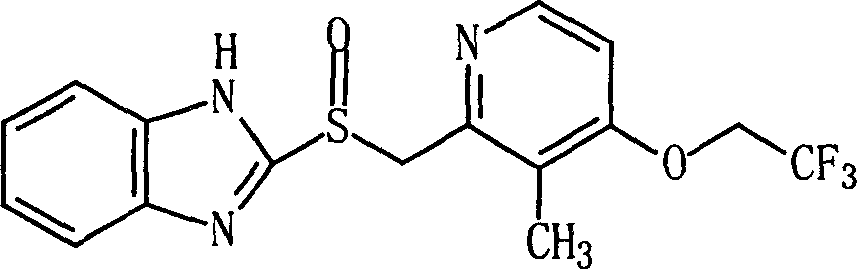
Lansoprazole lyophilized powder injection and its preparing method
A technology for freeze-dried powder injection and lansoprazole, which is applied to the lansoprazole freeze-dried powder injection containing ethylenediaminetetraacetic acid and/or its salts and the field of preparation thereof, and can solve the problem that lansoprazole is unstable, Low bioavailability, easy to be destroyed and other problems, to achieve the effect of easy quality control, simple production process, fast cure speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: 30mg / bottle specification, 1000 lansoprazole freeze-dried powder injection formulations:
[0031] Lansoprazole 30g
[0032] Disodium EDTA 5g
[0033] Mannitol 50g
[0035] Add water for injection to 2000ml, make 1000 tubes in total
[0036] Its preparation process is as follows:
[0037] a. Ingredients according to the formulation prescription, add 1600ml of water for injection, stir, adjust to pH 10.0-11.5 with pH regulator sodium hydroxide, stir to dissolve.
[0038] b. Filter and sterilize the prepared solution with a pH of 10.0 to 11.5 under sterile conditions with a 0.22 μm microporous membrane, store it in a liquid storage tank, measure the content of intermediates, and add 400 ml of water for injection to the prescribed amount.
[0039] c. Divide the medicinal liquid into 10ml vials, each bottle is filled with 2ml, half-tighten the stopper, put it into a p...
Embodiment 2
[0040] Embodiment 2: 10mg / bottle specification, 1000 lansoprazole freeze-dried powder injection formulations:
[0041] Lansoprazole 10g
[0042] Disodium EDTA 0.5g
[0043] Mannitol 100g
[0045] Add water for injection to 2000ml, make 1000 tubes in total
[0046] Its preparation process is as follows:
[0047] a. Ingredients according to the formulation prescription, add 1600ml of water for injection, stir, adjust to pH 10.0-11.5 with pH regulator sodium hydroxide, stir to dissolve.
[0048] b. Filter and sterilize the prepared solution with a pH of 10.0 to 11.5 under sterile conditions with a 0.22 μm microporous membrane, store it in a liquid storage tank, measure the content of intermediates, and add 400 ml of water for injection to the prescribed amount.
[0049]c. Divide the medicinal liquid into 10ml vials, each bottle is filled with 2ml, half-pressed, put on a plate, put into ...
Embodiment 3
[0050] Embodiment 3: 60mg / bottle specification, 1000 lansoprazole freeze-dried powder injection formulations:
[0051] Lansoprazole 60g
[0052] Disodium EDTA 30g
[0053] Mannitol 120g
[0054] Sodium hydroxide 120g
[0055] Add water for injection to 2000ml, make 1000 tubes in total
[0056] Its preparation process is as follows:
[0057] a. Ingredients according to the formulation prescription, add 1600ml of water for injection, stir, adjust to pH 10.0-11.5 with pH regulator sodium hydroxide, stir to dissolve.
[0058] b. Filter and sterilize the prepared solution with a pH of 10.0 to 11.5 under sterile conditions with a 0.22 μm microporous membrane, store it in a liquid storage tank, measure the content of intermediates, and add 400 ml of water for injection to the prescribed amount.
[0059] c. Divide the medicinal liquid into 10ml vials, each bottle is filled with 2ml, half-pressed, put on a plate, put into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com