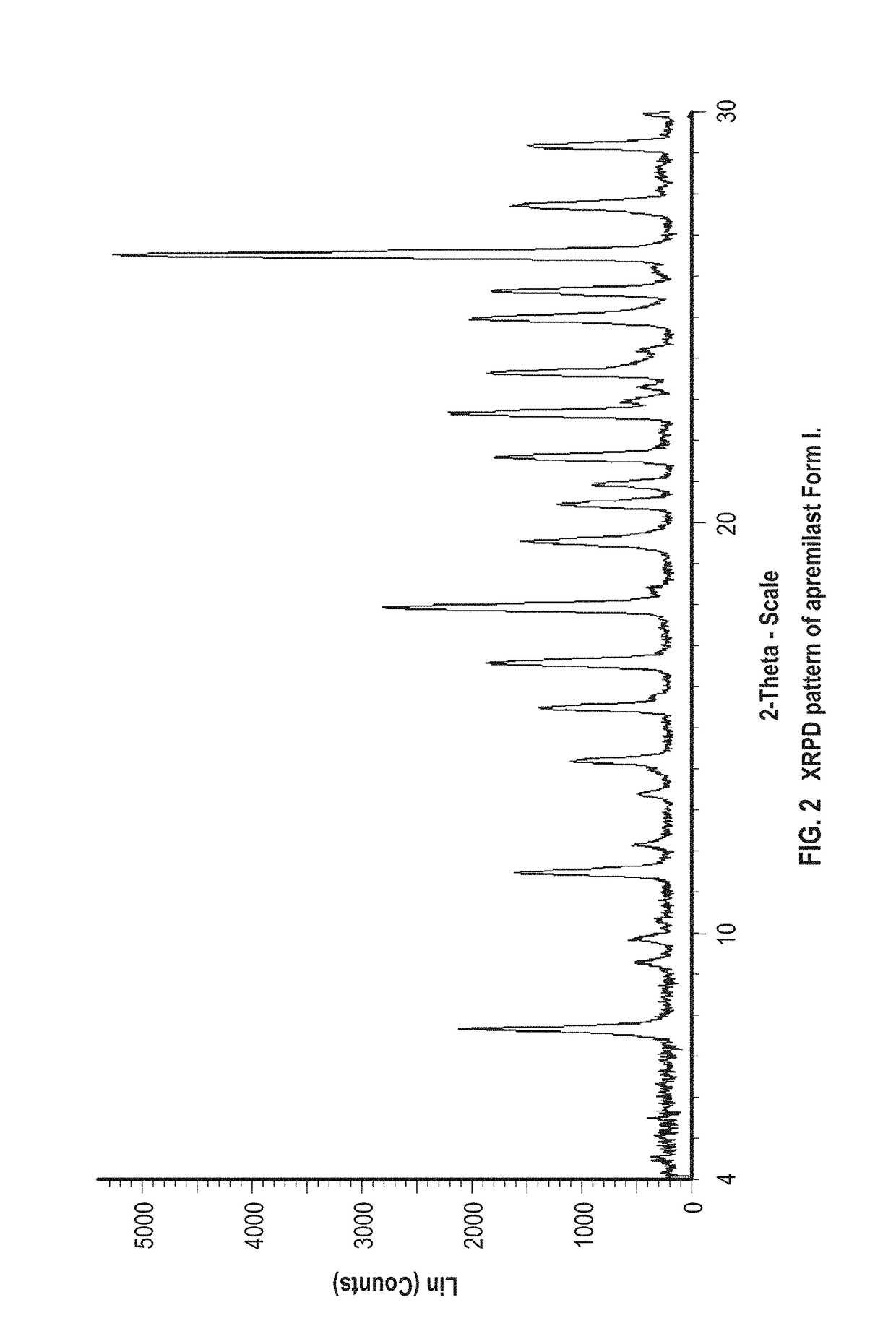
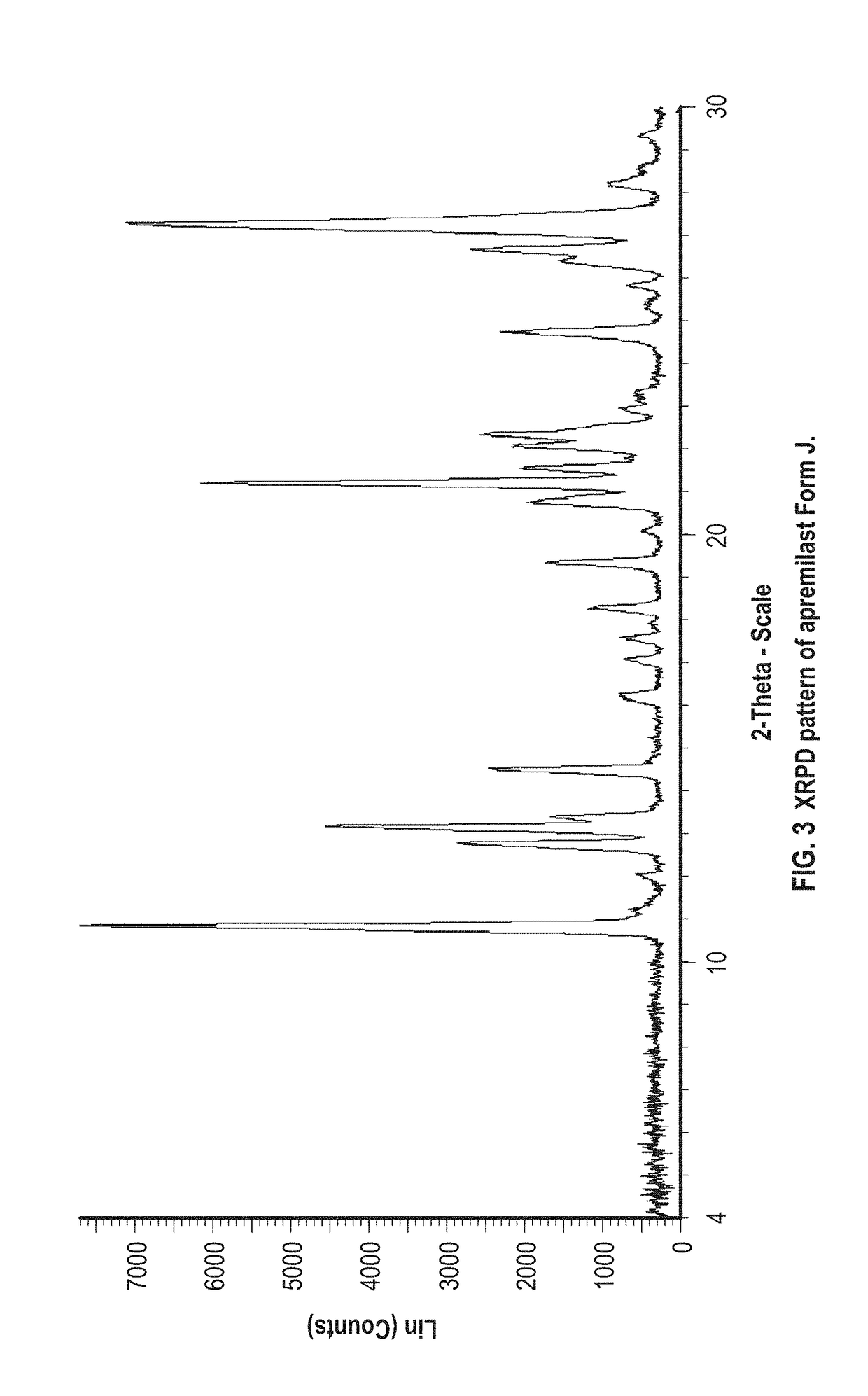
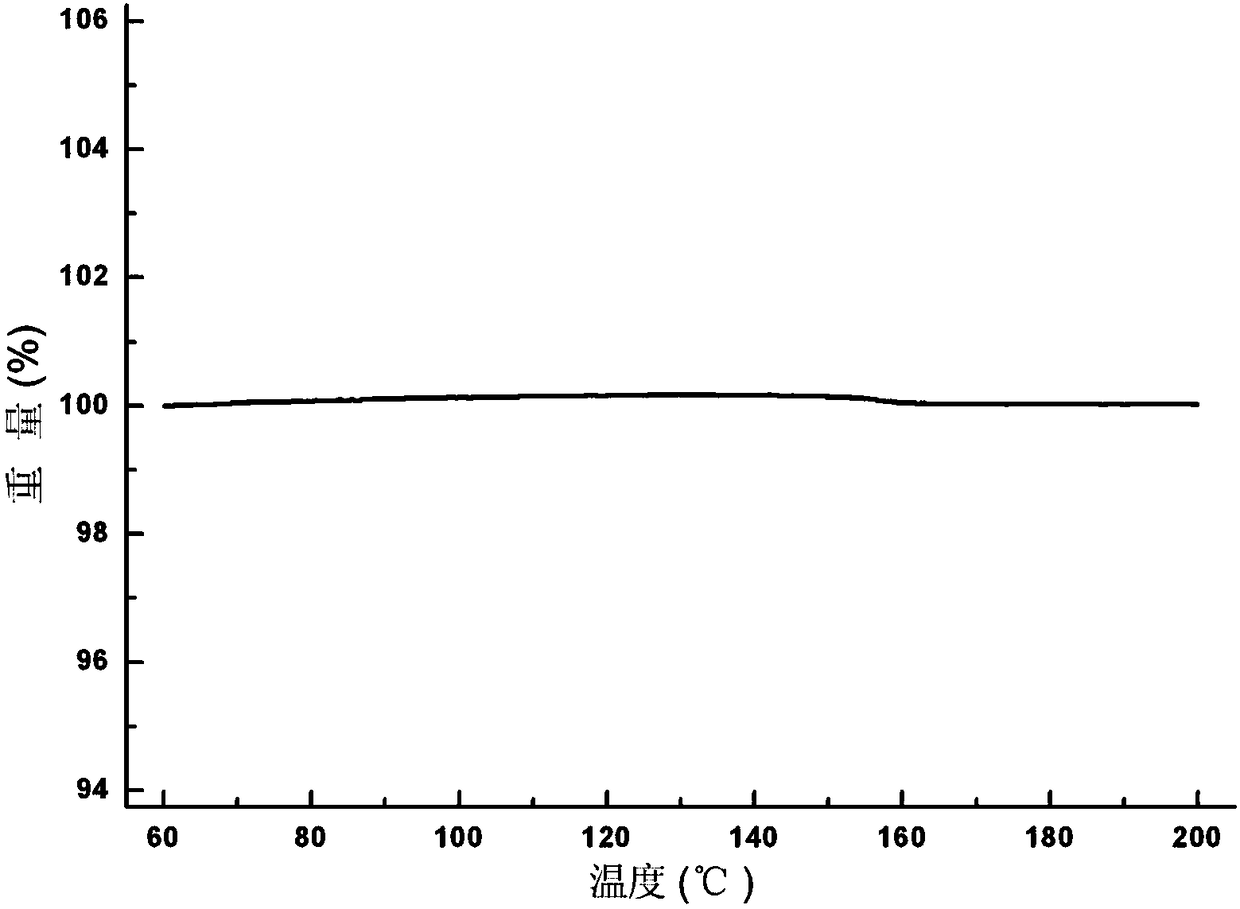
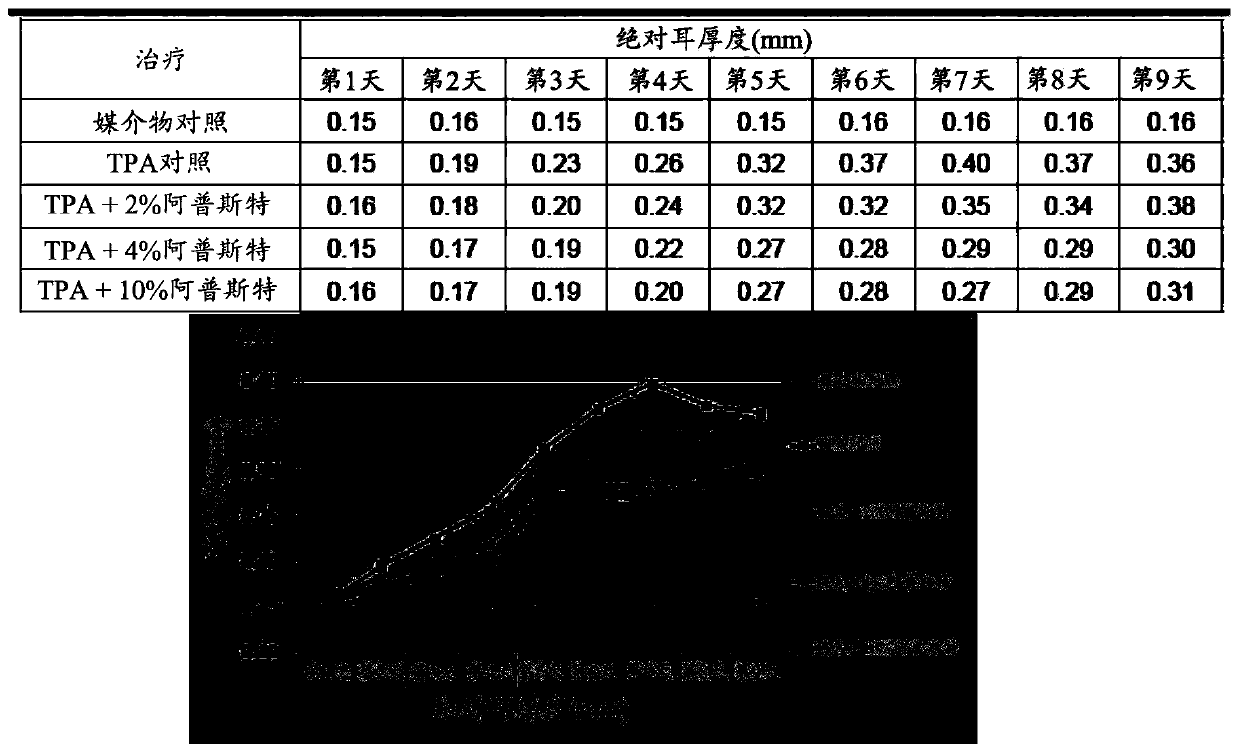
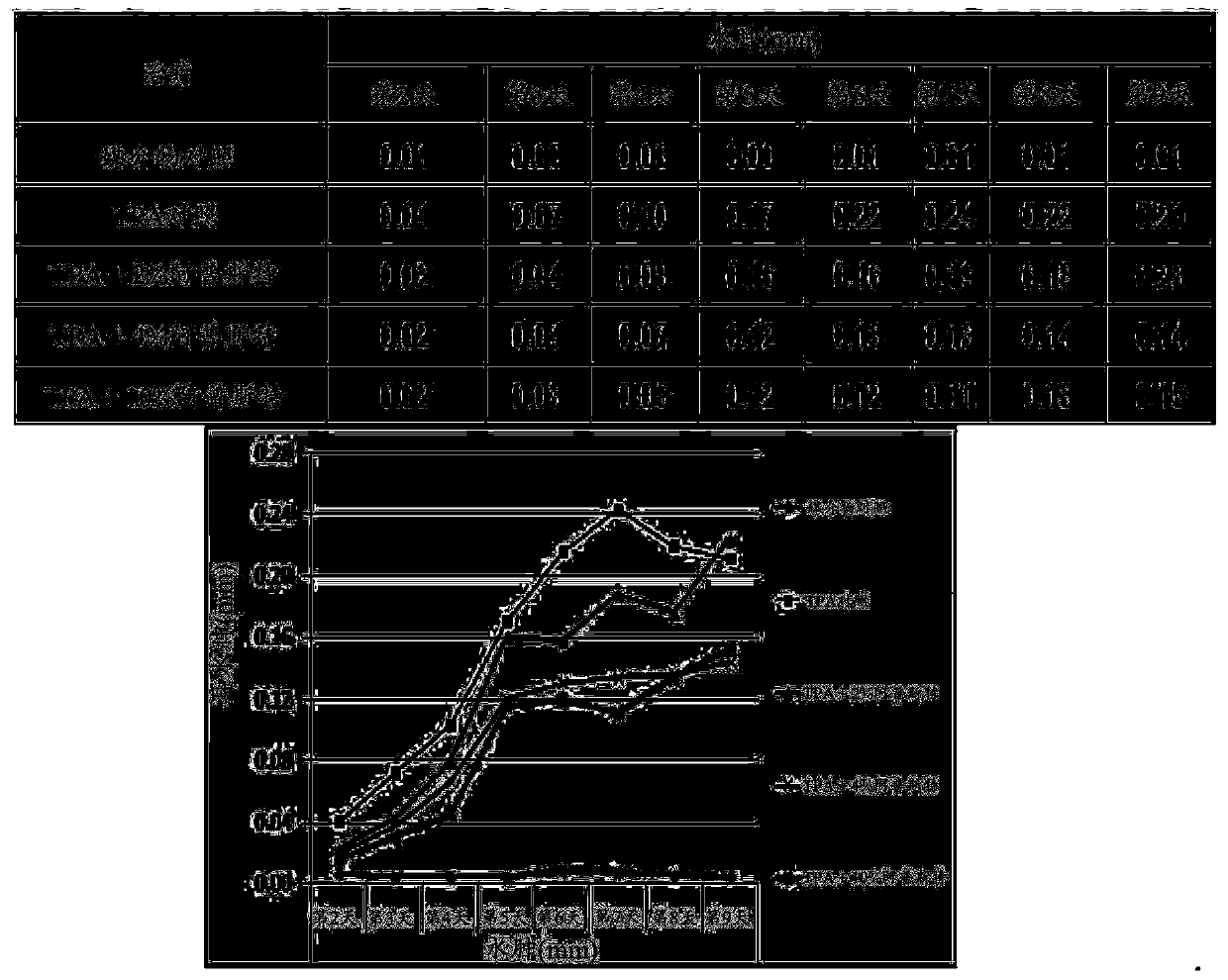
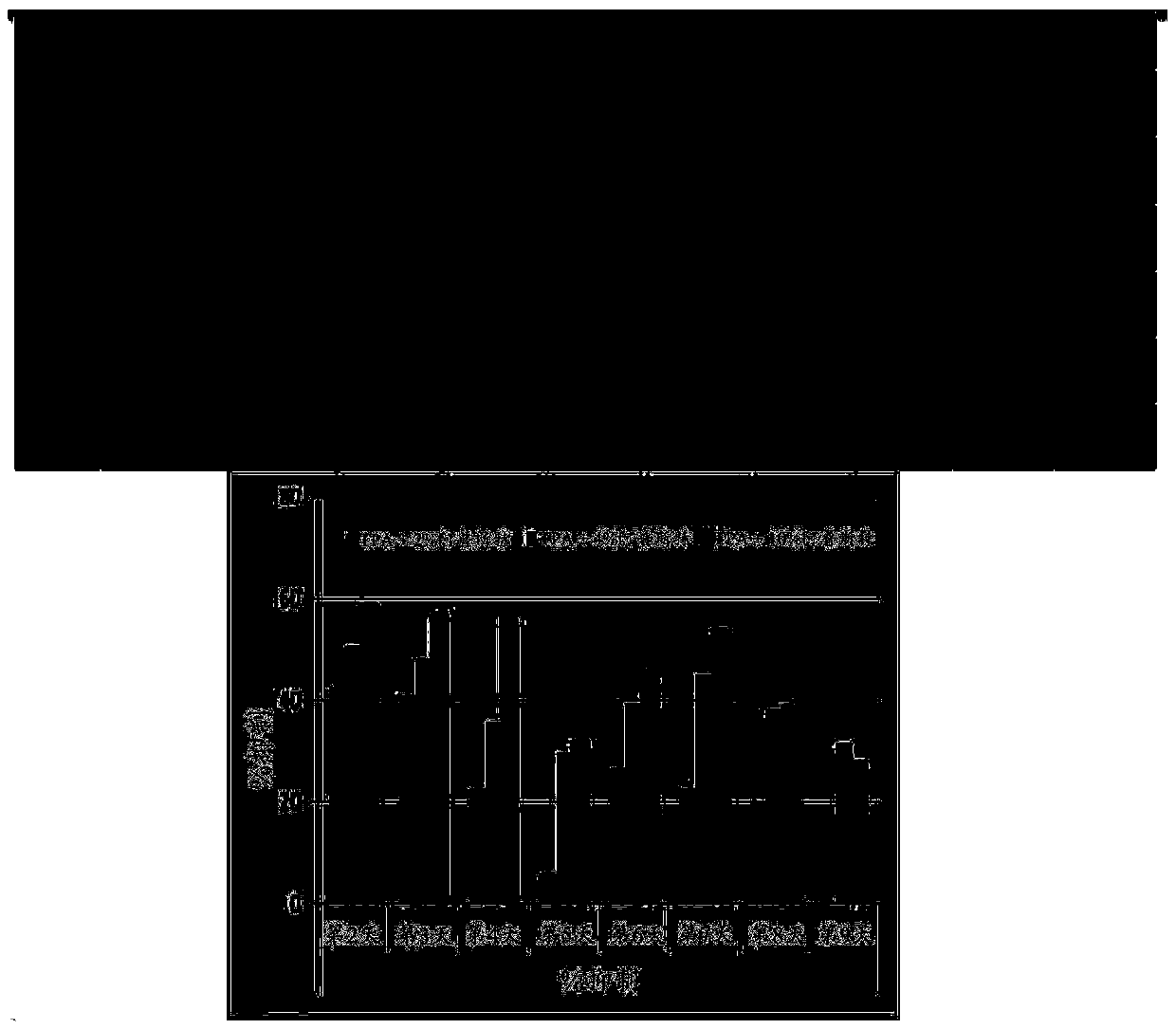
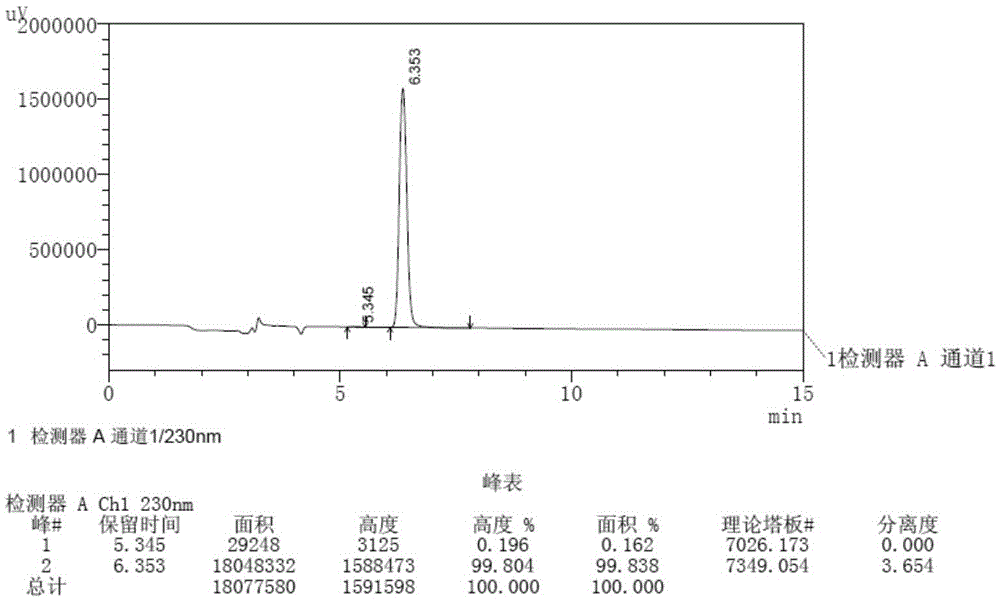
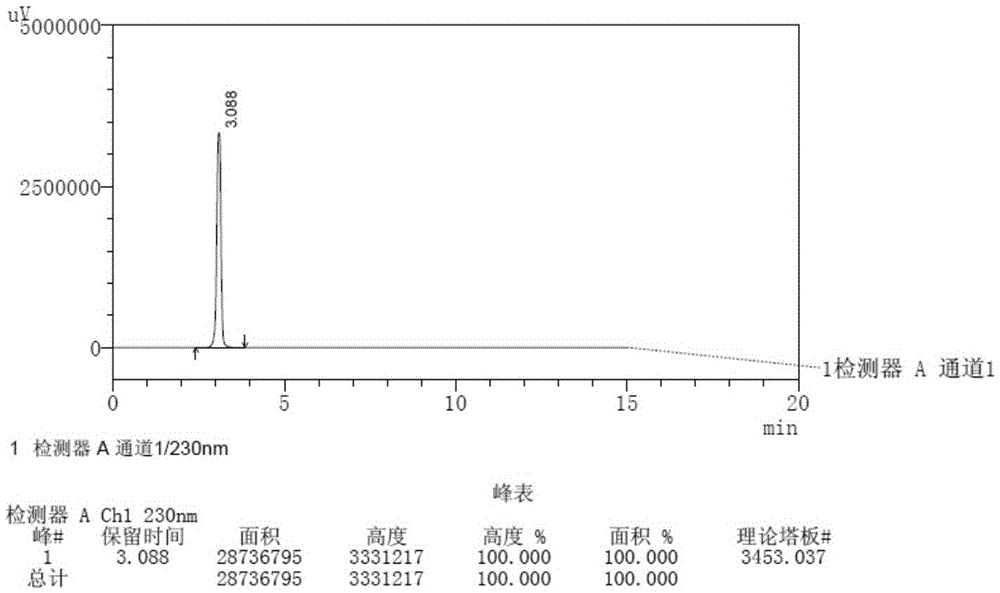
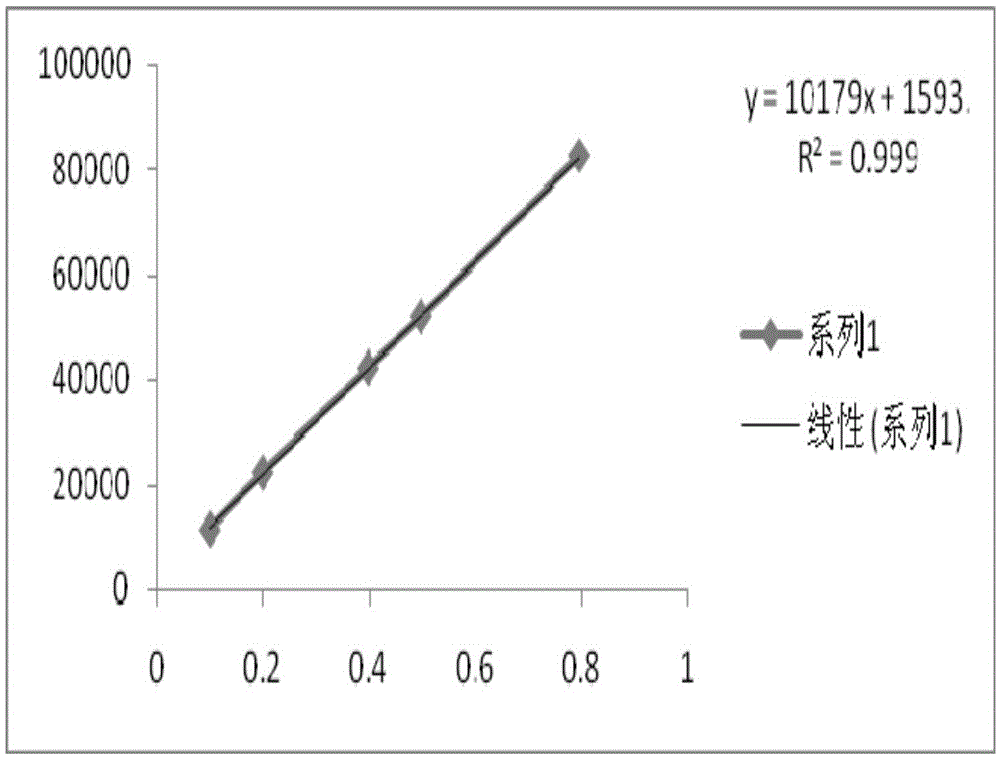
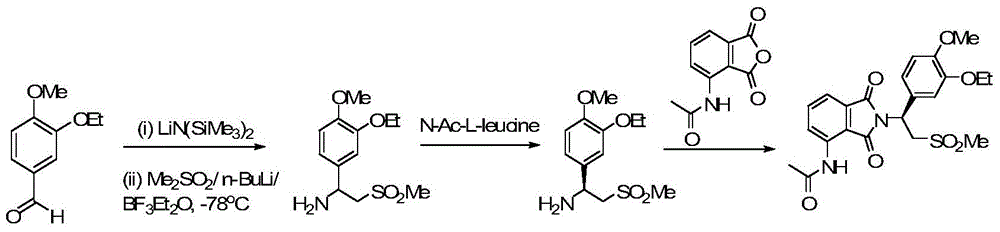
Patents
Literature
85 results about "Apremilast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
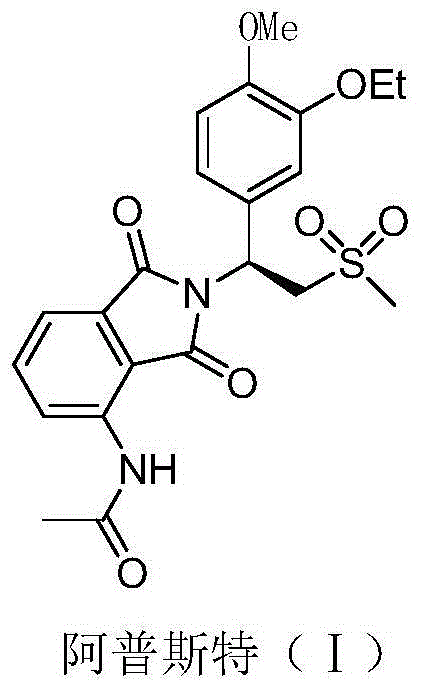
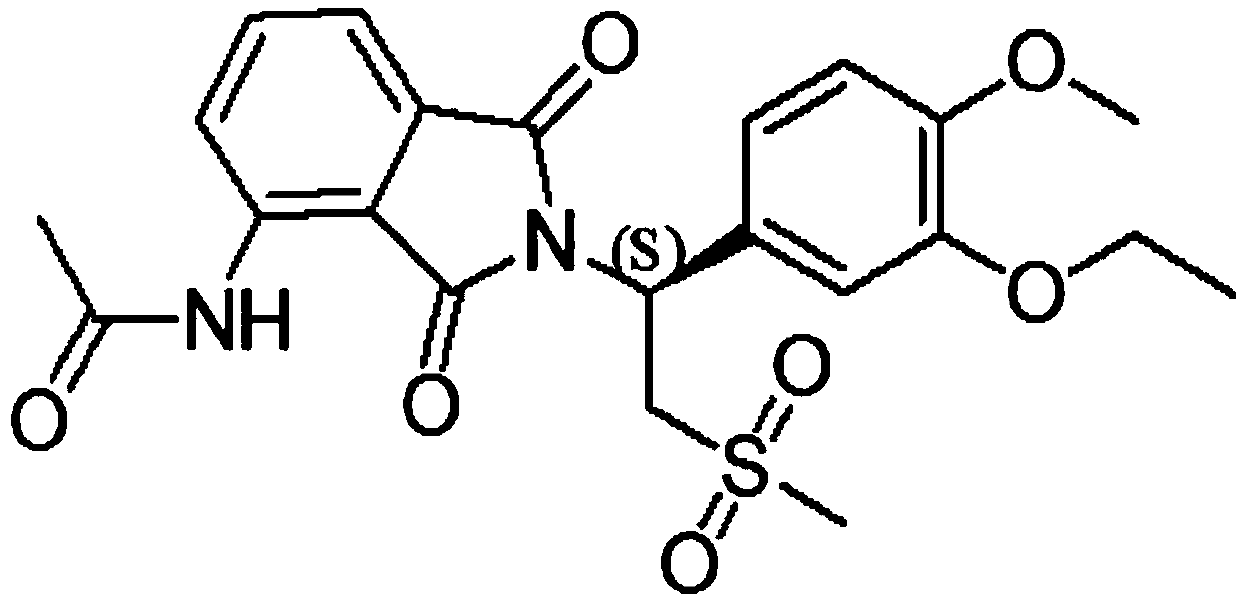
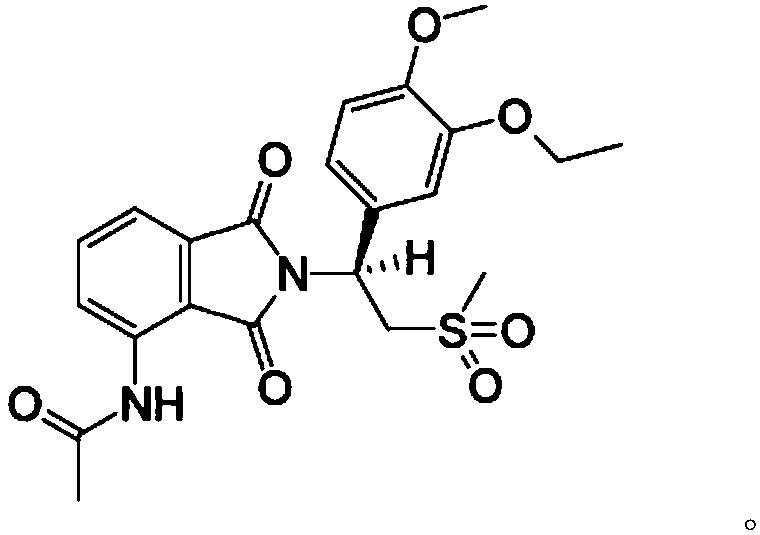
This medication is used to treat a certain type of arthritis (psoriatic arthritis). Apremilast is also used to treat a certain type of skin condition (moderate to severe plaque psoriasis).
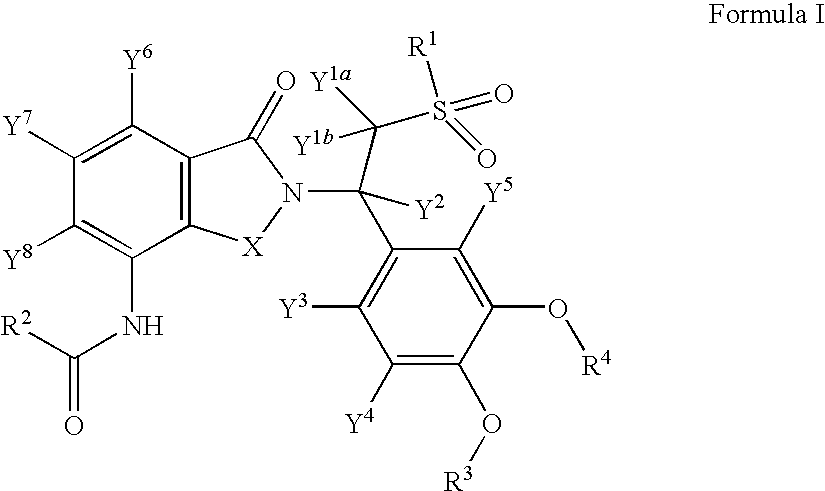
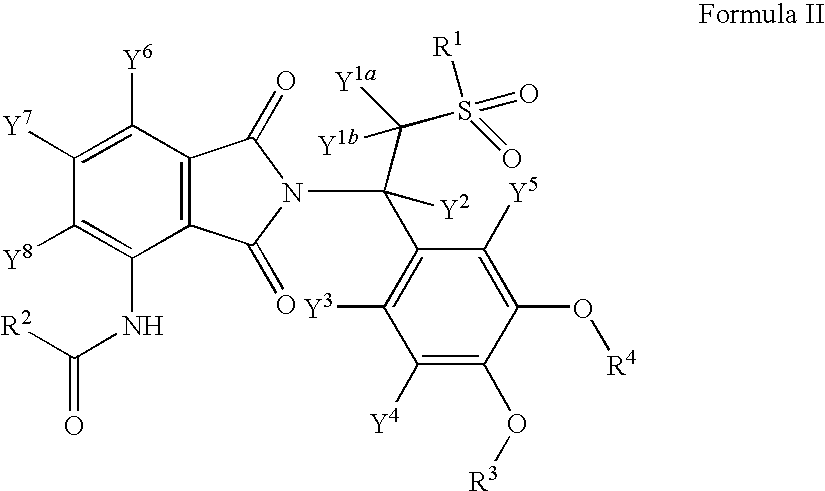
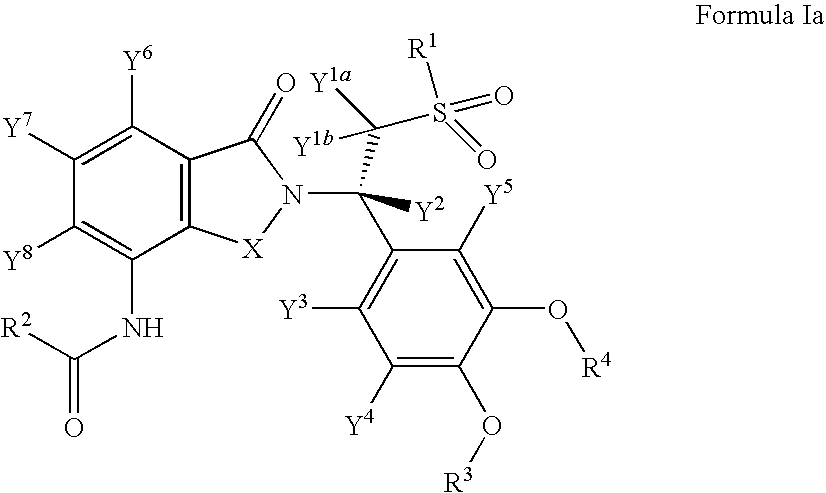
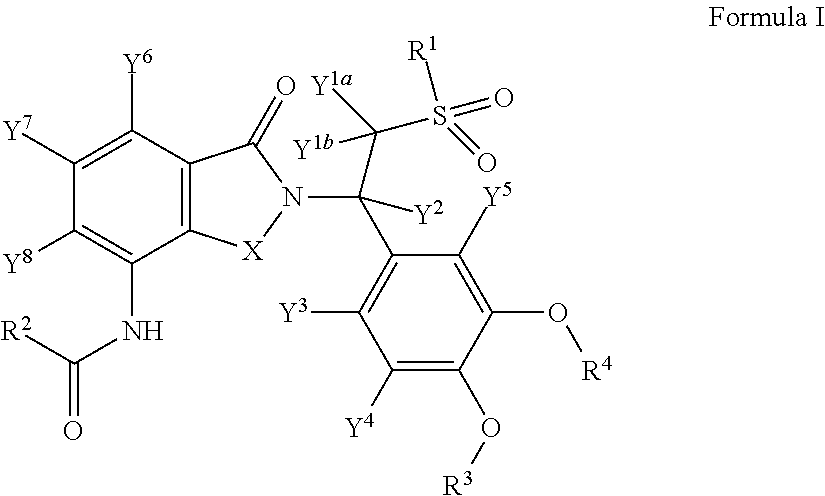
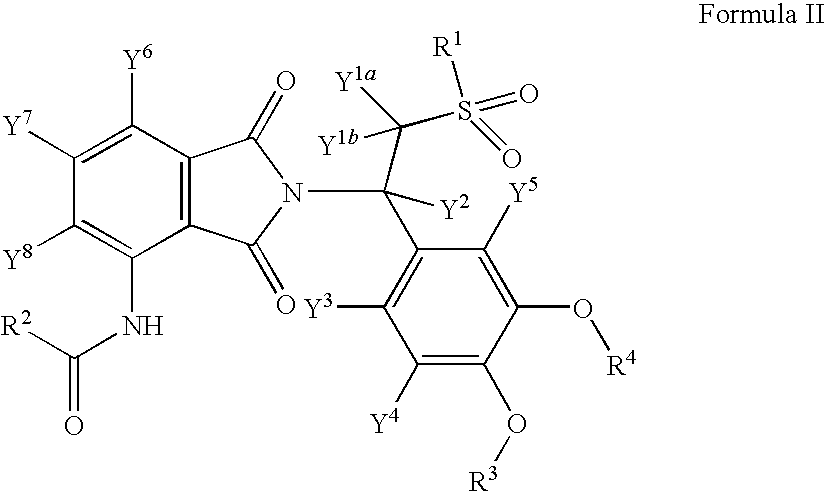
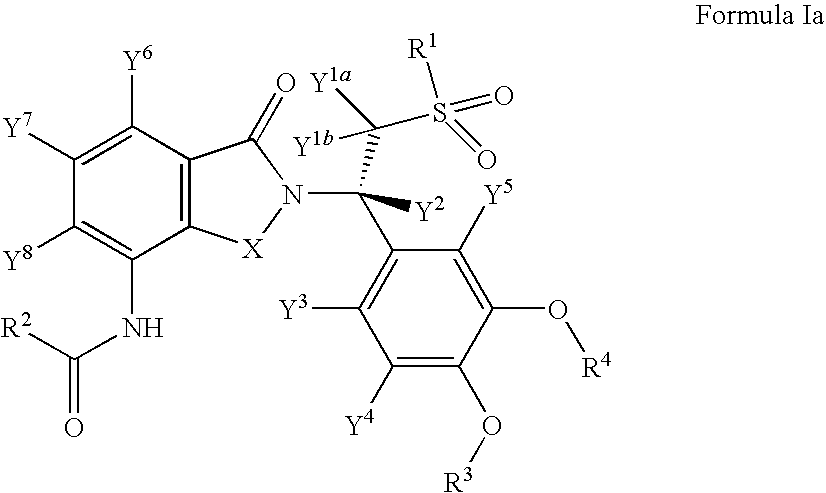
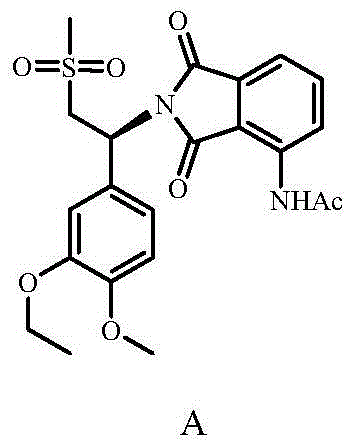
Substituted isoindoline-1,3-dione derivatives
This invention relates to novel substituted isoindoline-1,3-dione derivatives and pharmaceutically acceptable salts thereof. More specifically, the invention relates to novel substituted isoindoline-1,3-dione derivatives that are analogues of apremilast. This invention also provides compositions comprising a compound of this invention and a carrier and the use of disclosed compounds and compositions in methods of treating diseases and conditions that are beneficially treated by administering apremilast.
Owner:SUN PHARMA IND INC
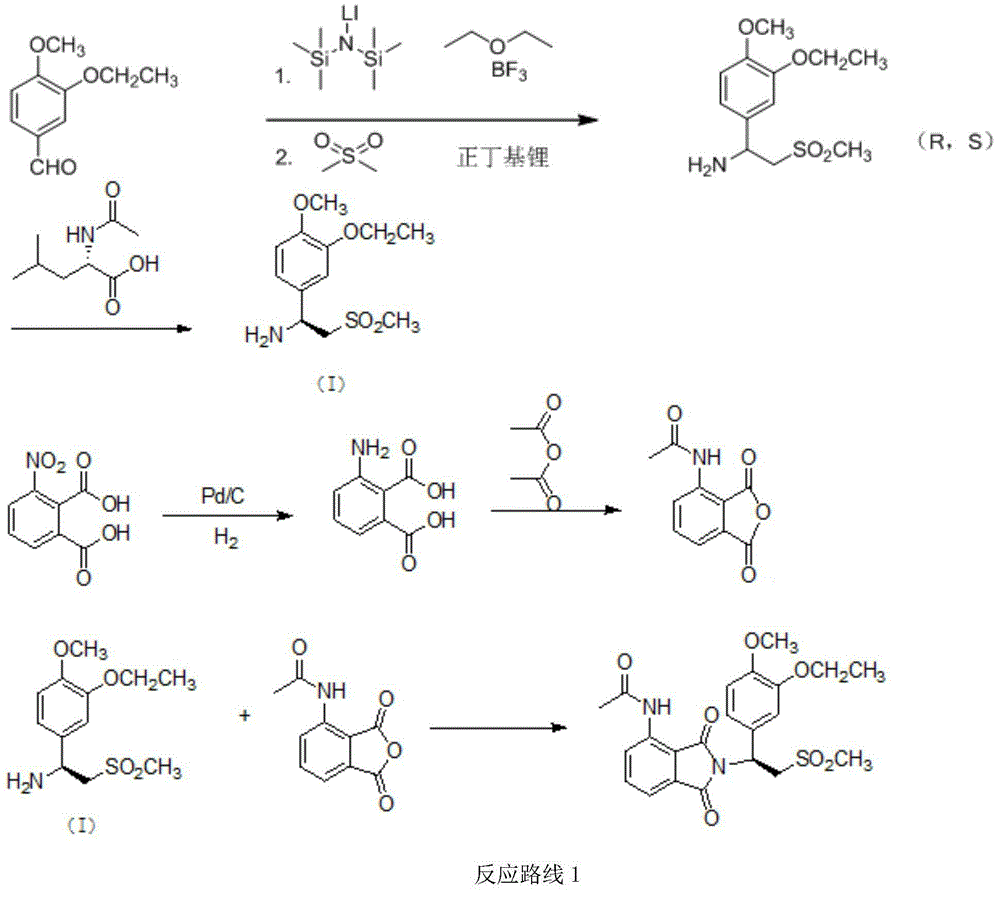
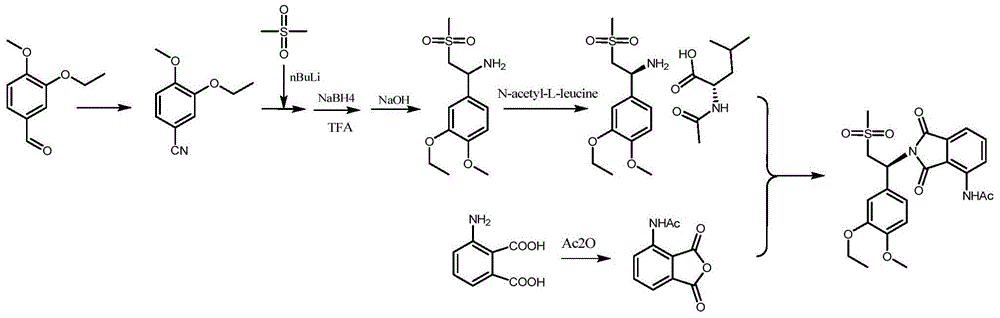
Preparation method for synthesizing apremilast intermediate
ActiveCN104447445AStable and cheapReaction is easy to controlOrganic chemistryOrganic compound preparationPhenyl groupSulfone
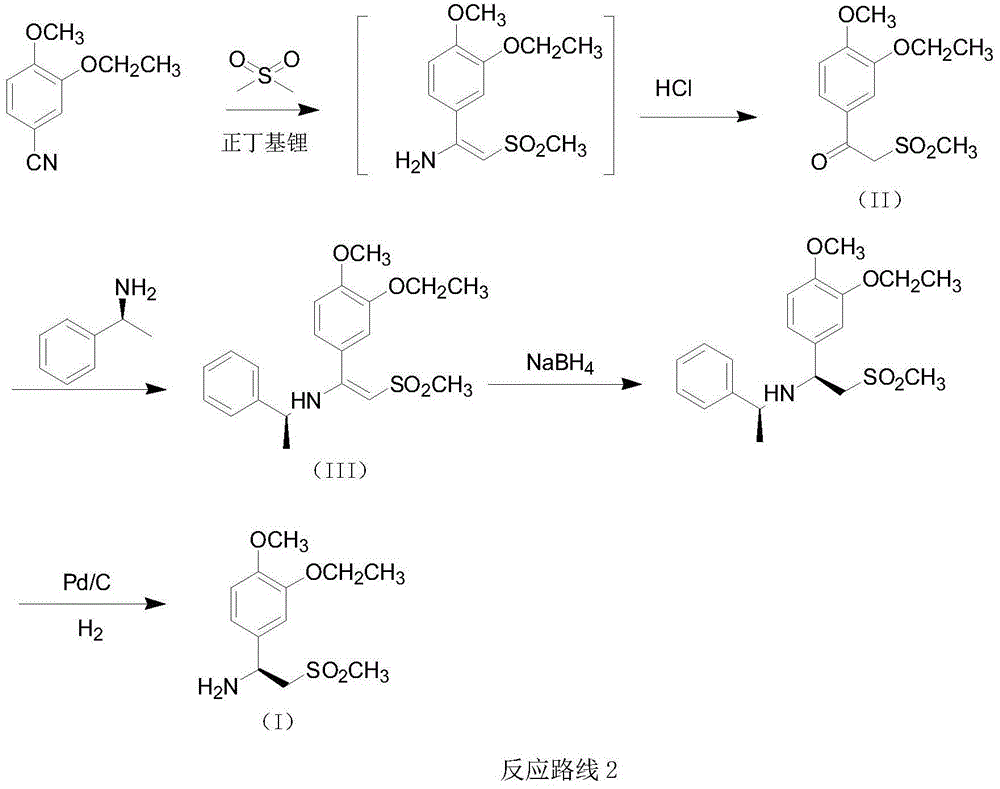
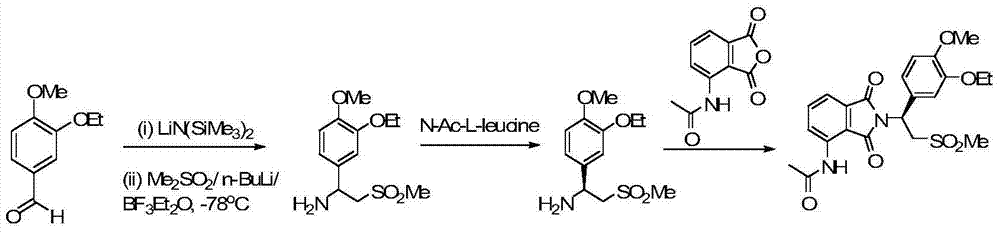
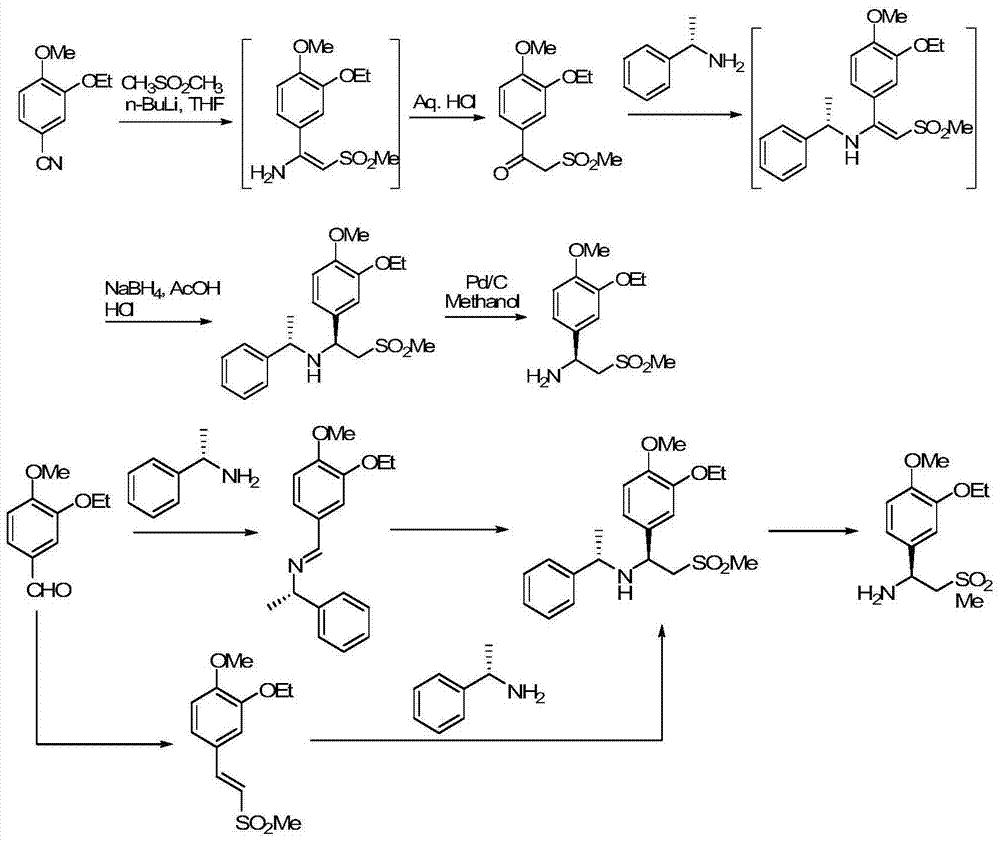
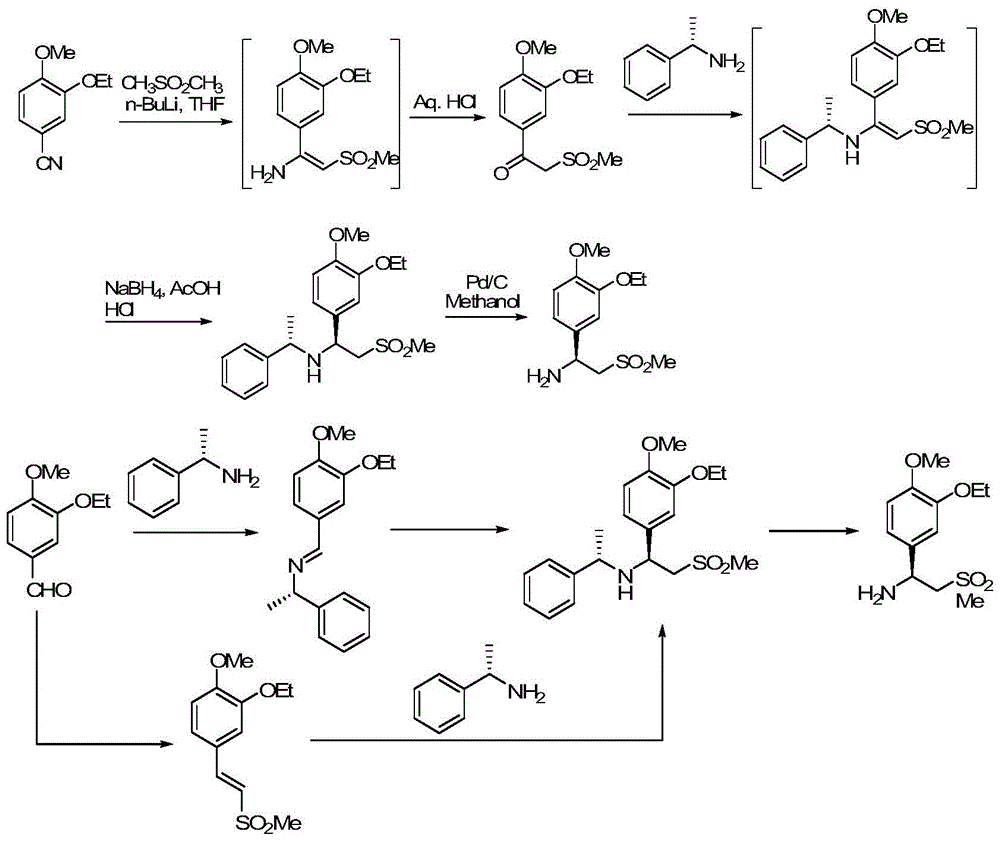
The invention relates to a preparation method for synthesizing an apremilast intermediate. The preparation method comprises the following steps of: carrying out condensation reaction on 3-ethoxyl-4-methoxyl-benzoate and dimethyl sulfone under an alkaline condition to generate 2-(3-ethoxy-4-methoxyphenyl)-1-methylsulfonyl acetone; reacting the compound II and chiral amine in the presence of an acidic catalyst to obtain 1-N-substituted amino-1-(3-ethoxyl-4-methoxyl) phenyl-2-methylsulfonyl ethylene (III), and directly hydrogenating the obtained compound III in the presence of a hydrogenation catalyst without separating the compound III to obtain a product (S)-1-(3-ethoxyl-4-methoxyl) phenyl-2-methanesulfonyl ethylamine (I), namely the apremilast intermediate, wherein the apremilast intermediate can be further prepared into N-acetyl L-leucinate. The invention also provides a preparation method of apremilast. The preparation method disclosed by the invention has the advantages of simple process flow, safety, environmental friendliness and low cost and is favorable to clean industrialized production.
Owner:XINFA PHARMA
Preparation method for apremilast and intermediate of apremilast
ActiveCN104447443AHigh purityRaw materials are easy to getOrganic chemistryOrganic compound preparationMannich reactionPhthalic anhydride
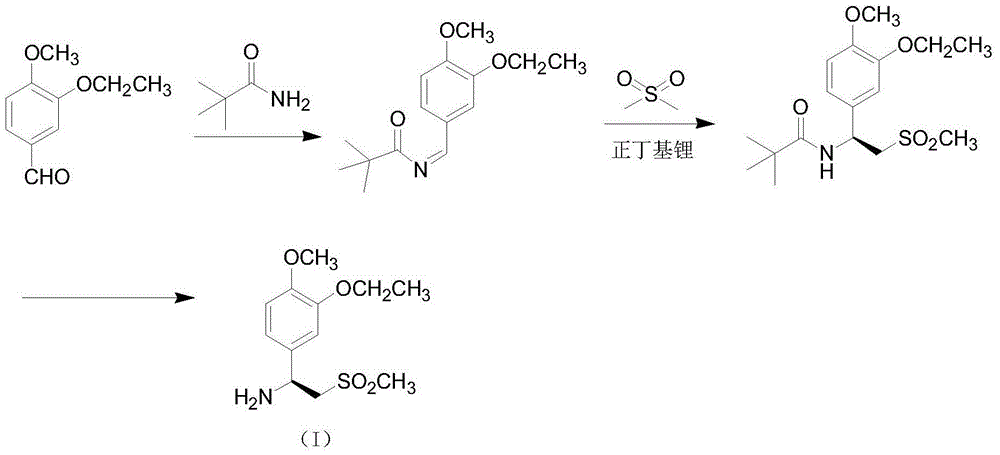
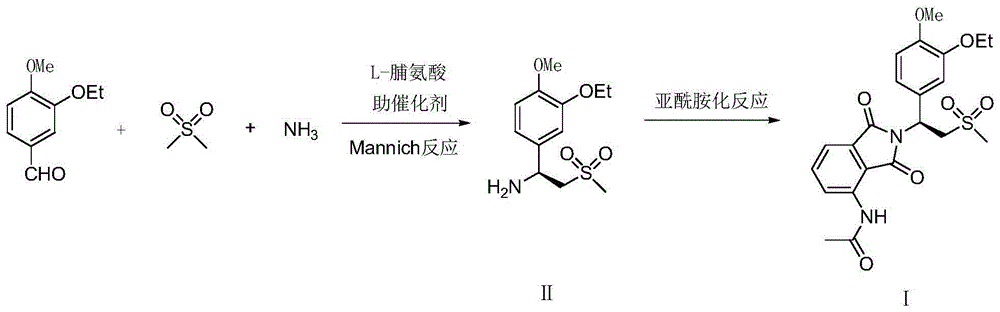
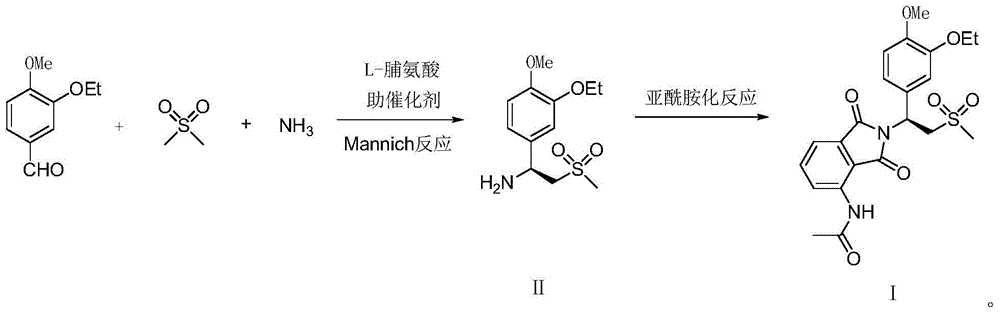
The invention relates to a preparation method for apremilast and an intermediate of the apremilast. The preparation method comprises the following steps: enabling 4-methoxy-3-ethoxybenzaldehyde to have Mannich reaction with methylsulfonylmethane and ammonia under the action of L-proline and a promoter to obtain an intermediate, (S)-1-(4-methoxy-3-ethyoxyl)phenyl-2-(methylsulfonyl)ethylamine (II), and then amidating the intermediate (II) and 3-acetamido-phthalic anhydride to prepare the apremilast (I). According to the preparation method for the apremilast and the intermediate of the apremilast, the raw materials are easily available, the flow path is short, the process is simple and convenient, the product has high optical purity, and the industrial production is safe and environmentally friendly.
Owner:XINFA PHARMA
Method for assaying impurities in apremilast and preparations thereof through liquid chromatography
ActiveCN105588886AAccurate measurementQuality is easy to controlComponent separationFluid phaseSilanes
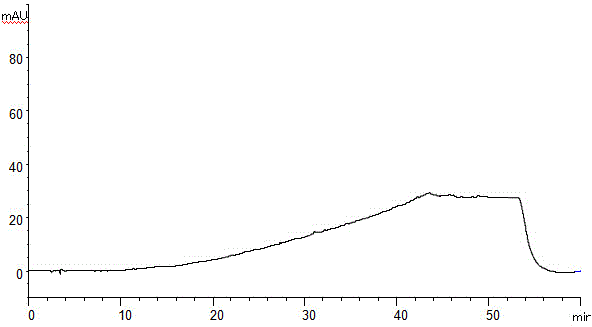
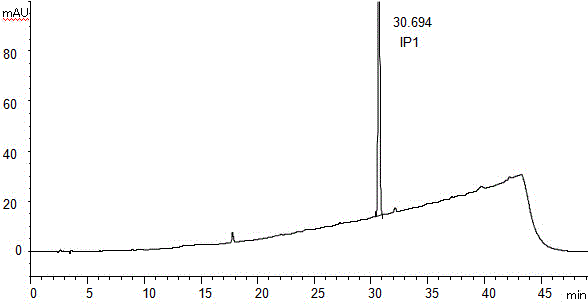
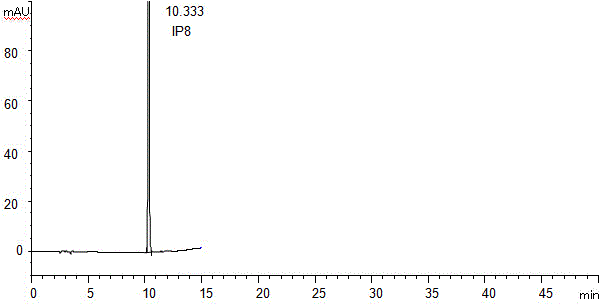
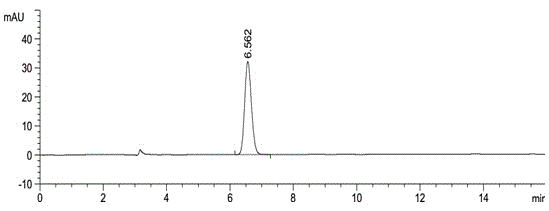
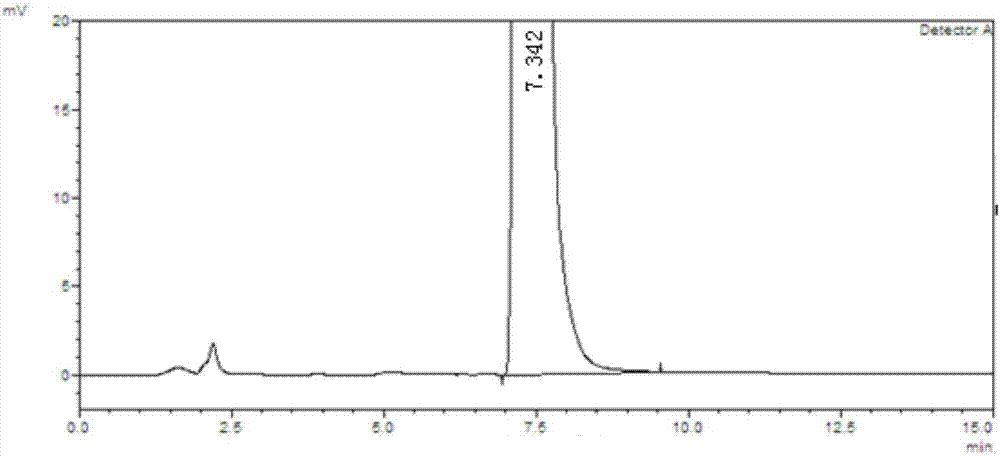
The invention discloses a method for separating and assaying impurities in apremilast and preparations thereof through liquid chromatography. In the method, octadecylsilane chemically bonded silica is employed as a filler in a chromatography column; a buffer solution is employed as a mobile phase A; and a methanol-acetonitrile mixture solvent is employed as a mobile phase B, wherein a gradient elution method is employed in the mobile phases to assay the impurities in apremilast and the preparations thereof. The method can effectively separate and assay unknown impurities and known impurities from the apremilast. The method has strong specificity, is high in accuracy, is easy to use and can be used for effectively control the quality of the apremilast and preparations thereof.
Owner:CHONGQING PHARMA RES INST
Synthetic method of apremilast chiral amine intermediate
ActiveCN104761474AChiral avoidanceReduce dosageOrganic chemistryOrganic compound preparationAlcoholAsymmetric hydrogenation
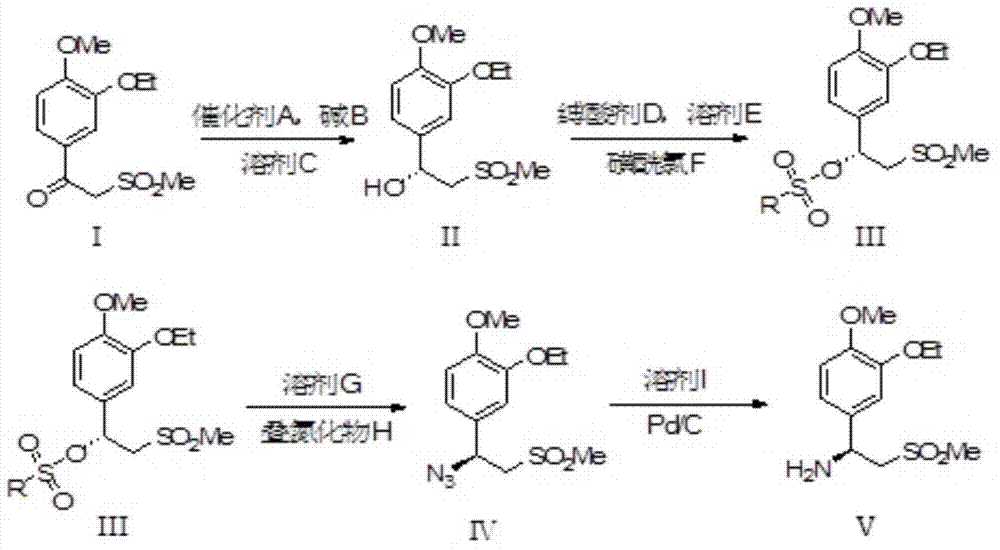
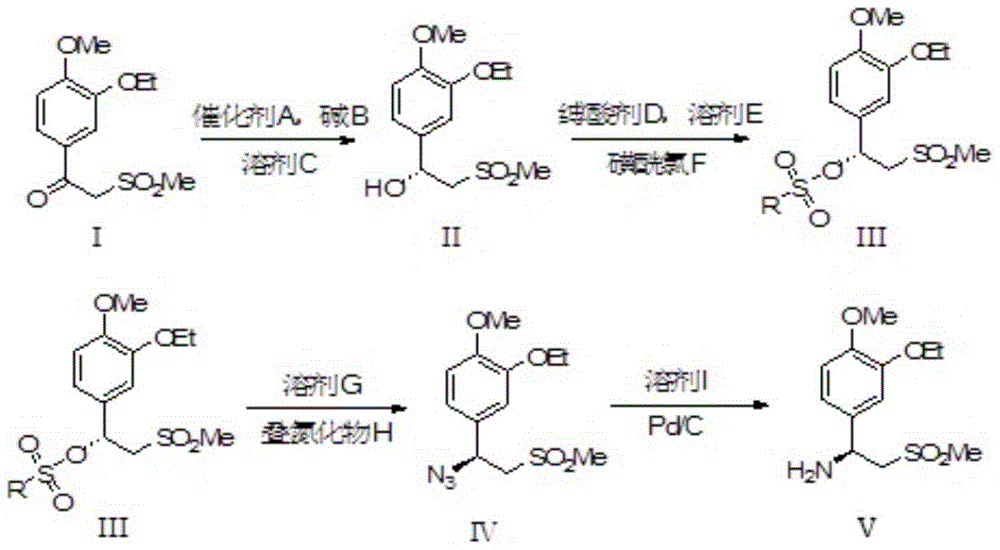
The invention relates to a synthetic method of an apremilast chiral amine intermediate (S)-2-[1-(3-ethoxyl-4-methoxylphenyl)]-1-methylsulfonyl-2-ethylamine (V). The synthetic method is characterized by including following steps: (1) with 1-(3-ethoxyl-4-methoxylphenyl)-2-(methylsulfonyl)ethyl ketone (I) as a raw material, performing asymmetric hydrogenation reduction to obtain methyl sulfonyl ethanol (II); (2) performing an esterification reaction to obtain methyl sulfonyl ester (III); (3) performing an azidation reaction to obtain an azide compound (IV); and (4) performing hydrogenation reduction to obtain the chiral amine intermediate (V) being high in chiral purity. The synthetic method is simple in process, is stable in reaction processes, and is environmental-protective and low-cost. In the synthetic method, the use amount of a catalyst during catalytic hydrogenation is less and the conversion rate reaches higher than 98%. The chiral amine can be prepared through chiral alcohol with very high yield and purity so that the synthetic method has a quite excellent commercial value and develops a new approach for synthesizing the apremilast chiral amine intermediate.
Owner:ENANTIOTECH CORP
Separation detection method of apremilast and apremilast enantiomer
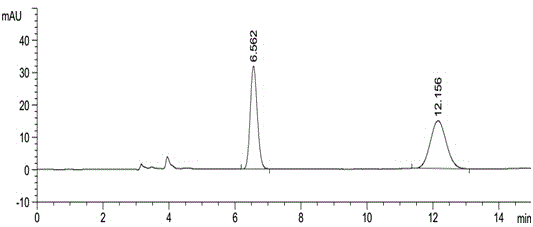
The invention discloses a high-efficient liquid phase chromatograph separation detection method of apremilast and apremilast enantiomer. A chromatographic condition is to adopt a direct-chain starch chiral column, and an n-hexane-low alcohol solution is adopted as a flow phase to carry out the separation determination for apremilast and apremilast enantiomer. By adopting the high-efficient liquid phase chromatograph separation detection method of the apremilast and apremilast enantiomer, the apremilast enantiomer can be effectively separated and determined, the specificity is high, the accuracy is high, and the method can be used for effectively controlling the quality of apremilast active ingredients.
Owner:SUZHOU YABAO PHARMA R&D CO LTD
Industrial method for preparing apremilast and intermediate thereof
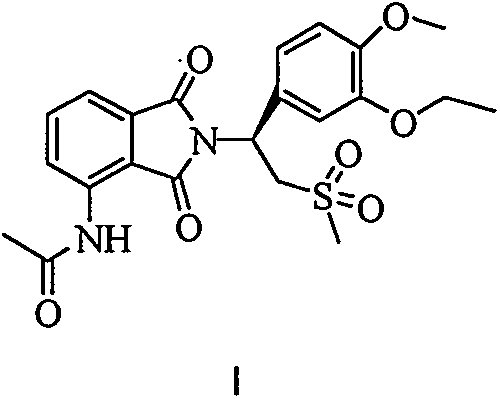
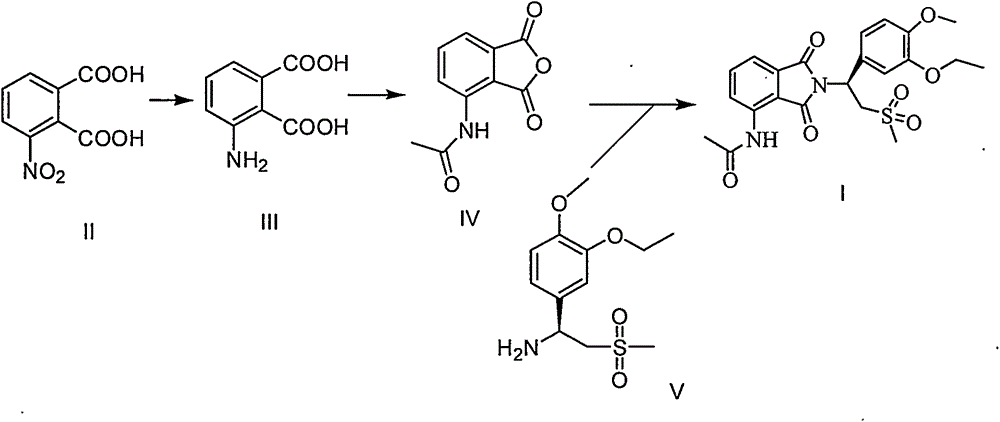
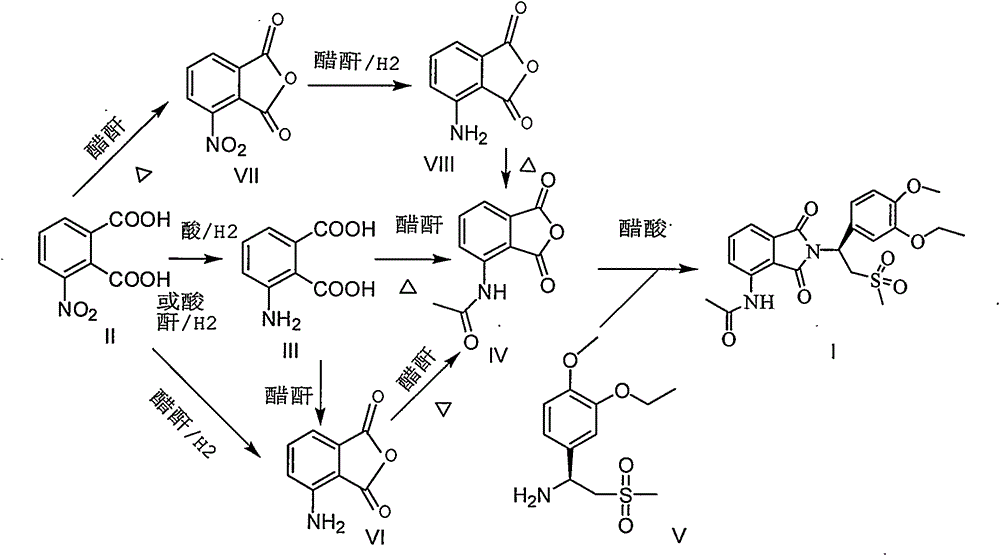
The invention discloses an industrial method for preparing high-purity apremilast (described in formula I) and an intermediate thereof. With 3-nitrophthalic acid (compound II) adopted as the starting raw material, and organic acid or acid anhydride adopted as the solvent, the method comprises the following steps: preparing high-purity 3-acetamido phthalic anhydride (compound IV) through different intermediates; directly enabling the obtained undried product to react with (S)-1-(3-oxethyl-4-methoxy phenyl)-2-mesyl) ethylamine (compound V) or the salt during reflux in glacial acetic acid, so as to obtain the apremilast. The preparation method is easy to operate, low in energy consumption, high in yield, and applicable to industrial production.
Owner:UTOPHARM SHANGHAI
Substituted isoindoline-1,3-dione derivatives
This invention relates to novel substituted isoindoline-1,3-dione derivatives and pharmaceutically acceptable salts thereof. More specifically, the invention relates to novel substituted isoindoline-1,3-dione derivatives that are analogues of apremilast. This invention also provides compositions comprising a compound of this invention and a carrier and the use of disclosed compounds and compositions in methods of treating diseases and conditions that are beneficially treated by administering apremilast.
Owner:SUN PHARMA IND INC
Apremilast solid dispersoid and preparation method thereof
ActiveCN104523574ASimple stepsProcess parameters are easy to controlOrganic active ingredientsPharmaceutical delivery mechanismDrug release ratePharmaceutical drug
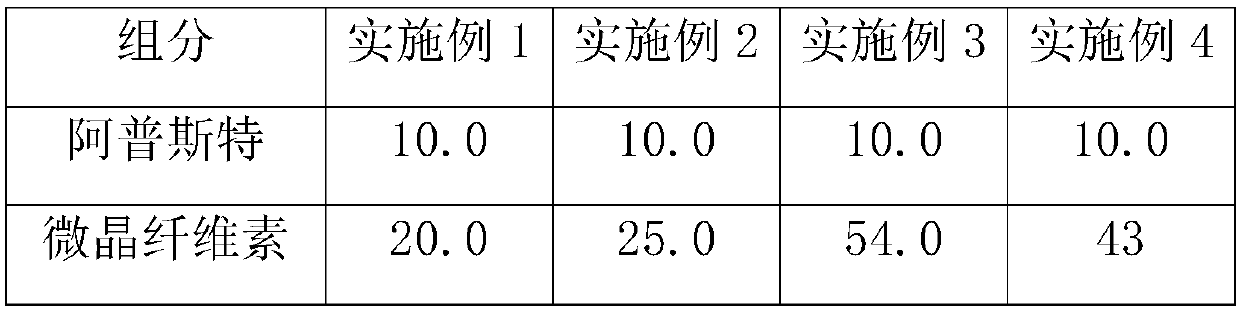
The invention provides an apremilast solid dispersoid and a preparation method thereof. The apremilast solid dispersoid is characterized by having a prescription comprising the following components in percentage by weight: 5-35% of apremilast, 5-70% of a carrier material and 2-30% of an adsorbing agent. The apremilast solid dispersoid has the advantages that drug releasing rate is increased, pharmaceutical function can be realized in short time, and pharmaceutical bioavailability is improved. The apremilast solid dispersoid is prepared by adopting a melting method, steps are simple, technological parameters are easy to control, and the apremilast solid dispersoid is applicable to large-scale production.
Owner:CHANGSHA BAISHUN BIOTECH
Substituted isoindoline-1,3-dione derivative
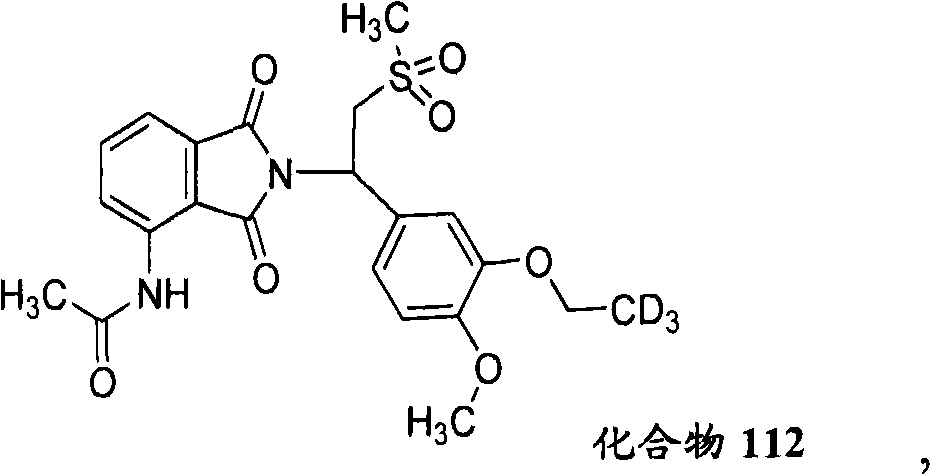
The invention relates to a novel substituted isoindoline-1,3-dione derivative and the pharmaceutically acceptable salts thereof. More particularly, the invention relates to a novel substituted isoindoline-1,3-dione derivative used as an analogue of apremilast. The invention further provides a composition containing the compound disclosed by the invention and a carrier, and the application of the disclosed compound and composition in a method for beneficially treating diseases and conditions by administering apremilast.
Owner:CONCERT PHARMA INC
Method for using liquid chromatography to separate and measure apremilast and enantiomer thereof
ActiveCN104820028AAccurate quality controlAccurate separation detectionComponent separationAlkaneEnantiomer
The invention relates to a method for using a liquid chromatography to separate and measure apremilast and enantiomer thereof. The method includes that using spherical silica gel coated with chiral polymer at surface as a chiral chromatographic column, using alkane-different concentrations of polarity organic solvent as mobile phase, wherein the polarity organic solvent is composed of first organic solvent and second organic solvent, the alkane is selected from normal hexane, normal heptane, cyclohexane and methylene dichloride, the first organic solvent is isopropanol, and the second organic solvent is selected from methanol, ethanol and acetonitrile. The method for using the liquid chromatography to separate and measure the apremilast and enantiomer thereof solves the problem that the apremilast and enantiomer thereof are difficult to separate for the separation and measurement, and accordingly the controllable quality of the apremilast and the preparation thereof is guaranteed.
Owner:CHONGQING PHARMA RES INST
Apremilast oral preparation and preparation method thereof
InactiveCN105343025AOrganic active ingredientsSkeletal disorderBULK ACTIVE INGREDIENTTraditional medicine
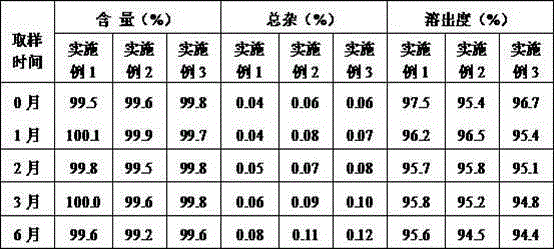
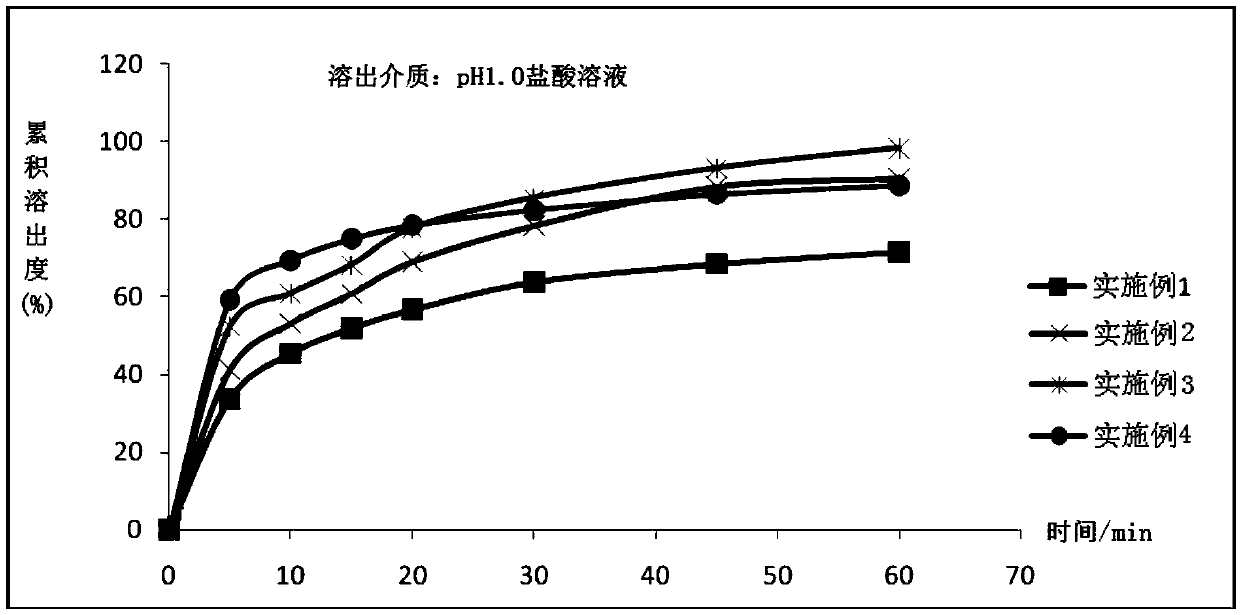
The invention provides an apremilast oral preparation and a preparation method thereof. The apremilast oral preparation consists of the following ingredients in percentage by weight: 2-20% of apremilast which serves as an active ingredient, 20-70% of a filling agent, 3-30% of a disintegrating agent, 1-10% of a binding agent, 0.2-5% of a lubricating agent and 2-6% of a coating material. The apremilast oral preparation prepared by the invention is above 95% in dissolution rate and high in bioavailability; the shortcomings of poor dissolution degree of the active ingredient and low bioavailability are overcome; and the oral preparation is stable and reliable in quality and is high in market development prospect.
Owner:CHANGSHA BAISHUN BIOTECH
Apremilast oral liquid and preparation method thereof
ActiveCN105919927AIncrease concentrationGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityAlcohol
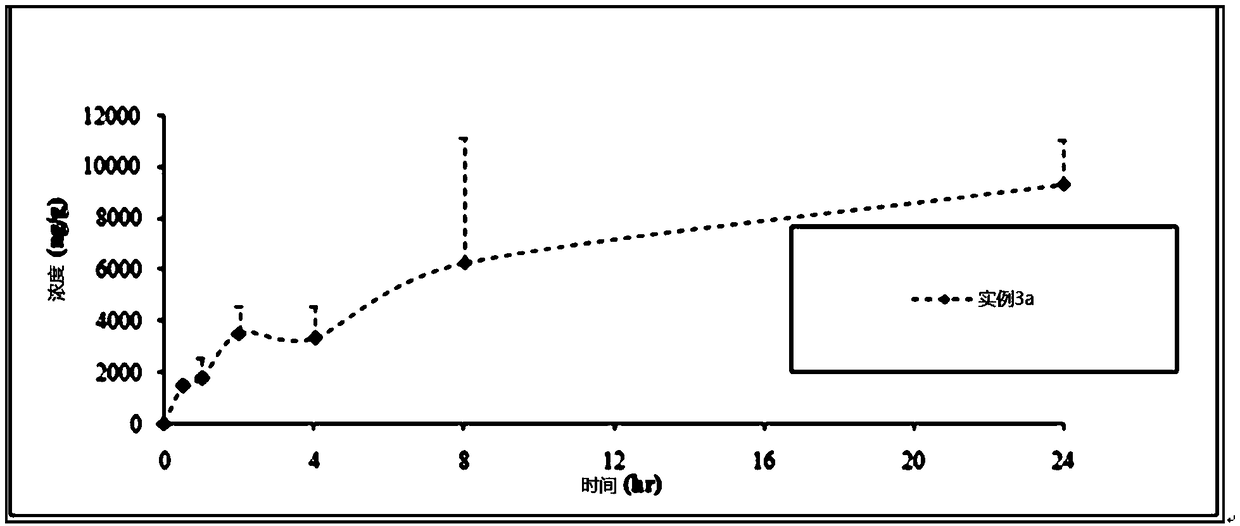
The invention provides an Apremilast oral liquid, including 0.05wt%-0.3wt% of Apremilast, 0.5wt%-20wt% of sulfobutyl- beta-cyclodextrin, 1wt%-30wt% of an alcohol solvent, 0-2wt% of surface active agent and the balance of water. The invention adopts sulfobutyl- beta-cyclodextrin for Apremilast inclusion, so that Apremilast is in an amorphous state, and has increased water solubility, and the concentration of Apremilast is increased to more than 1mg / ml. The prepared oral liquid is easy for the dosing, and has greatly improved bioavailability due to no disintegration and dissolution process of solid preparations. At the same time, and the stability of Apremilast is not affected due to production into oral liquid; and test shows that the stability of the oral liquid is equal to that of tablets.
Owner:LIANGJIANG MEDICINE CO LTD
Method of separation and detection of apremilast and enantiomer thereof by adopting HPLC (high performance liquid chromatography)
InactiveCN105136933AAccurate quality controlSimple Separation AssayComponent separationCarbamateAlcohol
The invention discloses a method of separation and detection of apremilast and enantiomer thereof by adopting HPLC (high performance liquid chromatography). The chromatographic condition of the method adopts a chiral column taking a silica gel of which the surface is coated with amylose-three(3,5-dimethyl phenyl carbamate) as filler; a mobile phase is prepared from hexane-ethyl alcohol-acetonitrile-diethylamine (the ratio is 45 to 45 to 10 to 0.1); the flow velocity is 0.8ml / min; the wavelength is 230nm; and the column temperature is 35 DEG C.
Owner:宜邦国创药物研究院(北京)有限公司
Separation and determination method of apremilast and potential genotoxic impurities thereof
ActiveCN107014910AEfficient separationAccurate determination of contentComponent separationElutionSilica gel
The invention belongs to the field of analytic chemistry, and concretely relates to a separation and determination method of apremilast and potential genotoxic impurities thereof. The method comprises the steps of adopting a chromatographic column taking octadecyl silane bonded silica gel as filler, and concretely comprises the steps of adding a diluting agent for dissolving each potential genotoxic impurity reference substance so as to prepare a reference substance solution with known concentration; adding a diluting agent for dissolving a sample for test so as to prepare a solution of the sample for test; taking the reference substance solution and the solution of the sample for test respectively for sample injection; adopting a moving phase for carrying out high performance liquid chromatography elution analysis, and recording a chromatogram map; comparing peak areas of impurities, corresponding to peak appearance times, in the reference substance solution and the solution of the sample for test, and calculating the contents of the apremilast and the potential genotoxic impurities contained in the sample for test. The method is high in specificity, high in sensitivity (the limit of detection of the potential genotoxic impurities of SM1 is 0.0004 percent), and simple to operate, and has the advantages of convenience and quickness.
Owner:CHONGQING HUAPONT PHARMA
Topical compositions of apremilast
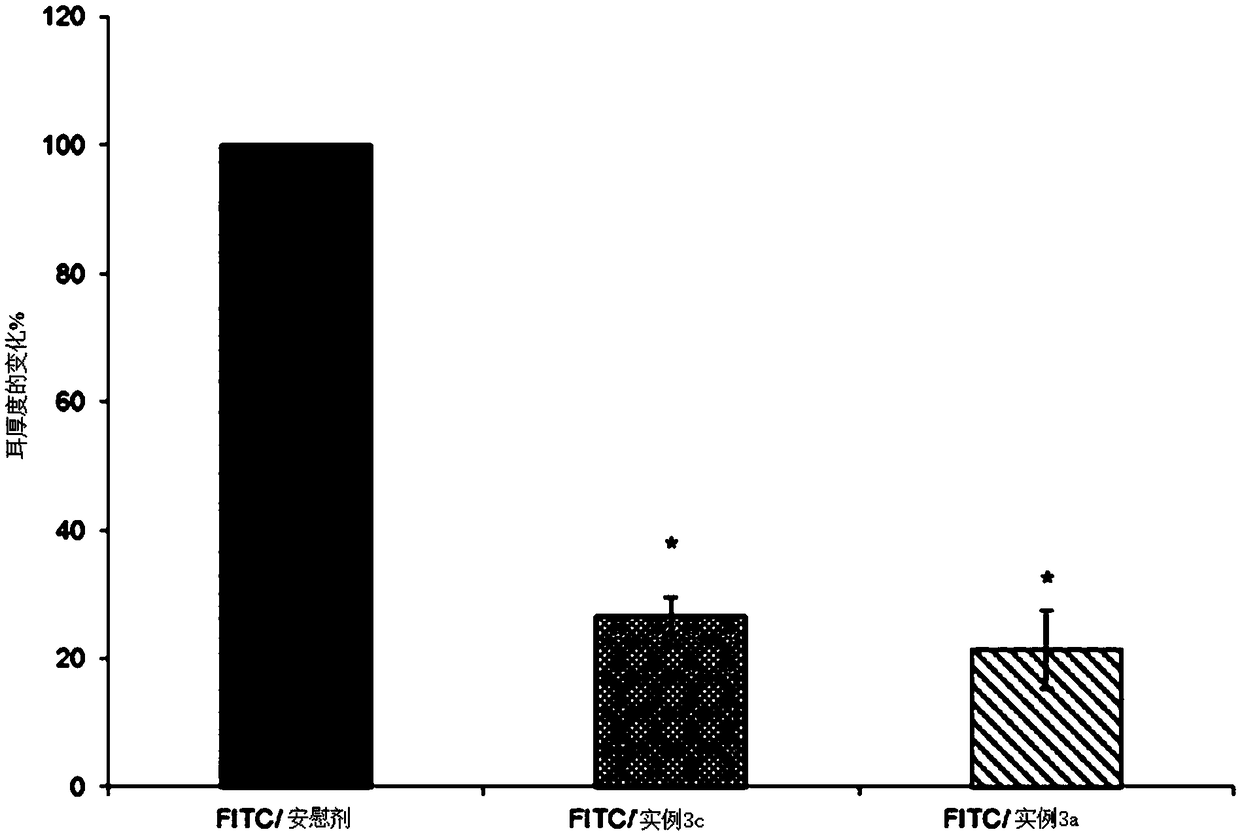
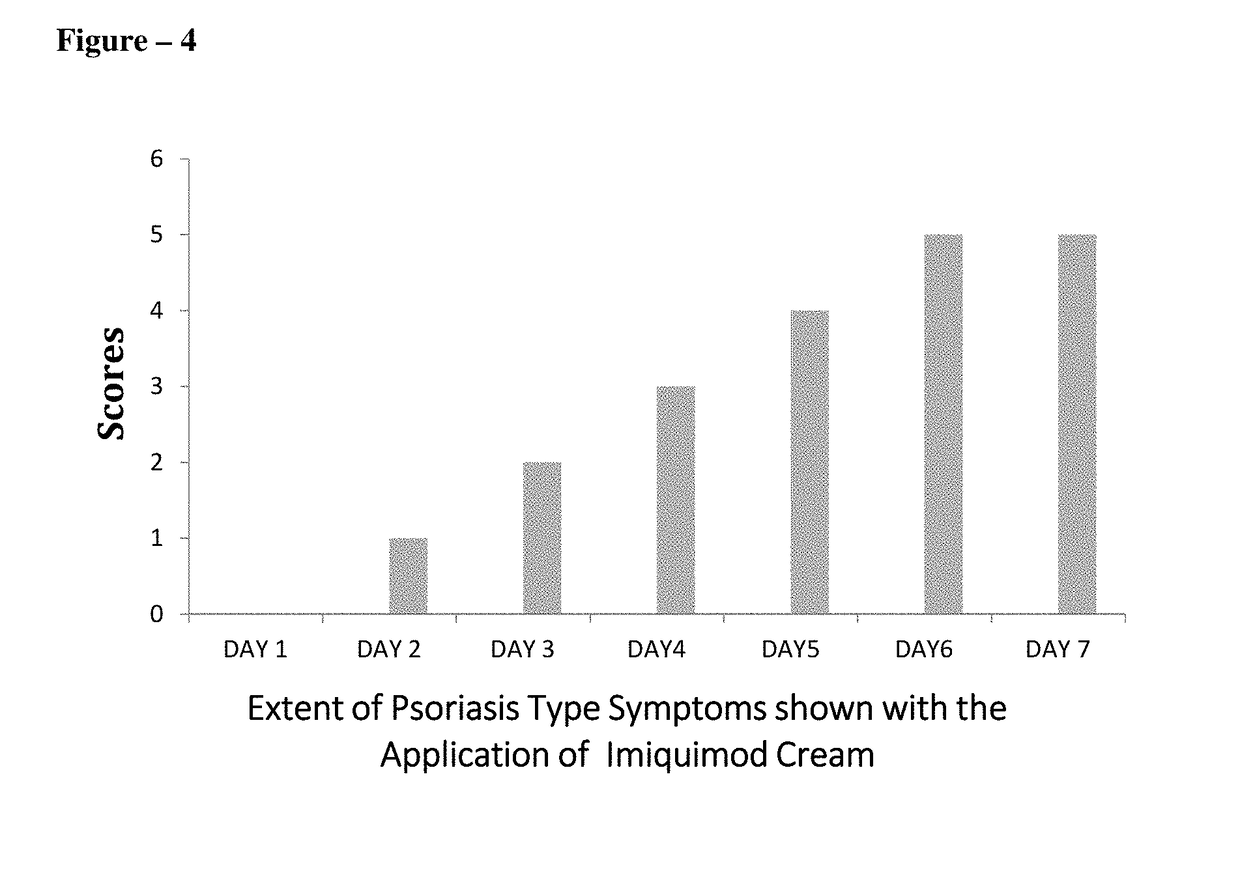
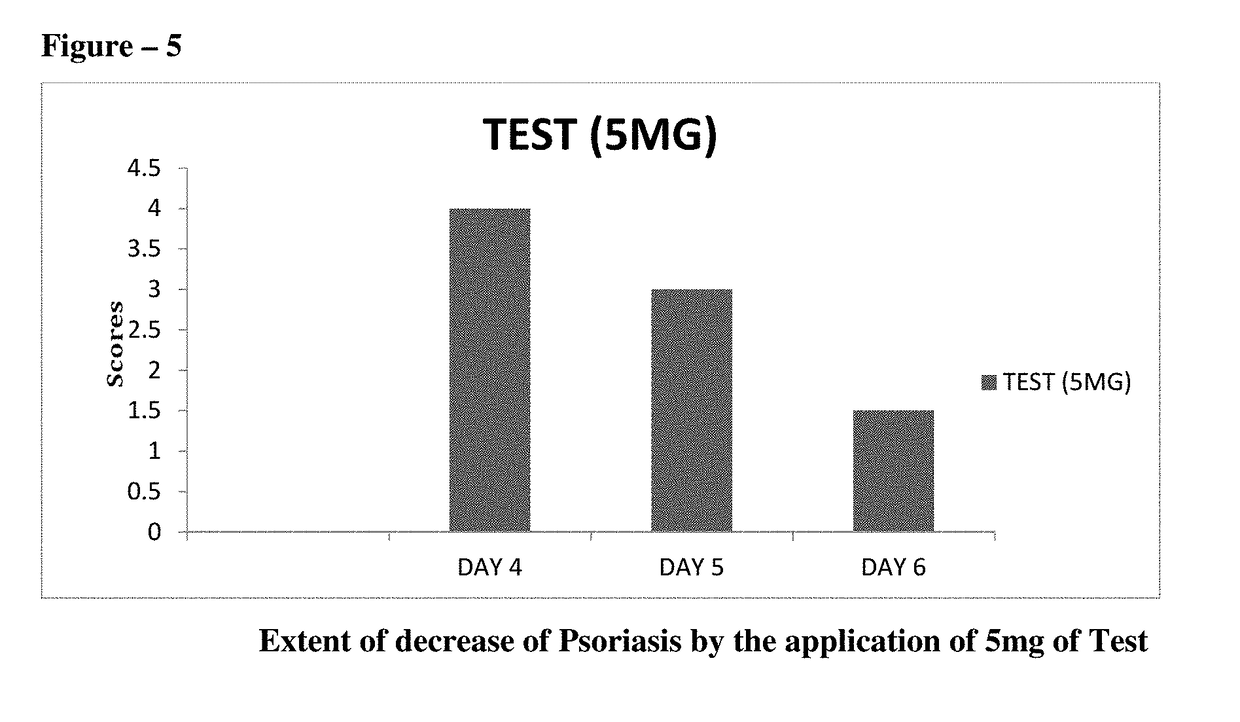
The present invention relates to topical pharmaceutical compositions comprising Apremilast in an amount of about 0.1 to 5 % w / w of the total composition and one or more pharmaceutically acceptable excipients and process for their preparation. The present invention further relates to method for treatment of skin diseases using topical pharmaceutical compositions comprising Apremilast.
Owner:TORRENT PHARMA LTD
Apremilast tablets and preparation method thereof
InactiveCN110548015AIncrease dissolution rateThe preparation process is simple and controllableOrganic active ingredientsSkeletal disorderActive componentMedicine
The invention discloses apremilast tablets. Plain tablets of the tablets comprise active components and an excipient, wherein the mass of the active components is 5%-15% of that of the plain tablets,the excipient comprises an excipient A, the excipient A is selected from one or a mixture of more of lactose, microcrystalline cellulose, Ludipress and Ludipress LCE, the mass of the excipient A is 80%-92% of that of the plain tablets, and the excipient A is further selected from 85%-90%. A preparation method of the apremilast tablets comprises the following steps of crushing the active componentsfor standby application, wherein the size distribution d(0.9) of the crushed active components is smaller than or equal to 15.0 [mu]m; screening the excipient for standby application; screening the active components and the excipient, and performing uniform mixing; and performing tabletting and coating. According to the apremilast tablets and the preparation method thereof disclosed by the invention, the dissolution rate of the apremilast in the tablets is greatly increased; and besides, the preparation technology is simple, controllable, environmentally-friendly and sustainable, the production cost is low, the production efficiency is high, and the apremilast tablets and the preparation method are suitable for large-scale industrialization big production.
Owner:CHENGDU BAIYU PHARMA CO LTD
Apremilast and nicotinamide co-crystal as well as preparation method and application thereof
InactiveCN107721902AStable co-crystallizationEasy to operateOrganic active ingredientsAntipyreticX-rayRepeatability
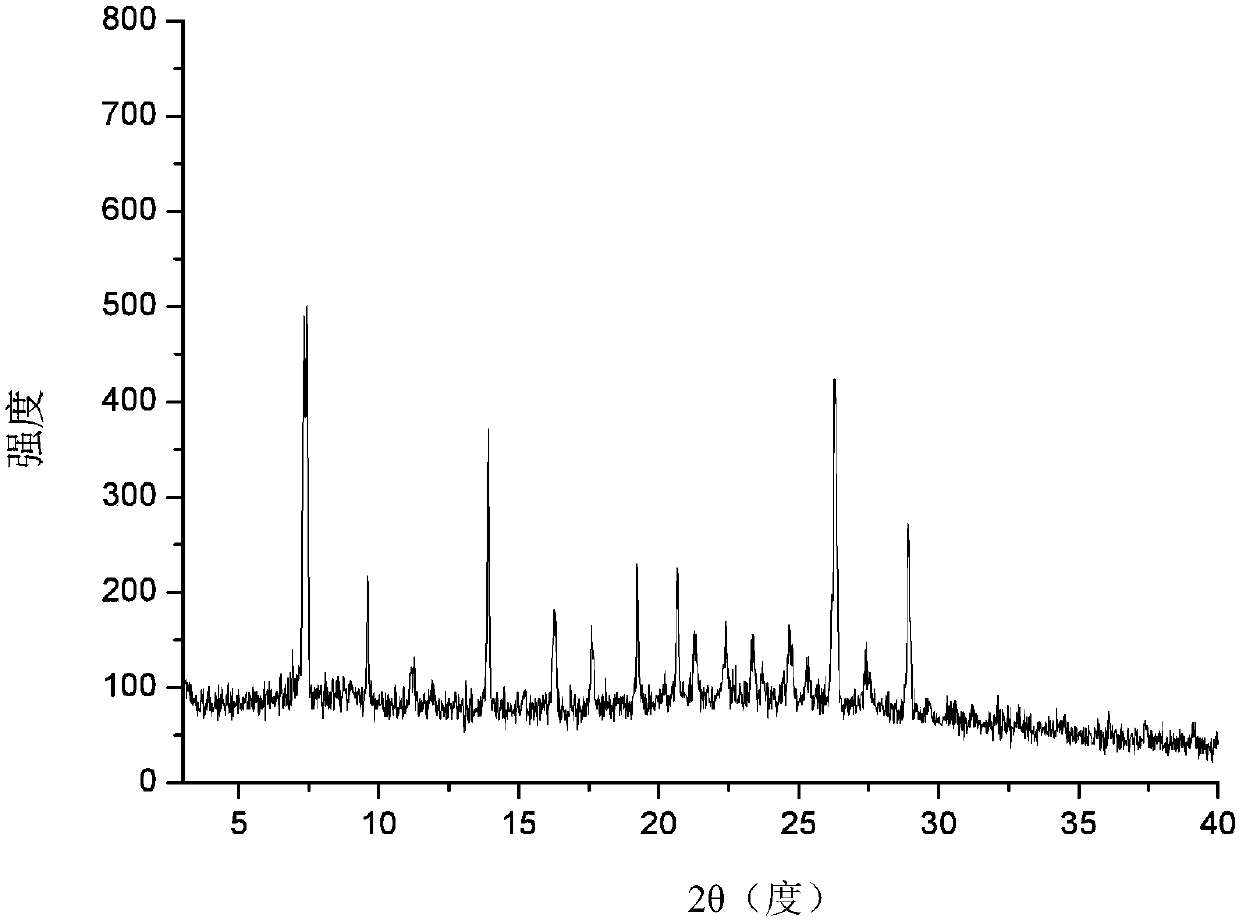
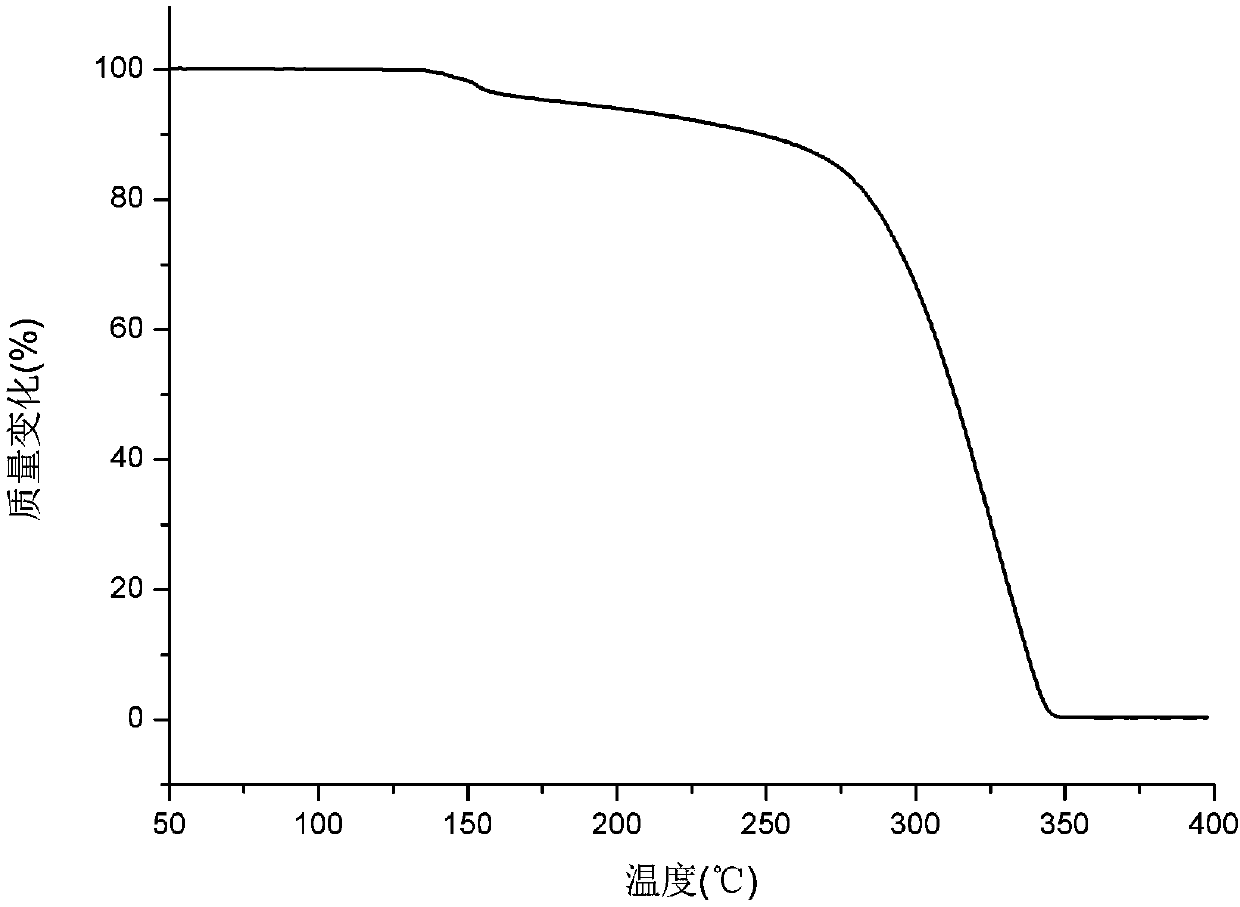
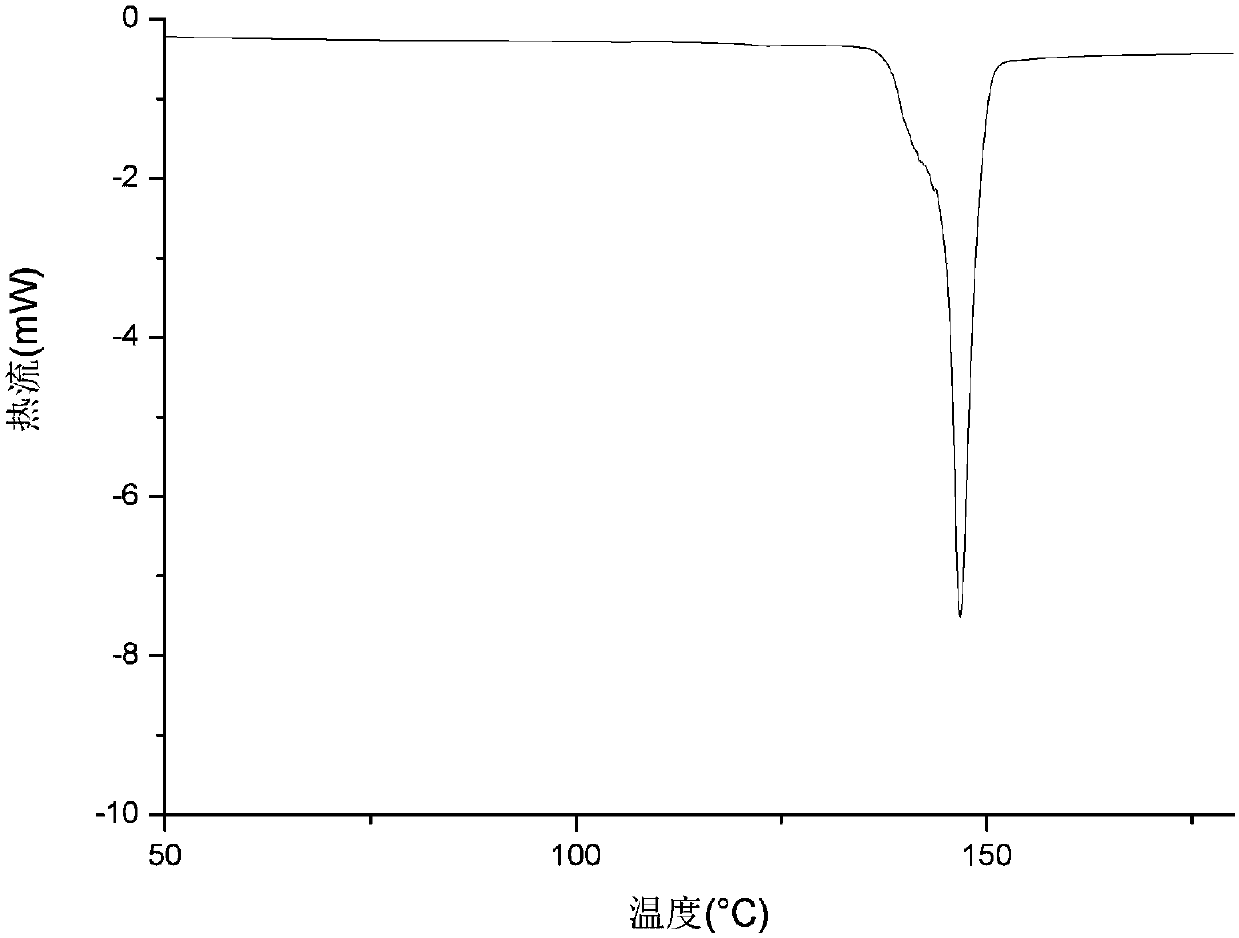
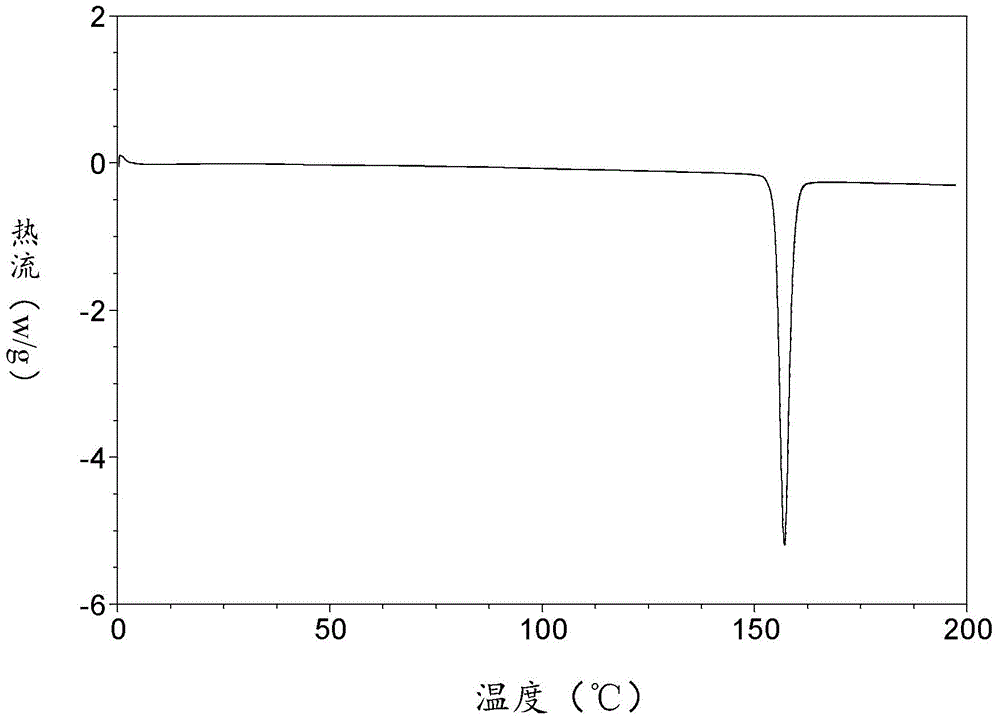
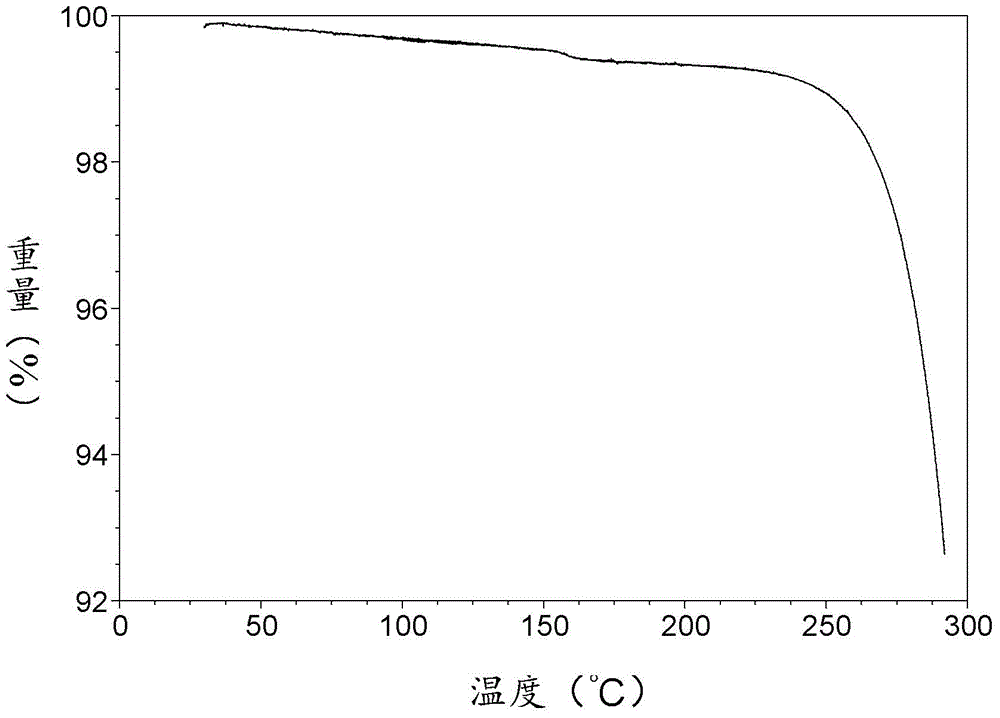
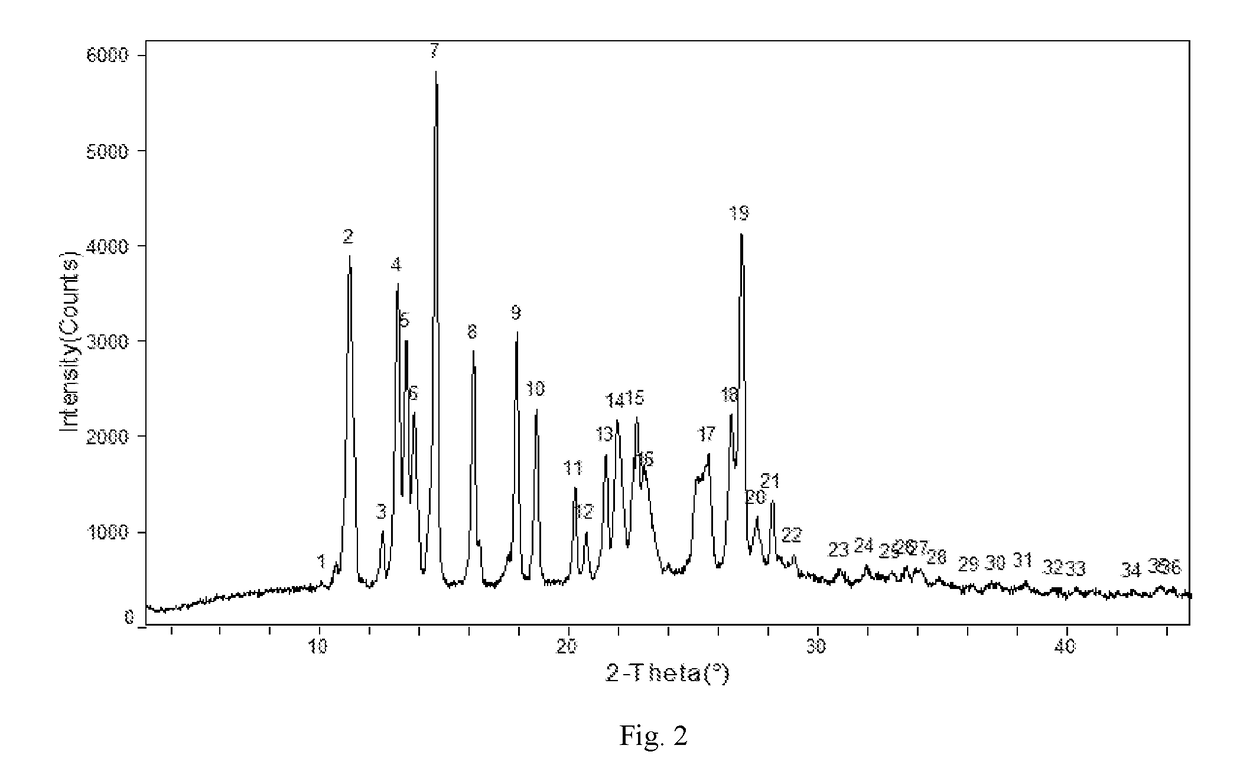
The invention relates to an apremilast and nicotinamide co-crystal and a preparation method thereof. The co-crystal is subjected to comprehensive characterization by applying means including X-ray powder diffraction analysis, thermogravimetric analysis, differential scanning amount thermal analysis and the like, and the comprehensive characterization finds out that compared with apremilast, the co-crystal has better physical and chemical and drug formation performance. The preparation method of the apremilast and nicotinamide co-crystal is simple, easy to control and good in repeatability; theapremilast and nicotinamide co-crystal can be stably obtained.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparation method of Apremilast and intermediate
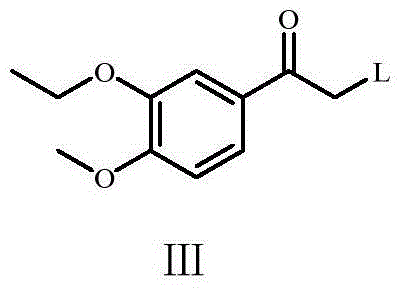
ActiveCN105622380ASuitable for industrial productionReduce usageOrganic compound preparationCarbonyl compound preparationN-ButyllithiumCombinatorial chemistry
The invention provides a preparation method of Apremilast and its intermediate. The invention provides an Apremilast intermediate as shown in the formula III. According to the preparation method, the compound as shown in the formula III reacts with methanesulfinate to obtain a compound as shown in the formula IV; the compound as shown in the formula IV undergoes reductive amination to obtain a compound as shown in the formula V; the compound as shown in the formula V and asymmetry acid undergo salifying to obtain a compound as shown in the formula VI; and the compound as shown in the formula VI reacts with 3-acetamido-phthalic anhydride to obtain Apremilast. By the method for synthesizing Apremilast, usage of an n-butyllithium hexane solution can be avoided. Production cost is reduced, and operation process is convenient. In addition, safety in the industrial production is raised to a great extent, and the preparation method is more suitable for industrial continuous production. According to the preparation method of 3-acetamido-phthalic anhydride, yield is raised to 81%. As the yield is high, the method provided by the invention is extremely suitable for industrial production of Apremilast.
Owner:NANJING ANYUAN BIO PHARMA TECH CO LTD +1
Apremilast gel for injection to articular cavity and preparation method of apremilast gel
InactiveCN106924174AImprove disadvantagesReduce adverse reactionsOrganic active ingredientsAerosol deliveryArticular cavityAqueous solution
The invention mainly provides apremilast gel for injection to the articular cavity and a preparation method of the apremilast gel. According to the apremilast gel and the preparation method, polymer with the temperature-sensitive reverse gel property is adopted as a carrier material, and medicines are dispersed or dissolved in an aqueous solution of the carrier material. When the temperature is lower than the body temperature, a sample exists in the form of sol, and is injected into the body through a common syringe; when the temperature of the injected part rises to the body temperature, the sol is transformed into semi-solid gel, along with the slow dissolving of the carrier material, the medicines are released from the gel at the low rate, and the release of the medicines can last for several weeks to several months. The release ratio of the medicines can be realized by changing the variety, concentration, proportion and molecular weight of the gel material. The dosing interval of the gel is long, the preparation process is simple, the preparation process is mature, and the method is suitable for industrial mass production.
Owner:CHONGQING UNIV
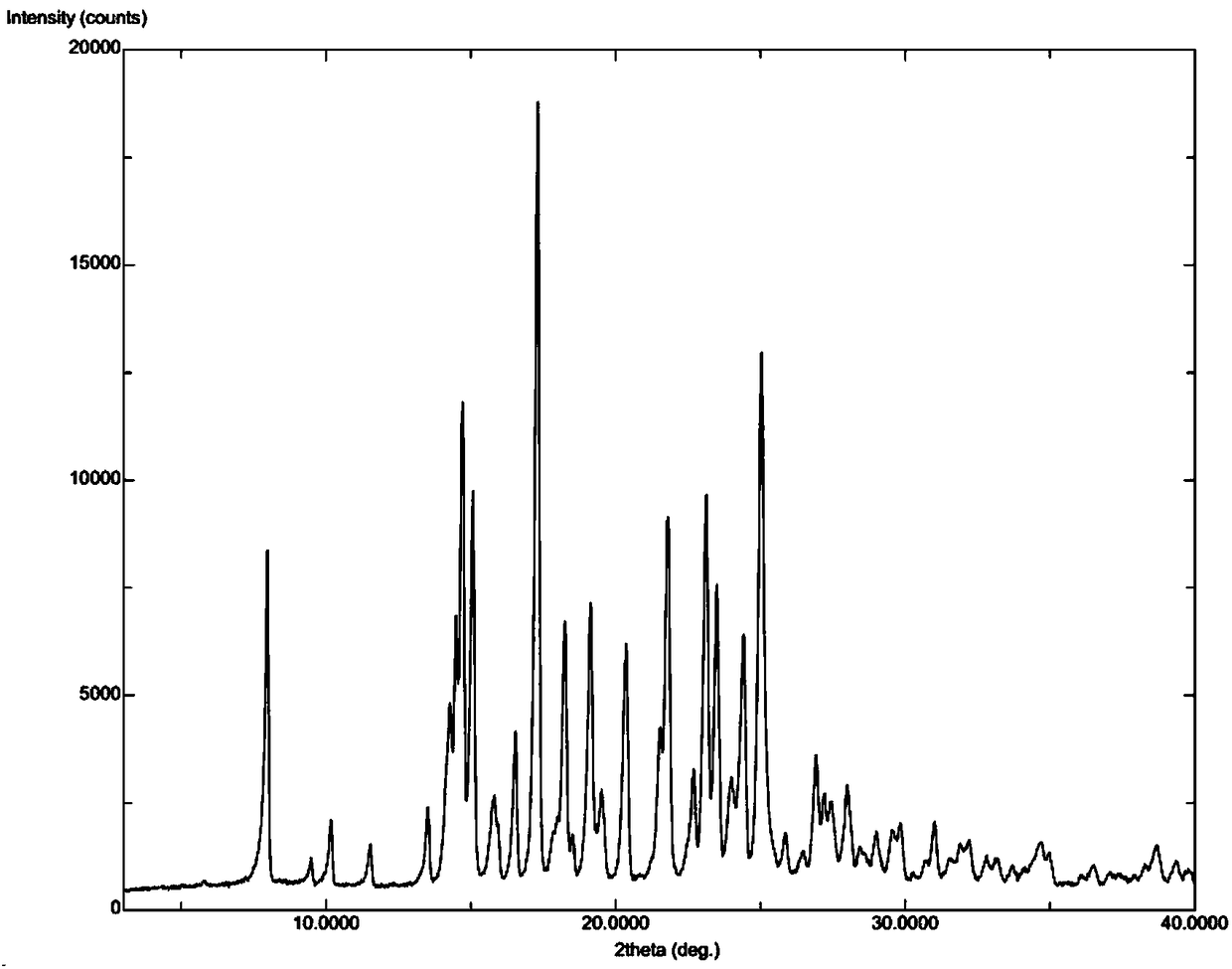
Apremilast crystal form, and preparation method, pharmaceutical composition and application thereof
ActiveCN105461610AGood compressibilityReduce toxic effectsAntibacterial agentsOrganic active ingredientsCombinatorial chemistryPsoriasis arthropathy
The invention relates to an apremilast novel crystal form. Compared to a known apremilast solid form, the novel crystal form provided by the invention has good stability and beneficial processing and handling characteristics. The crystal form is suitable for solid preparation application. The invention also relates to a preparation method and a pharmaceutical composition of the novel crystal form, as well as an application of the novel crystal form in preparing medicines used for treating psoriatic arthritis.
Owner:SOLIPHARMA
Apremilast solid composition and preparation method thereof
InactiveCN109925292AImprove bioavailabilityQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismBioavailabilityLubricant
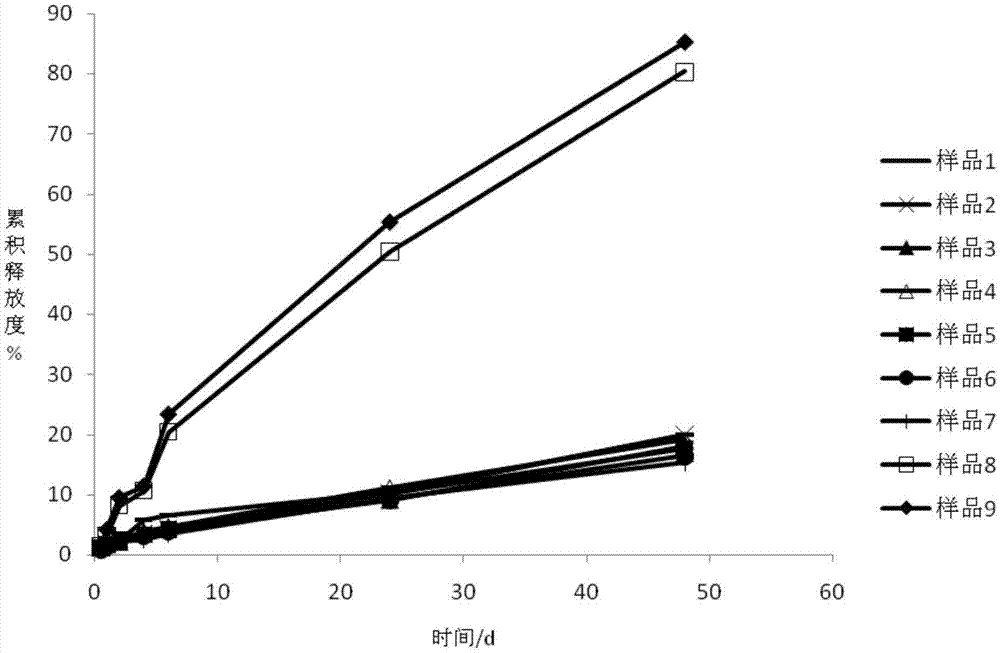
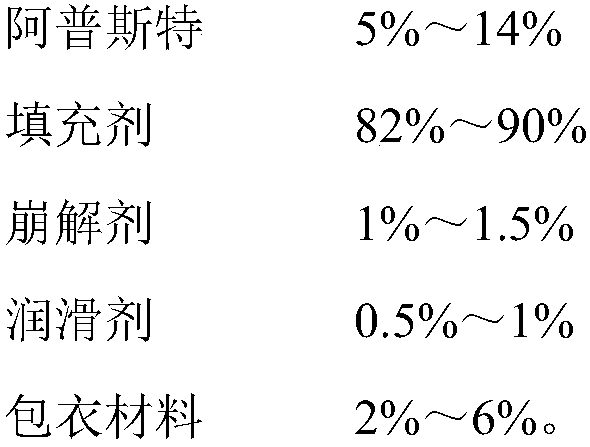
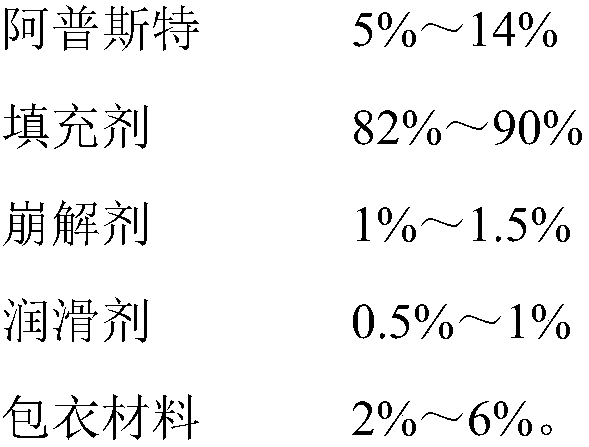
The invention provides an apremilast solid composition. The apremilast solid composition is prepared by the following components in parts by weight: 5% to 14% of apremilast; 82% to 90% of a filler; 1%to 1.5% of a disintegrant; 0.5% to 1% of a lubricant; and 2% to 6% of a coating material. The apremila solid composition prepared by the invention has a dissolution rate of more than 95%, has high bioavailability, and stable and reliable quality, and has wide clinical application prospects.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Stable apremilast crystalline form ii free of solvate and method of making the same
ActiveUS20170298018A1Easy to prepareIncreased formationSenses disorderNervous disorderPhysical chemistryPharmaceutical drug
A stable Crystalline Form II of non-solvate of Apremilast (Formula I), methods of making Form II, pharmaceutical compositions comprising Form II, and their uses are disclosed. Also discloses are mixed crystals comprising Form Hand Form B and methods of making the same. The crystalline forms are characterized using X-ray powder diffractometry (XRPD), infrared spectroscopy (IR), differential scanning calorimetry (DSC), and thermal gravimetric analysis (TG). As compared with Forms A, B, C, D, E, F, and G reported in prior art references, Apremilast Form II of the present invention is more stable to temperature, light, and humidity, and is more suitable for long term storage; the crystallization solvents are safe and can be easily removed; the Form II has a white or off white appearance, and can be directly used in preparation processing; the preparation methods are simple and easy to reproduce, and are suitable for industrialized production.
Owner:UTOPHARM SHANGHAI +1
Apremilast pharmaceutical compositions
ActiveUS20190046505A1Safe and viable processAcceptable shelf lifeOrganic active ingredientsAerosol deliveryPsoriasis arthropathyApremilast
The present invention relates to topical pharmaceutical compositions of apremilast used for the treatment of psoriasis and / or psoriatic arthritis. It further relates to processes of preparation of the compositions and the method of use for these compositions.
Owner:SARUDBHAVA FORMULATIONS PTE LTD
Method for separating and measuring Apremilast and enantiomer of Apremilast through liquid chromatography
ActiveCN105628841AEffective separation detectionAchieve quality controlComponent separationAmylaseCarbamate
The invention provides a method for separating and measuring Apremilast and enantiomer of the Apremilast through liquid chromatography, and relates to the technical field of analytical chemistry. The method comprises the steps that a chiral chromatographic column with silica gel as filler is adopted, the surface of the silica gel is in covalent bonding with amylase-tris(3-chlorobenzene carbamate), methyl tertiary butyl ether-ethyl alcohol-diethylamine (91-9-0.1) serves as a mobile phase, the flow speed is 1 ml / min, the wave length is 230 nm, and the column temperature is 30 DEG C. The high performance liquid chromatography separating and detecting method for the Apremilast and the enantiomer of the Apremilast is high in separation degree and high in specificity, the problem that the Apremilast and corresponding isomer of the Apremilast are hard to separate is solved, and therefore it is guaranteed that the quality of the Apremilast and a preparation of the Apremilast is controllable.
Owner:安徽瑞达健康产业有限公司
Novel forms of apremilast
The present disclosure is directed to novel forms of apremilast and pharmaceutical compositions comprising any of the novel forms of apremilast. Also provided are processes for the preparation of novel forms of apremilast.
Owner:MACFARLAN SMITH
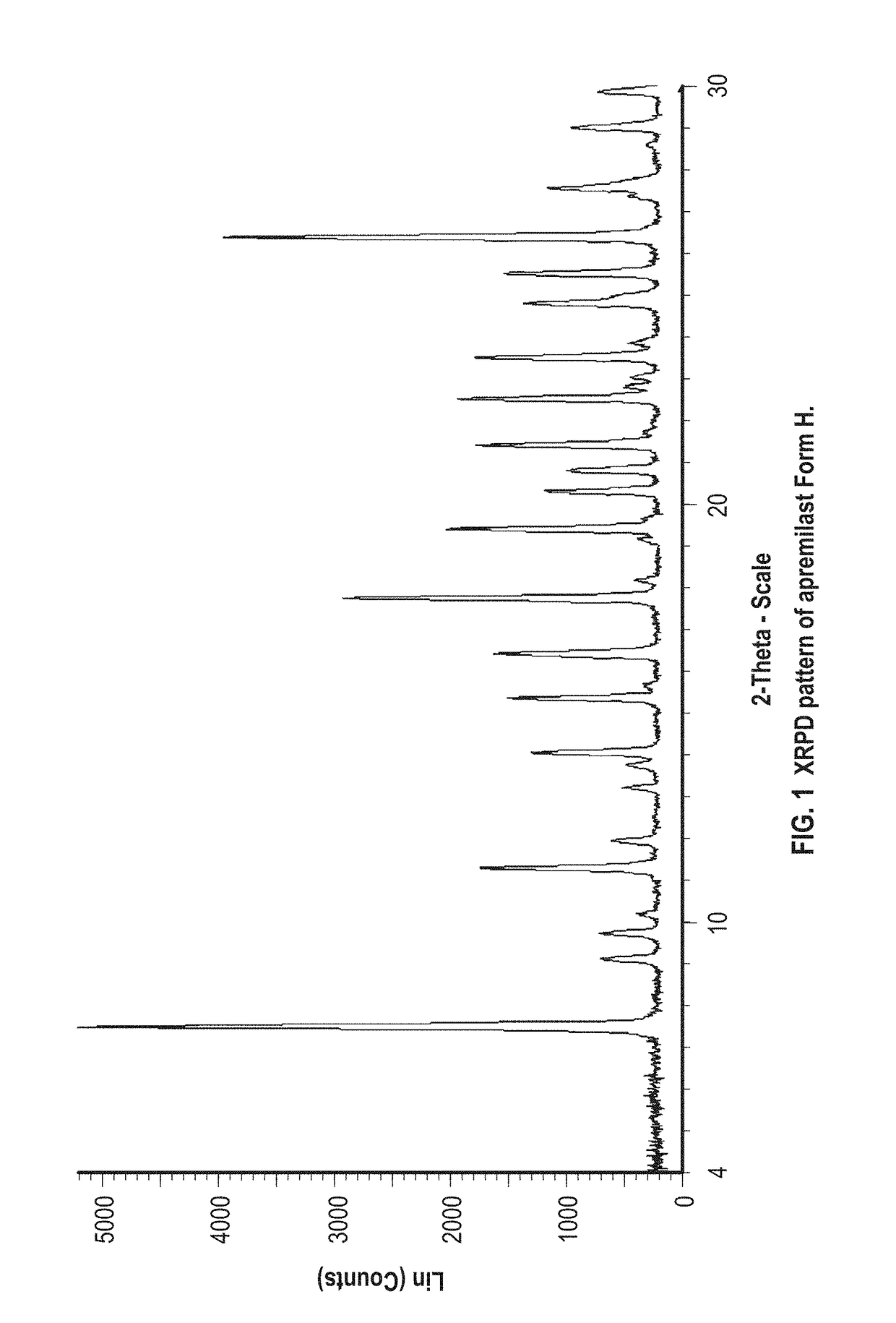
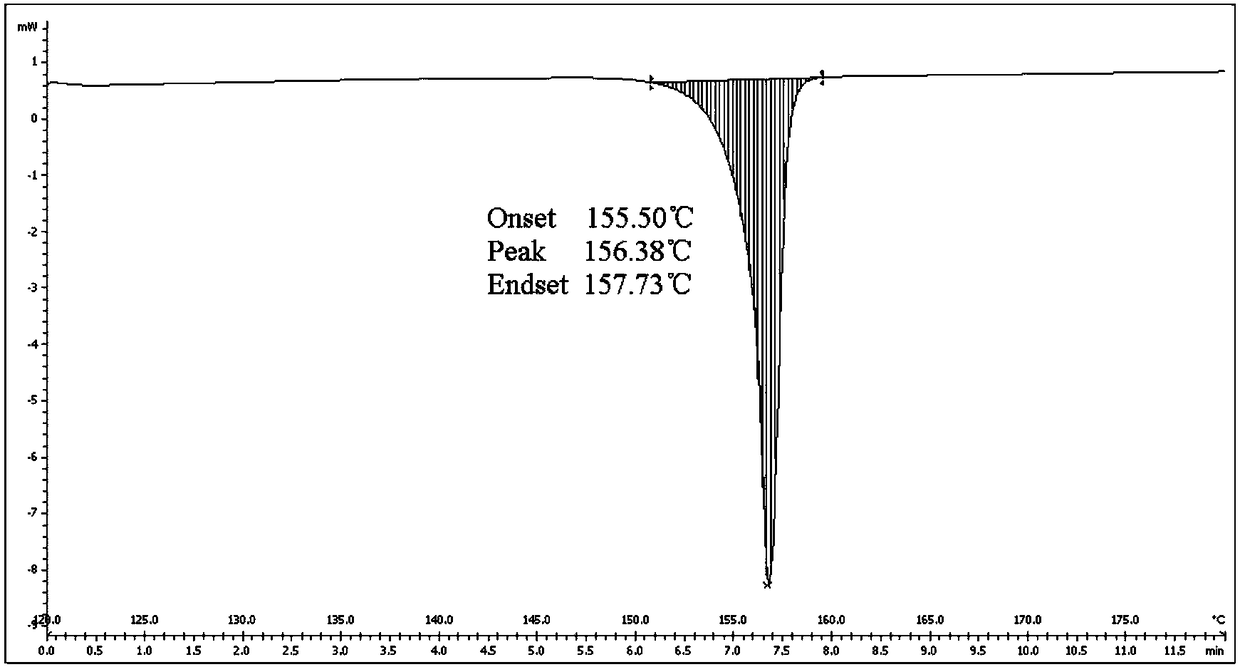
New apremilast crystal form H and preparation method thereof
InactiveCN108440381AImprove solubilityImprove stabilityOrganic chemistry methodsX-rayDifferential scanning calorimetry
The invention relates to the technical field of medicinal chemistry, in particular to a new apremilast crystal form H and a preparation method thereof. The crystal form H is characterized in that a. differential scanning calorimetry analysis shows that only one endothermic peak exists within a temperature range of 100-180 DEG C, and the endothermic peak top value is 156 + / -3 DEG C; b. the X-ray powder diffraction pattern has the following characteristic peaks at 2theta+ / -0.2: 7.980, 14.494, 14.723, 15.081, 17.317, 18.261, 19.147, 20.362, 21.809, 23.138, 23.505, 25.057; c. the X-ray powder diffraction pattern has no characteristic peak at the position where 2theta+ / -0.2 is 8.443 and 15.482. The invention provides the highly dissolved new apremilast crystal form H.
Owner:WEIHAI DISU PHARMA CO LTD +1
Therapeutic topical compositions of apremilast
The present invention relates to topical compositions comprising apremilast. It also relates to processes for preparing such compositions and methods of using them in management of skin diseases or disorders such as psoriasis, dermatosis, eczema, rosacea, acne vulgaris, allergies, contact and atopic dermatitis, pruritus, seborrhea, skin cancers, inflammation and other associated skin conditions.
Owner:SARUDBHAVA FORMULATIONS PTE LTD
Method for detecting enantiomer impurity in apremilast
InactiveCN105486785AEffective separation detectionLow priceComponent separationEnantiomerStructural formula
The invention discloses a method for detecting an enantiomer (I) in apremilast. A protein type chiral chromatographic column is adopted in the method; while the defect of shorter service life of an existing starch coating chiral column is overcome, the enantiomer in the apremilast is effectively separated and detected, and a detection result is accurate and reliable; the method has the multiple advantages such as excellent linear relation, high precision, good stability, good repeatability, good recovery rate, simple and convenient operation and low cost, and is suitable for popularization and application. A structural formula is shown in the description.
Owner:CHENGDU BAIYU JINGELAI PHARMA CO LTD
A kind of synthetic method of Apremilast chiral amine intermediate
ActiveCN104761474BChiral avoidanceReduce dosageOrganic chemistryOrganic compound preparationSynthesis methodsKetone
The invention relates to a synthetic method of an apremilast chiral amine intermediate (S)-2-[1-(3-ethoxyl-4-methoxylphenyl)]-1-methylsulfonyl-2-ethylamine (V). The synthetic method is characterized by including following steps: (1) with 1-(3-ethoxyl-4-methoxylphenyl)-2-(methylsulfonyl)ethyl ketone (I) as a raw material, performing asymmetric hydrogenation reduction to obtain methyl sulfonyl ethanol (II); (2) performing an esterification reaction to obtain methyl sulfonyl ester (III); (3) performing an azidation reaction to obtain an azide compound (IV); and (4) performing hydrogenation reduction to obtain the chiral amine intermediate (V) being high in chiral purity. The synthetic method is simple in process, is stable in reaction processes, and is environmental-protective and low-cost. In the synthetic method, the use amount of a catalyst during catalytic hydrogenation is less and the conversion rate reaches higher than 98%. The chiral amine can be prepared through chiral alcohol with very high yield and purity so that the synthetic method has a quite excellent commercial value and develops a new approach for synthesizing the apremilast chiral amine intermediate.
Owner:ENANTIOTECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com