Production method of high-purity human coagulation factor IX preparation
A technology of human coagulation factor and production method, which is applied in the field of high-purity human coagulation factor IX preparation production, can solve the problems of large gel filler consumption, high production cost, loss of target protein, etc. Time cost, the effect of reducing the amount of gel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
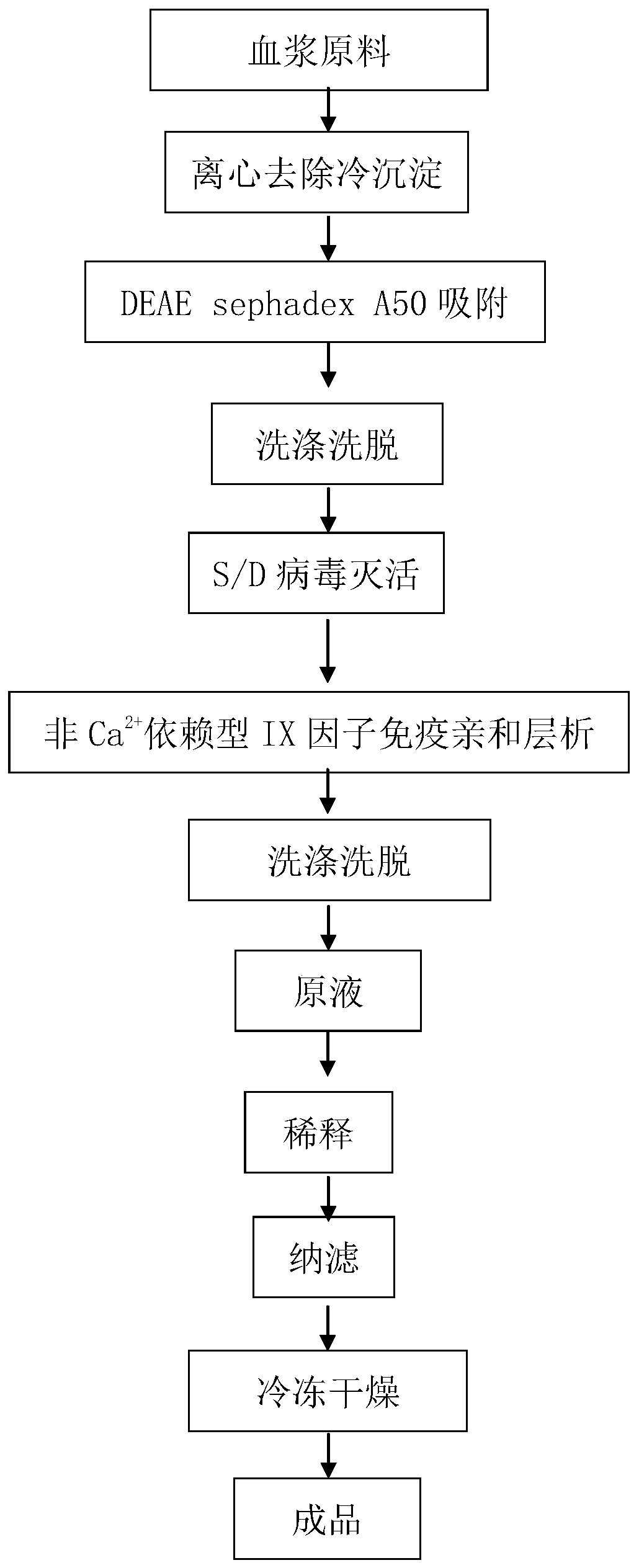
[0036] Removal of plasma cryoprecipitate: using fresh frozen human plasma as raw material, the cryoprecipitate in the plasma is removed through the processes of thawing, mixing, and continuous centrifugation to obtain 2 kg of plasma centrifugation supernatant without cryoprecipitation;
[0037] Gel batch adsorption: The plasma temperature is controlled at 15°C, according to 1.5g dry gel / L plasma, add the balanced DEAE A-50 gel to the cryoprecipitated plasma, stir and absorb for 45min, then turn off the stirring. The plasma after gel adsorption is filtered with a small closed filter, and the adsorbed A-50 gel is collected;
[0038] Column packing and elution: Pack the adsorbed A-50 gel into a small XK16 / 20 chromatographic column, and use the AKTA chromatographic system for online washing of the packed chromatographic column. The flow rate of the washing liquid is 2ml / min, and the washing time is 50min. The washed A-50 gel switches the eluent for online elution, the eluent flow...
Embodiment 2
[0044] Removal of plasma cryoprecipitate: Using fresh frozen human plasma as the raw material, the cryoprecipitate in the plasma is removed through the processes of thawing, mixing, and continuous centrifugation to obtain 4kg of plasma centrifugation supernatant without cryoprecipitation;
[0045] Gel batch adsorption: The plasma temperature is controlled at 10°C. According to 1.0g dry gel / L plasma, add the balanced DEAE A-50 gel to the cryoprecipitated plasma, stir and absorb for 45min, and then turn off the stirring. The plasma after gel adsorption is filtered with a small closed filter, and the adsorbed A-50 gel is collected;
[0046] Column packing and elution: Pack the adsorbed A-50 gel into a small XK16 / 20 chromatographic column, and use the AKTA chromatographic system for online washing of the packed chromatographic column. The flow rate of the washing liquid is 2ml / min, and the washing time is 60min. After washing, the A-50 gel switches the eluent for online elution, ...
Embodiment 3
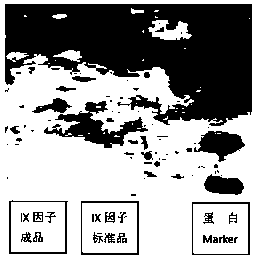
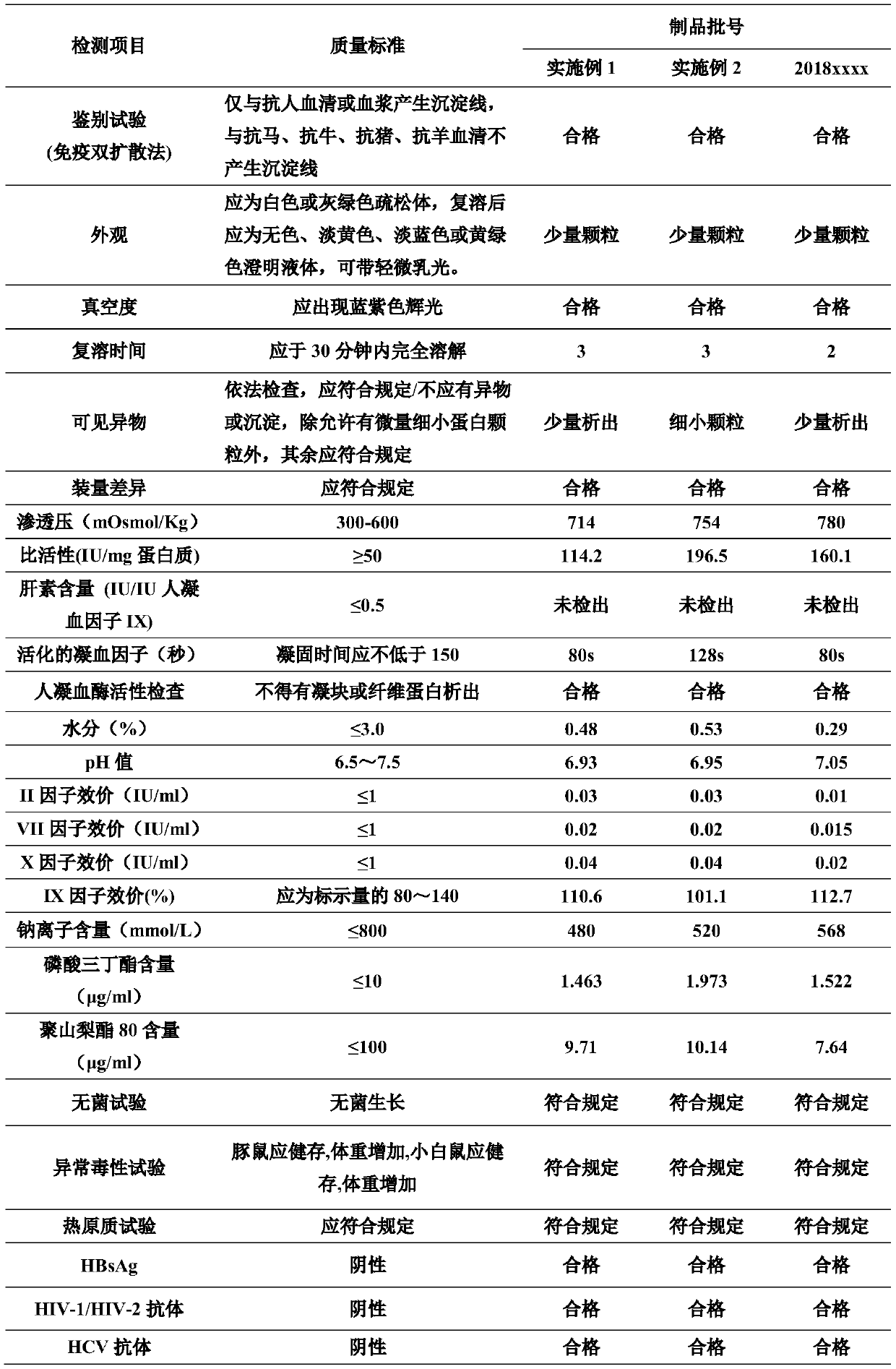
[0053] A testing laboratory is commissioned to perform SDS-page electrophoresis testing on the finished product of the high-purity human coagulation factor IX preparation prepared in Example 2, so as to determine the distribution of main protein types in the finished product. Detection operation steps:
[0054] (1) Wash and dry the glass plate;
[0055] (2) Fix the glass plate on the glue-filling bracket. When fixing, force evenly on both sides to prevent the glass plate from being pinched;
[0056] (3) Configure 8% separating gel in proportion, quickly add it with a pipette, and add it to about 1cm from the upper edge, then add a little water to seal it overnight, and let it stand for about 40 minutes;
[0057] (4) Pour out the water and dry the remaining water with filter paper, prepare 5% concentrated gel, add the concentrated gel continuously and steadily to a distance of 5mm from the edge, quickly insert the sample comb, and let stand for 30-40min;
[0058] (5) After ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com