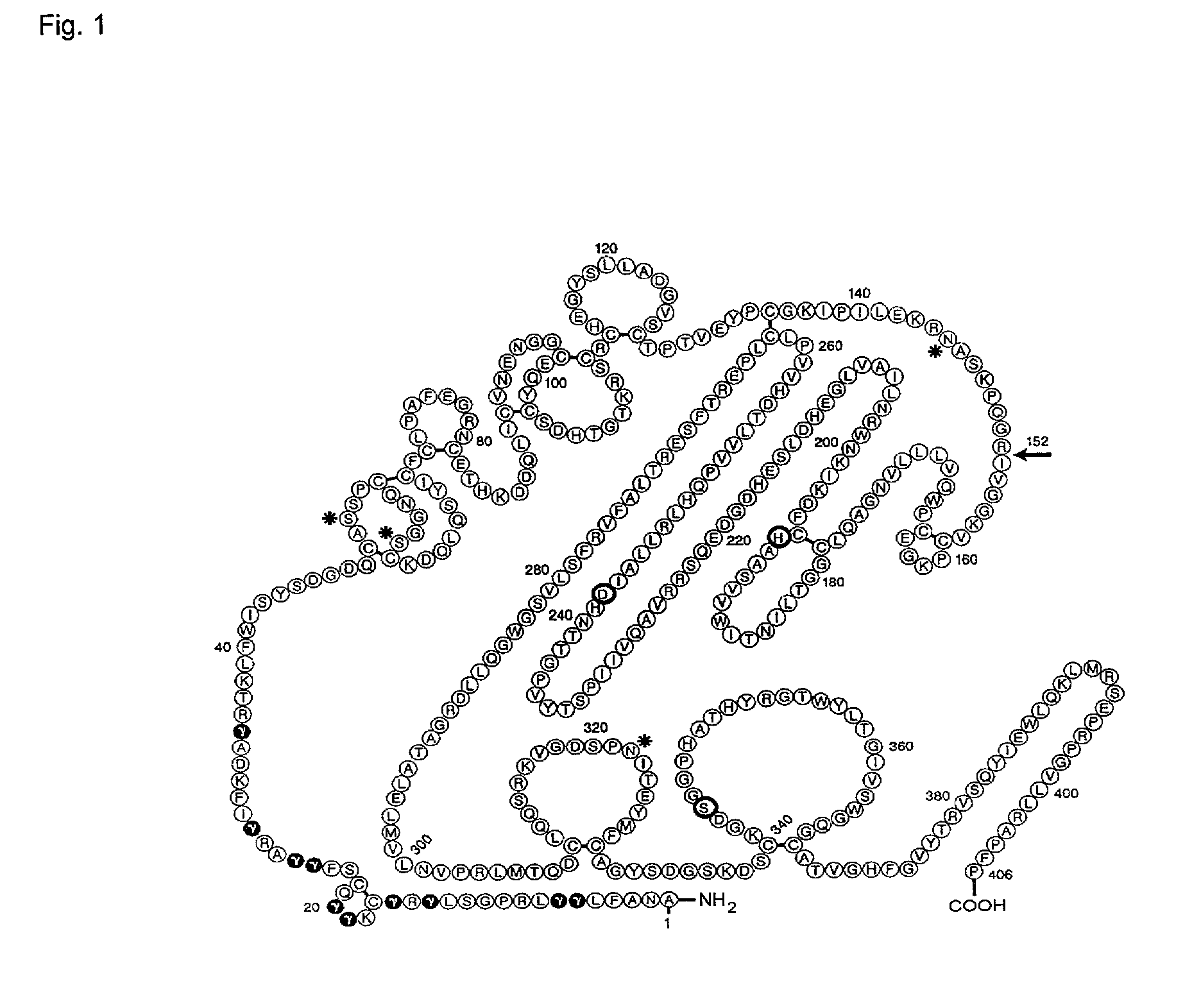
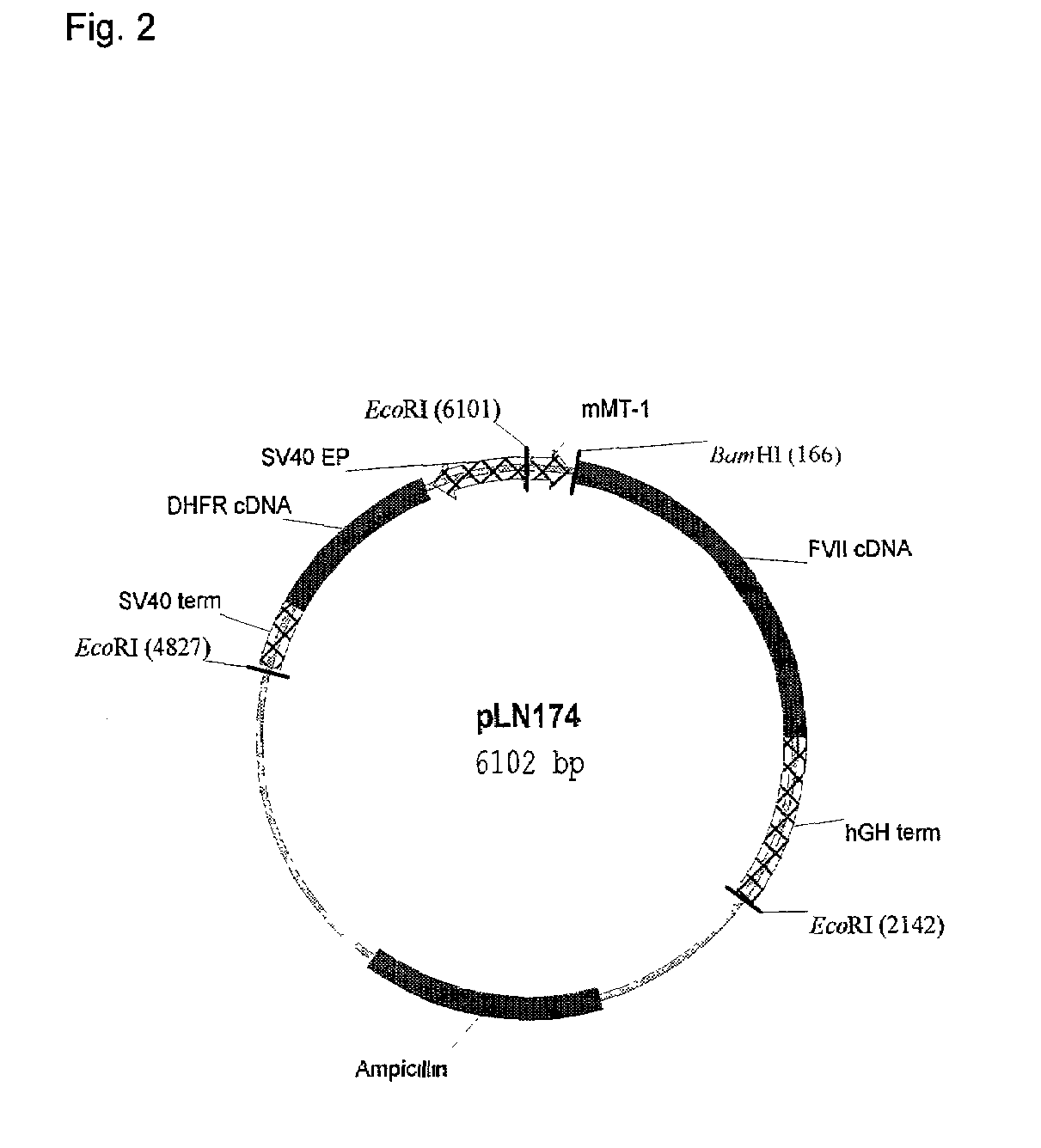
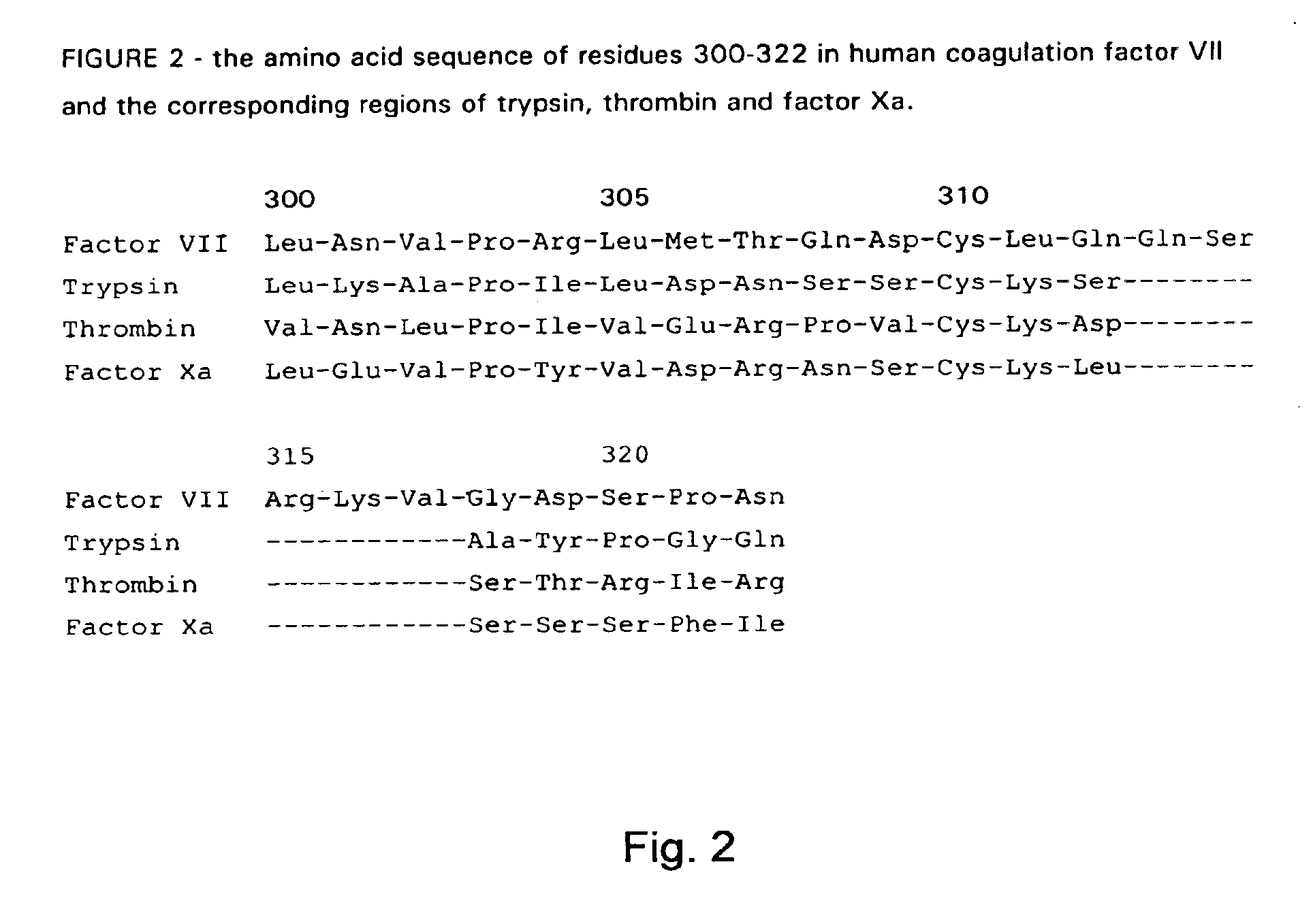
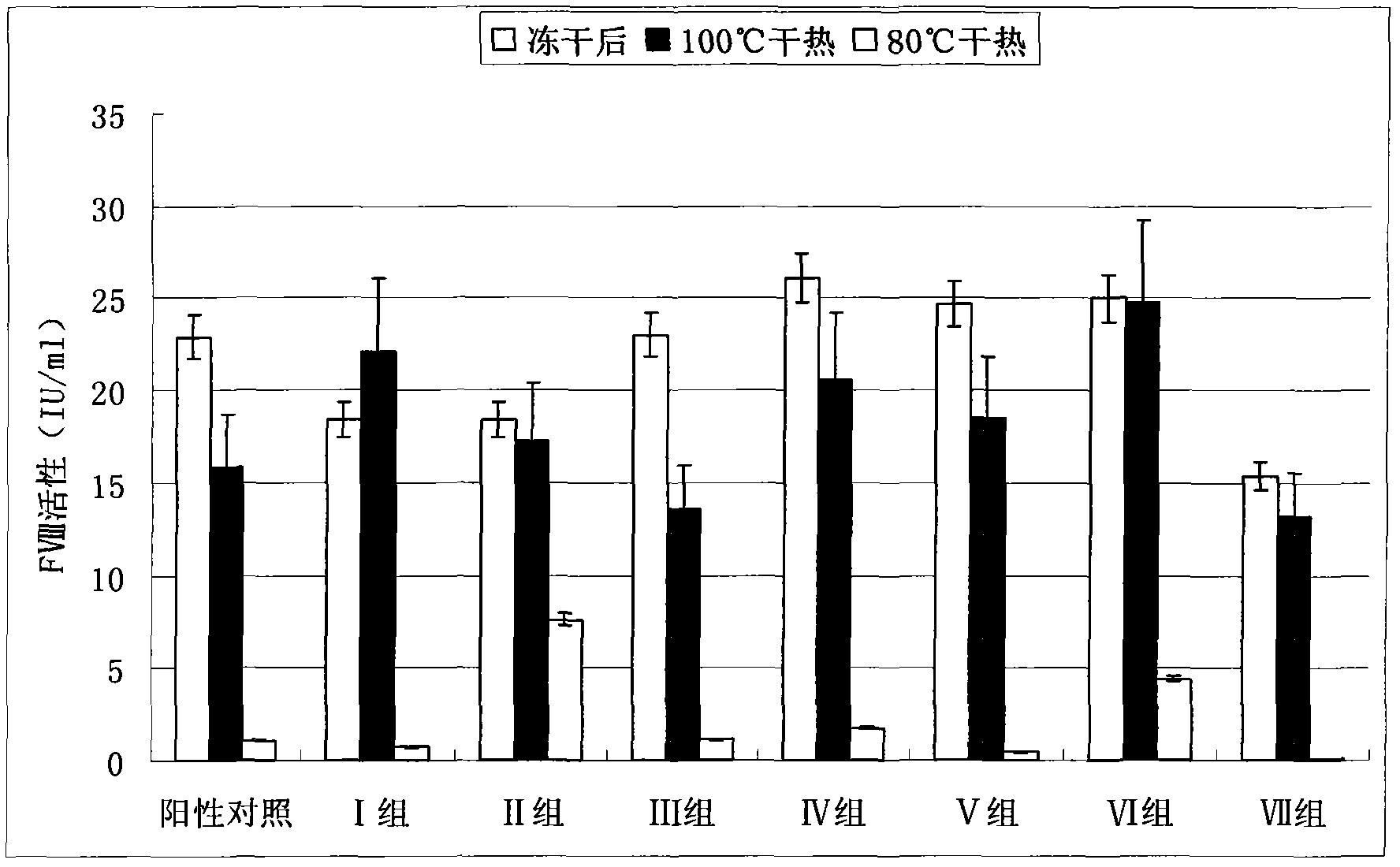
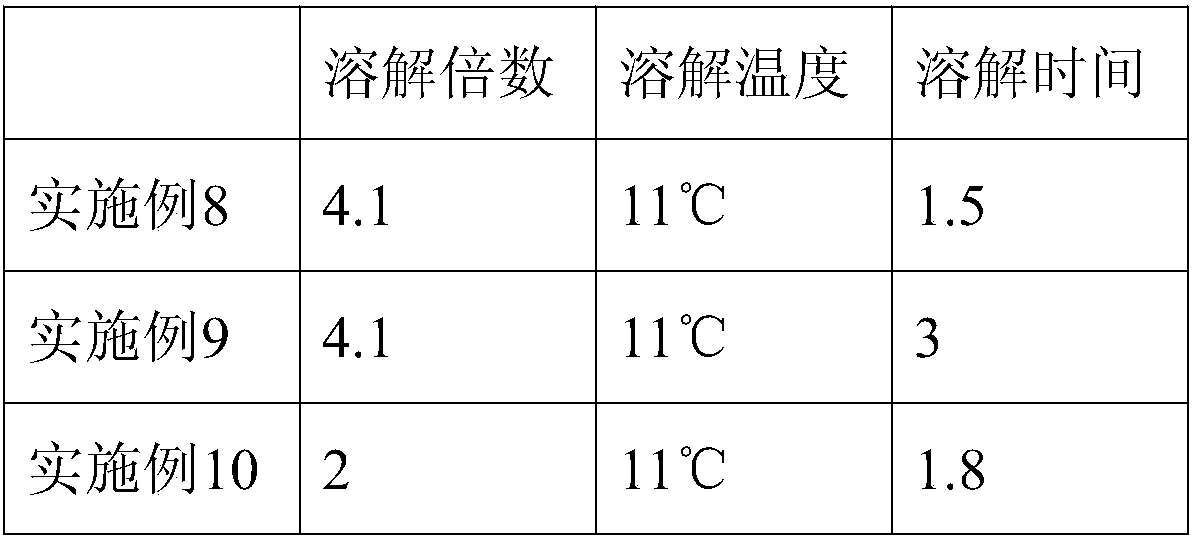
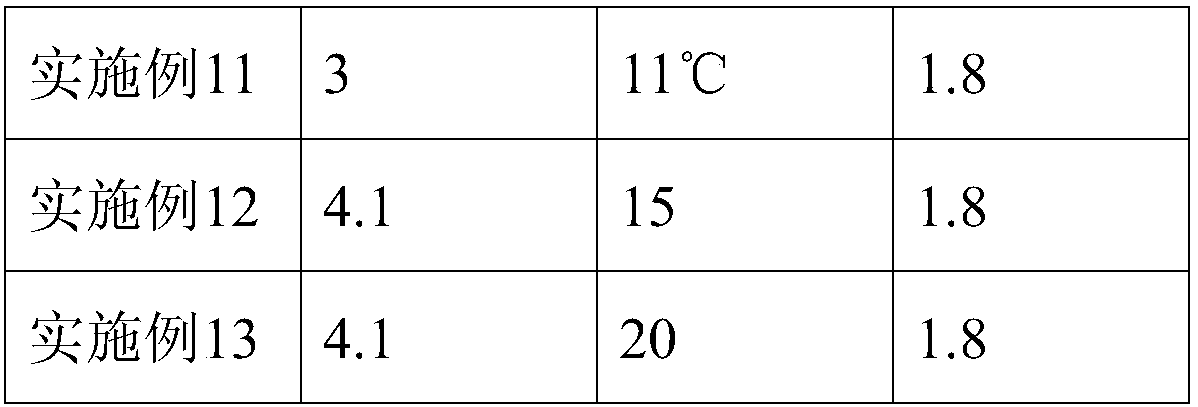
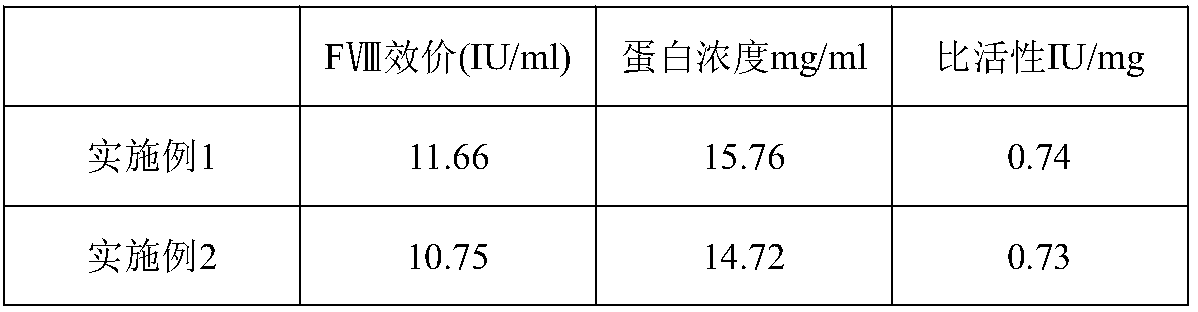
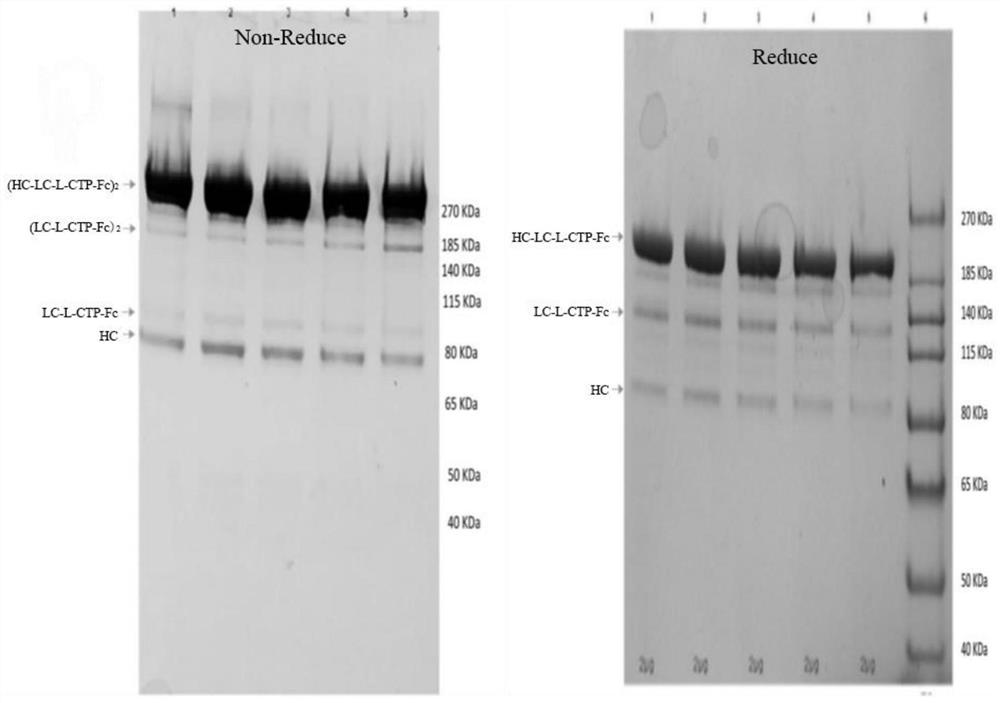
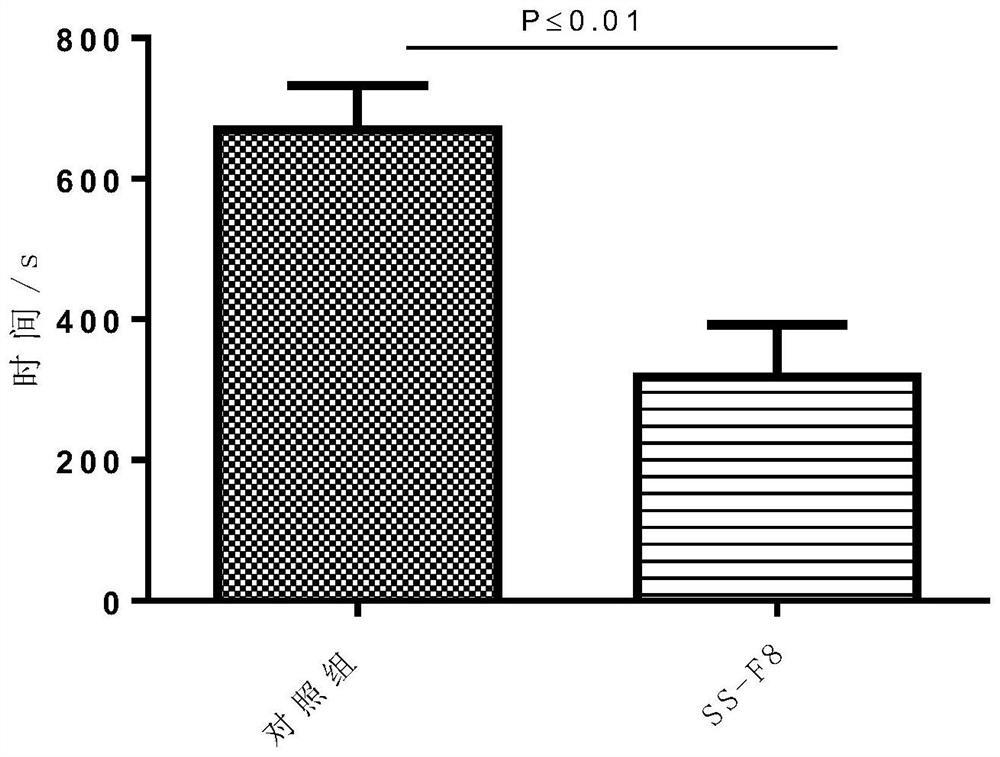
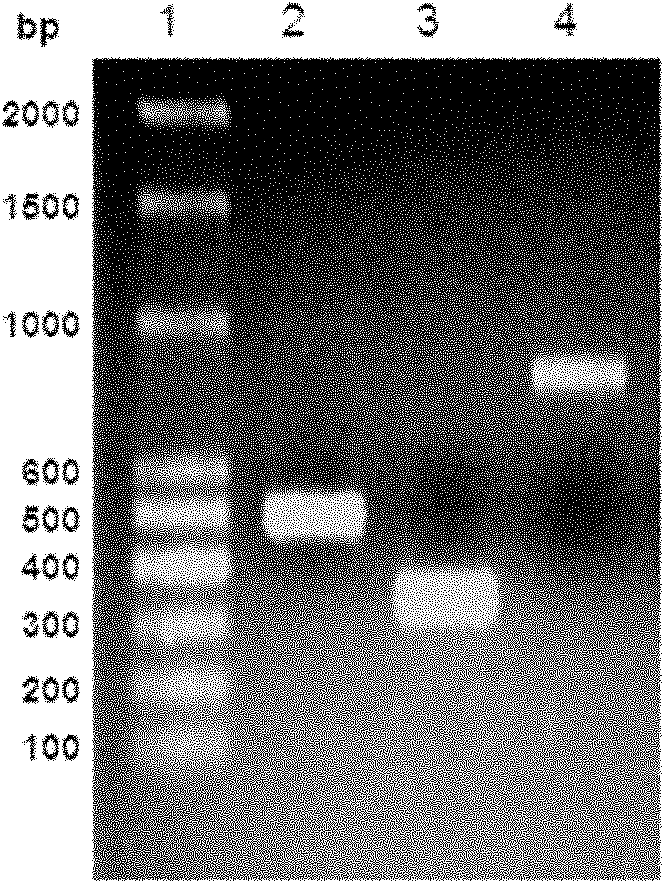
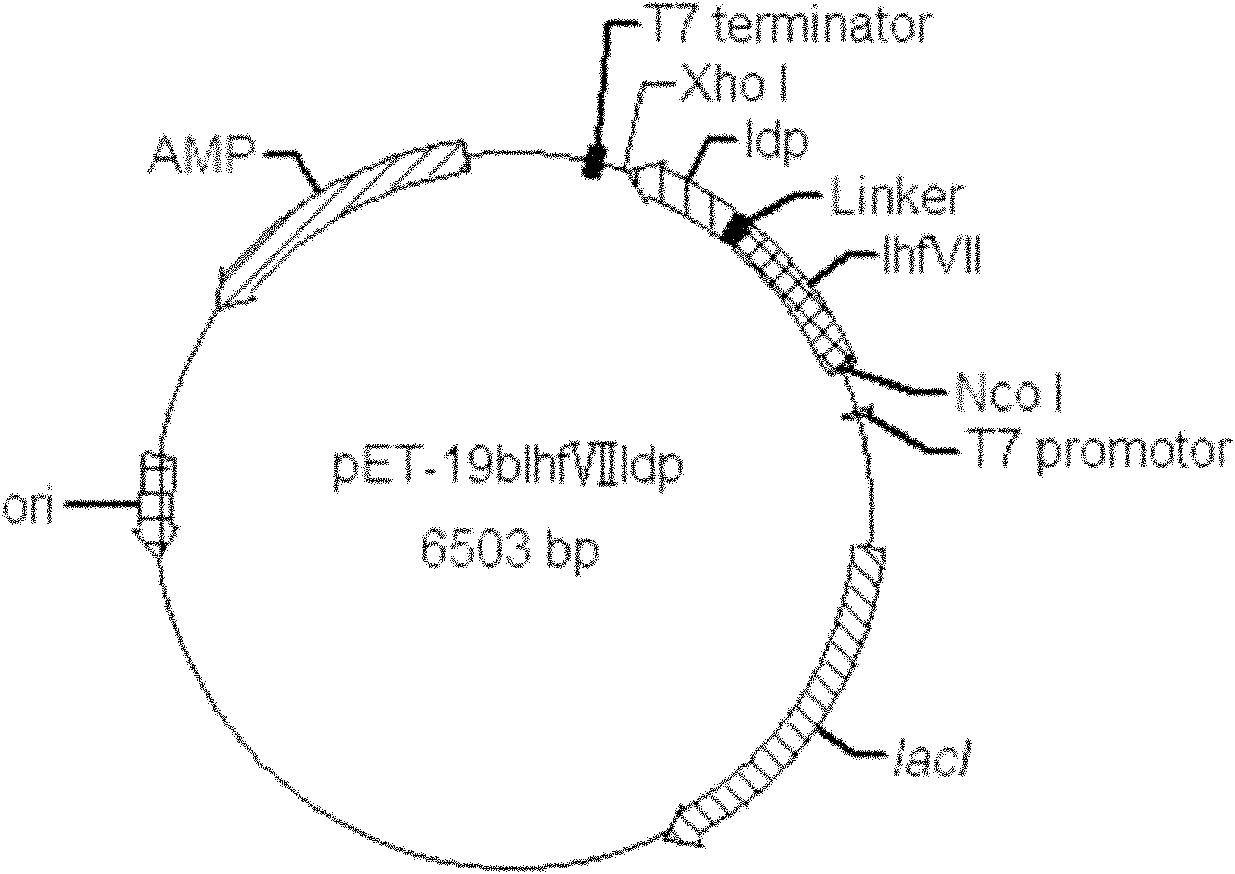
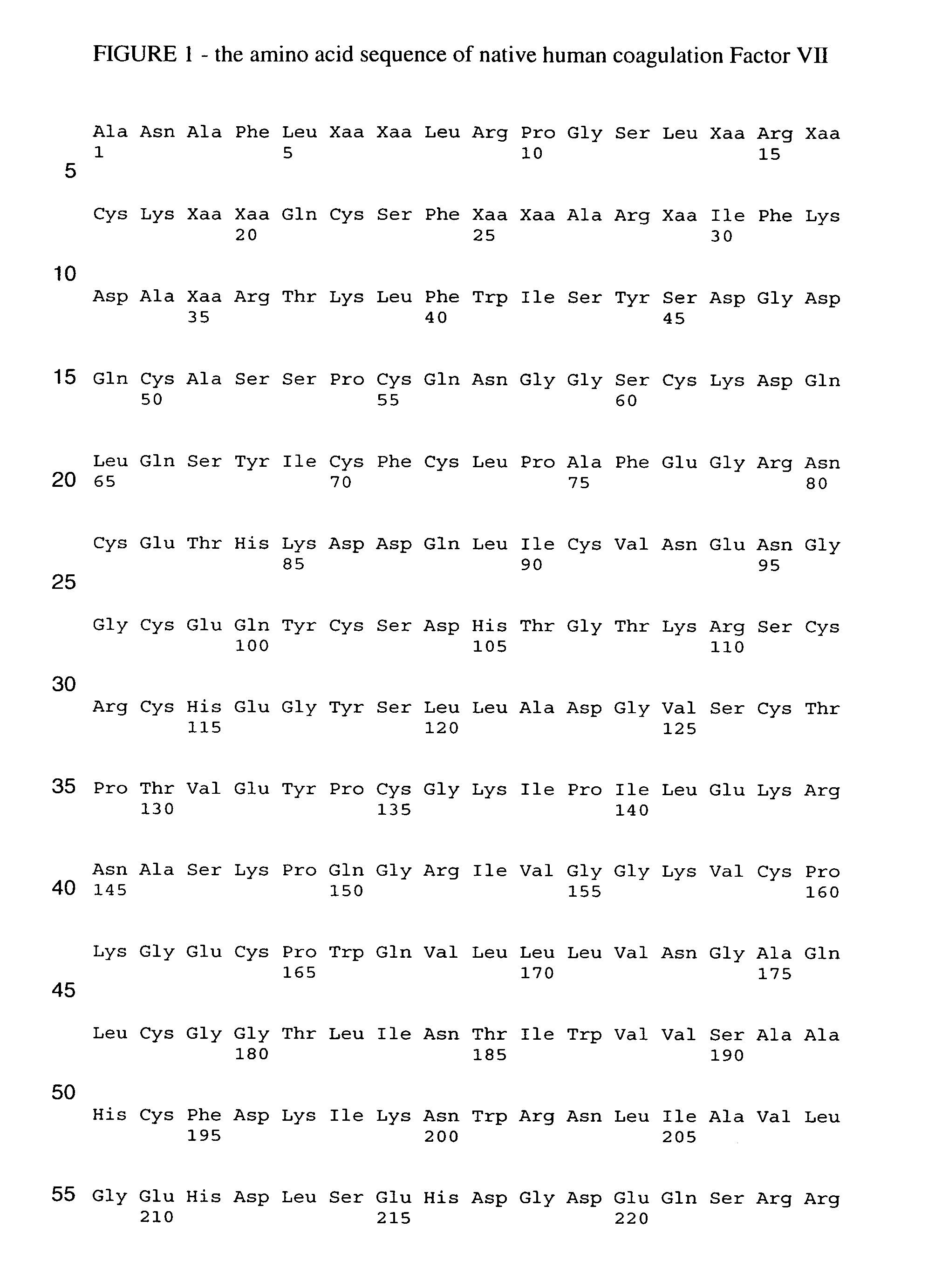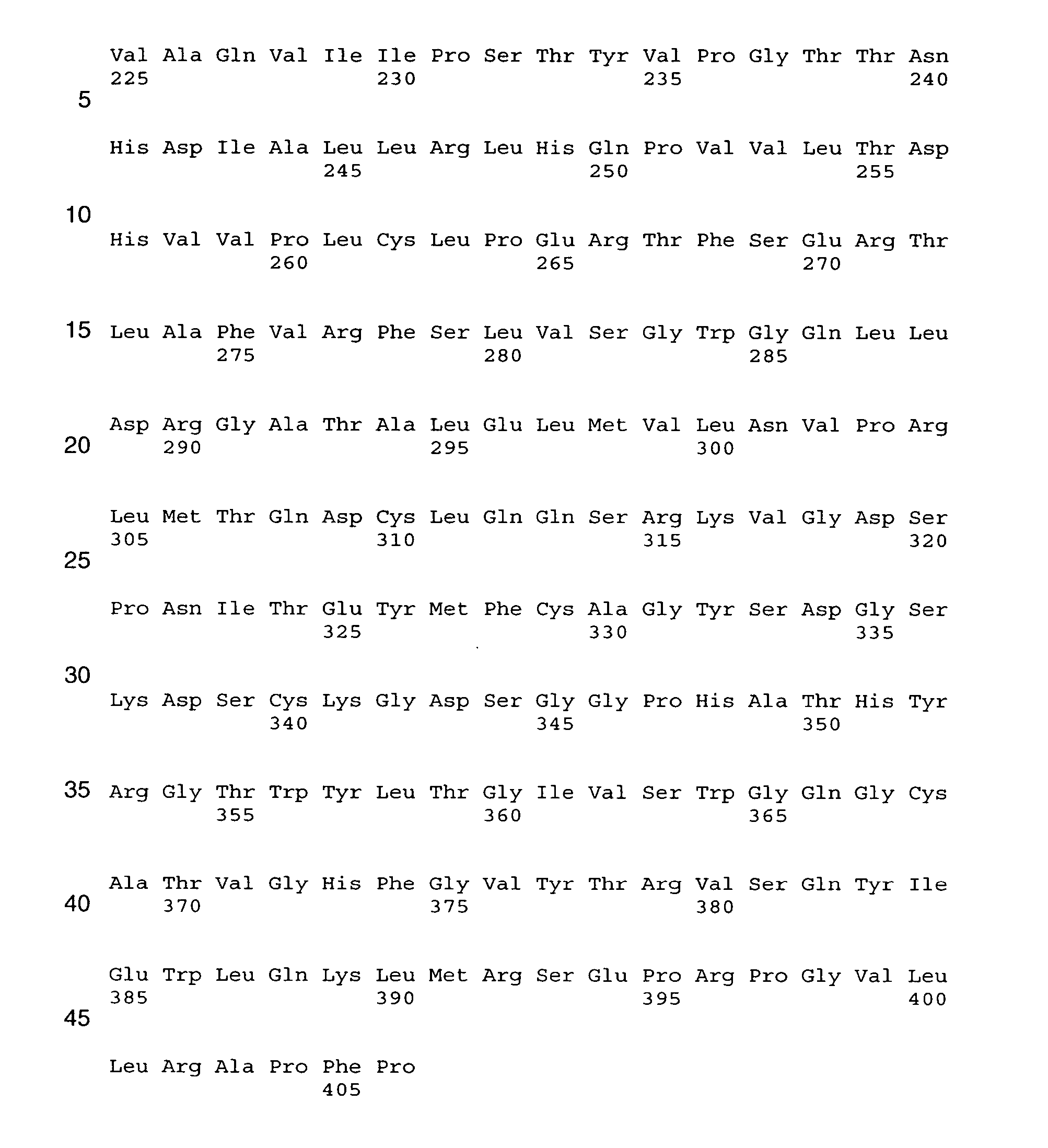Patents
Literature
63 results about "Human coagulation factor VIII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human coagulation factor VII variants
InactiveUS7026524B2Peptide/protein ingredientsMammal material medical ingredientsHuman coagulation factor VIIIHuman coagulation factor VII
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as nucleic acid constructs encoding such variants, vectors and host cells comprising and expressing the nucleic acid, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Coagulation factor VII derivatives
InactiveUS7235638B2Same and increased activityExtended half-lifeBiocidePeptide/protein ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VII polypeptides, Factor VII derivatives as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Human coagulation factor VII polypeptides
InactiveUS6911323B2Peptide/protein ingredientsMammal material medical ingredientsPolynucleotideHuman coagulation factor VIII
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Human coagulation factor VII variants
InactiveUS6905683B2Increased tissue factor-independent activityPeptide/protein ingredientsMammal material medical ingredientsProteinase activityCoagulation system
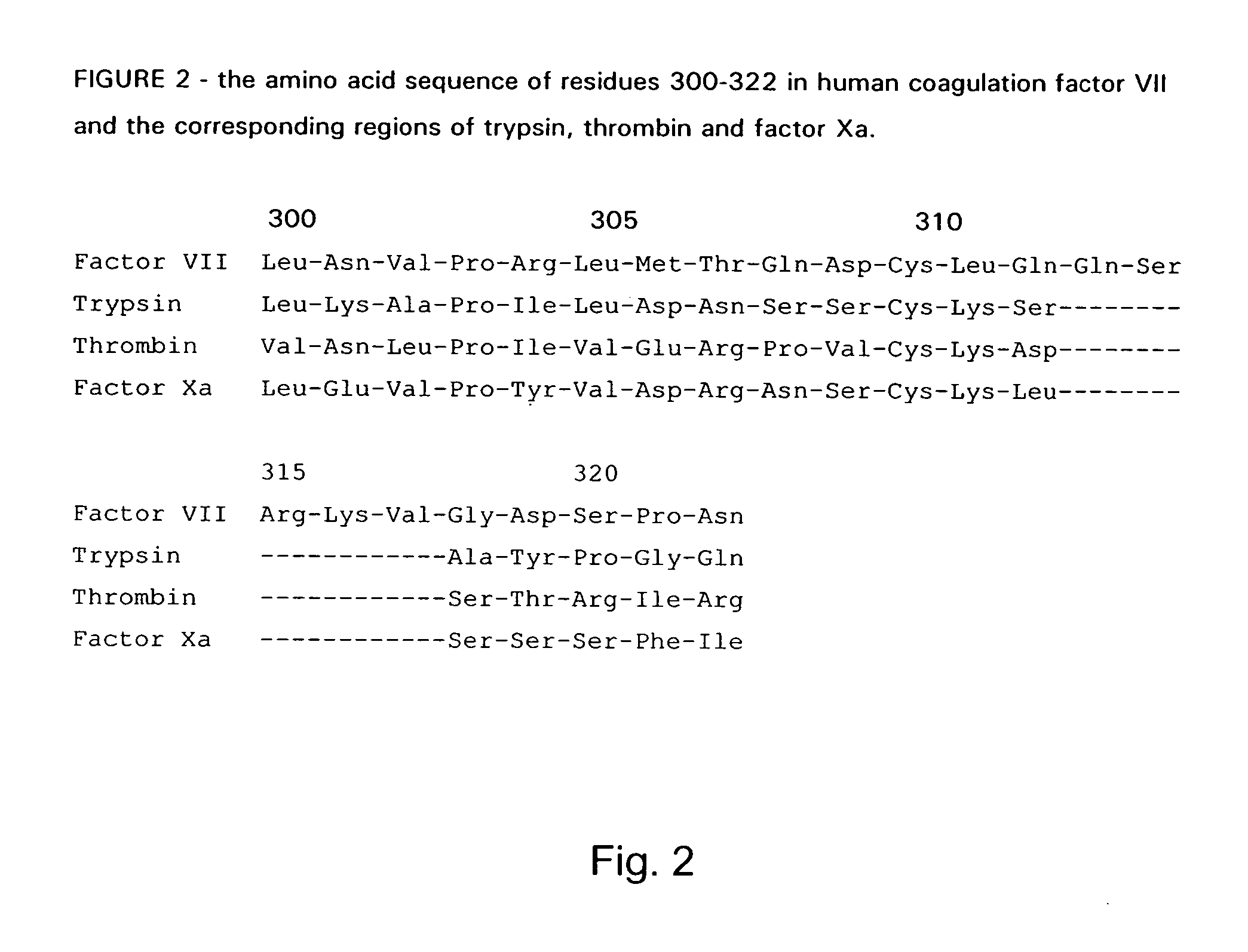
The invention concerns novel coagulation factor VII variants, wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs;with the proviso that the variant is not FVII(Ala305).The invention further concerns nucleic acids encoding the Factor VII variants; vectors and cells comprising the nucleic acid; methods for producing the variants; pharmaceutical compositions comprising a Factor VII variant wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs; use of the variants for producing a medicament for treatment or prophylaxis of bleeding disorders or enhancement of the coagulation system; and methods of treatment.
Owner:NOVO NORDISK AS
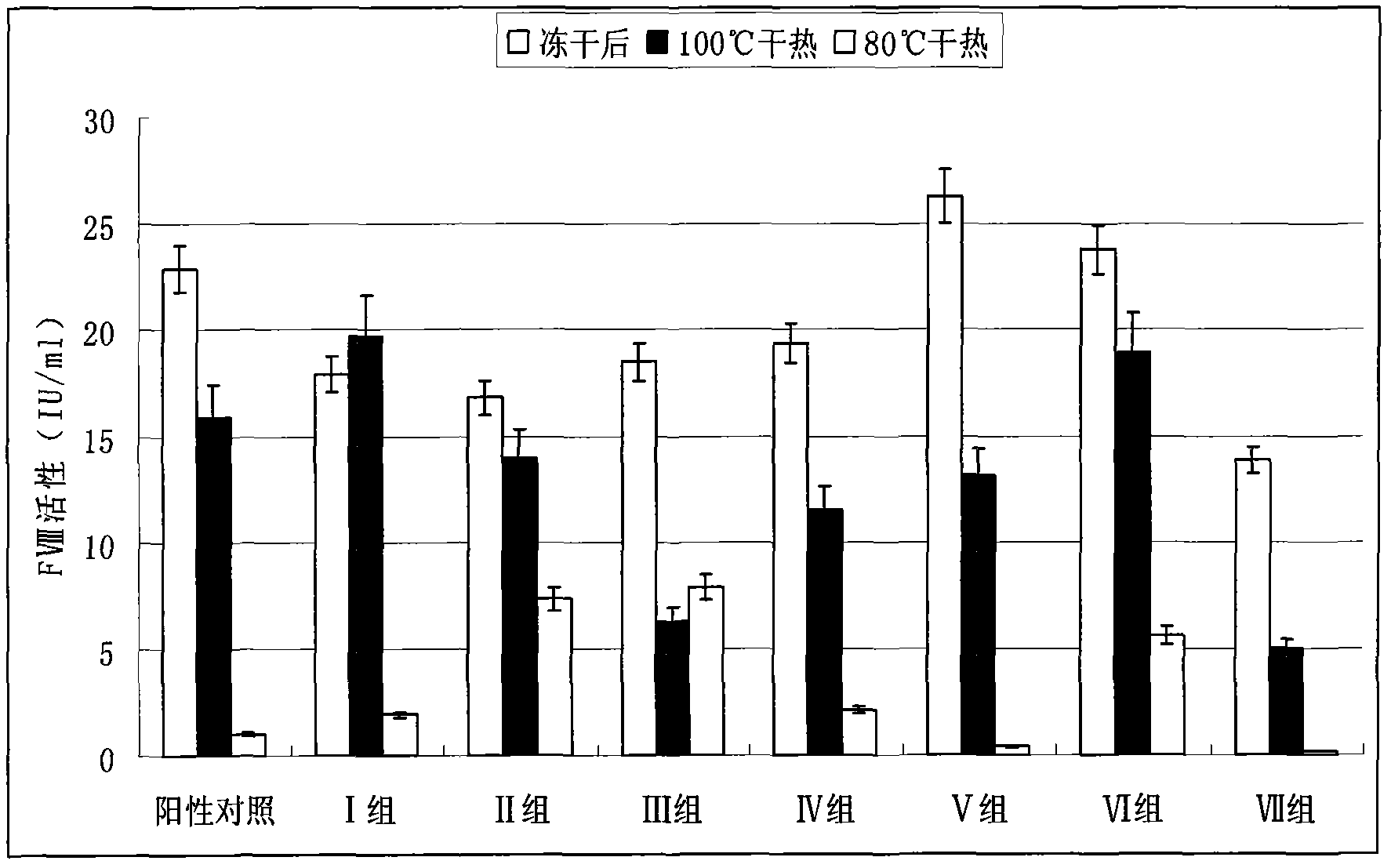
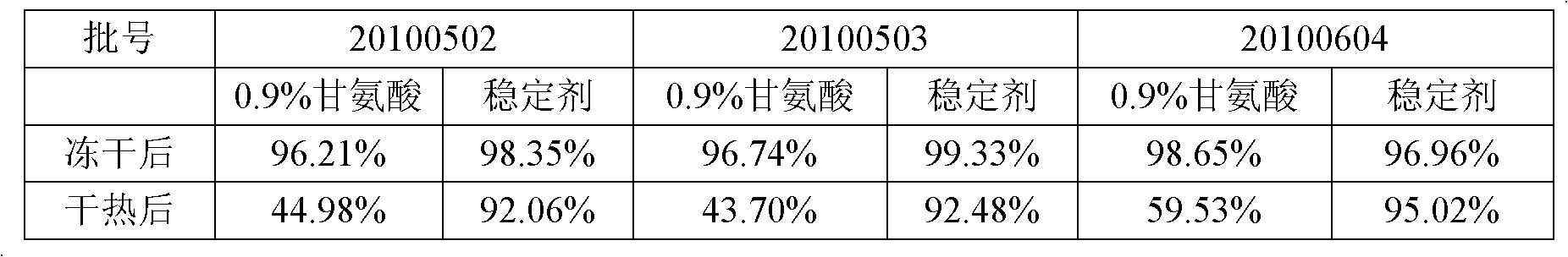
Dry heat treatment stabilizing agent for human coagulation factor VIII and vWF (von willebrand factor) compound or human coagulation factor VIII preparation
ActiveCN102580062ALittle loss of activityIncreased loss of activityPeptide/protein ingredientsPharmaceutical non-active ingredientsSucroseFreeze-drying
The invention discloses a dry heat treatment stabilizing agent for a human coagulation factor VIII and vWF compound or a human coagulation factor VIII preparation. The stabilizing agent of the invention comprises histidine or its salt, arginine or its salt, lysine or its salt, mannitol, mycose, and sucrose, and also can comprise one or several of common glycine, sucrose, common salt, calcium chloride, sodium citrate, and heparins. Experiments prove that the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation contains 0.1-10% of histidine or its salt, 0.1-10% of arginine or its salt, and one or several of 0.1-10% of lysine or its salt, 0.1-10% of glycine, 0.1-10% of mannitol, 0.1-10% of sucrose, and 0.1-10% of mycose, so the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation can effectively inactivate viruses under a 80-100DEG C dry heat environment, can effectively protect the activity of the human coagulation factor VIII, and has a qualified freeze-drying appearance and a redissolving appearance. So the stabilizing agent of the invention can be used as the dry heat treatment stabilizing agent for the human coagulation factor VIII and vWF (von willebrand factor) compound or the human coagulation factor VIII preparation.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
Hyperglycosylatedhuman coagulation factor VIII fusion protein and preparation method and application thereof
ActiveCN106279437AIncrease productionExtended half-life of activity in vivoFactor VIIPeptide/protein ingredientsHuman Chorionic Gonadotropin Beta SubunitHalf-life
The invention discloses a hyperglycosylated human coagulation factor VIII (FVIII) fusion protein and a preparation method and application thereof. The fusion protein successively contains human FVIII, a soft peptide connector, at least one human chorionic gonadotropin betasubunitcarboxyl terminal peptide rigid unit and prolonged half-life period portion (preferably ahumanIgG Fc variant) from an end N to an end C. The fusion protein has biological activity similar to that of recombinant FVIII and prolonged in-vivo activity half-life period, thereby improving the pharmacokinetics and drug efficacy.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC +3
Hybrid molecules having Factor VII/VIIa activity
InactiveUS7432352B2Same and increased activityIncreased serum half-lifePeptide/protein ingredientsMammal material medical ingredientsSynthetic analogueFactor ii
Owner:NOVO NORDISK HEALTH CARE AG
Preparation method of human coagulation factor VIII
ActiveCN104231073ASuitable for a wide range of peopleHigh potencyFactor VIIPeptide/protein ingredientsAlcohol sugarsDiabetic patient
The invention discloses a preparation method of a human coagulation factor VIII. The human coagulation factor VIII prepared by the method does not contain human serum albumin or other animal-derived protein, does not contain sugar or sugar alcohol, does not have the risk for transmitting other viruses or pathogene, and is wide in applicable crowd scope, and can be used by diabetic patients; the human coagulation factor VIII prepared by the method is fast to redissolve and good in redissolving effect, and still keeps high titer and high specific activity which are respectively larger than 80 percent and 40 IU / mg; in addition, the preparation method is simple, the cost is low, the human coagulation factor VIII is safe and effective, and has a good industrial application prospect.
Owner:广东双林生物制药有限公司
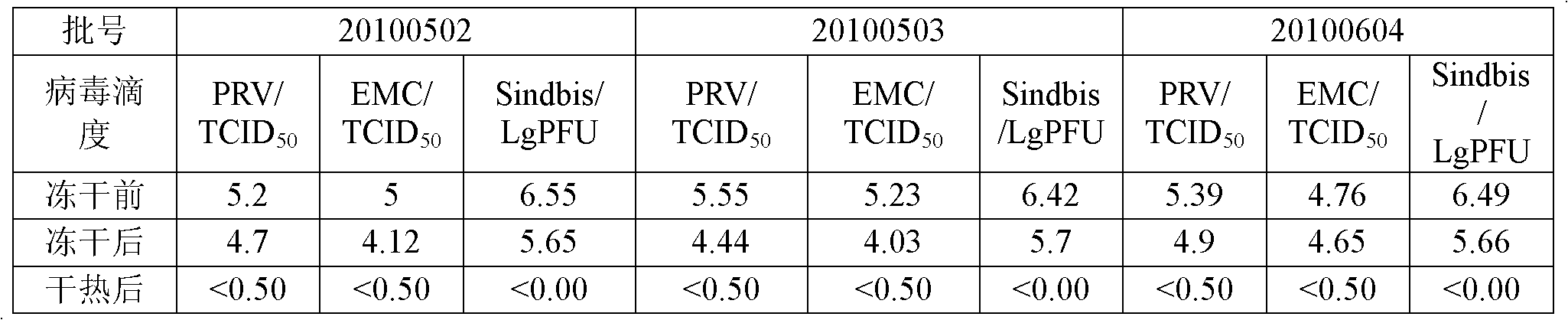
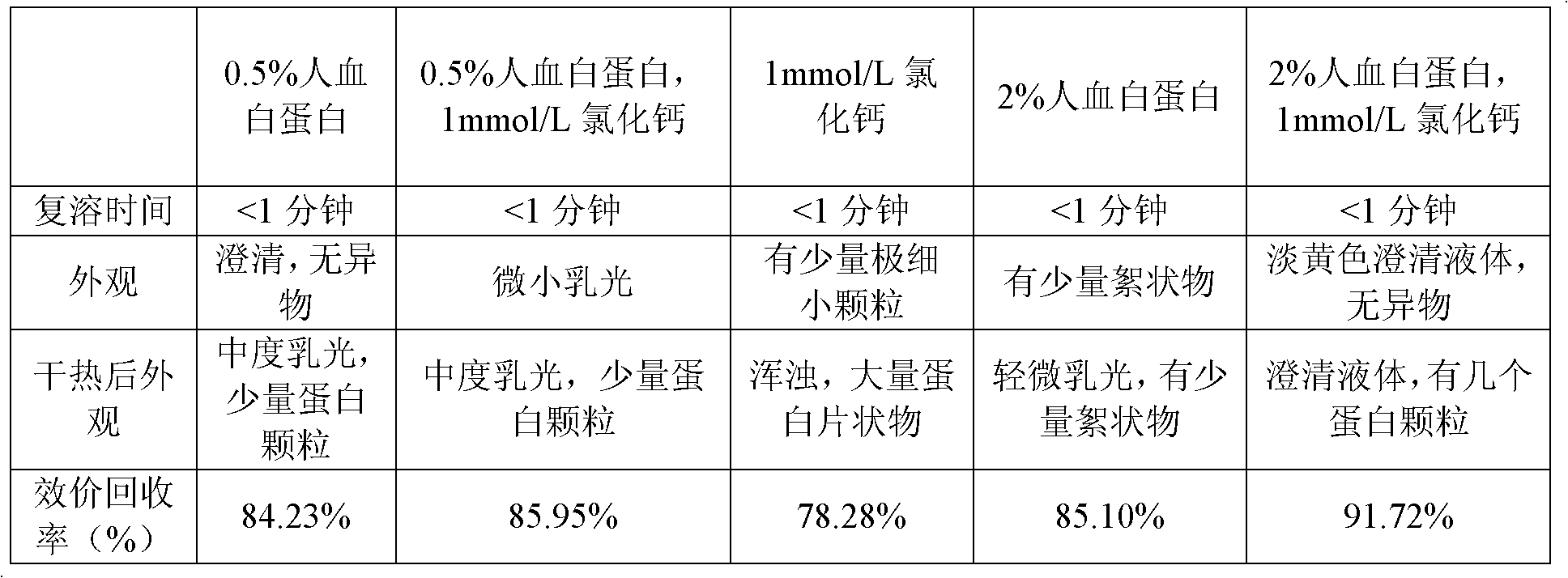
Dry heat treatment method for human coagulation factor VIII preparation and dry heat treatment stabilizer
InactiveCN102430116AReduce lossesAchieve the inactivation effectPeptide/protein ingredientsMacromolecular non-active ingredientsFreeze-dryingFactor ii
The invention discloses a dry heat treatment method for a human coagulation factor VIII preparation. Before a freeze-drying step, the stabilizer is added into human coagulation factor VIII solution, wherein the stabilizer comprises human serum albumin, Ca2+ soluble salt, amino acid, sodium citrate and sodium chloride; and the concentration percentage of the human serum albumin is 0.5 to 5 percent and the concentration of Ca2+ is 1mmol / L. The invention also discloses the dry heat treatment stabilizer for the human coagulation factor VIII preparation. The dry heat treatment method is performed for 72 hours at 80 DEG C and is effective on both lipid envelop virus and non-lipid envelop virus. Usually, heat treatment is the last step. The method is low in cost and higher in maneuverability and controllability.
Owner:SHANGHAI XINXING MEDICINE
Human coagulation Factor VII variants
InactiveUS20060252129A1Peptide/protein ingredientsMammal material medical ingredientsBiologyHuman coagulation factor VIII
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor VII variants
InactiveUS20050204406A1Peptide/protein ingredientsMammal material medical ingredientsDiseaseProteinase activity
Owner:NOVO NORDISK AS
Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
InactiveCN105315360AHigh yieldIncrease productivityFactor VIIFibrinogenDEAE SephadexVirus inactivation
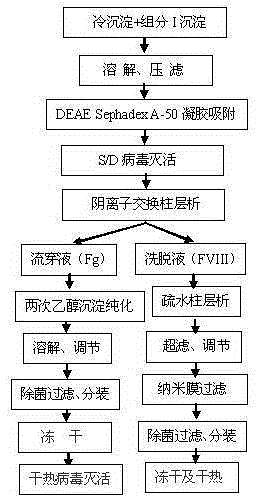
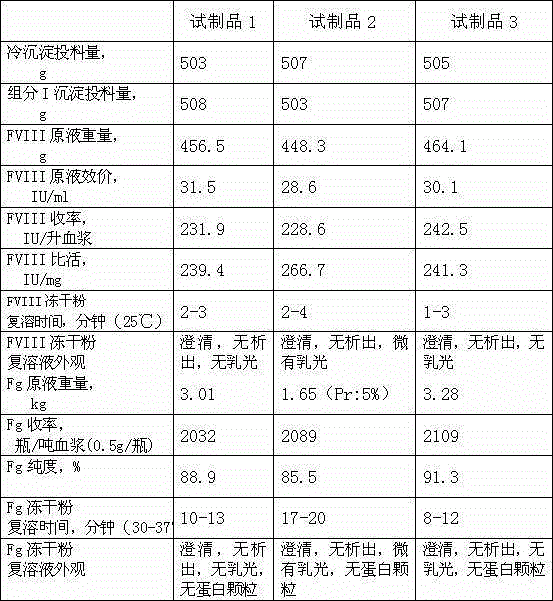
The invention discloses a method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen by cryoprecipitate and component I precipitation, mixing and feeding. The method comprises the following steps: (1) simultaneous feeding and dissolution of a cryoprecipitate and a component I; (2) DEAE Sephadex A-50 gel adsorption; (3) S / D virus inactivation; (4) anion exchange column chromatography; (5) two-step low-temperature ethanol precipitation and purification, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a chromatographic penetration liquid to obtain a human fibrinogen; (6) further hydrophobic column chromatography of a chromatographic eluant; (7) ultrafiltration, nanofilm filtration, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a hydrophobic eluant to obtain a high-purity human coagulation factor VIII. By the adoption of the process, FVIII and Fg in the two raw materials are extracted simultaneously, so that the yields of the two products are greatly improved, the yield of the human coagulation factor VIII can reach 200,000 IU / ton plasmas, the yield of the human fibrinogen exceeds 2,000 bottles / ton plasmas, and the yields are both far higher than those of a traditional process.
Owner:上海洲跃生物科技有限公司
Method for preparing high-purity human coagulation factor VIII
ActiveCN105348382AAvoid damageNo precipitationFactor VIIPeptide preparation methodsUltrafiltrationBlood plasma
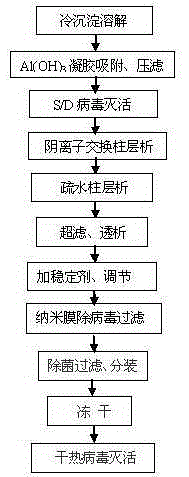
The invention discloses a method for preparing a high-purity human coagulation factor VIII from a cryoprecipitate of a human plasma fraction. The method comprises the following steps: (1) cryoprecipitate dissolution; (2) aluminum hydroxide gel adsorption and filter pressing; (3) S / D virus inactivation; (4) anion exchange resin column chromatography; (5) hydrophobic column chromatography; (6) ultrafiltration dialysis and concentration; (7) addition of one or more stabilizers and titer adjustment; (8) nano-membrane virus-removing filtration; (9) sterile filtration and sub-packaging; (10) freeze-drying; (11) dry-heat virus inactivation. The method has the advantages that a human coagulation factor VIII is purified through the two column chromatography steps, so that the prepared high-purity product can reach a specific activity of about 300 IU / mg, considerably higher than about 50 IU / mg in the prior art, and the product appearance and the heat stability are obviously improved; in the preparation process, three virus removing modes are adopted, so that the clinical use safety of the product can be greatly improved.
Owner:上海洲跃生物科技有限公司
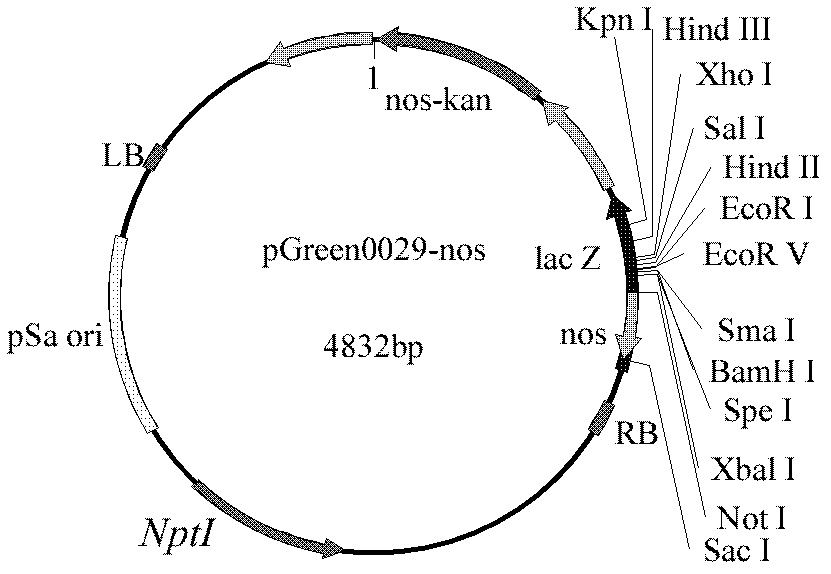
Production of human coagulation factor VIII from plant cells and whole plants
InactiveUS20050060775A1Mammal material medical ingredientsPeptide preparation methodsFactor VIII ActivityPlant tissue
The invention includes methods for production of a polypeptide having factor VIII activity by introduction of a polynucleotide construct into a plant cell. The construct includes an encoding sequence for a polypeptide of coagulation factor VIII or a functional variant thereof. The plant cell is cultured or regenerated into a plant and the polypeptide or functional variant of factor VIII is expressed therein. The invention also includes vectors, plant cells, plant tissues, plants and seeds containing a polynucleotide sequence encoding a functional variant of human coagulation factor VIII. The invention further includes a recombinant DNA molecule having a promoter which is functional in plants operably linked to a coding sequence which codes for a polynucleotide having coagulation factor VIII activity.
Owner:BATTELLE MEMORIAL INST
Activated human coagulation factor VII fusion protein and preparation method and application thereof
ActiveCN106279436APeptide/protein ingredientsAntibody mimetics/scaffoldsHuman Chorionic Gonadotropin Beta SubunitHalf-life
The invention discloses a hyperglycosylated activated human coagulation factor VII (FVIIa) fusion protein, and a preparation method and application thereof. The fusion protein comprises human FVIIa, a flexible peptide joint, at least one human chorionic gonadotropin beta-subunit carboxyl terminal peptide rigid unit and a half-life period prolonging part (a human IgG Fc variant preferred). The fusion protein has bioactivity similar to that of the natural human FVIIa and has a longer in-vivo active half-life period, so that the pharmacokinetics and the medicinal effect are improved.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC +3
Human coagulation factor VIII preparation method
ActiveCN107226859AImprove securityHigh potencyFactor VIIPeptide preparation methodsFreeze-dryingDissolution
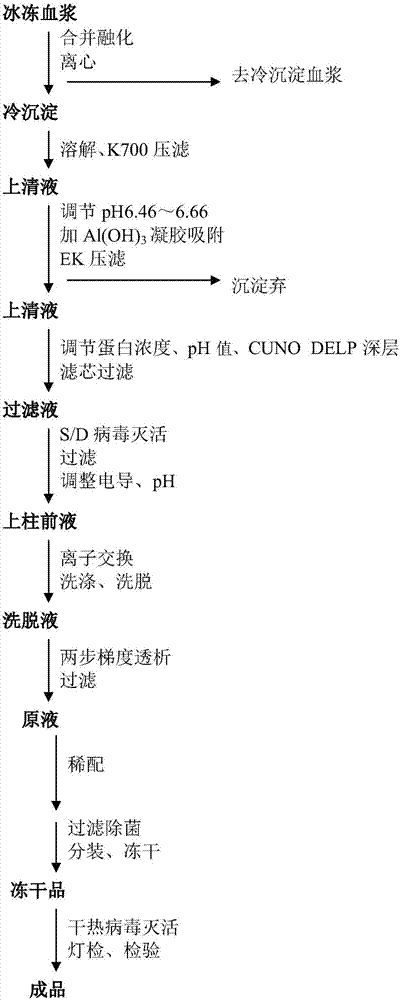
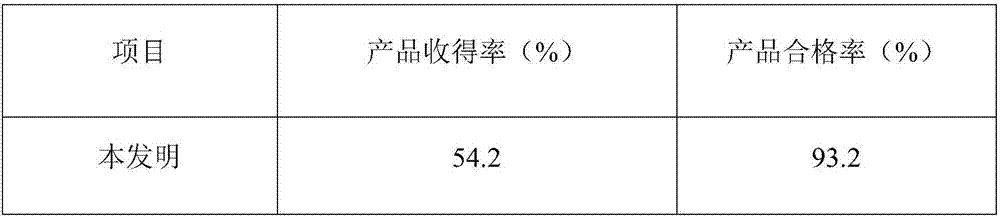
The invention discloses a human coagulation factor VIII preparation method. In the preparation process of a human coagulation factor VIII, a two-step filter press technique is adopted during treatment of human plasma initial materials, that is, a K700 filter plate is adopted for filter pressing after cryoprecipitate dissolution, the filter press liquor is collected, the pH value is adjusted, a 2% aluminium hydroxide gel is added for adsorption, and then an EK filter plate is adopted for filter pressing. The two-step filter press technique is adopted to improve the separation effect, meanwhile, the aluminium hydroxide gel adsorption method is used together to remove a coagulation factor on which vitamin K depends, the aluminium hydroxide gel does not adsorb the human coagulation factor VIII, and the product yield is high. In the preparation process, the two-step filter press technique replaces a traditional two-step high-speed centrifuging method, a CUNO DELP deep filter element is adopted for filtration, a two-step gradient dialysis method is adopted, a re-dissolution and re-freezing process is added to a freeze-drying process, and meanwhile, various virus removal / inactivation technologies are adopted in the production process, so that the product yield can be improved, the risk of virus spreading can be reduced, and the clinical medication security is improved.
Owner:华润博雅生物制药集团股份有限公司
Pichia yeast expressing recombinant human blood coagulation factor VII, and preparation and use thereof
InactiveCN101376875AHigh activityHigh expressionFungiPeptide/protein ingredientsFactor SevenFactor ii
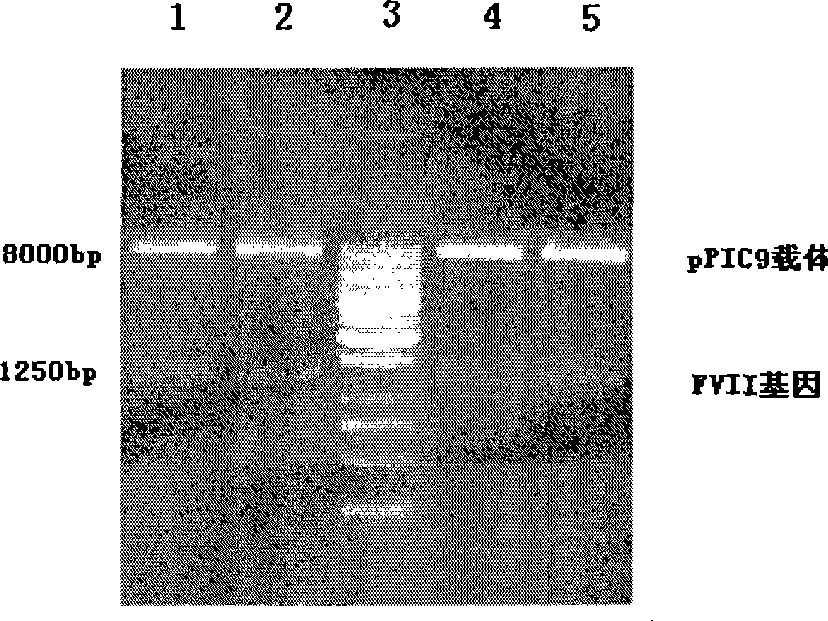
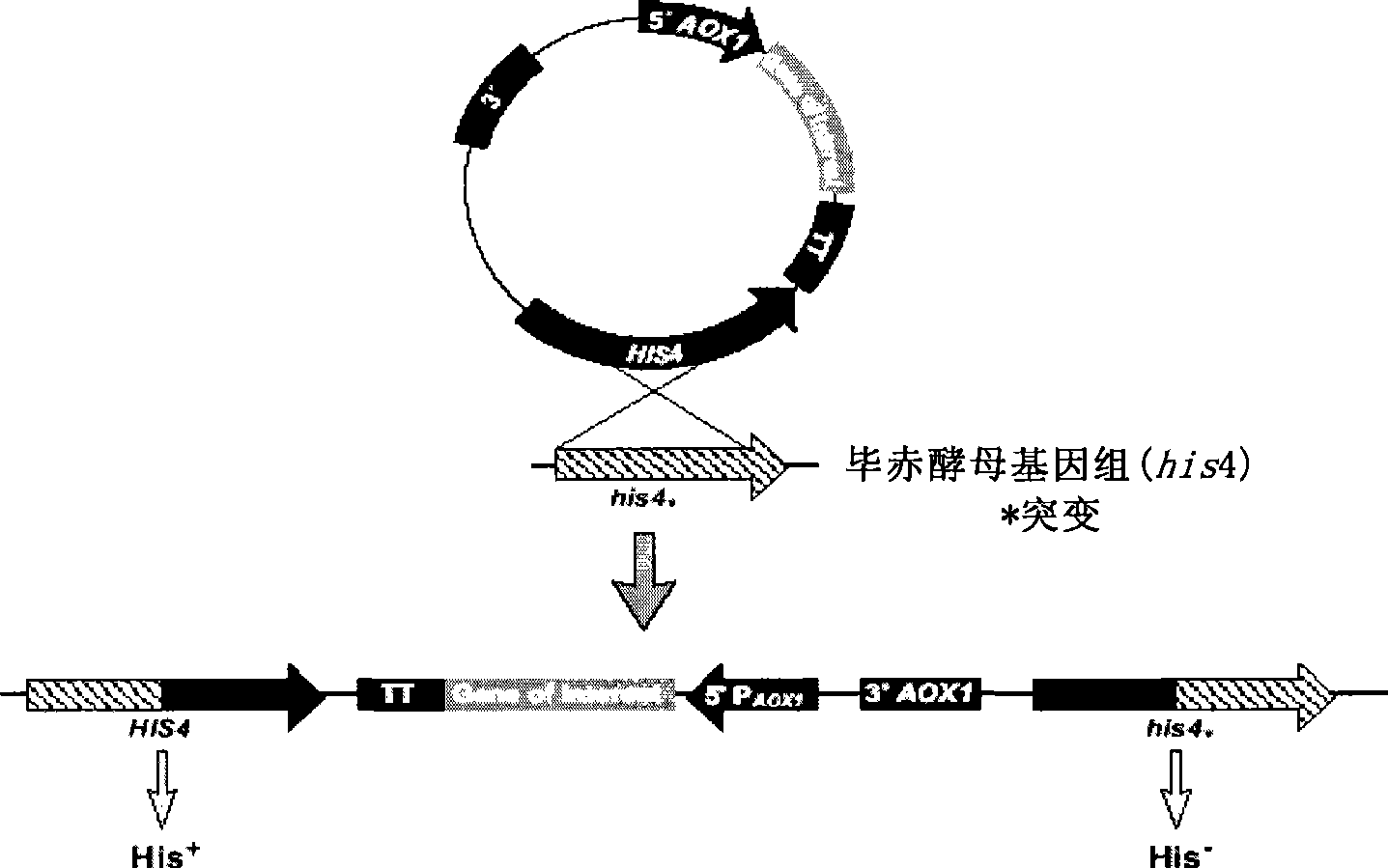
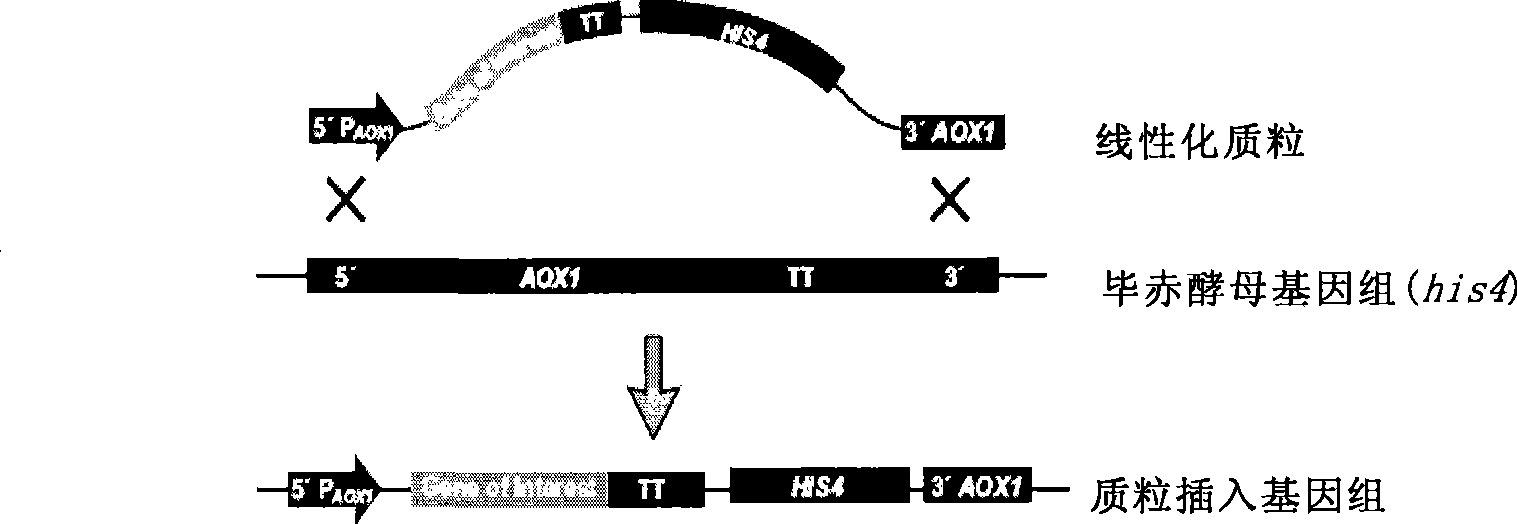
The invention provides a yeast cell which expresses a human coagulation factor seven; the yeast cell integrates a structure which expresses the human coagulation factor seven; the structure comprises two to fifteen human coagulation factor seven expression cassettes which are serially connected and lined; each expression cassette subsequently comprises the following elements from 5' to 3': (a) a start signal element AOX; (b) a human coagulation factor seven gene; (c) a stop signal element AOX (TT); and the yeast cell expresses the activated human coagulation factor seven under the induction of methanol. The invention also provides the structure and a preparation method thereof, a preparation method of the yeast cell and the human coagulation factor seven expressed by the yeast cell. The two to eighteen copied yeast cells structured by the invention can be used to improve yield and reduce cost, and are applicable in the mass production of human coagulation factor seven preparation.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Process for extracting human coagulation factor VIII from plasma
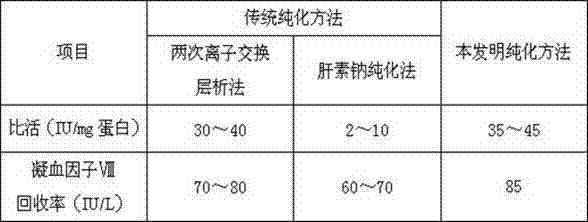
The invention discloses an economic and simple method for improving the separation efficiency, activity, specific activity and recovery rate of coagulation factor VIII. The method comprises washing cryoprecipitate twice with sodium heparin firstly, then carrying out ion exchange chromatography and eluting with an eluent to obtain higher-purity coagulation factor VIII. Compared with traditional methods, the method has the advantages of simple process and low cost; the separation efficiency, activity, specific activity and recovery rate of the coagulation factor VIII are also improved.
Owner:新疆德源生物工程有限公司
Washing buffer solution for ion-exchange chromatography for preparation of FVIII (human coagulation factor VIII) and application of washing buffer solution
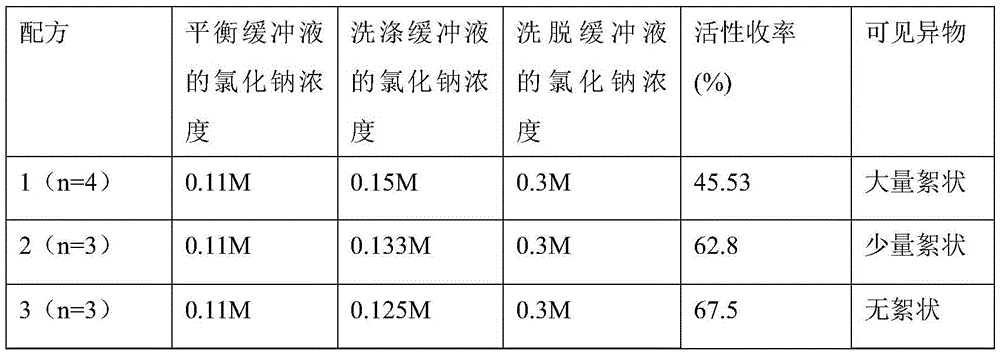
ActiveCN105481976ASimple recipeLow costFactor VIIPeptide preparation methodsForeign matterIon exchange
The invention discloses a washing buffer solution for ion-exchange chromatography for preparation of an FVIII (human coagulation factor VIII). The washing buffer solution contains sodium chloride with the final concentration being 0.121-0.129 M. The invention further provides an application of the washing buffer solution. The invention further provides a method for preparing the FVIII and a prepared product. The washing buffer solution can better maintain the activity of the FVIII, the activity yield of the FVIII is high, the purity is high, the visible foreign matters are qualified, the stability is good, the formula of the washing buffer solution is simple, the cost is low, and the washing buffer solution has good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
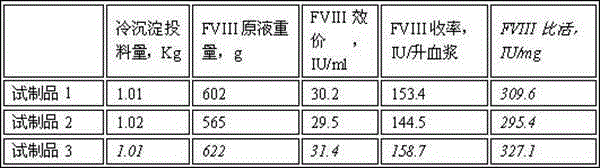
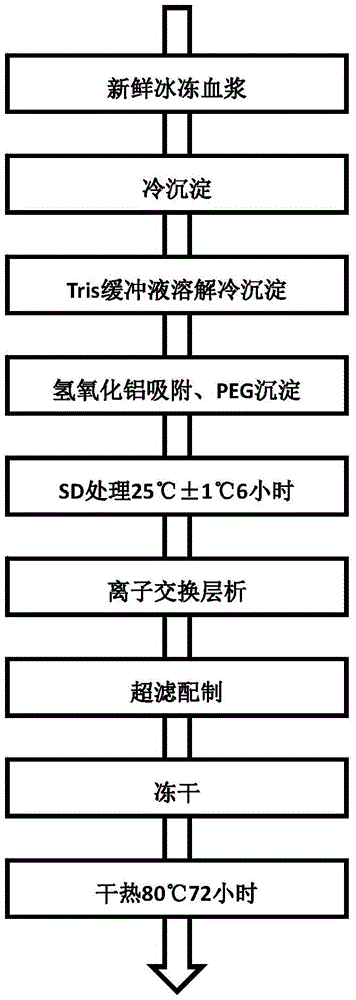
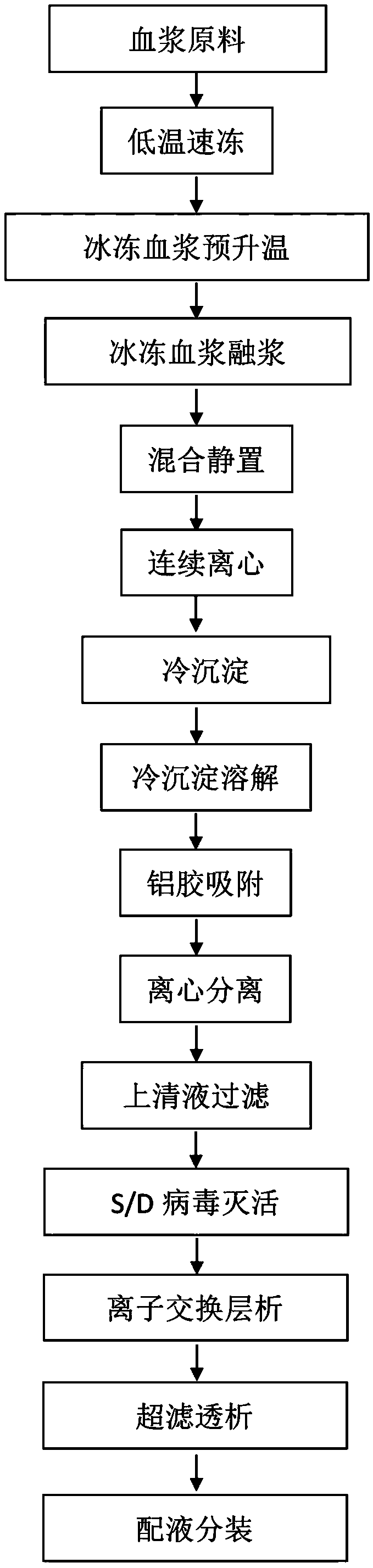
Preparation method of cold sediment and application of cold sediment to production of human coagulation factor VIII
InactiveCN108976297AActive Decay ControlLittle loss of activityFactor VIIPeptide preparation methodsQuick FreezeMedicine
The invention discloses a preparation method of cold sediment and application of cold sediment to production of a human coagulation factor VIII. The preparation method comprises the following steps: (1) quickly freezing blood plasma: after collecting the blood plasma, freezing and molding through a low-temperature quick-freezing technology; (2) transporting the blood plasma; (3) melting the bloodplasma: pre-heating at -5 to -3 DEG C and treating for 24 to 36 hours; then melting the blood plasma at the constant temperature of 0 to 4 DEG C until the blood plasma is completely melted; (4) mixingthe blood plasma and standing: mixing the blood plasma under mild stirring; standing for 4 to 8 hours at the constant temperature of 0 to 4 DEG C; (5) centrifuging the blood plasma: continuously centrifuging the blood plasma at low temperature to prepare the cold sediment. According to the preparation method disclosed by the invention, the activity of the factor VIII in the blood plasma is effectively protected through the low-temperature quick-freezing technology, and the activity loss of the factor VIII is reduced by 30 percent or more when being compared with the activity loss of a conventional method. The cold sediment is used as a raw material to produce a human coagulation factor VIII product with high potency and high specific activity; the potency of the obtained product is greater than 80 percent and the specific activity of the product reaches 90IU / mg or more.
Owner:广东双林生物制药有限公司
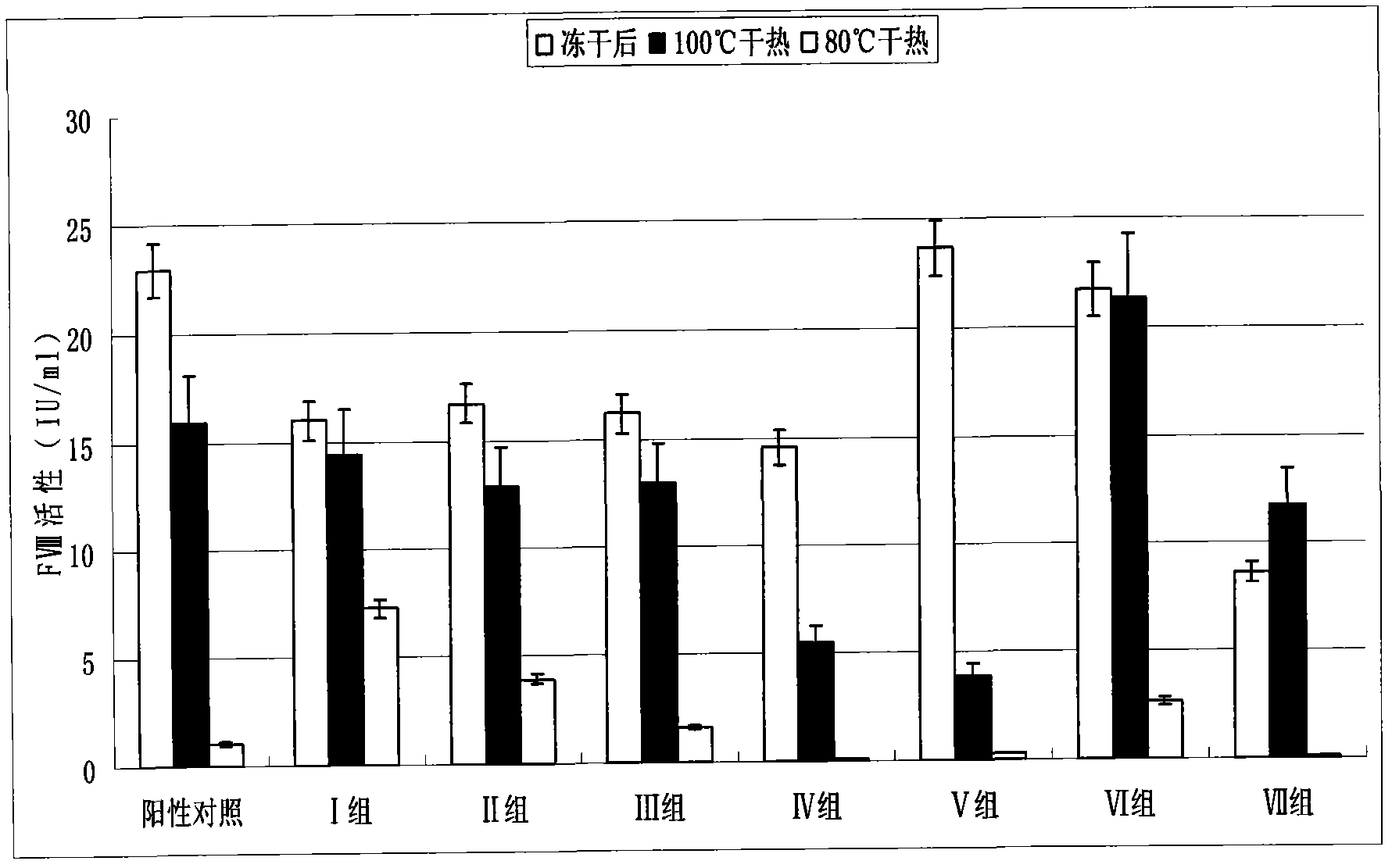
Freeze-dry and dry heat treatment protecting agent for human blood coagulation factor VIII
ActiveCN104225601ASuitable for a wide range of peopleHigh potencyPharmaceutical non-active ingredientsActive agentAlcohol sugars
The invention discloses a freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII. The freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII does not contain human serum albumin or other animal-source proteins, sugar or sugar alcohol, is composed of soluble salt, amino acid and a surface active agent, is free from the risk of spreading other viruses or causative agents, and is applicable to a wide crowd range, including diabetics; after being freeze-dried, a product of the human coagulation factor VIII using the protecting agent is quick in redissolving and has a good redissolving effect, still keeps high potency and high specific activity, and has the potency higher than 80 percent and the specific activity higher than 40 IU / mg; in addition, the freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII is low in cost, simple to prepare, has an obvious protecting effect, is safe and effective, and has an excellent industrial application prospect.
Owner:广东双林生物制药有限公司
Method for preparing coagulation factor VIII by using rabbit mammary gland bioreactor
InactiveCN103555759ARapid population reproductionImprove securityFermentationVector-based foreign material introductionCvd riskBlood plasma
The present invention discloses a method for preparing coagulation factor VIII by using a rabbit mammary gland bioreactor. The method comprises: inserting cDNA of B-domain deleted recombinant human coagulation factor VIII externally connected with a signal peptide into a plasmid vector to form a coagulation factor VIII mammary gland expression component, and performing microinjection or a somatic cell transplantation technology to obtain transgenic rabbit, wherein the present generation or future generation female rabbit of the obtained transgenic rabbit produces the recombinant human coagulation factor VIII in breast milk at the lactation period. According to the present invention, expression of the coagulation factor VIII mammary gland expression component in the transgenic rabbit mammary gland bioreactor can be achieved so as to prepare rhFVIII, and the transgenic rabbit is subjected to rapid population breeding so as to secrete a large amount of rhFVIII-containing breast milk, achieve batch production of the rhFVIII, reduce production cost to a great degree, avoid risk of virus infection on hFVIII extracted from blood plasma, and increase medication safety.
Owner:LANNUO BIOTECH WUXI
Method for improving high-efficiency expression of recombinant human coagulation factor VIII
InactiveCN109929029AStable and efficient expressionStable and efficient secretionFactor VIIVector-based foreign material introductionSerum igeCell strain
The invention relates to the technical field of genetic engineering, in particular to a method for improving the high-efficiency expression of a recombinant human coagulation factor VIII (rhFVIII), and in particular to the method for increasing expression amount of the recombinant human coagulation factor VIII in mammalian cells. The method promotes the high-efficiency expression of the recombinant human coagulation factor VIII by the selection and design of a recombinant human coagulation factor VIII gene sequence, the construction and screening of the recombinant expression plasmid and the engineering cell, and the culture of the positive cells by using a certain concentration of the serum medium. The method overcomes the shortcomings of the existing cell strains, such as difficulty in construction, complicated production, low expression, and the like, and has the characteristics of being fast, simple, and stable while reducing the research and development cost. The method provides asimple method for the industrial transfer of the recombinant human factor VIII, and has important research and application value for the development of biological products and therapeutic research.
Owner:SUNSHINE LAKE PHARM CO LTD
Anti-human coagulation factor VII monoclonal antibody, preparation method thereof and use thereof
InactiveCN101906161AStrong specificityHigh affinityImmunoglobulins against blood coagulation factorsTissue cultureAntigen bindingClotting factor
The invention provides an anti-human coagulation factor VII monoclonal antibody, a preparation method thereof and use thereof. The monoclonal antibody has a characteristic of high human coagulation factor VII antigen binding specificity. The invention also provides a corresponding monoclonal antibody fragment and an immunoconjugate. The invention also provides a detection kit for detecting the human coagulation factor VII.
Owner:SUZHOU ZELGEN BIOPHARML
Expression vector for expressing blood coagulation factor VIII and application thereof
InactiveCN102277379APromote safe productionEfficient productionUnicellular algaeMicroorganism based processesBlood coagulation factor VIIIFactor VIII preparation
The invention discloses an expression vector for expressing a coagulation factor VIII and application thereof. In the invention, a plant expression vector of a B domain delete recombinant human coagulation factor VIII (BDD-rhFVIII) is constructed by using a nitrate reducase promoter (NRpro) as a starting element; and the B domain delete recombinant human coagulation factor VIII (BDD-rhFVIII) is expressed by taking chlorella as a receptor. The recombinant human coagulation factor VIII expressed in the chlorella has a strong blood coagulation effect. The result can be applied to the preparationof coagulation factor VIII preparations.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
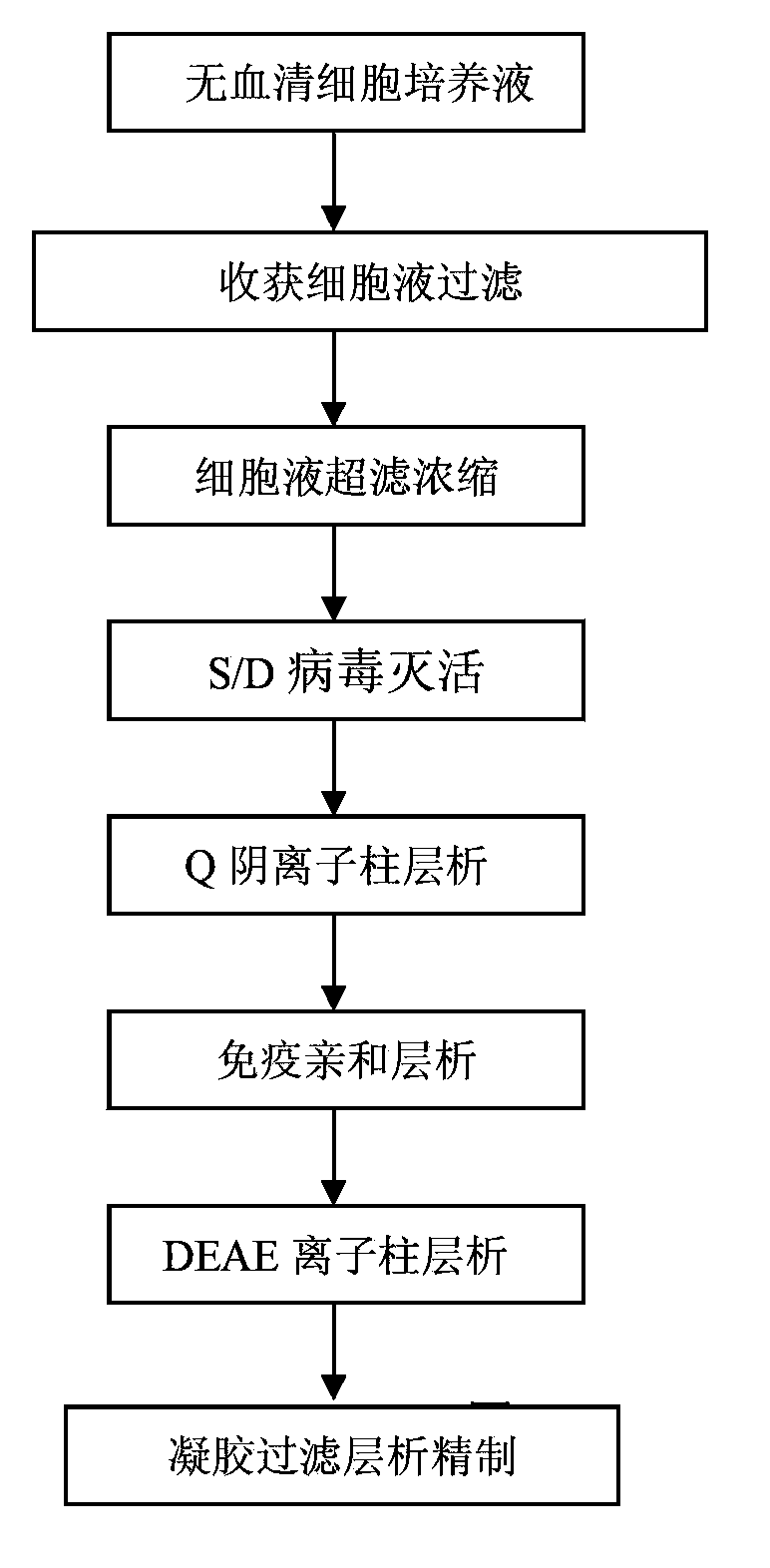
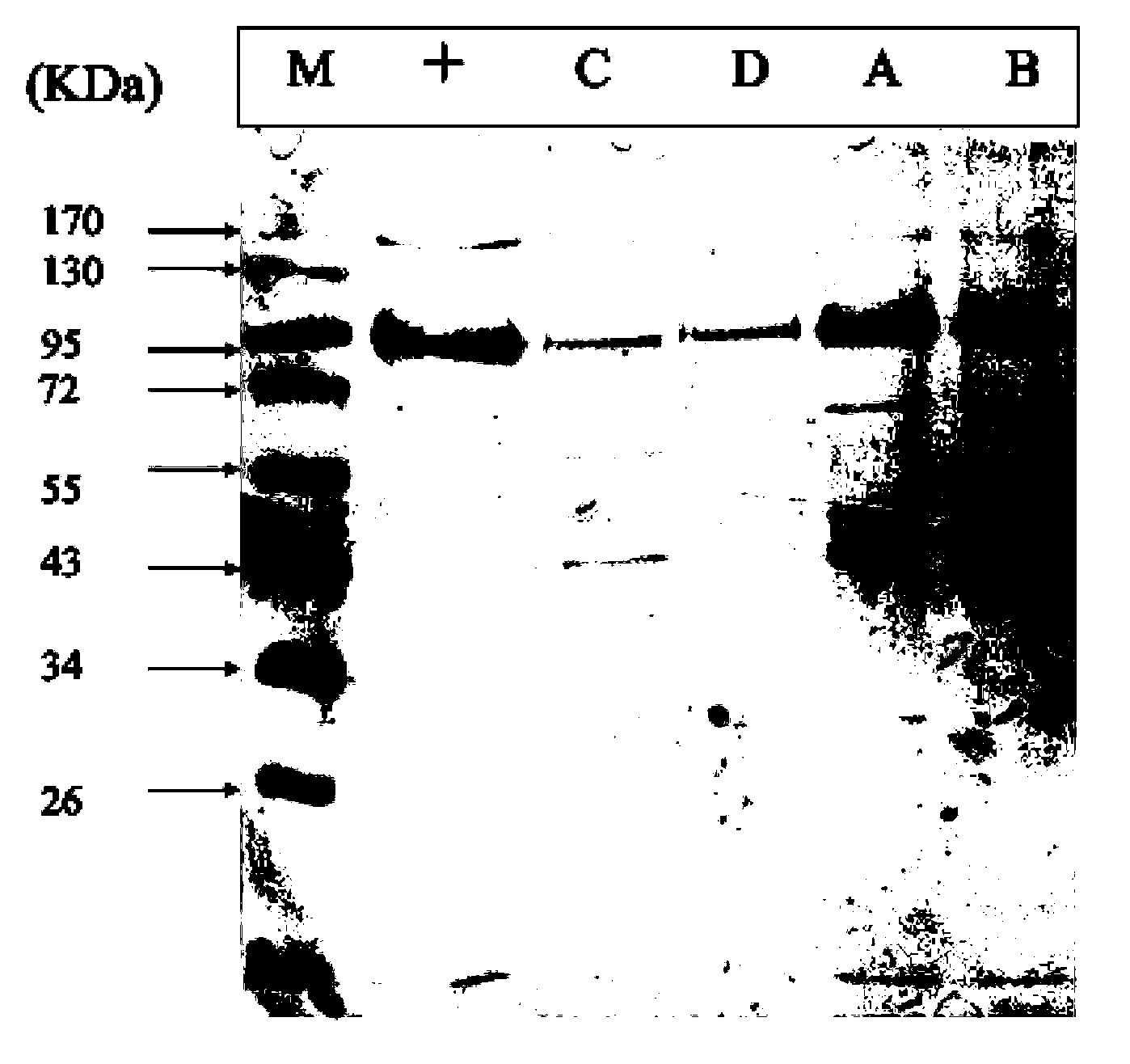
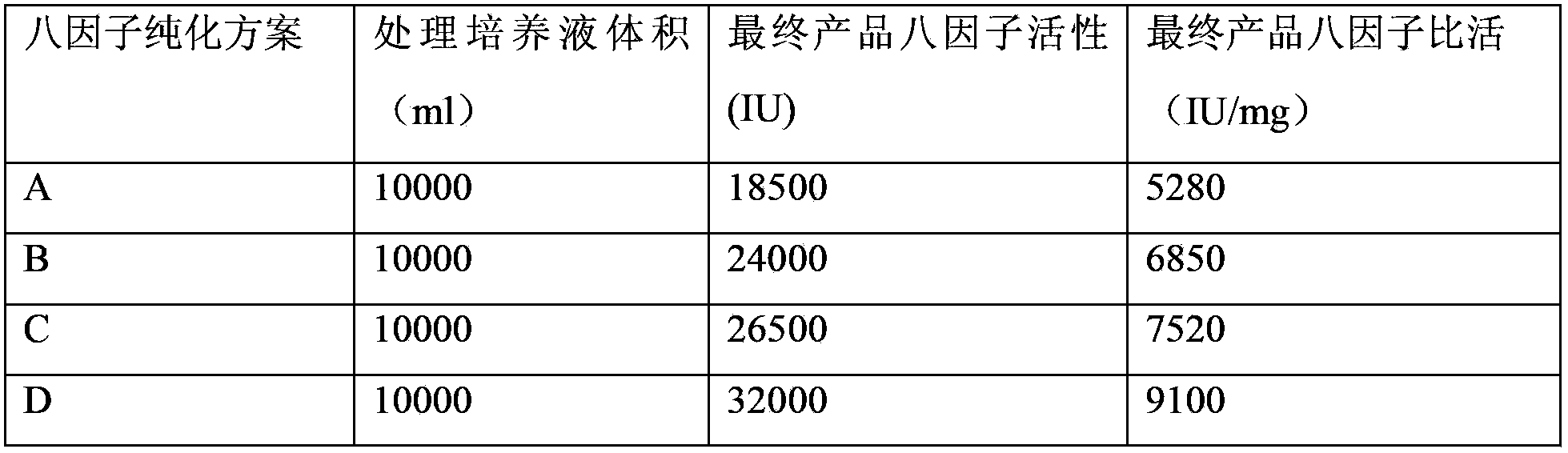
Method for separating and purifying recombinant human coagulation factor VIII from cell culture fluid
InactiveCN103539852AImprove stabilityWill not degradeFactor VIIPeptide preparation methodsUltrafiltrationFiltration
The invention discloses a method for separating and purifying recombinant human coagulation factor VIII from cell culture fluid. The method comprises the following steps: removing cells in the cell culture fluid of recombinant human coagulation factor VIII; ultrafiltration concentrating the culture fluid with cells removed; purifying the ultrafiltration concentrated culture fluid through one or more of anion exchange chromatography, immunoaffinity chromatography or gel filtration chromatography to obtain purified recombinant human coagulation factor VIII, wherein equilibration buffer and elution buffer adopted in the anion exchange chromatography, immunoaffinity chromatography and the gel filtration chromatography contain calcium ions with mol concentration of 1-100 mM and zinc ions with mol concentration of 1-100 mM. According to the method disclosed by the invention, the calcium ions and the zinc ions are added in the buffer solutions adopted in the whole chromatography process to effectively solve the degradation problem of the factor VIII and carry out the whole purification operation under normal temperature, thereby greatly improving the working efficiency and improving the specific activity of the final product.
Owner:上海泰龙生物医药科技有限公司
Preparation method of human coagulation factor VIII
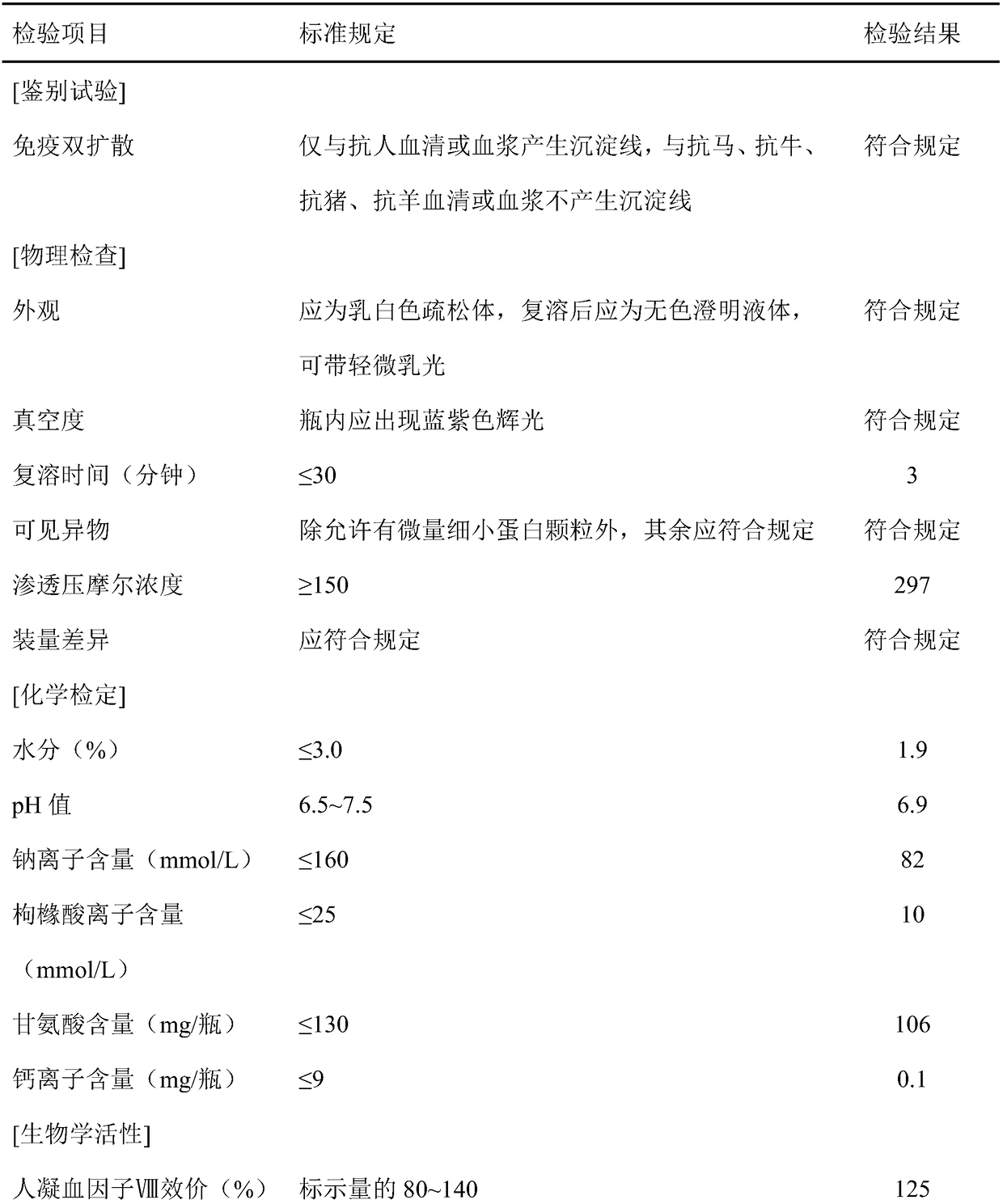
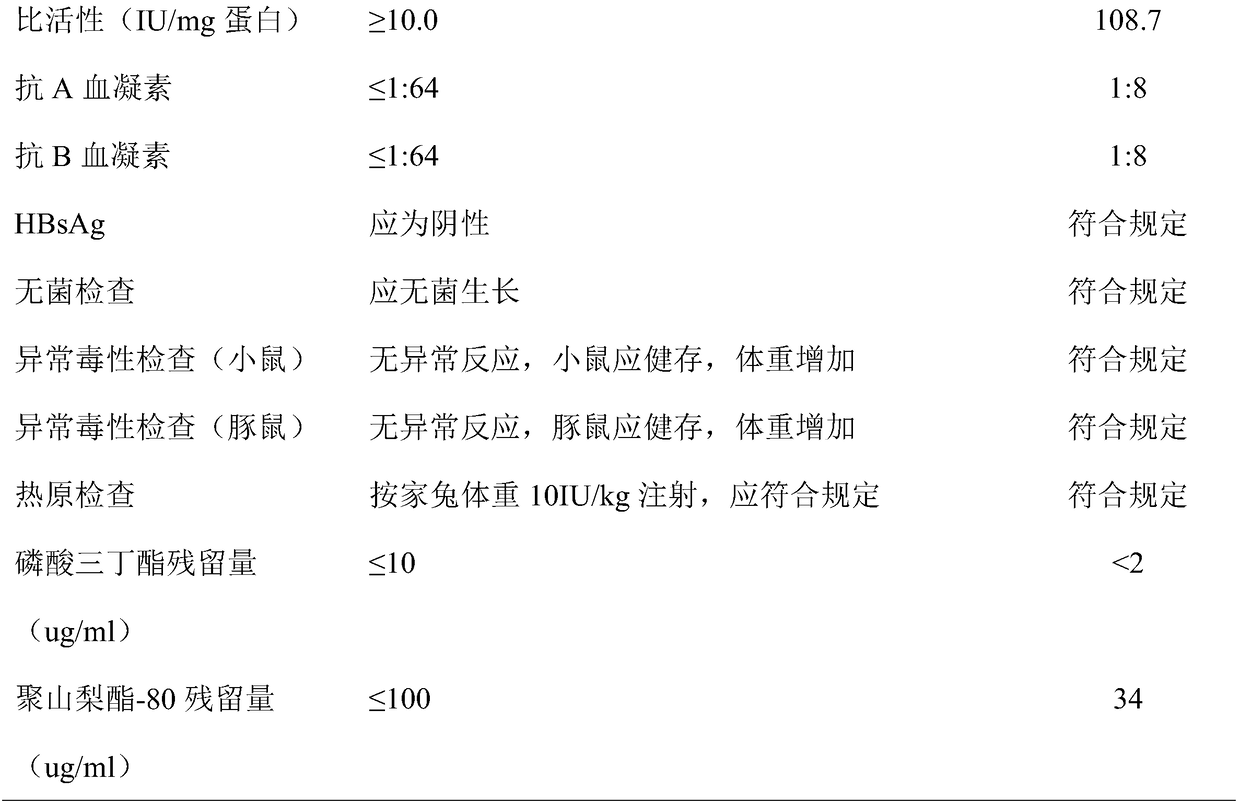
ActiveCN108218981AIncrease the efficiency of preparing FⅧImprove efficiencyFactor VIIPeptide preparation methodsWhole blood productDry heat
The invention belongs to the technical field of biological pharmacy and blood products, and especially relates to a preparation method of a human coagulation factor VIII. According to the invention, apreparation process of the human coagulation factor VIII is subjected to total research, cryoprecitation preparation, dissolution, washing liquid and an eluate are screened, after elution, the residual quantity of sorbate 80 and tributyl phosphate can achieve the requirement of current pharmacopeia; a dialysate is screened, the finally obtained product has the advantages of no collapse, no atrophy, and fast redissolving time, and FVIII titer after dry heat inactivation is 28.9 IU / ml. The processes for preparing the human coagulation factor VIII are optimized and screened, an optimum scheme isobtained, the recovery rate of each process is calculated, the recovery rate of FVIII is 28.91%, the efficiency for preparing FVIII by blood plasma is effectively increased, and a rear blood plasma resource is indirectly saved.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Application of mutant single-chain human coagulation factor VIII in preparation of fusion protein
ActiveCN113105562AModulating binding affinityNon-cytotoxicFactor VIIPeptide/protein ingredientsDiseaseHuman Chorionic Gonadotropin Beta Subunit
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC +1
Fusion protein lhfvii-ldp and enhanced fusion protein lhfvii-ldp-ae and applications thereof
InactiveCN102277364AStrong targetingLow immunogenicityPeptide/protein ingredientsPharmaceutical non-active ingredientsNucleotideEukaryotic plasmids
A fusion gene encoding the fusion protein LhFVII-LDP is formed by linking the gene encoding the light chain of human blood coagulation factor VII and the gene encoding lidamycin prosthetic protein, and its nucleotide sequence is shown in SEQ ID NO. 3. A recombinant plasmid expressing the fusion protein LhFVII-LDP contains a fusion gene encoding the fusion protein LhFVII-LDP. The fusion protein LhFVII-LDP is expressed from the above-mentioned recombinant plasmid, and its amino acid sequence is shown in SEQ ID NO.4 in the sequence listing. The enhanced fusion protein LhFVII-LDP-AE is composed of the fusion protein LhFVII-LDP whose amino acid sequence is shown in SEQ ID NO.4 in the sequence listing and the enediyne chromophore AE. The fusion protein LhFVII-LDP and the strengthened fusion protein LhFVII-LDP-AE can be used in the preparation of medicines for treating tumors.
Owner:SICHUAN UNIV +1
A kind of preparation method of human coagulation factor VIII
ActiveCN104231073BSuitable for a wide range of peopleHigh potencyFactor VIIPeptide/protein ingredientsAlcohol sugarsCrowds
The invention discloses a preparation method of a human coagulation factor VIII. The human coagulation factor VIII prepared by the method does not contain human serum albumin or other animal-derived protein, does not contain sugar or sugar alcohol, does not have the risk for transmitting other viruses or pathogene, and is wide in applicable crowd scope, and can be used by diabetic patients; the human coagulation factor VIII prepared by the method is fast to redissolve and good in redissolving effect, and still keeps high titer and high specific activity which are respectively larger than 80 percent and 40 IU / mg; in addition, the preparation method is simple, the cost is low, the human coagulation factor VIII is safe and effective, and has a good industrial application prospect.
Owner:广东双林生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com