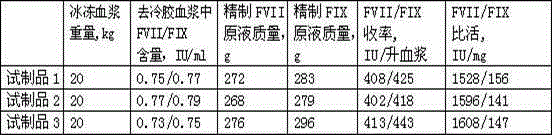
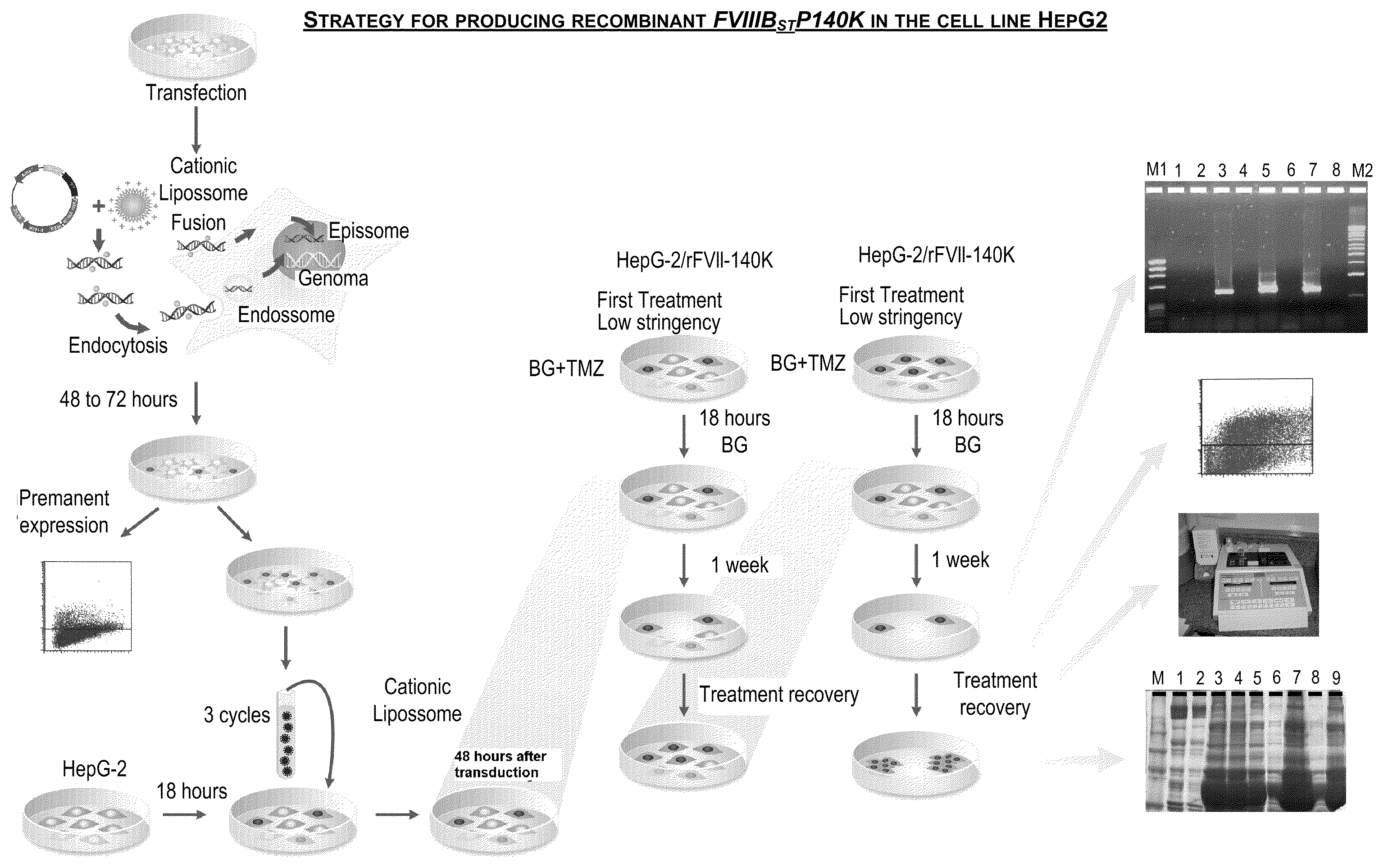
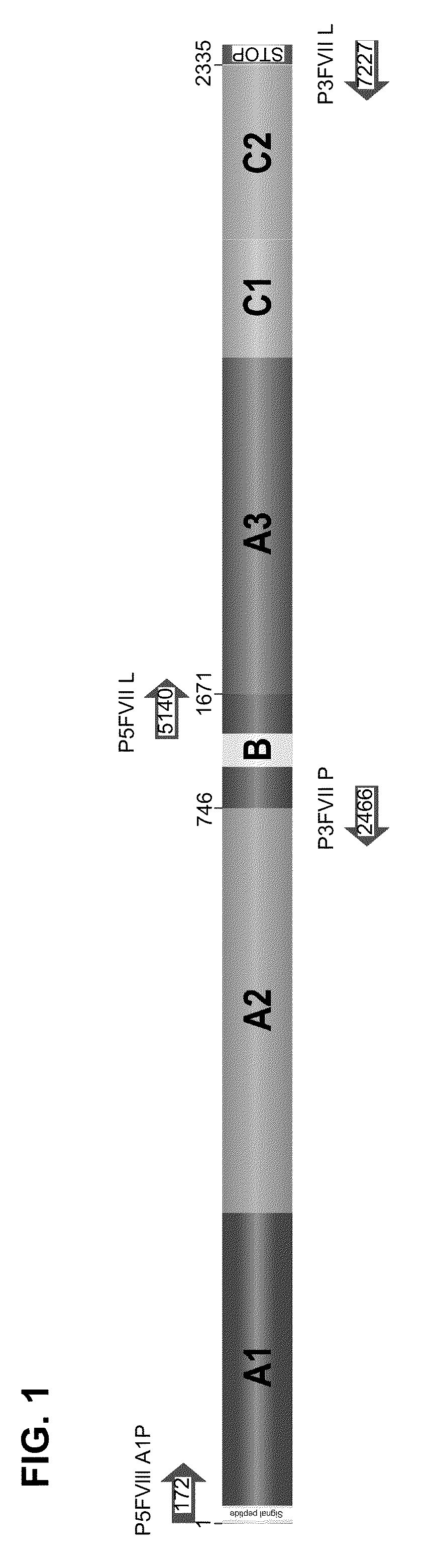
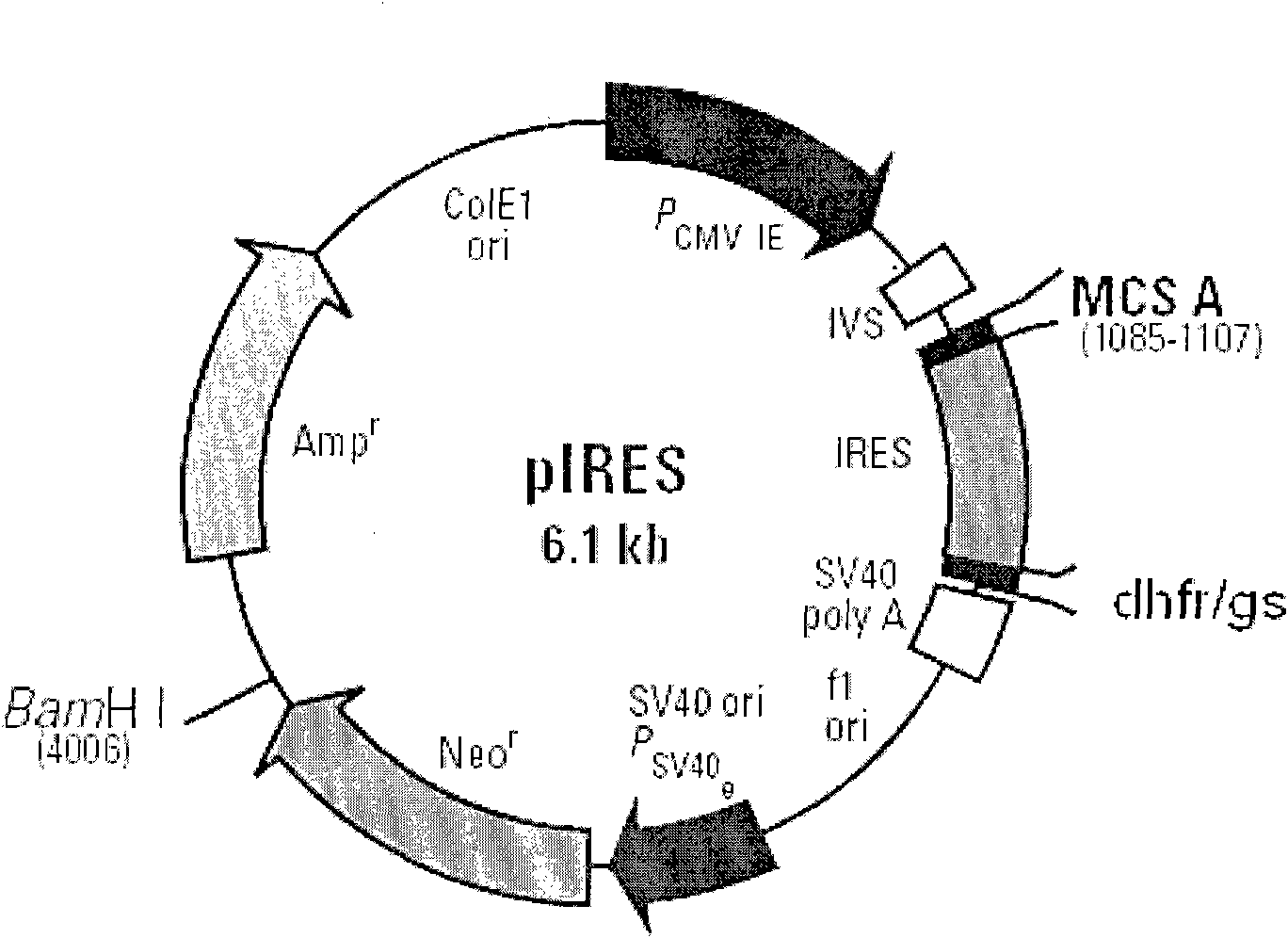
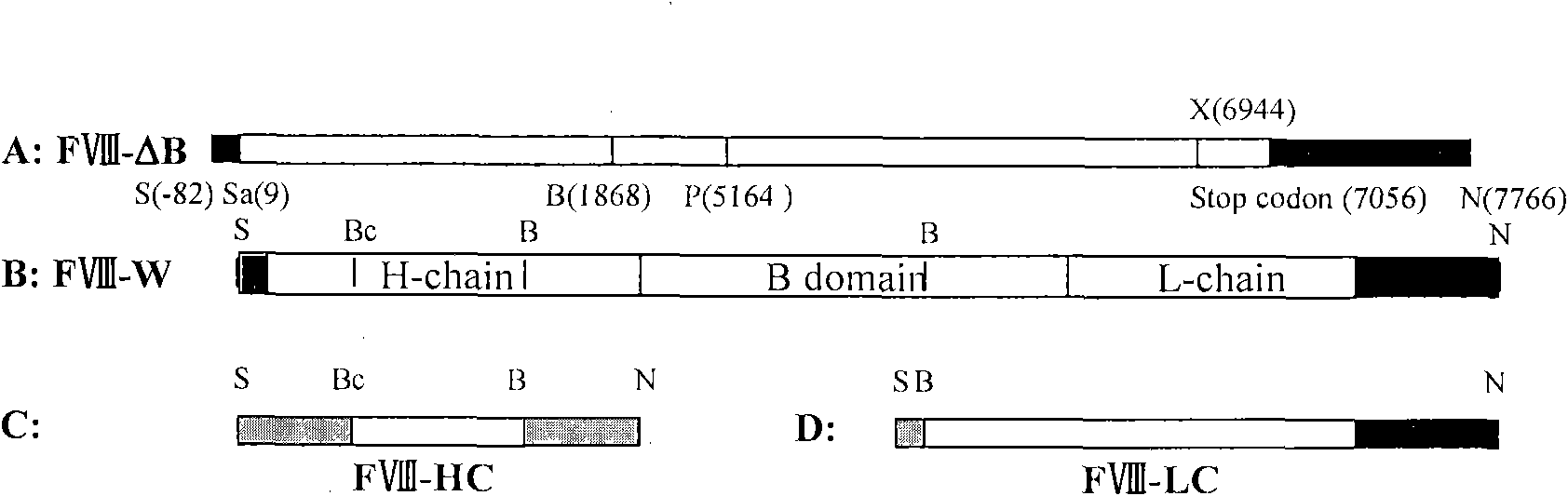
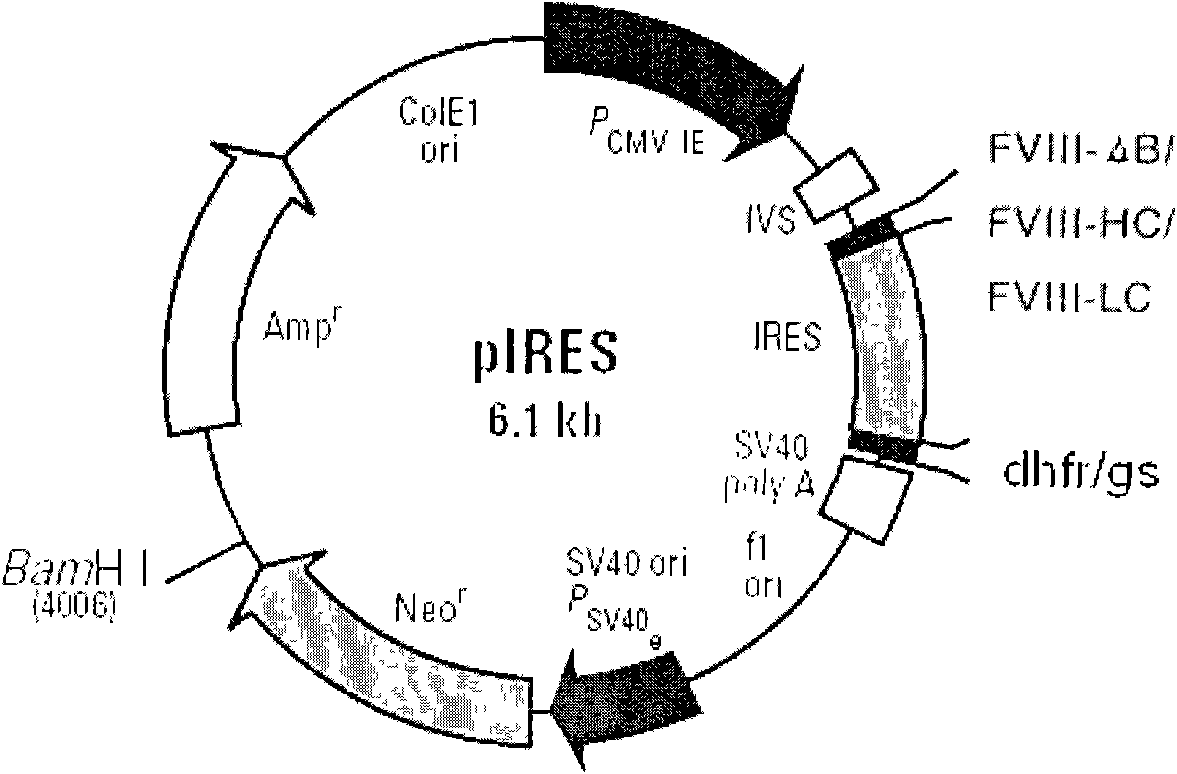
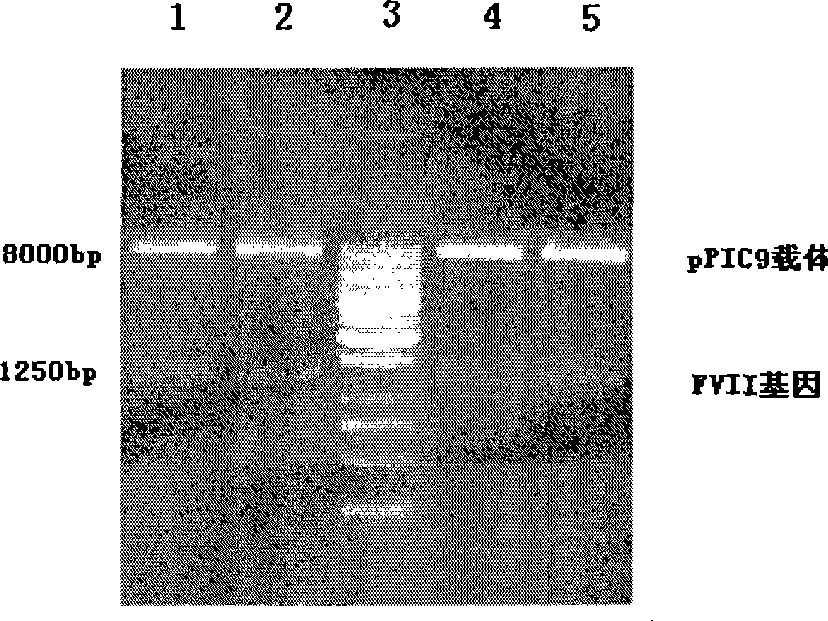
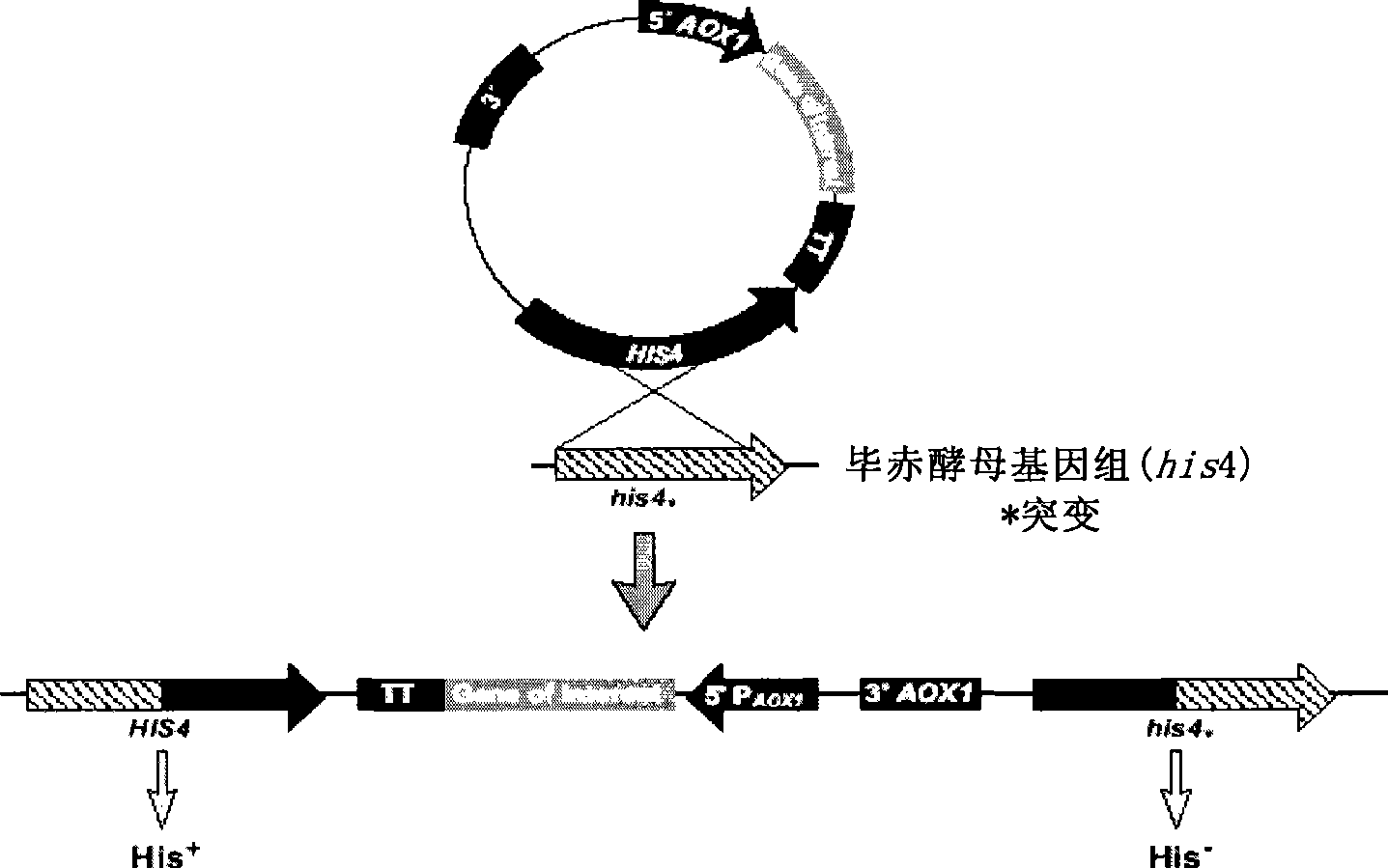
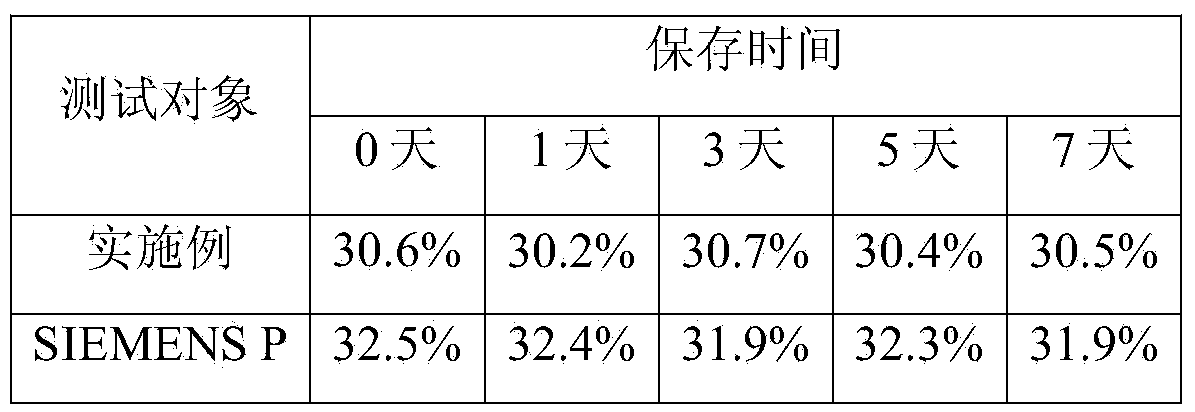
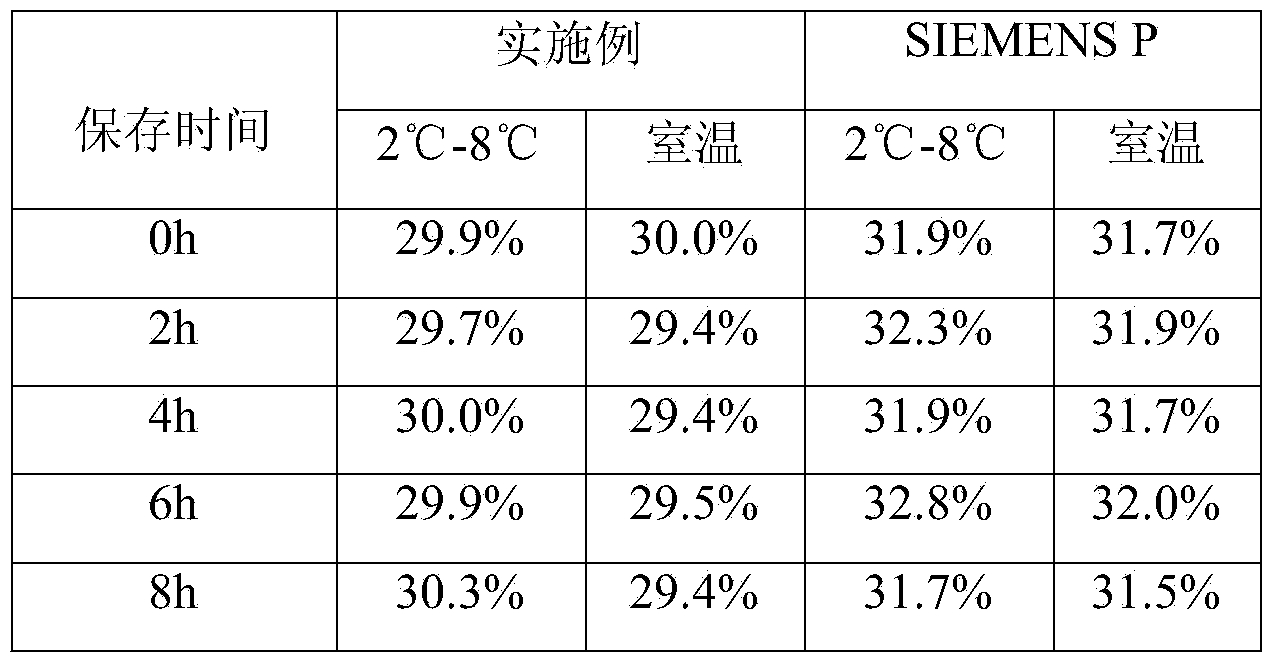
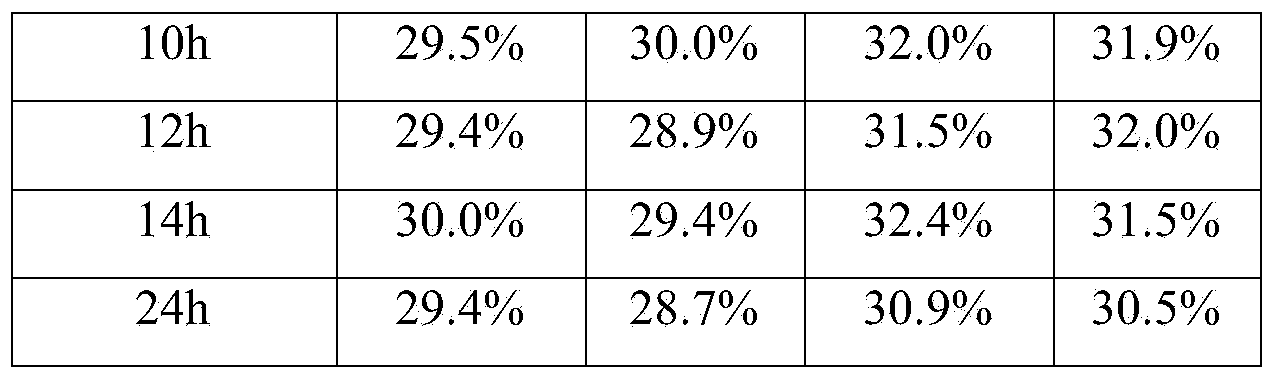
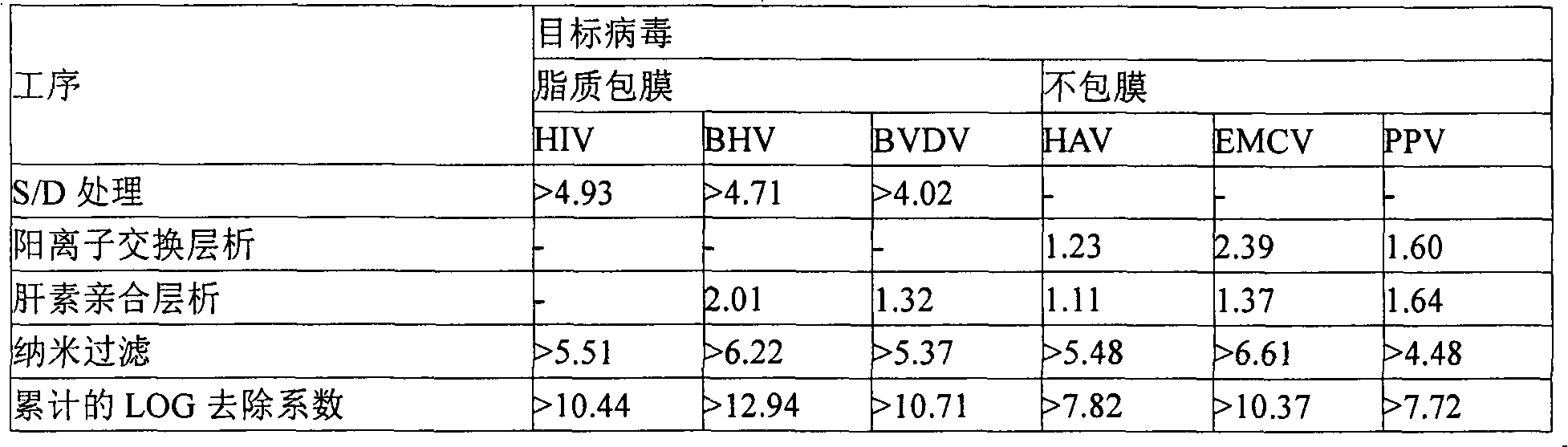
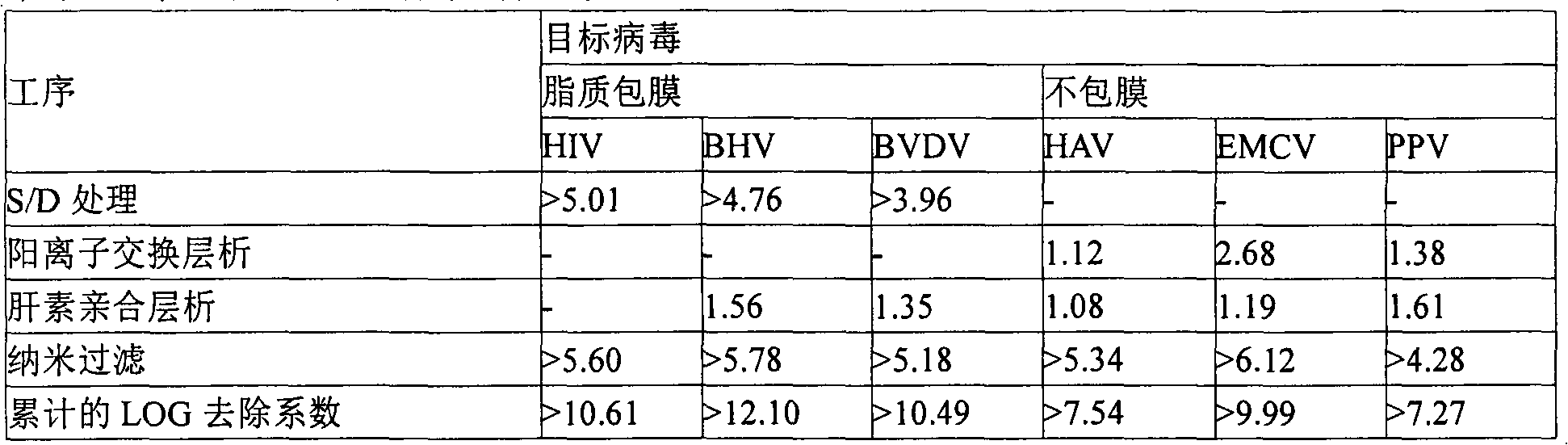
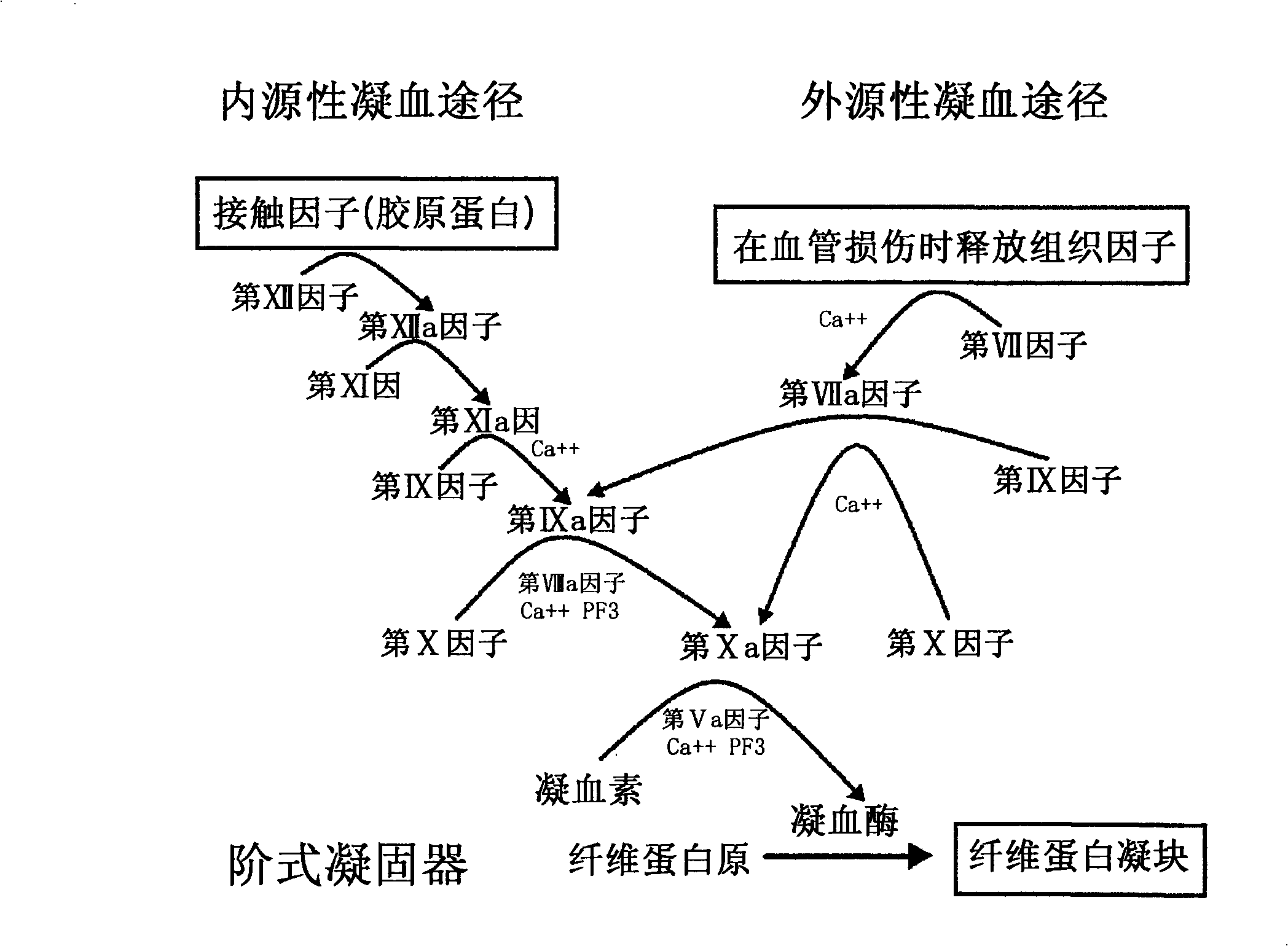
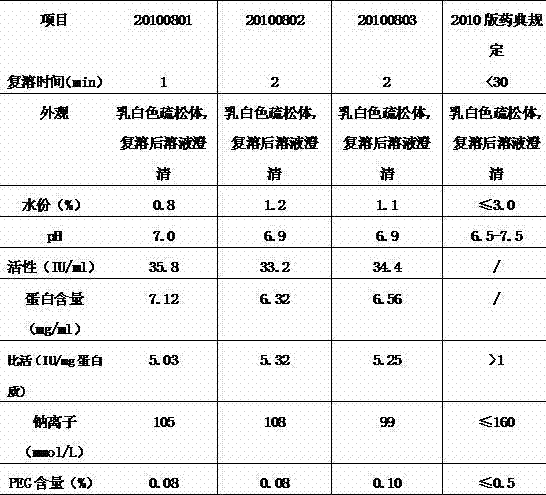
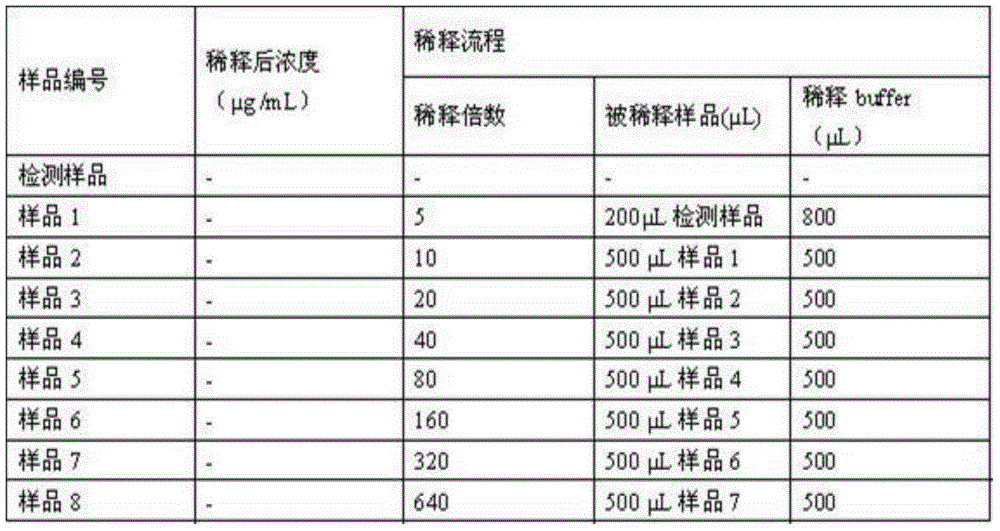
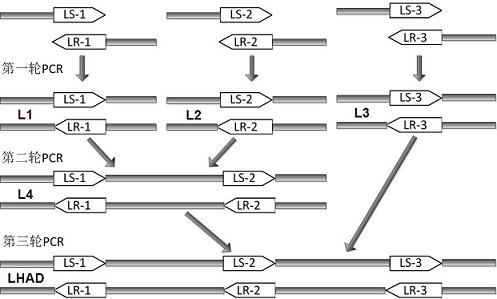
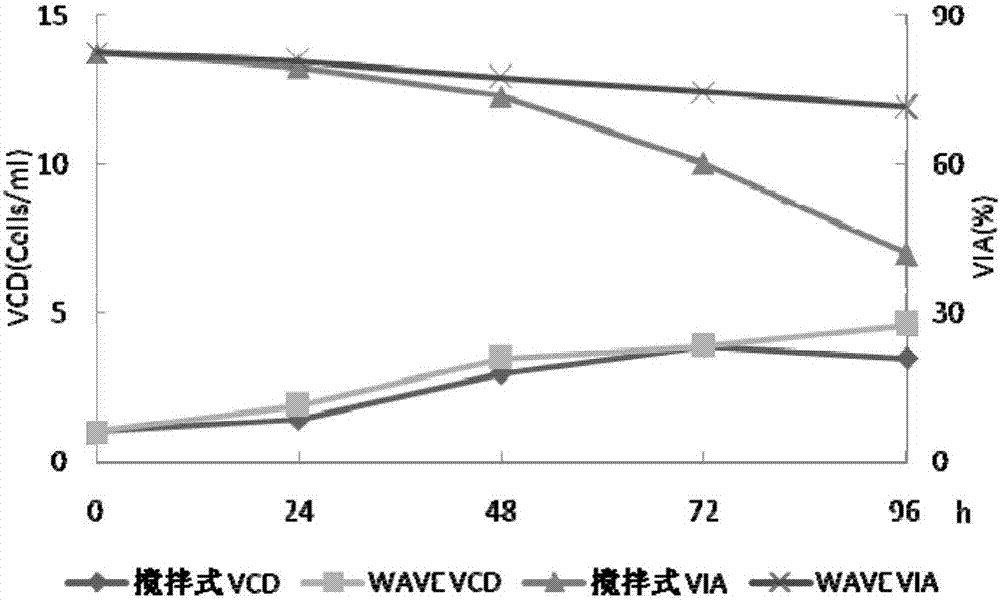
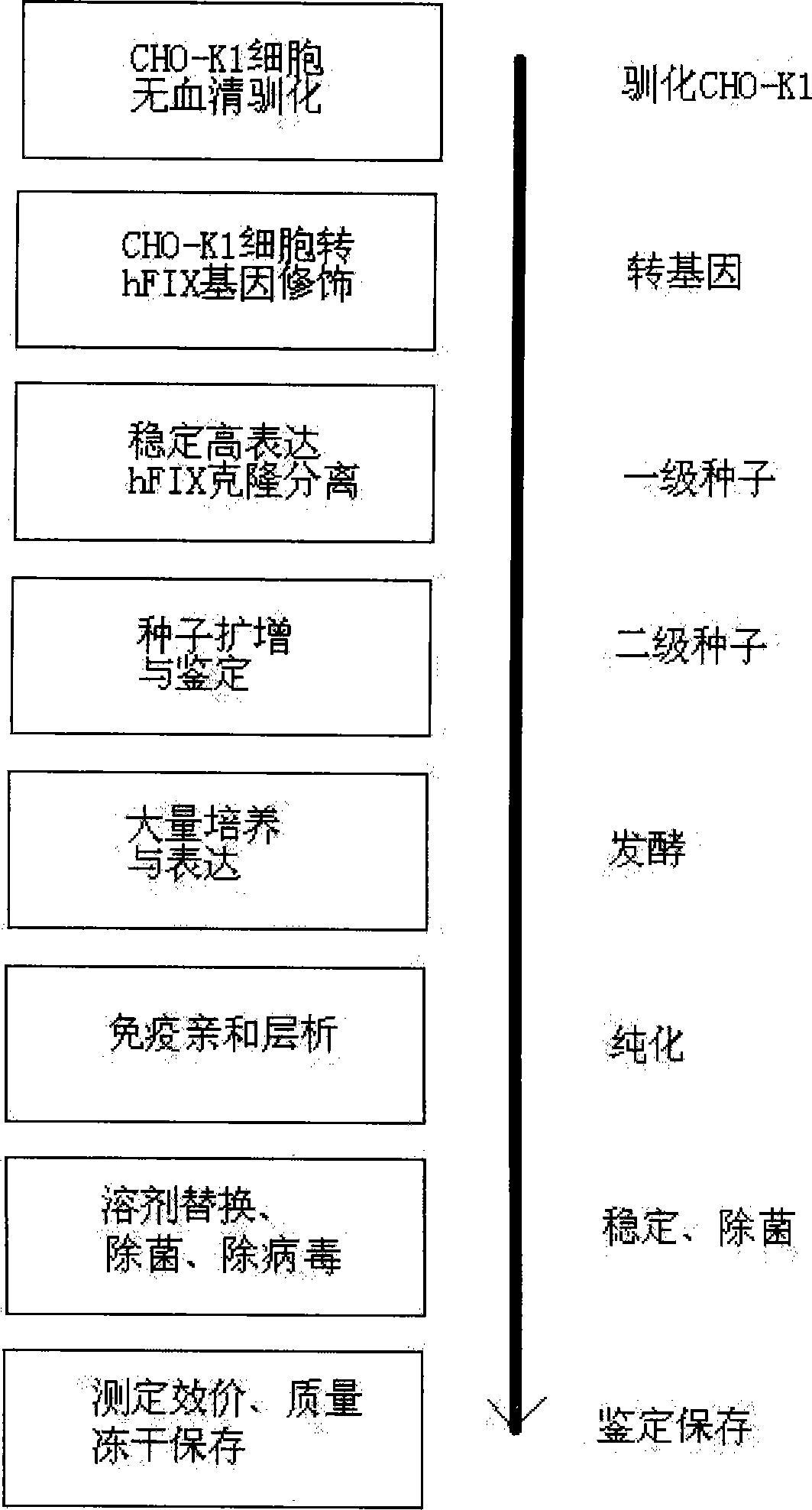
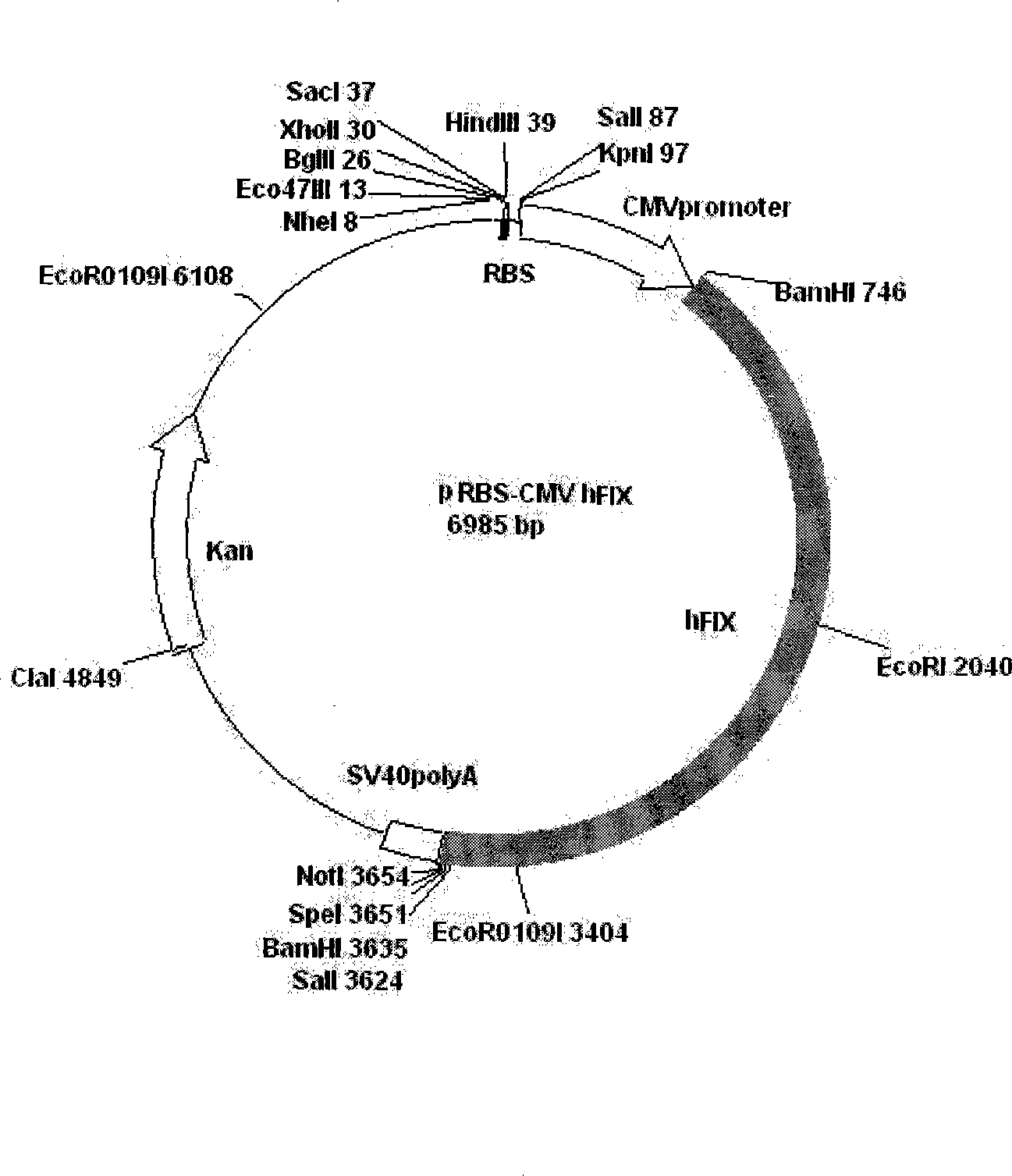
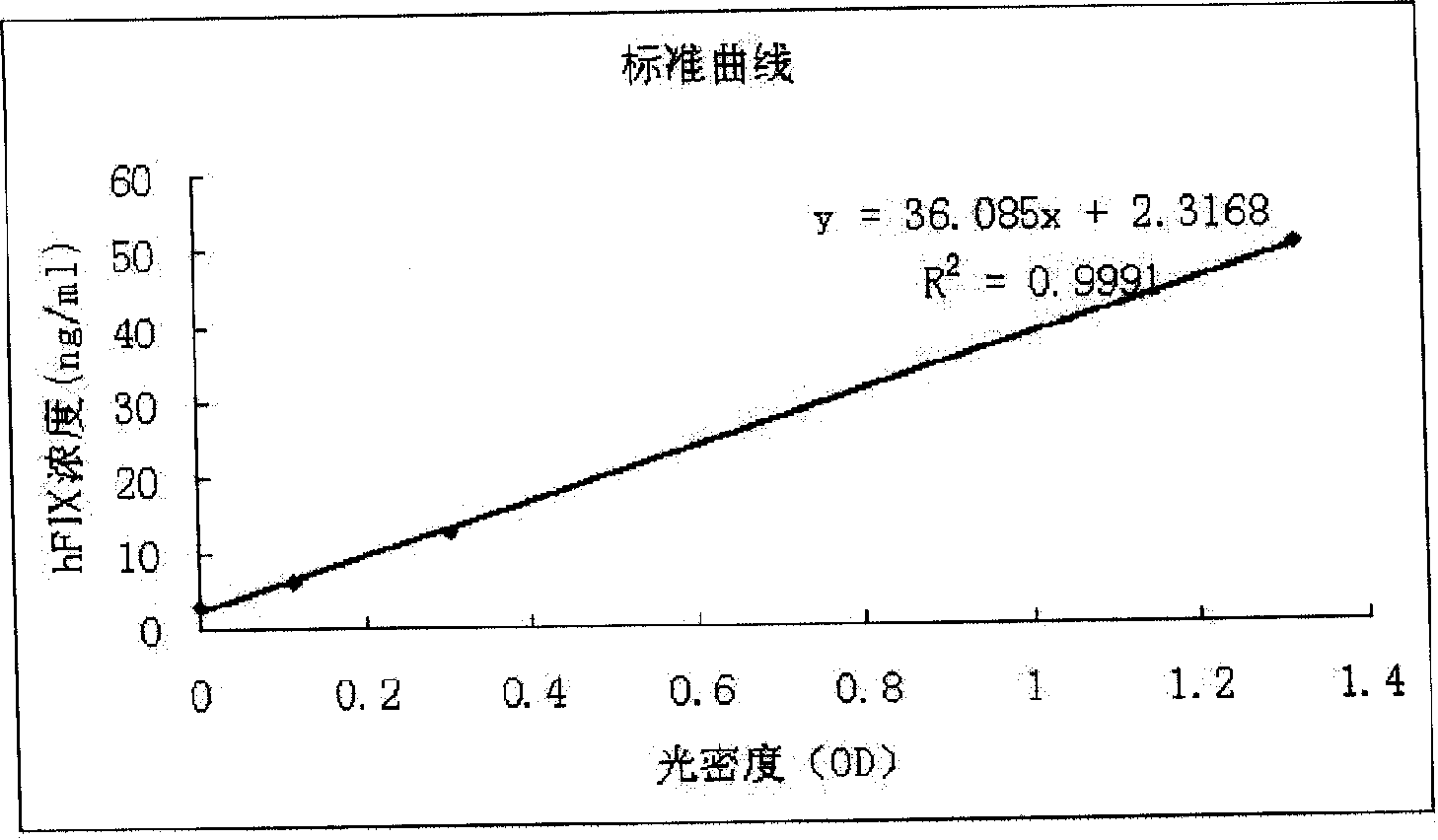
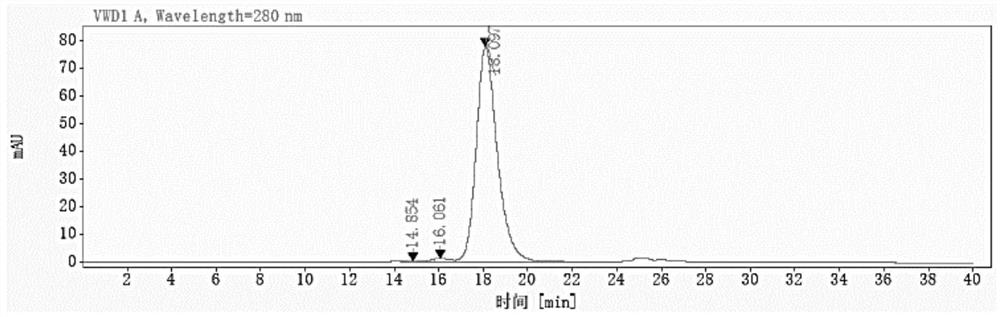
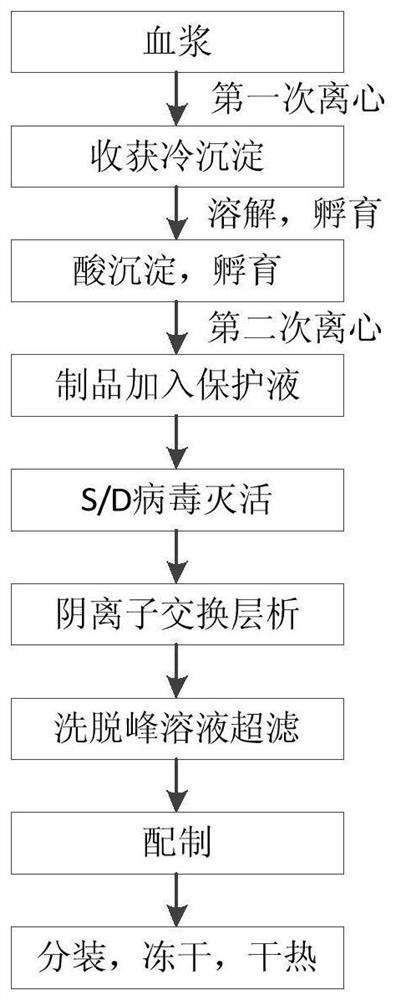
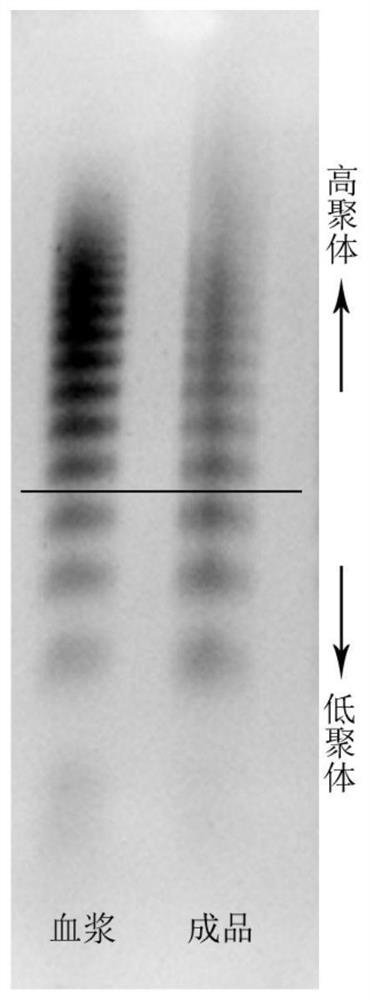
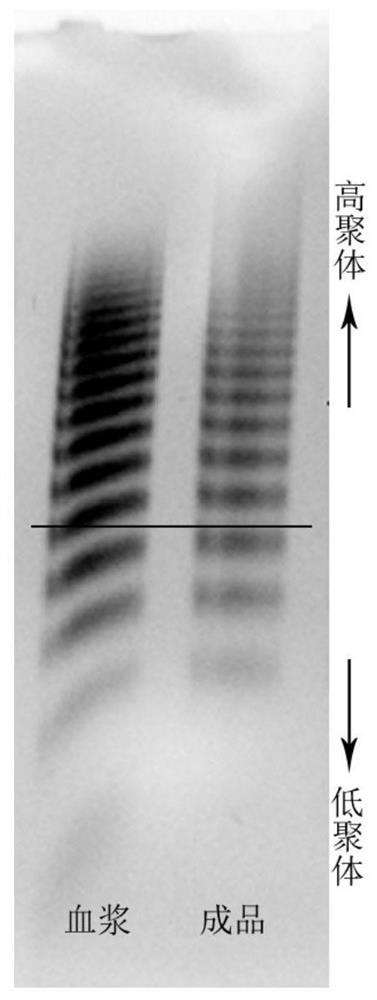
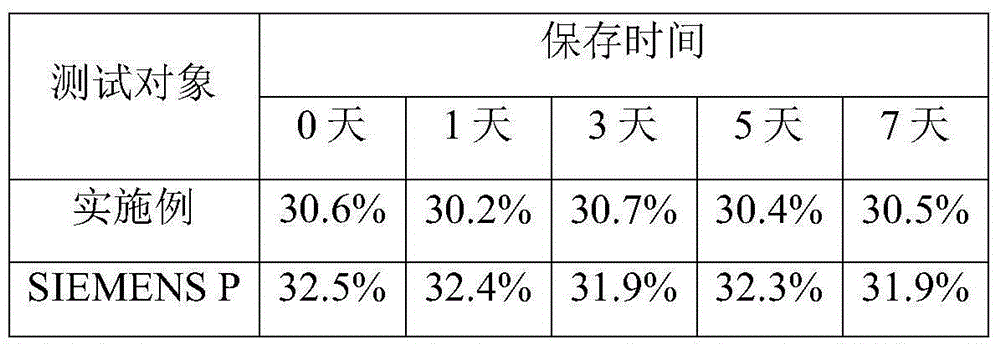
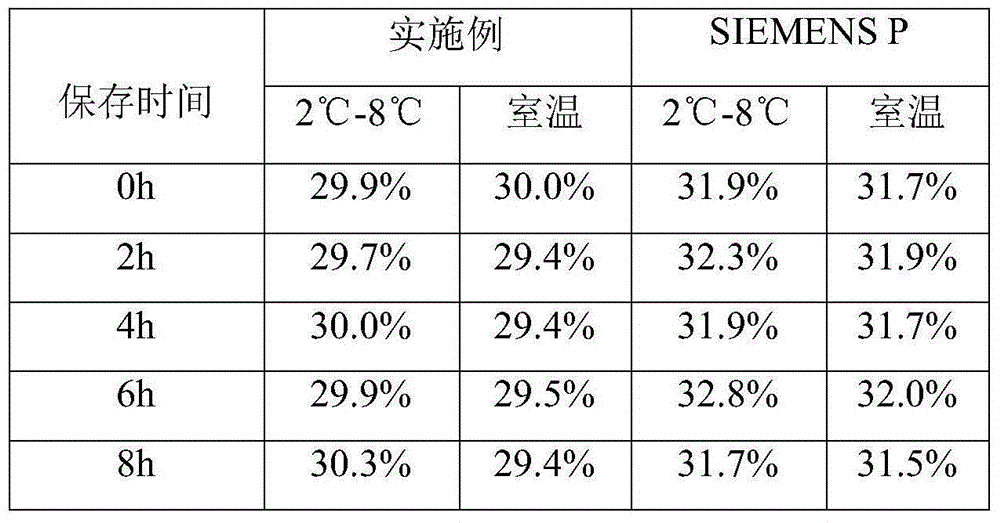
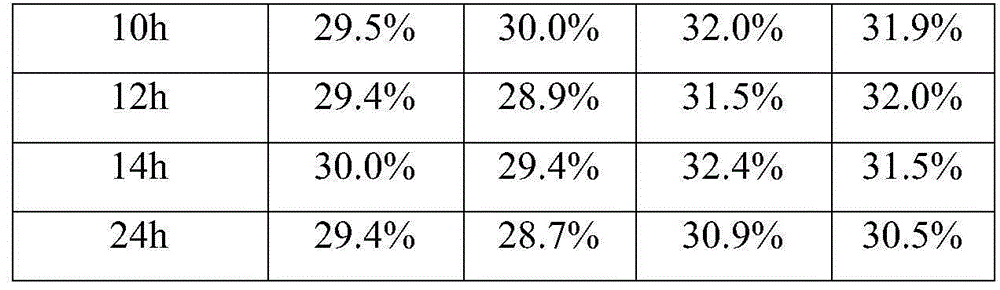
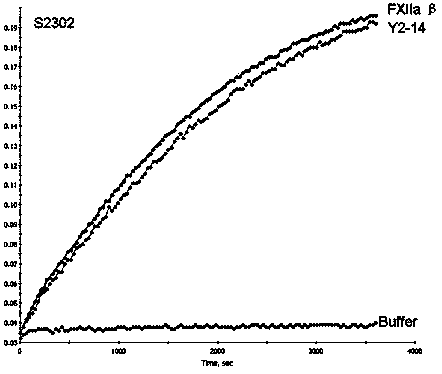
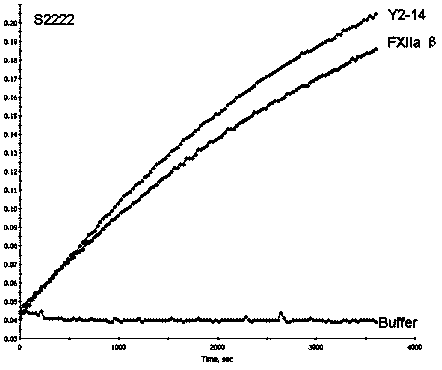
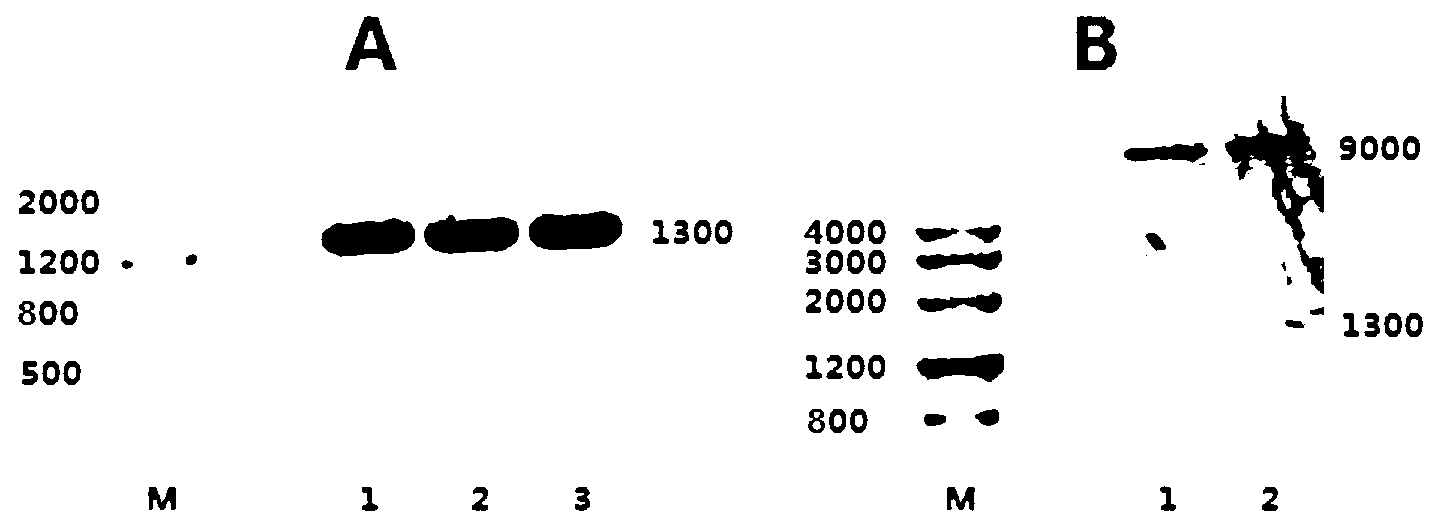Patents
Literature
54 results about "Human Blood Coagulation Factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing freeze-dried human blood coagulation factor VIII
ActiveCN102228683AEasy to cleanGuaranteed stabilityPowder deliveryPeptide/protein ingredientsUltrafiltrationFreeze-drying
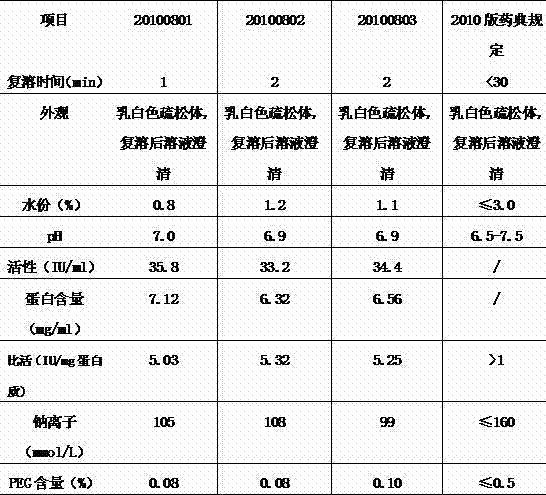
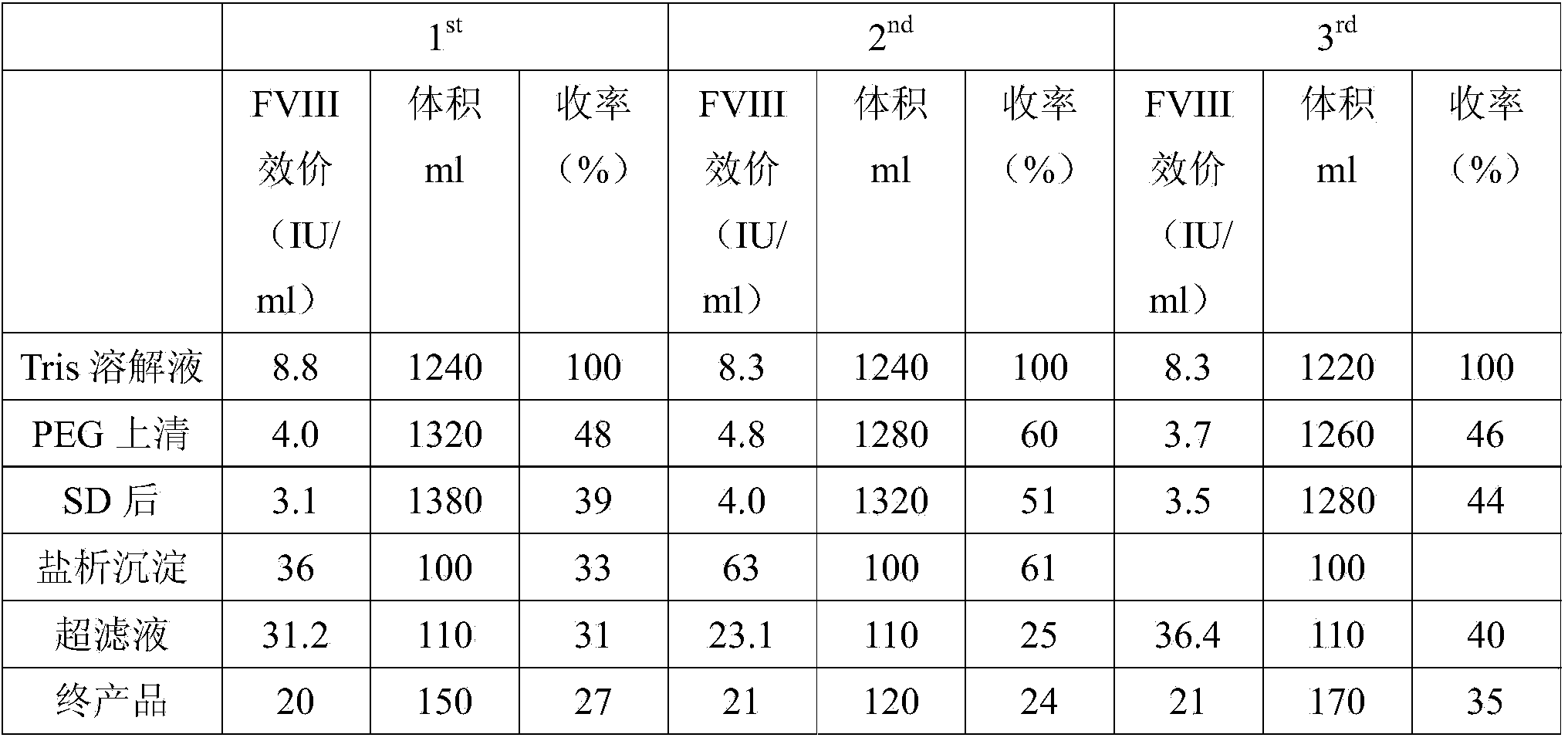
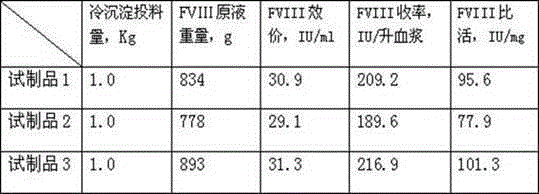
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII. The method comprises the following process of: dissolving by taking water for injection, comprising 3-10 IU (International Unit) / ml of heparin, as a heparin sodium solution and cryoprecipitating; performing PEG (Polyethylene Glycol) precipitation and taking supernatant; performing centrifugal filtration; performing S / D (Solvent / Detergent) inactivation at the temperature of 24-26 DEG C; performing DEAE (Diethylaminoethyl) Sepharose Fast Flow chromatographic column balance, adsorption, washing andelution; performing molecular membrane ultrafiltration; preparing, removing bacteria, sub-packaging, freeze-drying and capping; and dry-heating at the temperature of 99.5-100.5 DEG C and inactivating. In the invention, the process method of combining the PEG precipitation and an ion exchange chromatography technology is adopted; the method is easy and convenient to operate; the F VIII active recovery rate is increased; miscellaneous proteins can be removed on a large scale; the product yield reaches over 60 percent; and the specific activity of the product reaches 5 IU / mg and is obviously greater than a value which is not less than 1 IU / mg stipulated in the pharmacopeia. Meanwhile, the PEG residue is 0.08g / L which is obviously less than the value which is less than or equal to 0.5 IU / mg stipulated in the pharmacopeia, so that Al<3+> residues in the final preparation of an Al(OH)3 gel method are avoided; the product has high purity and high safety; and the quality of the final product is obviously improved.
Owner:NANYUE BIOPHARMING
Technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation
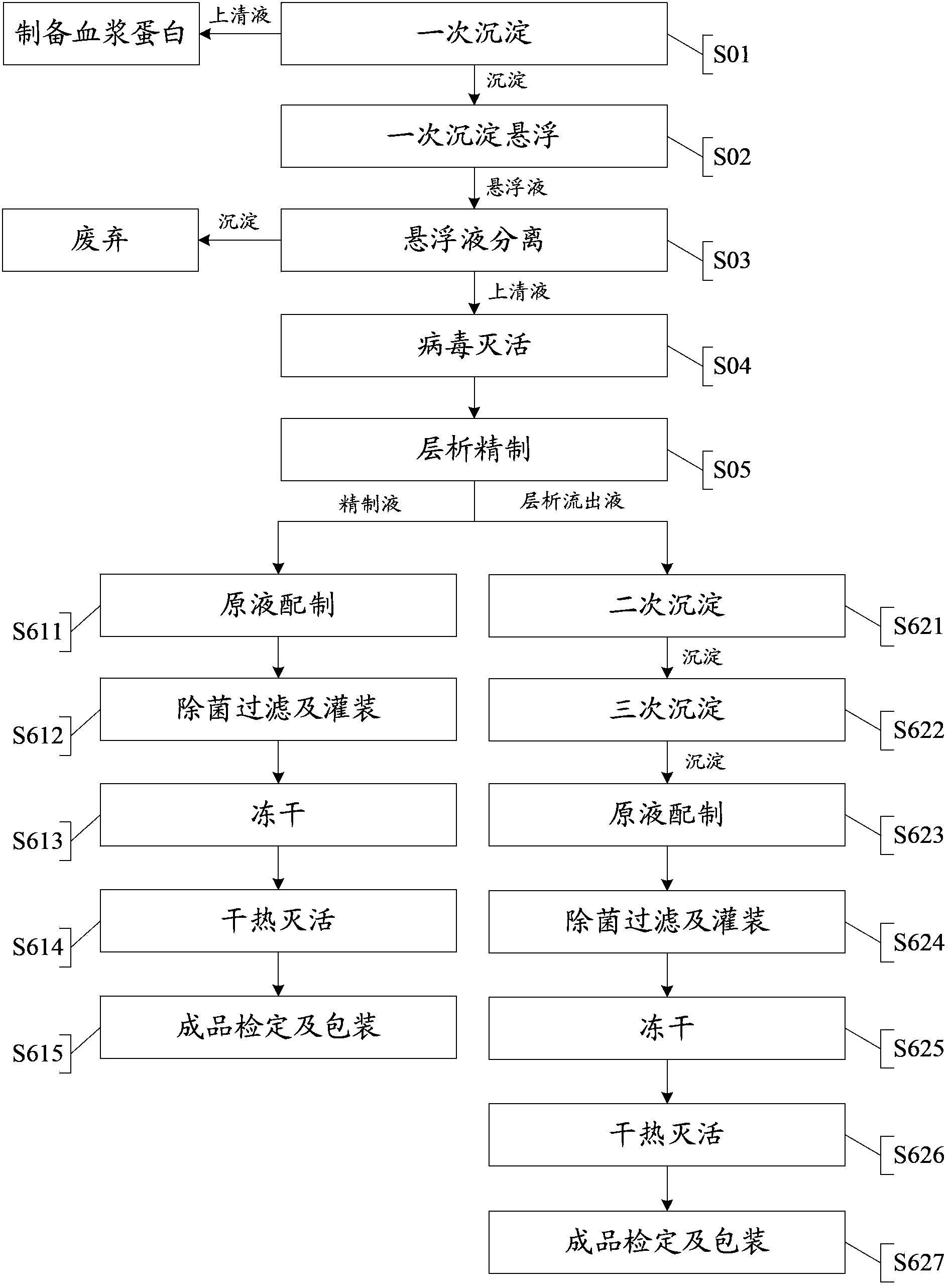
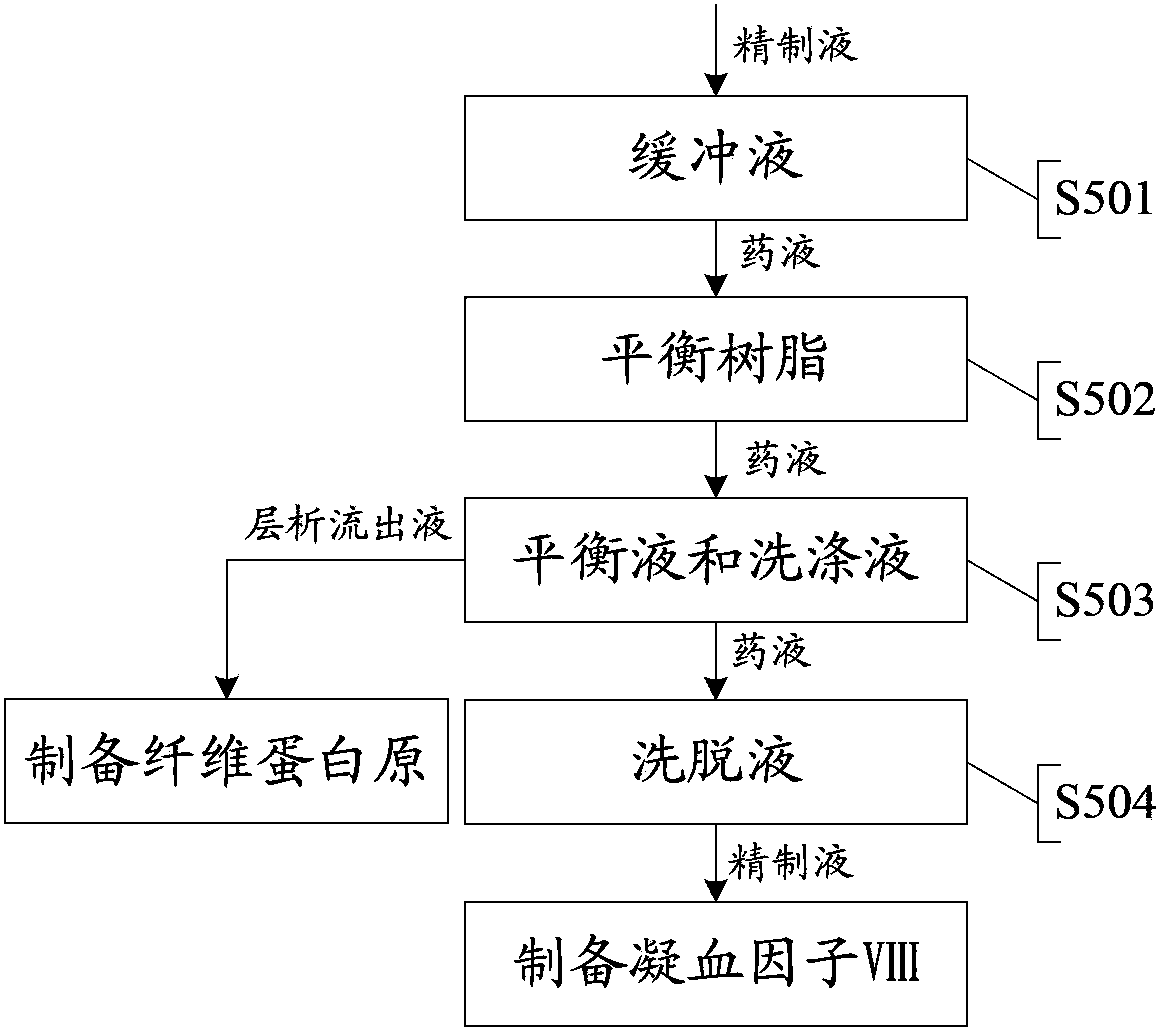
The invention provides a technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation. The preparation technology comprises the following steps: fresh plasma is subjected to primary sedimentation, so that blood coagulation factor VIII and fibrinogen precipitation can be jointly precipitated from the plasma; the primary precipitation is subjected to suspension; the suspension liquid is subjected to centrifugal separation to obtain supernatant; the centrifugally separated supernatant is subjected to virus inactivation and chromatography refining to respectively obtain human blood coagulation factor VIII refined liquid used for preparing human blood coagulation factor VIII products and chromatography effluent used for preparing human fibrinogen products. According to the invention, the human blood coagulation factor VIII and the human fibrinogen are precipitated through the one-step plasma constituent precipitation, and an ion-exchange column chromatography technology is adopted to perform purification preparation of the human blood coagulation factor VIII and the human fibrinogen, so that the deficiency that the human fibrinogen cannot be normally produced as the human blood coagulation factor VIII is prepared through cryoprecipitation is overcome.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Dry heat treatment stabilizer for human blood coagulation factor VIII and application thereof
ActiveCN102924562AEffectively maintain activityHigh activityFactor VIIPeptide/protein ingredientsDry heatChloride
The invention discloses a dry heat treatment stabilizer for human blood coagulation factor VIII, which comprises the following proportions of components: 0.005M-0.015M of sodium citrate, 0.001M-0.002M of calcium chloride, 0. 15M-0.25M of arginine hydrochloride, and 6-10g / L of human serum albumin. The invention also discloses a human blood coagulation factor VIII preparation and a preparation method of the human blood coagulation factor VIII preparation. The dry heat treatment stabilizer for human blood coagulation factor VIII provided by the invention can maintain the activity of the human blood coagulation factor VIII, and is low in cost, thus having good prospect in clinical application.
Owner:CHENGDU RONGSHENG PHARMA
Method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma
InactiveCN105330736AIncrease profitSimple processPeptide preparation methodsBlood coagulation/fibrinolysis factorsUltrafiltrationEngineering
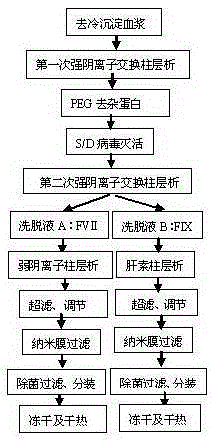
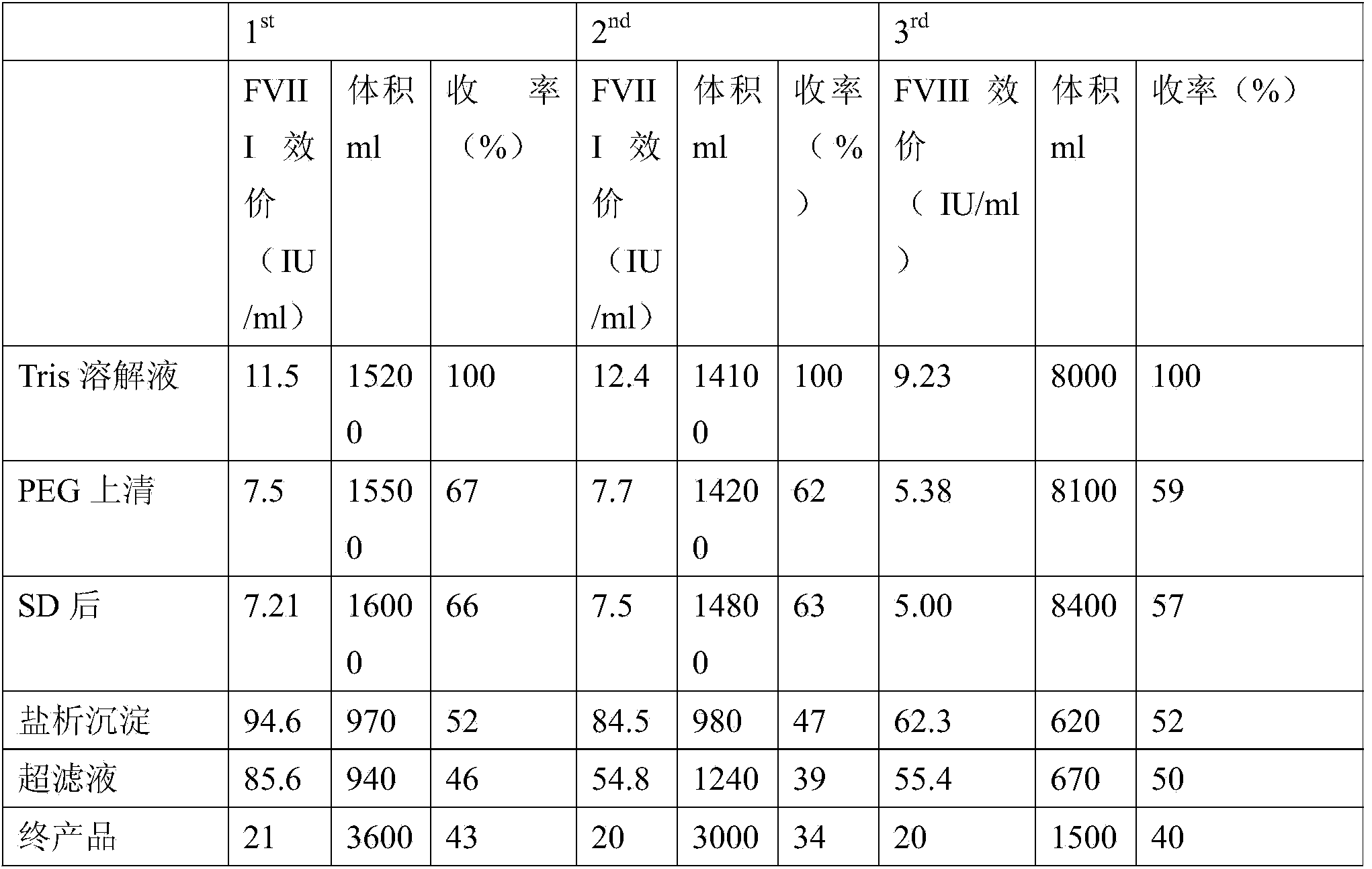
The invention discloses a method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma. The method comprises the following steps: 1, removing cold glue from blood plasma; 2, conducting strong anion-exchange column chromatography the first time; 3, conducting PEG sedimentation for removing impure protein; 4, conducting S / D viral inactivation; 5, conducting strong anion-exchange column chromatography the second time, and obtaining FVII eluent and FIX eluent; 6, conducting weak anion-exchange column chromatography, and concentration for purifying blood coagulation FVII; 7, conducting heparin affinity column chromatography for purifying blood coagulation FIX; 8, conducting ultrafiltration; 9, adding a stabilizing agent, and conducting adjustment; 10, conducting virus-removal filtration through nanofilms; 11, conducting sterilization, filtration and subpackage; 12, conducting freeze-drying; 13, conducting dry-hot viral inactivation. According to the method, PEG sedimentation is adopted for removing the impure protein, the target of preparing high-purity FVII and FIX simultaneously is achieved through combination of an ion-exchange column chromatography technology and an affinity chromatography technology, the process flow is simple, the production cycle is short, a product is subjected to three steps of virus eradicating measures, and use safety is high.
Owner:上海洲跃生物科技有限公司
Human blood coagulation factor VIII resisting monoclonal antibody as well as preparation method and application thereof
InactiveCN101906160AStrong specificityHigh affinityImmunoglobulins against blood coagulation factorsTissue cultureAntigenClotting factor
The invention provides a human blood coagulation factor VIII resisting monoclonal antibody as well as a preparation method and application thereof. The monoclonal antibody has the characteristic of strong specificity in combination with the human blood coagulation factor VIII antigen. The invention also provides a corresponding monoclonal antibody segment, immune conjugate and a detection kit for detecting the human blood coagulation factor VIII.
Owner:SUZHOU ZELGEN BIOPHARML
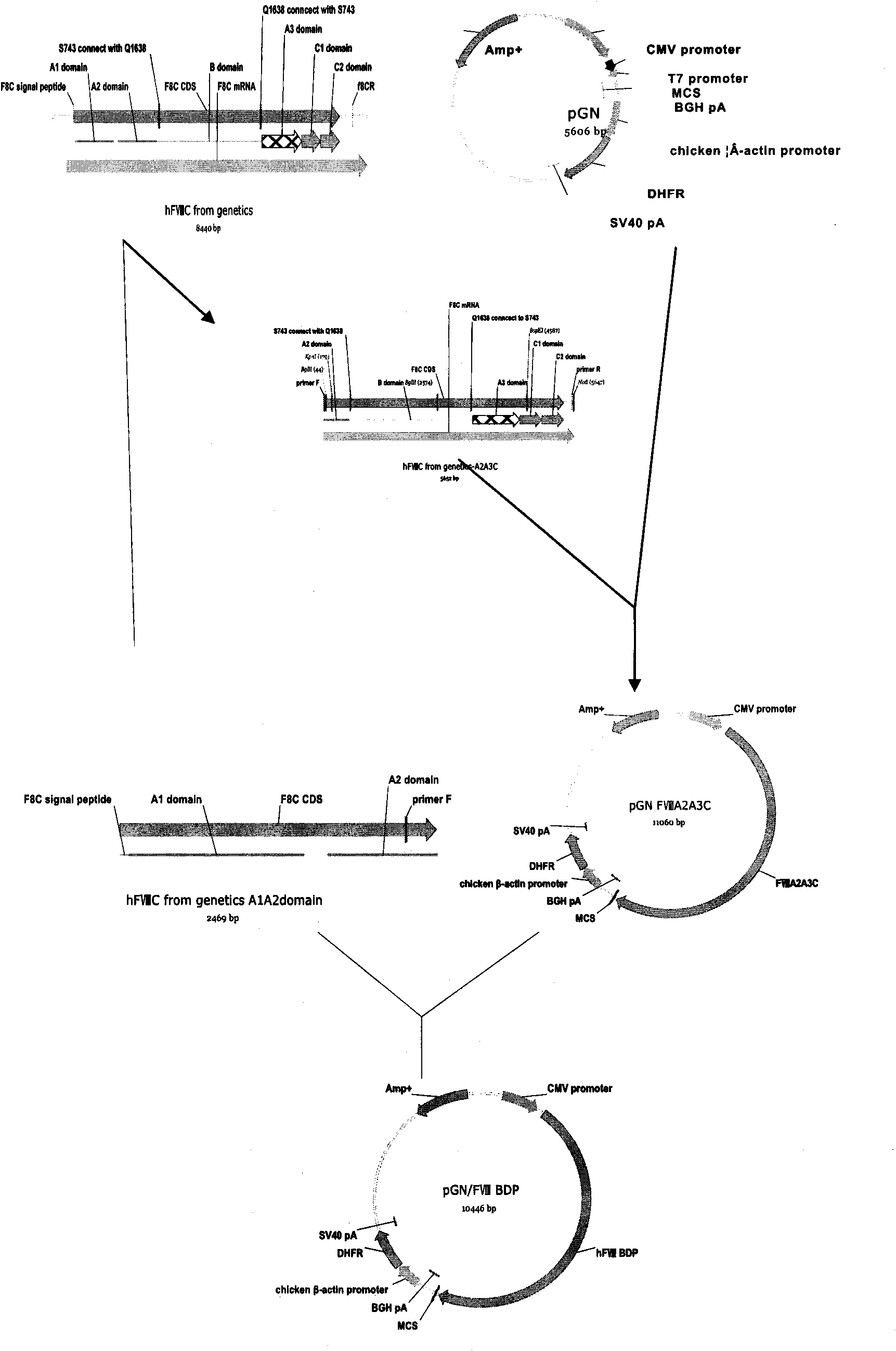
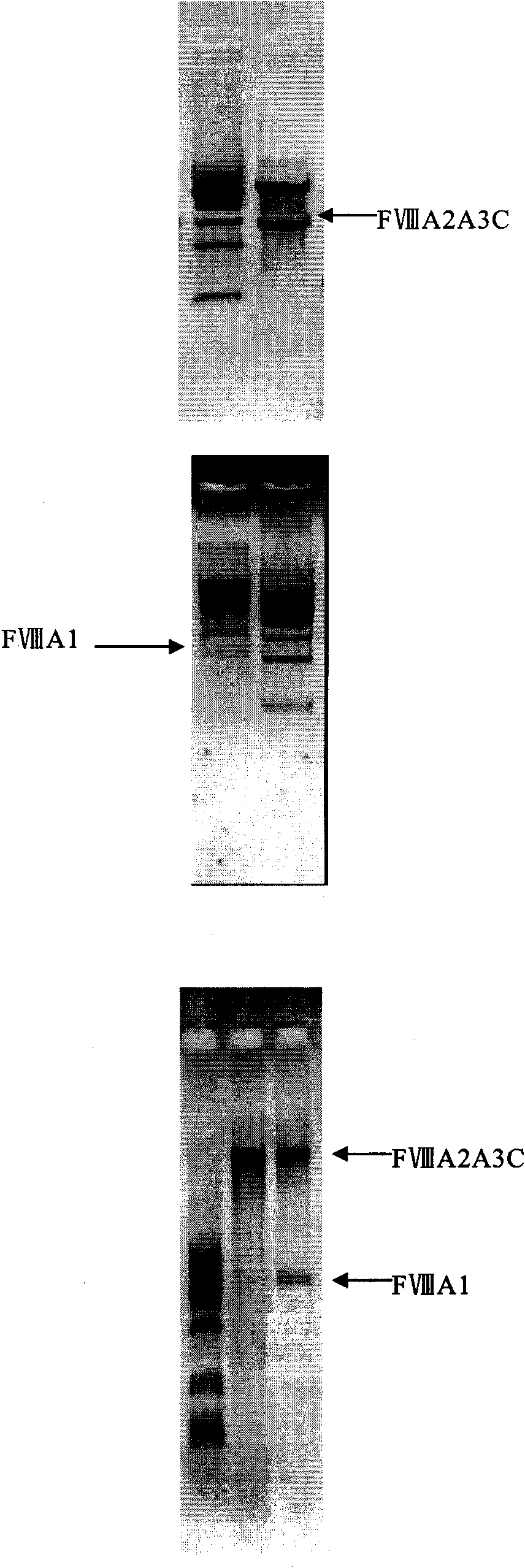
Recombinant human blood coagulation factor VIII protein, composition, use of a recombinant factor VIII protein, use of a composition, method of obtaining a recombinant human blood coagulation factor VIII protein and use thereof
InactiveUS20100172891A1Improve biological activityImprove the level ofFactor VIIPeptide/protein ingredientsHemophiliasHuman Blood Coagulation Factor
The present invention refers to a recombinant human blood coagulation factor VIII protein and a composition containing it. The present invention also refers to the use of the protein or composition of the invention for manufacturing a medicine for treating hemophilia A. Additionally, the present invention refers to the method of obtaining a recombinant human blood coagulation factor VIII protein. A further object of the present invention is a recombinant protein obtained by the method described herein, and its use in the preparation of a medicine for the treatment of hemophilia A.
Owner:FUNDACAO HEMOCENT DE RIBEIRAO PRETO +1
Novel recombinant human blood coagulation factor VIII and production method thereof
InactiveCN102311495AHigh expressionHigh activityFactor VIIFermentationInternal ribosome entry siteClotting factor
The invention provides a novel recombinant human blood coagulation factor VIII and a production method thereof. Particularly, the invention relates to reconstruction of a human blood coagulation factor VIII gene. A novel expression vector containing a weakened internal ribosome entry site (IRES for short) sequence and double screening marks is constructed. The vector can be used for high-efficiency recombinant expression of the human blood coagulation factor VIII, so that the expression of the vector in a mammalian cell expression system is increased.
Owner:SHANGHAI TONEKER BIOTECH
Pichia yeast expressing recombinant human blood coagulation factor VII, and preparation and use thereof
InactiveCN101376875AHigh activityHigh expressionFungiPeptide/protein ingredientsFactor SevenFactor ii
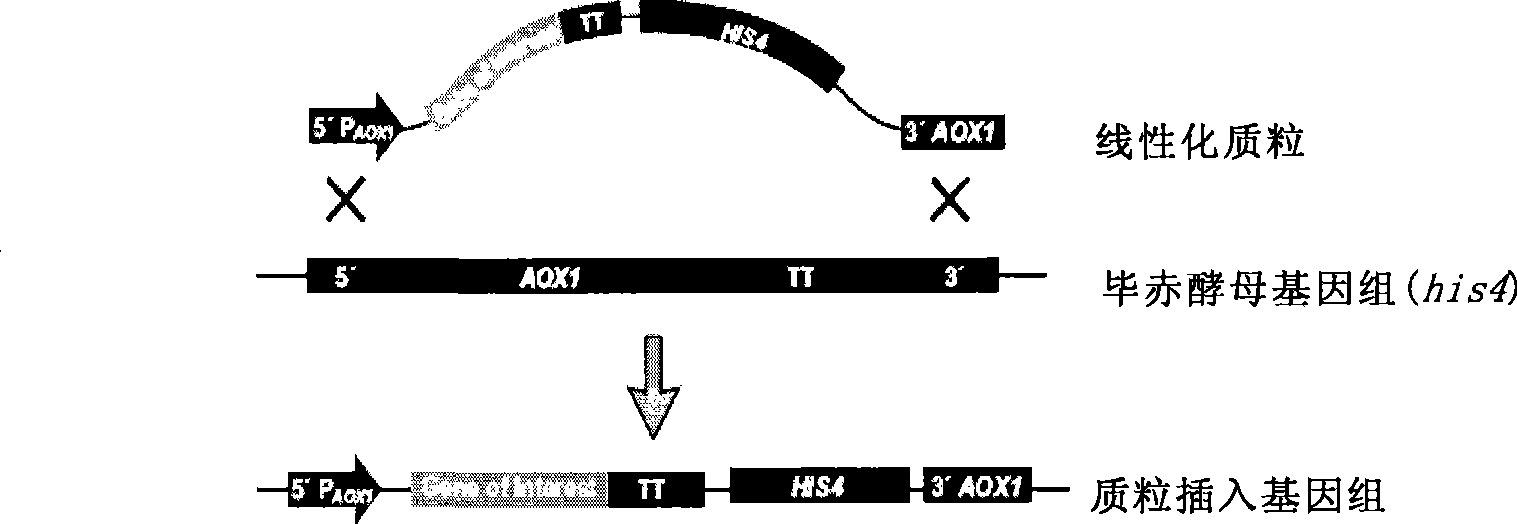
The invention provides a yeast cell which expresses a human coagulation factor seven; the yeast cell integrates a structure which expresses the human coagulation factor seven; the structure comprises two to fifteen human coagulation factor seven expression cassettes which are serially connected and lined; each expression cassette subsequently comprises the following elements from 5' to 3': (a) a start signal element AOX; (b) a human coagulation factor seven gene; (c) a stop signal element AOX (TT); and the yeast cell expresses the activated human coagulation factor seven under the induction of methanol. The invention also provides the structure and a preparation method thereof, a preparation method of the yeast cell and the human coagulation factor seven expressed by the yeast cell. The two to eighteen copied yeast cells structured by the invention can be used to improve yield and reduce cost, and are applicable in the mass production of human coagulation factor seven preparation.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Method for preparing cryoprecipitate and method for preparing blood coagulation factor VIII preparation by using cryoprecipitate
ActiveCN103848886AReduce the amount of solutionFactor VIIPeptide preparation methodsBlood coagulation factor VIIICryoprecipitate
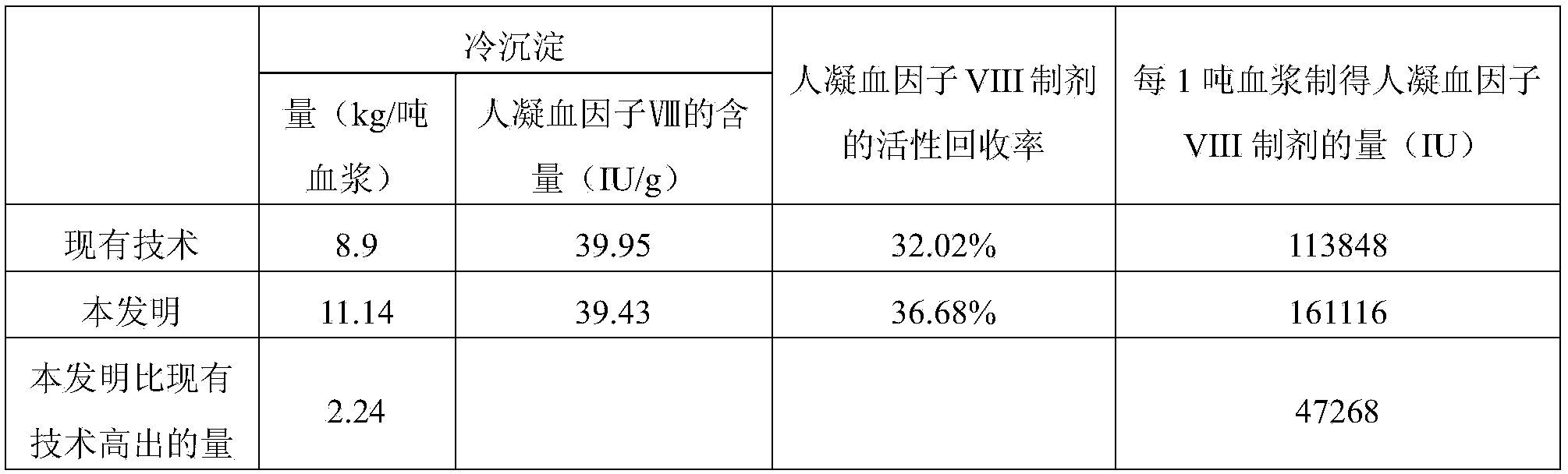
The invention discloses a method for preparing a cryoprecipitate. The method comprises the steps of (1) thawing: selecting fresh frozen plasma, and heating to obtain thawed plasma of which the temperature is 0-5 DEG C; (2) filtering: filtering under the condition that the temperature of the thawed plasma is 0-5 DEG C to obtain filtrate and filter residues; (3) centrifuging: centrifuging under the condition that the temperature of the filtrate is 0-5 DEG C to obtain a precipitate; (4) combining the filter residues obtained in step (2) and the precipitate obtained in step (3) to obtain the cryoprecipitate. The preparation method disclosed by the invention is simple, and the prepared cryoprecipitate is high in yield and human blood coagulation factor VIII content, so the preparation method has a good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
Method for preparing freeze-dried human blood coagulation factor VIII
InactiveCN105294858AReduce lossesPrevent denaturation and inactivationFactor VIIPeptide preparation methodsUltrafiltrationFreeze-drying
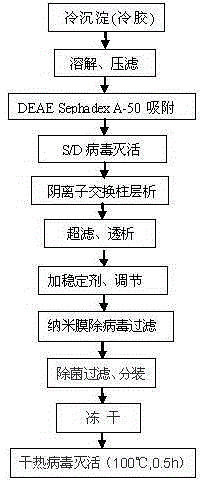
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII through cryoprecipitation. The method comprises the following steps of (1) dissolving and carrying out filter pressing in cryoprecipitation; (2) deactivating S / D viruses; (3) removing a blood coagulation factor depended by vitamin K by using DEAE SephadexA-50 gel; (4) purifying FVIII through carrying out column chromatography on anion exchange resin; (5) carrying out ultrafiltration and dialysis, and concentrating an eluent; (6) adding a stabilizing agent in a concentrated solution, and regulating the titer and PH value of FVIII; (7) removing viruses on a nanofilm, and filtering; (8) degerming, filtering and subpackaging; (9) carrying out freeze drying; and (10) carrying out dry heat deactivation on the viruses. According to the method disclosed by the invention, the blood coagulation factor depended by vitamin K is removed through adsorption of the DEAE SephadexA-50 gel, the traditional aluminum hydroxide and PEG sedimentation way is replaced, and therefore, the method has the advantages of production stability, high yield and good quality; and the safety of the product is greatly improved due to the adoption of three-step virus removing measures.
Owner:上海洲跃生物科技有限公司
Human blood coagulation factor IX mutant pichia pastoris expression vector and construction method and application thereof
InactiveCN103060366AHigh activityHigh yieldMicroorganism based processesVector-based foreign material introductionPichia pastorisBiotechnology
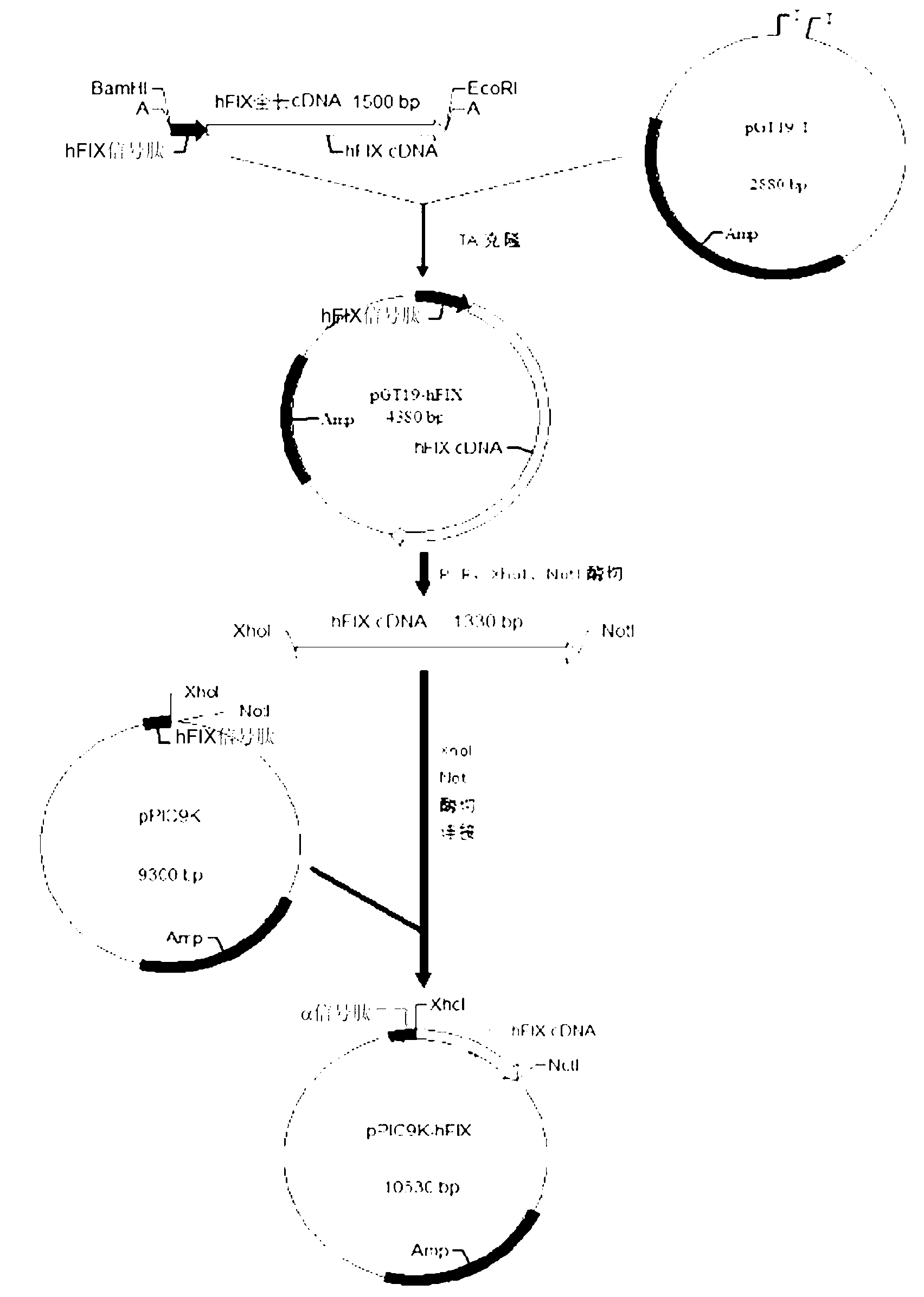
The invention discloses a human blood coagulation factor IX mutant pichia pastoris expression vector and a construction method and application thereof. The procedures of the construction method of the human blood coagulation factor IX mutant pichia pastoris expression vector includes that according to a hFIX gene sequence, the reverse transcription-polymerase chain reaction (RT-PCR) technology is used for cloning hFIX overall length complementary deoxyribonucleic acid (cDNA) from human hepatic cells, signal peptide mixed with yeast Alpha -factor and yeast expression plasmids pPIC9K-hFIX are constructed to express pichia pastoris, and then based on that the yeast expression plasmids pPICZ Alpha A-hFIX are constructed, four saccharomycetes hFIX high-activity mutants are constructed. Cruor activity of the achieved hFIX yeast expression vector is apparently higher than that of standard hFIX. One of the mutants is through 50-litre pilot scale test of fermentation, protein purification product secretory expression quantity is 702 milligrams / litre, so that a good foundation is laid for producing efficient hFIX products used for treating hemophilia B in a low cost and industrialized mode.
Owner:WUHAN UNIV OF SCI & TECH
Preparation method of blood coagulation factor IX quality control product
InactiveCN104181313AEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Method for manufacturing high purified factor ó¨
ActiveCN101291951APeptide preparation methodsPeptidasesCell culture mediaAnion-exchange chromatography
The present invention discloses a preparation method of a highly purified human blood coagulation factor IX. The human blood coagulation factor IX is prepared by performing anion exchange chromatography, cation exchange chromatography, heparin affinity chromatography to the material containing human blood coagulation factor IX (taken from human blood serum or recombined cell culture medium), which includes the period of passivating virus through S / D treatment (Solvent / Detergent treatment) or removing virus through nanofiltration. The highly purified safe coagulation factor IX preparation having a specific activity of above 150 IU / mg, with substantially undoped proteins can be prepared by the preparation method of the invention.
Owner:THE GREEN CROSS CORP
Method for preparing freeze-dried human blood coagulation factor VIII
ActiveCN102228683BImprove securityHigh recovery rate of activityPowder deliveryPeptide/protein ingredientsFreeze-dryingUltrafiltration
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII. The method comprises the following process of: dissolving by taking water for injection, comprising 3-10 IU (International Unit) / ml of heparin, as a heparin sodium solution and cryoprecipitating; performing PEG (Polyethylene Glycol) precipitation and taking supernatant; performing centrifugal filtration; performing S / D (Solvent / Detergent) inactivation at the temperature of 24-26 DEG C; performing DEAE (Diethylaminoethyl) Sepharose Fast Flow chromatographic column balance, adsorption, washing andelution; performing molecular membrane ultrafiltration; preparing, removing bacteria, sub-packaging, freeze-drying and capping; and dry-heating at the temperature of 99.5-100.5 DEG C and inactivating. In the invention, the process method of combining the PEG precipitation and an ion exchange chromatography technology is adopted; the method is easy and convenient to operate; the F VIII active recovery rate is increased; miscellaneous proteins can be removed on a large scale; the product yield reaches over 60 percent; and the specific activity of the product reaches 5 IU / mg and is obviously greater than a value which is not less than 1 IU / mg stipulated in the pharmacopeia. Meanwhile, the PEG residue is 0.08g / L which is obviously less than the value which is less than or equal to 0.5 IU / mg stipulated in the pharmacopeia, so that Al<3+> residues in the final preparation of an Al(OH)3 gel method are avoided; the product has high purity and high safety; and the quality of the final product is obviously improved.
Owner:NANYUE BIOPHARMING
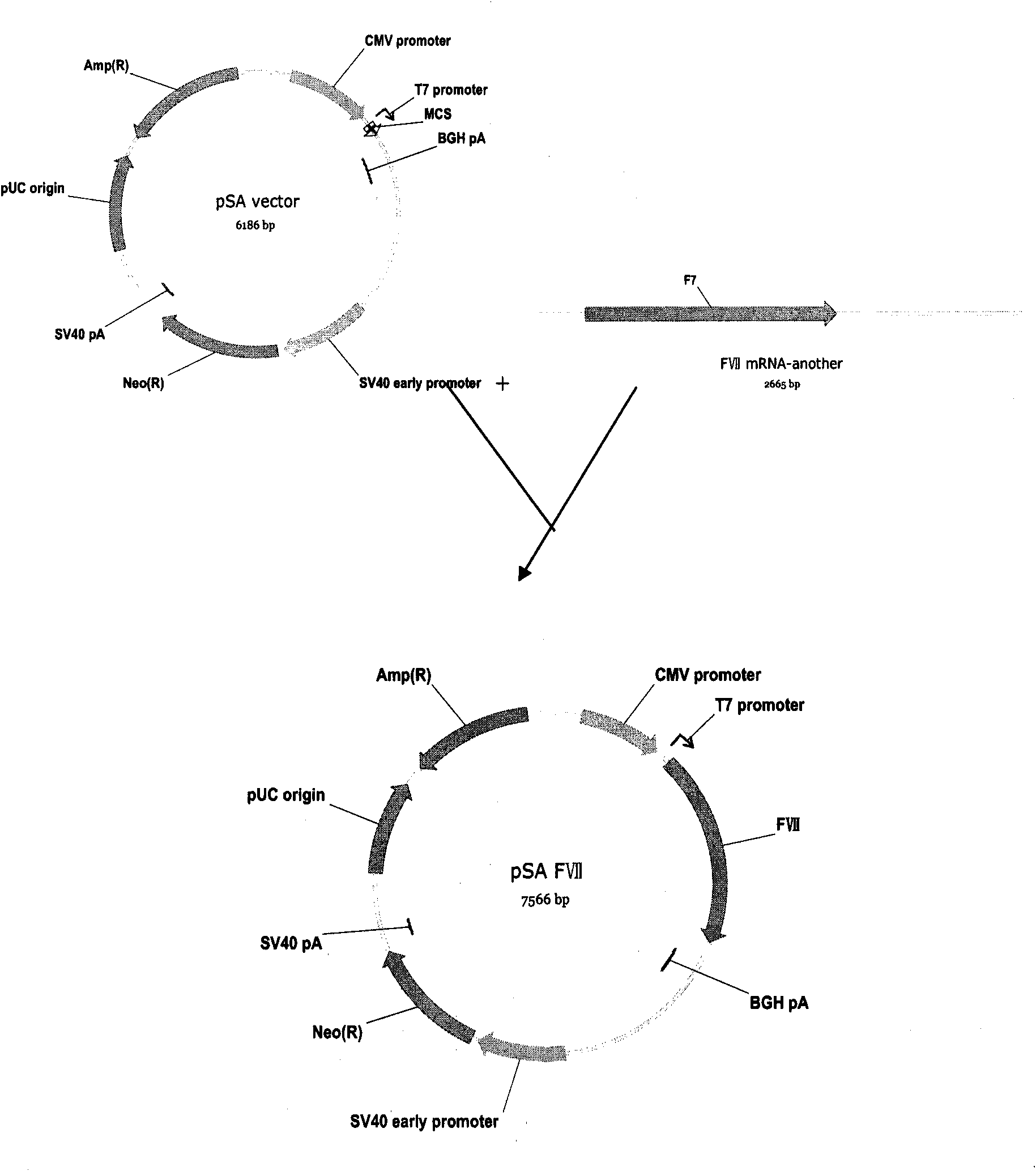
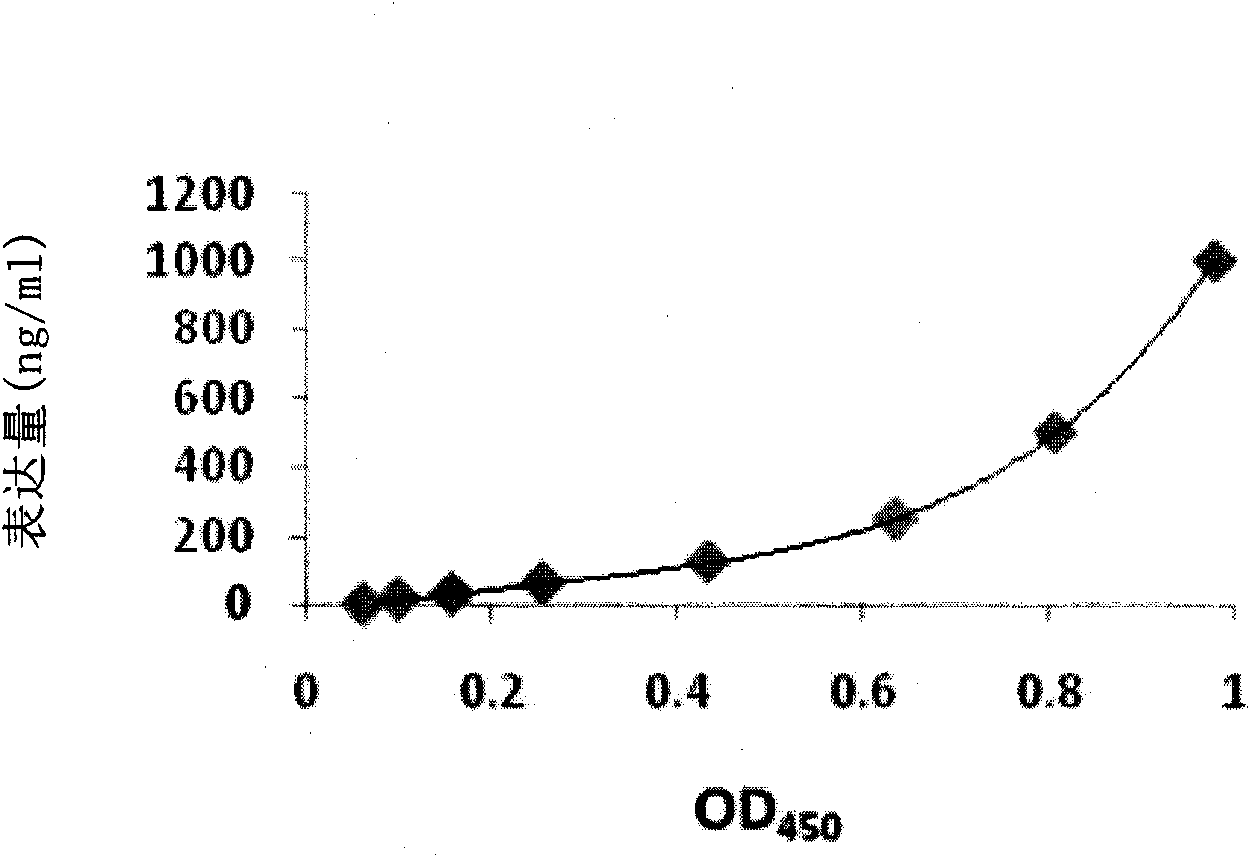
Method for expressing and producing recombinant human blood coagulation factors VII in animal cells
ActiveCN101906438AFermentationVector-based foreign material introductionMultiple cloning siteClotting factor
The invention provides a method for expressing and producing recombinant human blood coagulation factors VII in animal cells, in particular an expression vector for expressing exogenous protein in the animal cells. The expression vector contains an expression cassette for expressing the exogenous protein, wherein the expression cassette comprises the following elements from 5' to 3': (a) a first promoter; (b) a polyclone locus and an optional coding sequence of the exogenous protein; (c) a first polyA sequence; (d) a second promoter; (e) a selected marker gene; and (f) a second polyA signal sequence. The expression vector is particularly suitable for expressing the FVII efficiently in the animal cells.
Owner:SUZHOU ZELGEN BIOPHARML
Human blood coagulation factor VIII gene intron 22 inversion mutation in-situ remediation plasmid, kit and method
ActiveCN104762318APrecise point importNo uncertaintyFermentationVector-based foreign material introductionIn situ remediationIntron 22 inversion
The invention discloses a human blood coagulation factor VIII gene intron 22 inversion mutation in-situ remediation plasmid, kit and method. The method constructs specific in-situ remediation treatment and can specifically repair the type of F8 mutation, and the in-situ remediation strategy is verified in hemophilia patient specific iPSCs by combination with a TALEN technology. The in-situ remediation strategy is a first remediation strategy for remediation of intron 22 inversion as common HA mutation. The in-situ remediation strategy utilizes a sequence accurate fixed-point introduction method, is relatively safe and has no nondeterminacy of the prior art.
Owner:SHANGHAI PINPOINT MEDICAL TECH CO LTD
Freeze-dry and dry heat treatment protecting agent for human blood coagulation factor VIII
ActiveCN104225601ASuitable for a wide range of peopleHigh potencyPharmaceutical non-active ingredientsActive agentAlcohol sugars
The invention discloses a freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII. The freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII does not contain human serum albumin or other animal-source proteins, sugar or sugar alcohol, is composed of soluble salt, amino acid and a surface active agent, is free from the risk of spreading other viruses or causative agents, and is applicable to a wide crowd range, including diabetics; after being freeze-dried, a product of the human coagulation factor VIII using the protecting agent is quick in redissolving and has a good redissolving effect, still keeps high potency and high specific activity, and has the potency higher than 80 percent and the specific activity higher than 40 IU / mg; in addition, the freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII is low in cost, simple to prepare, has an obvious protecting effect, is safe and effective, and has an excellent industrial application prospect.
Owner:广东双林生物制药有限公司
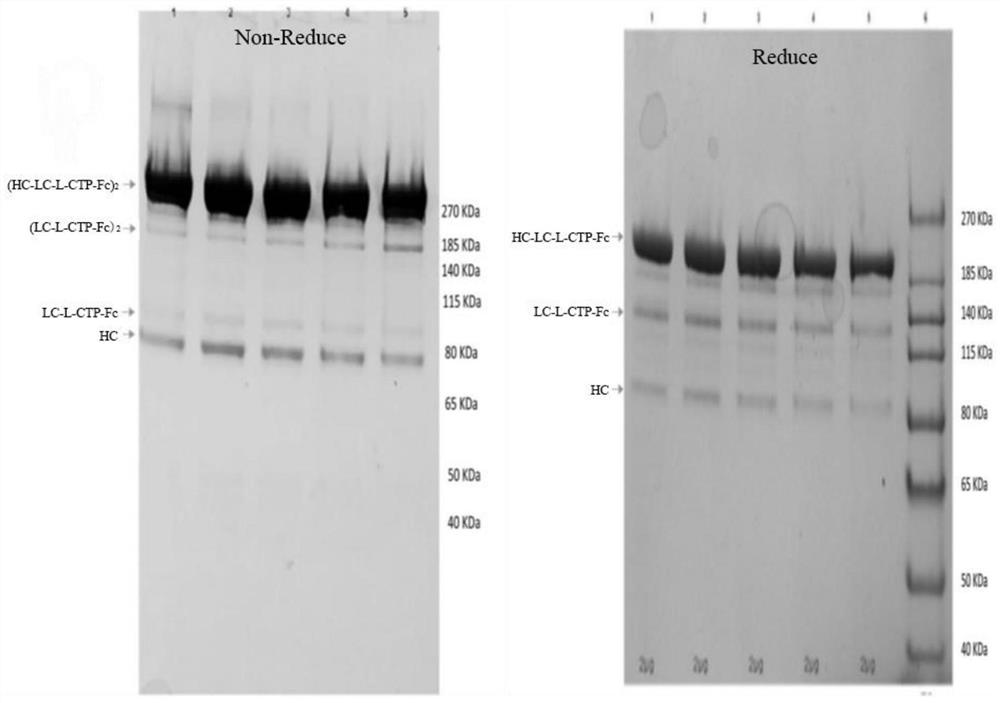
Improved human blood coagulation factor FVII-Fc fusion protein and preparation method and application thereof
ActiveCN104774269APeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectPharmaceutical drug
Disclosed are a recombinant fusion protein of human blood coagulation factor FVII-Fc, preparation method therefor and use thereof. The fusion protein comprises a human FVII, flexible peptide linker, and IgG2 Fc variant sequentially from the N-terminal to the C-terminal. The Fc variant has on lytic activity, and shows minimal Fc-mediated adverse side-effects. The fusion protein has a bioactivity similar to or higher than that of human FVII and a greatly prolonged plasma half-life, thereby improving the pharmacokinetics and pharmaceutical effect.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Zone B partially-deleted type recombinant human blood coagulation factor VIII
ActiveCN105017410AConsistent biological activityImprove stabilityFactor VIIPeptide/protein ingredientsBlood coagulation factor VIIIClotting factor
The invention belongs to the field of biological medicines, and in particular relates to a gene recombinant human blood coagulation factor VIII for treating hemophilia A. By performing zone B partially-deleted type mutation on a full-length blood coagulation factor VIII, the obtained novel gene recombinant human blood coagulation factor VIII product has a characteristic that the structure is more stable.
Owner:BEIJING NORTHLAND BIOTECH
Target multifunctional anti-embolism fusion protein as well as preparation method and application thereof
InactiveCN102180973AInhibition formationInhibition of developmentFungiPeptide/protein ingredientsArginineCoagulation Factor Xa
The invention belongs to the technical field of genetic engineering, relating to a target multifunctional anti-embolism fusion protein an amino acid sequence of which is as shown in SEQ ID NO.1. The invention also relates to a gene for encoding the fusion protein, a recombinant expression vector containing the gene, a transformant containing the recombinant expression vector and a method for preparing the fusion protein. The fusion protein can reasonably splice human antibacterial peptide LL-37, leech peptide Hirudin-12, Agkistrodon acutus peptide (AAP), arginine-glycin-aspartate (RGD) and human blood coagulation factor Xa identification sites, so that the functions among a target component, an antibacterial component, a thrombin resisting component and a platelet aggregation resisting component are complementary and in synergistic effect; and the fusion protein can well exert target anti-inflammatory and anticoagulation activities at a thrombus position, and simultaneously can repair a vessel endothelial cell, can commonly inhibit the formation and development of thrombus by virtue of multiple ways, and can be used for preparing a medicament for preventing and treating thrombotic diseases.
Owner:CHONGQING UNIV
Method for preparing recombinant human blood coagulation factor VIII
ActiveCN107287265AImprove mixing efficiencyFull gas-liquid exchangeFactor VIIFermentationFactor iiProtein activity
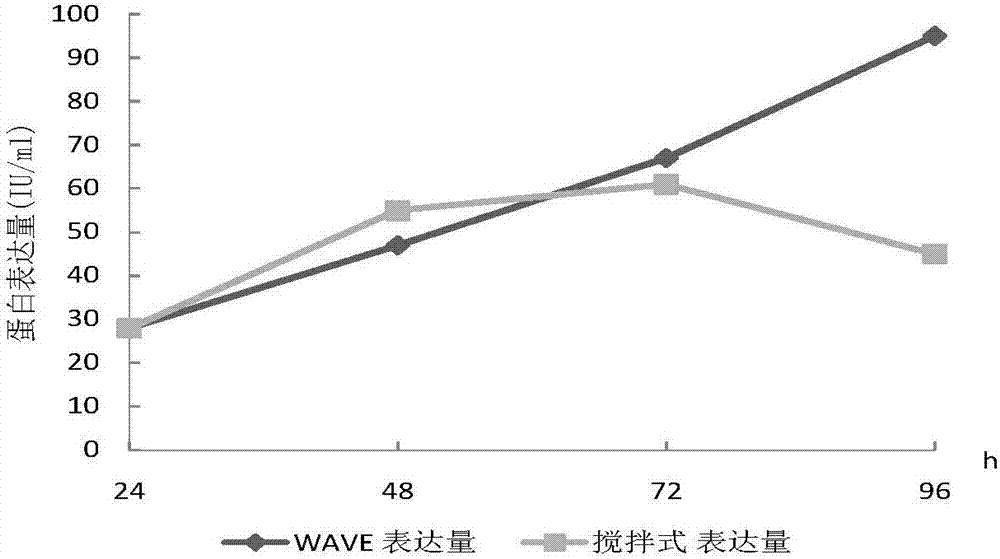
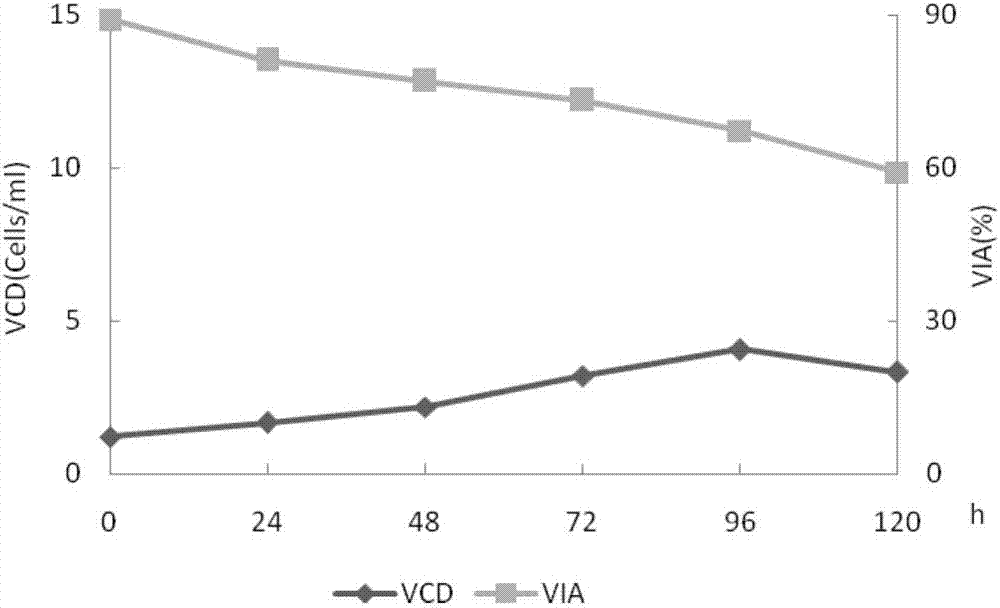
The invention belongs to the technical field of biological engineering, and relates to a method for preparing a recombinant human blood coagulation factor VIII, in particular to a method for culturing cells for expressing a recombinant human blood coagulation factor VIII by using a wave type bioreactor and separating and purifying the recombinant human blood coagulation factor VIII from a cell culture liquid. The WAVE bioreactor is high in mixing efficiency, sufficient in gas-liquid exchange, small in foam amount and low in shearing force, damage of paddles of a stirring type stainless steel reactor and foams to cells is avoided, and thus the cell state, the cell survival rate and the protein activity of the WAVE bioreactor are all superior to those of the stirring type stainless steel reactor.
Owner:NANJING SHUNXIN PHARM CO LTD OF CHIATAI TIANQING PHARM GRP +1
Preparation method of human blood coagulation factor VIII and human blood coagulation factor VIII product
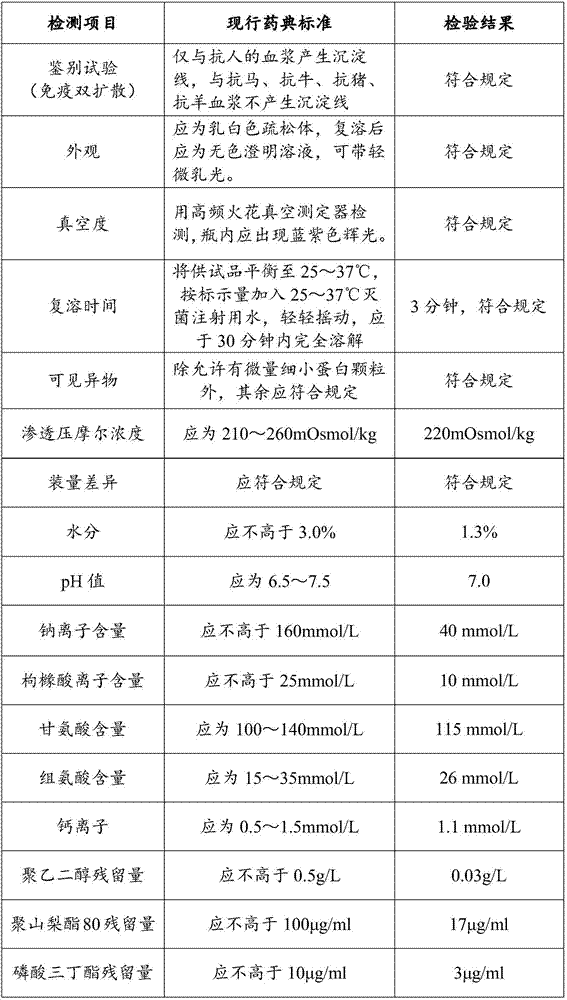
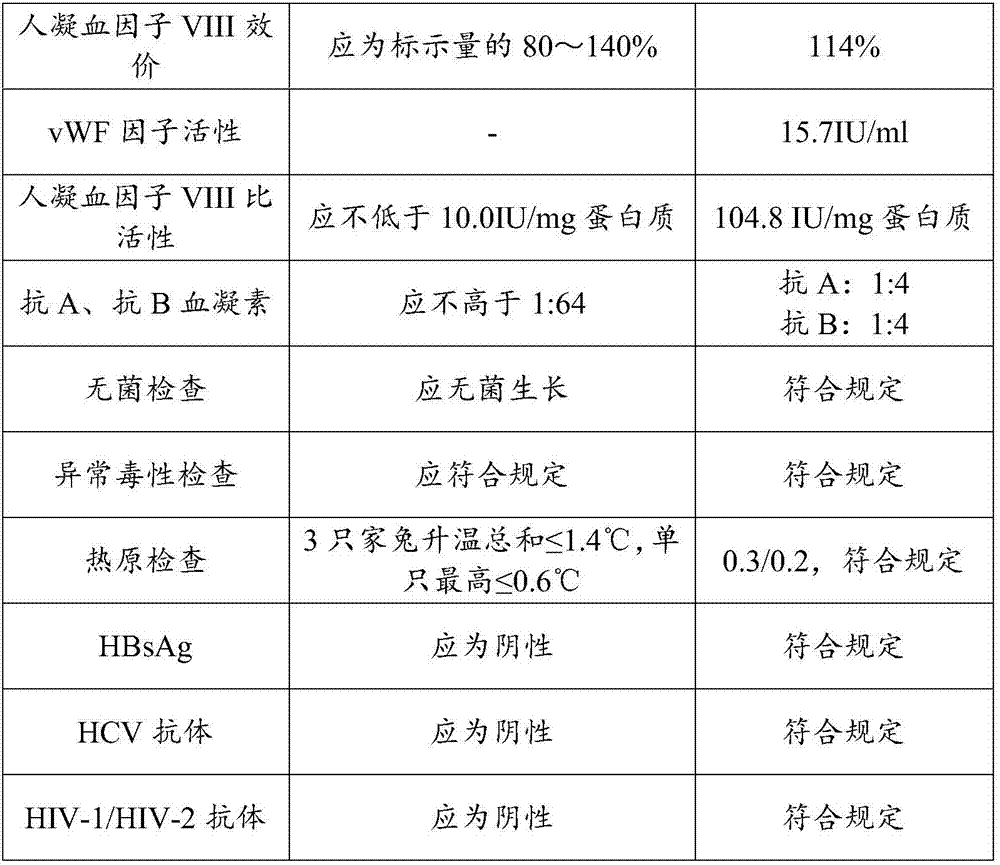
ActiveCN107880112AHigh recovery rateTaller than aliveFactor VIIPeptide preparation methodsWhole blood productIon chromatography
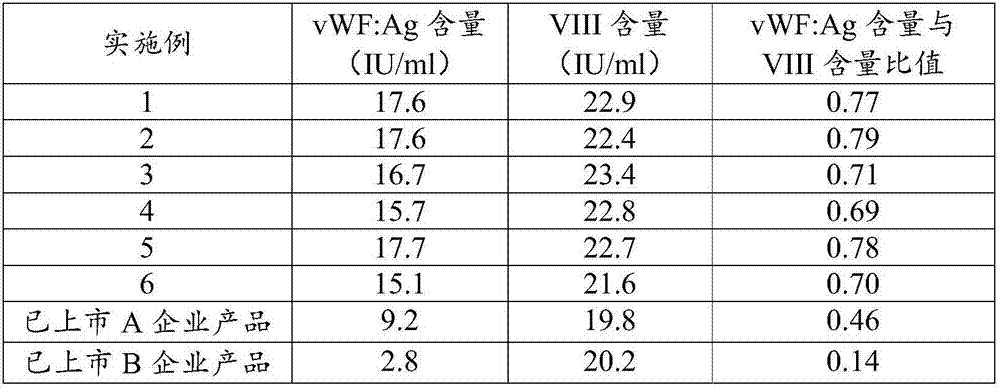
The invention discloses a preparation method of a human blood coagulation factor VIII and a human blood coagulation factor VIII product and relates to the field of blood products. The preparation method of the human blood coagulation factor VIII comprises the following steps: dissolving a cryoprecipitate with a 0.015-0.025mol / L tromethamine solution in a mass ratio of (3-5): 1 by reasonably processing the cryoprecipitate; and carrying out separation and purification by means of a polyethylene glycol precipitating method combined with ion exchange chromatography, wherein the recovery ratio of the human blood coagulation factor VIII and the specific activity of a final product can be improved effectively, the yield reaches 180-240IU / L plasma, and the specific activity is not lower than 100IU / mg proteins. The prepared human blood coagulation factor VIII product is rich in vWF factors and the proportion of the vWF factors and the human blood coagulation factor VIII is close to 1: 1. Besides treating hemophiliac A, the human blood coagulation factor VIII can be also used for treating patients with angiohemophilia, and the human blood coagulation factor VIII has good stability and heat resistance.
Owner:HUALAN BIOLOGICAL ENG INC +2
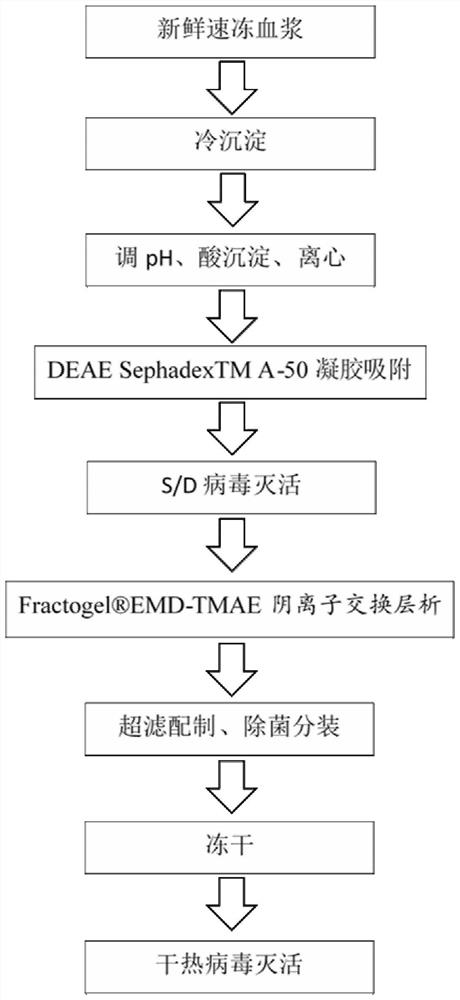
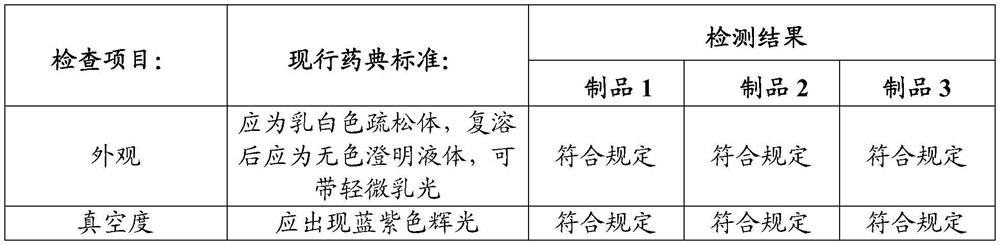
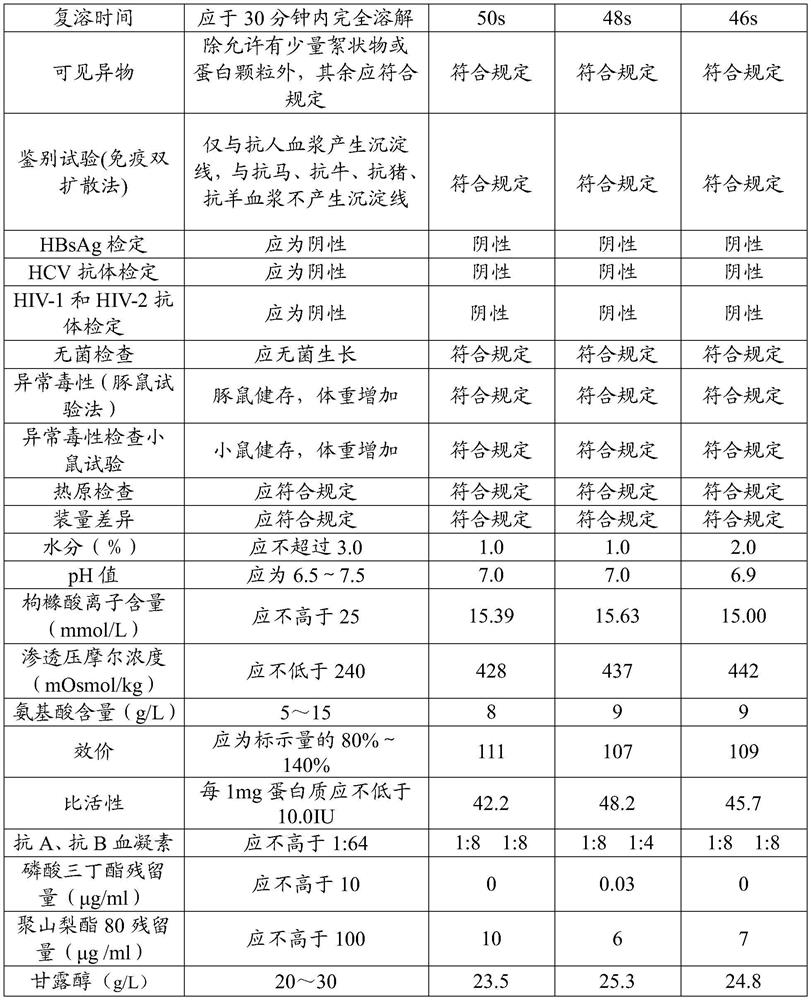
Preparation method of freeze-dried human blood coagulation factor VIII
PendingCN112028988AHigh purityHigh activityFactor VIIPeptide preparation methodsCryoprecipitateVirus inactivation
The invention relates to a preparation method of a freeze-dried human blood coagulation factor VIII. The preparation method comprises the following steps of collecting plasma and quick-frozen plasma;performing plasma melting and separating cryoprecipitate; dissolving cryoprecipitate; performing acid precipitation; performing gel adsorption; inactivating S / D virus; performing anion exchange chromatography; performing ultrafiltration; preparing a semi-finished product; carrying out sterilizing and sub-packaging; performing freeze-drying; and carrying out dry heat virus inactivation. The preparation method disclosed by the invention is short in product remelting time, high in specific activity, stable in quality and simple to operate, and after industrialization, the utilization rate of plasma resources can be greatly improved, market supply is increased, and the situation of FVIII medication tension in the Chinese market is relieved.
Owner:哈尔滨派斯菲科生物制药有限公司
Non-blood serum preparation of recombinant human blood coagulation factor IX
ActiveCN101481692AReduce pollutionHigh production yieldPeptide/protein ingredientsBiological testingDiseaseFactor ii
The invention relates to the preparation of recombinant human coagulation factor IX and an application thereof. The recombinant hFIX protein and hFIX of human blood source have the same molecular composition and biological activity and can be eased by being used for hemophilia B mouse model. The recombinant hFIX protein can be used as a standard in the tests for clinically detecting hFIX and the like, and can also cure diseases requiring supplementing hFIX, such as hemophilia B, etc.
Owner:浙江耶大生物医药有限公司
Application of mutant single-chain human coagulation factor VIII in preparation of fusion protein
ActiveCN113105562AModulating binding affinityNon-cytotoxicFactor VIIPeptide/protein ingredientsDiseaseHuman Chorionic Gonadotropin Beta Subunit
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC +1
Method for expressing and producing recombinant human blood coagulation factors VIII in animal cells
InactiveCN101906437AFermentationVector-based foreign material introductionMultiple cloning siteClotting factor
The invention provides a method for expressing and producing recombinant human blood coagulation factors VIII in animal cells, in particular an expression vector for expressing exogenous protein in the animal cells. The expression vector contains an expression cassette for expressing the exogenous protein, wherein the expression cassette comprises the following elements from 5' to 3': (a) a first promoter; (b) a polyclone locus and an optional coding sequence of the exogenous protein; (c) a first polyA sequence; (d) a second promoter; (e) a selected marker gene; and (f) a second polyA signal sequence. The expression vector is particularly suitable for expressing the VIII efficiently in the animal cells.
Owner:SUZHOU ZELGEN BIOPHARML
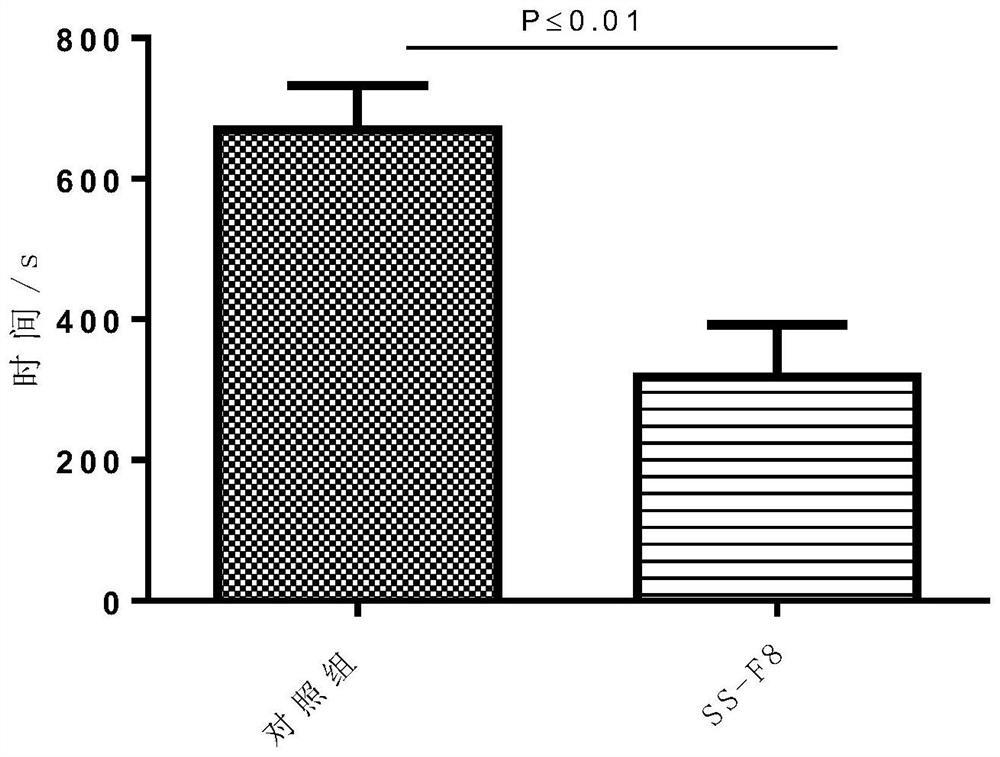
Thrombin generation inhibitors
InactiveUS7074892B2Excellent blood anticoagulation propertyLittle and no adverse side effectTetrapeptide ingredientsTripeptide ingredientsDiseaseHeavy chain
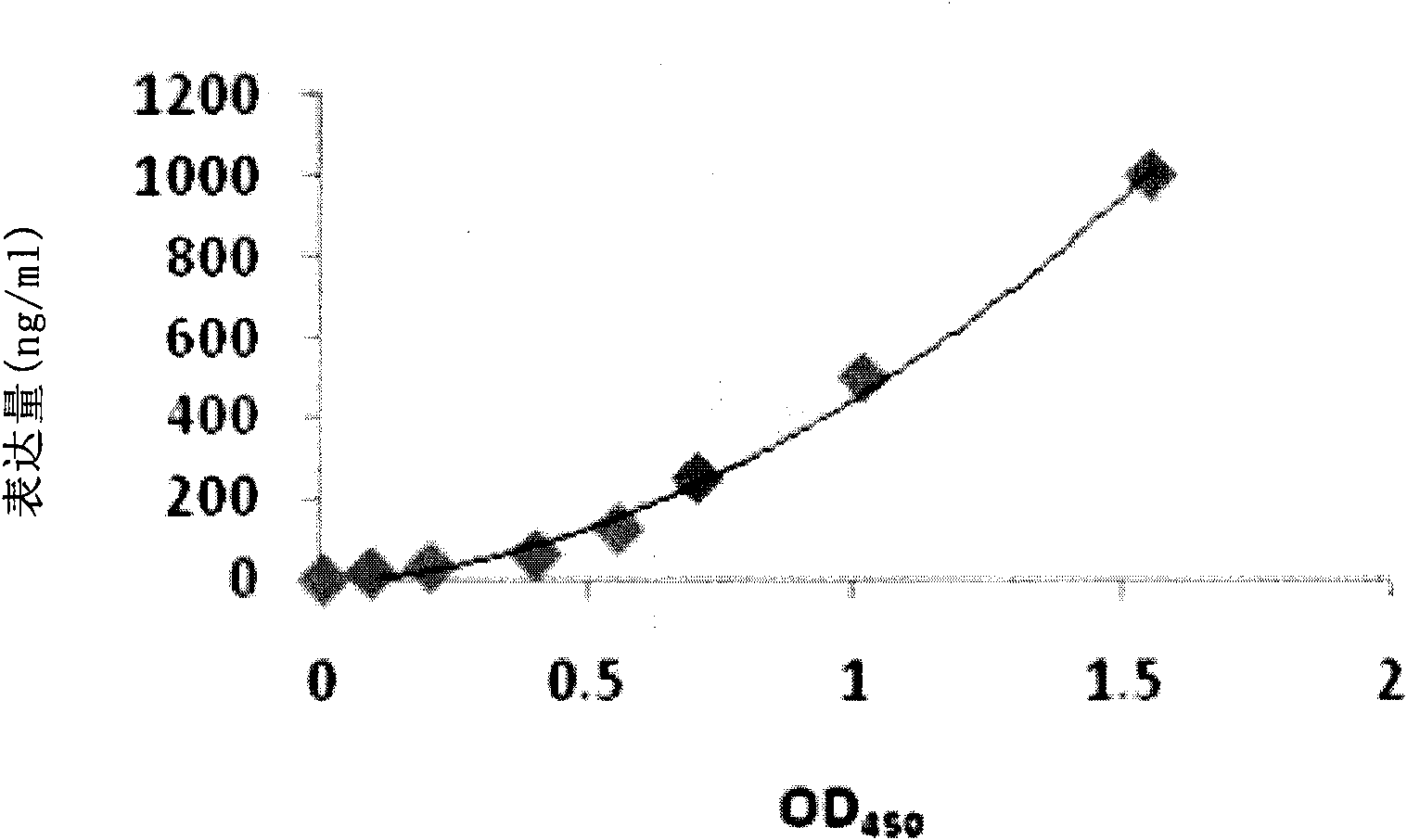
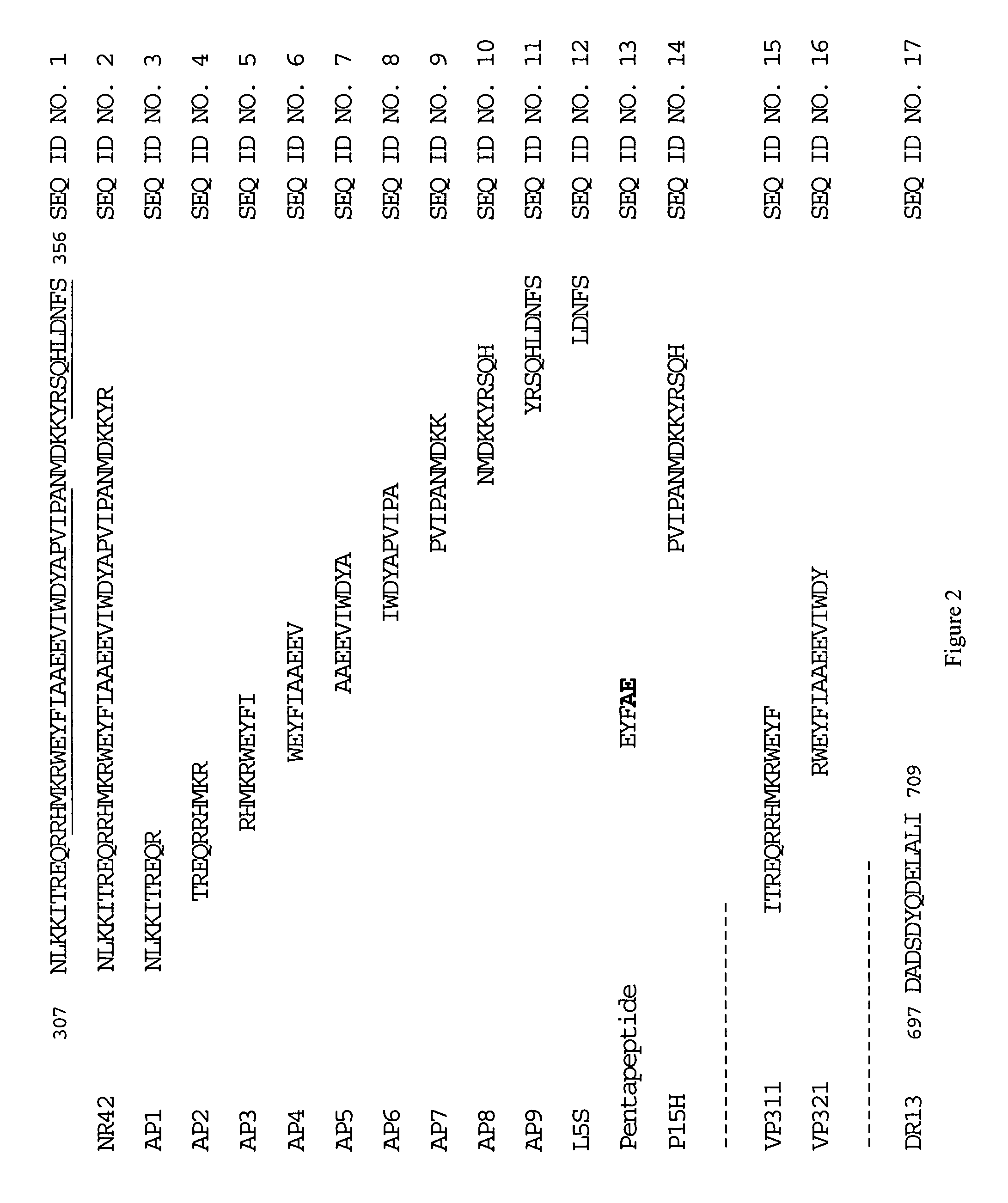
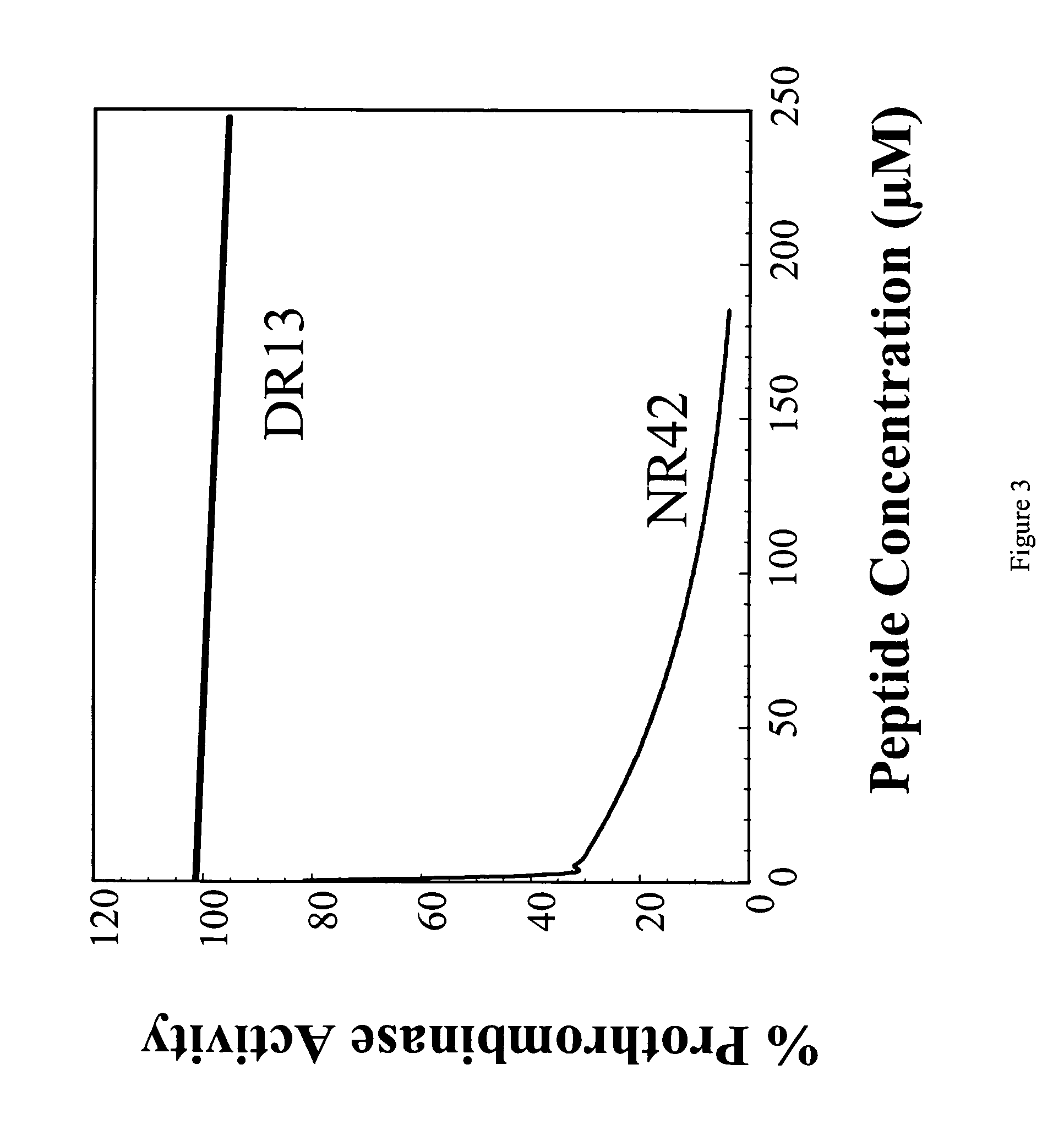
Peptides derived from amino acids 307 to 356 of the human blood coagulation factor Va are provided. Such peptides comprise: i) a length of between 3 and 50 amino acids, ii) a minimum of 3 contiguous amino acids from the 307–356 heavy chain region of factor Va, excluding peptide segments comprising amino acids 311 to 325 and amino acids 321 to 335, iii) optional additional amino acids at one or both ends of the contiguous amino acids such that the entire peptide is at least 60% identical to a sequence within 307 to 356 of factor Va, and iv) have an IC50 of between 50 nM to 500 μM for inhibition of prothrombinase. The present invention also provides a pharmaceutical composition comprising one or more prothrombinase-inhibiting peptide segments. The present invention also provides administration of the pharmaceutical composition to human subjects for the purpose of preventing thrombotic disorders.
Owner:CLEVELAND STATE UNIVERSITY
Production process of blood-derived human blood coagulation factor VIII/von willebrand factor compound
ActiveCN114106145AEnsure safetyEffective inactivationFactor VIIPeptide preparation methodsWhole blood productUltrafiltration
The invention relates to the technical field of blood products, in particular to a blood-source human blood coagulation factor VIII / von willebrand factor compound production process which comprises the following steps: (1) preparing cryoprecipitate from plasma, dissolving the cryoprecipitate and removing crude impurities; (2) product amino acid protection incubation; (3) S / D virus inactivation; (4) ion exchange chromatography: incubating a chromatographic filler by using a solution containing protective components before loading; (5) ultrafiltration preparation; and (6) sub-packaging, freeze-drying and dry-heating. According to the production process, only two steps of centrifugation, one step of chromatography and one step of ultrafiltration are needed from plasma to a finished product, the process steps are simple, and aluminum hydroxide, PEG and other allogenic substances which are not contained in a human body are not introduced; the purity of the product is effectively improved through amino acid protection in the incubation step and the chromatography step after cryoprecipitation dissolution and crude impurity removal.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Coagulation factor ⅸ quality control product preparation method
InactiveCN104181313BEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Production method of recombinant human blood coagulation factor XIIa
ActiveCN103374588AHigh activityHigh yieldVector-based foreign material introductionBlood coagulation/fibrinolysis factorsPichia pastorisYeast
The invention belongs to the field of genetic engineering and in particular relates to a production method of a recombinant human blood coagulation factor XIIa. The production method of the recombinant human blood coagulation factor XIIa comprises the following steps of: acquiring DNA (deoxyribonucleic acid) coding an active region of a human blood coagulation factor XIIa, amplifying by virtue of pichia pastoris, centrifuging and dialyzing a yeast culture solution and carrying out filter pressing by adopting a filter membrane on the yeast culture solution, and then carrying out column chromatographic purification by taking Ni-NTA (Ni-nitrilotriacetic acid) as medium, so that the recombinant human blood coagulation factor XIIa is obtained. By adopting the production method of the recombinant human blood coagulation factor XIIa, production cost of the human blood coagulation factor XIIa is reduced, and product activity is higher than that of blood coagulation factor XIIa extracted from blood.
Owner:VIVA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com