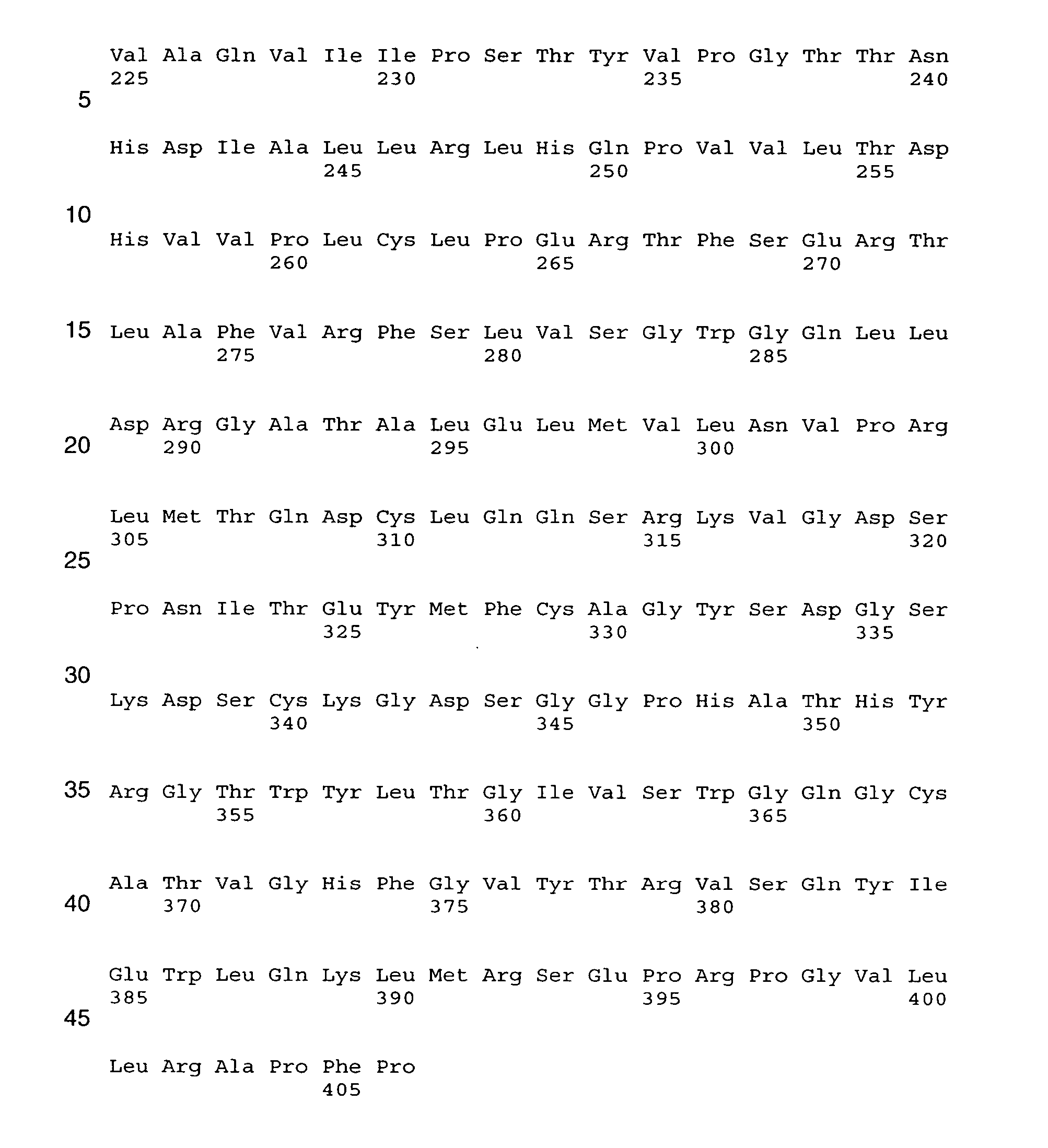Patents
Literature
33 results about "Human coagulation factor VII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
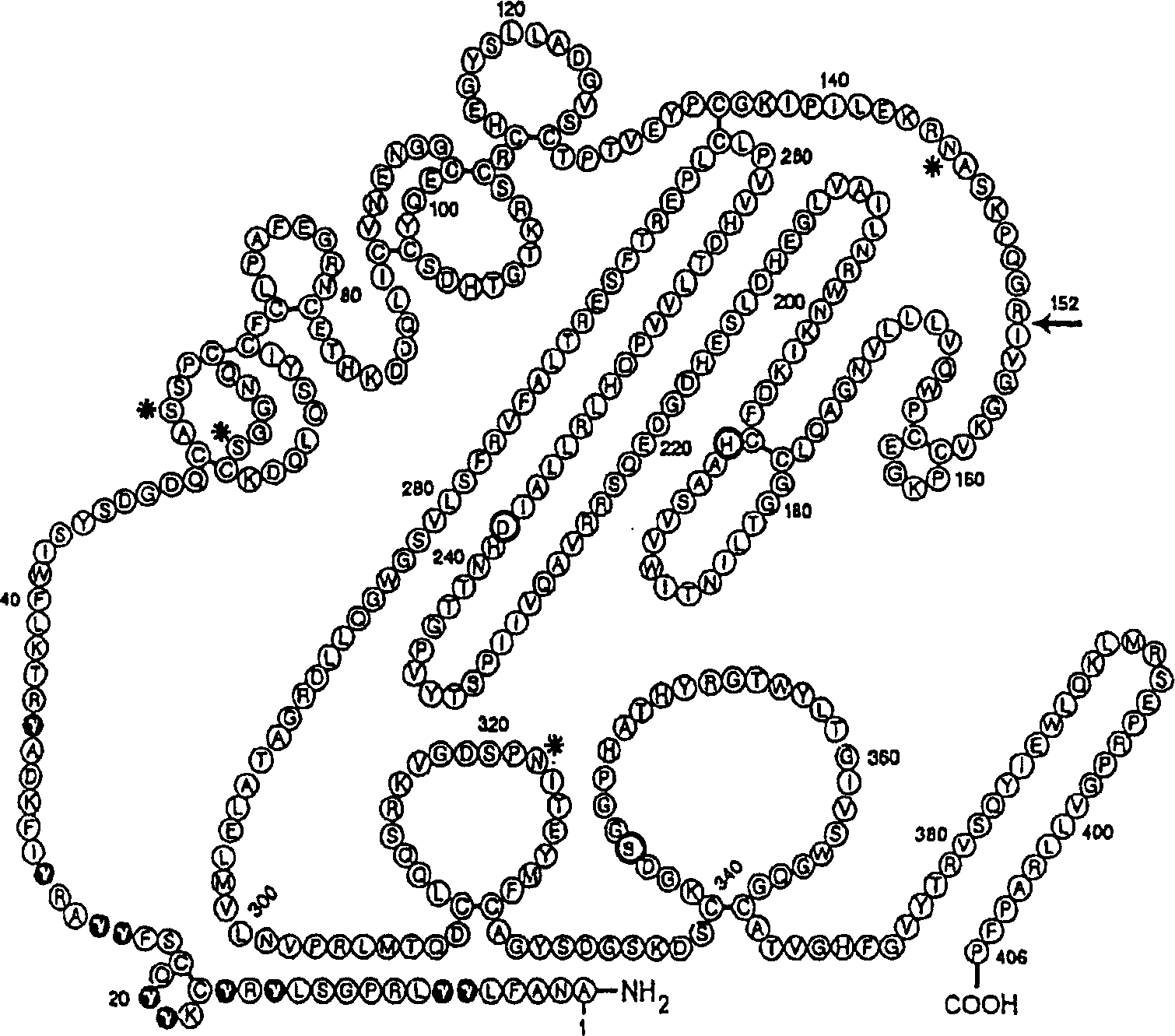
Human Coagulation Factor VII Polypeptides
InactiveUS20090055942A1Prolong half-life in vivoPeptide/protein ingredientsFermentationFactor VIIClotting factor
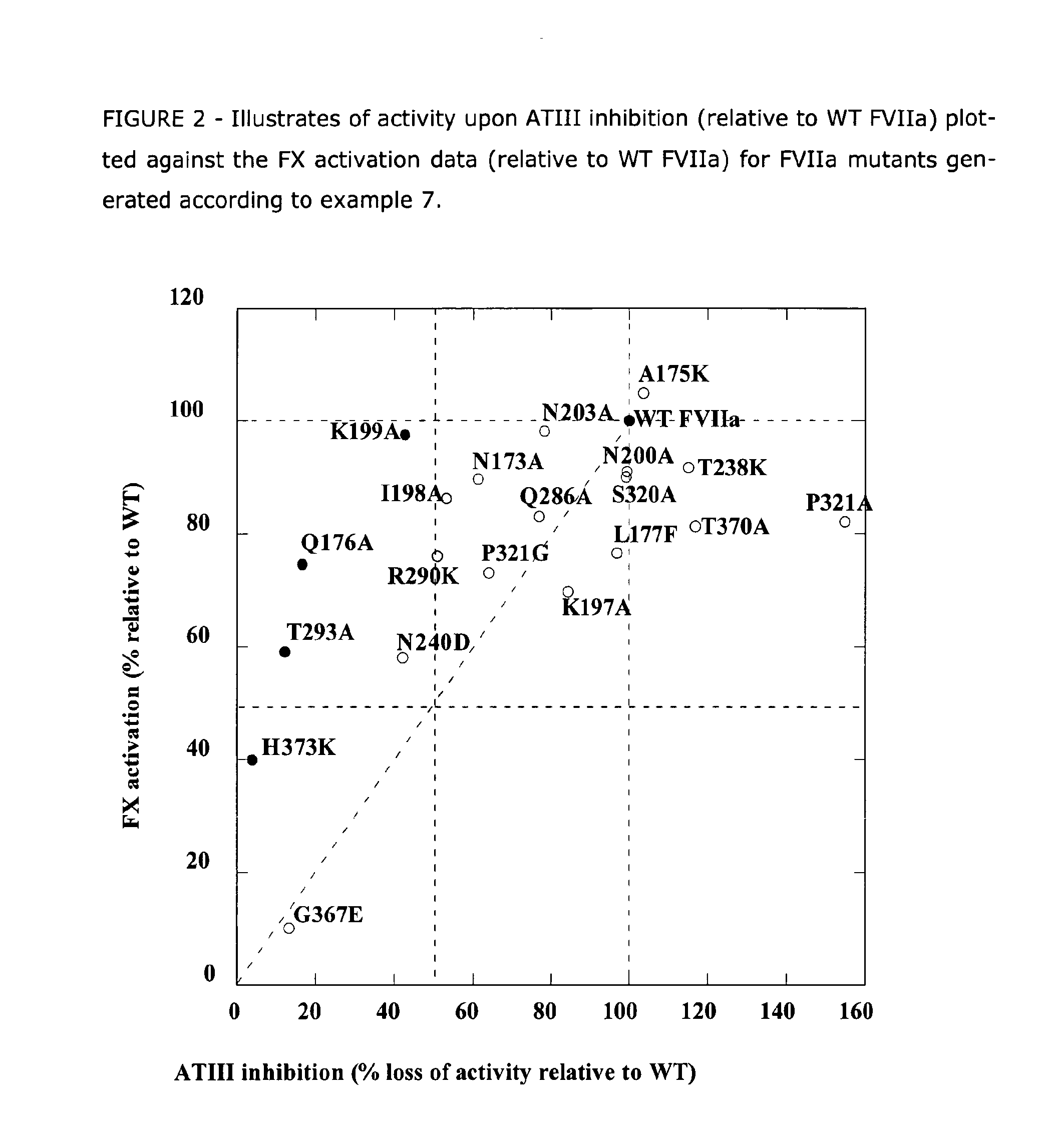
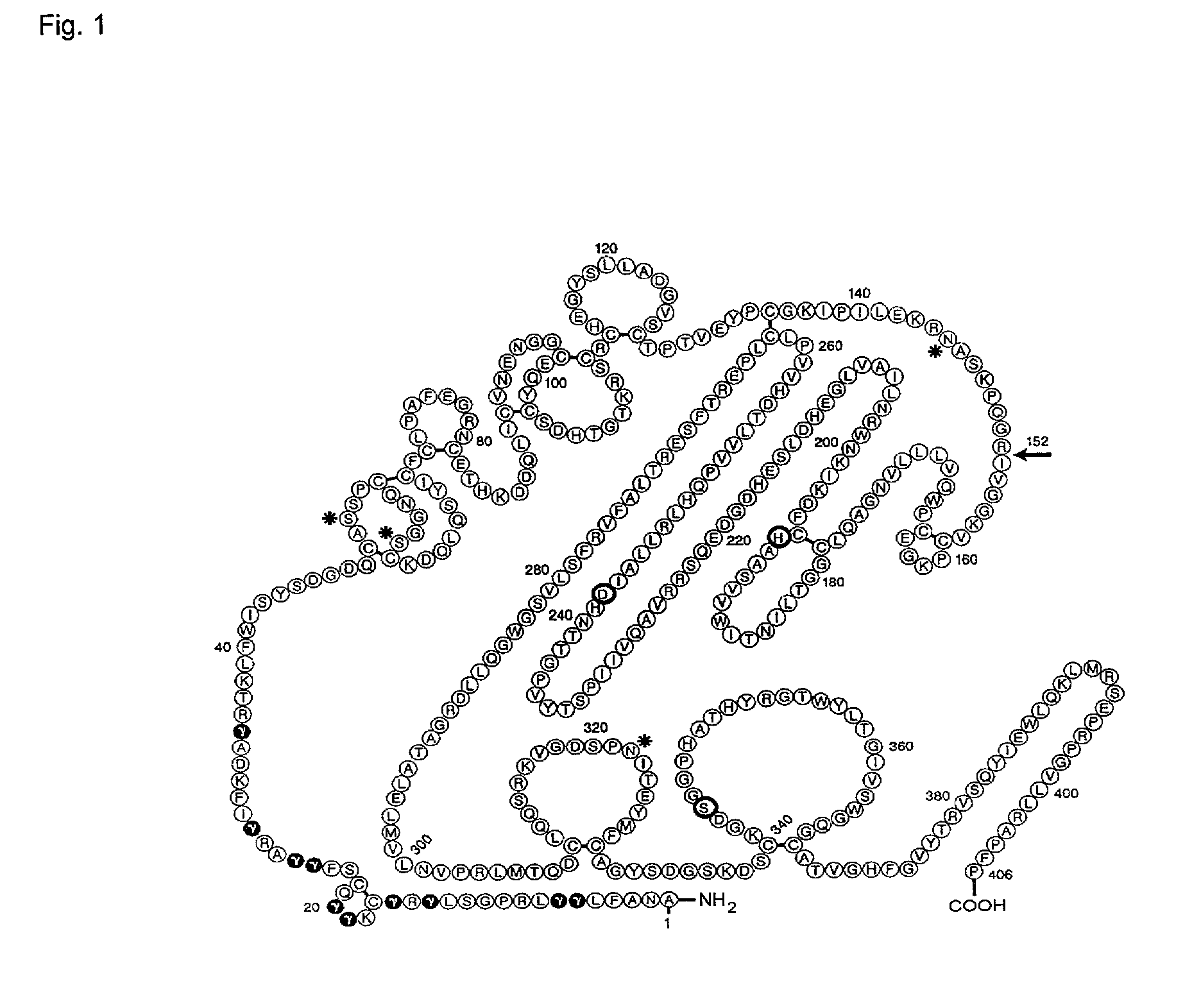
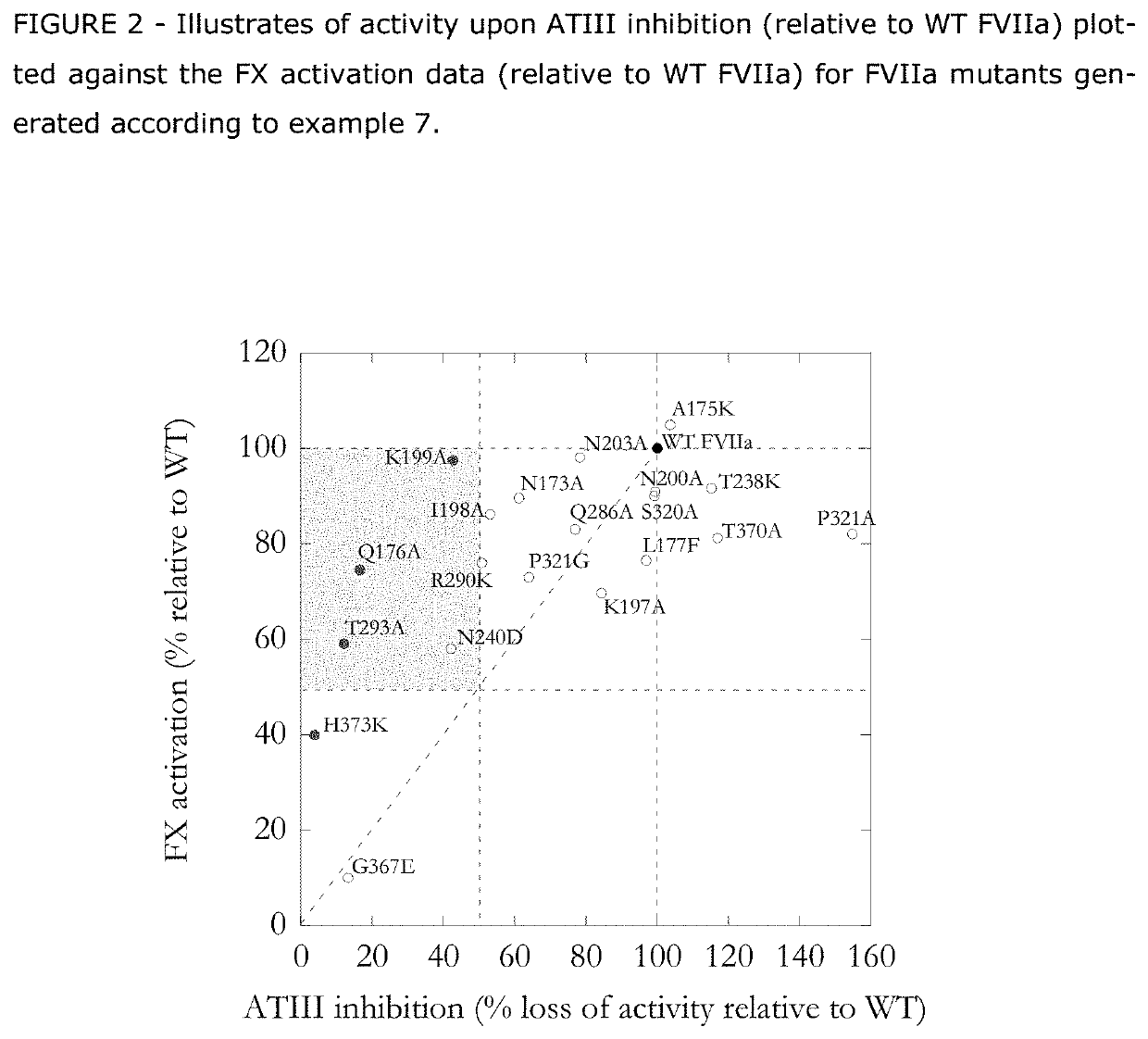
The present invention relates to novel human coagulation Factor Vila variants having substitutions of one or more amino acids at a position selected from the group consisting of position 172, 173, 175, 176, 177, 196, 197, 198, 199, 200, 203, 235, 237, 238, 239, 240, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 297, 299, 319, 320, 321, 327, 341, 363, 364, 365, 366, 367, 370, 373 corresponding to amino acid positions of SEQ ID NO:1 and wherein said Factor VII polypeptide exhibits increased resistance to inactivation by an endogenous inhibitor of said FVII polypeptide relative to wild-type human FVIIa.
Owner:NOVO NORDISK AS
Human coagulation factor VII variants
InactiveUS7026524B2Peptide/protein ingredientsMammal material medical ingredientsHuman coagulation factor VIIIHuman coagulation factor VII
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as nucleic acid constructs encoding such variants, vectors and host cells comprising and expressing the nucleic acid, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Coagulation factor VII derivatives
InactiveUS7235638B2Same and increased activityExtended half-lifeBiocidePeptide/protein ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VII polypeptides, Factor VII derivatives as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
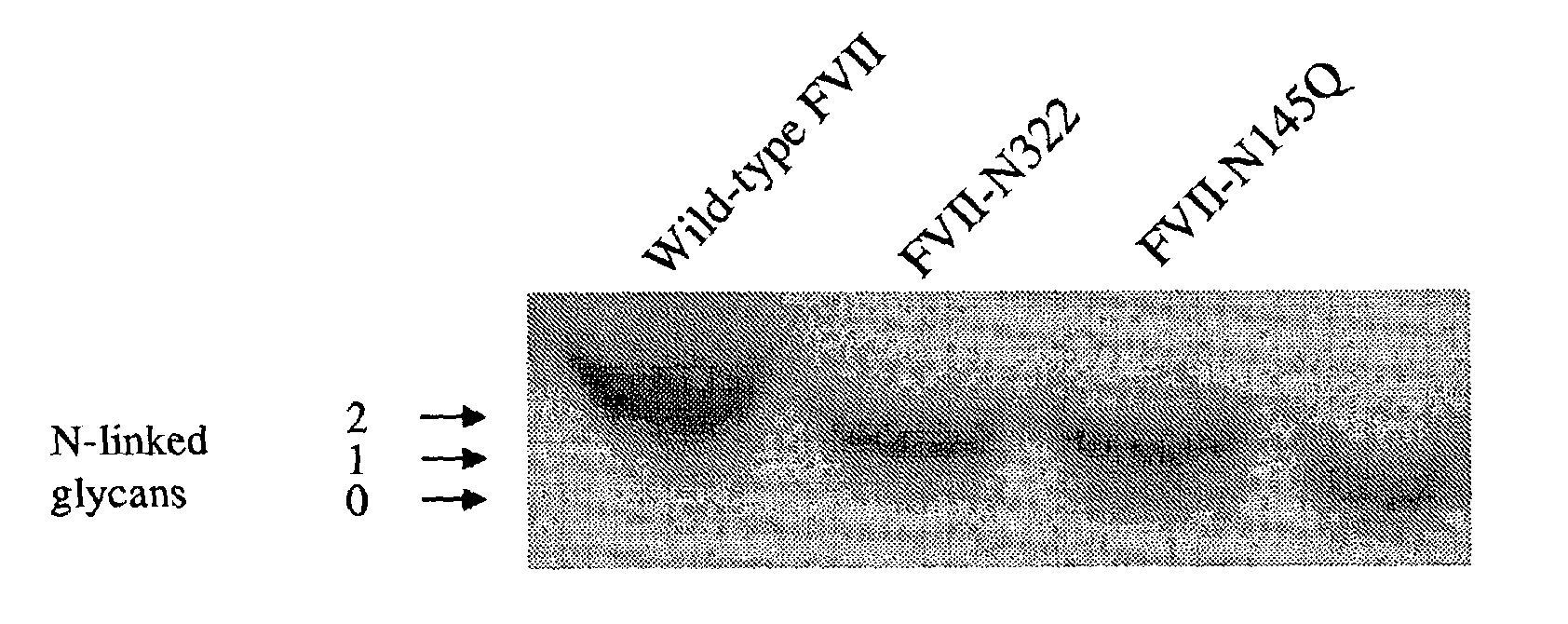
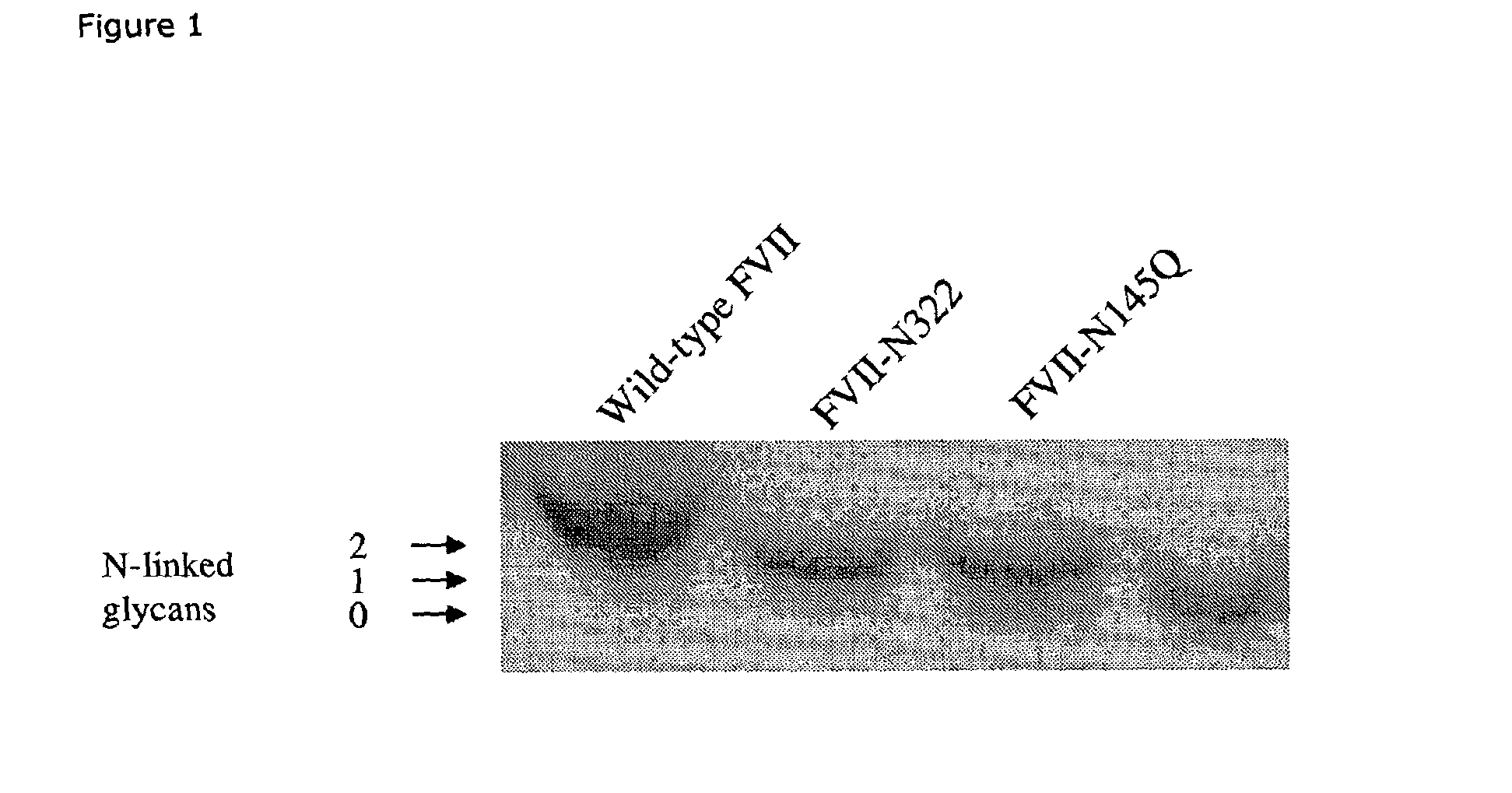
Glycosylation-Disrupted Factor VII Variants
InactiveUS20080058255A1Improve biological activityRapid clearancePeptide/protein ingredientsSurgical drugsNucleotideFactor VII
The present invention relates to human coagulation Factor VII polypeptides, as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions comprising Factor VII polypeptides, uses and methods of treatment; and any additional inventive features related thereto.
Owner:NOVO NORDISK AS
Human coagulation factor VII polypeptides
InactiveUS6911323B2Peptide/protein ingredientsMammal material medical ingredientsPolynucleotideHuman coagulation factor VIII
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Human coagulation factor VII polypeptides
InactiveUS6960657B2High activityPromote formationPeptide/protein ingredientsMammal material medical ingredientsPharmaceutical drugPolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Human coagulation factor VII polypeptides
InactiveUS20060166915A1High activityPromote formationPeptide/protein ingredientsGenetic material ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor VII variants
InactiveUS6905683B2Increased tissue factor-independent activityPeptide/protein ingredientsMammal material medical ingredientsProteinase activityCoagulation system
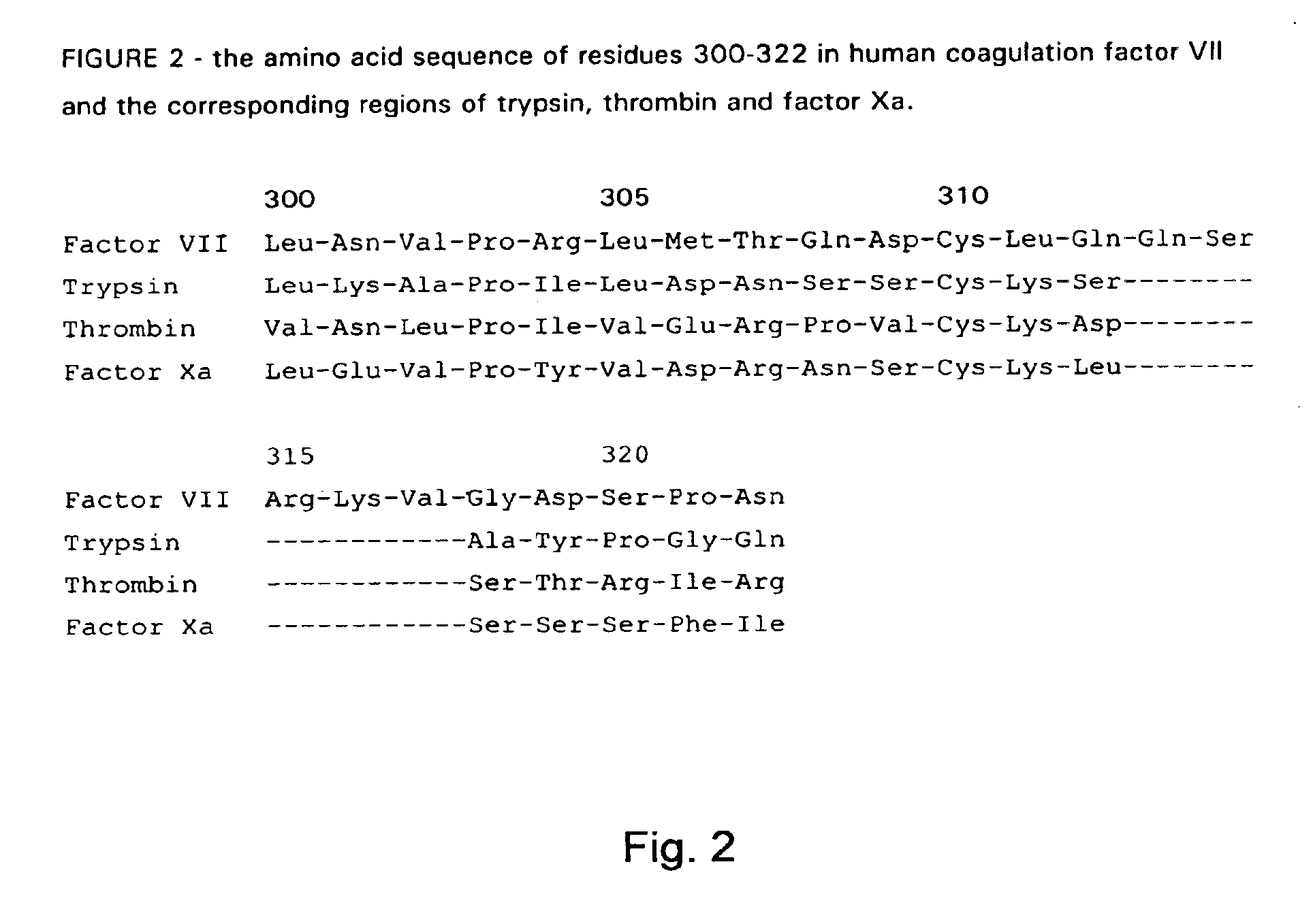
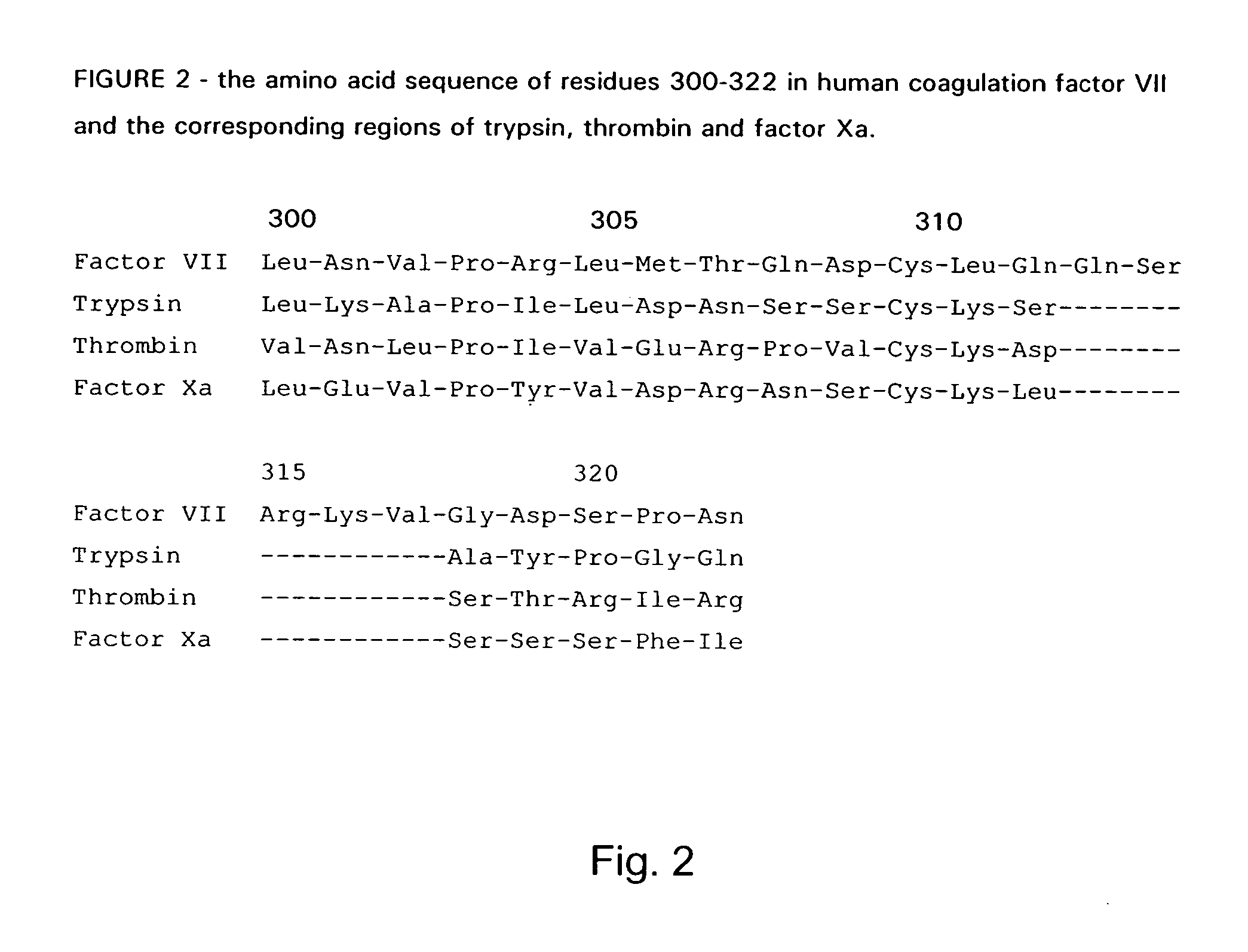
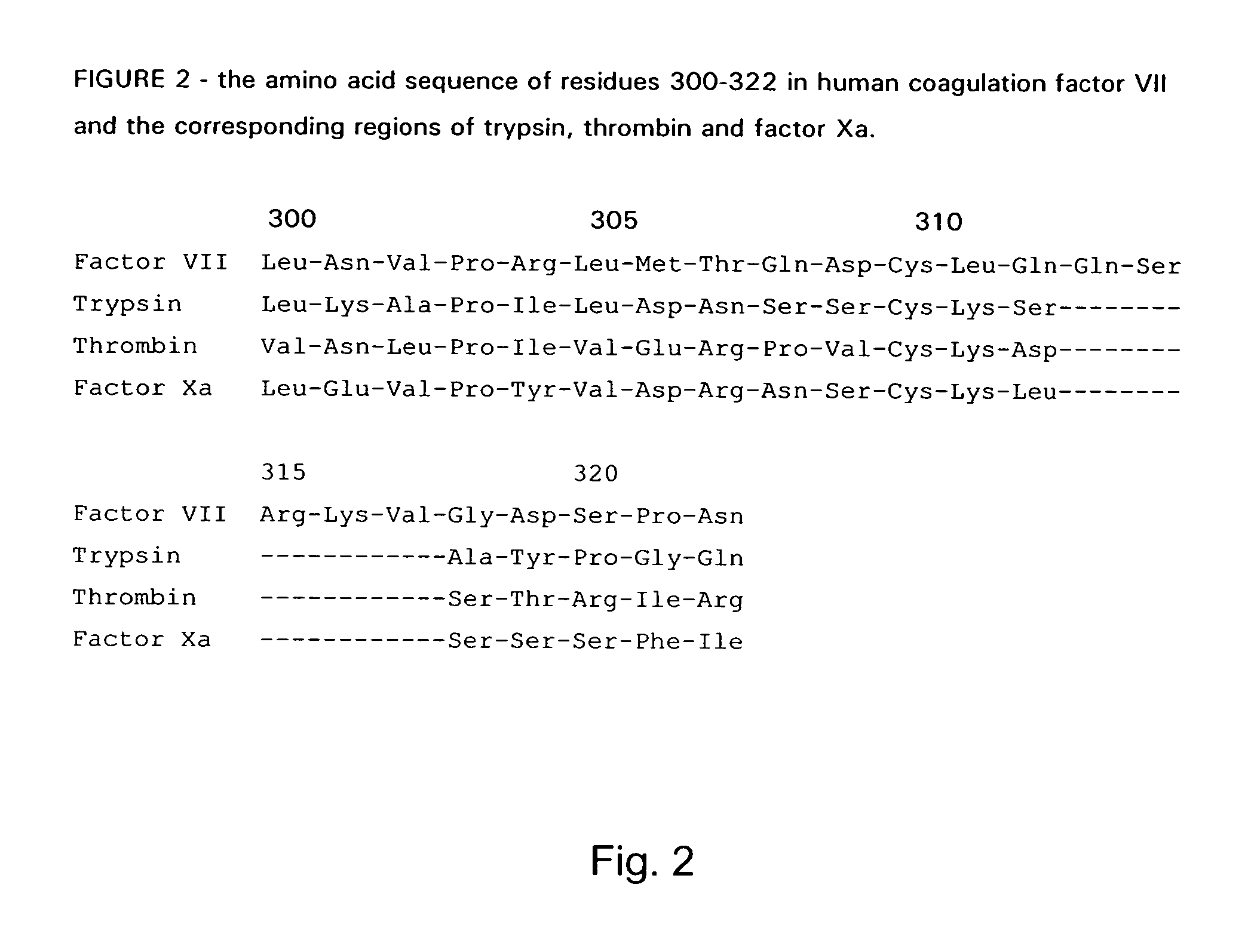
The invention concerns novel coagulation factor VII variants, wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs;with the proviso that the variant is not FVII(Ala305).The invention further concerns nucleic acids encoding the Factor VII variants; vectors and cells comprising the nucleic acid; methods for producing the variants; pharmaceutical compositions comprising a Factor VII variant wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs; use of the variants for producing a medicament for treatment or prophylaxis of bleeding disorders or enhancement of the coagulation system; and methods of treatment.
Owner:NOVO NORDISK AS
Hybrid molecules having Factor VII/VIIa activity
InactiveUS7432352B2Same and increased activityIncreased serum half-lifePeptide/protein ingredientsMammal material medical ingredientsSynthetic analogueFactor ii
Owner:NOVO NORDISK HEALTH CARE AG
Hybird molecules having factor VII/VIIa activity
InactiveUS20060258851A1Same and increased activityIncreased serum half-lifeFibrinogenPeptide/protein ingredientsSemi syntheticFactor VII
The present invention relates to novel human coagulation Factor VII / VIIa proteins having coagulant potential / activity as well as pharmaceutical compositions comprising the polypeptides, uses and methods of treatment. In particular, the present invention relates to novel, semi synthetic analogues of human coagulation Factor VII and VIIa (FVII and FVIIa) as well as to a method of their production.
Owner:NOVO NORDISK AS
Human coagulation factor VII polypeptides
InactiveUS7052868B2High activityInduce and stabilisationPeptide/protein ingredientsMammal material medical ingredientsPharmaceutical drugPolynucleotide
Owner:NOVO NORDISK HEALTH CARE AG
Human Coagulation Factor VII Polypeptides
InactiveUS20090011992A1Increased coagulant activityFactor VIIPeptide/protein ingredientsCoagulation factor VIIaHuman coagulation factor VII
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Human coagulation Factor VII variants
InactiveUS20060252129A1Peptide/protein ingredientsMammal material medical ingredientsBiologyHuman coagulation factor VIII
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor VII variants
InactiveUS20050204406A1Peptide/protein ingredientsMammal material medical ingredientsDiseaseProteinase activity
Owner:NOVO NORDISK AS
Activated human coagulation factor VII fusion protein and preparation method and application thereof
ActiveCN106279436APeptide/protein ingredientsAntibody mimetics/scaffoldsHuman Chorionic Gonadotropin Beta SubunitHalf-life
The invention discloses a hyperglycosylated activated human coagulation factor VII (FVIIa) fusion protein, and a preparation method and application thereof. The fusion protein comprises human FVIIa, a flexible peptide joint, at least one human chorionic gonadotropin beta-subunit carboxyl terminal peptide rigid unit and a half-life period prolonging part (a human IgG Fc variant preferred). The fusion protein has bioactivity similar to that of the natural human FVIIa and has a longer in-vivo active half-life period, so that the pharmacokinetics and the medicinal effect are improved.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC +3
Human coagulation factor VII polypeptides
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor VII polypeptides
InactiveUS20050204411A1High activityInduce and stabilisationHydrolasesPeptide/protein ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Nucleic acids encoding human coagulation factor VII variants
InactiveUS7416860B2Peptide/protein ingredientsMammal material medical ingredientsDrug biological activityHuman coagulation factor VII
The invention concerns nucleic acids encoding coagulation Factor VII variants in which the Leu residue in position 305 has been replaced by another amino acid residue. The invention further concerns the expression of such nucleic acids resulting in a Factor VII variant having increased biological activity.
Owner:NOVO NORDISK AS
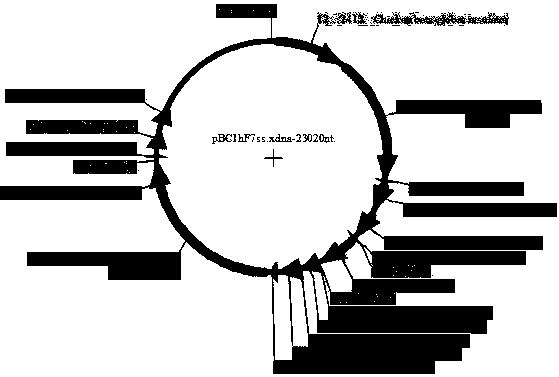
Method for producing recombinant human coagulation factor VII by using rabbit mammary gland reactor platform
ActiveCN103484497AEasy to produceHigh activityVector-based foreign material introductionEuropean rabbitComplementary deoxyribonucleic acid
The invention provides a method for producing a recombinant human coagulation factor VII by using a rabbit mammary gland reactor platform. CDNA (Complementary deoxyribonucleic acid) of the recombinant human coagulation factor VII is guided or integrated to a chromosome gene of a rabbit, and the obtained transgenic rabbit expresses recombinant human coagulation factor VII protein in a mammary gland reactor. The transgenic rabbit has relatively high recombinant human coagulation factor VII protein expression level in the mammary gland reactor, the protein activity is good, and the production cost is reduced.
Owner:LANNUO BIOTECH WUXI
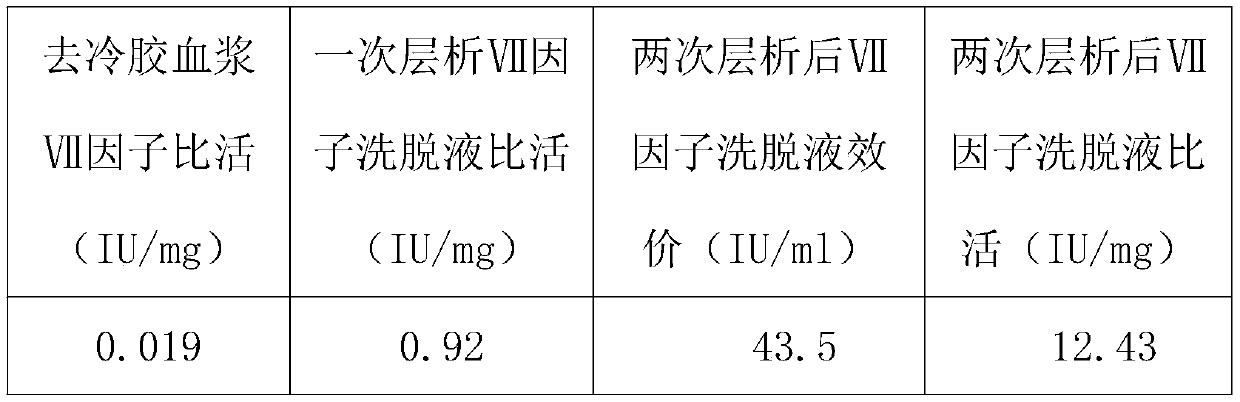
Method for purifying human coagulation factor VII from human plasma
ActiveCN111500563ATaller than aliveIncrease profitPeptidasesMonosodium glutamateAnion-exchange chromatography
The invention discloses a method for purifying a human coagulation factor VII from human plasma. The method comprises the following steps: (1) removing cold gel plasma, (2) performing anion exchange chromatography for the first time, (3) filtering the eluent, and adjusting the conductivity and pH value of the product, and (4) carrying out ion exchange chromatography for the second time. Accordingto the method, the human coagulation factor VII can be separated from the cold-gel-free plasma by adopting sodium glutamate as an eluent through first ion exchange chromatography, and the human coagulation factor VII can be further purified through two times of chromatography, so that the utilization rate of the plasma is greatly improved, and the human coagulation factor VII can achieve relatively high specific activity.
Owner:HUALAN BIOLOGICAL ENG CHONGQING
Coagulation factor vii derivatives
The present invention relates to novel human coagulation Factor VII polypeptides, Factor VII derivatives as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Anti-human coagulation factor VII monoclonal antibody, preparation method thereof and use thereof
InactiveCN101906161AStrong specificityHigh affinityImmunoglobulins against blood coagulation factorsTissue cultureAntigen bindingClotting factor
The invention provides an anti-human coagulation factor VII monoclonal antibody, a preparation method thereof and use thereof. The monoclonal antibody has a characteristic of high human coagulation factor VII antigen binding specificity. The invention also provides a corresponding monoclonal antibody fragment and an immunoconjugate. The invention also provides a detection kit for detecting the human coagulation factor VII.
Owner:SUZHOU ZELGEN BIOPHARML
Host cell containing vector for expressing functional recombinant human coagulation factor VII and high-level expression method of functional recombinant human coagulation factor VII
ActiveCN104293737AAvoid interactionImprove expression levelHydrolasesVector-based foreign material introductionVKORC1High level expression
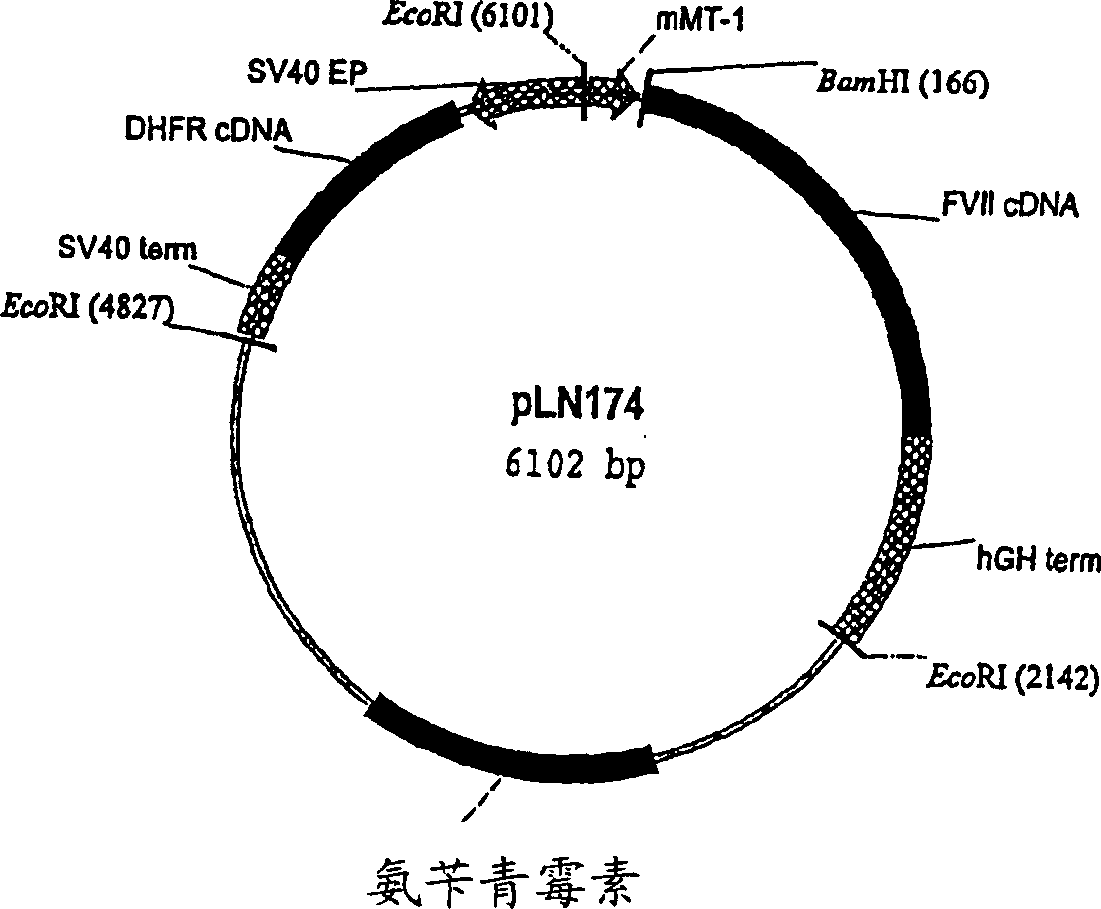
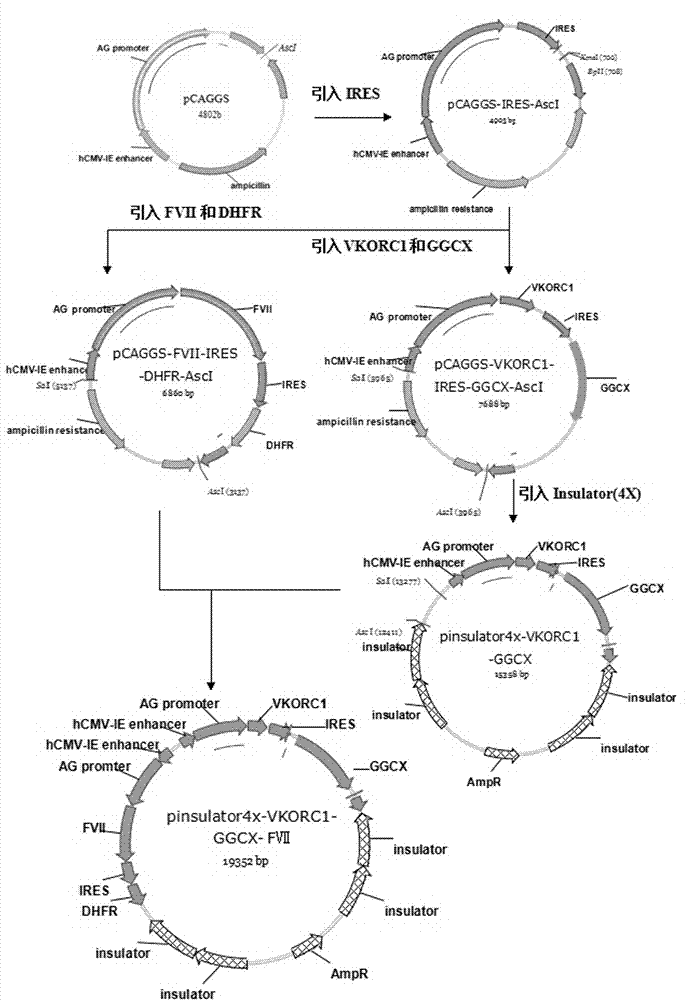
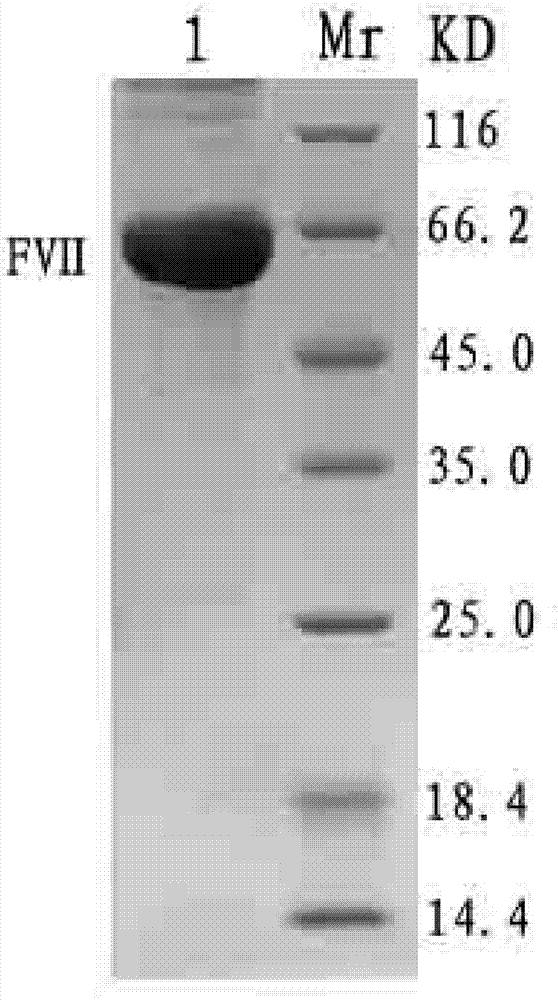
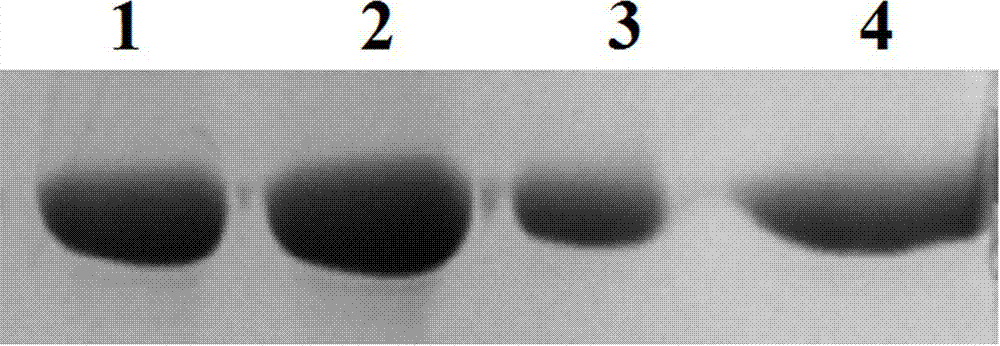
The invention provides a host cell containing a vector for expressing a functional recombinant human coagulation factor VII and a high-level expression method of the functional recombinant human coagulation factor VII, and aims at establishing an expression vector containing F VII, GGCX and VKORC1 recombinant nucleic acids and improving the g-carboxylation modification of the recombinant F VII by virtue of coordinated expression of the GGCX and the VKORC1. The host cell contains the F VII recombinant nucleic acid (human coagulation factor VII), depending on which the vitamin K is coded, a VKORC1 (mouse vitamin K epoxide reductase complex subunit 1) recombinant nucleic acid and a GGCX (mouse g glutamyl carboxylase) recombinant nucleic acid, as well as an insulator (4X) recombinant nucleic acid; the high-level expression method of the functional recombinant human coagulation factor VII comprises the steps of establishing an expression vector, mediating the expression vector into a DHFR deficient CHO animal cell by use of a lipidosome method and screening out positive clones by virtue of a DMEM culture medium, performing serum-free acclimation and culture on the host cell strain, purifying the h FVII recombinant protein by virtue of nickel ion affinity chromatography and performing SDS-PAGE and Westernblot detection, and finally, determining the procoagulant activity of the FVII recombinant protein according to the prothrombin time (PT).
Owner:山西省博奥特医学检验有限公司
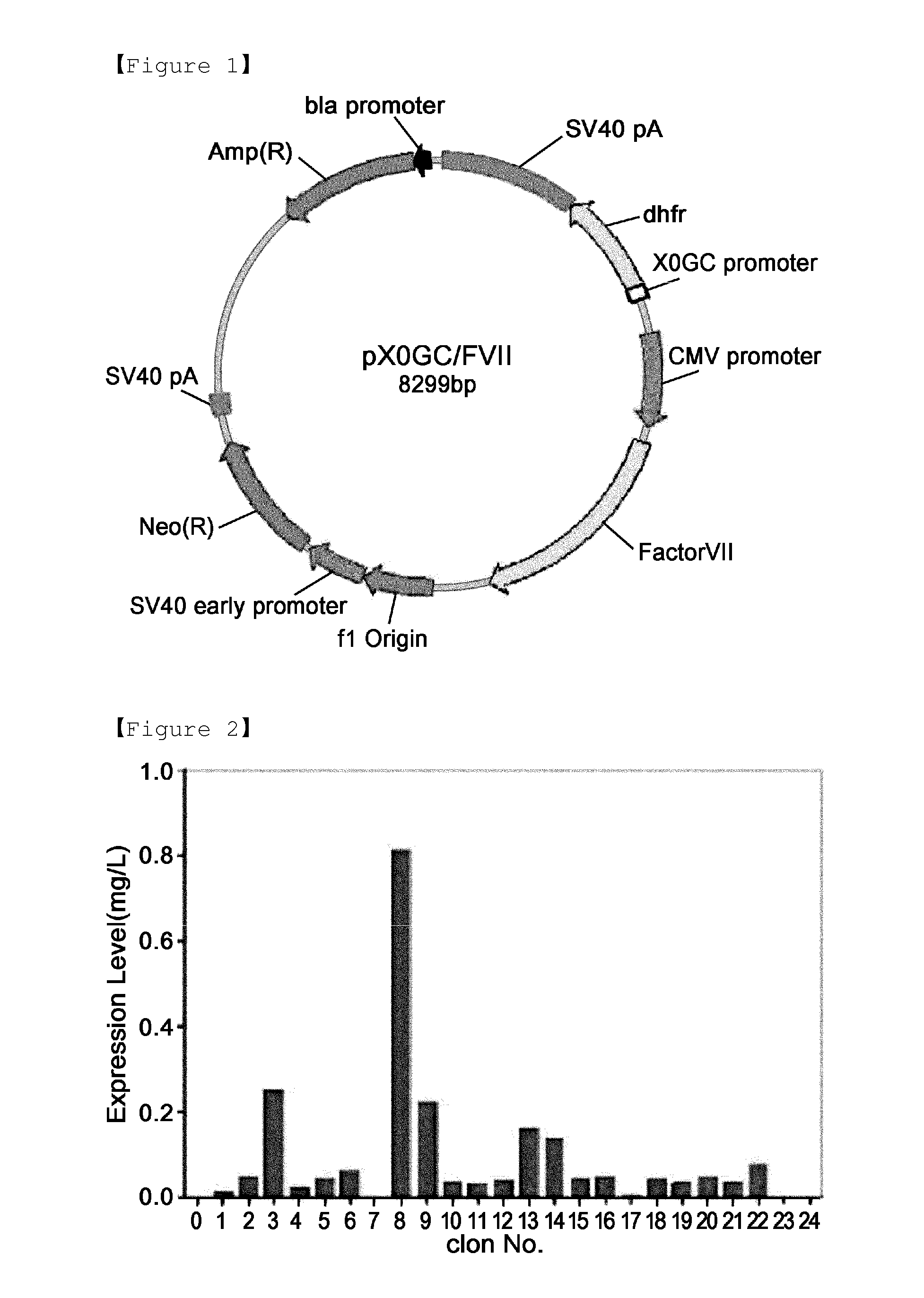
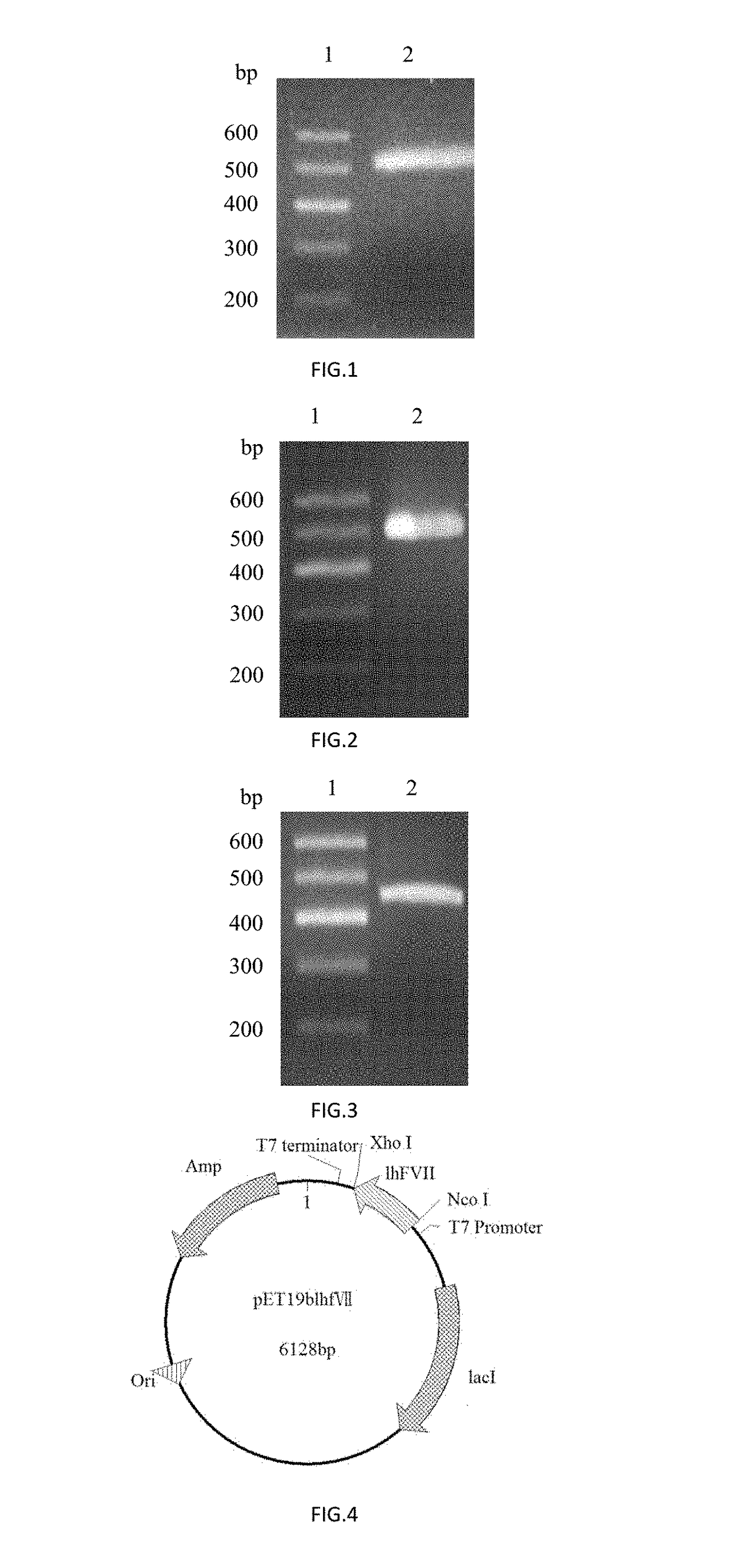
Method for mass production of factor vii/viia
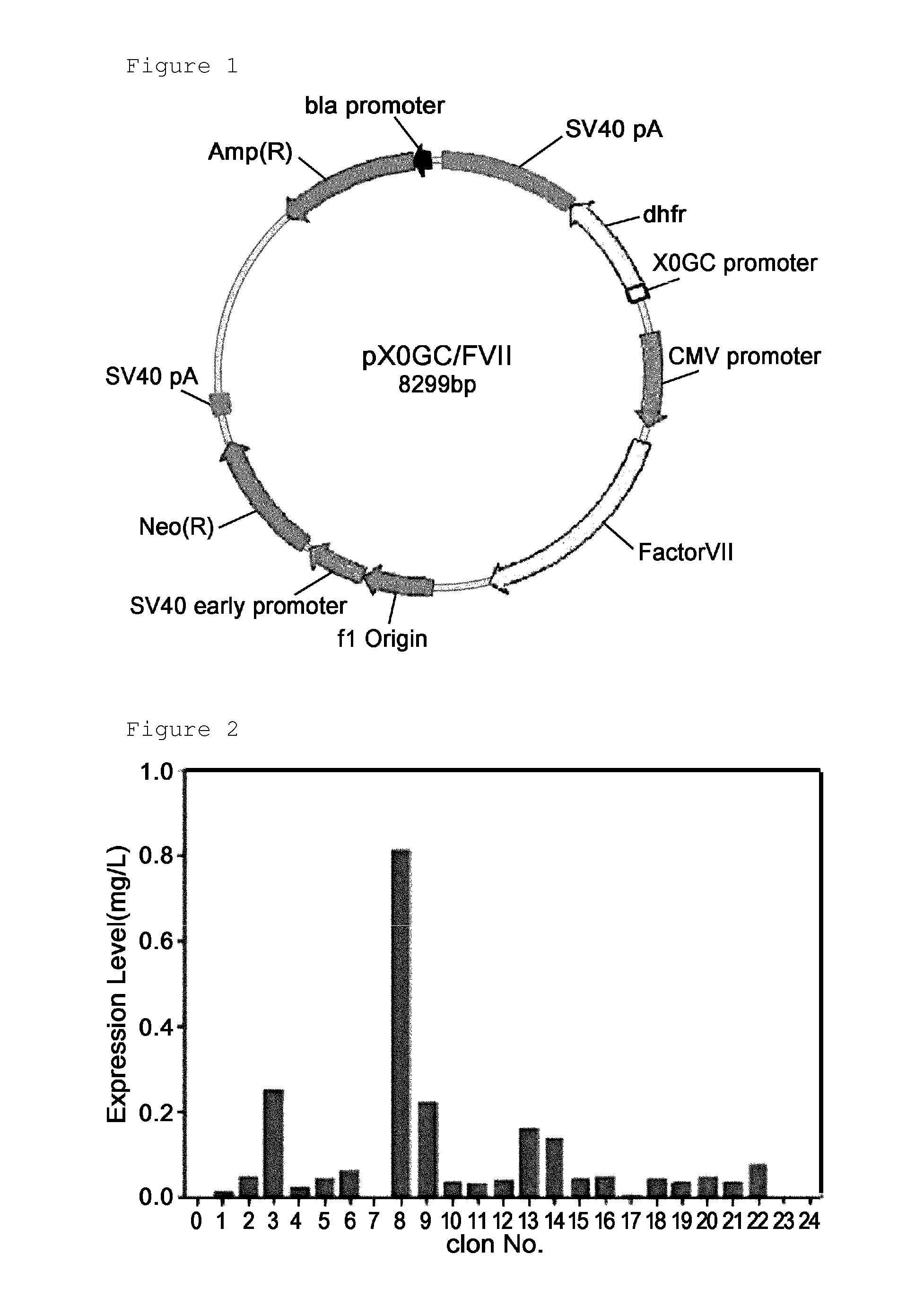
A method for the mass production of human coagulation factor VII. The method includes a) providing an expression vector carrying i) a dihydrofolate reductase promoter devoid of one or more CCGCC repeat sequences from the GC-rich region thereof and a dihydrofolate reductase (DHFR) gene operably linked thereto and ii) a cytomegalovirus (CMV) promoter and a human coagulation factor VII gene operably linked thereto; b) obtaining a transformed a host cell line containing the expression vector; and c) culturing the transfected host cell in the presence of a dihydrofolate reductase inhibitor to select cells which express human coagulation factor VII with high efficiency; and d) adding sodium butyrate to the selected host cells.
Owner:HANMI SCI CO LTD
Human coagulation factor light chain protein and use of the same
ActiveUS9782461B2Disrupting stabilityEnhanced inhibitory effectAntibacterial agentsPeptide/protein ingredientsMinimum inhibitory concentrationAntibacterial activity
In the invention, the minimum inhibitory concentrations of human coagulation factor light-chain proteins against different Gram-negative bacteria are detected with the in vitro antibacterial activity and the inhibiting effect of the human coagulation factor light-chain proteins against different Gram-negative bacteria is detected with the in vivo antibacterial activity. It has been shown that human coagulation factor light-chain proteins have an obvious inhibitory effect on the Gram-negative bacteria, so as to develop a novel class of medicaments for treating Gram-negative bacteria infection. It has been demonstrated by mass spectrometry and silver staining that human coagulation factor light-chain proteins have the effect on hydrolyzing and eliminating the endotoxin, which facilitates the development of a novel class of medicaments for treating endotoxemia. The human coagulation factor light-chain proteins are light chain proteins of human coagulation factors VII, IX, and X, as well as a protein having homology of more than 50% thereof.
Owner:CHENGDU SOURCEBIO LIMITED LIABILITY
Method for mass production of factor VII/VIIA
A method for the mass production of human coagulation Factor VII. The method includes a) providing an expression vector carrying i) a dihydrofolate reductase promoter devoid of one or more CCGCC repeat sequences from the GC-rich region thereof and a dihydrofolate reductase (DHFR) gene operably linked thereto and ii) a cytomegalovirus (CMV) promoter and a human coagulation Factor VII gene operably linked thereto; b) obtaining a transformed host cell line containing the expression vector; and c) culturing the transfected host cell in the presence of a dihydrofolate reductase inhibitor to select cells which express human coagulation Factor VII with high efficiency; and d) adding sodium butyrate to the selected host cells.
Owner:HANMI SCI CO LTD
Human coagulation factor vii polypeptides
InactiveUS20200010820A1Prolong half-life in vivoPeptide/protein ingredientsBlood disorderPharmaceutical drugPolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor light chain protein and use of the same
ActiveUS20160106818A1Disrupting stabilityEnhanced inhibitory effectAntibacterial agentsPeptide/protein ingredientsMinimum inhibitory concentrationFactor ii
In the invention, the minimum inhibitory concentrations of human coagulation factor light-chain proteins against different Gram-negative bacteria are detected with the in vitro antibacterial activity and the inhibiting effect of the human coagulation factor light-chain proteins against different Gram-negative bacteria is detected with the in vivo antibacterial activity. It has been shown that human coagulation factor light-chain proteins have an obvious inhibitory effect on the Gram-negative bacteria, so as to develop a novel class of medicaments for treating Gram-negative bacteria infection. It has been demonstrated by mass spectrometry and silver staining that human coagulation factor light-chain proteins have the effect on hydrolyzing and eliminating the endotoxin, which facilitates the development of a novel class of medicaments for treating endotoxemia. The human coagulation factor light-chain proteins are light chain proteins of human coagulation factors VII, IX, and X, as well as a protein having homology of more than 50% thereof.
Owner:CHENGDU SOURCEBIO LIMITED LIABILITY
Method for producing recombinant human coagulation factor VII by using rabbit mammary gland reactor platform
ActiveCN103484497BEasy to produceHigh expressionVector-based foreign material introductionComplementary deoxyribonucleic acidProtein activity
The invention provides a method for producing a recombinant human coagulation factor VII by using a rabbit mammary gland reactor platform. CDNA (Complementary deoxyribonucleic acid) of the recombinant human coagulation factor VII is guided or integrated to a chromosome gene of a rabbit, and the obtained transgenic rabbit expresses recombinant human coagulation factor VII protein in a mammary gland reactor. The transgenic rabbit has relatively high recombinant human coagulation factor VII protein expression level in the mammary gland reactor, the protein activity is good, and the production cost is reduced.
Owner:LANNUO BIOTECH WUXI
Human Coagulation Factor VII Polypeptides
InactiveUS20170198274A1Prolong half-life in vivoPeptide/protein ingredientsBlood disorderNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com