Fusion protein lhfvii-ldp and enhanced fusion protein lhfvii-ldp-ae and applications thereof
A fusion protein, lhfvii-ldp-ae technology, applied in the fusion protein LhFVII-LDP and strengthened fusion protein LhFVII-LDP-AE and its application field, can solve the problem of strengthened fusion protein and achieve good targeting, Low production cost and reduced immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Cloning of the fusion gene encoding the fusion protein LhFVII-LDP
[0028] Primer 1: 5'-TAA CCATGG GCCATCATCATCATCATCACGCCAACGCGTTCCTGGAGGAGCTGCG-3'
[0029] Nco I
[0030] Primer 2: 5'- CCACCACTGCCGCCGCCGCCGCTACCACCACCACCACC TCGGCCTTGGGGTTTGCTGGCATT-3'
[0031] Linker: GGGGS×3 connecting peptide
[0032] Primer 3: 5'- GTGGTGGTAGCGGCGGCGGCGGCAGTGGTGGCGGTGGCTCT GCGCCCGCCTTCTCCGTC-3'
[0033] Linker: GGGGS×3 connecting peptide
[0034] Primer 4: 5'-TAT CTCGAG TTAGCCGAAGGTCAGAGCCACGT-3'
[0035] Xho I
[0036] The above primers were synthesized by Handsome Biotechnology Co., Ltd.
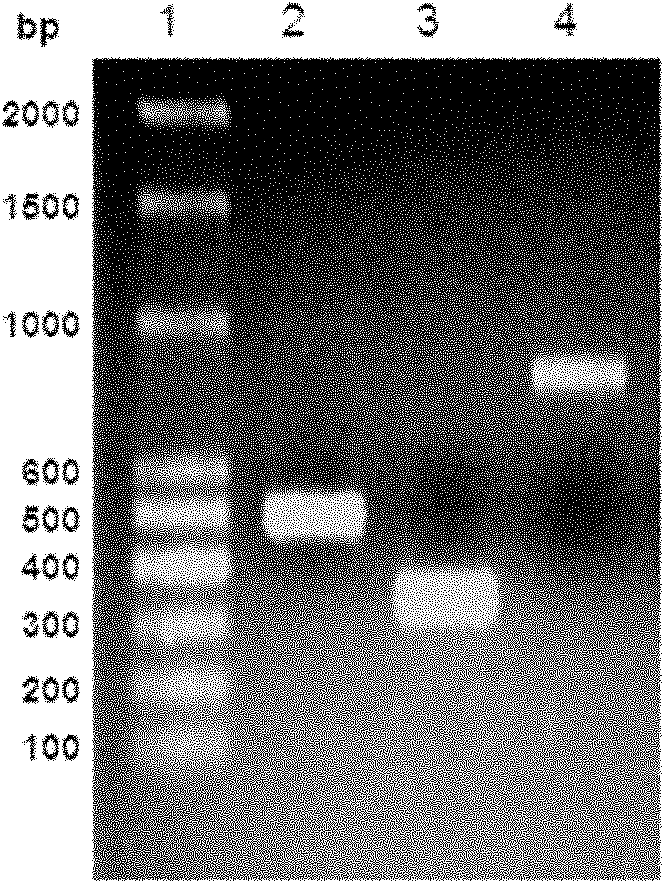
[0037] Using human coagulation factor VII cDNA (Guangzhou Funeng Gene Co., Ltd.) as a template, PCR amplification was performed with primers 1 and 2 to obtain a 521bp DNA fragment containing the coding sequence of human coagulation factor VII light chain and GGGGS×3 connecting peptide ( See figure 1...
Embodiment 2
[0062] Embodiment 2: Construction of the recombinant plasmid expressing fusion protein LhFVII-LDP
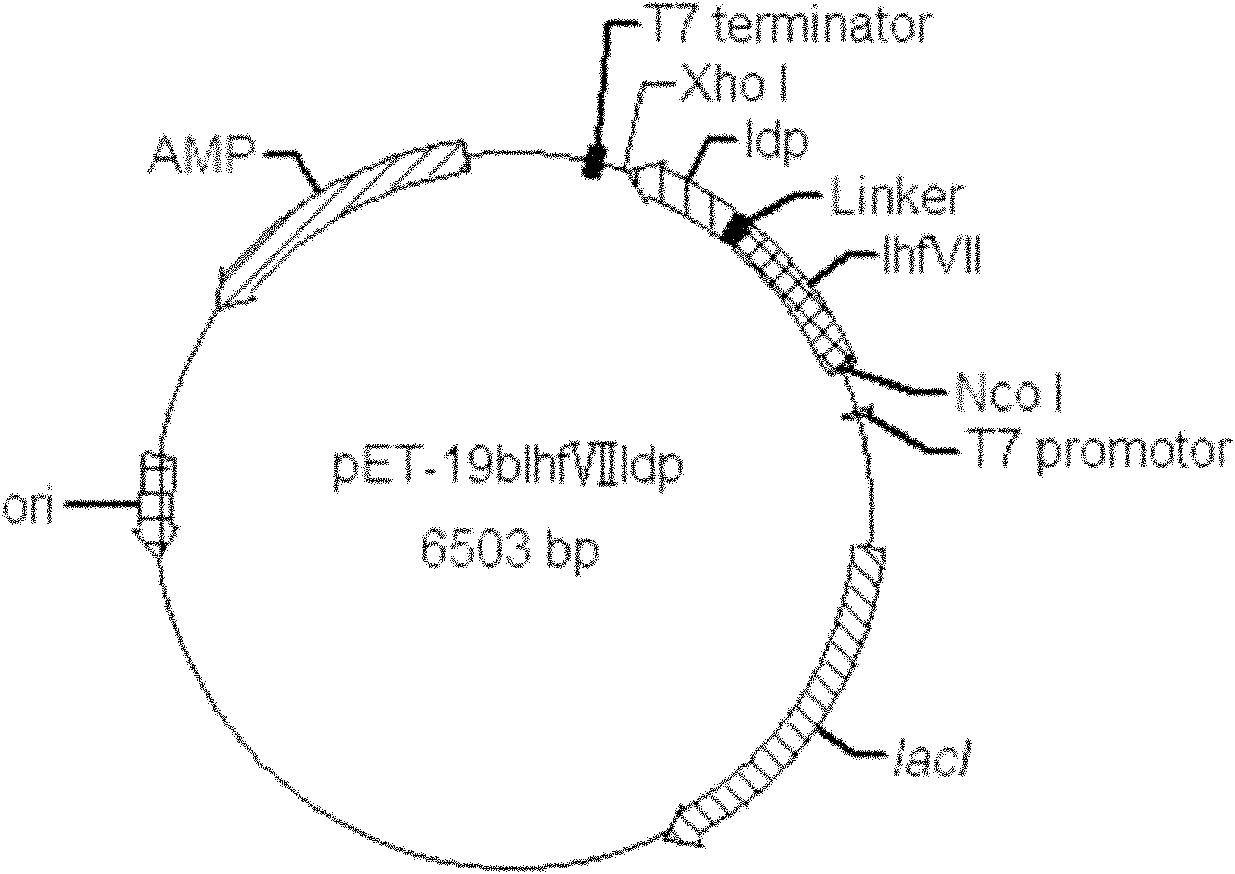
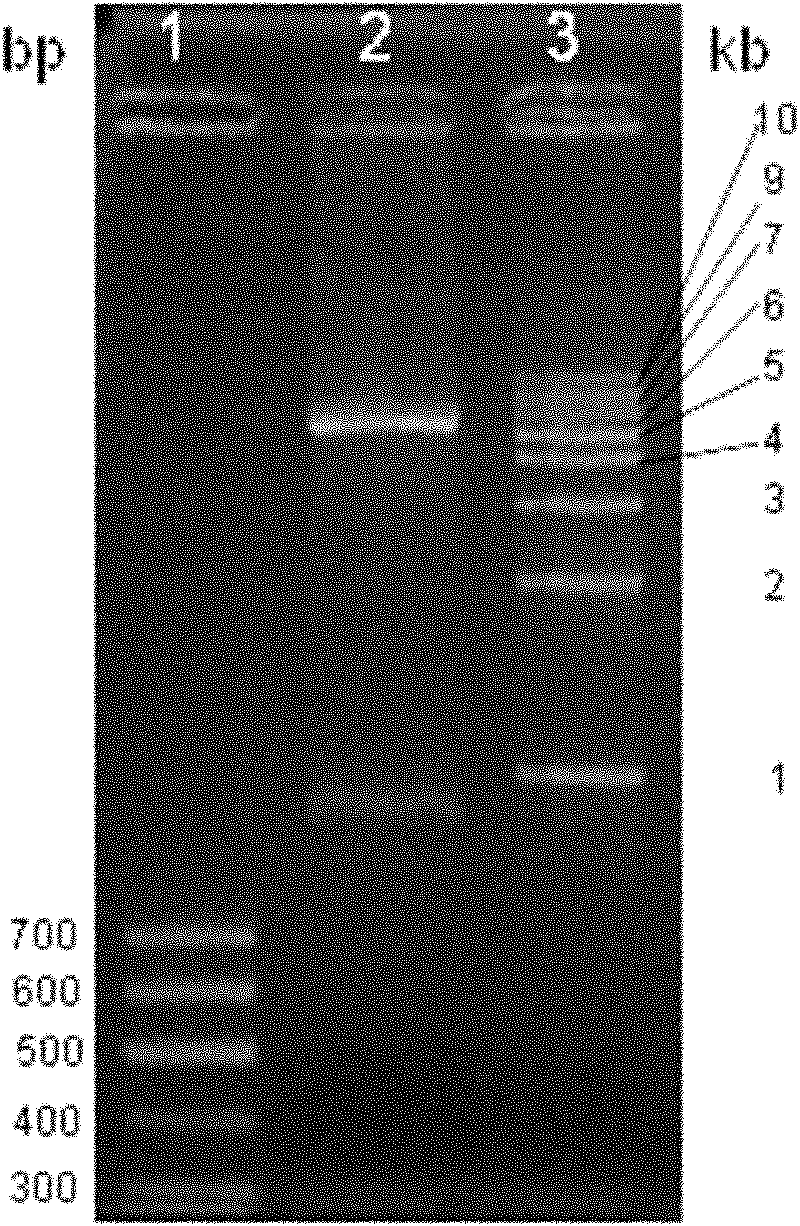
[0063] The lhfVIIldp fusion gene fragment cloned in Example 1 was subjected to NcoI / XhoI double digestion, and the restriction gene fragment was connected to the pET-19b vector (Novagen company product) through the same digestion (see figure 2 ), and then transform the ligated product into E.coli DH5α After culturing at 37°C, single clones were picked, plasmids were extracted after a small amount of culture, and identified by NcoI / XhoI double enzyme digestion (for the identification results, see image 3 ). The identified plasmid was sent to Beijing Huada Gene Research Center to sequence the inserted fusion gene fragment, and its nucleotide sequence is shown in SEQ ID NO.3 in the sequence list. The recombinant plasmid containing lhfVIIldp fusion gene with correct sequencing results was named pET19blhfVIIldp.
Embodiment 3
[0064]Example 3: Expression of recombinant plasmids in Escherichia coli
[0065] The recombinant plasmid pET19blhfVIIldp constructed in Example 2 was transformed into Escherichia coli Rosetta-gami B(DE3)pLysS (product of Novagen), and a single clone was picked in LB medium, and cultured with shaking at 37°C until OD 600 ≈0.6, take 15ul bacterial liquid and freeze it at -20°C, then add IPTG (isopropyl-β-D-thiogalactoside) with a final concentration of 0.8mM to continue culturing for 6h, take 7.5ul bacterial liquid and freeze it at -20°C . Take another 5ml of the induced bacterial solution, centrifuge at 4000rpm×3min, discard the supernatant, and use 600ulPBS (preparation method: weigh 8g of NaCl, 0.2g of KCl, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.24g, dissolved in 900ml of double-distilled water, adjusted the pH value to 7.4 with hydrochloric acid, added water to make up to 1L) and resuspended, the resuspended bacterial solution was ultrasonically crushed, centrifuged at 16000g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com