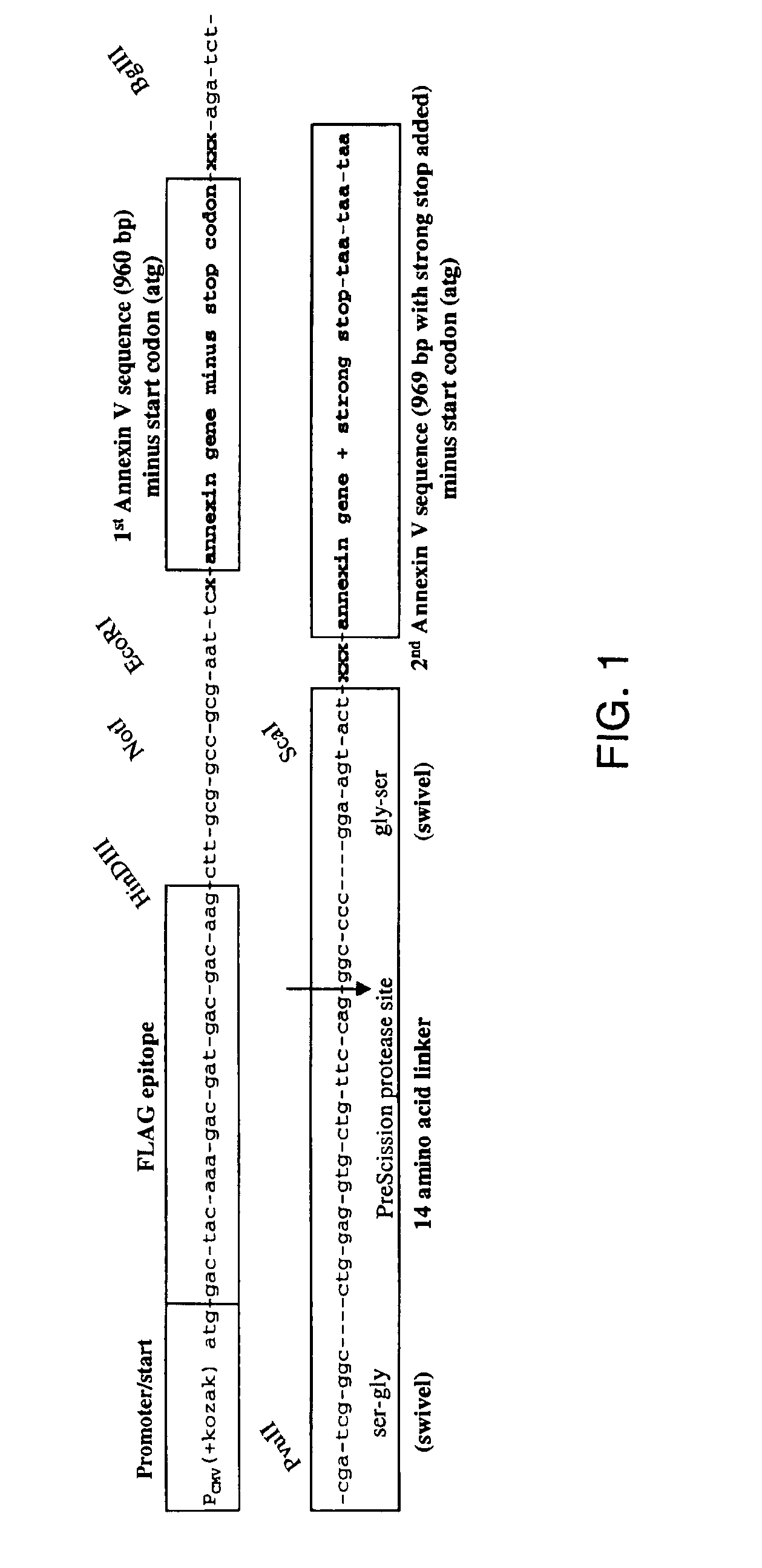
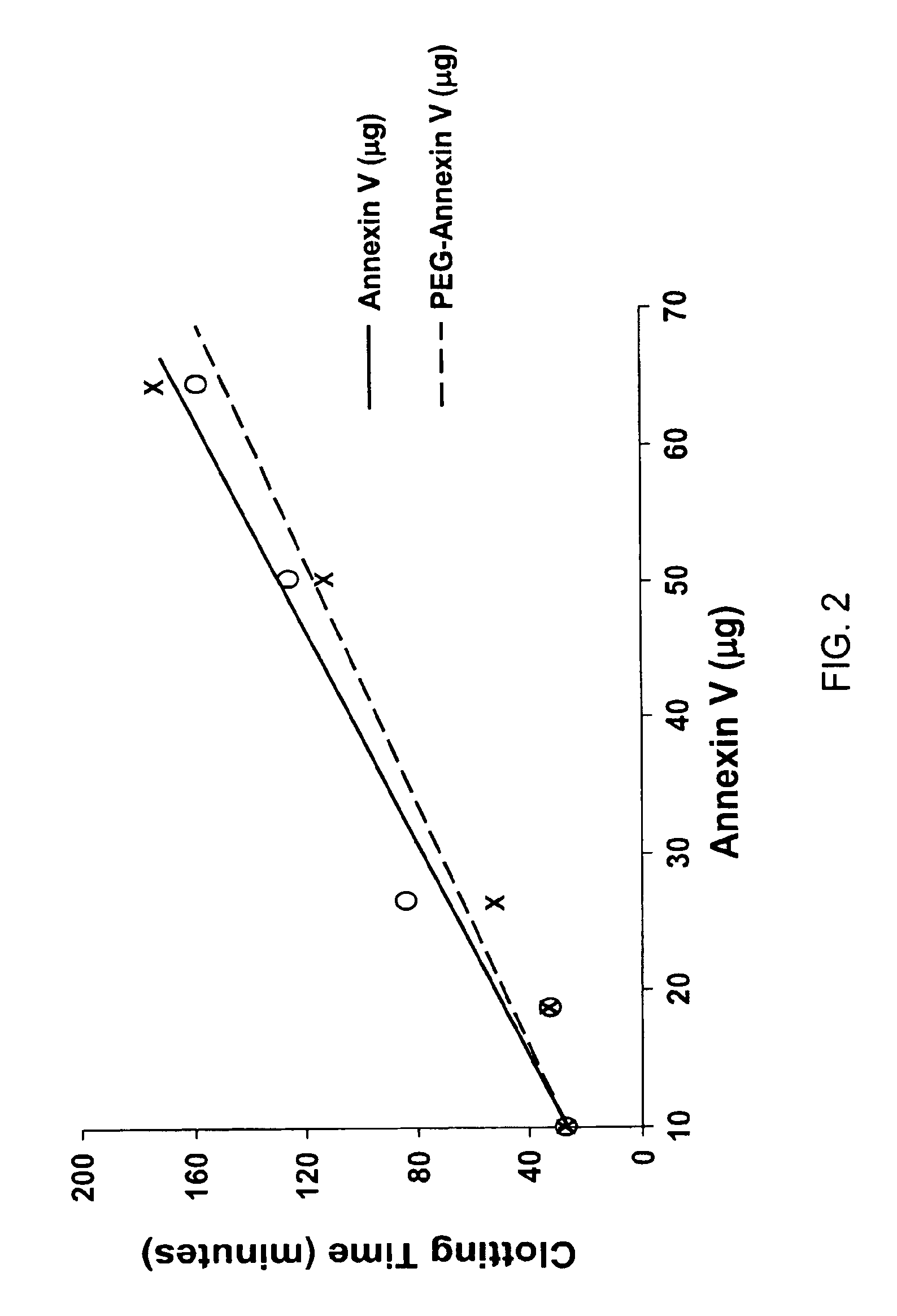
Modified annexin proteins and methods for preventing thrombosis
a technology of annexin proteins and thrombosis, which is applied in the direction of drug compositions, peptides, cardiovascular disorders, etc., can solve the problems of increased intracerebral hemorrhage risk, increased risk of cerebral hemorrhage, and no benefit of antibody, so as to prevent arterial or venous thrombosis without increasing hemorrhage, prolong the half-life in the vascular compartment, and prevent thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Modified Annexin Preparation
[0089]Annexins can be purified from human tissues or produced by recombinant technology. For instance, annexin V can be purified from human placentas as described by Funakoshi et al. (1987). Examples of recombinant products are the expression of annexin II and annexin V in Escherichia coli (Kang, H.-M., Trends Cardiovasc. Med. 9:92-102 (1999); Thiagarajan and Benedict, 1997, 2000). A rapid and efficient purification method for recombinant annexin V, based on Ca2+-enhanced binding to phosphatidylserine-containing liposomes and subsequent elution by EDTA, has been described by Berger, FEBS Lett. 329:25-28 (1993). This procedure can be improved by the use of phosphatidylserine coupled to a solid phase support.
[0090]Annexins can be coupled to polyethylene glycol (PEG) by any of several well-established procedures (reviewed by Hermanson, 1996) in a process referred to as pegylation. The present invention includes chemically-derivatized annexin molecules having...
example 2
[0098]In vitro assays determine the ability of modified annexin proteins to bind to activated platelets. Annexin V binds to platelets, and this binding is markedly increased in vitro by activation of the platelets with thrombin (Thiagarajan and Tait, 1990; Sun et al., 1993). Preferably, the modified annexin proteins of the present invention are prepared in such a way that perform the function of annexin in that they bind to platelets and prevent protein S from binding to platelets (Sun et al., 1993). The modified annexin proteins also perform the function of exhibiting the same anticoagulant activity in vitro that unmodified annexin proteins exhibit. A method for measuring the clotting time is the activated partial thromboplastin time (Fritsma, in Hemostasis and thrombosis in the clinical laboratory (Corriveau, D. M. and Fritsma, G. A., eds) J. P. Lipincott Co., Philadelphia (1989), pp. 92-124, incorporated herein by reference).
[0099]In vivo assays determi...
example 3
[0100]The anticoagulant ability of human recombinant annexin V and pegylated human recombinant annexin V were compared in vitro.
[0101]Annexin V production. The polymerase chain reaction was used to amplify the cDNA from the initiator methionine to the stop codon with specific oligonucleotide primers from a human placental cDNA library. The forward primer was 5′-ACCTGAGTAGTCGCCATGGCACAGGTTCTC-3′ (SEQ ID NO:7) and the reverse primer was 5′-CCCGAATTCACGTTAGTCATCTTCTCCACAGAGCAG-3′ (SEQ ID NO:8). The amplified 1.1-kb fragment was digested with Nco I and Eco RI and ligated into the prokaryotic expression vector pTRC 99A. The ligation product was used to transform competent Escherichia coli strain JM 105 and sequenced.
[0102]Recombinant annexin V was isolated from the bacterial lysates as described by Berger et al., 1993, with some modification. An overnight culture of E. coli JM 105 transformed with pTRC 99A-annexin V was expanded 50-fold in fresh Luria-Bertrani medium containing 100 mg / L ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com