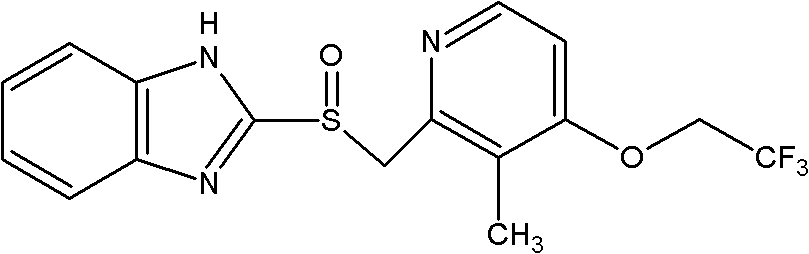
Lansoprazole composition freeze-dried powder for injection
A technology of lansoprazole and its composition, which is applied in the direction of freeze-drying delivery, drug combination, powder delivery, etc., can solve the problems of insufficient excipient formation and unfull appearance, reduce reconstitution time and increase sublimation efficiency , Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
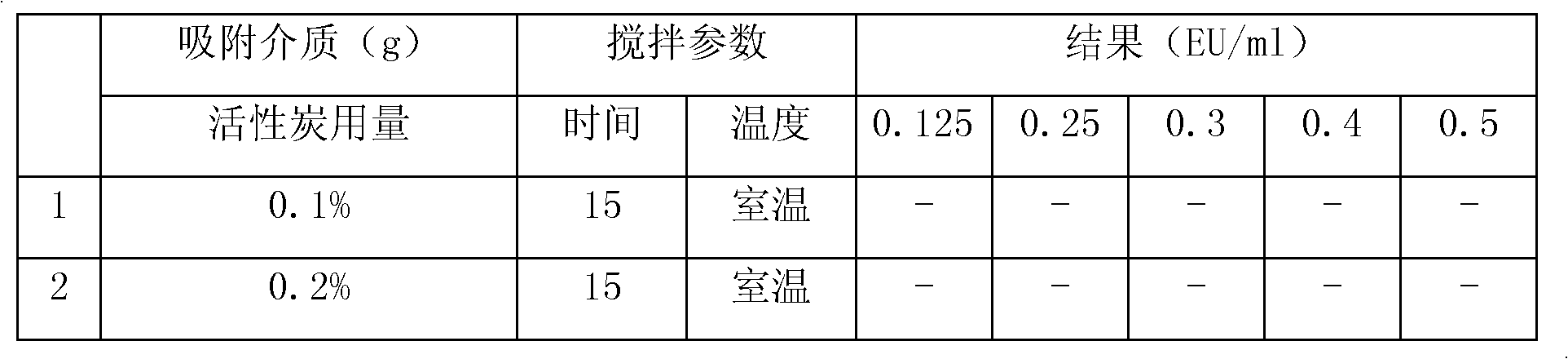
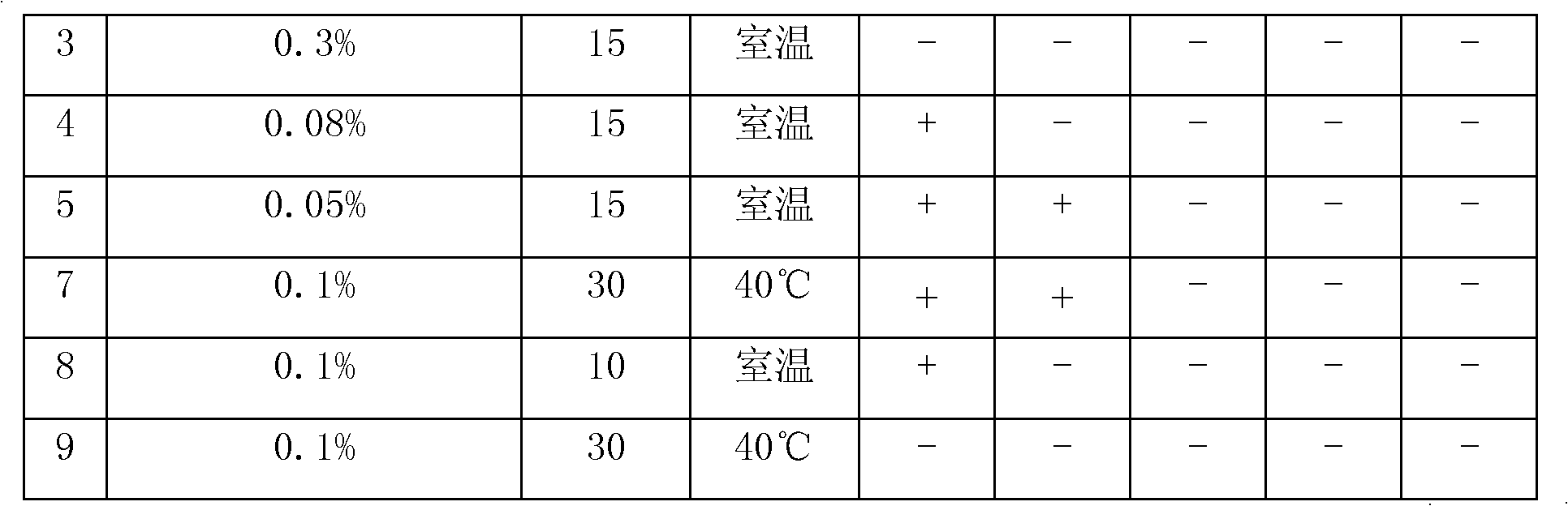
[0047] Add 30g of lansoprazole, 10g of meglumine, 200g of mannitol, 2g of sodium bisulfite and 2g of disodium edetate into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust with sodium hydroxide pH10~12, add water for injection to 3000ml, add medical activated carbon to the prepared liquid medicine, wherein the weight of medical activated carbon is 0.1% of the solution volume, stir at room temperature for 15 minutes, filter and decarbonize, and then sterilize the filtrate with 0.22μm Fine filter with microporous membrane, measure the pH value and content of the filtrate, add water for injection until the volume of the solution is 630 times that of lansoprazole, pack into 1000 bottles, stopper half, cool the freeze-drying box to 3 ° C, put The packaged medicinal solution is placed in the freeze-drying box for 30 minutes, then the freeze-drying box is cooled to -40°C at a rate of 40°C / min, then heated to -13°C, kept for 0.7 hour...
Embodiment 2
[0049] Add 30g of lansoprazole, 10g of meglumine, 180g of mannitol, 2g of sodium bisulfite and 2g of disodium edetate into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust with sodium hydroxide pH10~11, add water for injection to 3000ml, add medical activated carbon to the prepared liquid medicine, wherein the weight of medical activated carbon is 0.1% of the solution volume, stir at room temperature for 15 minutes, filter and decarbonize, and then sterilize the filtrate with 0.22μm Fine filter with microporous membrane, measure the pH value and content of the filtrate, add water for injection until the volume of the solution is 630 times that of lansoprazole, pack into 1000 bottles, stopper half, cool the freeze-drying box to 3 ° C, put The packaged medicinal solution is placed in the freeze-drying box for 30 minutes, then the freeze-drying box is cooled to -40°C at a rate of 40°C / min, then heated to -13°C, kept for 0.7 hour...
Embodiment 3
[0051] Add 30g of lansoprazole, 10g of meglumine, 220g of mannitol, 1g of sodium bisulfite and 2g of disodium edetate into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust with sodium hydroxide pH11~12, add water for injection to 3000ml, add medical activated carbon to the prepared drug solution, wherein the weight of medical activated carbon is 0.1% of the solution volume, stir at room temperature for 15 minutes, filter and decarbonize, and then sterilize the filtrate with 0.22μm Fine filter with microporous membrane, measure the pH value and content of the filtrate, add water for injection until the volume of the solution is 630 times that of lansoprazole, pack into 1000 bottles, stopper half, cool the freeze-drying box to 3 ° C, put The packaged medicinal solution is placed in the freeze-drying box for 30 minutes, then the freeze-drying box is cooled to -40°C at a rate of 40°C / min, then heated to -13°C, kept for 0.7 hours,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com