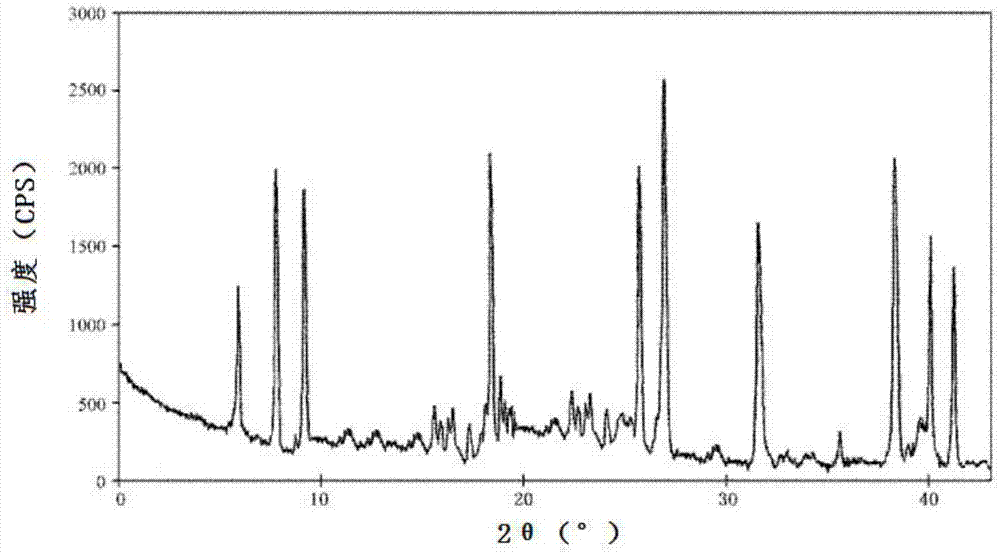
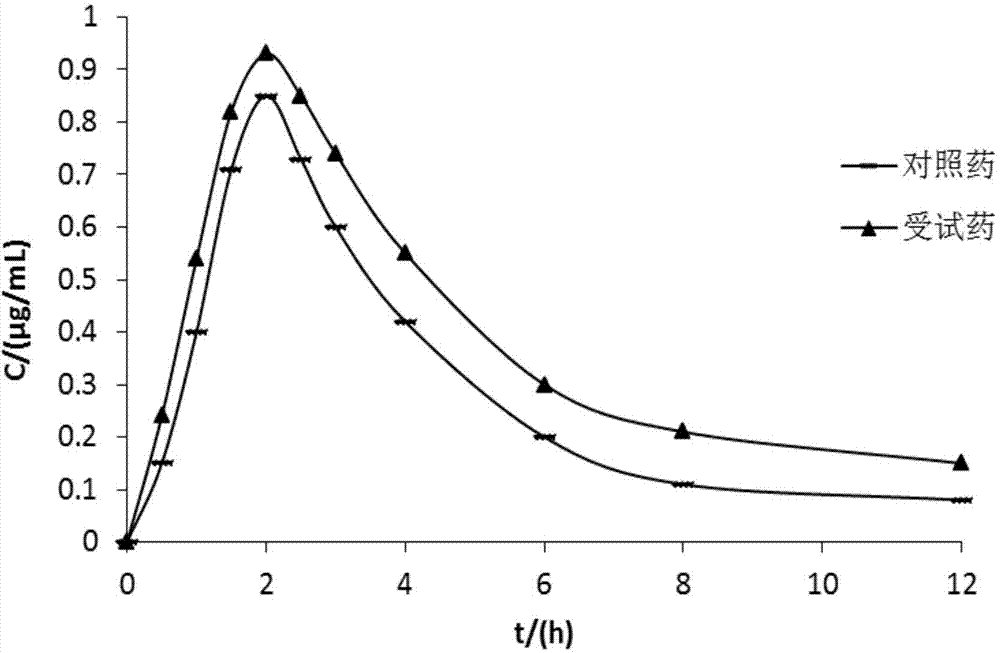
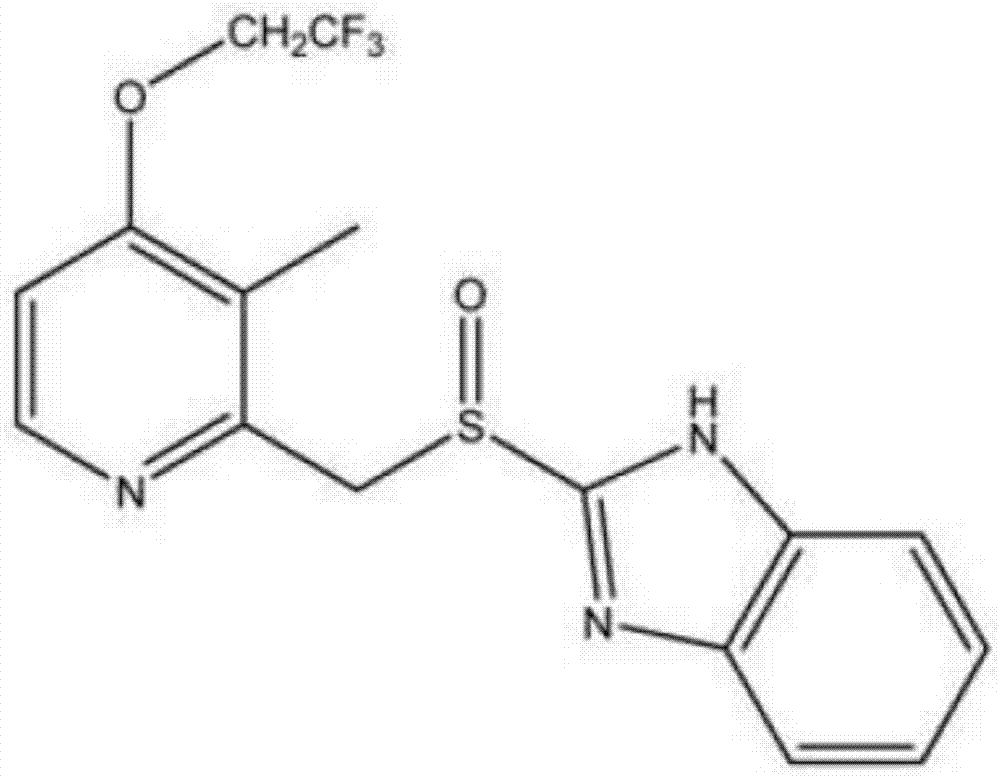
Lansoprazole compound
A technology of lansoprazole and compounds, applied in the field of lansoprazole compounds, can solve the problems of fluid crystal form change, unfavorable operation, time-consuming, etc., and achieve the effects of low hygroscopicity, good water solubility, and quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] [embodiment 1] the preparation of lansoprazole compound
[0037] 1) Take 10kg of crude lansoprazole and dissolve it in 12L of acetone at 50°C to obtain acetone solution of lansoprazole;
[0038] 2) Add 0.01 g of activated carbon to the lansoprazole acetone solution obtained in step 1), stir for 15 minutes, filter with suction, and take the filtrate;
[0039] 3) Cool the filtrate to room temperature naturally, and then add 24L of organic solvent A to the filtrate at a speed of 13L / min under stirring at a speed of 50r / min to form a cloudy solution, wherein the organic solvent A is methanol and A mixed solvent composed of methyl chloride at a volume ratio of 2:1;
[0040] 4) Adjust the stirring speed to 25r / min, then add 36L of organic solvent B to the turbid solution in step 3) at a speed of 12L / min, after the addition is completed, cool down to 0°C, and crystals are precipitated. Solvent B is a mixed solvent composed of ethyl acetate and dimethylformamide at a volume r...
Embodiment 2
[0043] [embodiment 2] the preparation of lansoprazole compound
[0044] 1) Take 10kg of crude lansoprazole and dissolve it in 26L of acetone at 60°C to obtain acetone solution of lansoprazole;
[0045] 2) Add 0.01 g of activated carbon to the lansoprazole acetone solution obtained in step 1), stir for 15 minutes, filter with suction, and take the filtrate;
[0046] 3) Cool the filtrate to room temperature naturally, then add 130L of organic solvent A to the filtrate at a speed of 20L / min under stirring at a speed of 60r / min to form a cloudy solution, wherein the organic solvent A is methanol and A mixed solvent composed of methyl chloride at a volume ratio of 5:1;
[0047] 4) Adjust the stirring speed to 25r / min, then add 78L of organic solvent B to the turbid solution in step 3) at a rate of 6L / min, after the addition is completed, cool down to 5°C, and crystals are precipitated. Solvent B is a mixed solvent composed of ethyl acetate and dimethylformamide at a volume ratio of...
Embodiment 3
[0050] [embodiment 3] the preparation of lansoprazole compound
[0051] 1) Take 10kg of crude lansoprazole and dissolve it in 20L of acetone at 55°C to obtain acetone solution of lansoprazole;
[0052] 2) Add 0.01 g of activated carbon to the lansoprazole acetone solution obtained in step 1), stir for 15 minutes, filter with suction, and take the filtrate;
[0053] 3) Cool the filtrate to room temperature naturally, and then add 60L of organic solvent A to the filtrate at a speed of 18L / min under stirring at a speed of 55r / min to form a cloudy solution, wherein the organic solvent A is methanol and A mixed solvent composed of methyl chloride at a volume ratio of 4:1;
[0054] 4) Adjust the stirring speed to 20r / min, then add 160L of organic solvent B to the turbid solution in step 3) at a speed of 8L / min, after the addition is completed, cool down to 3°C, and crystals are precipitated. Solvent B is a mixed solvent composed of ethyl acetate and dimethylformamide in a volume r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com