Patents
Literature
47results about How to "Short reconstitution time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Panax notoginseng saponins freeze-dried powder injection and preparation method thereof
ActiveCN102512466AGood resolubilityReduce moisture contentPowder deliveryPharmaceutical product form changePANAX NOTOGINSENG ROOTFreeze-drying
The invention relates to panax notoginseng saponins freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is prepared by panax notoginseng saponins and water for injection. The panax notoginseng saponins freeze-dried powder injection is free of auxiliary materials or stent agents, good in re-dissolubility, low in water content and good in appearance forming.
Owner:GUANGXI WUZHOU PHARMA GRP
Method for preparing high-purity human coagulation factor VIII
ActiveCN105348382AAvoid damageNo precipitationFactor VIIPeptide preparation methodsUltrafiltrationBlood plasma
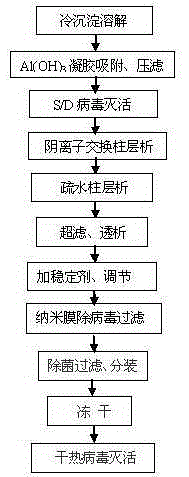
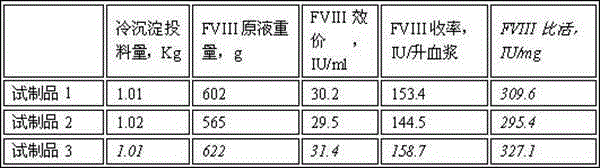
The invention discloses a method for preparing a high-purity human coagulation factor VIII from a cryoprecipitate of a human plasma fraction. The method comprises the following steps: (1) cryoprecipitate dissolution; (2) aluminum hydroxide gel adsorption and filter pressing; (3) S / D virus inactivation; (4) anion exchange resin column chromatography; (5) hydrophobic column chromatography; (6) ultrafiltration dialysis and concentration; (7) addition of one or more stabilizers and titer adjustment; (8) nano-membrane virus-removing filtration; (9) sterile filtration and sub-packaging; (10) freeze-drying; (11) dry-heat virus inactivation. The method has the advantages that a human coagulation factor VIII is purified through the two column chromatography steps, so that the prepared high-purity product can reach a specific activity of about 300 IU / mg, considerably higher than about 50 IU / mg in the prior art, and the product appearance and the heat stability are obviously improved; in the preparation process, three virus removing modes are adopted, so that the clinical use safety of the product can be greatly improved.
Owner:上海洲跃生物科技有限公司
Sotalol hydrochloride for injection
InactiveCN104352453AOvercome oralOvercome absorbencyPowder deliveryPharmaceutical non-active ingredientsChitosan nanoparticlesMedicine
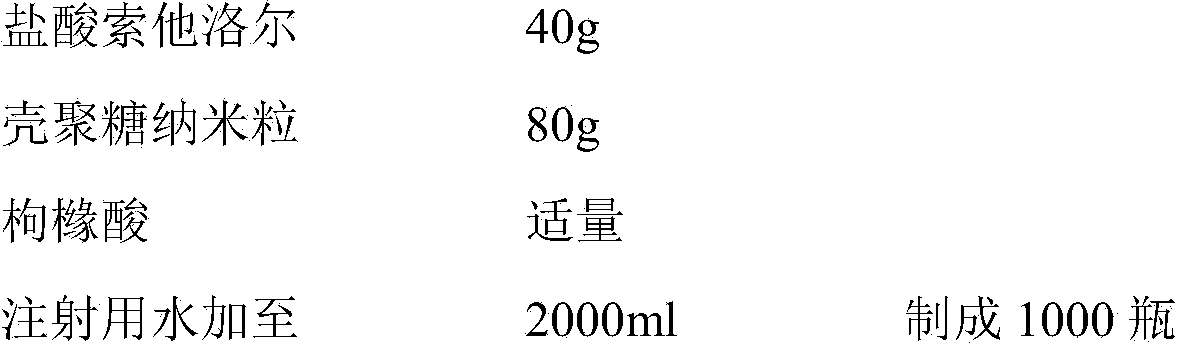
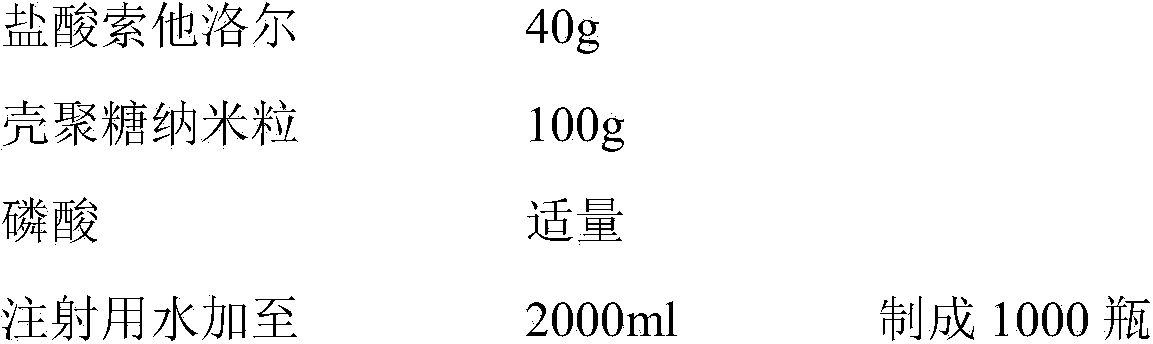
The present invention belongs to the field of chemical drug preparations and particularly relates to sotalol hydrochloride powder injection for injection and a preparation method thereof. The sotalol hydrochloride powder injection comprises sotalol hydrochloride, chitosan nanoparticles and a PH (power of hydrogen) adjusting agent, wherein the chitosan nanoparticles adopted can significantly shorten the redissolving time of the sotalol hydrochloride, and improve the dissolution rate of the sotalol hydrochloride in injection. The sotalol hydrochloride provided by the invention has the advantages of good redissolving effect, more stable quality, high safety, high reliability, good convenience in transportation, and good application to industrial production, and the like.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of carbazochrome sodium sulfonate freeze-dried powder injection
ActiveCN105343019AControl contentShort reconstitution timePowder deliveryOrganic active ingredientsCarbazochrome Sodium SulfonateFreeze-drying
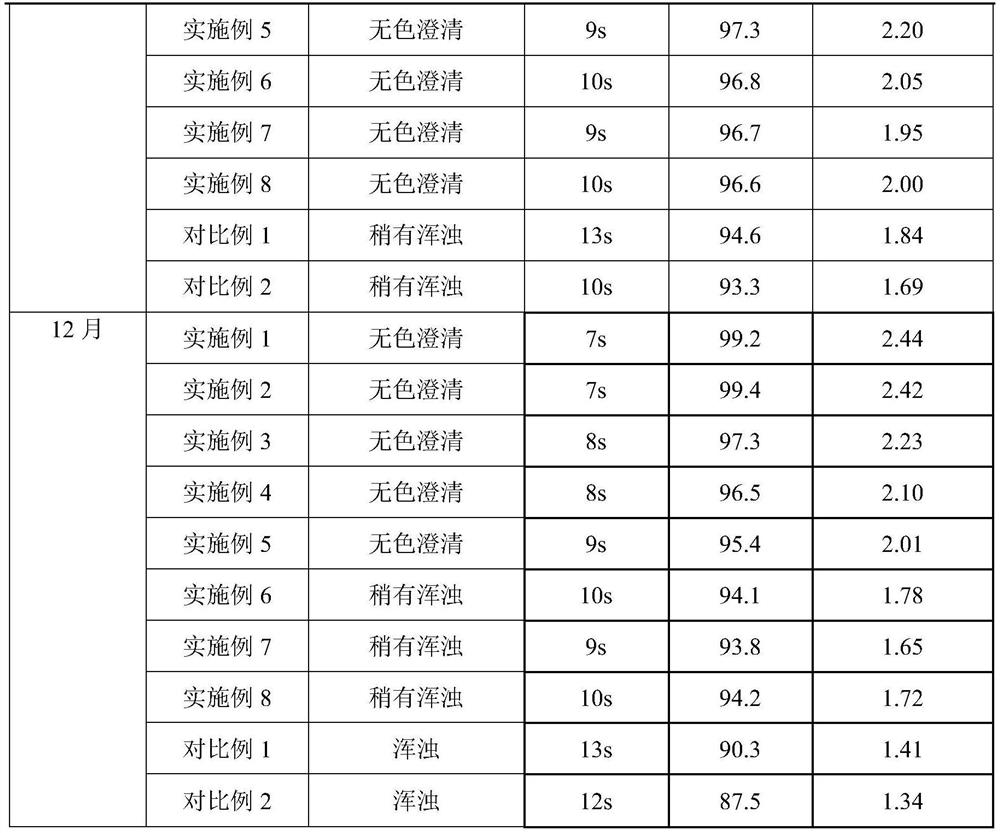
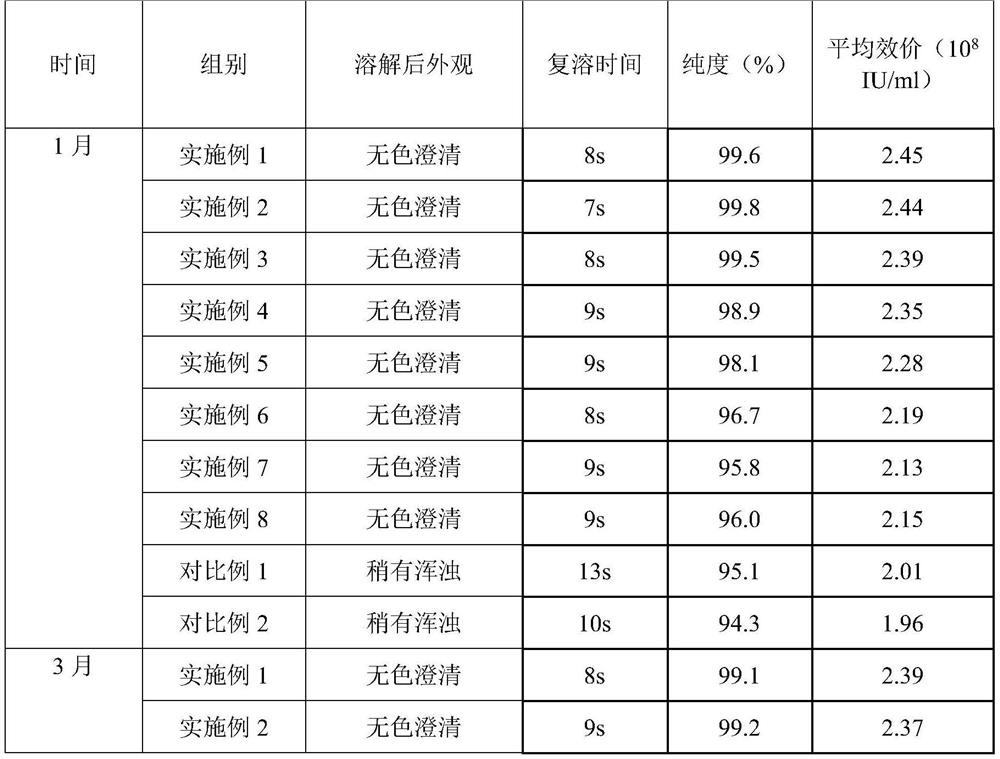
The invention provides a preparation method of a carbazochrome sodium sulfonate freeze-dried powder injection. The method comprises the following steps: (a) mixing carbazochrome sodium sulfonate with first injection water, and sequentially carrying out pH value adjustment and adsorption treatment to obtain a mixed solution A; and (b) mixing the mixed solution A with second injection water, and sequentially freezing and drying to obtain the carbazochrome sodium sulfonate freeze-dried powder injection, wherein the volume ratio of the first injection water to the second injection water is 6 to (3-5). Compared with the prior art, according to the preparation method provided by the invention, the content of impurities in the carbazochrome sodium sulfonate freeze-dried powder injection can be effectively controlled, and the re-dissolving time of a product is shortened. An experiment result shows that the content of the impurities in the carbazochrome sodium sulfonate freeze-dried powder injection prepared by the preparation method provided by the invention is lower than 0.5%, and the re-dissolving time is within 8 seconds.
Owner:HUNAN KELUN PHARMA
Method for preparing human fibrinogen by bilayer chromatography
PendingCN107540743AHigh activityImprove appearance qualityFibrinogenHydrolasesUltrafiltrationFiltration
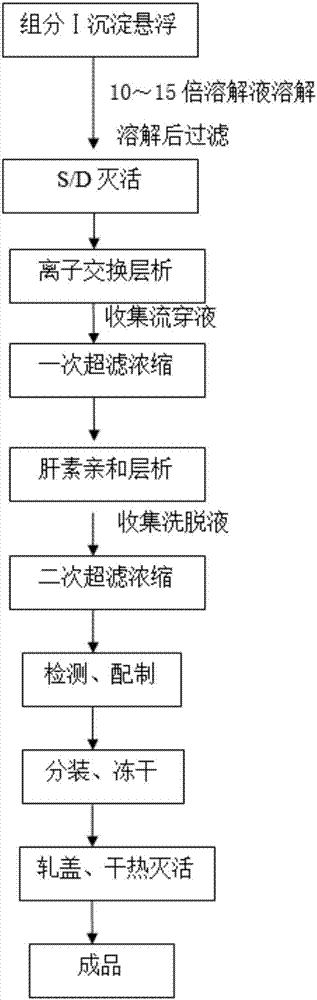
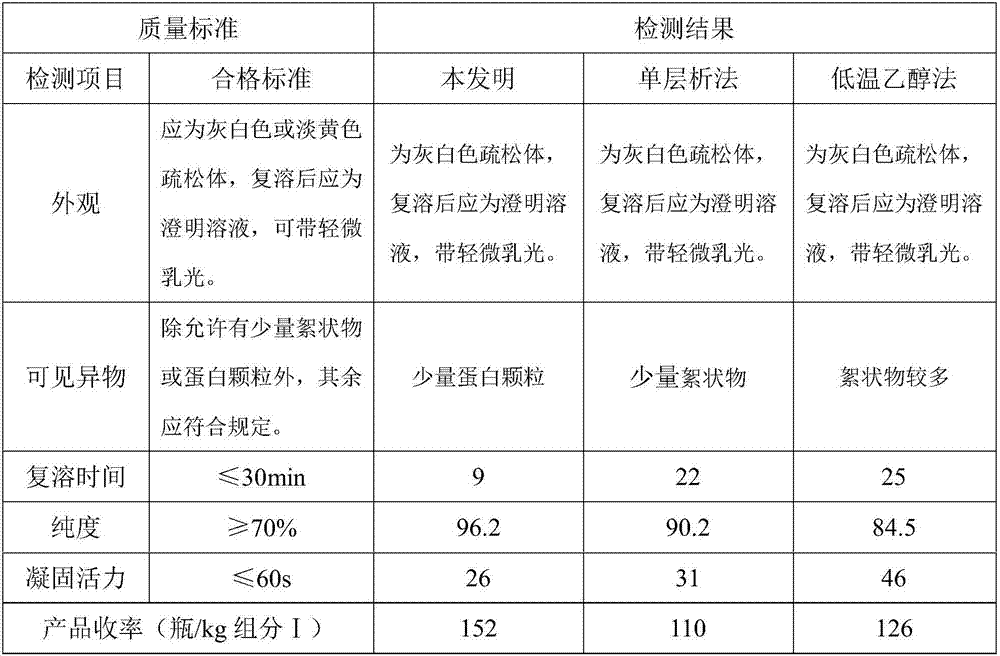
The invention discloses a method for preparing human fibrinogen by bilayer chromatography. The method comprises the following steps: precipitation, dissolution and filtration of a component I; inactivation of S / D virus; ion exchange chromatography; primary ultrafiltration concentration; heparin affinity chromatography; secondary ultrafiltration concentration; detection and preparation; packaging and freeze drying; capping and dry heating deactivation. According to the method disclosed by the invention, the ion exchange chromatography is combined with the heparin affinity chromatography, and the whole process is produced in a normal-temperature environment; the method has the advantages of short process flow, high purity of a product, high yield and short redissolving time.
Owner:NANYUE BIOPHARMING
Preparation method of biapenem bulk drug
ActiveCN111875622AHigh purityThe status of impurities is clearAntibacterial agentsOrganic chemistryActivated carbonBiapenem
The invention provides a biapenem bulk drug preparation method, which comprises: 1) dissolving a biapenem crude product in water at a certain dissolving temperature T1 to prepare a biapenem crude product aqueous solution; (2) controlling the temperature of the biapenem crude product aqueous solution obtained in the step (1) to be T1 or T2, adding activated carbon with stirring for decolorization,filtering the liquid, and cooling the filtrate to T3 for later use; 3) dropwise adding the filtrate obtained in the step 2) into a mixed solvent of acetone and ethanol, which is cooled to T4 in advance, and crystallizing the filtrate; 4) growing crystal; and 5) separating, washing and drying the crystals separated out in the step 4) to obtain the biapenem bulk drug.
Owner:SHENZHEN HAIBIN PHARMA +2
Preparation method of betamethasone sodium phosphate freeze-dried powder injection
ActiveCN105796509AShorten production timeSave energyPowder deliveryOrganic active ingredientsBetamethasone Sodium PhosphateMedicine
The invention relates to a preparation method of a cortical hormone preparation, and concretely relates to a preparation method of a betamethasone sodium phosphate freeze-dried powder injection. The method comprises the following steps: dissolving betamethasone sodium phosphate and auxiliary materials in injection, filling the obtained solution, and freeze-drying the filled solution, wherein nitrogen is introduced in the whole sublimation drying stage and desorption drying stage of freeze-drying. The method solves the problem of increase of the content of impurities in the storage process in the prior art, substantially improves the stability of the betamethasone sodium phosphate freeze-dried powder, and also solves the problem of low production efficiency in the prior art.
Owner:ZHEJIANG XIANJU PHARMA
Preparation method of voriconazole for injection
ActiveCN111700864AImprove uniformityHigh clarityOrganic active ingredientsPowder deliveryAssayPhysical chemistry
The invention discloses a preparation method of voriconazole for injection. A sample solution containing voriconazole is sublimated to the end point after supercooling, pre-freezing and annealing treatment, and the voriconazole for injection is obtained through desorption drying; sublimation is divided into a first stage and a second stage, wherein the temperature of the first stage is higher thanthat of the second stage, and the pressure of first stage is lower than that of the second stage; and the first stage and the second stage can be conducted under the conditions that vacuum degrees are not higher than 20 Pa and the temperature is not higher than 15 DEG C. Through the adoption of the sublimation way which is performed with high temperature and low pressure at an earlier stage and with low temperature and high pressure at a later stage, heat and mass transfer processes can be improved, the uniformity of crystallization can be promoted, intra assay variance can be avoided, re-dissolution time can be reduced, and the clarity of re-dissolution liquid medicine can be increased; and sublimation efficiency can be enhanced, freeze-drying periods can be shortened, and production costs can be reduced. Through the optimizing of the pre-freezing technology, crystal morphology can be further improved, the comprehensive advantages of products can be enhanced, and therefore, the advantages of clinical medication can be enhanced.
Owner:HAINAN LEVTEC PHARMA
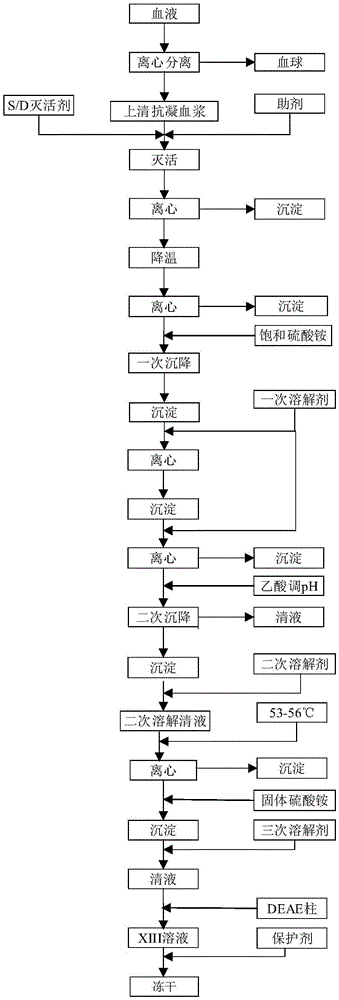
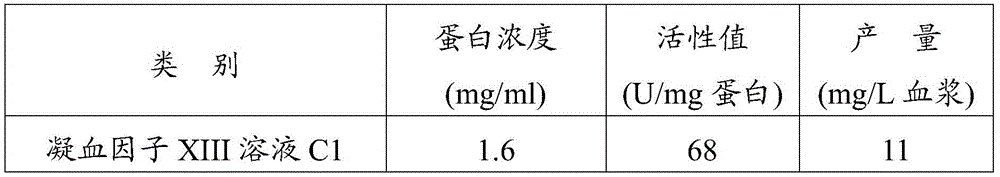
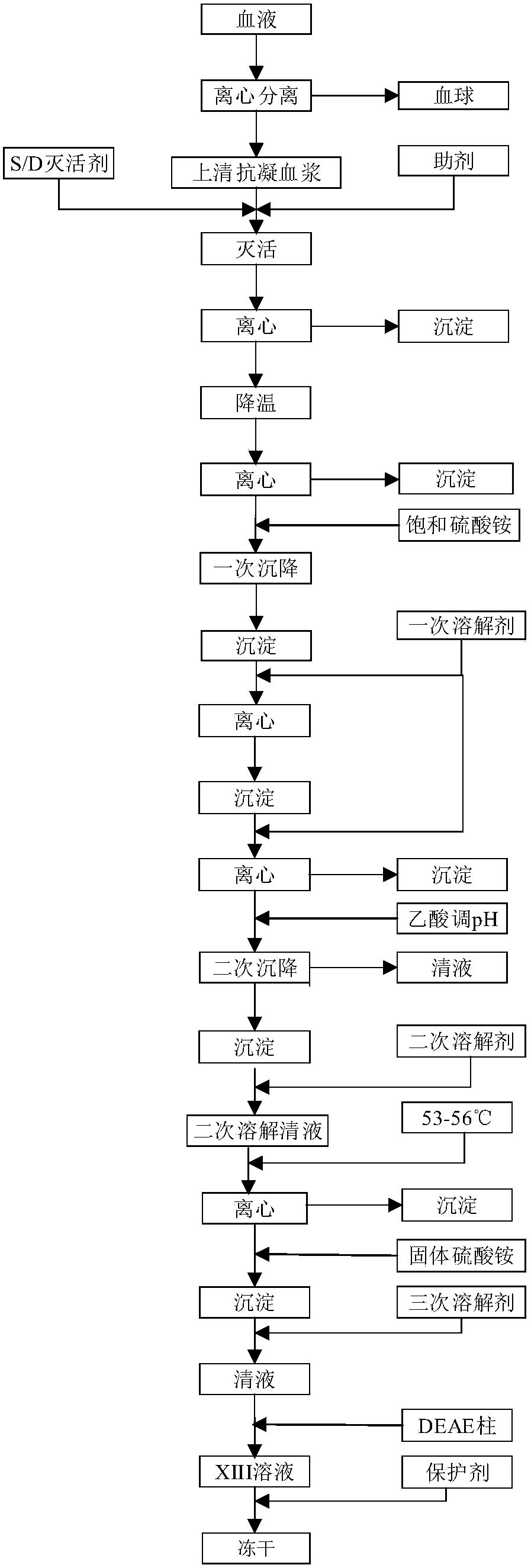
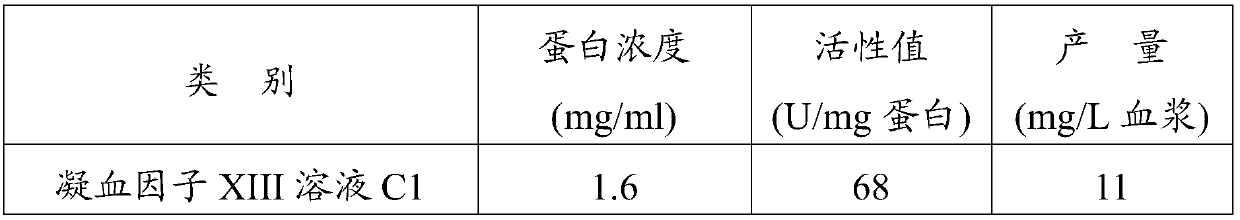
Preparation method of blood coagulation factor XIII
ActiveCN105524894AShort reconstitution timeShort storage timeAcyltransferasesAmmonium sulfateChromatographic column
The invention provides a preparation method of a blood coagulation factor XIII. The preparation method has the advantages of high yield, high purity and short production period, and comprises the steps of blood plasma and blood cell separation, virus inactivation, ammonium sulfate graded sedimentation, DEAE-FF chromatographic column treatment, split charging and freeze drying, wherein an auxiliary agent is added during the virus inactivation; the graded sedimentation and dissolution process of the blood coagulation factor XIII is performed in an environment being 0 to 4 DEG C; different dissolving agents are added for performing graded dissolution on precipitates; the dissolution efficiency is improved; the production period is shortened; the bacteria breeding is reduced; and the yield and the purity of the blood coagulation factor XIII are improved. The preparation method of the blood coagulation factor XIII provided by the invention has the beneficial effects that the extraction efficiency is high; more than 10mg of the blood coagulation factor XIII can be extracted from per liter of blood plasma; in a prepared blood coagulation factor XIII freeze-drying product, the purity of the blood coagulation factor XIII is higher than 90 percent; the operation is simple; the production period is short; and the preparation of the blood coagulation factor XIII can be completed in two days.
Owner:FUJIAN HUAGENE BIOSCI CO LTD
Freeze-drying method of pseudo-ginseng total saponin freeze-dried injection powder with filling amount more than 500 mg
ActiveCN102429942AShorten drying timeShorten the timePowder deliveryPharmaceutical product form changeFreeze-dryingMedicine
The invention relates to a freeze-drying method of pseudo-ginseng total saponin freeze-dried injection powder with a filling amount more than 500 mg. In the method, freeze-dried is carried out mainly through specific refrigerating parameters. The freeze-drying method provided by the invention has good drying effect and significantly shortened freeze-drying time, which is 20-50% of common freeze-drying time.
Owner:GUANGXI WUZHOU PHARMA GRP
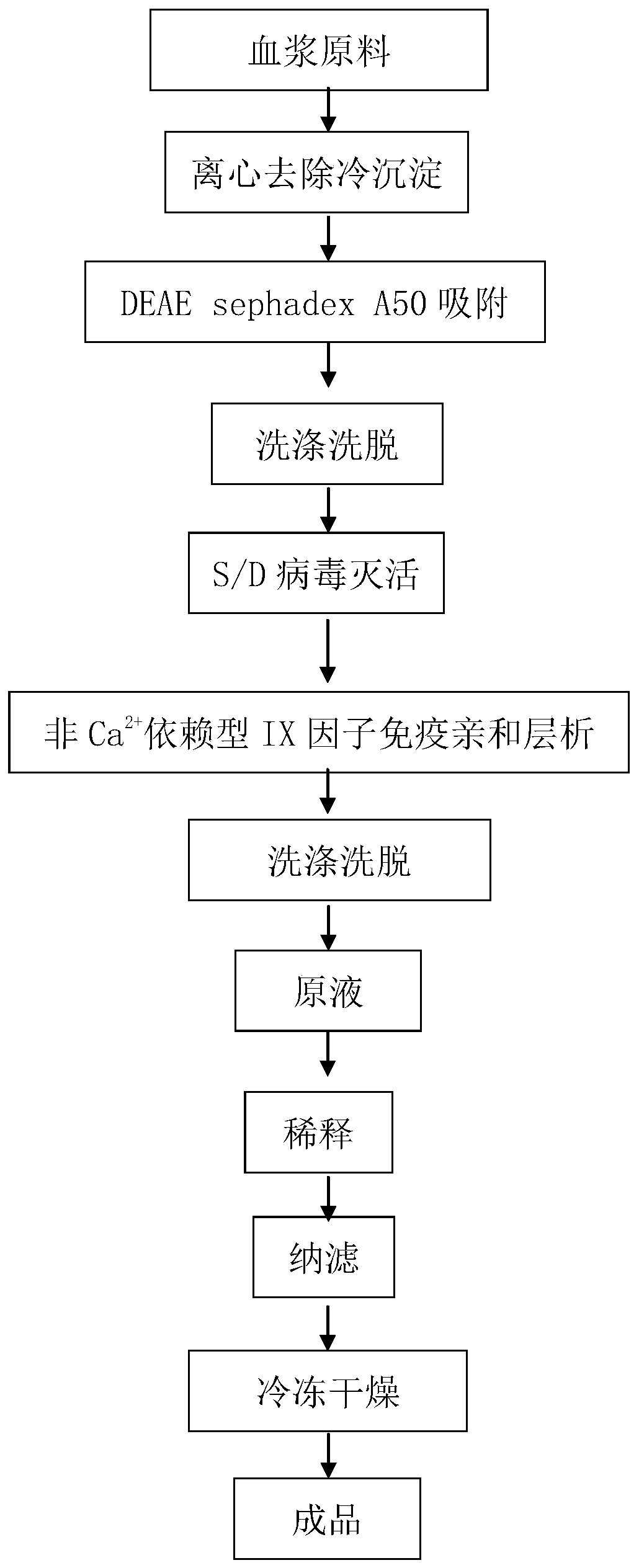
Production method of high-purity human coagulation factor IX preparation
PendingCN110257358AReduce the amount of gel usedReduce material and time costsPeptide/protein ingredientsBlood disorderChemistryTiter
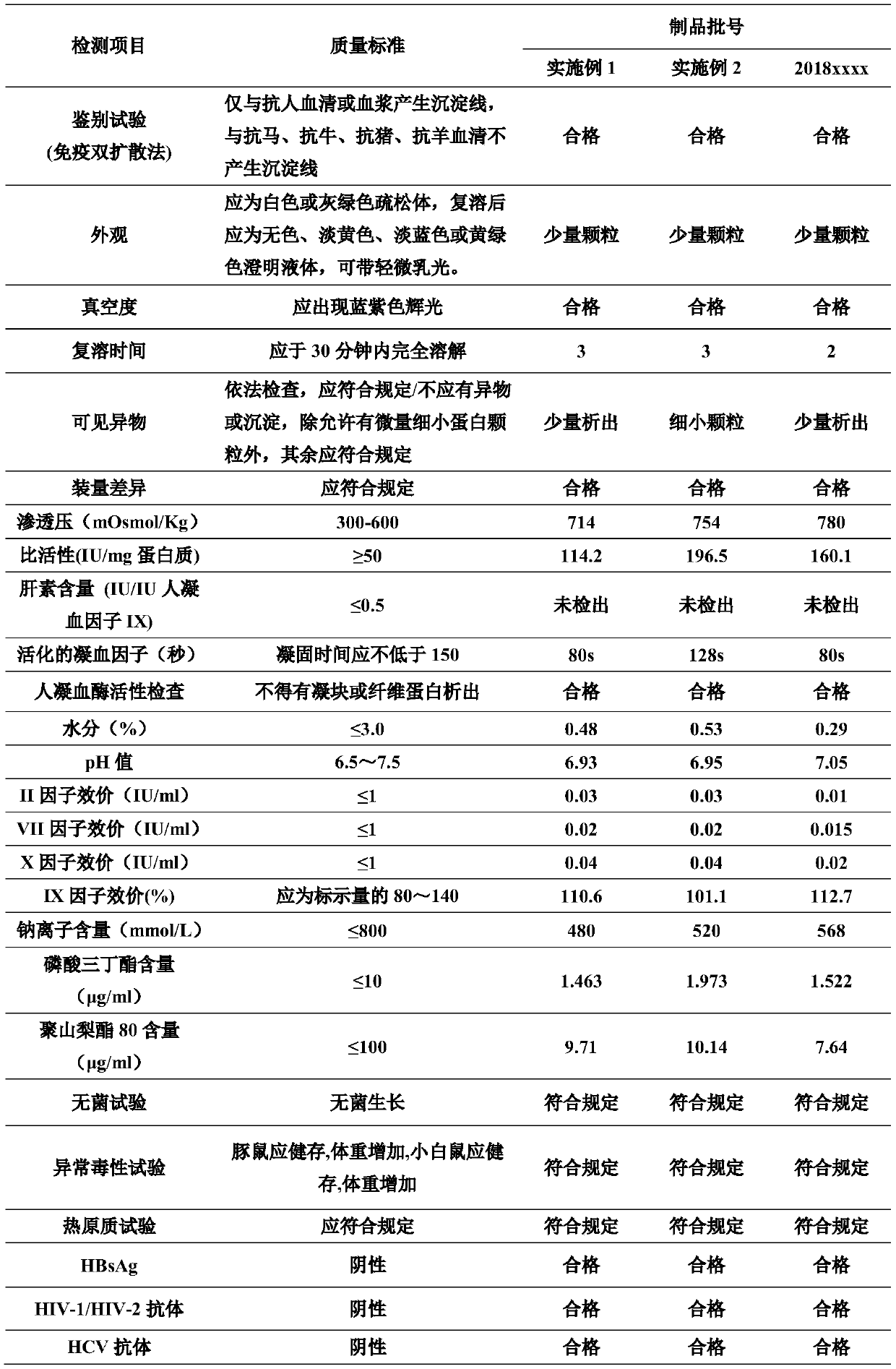
The invention discloses a production method of a high-purity human coagulation factor IX preparation, wherein the production method is les sin process step, low in production cost and high specific activity. The method includes the steps: (1) plasma cryoprecipitation removal; (2) DEAE sephadex A50 anion exchange chromatography; (3) S / D inactivated virus; (4) non-Ca2+dependent IX factor immune affinity chromatography; (5) adding of protective agents in matched liquid; (6) virus removal by a nano-filtration membrane; (7) sub-packaging and freeze drying. According to the method, a human coagulation factor IX production process is greatly simplified by the aid of novel high-adsorption specific immune affinity chromatography, the titer of a prepared high-purity human coagulation factor IX finished product can reach 114.2IU / ml, and the specific activity of an IX factor reaches up to 196.5IU / mg protein or more. Two-step virus inactivation is implemented by an S / D method with high maturity and safety and a nano-filtration method, and safety of the finished product is effectively ensured.
Owner:广东双林生物制药有限公司
Preparation method of human coagulation factor VIII
ActiveCN108218981AIncrease the efficiency of preparing FⅧImprove efficiencyFactor VIIPeptide preparation methodsWhole blood productDry heat
The invention belongs to the technical field of biological pharmacy and blood products, and especially relates to a preparation method of a human coagulation factor VIII. According to the invention, apreparation process of the human coagulation factor VIII is subjected to total research, cryoprecitation preparation, dissolution, washing liquid and an eluate are screened, after elution, the residual quantity of sorbate 80 and tributyl phosphate can achieve the requirement of current pharmacopeia; a dialysate is screened, the finally obtained product has the advantages of no collapse, no atrophy, and fast redissolving time, and FVIII titer after dry heat inactivation is 28.9 IU / ml. The processes for preparing the human coagulation factor VIII are optimized and screened, an optimum scheme isobtained, the recovery rate of each process is calculated, the recovery rate of FVIII is 28.91%, the efficiency for preparing FVIII by blood plasma is effectively increased, and a rear blood plasma resource is indirectly saved.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Preparation method of furosemide freeze-dried powder injection
InactiveCN105030700AControl UniformityShort reconstitution timePowder deliveryOrganic active ingredientsMass ratioFreeze-drying
The invention provides a preparation method of furosemide freeze-dried powder injection. The method comprises the following steps: a, mixing furosemide, an alkaline solution and first water for injection, and then mixing with an excipient after performing pH value adjustment, so as to obtain a mixed solution A; b, mixing the mixed solution A with second water for injection, and sequentially filtering, freezing and drying, so as to obtain the furosemide freeze-dried powder injection; the mass ratio of the first water for injection and the second water for injection is 5:(4 to 6). Compared with the prior art, the preparation method provided by the invention can effectively control the uniformity of the furosemide in the furosemide freeze-dried powder injection, and the redissolving time of products can be reduced. Experimental results show that, the redissolving time of furosemide freeze-dried powder injection obtained by adopting the preparation method provided by the invention is within 3 seconds.
Owner:HUNAN KELUN PHARMA
Preparation method of cytidine disdoium triphosphate freeze-dried powder injection
ActiveCN104906055AControl UniformityShort reconstitution timePowder deliveryOrganic active ingredientsFreeze-dryingMass ratio
The invention provides a preparation method of a cytidine disdoium triphosphate freeze-dried powder injection. The preparation method comprises the following steps of: (a) mixing cytidine disdoium triphosphate, an excipient with first water for injection, and adjusting the pH value to obtain mixed solution A; and (b) mixing the mixed solution A with second water for injection, and filtering, freezing and drying in sequence so as to obtain the cytidine disdoium triphosphate freeze-dried powder injection, wherein the mass ratio of the first water for injection to the second water for injection is 6:(3-5). Compared with the prior art, the preparation method provided by the invention can be used for effectively controlling the content of impurities in the cytidine disdoium triphosphate freeze-dried powder injection and increasing the mass stability and the yield of products; and experimental results show that the impurity content of the cytidine disdoium triphosphate freeze-dried powder injection prepared through the preparation method provided by the invention is below 0.41%.
Owner:HUNAN KELUN PHARMA
Lyophilized preparation of temozolomide and its preparation method
InactiveCN102949350AShort reconstitution timeQuality improvementPowder deliveryLyophilised deliveryActive componentDissolution
The invention discloses a temozolomide lyophilized powder preparation, which includes an antitumor active component temozolomide and its pharmaceutically acceptable salt, an aqueous diluent, a filler, a pH regulator and at least one solubilizing agent. Specifically, the solubilizing agent is one of niacinamide and biuret or is a mixture of them. After optimization, niacinamide or biuret is adopted as the solubilizing agent in the invention. The prepared temozolomide lyophilized powder preparation has the advantages of rapid re-dissolution and good stability during placing. The invention also discloses a preparation method of the temozolomide lyophilized powder preparation.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD
Panax notoginseng saponins freeze-dried powder injection and preparation method thereof
ActiveCN102512466BGood resolubilityReduce moisture contentPowder deliveryPharmaceutical product form changePANAX NOTOGINSENG ROOTMedicine
The invention relates to panax notoginseng saponins freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is prepared by panax notoginseng saponins and water for injection. The panax notoginseng saponins freeze-dried powder injection is free of auxiliary materials or stent agents, good in re-dissolubility, low in water content and good in appearance forming.
Owner:GUANGXI WUZHOU PHARMA GRP
Sugammadex sodium freeze-dried powder injection and preparation method thereof
PendingCN113456598ALess impuritiesSolve the problem of easy generation of large amounts of impuritiesPowder deliveryOrganic active ingredientsFreeze-dryingMedicine
The invention relates to the technical field of medicine technology, in particular to sugammadex sodium freeze-dried powder injection and a preparation method thereof. The preparation method of the sugammadex sodium freeze-dried powder injection comprises the following steps: adding sugammadex sodium into water for injection according to a prescription dosage, and stirring until sugammadex sodium is completely dissolved; adding a prescription amount of freeze-drying protection solution into the solution, stirring until the solution is completely dissolved, adjusting the pH value, stirring, filtering, filling and freeze-drying to obtain the target product. according to the method, the sugammadex sodium freeze-dried powder injection for injection is provided, and the problems that API serving as a cyclic molecular structure is unstable, and a large number of impurities are easily generated after high-temperature sterilization are solved; inert gas (nitrogen) is filled for protection after freeze-drying is finished, so that oxygen is effectively isolated, and the prepared sugammadex sodium freeze-dried powder injection has fewer impurities and is more stable; through optimization of a freeze-drying process, the prepared freeze-dried powder is shortest in redissolution time, the clarity and insoluble particles meet the requirements, the stability is higher, and the freeze-dried powder is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Porcine fibrinogen freeze-dried preparation, preparation process and application of porcine fibrinogen freeze-dried preparation
PendingCN111956788AGood instant solubilityShort reconstitution timePowder deliverySurgical adhesivesAdjuvantActive agent
The invention belongs to the field of biopharmaceuticals, and particularly relates to a porcine fibrinogen freeze-dried preparation, a preparation process and application of the porcine fibrinogen freeze-dried preparation. By per mL of preparation, the porcine fibrinogen freeze-dried preparation comprises: porcine fibrinogen 100 mg, sodium chloride 4-12 mg, sodium citrate 5-15 mg, arginine hydrochloride 60-90 mg and an adjuvant 2-10 mg; the adjuvant includes: one or more of a polyhydroxy compound, a non-ionic surface active agent, saccharides and a disintegrating agent. The preparation processis shown as follows: after the porcine fibrinogen is dissolved with a buffer solution containing the sodium citrate, the sodium chloride and the arginine hydrochloride, the adjuvant is added, stirring and dissolving are performed to obtain a porcine fibrinogen freeze-dried solution, and the porcine fibrinogen freeze-dried solution is aseptically filtered with a filter membrane of 0.22[mu]m, and is split-charged, and freeze-dried to obtain the porcine fibrinogen freeze-dried preparation. The porcine fibrinogen freeze-dried preparation of the invention has high instant solubility, short re-dissolving time, high re-dissolving solution clarity, and no protein precipitation.
Owner:SHANGHAI LIKANGRUI BIOLOGICAL ENG
Poly-L-lactic acid (PLLA) filler for injection and preparation method thereof
ActiveCN112755245AGood suspensionShort reconstitution timePharmaceutical delivery mechanismProsthesisPolymer scienceOrganic chemistry
The invention provides poly-L-lactic acid (PLLA) particles and a PLLA filler for injection prepared from the particles. The PLLA particles with different molecular weights and granularities are mixed to prepare the PLLA for injection, and the prepared PLLA for injection can achieve the beautifying effects of quick response and long maintenance time. Compared with an existing product, the PLLA filler has better redissolving and suspending effects, can reduce subcutaneous nodules, remarkably improves the comfort of a user, and provides a new choice for the field of medical cosmetology.
Owner:BEIJING JINGYU YIMEI BIOTECHNOLOGY CO LTD
Recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation for injection and preparation method thereof
PendingCN113368063AReduce degradationReduce aggregationPowder deliveryPeptide/protein ingredientsWhite blood cellChimera Protein
The invention belongs to the technical field of protein and polypeptide drugs, and particularly relates to a recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation and a preparation method thereof. The lyophilized powder for injection comprises the recombinant leukocyte inhibitory factor and leech peptide chimeric protein, an excipient, a cryoprotectant, and a buffer system. By researching different excipients, cryoprotectants, buffer systems and freeze-drying curves, the recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-drying preparation for injection is provided, which is good in appearance, good in resolubility, high in activity, less in impurity, low in side effect, high in safety and stable in quality. The problems of unstable protein, easy aggregation and denaturation, reduced activity and the like are solved. The preparation is convenient to use, quick to absorb and convenient for storage and transport.
Owner:LUNAN PHARMA GROUP CORPORATION
Cetrorelix pharmaceutical composition and preparation method thereof
PendingCN112717119AImprove stabilityShort reconstitution timePeptide/protein ingredientsInorganic non-active ingredientsUse medicationMedicine
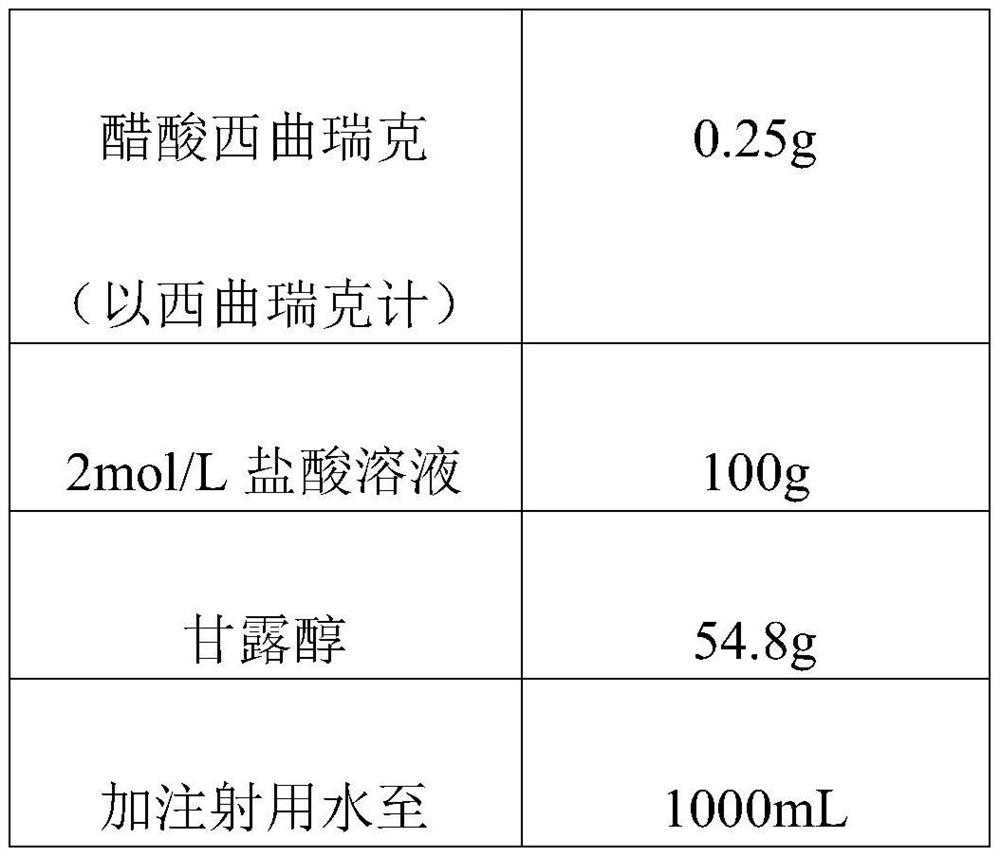
The invention discloses a cetrorelix pharmaceutical composition and a preparation method thereof, and relates to the technical field of biological medicine. The preparation method comprises the steps that cetrorelix acetate is dissolved with hydrochloric acid, and a cetrorelix acetate hydrochloric acid solution is obtained; wherein in the cetrorelix acetate hydrochloric acid solution, the final concentration of hydrochloric acid is 0.05-3 mol / L. The cetrorelix medicine prepared by the preparation method provided by the invention has higher stability, can be stored for a long time at room temperature or ambient temperature, obviously shortens the redissolution time, and improves the safety during clinical medication.
Owner:南京康舟医药科技有限公司
A kind of preparation method of anti-hepatitis B immune ribonucleic acid freeze-dried powder injection
ActiveCN104940233BControl UniformityShort reconstitution timePowder deliveryDigestive systemBiochemistryRNA - Ribonucleic acid
The invention provides a preparation method of an anti-hepatitis B immune ribonucleic acid freeze-dried powder injection. The preparation method of the anti-hepatitis B immune ribonucleic acid freeze-dried powder injection comprises the following steps: a) mixing anti-hepatitis B immune ribonucleic acid, an excipient and water for injection, so that a mixed solution A is obtained; b) filtering, and freeze-drying the mixed solution A, so that the anti-hepatitis B immune ribonucleic acid freeze-dried powder injection is obtained, wherein mass ratio of the anti-hepatitis B immune ribonucleic acid to the excipient to the water for injection is 1:(2-15):(200-600). Compared with the prior art, the preparation method provided by the invention has the advantages that uniformity of anti-hepatitis B immune ribonucleic acid in the anti-hepatitis B immune ribonucleic acid freeze-dried powder injection can be effectively controlled, and redissolution time of a product is reduced; and experiment results show that the redissolution time of the anti-hepatitis B immune ribonucleic acid freeze-dried powder injection obtained by adopting the preparation method provided by the invention is within 12 seconds.
Owner:HUNAN KELUN PHARMA
Preparation method of freeze-dried powder
ActiveCN113750054AKeep the original volumeNo collapsePowder deliveryDrying solid materials without heatCrystallographyPhysical chemistry
The invention belongs to the technical field of preparation of freeze-dried powder and particularly relates to a preparation method of the freeze-dried powder. The method comprises the following steps: placing a traditional Chinese medicine extract at a pre-freezing temperature, and then, heating the temperature to 25-35 DEG C to obtain traditional Chinese medicine freeze-dried powder, wherein the pre-freezing temperature is 5-14 DEG C lower than an eutectic point of the traditional Chinese medicine extract; and the process of raising the pre-freezing temperature to 25-35 DEG C at least comprises 8 heat preservation processes. The freeze-dried powder obtained by the method can keep the original volume, does not collapse, has a smooth surface, can integrally fall off without being broken, has uniform color and luster, is free of spots and has fine texture. In addition, the freeze-dried powder prepared by the method provided by the invention is low in water content and short in redissolution time, the specific surface area and porosity are increased, and the redissolution time of the freeze-dried powder is effectively shortened.
Owner:BEIJING KANGRENTANG PHARMA
PEGylated recombinant human granulocyte colony stimulating factor freeze-dried preparation
ActiveCN113797171AImprove medication safetyImprove stabilityPowder deliveryPeptide/protein ingredientsGranulocyte colony-stimulating factorFreeze-drying
The invention provides a PEGylated recombinant human granulocyte colony stimulating factor (PEG-rhG-CSF) freeze-dried preparation. The PEG-rhG-CSF and an excipient are subjected to spray freeze-drying treatment, when the prepared PEG-rhG-CSF freeze-dried preparation is clinically used, a special solvent containing sodium chloride and acetate is prepared, and the freeze-dried preparation can be used after being mixed and dissolved with the special solvent. The PEG-rhG-CSF freeze-dried preparation prepared by the invention does not contain a surfactant namely Tween 20, is high in medication safety and good in stability, can be stored for a long time, and is simple in preparation process and short in production period.
Owner:SHANDONG NEWTIME PHARMA
A kind of carrimycin freeze-dried powder preparation and preparation method thereof
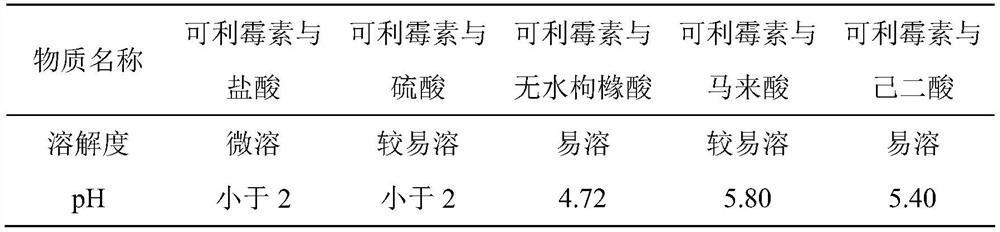
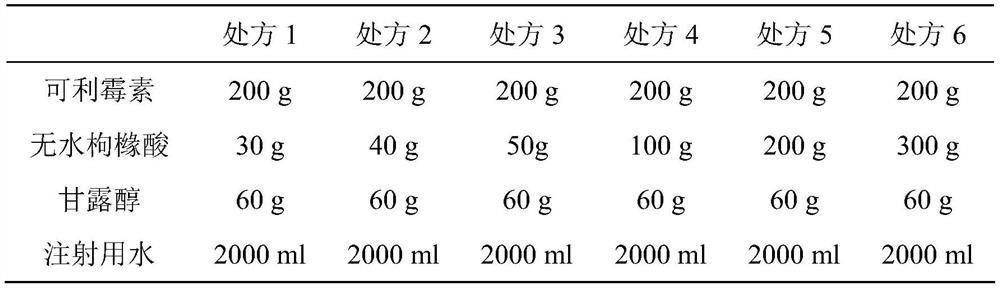
ActiveCN111450066BEnhanced inhibitory effectImprove securityAntibacterial agentsOrganic active ingredientsO-Phosphoric AcidEthylic acid
The invention belongs to the technical field of medicine, and relates to a corimycin freeze-dried powder preparation and a preparation method thereof. The freeze-dried powder preparation is prepared by corimycin, an organic acid or an inorganic acid, a proppant and water for injection. to make. Wherein, every 1000ml of water for injection contains 40-115g of corimycin, 20-100g of organic acid or inorganic acid, and 15-30g of proppant. The weight ratio of carrimycin, organic acid or inorganic acid is: 1:1-5:1, preferably 2:1-4:1. Inorganic acid includes hydrochloric acid, sulfuric acid or phosphoric acid; described organic acid includes citric acid, anhydrous citric acid, maleic acid, adipic acid, acetic acid, methanesulfonic acid, ethanesulfonic acid, fumaric acid, tartaric acid, apple Acid, pyroglutamic acid, lactic acid, succinic acid or C1-C4 straight chain or branched chain alkane sulfonate unsubstituted or substituted by any position of 1-3 hydroxyl groups. The freeze-dried powder injection of the invention has the characteristics of short reconstitution time, few insoluble particles, easy preparation and relatively stable characteristics.
Owner:沈阳信达泰康医药科技有限公司
A kind of preparation method of biapenem bulk drug
ActiveCN111875622BHigh purityThe status of impurities is clearAntibacterial agentsOrganic chemistryActivated carbonSolvent
The invention provides a method for preparing a biapenem bulk drug, the method comprising the following steps: 1) at a certain dissolution temperature T 1 2) The crude biapenem aqueous solution obtained in step 1) is controlled at T 1 or cool down to T 2 , add activated carbon, stir to decolorize, filter, and cool the filtrate to T 3 Standby; 3) the filtrate of step 2) gained is added dropwise to pre-cooling to T 4 4) growing crystals; 5) separating, washing and drying the crystals precipitated in step 4) to obtain the biapenem bulk drug.
Owner:SHENZHEN HAIBIN PHARMA +2
Preparation method of coagulation factor xiii
The invention provides a preparation method of a blood coagulation factor XIII. The preparation method has the advantages of high yield, high purity and short production period, and comprises the steps of blood plasma and blood cell separation, virus inactivation, ammonium sulfate graded sedimentation, DEAE-FF chromatographic column treatment, split charging and freeze drying, wherein an auxiliary agent is added during the virus inactivation; the graded sedimentation and dissolution process of the blood coagulation factor XIII is performed in an environment being 0 to 4 DEG C; different dissolving agents are added for performing graded dissolution on precipitates; the dissolution efficiency is improved; the production period is shortened; the bacteria breeding is reduced; and the yield and the purity of the blood coagulation factor XIII are improved. The preparation method of the blood coagulation factor XIII provided by the invention has the beneficial effects that the extraction efficiency is high; more than 10mg of the blood coagulation factor XIII can be extracted from per liter of blood plasma; in a prepared blood coagulation factor XIII freeze-drying product, the purity of the blood coagulation factor XIII is higher than 90 percent; the operation is simple; the production period is short; and the preparation of the blood coagulation factor XIII can be completed in two days.
Owner:FUJIAN HUAGENE BIOSCI CO LTD
Freeze-drying method of pseudo-ginseng total saponin freeze-dried injection powder with filling amount more than 500 mg
ActiveCN102429942BShorten drying timeShorten the timePowder deliveryPharmaceutical product form changeMedicine
Owner:GUANGXI WUZHOU PHARMA GRP
A kind of preparation method of cytidine triphosphate disodium freeze-dried powder injection
ActiveCN104906055BControl UniformityShort reconstitution timeOrganic active ingredientsPowder deliveryDisodium phosphateMass ratio
The invention provides a preparation method of a cytidine disdoium triphosphate freeze-dried powder injection. The preparation method comprises the following steps of: (a) mixing cytidine disdoium triphosphate, an excipient with first water for injection, and adjusting the pH value to obtain mixed solution A; and (b) mixing the mixed solution A with second water for injection, and filtering, freezing and drying in sequence so as to obtain the cytidine disdoium triphosphate freeze-dried powder injection, wherein the mass ratio of the first water for injection to the second water for injection is 6:(3-5). Compared with the prior art, the preparation method provided by the invention can be used for effectively controlling the content of impurities in the cytidine disdoium triphosphate freeze-dried powder injection and increasing the mass stability and the yield of products; and experimental results show that the impurity content of the cytidine disdoium triphosphate freeze-dried powder injection prepared through the preparation method provided by the invention is below 0.41%.
Owner:HUNAN KELUN PHARMA
A kind of preparation method of betamethasone sodium phosphate freeze-dried powder injection
ActiveCN105796509BShorten production timeSave energyPowder deliveryOrganic active ingredientsBetamethasone Sodium PhosphateDesorption
The invention relates to a preparation method of a cortical hormone preparation, and concretely relates to a preparation method of a betamethasone sodium phosphate freeze-dried powder injection. The method comprises the following steps: dissolving betamethasone sodium phosphate and auxiliary materials in injection, filling the obtained solution, and freeze-drying the filled solution, wherein nitrogen is introduced in the whole sublimation drying stage and desorption drying stage of freeze-drying. The method solves the problem of increase of the content of impurities in the storage process in the prior art, substantially improves the stability of the betamethasone sodium phosphate freeze-dried powder, and also solves the problem of low production efficiency in the prior art.
Owner:ZHEJIANG XIANJU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com