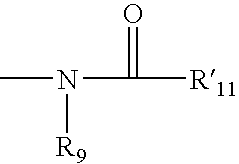
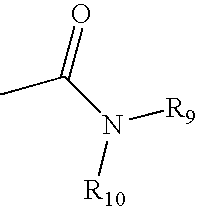
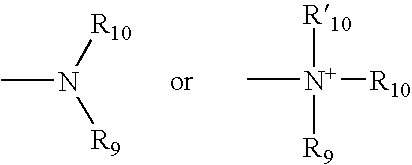Patents
Literature
225 results about "Thrombosis prevention" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thrombosis prevention or thromboprophylaxis is medical treatment to prevent the development of thrombosis (blood clots inside blood vessels) in those considered at risk for developing thrombosis. Some people are at a higher risk for the formation of blood clots than others. Prevention measures or interventions are usually begun after surgery as people are at higher risk due to immobility. There are medication-based interventions and non-medication-based interventions. The risk of developing blood clots can be modified by life style modifications, the discontinuation of oral contraceptives, and weight loss. In those at high risk both interventions are often used. The treatments to prevent the formation of blood clots is balanced against the risk of bleeding.
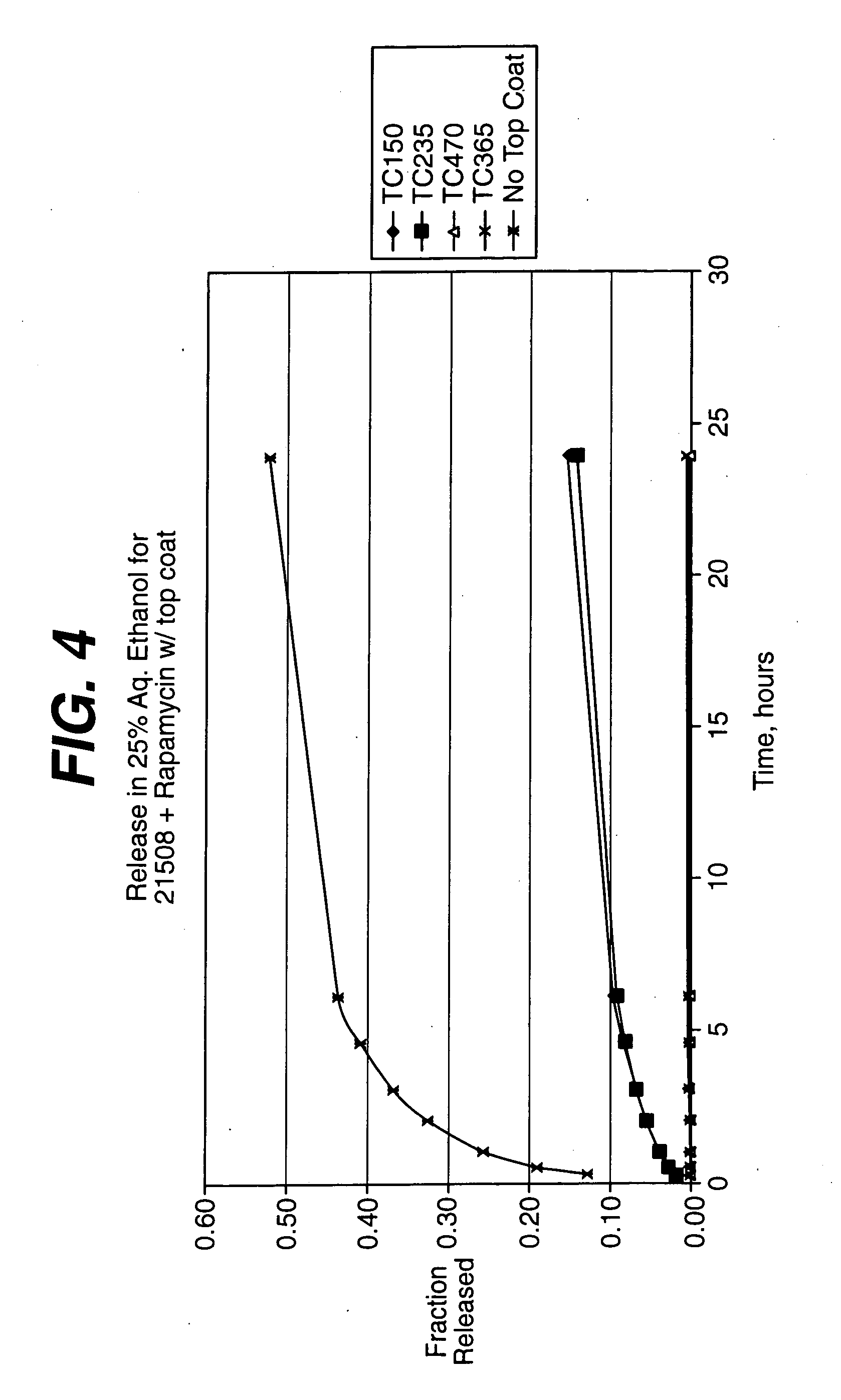
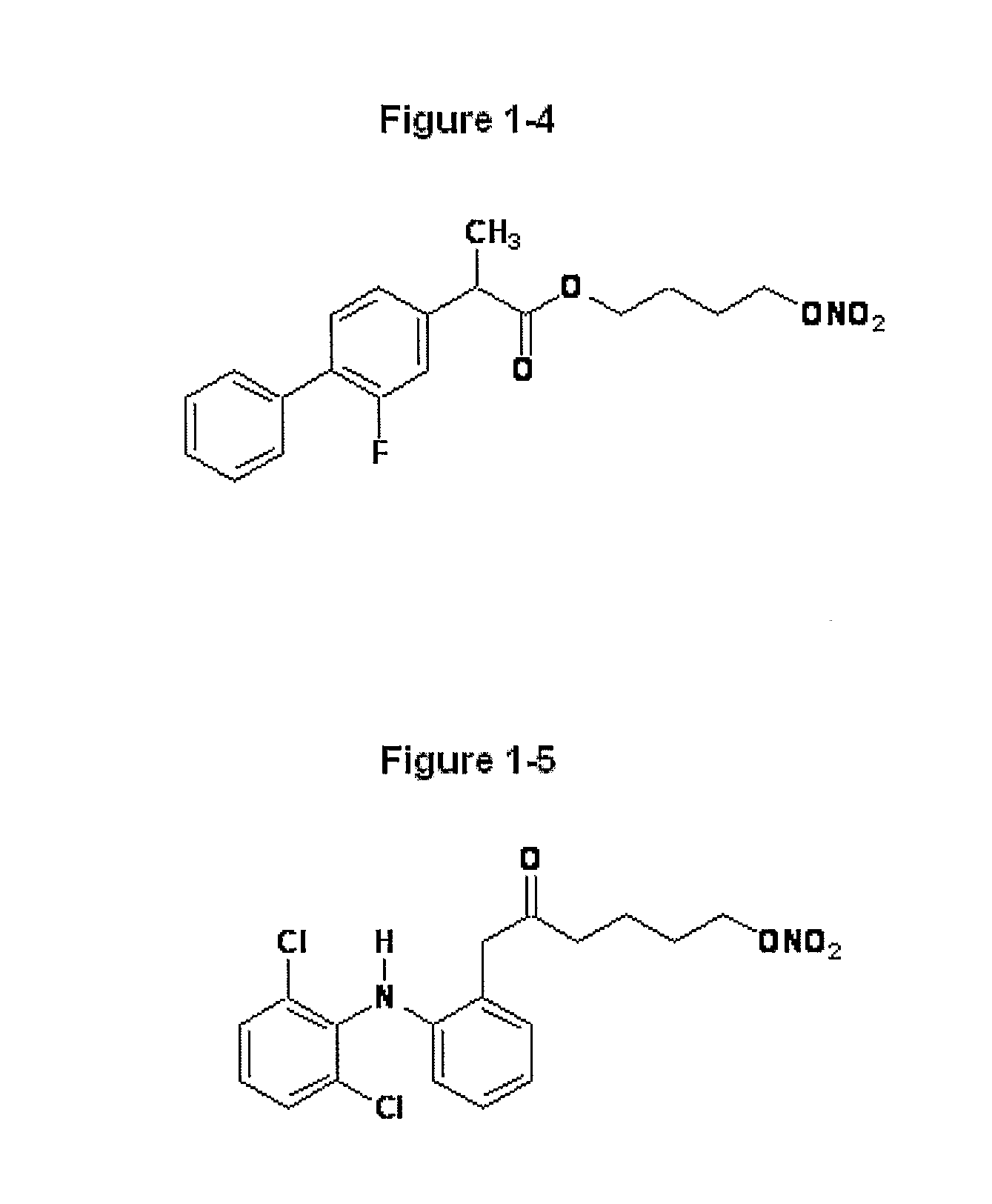
Conjugates comprising a biodegradable polymer and uses therefor
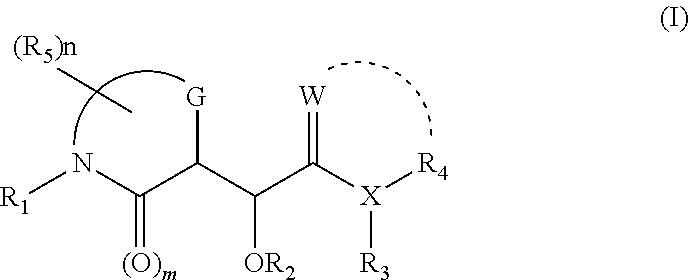
InactiveUS20050169968A1Improve biological activityProlong half-life in vivoPeptide/protein ingredientsPharmaceutical non-active ingredientsActive agentThrombosis prevention
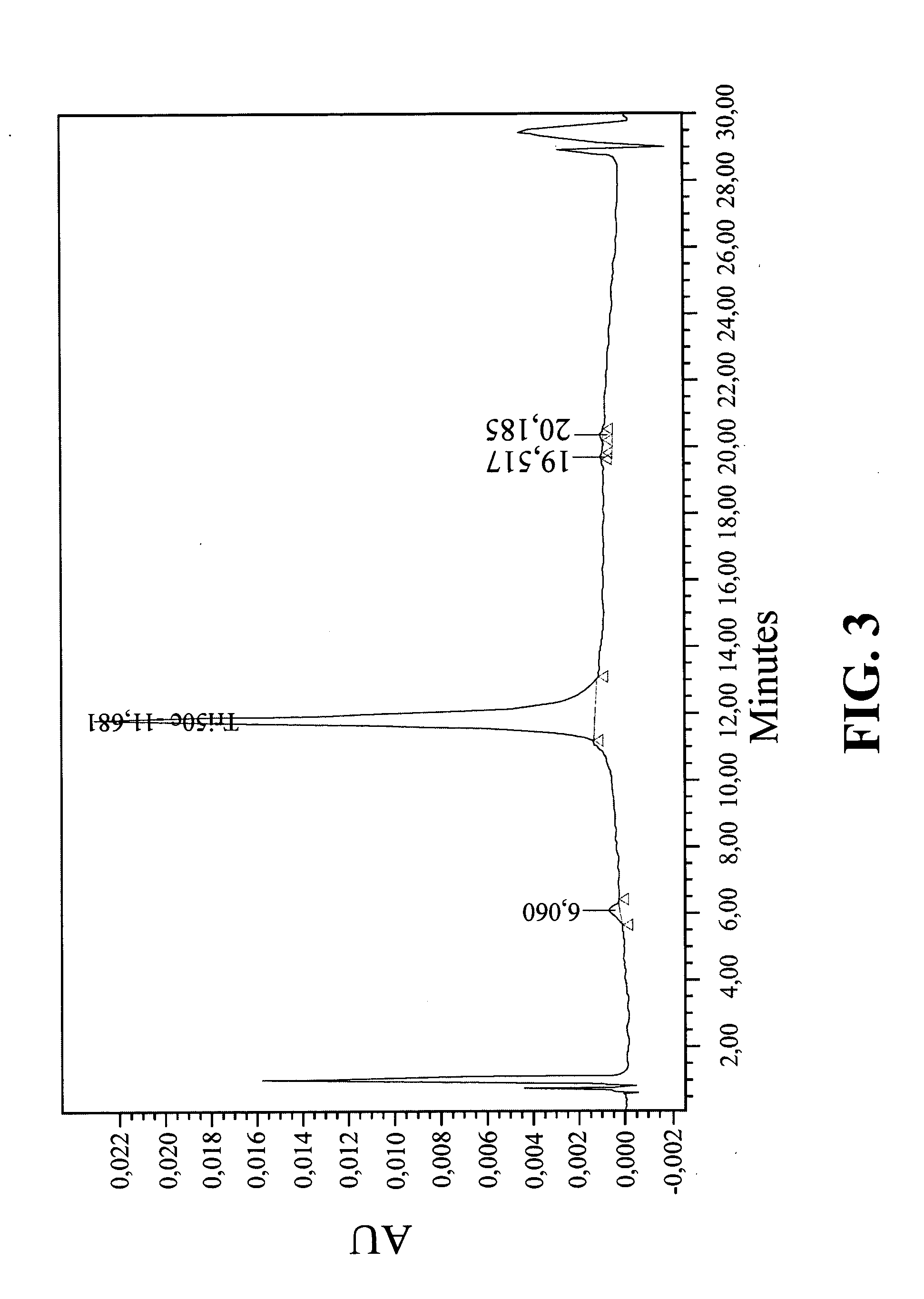
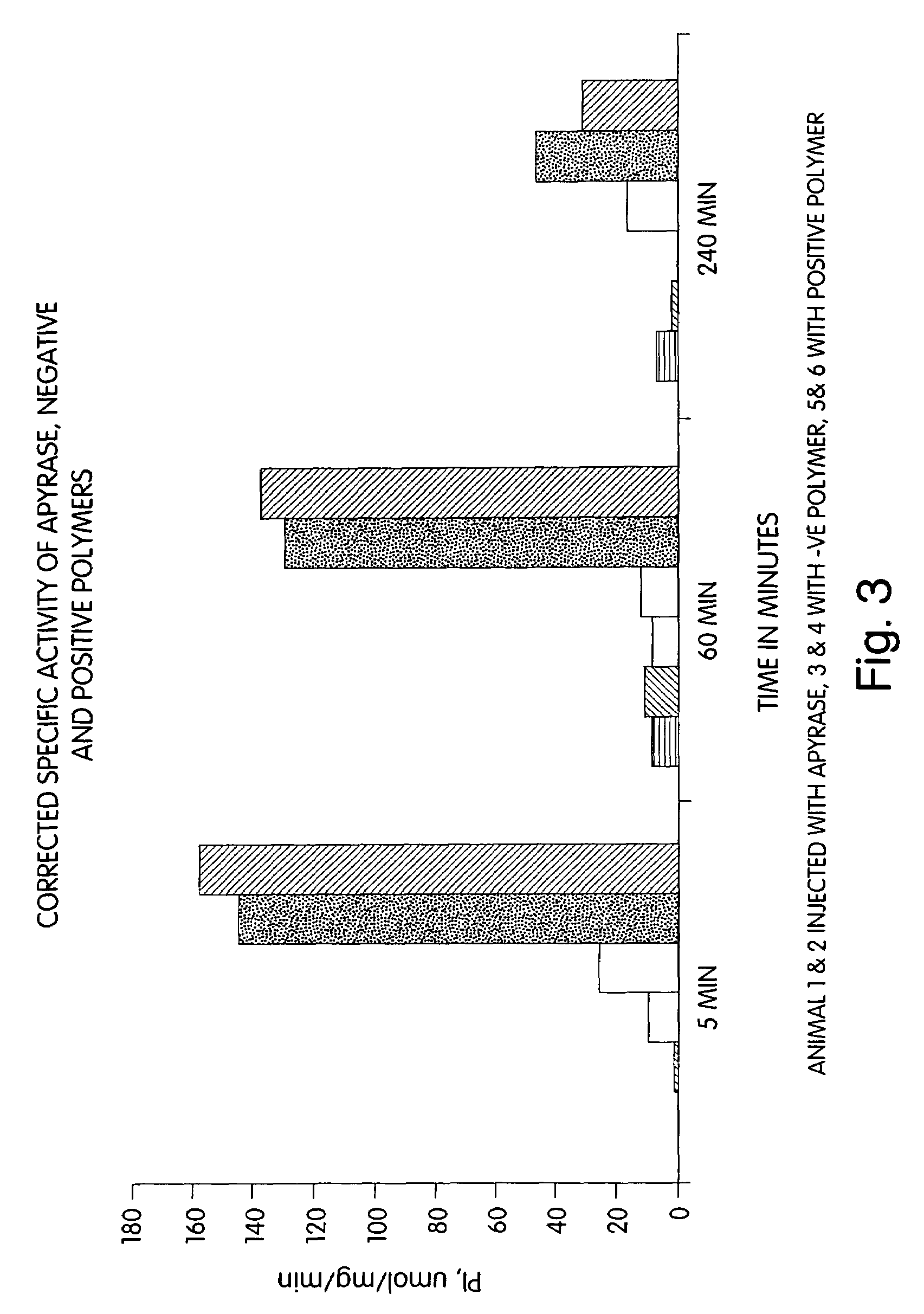
Biologically active agents covalently linked to a polymer. The polymer is preferably a biodegradable polymer are provided. The biologically active agent is preferably a protein, such as an extracellular soluble protein, e.g., an extracellular enzyme. The enzyme can be an apyrase, e.g., NTPDase. Conjugates of the invention can be used as therapeutics in subjects. For example, a conjugate comprising an apyrase can be used for treating and preventing thrombosis, atherosclerotic plaque complications and vascular disorders.
Owner:MERSANA THERAPEUTICS INC +1
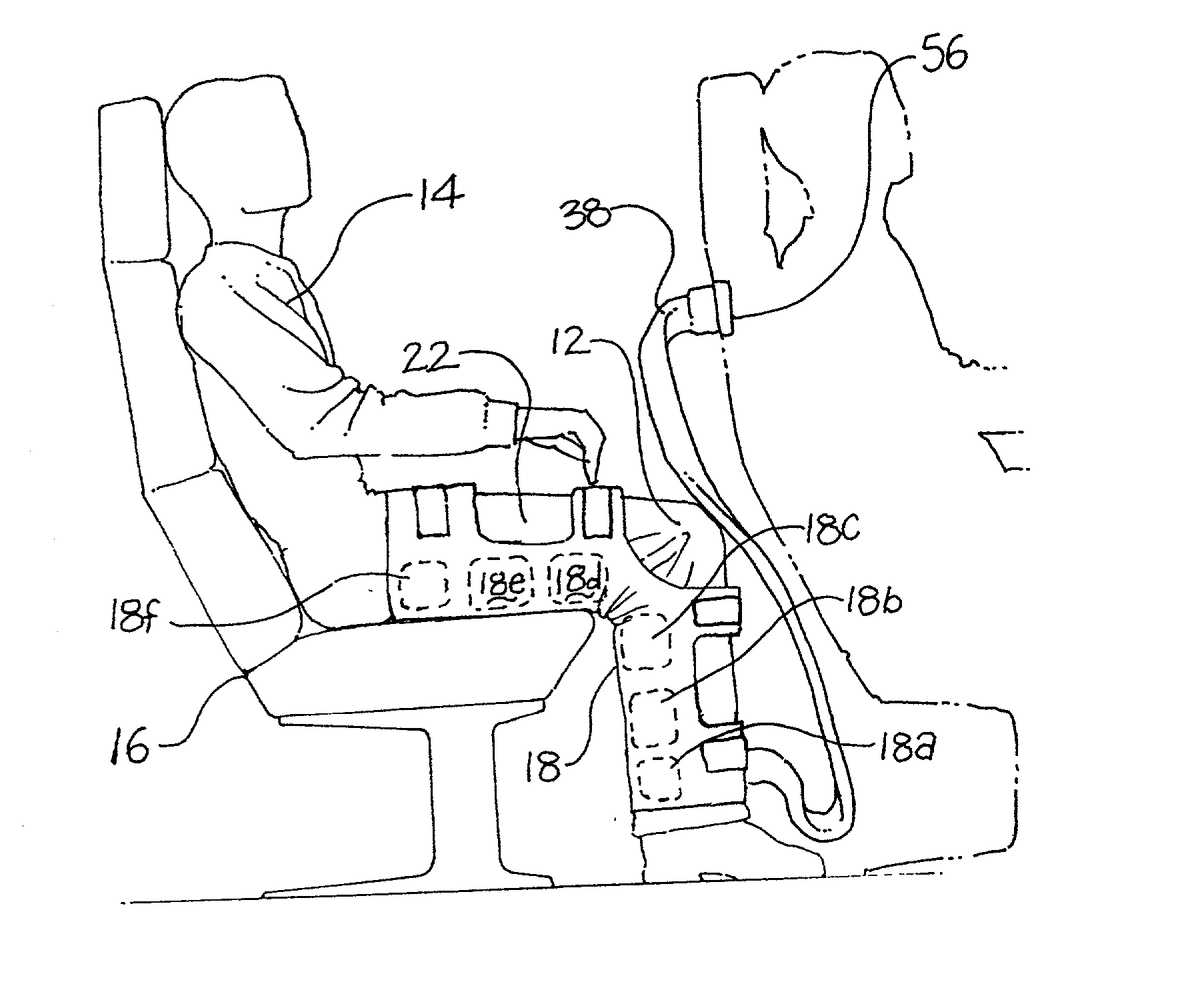
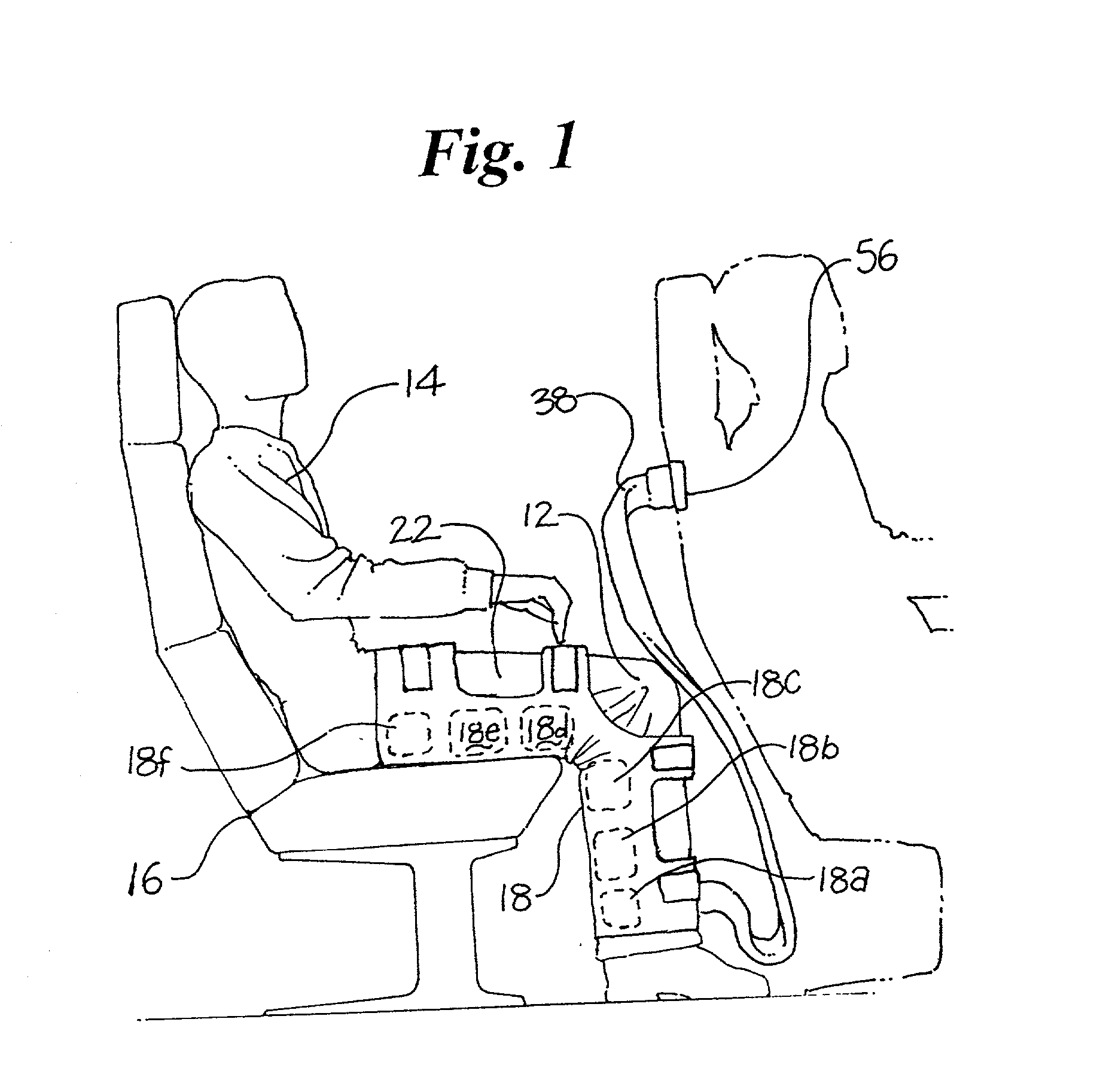
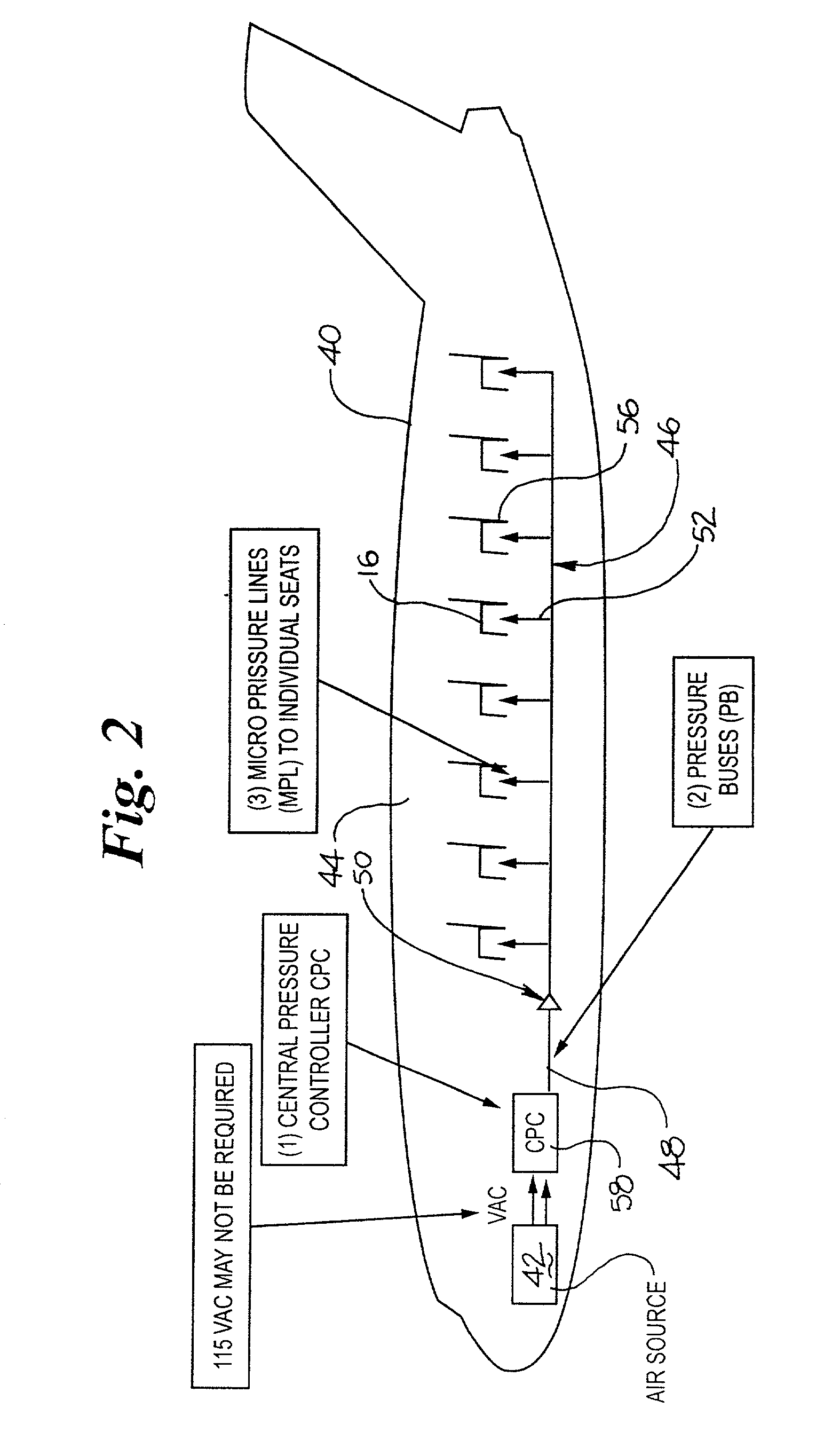
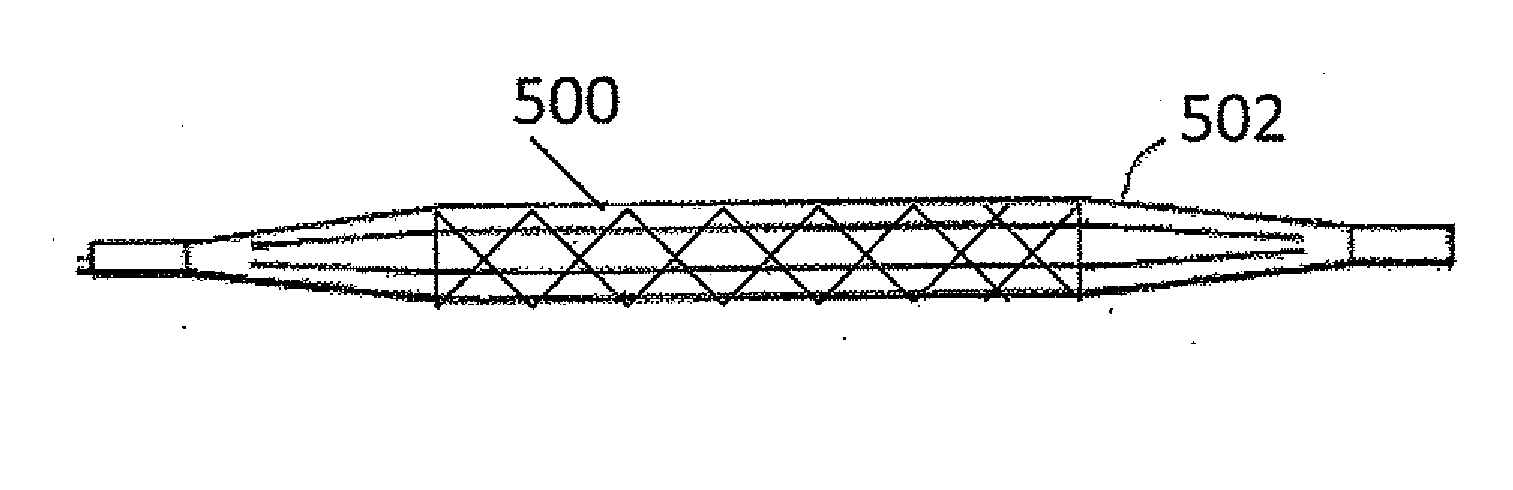
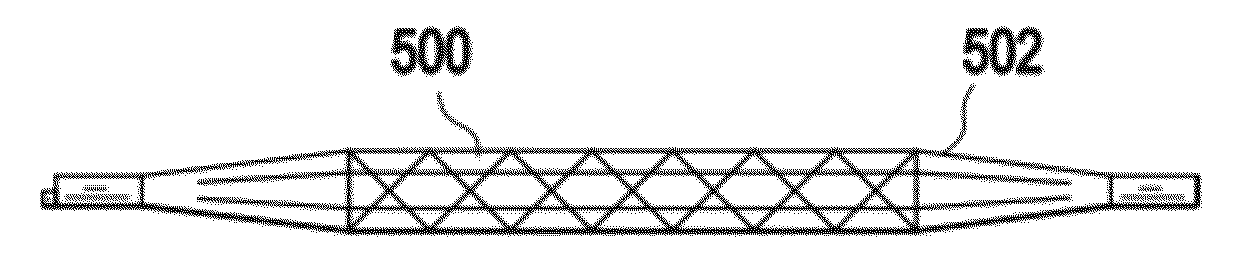
Pressure device and system for preventing thrombosis
A system for providing prophylaxis against thrombosis comprises in combination a vehicle and a plurality of inflatable compression sleeves. The vehicle has a pneumatic pressure source for supplying a predetermined flow of pneumatic fluid to a plurality of passenger positions. The pneumatic pressure source has a plurality of leads, which extend to a connection port located in each of the passenger positions. Each of the inflatable compression sleeves has a plurality of inflatable chambers therein. The sleeves are constructed to be engaged to a connection port, thereby providing fluid communication between the pneumatic pressure source and the plurality of chambers. The sleeves may be worn by a passenger to help prevent the occurrence of deep vein thrombosis. Individual sleeves comprise at least two inflatable chambers wherein a second inflatable chamber is subsequent to the inflation of a first inflatable chamber.
Owner:KUSLICH STEPHEN D +1
Device for local and/or regional delivery employing liquid formulations of therapeutic agents
InactiveUS20080181927A1Elimination reactionReduce riskBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
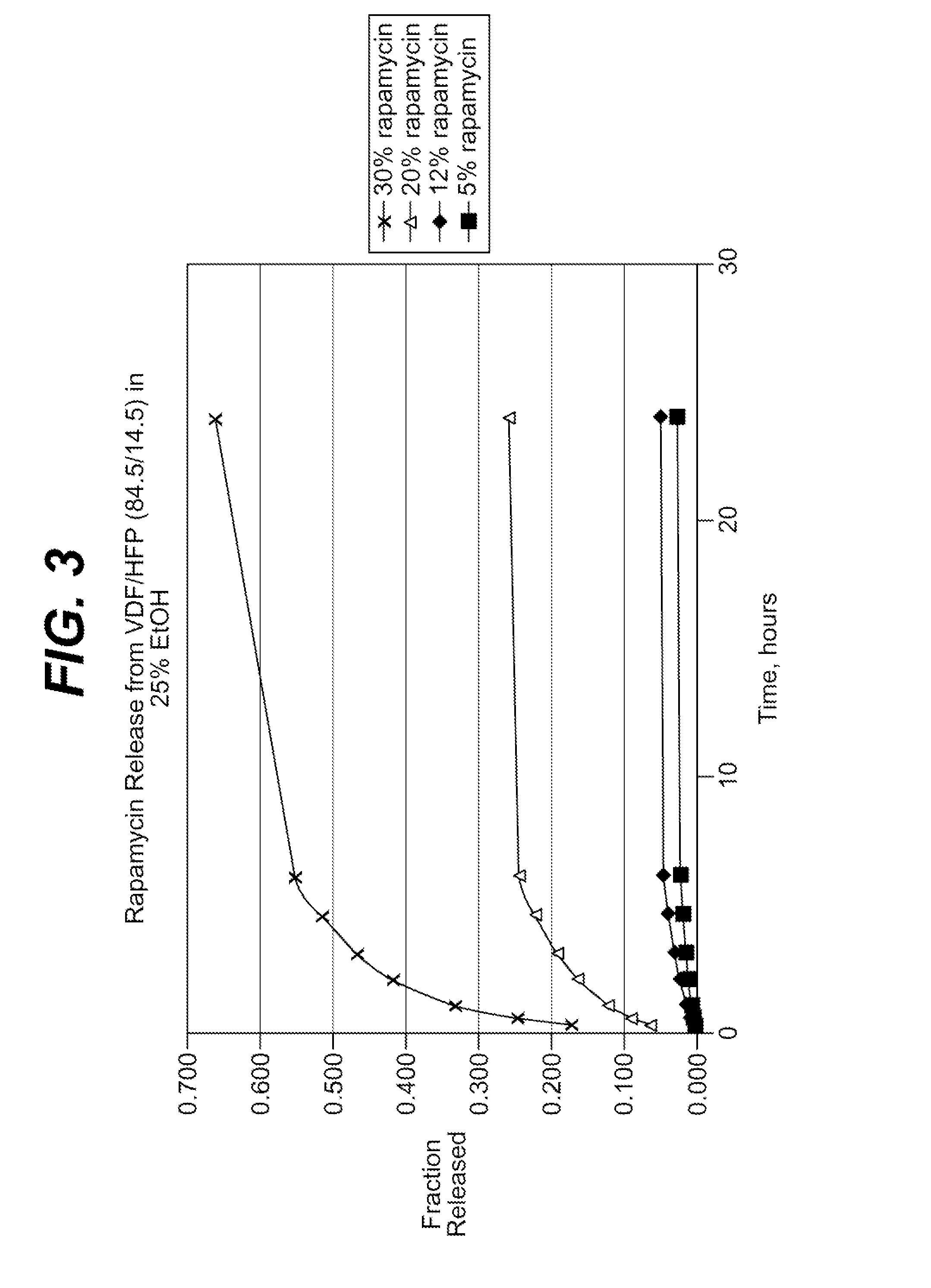
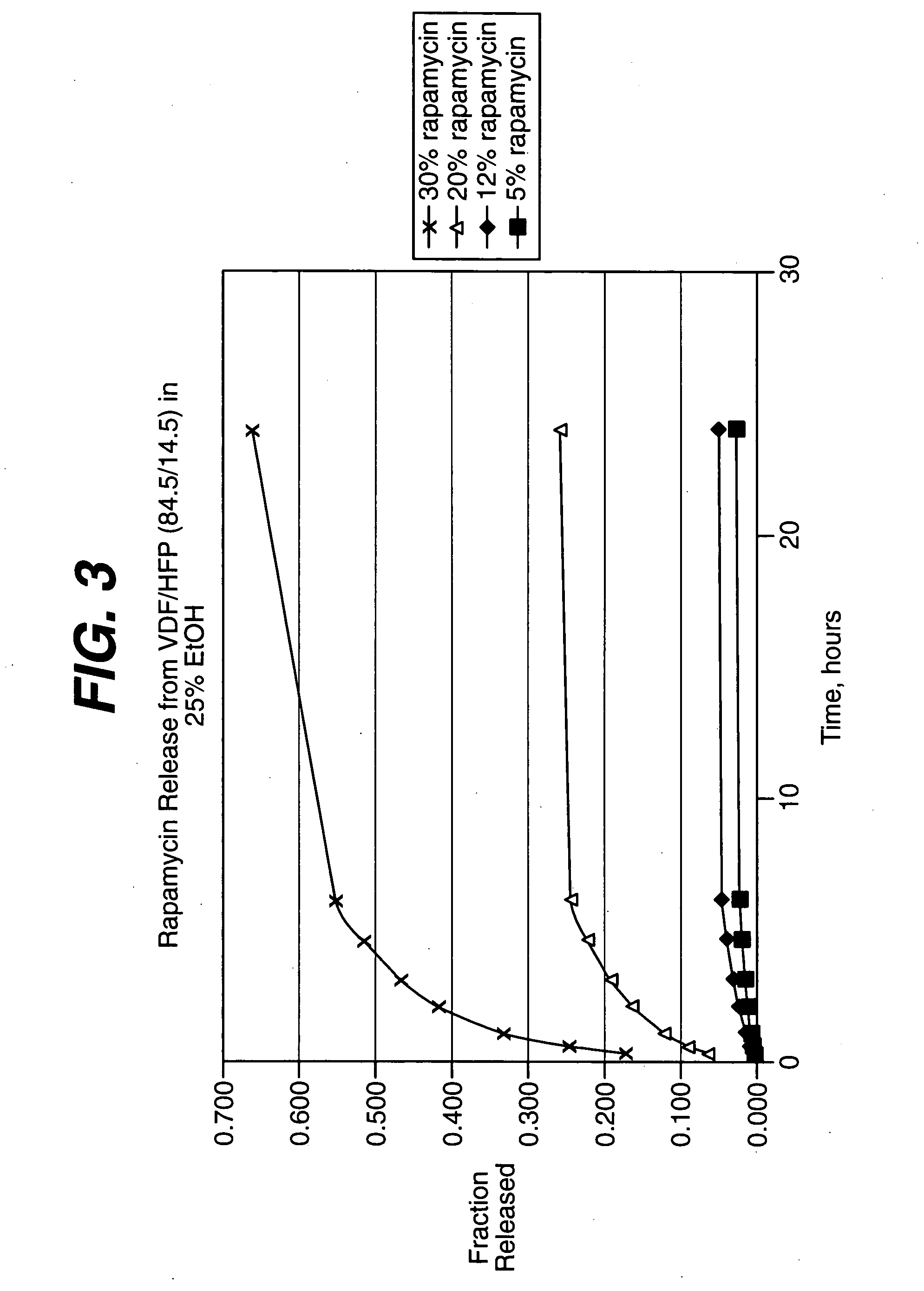
Rapamycin coated expandable devices
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Drug coated expandable devices
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
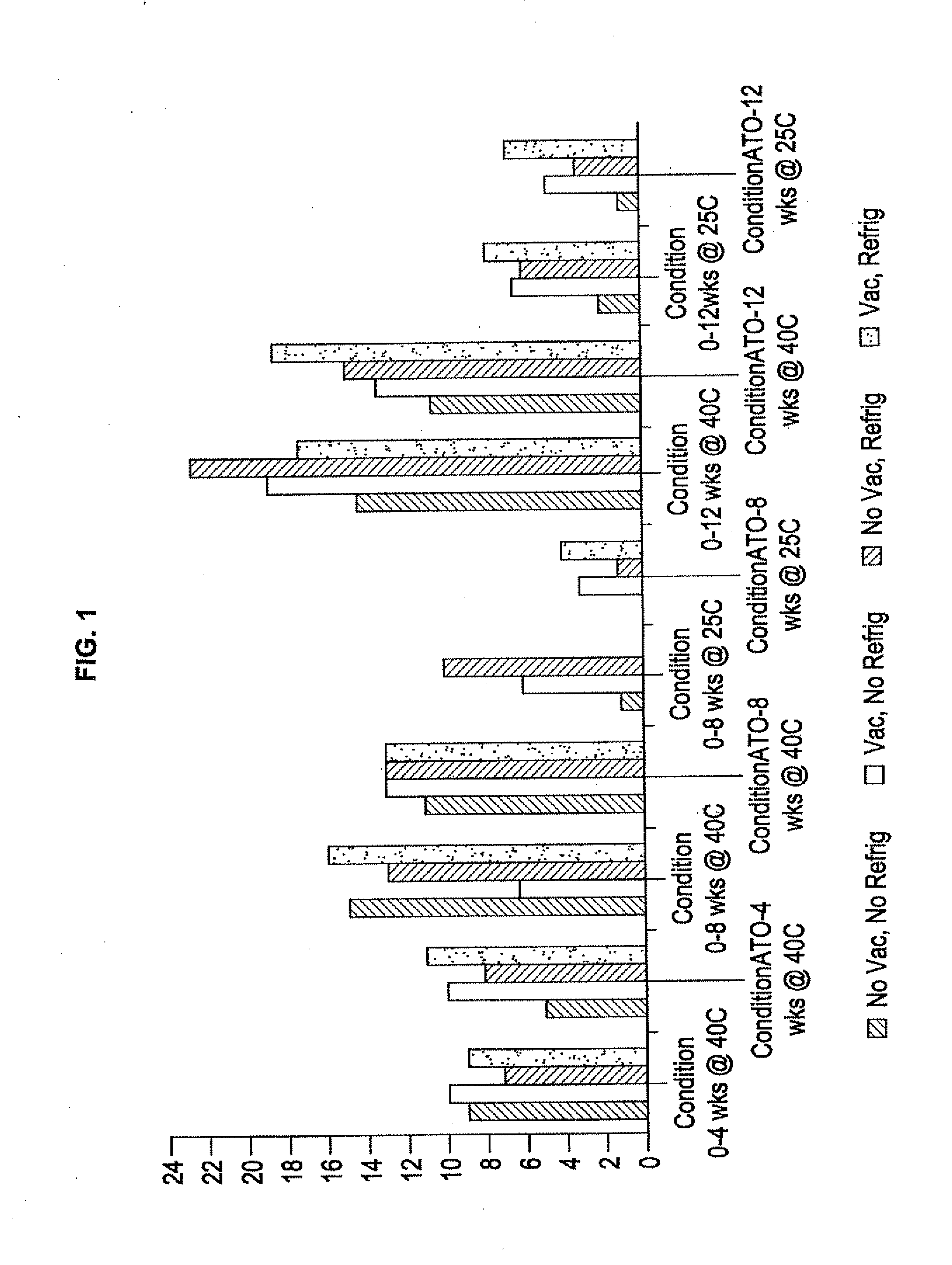
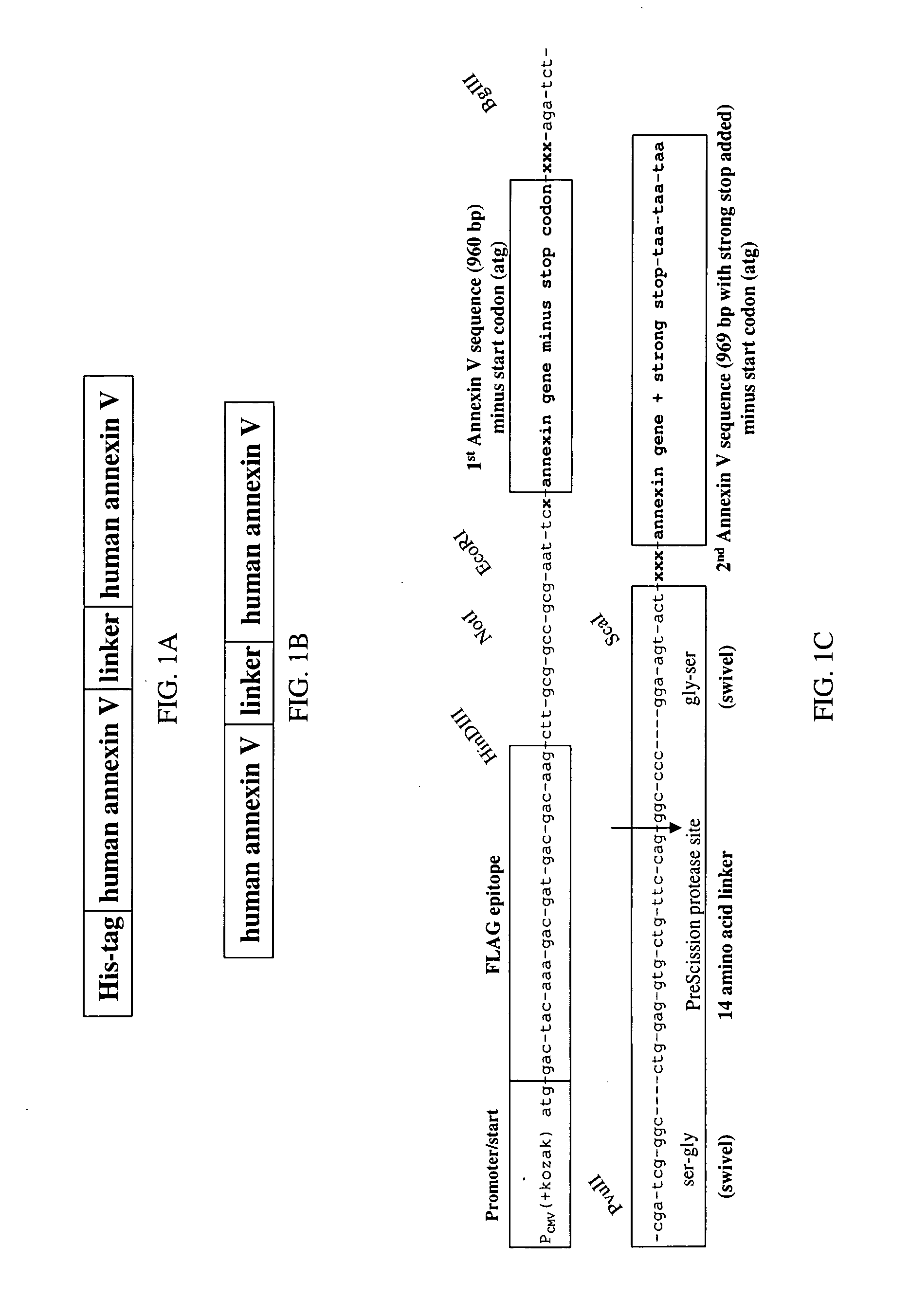
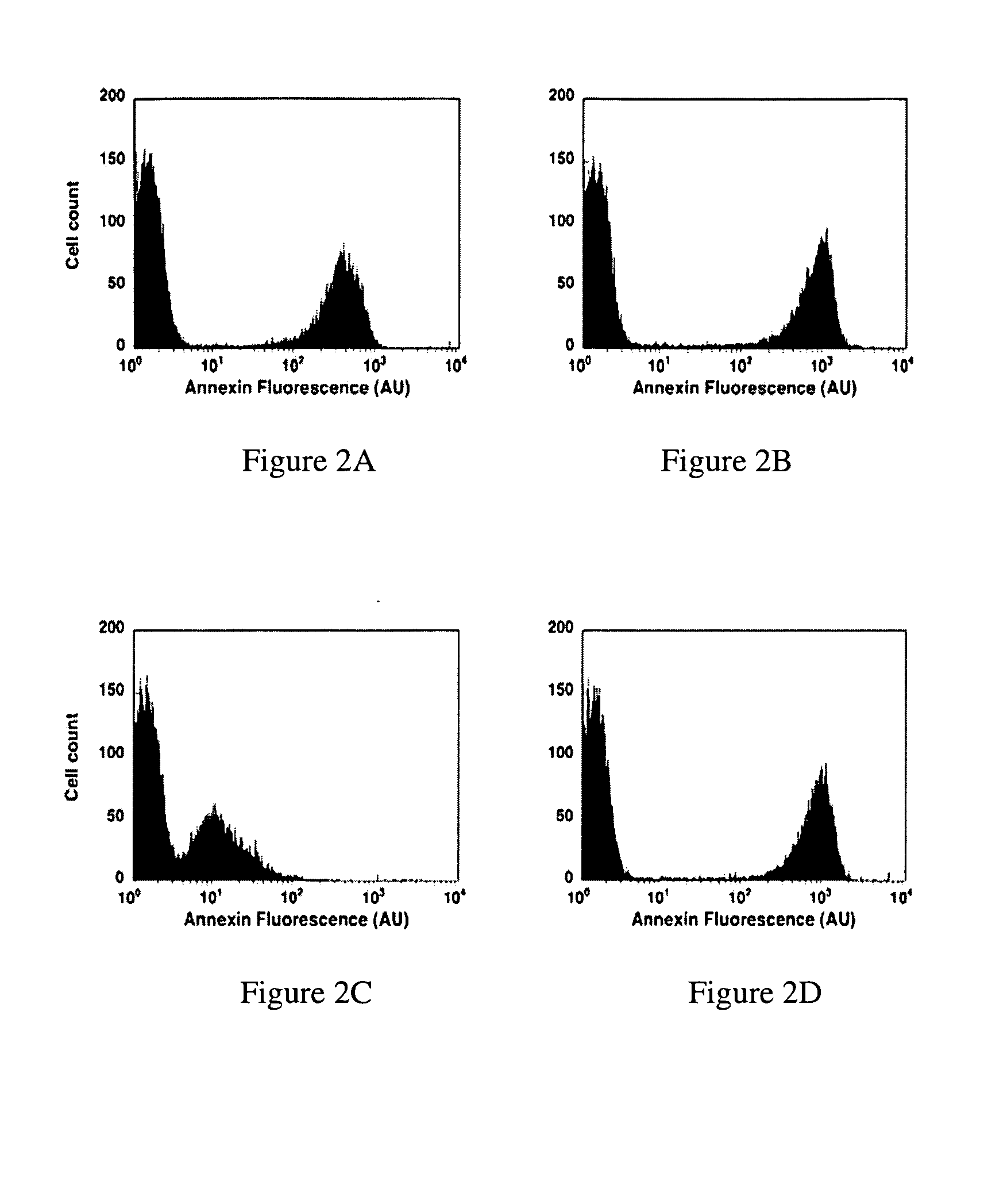
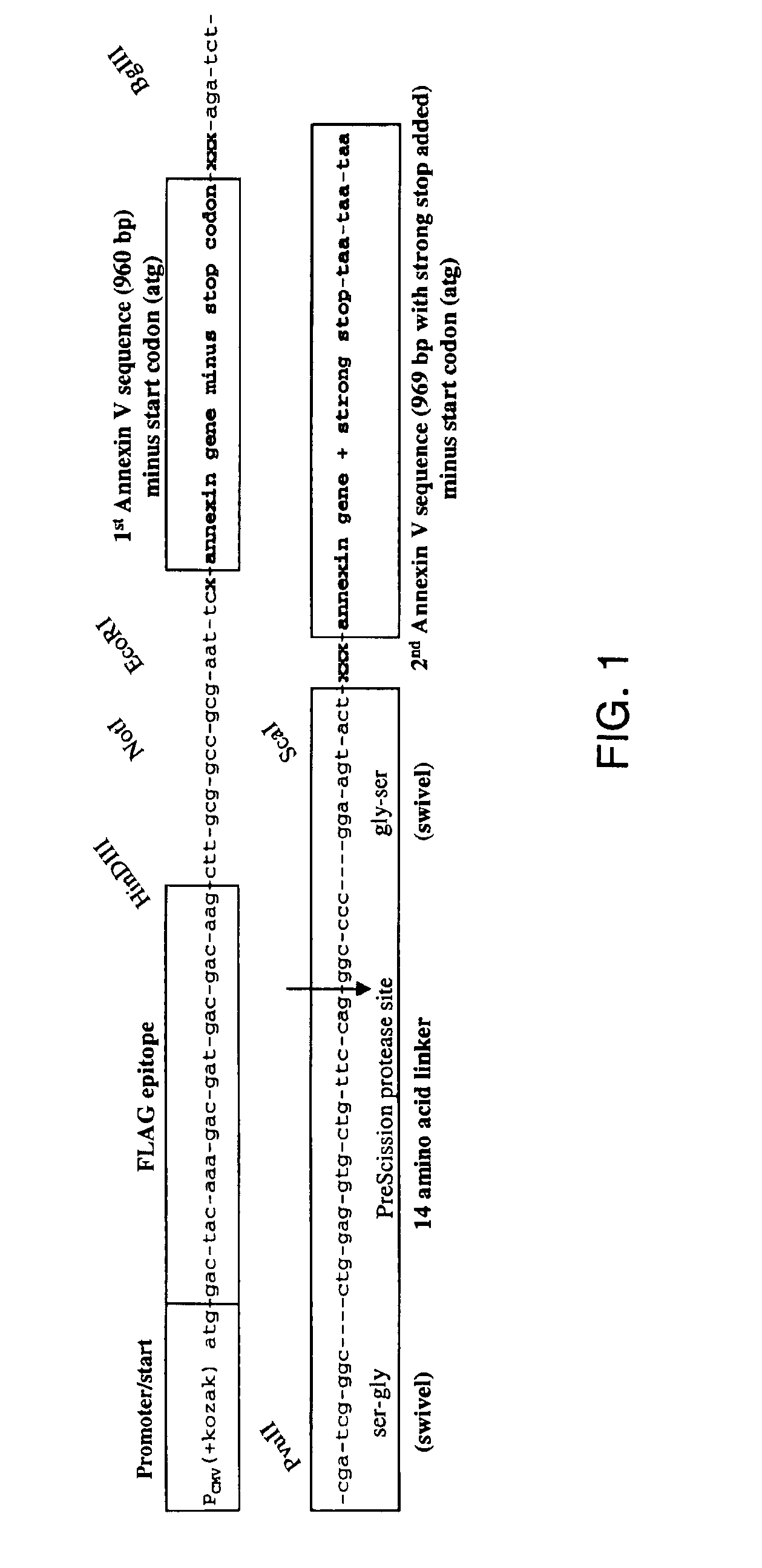
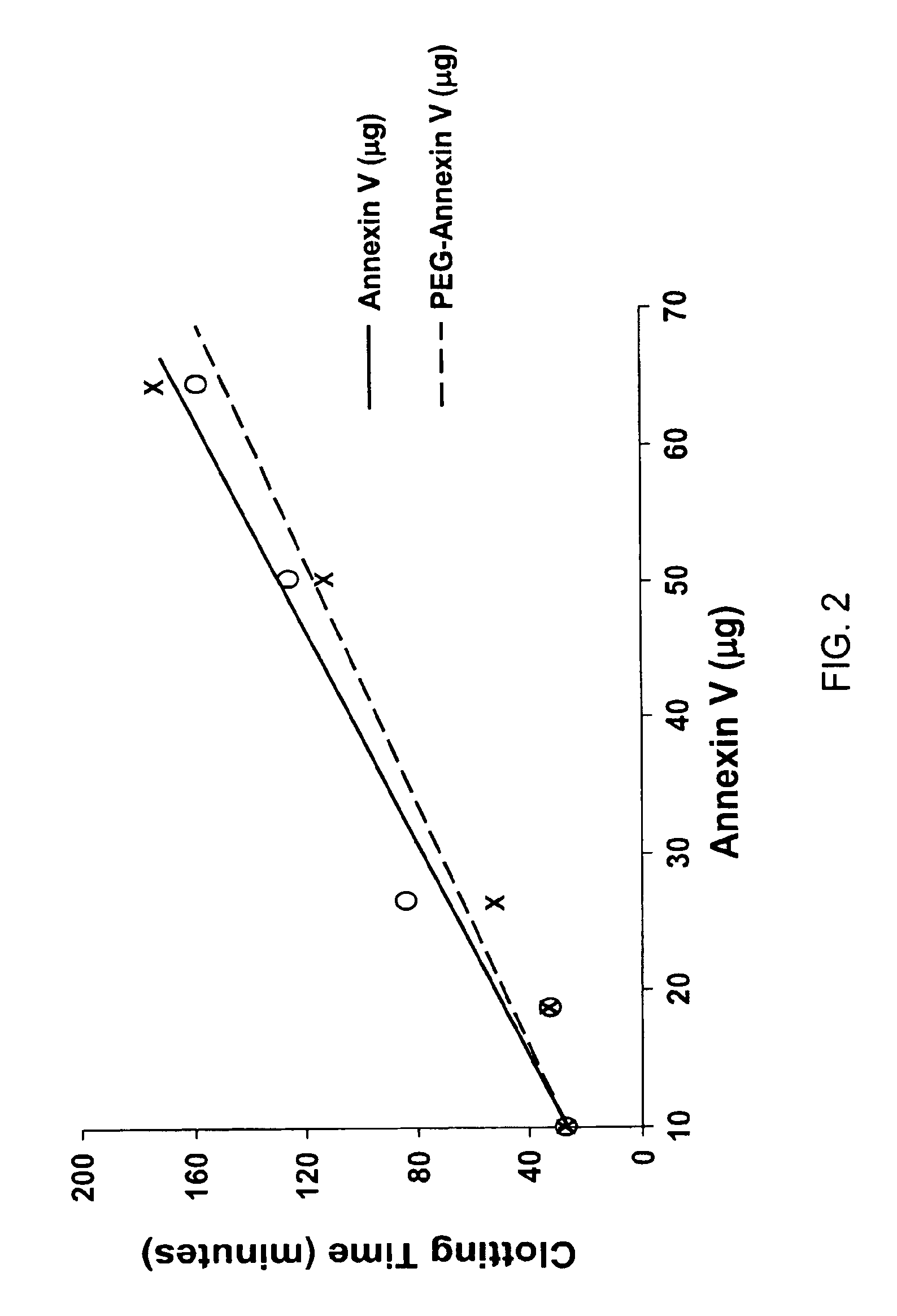
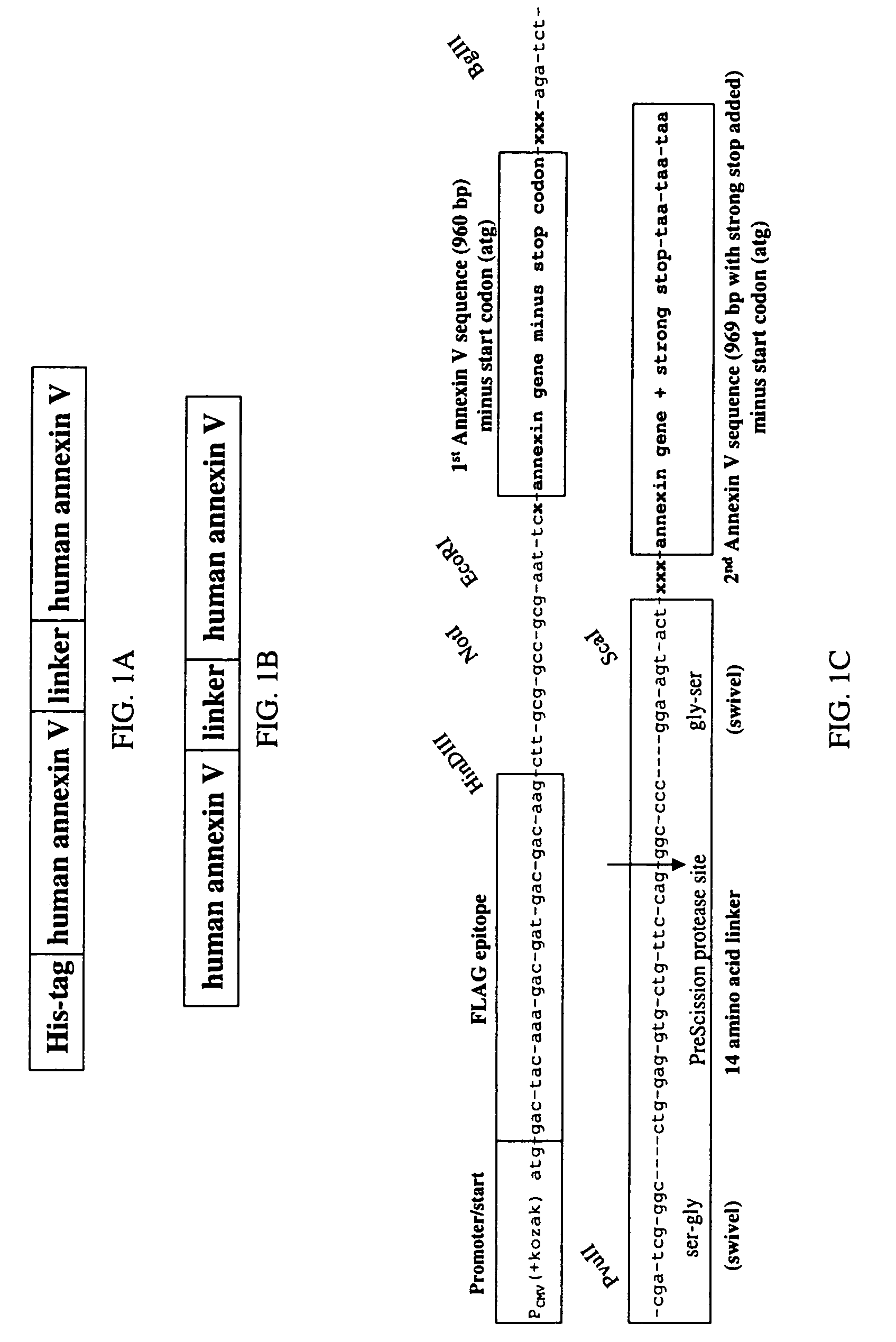
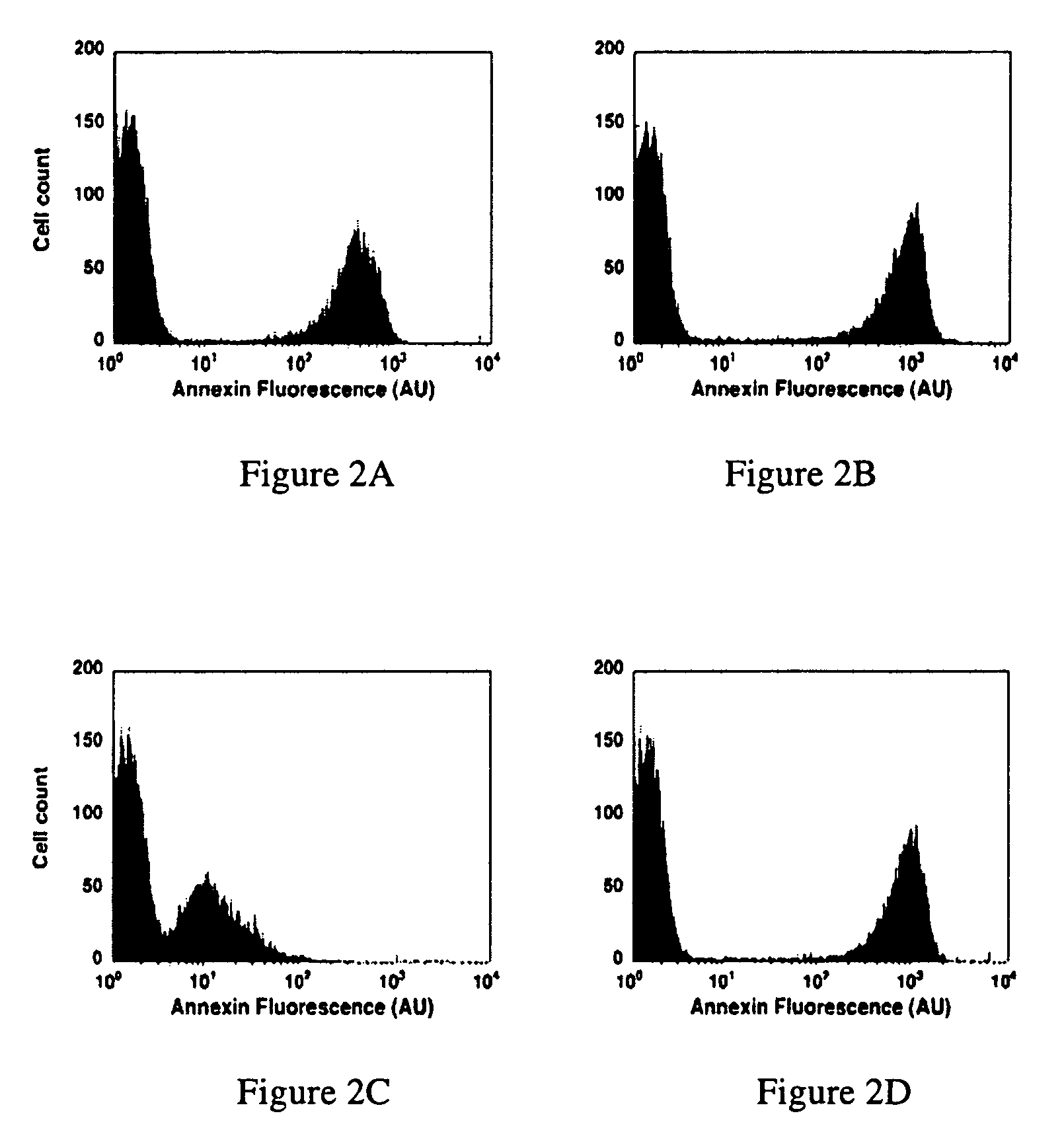
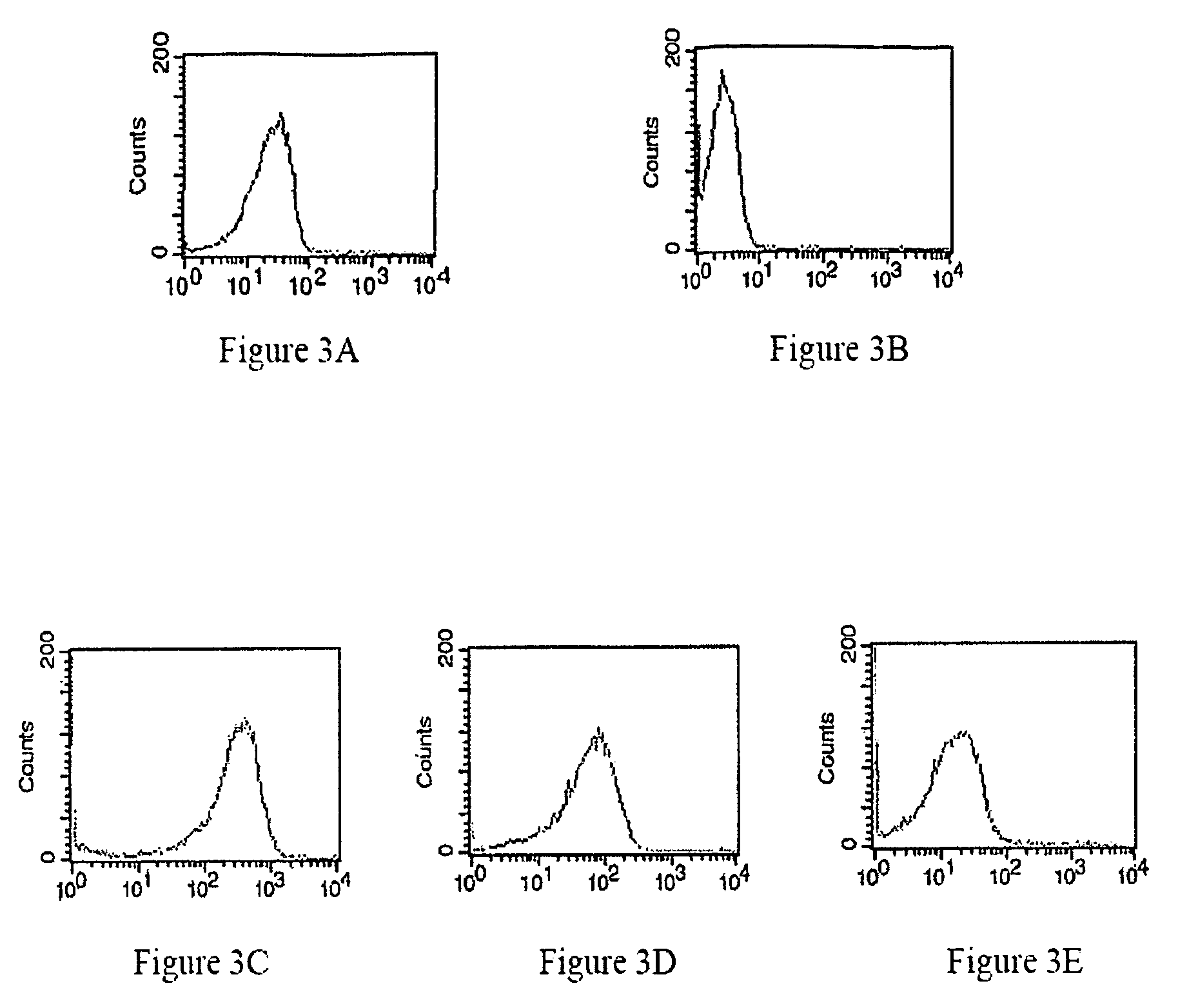
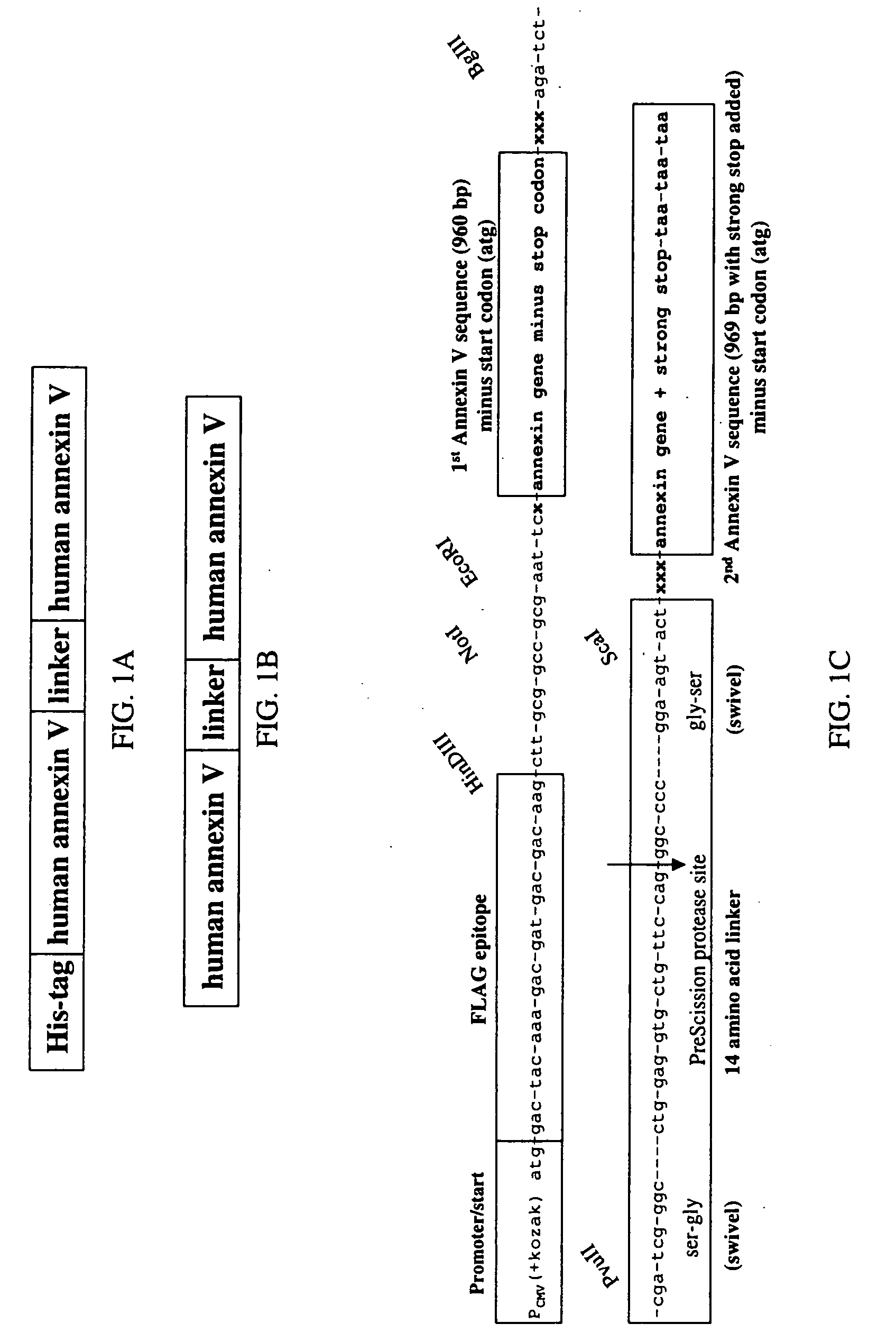
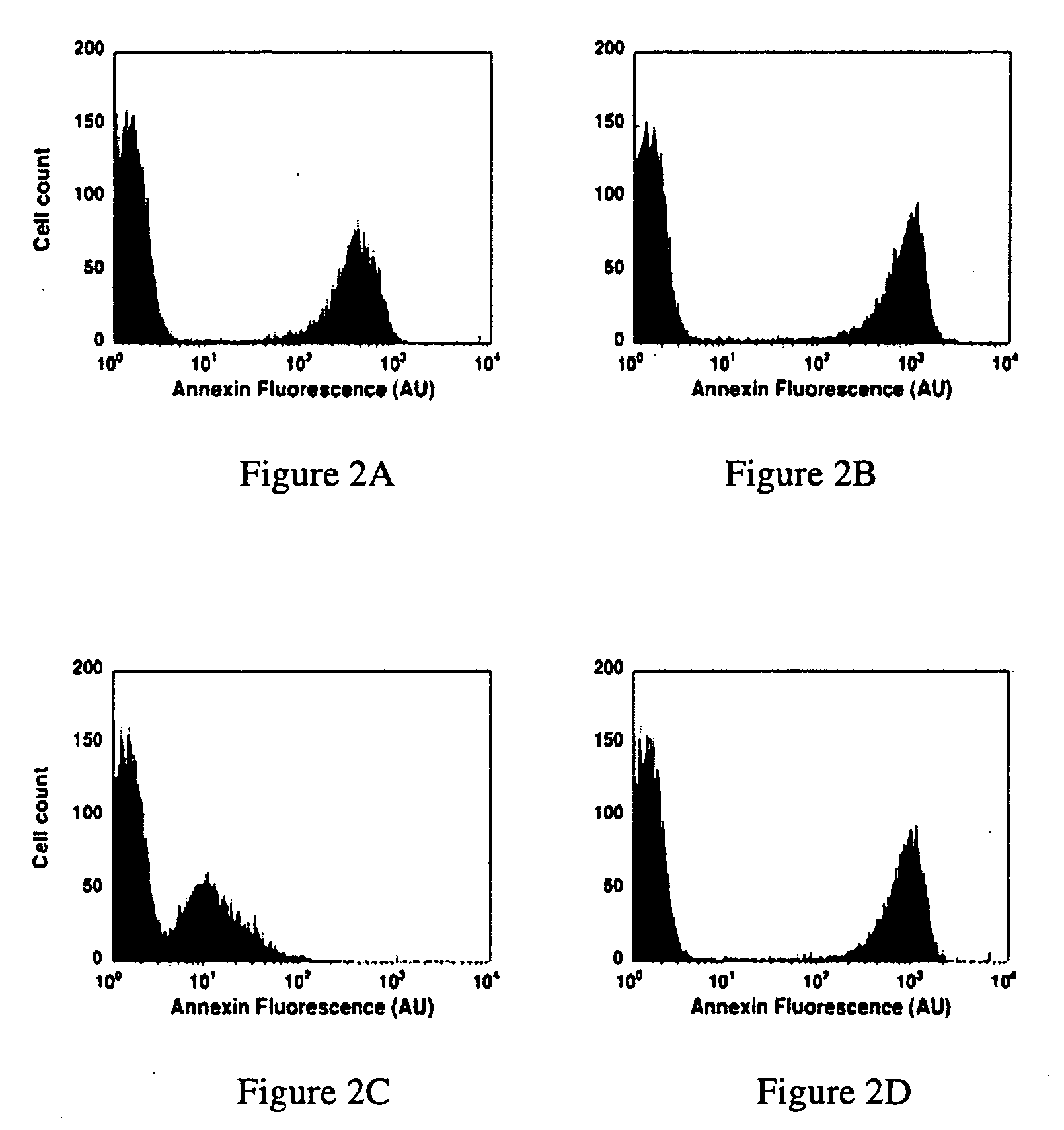
Modified annexin proteins and methods for preventing thrombosis
InactiveUS20050222030A1Inhibition of attachmentReducing endothelial cell damagePeptide/protein ingredientsAntibody mimetics/scaffoldsTreatment effectSufficient time
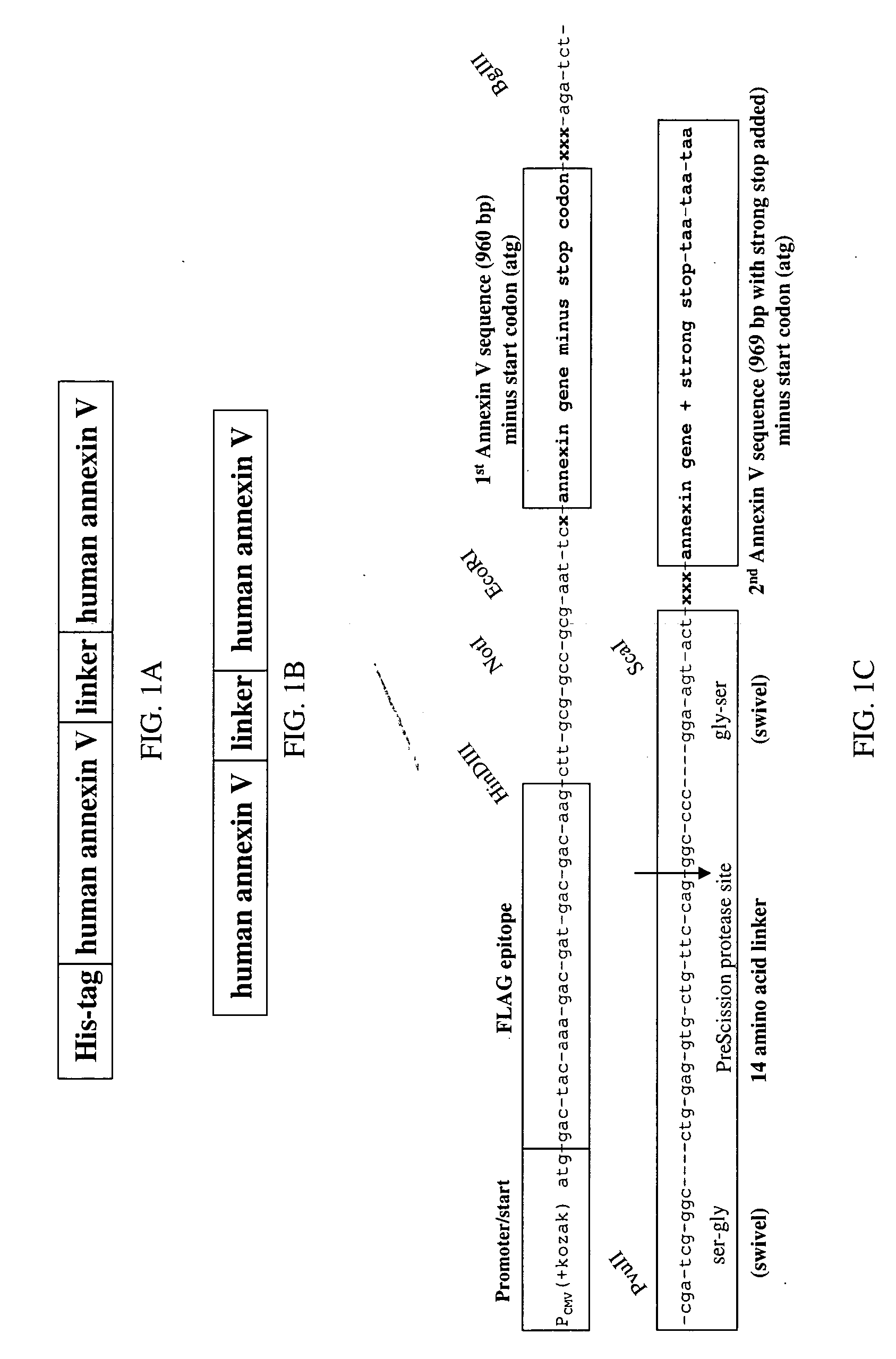
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:ALAVITA PHARMA
Local vascular delivery of probucol alone or in combination with sirolimus to treat restenosis, vulnerable plaque, aaa and stroke
InactiveUS20080241215A1Minimize potential risk of damageReduce frictionBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Layer - by - layer stereocomplexed polymers as drug depot carriers or coatings in medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque, and atherosclerosis in type 2 diabetic patients. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Methods of coating a device using anti-thrombin heparin
Novel conjugates of glycosaminoglycans, particularly heparin and dermatan sulfate, and amine containing species and therapeutic uses thereof are described. In particular, mild methods of conjugating heparins to proteins, such as antithrombin III and heparin cofactor II, which provide covalent conjugates which retain maximal biological activity are described. Uses of these conjugates to prevent thrombogenesis, in particular in lung airways, such as found in infant and adult respiratory distress syndrome, and on surfaces in contact with blood are also described.
Owner:MCMASTER UNIV
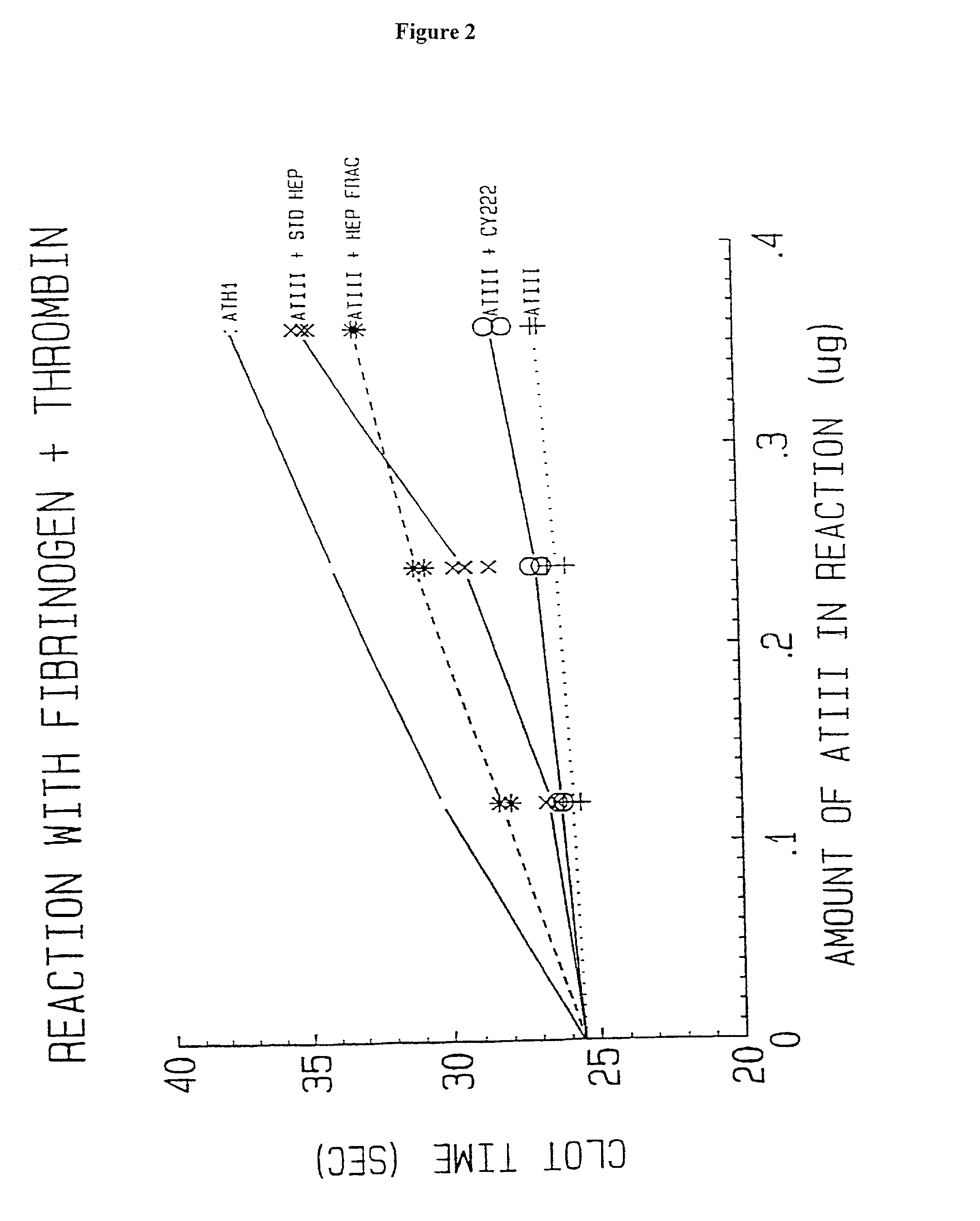
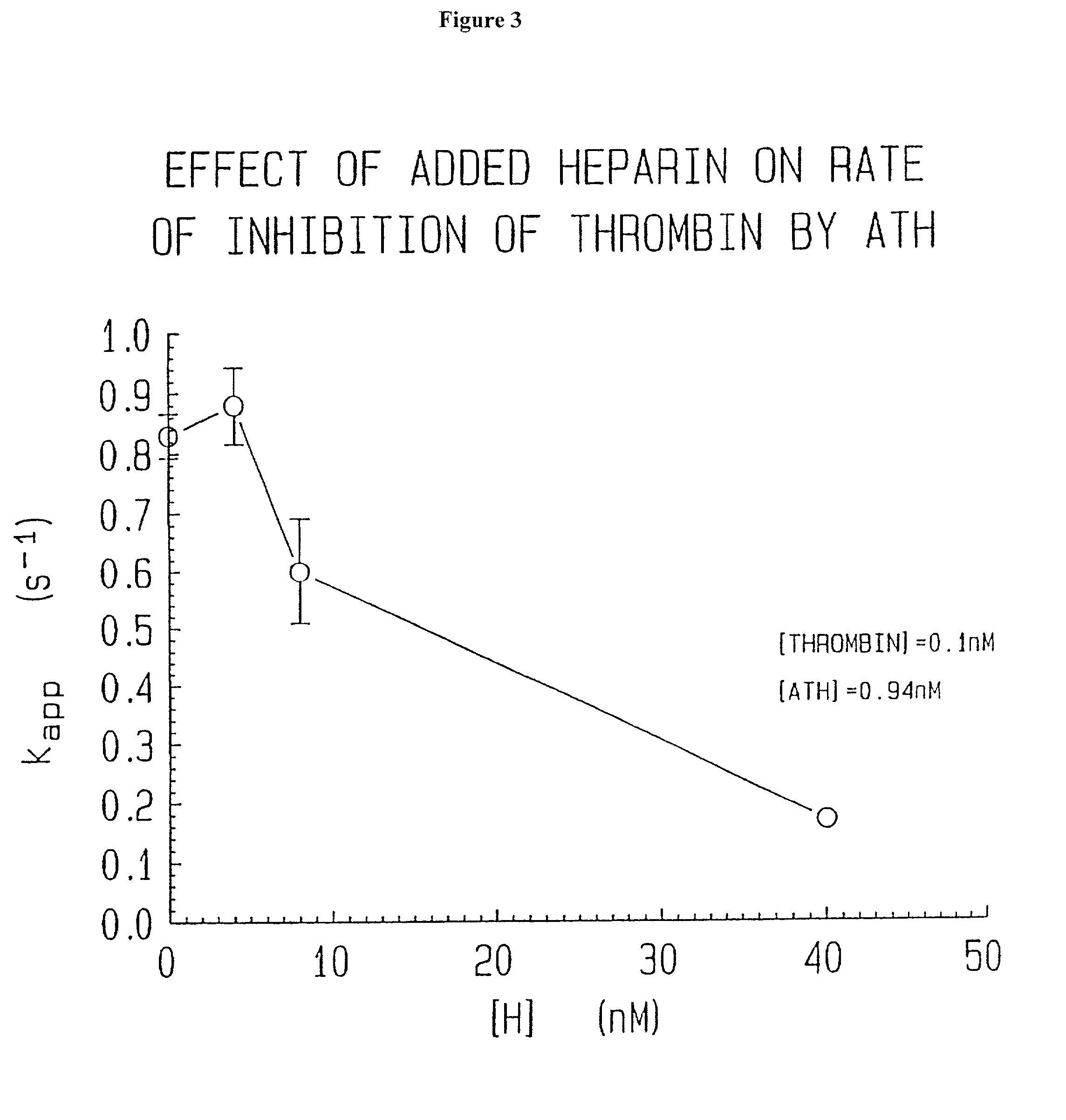
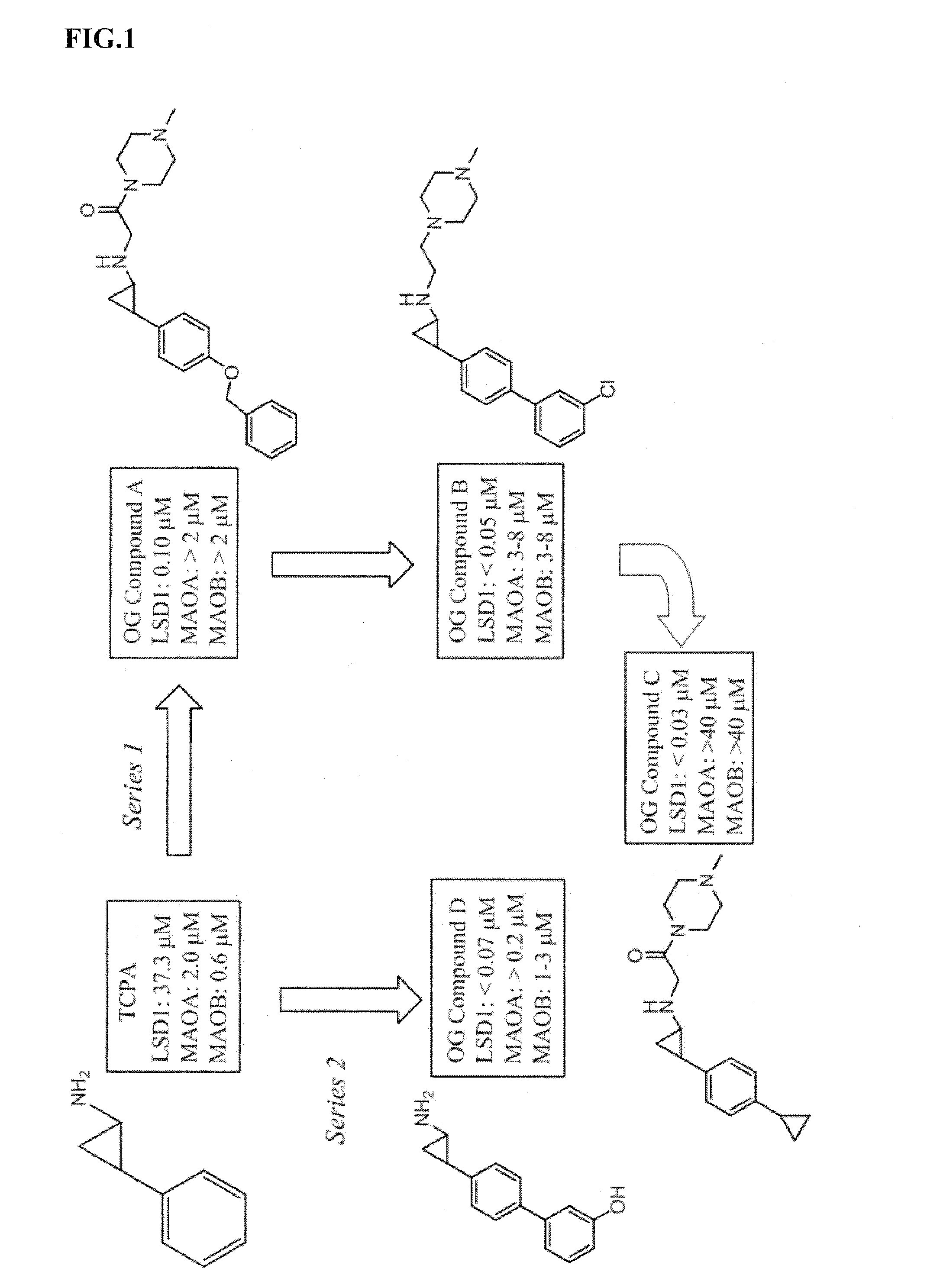
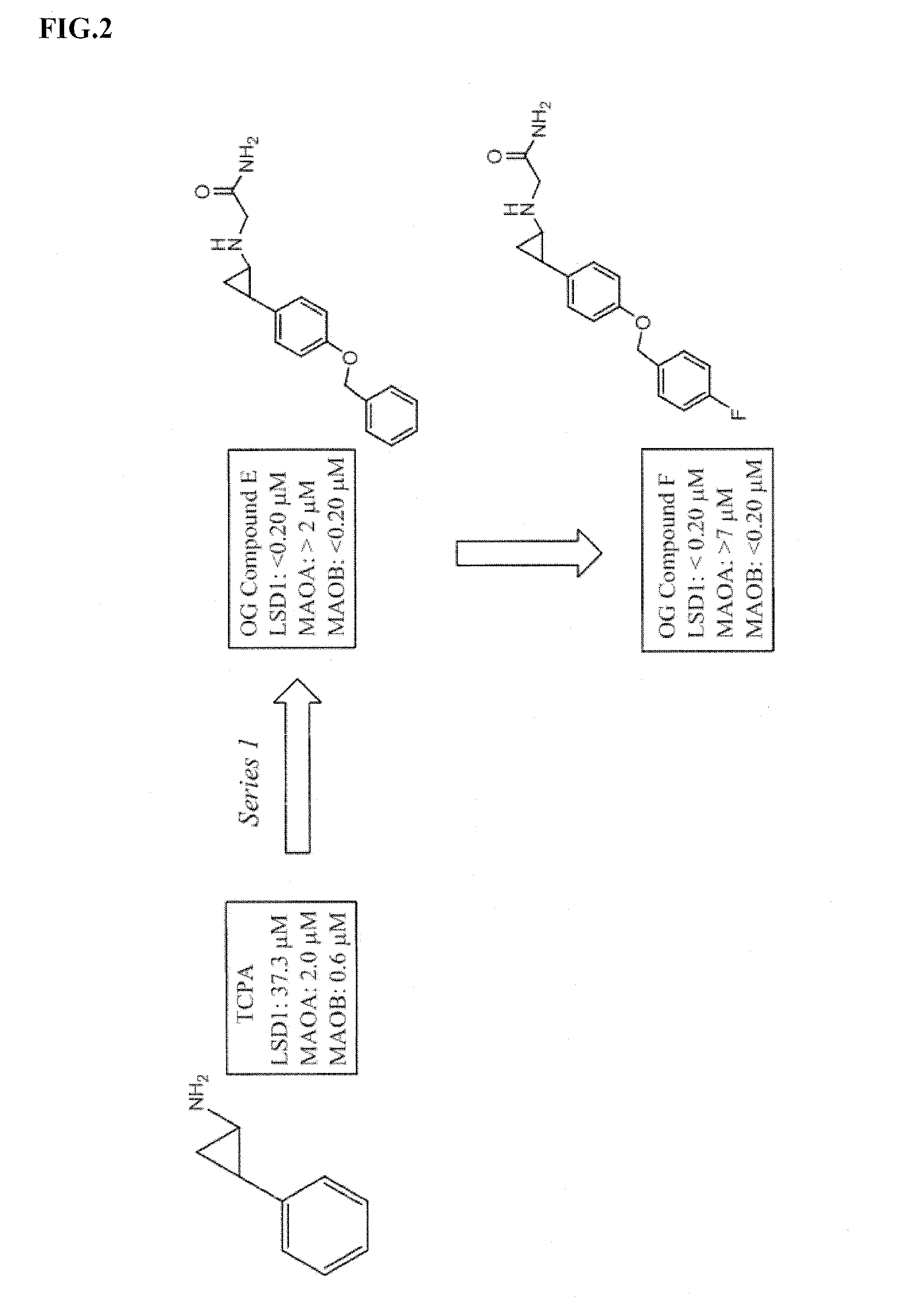
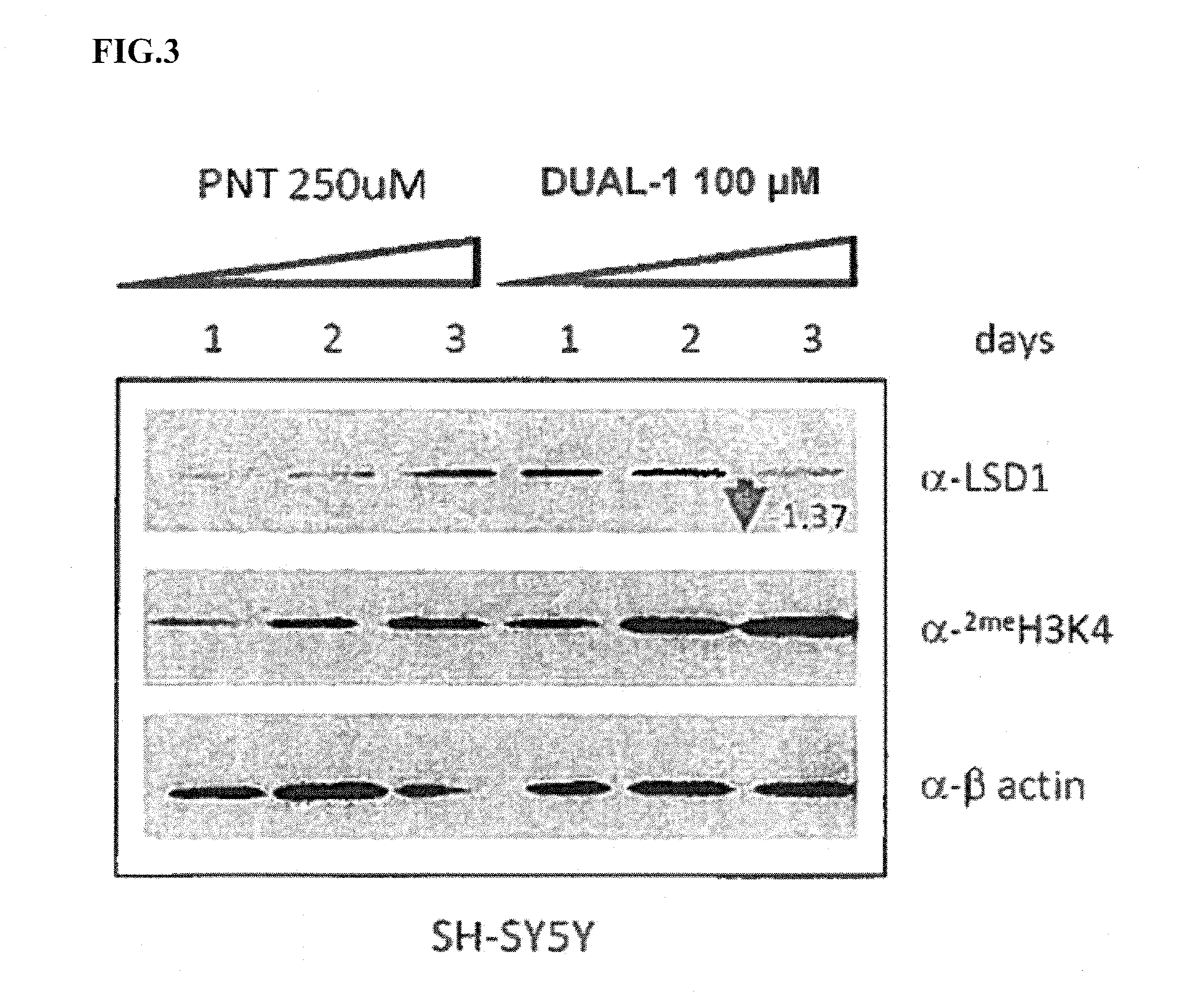
Lysine demethylase inhibitors for thrombosis and cardiovascular diseases
InactiveUS20140296255A1Reduce plateletAvoids side-effectsBiocideAmide active ingredientsThrombusThrombosis prevention
The invention relates to methods and compositions for the treatment or prevention of thrombosis, thrombus formation, a thrombotic event or complication, or a cardiovascular disease or event. In particular, the invention relates to an LSD inhibitor such as a 2-cyclylcyclopropan-1-amine derivative, a phenelzine derivative and a propargylamine derivative, for use in treating or preventing thrombosis, thrombus formation, a thrombotic event or complication, or a cardiovascular disease or event.
Owner:ORYZON GENOMICS SA
Drug-eluting stents coated with P2Y12 receptor antagonist compound
InactiveUS20060122143A1Prevent restenosisPrevent thrombosisBiocideSugar derivativesPercent Diameter StenosisThrombus
The present invention provides a P2Y12 receptor antagonist compound-eluting stent, wherein the stent is coated with one or more P2Y12 receptor antagonist compounds or a pharmaceutically acceptable salt, solvate, or hydrate thereof. When the stent is placed in a narrowed or damaged arterial vessel, a therapeutically effective amount of the P2Y12 receptor antagonist compound is eluted continuously from the stent to the local environment of the stent. The P2Y12 receptor antagonist compound-eluting stents are useful in preventing thrombosis and restenosis, and are effective in inhibiting the contraction of vascular smooth muscle cells, inhibiting cell proliferation, and reducing inflammation.
Owner:INSPIRE PHARMA +1
Targeting recombinant therapeutics to circulating red blood cells
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
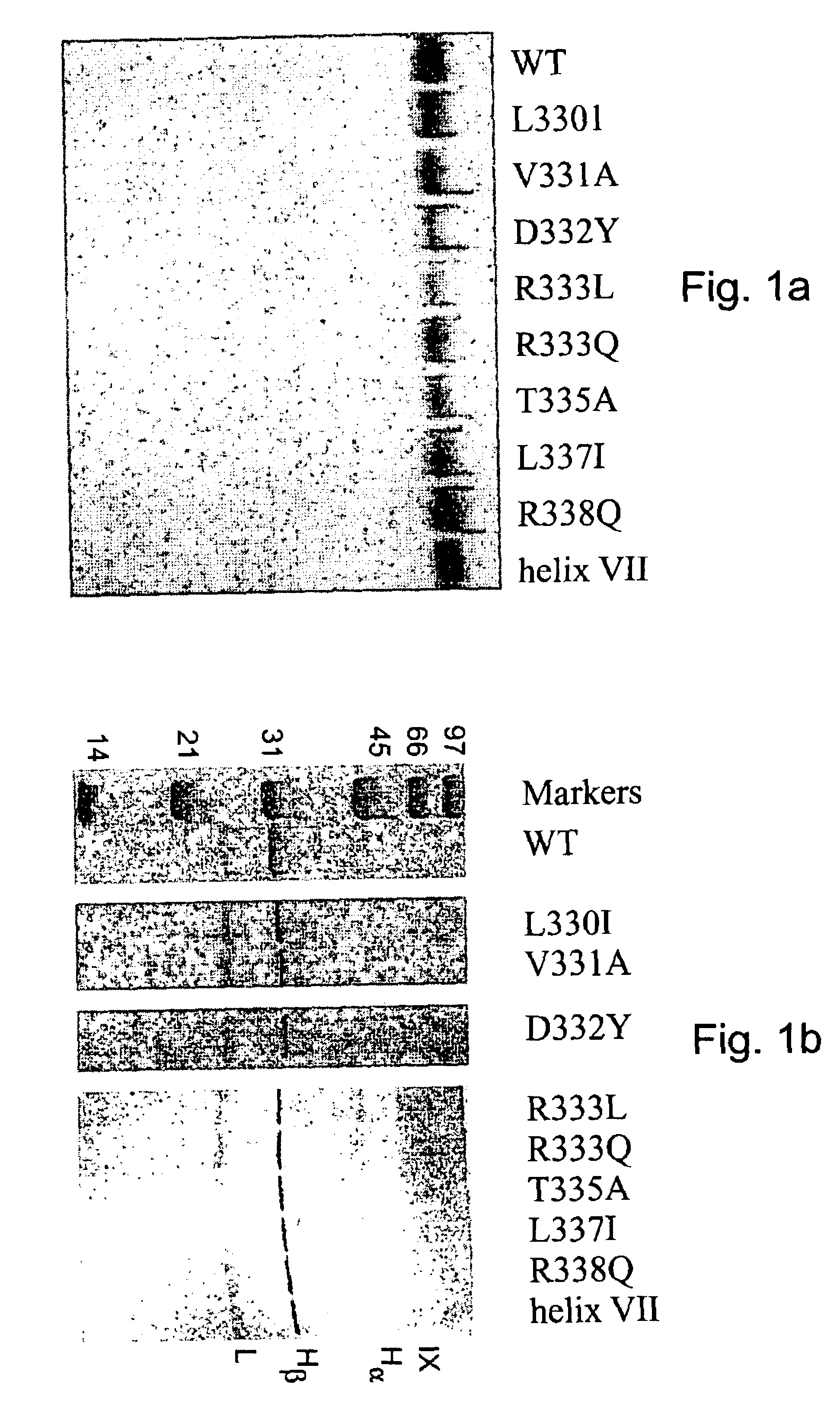
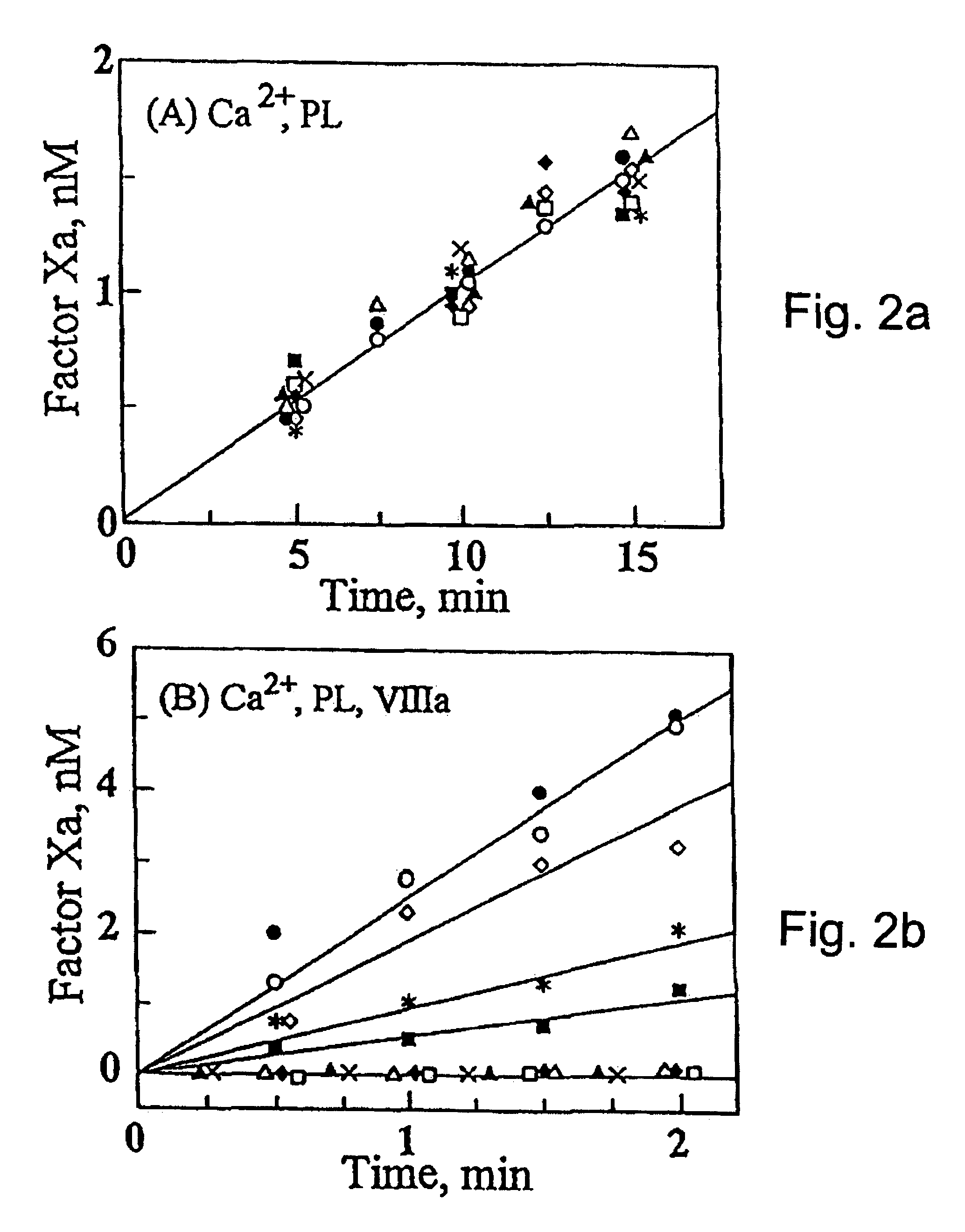
Region of factor IXa protease domain that interacts with factor VIIIa and methods therefor
Novel polypeptides or derivatives comprising the factor VIIIa binding site on factor IXa are disclosed. The novel polypeptides or derivatives have anti-coagulation activity. Nucleic acids encoding those polypeptides are also disclosed. Methods for identifying an agent having anti-coagulation activity are also disclosed. These methods comprise determining whether the agent displaces the polypeptide or derivative from its factor VIIa binding site. The agent identified in these methods is also useful in methods for treating a patient to prevent thrombosis. The treatment methods comprise administration of the agent to the patient. Additional methods are also disclosed for treating a patient to prevent thrombosis, comprising treating the patient with a polypeptide or derivative comprising the factor VIIIa binding site on factor IXa. Methods of preventing coagulation in a blood sample are also disclosed, comprising adding the polypeptides or derivatives described above to the blood sample. Methods of detecting factor VIIIa in a sample are also disclosed. Those methods comprise contacting the sample with the above-described polypeptide or derivative, wherein the polypeptide or derivative also comprises a covalently attached detectable moiety, then determining whether the polypeptide or derivative is binding factor VIIIa from the sample.
Owner:SAINT LOUIS UNIVERSITY
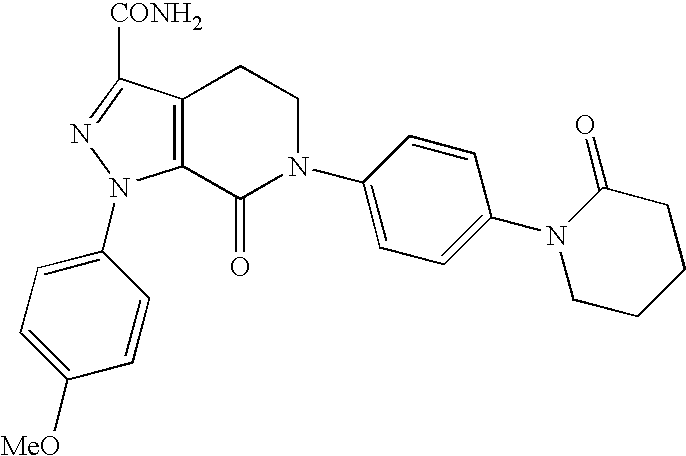
Prophylaxis of thromboembolic events in cancer patients
The present invention generally relates a method of prophylaxis of thrombotic and thromboembolic events in a cancer patient in need thereof comprising administering to said patient a therapeutically effective amount of a factor Xa inhibitor, especially apixaban or a polymorph or pharmaceutically acceptable solvate form thereof. The factor Xa inhibitor may be used in combination with therapeutic agents.
Owner:BRISTOL MYERS SQUIBB CO
Morpholinone compounds as factor ixa inhibitors
Owner:MOCHIDA PHARM CO LTD +1
Modified annexin proteins and methods for preventing thrombosis
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:SURROMED
Targeting recombinant therapeutics to circulating red blood cells
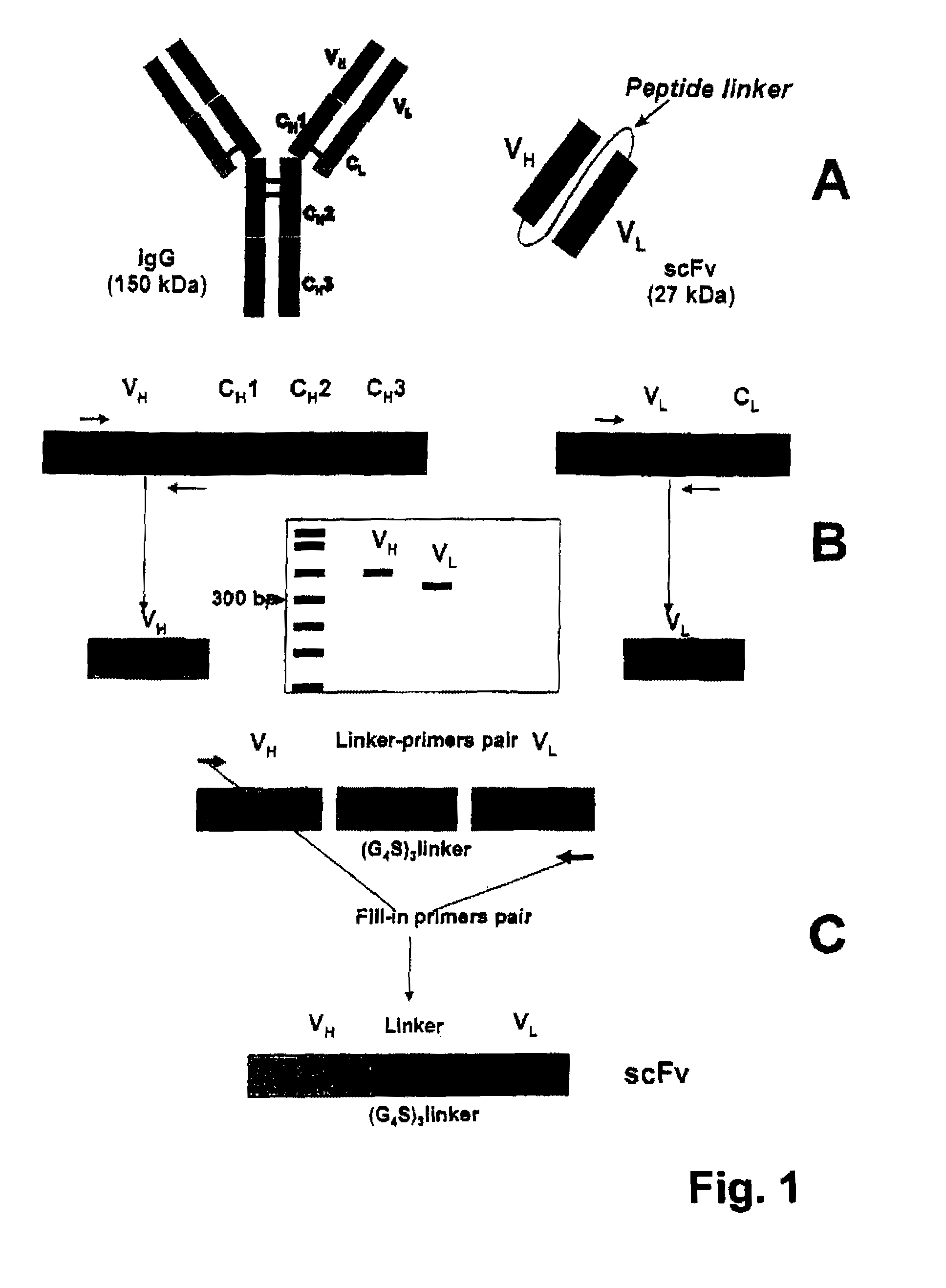
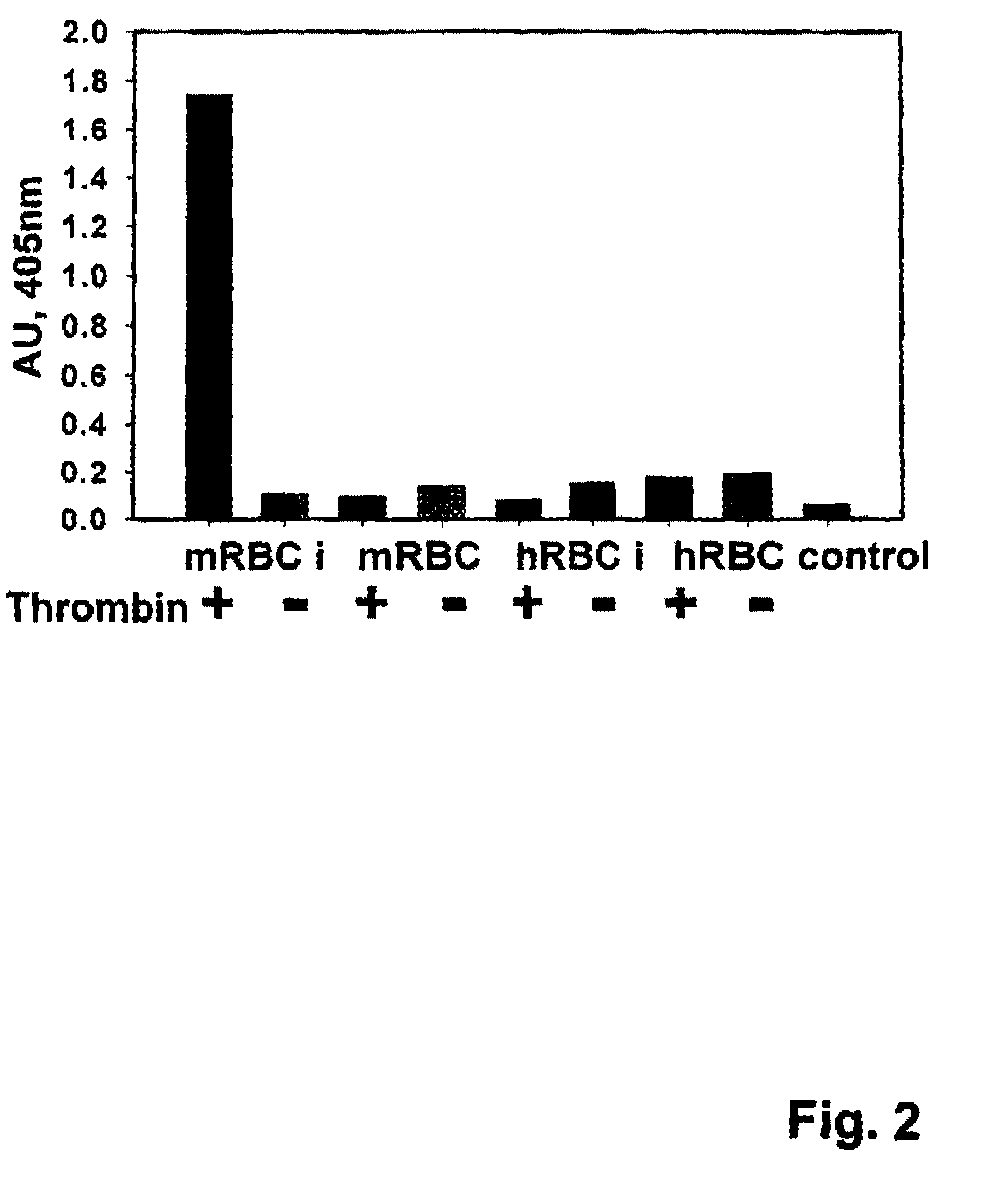
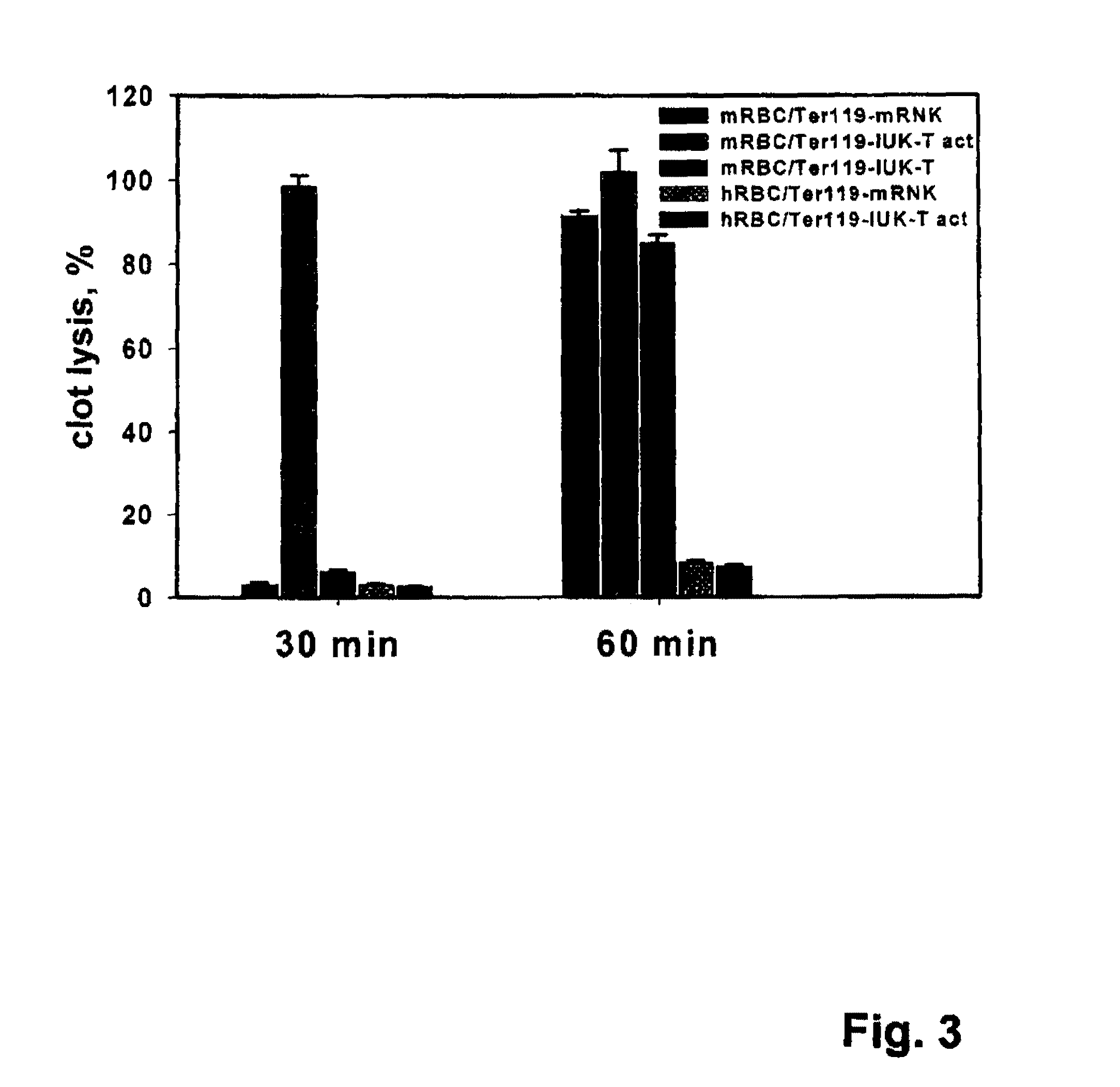
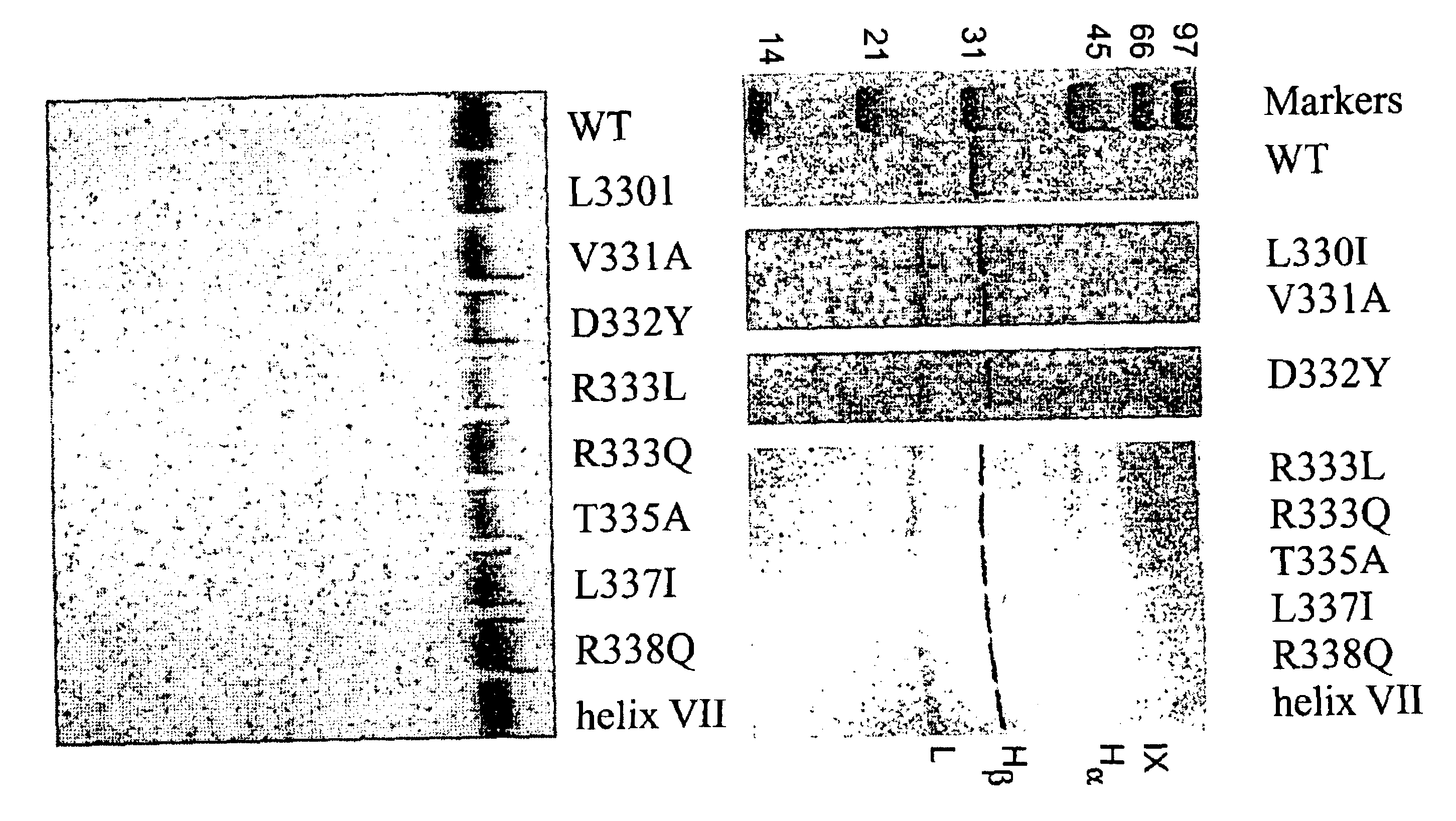
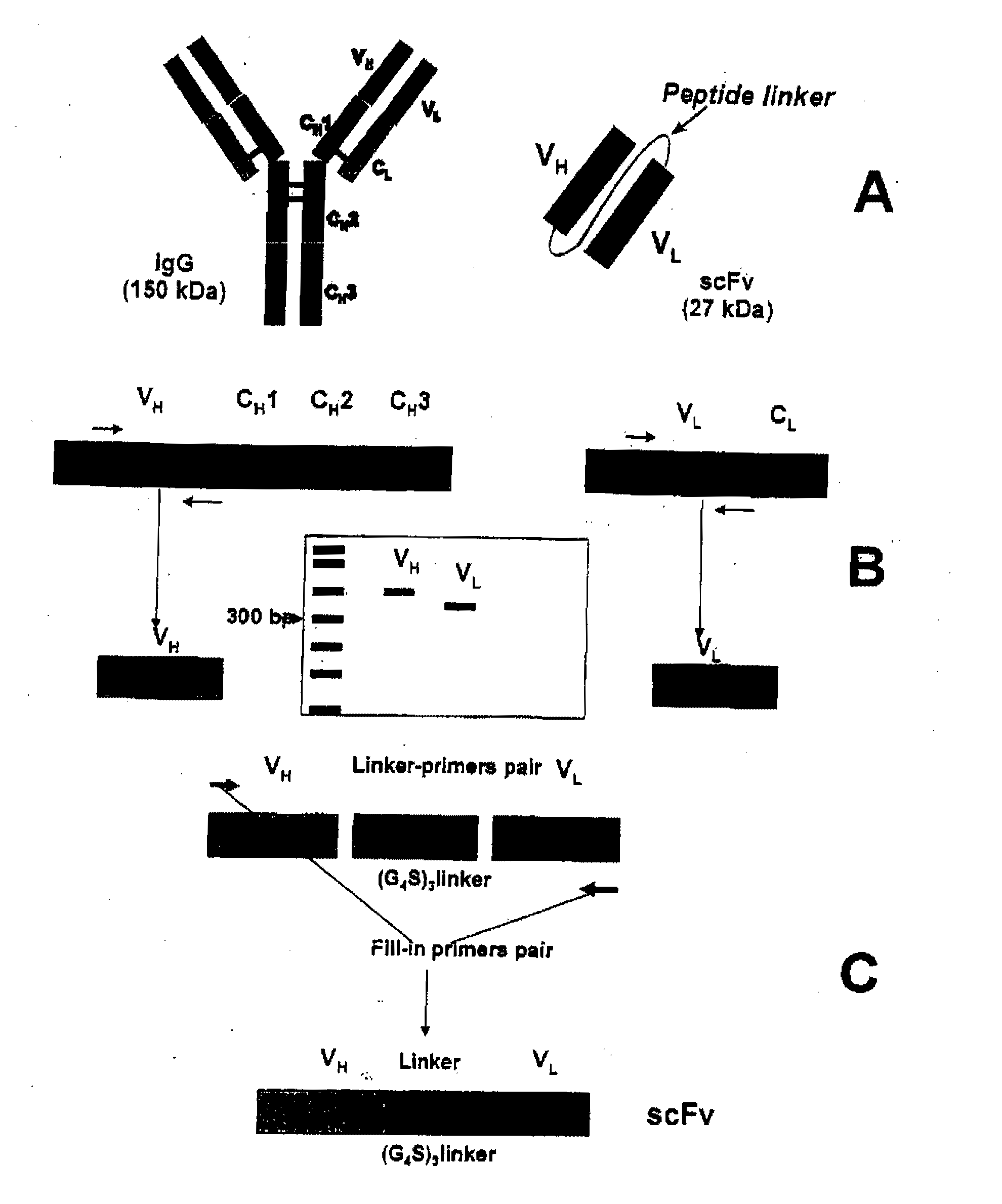
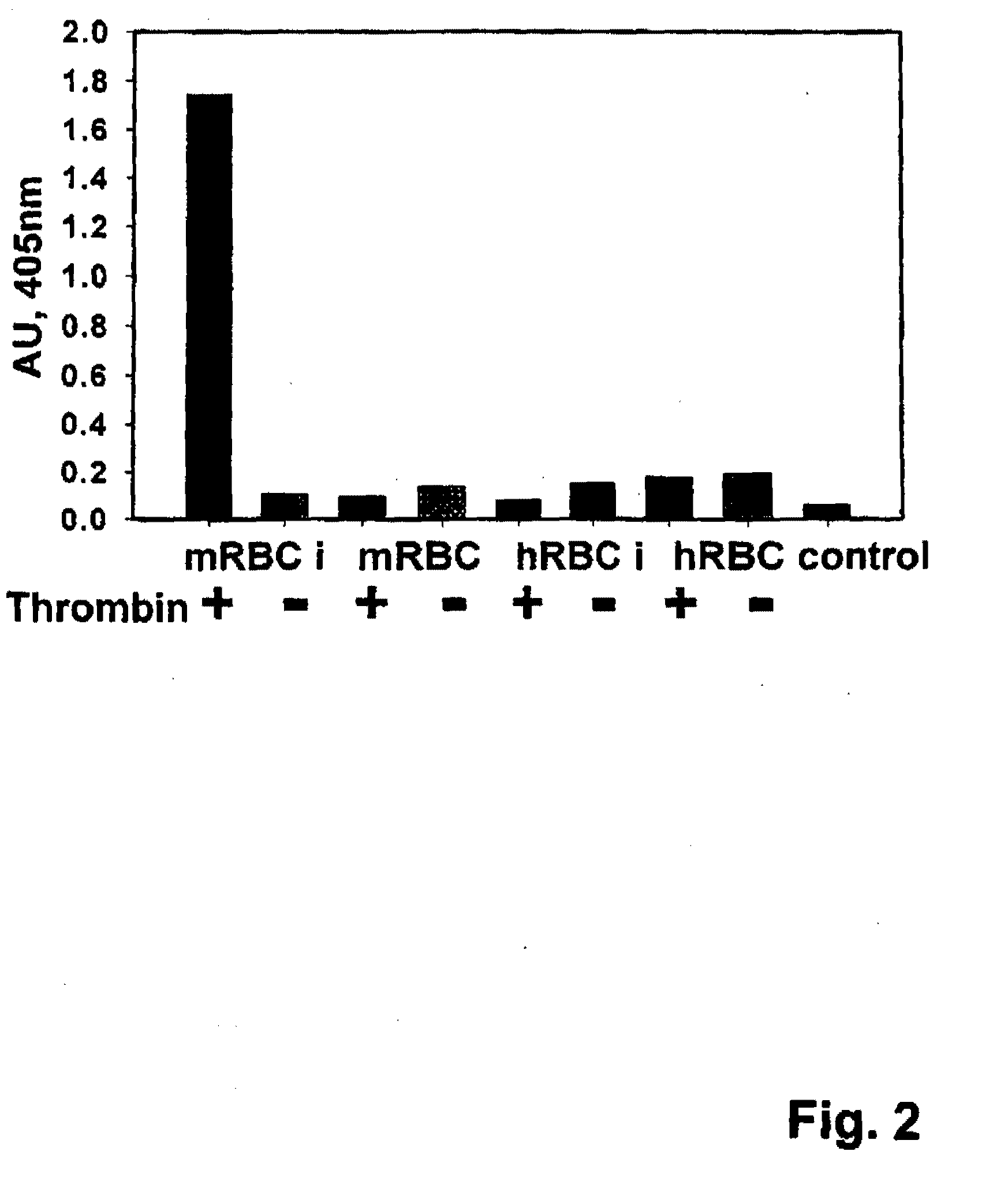
Fusion proteins comprising a single chain antigen-binding domain (scFv) of a monoclonal antibody, linked to an anti-thrombotic agent, anti-inflammatory agent, or a pro-drug thereof are provided, where the polypeptide binds to a binding site (antigen) expressed on the surface of a red blood cell at a density greater than 5,000 copies per red blood cell. Pharmaceutical compositions comprising these fusion proteins, and methods of delivering an anti-thrombotic agent to the surface of a red blood cell via delivery of these fusion proteins, and methods of treating or preventing thrombosis, tissue ischemia, acute myocardial infarction (AMI), ischemic stroke, cerebrovascular disease, pulmonary embolism, or ischemic peripheral vascular disease via administration of the fusion proteins or compositions comprising same are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Modified annexin proteins and methods for their use in organ transplantation
InactiveUS20060105952A1Preventing arterial or venous thrombosisExtended half-lifePeptide/protein ingredientsDead animal preservationReperfusion injuryWhite blood cell
Modified annexin proteins, including a homodimer of human annexin V, are provided. Methods for their use, such as to prevent thrombosis without increasing hemorrhage and to attenuate ischemia-reperfusion injury (IPI), are also provided. The modified annexins bind phosphatidylserine (PS) on cell surfaces, thereby preventing the assembly of the prothromkinase complex. The modified annexin decreases the binding of leukocytes and platelets during post-ischemic reperfusion, thereby restoring microvascular blood flow and decreasing organ damage.
Owner:ALAVITA PHARMA
Therapeutic agent elution control process
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Implantable coated medical devices may be processed through annealing to better control the elution characteristics of the therapeutic agents. Furthermore, annealing leads to better therapeutic agent stability and a larger device shelf-life.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
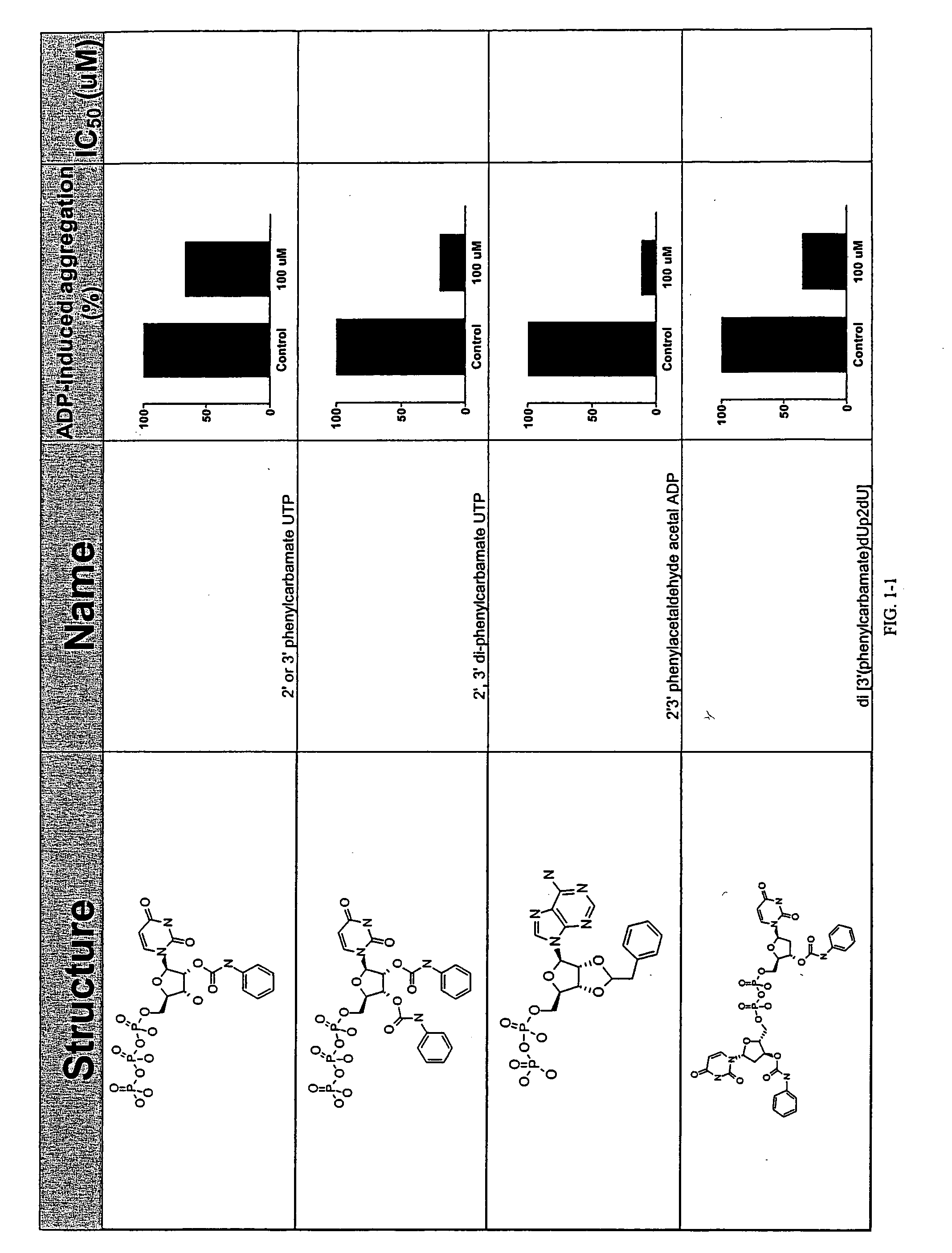
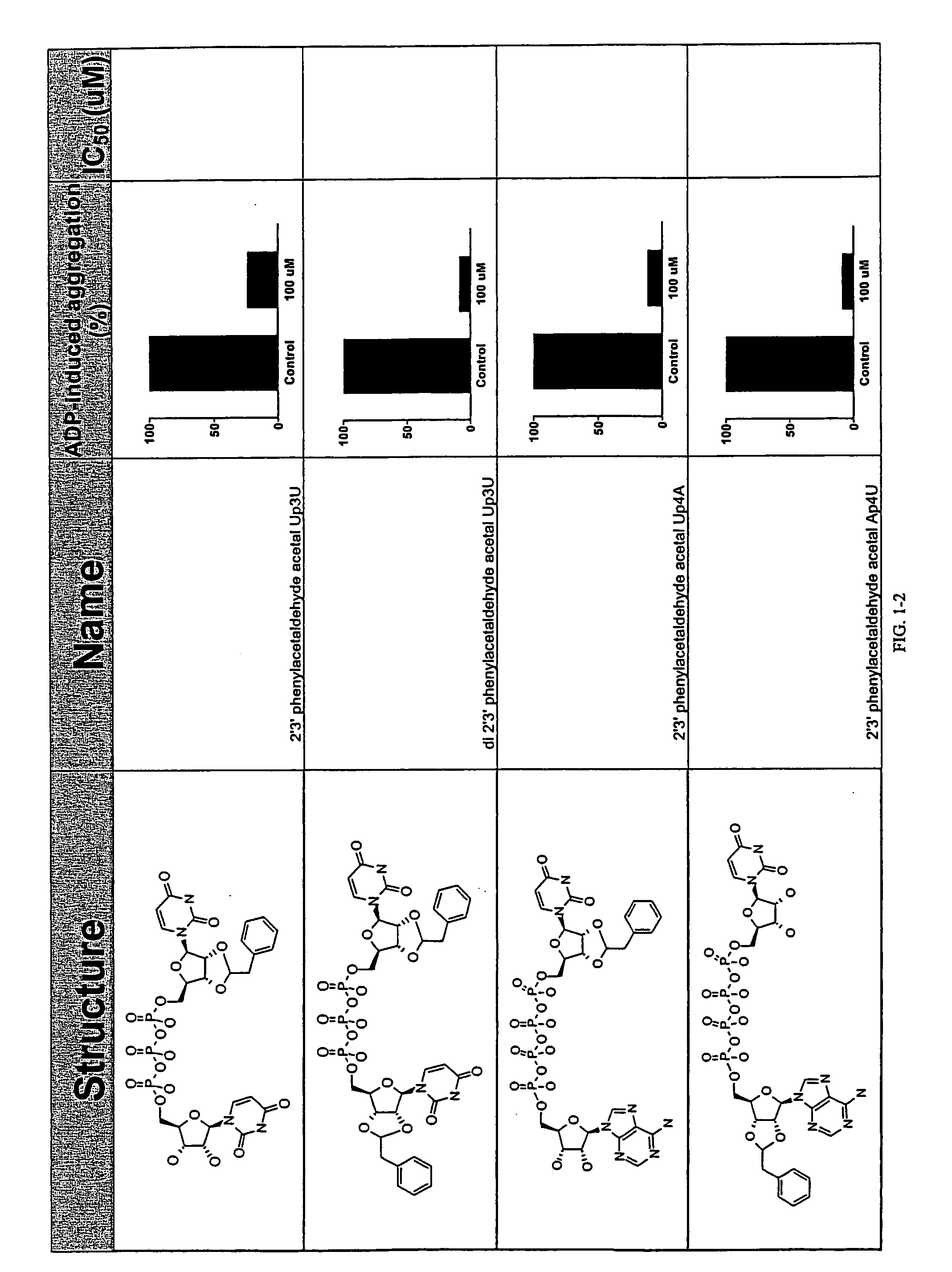
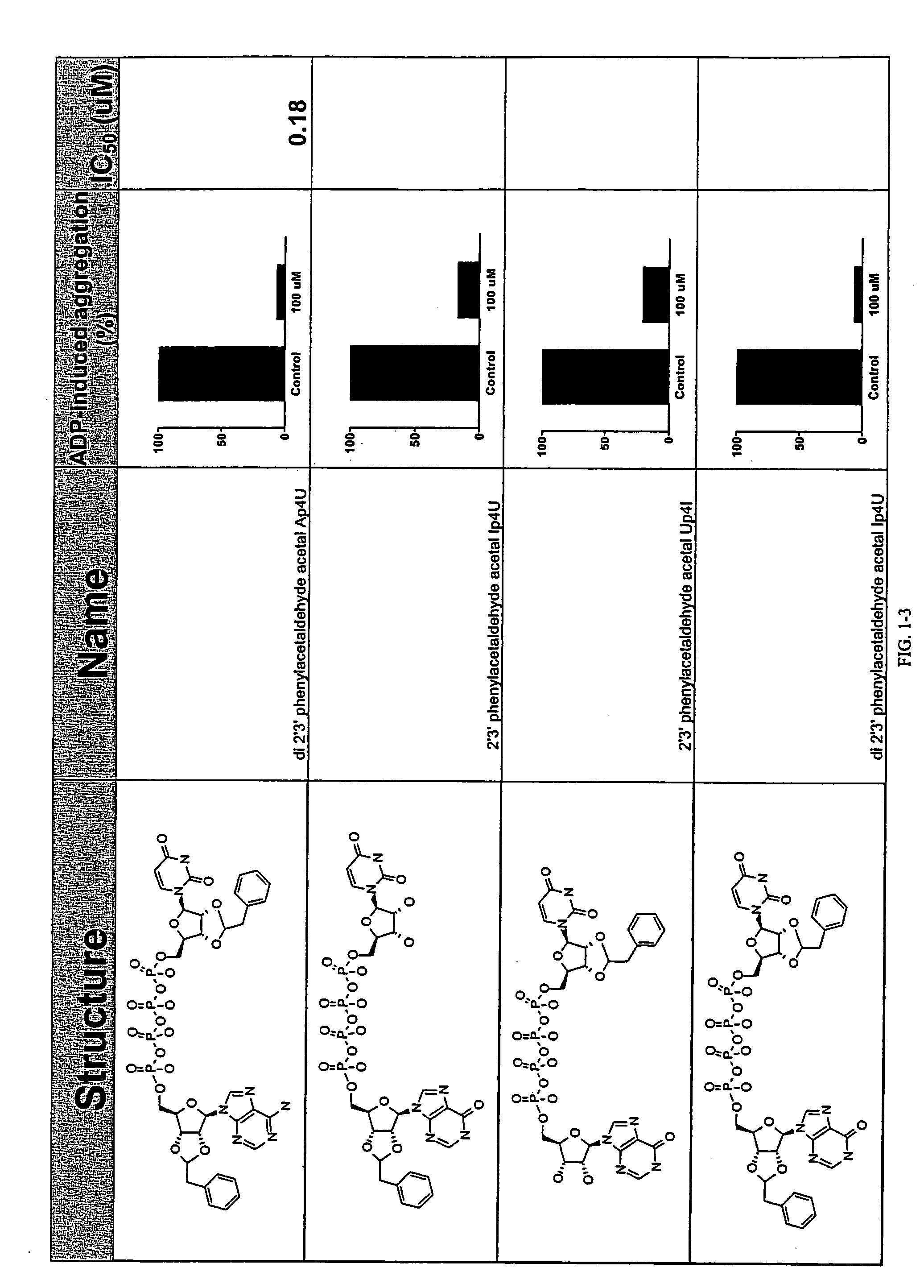
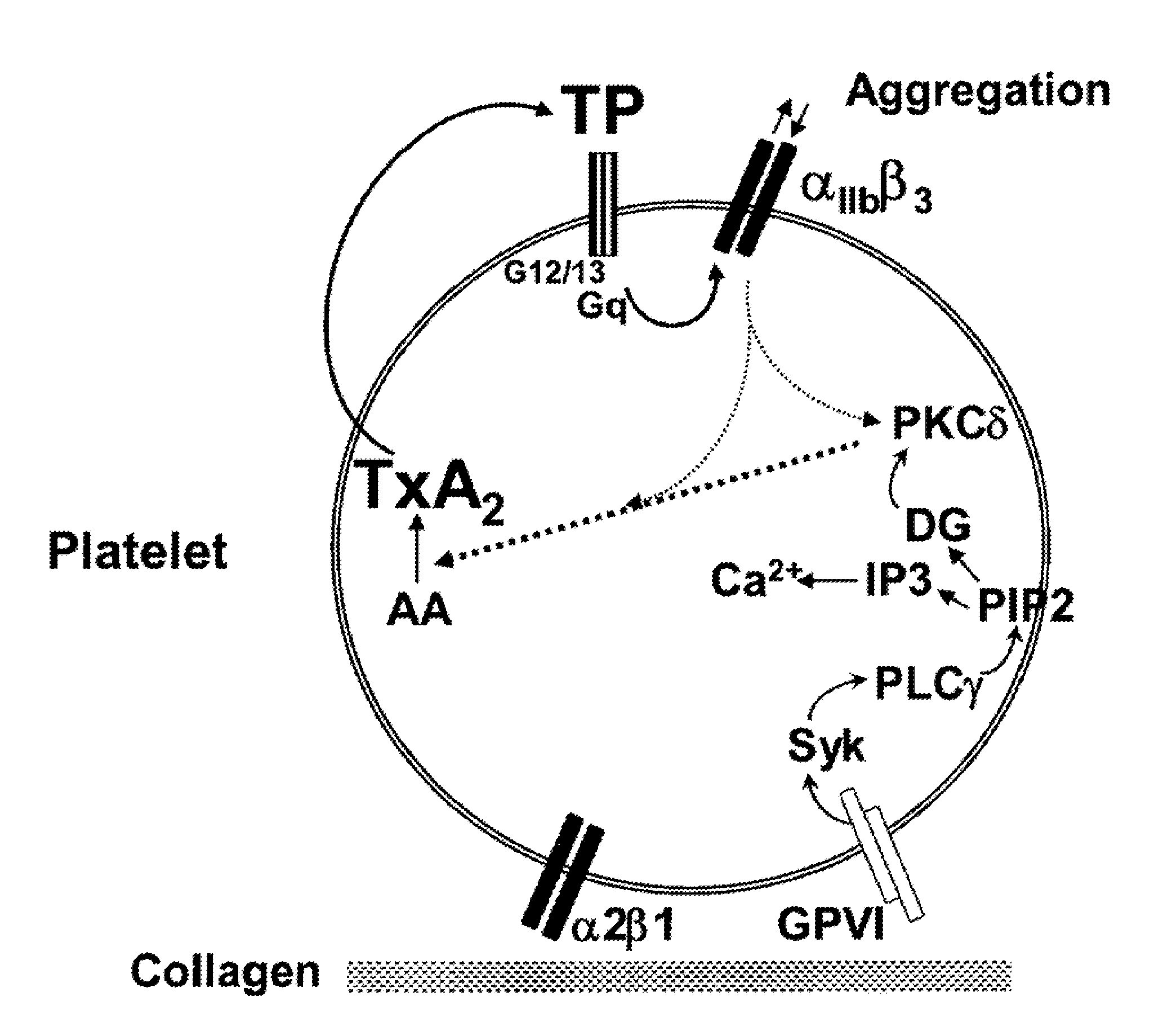
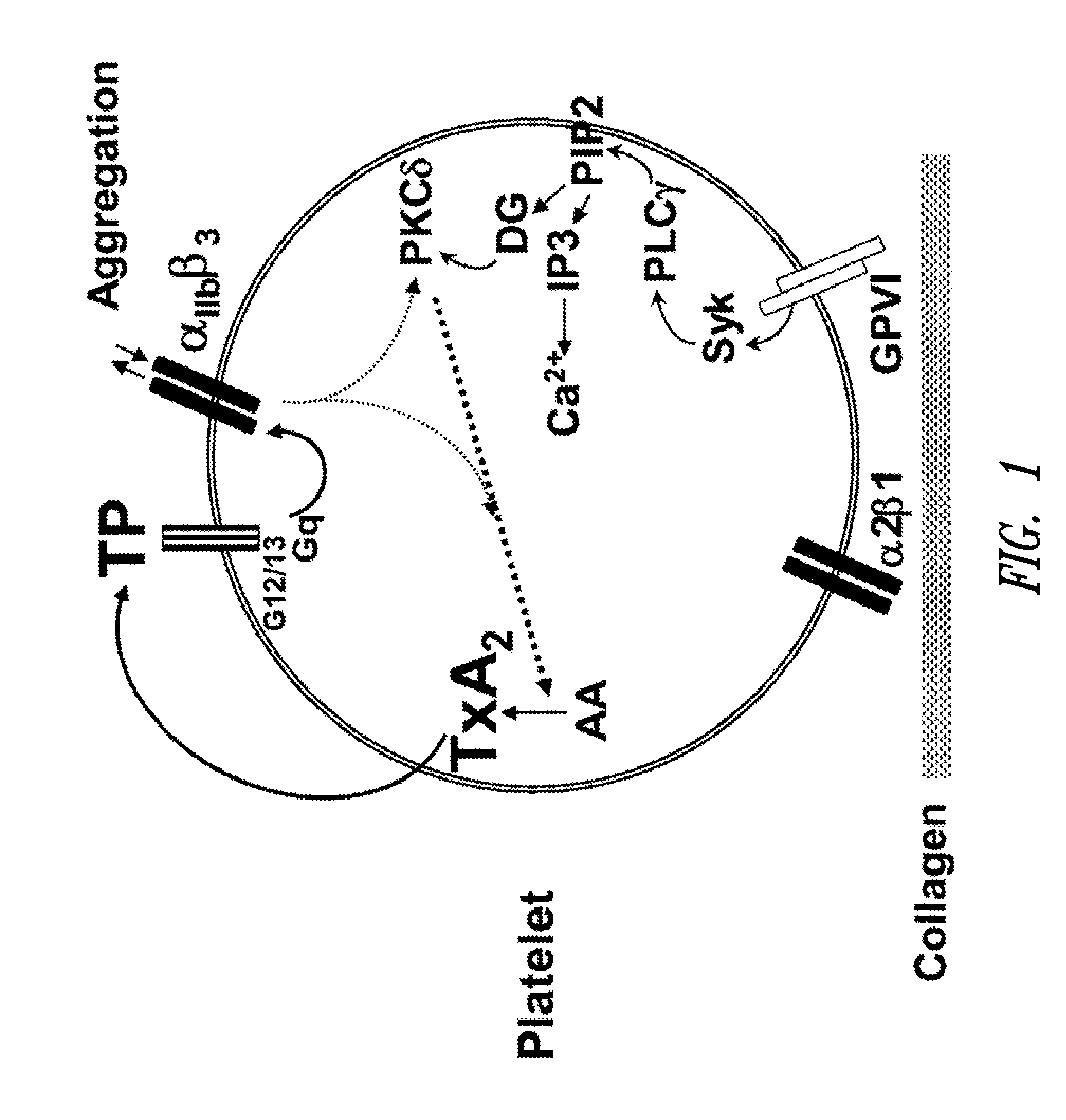
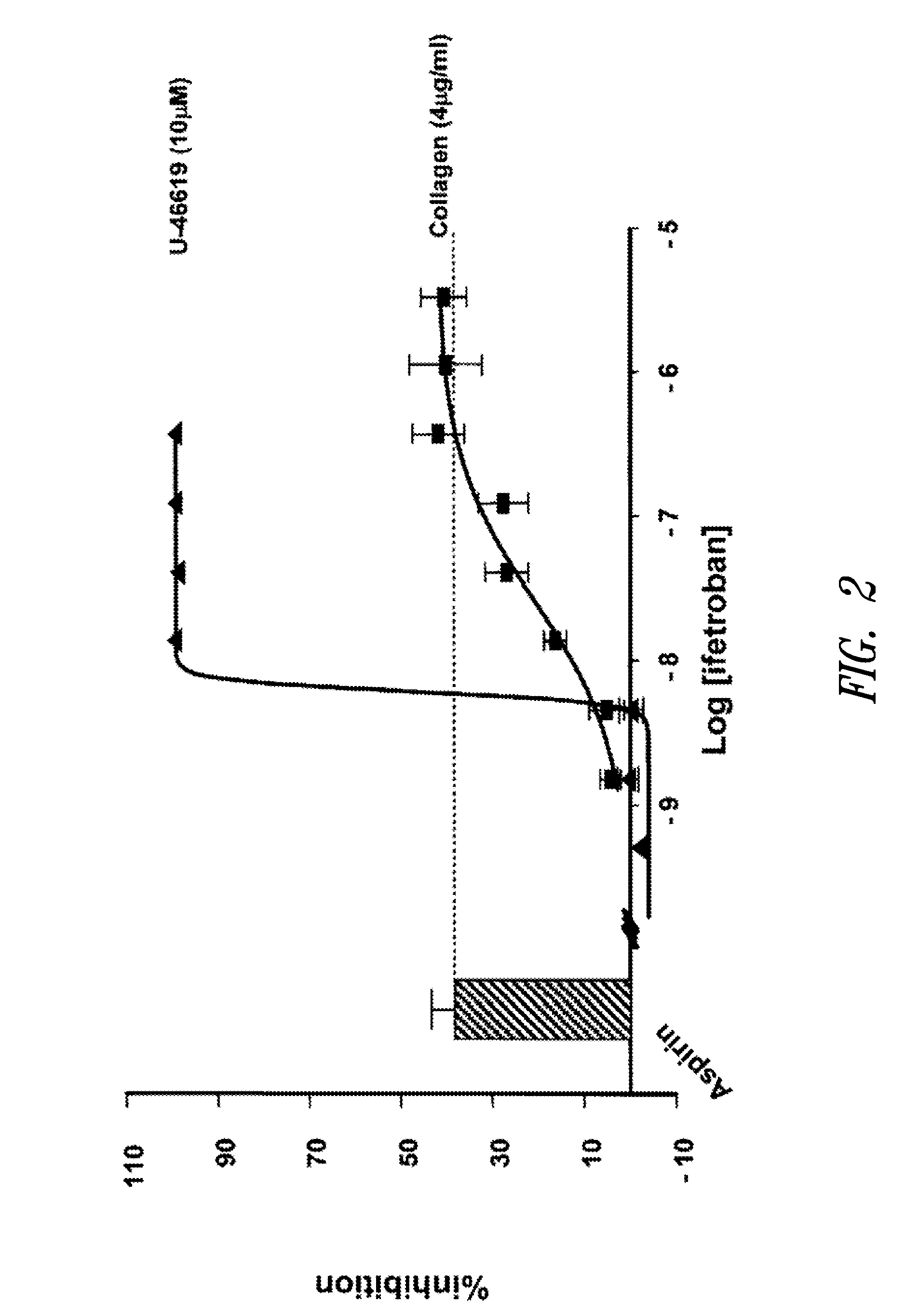
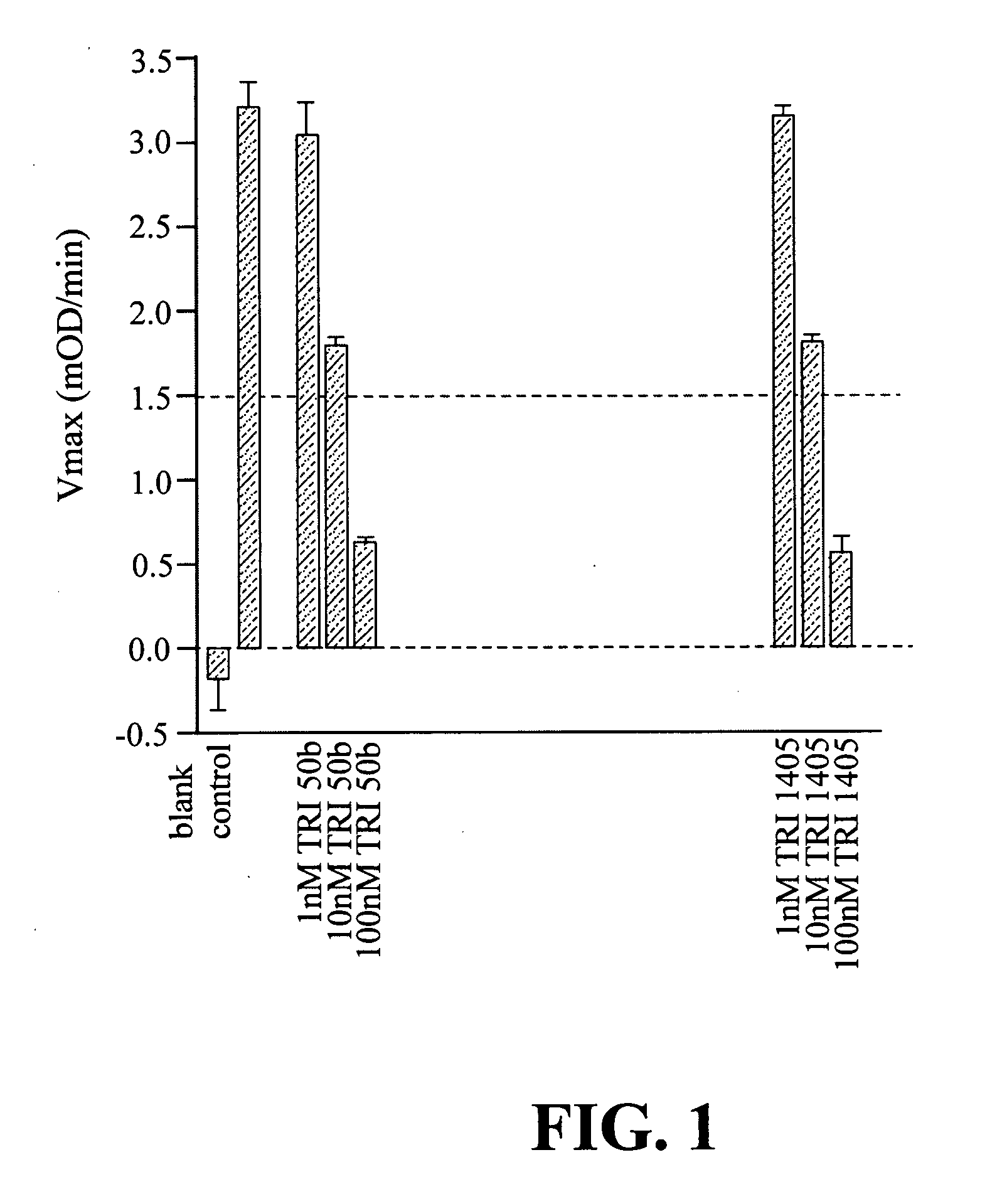
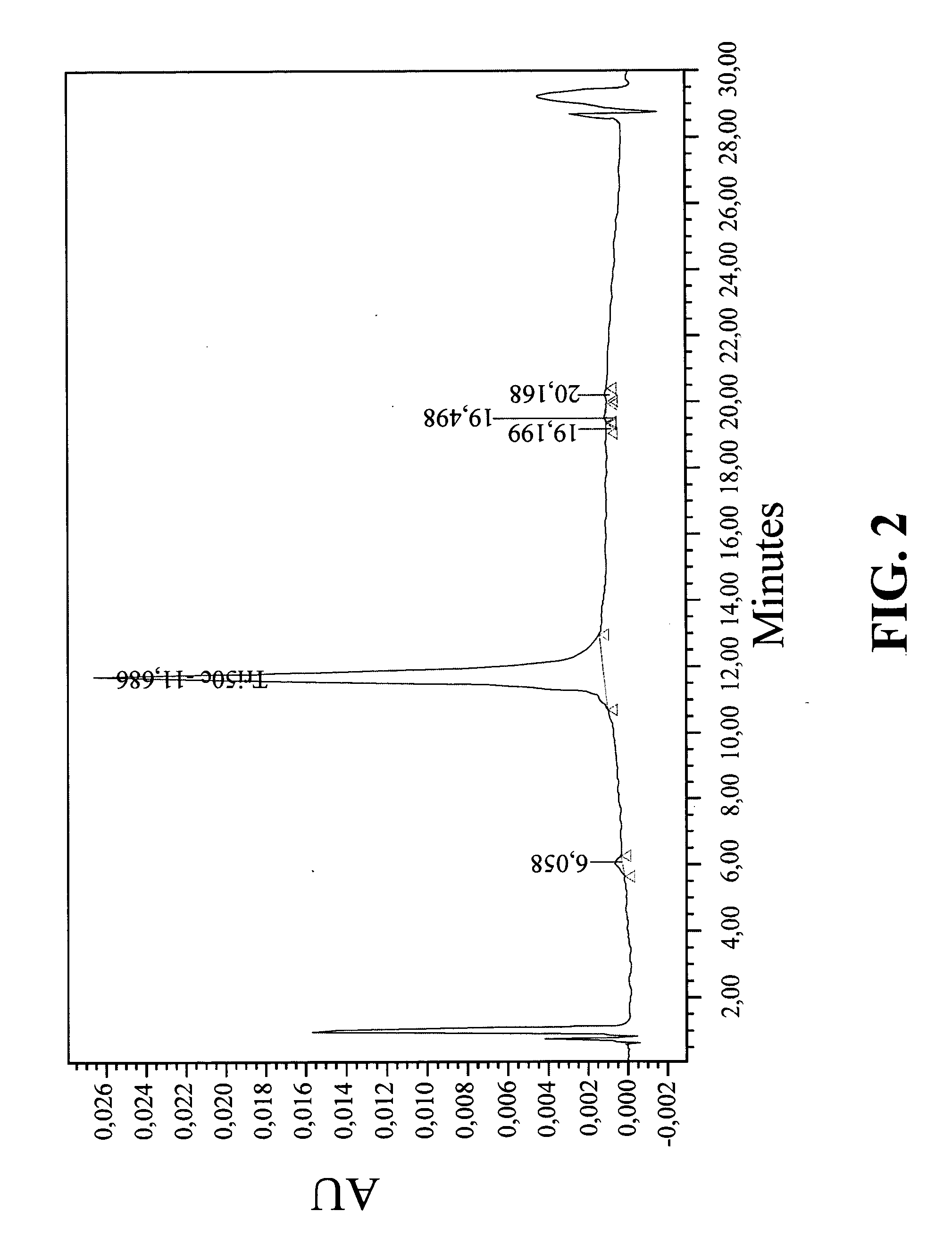
Unit dose formulations and methods of treating and preventing thrombosis with thromboxane receptor antagonists
InactiveUS20090012136A1Inhibit aggregationInhibits platelet aggregationAntibacterial agentsBiocideAntithrombotic AgentTP Receptors
The present invention provides new methods of treating thrombosis and cardiovascular diseases using of antithrombotic agents, as well as methods of determining therapeutically effective amounts of antithrombotic agents and unit dose formulations thereof.
Owner:ALEXION PHARMA INC
Expandable devices coated with a paclitaxel composition
InactiveUS20120302954A1Reduced responsePromote healingOrganic active ingredientsBalloon catheterDiseaseThrombus
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients.
Owner:CORDIS CORP
Modified annexin compositions and methods of using same
InactiveUS7645739B2Preventing arterial or venous thrombosisExtended half-lifePeptide/protein ingredientsAntinoxious agentsReperfusion injurySurvivability
Modified annexin proteins, including a homodimer of human annexin V, are provided. Methods for their use, such as to prevent thrombosis without increasing hemorrhage, enhancing the survivability of platelets during storage or transfusion and to attenuate ischemia-reperfusion injury (IPI), are also provided. The modified annexins bind phosphatidylserine (PS) on cell surfaces, thereby preventing the assembly of the prothromkinase complex. The modified annexin decreases the binding of leukocytes and platelets during post-ischemic reperfusion, thereby restoring microvascular blood flow and decreasing organ damage. In addition, the modified annexin prevents lipid loss from platelets during storage.
Owner:ALAVITA PHARMA
Peptide boronic acid compounds useful in anticoagulation
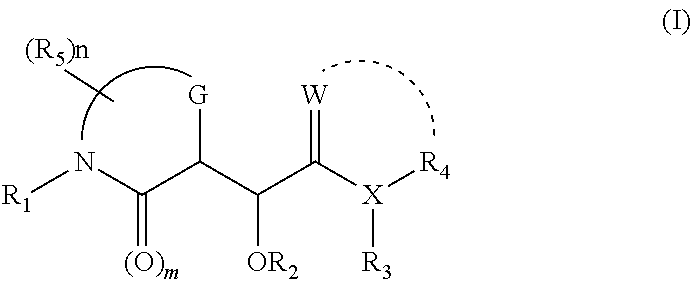
InactiveUS20050282757A1Reduce the amount of solutionImprove consistencyBiocideDipeptide ingredientsThrombin activityBoronic acid
A method for preventing thrombosis in a setting where rapid onset and / or rapid offset of anticoagulation is required, comprising administering a compound selected from the group consisting of boronic acids which have a neutral thrombin P1 domain linked to a hydrophobic moiety capable of binding to the thrombin S2 and S3 subsites, and pharmaceutically acceptable salts, prodrugs and pharmaceutically acceptable prodrug salts of such acids.
Owner:PAION GMBH
Conjugates comprising a biodegradable polymer and uses therefor
InactiveUS7785618B2Improve biological activityProlong half-life in vivoPeptide/protein ingredientsPharmaceutical non-active ingredientsVascular diseaseActive agent
Biologically active agents covalently linked to a polymer. The polymer is preferably a biodegradable polymer are provided. The biologically active agent is preferably a protein, such as an extracellular soluble protein, e.g., an extracellular enzyme. The enzyme can be an apyrase, e.g., NTPDase. Conjugates of the invention can be used as therapeutics in subjects. For example, a conjugate comprising an apyrase can be used for treating and preventing thrombosis, atherosclerotic plaque complications and vascular disorders.
Owner:MERSANA THERAPEUTICS INC +1
Section-by-section pressurization lower limb vein auxiliary backflow air sac
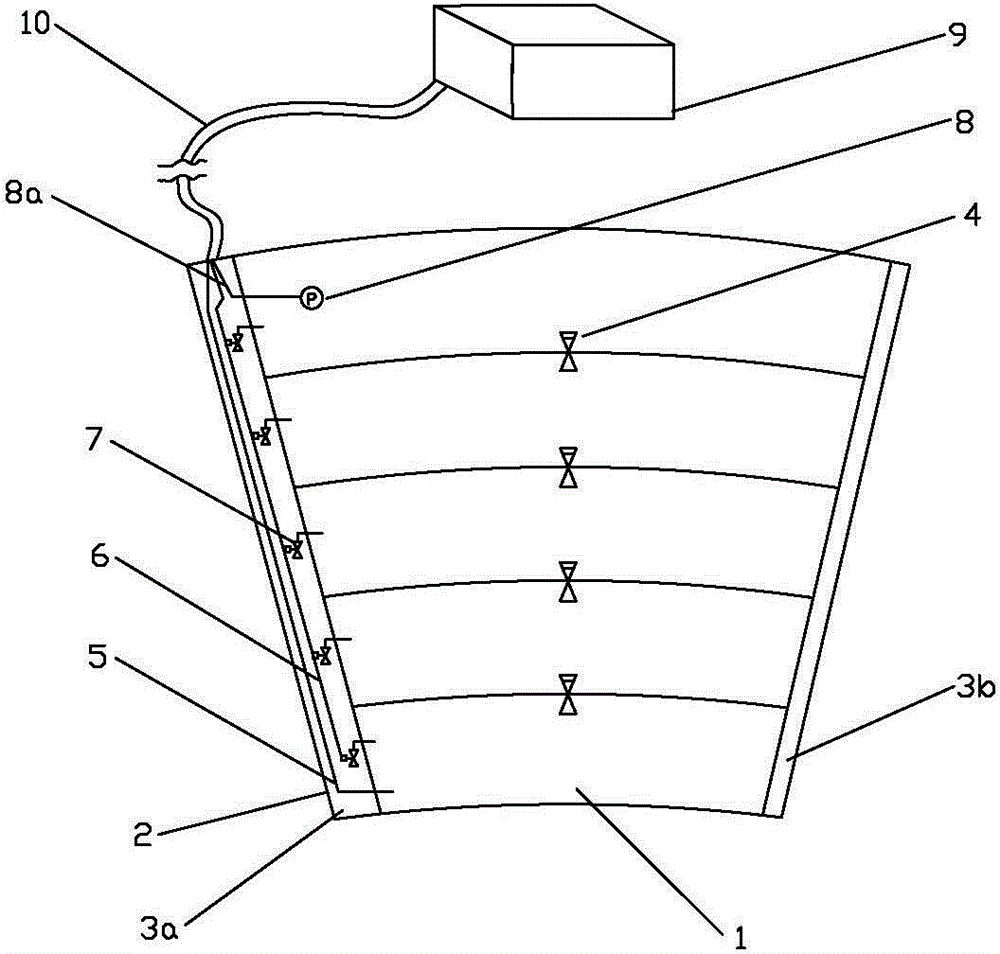
The invention relates to a section-by-section pressurization lower limb vein auxiliary backflow air sac which is capable of solving the problem of unsmooth lower limb vein backflow or thrombosis effectively. According to the technical scheme, the sectorial air sac is divided into a plurality of section air sac bodies which are adjacent and sealed respectively along the radial direction. A pressure check valve is arranged between every two section air sac bodies. An air inlet is formed in one end of the air sac body in the bottom section and is connected with a compression air pipeline. A pressure sensor is arranged in the air sac body in the top section, and a signal line of the pressure sensor stretches out one end of the air sac body. An air leakage hole is formed in one end of the air sac body in each section and is connected with a mini solenoid valve, and all mini solenoid valves are connected in parallel into an air leakage execution line. By means of the air sac, the defect that lower limbs are squeezed and massaged by applying overall equal pressure of some passive measures in the prior art is overcome, and the air sac is innovative as for unsmooth lower limb vein backflow or thrombosis prevention and auxiliary treating devices.
Owner:HENAN UNIV OF CHINESE MEDICINE
Layer-by-layer stereocomplexed polymers as drug depot carriers or coatings in medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque, and atherosclerosis in type 2 diabetic patients. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Production process of natural marine organism health food with deep sea clam worm as material
InactiveCN1436490ANo pollution in the processExtraction method scienceCapsule deliveryFood preparationFreeze-dryingPhosphate
The production process of natural marine organism health food includes the process of culturing deep sea clam worm in salt water for 3-4 days to make it spit out content; homogenizing and centrifuging after adding physiological saline or phosphate buffering liquid; salting out, dialysis or column chromatography and ultrafiltration to eliminate impurities and purify solution; spray drying or freezing drying to obtain powder; Co-60 ray irradiatino to sterilize to obtain food powder; and capsulizing. The health food contains rich amino acids, fatty acids, trace elements, vitamines and bioactive enzymes, especially great amount of plasmin. Experiments show that the health food has the health functions of regulating blood pressure and blood fat, preventing thrombus, resisting senility and strengthening immunity.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Modified annexin proteins and methods for their use in platelet storage and transfusion
InactiveUS20070015705A1Preventing arterial or venous thrombosisExtended half-lifePeptide/protein ingredientsAntinoxious agentsLipid formationReperfusion injury
Modified annexin proteins, including a homodimer of human annexin V, are provided. Methods for their use, such as to prevent thrombosis without increasing hemorrhage, enhancing the survivability of platelets during storage or transfusion and to attenuate ischemia-reperfusion injury (IPI), are also provided. The modified annexins bind phosphatidylserine (PS) on cell surfaces, thereby preventing the assembly of the prothromkinase complex. The modified annexin decreases the binding of leukocytes and platelets during post-ischemic reperfusion, thereby restoring microvascular blood flow and decreasing organ damage. In addition, the modified annexin prevents lipid loss from platelets during storage.
Owner:ALAVITA PHARMA
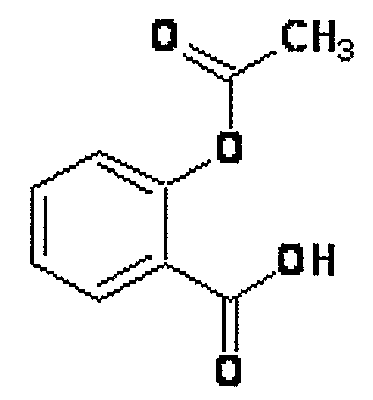
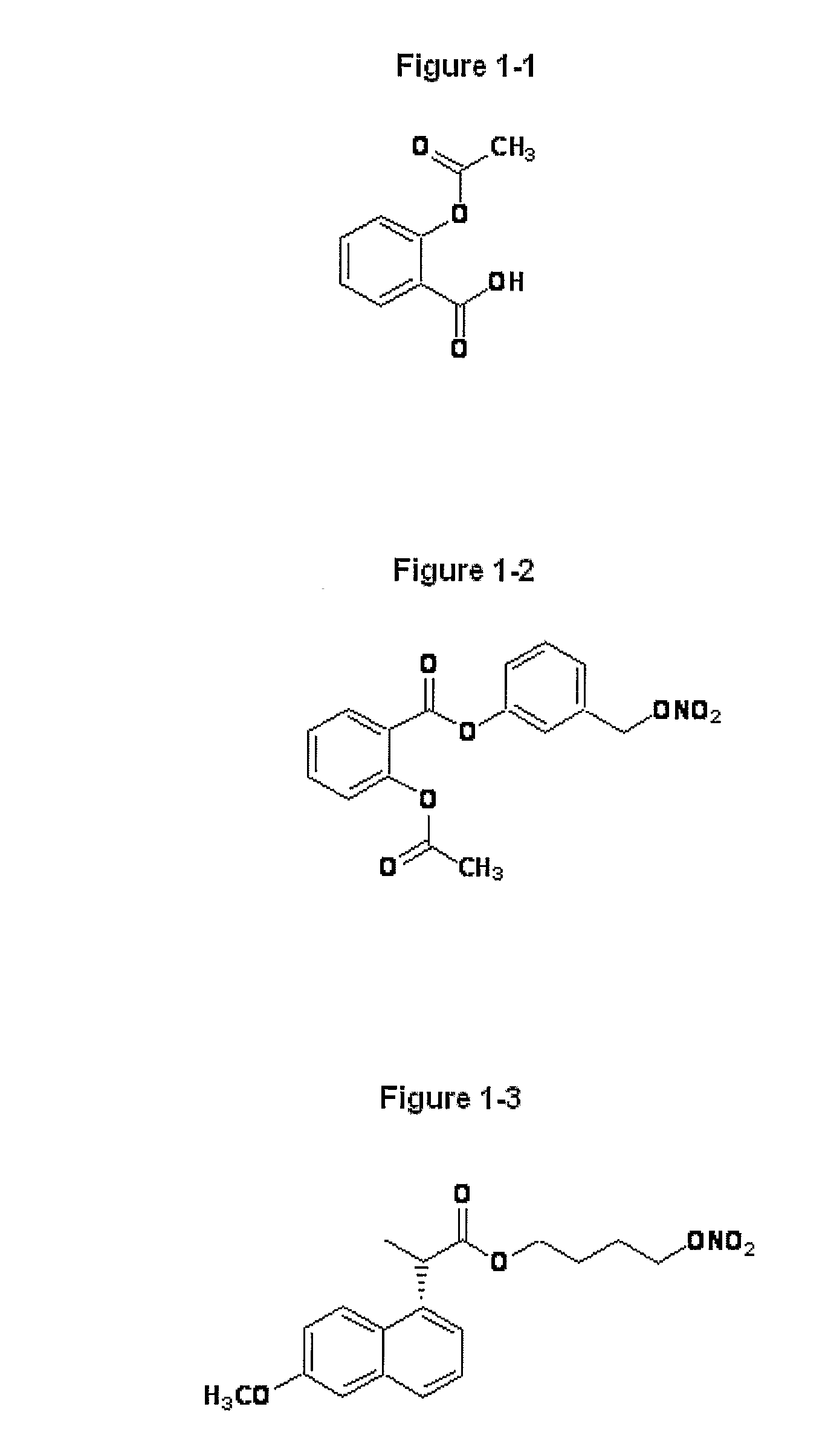
Novel Nsaids Possessing a Nitric Oxide Donor Diazen-1-Ium-1,2-Diolate Moiety
InactiveUS20080214646A1Prevent or ameliorate gastrointestinal upsetBiocideOrganic chemistryEster prodrugRadiation equivalent dose
This invention provides a prodrug that help arthritis patients without increasing cardiovascular and gastrointestinal risk. A novel group of hybrid nitric oxide-releasing non-steroidal anti-inflammatory drugs (NO-NSAIDs), moiety attached via a one -carbon methylene spacer to the carboxylic acid group of the traditional NSAIDs aspirin, ibuprofen and indomethacin were synthesized. The ester prodrugs showed equipotent anti-inflammatory activities in vivo to that of the parent aspirin, ibuprofen and indomethacin. The simultaneous release of parent drug and nitric oxide from the NO- prodrugs constitutes a potentially beneficial property for the prophylactic prevention of thrombus formation and adverse cardiovascular events such as stroke and myocardial infarction. Data acquired in an in vivo ulcer index (UI) assay showed that this group of ester prodrugs in which no lesions were observed when compared to the parent drugs at equivalent doses. Accordingly, these hybrid NO-NSAID prodrugs possessing a diazen-1-ium-1,2-diolate moiety, represents a new approach for the rational design of anti-inflammatory drugs with reduced gastric ulcerogenicity.
Owner:THE UNIV OF ALBERTA
Local vascular delivery of PI3 kinase inhibitors alone or in combination with sirolimus to prevent restinosis following vascular injury
InactiveUS20070116736A1Reduce frictionLittle strengthStentsCoatingsPI3-Kinase InhibitorsDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com