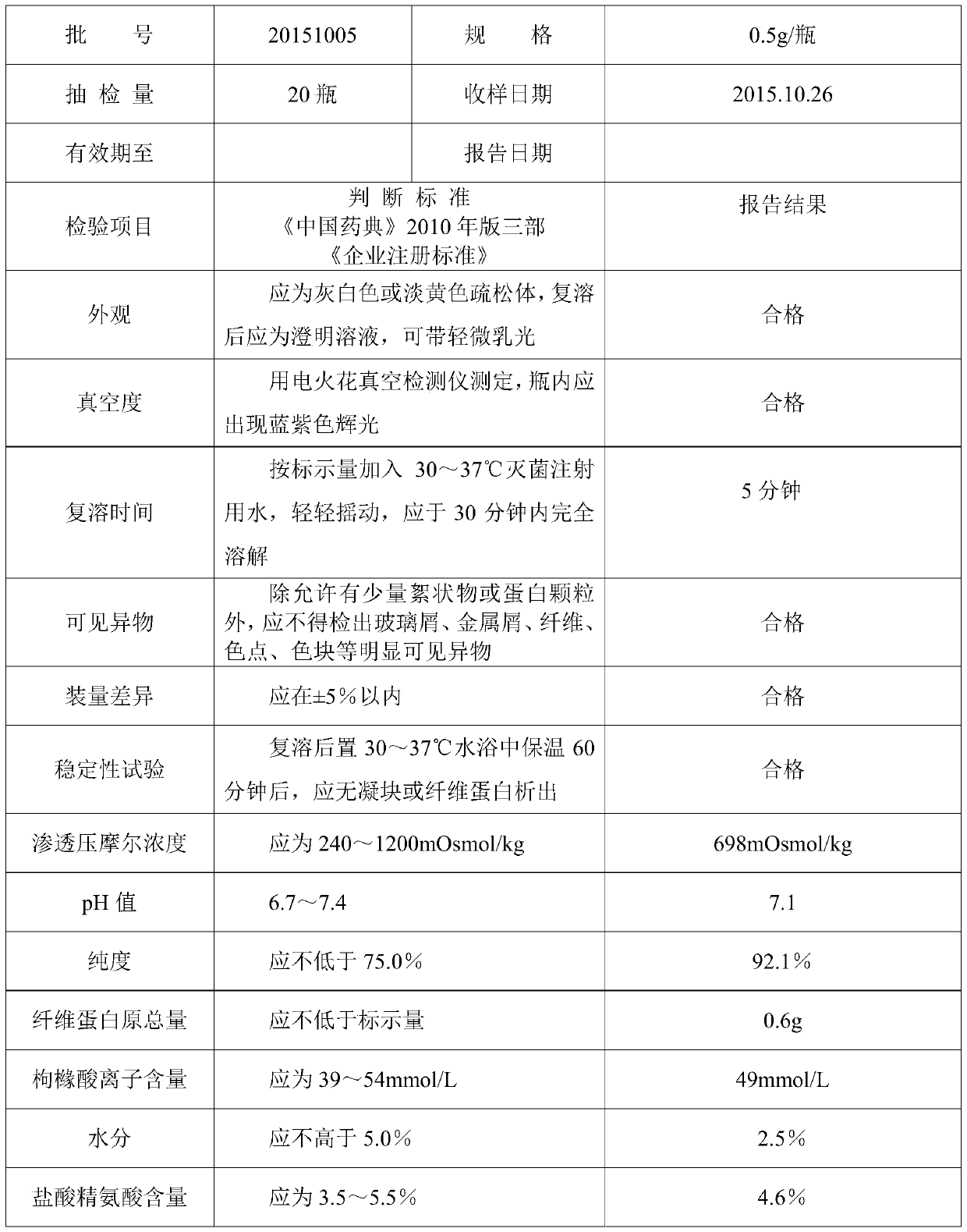
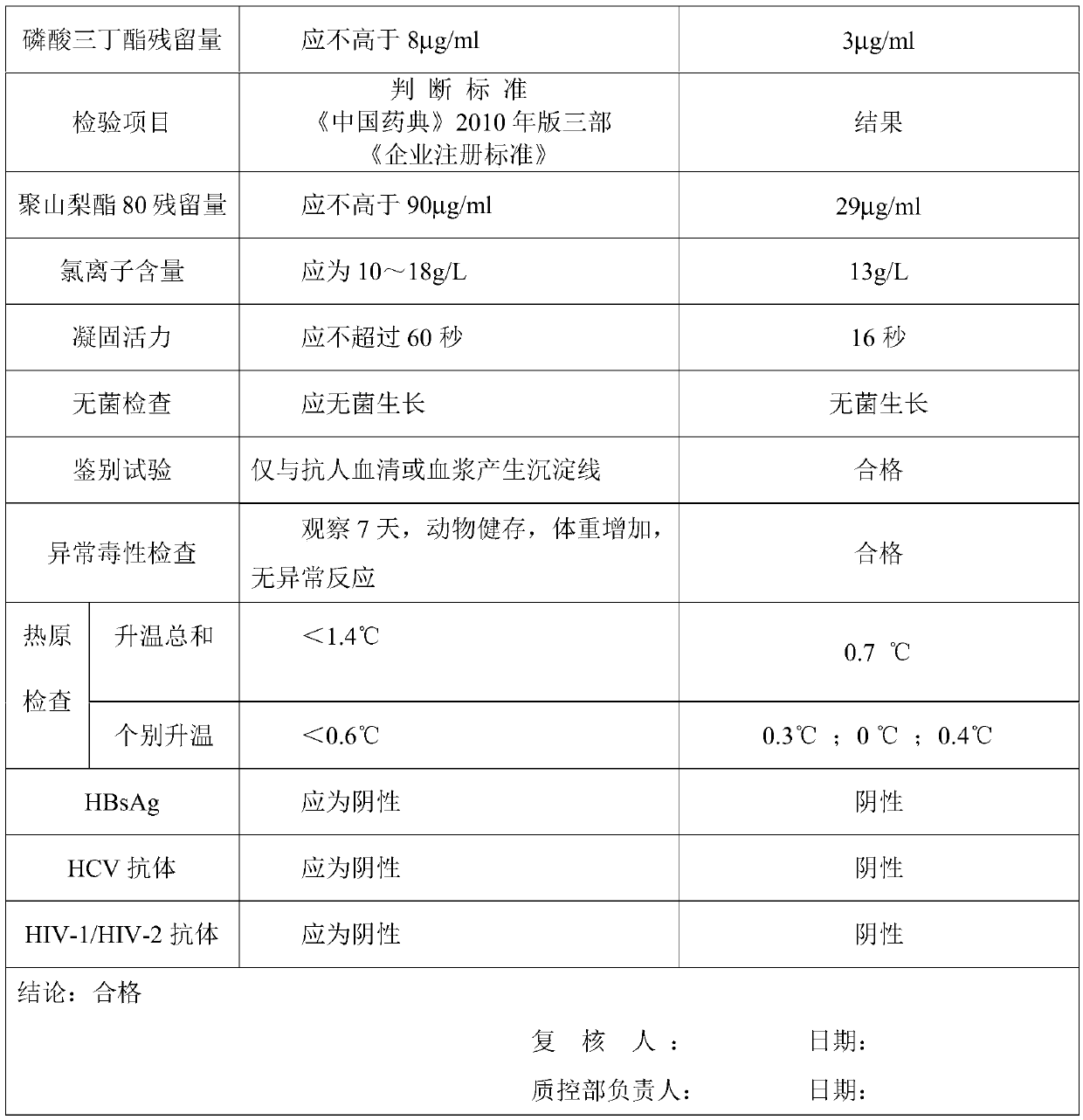
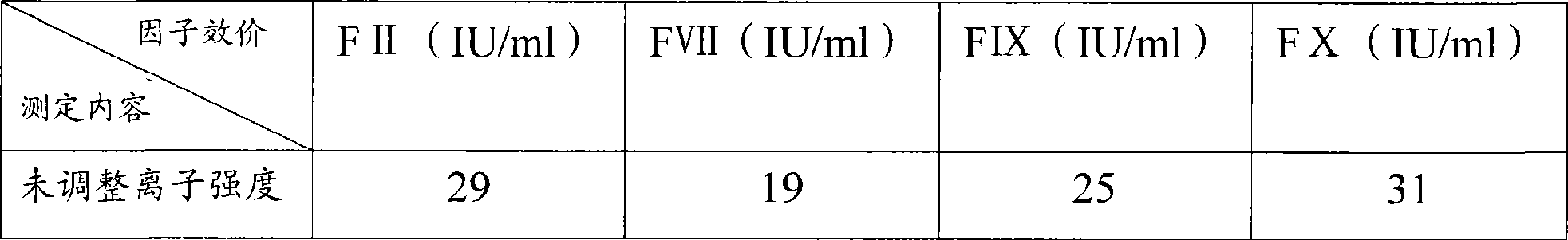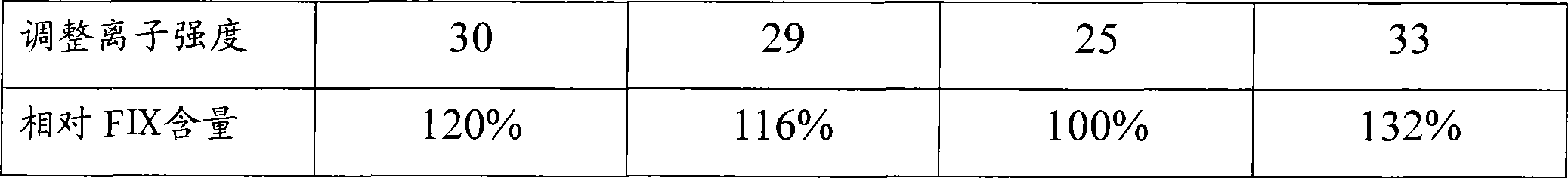Patents
Literature
63 results about "Cryoprecipitate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cryoprecipitate, also called cryo for short, is a frozen blood product prepared from blood plasma. To create cryoprecipitate, fresh frozen plasma thawed at 1–6 °C, is then centrifuged and the precipitate is collected. The precipitate is resuspended in a small amount of residual plasma (generally 10–15 mL) and is then re-frozen for storage. It is often transfused to adults as two 5-unit pools instead of as a single product. One of the most important constituents is factor VIII (also called antihaemophilic factor or AHF), which is why cryoprecipitate is sometimes called cryoprecipitated antihaemophilic factor or cryoprecipitated AHF. In many clinical contexts, use of whole cryoprecipitate has been replaced with use of clotting factor concentrates made therefrom (where available), but the whole form is still routinely stocked by many, if not most, hospital blood banks. Cryo can be stored at −18 °C or colder for 12 months from the original collection date. After thawing, single units of cryo (or units pooled using a sterile method) can be stored at 20–24 °C for up to 6 hours. If units of cryo are pooled in an open system, they can only be held at 20–24 °C for up to 4 hours. Presently cryo cannot be re-frozen for storage after it is thawed for use if it is not transfused.
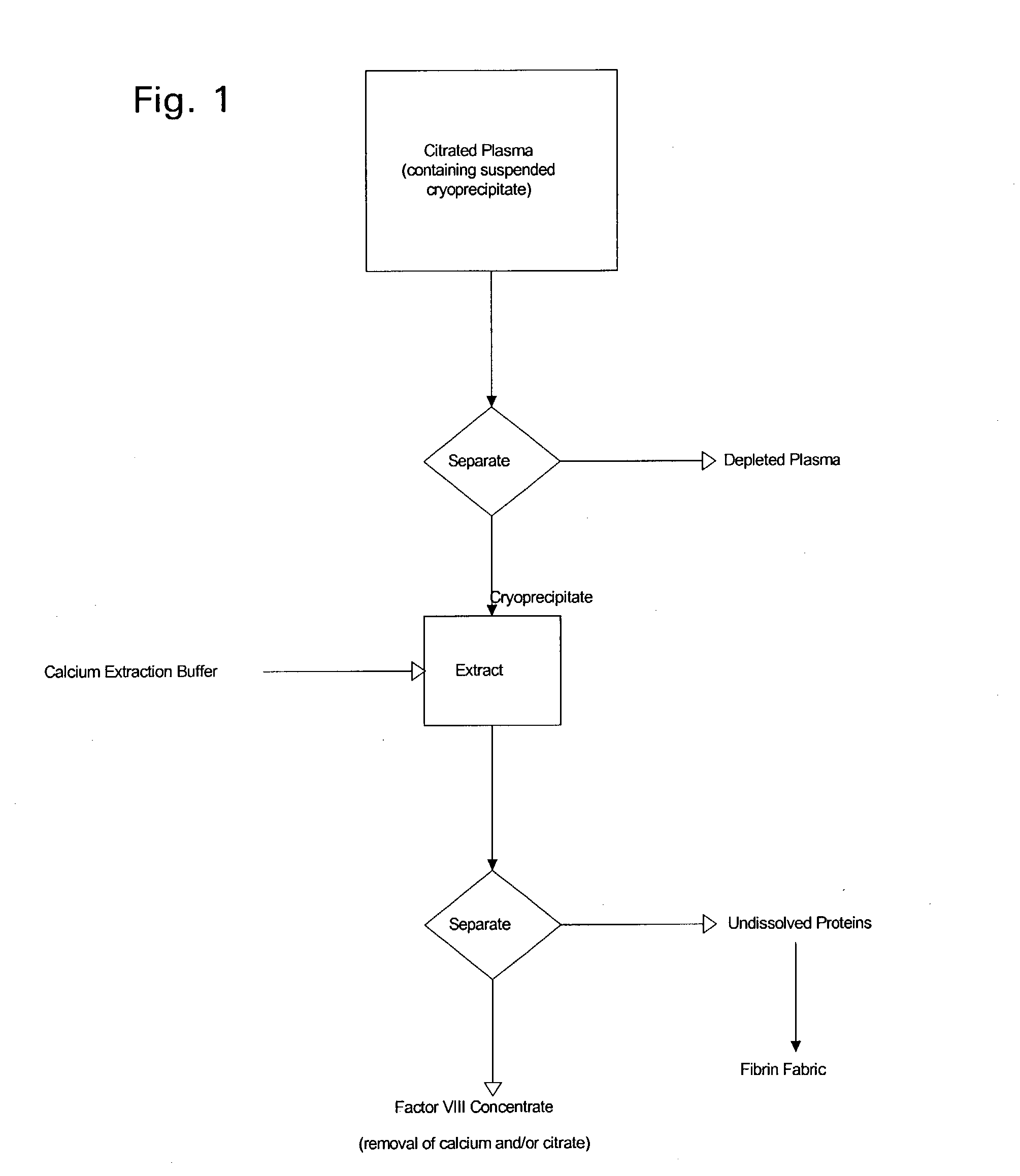
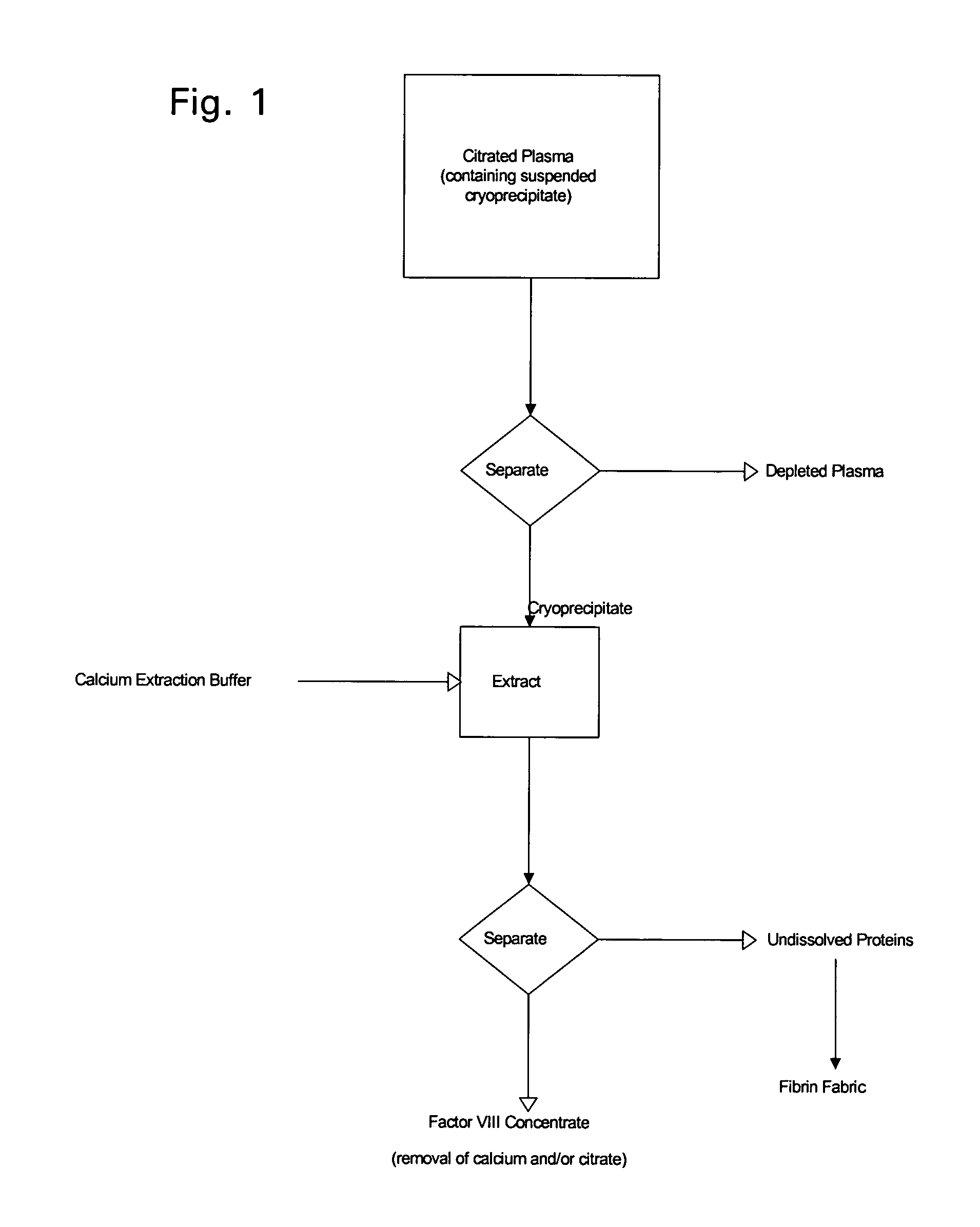
Enhanced production of blood clotting factors and fibrin fabric
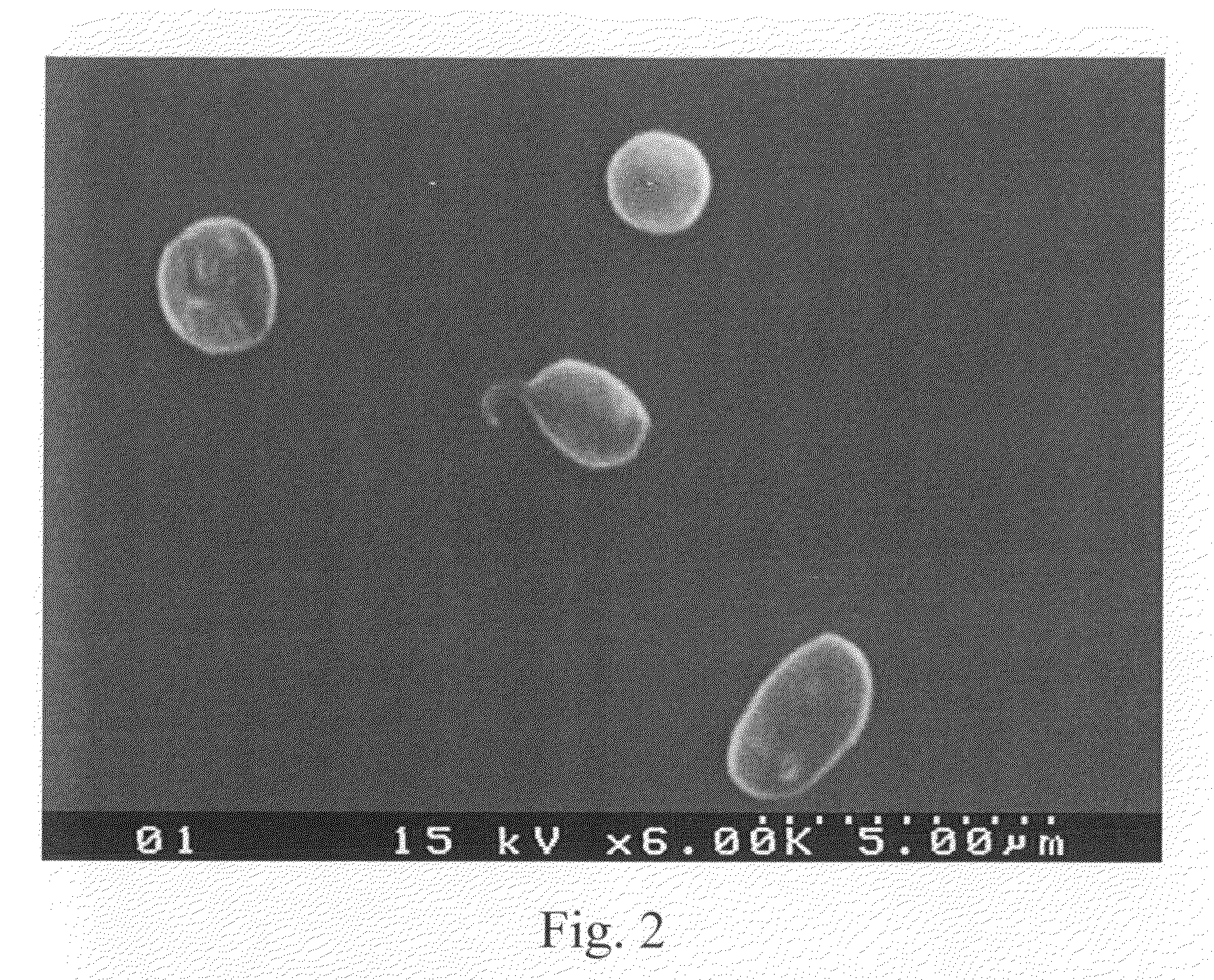
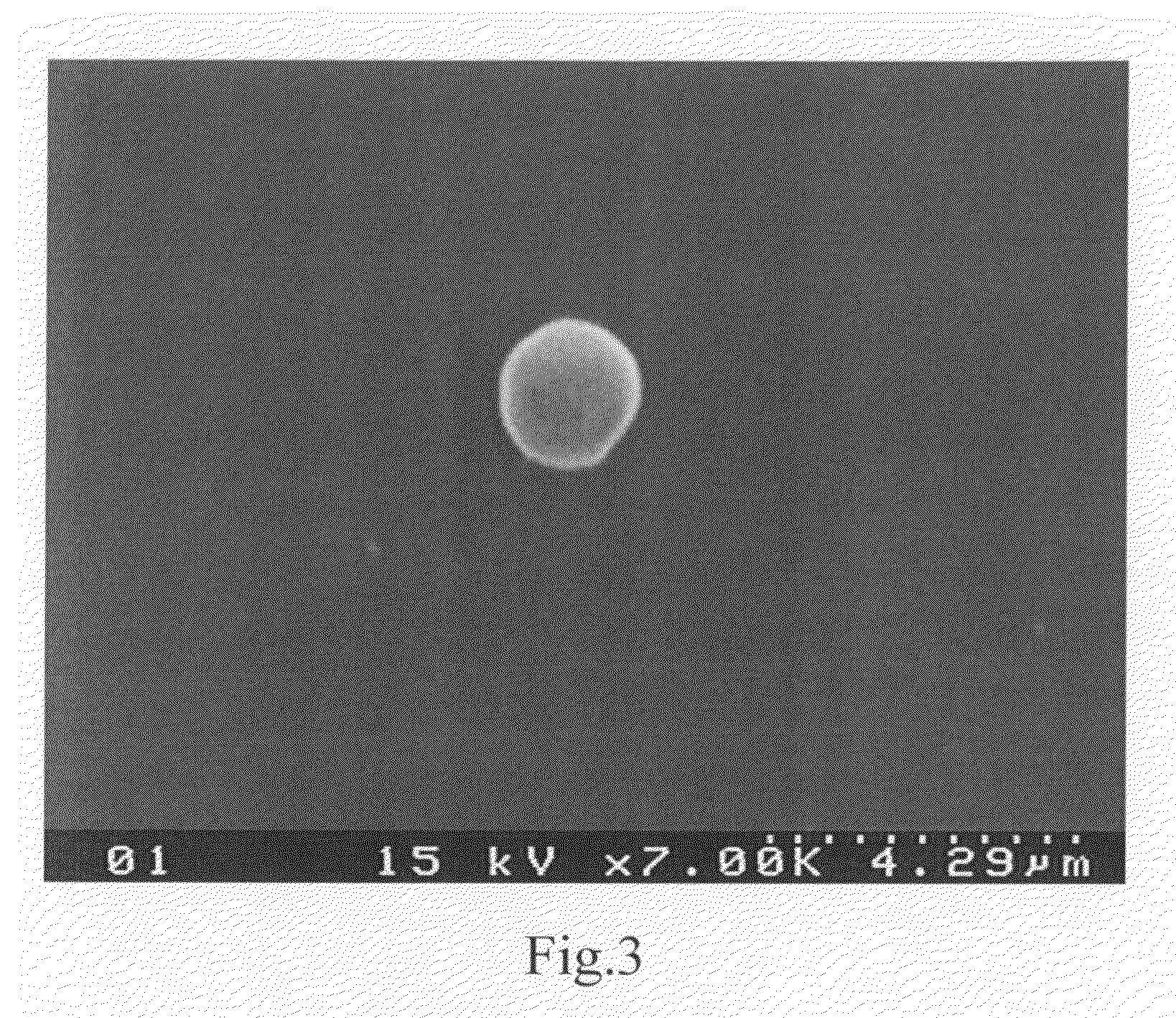
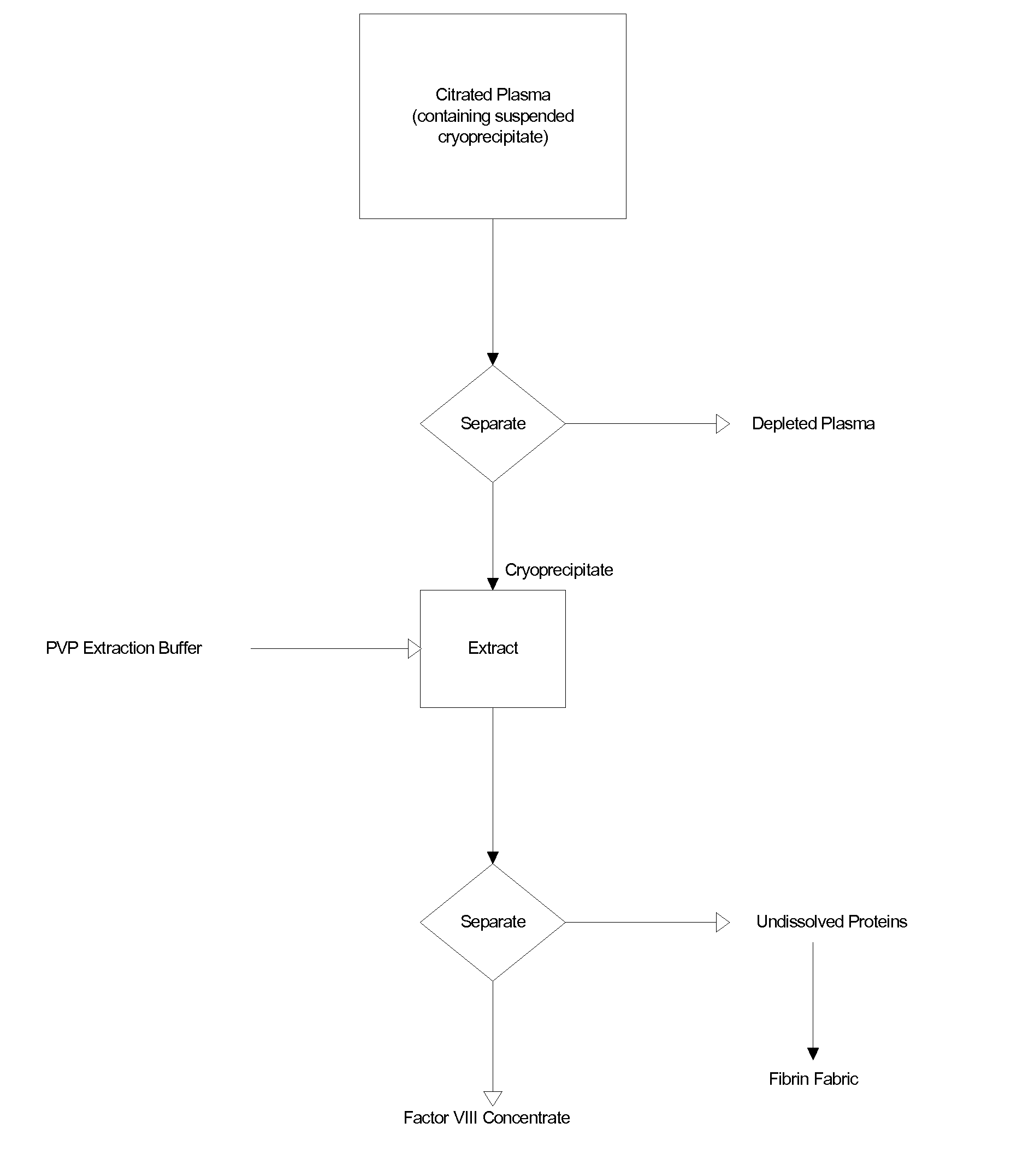
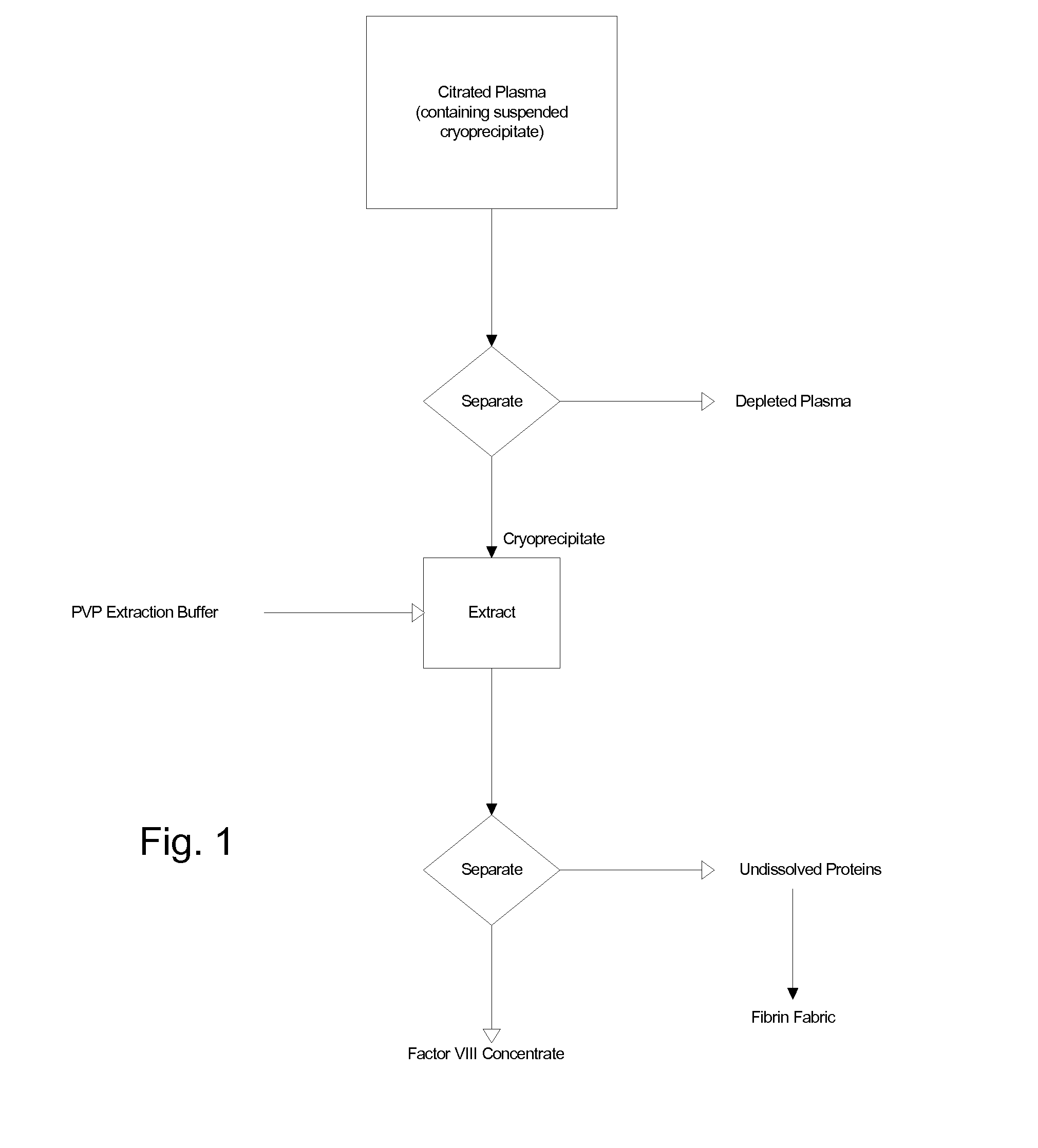
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds
Owner:SHANBROM TECH
Enhanced production of blood clotting factors and fibrin fabric
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds.
Owner:SHANBROM TECH
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS20060247426A1Shorten the timeImprove protectionPowder deliveryPeptide/protein ingredientsCryoprecipitateMedicine
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Compositions Suitable for Treatment of Spinal Disease, Disorder or Condition
InactiveUS20100310524A1Raise the ratioRestore disc heightBiocideNervous disorderCryoprecipitateMedicine
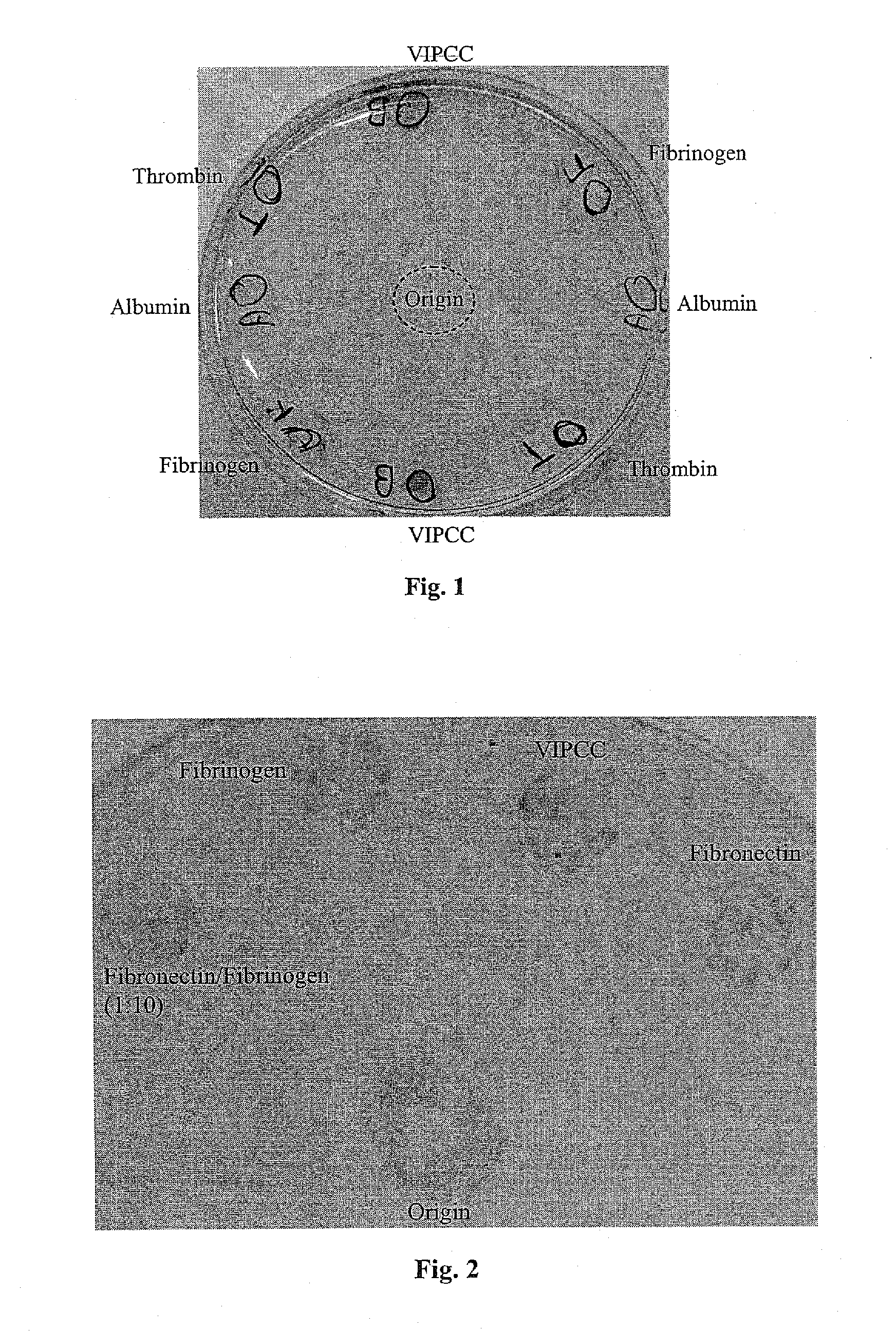
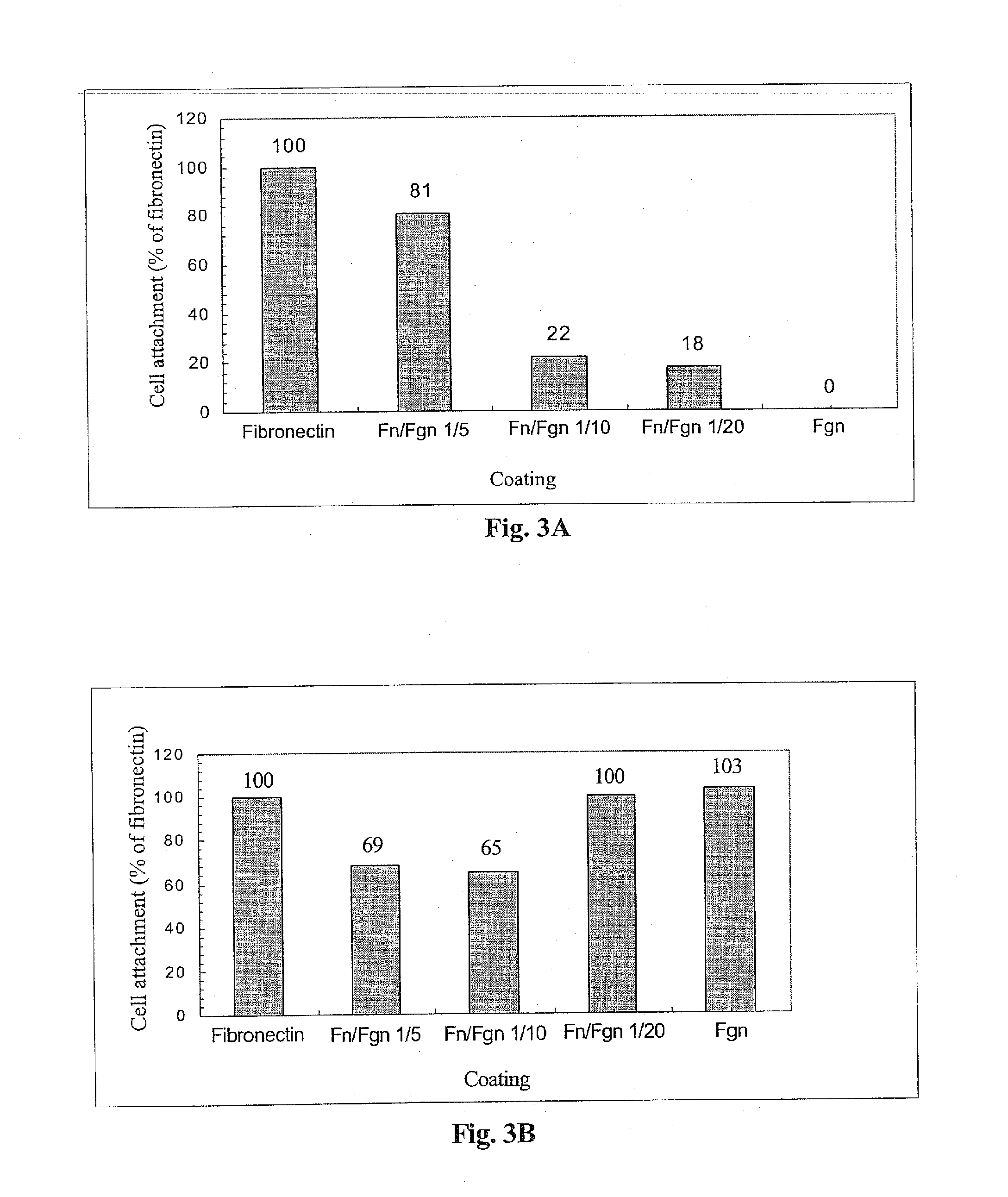
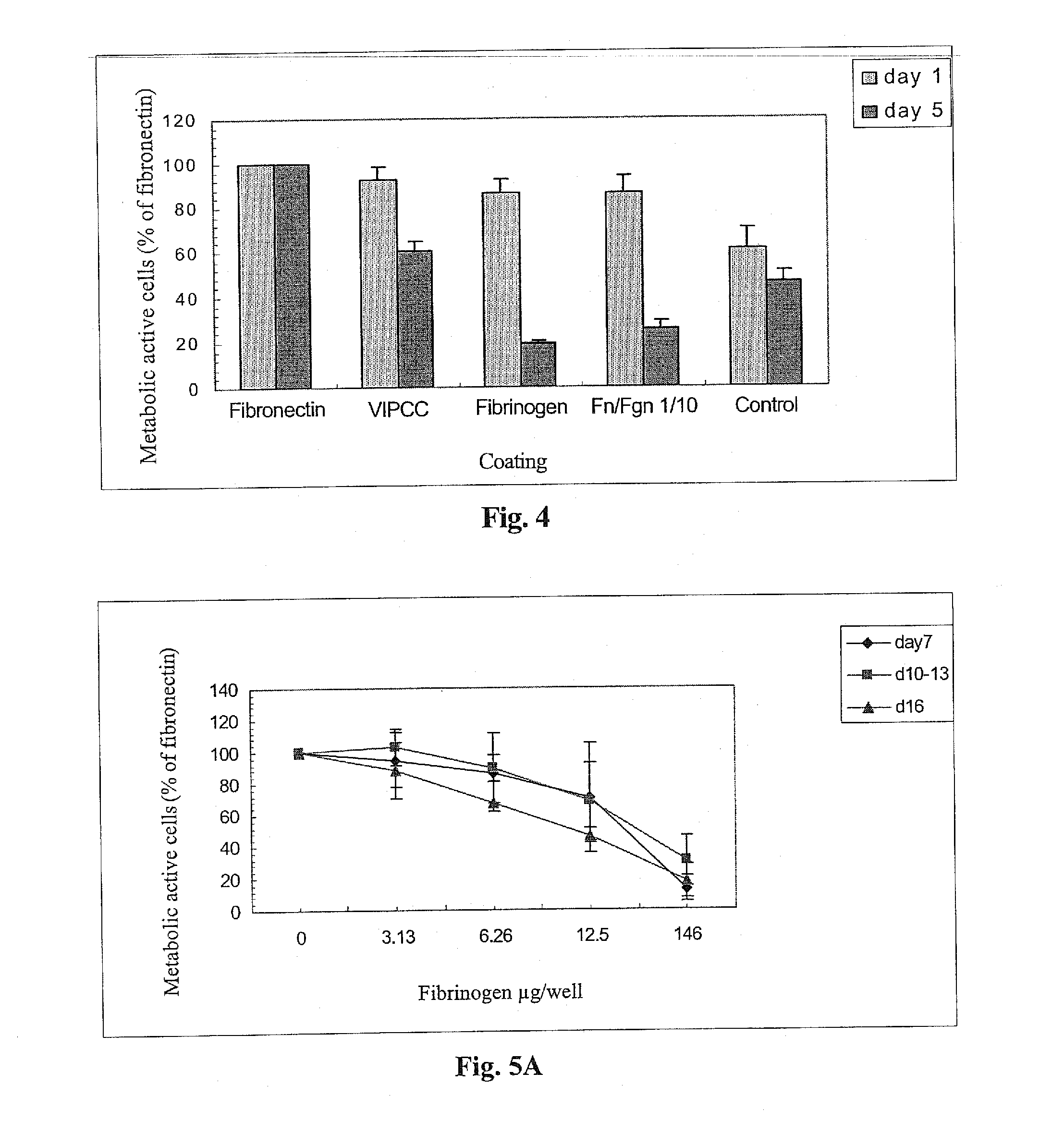
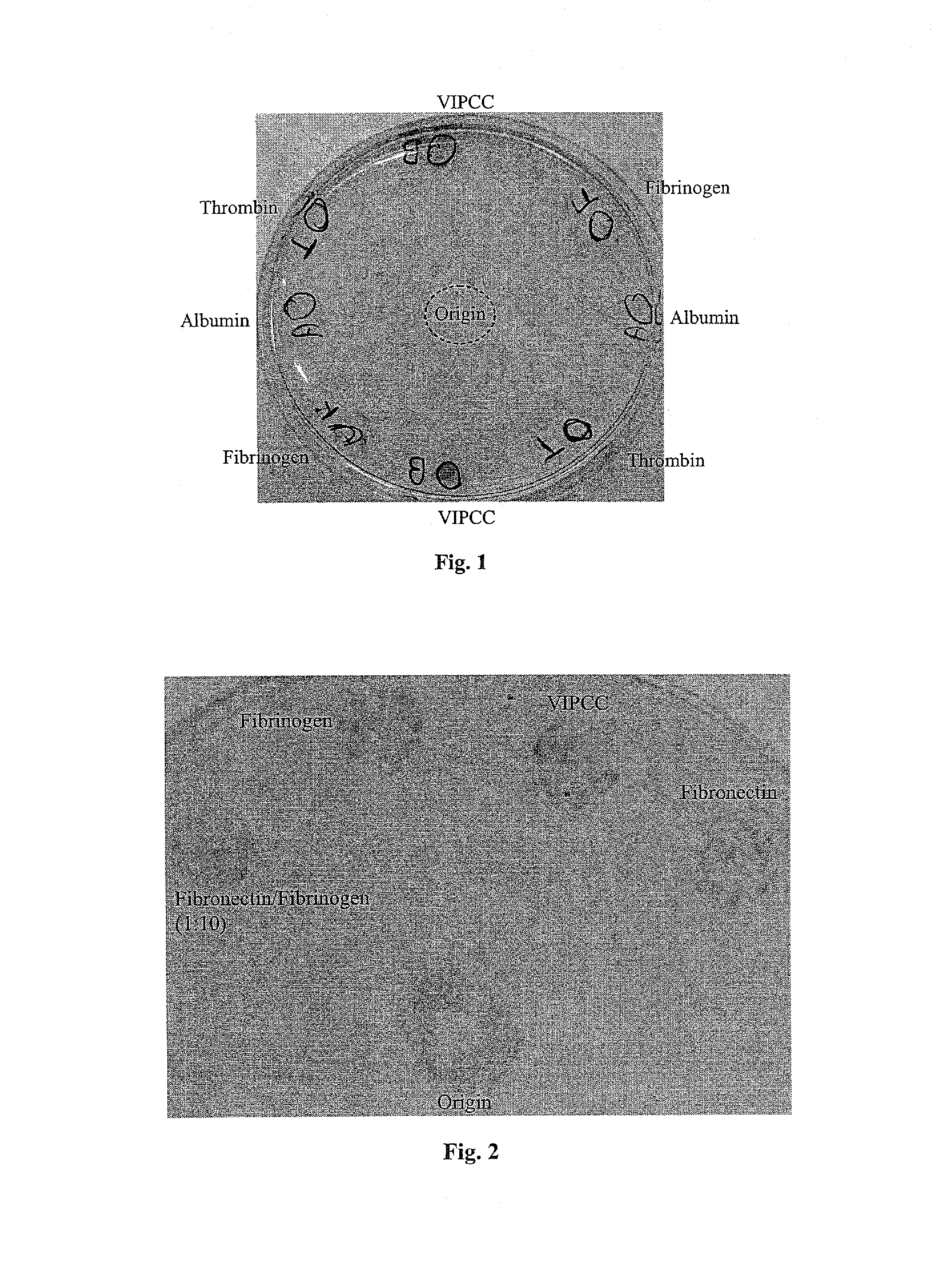
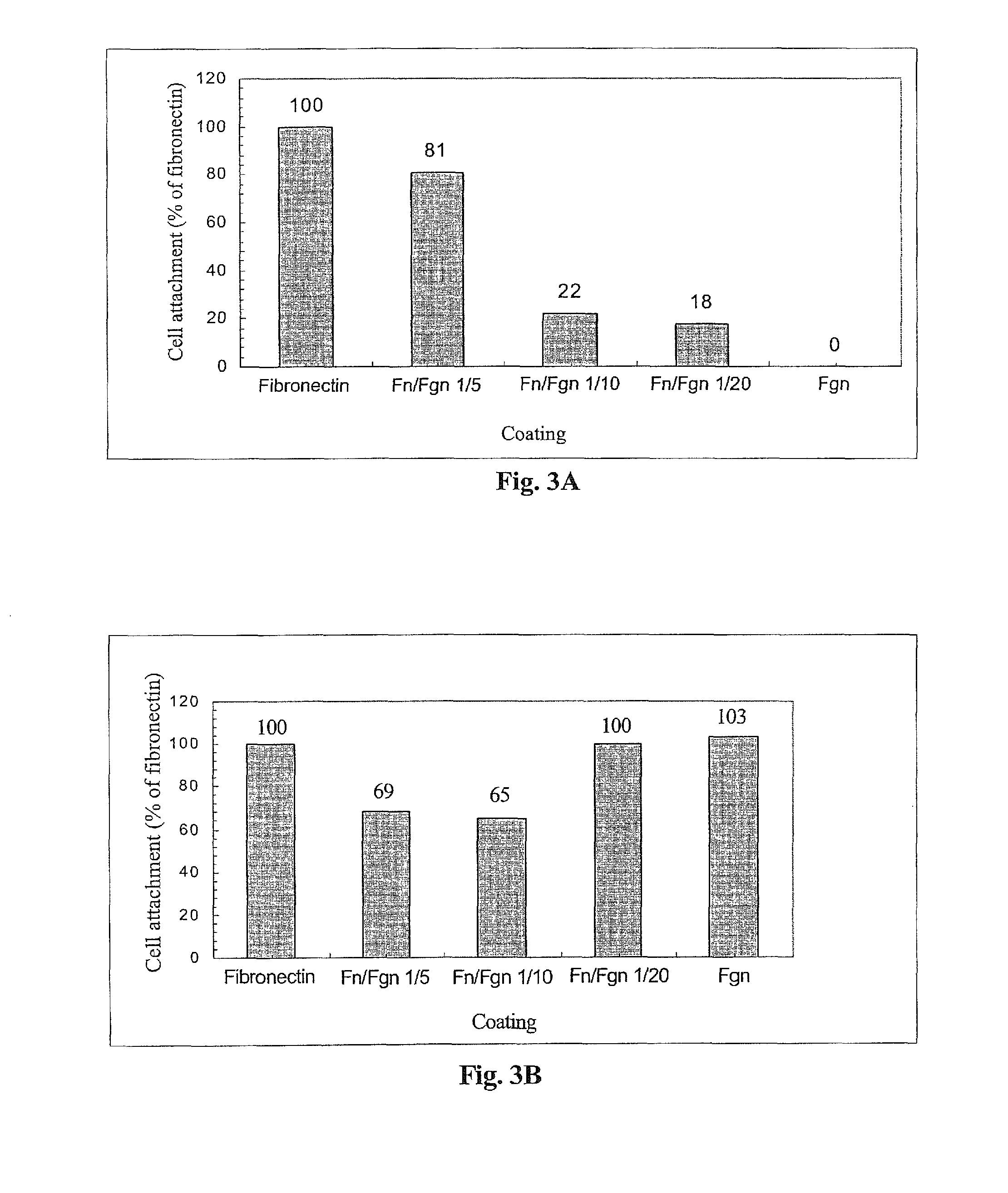
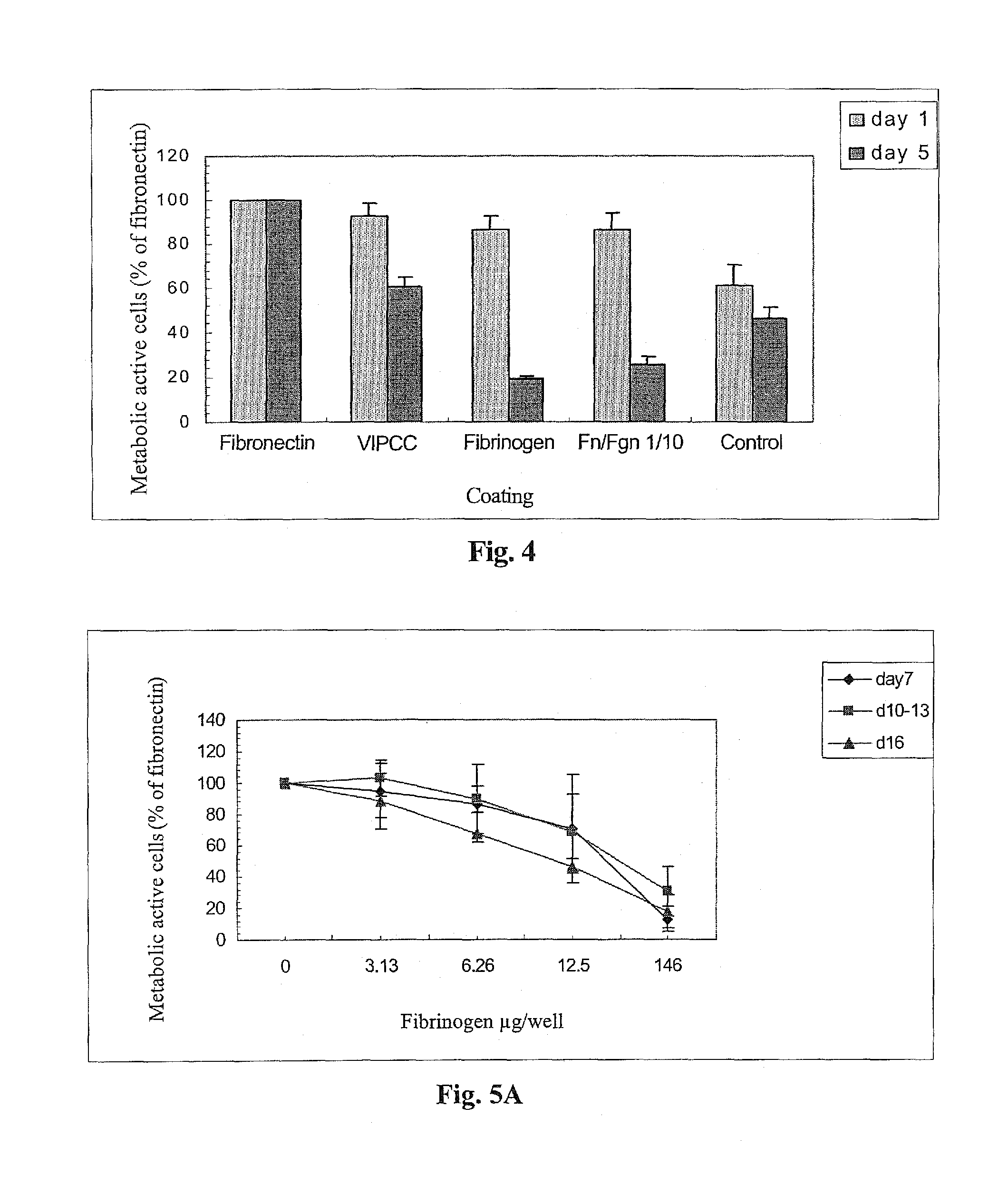
The invention relates to the use of viral inactivated-plasma cryoprecipitate concentrate (VIPCC) comprising a suitable fibronectin / fibrinogen ratio for treating a spine disease, disorder or condition such as intervertebral disc degeneration.
Owner:OMRIX BIOPHARM
Prothrombic complex composition
InactiveCN102459583AMicrobiological testing/measurementInactivation/attenuationCryoprecipitateIon-exchange resin
The present invention relates to a method for preparing a composition or a concentrate of a prothrombic complex that includes the II, VII, IX and X coagulation factors, wherein said method includes the steps of providing a supernatant of a plasma cryoprecipitate, applying said supernatant on an anion-exchange resin in order to produce an eluate containing said complex and proteins having a high molecular weight, and applying said eluate on a hydroxyapatite column in order to produce a second eluate containing said complex. The invention also relates to a composition that can be produced by said method.
Owner:法国分馏学和生物学实验室
Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
InactiveCN105315360AHigh yieldIncrease productivityFactor VIIFibrinogenDEAE SephadexVirus inactivation
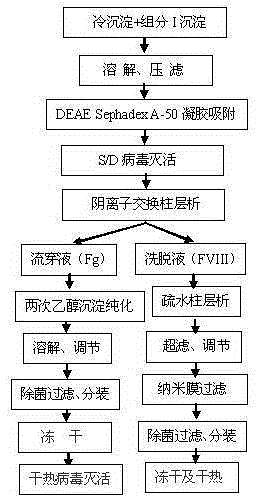
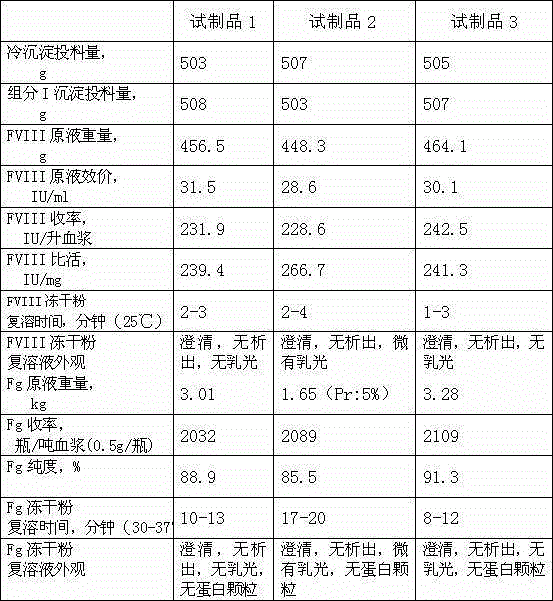
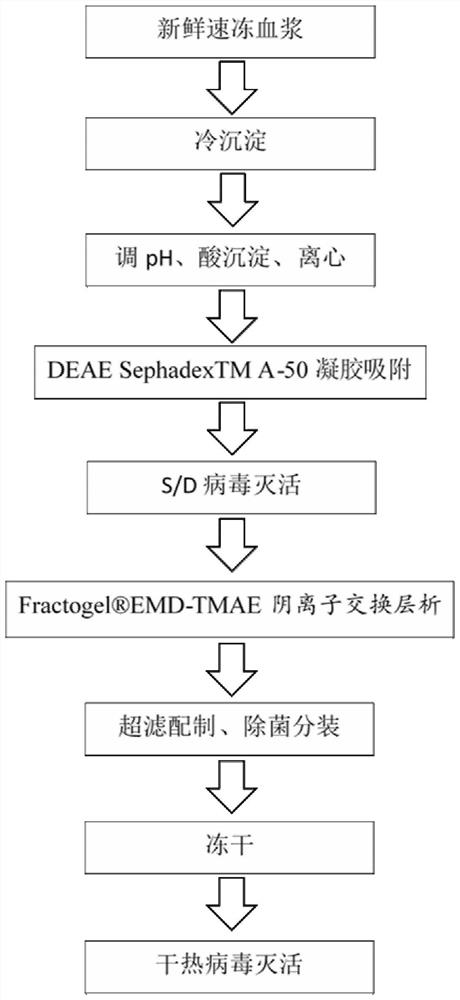
The invention discloses a method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen by cryoprecipitate and component I precipitation, mixing and feeding. The method comprises the following steps: (1) simultaneous feeding and dissolution of a cryoprecipitate and a component I; (2) DEAE Sephadex A-50 gel adsorption; (3) S / D virus inactivation; (4) anion exchange column chromatography; (5) two-step low-temperature ethanol precipitation and purification, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a chromatographic penetration liquid to obtain a human fibrinogen; (6) further hydrophobic column chromatography of a chromatographic eluant; (7) ultrafiltration, nanofilm filtration, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a hydrophobic eluant to obtain a high-purity human coagulation factor VIII. By the adoption of the process, FVIII and Fg in the two raw materials are extracted simultaneously, so that the yields of the two products are greatly improved, the yield of the human coagulation factor VIII can reach 200,000 IU / ton plasmas, the yield of the human fibrinogen exceeds 2,000 bottles / ton plasmas, and the yields are both far higher than those of a traditional process.
Owner:上海洲跃生物科技有限公司
Method for preparing high-purity human coagulation factor VIII
ActiveCN105348382AAvoid damageNo precipitationFactor VIIPeptide preparation methodsUltrafiltrationBlood plasma
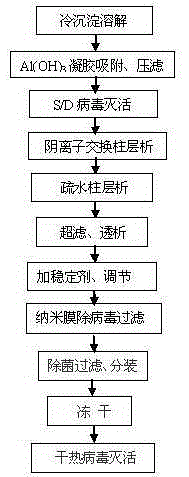
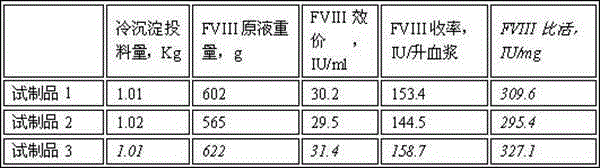
The invention discloses a method for preparing a high-purity human coagulation factor VIII from a cryoprecipitate of a human plasma fraction. The method comprises the following steps: (1) cryoprecipitate dissolution; (2) aluminum hydroxide gel adsorption and filter pressing; (3) S / D virus inactivation; (4) anion exchange resin column chromatography; (5) hydrophobic column chromatography; (6) ultrafiltration dialysis and concentration; (7) addition of one or more stabilizers and titer adjustment; (8) nano-membrane virus-removing filtration; (9) sterile filtration and sub-packaging; (10) freeze-drying; (11) dry-heat virus inactivation. The method has the advantages that a human coagulation factor VIII is purified through the two column chromatography steps, so that the prepared high-purity product can reach a specific activity of about 300 IU / mg, considerably higher than about 50 IU / mg in the prior art, and the product appearance and the heat stability are obviously improved; in the preparation process, three virus removing modes are adopted, so that the clinical use safety of the product can be greatly improved.
Owner:上海洲跃生物科技有限公司
Human coagulation factor VIII preparation method
ActiveCN107226859AImprove securityHigh potencyFactor VIIPeptide preparation methodsFreeze-dryingDissolution
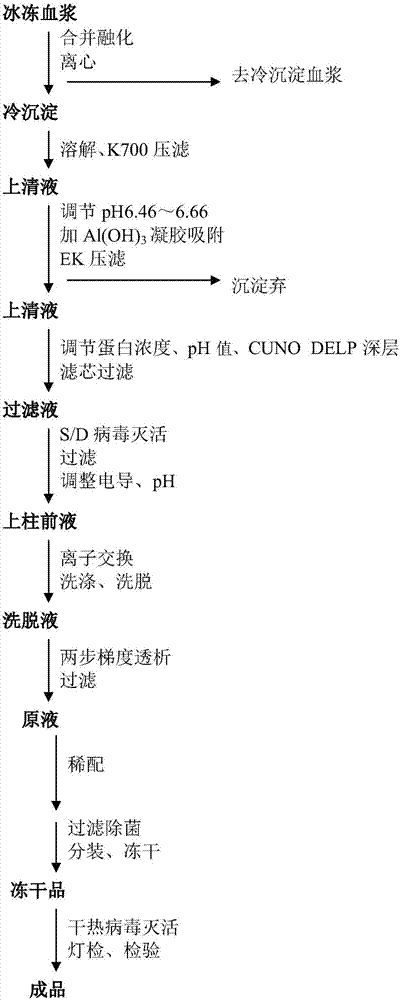
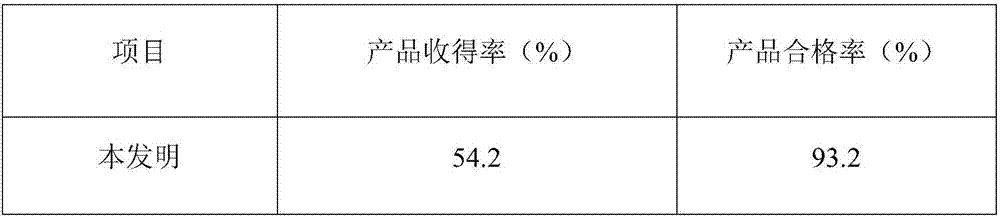
The invention discloses a human coagulation factor VIII preparation method. In the preparation process of a human coagulation factor VIII, a two-step filter press technique is adopted during treatment of human plasma initial materials, that is, a K700 filter plate is adopted for filter pressing after cryoprecipitate dissolution, the filter press liquor is collected, the pH value is adjusted, a 2% aluminium hydroxide gel is added for adsorption, and then an EK filter plate is adopted for filter pressing. The two-step filter press technique is adopted to improve the separation effect, meanwhile, the aluminium hydroxide gel adsorption method is used together to remove a coagulation factor on which vitamin K depends, the aluminium hydroxide gel does not adsorb the human coagulation factor VIII, and the product yield is high. In the preparation process, the two-step filter press technique replaces a traditional two-step high-speed centrifuging method, a CUNO DELP deep filter element is adopted for filtration, a two-step gradient dialysis method is adopted, a re-dissolution and re-freezing process is added to a freeze-drying process, and meanwhile, various virus removal / inactivation technologies are adopted in the production process, so that the product yield can be improved, the risk of virus spreading can be reduced, and the clinical medication security is improved.
Owner:华润博雅生物制药集团股份有限公司
A brewing process of red yeast large perfume pear wine
The invention discloses a brewing process of red yeast large perfume pear wine, including the following steps: firstly preparing red yeast wine mash, performing selection, cleaning, quickly crushing to the pear raw material and adding appropriate amount of sulfur dioxide for beating, then adding red yeast wine mash, adding pectinase, preliminary filtering, clarifying, sugar acid modulating, inoculating composite saccharomyces cerevisiae for main fermentation under 20-35 DEG C, inverting barrels (tanks), rear fermentation, inverting barrels (tanks), northern winter outdoor frozen precipitation(southern low-temperature treatment), filtering, aging, filtering, storage, deployment, diatomite fine filtering, high-temperature instantaneous sterilization, sterilized canning, finished product, inspection, warehousing storage, finished product. The invention has unique process, antioxidant browning control of pear, red yeast wine mash adding for not only increasing scent but also improving the wine filtration and reducing sugar adding amount, so that the pear wine has thick scent and has dual nutrition of pear wine and red yeast wine.
Owner:吉林森工集团泉阳泉饮品有限公司
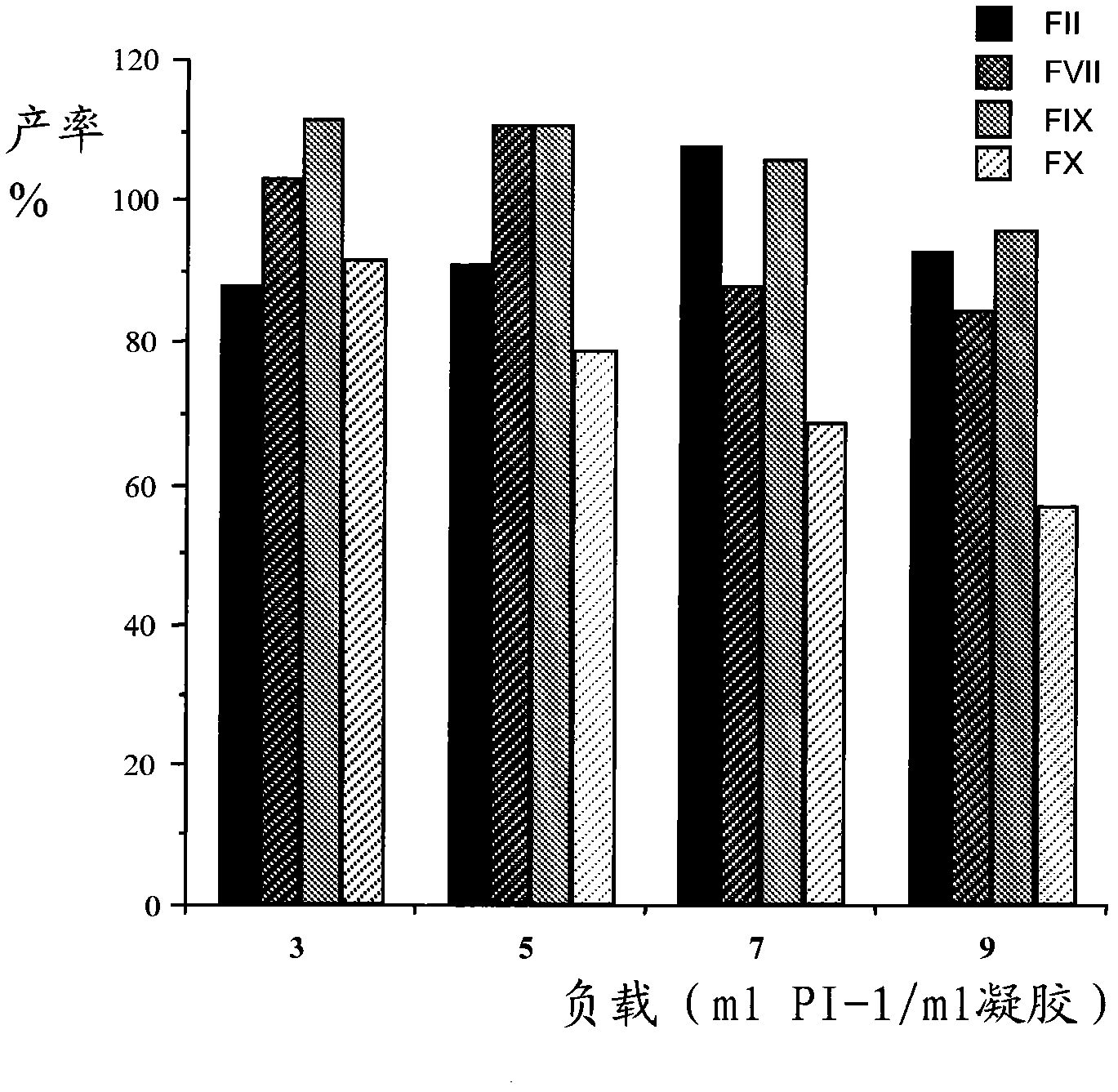
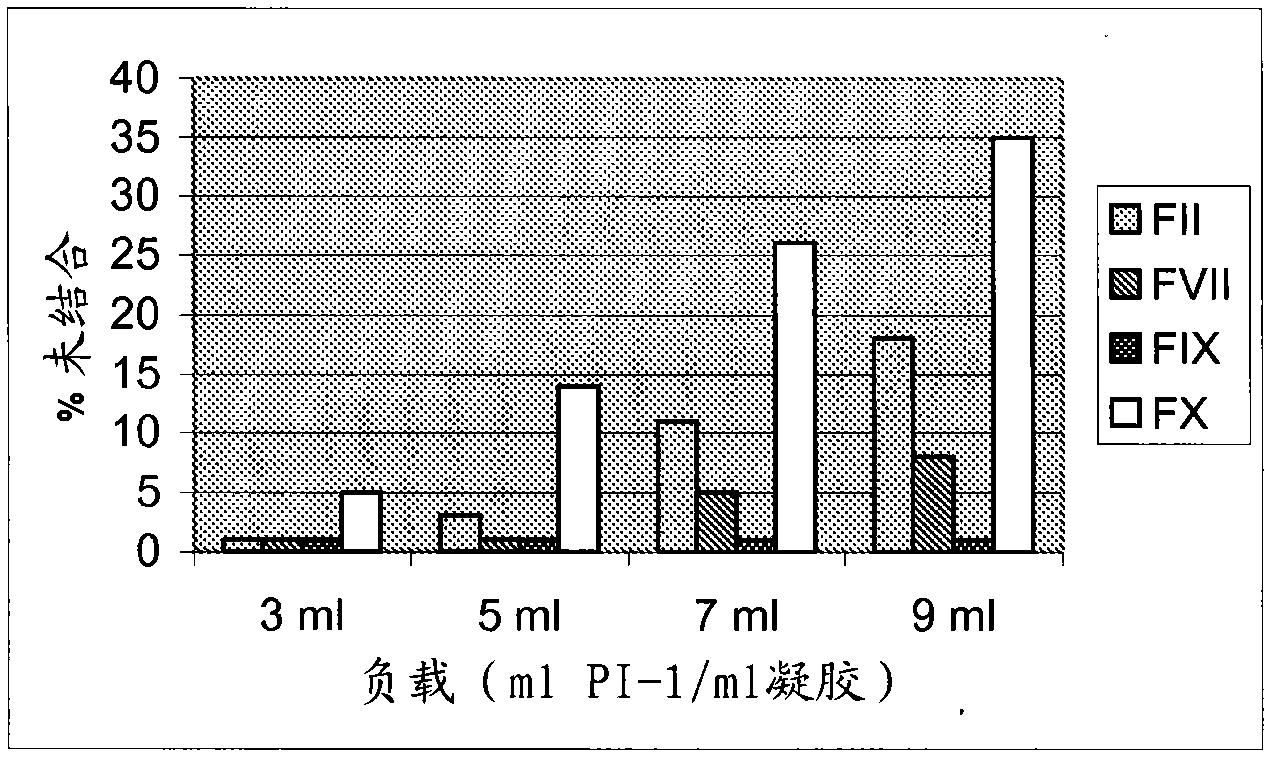
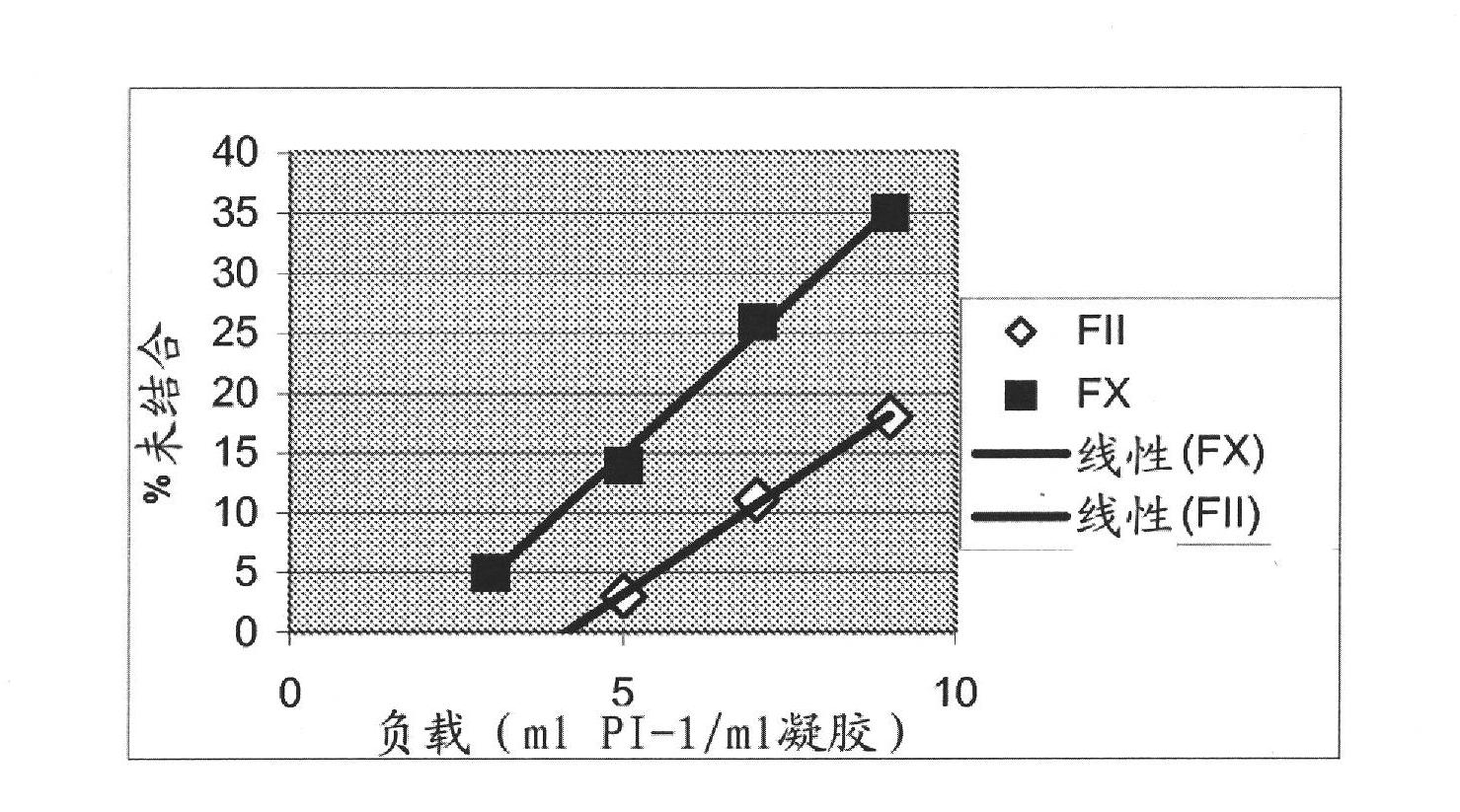
Technological process for improving stable FVII yield of human prothrombin complexes
ActiveCN101439047AImprove bindingHigh yieldPowder deliveryPeptide/protein ingredientsFiltrationUltrafiltration
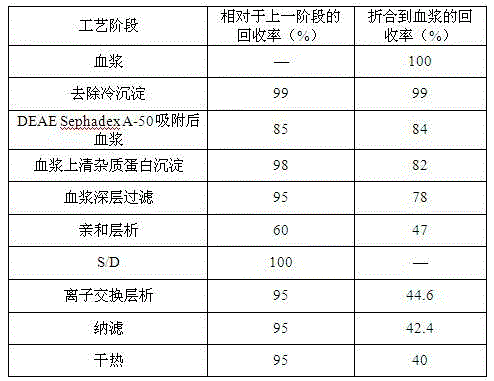
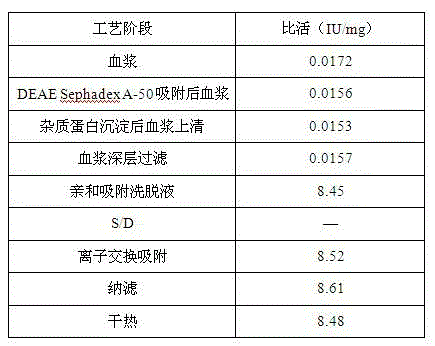
The invention relates to the field of bio-pharmaceutical technology, in particular to a technical method for improving stable F VII yield rate of human thrombogen complex. The following procedures are included: freshly freezing blood plasma of a healthy person at a low temperature; removing cryoprecipitate; carrying out clarification filtration; adjusting ionic strength; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; killing S / D virus; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; carrying out aseptic filtration and subpackage; carrying out freeze-drying; and carrying out hot-air sterilization.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD +1
Production method of human antithrombin III
ActiveCN104672328AHigh purityIncrease capacityPeptide preparation methodsProtease inhibitorsDEAE SephadexVirus inactivation
The invention relates to a production method of human antithrombin III. The production method comprises the following steps: removing cryoprecipitate from fresh frozen human plasma to obtain a plasma supernatant, and carrying out adsorption treatment on the plasma supernatant by using a DEAE Sephadex-A50 gel; precipitating impurity protein of the plasma supernatant, and deeply filtering plasma; carrying out Capto Heparin affinity chromatography; carrying out S / D virus inactivation and Capto Q ion exchange chromatography, and removing an S / D reagent; and filtering by virtue of a nanometer film to remove viruses, preparing, freeze-drying and dry-heating. The process is capable of preparing the antithrombin III by virtue of affinity chromatography with only one step. According to the production method disclosed by the invention, the recovery rate can reach above 40 percent, and the specific activity can reach 8-10 IU / mg.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
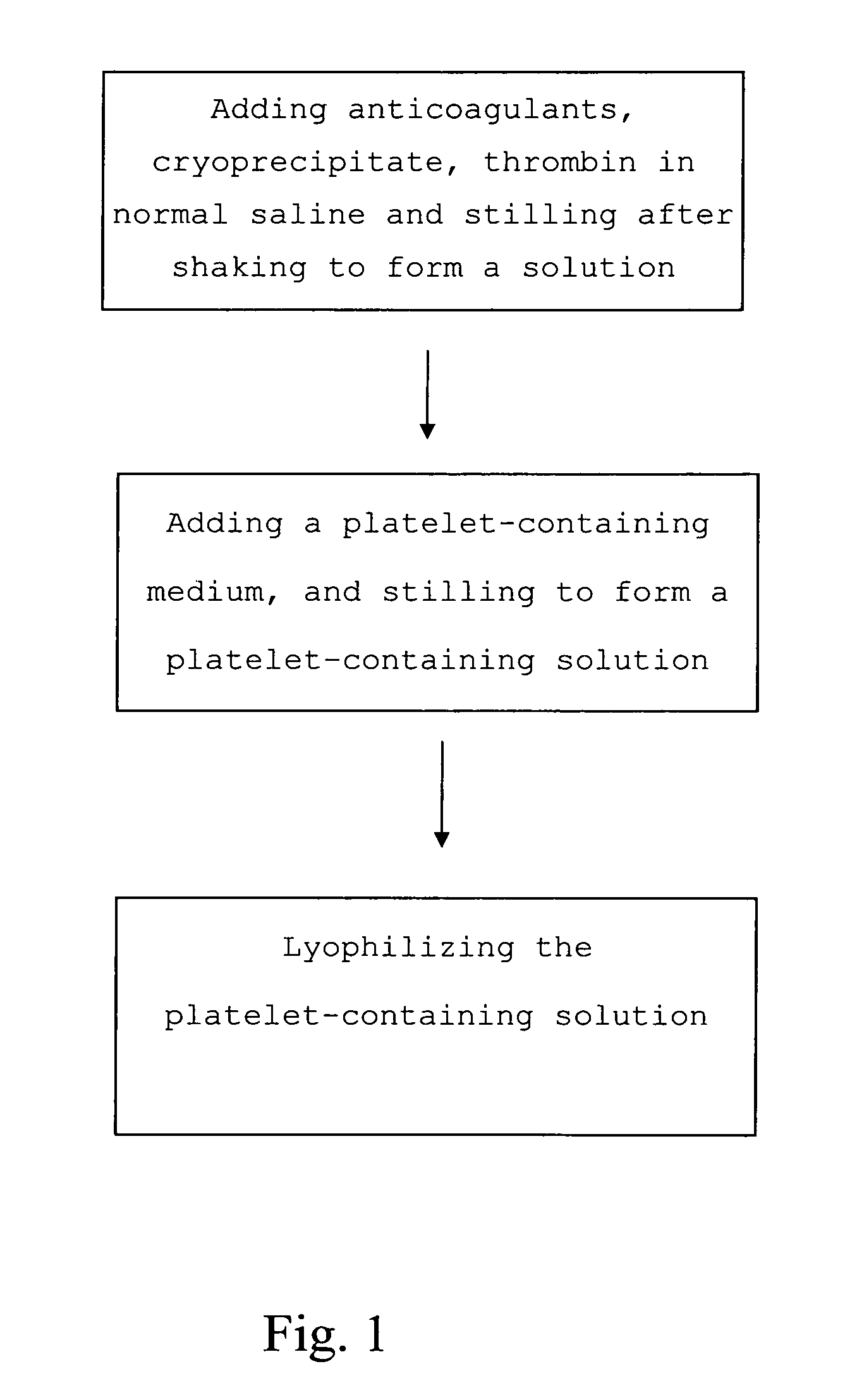
Medium comprising cryoprecipitate and method for preserving platelets, red blood cells and other cells without a nucleus
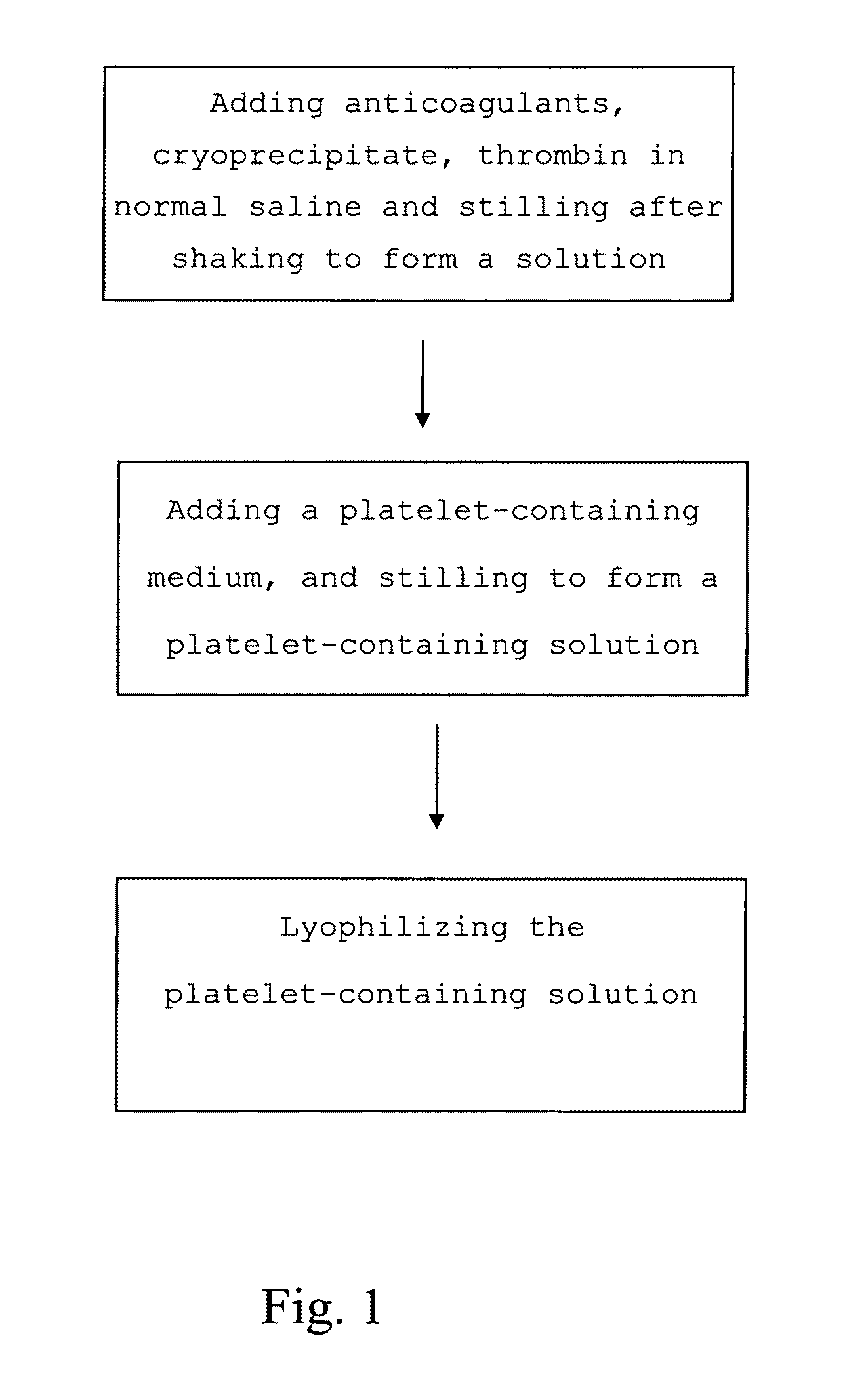
The present invention provides a platelet-containing composition prepared by contacting platelets with a medium for preserving. The medium comprises anticoagulant, cryoprecipitate and thrombin. The present invention also provides a method for long-term preservation of platelets, comprising the steps of: (a) adding an anticoagulant, cryoprecipitate, thrombin in normal saline; (b) adding a platelet-containing medium into the solution formed in step (a); and (c) lyophilizing the platelet-containing solution formed in step (b). Moreover, the present invention yet provides a medium for preserving non-nucleus cells.
Owner:LIN CHENG CHIH +1
Polyvinyl Pyrollidone Cryoprecipitate Extraction of Clotting Factors
InactiveUS20080281081A1Increase productionEliminate and suppressFactor VIISurgical adhesivesBlood collectionCITRATE ESTER
Blood collection, processing and transfer leads to the separation of discrete fractions by adding additional citrate (trisodium citrate) to bring the citrate concentration to 10%-15% w / v thereby leading to enhanced yield and purity of cryoprecipitate. The improved cryoprecipitate then yields concentrated clotting factors by an improved extraction process which uses polyvinyl pyrollidone to reduce the extraction of fibrinogen. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds
Owner:SHANBROM TECH
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS7727743B2Improve protectionImprove solubilityPowder deliveryPeptide/protein ingredientsProtein compositionCryoprecipitate
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Method for preparing cryoprecipitate and method for preparing blood coagulation factor VIII preparation by using cryoprecipitate
ActiveCN103848886AReduce the amount of solutionFactor VIIPeptide preparation methodsBlood coagulation factor VIIICryoprecipitate
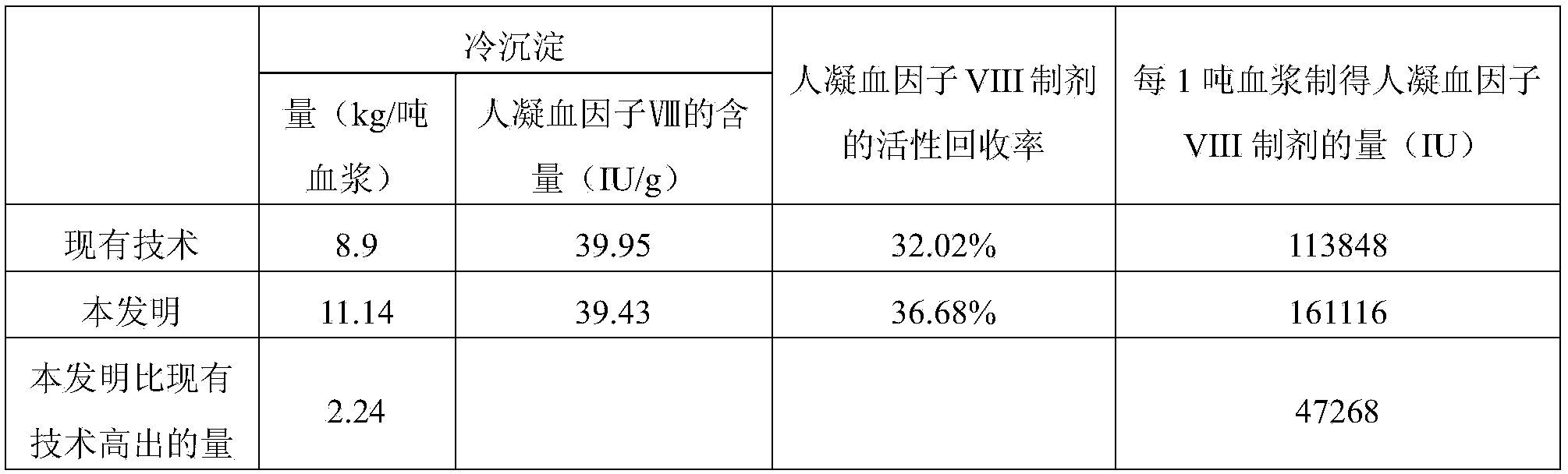
The invention discloses a method for preparing a cryoprecipitate. The method comprises the steps of (1) thawing: selecting fresh frozen plasma, and heating to obtain thawed plasma of which the temperature is 0-5 DEG C; (2) filtering: filtering under the condition that the temperature of the thawed plasma is 0-5 DEG C to obtain filtrate and filter residues; (3) centrifuging: centrifuging under the condition that the temperature of the filtrate is 0-5 DEG C to obtain a precipitate; (4) combining the filter residues obtained in step (2) and the precipitate obtained in step (3) to obtain the cryoprecipitate. The preparation method disclosed by the invention is simple, and the prepared cryoprecipitate is high in yield and human blood coagulation factor VIII content, so the preparation method has a good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
Process for extracting human coagulation factor VIII from plasma
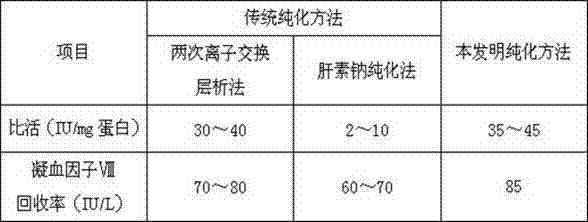
The invention discloses an economic and simple method for improving the separation efficiency, activity, specific activity and recovery rate of coagulation factor VIII. The method comprises washing cryoprecipitate twice with sodium heparin firstly, then carrying out ion exchange chromatography and eluting with an eluent to obtain higher-purity coagulation factor VIII. Compared with traditional methods, the method has the advantages of simple process and low cost; the separation efficiency, activity, specific activity and recovery rate of the coagulation factor VIII are also improved.
Owner:新疆德源生物工程有限公司
Solubilization technology for producing human fibrinogen
ActiveCN103405754AImprove utilizationEffective inactivationPowder deliveryFibrinogenSolubilityLipid formation
The invention relates to a solubilization technology for producing human fibrinogen. The solubilization technology comprises following main steps: (1) source plasma treatment; (2) dissolving and centrifuging of cryoprecipitate; (3) inactivation of viruses; (4) chromatography purification; (5) centrifugal separation; (6) dissolving of sediments; (7) degerming and filtering; (8) split charging and freeze drying. The solubilization technology has the advantages that the purity of the extracted product can be up to over 95%; the content of foreign protein is low; a lipid envelope virus and a non-lipid envelope virus can be effectively activated; the safety of the product is ensured; arginine hydrochloride and glycine are adopted as stabilizers; the product can be fully protected in a freeze-drying process; the temperature change of the product in the freeze-drying process is stable by prolonging the freeze-drying time (about 4-8 days, and about 3 days for general factories); a freeze-dried product with a uniform structure can be obtained; the solubility of the human fibrinogen is increased; the product is quick to dissolve after being re-dissolved; no highly visible denatured protein is generated.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Preparation method of human VIII blood coagulation factor
ActiveCN103613658AEasy to manufactureQuality is easy to controlFactor VIIPeptide preparation methodsFreeze-dryingDissolution
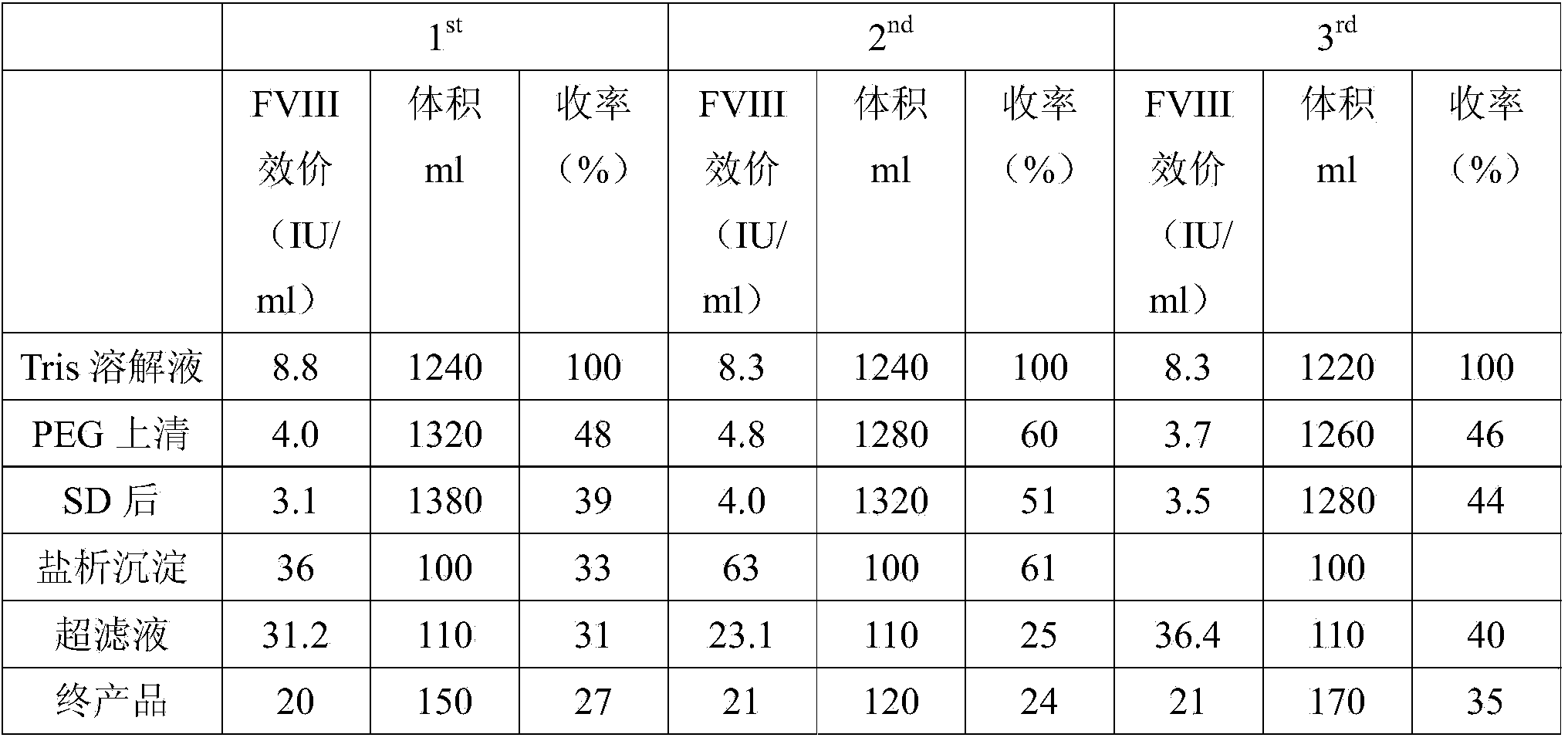
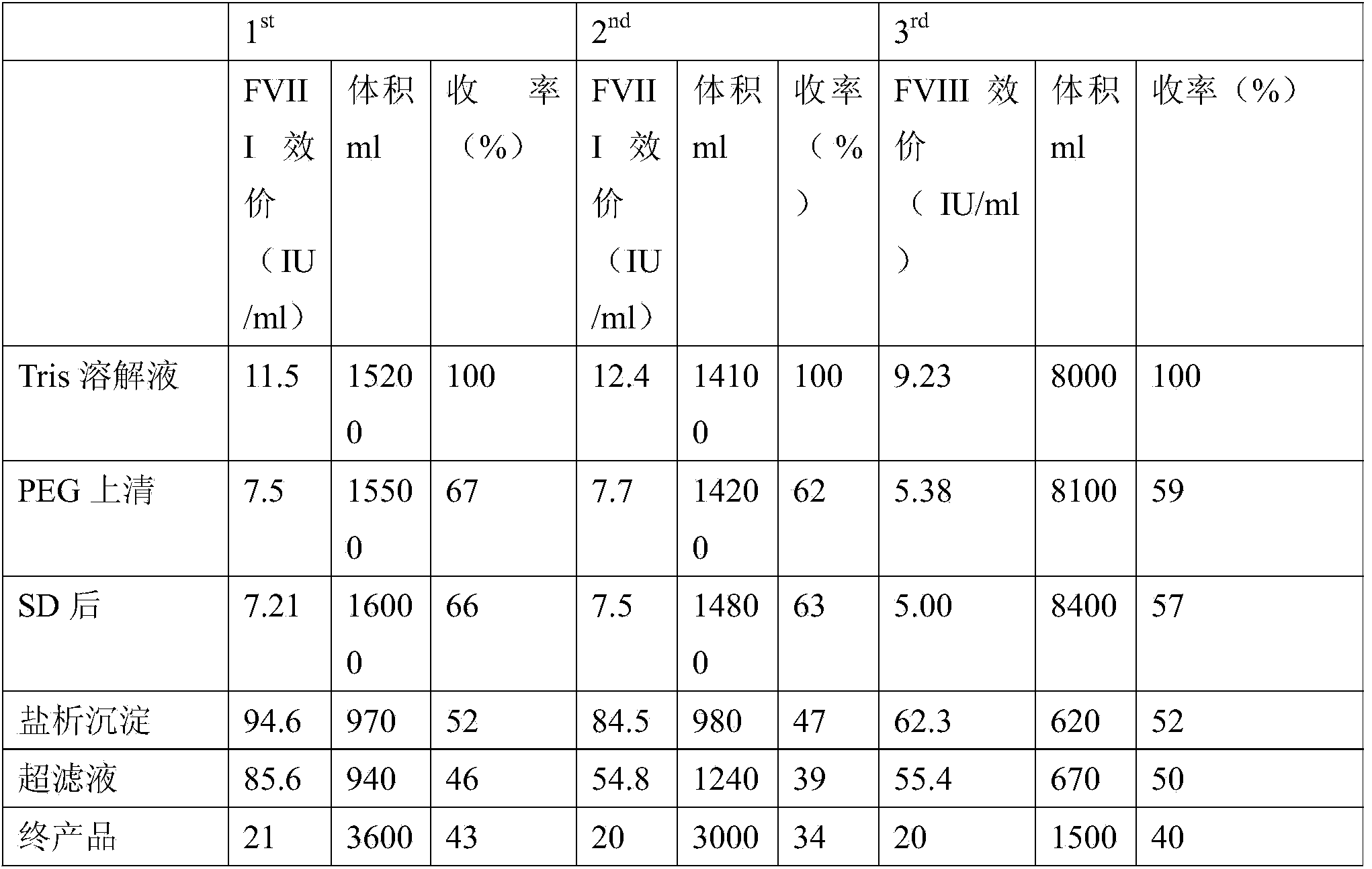
The invention discloses a preparation method of a human VIII blood coagulation factor. The preparation method comprises the following steps: (1) separating cryoprecipitates from human plasma, and adding a Tris-HCl dissolving solution to dissolve; (2) primary PEG (Polyethylene Glycol) precipitation: adding a PEG 1 solution into the dissolving solution, stirring, standing, and then, centrifuging; (3) collecting a centrifuged suspension liquid, and adding an S / D solution to inactivate; (4) secondary PEG precipitation: clarifying the inactivated suspension liquid, filtering to obtain a filtrate, transferring the filtrate into a low-temperature reaction tank, adding a PEG 2 solution under the condition of stirring, stirring, standing, and then, centrifuging; and (5) cleaning a precipitate, dissolving the sediment by using the dissolving solution, centrifuging after complete dissolution, collecting the suspension liquid, filtering, preparing, degerming, split charging, freeze-drying, capping, and carrying out dry heat inactivation to obtain the human VIII blood coagulation factor. A two-step PEG precipitation method is adopted in the method disclosed by the invention, so that the production process is simplified, and the requirement for large equipment is reduced; the preparation method is small in floor area, simple in operation, low in cost and capable of increasing the labor efficiency and economic benefit.
Owner:TONROL BIOLOGICAL PHARM CO LTD
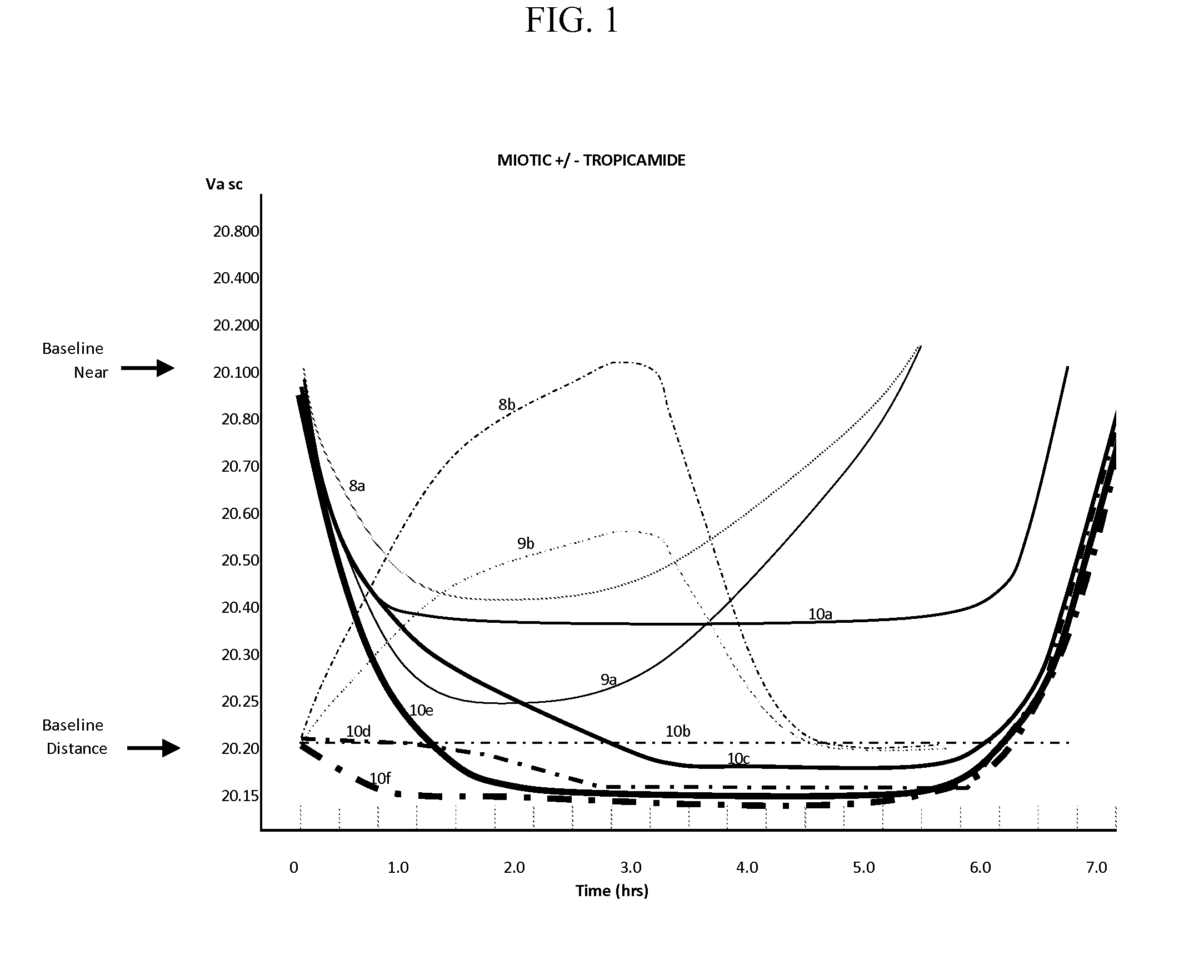
Compositions and Methods for the Improvement of Distance Vision and the Treatment of Refractive Errors of the Eye
ActiveUS20150290126A1Eliminating optical aberrationImprove visual acuityBiocideInorganic non-active ingredientsRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
Preparation method of human fibrinogen
InactiveCN105504046AEasy extractionImprove utilizationFibrinogenPeptide preparation methodsFiberFiltration
The invention discloses a preparation method of human fibrinogen. The method comprises the following steps that 1, cryoprecipitate serves as a staring material and is dissolved; 2, clarification and filtration are conducted; 3, solvent / detergent (S / D) inactivation is conducted; 4, Q Sepharose fastflow gel adsorption chromatographic processing is conducted; 5, centrifugation is conducted, precipitation is collected, sterilizing, subpackaging, freeze-drying, capping and dry-heating inactivating are conducted, and the product is obtained. The preparation method of the human fibrinogen is simple, easy to operate, low in cost and capable of increasing the comprehensive utilization ratio of blood plasma, and the obtained product is high in purity, activity and safety.
Owner:TONROL BIOLOGICAL PHARM CO LTD
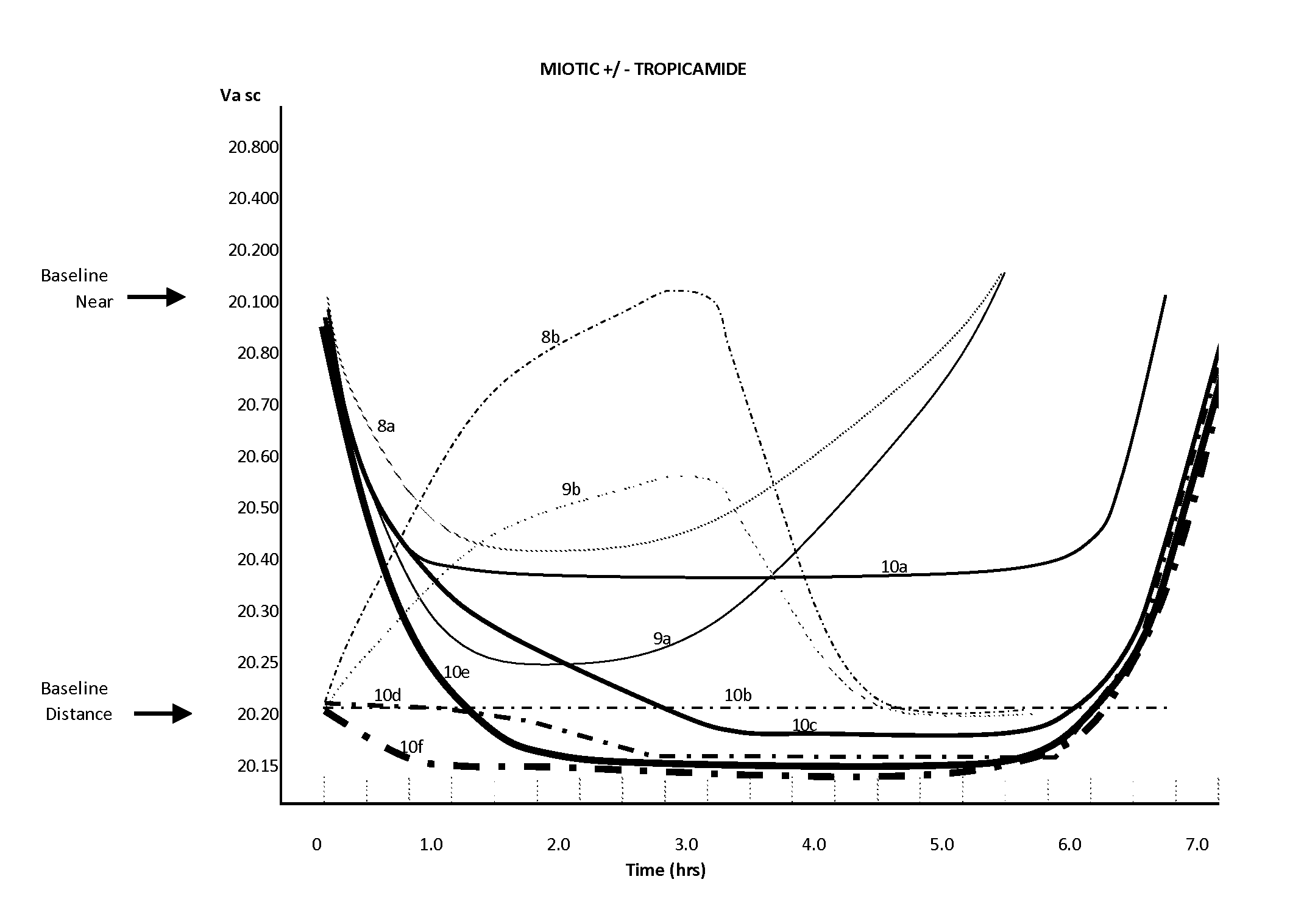
Compositions and methods for the improvement of distance vision and the treatment of refractive errors of the eye
ActiveUS9314427B2Organic active ingredientsPharmaceutical delivery mechanismRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
Preparation method of human blood coagulation factor VIII and human blood coagulation factor VIII product
ActiveCN107880112AHigh recovery rateTaller than aliveFactor VIIPeptide preparation methodsWhole blood productIon chromatography
The invention discloses a preparation method of a human blood coagulation factor VIII and a human blood coagulation factor VIII product and relates to the field of blood products. The preparation method of the human blood coagulation factor VIII comprises the following steps: dissolving a cryoprecipitate with a 0.015-0.025mol / L tromethamine solution in a mass ratio of (3-5): 1 by reasonably processing the cryoprecipitate; and carrying out separation and purification by means of a polyethylene glycol precipitating method combined with ion exchange chromatography, wherein the recovery ratio of the human blood coagulation factor VIII and the specific activity of a final product can be improved effectively, the yield reaches 180-240IU / L plasma, and the specific activity is not lower than 100IU / mg proteins. The prepared human blood coagulation factor VIII product is rich in vWF factors and the proportion of the vWF factors and the human blood coagulation factor VIII is close to 1: 1. Besides treating hemophiliac A, the human blood coagulation factor VIII can be also used for treating patients with angiohemophilia, and the human blood coagulation factor VIII has good stability and heat resistance.
Owner:HUALAN BIOLOGICAL ENG INC +2
Medium and method for preserving platelets, red blood cells, and other non-nucleus cells and platelets-containing composition
The present invention provides a platelet-containing composition prepared by contacting platelets with a medium for preserving. The medium comprises anticoagulant, cryoprecipitate and thrombin. The present invention also provides a method for long-term preservation of platelets, comprising the steps of: (a) adding an anticoagulant, cryoprecipitate, thrombin in normal saline; (b) adding a platelet-containing medium into the solution formed in step (a); and (c) lyophilizing the platelet-containing solution formed in step (b). Moreover, the present invention yet provides a medium for preserving non-nucleus cells.
Owner:LIN CHENG CHIH +1
Preparation method of freeze-dried human blood coagulation factor VIII
PendingCN112028988AHigh purityHigh activityFactor VIIPeptide preparation methodsCryoprecipitateVirus inactivation
The invention relates to a preparation method of a freeze-dried human blood coagulation factor VIII. The preparation method comprises the following steps of collecting plasma and quick-frozen plasma;performing plasma melting and separating cryoprecipitate; dissolving cryoprecipitate; performing acid precipitation; performing gel adsorption; inactivating S / D virus; performing anion exchange chromatography; performing ultrafiltration; preparing a semi-finished product; carrying out sterilizing and sub-packaging; performing freeze-drying; and carrying out dry heat virus inactivation. The preparation method disclosed by the invention is short in product remelting time, high in specific activity, stable in quality and simple to operate, and after industrialization, the utilization rate of plasma resources can be greatly improved, market supply is increased, and the situation of FVIII medication tension in the Chinese market is relieved.
Owner:哈尔滨派斯菲科生物制药有限公司
A kind of production method of human antithrombin III
ActiveCN104672328BIncrease capacityDoes not affect productionPeptide preparation methodsProtease inhibitorsDEAE SephadexVirus inactivation
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Production method of fibrinogen
PendingCN111518197AHigh purityLow purityFibrinogenPeptide preparation methodsBiotechnologyVirus inactivation
The invention relates to a production method of fibrinogen. The production method comprises the following steps of collecting plasma and quickly freezing plasma; melting plasma and removing cryoprecipitate; preparing a component I precipitate; dissolving the component I precipitate; inactivating virus by adopting an S / D virus inactivation method; performing ethanol precipitation for the first time, and performing centrifuging and dissolving; performing ethanol precipitation for the second time, performing centrifuging and separating a precipitate; preparing a semi-finished product; performingsterilizing and sub-packaging; performing freeze drying; and carrying out dry heat inactivation of viruses. The method has the advantages that the purity of the obtained fibrinogen is greatly improvedand can reach 92% or above; the solidification activity is high and is 18-22 seconds; the re-melting time is short and is 5-10 minutes; after freeze-drying, the appearance is more uniform and stable,the yield is more than 2000 bottles per ton of plasma, and the yield is obviously improved; the long-term stability of the product is good, and the good inherent quality can still be guaranteed after36-month long-term stability test; and due to double virus inactivation, the clinical medication is safer, and no adverse reaction is caused.
Owner:哈尔滨派斯菲科生物制药有限公司
Method for chromatography
ActiveUS11352388B2High purityHigh activityFactor VIICation exchanger materialsFactor VIII vWFCryoprecipitate
The present invention relates to the field of chromatography. More closely, the invention relates to a chromatographic method for purification of Factor VIII and von Willebrand factor from a cryoprecipitate of plasma. The chromatographic method is performed on a matrix comprising an inner porous core and outer porous lid surrounding said core.
Owner:CYTIVA BIOPROCESS R&D AB
Compositions suitable for treatment of spinal disease, disorder or condition
InactiveUS8968724B2Raise the ratioIncreased proliferationBiocideNervous disorderDiseaseCryoprecipitate
Owner:OMRIX BIOPHARM
Method for Chromatography
ActiveUS20180127459A1High purityHigh activityFactor VIICation exchanger materialsCryoprecipitateFactor VIII vWF
The present invention relates to the field of chromatography. More closely, the invention relates to a chromatographic method for purification of Factor VIII and von Willebrand factor from a cryoprecipitate of plasma. The chromatographic method is performed on a matrix comprising an inner porous core and outer porous lid surrounding said core.
Owner:CYTIVA BIOPROCESS R&D AB
Method for preparing cryoprecipitate coagulation factor
InactiveCN107281222AShort preparation timeAccurate capacityPharmaceutical delivery mechanismMammal material medical ingredientsCryoprecipitateFrequency conversion
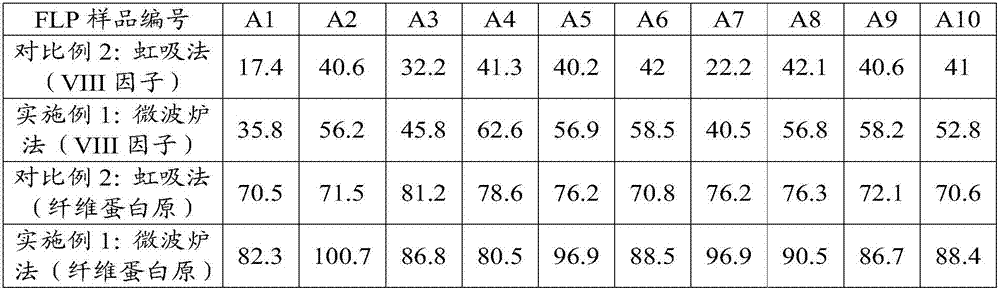
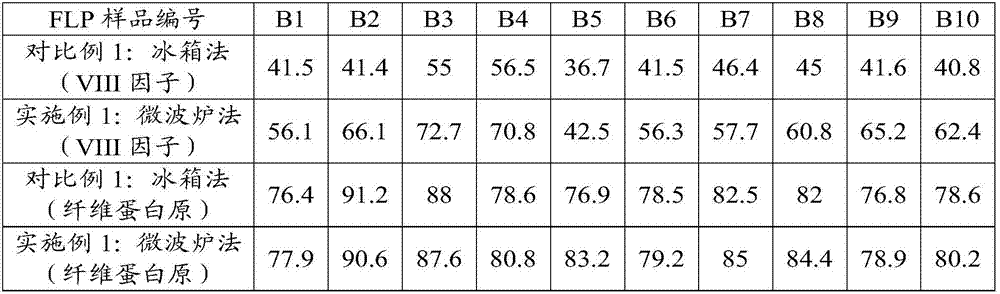
The invention provides a method for preparing a cryoprecipitate coagulation factor. The method for preparing the cryoprecipitate coagulation factor, provided by the invention, comprises the following steps: a) providing fresh lyophilized plasma; b) unfreezing: unfreezing the fresh lyophilized plasma through microwaves by adopting a frequency conversion microwave heating device to unfreezing temperature, so as to obtain an ice-block-shaped mixture; c) separating: separating a precipitated substance, which is not dissolved coldly, from the ice-block-shaped mixture, so as to obtain the cryoprecipitate coagulation factor. The method provided by the invention has the advantages of simple equipment and convenience for operation, short preparation time and accurate preparation capacity, more content of obtained VIII factors, good clinical transfusion effect and the like.
Owner:侯广安 +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com