JAB1 as a prognostic marker and a therapeutic target for human cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
JAB1 and p27 Expression Profiles in Breast Tumors
[0236] Breast tumor samples were obtained from a study group of 53 women with invasive breast carcinoma. The women were 35 to 90 years old and had a mean age of 63.2±13.3 years and a median age of 65. None of the women had a family history of breast cancer. The patients had not undergone any chemotherapy or radiotherapy before surgery. Patient selection was based on the availability of archived paraffin blocks for immunohistochemical studies, which are described below. Six cases (11%) were stage I, 28 (53%) were stage II, 13 (25%) were stage III, and 6 (11%) were stage IV. Five tumors (9%) were grade 1, 29 (55%) were grade 2, and 19 (36%) were grade 3. The tumors were surgically staged according to the American Joint Committee on Cancer's tumor-nodes-metastasis system and graded according to the Nottingham modification of the Bloom-Richardson system. All of tumors excepted for one had a maximum diameter larger than 1 cm. 47 of the br...
example 2
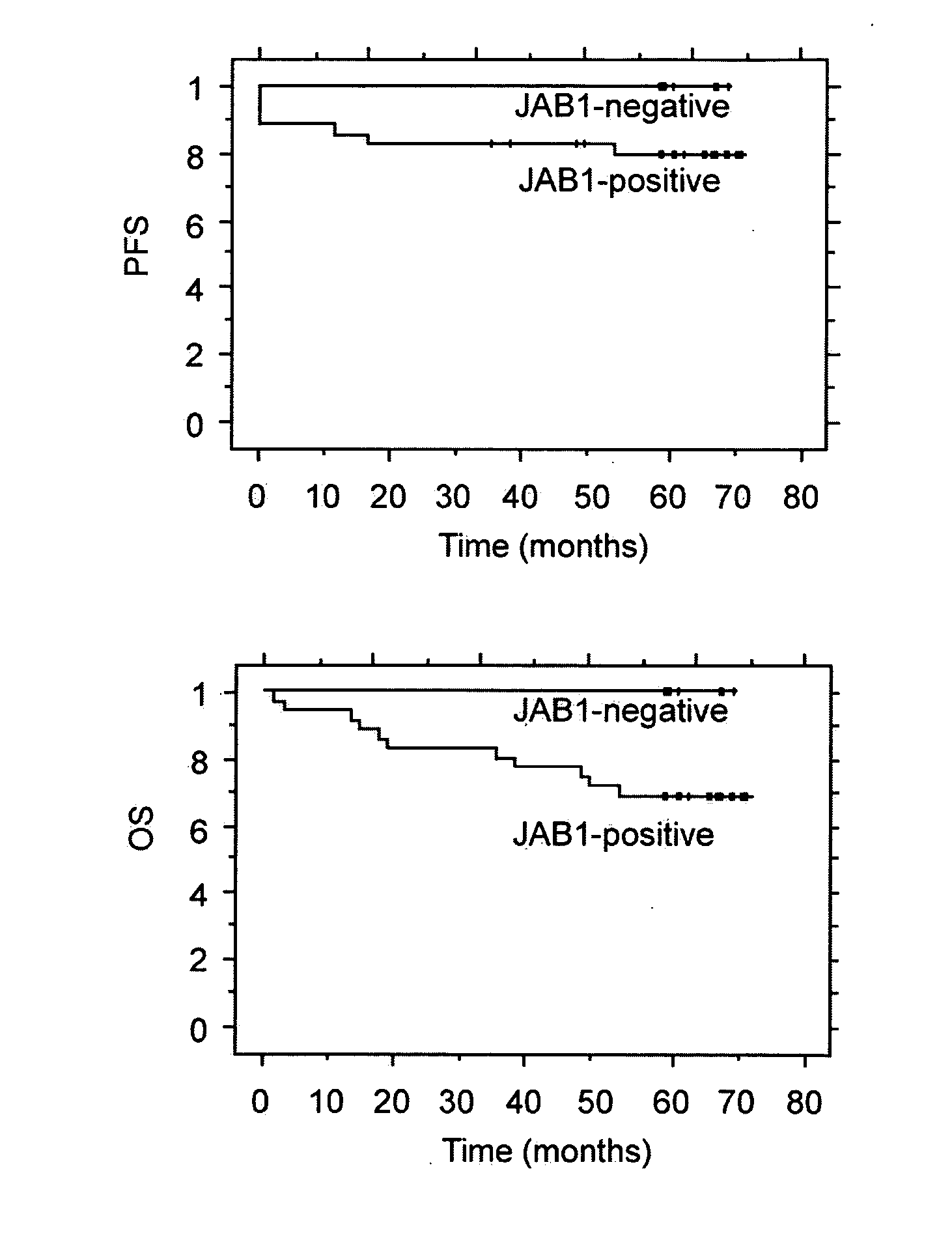
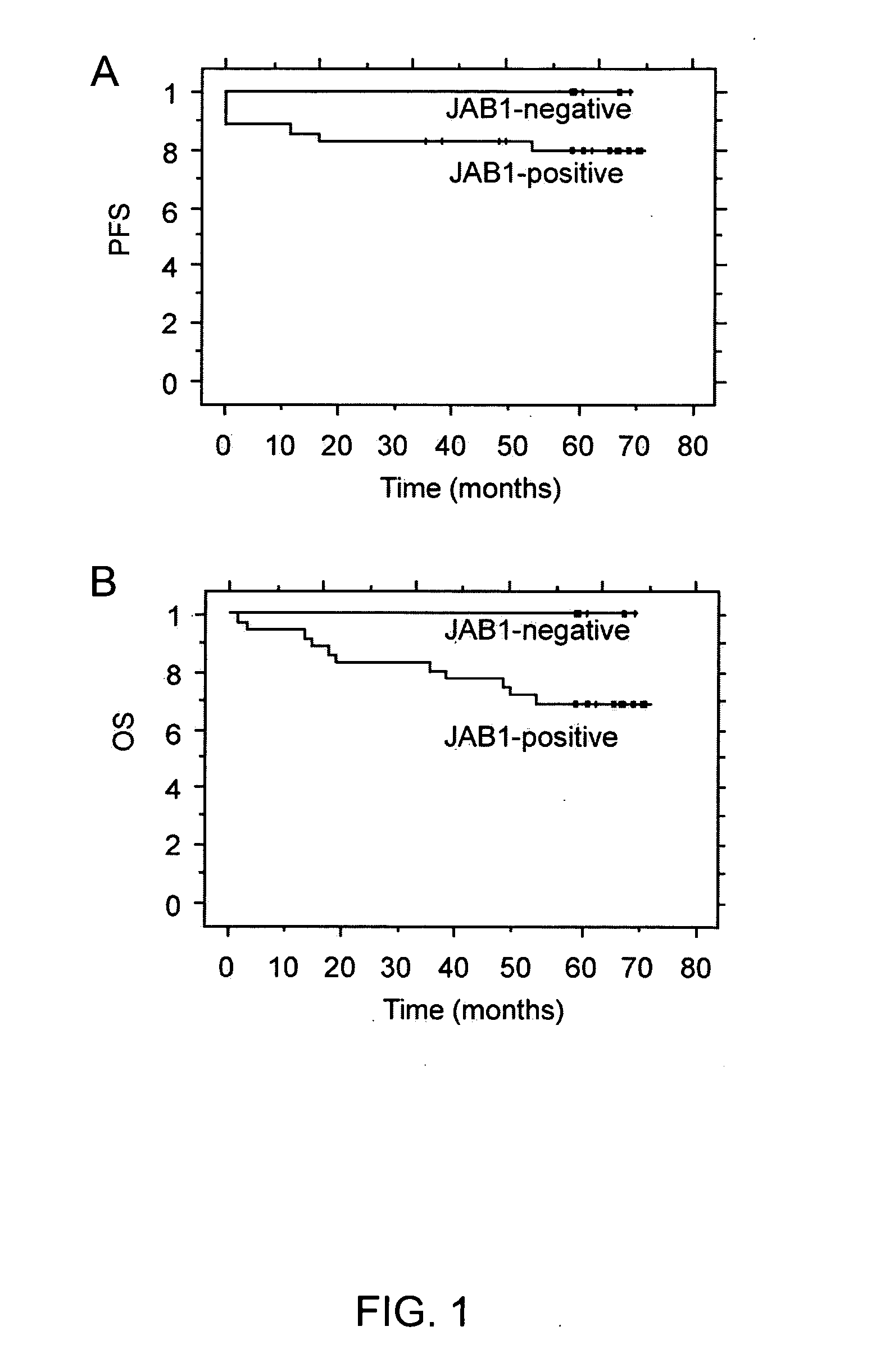
Survival Rates
[0244] The available clinical data on the survival rate of the female patients with breast carcinomas discussed above was examined and is summarized in FIGS. 1A and 1B. FIG. 1B shows that a significant difference in overall survival rates was found between women with breast tumors that had a detected level of JAB1 protein and women with breast tumors that did not have a detected level of JAB1 protein. As defined herein, the “5-year overall survival rate” is the percentage of surviving patients five years after the patients' treatment or cancer diagnosis. The 5-year survival rate includes patients that have experienced relapses or cancer progression. After an average of 70 months, there was a 69% 5-year overall survival rate among the women with breast tumors that had a detected level of JAB1 protein, while there was a 100% 5-year overall survival rate among the women with breast tumors that did not have a detected level of JAB1 protein. In addition, there was also a d...
example 3
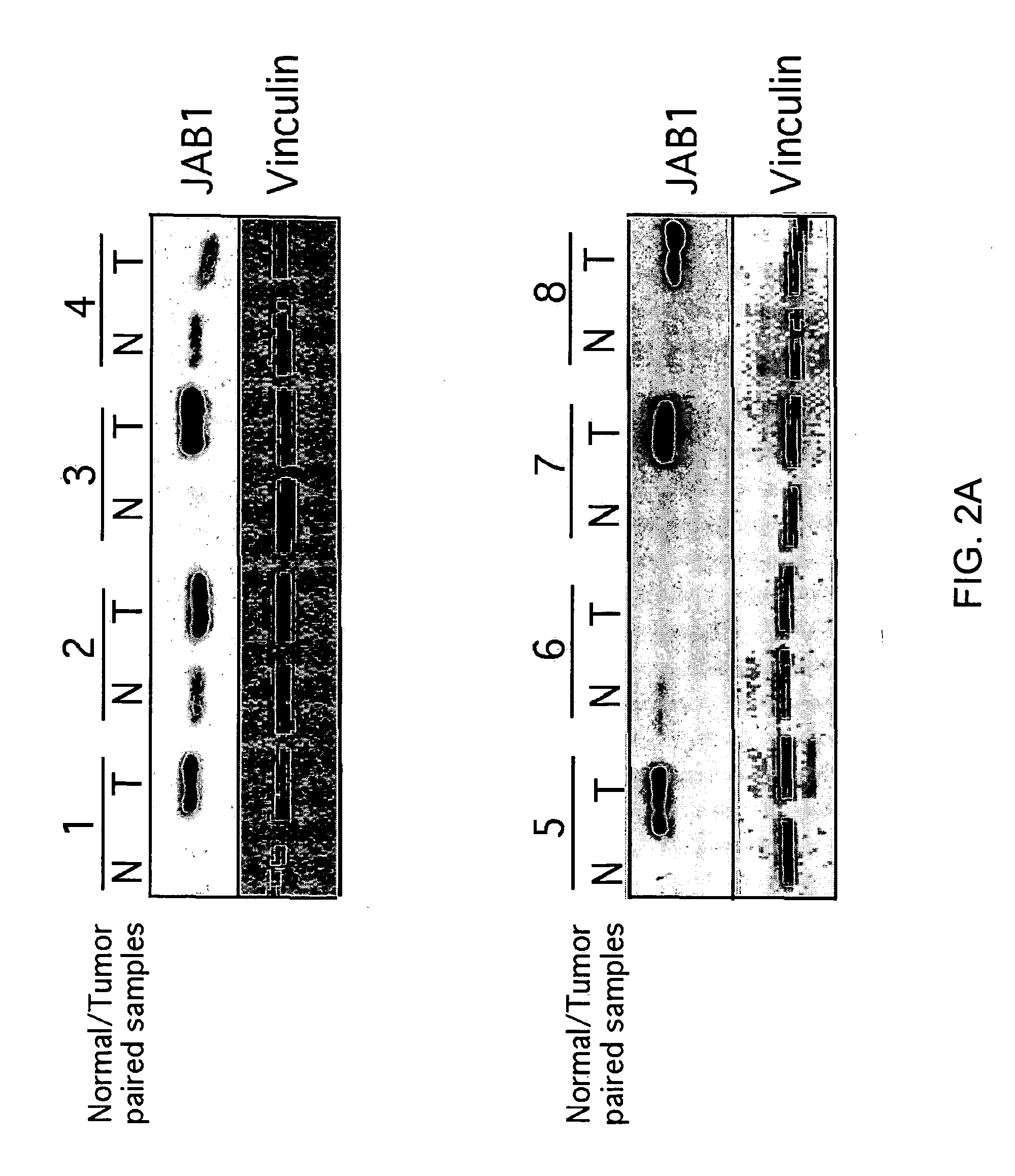
High JAB1 Expression is Directly Proportional to HER-2 Expression
[0246] JAB1 protein levels were examined in eight pairs of non-cancerous and cancerous breast tissue samples from eight of the patients of Example 1. The samples were obtained in a biopsy. The samples were washed twice in cold 1×PBS that was diluted from 10×PBS, (Catalog #M6505, available from Fisher) and lysed at 4° C. in lysis buffer (25 mM Hepes, pH 7.7, 400 mM NaCl, 0.5% Triton X-100, 1.5 mM MgCl2, 2 mM EDTA, 2 mM DTT, 0.1 mM PMSF, protease inhibitors [including the following protease inhibitors at the following final concentrations: leupeptin 10 μg / ml, peptstatin 2 μg / ml, antipain 50 μg / ml, aprotinin 2 μg / ml, chymostatin 20 μg / ml, and benzamidine 2 μg / ml] and phosphatase inhibitors [including the following phosphatase inhibitors at the following final concentrations: 50 mM NaF, 0.1 mM Na3VO4, and 20 mM P-glycerophosphate]). Aliquots of cell lysates containing about 70 mg of total protein were run on 10-12% SDS-PA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com