Host cell containing vector for expressing functional recombinant human coagulation factor VII and high-level expression method of functional recombinant human coagulation factor VII
A technology of human coagulation factor and host cell, which is applied to the host cell and the high-level expression field of the vector expressing functional recombinant human coagulation factor VII, can solve the problems of excessive exogenous gene fragment and low productivity of functional FVII, Achieve the effect of improving expression level, increasing synergy, and avoiding mutual influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
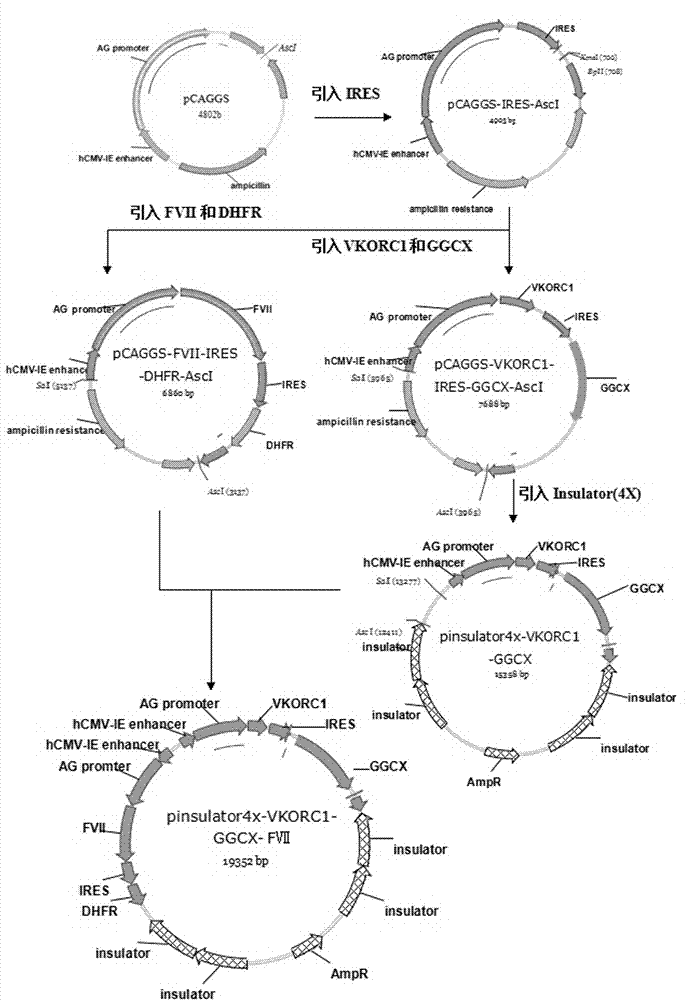
[0029] Example 1: Functional recombinant human coagulation factor VII expression vector pInsulator4x-CAGGS-VKORC1-GGCX-
[0030] Construction of FVII;
[0031] (1) Gene cloning of human coagulation factor VII (FVII), mouse vitamin K epoxide reductase complex subunit 1 (VKORCl) and mouse γ-glutamyl carboxylase (GGCX).
[0032] Human fetal liver tissue and mouse liver tissue were taken respectively, and total RNA was extracted and prepared using TRIZOLLS kit, and the integrity of total RNA was detected by 1% formaldehyde-denatured agarose gel electrophoresis; total RNA was used as a template, and Oligo (dT) was used as a primer According to the instructions of the kit, the first strand of cDNA was synthesized; using the respective reverse transcription reaction products as templates, the target fragments were amplified with the designed and synthesized FVII, GGCX and VKORC primers. The PCR reaction system is as follows: cDNA template 10 ul, 25mmol / L 10×PCR Buffer 5 ul, 25mmol / L...
Embodiment 2
[0073] Example 2: Transfection, screening and cloning of CHO mammalian cells
[0074] (1) Linearization of the pIsulator4x-VOKRC1-GGCX-FVII plasmid constructed in Example 1
[0075] The plasmid was digested with Swa I, digested at 25 °C for 8 h, then added 1 / 10 volume of 3 M sodium acetate and 2.5 volumes of absolute ethanol, incubated at -20 °C for 2 h, and centrifuged at 14000 rpm for 10 min at low temperature. Discard the supernatant, add 500 uL of 70% ethanol, and centrifuge at 14000 rpm for 1 min at low temperature. Repeat the above steps, discard the supernatant, and add 10 uL sterile water.
[0076] (2) Plasmid transfection
[0077] CHO cells were transfected with lipofectin liposomes. Routinely culture DHFR-deficient CHO cells in DMEM medium (HT selection medium) containing 4 g / L hypoxanthine (H) and thymine (T). 5 / L inoculated into a 6-well plate at 2 ml / L, and cultured until the cells grew into a 50%-70% monolayer, the culture medium was discarded, the cells wer...
Embodiment 3
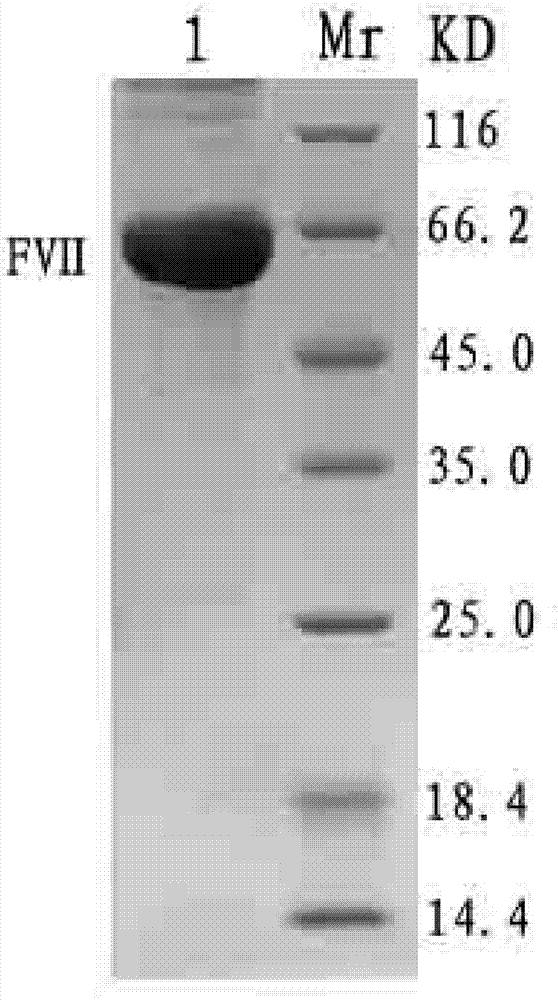
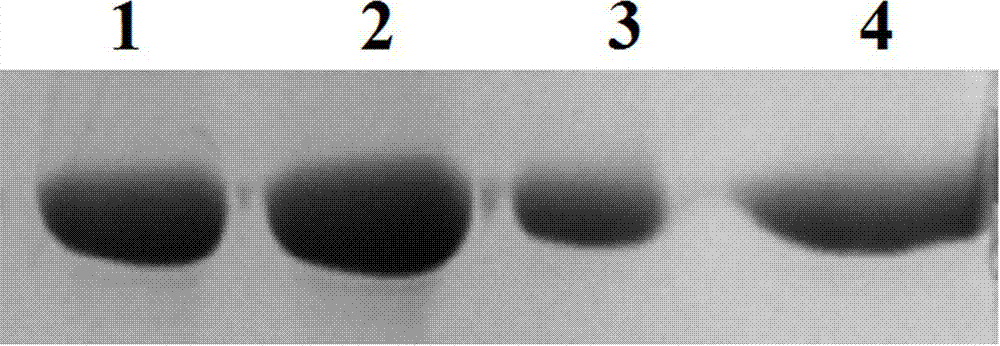
[0080] Example 3: Expression, purification and identification of human recombinant coagulation factor VII
[0081] (1) Expression and purification of FVII
[0082] The high-expressing cell lines obtained by limiting dilution were acclimatized in the absence of serum, expanded and cultured under MTX pressure, and the culture supernatant was collected. After centrifugation at 1000rpm at 4°C, the supernatant was filtered through a 0.22 μM filter membrane and Ni sepharose TM 6 Fast Flow separation and purification. Equilibration buffer (20 mM Tris, 500 mM NaCl, 10 mM imidazole pH 7.8) to equilibrate Ni sepharose TM 6 Fast Flow column, and then put the filtered supernatant on the column. After sample loading, wash with equilibration buffer to baseline, then use elution buffer (20 mM Tris, 500 mM NaCl, 50 mM imidazole pH 7.8) to elute impurities, and finally wash with elution buffer (20 , 500 mM sodium chloride, 250 mM imidazole (pH 7.8) to elute the target protein peak.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com