Method for detecting fasudil hydrochloride and related substances thereof
A technology for fasudil hydrochloride and related substances, applied in the field of medicine, can solve the problems of poor sensitivity, poor detection peak shape, inability to separate and detect isoquinoline and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In this embodiment, fasudil hydrochloride injection is detected according to the following method:
[0054] 1. Prepare the sample solution
[0055] Blank solution: water.
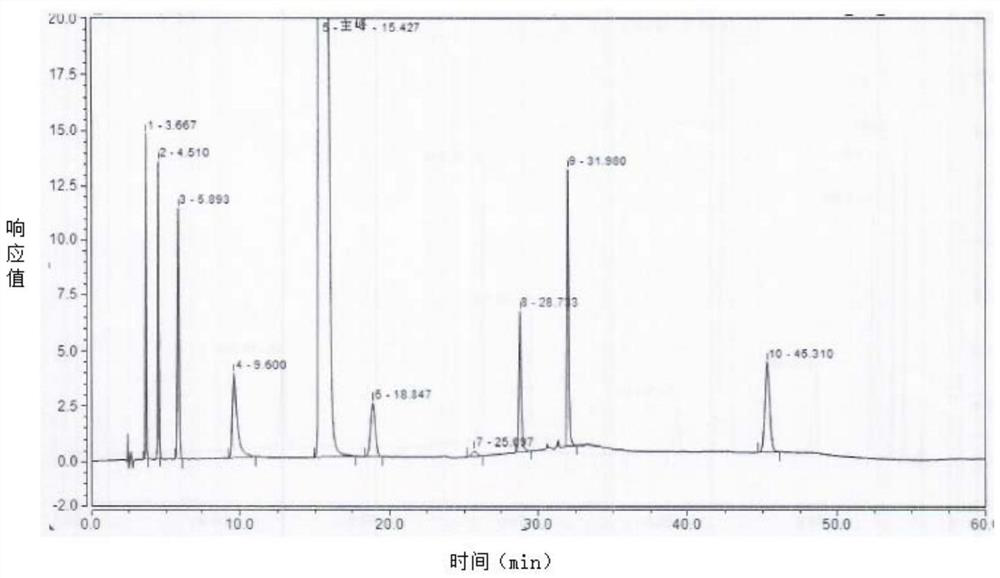
[0056] Mixed reference solution: Take about 10 mg of isoquinoline and place it in a 20 ml volumetric flask, dilute to the mark with methanol, shake well, and use it as the isoquinoline reference solution. Take 4 mg of impurity 15, fasudil dimer, impurity 10, impurity A, impurity B, impurity D, and impurity E respectively and place them in different 10ml volumes, add methanol to dilute to the mark, shake well, and serve as the control of each impurity product solution. Take 100 μl of the above 8 impurity reference substance solutions and place them in the same 10ml volumetric flask, add 4 mg of fasudil hydrochloride reference substance, dissolve in water and dilute to the mark, shake well, and use it as a mixed control solution. (See Table 1 for the names of impurities)
[0057] Table 1 Impurity n...
Embodiment 2
[0088] In this embodiment, the influence of different chromatographic columns on the detection of fasudil hydrochloride injection is studied, and the specific steps are as follows:
[0089] 1. Prepare the sample solution
[0090] Blank solution: water.
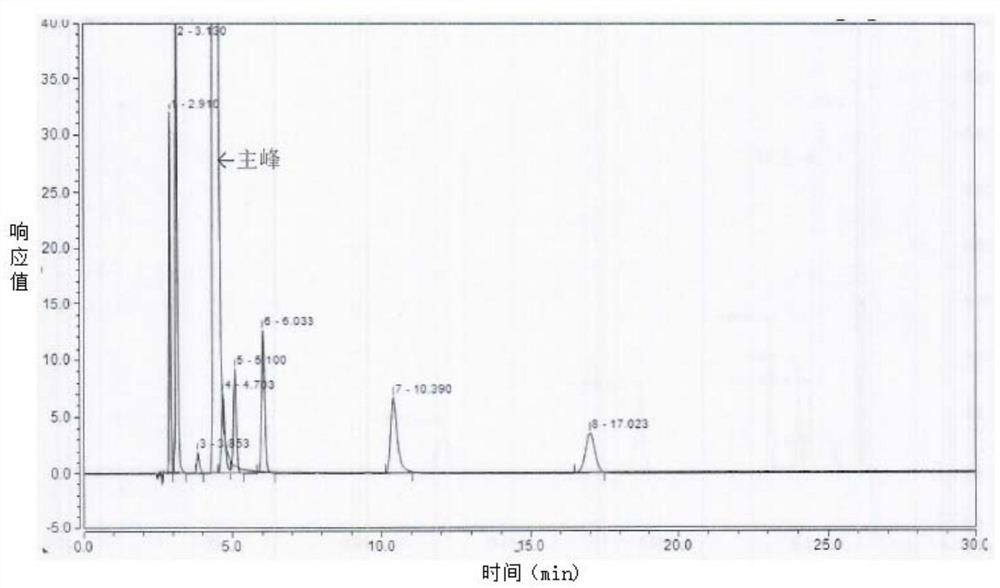
[0091] Oxidative destruction of the test solution: take 1.00ml of Fasudil hydrochloride injection and put it in a 50ml volumetric flask, add 1.0ml of 30% hydrogen peroxide, shake well, leave it for 30 minutes, dilute to the mark with water, and shake well. As the oxidative destruction of the test solution.
[0092] 2. Perform high-performance liquid chromatography detection on the blank solution and the oxidatively damaged test solution respectively. The detection conditions are as follows:
[0093] Chromatographic column: Welch Ultimate AQ-C18 150×4.6mm, 5μm or Welch Xtimate C18 250×4.6mm, 5μm;
[0094] Detection wavelength: 275nm;
[0095] Injection volume: 20μl;
[0096] Column temperature: 30℃;
[0097] Flow rate: 1....
Embodiment 3
[0103] In this embodiment, the influence of different elution conditions on the detection of fasudil hydrochloride injection is studied, and the specific steps are as follows:
[0104] 1. Prepare the sample solution
[0105] Blank solution: water.
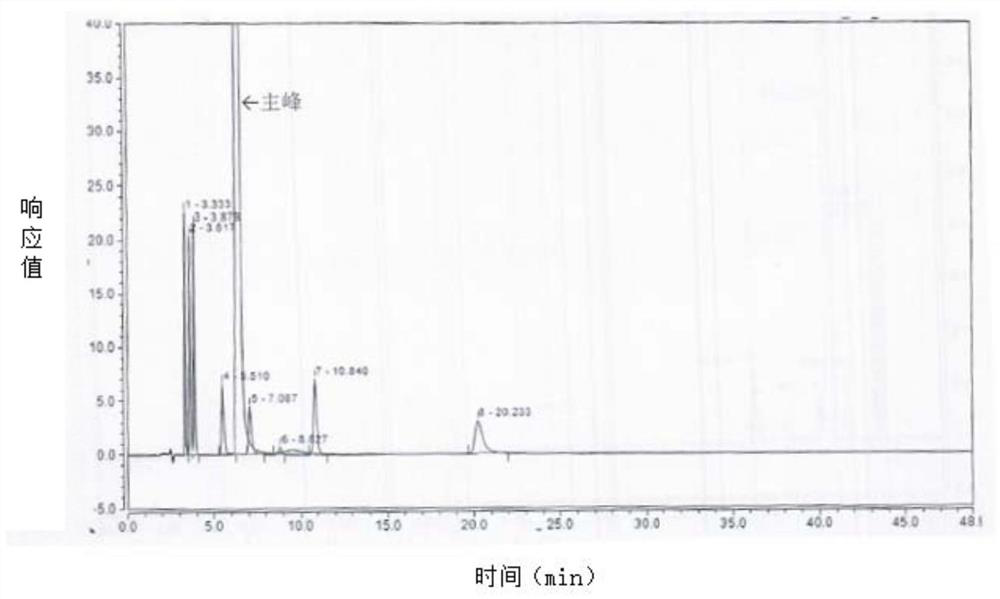
[0106] Mixed reference solution: take about 10 mg of isoquinoline and place it in a 20 ml volumetric flask, dilute to the mark with methanol, shake well, and use it as the isoquinoline reference solution. Take 4 mg of impurity 15, fasudil dimer, impurity 10, impurity A, impurity B, impurity D, and impurity E respectively and place them in different 10ml volumes, add methanol to dilute to the mark, shake well, and serve as the control of each impurity product solution. Take 100 μl of the above 8 impurity reference substance solutions and put them in the same 10ml volumetric flask, add 4 mg of fasudil hydrochloride reference substance, dissolve in water and dilute to the mark, shake well, and use it as a mixed control solution. (S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com