Patents
Literature
306 results about "Lansoprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
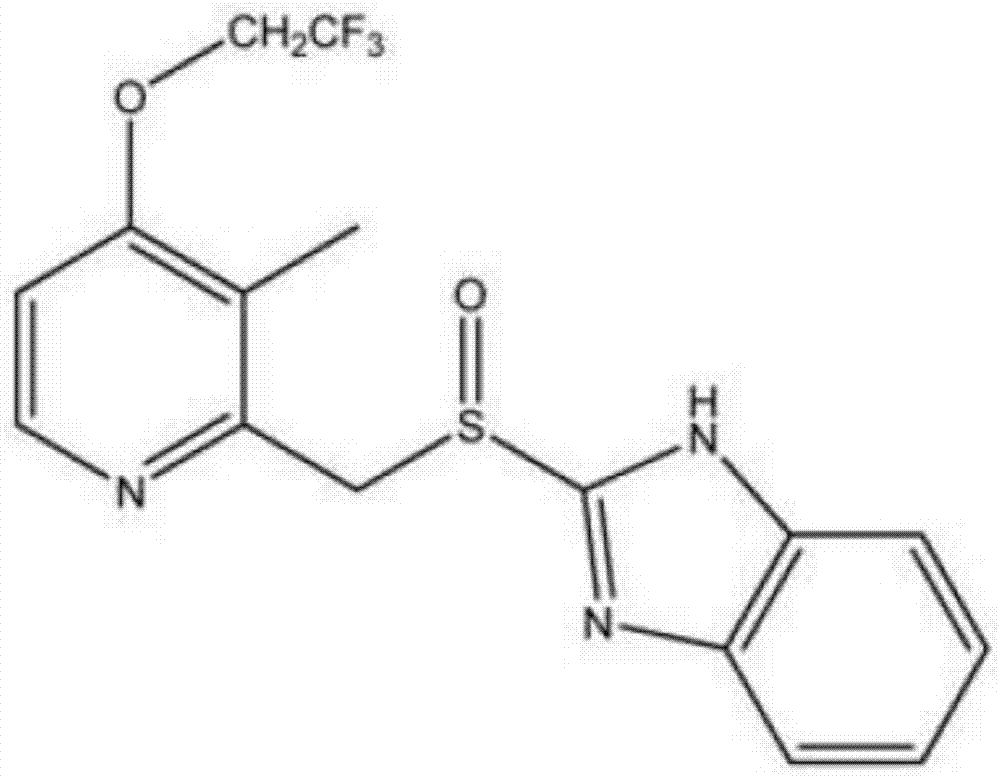
Lansoprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers).
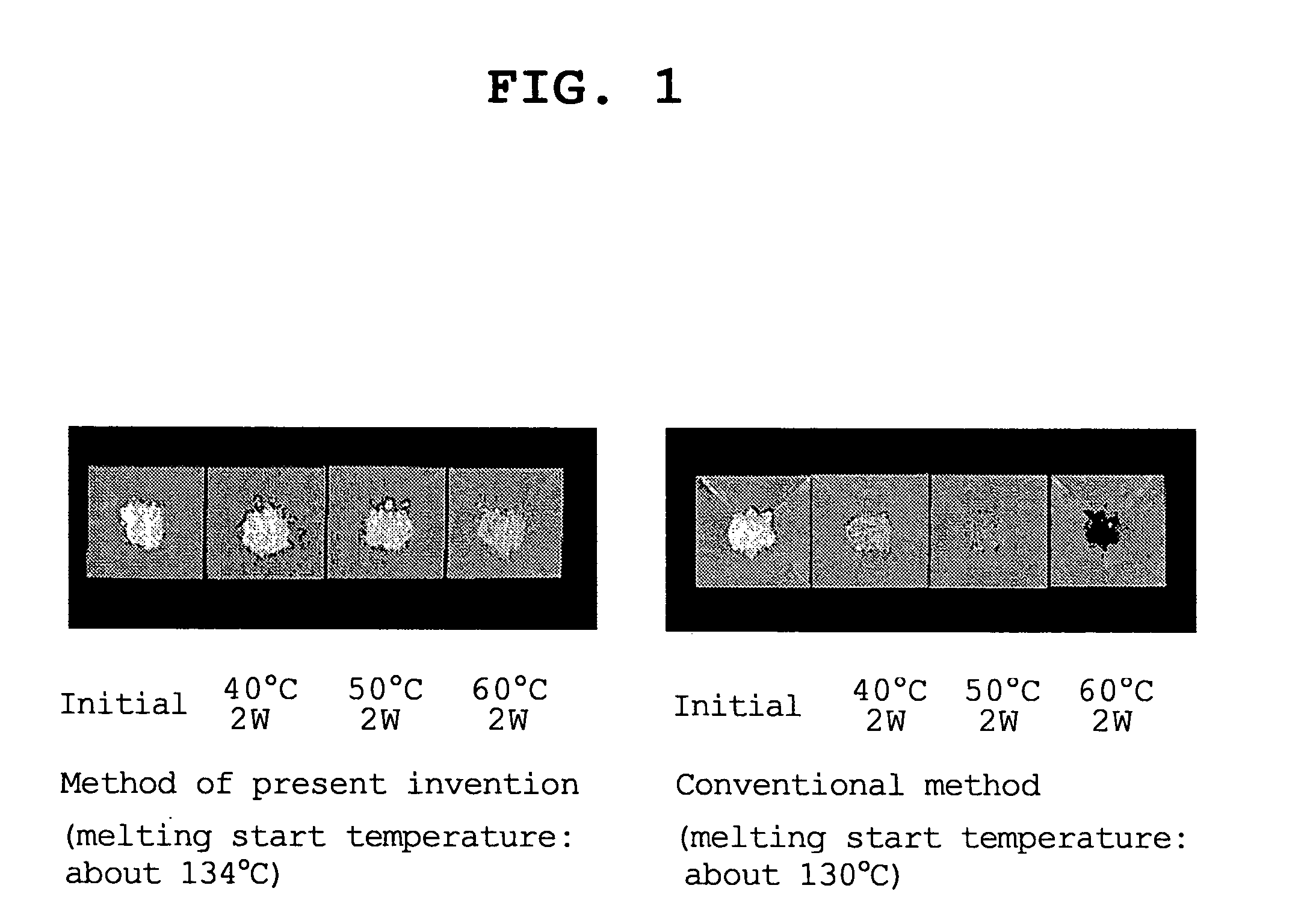
Process for the crystallization of (R)- or (S)-lansoprazole
Owner:TAKEDA PHARMA CO LTD
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Process for the crystallization of (r)-or (s)-lansoprazole
InactiveUS20040049045A1Good storage stabilityLow toxicityAntibacterial agentsOrganic chemistryLansoprazoleCrystallization
Owner:TAKEDA PHARMA CO LTD
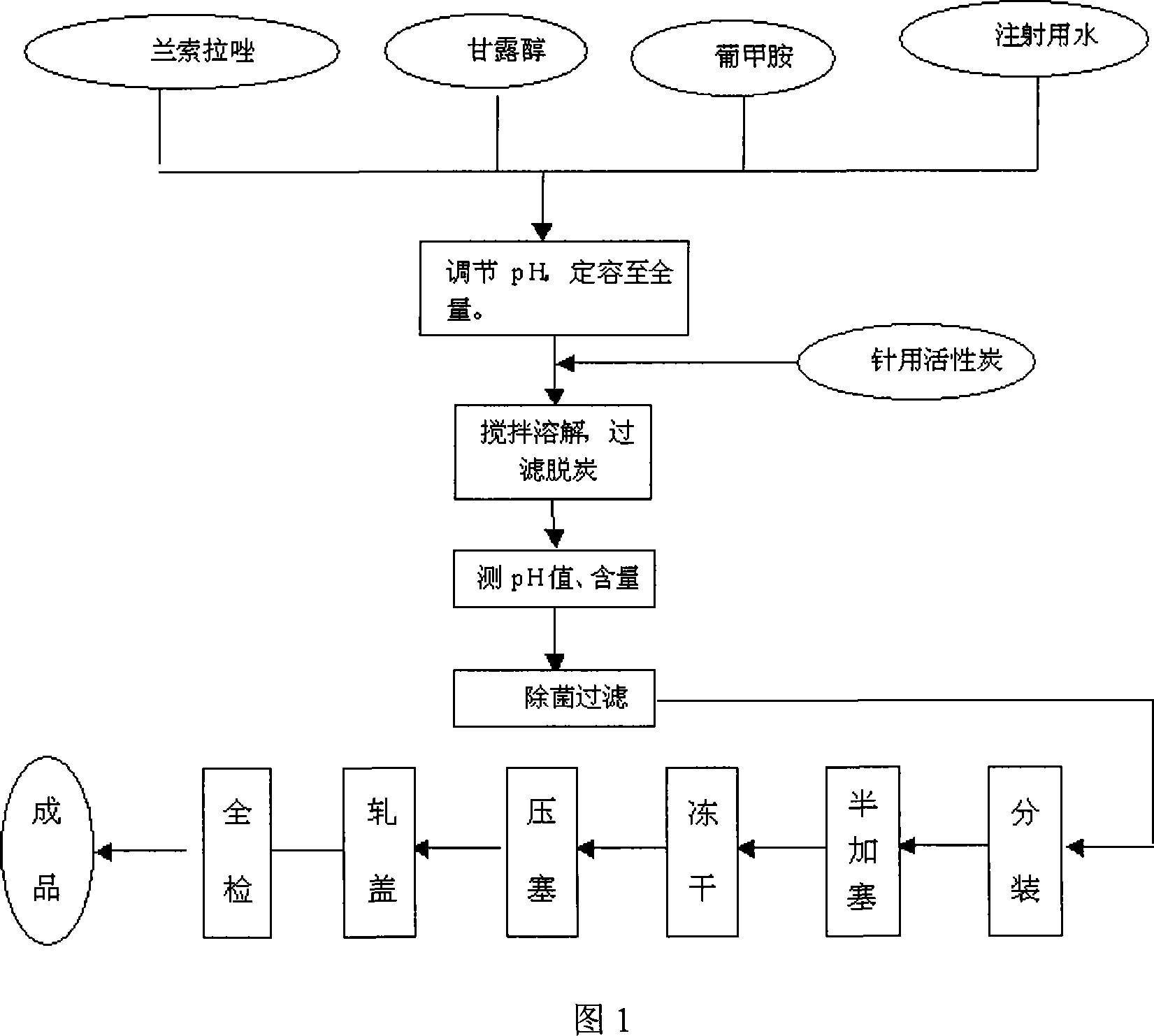
Lansoprazole for injecting and its preparation method
InactiveCN101057846AConvenient for clinical operationReduce dosageOrganic active ingredientsPowder deliveryLansoprazoleIothalamate Meglumine
The invention discloses a Lansoprazole preparation for injection comprising (1) Lansoprazole 10-50 weigh parts, (2) meglumine 2-20 weight parts, (3) sodium hydroxide 1-5 weight parts, (4) mannitol 20-100 weight parts. The invention also discloses the process for preparing the preparation.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Lansoprazole orally disintegrating tablets
InactiveUS20070141151A1Dissolve fastPrevent degradationBiocidePill deliveryLansoprazoleOrally disintegrating tablet
The invention provides orally disintegrating tablets that readily disintegrates in the mouth, releasing enteric coated drug sub-tablets.
Owner:TEVA PHARM USA INC
Freeze dried combination of Lansoprazole available for linjection and preparation method
InactiveCN1660091AImprove product qualityDoes not reduce efficacyPowder deliveryOrganic active ingredientsLansoprazoleFreeze-drying
A freeze-dried powder injection of lansoprazole is prepared from lansoprazole, cosolvent, alkaline amino acid and water proportionally.
Owner:龙蓓
Novel method for preparing chiral sulphoxide compound
The invention provides a novel method for preparing optically pure substituted [(pyridyl methylene) sulfinyl]-1H-benzimidazole sulphoxide compound by enantioselective synthesis. The method requiring protection is to directly and asymmetrically oxidize prochiral thioether into a corresponding optically pure sulphoxide compound or a sulphoxide compound rich in single enantiomer by a mild and cheap oxidizing agent in the presence of a complex compound catalyst formed by an accessible and stable (+)- or (-)- tartaric acid diamide ligand shown in a general formula and titanium. Therefore, optically pure omeprazole, lansoprazole and pantoprazole can be obtained, wherein R8, R9, R10 and R11 are the same or different, and are selected from hydrogen, alkyl, aralkyl, aryl, organic polymers or a silica loading body.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
Lansoprazole lyophilized powder injection and its preparing method
The present invention relates to a lansoprazole freeze-dried powder injection and its preparation method. Said lansoprazole freeze-dried powder injection contains ethylene diamine tetraacetic acid (EDTA) and / or its salt, and is made up by using active component lansoprazole, ethylene diamine tetraacetic acid and / or its salt, stabilizing agent, excipient, pH regulator and injection water through a certain preparation process. Besides, said invention also provides the concrete steps of said preparation process.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
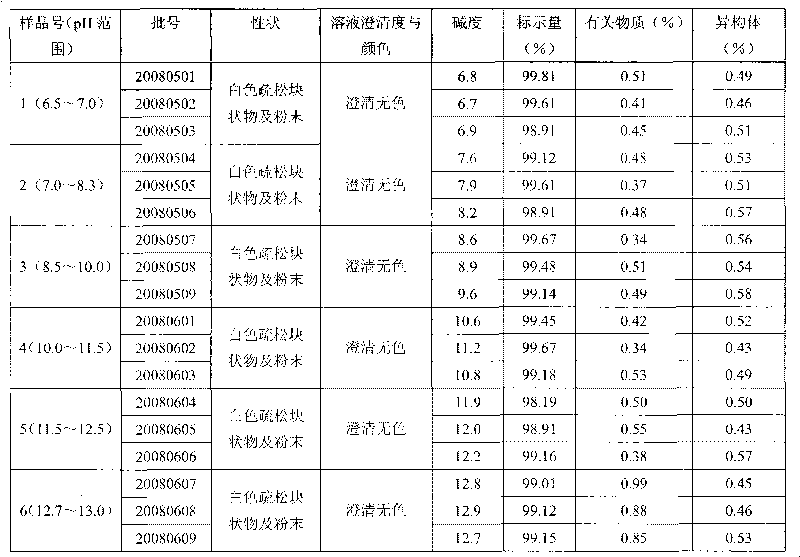
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
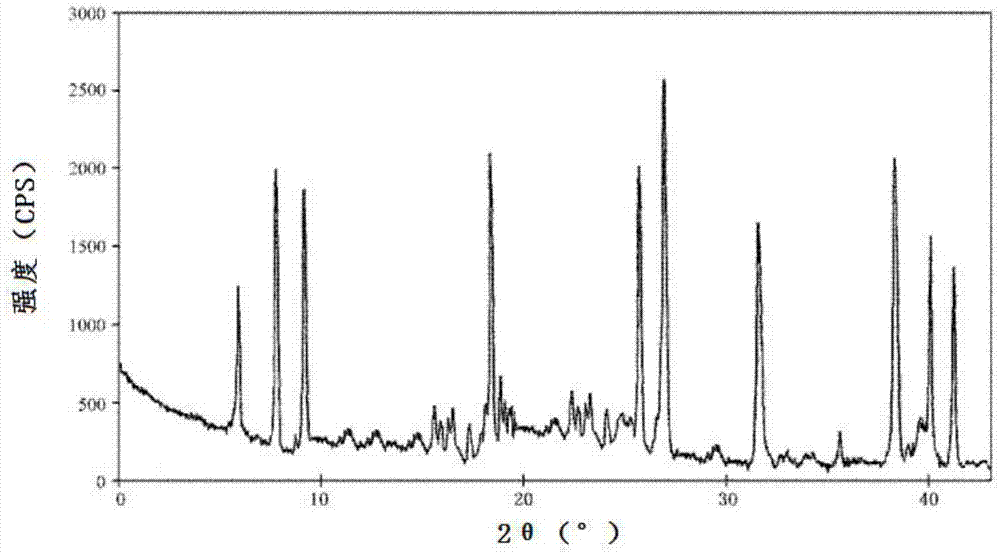
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
Freeze-dried powder needle containing lansoprazole
ActiveCN101129368ASolve the painEasy to producePowder deliveryOrganic active ingredientsLansoprazoleSolubility
The invention discloses a freeze dried of lansuolazole and making method, which comprises the following parts: 1% lansuolazole, 0. 05-50% excipient, and 0. 1-10% carbowax. The making method of the freeze dried comprises the following steps: weighing lansuolazole; adding injecting water and pH value adjuster to adjust the pH value to 10-12. 5; stirring until the lansuolazole is dissolved completely; adding carbowax and excipient; supplementing the injecting water to full quantity to pack in the Schering bottle quantitatively; freezing; drying. The invention solves the problem of stability and solubility of the agent simultaneously, which has good double dissolving property.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4
Lansoprazole microtablets
A pharmaceutical composition comprising microtablets, wherein said microtablets comprise lansoprazole, a lubricant, optionally one or more excipients, and an enteric coating, wherein the weight ratio of lansoprazole to lubricant is from about 1:4 to about 8:1, wherein said microtablets have a tablet size of about 1 mm to about 4 mm, and a tablet weight of 1 to 50 mg, and said microtablets are free of a separating or intermediate layer between the lansoprazole and enteric coating.
Owner:PATEL SATISHKUMAR AMBALAL +3
High-yield method for preparing lansoprazole
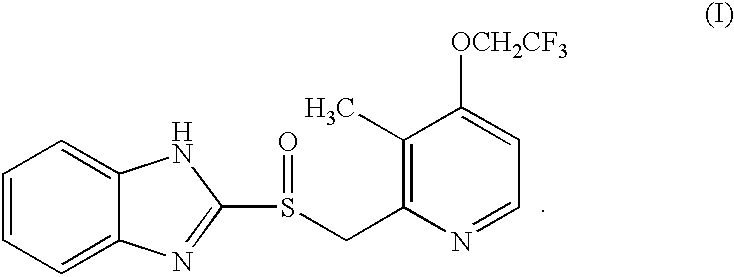
Lansoprazole of formula (I) can be produced economically in a high yield using a simple, two-step method comprising reacting hydroxymethylpyridine and benzimidazole starting materials in the presence of a phosphine compound and a dialkyl azodicaboxlate and oxidizing the product of the first step in the presence of an organic radical catalyst:
Owner:HANMI PHARMA
Orally disintegrating tablet
InactiveUS20130273157A1Avoid breakingControl releaseBiocideDigestive systemLansoprazoleControlled release
A orally disintegrating tablet is obtained by tableting fine granules showing controlled release of lansoprazole and an additive, which is capable of suppressing breakage of the fine granules during tableting, and can control the release of lansoprazole for a long time, and can maintain a therapeutically effective concentration for a prolonged time, and shows superior disintegration property in the oral cavity.
Owner:TAKEDA PHARMA CO LTD
Stable oral pharmaceutical dosage forms
InactiveUS7041316B2Effective protectionEfficient deliveryPowder deliveryOrganic active ingredientsLansoprazoleEnteric coated
The present invention relates to new stable enteric coated pharmaceutical dosage forms for oral use containing Omeprazole or Lansoprazole, to a formulation and a method for the manufacture of such a dosage forms, and to a method of gastric acid pump inhibition and providing gastrointestinal cytoprotective benefit by using them.
Owner:SAGE PHARMA
Lansoprazole freeze-dried powder for injection and preparing method thereof
ActiveCN101229136AFull appearanceImprove solubilityPowder deliveryOrganic active ingredientsLansoprazoleActivated carbon
The invention provides a freeze-dried powder of lansoprazole for injection and a preparation method of the freeze-dried powder which consists of lansoprazole, meglumine and mannitol with the weight ratio of 3:1:18 to 22. The invention provides the preparation method: dissolving raw and supplemental materials with water and adjusting the pH, and adding activated carbon to remove the color and filtering to remove the carbon; making fine filtration with a filtering film and loading separately and cooling rapidly to minus 50 to minus 46 DEG C with the speed of 1 to 1.2 DEG C per minute; preserving the heat and freezing for 3 hours and pumping the vacuum to 15Pa; heating uniformly to minus 22 to minus 18 DEG C in 7 to 9 hours and preserving the heat for 1 hour to 2 hours; heating rapidly to 3 to 7 DEG C in 4 to 6 hours and then heating to 40 DEG C in 4 hours; preserving the heat and drying for 3 hours and packaging and warehousing after the measuring there. Products prepared by the method are low in water content, beautiful in appearance and easy in storage and transportation.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
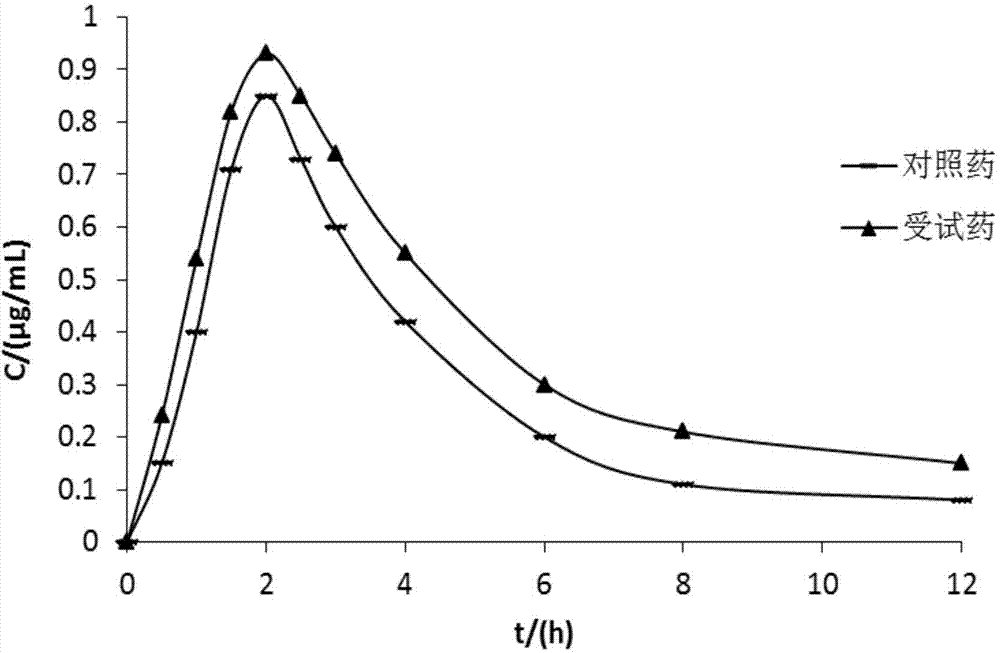
R-lansoprazole for injection and preparation method thereof
InactiveCN101716176AAvoid destructionQuick effectOrganic active ingredientsPowder deliveryDuodenal ulcerExcipient
The invention provides an r-lansoprazole for injection and a preparation method thereof. The medicament comprises active components of r-lansoprazole, or r-lansoprazole salt or r-lansoprazole water crystal as well as an excipient, a pH regulator and an antioxidizer. The preparation method comprises the following steps of: dissolving raw and auxiliary materials in water; adjusting the pH value; adding active carbon to decolor; filtering with a filter membrane; sub-packaging; freezing and drying to obtain the r-lansoprazole. The medicament is clinically used for treating gastric ulcer, duodenal ulcer, erosive gastro-esophageal reflux disease, helicobacter pylori, zollinger-ellison syndrome, and the like, can prevent the orally taken r-lansoprazole from being damaged in gastric acid and reach the aims of fast onset and improving the bioavailability.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
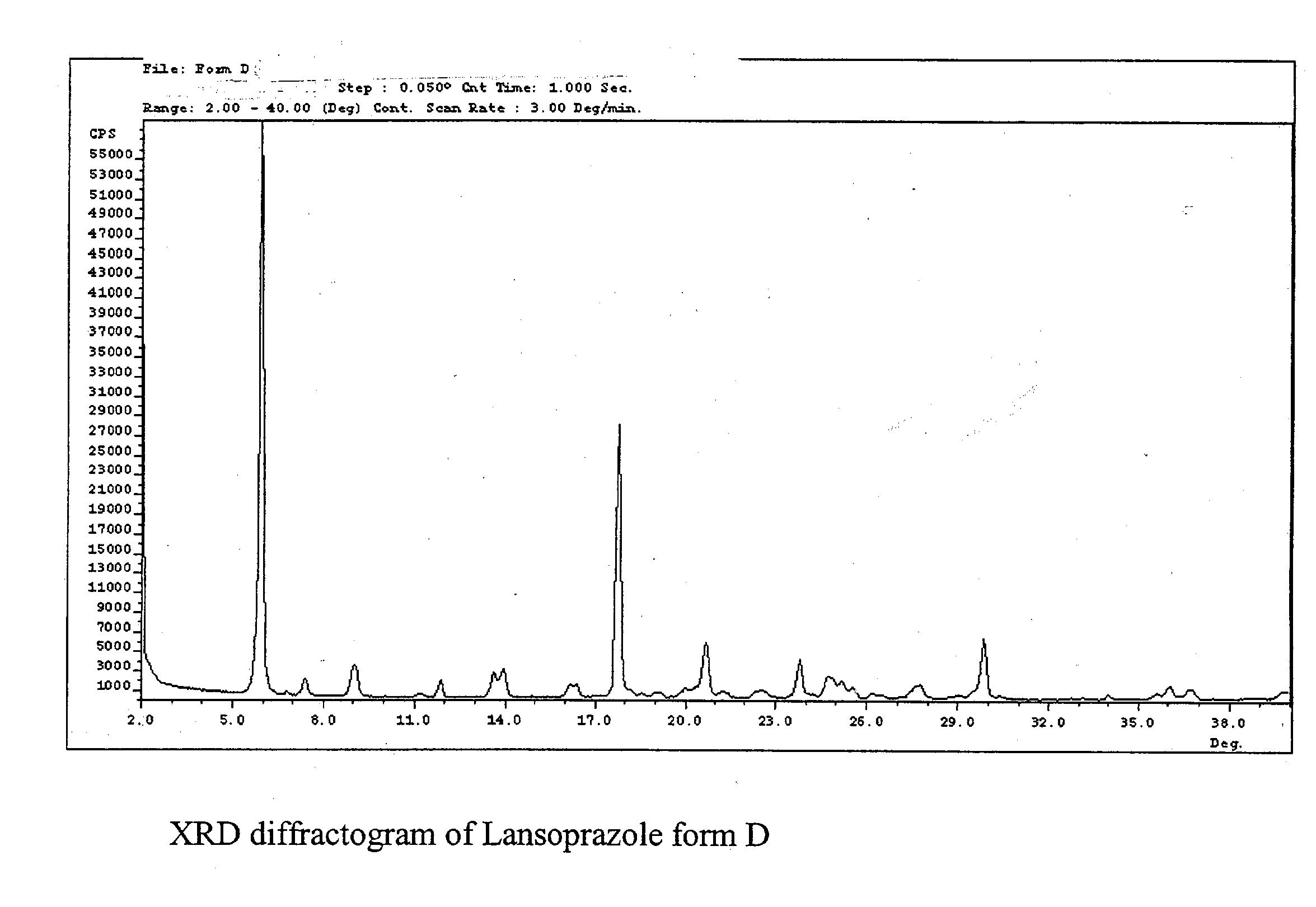
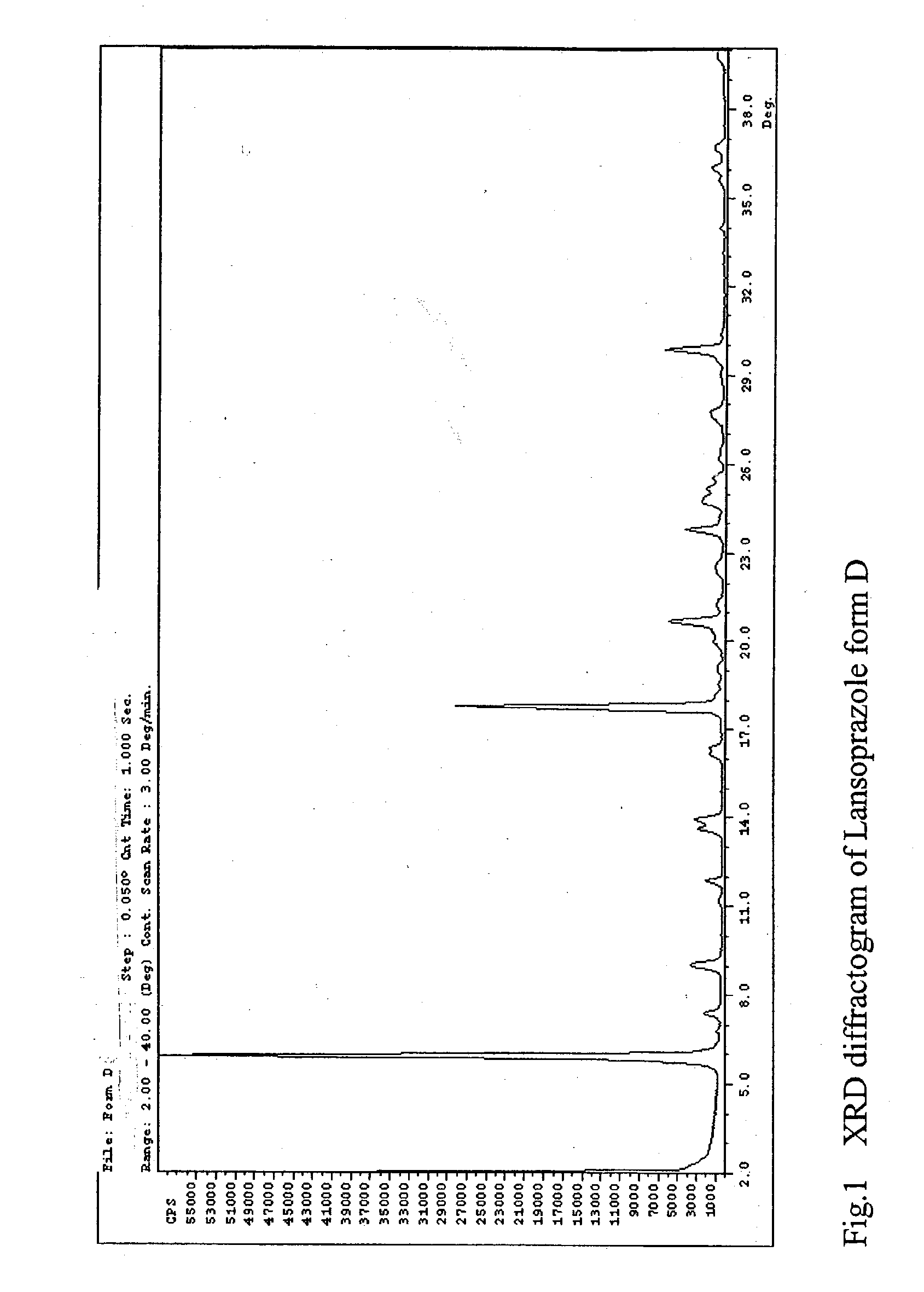
Lansoprazole polymorphs and processes for preparation thereof
The present invention relates to three crystalline solid forms of lansoprazole denominated as forms D, E and F. Processes for preparing these crystalline solid forms of lansoprazole are disclosed.
Owner:TEVA PHARM USA INC
Process For Preparing Crystalline Form A Of Lansoprazole
The present invention relates to a process for preparing Crystalline Form A of Lansoprazole. Specifically, the present invention relates to a process for preparing highly pure Crystalline Form A of Lansoprazole in a large scale without any additional conversion step, even by using ethanol in which Crystalline Form B is easily formed.
Owner:DAEWOONG PHARM CO LTD +1
Lansoprazole-contained freeze-dried powder injection
ActiveCN101874789AOvercome deficienciesFix stability issuesOrganic active ingredientsPowder deliverySolubilityLansoprazole
The invention provides a lansoprazole-contained freeze-dried powder injection and a preparation method thereof, wherein the freeze-dried powder injection comprises 1 part of lansoprazole, 0.05-50 parts of excipient and 0.1-10 parts of polyethylene glycol; the preparation method of the freeze-dried powder injection comprises the following steps of: weighing the lansoprazole, adding into water for injection, adding a pH regulator for regulating the pH value of a solution to be 10-12.5, stirring until the lansoprazole in the solution is completely dissolved, adding the polyethylene glycol and the excipient, adding the water for injection until reaching the whole volume, quantitatively filling into a cillin bottle, and carrying out freeze-drying; and the freeze-dried powder injection can simultaneously solve the problem of stability and solubility puzzling the preparation and has good redissolution property.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Method for refining lansoprazole bulk drug
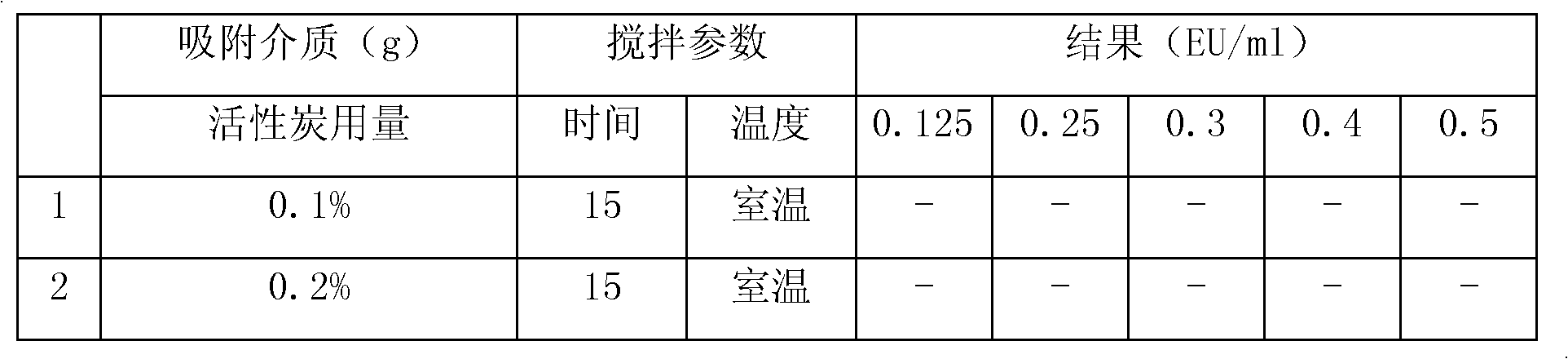
InactiveCN101289443AHigh refining yieldHigh clarityOrganic chemistryDigestive systemActivated carbonLansoprazole
The invention relates to a refining method for a lansoprazole crude drug, which is characterized in that the lansoprazole sample is dissolved in anhydrous alcohol and stirred to complete dissolution, and activated carbon is added to be stirred, pumped and filtered; then the solution which is placed in a freezing chamber to freeze and crystallize is pumped and filtered to be dry, the filter cake is washed to be dry by the anhydrous alcohol, and white solid can be obtained; finally, the obtained white solid is dried in an oven, and the solid is turned over discontinuously during the drying until reaching constant weight. The preparation method for the lansoprazole crude drug is simple and convenient, high in refining yield, low in the total impurities of the refined lansoprazole and good in clarity after being prepared into injections.
Owner:上海慈瑞医药科技股份有限公司
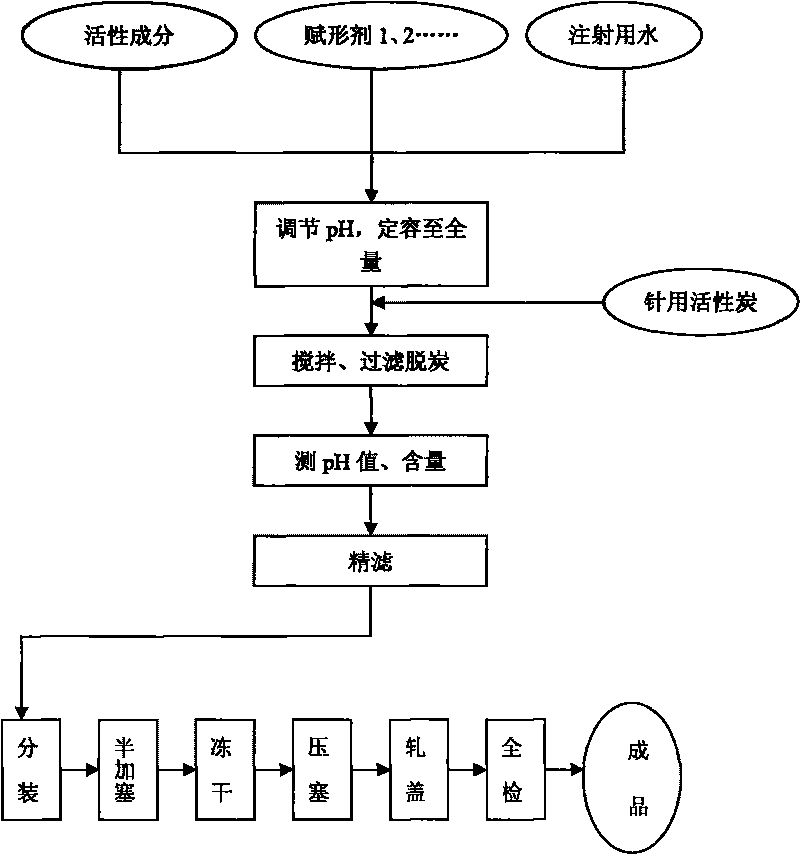
Lansoprazole preparation for injection and preparation method thereof
Lansoprazole is a second generation proton pump inhibitor developed by Takeda in Japan, and is clinically and widely applied for treatment of acid related diseases and helicobacter pylori eradiation. The invention provides a preparation method of a Lansoprazole lyophilized powder injection preparation, creatively screens and tests the composition and dosage as well as the technology of the Lansoprazole lyophilized powder injection, obtains the optimal experiment condition, and gains good effect. The weight ratio of all the components of the Lansoprazole lyophilized powder injection satisfies the relation that Lansoprazole: meglumine: mannitol: sodium hydroxide equals to 30:10:60:4-4.1, the optimal weight ratio of all the components meets the relation that Lansoprazole: meglumine: mannitol: sodium hydroxide equals to 30:10:60:4.
Owner:JUMPCAN PHARMA GRP
Lansoprazole nano-particle frozen preparation for injection and preparation method thereof
ActiveCN102198106APromote absorptionImprove bioavailabilityPowder deliveryOrganic active ingredientsLansoprazoleSulfite salt
The invention discloses a lansoprazole nano-particle frozen preparation for injection capable of simultaneously improving the stability and dissolubility, and a preparation method thereof. The preparation comprises the following components in parts by weight: 20-40 parts of lansoprazole, 5-50 parts of dextran, 5-40 parts of sodium sulfite, 5-60 parts of solubilizer, 10-100 parts of nano-carrier material and 10-100 parts of freeze drying excipient. The preparation method comprises the steps of: adding the dextran, the solubilizer and the sodium sulfite into a liquid preparation tank, adding water for injection and stirring until dissolved, regulating the pH value, adding the lansoprazole and the nano-carrier material, continuing to stir evenly, adding the freeze drying excipient and stirring until dissolved, supplementing the water for injection to the full dose, decoloring, finely filtering, subpackaging and freeze drying to obtain the frozen preparation.
Owner:WUHAN PUSHENG PHARMA
Lansoprazole intestine solution capsule and its preparation method
ActiveCN101156852AHigh dissolution rateSolve easy aggregationOrganic active ingredientsDigestive systemIntestinal structureVegetable oil
The invention discloses a lansoprazole enteric liquid capsule which has a simple manufacturing process, avoids water from being led in during the manufacturing process, and has good storage stability and a manufacturing method. The lansoprazole enteric liquid capsule consists of lansoprazole, dispersant, edible vegetable oil, magnesium oxide or magnesium carbonate, an emulsifier, poloxamer, animal and vegetable glue, and sodium citrate. After being mixed uniformly, raw materials enter into a colloid mill, and then the raw materials are infused into a hollow capsule, and polyacrylic resin ethanol solution is used as the sealing material to sleeve and seal the infused capsule. The invention suspends the lansoprazole into hydrophobic excipient oil, forms a protective film around the lansoprazole powder, does not lead in water during the manufacturing process, avoids the degradation of the lansoprazole, does not need to wrap an isolation layer and an enteric coating, and has short production cycle, simple production process, low cost, and good product quality and stability.
Owner:INCREASEPHARM TIANJIN INST CO LTD
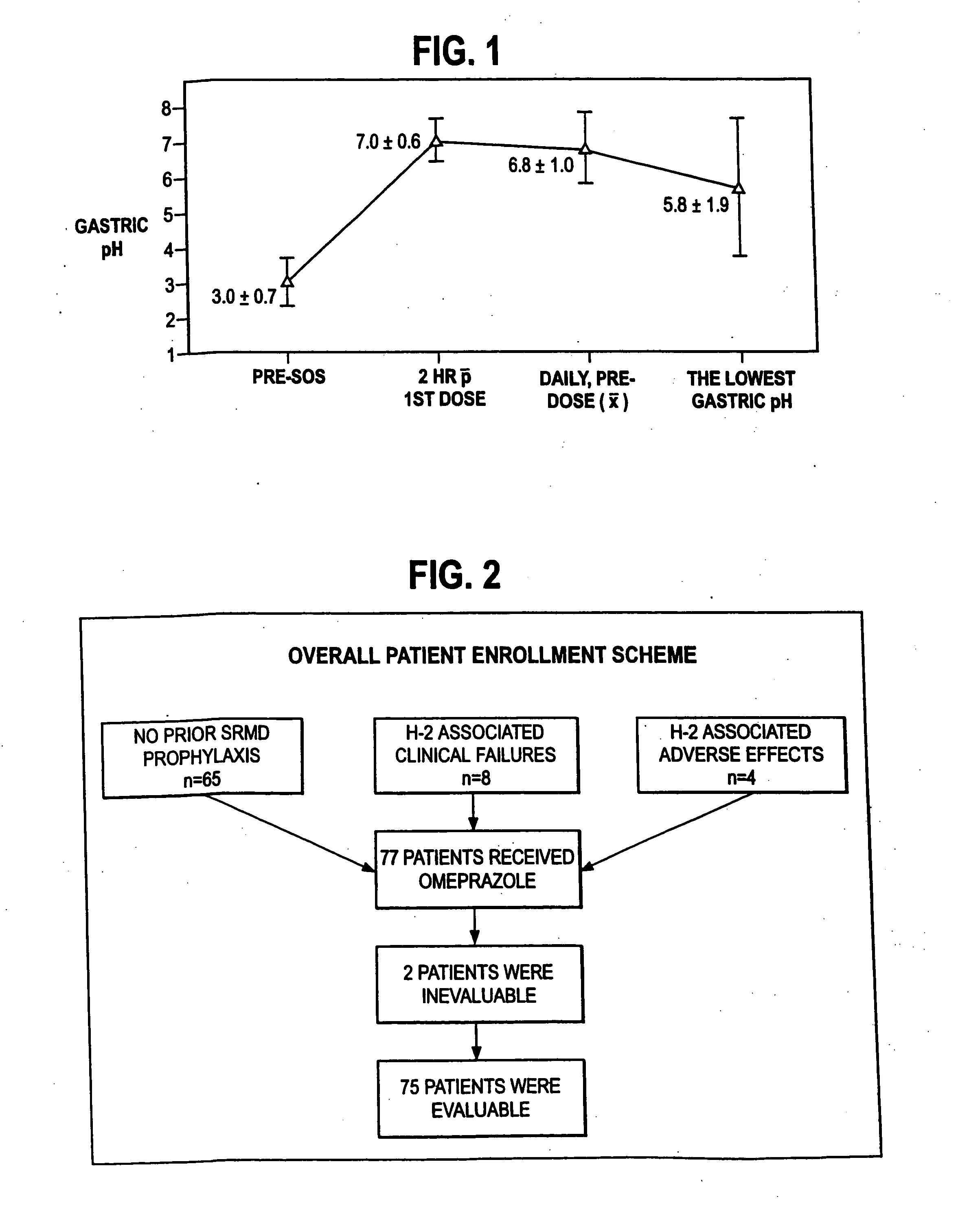
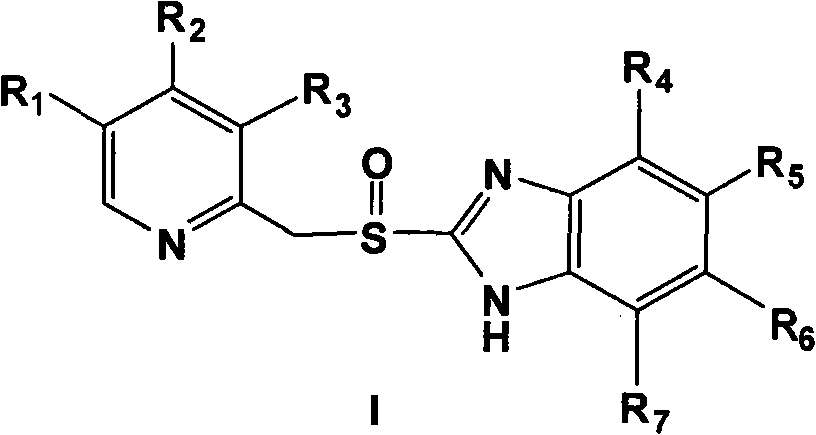
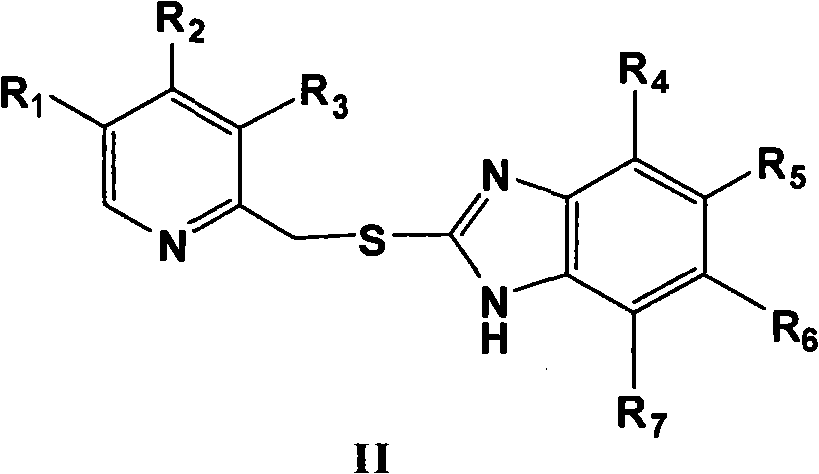
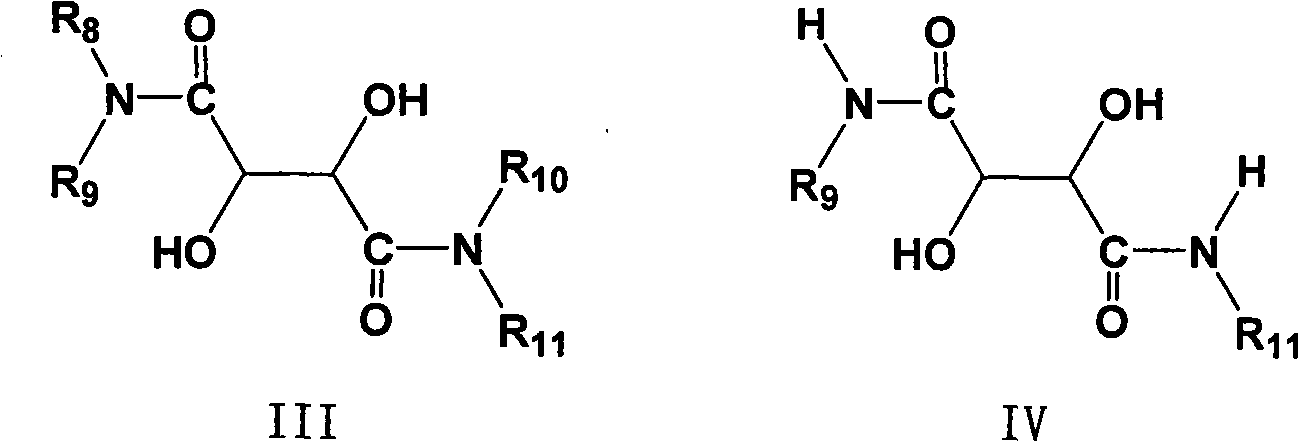
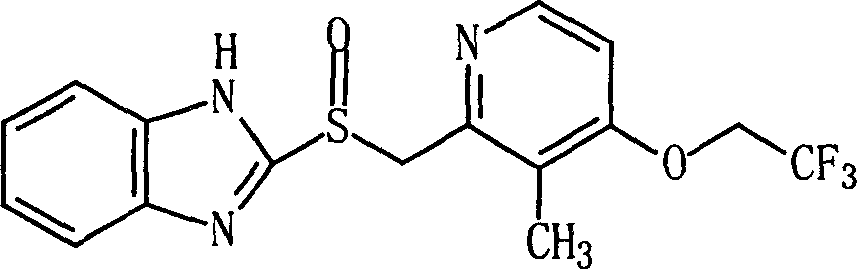
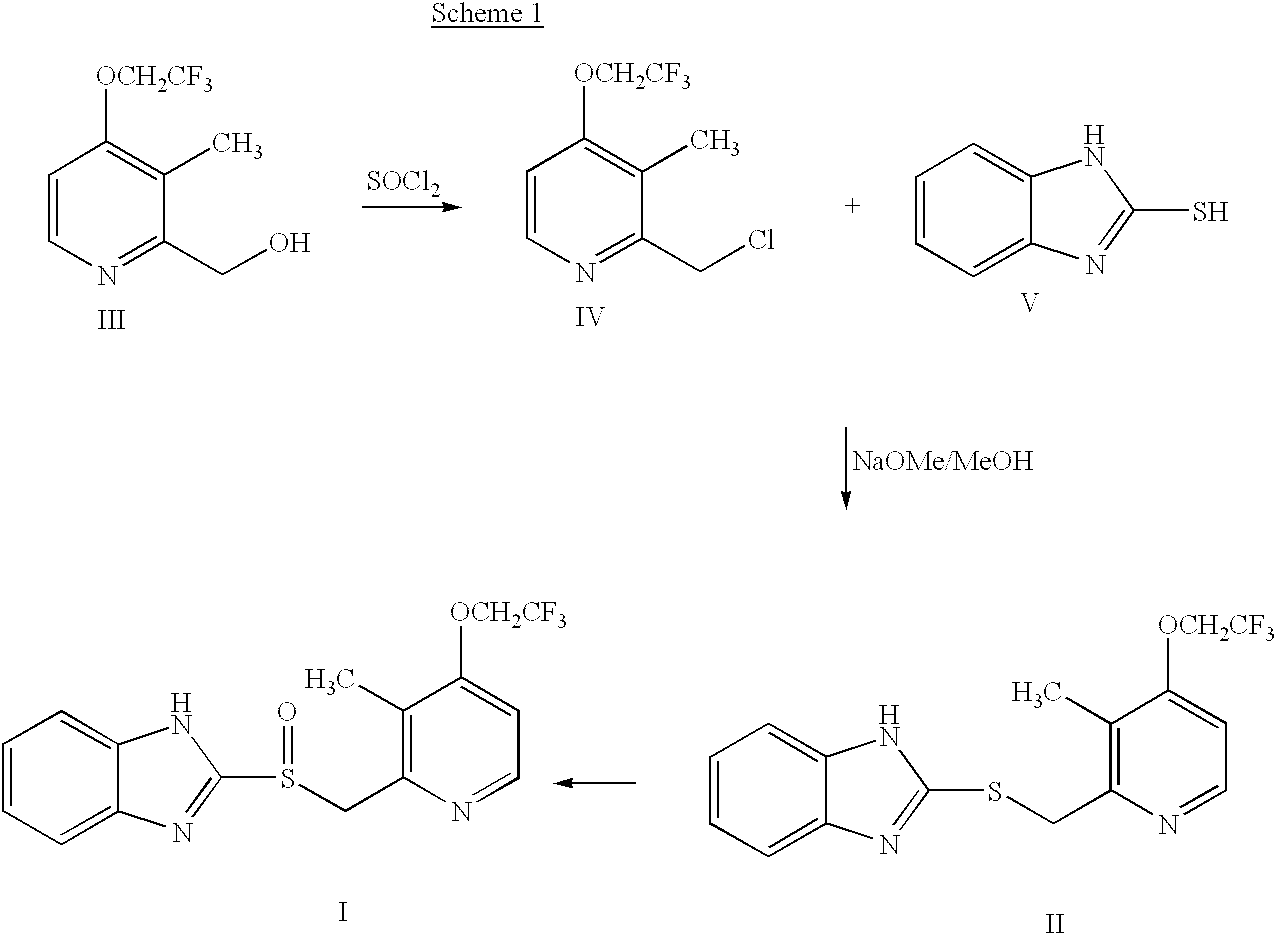
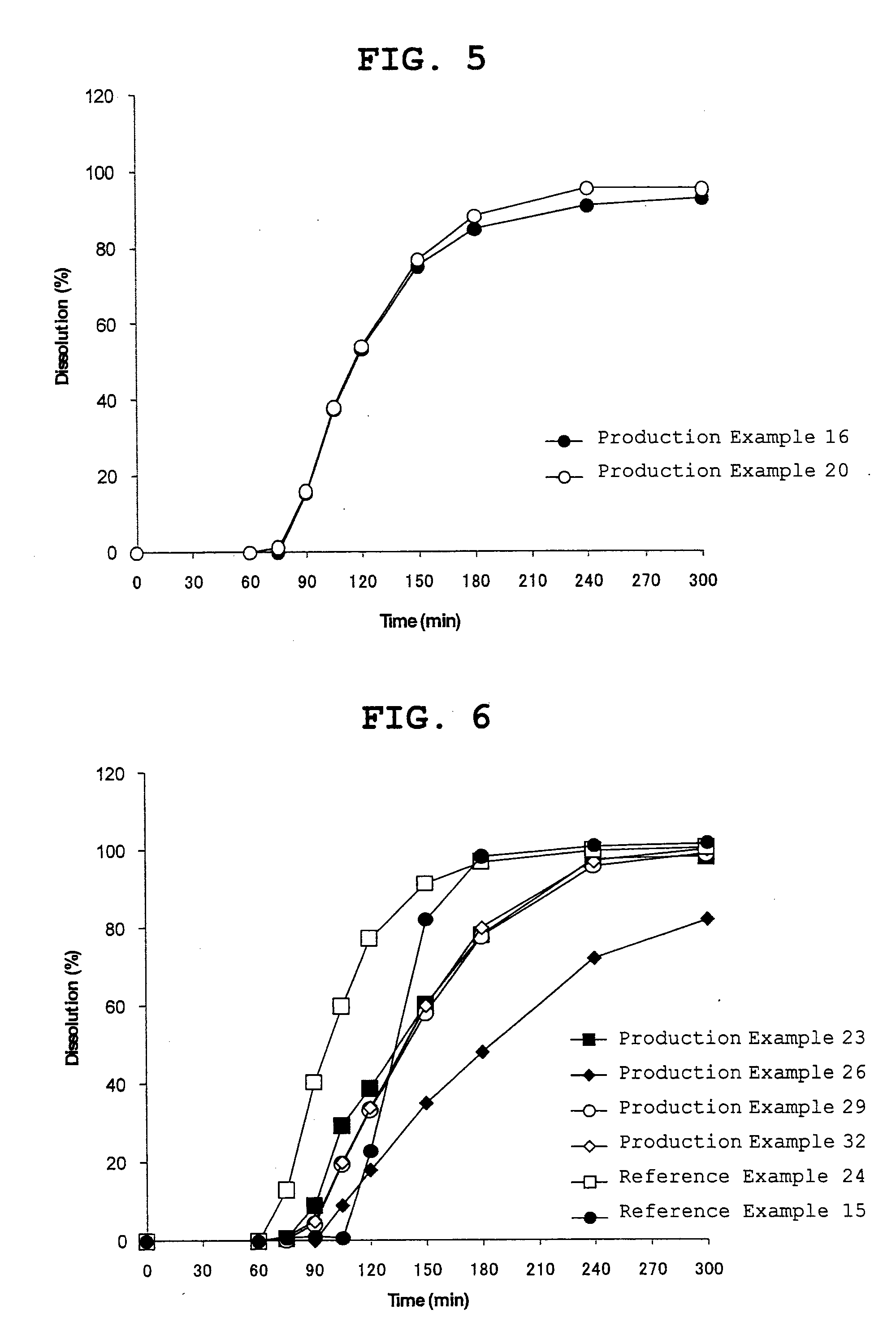
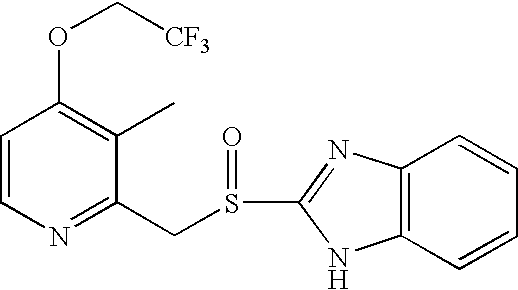
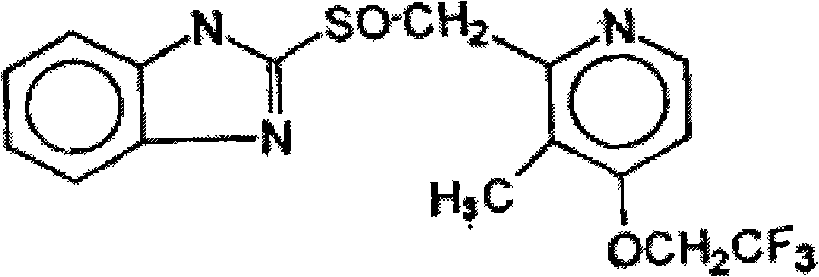
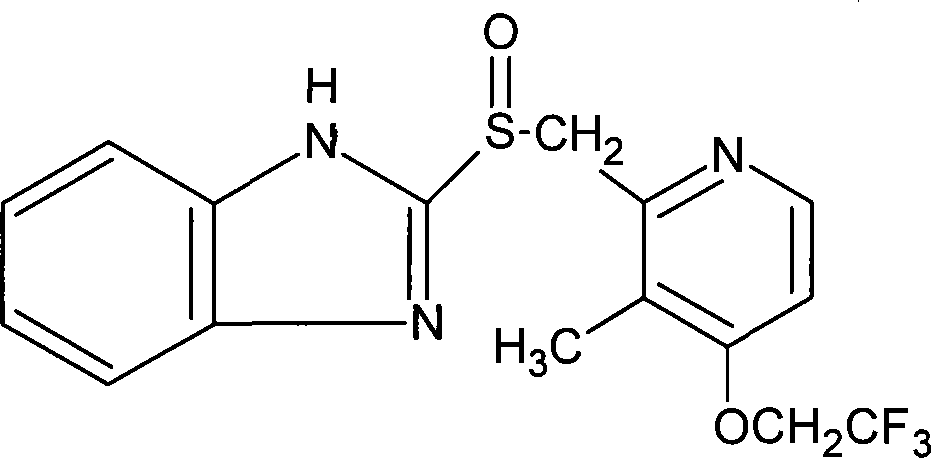
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
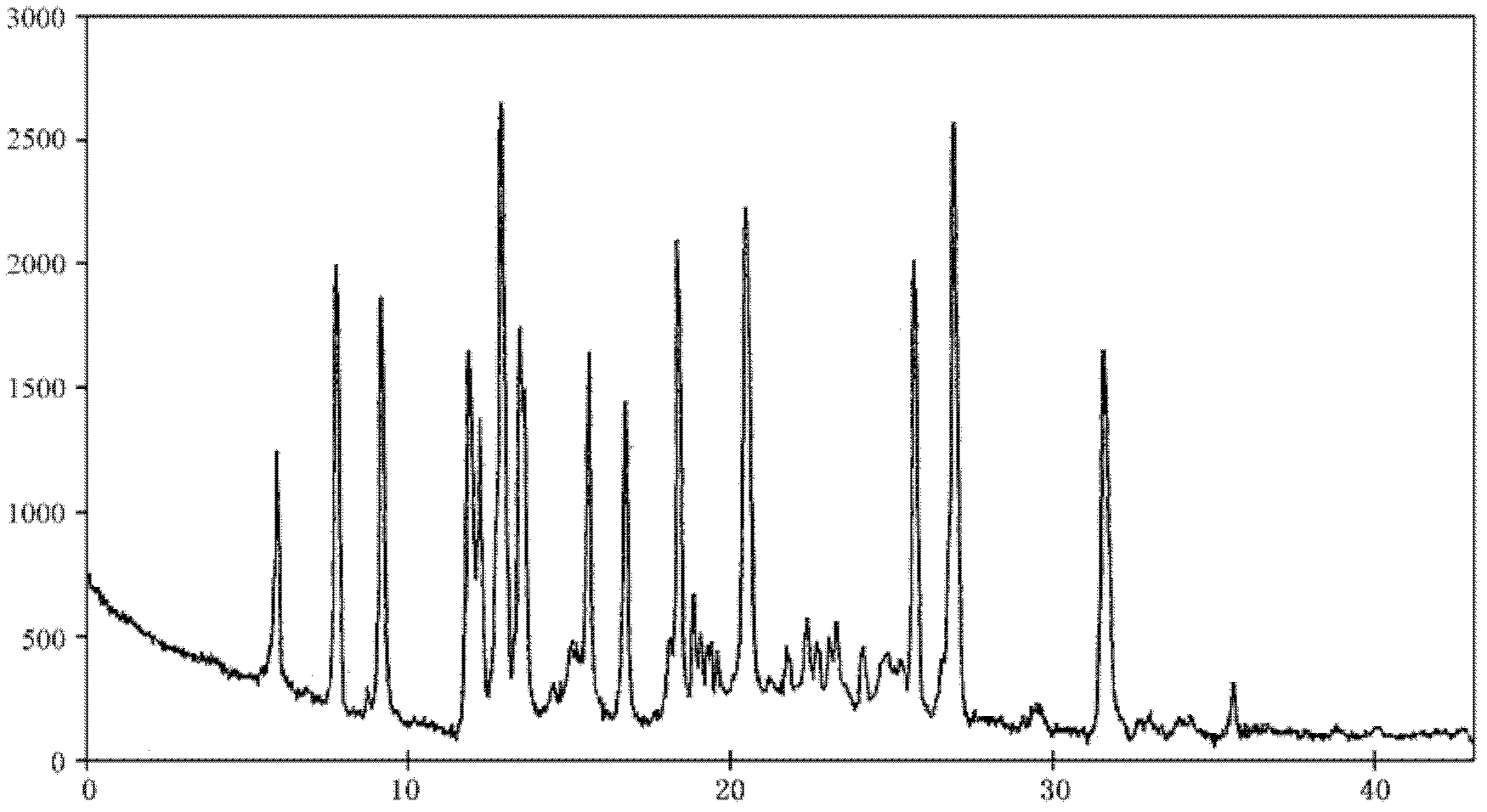
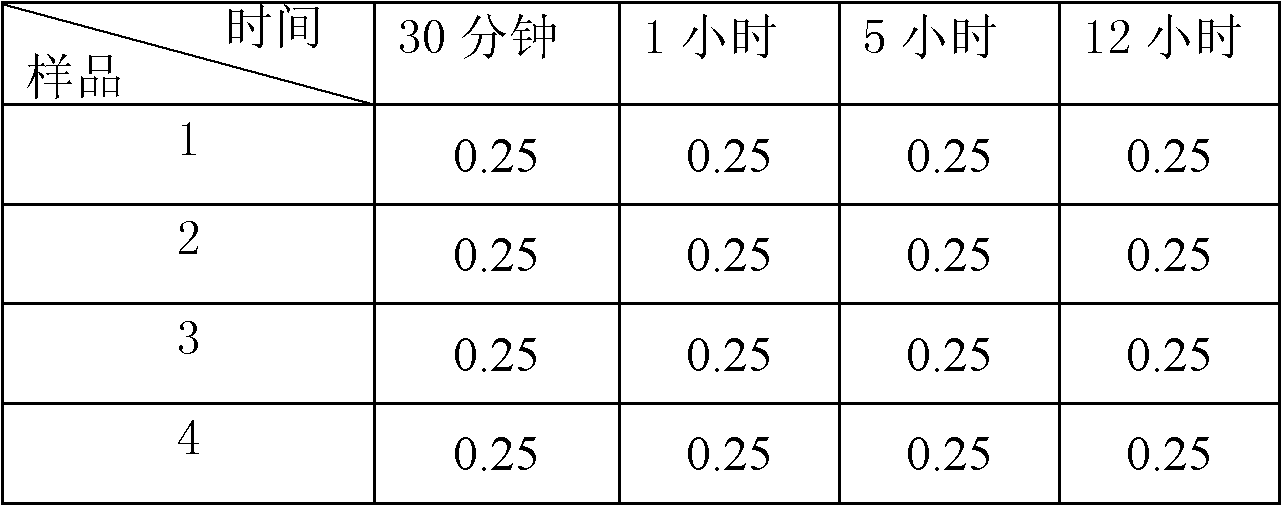
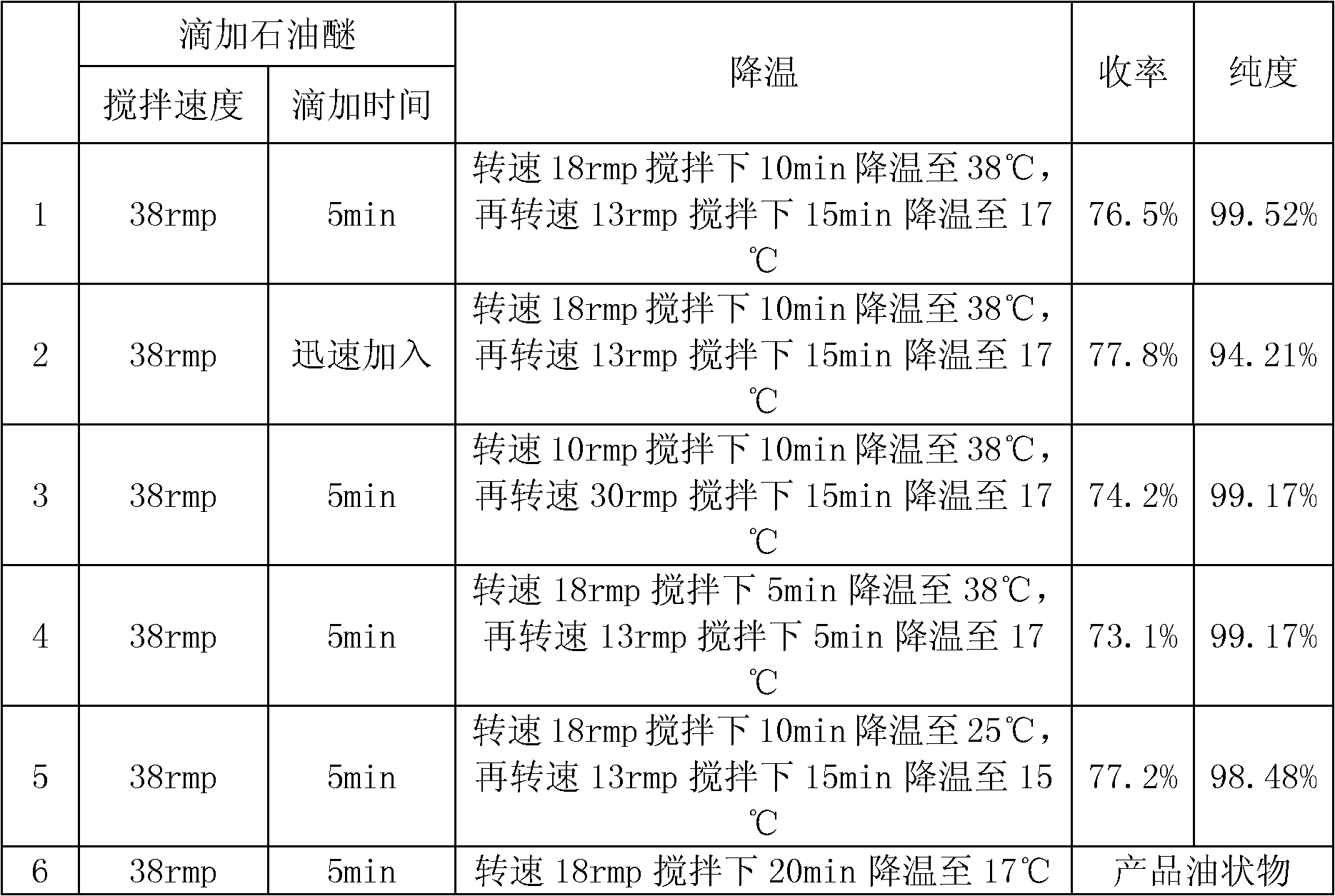
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H- benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)chlorohydroxymethyl] sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:TEVA PHARMA IND LTD
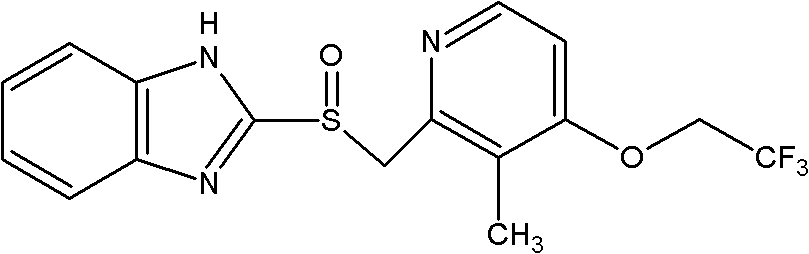
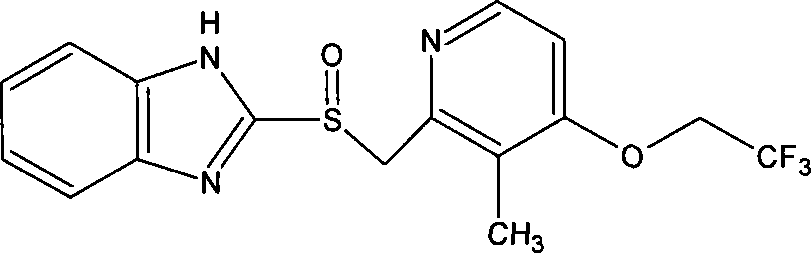
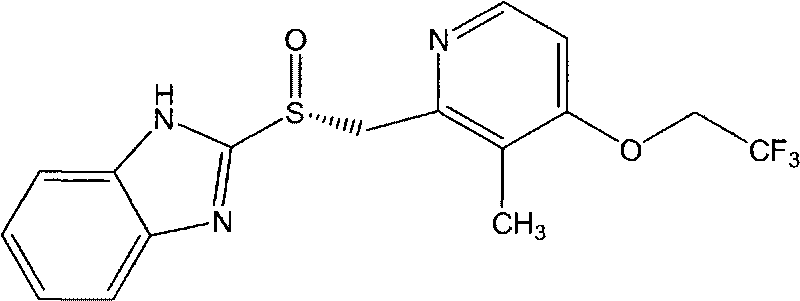
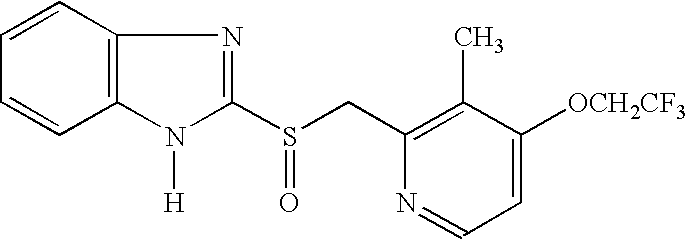
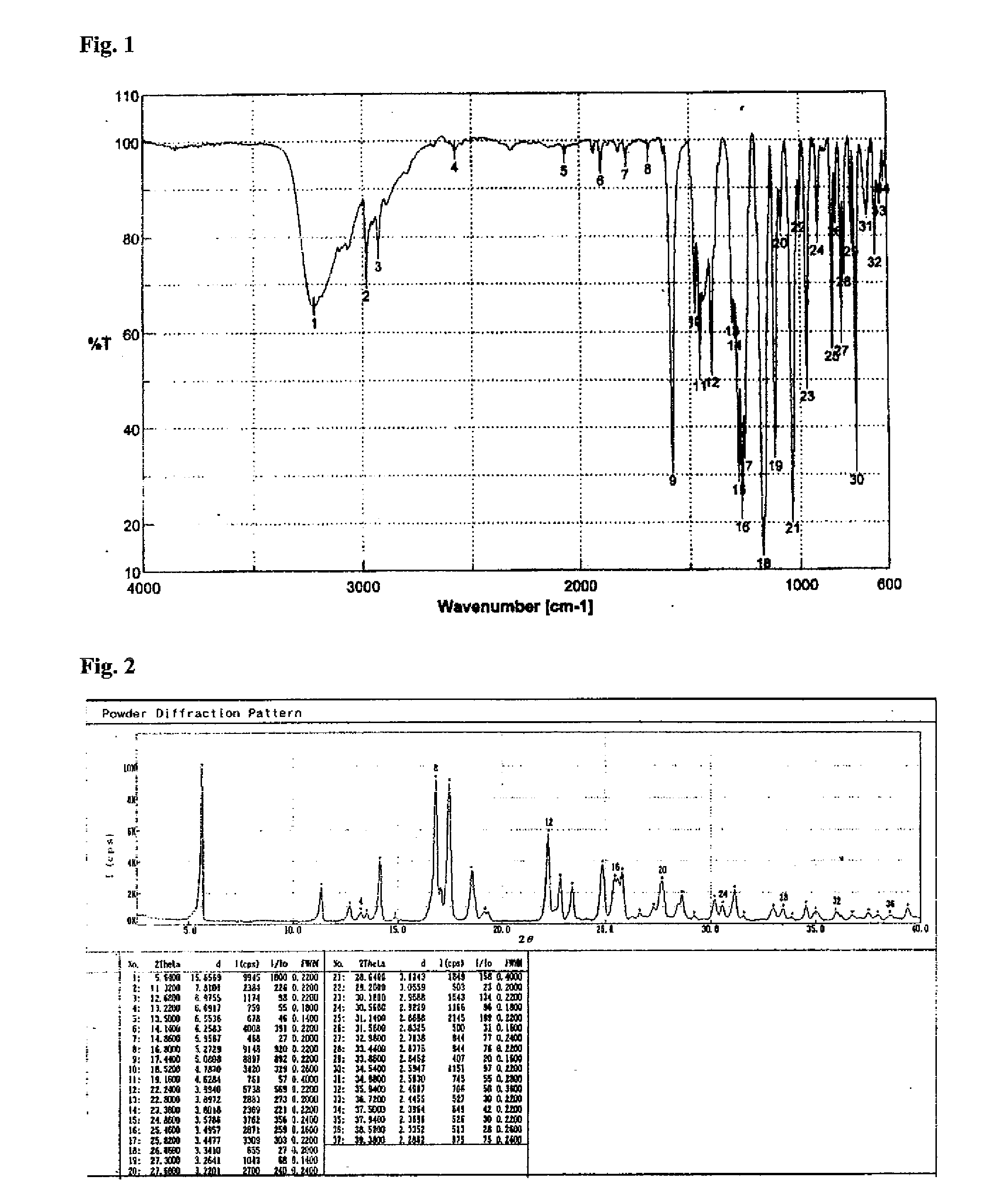
Lansoprazole compound
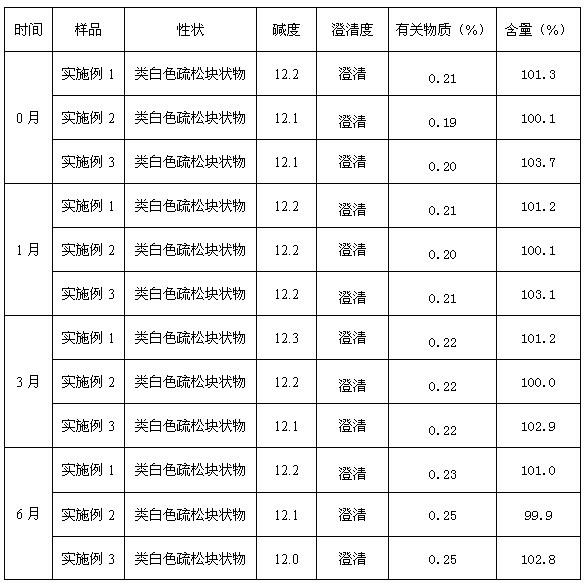
The invention belongs to the technical field of medicines and in particular relates to a lansoprazole compound. The lansoprazole compound has a chemical structural formula and is measured by a powder X-ray diffraction measurement method, and an X-ray diffraction pattern represented by a diffraction angle of 2theta 0.2 degrees is as shown in a figure 1. The lansoprazole compound provided by the invention is a novel lansoprazole crystal form different from that in the prior art, and the crystal form is lower in moisture absorptivity and better in solubility.
Owner:北京科创鼎诚医药科技有限公司
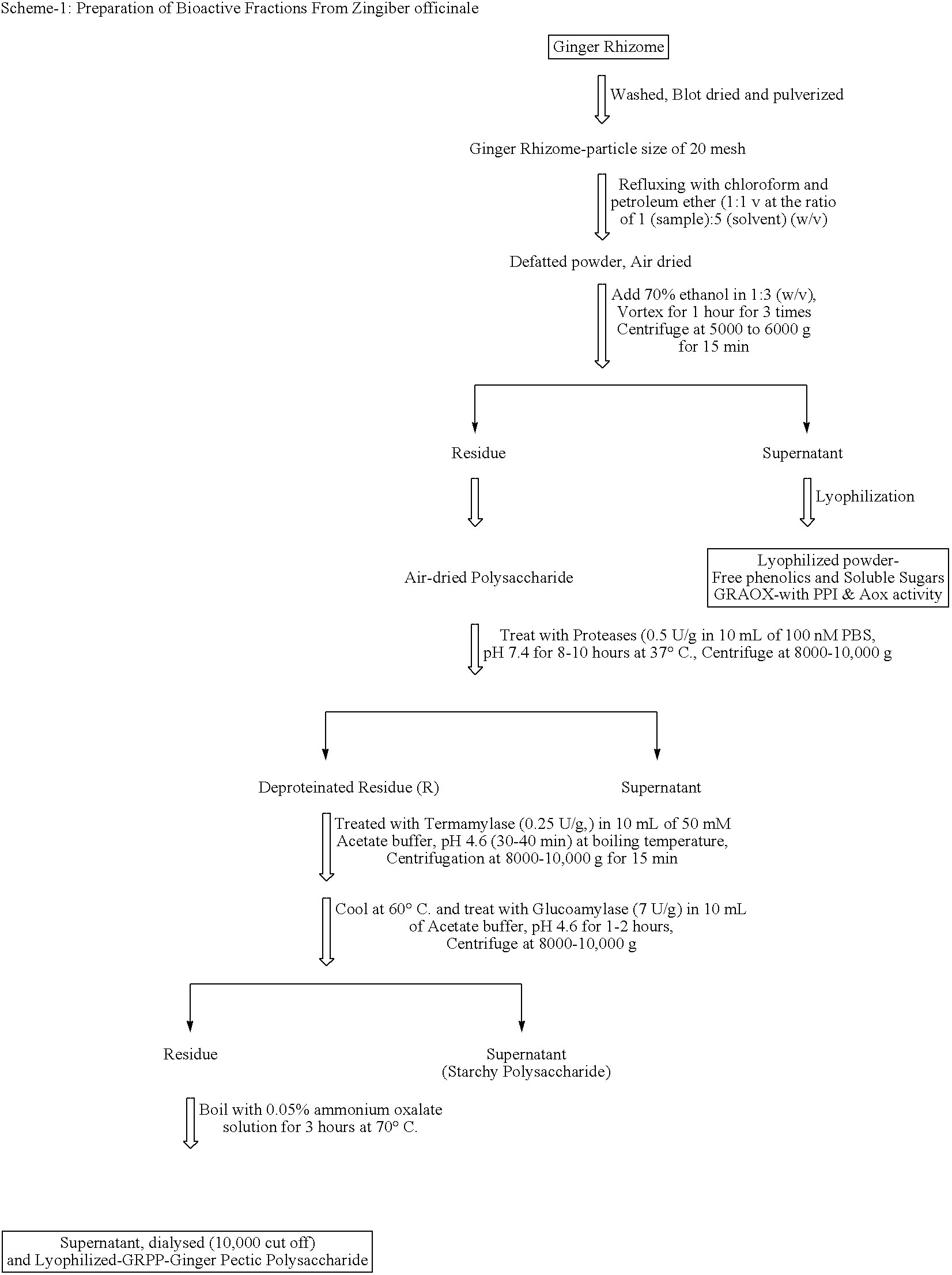
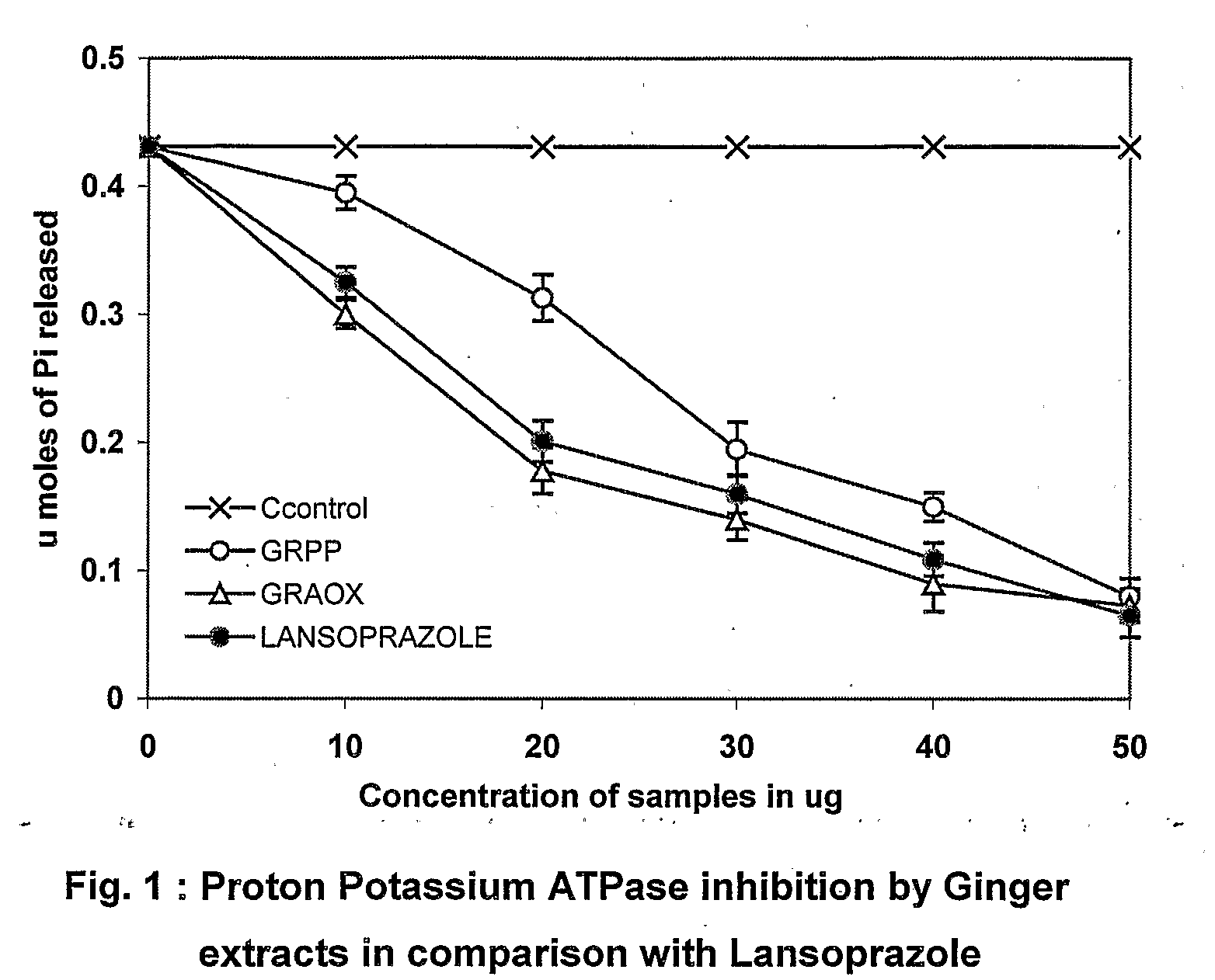
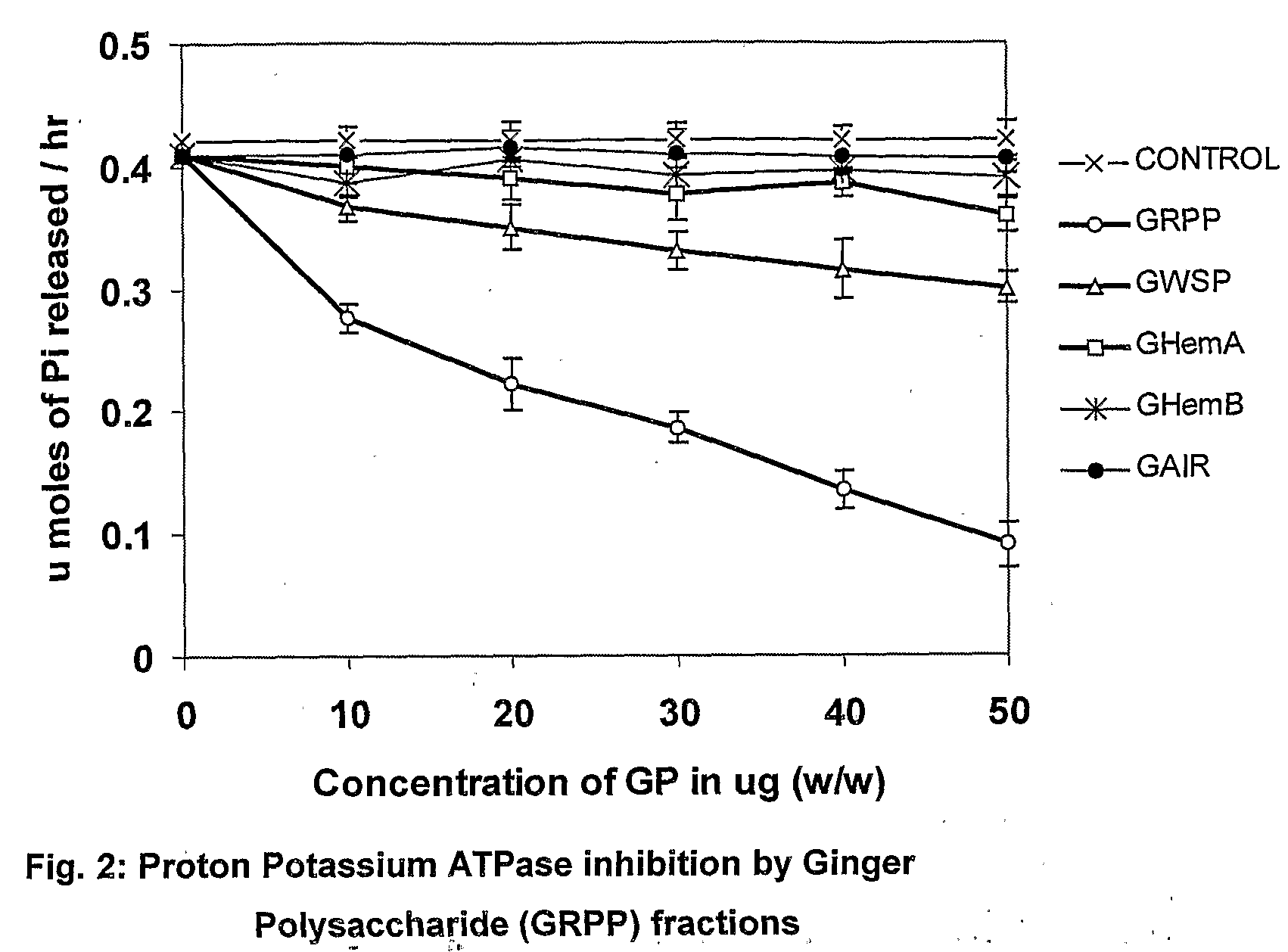
Bioactive fraction from zingier officinale and a process for the preparation thereof
Ulcer is a serious oxidative stress induced disease with complex pathologic events including upregulation of H+K+ ATPase of parietal cell (PC) membrane, damage of mucin layer around PC, PC-DNA damage etc. The polysaccharide (GRPP) fraction and antioxidant extract (GRAOX) of ginger was examined for their ability to inhibit H+ K+ ATPase. Results indicated that the inhibition of H+ K+ ATPase activity which causes acidity in the lumen of the stomach, also exhibited better H+K+ ATPase inhibition (PPI) at the IC50 of 27.2 μg (GRPP) and 16.5 μg GAE (AOX) respectively than the known antiulcer proton pump blocker Lansoprazole (19.3 μg). Further the antioxidant activity in antioxidant extract (GRAOX) by various assay systems was examined such as Reducing power, Free radical scavenging (FRS), DNA and cytoprotection systems etc. GRAOX exhibited concentration dependent reducing power ability at 5-25 g equivalent of phenol. FRS activity with IC50 of 6.8 μg equivalent of phenol etc. At 0.3 μg level GRAOX offered >80% protection to DNA and against FeSO4-Asc'orbate induced oxidation. The major active phenolic components were identified as Gallic acid / Tannic acid (46%), and Cinnamic acid (51%).
Owner:COUNCIL OF SCI & IND RES
Lansoprazole composition freeze-dried powder for injection
ActiveCN101829065ASmall particle sizeHigh porosityPowder deliveryOrganic active ingredientsAcetic acidEthylene diamine
The invention relates to lansoprazole composition freeze-dried powder for injection. The lansoprazole composition freeze-dried powder is characterized by comprising lansoprazole used as a main material, meglumine, mannitol, sodium hydrogensulfite and ethylene diamine tetraacetic acid, wherein the proportion of the components is 3:(0.1-1):(1-20):(0.01-0.5):(0.01-0.5); preferably, the proportion is 3:(0.5-1):(10-20):(0.1-0.3):(0.05-0.3); and more preferably, the proportion is 3:1:20:0.2:0.2. The preparation method comprises the following steps of: dissolving the raw material and the auxiliary materials by adding water; regulating the pH value; adding active carbon for decolorizing; filtering for decarburizing; fine filtering by using a filter membrane; sub-packaging; cooling to -50 to -46 DEG C according to a speed of 1-1.2 DEG C / minute; preserving heat and freezing for 3 hours; vacuumizing to 15Pa; uniformly heating up to -22 to -18 DEG C within 7-9 hours and then preserving heat for 1-2 hours; uniformly heating up to 3-7 DEG C within 4-6 hours and then heating up to 40 DEG C within 4 hours; preserving heat and drying for 3 hours; and packaging and storing after inspection.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Compound capsule and preparation method thereof
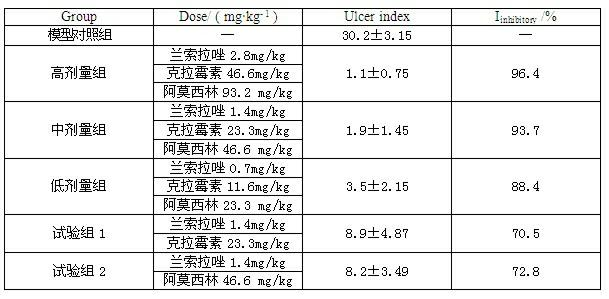
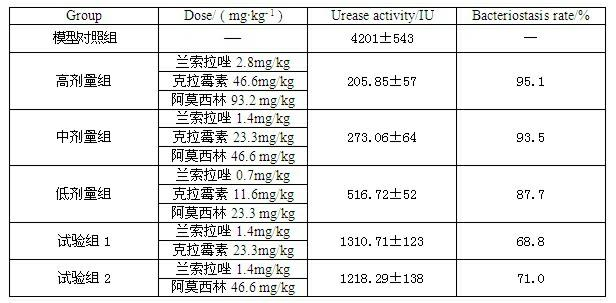
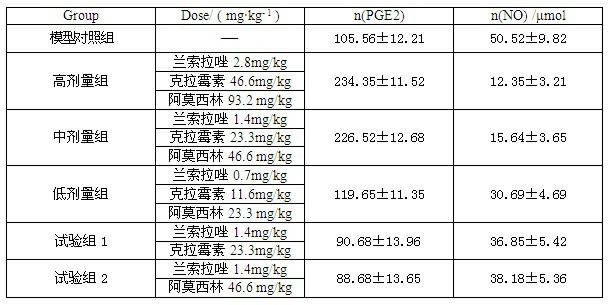
InactiveCN102091084AQuick effectReduce adverse reactionsAntibacterial agentsDigestive systemLansoprazolePeptic ulcer
The invention relates to a compound capsule and a preparation method thereof. The capsule comprises lansoprazole enteric coated pellets, clarithromycin stomach-soluble pellets and amoxicillin stomach-soluble pellets. The compound capsule is administered twice one day based on the following dose each time: lansoprazole 20-40 mg, clarithromycin 400-600 mg, and amoxicillin 900-1100 mg. The capsule provided by the invention has a distinct effect on peptic ulcer, is used for thoroughly killing helicobacter pylori, and has the advantages of quick action, strong effect, improved bioavailability, convenience in administration and low cost.
Owner:王勇
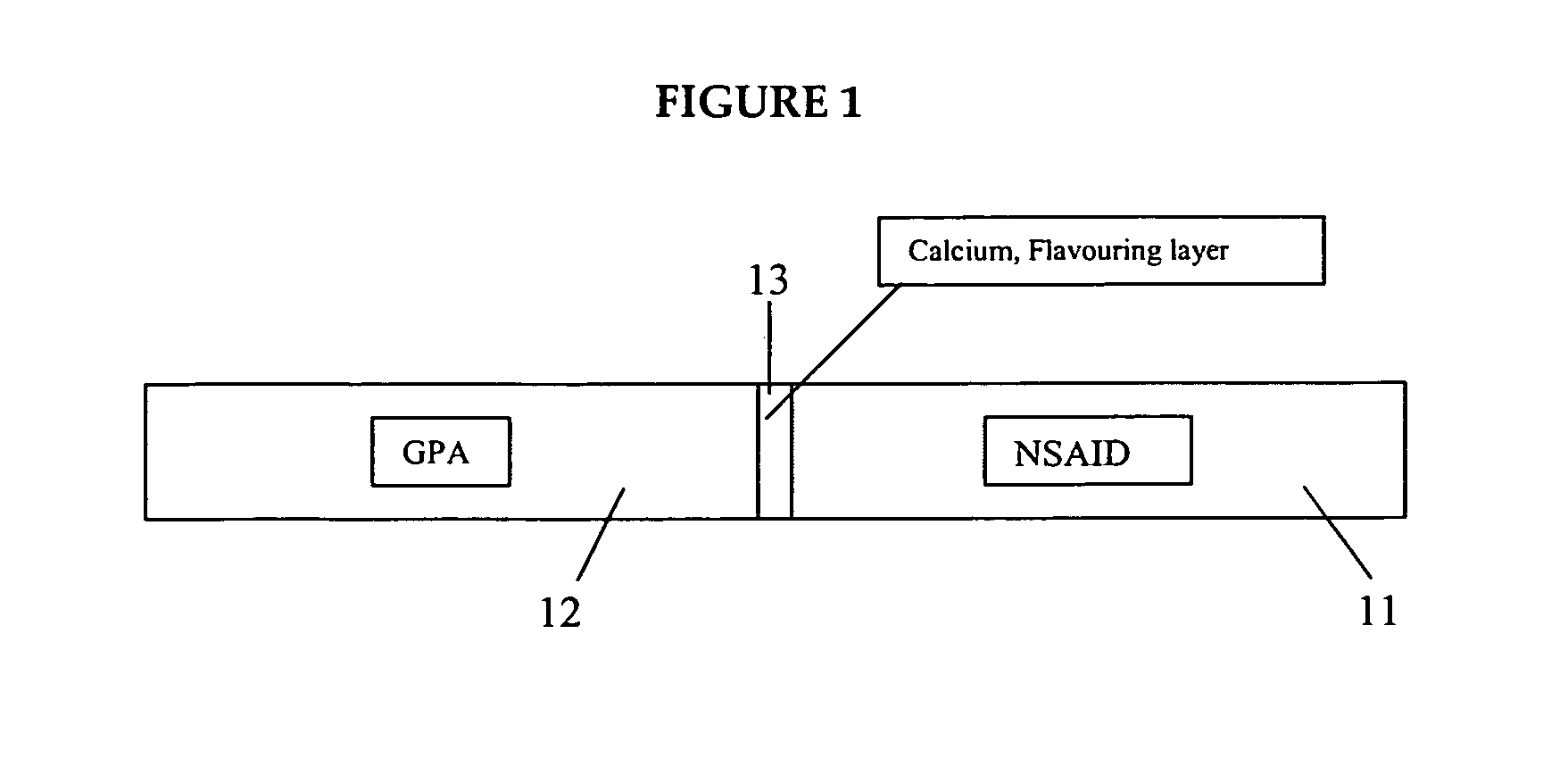
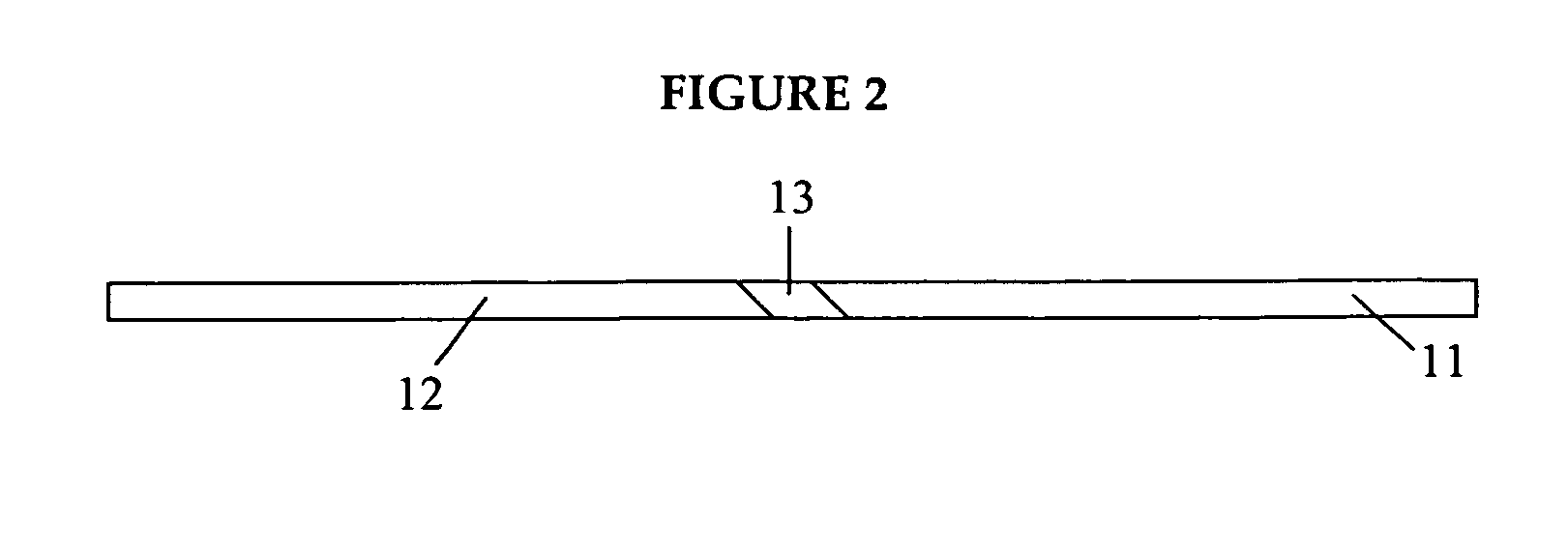
Medicated gumstick for treatment in anti-inflammatory conditions and prophylaxis against NSAID gastropathy
InactiveUS20070003490A1Promote absorptionMinimize contactAntipyreticAnalgesicsDiseaseMedicated chewing-gum
A stick of gum is provided containing therapeutic benefits of non-steroid anti-inflammatory drugs for inflammation in conditions such as arthritis, and also alleviates subsequent side effects of NSAID administration, as well as antacid effects from compounds such as an H2 antagonist (ranitidine, cimetidine, famotidine) and / or a proton pump inhibitor (such as lansoprazole, pantoprazole, omeprazole, esomeprazole or rabeprazole) and / or an acid pump antagonist selected from the group of soraprazan, AZD0865, YH1885 and CS-526.
Owner:MEDICAL FUTURES
Lansoprazole liposome and its preparation
InactiveCN1745750AImprove poor oral absorption and low bioavailabilityImprove the deficiency of low bioavailabilityOrganic active ingredientsDigestive systemReflux esophagitisCholesterol
A liposome of lansoprazole is prepared from lansoprazole, phosphatide, cholesterol and the supporting agent chosen from sorbitol, mannitol, cane sugar, sodium chloride, water-soluble starch, etc. It is used for treating gastric ulcer, duodenal ulcer, reflux esophagitis, etc. Its preparing process is also disclosed.
Owner:胡才忠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900401.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900411.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900421.PNG)