Lansoprazole nano-particle frozen preparation for injection and preparation method thereof
A technology of lansoprazole and freeze-dried preparations, which is applied in the field of lansoprazole nanoparticle freeze-dried preparations for injection and its preparation, can solve the problems of submicron emulsion thermodynamic instability and emulsion droplet enlargement, and improve biological Utilization, increase absorption, effect of ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
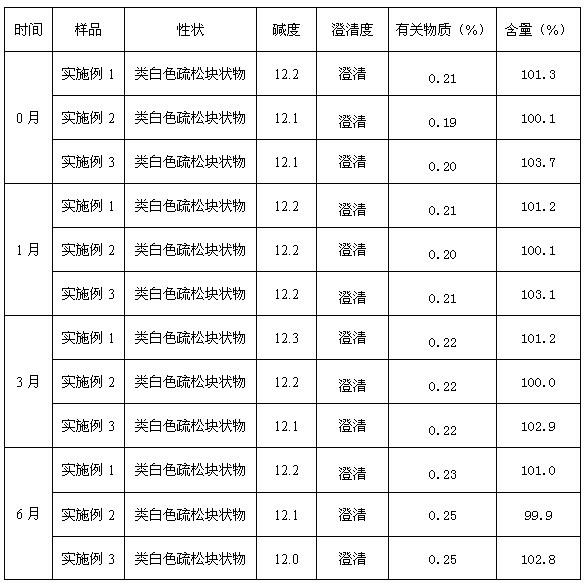
[0027] Formula: Lansoprazole 30g, dextran 10g, sodium sulfite 10g, hydroxypropyl-β-cyclodextrin 30g, polyethylene glycol-polylactic acid block copolymer 60g, mannitol 60g.
[0028] Preparation:
[0029] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0030] (2) Dosing: First weigh the prescribed amount of dextran, solubilizer, and sodium sulfite into the dosing tank, add 80% of the prescribed amount of water for injection and stir to dissolve, then adjust the pH value with 10mol / L sodium hydroxide solution To 12.3; Add the prescribed amount of lansoprazole into the above liquid, stir to dissolve (if necessary, use 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution to adjust the pH value); add the prescribed amount under stirring The polyethylene glycol-polylactic ...
Embodiment 2
[0036] Formula: 30 g of lansoprazole, 5 g of dextran, 5 g of sodium sulfite, 20 g of hydroxypropyl-β-cyclodextrin, 50 g of polyethylene glycol-polylactic acid block copolymer, and 50 g of mannitol.
[0037] Preparation:
[0038] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0039] (2) Dosing: First weigh the prescribed amount of dextran, solubilizer, and sodium sulfite into the dosing tank, add 80% of the prescribed amount of water for injection and stir to dissolve, then adjust the pH value with 10mol / L sodium hydroxide solution to 12; add the prescription amount of lansoprazole into the above liquid, stir to dissolve (if necessary, use 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution to adjust the pH value); add the prescription amount under stirring The pol...
Embodiment 3
[0045] Formula: 30 g of lansoprazole, 20 g of dextran, 20 g of sodium sulfite, 40 g of hydroxypropyl-β-cyclodextrin, 70 g of polyethylene glycol-polylactic acid block copolymer, and 70 g of mannitol.
[0046] Preparation:
[0047] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0048] (2) Dosing: First weigh the prescribed amount of dextran, solubilizer, and sodium sulfite into the dosing tank, add 80% of the prescribed amount of water for injection and stir to dissolve, then adjust the pH value with 10mol / L sodium hydroxide solution to 13; another weighed prescription amount of lansoprazole, added to the above liquid, stirred to dissolve (if necessary, use 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution to adjust the pH value); add the prescription under stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com