat developmentSpecial data processing applicationsSilicone rubber insulatorsCalculation methods
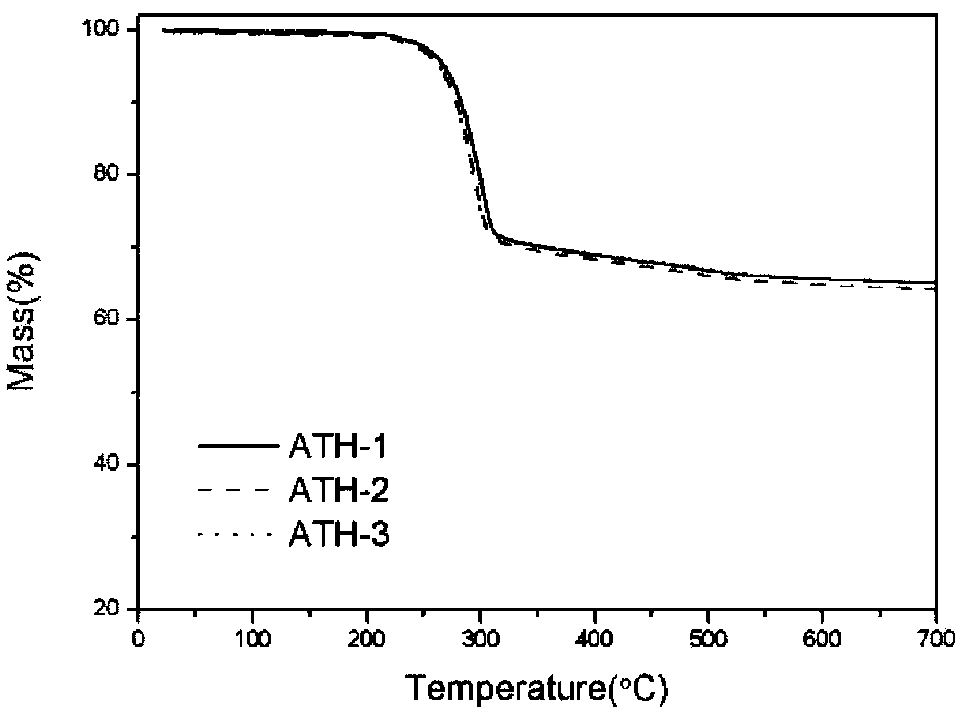
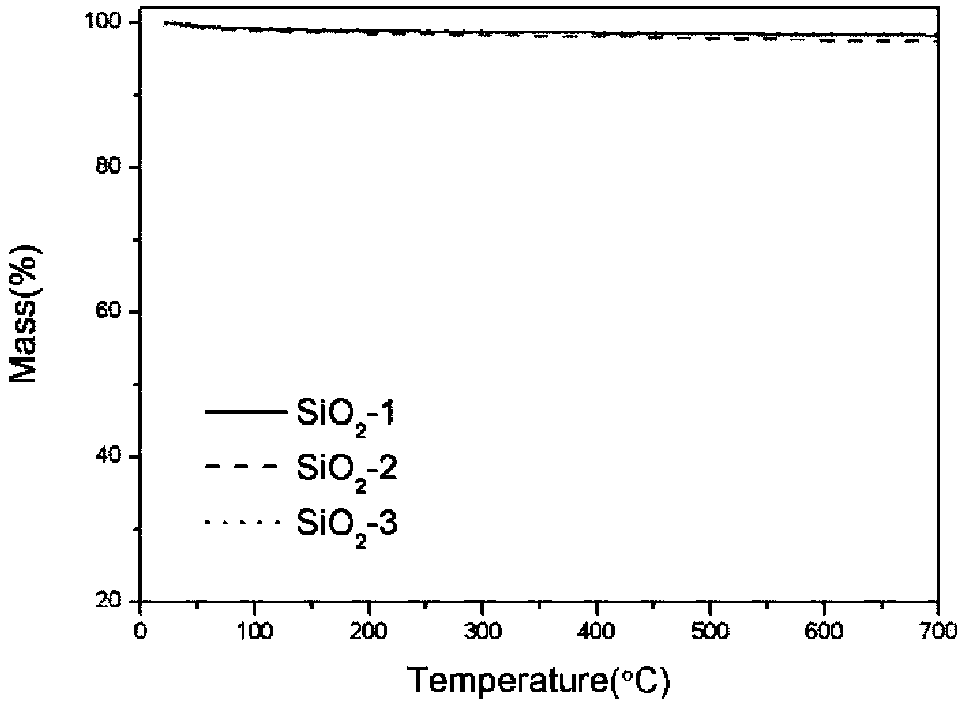
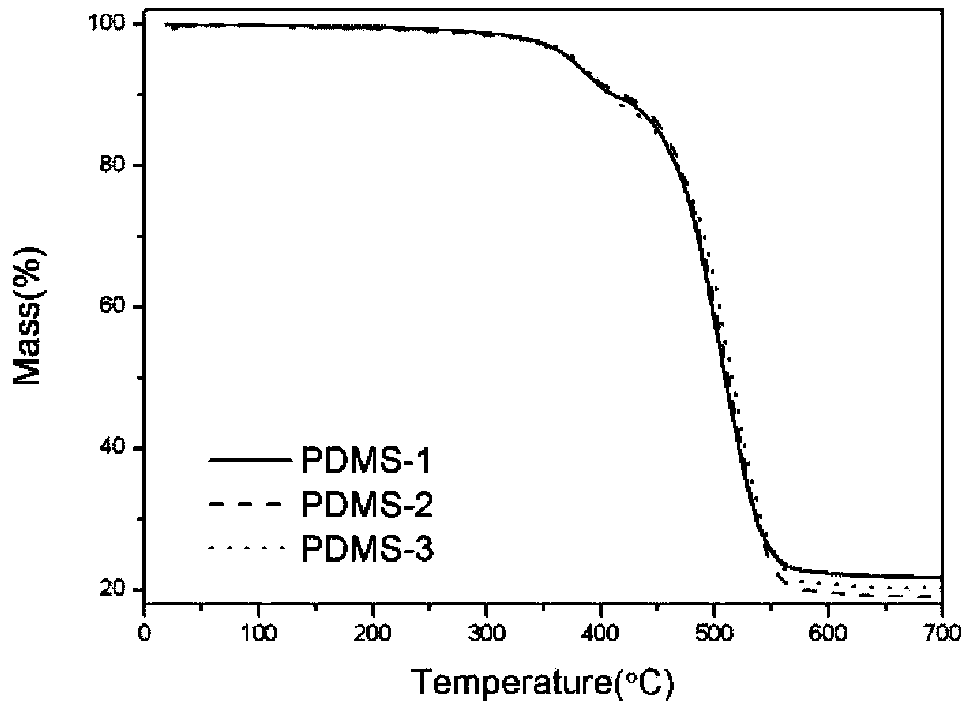
The invention relates to a thermal analysis method for measuring the contents of polydimethylsiloxane (PDMS), SiO2 and aluminum hydroxide (ATH) in a silicone rubber composite insulator. The method comprises the steps of S1, respectively measuring the thermal gravity loss (TG) curves of the PDMS, the ATH and the SiO2 for three times by a thermal gravity loss-differential thermal analysis combination comprehensive thermal analyzer, and averaging; S2, cutting three parts from the silicone rubber insulator randomly, wherein the cut parts have the sizes of 1cm*1cm and the masses within the range of 5-50mg; pretreating, then measuring the TG curves of the silicone rubber insulator for three times, and averaging; S3, calculating the thermal weight loss rates of the PDMS, the ATH, the SiO2 and the silicone rubber insulator in the ranges of 20-360 DEG C and 360-700 DEG C according to the TG curves; S4, substituting the obtained numerical values into the formula delta M=(m(ATH)*deltam(ATH)+m(PDMS)*delta m(PDMS)+m(SiO2)*delta m(SiO2)) / (m(ATH)+m(PDMS)+m(SiO2)); and S5, working out the equation in the S4, and calculating the contents of the ATH and the SiO2 relative to the PDMS. According to the method, the contents of the three components of the silicone rubber composite insulator can be worked out by utilizing the thermal weight loss rates of the three components in the respective specific thermal decomposition temperature ranges, and a concrete calculation method is provided.
Owner:ELECTRIC POWER RES INST OF GUANGDONG POWER GRID +1
Crystalline resin cured product, crystalline resin composite material, and method for producing the same
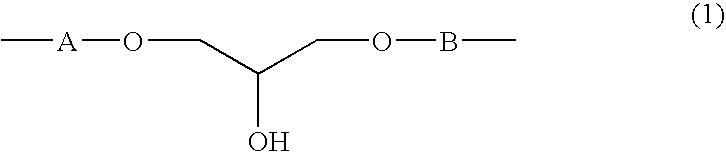
Provided are a crystalline resin cured product which shows high thermal conductivity, low thermal expansion, high heat resistance, low moisture absorption, and good gas barrier properties and a crystalline resin composite material produced therefrom. Further provided is a method for producing the crystalline resin cured product and the crystalline resin composite material. The crystalline resin cured product is obtained by the reaction of an aromatic diglycidyl compound or a diglycidyl resin with an aromatic dihydroxy compound or with a dihydroxy resin and it shows a heat of melting of 10 J / g or more in differential thermal analysis while the endothermic peak corresponding to the melting appears in the range of 120 to 320° C. The crystalline resin composite material is obtained by combining the crystalline resin cured product with a filler or a base material. The crystalline resin cured product has a unit represented by -A-O—CH2—CH(OH)—CH2—O—B—, wherein A and B are divalent aromatic groups.
Owner:NIPPON STEEL CHEMICAL CO LTD
A stable Apremilast crystal form II free of solvates and a preparing method thereof
The invention discloses a stable Apremilast (shown as a formula I) crystal form II free of solvates, and a preparing method, pharmaceutical compositions and pharmaceutical uses thereof, and discloses a mixed crystal of the crystal form II and a crystal form B and a preparing method thereof. The crystal form II is confirmed by a melting point, an X-ray powder diffraction pattern (XRPD), an infrared spectrum (IR), a differential thermal analysis graph (DSC) and a thermogravimetric curve (TG). Compared with crystal forms A, B, C, D, E, F and G reported in the present literature, the Apremilast crystal form II is more stable to temperature, light and humidity and is beneficial to long-term storage, a crystallization solvent used is safe and easy to remove, the crystal form II is white or off-white and can be directly used for preparation processing, and the preparing method of the crystal form II is simple in operation, easy to repeat and suitable for industrial production.
Owner:UTOPHARM SHANGHAI
Preparation method of solar energy power generation heat-preserving material such as Al-Si alloy in which percentage of Si is 12.07
InactiveCN102191392AInhibition of premature productionExtended service lifeEnergy inputHeat-exchange elementsNew energySilicon alloy
The invention relates to a preparation method of a solar energy power generation heat-preserving material such as Al-Si alloy in which percentage of Si is 12.07. The new energy-storing material, namely the aluminium-silicon alloy, is mainly applied to solar energy power generation to store heat, so that new energy is reasonably used. In the Al-Si alloy in which percentage of Si is 12.07, AlTi10 intermediate alloy and a phosphor salt serve as a composite alterant and commercial-purity aluminium of which the mass fraction is 99.9 percent and crystal silicon of which the mass fraction is 99.7 percent serve as substrates to prepare the new energy-storing material according with use of solar energy power generation. Results of metallographic analysis and differential thermal analysis show that: in the alloy subjected to composite modification of phosphor and AlTi10, the coarse block and stripe primary crystal silicon is obviously reduced, the edges and corners are passivated, and the relatively big needlelike eutectic silicon becomes particles. Composition segregation appearing when the traditional material is subjected to smelt-solidification circulation for about 1,000 times at the temperature of between 480 and 620 DEG C is improved and the utilization rate of new energy is improved.
Owner:GUANGDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com