Patents
Literature
132 results about "Tobramycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat eye infections.
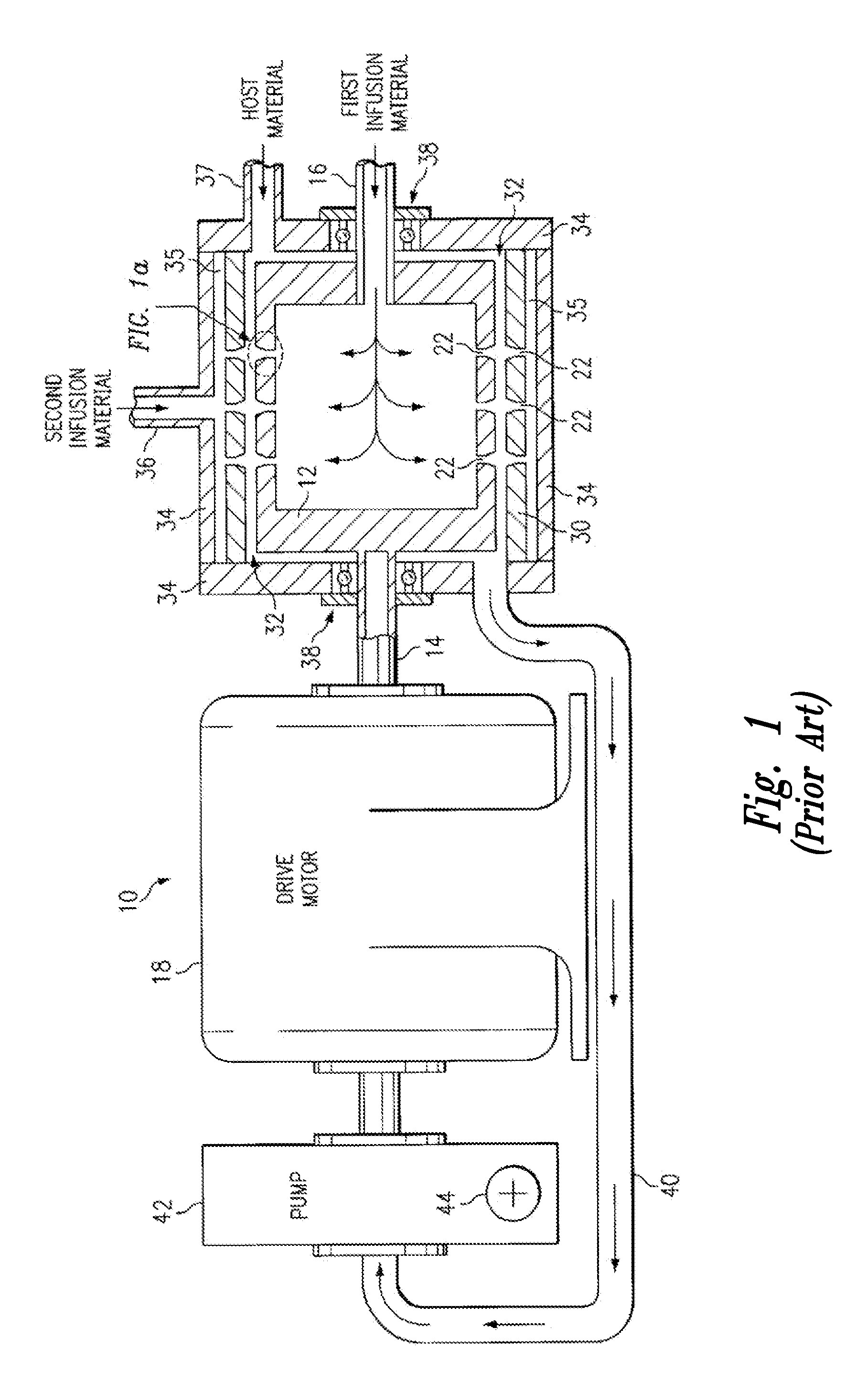
Method and a tobramycin aerosol formulation for treatment prevention and containment of tuberculosis
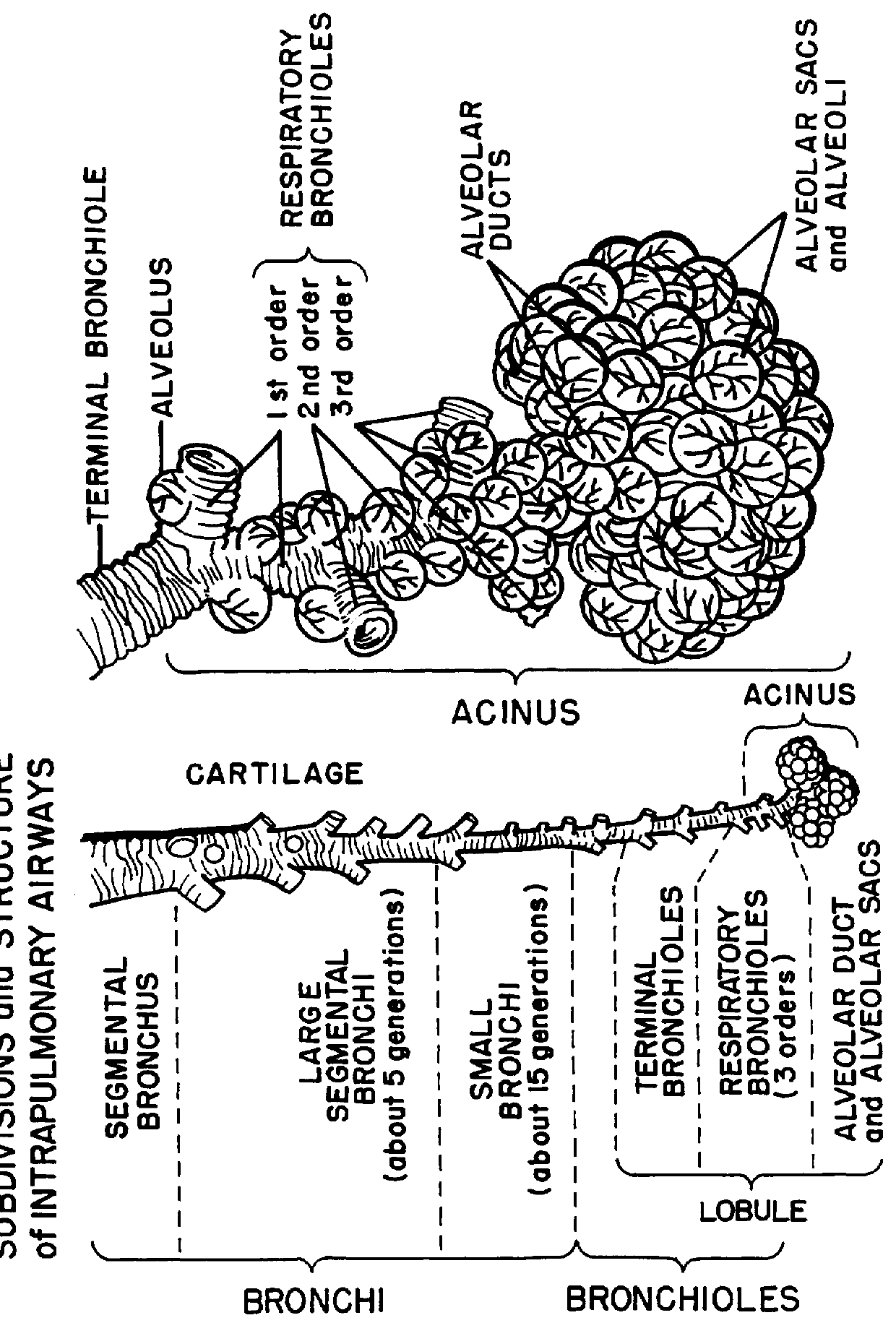
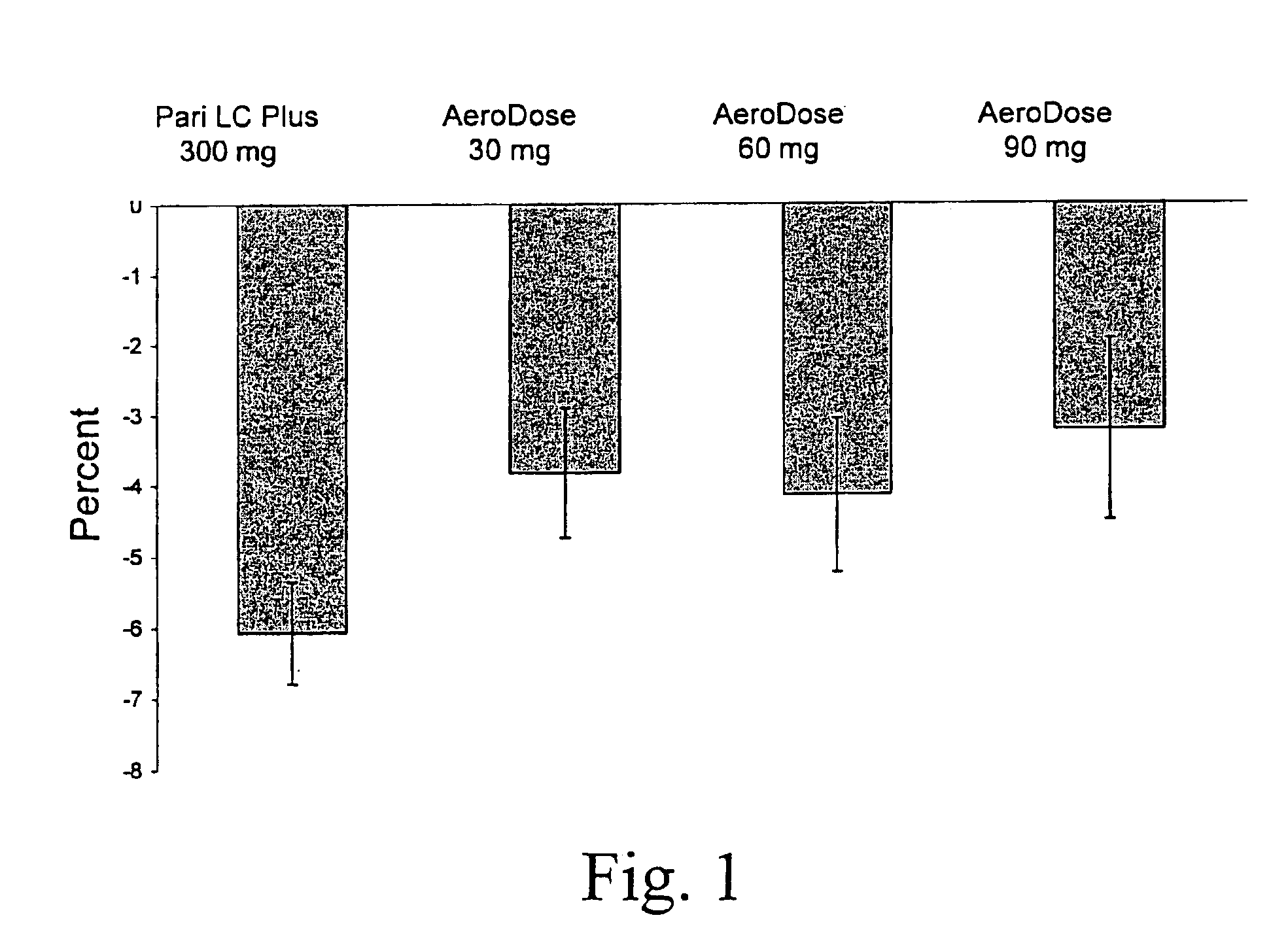
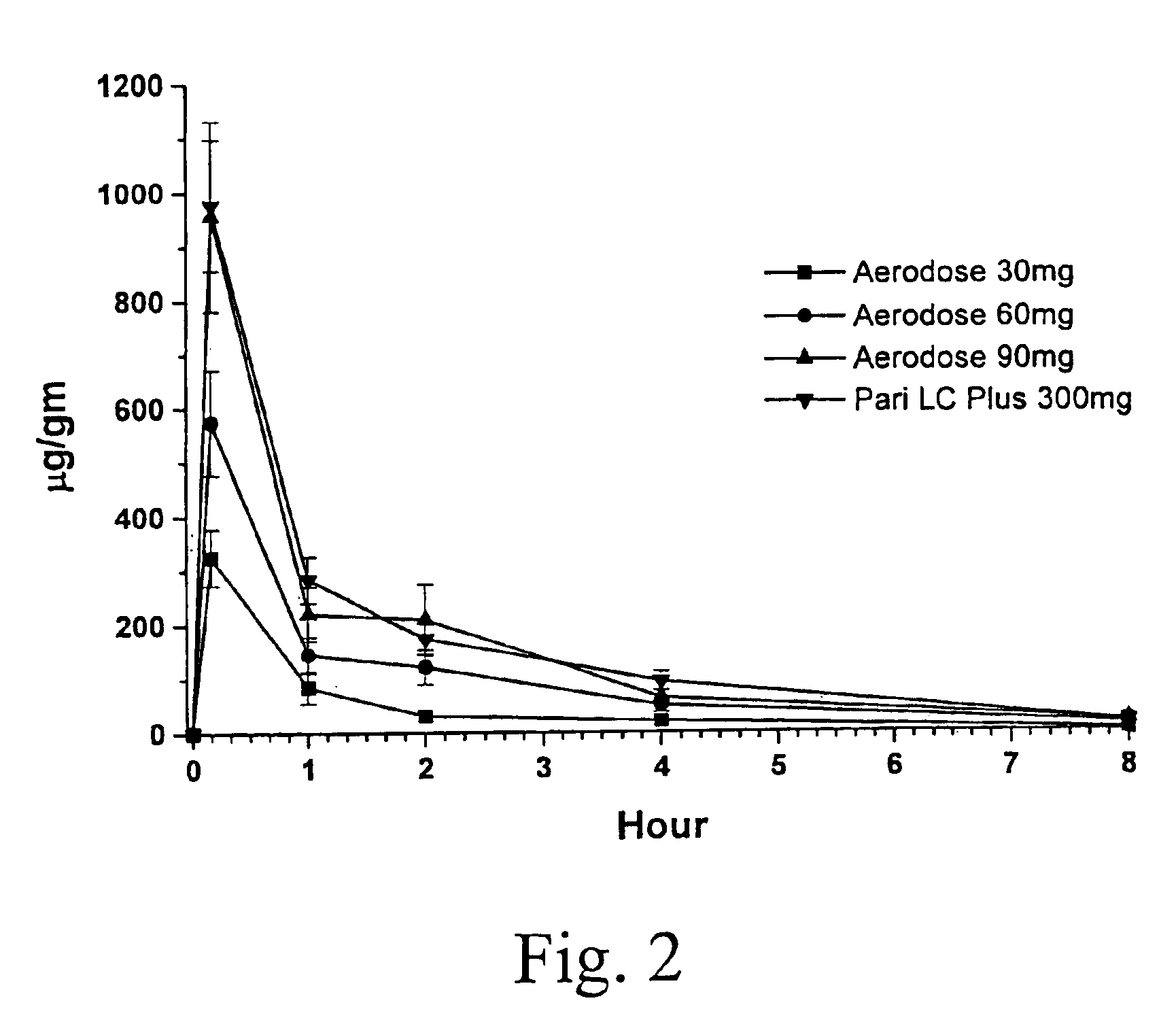
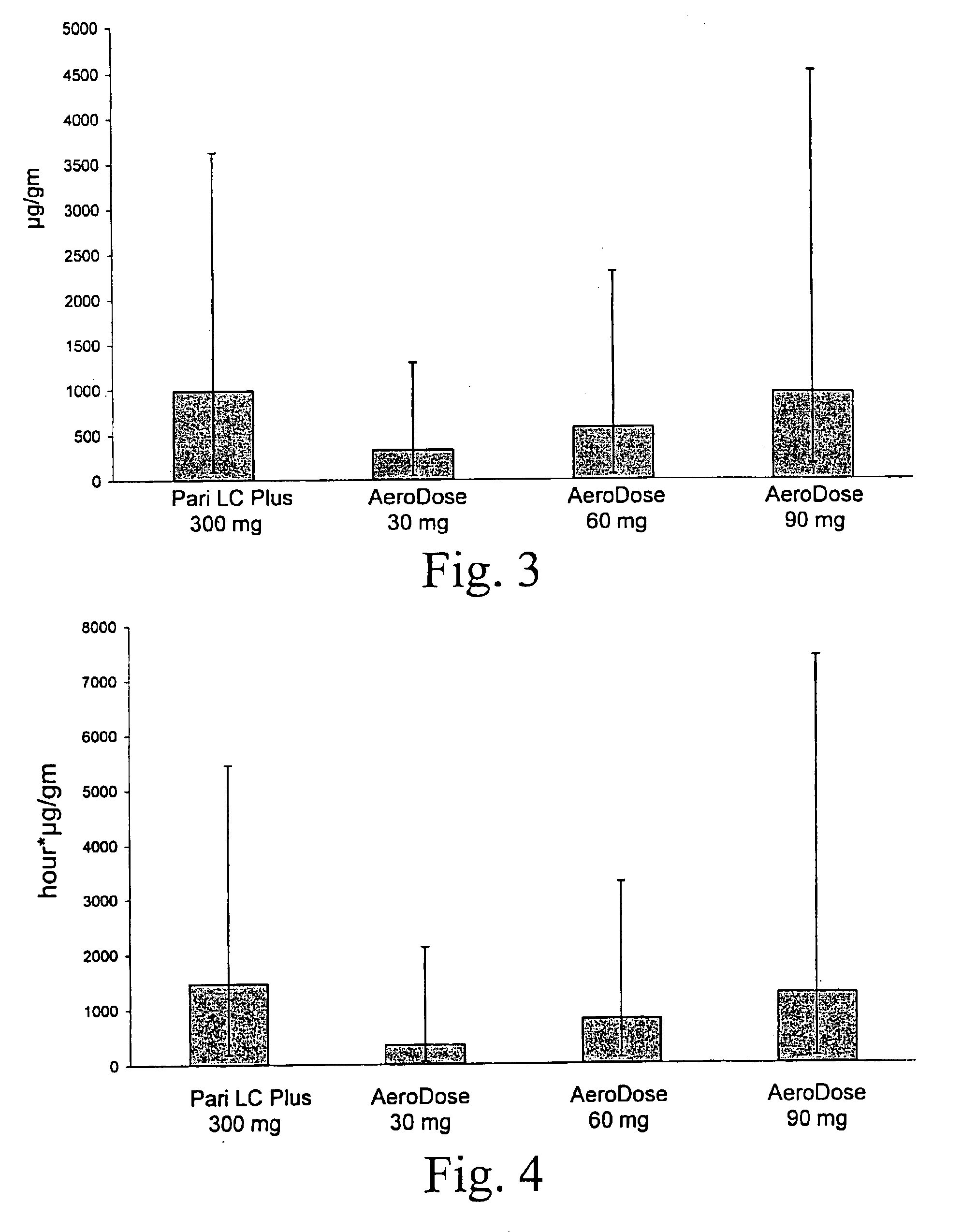
A method for treatment, prevention and containment of acute and chronic tuberculosis using a preservative-free concentrated tobramycin aerosol formulation delivering tobramycin to the lung endobronchial space including alveoli in an aerosol having mass medium average diameter predominantly between 1 to 5 mu . The method comprises administration of tobramycin in concentration one to ten thousand times higher than the minimal inhibitory concentration of Mycobacterium tuberculosis. A method for containment of and decreasing infectivity periods of tuberculosis patients to shorter periods of time.
Owner:CHIRON CORP
Compositions and methods for treating cystic fibrosis
InactiveUS20100303917A1Increased mucus secretionReduce airflowBiocidePowder deliveryTobramycinCell membrane
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating cystic fibrosis or a symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered fluids optionally in combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). Particular embodiments comprise use and / or synergy with tobramycin for treating bacterial infection, and use and / or synergy with a bronchiodilator. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (like, membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions).
Owner:REVALESIO CORP
Compositions and methods for treating cystic fibrosis
Particular aspects provide electrokinetically-generated fluids (e.g., electrokinetically-generated gas-enriched fluids and solutions), and therapeutic compositions and methods comprising use thereof in treating at least one symptom of cystic fibrosis. In particular embodiments, at least one symptom of cystic fibrosis treated by the present invention include inhibition of Pseudomonas infection, synergy with tobramycin (including TOBI) for use against bacterial infection, and synergy with a bronchiodilator. In particular embodiments, the electrokinetically-generated fluids or therapeutic compositions and methods comprise combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). In certain aspects, the methods comprise regulating or modulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes).
Owner:REVALESIO CORP
Compositions and methods for treating cystic fibrosis
InactiveUS20100004189A1Increased mucus secretionReduce airflowBiocideCarbohydrate active ingredientsTobramycinCell membrane
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating cystic fibrosis or a symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered fluids optionally in combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). Particular embodiments comprise use and / or synergy with tobramycin for treating bacterial infection, and use and / or synergy with a bronchiodilator. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (like, membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions).
Owner:REVALESIO CORP
Methods and unit dose formulations for the inhalation administration of tobramycin
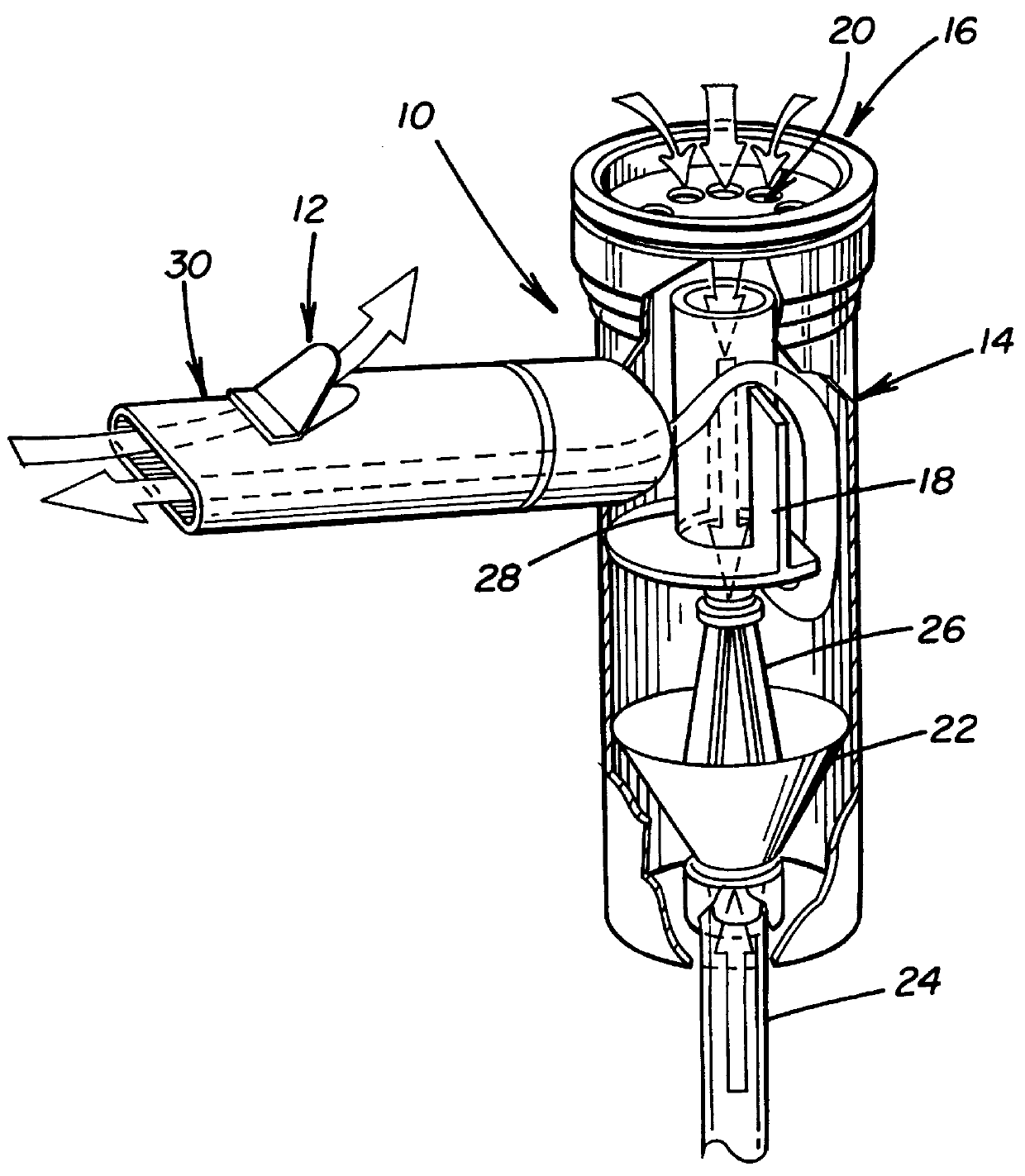
A patient suffering from an endobronchial infection is treated by administering to the patient for inhalation a dose of less than about 4.0 ml of a nebulized aerosol formulation comprising from about 60 to about 200 mg / ml of an aminoglycoside antibiotic, such as tobramycin, in a physiologically acceptable carrier in a time period of less than about 10 minutes. Unit dose devices for storage and delivery of the aminoglycoside antibiotic formulations are also provided.
Owner:NOVARTIS AG
Beaded Wound Spacer Device
InactiveUS20080188819A1Good adhesionImproved handling of deviceSuture equipmentsAntibacterial agentsMedical unitElution
A wound spacer device comprising multiple beads connected by non-absorbable suture material is disclosed. The device can be applied, for example, by a first responder to an injured individual, or can be applied by a trauma treatment facility, such as a Level 2 medical unit. In typical embodiments the device allows for site-specific controlled elution of an antimicrobial agent, such as Tobramycin, including defined elution over a period of time, such as 48 or 72 hours.
Owner:SURMODICS INC
Combination of loteprednol etabonate and tobramycin for topical ophthalmic use
InactiveUS20050095205A1Improve storage characteristicsImprove featuresAntibacterial agentsPowder deliveryTobramycinAntibiotic Y
This invention relates to formulations for topical use comprising antibiotics in combination with anti-inflammatory steroids for treating ophthalmic infections and attendant inflammation. More specifically, this invention relates to pharmaceutical ophthalmic formulations comprising a pH stabilizing amount of tobramycin and the soft steroid loteprednol etabonate.
Owner:BAUSCH & LOMB INC
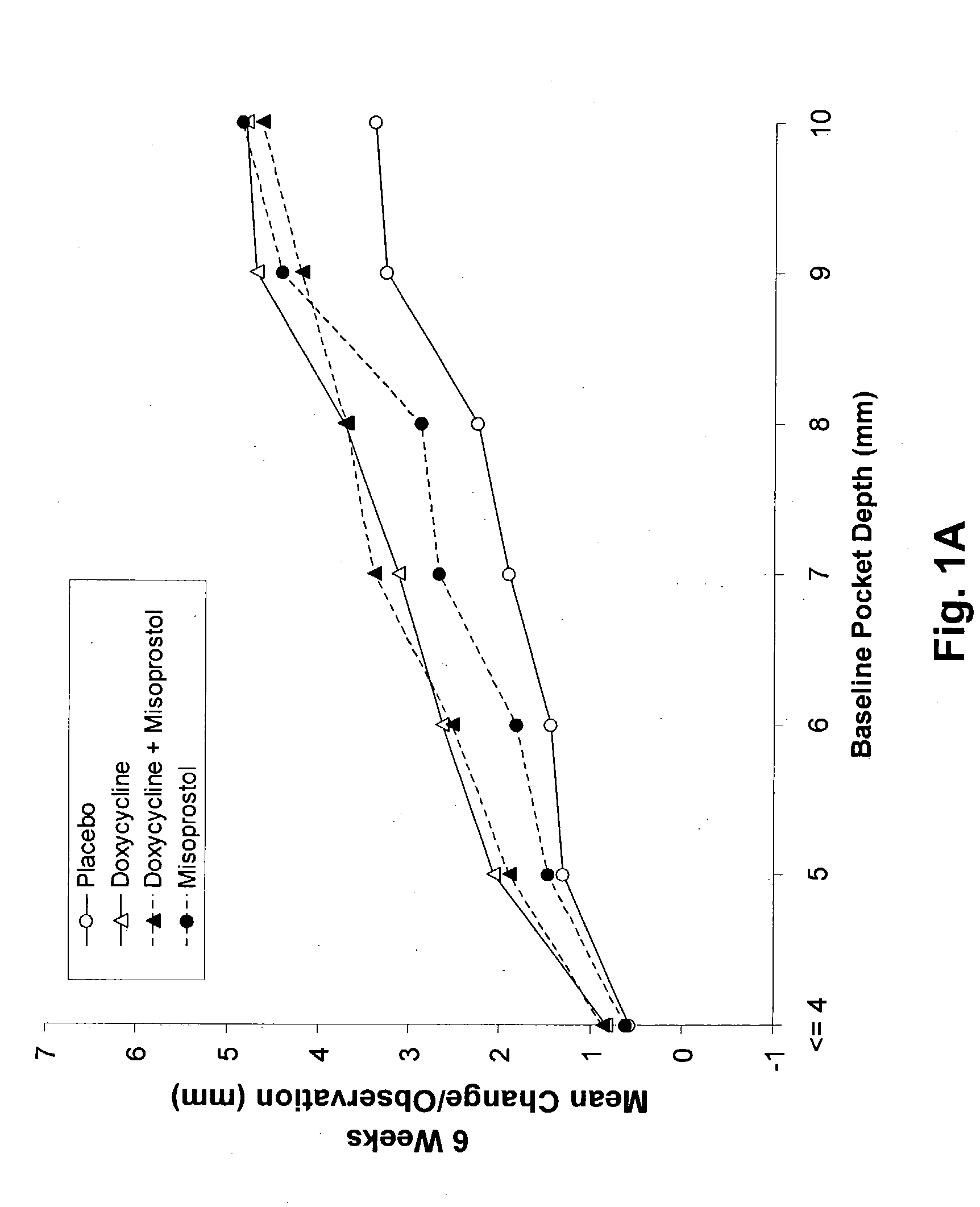
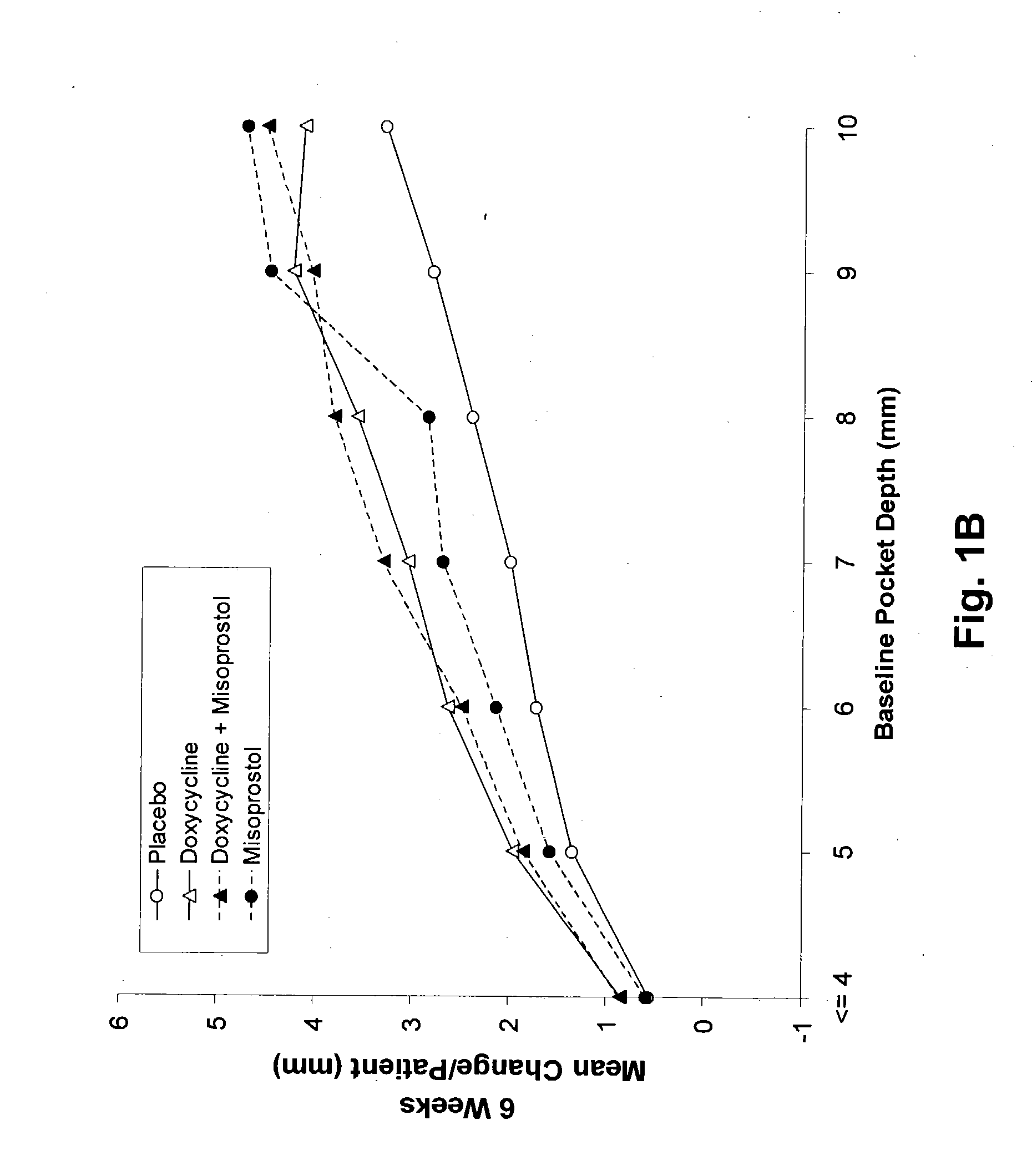
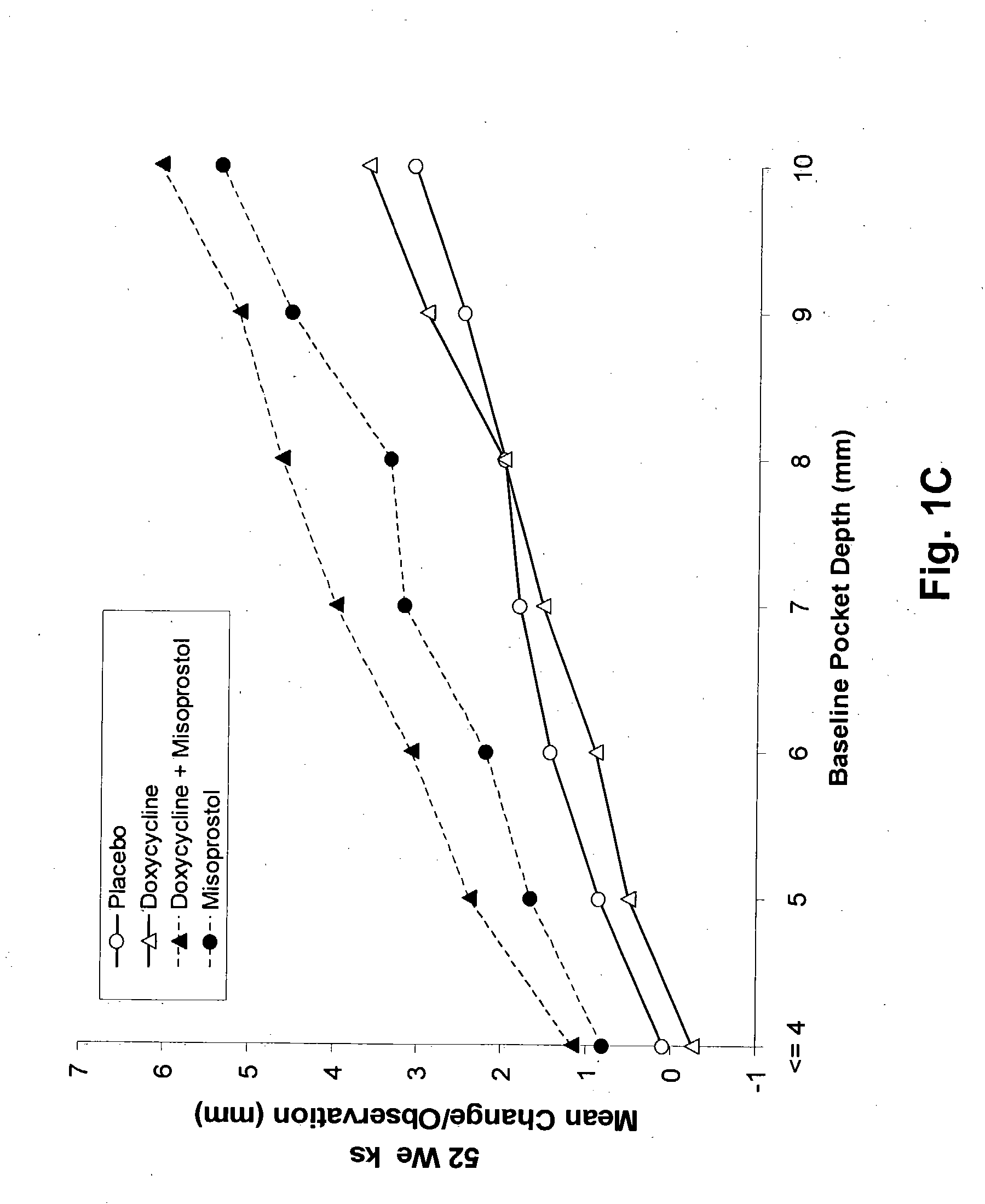
Method and composition for treating peridontal disease
InactiveUS20050032720A1Reduce additionalReduce inflammationBiocideElcosanoid active ingredientsClarithromycinAntibiotic Y
The present invention is directed to a pharmaceutical composition comprising a therapeutically effective amount of misoprostol and an effective amount of an antibiotic. A suitable antibiotic is selected from the group consisting of doxycycline, gentamicin, tobramicin, ciprofloxacin, clindamycin, clarithromycin, azithromycin and metronidazole. Preferred antibiotics are doxycycline and ciprofloxacin. More preferably, the antibiotic is doxycycline. In its second aspect, the present invention is directed to a method for treating periodontal disease in a mammalian patient comprising administering to a mammalian patient in need of such treatment a therapeutically effective amount of misoprostol and an effective amount of an antibiotic. Typically, the mammalian patient is a human.
Owner:REGENA THERAPEUTICS LC
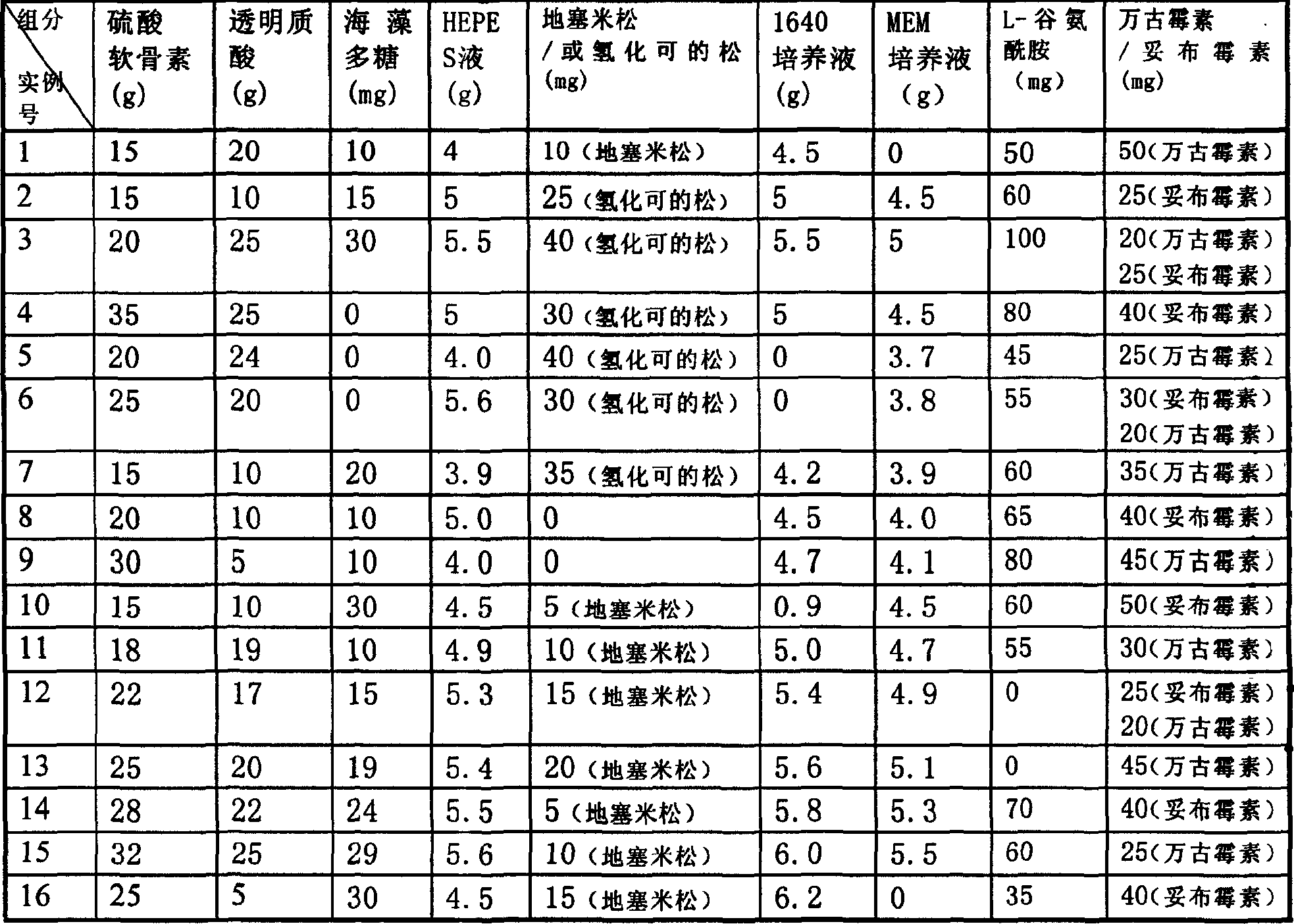
Cornea middle term preserving fluid
Disclosed is a cornea middle term preserving fluid which comprises RPMI 1640 culture liquid, chondroitin sulfate, hyaluronic acid, HEPES cushioning liquid, dexamethasone, low-molecular-weight seaweed polysaccharides, Tobramycin or vancomycin, the preserving fluid has stabilized cell activity during storage period, and higher endothelial cell metabolizing function can be maintained than the US's Opthiol.
Owner:SHENZHEN WATSIN GENETECH
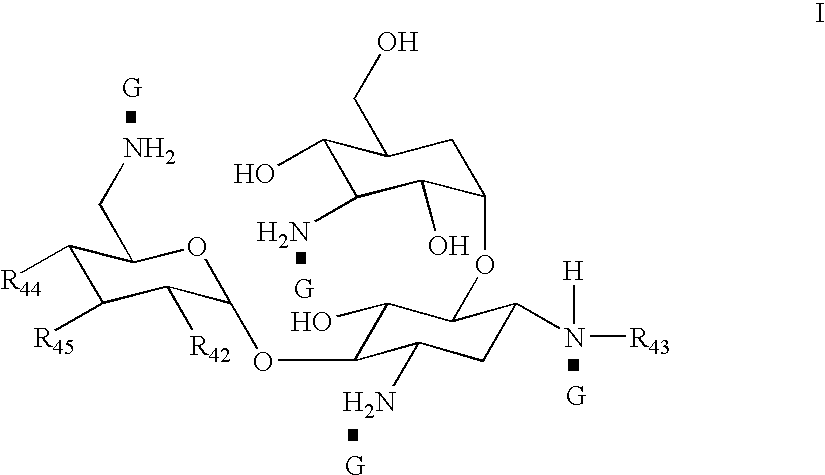
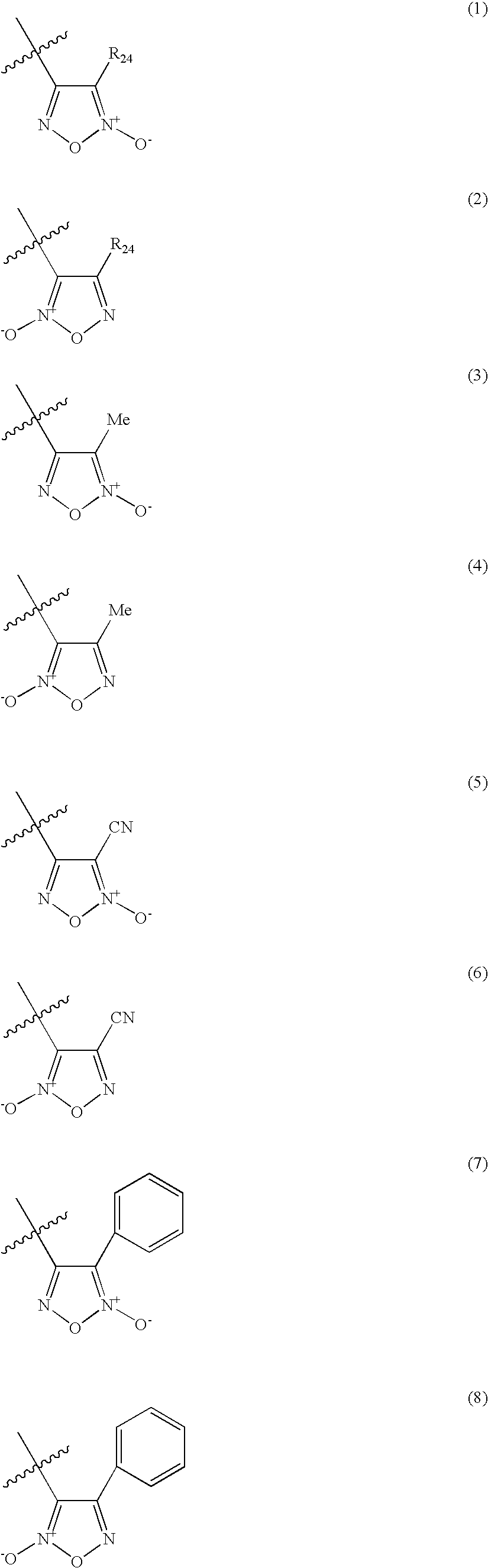
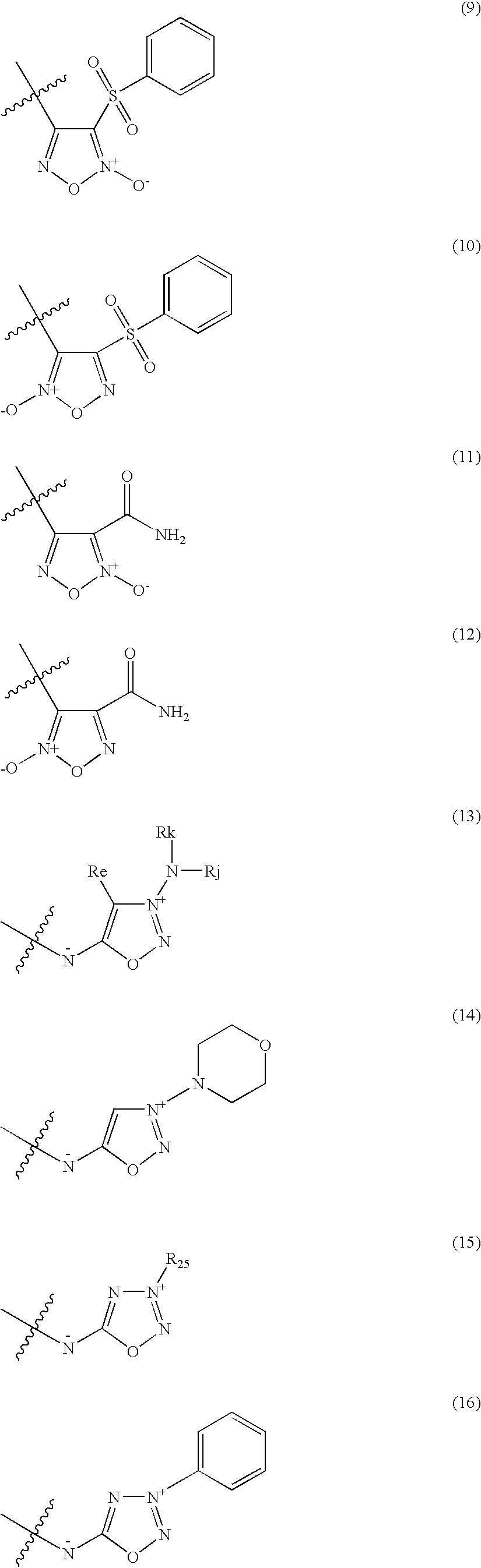
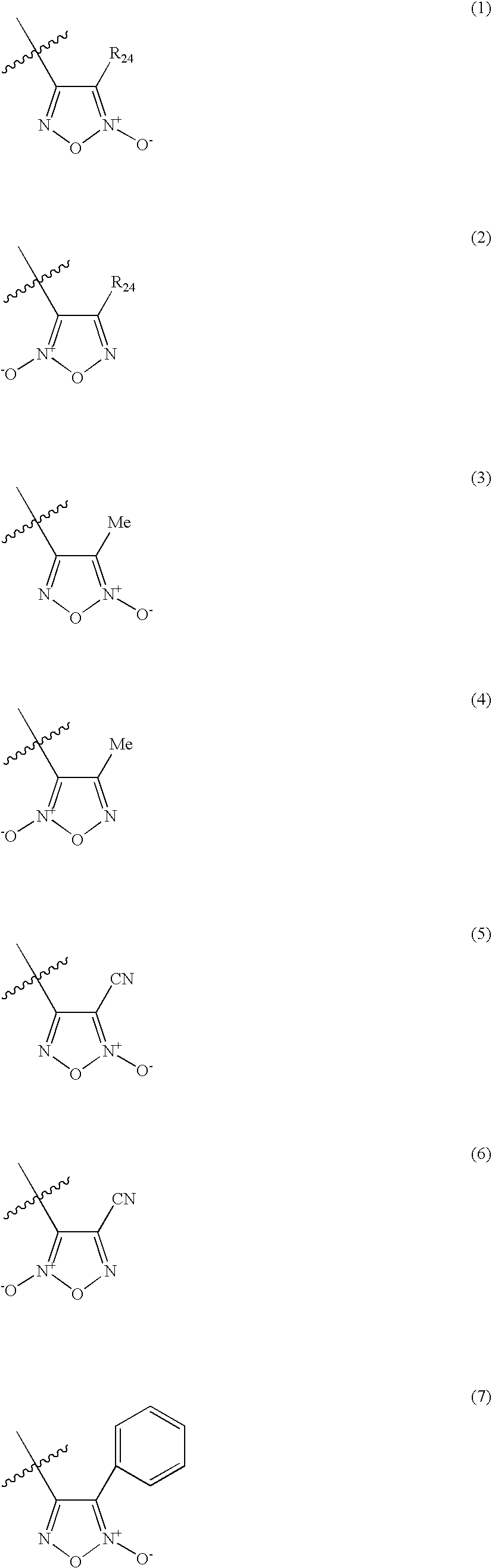
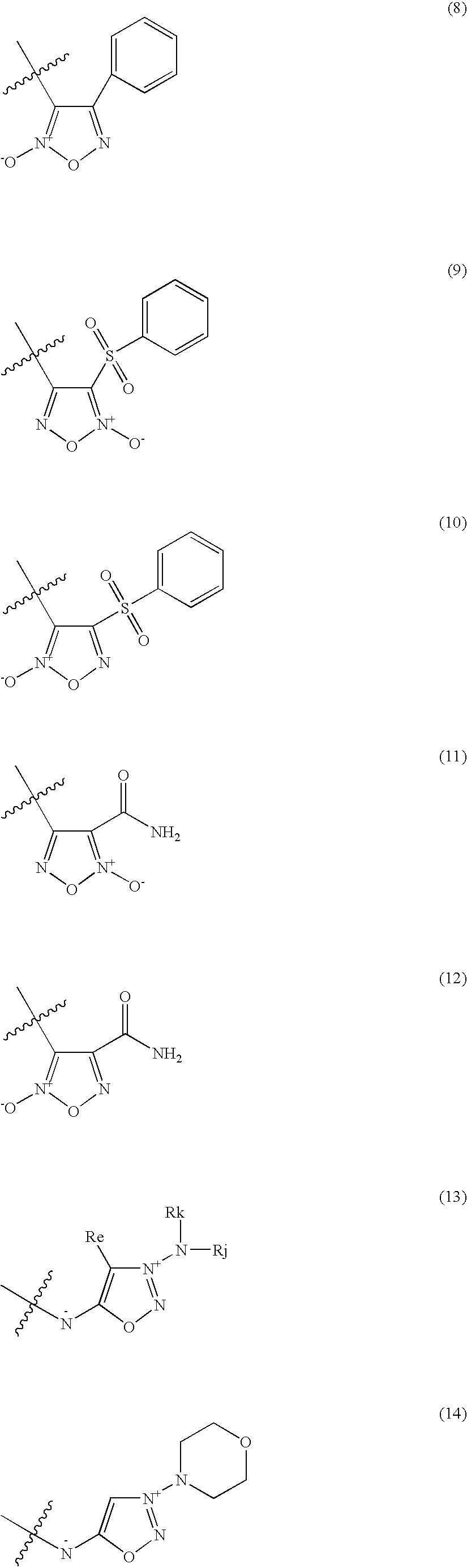
Organic nitric oxide donor salts of antimicrobial compounds, compositions and methods of use
The invention describes novel organic nitric oxide donor salts of a antimicrobial compounds, and novel compositions and kits comprising at least one organic nitric oxide donor salt of an antimicrobial compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating bacterial infections; (b) treating viral infections; (c) treating fungal infections; and (d) treating lesions. In one embodiment the antimicrobial compounds of the invention are aztreonam, ciprofloxacin, doripenam, duramycin and tobramycin. The organic nitric oxide donors that form salts are preferably organic nitrates, organic nitrites, nitrosothiols, thionitrites and heterocyclic nitric oxide donors. The heterocyclic nitric oxide donors are preferably furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines. The methods of the invention are preferably for the treatment of bacterial infections associated with pulmonary diseases such as cystic fibrosis and for treating Bacillus anthracis infections.
Owner:NICOX SA
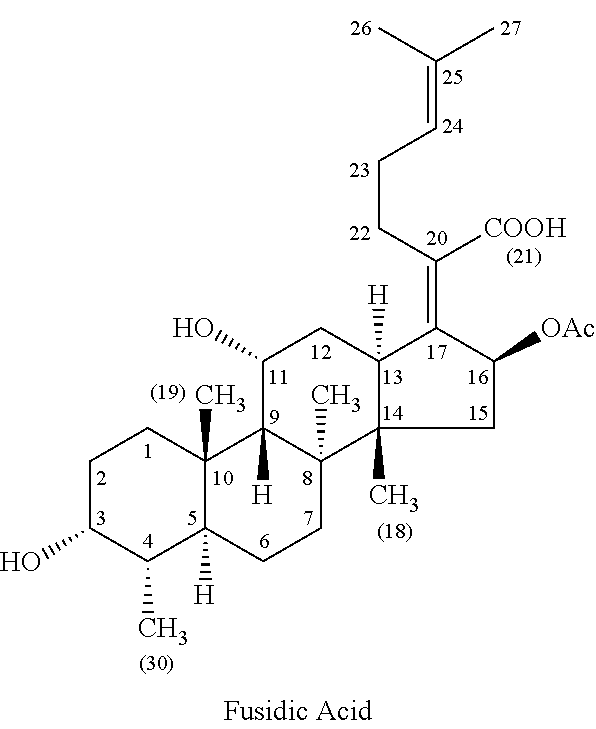
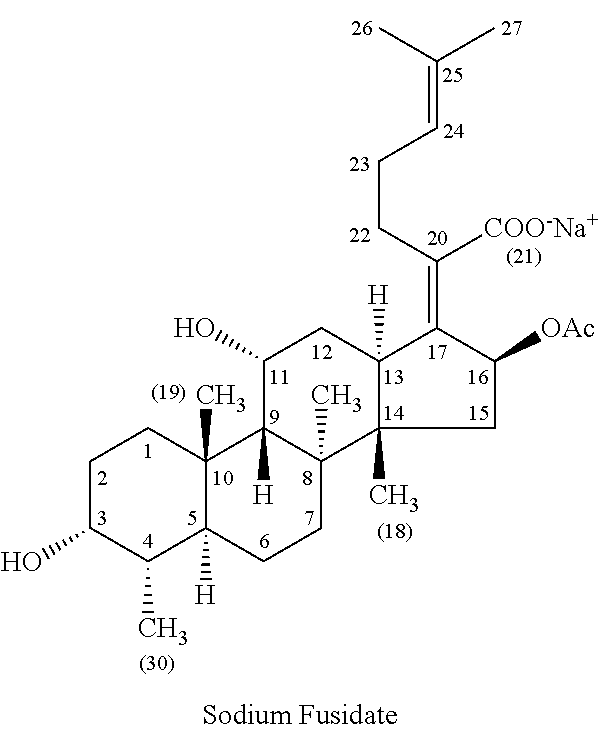
Methods of treating bacterial infections through pulmonary delivery of fusidic acid
Methods for the treatment of bacterial infections in the respiratory system of a subject, such as the lungs of a subject, using fusidic acid alone or in combination with a second bacterial agent such as tobramycin, amikacin, fosfomycin or levofloxacin are described.
Owner:CEMPRA PHARMA INC
Coating composition
ActiveUS20120021038A1Good adhesionImprove handlingAntibacterial agentsSuture equipmentsMedical unitMedicine
Owner:SURMODICS INC
Nitric Oxide Enhancing Antimicrobial Compounds, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one nitric oxide enhancing group antimicrobial compound, or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitric oxide enhancing antimicrobial compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating bacterial infections; (b) treating viral infections; (c) treating fungal infections; and (d) treating lesions. The antimicrobial compounds of the invention are preferably tobramycin, aztreonam, ciprofloxacin and doripenam. The nitric oxide enhancing antimicrobial compounds are substituted with at least one heterocyclic nitric oxide donor group and / or at least one nitroxide group. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors. The heterocyclic nitric oxide donors are furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines. In one embodiment the methods of the invention are for the treatment of bacterial infections associated with pulmonary diseases such as cystic fibrosis and for treating Bacillus anthracis infections.
Owner:NICOX SA
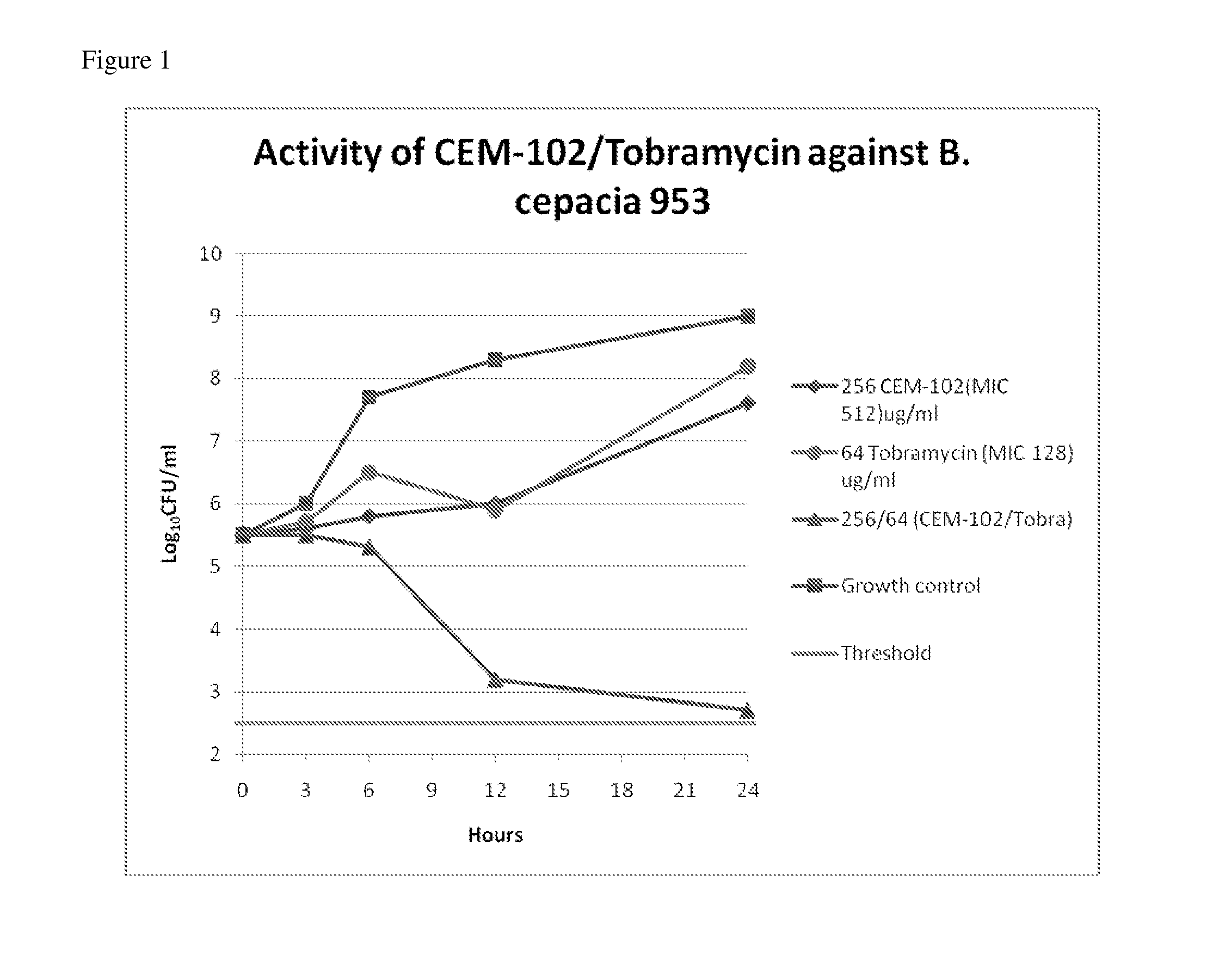
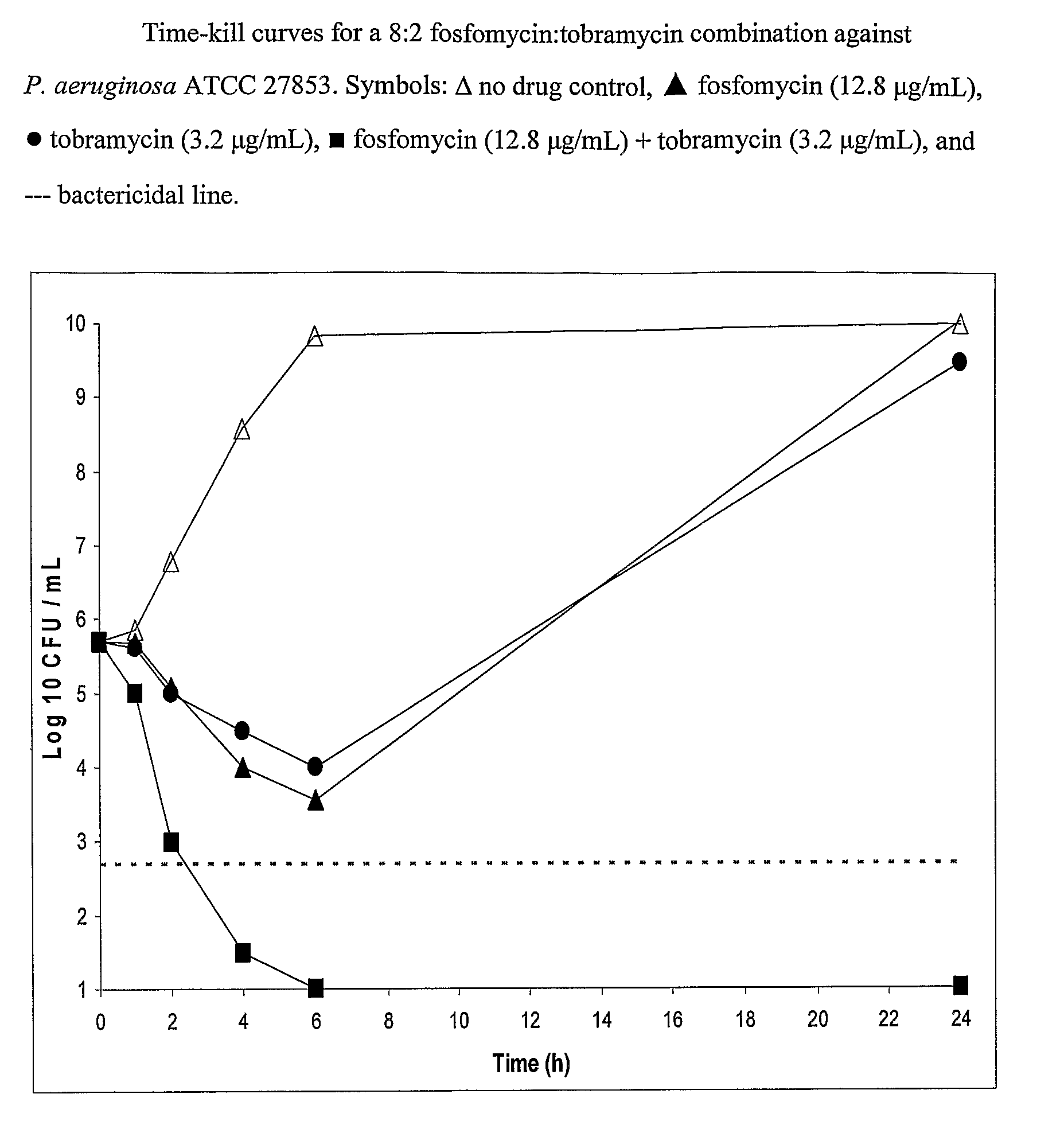
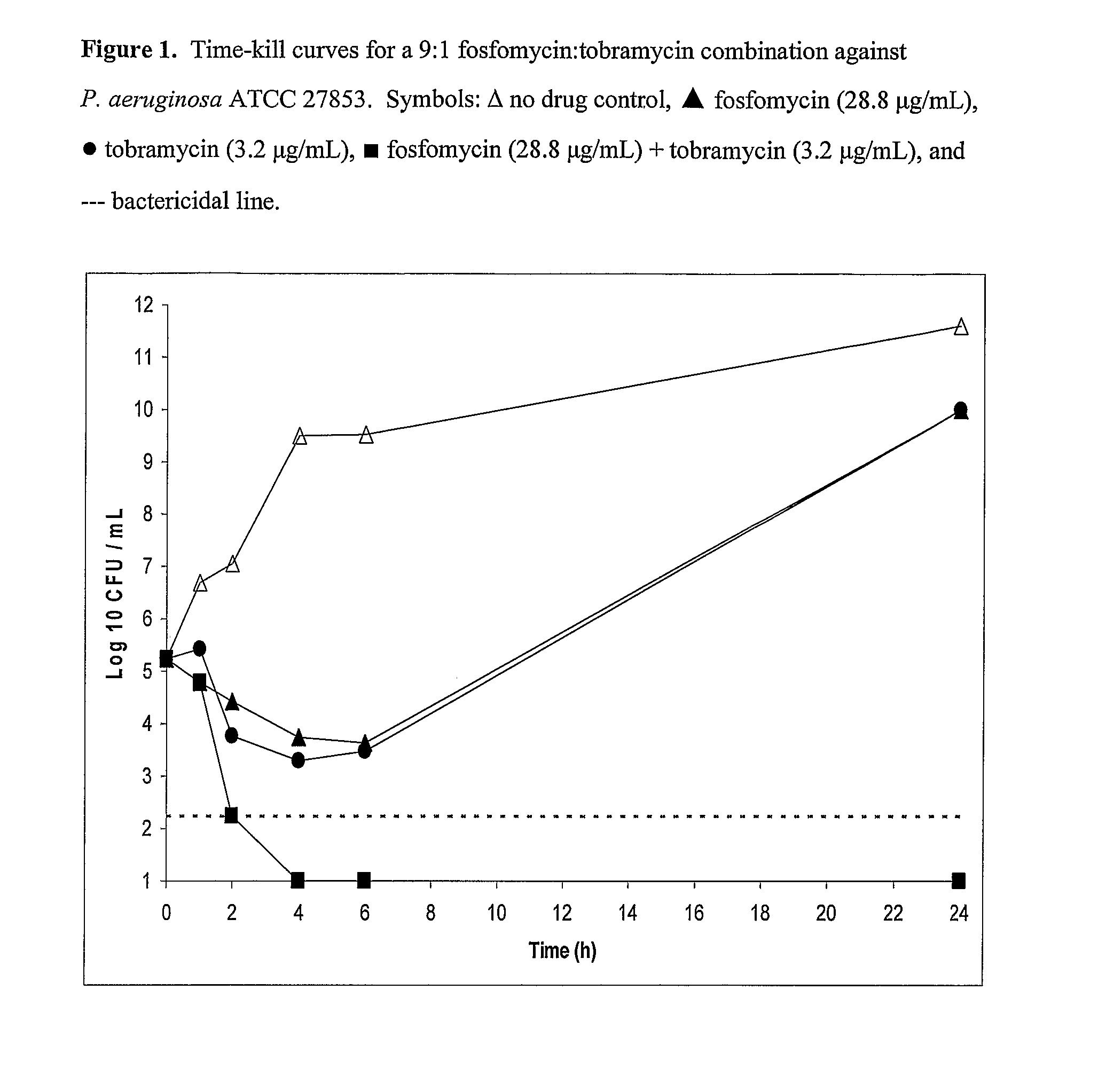
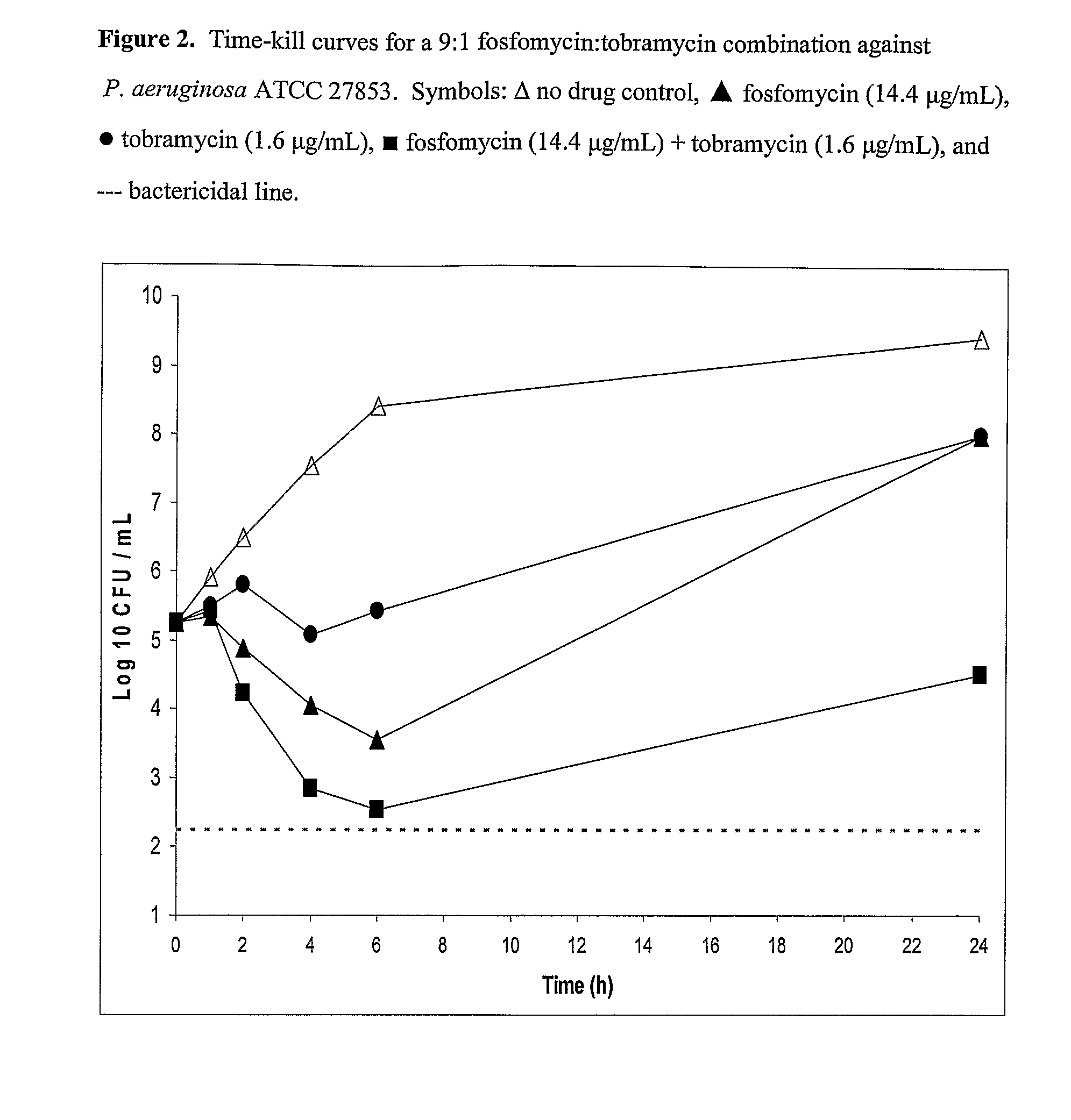
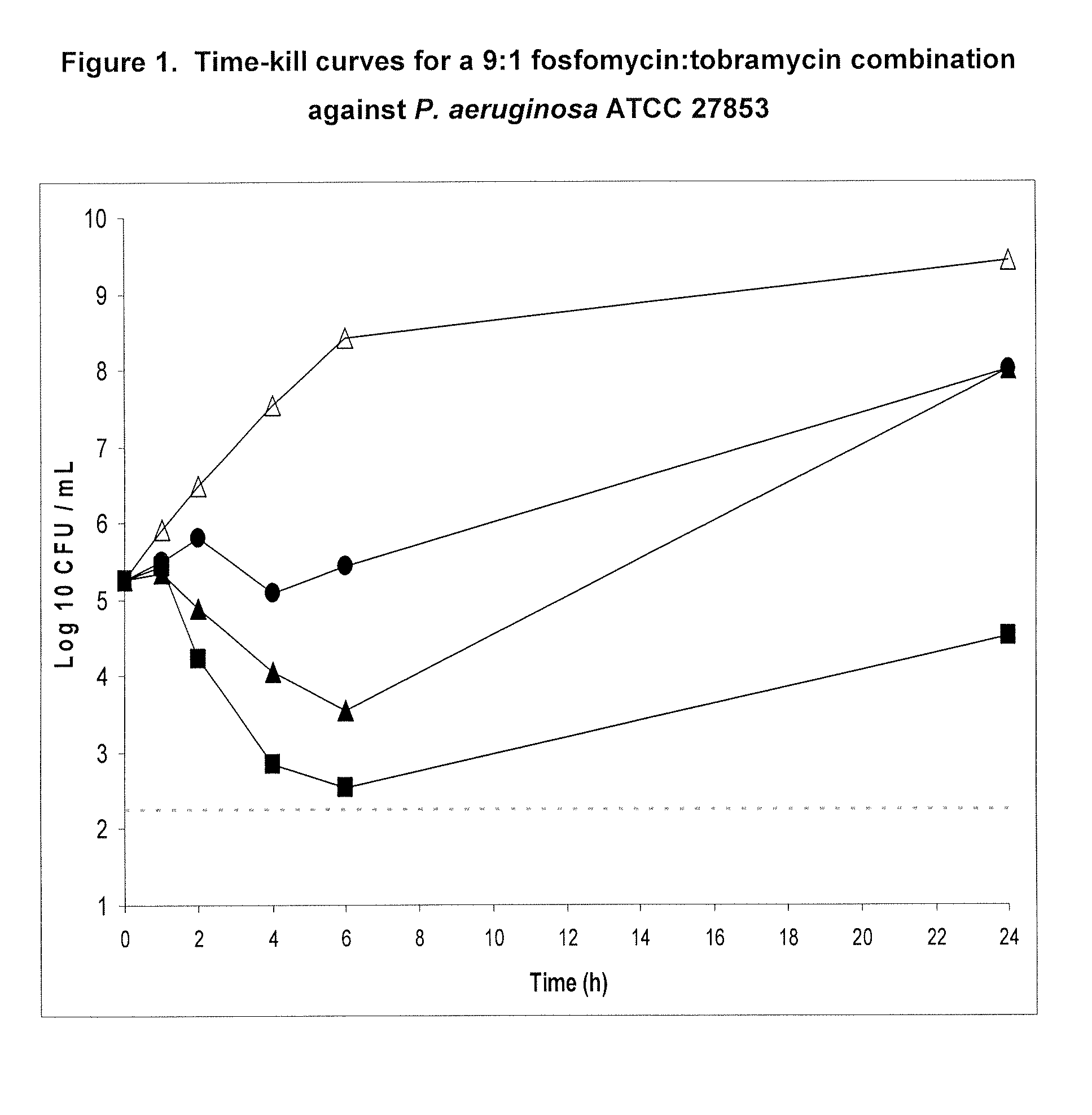
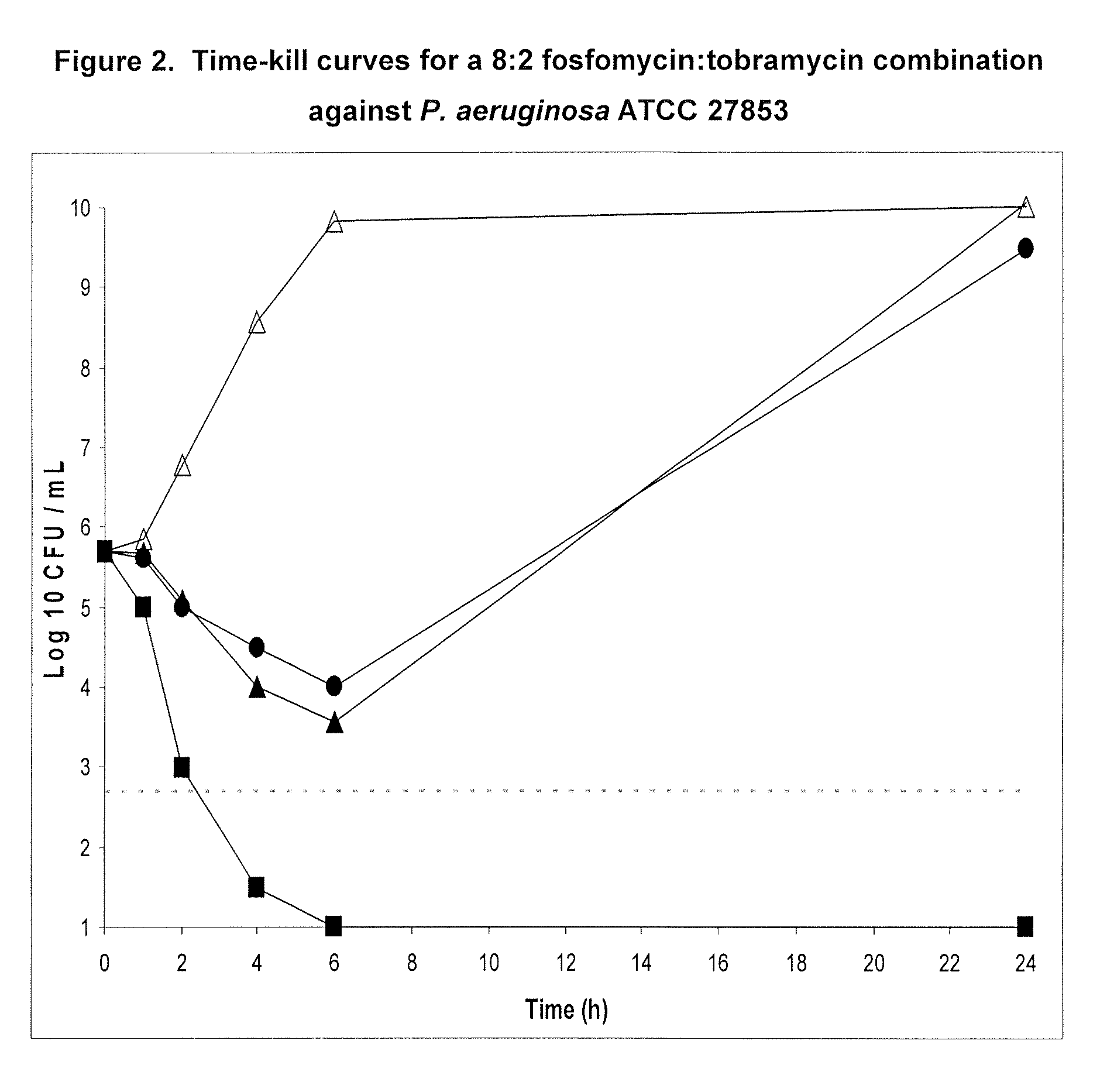
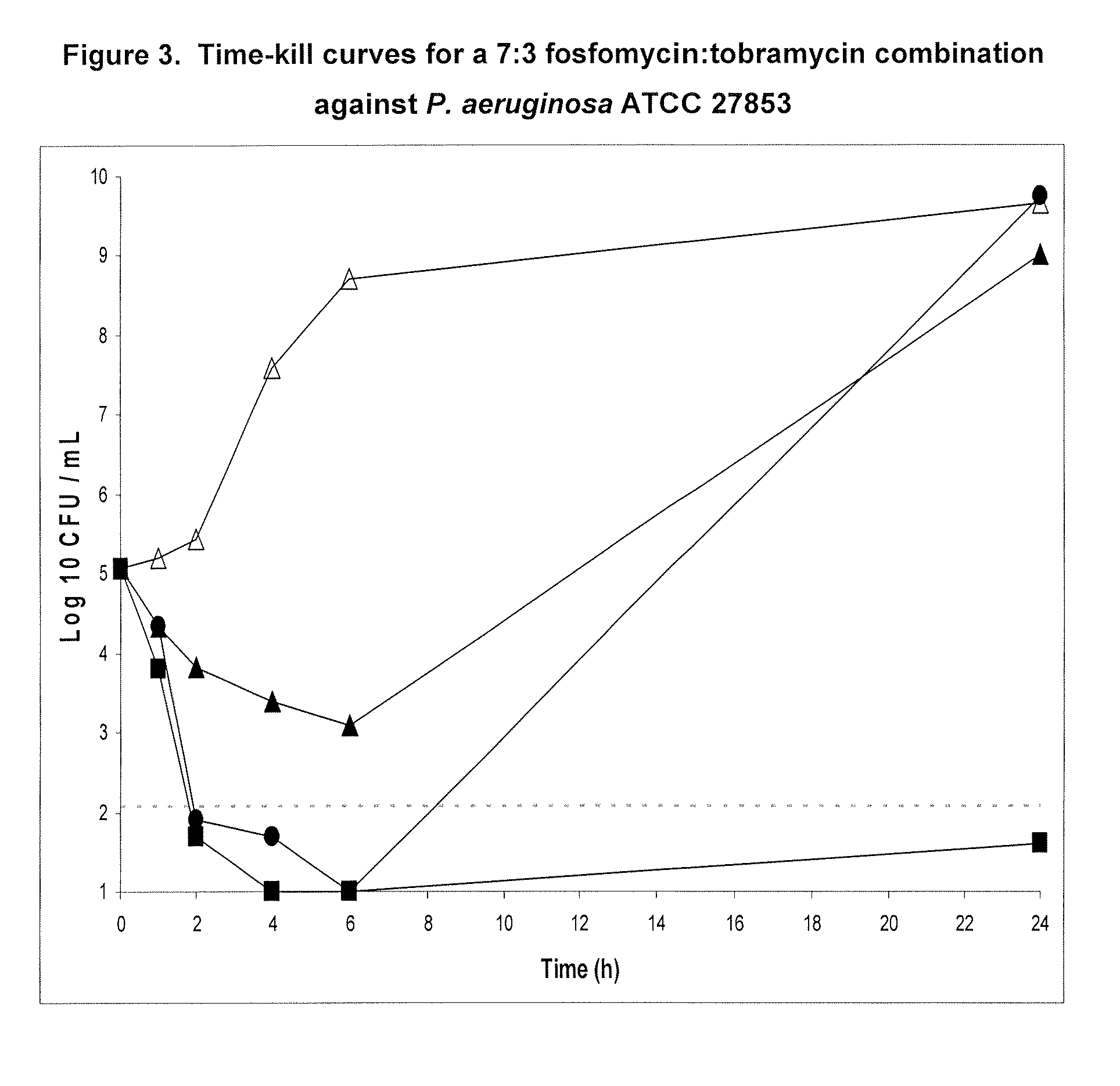
Aerosolized fosfomycin/aminoglycoside combination for the treatment of bacterial respiratory infections
InactiveUS7943118B2Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideBacterial respiratory infectionTobramycin
Owner:GILEAD SCI INC
Amniotic membrane long-term preserving fluid and preparation method thereof
ActiveCN102132697AKeep aliveEasy batch processingDead animal preservationHydroxyethyl starchCulture fluid
The invention relates to an amniotic membrane long-term preserving fluid which is characterized in that 1000ml of preserving fluid contains 10g-200g of chondroitin sulfate and low-molecular-weight dextranum, 10g-50g of hydroxyethyl starch, 5ml-20ml of amino acid, 5ml-20ml of tobramycin, 10ml-50ml of HEPES, 5ml-30ml of ascorbic acid, 5ml-20ml of sodium pyruvate, and 860ml-970ml of culture solution. A preparation method of the amniotic membrane long-term preserving fluid comprises the following steps: adding the chondroitin sulfate, the low-molecular-weight dextranum, the amino acid, the tobramycin, the hydroxyethyl starch, the ascorbic acid and the sodium pyruvate into the culture solution and then uniformly mixing the mixture; using 1%-5% of HEPES to adjust pH value till the pH value is 7.2-7.4; using an osmotic pressure buffering agent to adjust the osmotic pressure till the osmotic pressure is 350-380mOsm / L; and performing membrane filtration and degerming, thereby acquiring the amniotic membrane long-term preserving fluid.
Owner:周海华
Composite collagen eye drops
ActiveCN1927392AKeep aliveMaintain adhesionOrganic active ingredientsSenses disorderTobramycinAntibiotic Y
Owner:GUANGZHOU TRAUER BIOTECH
Self-Preserved Ophthalmic Pharmaceutical Compositions Containing Tobramycin
InactiveUS20080089953A1Good antifungal activityHigh activityAntibacterial agentsBiocideMedicinePreservative
Self-preserved, multi-dose ophthalmic compositions containing tobramycin are described. The compositions do not contain a conventional antimicrobial preservative, such as benzalkonium chloride. Rather, the compositions are self-preserved as a result of the inherent antimicrobial activity of tobramycin. The compositions preferably also contain either boric acid or, more preferably, a borate / polyol complex selected from the group consisting of borate / propylene glycol, borate / glycerol, and combinations thereof.
Owner:ALCON INC
Optimised formulation of tobramycin for aerosolization
InactiveUS20050163722A1Improve complianceMaximum efficacyAntibacterial agentsBiocideTobramycinCystic fibrosis lungs
The invention provides a tobramycin formulation for delivery by aerosolization in the form of additive-free, isotonic solution whose pH has been optimised to ensure adequate shelf-life at room temperature. Said formulation can be advantageously used for the treatment and prophylaxis of acute and chronic endobronchial infections, in particular those caused by the bacterium Pseudomonas aeruginosa associated to lung diseases such as cystic fibrosis.
Owner:CHIESI FARM SPA
Lotepredenol etabonate gernebcin suspension solution and method for preparing the same
The invention discloses an eye use compound drug combination containing lotepredenol etabonate and tobramycin. The combination contains less than 0.1 percent of tetra chloro metlbond. The preparation method of the drug combination is simple and is suitable for industrial production.
Owner:BEIJING D VENTUREPHARM TECH DEV
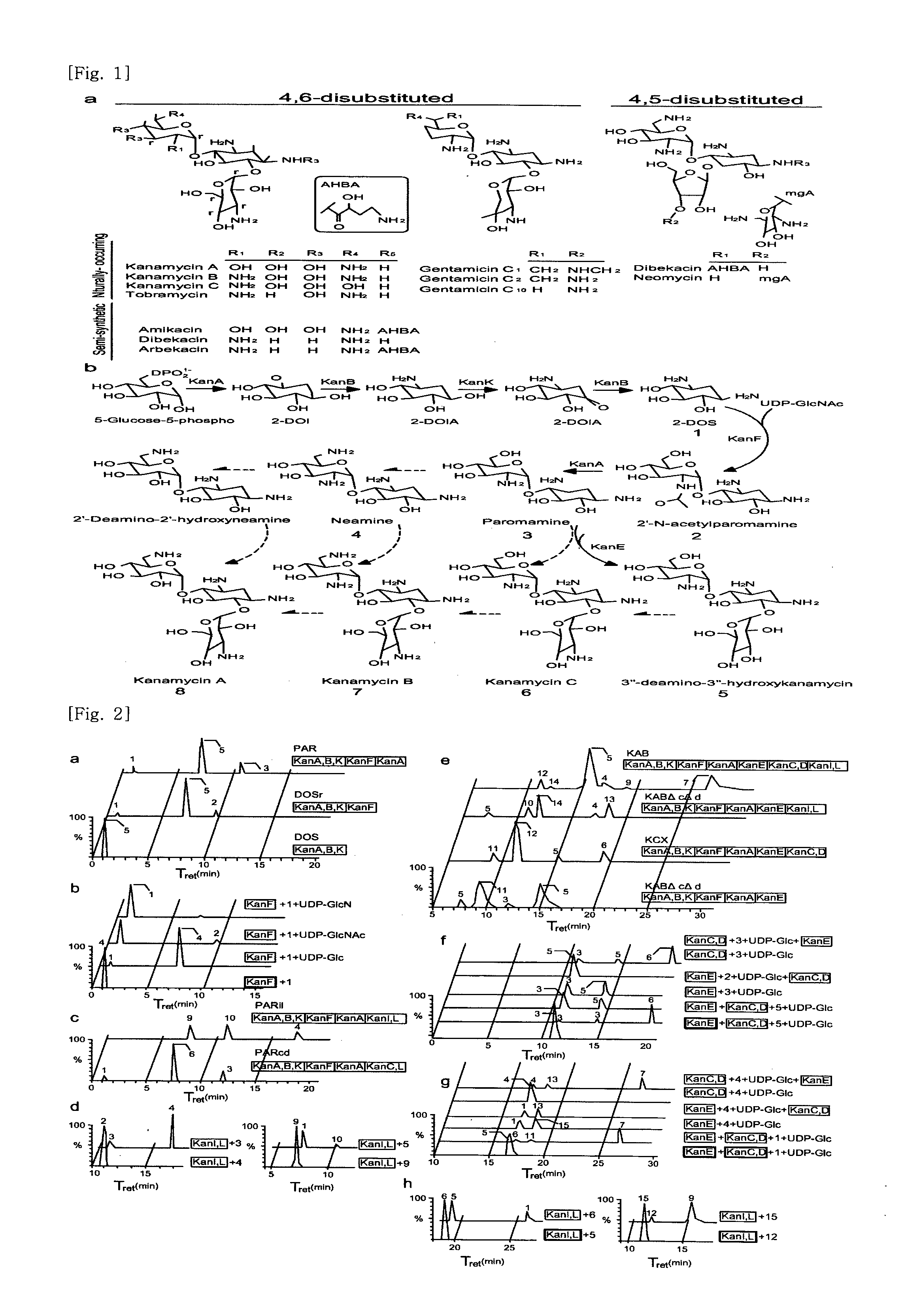
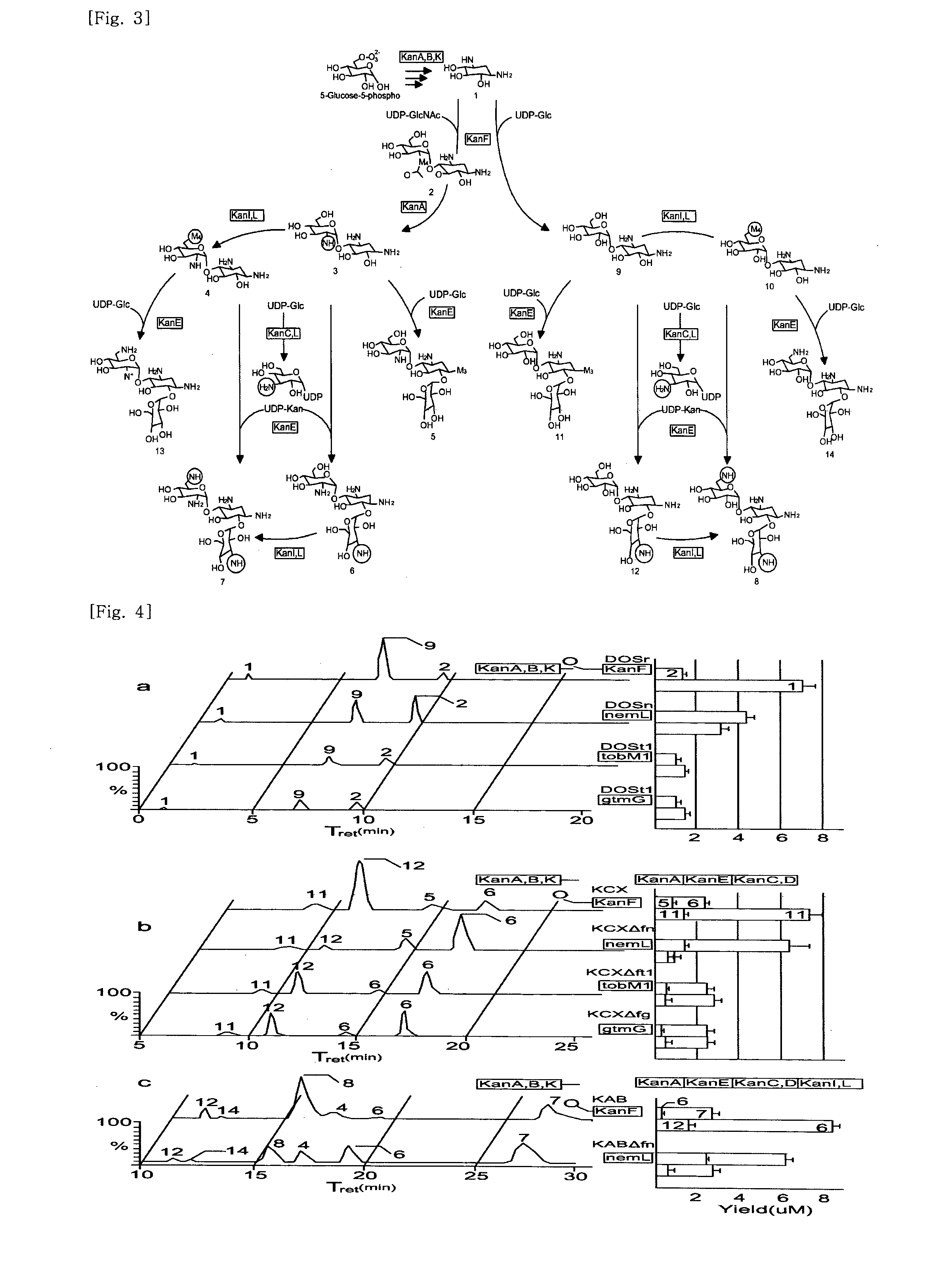
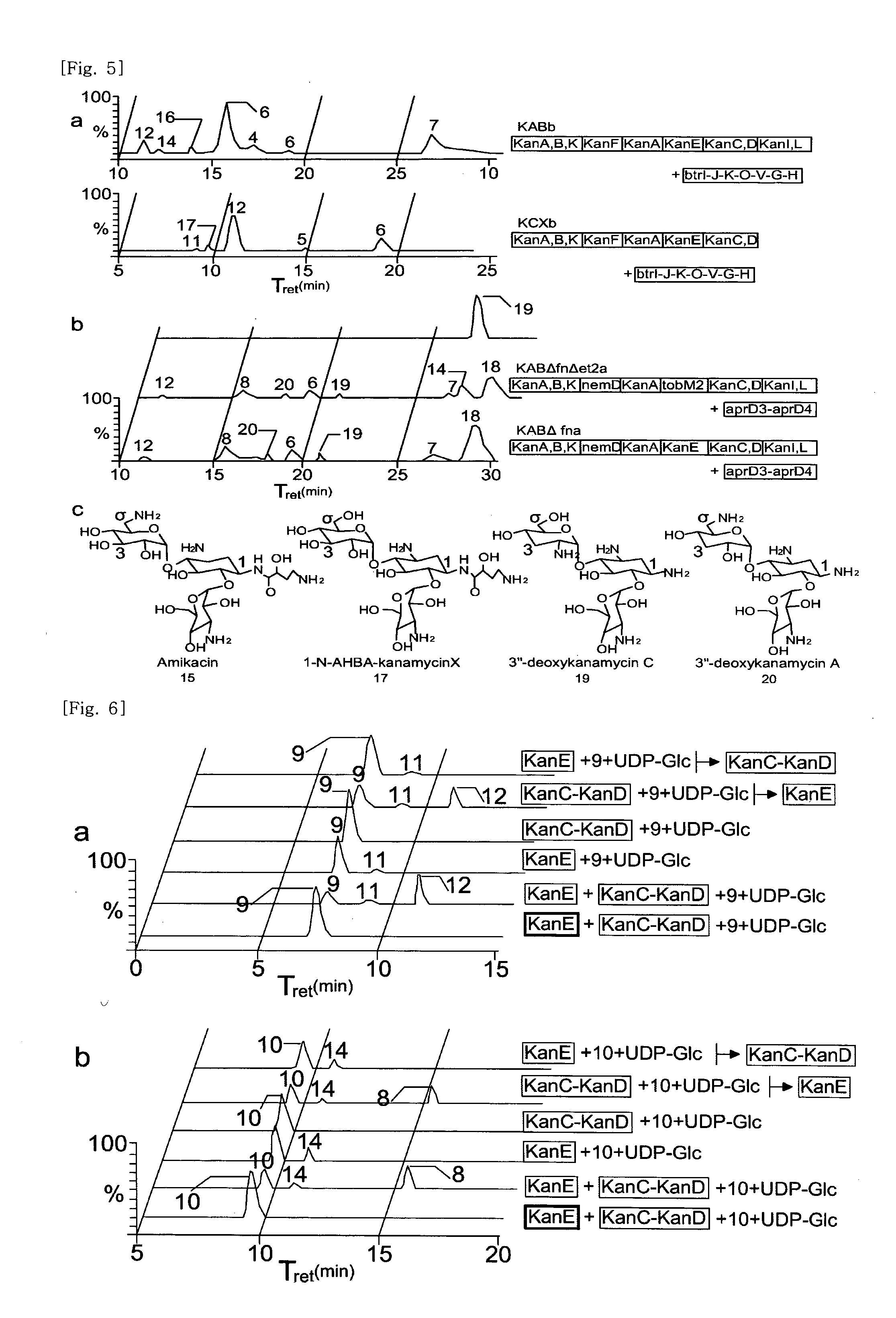
New kanamycin compound, kanamycin-producing streptomyces species bacterium, and method of producing kanamycin
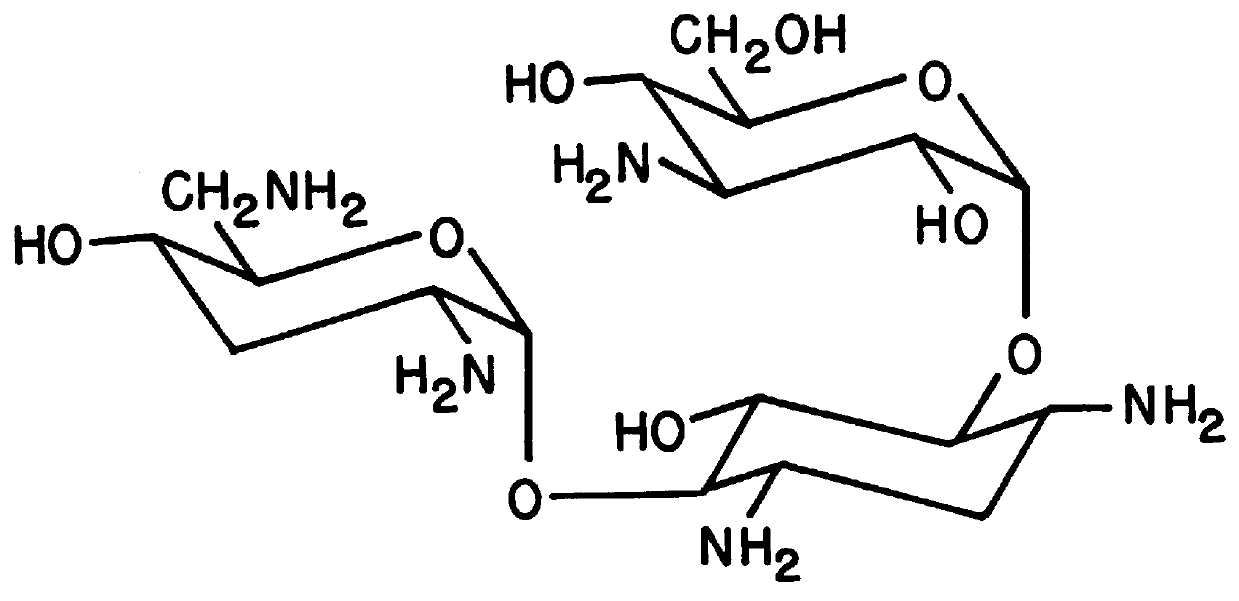
Vectors expressing kanA-kanB-kanK and other kanamycin production-related genes, Streptomyces<i / > species recombinant bacteria transformed with the vectors, a method of producing kanamycin antibiotics by the bacteria, and a new kanamycin compound produced by the bacterium are provided. With the use of the recombinant bacteria of the present invention, the direct fermentative biosynthesis of amikacin and tobramycin as semi-synthetic kanamycins is possible, and the yield of kanamycin B as a precursor of the semi-synthetic kanamycin is improved.
Owner:INTRON BIOTECHNOLOGY INC
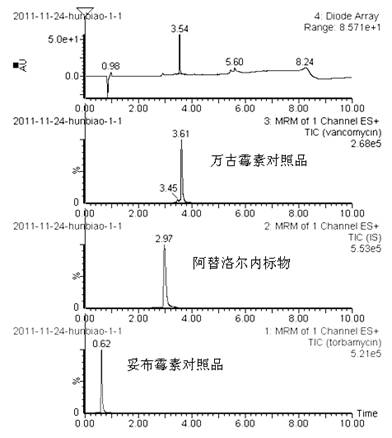
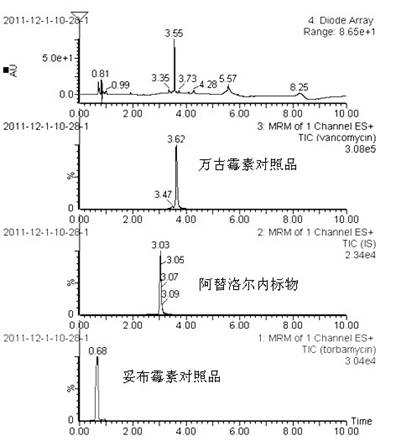
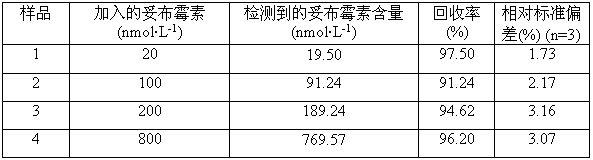
Method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling technique
The invention relates to the field of drugs, in particular to a detection method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid. A method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through an ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling techniquecomprises the following steps of: 1) preparing reference samples; 2) preparing internal standard solution; 3) preparing sample solution; 4) preparing mobile-phase solution; 5) setting chromatographicconditions; 6) optimizing mass spectrometric conditions; 7) determining the samples; 8) preparing gradient-concentration reference sample solution; 9) preparing a standard curve; and 10) analyzing and computing data. The method has the characteristics that: 1) the method is advanced; 2) the method is rapid and high-efficiency the loss is low; and 3) the clinical application prospect and the effect are good.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Inhaled fosfomycin/tobramycin for the treatment of chronic obstructive pulmonary disease
InactiveUS20110124589A1Reduce frequencyReduce severityAntibacterial agentsPowder deliveryTobramycinObstructive Pulmonary Diseases
The present invention provides the use of an aerosol formulation comprising fosfomycin and tobramycin in the treatment of patients with chronic obstructive pulmonary disease (COPD) who are experiencing or are at risk of experiencing acute exacerbations of COPD. Formulations for such use and methods of treating humans with COPD are also provided.
Owner:GILEAD SCI INC
Method for preparing decellularized cornea
ActiveCN106421908ALow transparencyAvoid breedingTissue regenerationProsthesisCell-Extracellular MatrixECM Protein
The invention provides a method for preparing decellularized cornea. Due to the selection of components and proportions, colloid osmotic pressure of a decellularized cornea preparation liquid can be maintained by using a prepared protection liquid, and compared with a conventional decellularized cornea preparation liquid, the decellularized cornea preparation liquid is capable of remarkably alleviating the situation that the cornea transparency is degraded as an extracellular matrix such as collagen is excessively swelled and lost in the later cell treatment process; in addition, due to the addition of antibiotics such as tobramycin, bacterium propagation of susceptible cornea can be inhibited, and the contamination rate in the treatment process can be reduced. By adopting the method provided by the invention, cells can be crushed, and the situation that the trenchancy is degraded as cornea matrix collagen is damaged by long-term high pressure can be avoided. Secondly, as a detergent and nuclease act up simultaneously, cell debris and nucleic acid components can be relatively rapidly and effectively removed, and the situation that the trenchancy of the cornea is degraded as protein components such as cornea collagen are damaged in long-term treatment of the detergent and other components can be avoided.
Owner:SHENZHEN AINEAR CORNEA ENG
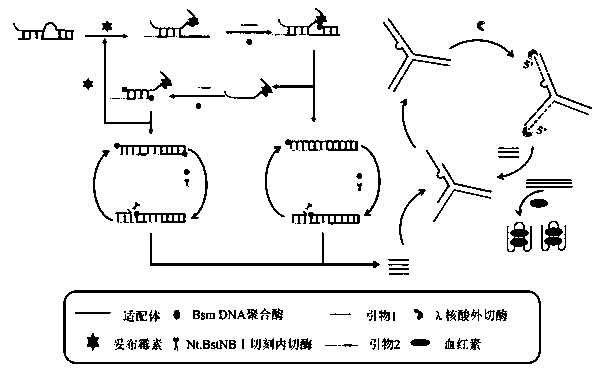
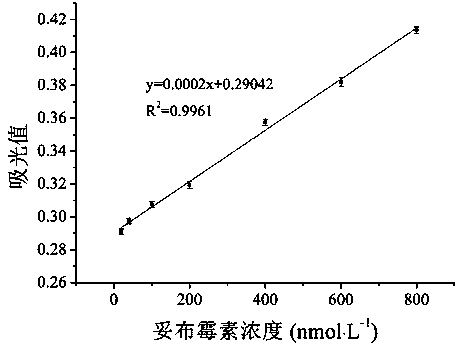
Colorimetric method for detecting tobramycin based on double strand displacement and three-dimensional DNA structure
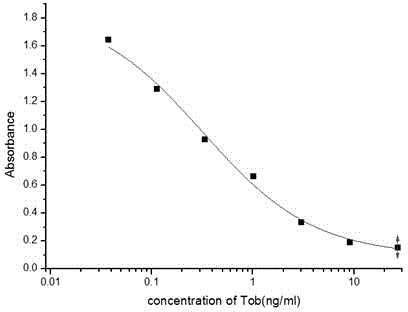
ActiveCN110592187AAchieving multiple magnificationsExpand the scope of detectionMicrobiological testing/measurementLinear relationshipGenetics
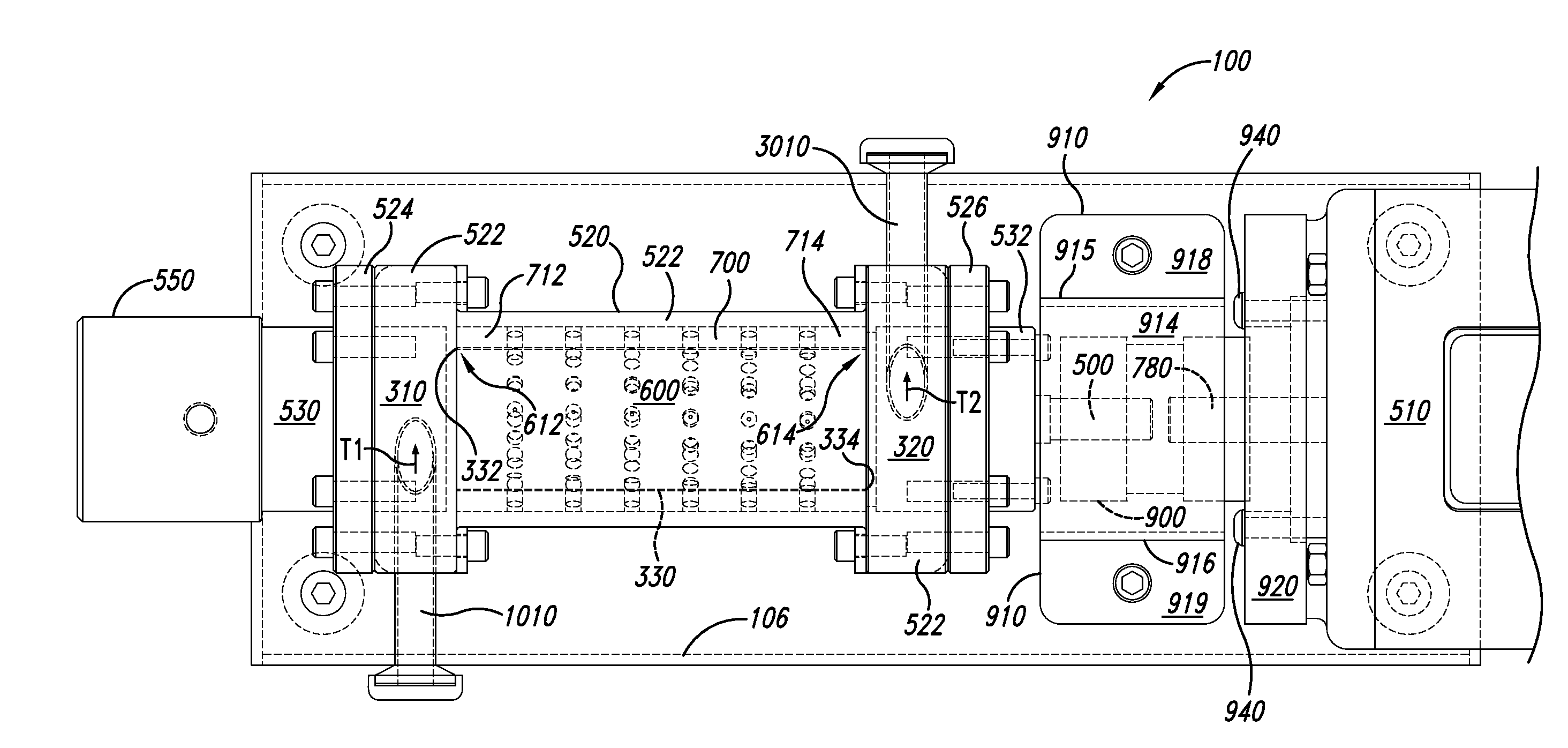
The invention discloses a colorimetric method for detecting tobramycin based on double strand displacement and a three-dimensional DNA structure, and belongs to the field of food safety, medical analysis and environmental pollution detection. The method comprises the following steps: firstly, double strands T1 / T2 are designed; when tobramycin exists, Bsm DNA polymerase synthesizes double strands which are completely complementary through a strong strand displacement reaction, and Nt.BstNBI incision endonuclease cuts recognition sites on the double strands; the three-way DNA structure capture reporter probes, and regenerates and replaces a large number of S1 strands containing G-quadruplex forming sequences. Thereafter, the G-quadruplex / heme catalyzes ABTS<2-> / H2O2 chromogenic reaction, andthe tobramycin content can be determined by using the linear relationship between light absorption value and tobramycin concentration. According to the invention, an aptamer captures tobramycin to trigger double strand displacement reaction which is mediated by the Nt.BstNBI incision endonuclease and the Bsm DNA polymerase so as to generate a large number of reporter probes. Meanwhile, the reporter probes trigger lambda exonuclease-assisted loop amplification, so that multiple amplifications of colorimetric signals are realized, the detection range is widened, and the detection sensitivity isimproved.
Owner:JIANGNAN UNIV
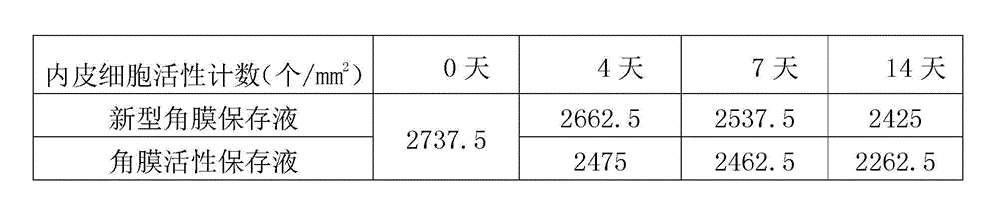
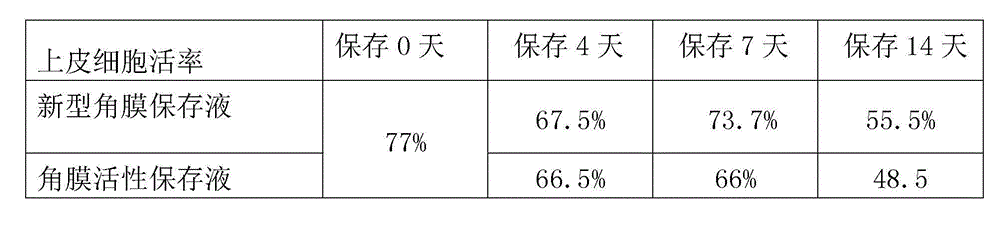
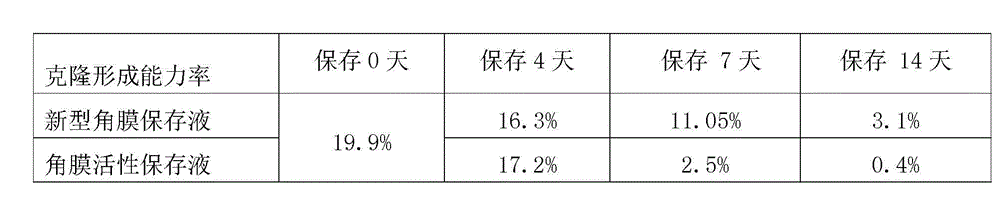
Cornea metaphase preservation solution, and preparing and using methods thereof
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
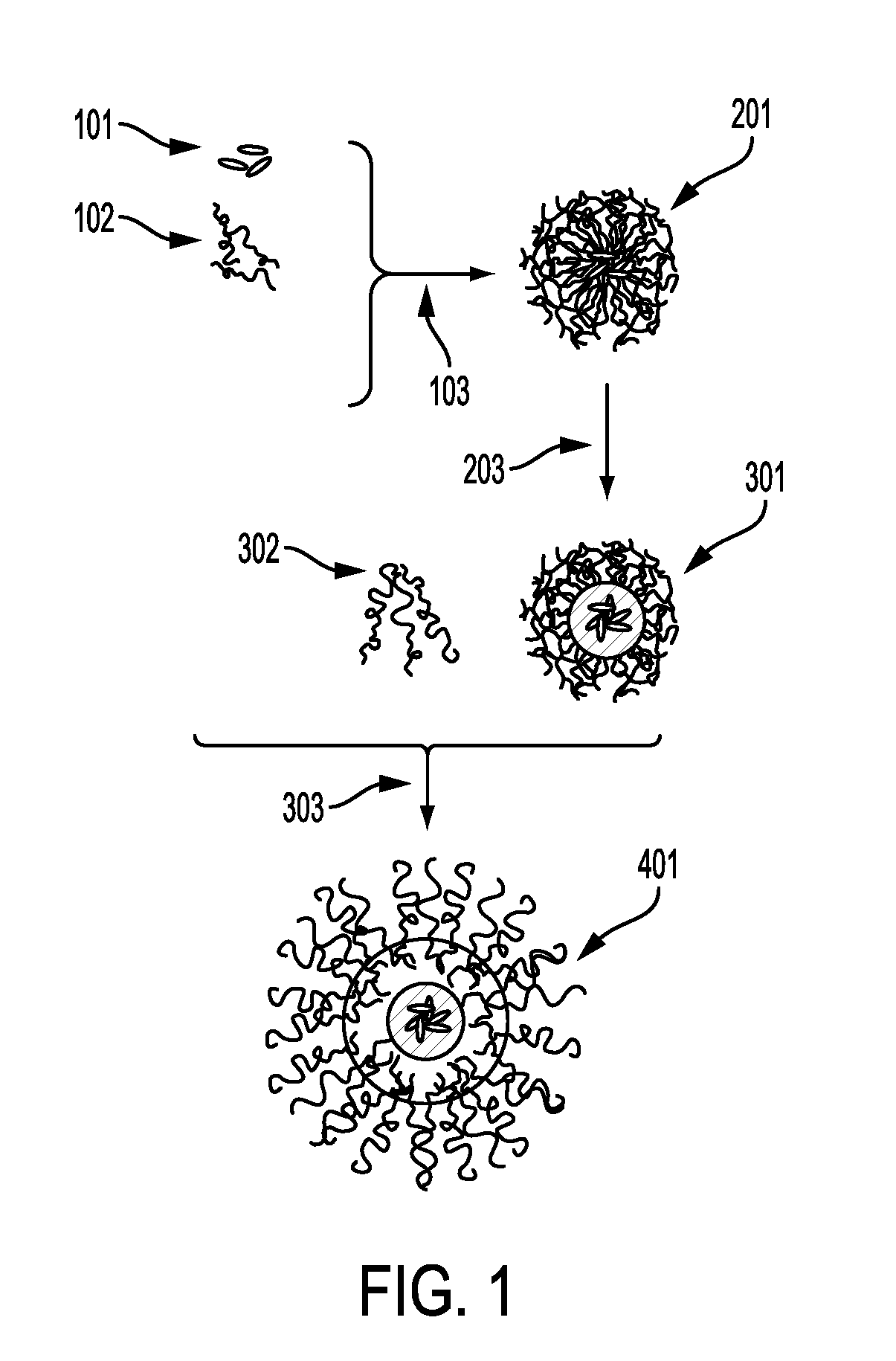
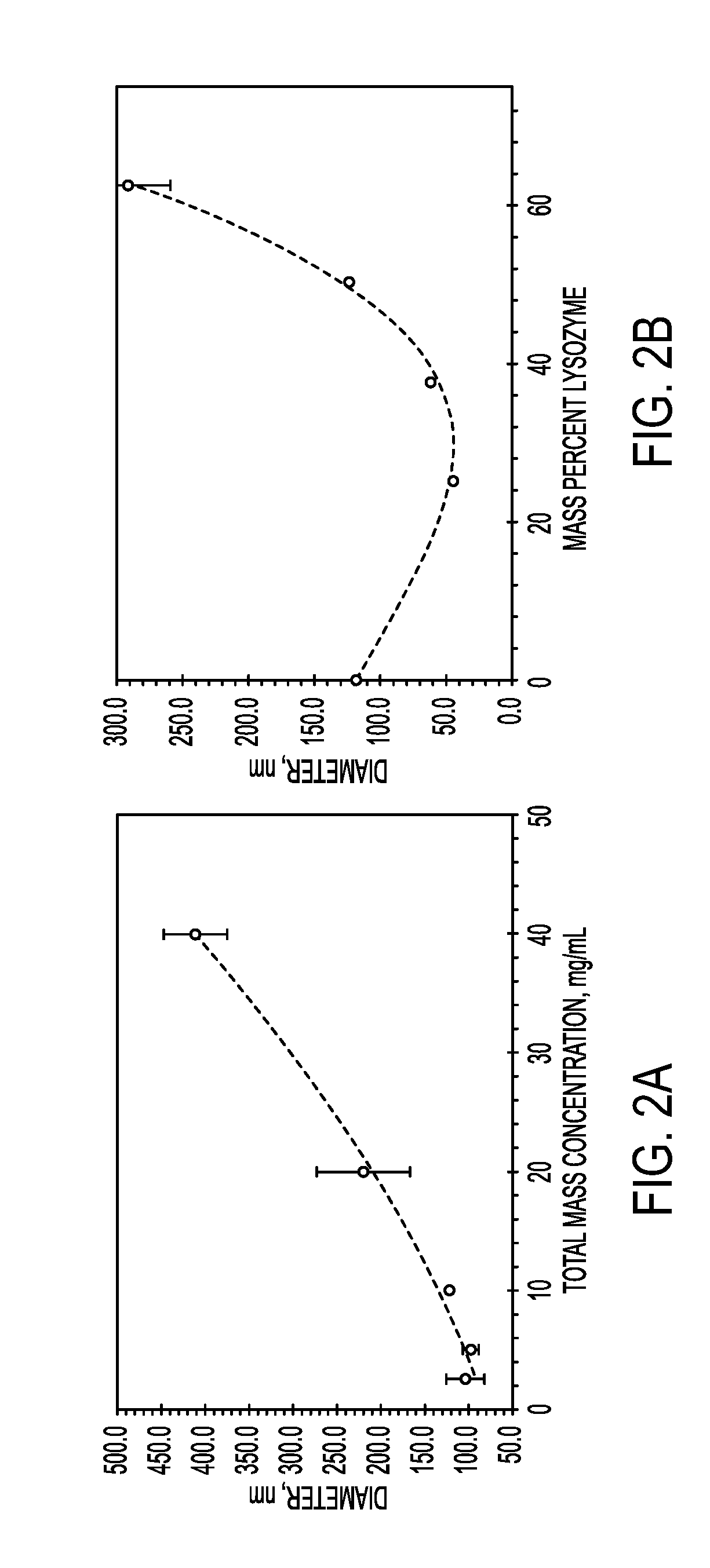
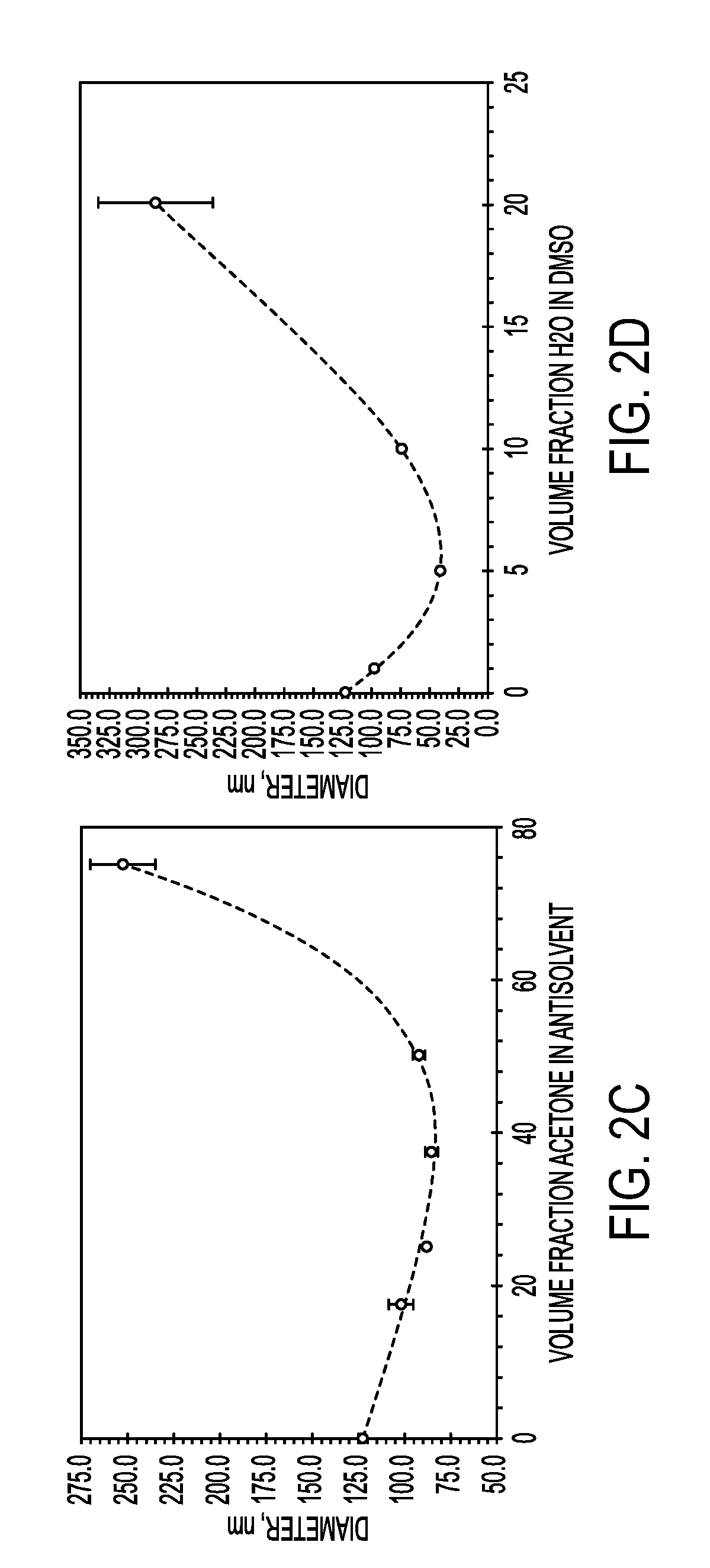
Process for encapsulating soluble biologics, therapeutics, and imaging agents
An “inverse” precipitation route to precipitate aqueous soluble species with copolymers as nanoparticles having a hydrophilic, polar core and a less polar shell is described. A method of the invention for encapsulating water soluble molecules using rapid, controlled precipitation is presented. Water soluble molecules—including peptides, proteins, DNA, RNA, non-biologic therapeutics, polysaccharide-based therapeutics (e.g., tobramycin) and imaging agents—precipitate into nano articles that are protected by a copolymer stabilizing agent. These particles may be covalently or non-covalently stabilized. The particles may be coated with an amphiphilic polymer, or processed into microparticles or larger monoliths. Post processing on the final construct may be conducted.
Owner:THE TRUSTEES FOR PRINCETON UNIV
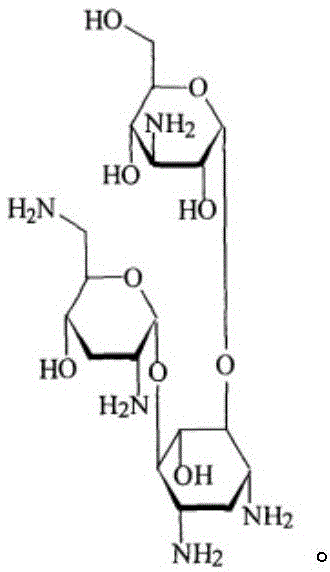
Tobramycin monoclonal antibody as well as preparation method and application of tobramycin monoclonal antibody
ActiveCN104312978AEasy to prepareHigh sensitivityEgg immunoglobulinsMicroorganism based processesTobramycinCarrier protein
The invention relates to a tobramycin monoclonal antibody as well as a preparation method and application of the tobramycin monoclonal antibody and belongs to the field of food safety immunodetection. The monoclonal antibody is generated by mouse hybridoma cell line No. G (A10) with the preservation number of CGMCC No.9307. The preparation method comprises the following steps: (1) activating amino of tobramycin with a glutaraldehyde method, coupling the activated amino of the tobramycin with the amino of carrier protein, reducing C=N of Schiff base formed by glutaraldehyde and amino of the obtained conjugate into a stable C-N structure to obtain final stable conjugate serving as complete antigen; (2) immunizing, fusing and sieving the complete antigen obtained in the step (1), carrying out enlarged cultivation and mouse enterocoelia induction to generate ascites to obtain the monoclonal antibody used for specific detection of tobramycin; and (3) activating carboxyl of succinic acid with a DCC method, reacting activate fluid with ovalbumin amidogen to obtain carboxylation protein of ovalbumin-succinic acid, carrying out dialysis and condensing the tobramycin and the carboxyl of the protein by using an EDC method to prepare peridium of screened antibody.
Owner:JIANGNAN UNIV
Tobramycin inhalation composition and preparation method and application thereof
ActiveCN105616345AMeets requirements for inhalation solutionsInhalation solution requirements addressedAntibacterial agentsOrganic active ingredientsInhalationTobramycin
The invention relates to a tobramycin inhalation composition and a preparation method and application thereof. The composition is prepared from tobramycin 4%-8% (w / v), sodium chloride 0.1%-0.9% (w / v), weak-reducibility organic weak acid serving as a stabilizer 0.1%-1.0% (w / v), strong acid for regulating the pH value of the composition and the balance water, wherein the pH value of the composition is 5.6-7.5. The tobramycin inhalation composition prepared by adopting the technical scheme has the pH value closer to the physiological pH of a human body and has significantly improved stability at room temperature.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
Eye sterile suspension containing loteprednol etabonate and preparation method thereof
The invention relates to an ophthalmic sterile suspension containing loteprednol etabonate, which contains 0.5 percent of loteprednol etabonate minicrystal particles and takes 0.3 percent to 0.8 percent of chlorobutanol as a preservative, 0.1 percent to 0.3 percent of tween-80 as a surface active agent, 0.1 percent to 0.5 percent of hydroxypropylmethyl cellulose as a suspending agent, 0.03 percent to 0.08 percent of natrium adetate as a metal ion complexing agent, and glycerin and sodium chloride as an osmotic pressure regulator. The ophthalmic sterile suspension is prepared by respectively adopting different sterilization methods to the loteprednol etabonate and other components. Apart from the components, the ophthalmic sterile suspension also contains 0.3 percent of tobramycin.
Owner:马晶
Cornea metaphase preservation liquid and preparation method thereof
ActiveCN105284788AImprove survivabilityLess fusionDead animal preservationCorneal endothelial cellTobramycin
The invention discloses a cornea metaphase preservation liquid and a preparation method thereof. The cornea metaphase preservation liquid is an MEM cell culture medium, which comprises the following components: chondroitin sulfate (20-26 g / L), low molecular dextran (8-12 g / L), L-glutamine (0.37-0.38 mg / L), fetal calf serum (2-5.5%), tobramycin (0.08-0.12 g / L), Hepes (9-10 g / L), and rapamycin (0.9-1.1 nM / L). The provided cornea metaphase preservation liquid can protect corneal endothelial cells, can avoid the phenomenon of necrosis and fusion of endothelial cells due to long time preservation, and prolongs the cornea preservation time more effectively.
Owner:XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com