Method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma
A technology to remove cold gelatin plasma and human coagulation factors, which is applied in coagulation/fibrinolytic factors, chemical instruments and methods, animal/human proteins, etc. It can solve the problems of incomplete product safety, single virus inactivation method, and thrombin activation. and other problems, to achieve the effect of reducing the probability of activation, high plasma utilization rate, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
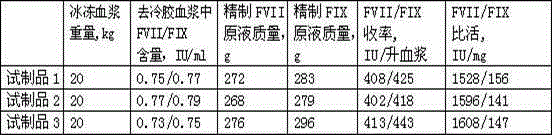
Examples
Embodiment 1
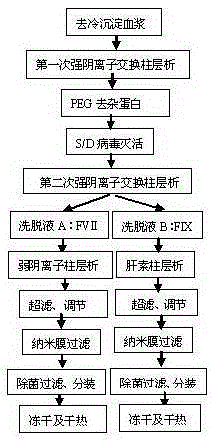
[0051] 1. Preparation of decolonized plasma: Take 35 bags of frozen plasma, sterilize with 70% ethanol and rinse with water for injection, cut the bag, melt the frozen plasma in a jacketed tank, weigh 20kg, and centrifuge continuously at 0-3°C , remove the cold glue, collect the supernatant, and filter it with a Supradur50P filter plate produced by Pall in series with a 0.45 μm filter element to obtain clarified cold glue-free plasma; before filtering, use buffer solution (PH6.90-7.10, 0.02M Sodium, 0.1M sodium chloride) pre-wash the filter element;
[0052] 2. The first strong anion exchange column chromatography: put the clarified cold gelatinized plasma obtained in step 1 on the QSepharose4FF column, and the column is pre-filled with equilibrium buffer (PH6.90-7.10, 0.02M sodium citrate, 0.1M sodium chloride ,) equilibration; after loading the column, wash the column with the above equilibration buffer, and then wash with elution buffer (PH6.90-7.10, 0.02M sodium citrate, 0...
Embodiment 2
[0065] 1, the preparation of cold gelatin-free plasma: the same as in Example 1;
[0066] 2. The first strong anion exchange column chromatography: Put the clarified cold gel plasma obtained in step 1 on the Capto-Q column, and the column is pre-filled with an equilibration buffer (PH7.30-7.40, 0.02M sodium citrate, 0.1M chloride NaCl,) equilibrate; after loading the column, wash the column with the above equilibration buffer, and then wash the column with elution buffer (PH7.30-7.40, 0.02M sodium citrate, 0.8M sodium chloride, 0.02M arginine hydrochloride ) to elute the column and collect the eluate (rich in coagulation FVII and FIX);
[0067] 3. PEG precipitation to remove impurities: add 50% PEG solution to the above eluent to make the final PEG concentration to 6%, stir for 0.5 hours, and then filter with a 1.0 μm filter element in series with 0.45 μm to obtain a clear filtrate;
[0068] 4, S / D virus inactivation: with embodiment one;
[0069] 5. The second strong anion ...
Embodiment 3
[0075] 1, the preparation of cold gelatin-free plasma: the same as in Example 1;
[0076] 2. The first strong anion exchange column chromatography: Put the clarified cold gel plasma obtained in step 1 on the Capto-Q column, and the column is pre-filled with equilibration buffer (PH6.50-6.60, 0.02M sodium citrate, 0.15M chloride NaCl,) equilibrate; after loading the column, wash the column with the above equilibration buffer, and then wash the column with elution buffer (PH7.30-7.40, 0.02M sodium citrate, 0.6M sodium chloride, 0.02M arginine hydrochloride ) to elute the column and collect the eluate (rich in coagulation FVII and FIX);
[0077] 3. PEG precipitation to remove impurities: add 50% PEG solution to the above eluent to make the final PEG concentration to 4%, stir for 1 hour, and then filter with a 1.0 μm filter element in series with 0.45 μm to obtain a clear filtrate;
[0078] 4, S / D virus inactivation: with embodiment one;
[0079] 5. The second strong anion colum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com