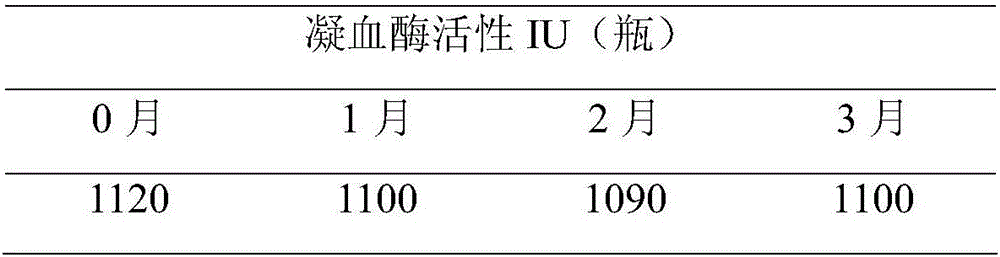
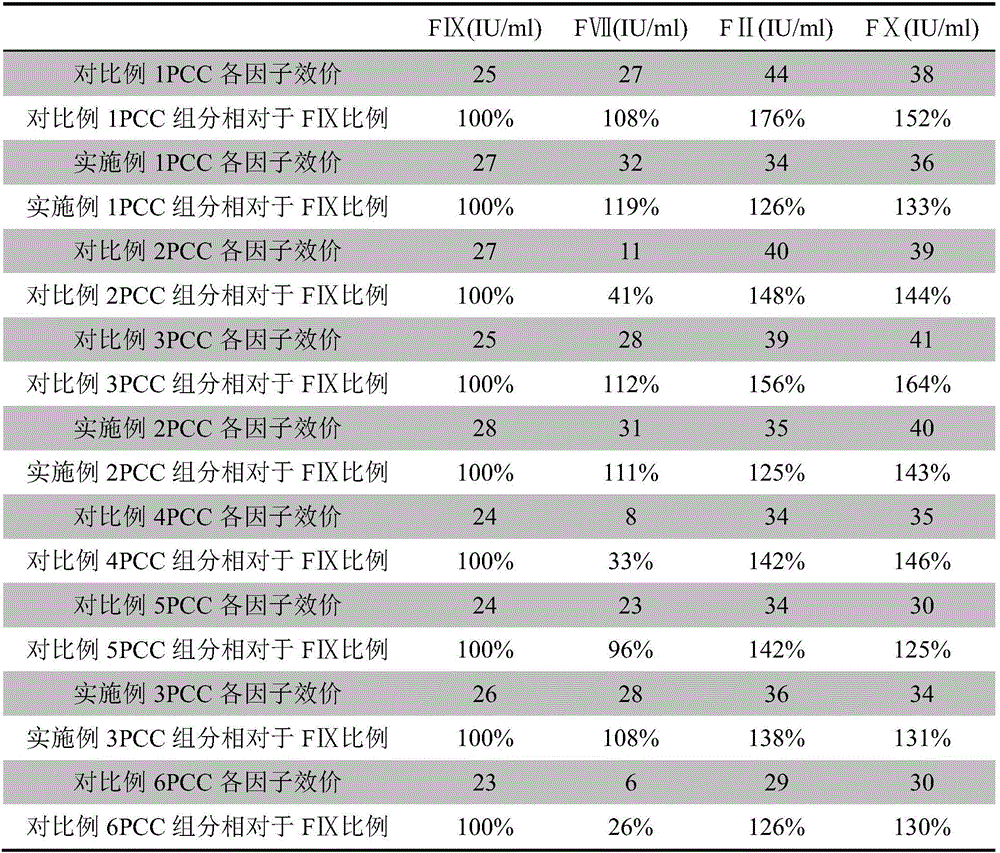
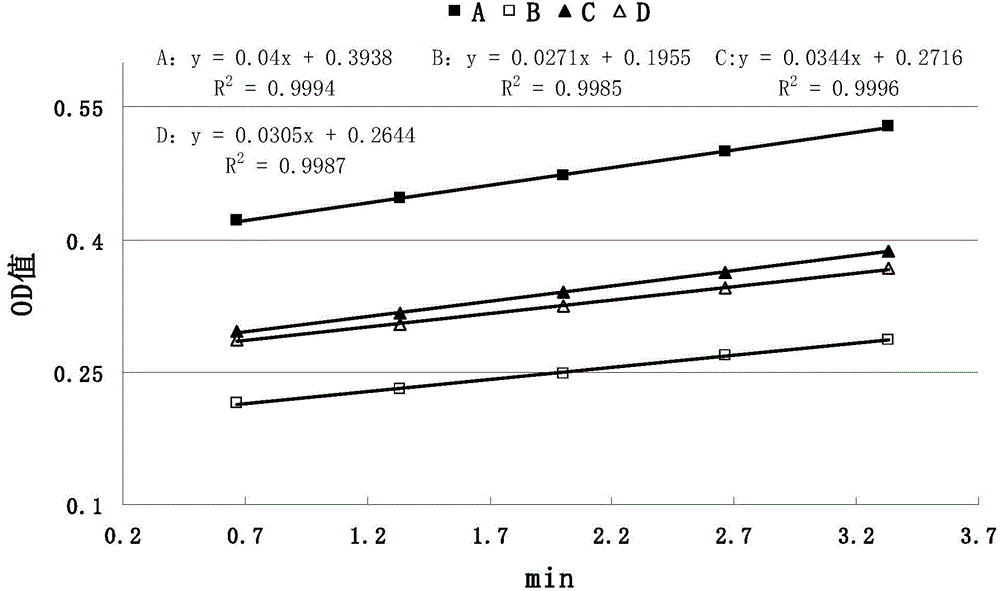
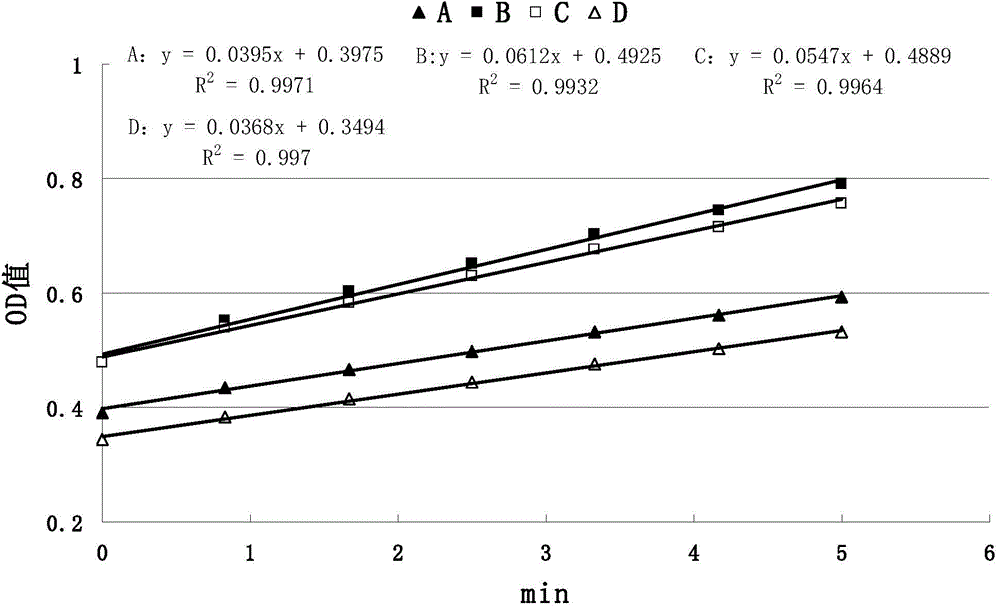
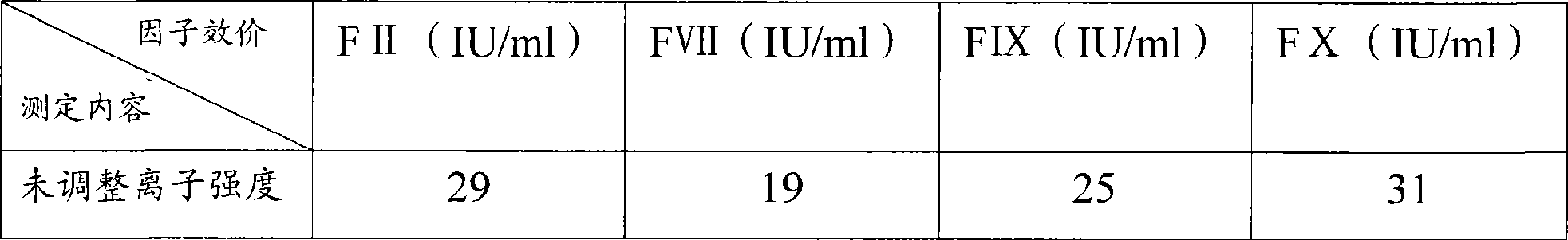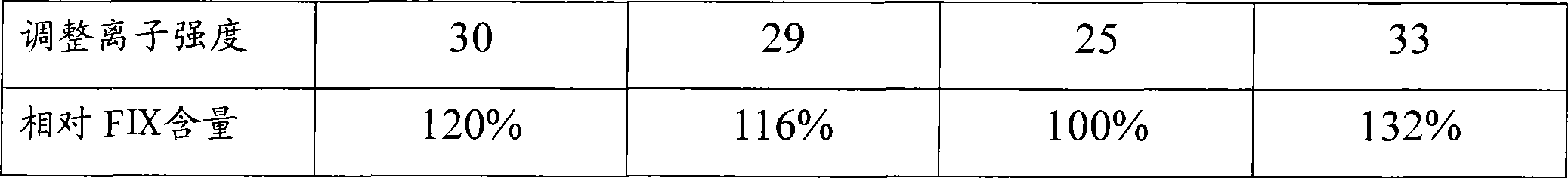Patents
Literature
47 results about "PROTHROMBIN COMPLEX" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
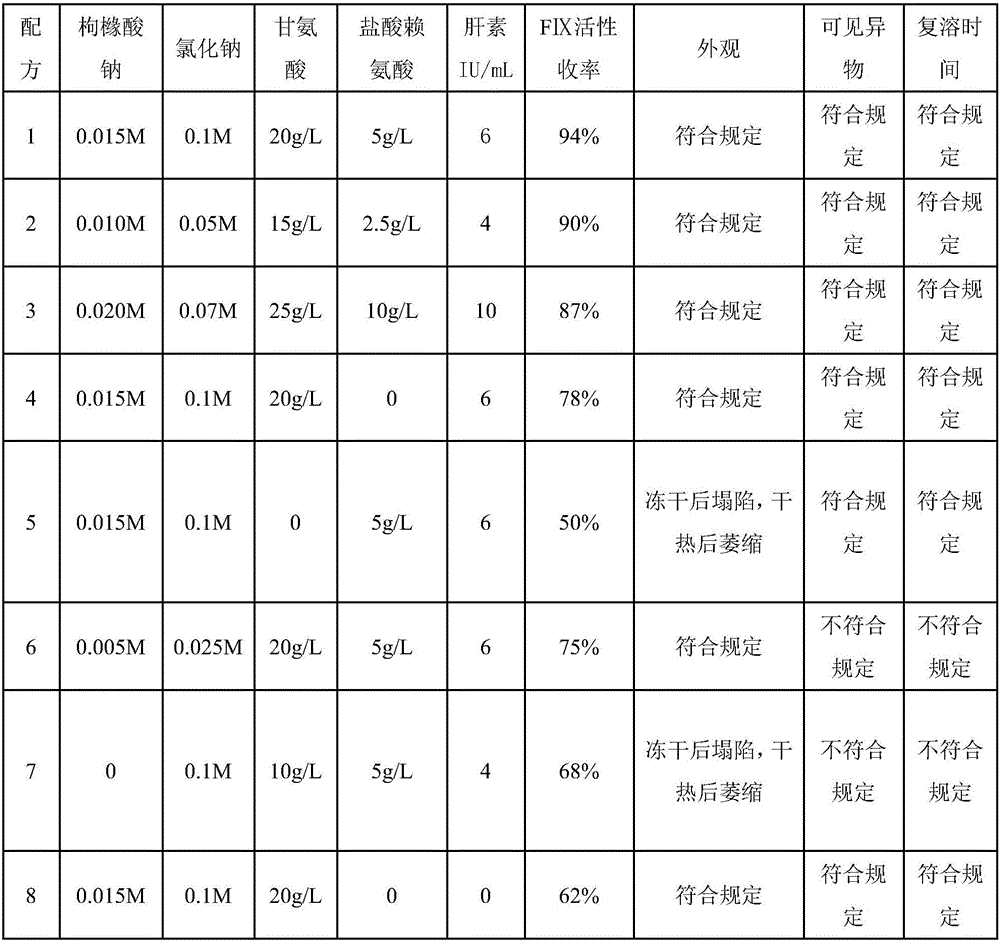
Prothrombin complex concentrate (PCC), also known as factor IX complex, is a medication made up of blood clotting factors II, IX, and X. Some versions also contain factor VII. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available.
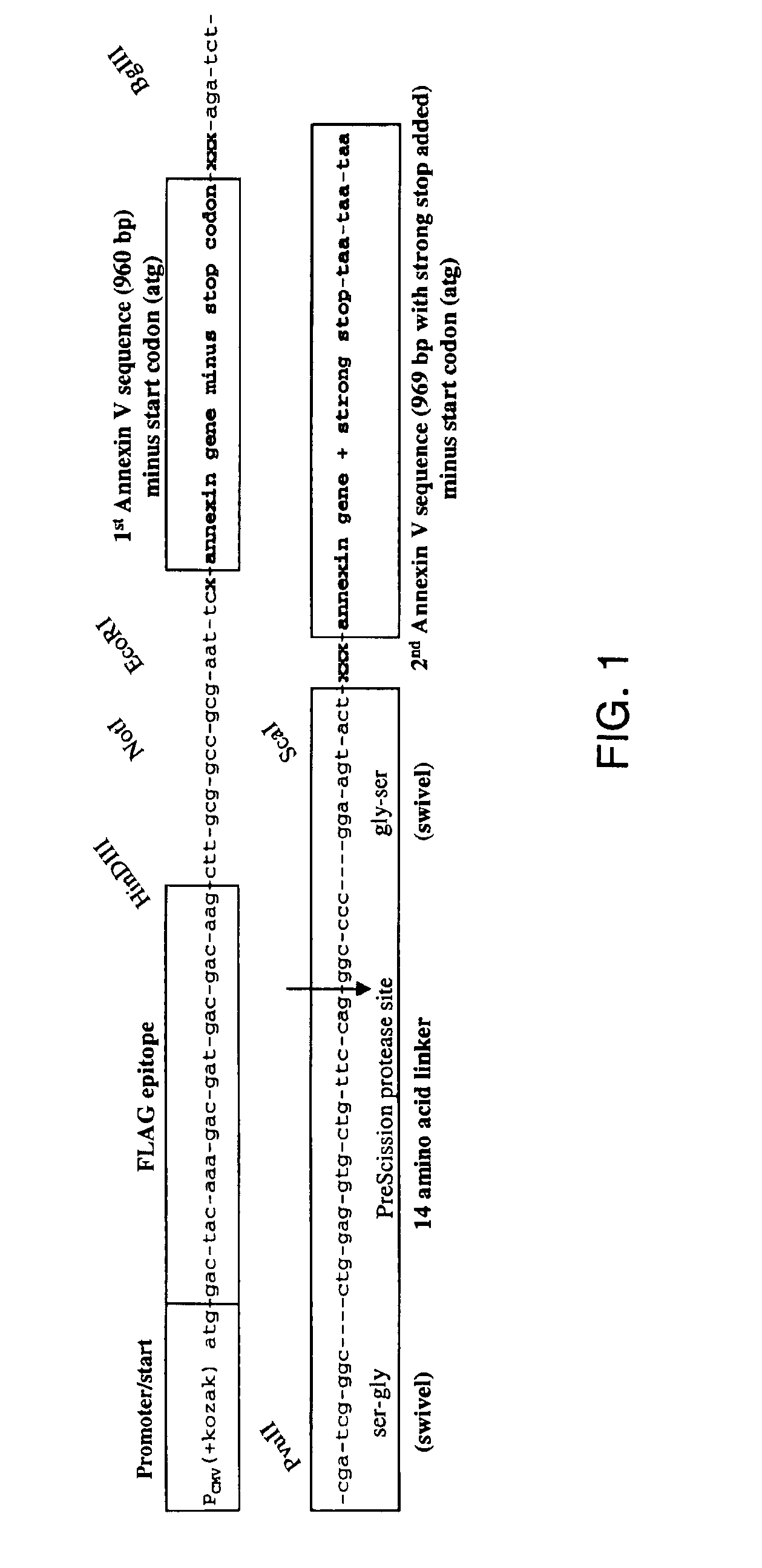
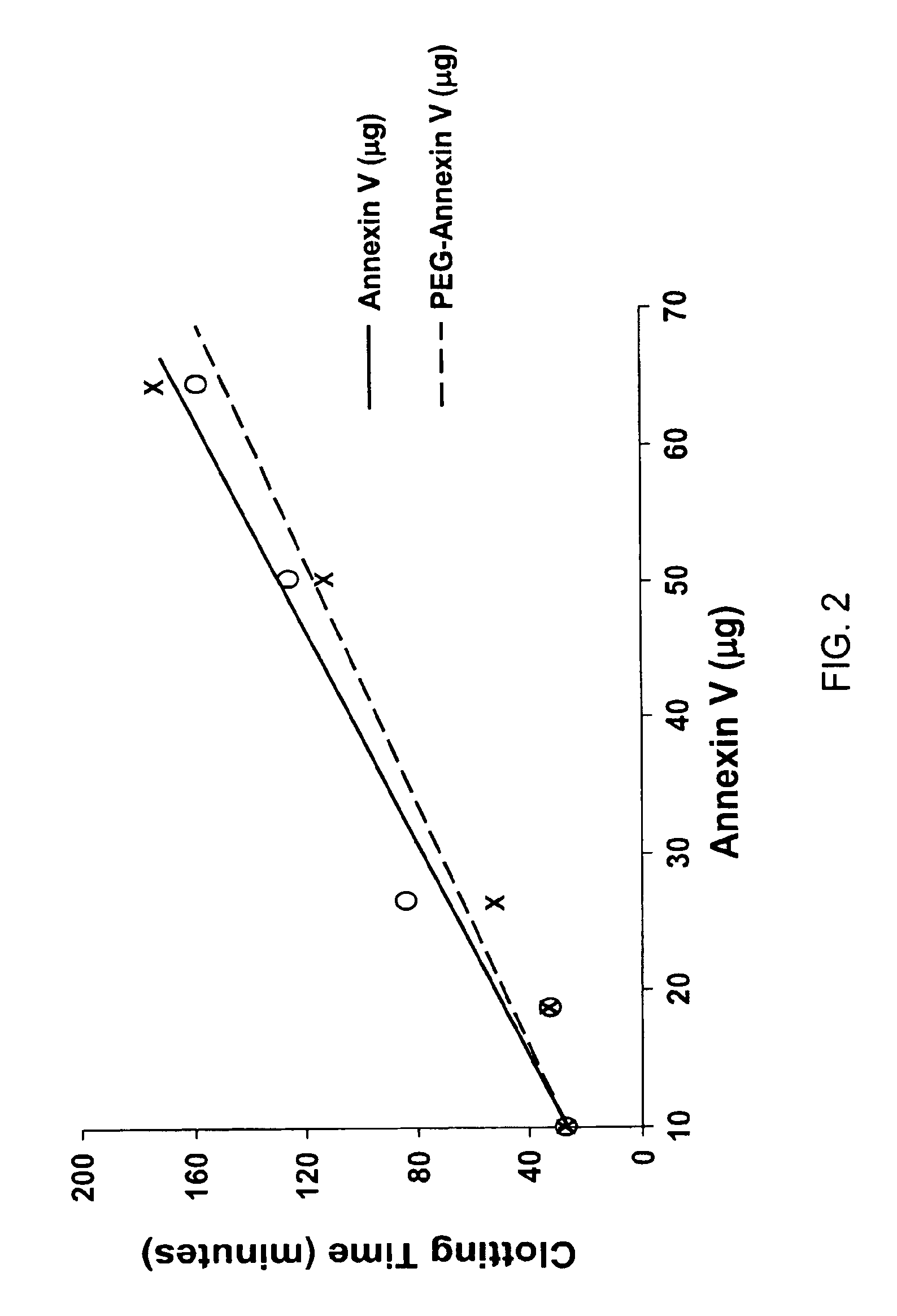
Modified annexin proteins and methods for preventing thrombosis
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:SURROMED
Method for adsorbing human prothrombin complex from plasma
ActiveCN104109202AHigh yieldHigh activityPeptide preparation methodsPeptidasesProthrombin complex concentrateCellulose
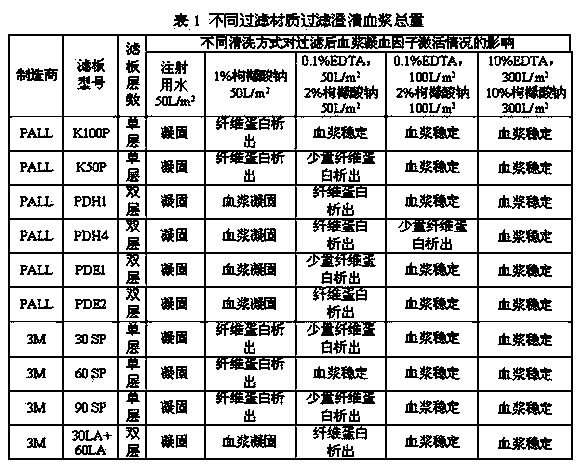
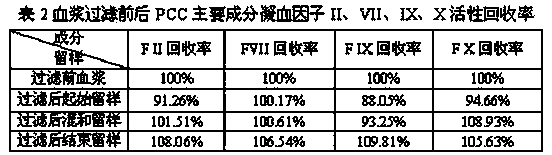
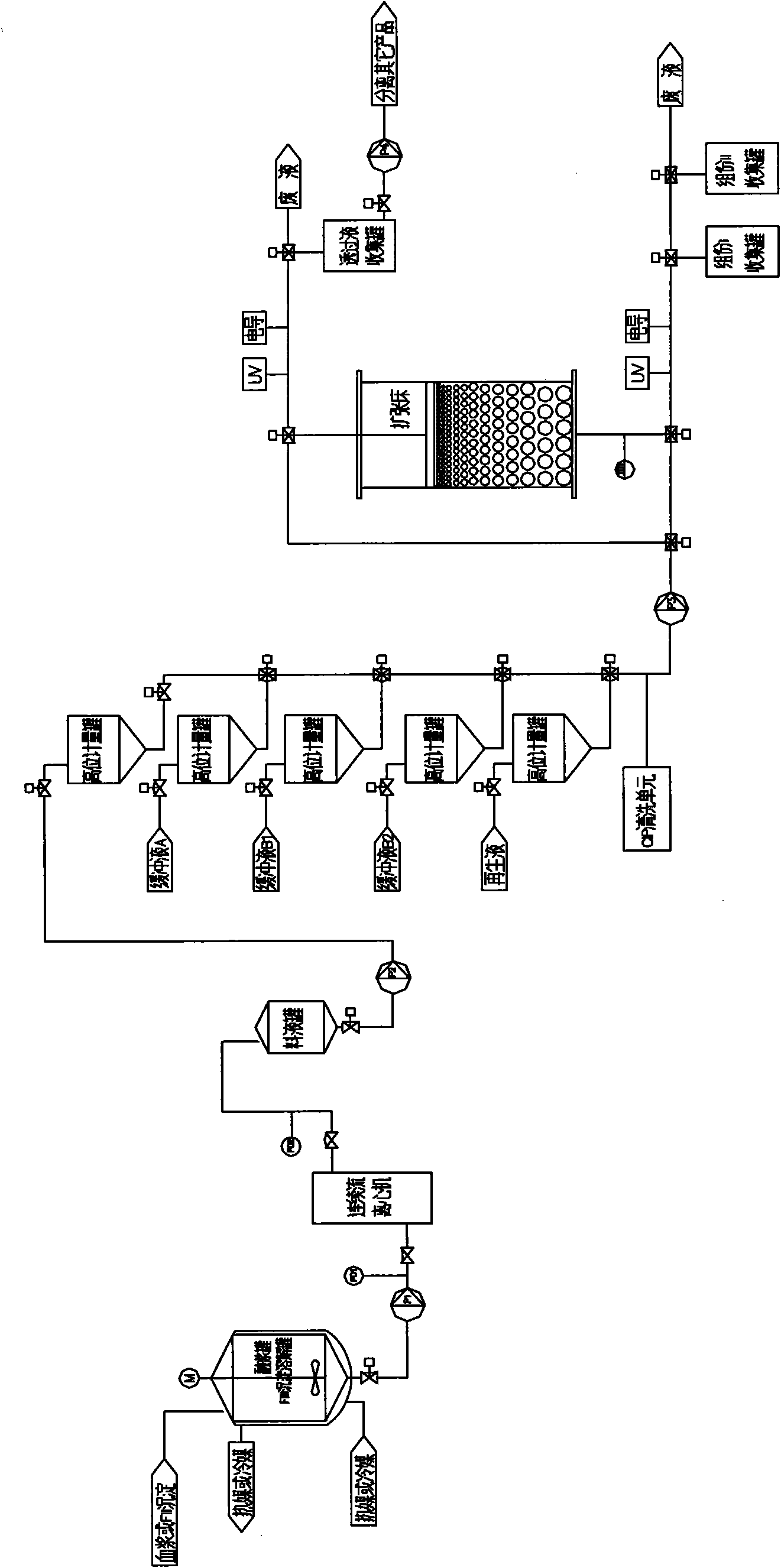
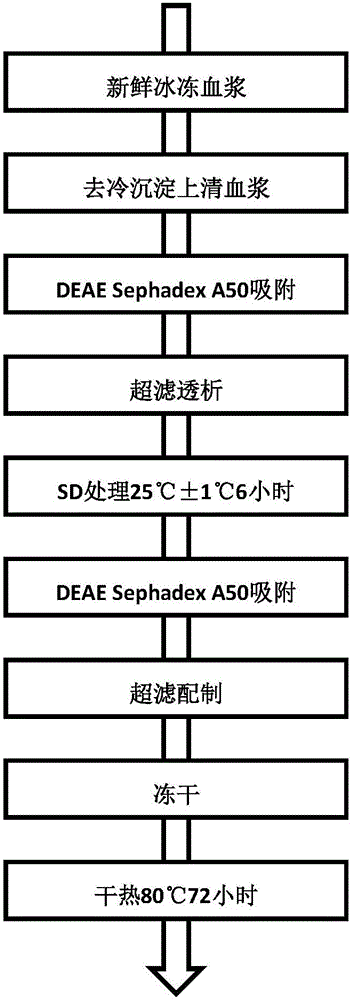
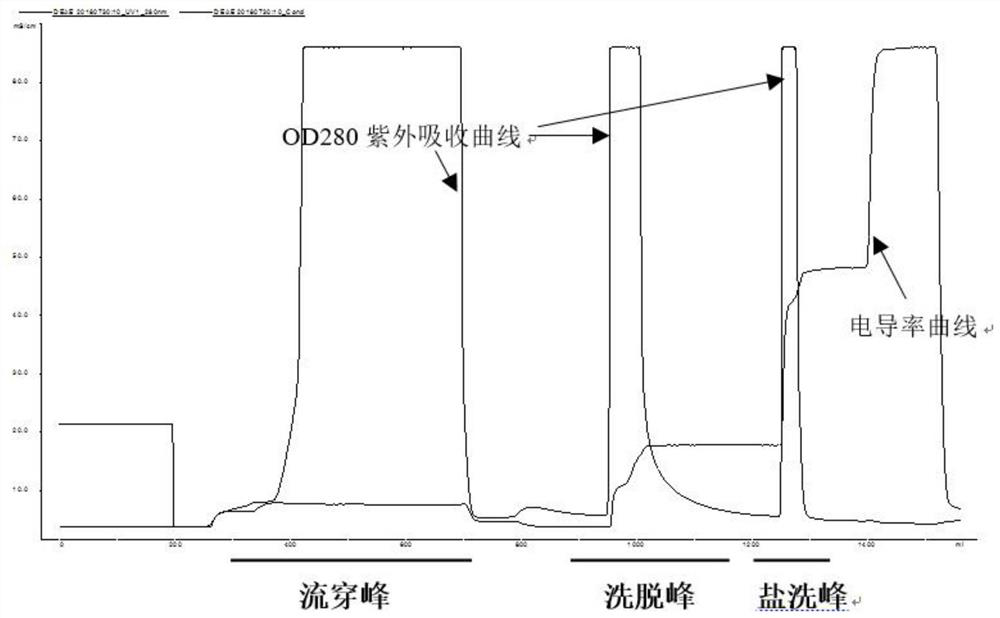
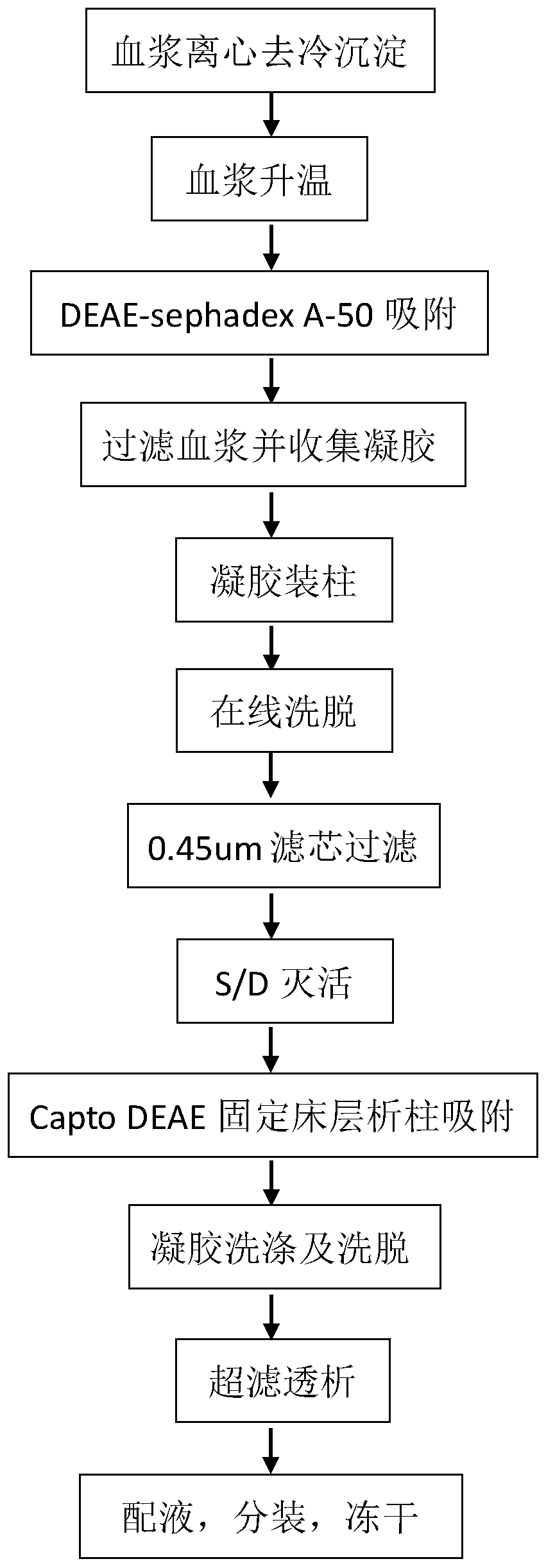
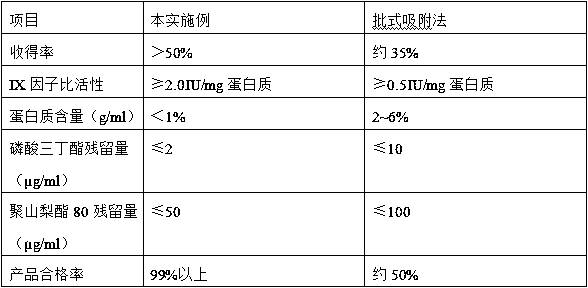
The invention relates to a production method for adsorbing a human complex from plasma by a fixed bed column chromatography technique, which comprises the following steps: (1) cryoprecipitation plasma removal: filtering by using a cellulose deep filter plate which is cleaned by an EDTA (ethylene diamine tetraacetic acid) solution and a sodium citrate solution; (2) filtering the plasma subjected to deep filtration through a 0.2 mu m filter element membrane while fixed bed loading; (3) balancing 2-5 column volumes in a fixed bed chromatographic column filled with anion exchange gel Capto DEAE by using a buffer solution A at the plasma loading flow rate of 60-120 cm / hour, washing the chromatographic column with a buffer solution B, and eluting the chromatographic column with a buffer solution C to obtain a PCC (prothrombin complex concentrate) product. When the calculation is based on coagulation factor IX, the yield of the PCC can reach 75-90%, and the specific activity can reach 5.5 IU / mg above.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Preparation process of human prothrombin compound
ActiveCN101974070AImprove securitySimplify production stepsPeptide preparation methodsPharmacyVirus inactivation
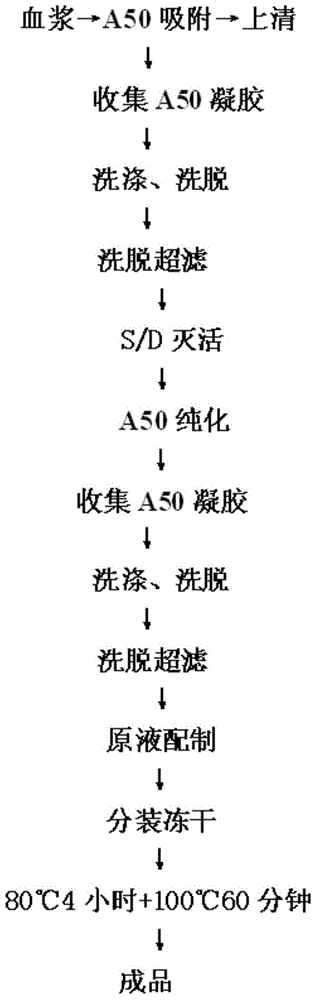
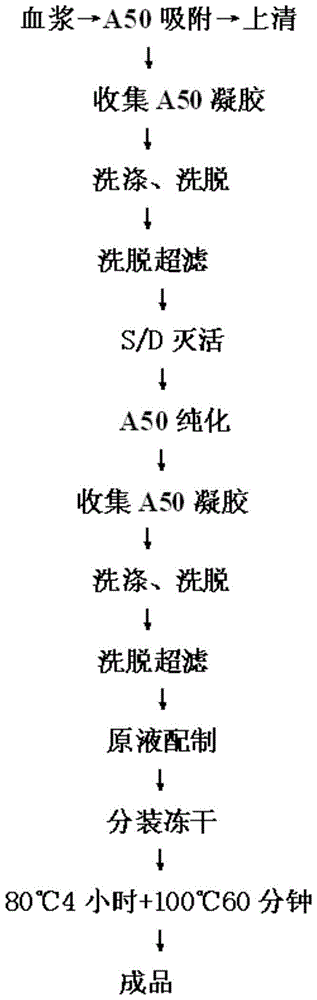
The invention relates to a preparation process of a human prothrombin compound, belonging to the field of biological pharmacy. The preparation process comprises the following steps of: absorbing blood plasma, washing, eluting and clarifying by filtration; inactivating viruses by using the S / D (Solvent / Detergent) method; purifying by absorption; subpackaging; freeze-drying; and inactivating viruses by the dry heat method. The human prothrombin compound is directly absorbed from blood plasma by using gel, only the gel chromatography technology is used in the entire extraction process, so that the production steps are simplified, the pollution of various factors on the production process of the product is reduced, meanwhile, the yield of the product is increased by 25-30%. In addition, the S / D method is used for removing lipid-enveloped viruses and the dry heat method is used for removing non lipid-enveloped viruses in the production process, and the safety of clinic medication is obviously increased through the two virus inactivation steps.
Owner:华润博雅生物制药集团股份有限公司
Method for producing human prothrombin complex
ActiveCN102151289AInhibition of activationHigh yieldPeptide/protein ingredientsMammal material medical ingredientsActivation methodVirus inactivation
The invention relates to a method for producing a human prothrombin complex. The method is characterized in that the following steps of direct separation and extraction from blood plasma, virus inactivation, refining and secondary virus inactivation are adopted to obtain finished human prothrombin complex. As the method adopts the step of direct separation and extraction from the blood plasma, the separation condition is mild, the batch-to-batch difference of the products is small, the blood coagulation factor activity is stable, the yield rate is high, and the activation phenomenon basicallydoes not exist. The virus inactivation process adopts a method of combining an S / D (organic solvent / detergent) method and a dry and thermal activation method and fully ensures that the virus safety of the human prothrombin complex.
Owner:哈尔滨派斯菲科生物制药有限公司
Production method of freeze dried human prothrombin compound
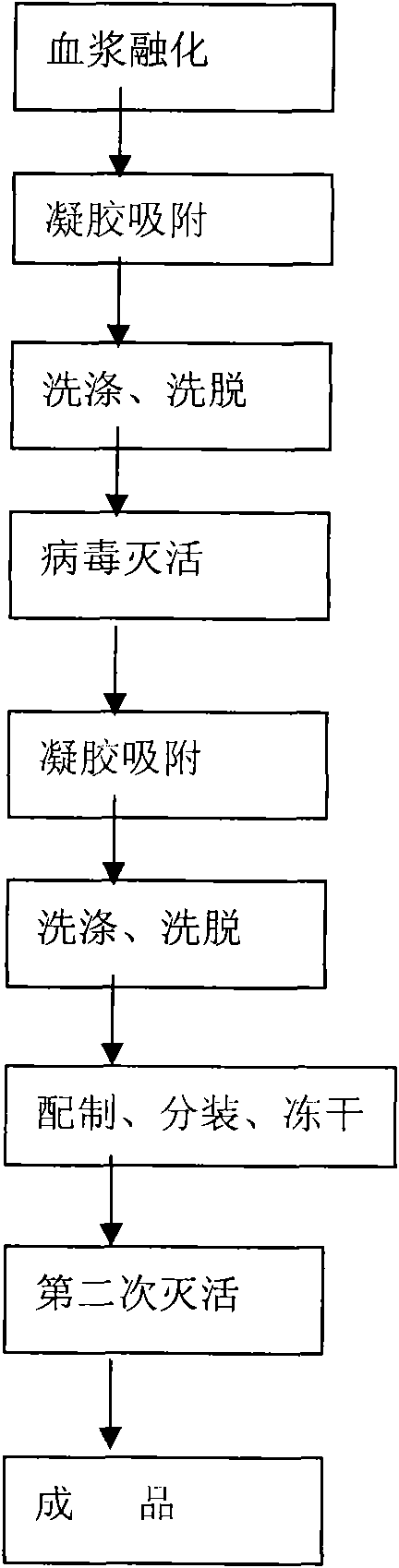
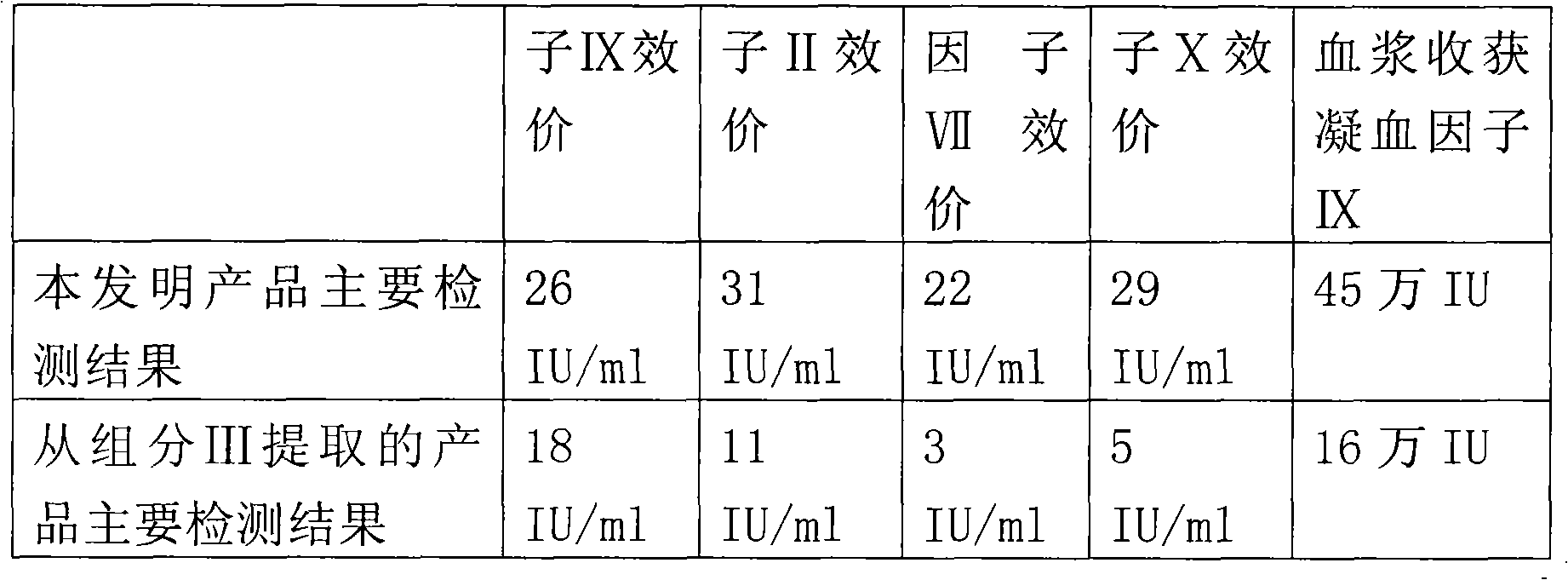
A process for preparing freeze dried human thrombinogen compound includes dissolving FIII deposit, low-temp centrifugal separation, filtering, gel adsorption, washing, eluting gel, superfiltering, concentrating, S / D deactivation of virus, secondary gel adsorption, washing, eluting gel, superfiltering, dialysis, removing bacteria by miniporous membrane, filtering and freeze drying. It features that said compound is deactivated in boiling water bath at 98-100 deg.C for 30 min. It has high content (2010 / ml) of factor IX and high specific activity of factor IC (>0.610 / ag).
Owner:HUALAN BIOLOGICAL ENG INC
Method for preparing human prothrombin complex from plasma
The invention discloses a method for preparing a human prothrombin complex from plasma. The method comprises: directly adsorbing the human prothrombin complex from the plasma by using DEAE A-50 gel, filling the adsorbed A-50 gel into a chromatographic column of a fixed bed, pumping eluant into the filled chromatographic column by using a peristaltic pump for online washing and elution, performing S / D inactivation on the eluant, and performing secondary chromatographic purification by using the chromatographic column of the fixed bed to obtain a high-purity human prothrombin complex product. According to the method, elution flow and speed can be accurately controlled by online elution after filling the gel into the column, the problems of contamination, cross contamination, gel leakage and the like caused by an open operation are reduced, an obtained product is high in purity, an IX factor titer can exceed 27IU / ml, and the IX factor specific activity exceeds 0.8IU / mg protein. Meanwhile, a self-flushing type filter with a pressure difference controller and an automatic solid matter stripping system is adopted, so the pressure difference in the whole filtering process is stable and controllable, gel particles can be well protected, broken colloidal particles flowing into a plasma tank in the filtering process are remarkably reduced, and gel losses in a production process can be reduced by above 20%.
Owner:广东双林生物制药有限公司
Technological process for improving stable FVII yield of human prothrombin complexes
ActiveCN101439047AImprove bindingHigh yieldPowder deliveryPeptide/protein ingredientsFiltrationUltrafiltration
The invention relates to the field of bio-pharmaceutical technology, in particular to a technical method for improving stable F VII yield rate of human thrombogen complex. The following procedures are included: freshly freezing blood plasma of a healthy person at a low temperature; removing cryoprecipitate; carrying out clarification filtration; adjusting ionic strength; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; killing S / D virus; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; carrying out aseptic filtration and subpackage; carrying out freeze-drying; and carrying out hot-air sterilization.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD +1
Method for absorbing and separating human prothrombin complex by utilizing expansion bed
InactiveCN101838304AAdsorption separation stabilityEfficient Adsorption SeparationPeptide preparation methodsContinuous flow centrifugationPROTHROMBIN COMPLEX
The invention relates to a method for absorbing and separating a human prothrombin complex by utilizing an expansion bed, comprising the following steps: (a) separating cryoprecitation from melted refrigerated plasma by using a refrigeration type continuous flow centrifuge, and collecting supernate as a raw material; (b) carrying out stable expansion on an expansion bed by using a buffer solution A, wherein the buffer solution A is 0.05mol / L1tris-citric acid with the PH value of 7.0; (c) rapidly switching into a feed solution after the expansion bed is in stable balance, stopping feeding and rapidly switching into the buffer solution A when breakthrough points reaches 5-10%, and washing in an expanded mode; and (d) switching into buffer solutions of 0.4mol / L NaCl and 1.6mol / L NaCl after washing, carrying out gradient elution in a stationary bed mode, and collecting the protein elution peak of the buffer solution of 1.6mol / L NaCl to obtain a prothrombin complex crude product.
Owner:成都英德生物工程有限公司
Preparation method of human thrombinogen compound
ActiveCN108048433AReduce open handling contaminationHigh purityPeptidasesWhole blood productFreeze-drying
The invention belongs to the technical fields of bio-pharmaceuticals and blood products, and particularly relates to a preparation method of a human thrombinogen compound. According to the human thrombinogen compound prepared by the method, an excellent production process is obtained by improving and researching adsorbing balance liquid, subsequent washing liquid and eluent and a freeze-drying process, four blood coagulation factors that the PCC product contain have high active ratio, the yield of each component is high, the IX specific activity is up to 3.13 IU / mg, the specific activity is higher than that of the pharmacopeia standard, the thrombosis probability is effectively reduced and activation of the blood coagulation factors is reduced.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
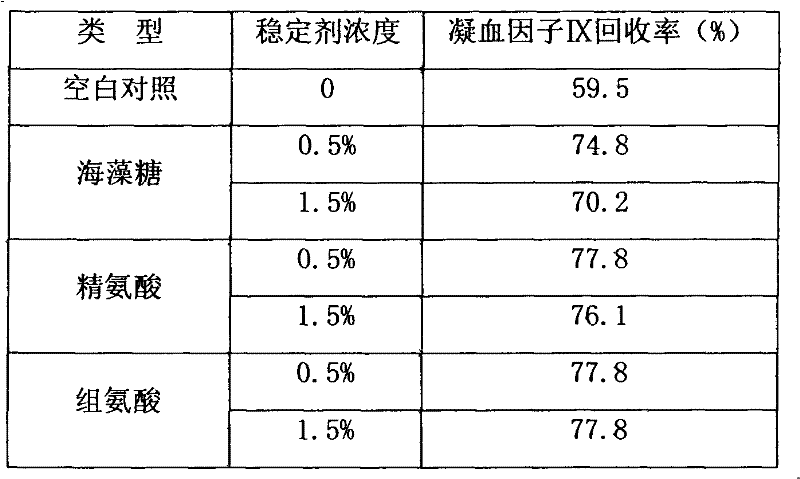
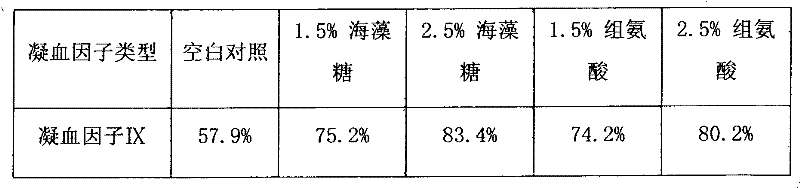
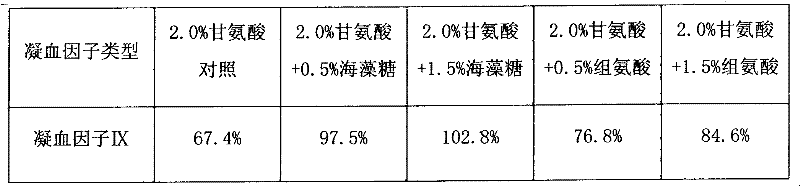
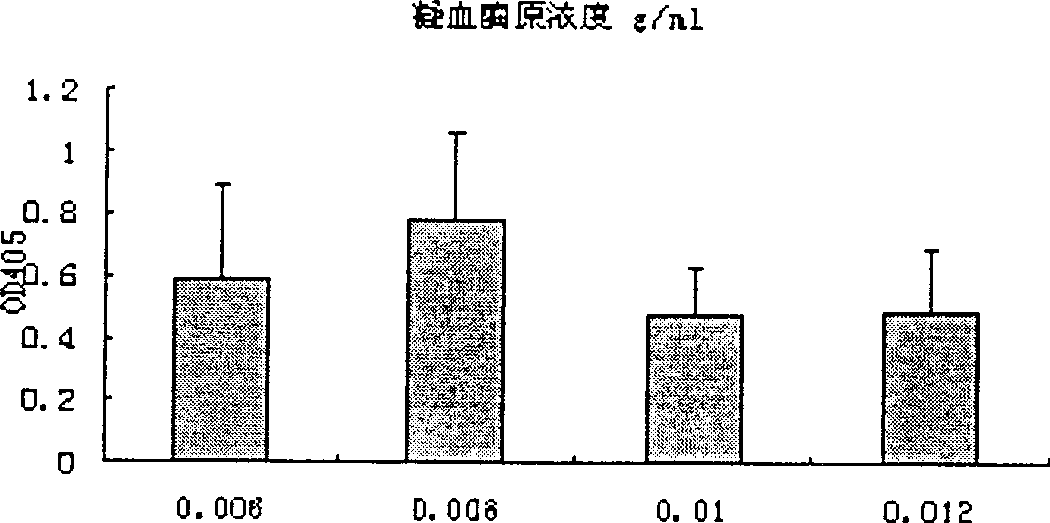
High-purity prothrombin complex product freeze-drying stabilizer
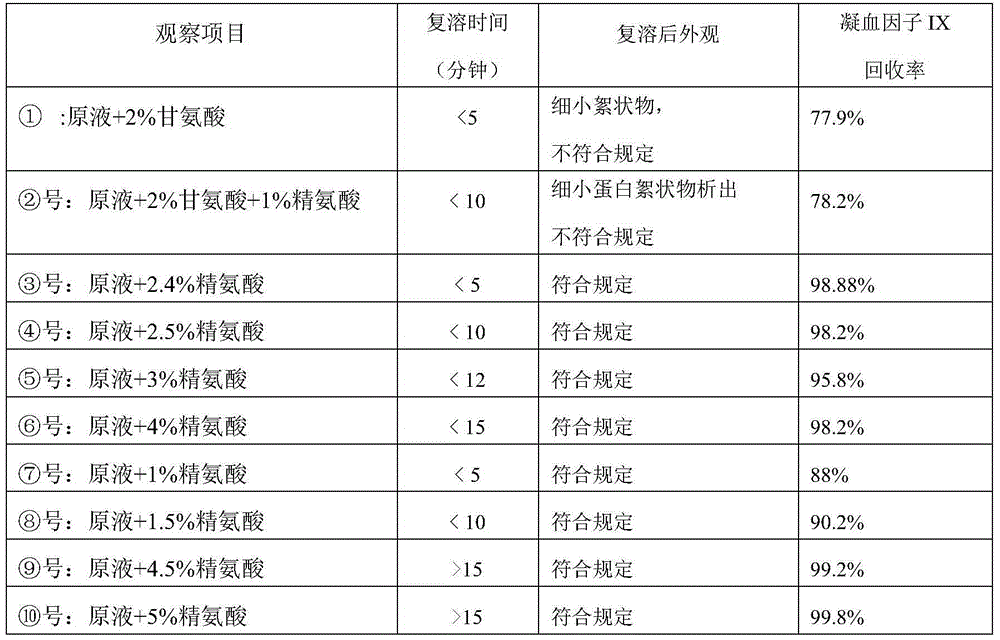
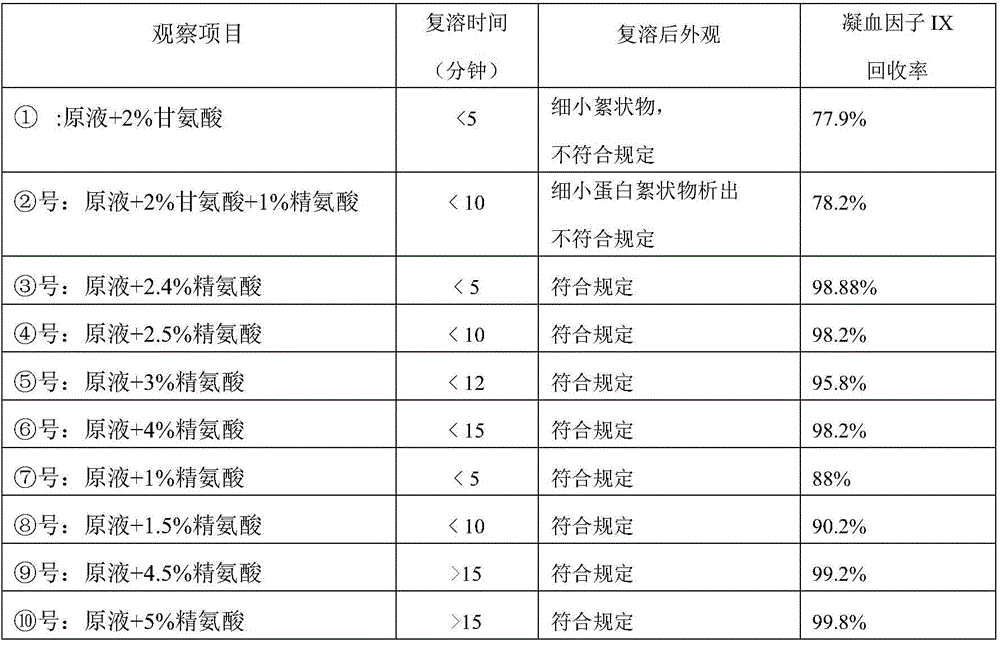
InactiveCN102441172AImprove protectionPeptide/protein ingredientsPharmaceutical non-active ingredientsZymogenArginine
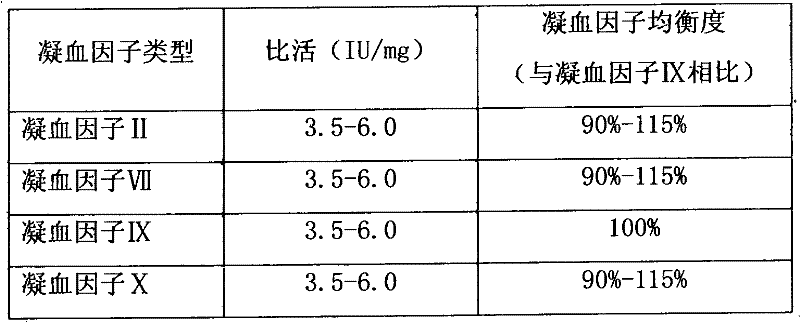
The invention relates to the field of medicinal biotechnology, and in particular to a high-purity prothrombin complex product stabilizer capable of effectively preventing the inactivation of a blood coagulation factor IX with a main effect and blood coagulation factors II, VII and X in a product in a freeze-drying process, wherein the specific activity of each of four blood coagulation factors II, VII, IX and X is greater than or equal to 3.5IU / mg protein. The stabilizer provided by the invention contains arginine or / and trehalose or / and histidine or / and glycine. The tests show that so long as the high-purity prothrombin complex contains 0.1-10% of arginine or / and 0.1-10% of trehalose or / and 0.1-10% of histidine, the activity of the blood coagulation factor IX and the activity of the blood coagulation factors II, VII and X can be effectively protected in the freeze-drying process. Thus, the stabilizer provided by the invention can be applied to the freeze-drying process of the high-purity prothrombin complex.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +3
Process for purifying prothrombin compound
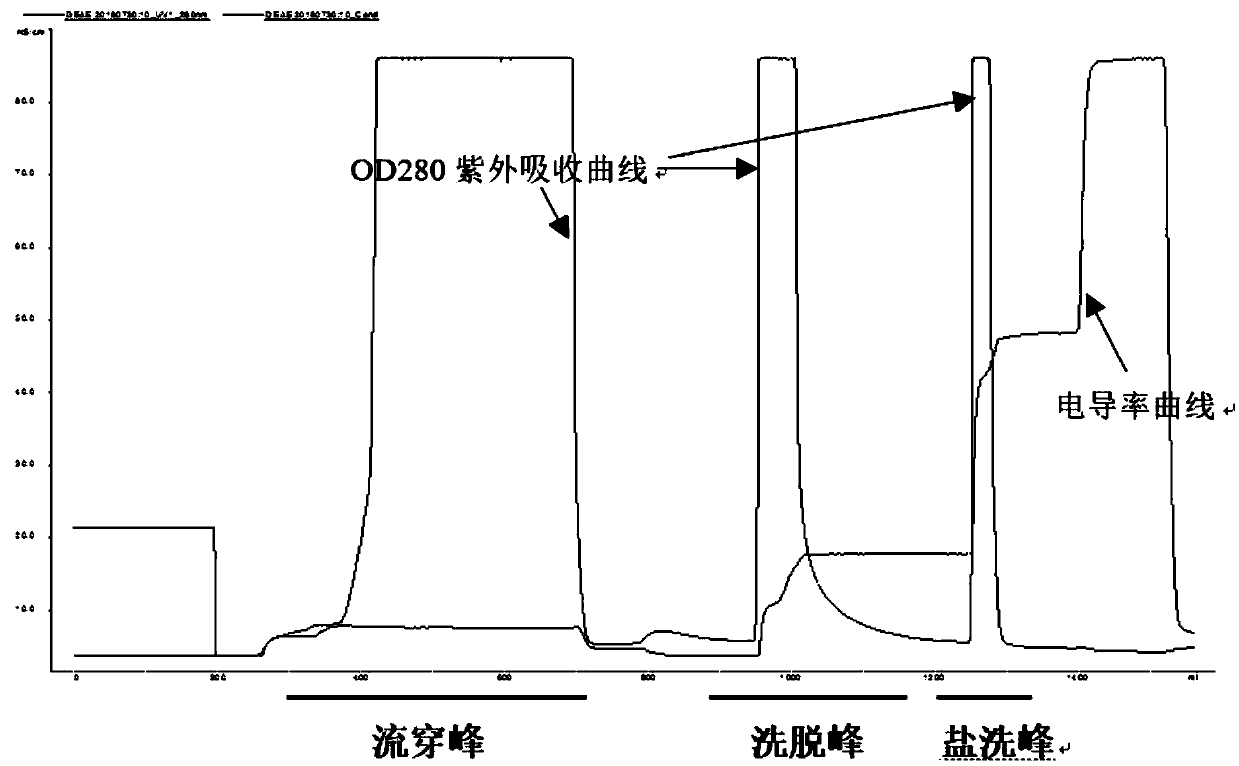
ActiveCN106497903AHigh purityImprove balanceFibrinogenPeptide preparation methodsDEAE SephadexCentrifugation
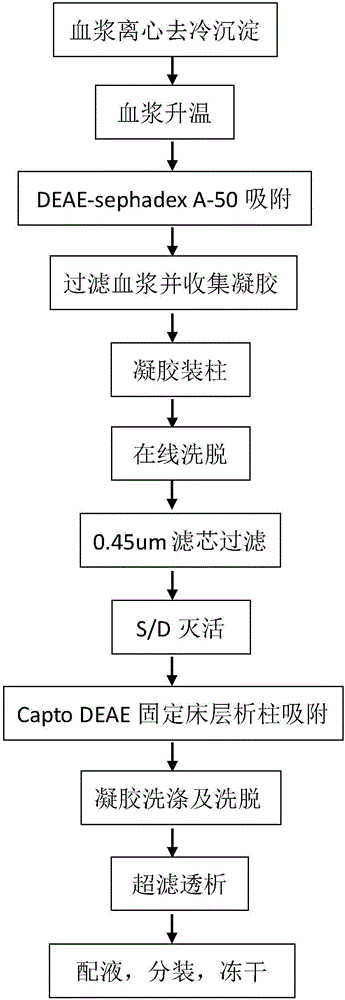
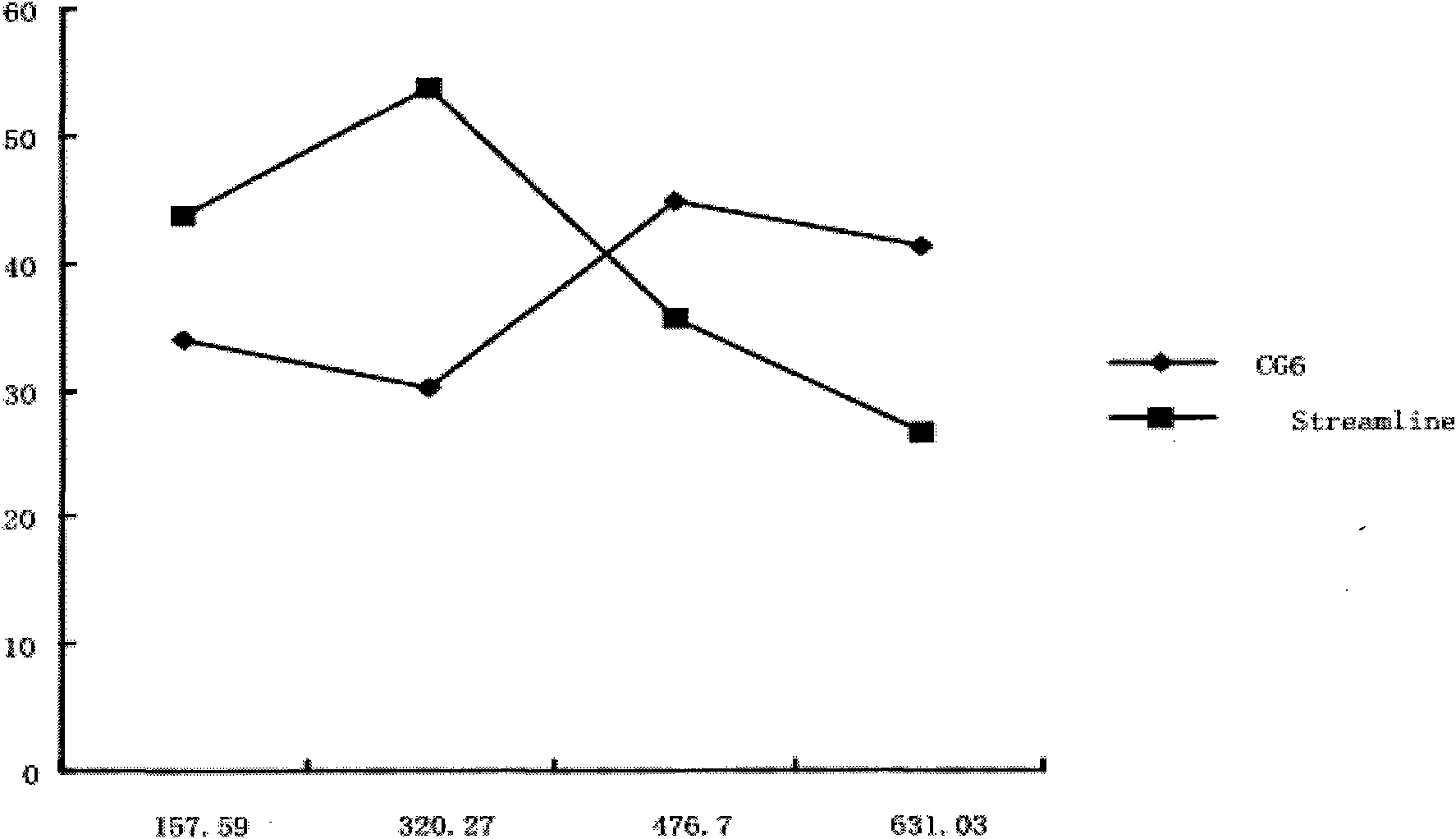
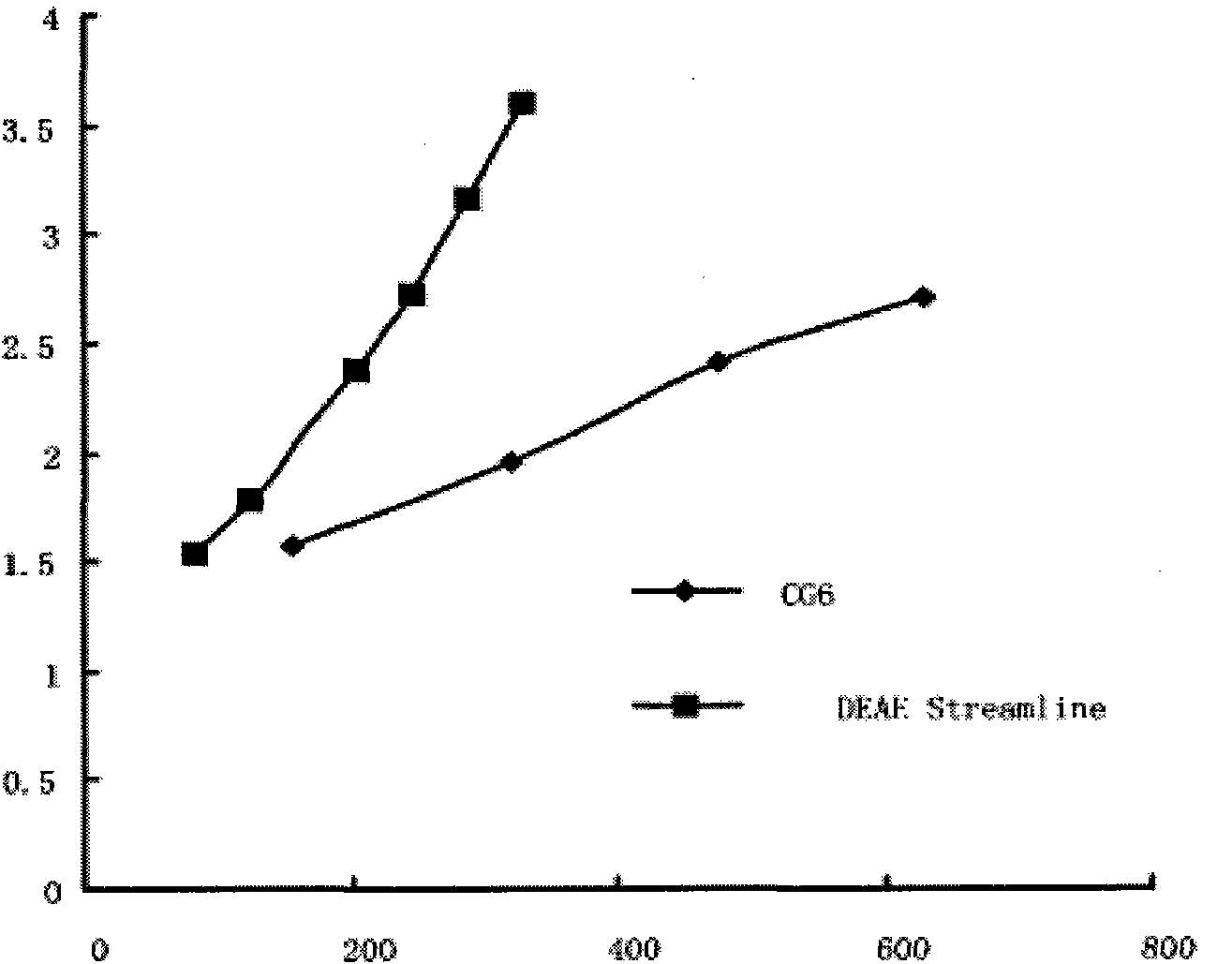
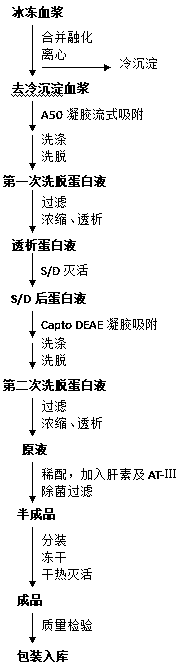
The invention provides a process for purifying a prothrombin compound. The process comprises the following steps: subjecting qualified detected plasma of a healthy person to centrifugation for removal of sediments so as to a raw plasma material; subjecting the raw plasma material to adsorption with DEAE-Sephadex A-50 gel and carrying out washing and elution so as to obtain crude extract of the prothrombin compound; and subjecting the crude extract of the prothrombin compound to further adsorption by using Capto DEAE gel column chromatography and carrying out washing and elution so as to obtain the liquid prothrombin compound. According to the invention, the prothrombin compound is prepared through two-step operations via the DEAE-Sephadex A-50 gel and Capto DEAE gel; and under the designed process conditions, the prepared prothrombin compound has a purity far higher than the purity of a prothrombin compound product prepared by using a traditional process, and blood coagulation factors II, IX and X are good in equalization.
Owner:DAAN PHARMA +1
Preparation method of human thrombin
The invention relates to the field of blood products and particularly relates to a preparation method of human thrombin. The invention provides a preparation method of human thrombin, which comprises the following steps: 1) performing redissolution and virus inactivation of a component III obtained by Cohn fraction of human plasma; and purifying the obtained liquid through anion exchange resin to obtain prothrombin complex eluent; 2) adding calcium ions into the eluent obtained in the step 1), and incubating until the prothrombin is effectively activated; 3) separating and purifying the liquid obtained in the step 2) through cation exchange resin; and 4) performing nano membrane filtration of the liquid obtained in the step 3) to obtain a finished product of human thrombin. In the preparation method provided by the invention, by adopting the thrombin product of the technology market, the human thrombin has high vigor, high yield and good long-term stability, meets the requirements on safety and effectiveness of clinical application and is of relatively obvious economic value and practical significance.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD
Method for increasing yield of F VII in human prothrombin compound
InactiveCN106085992AHigh purityFully elutedFibrinogenPeptidasesBlood Coagulation Factor VIIPROTHROMBIN COMPLEX
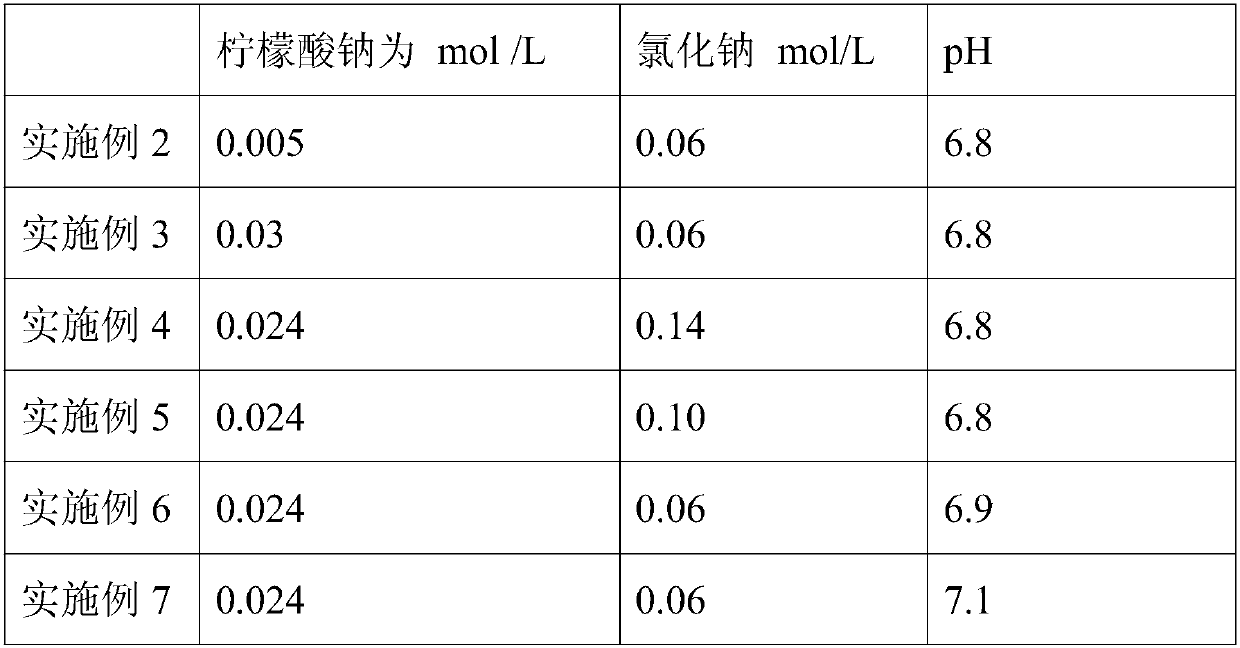
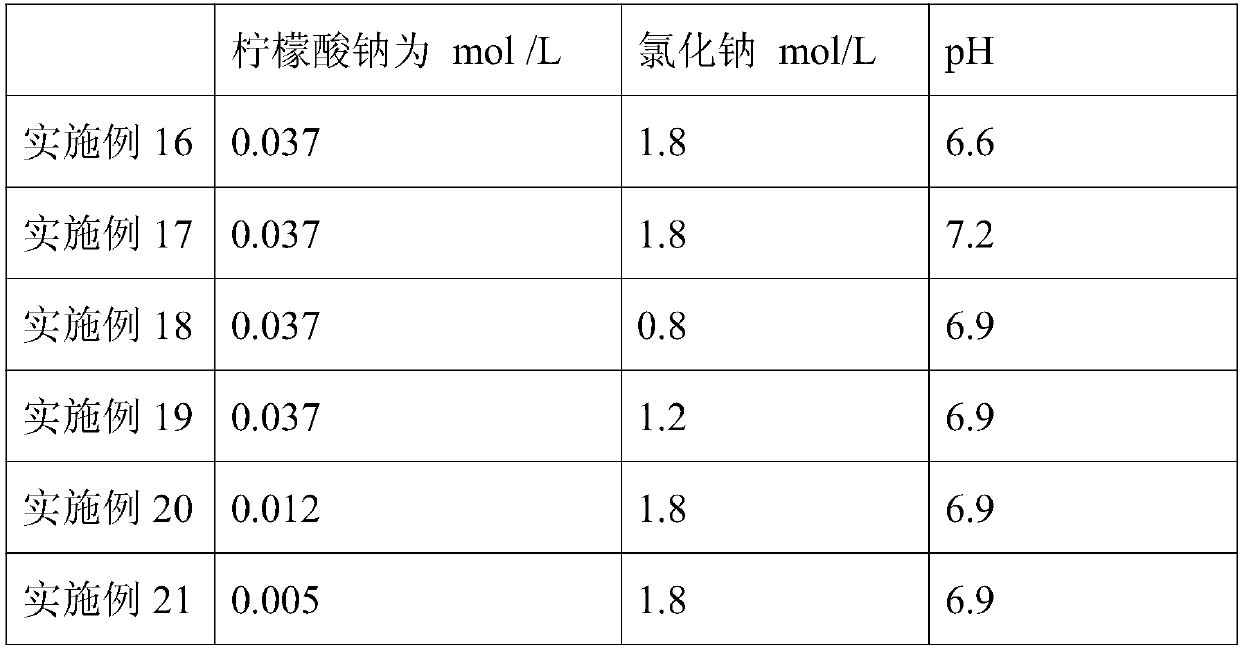
The invention discloses a method for extracting equilibrium liquid in a human prothrombin compound and extracting the human prothrombin compound. The equilibrium liquid for extracting the human prothrombin compound contains 0.10mol / L to 0.15mol / L of sodium chloride and 0.01mol / L to 0.02mol / L of sodium citrate, and the pH value of the equilibrium liquid is 6.8 to 7.2. By utilizing the equilibrium liquid, the human prothrombin compound can be effectively extracted, the yield and purity are relatively high, and particularly the yield of a blood coagulation factor VII (F VII) is relatively high. In addition, the stability of the obtained human prothrombin compound is relatively good.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
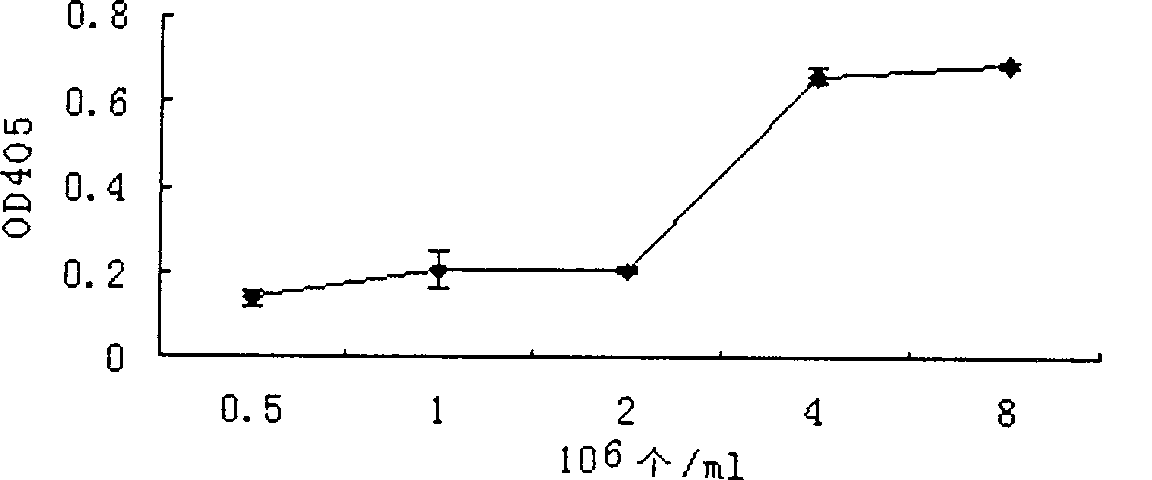
Method for detecting anticoagulant capacity of human prothrombin complex
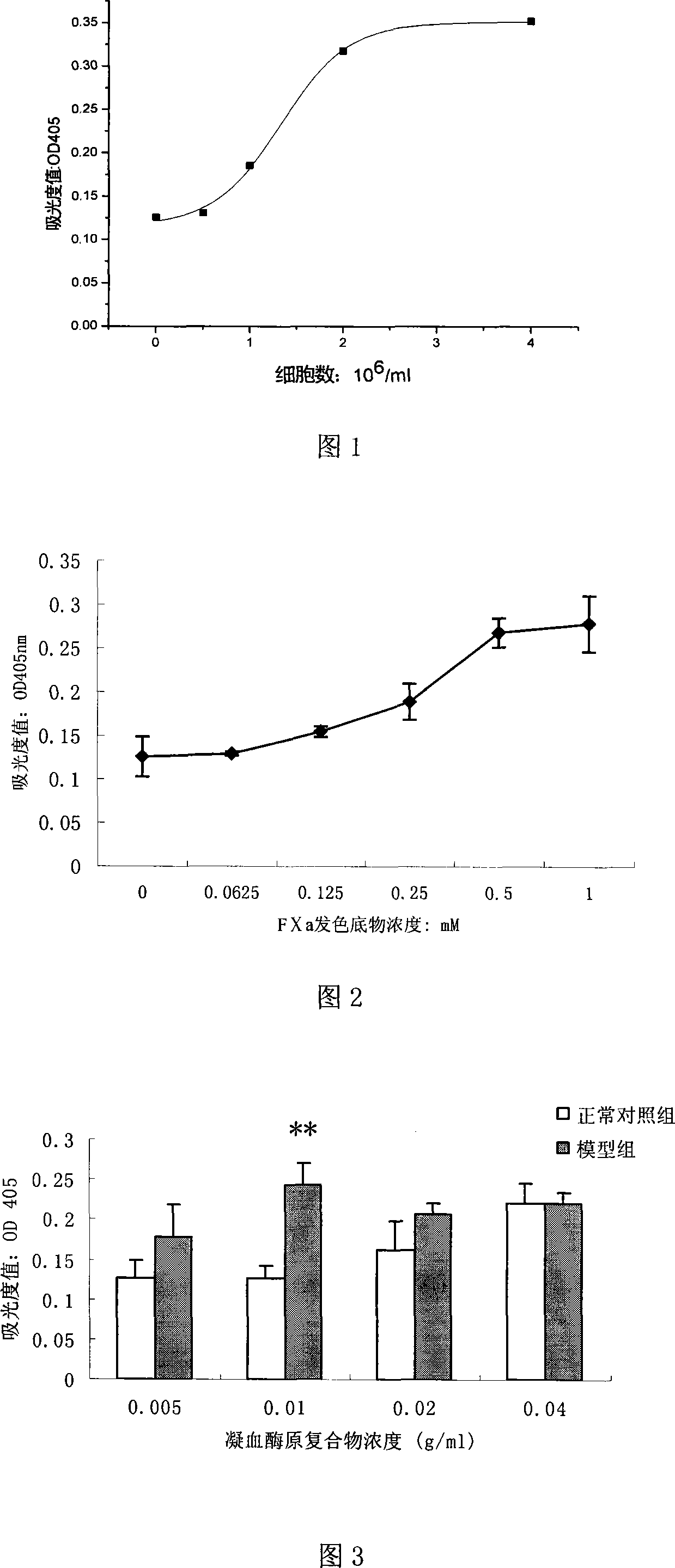
InactiveCN104459165AQuantitative determination of comprehensive anticoagulant capacitySimple and fast operationMaterial analysis by observing effect on chemical indicatorBiological testingWavelengthChemistry
The invention discloses a method for detecting anticoagulant capacity of a human prothrombin complex. The method comprises the following steps: (1) taking the human prothrombin complex, diluting until a blood coagulation factor IX is 0.8-1.3IU / ml, adding a human prothrombin solution with the isovolumetric concentration being 4-6IU / ml, mixing evenly, and standing at room temperature for 1-2 minutes; (2) adding a thrombin chromogenic substrate solution which is isovolumetric to the human prothrombin solution, mixing evenly, and incubating at 22-28 DEG C for 4-6 minutes, wherein the concentration of the chromogenic substrate solution is 0.5-0.7mg / ml; (3) determining a light absorption value at the wavelength of 405nm, and determining once 30-50 seconds for 5-8 times in all; and (4) drawing the time changing trend along with the light absorption value by taking the determined light absorption value as a longitudinal coordinate (y) and time (s) as a cross coordinate (x), building a linear equation y=kx+a, wherein k is slope, and calculating the value of 1 / k. The method for detecting the anticoagulant capacity of the human prothrombin complex is simple and convenient to operate and good in repeatability; the comprehensive anticoagulant ability of the PCCs product can be quantitatively detected; and the safety of the PCCs product can be evaluated.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
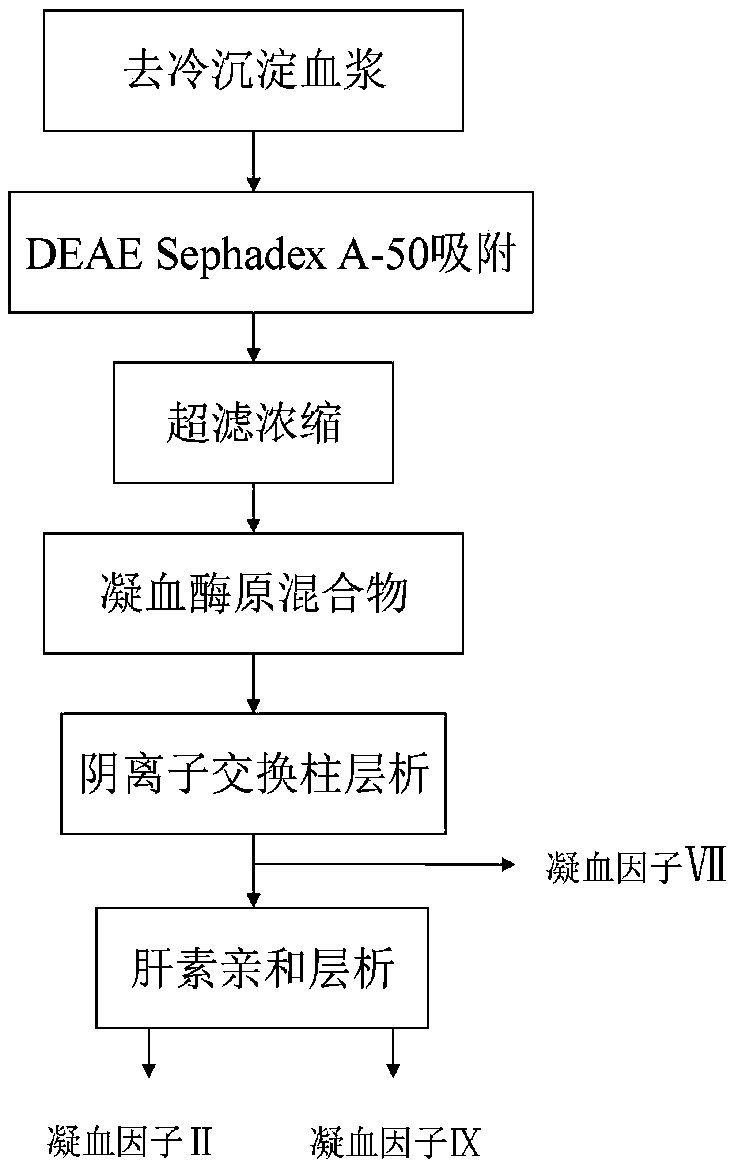
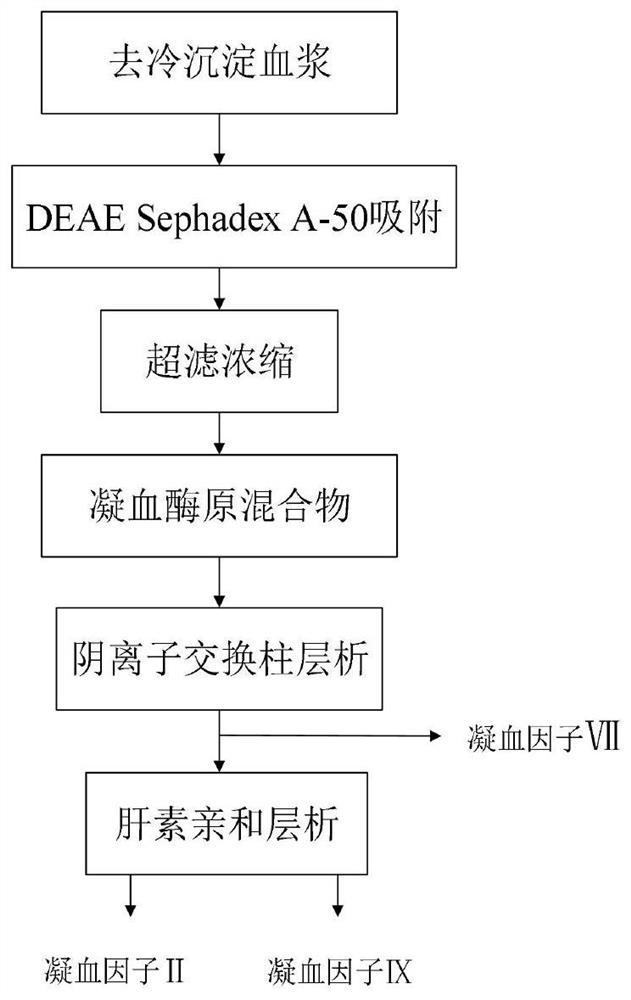
Method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma
ActiveCN109651502AAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
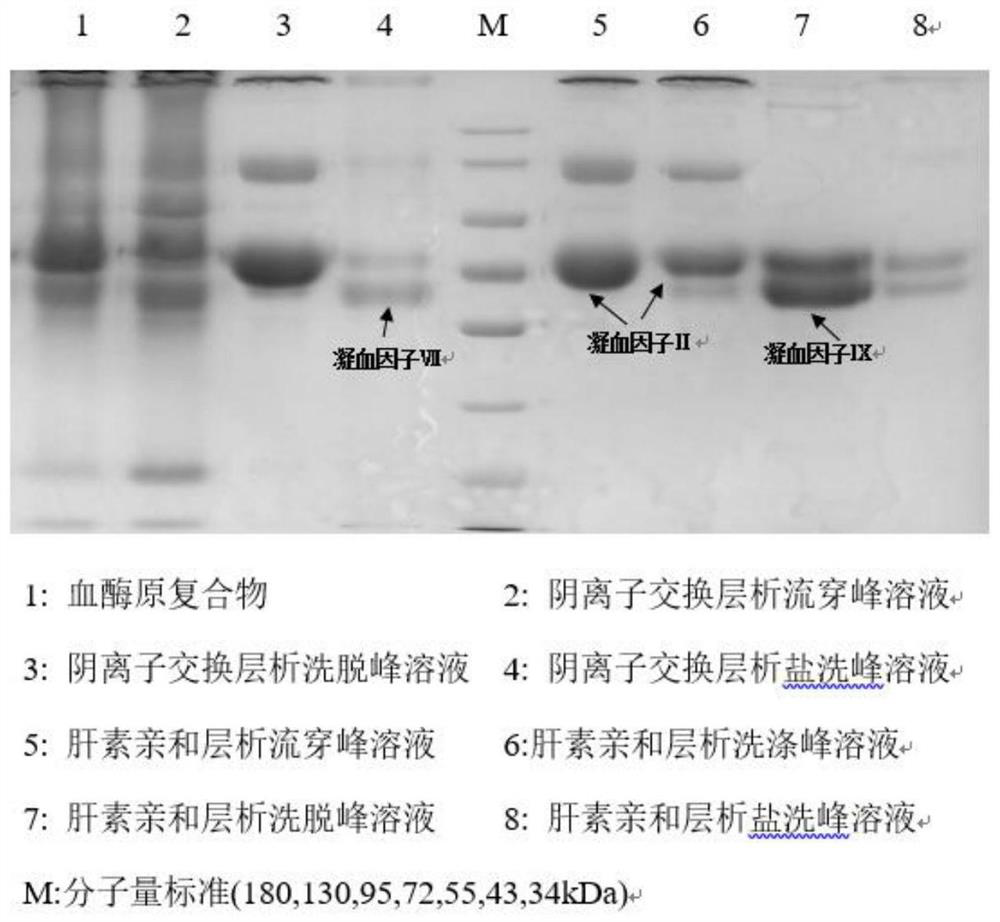
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Protective agent in process for performing dry heat virus inactivation on high-purity prothrombin complex concentrate products
InactiveCN102416171AHigh activity yieldPeptide/protein ingredientsPharmaceutical non-active ingredientsProthrombin complex concentrateZymogen
The invention relates to the field of medicinal biotechnologies, and discloses a protective agent which can effectively prevent a mainly acting blood coagulation factor IX and other blood coagulation factors II, VII and X in high-purity prothrombin complex concentrate products (the specific activities of the blood coagulation factors II, VII, IX, and X are more than and equal to 3.5IU / mg protein) from being inactivated in the process of performing dry heat virus inactivation at the temperature of 100 DEG C for 30 minutes. The protective agent is trehalose or / and histidine and also contains two or three of common glycine, sodium citrate and NaCl. Experiments prove that if only high-purity prothrombin complex concentrate products contain 0.1 to 8 percent of trehalose or / and 0.1 to 8 percent of histidine, the activities of the blood coagulation factors IX, II, VII and X can be effectively protected in the process of performing dry heat virus inactivation at the temperature of 100 DEG C for 30 minutes. Therefore, the invention can be used as the protective agent in the process for performing dry heat virus inactivation on the high-purity prothrombin complex concentrate products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +3
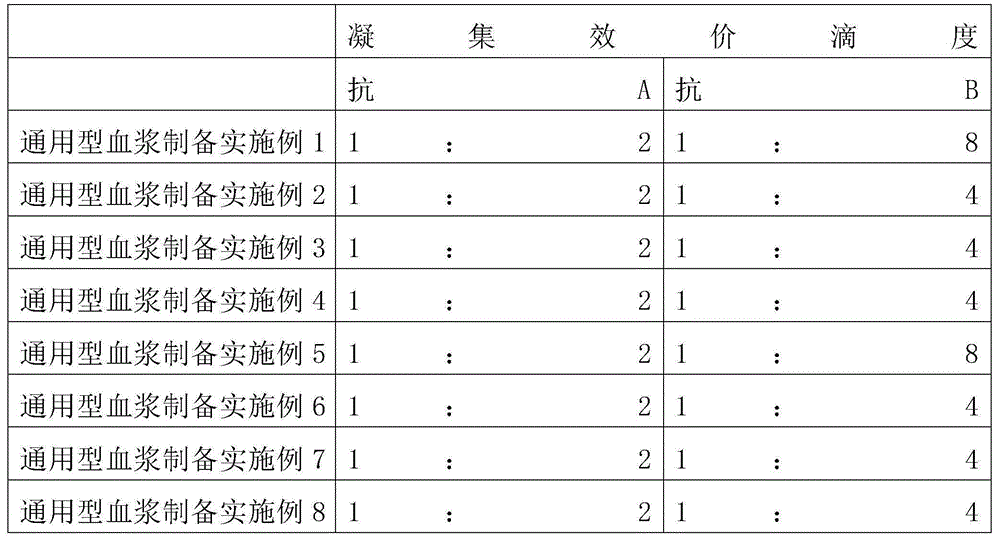
Human plasma with multiple functions and preparation method thereof
InactiveCN105832768ASave transfusion timeIncreased flexibility of usePeptide/protein ingredientsMammal material medical ingredientsPatient needBlood Coagulation Factor X
The invention provides a human plasma with multiple functions and a preparation method thereof, including general-purpose plasma and blood component additives; the general-purpose plasma is general-type fresh frozen plasma or general-purpose freeze-dried plasma; the blood component additive is selected from albumin, C Globulins, platelets, fibrinogen, factor II, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, and prothrombin complex any one or more of them. The advantages are: for patients who need to supplement plasma and at least one blood component additive at the same time, only the plasma including the corresponding blood component additive needs to be transfused, thereby increasing the scope and flexibility of use, saving the patient's blood transfusion time, and saving Blood transfusion equipment.
Owner:杜祖英
Anti-tumor metastasis medicament high flux screening model
InactiveCN101376907AHigh sensitivitySimple and fast operationMicrobiological testing/measurementMaterial analysis by optical meansAbnormal tissue growthHigh-Throughput Screening Methods
The invention belongs to the field of medicine and discloses an anti-tumor transfer medicine high throughput screening model. The screening method of the model comprises the following steps: an MDA-MB-435 cell is inoculated to a culture plate; a chemical compound to be screened is added into culture fluid and no inhibitor is added in; the chemical compound is cultured for an appropriate time of period and repeatedly melted, to obtain cell lysis solution; the cell lysis solution is added into human thrombinogen compound solution to be incubated together; a chromogenic substrate is added in; at 405nm, absorbency is determined and inhibitory rate is calculated; the chemical compound with the inhibitory rate higher than 20 percent is a positive compound. In the invention, the human breast cancer cell MDA-MB-435 is selected to build the anti-tumor transfer medicine screening model which is quick and stable, has high sensitivity and is convenient to be operated; the model is characterized by trace quantity and accuracy, and is applicable in screening anti-tumor transfer medicine with high throughput.
Owner:CHINA PHARM UNIV
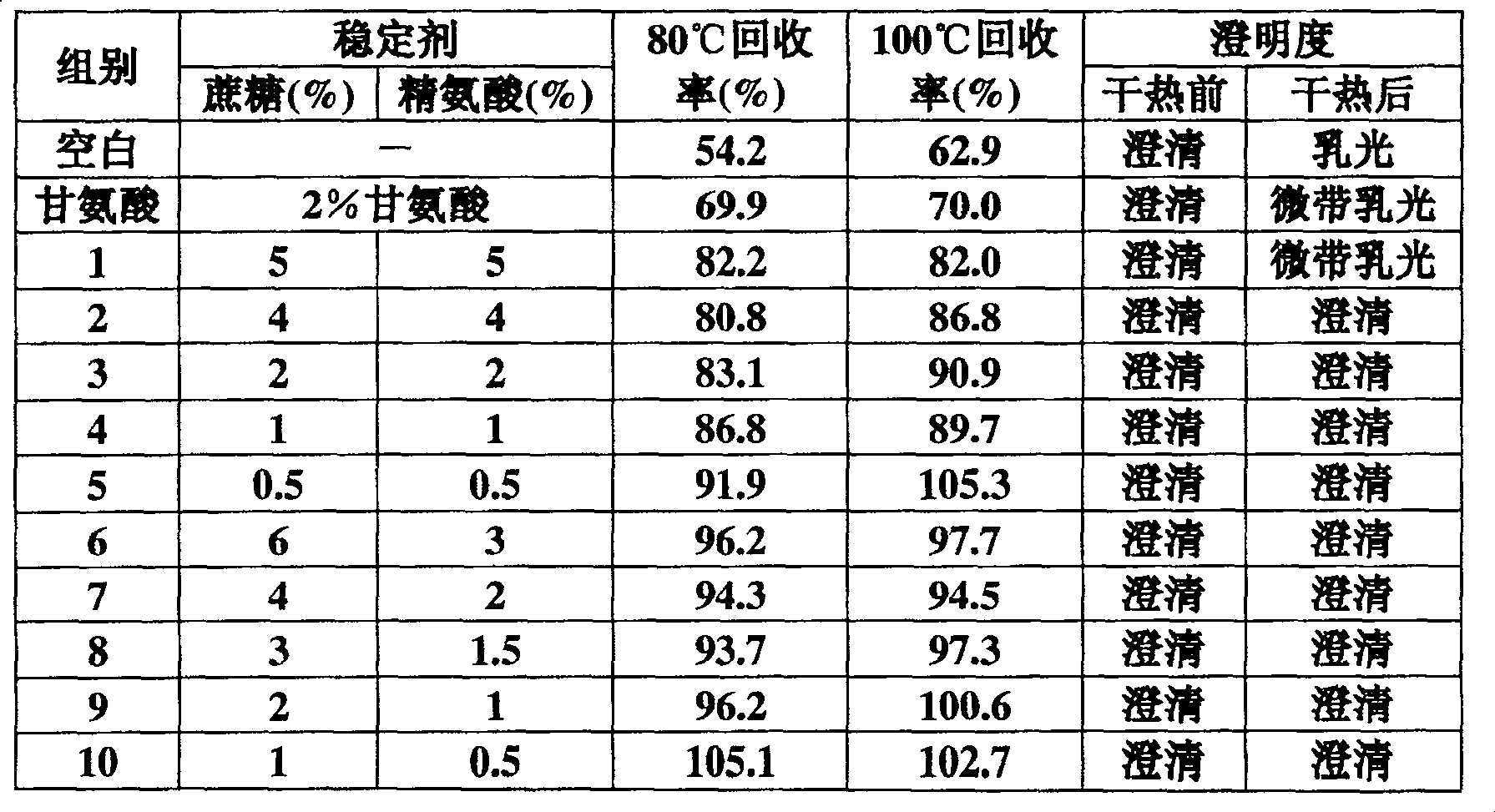
Dry heat processing stabilizer for prothrombin complex or factor v a IX preparation
InactiveCN100482272CLittle loss of activityAvoid damagePeptide/protein ingredientsPharmaceutical non-active ingredientsZymogenArginine
The invention relates to a stabilizing agent for preventing blood clotting factor reactive loss for prothrombin composite or blood clotting factor IX preparation during virus animatum eradication by dry heat, wherein the stabilizing agent is sucrose or / and arginine or its salt, it also can contain one or more of the conventional glycine, NaCl, citric acid trisodium and hamocura.
Owner:SHANGHAI XINXING MEDICINE
Method for screening micropore plate of tissue factor approach restrainer and uses thereof
InactiveCN101201314AReduce dosageLow costMicrobiological testing/measurementMaterial analysis by optical meansFreeze thawingScreening method
The invention relates to the field of medicament, in particular to an in-vitro microplate-based screening method of tissue factor (TF) pathway inhibitors, and is characterized in that the invention comprises the following steps: incubating cells and the medicament to be measured together; getting cell lysis solution after freeze thaw, blowing and beating; putting the cell lysis solution on the microplate for incubation; adding human prothrombin complex solution for incubation; adding chromogenic substrate solution in the reactant liquor for incubation; measuring the value of absorbance with a micro-plate reader of 405nm. The invention also discloses some tissue factor pathway inhibitors screened out by using the method, such as hydroxygenkwanin, ruscogenin, quercetinic acid, ginsenoside Rb1, ginsenoside or diosgenin and so on.
Owner:CHINA PHARM UNIV
Preparation process of human prothrombin compound
ActiveCN101974070BImprove securitySimplify production stepsPeptide preparation methodsVirus inactivationFiltration
Owner:华润博雅生物制药集团股份有限公司
Dry-heat treatment stabilizer for preparing human prothrombin complex and application of dry-heat treatment stabilizer
ActiveCN105879038AEffectively maintain activitySimple recipePeptide/protein ingredientsInorganic non-active ingredientsVirus inactivationMedicine
The invention discloses a dry-heat treatment stabilizer for a human prothrombin complex, and further provides application of the dry-heat treatment stabilizer and the human prothrombin complex prepared through the dry-heat treatment stabilizer. The dry-heat treatment stabilizer can not only effectively keep the activity of the human prothrombin complex but also ensure the virus inactivation effect of dry-heat treatment, is simple in formula and low in preparation cost and has the good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
A device for adsorption and separation of human prothrombin complex
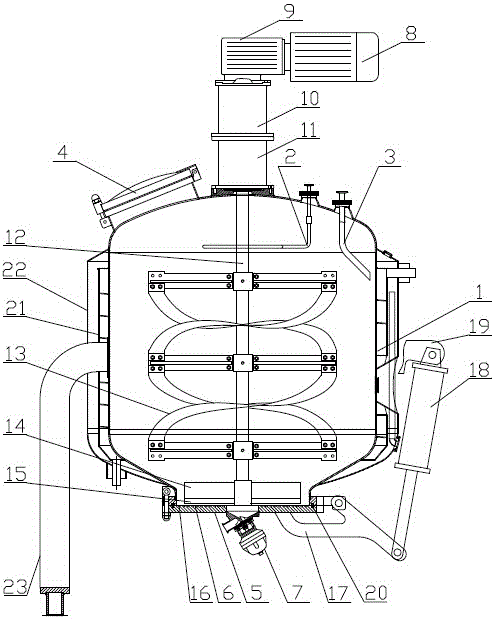
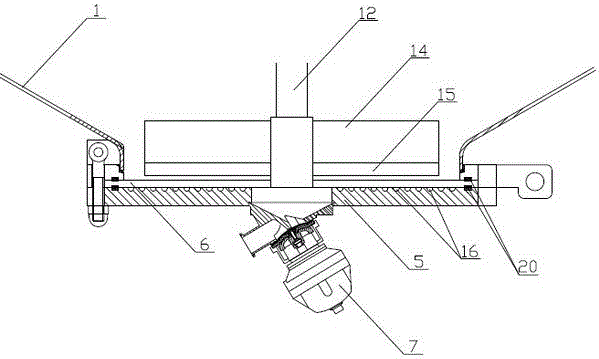
ActiveCN104328036BReduce the risk of contaminationReduce lossesBioreactor/fermenter combinationsBiological substance pretreatmentsChemical physicsPhysical chemistry
The invention relates to an adsorption and separation device for human prothrombin complex, belonging to the technical fields of biochemical industry and biopharmaceuticals. The present invention includes a tank body and a stirring part, the tank body is provided with a buffer distribution pipe and a feed pipe, and the buffer liquid distribution pipe and the feed pipe extend from the top of the tank body to Inside the tank, the top of the tank is also provided with a manhole; the bottom of the tank is provided with an openable tank bottom, the top of the tank bottom is provided with a filter plate, and the bottom of the tank bottom is A tank bottom valve is provided. The adsorption and separation device for human prothrombin complex of the present invention has comprehensively improved the adsorption tank, and realizes that the adsorption and separation of human prothrombin complex can be completed in the same device.
Owner:成都英德生物医药装备技术有限公司
Method for effectively inactivating parvovirus in prothrombin complex and preparation obtained by method
ActiveCN104623701AGuaranteed potency recoveryEnsure inactivationPeptide/protein ingredientsMammal material medical ingredientsPROTHROMBIN COMPLEXVirus safety
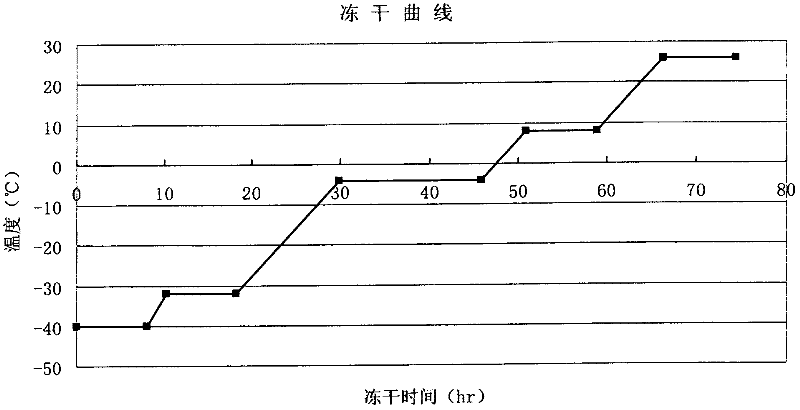
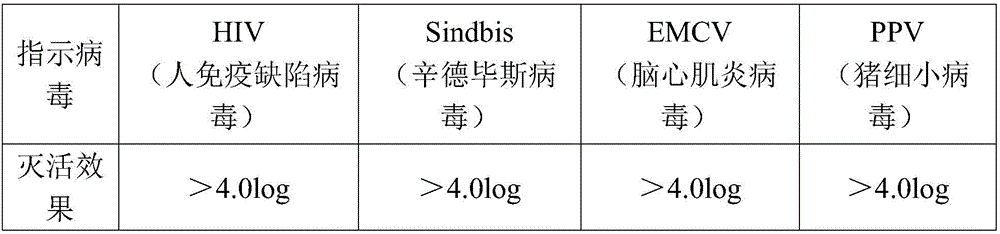
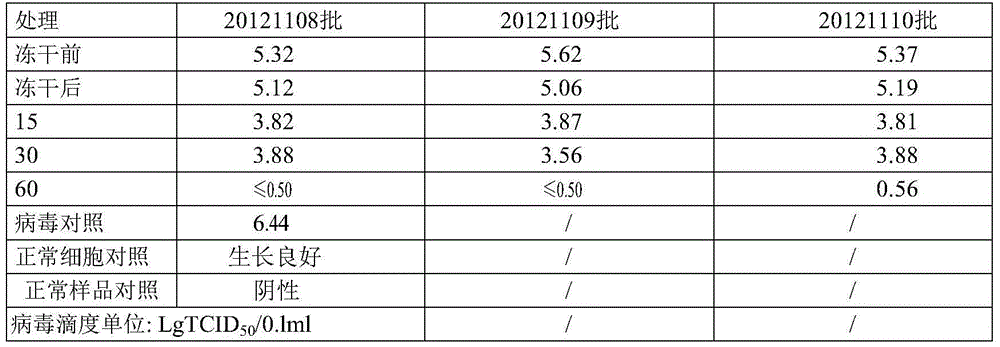
The invention discloses a method for effectively inactivating parvovirus (PPV) in a prothrombin complex. The method comprises the following steps: lyophilizing an S / D inactivated parvovirus in prothrombin complex preparation, carrying out dry-heat treatment for 2-10 hours at 80 DEG C and for 30-120 hours at 100 DEG C. The invention further discloses a prothrombin complex preparation prepared by the method. The inactivating method can be used for effectively inactivating PPV over 4log; the prothrombin complex preparation prepared by the method has high virus safety and good long-time stability, is qualified in full-inspections after being placed for 3 years at 2-8 DEG C, and has an FIX recovery rate of over 90 percent; and the II, VII, IX and X blood coagulation factors are not remarkably lost, and an excellent proportion is maintained. Furthermore, the preparation method has low cost which is lower than that of traditional 80-DEG C 72-hour dry-heat inactivation.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD
Method for liquid preservation of intermediate product of human prothrombin complex
ActiveCN109593747AReduce stabilityReduce the risk of contaminationEnzyme stabilisationPeptidasesCryoprecipitateLiquid state
The invention relates to a method for liquid preservation of an intermediate product of a human prothrombin complex. The method comprises the following steps: (1) carrying out plasma adsorption, namely stirring and adsorbing plasma of which cryoprecipitate is removed by using balanced gel; (2) washing the gel, and removing protein components in a non-human prothrombin complex; (3) eluting the gel,and collecting an elution liquid, namely eluting the gel washed in the step (2) by using an elution liquid, and collecting the elution liquid; (4) carrying out ultrafiltration on the elution liquid;(5) preserving an intermediate product, namely sealing and preserving a concentrated liquid at a non-frozen state of the intermediate product; (6) carrying out detection. By adopting the method, the intermediate product of the human prothrombin complex is preserved, and good activity of the intermediate product can be maintained; control on the production process is facilitated, and the risks of instability and contamination of a product at switched solid and liquid states can be reduced; cryopreservation and remelting processes are avoided, and the production efficiency can be improved.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
A method for simultaneously separating and purifying blood coagulation factors ix, x and ⅶ from human plasma
ActiveCN109651502BAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
A method for preparing human prothrombin complex from plasma
The invention discloses a method for preparing a human prothrombin complex from plasma. The method comprises: directly adsorbing the human prothrombin complex from the plasma by using DEAE A-50 gel, filling the adsorbed A-50 gel into a chromatographic column of a fixed bed, pumping eluant into the filled chromatographic column by using a peristaltic pump for online washing and elution, performing S / D inactivation on the eluant, and performing secondary chromatographic purification by using the chromatographic column of the fixed bed to obtain a high-purity human prothrombin complex product. According to the method, elution flow and speed can be accurately controlled by online elution after filling the gel into the column, the problems of contamination, cross contamination, gel leakage and the like caused by an open operation are reduced, an obtained product is high in purity, an IX factor titer can exceed 27IU / ml, and the IX factor specific activity exceeds 0.8IU / mg protein. Meanwhile, a self-flushing type filter with a pressure difference controller and an automatic solid matter stripping system is adopted, so the pressure difference in the whole filtering process is stable and controllable, gel particles can be well protected, broken colloidal particles flowing into a plasma tank in the filtering process are remarkably reduced, and gel losses in a production process can be reduced by above 20%.
Owner:广东双林生物制药有限公司
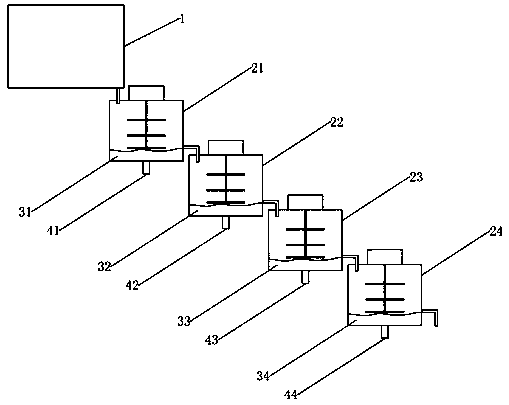
Process for preparing human prothrombin complex concentrate by adopting flow adsorption method
ActiveCN108441490ALow purityHigh purityBioreactor/fermenter combinationsBiological substance pretreatmentsProthrombin complex concentrateDynamic balance
The invention discloses a process for preparing a human prothrombin complex concentrate by adopting a flow adsorption method. The process is characterized in that a plurality of cylindrical containerswith stirrers are connected in series to form a natural flowing way; gels which are balanced in advance are placed in the various containers; a certain batch of to-be-processed blood plasma is storedabove the first container; the gels are injected quantitatively firstly in the first container, then the gels are stirred and adsorbed for a unit time, and the adsorbed blood plasma is discharged into the second container at a certain flow speed; simultaneously, other to-be-processed blood plasma flows into a first adsorption tank at the same flow speed, so that dynamic balance of the blood plasma quantity in the first container is kept, and at this time, adsorption and separation of the blood plasma in the first container are carried out simultaneously; by analogy, after the all blood plasmapasses through, outlets of the various containers are closed, and washing and elution on the gels are carried out. The blood plasma quantity is not limited by the volume of the containers, the process is easily controlled, an FIX activety recycling ratio is higher, and the risks of leakage of the gels and crossed pollution are avoided.
Owner:华润博雅生物制药集团股份有限公司
A method for liquid preservation of intermediate product of human prothrombin complex
ActiveCN109593747BReduce stabilityReduce the risk of contaminationEnzyme stabilisationPeptidasesUltrafiltrationLiquid state
The invention relates to a method for liquid preservation of an intermediate product of human prothrombin complex. The steps are: (1) plasma adsorption, using a balanced gel to stir and absorb the plasma from which cryoprecipitation has been removed; (2) gel washing, washing Gel, remove the protein components in the non-human prothrombin complex; (3) Gel elution, collect the eluate, use the eluent to elute the gel after washing in step (2), and collect the eluate ; (4) Ultrafiltration of the eluent; (5) Preservation of the intermediate product, sealing and storing the concentrated solution in a non-frozen state of the intermediate product; (6) Detection. Preserving the intermediate product of human prothrombin complex under the conditions of the present invention enables the intermediate product to better maintain its activity; it is beneficial to the control of the production process and reduces the risk of instability and contamination of the product under the conversion of different solid-liquid states; no need The process of freezing and rethawing is conducive to improving production efficiency.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
A method for effectively inactivating parvovirus in prothrombin complex and the obtained preparation
ActiveCN104623701BPeptide/protein ingredientsMammal material medical ingredientsZymogenPROTHROMBIN COMPLEX
The invention discloses a method for efficiently inactivating parvovirus PPV in a prothrombin complex. The prothrombin complex preparation inactivated by S / D is lyophilized and then subjected to dry heat treatment. The dry heat treatment method is 80°C. 2 to 10 hours, and then treated at 100°C for 30 to 120 minutes. The invention also discloses the blood zymogen complex preparation prepared by the method. The inactivation method of the present invention can effectively inactivate porcine parvovirus (PPV) greater than 4 log, and the prothrombin complex preparation prepared by the method of the present invention has higher virus safety and better long-term stability, and can be placed at 2-8°C The 3-year full inspection was qualified, the recovery rate of FIX was over 90%, and the coagulation factors II, VII, IX, and X had no significant loss and maintained a good ratio. At the same time, the preparation method has low cost, which is lower than the cost of traditional dry heat inactivation at 80° C. for 72 hours.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com