Method for preparing coagulation factor VIII by using rabbit mammary gland bioreactor
A bioreactor, coagulation factor technology, applied in biochemical equipment and methods, botanical equipment and methods, plant genetic improvement, etc., can solve problems such as increasing the risk of virus infection in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of B-domain deleted recombinant human coagulation factor eighth cDNA (B-deleted rhFVIII cDNA)
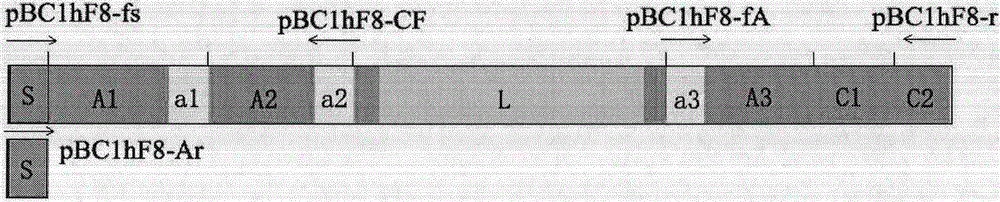
[0028] Recombinant human full sequence coagulation factor VIII cDNA (rhFVIII cDNA), including A1, A2, B, A3, C1 and C2 domains, figure 1 It is a schematic diagram of the structure of B-deleted rhFVIII cDNA connected with bGHployA, "S" in the figure indicates the signal gene bGHployA, and B-deleted rhFVIII cDNA is artificially synthesized. The primers used to detect B-deleted rhFVIII cDNA by PCR detection method are pBClhF8-fs, pBClhF8-Ar, pBClhF8-CF, pBClhF8-r, pBClhF8-fA, figure 1 The direction indicated by the middle arrow is the direction of the primer.
[0029] Infect the B-deleted rhFVIII cDNA fragment into the competent cells of Zero Blunt TOPO bacteria (source: Invitrogen, Cat: K2800-20), the size of the B-deleted rhFVIII cDNA fragment is 4355bp, and then make the bacteria proliferate and extract the plasmid for Electrophoretic determination, th...
Embodiment 2
[0030] Example 2 Preparation of the Mammary Gland Expression Component pBCl-rhFVIII of the Eighth Coagulation Factor
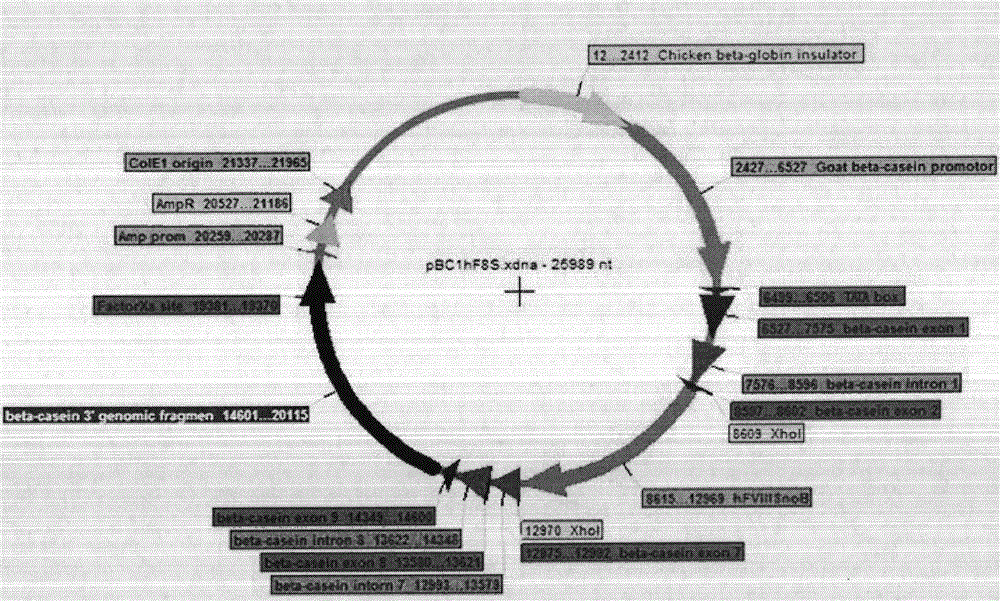
[0031] Such as image 3 As shown, the pBCl plasmid is connected with chicken β-globulin insulator, goat β-casein promoter, TATA box, β-casein exon 1, β-casein intron 1, β-casein exon 2, Insert the B-deleted rhFVIII cDNA of the external signal gene into the restriction endonuclease Xho I multiple cloning site of the plasmid vector, and then connect -casein exons 7, 8, 9, β-casein introns 7, 8 , loading the β-casein genome fragment at the 3' end to form the mammary gland expression component pBCl-rhFVIII of the eighth blood coagulation factor, and the gene sequence is 25989bp long. The pBCl plasmid DNA contains the bacterial (EolEl) initiation fragment, the resistance gene AmpR promoter and the resistance gene AmpR.
[0032]Among them, chicken beta-globin insulator (Chicken beta-globin insulator), goat beta-casein promoter (Goat beta-casein promoter), TATA box...
Embodiment 3
[0033] Example 3 Amplification and detection of the mammary gland expression component pBCl-rhFVIII of the eighth coagulation factor
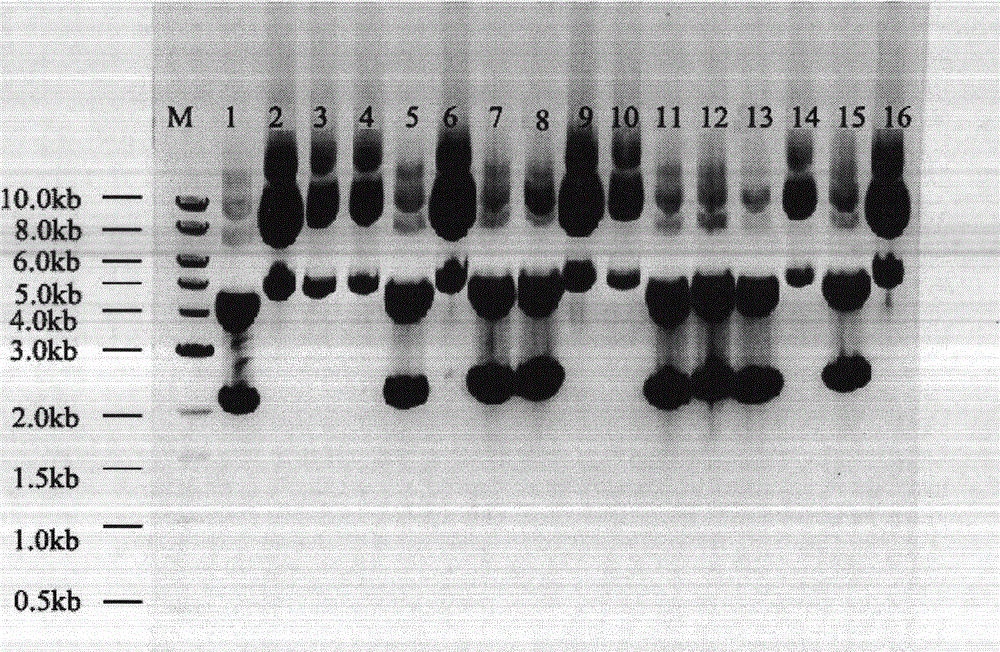
[0034] The mammary gland expression component pBCl-rhFVm of the eighth blood coagulation factor was transferred into the competent cells of the recipient strain DH5α, and the obtained recipient strain was multiplied to realize the amplification of pBCl-rhFVIII, and positive clones were screened out by PCR method, The primers are pBClhF8S-SNf: 5′-TGTGGTGAACTTCTCTAGACCCACCGTT-3′, pbCl-sr: 5′-CATCAGAAGTTAACAGCACAGTTAG-3′, and the PCR fragment size is 197bp. Then electrophoresis was carried out, and the results were as follows: Figure 4 As shown, among the 120 clones screened, numbers 38 and 47 are positive clones, wherein M is 100 bp DNA ladder (source: NEB, Cat. N0467S). Clones #38 and 47 were subjected to DNA sequencing, and clone #47 was completely consistent with the sequence of the gene bank cDNA (NM000132.3). The comparison results are as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com