Method for detecting enantiomer impurity in apremilast
A technology for enantiomers and detection methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of reduced column efficiency, chromatographic column damage, and short service life, and achieves excellent linearity and long service life. Long, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Determination of detection wavelength
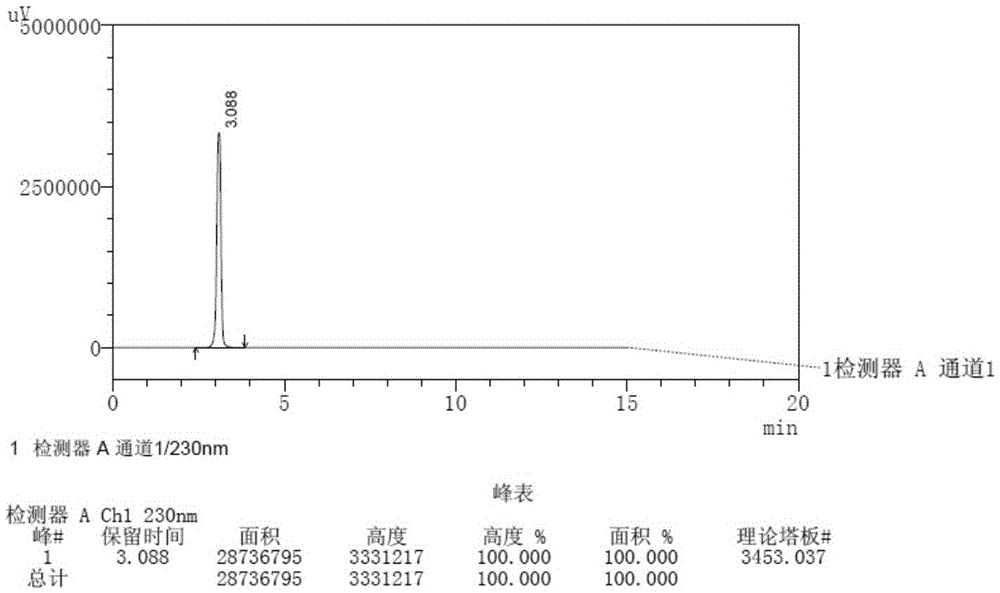
[0053] Take Apremilast tablets, grind finely, accurately weigh an appropriate amount, and use mobile phase (methyl tert-butyl ether-n-butanol=75:25) to prepare a solution with a concentration of 20 μg / mL as the test solution.
[0054] Accurately weigh an appropriate amount of the reference substance (enantiomer), and use a mobile phase (methyl tert-butyl ether-n-butanol=75:25) to prepare a solution with a concentration of 20 μg / mL as the reference substance solution.
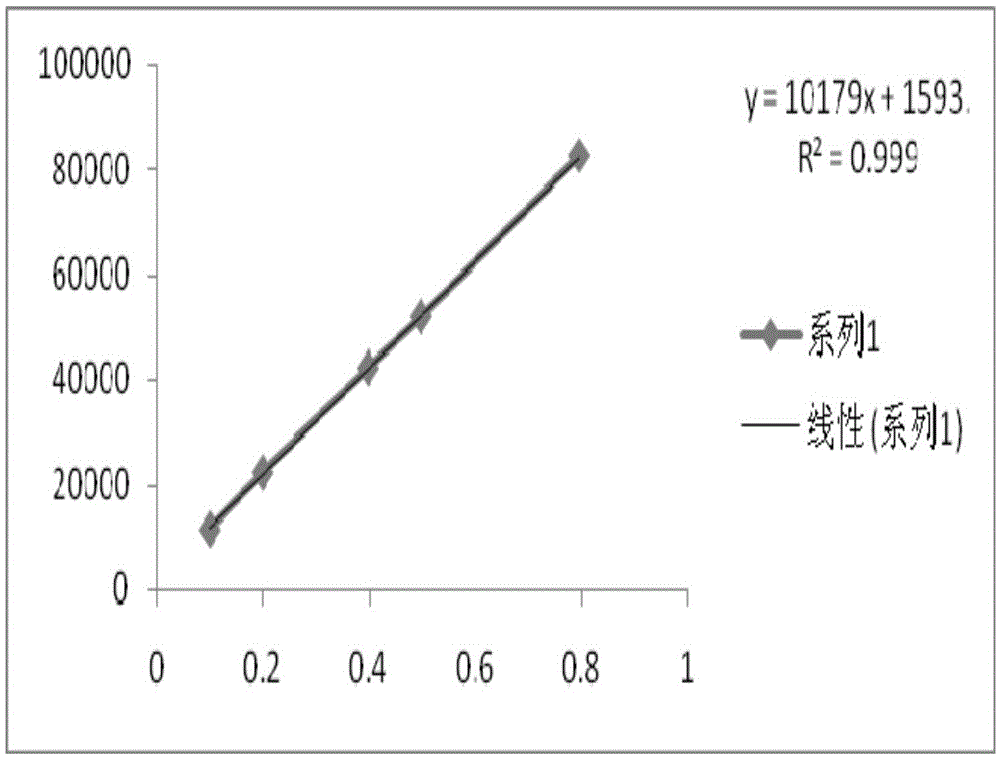
[0055] Take the above-mentioned test solution and reference solution, and scan in the wavelength range of 200nm to 400nm. The test results are shown in Table 1.
[0056] Table 1, the ultraviolet scanning result of the present invention
[0057] project
Peak wavelength(nm)
peak
Valley wavelength(nm)
valley
230
0.617
215
0.226
Reference solution
230
0.775
296
0.010
[0058]...
Embodiment 2
[0118] Detection by normal phase high performance liquid chromatography:
[0119] Chromatographic column: a chromatographic column filled with α-acid glycoprotein-bonded silica gel;
[0120] The model of the chromatographic column is CHIRALAGP, and the specifications are: the inner diameter is 4.6mm, the length is 150mm, and the filler particle size is 5μm;
[0121] Mobile phase: methyl tert-butyl ether-n-butanol (75:25);
[0122] Column temperature: 35°C;
[0123] Flow rate: 1.0mL / min;
[0124] Detection wavelength: 230nm;
[0125] Injection volume: 20 μL.
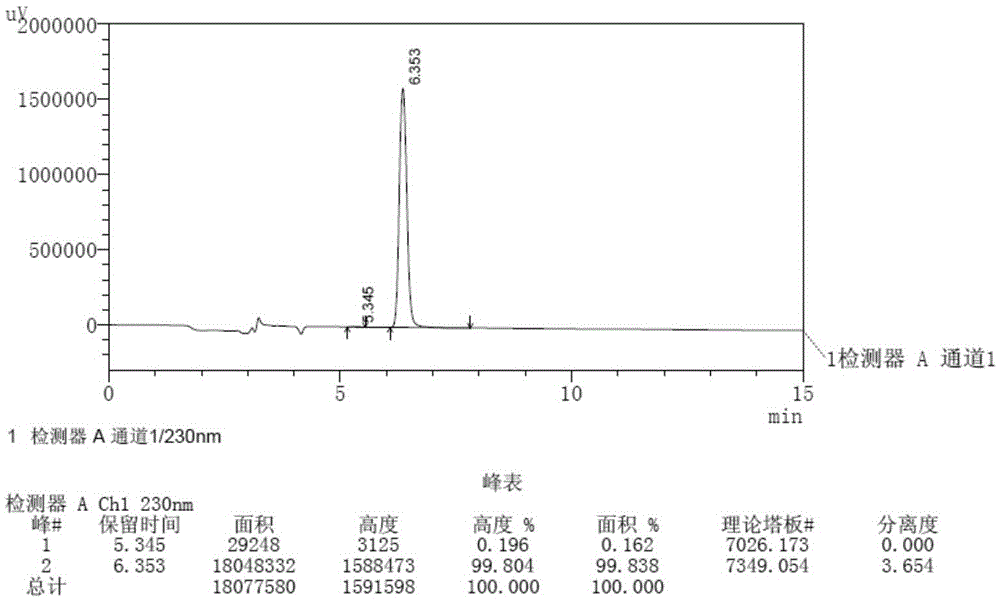
[0126] Carry out system suitability test according to the method for embodiment 1, the result shows: apremilast (retention time is 6.353min) and enantiomer (for its retention time is 5.345min) the degree of separation is 3.654, theoretical The number of plates (calculated by Aprester peak) is 7349, and the tailing factor of Aprester peak is 0.975.
[0127] It can be seen that the chromatographic conditions can accur...
Embodiment 3
[0129] Detection by normal phase high performance liquid chromatography:
[0130] Chromatographic column: a chromatographic column filled with α-acid glycoprotein-bonded silica gel;
[0131] The model of the chromatographic column is CHIRALAGP, and the specifications are: the inner diameter is 4.6mm, the length is 150mm, and the filler particle size is 5μm;
[0132] Mobile phase: methyl tert-butyl ether-n-butanol (70:30);
[0133] Column temperature: 35°C;
[0134] Flow rate: 1.0mL / min;
[0135] Detection wavelength: 230nm;
[0136] Injection volume: 20 μL.
[0137] Carry out system suitability test according to the method for embodiment 1, the result shows: the separation degree between apremilast (retention time is 10.115min) and enantiomer (for its retention time is 8.725min) is 4.128, theoretical The plate number (according to Aprester peak calculation) is 8107, and the tailing factor of Aprester peak is 1.08.
[0138] It can be seen that the chromatographic conditio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com