Patents
Literature
60 results about "Corneal limbus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
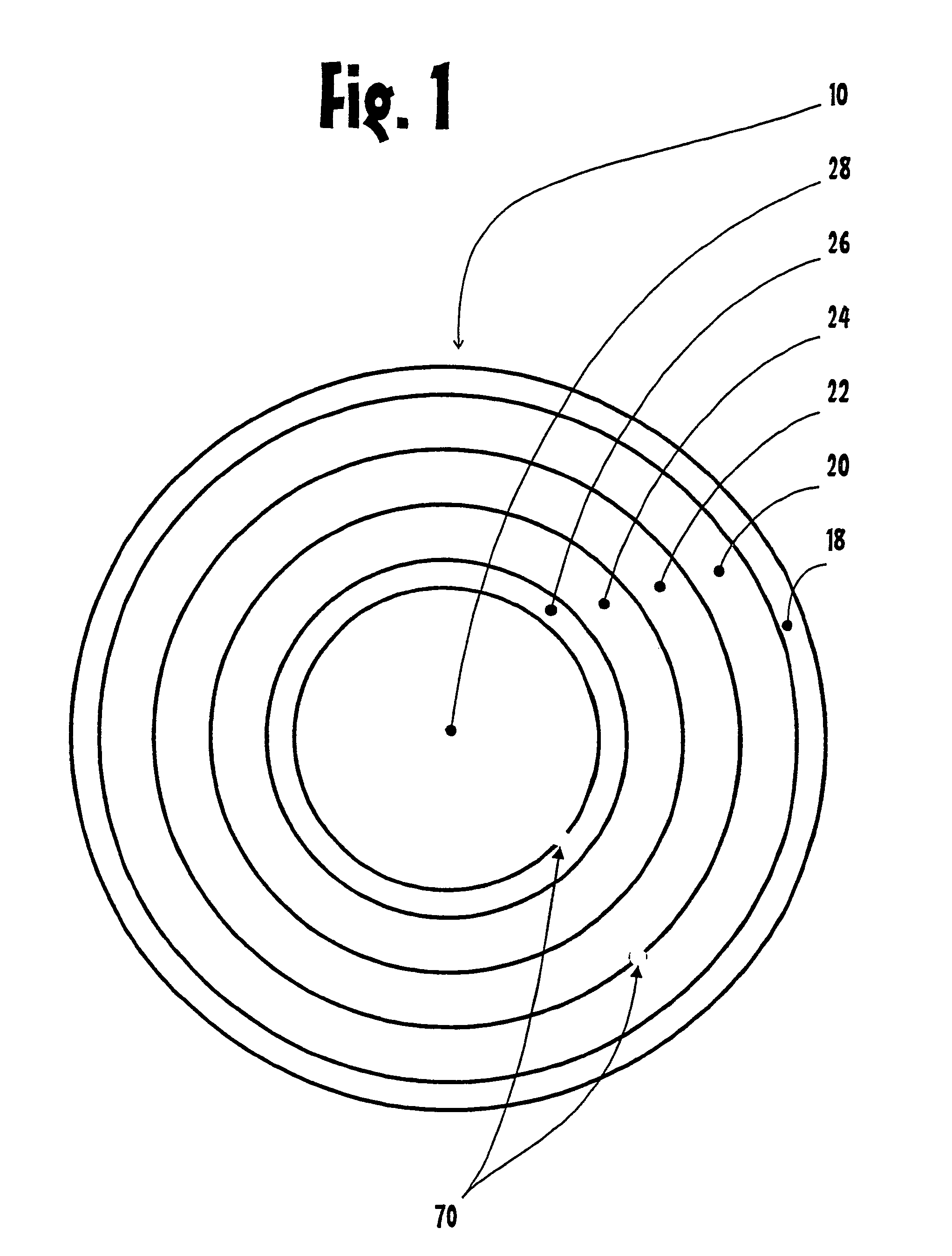
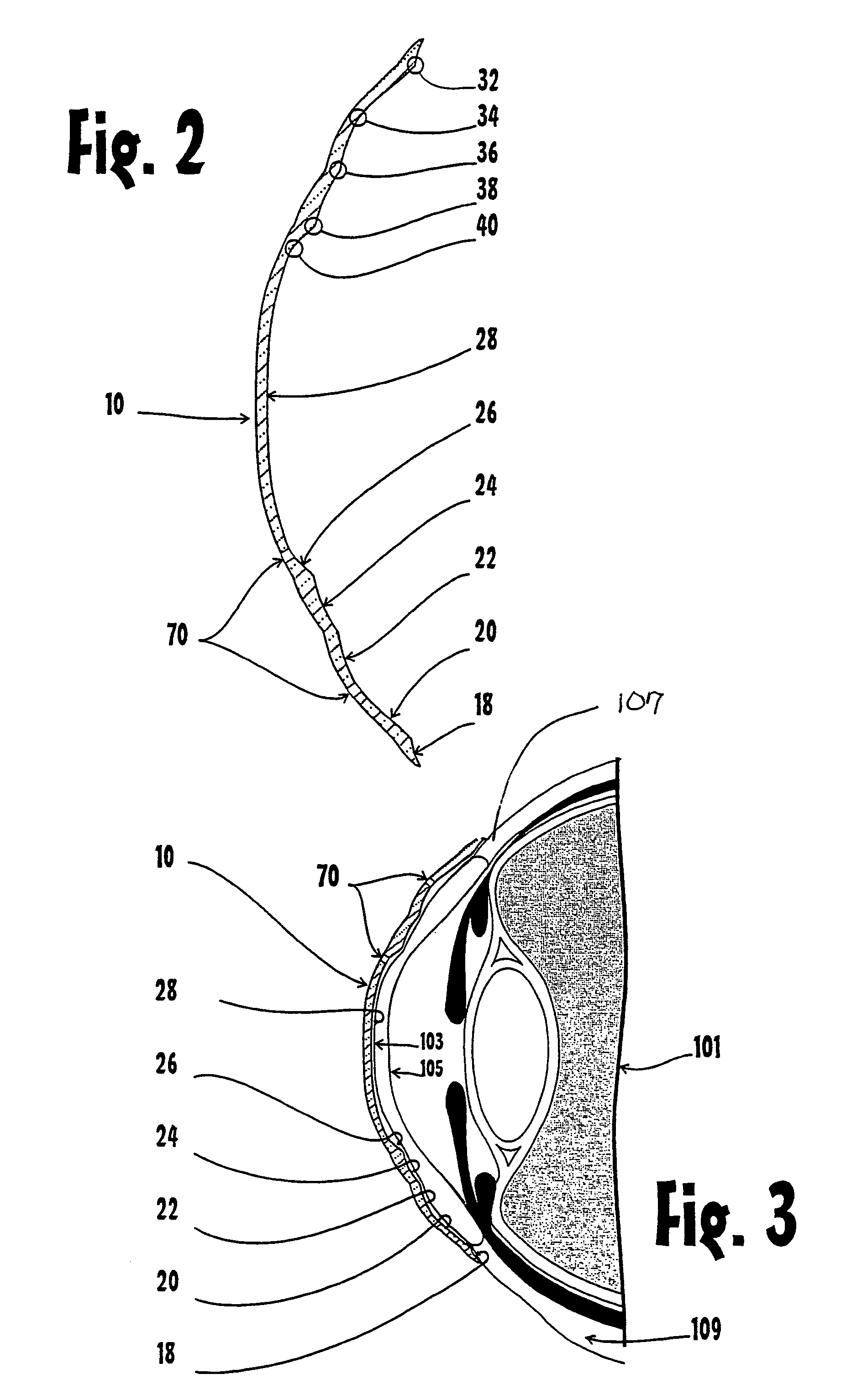
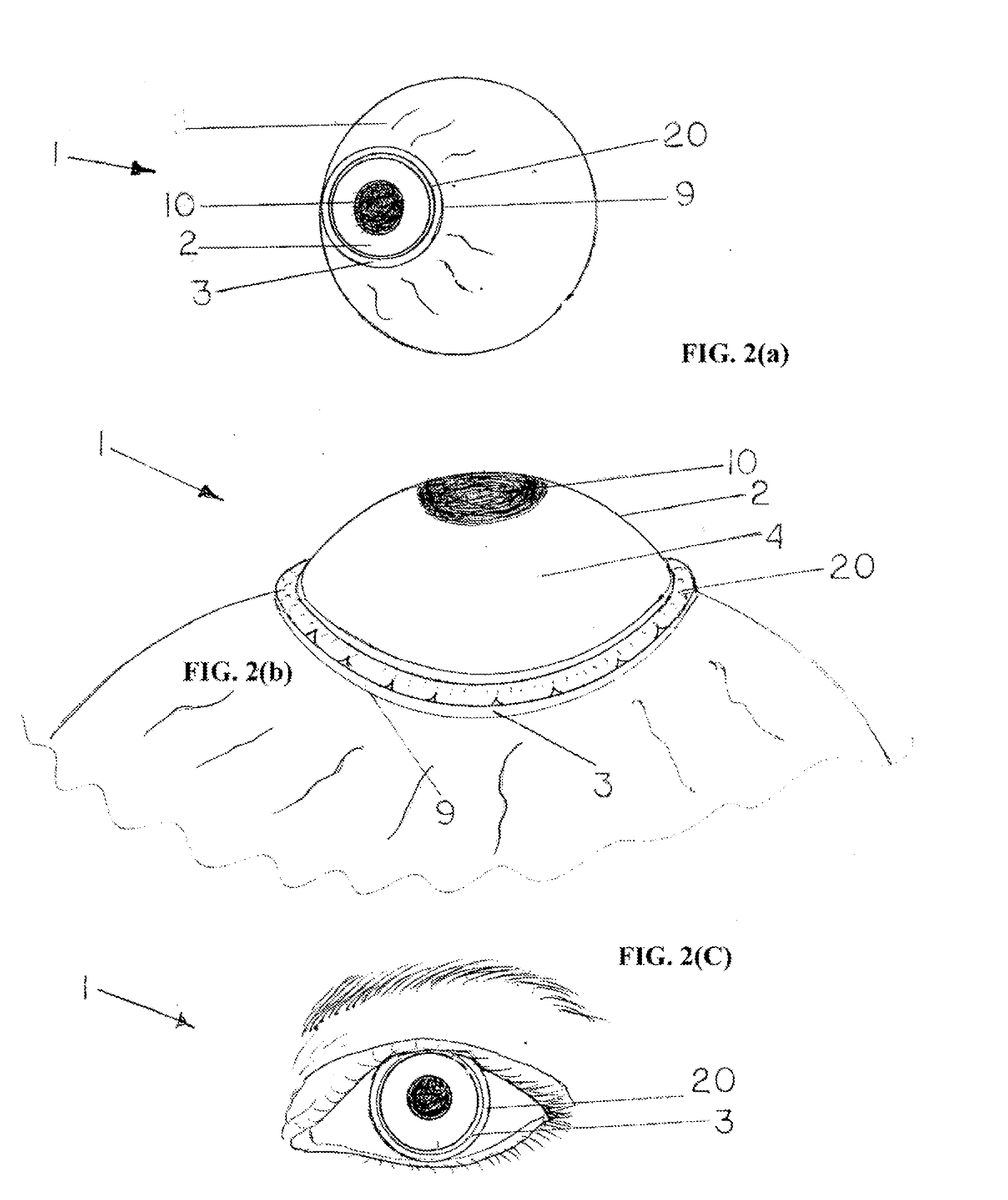
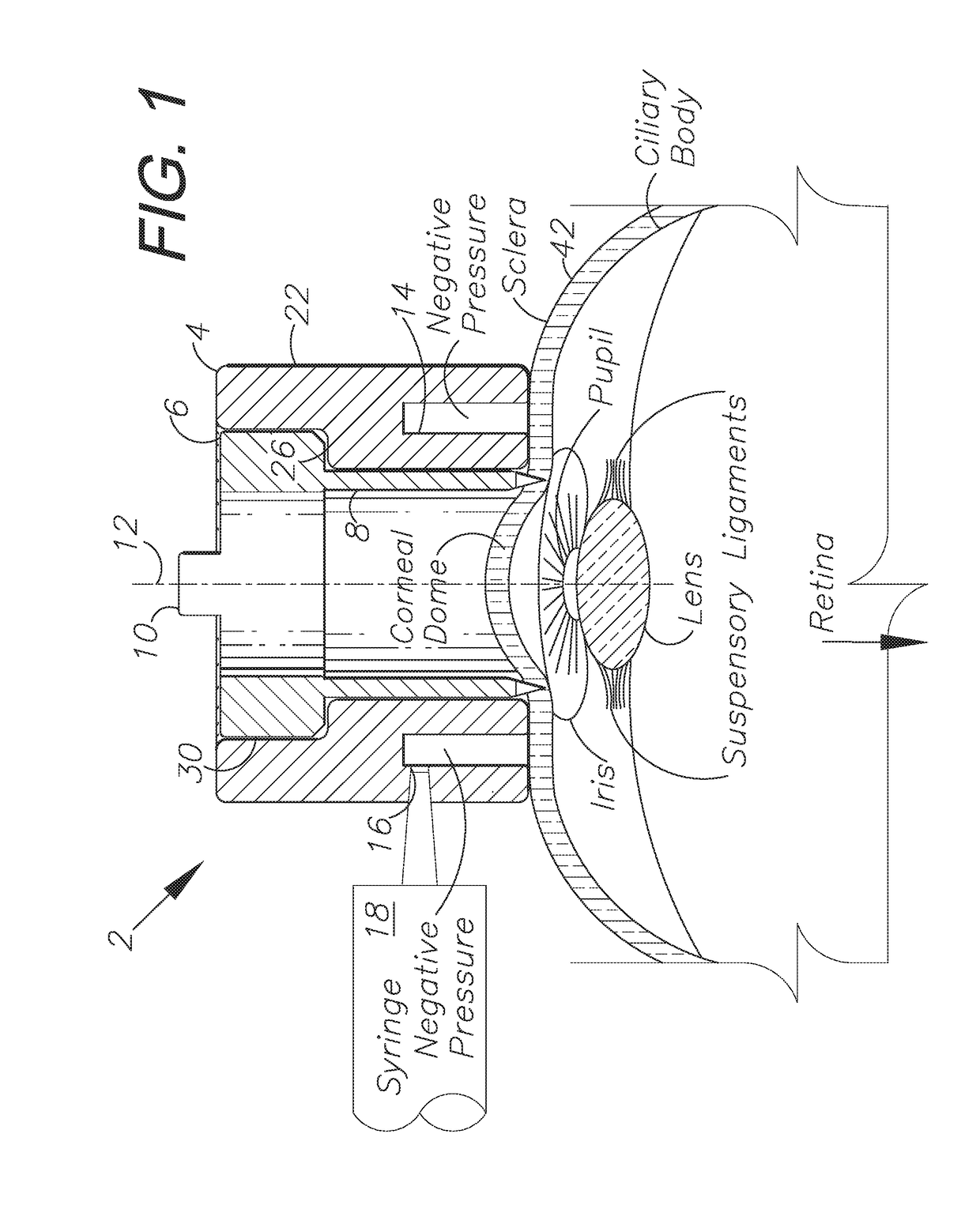
The corneal limbus is the border of the cornea and the sclera (the white of the eye). The limbus is a common site for the occurrence of corneal epithelial neoplasm. The limbus contains radially-oriented fibrovascular ridges known as the palisades of Vogt that may harbour a stem cell population. The palisades of Vogt are more common in the superior and inferior quadrants around the eye. Aniridia, a developmental anomaly of the iris, disrupts the normal barrier of the cornea to the conjunctival epithelial cells at the limbus.
Apparatus and methods for prevention of age-related macular degeneration and other eye diseases
InactiveUS20030105456A1Minor side effectsIncrease flexibilityLaser surgerySurgical instruments for heatingRadio frequencyLaser beams
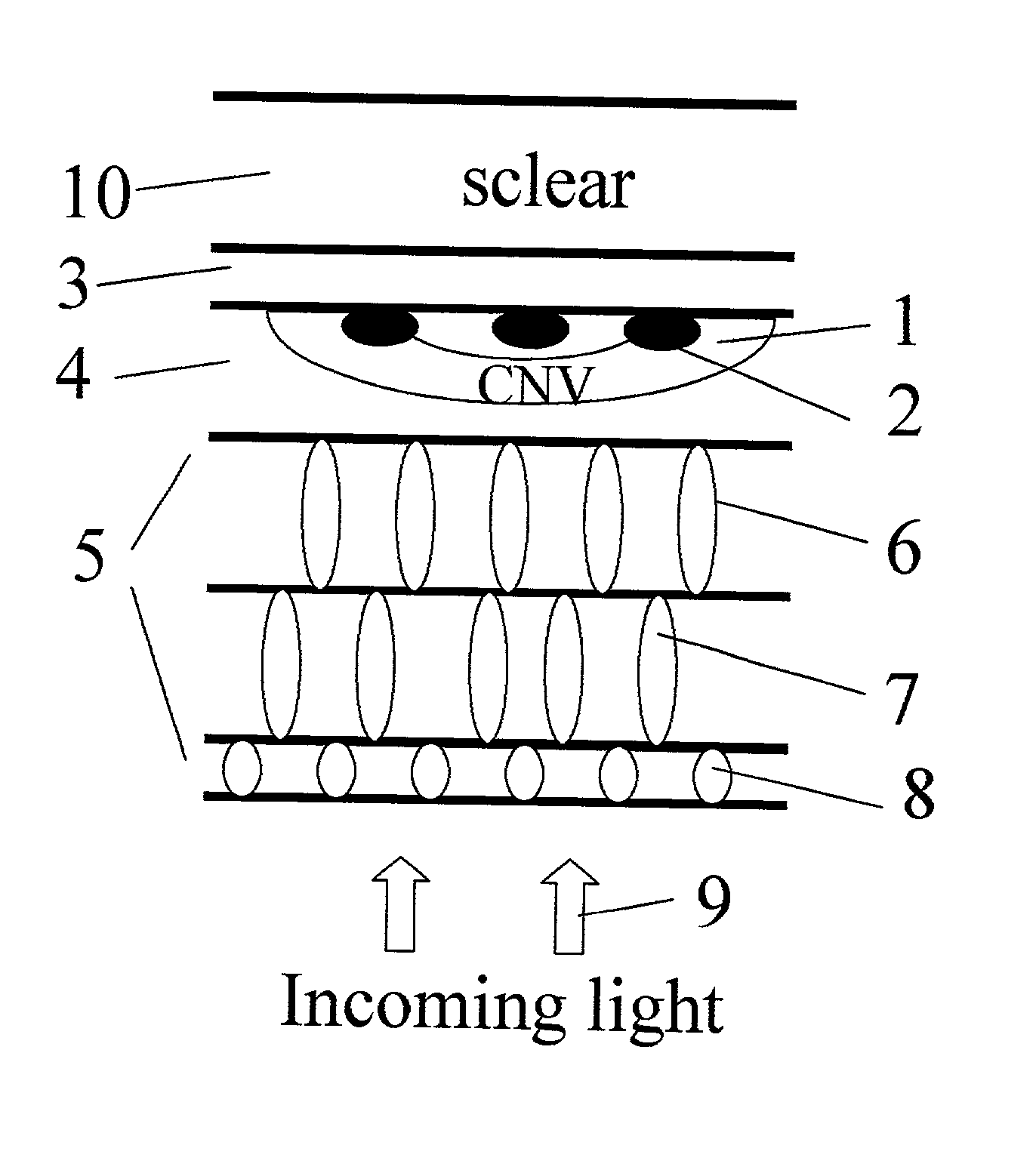
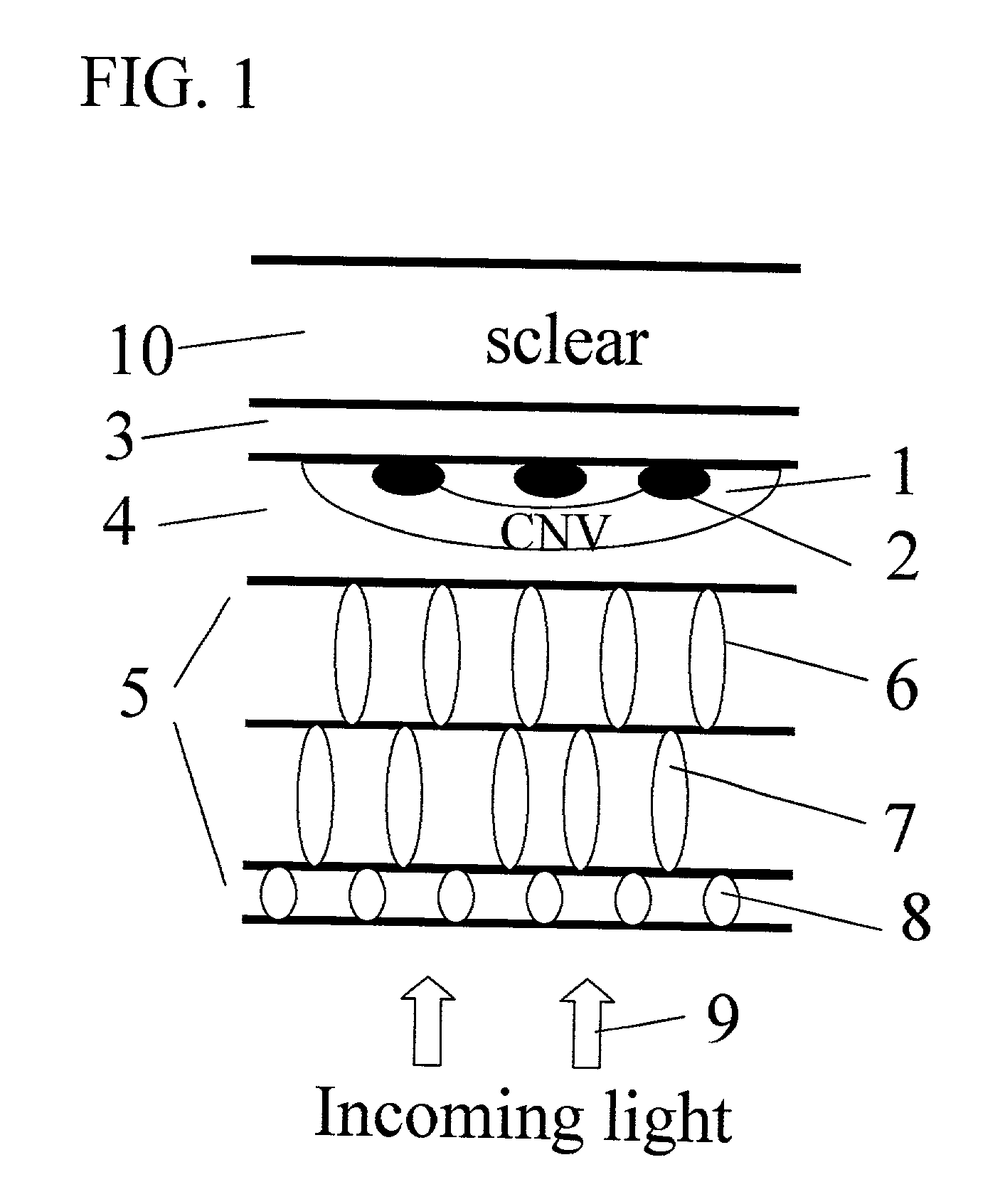
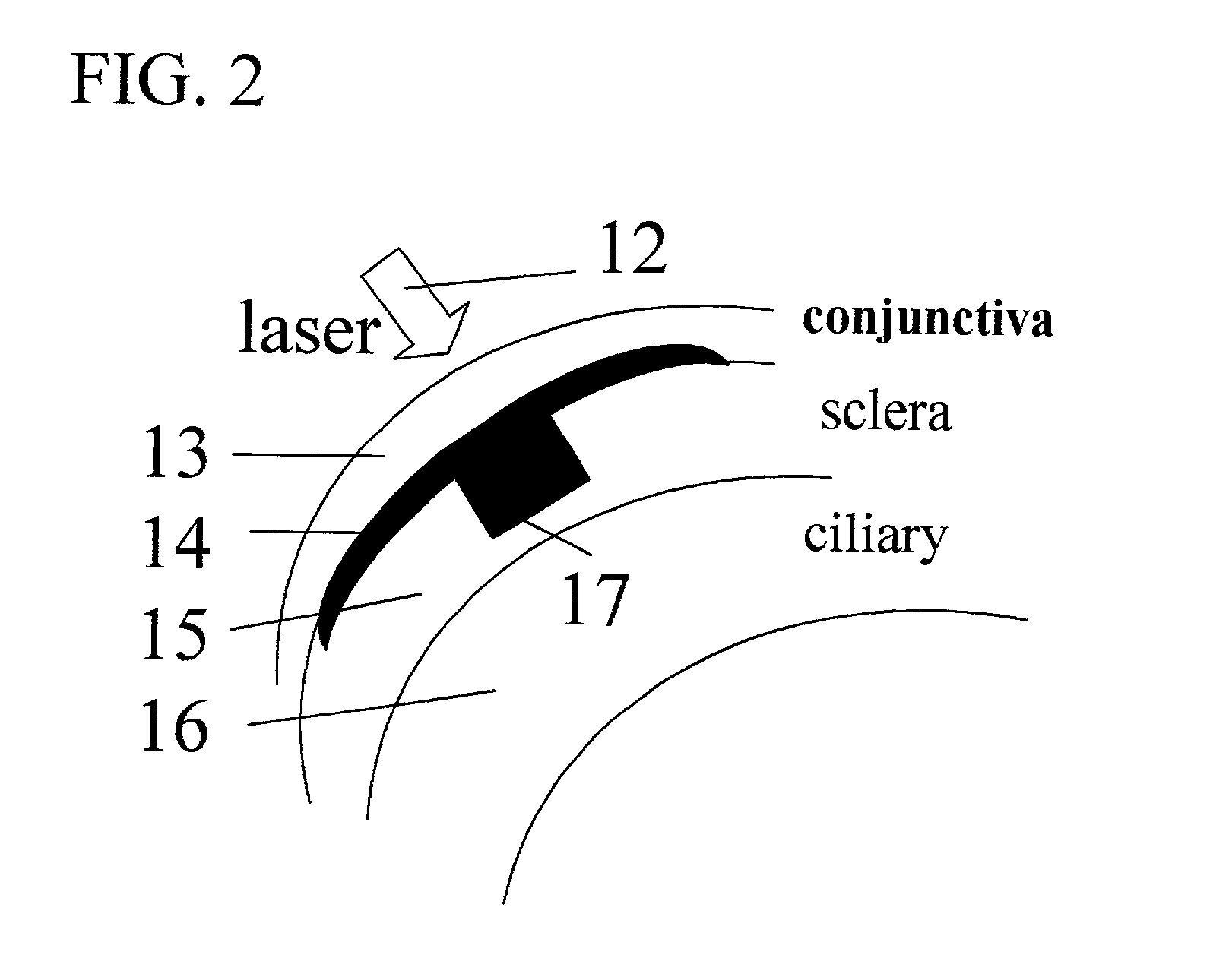
Surgical apparatus and surgical methods are proposed for the prevention of age-related macular degeneration (AMD) and choroidal neovascularization (CNV), and other eye diseases such as glaucoma by removal of the sclera tissue to reduce its rigidity and increase the flood flow and decrease pressure in the choriocapillaris. The disclosed preferred embodiments of the system consists of a tissue ablation means and a control means of ablation patterns and a fiber delivery unit. The basic laser beam includes UV lasers and infrared lasers having wavelength ranges of (0.15-0.36) microns and (0.5-3.2) microns and diode lasers of about 0.98, 1.5 and 1.9 microns. AMD and CNV are prevented, delayed or reversed by using an ablative laser to ablate the sclera tissue in a predetermined patterns outside the limbus to increase the elasticity of the sclera tissue surrounding the eye globe The surgery apparatus also includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device
Owner:LIN J T
Corneal-scleral orthokeratology contact lens
ActiveUS7559649B2Fast and reliable and comfortable resultAccurately determineOptical partsCamera lensCorneal curvature
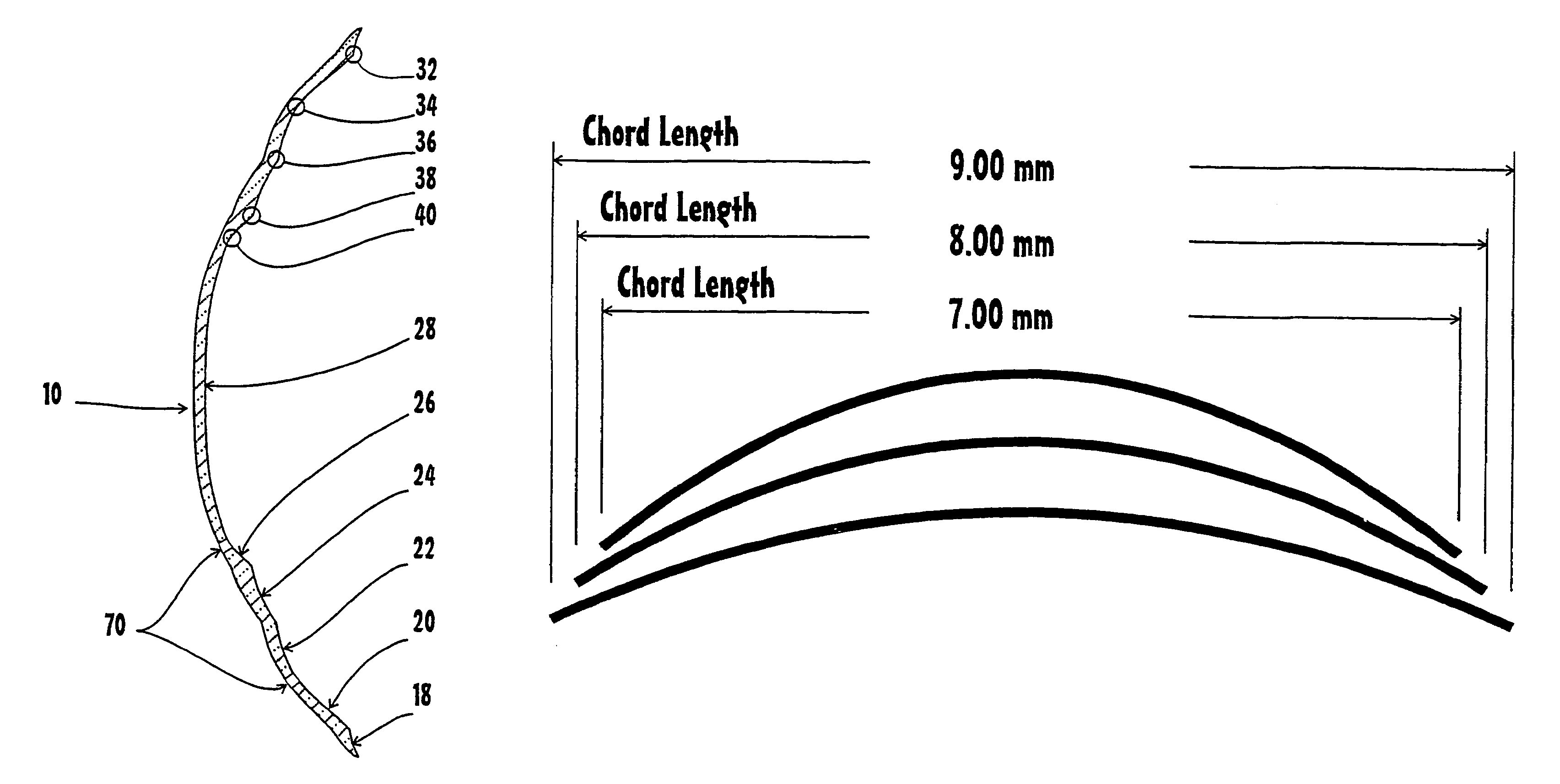
A corneal-scleral orthokeratology contact lens is formed of rigid gas permeable material with uniform arc lengths in the treatment area even when the corneal curvature varies and asymmetric blends or splines called minor zones, where the portion of the blend or spline is shorter toward the lens center and broader toward the lens edge, and relieve the treatment area of all centering responsibility, transferring it to the limbal-scleral region. The method of determining the total sagittal height of an eye at a given chord diameter to predetermine exact fitting parameters and predict unaided visual outcome trial lenses, with exact chord and sagittal height values used to match the sagittal height of an eye, the sagittal value may be obtained from some ocular topographers. A computer program can easily take the sagittal information and design an optimum lens.
Owner:DAKOTA SCI
Pluripotent embryonic-like stem cells derived from corneal limbus, methods of isolation and uses thereof
InactiveUS20060216821A1Increase telomerase activityCulture processNervous system cellsGerm layerDisease
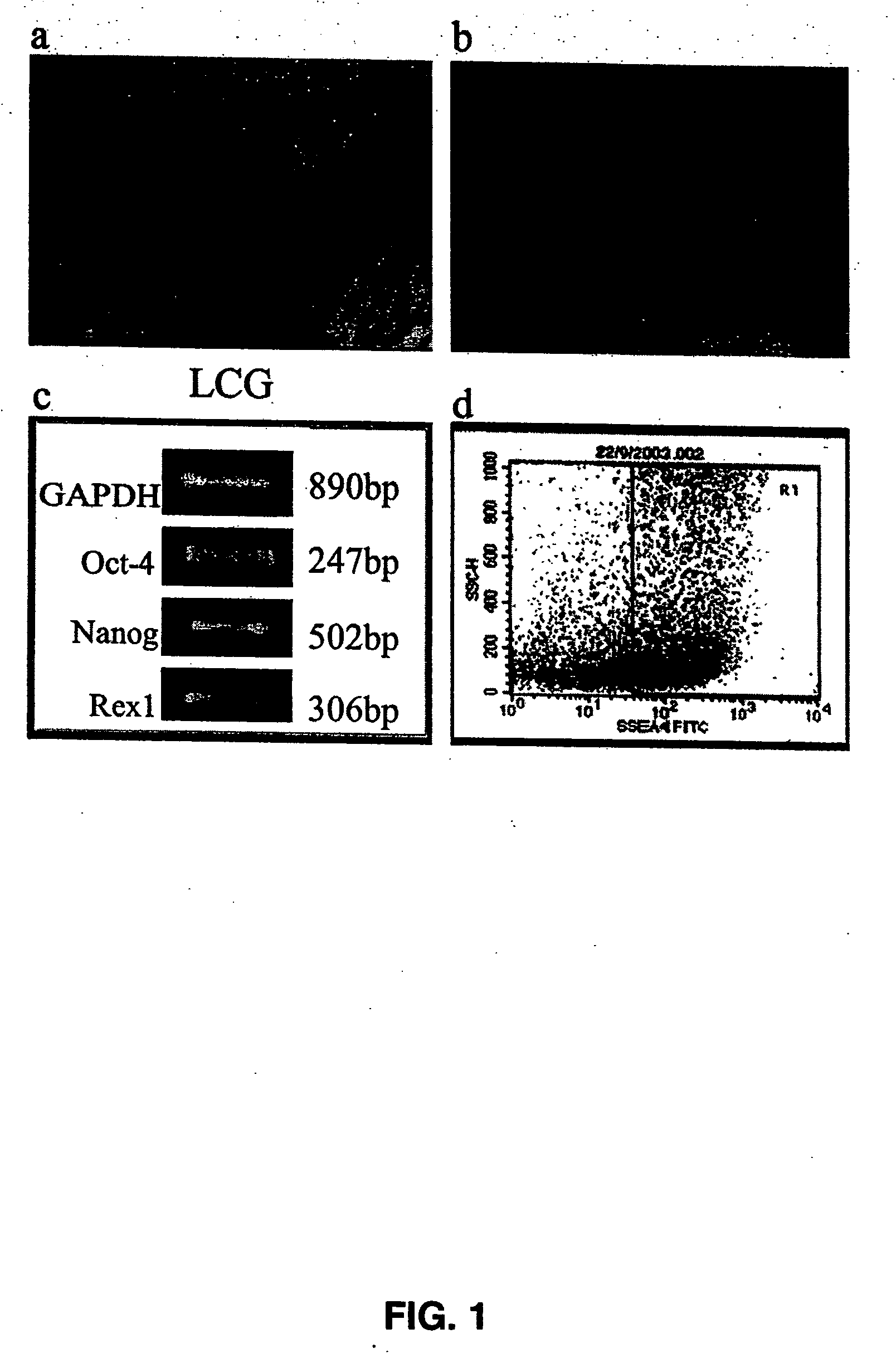
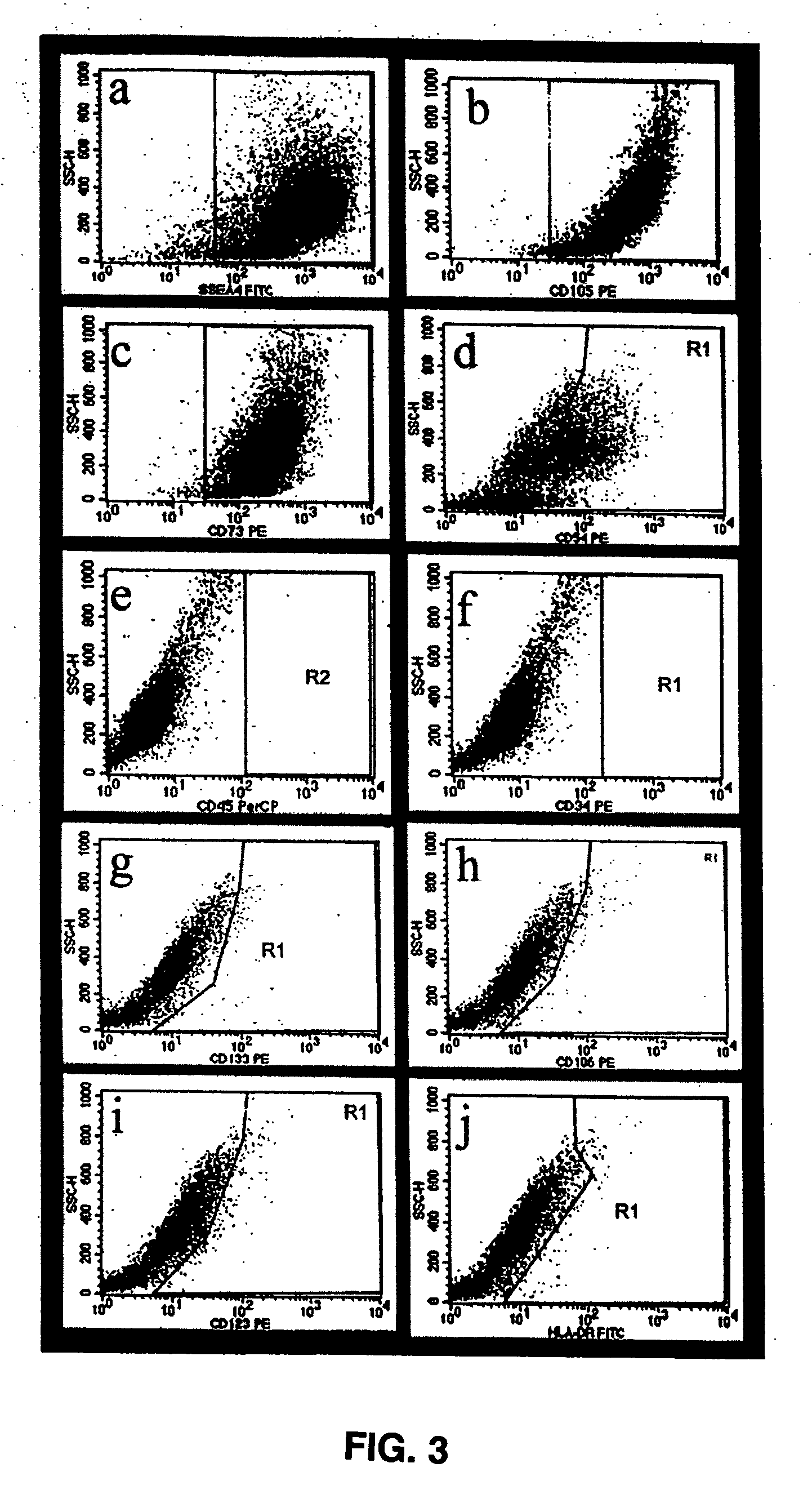
The present disclosure describes mammalian pluripotent embryonic-like stem cells (ELSCs) isolated from corneal limbal tissue, a non-embryonic tissue. The ELSCs of the present disclosure are capable of proliferating in an in vitro culture, maintain the potential to differentiate into cells of endoderm, mesoderm, and ectoderm lineage in culture, and are capable of forming embryoid-like bodies when placed in suspension culture. Thus, these cells possess multi-lineage differentiation potential and self-renewing capability. ELSCs may be a promising therapeutic tool, and may provide new therapeutic alternatives for various diseases, conditions, and injuries.
Owner:RELIANCE LIFE SCI PVT
Tissue system with undifferentiated stem cells derived from corneal limbus
InactiveUS20050186672A1Senses disorderCulture processSurface markerStage-Specific Embryonic Antigens
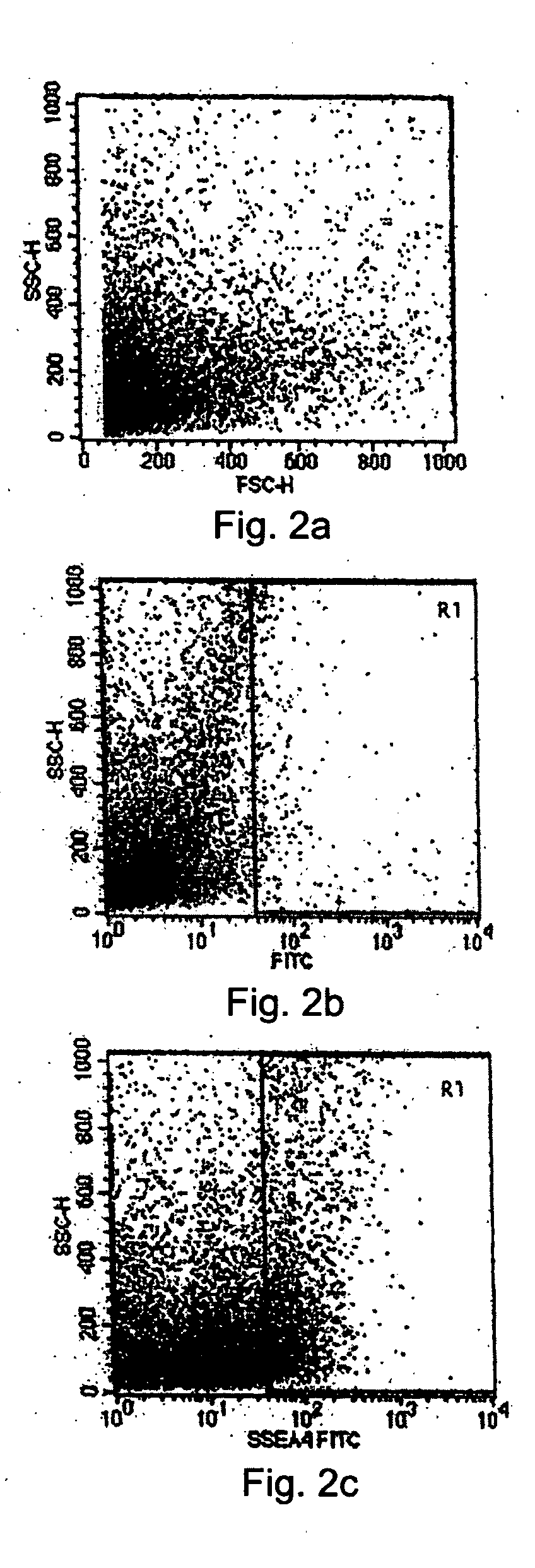
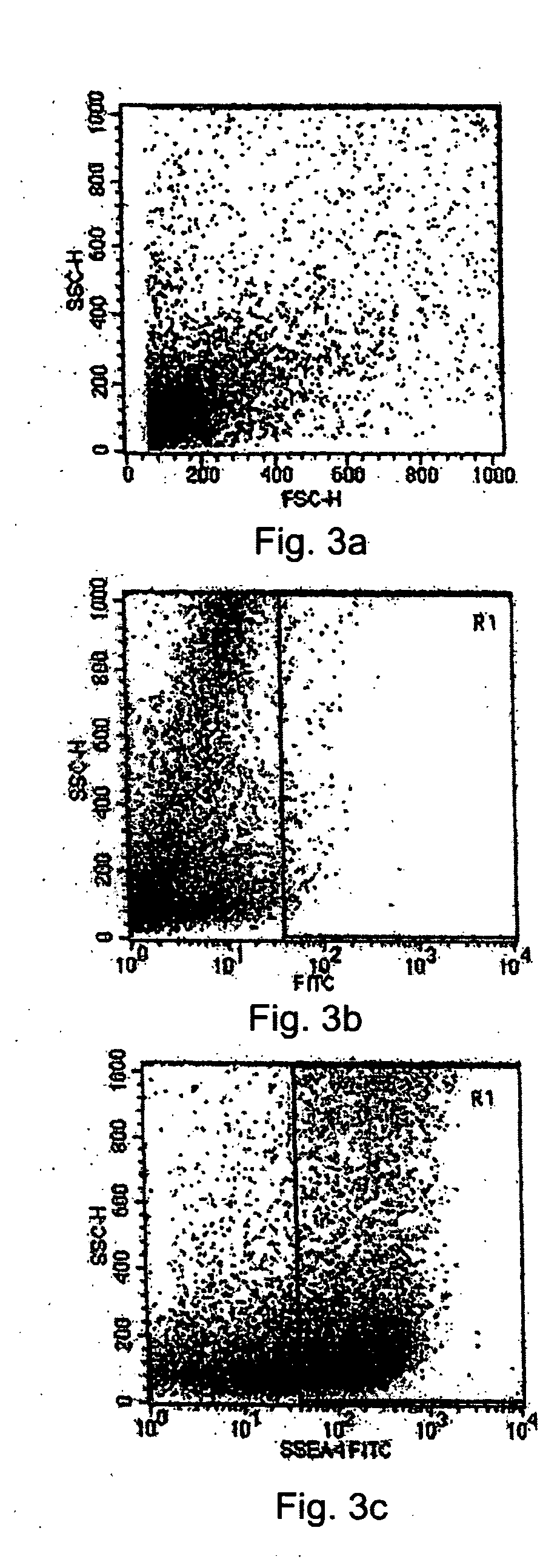
The present disclosure describes a tissue system with self-regenerating limbal stem cells, wherein the limbal stem cells are primarily undifferentiated stem cells (USCs). The tissue system is derived from isolated corneal limbal tissue, and is suitable for restoring ocular surface impairments, particularly those that result from limbal stem cell deficiencies. The tissue system is generated by selectively augmenting the tissue system for USCs, for example by selecting and sorting cells that express stem cell-specific surface markers, such as stage specific embryonic antigen marker 4 (SSEA-4). After isolation, the USCs are cultured on a tissue base in the presence of enriched medium to generate the tissue system, which is suitable for transplantation, implantation, or grafting.
Owner:RELIANCE LIFE SCI PVT
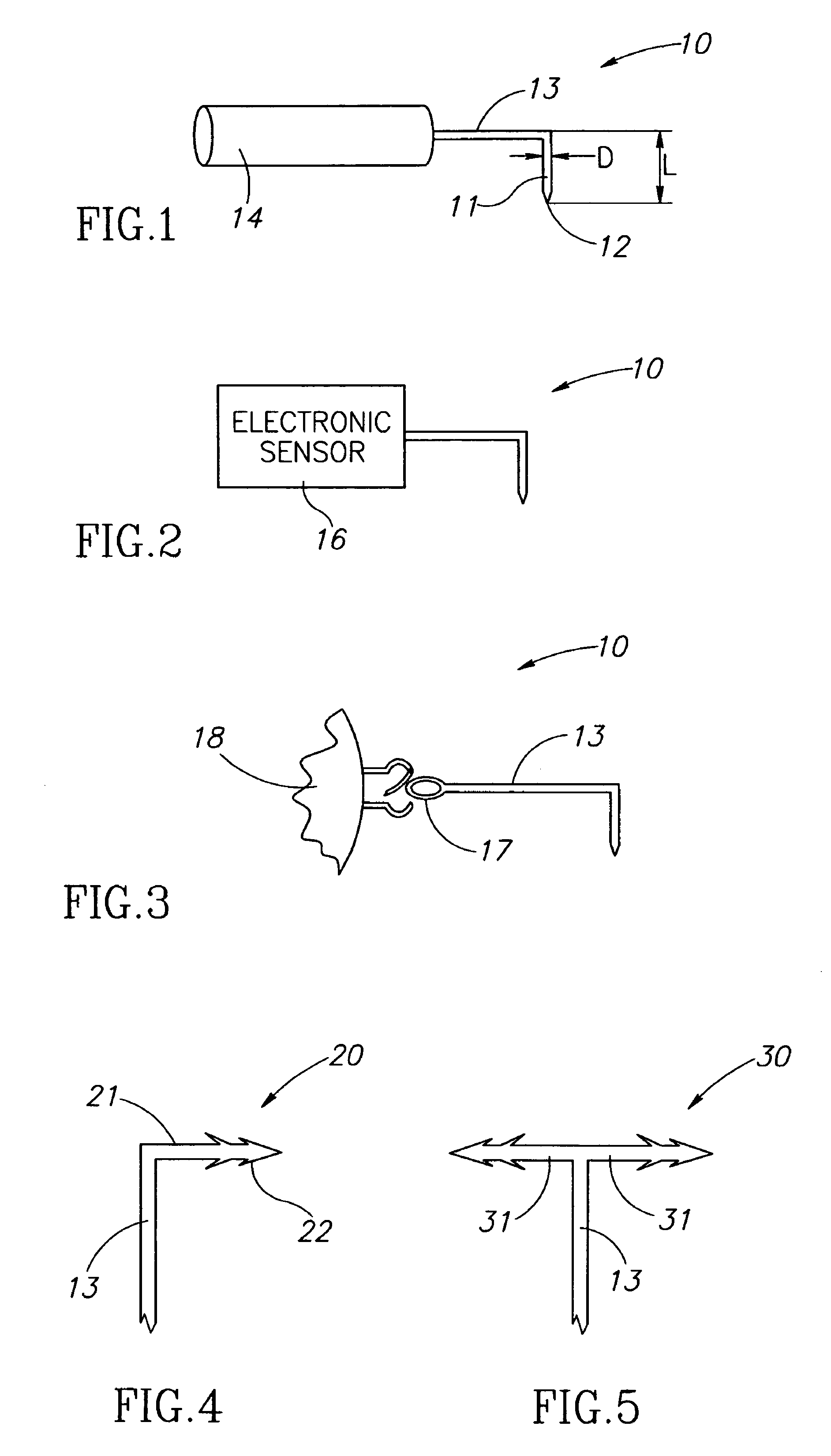
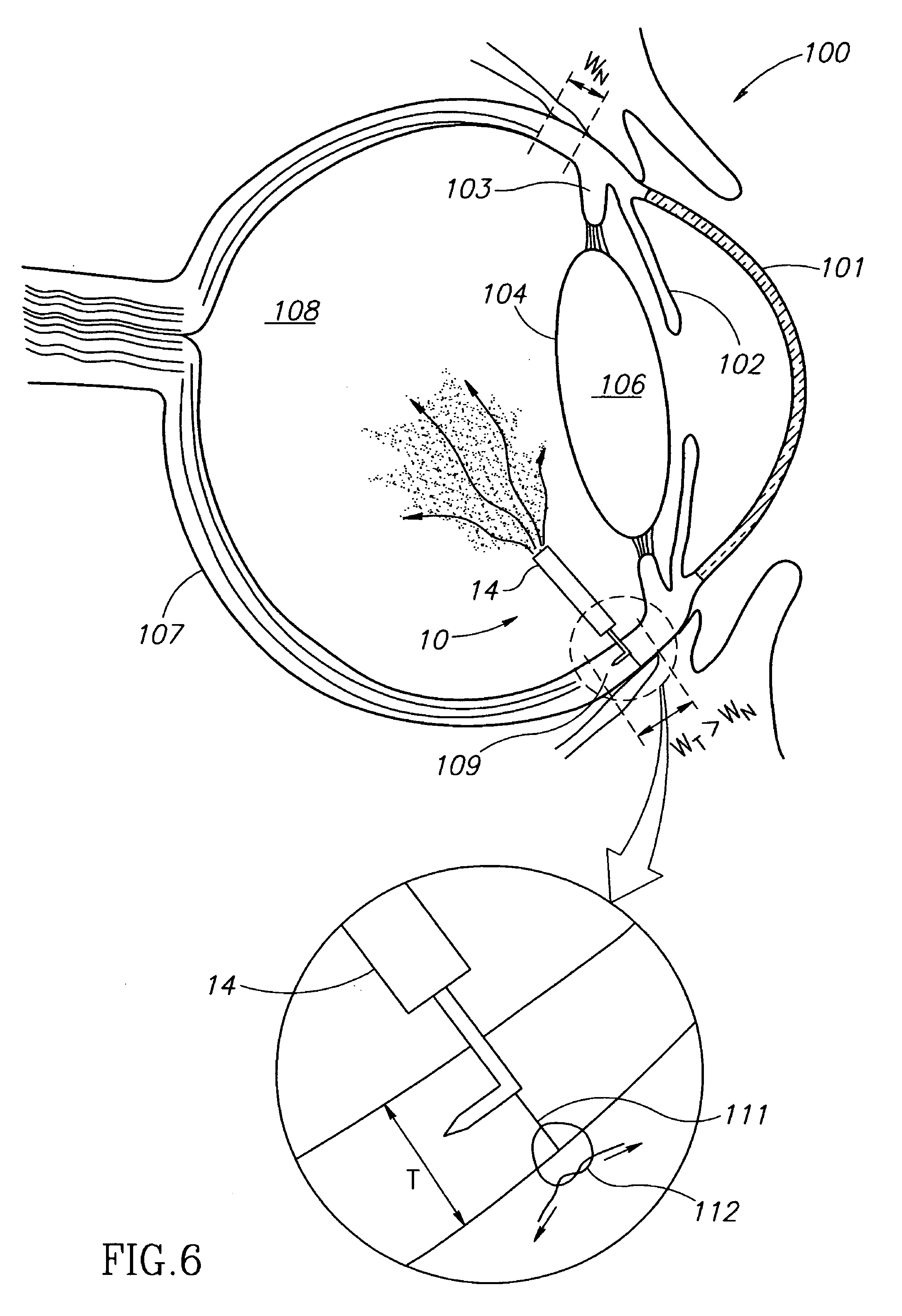
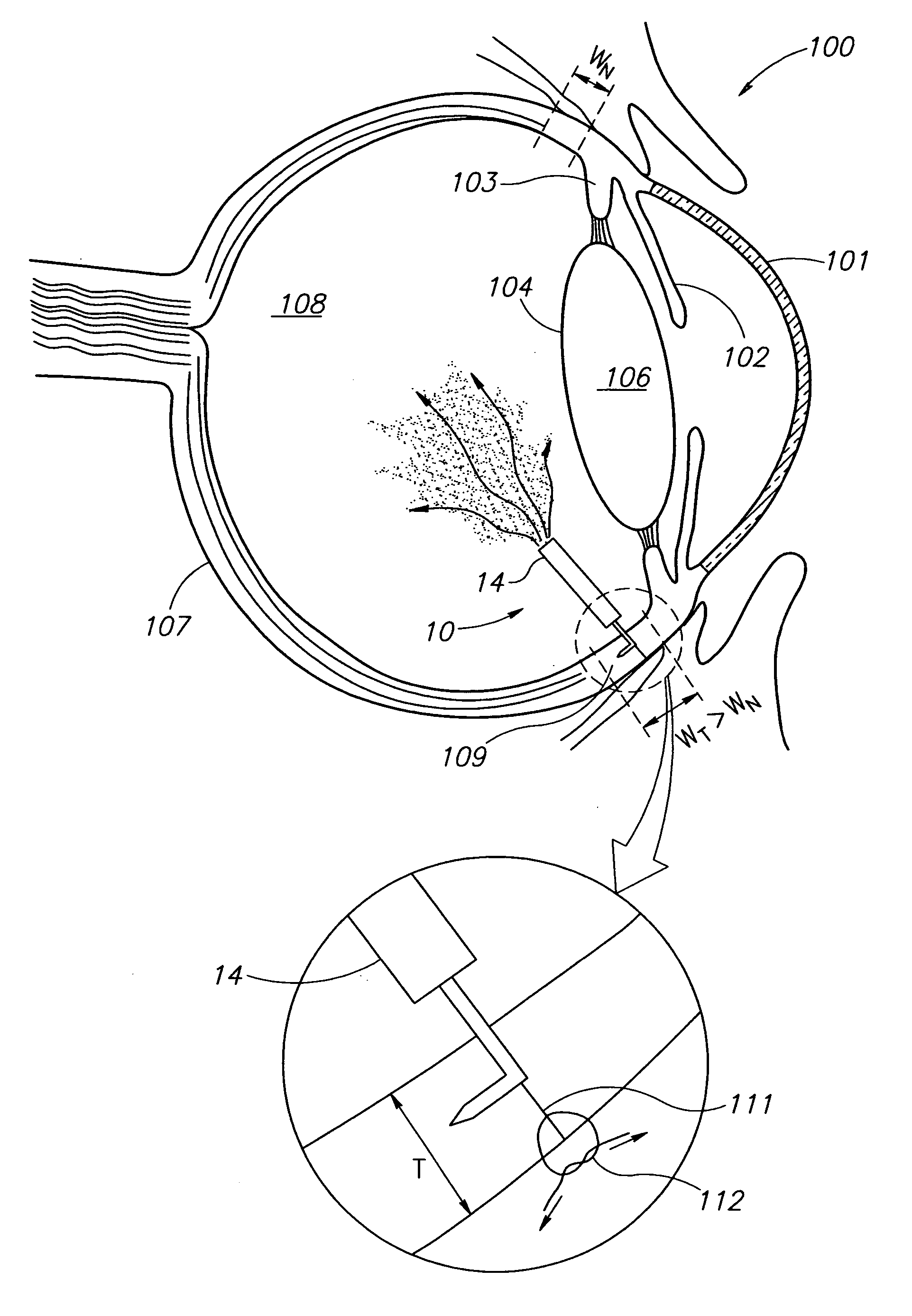
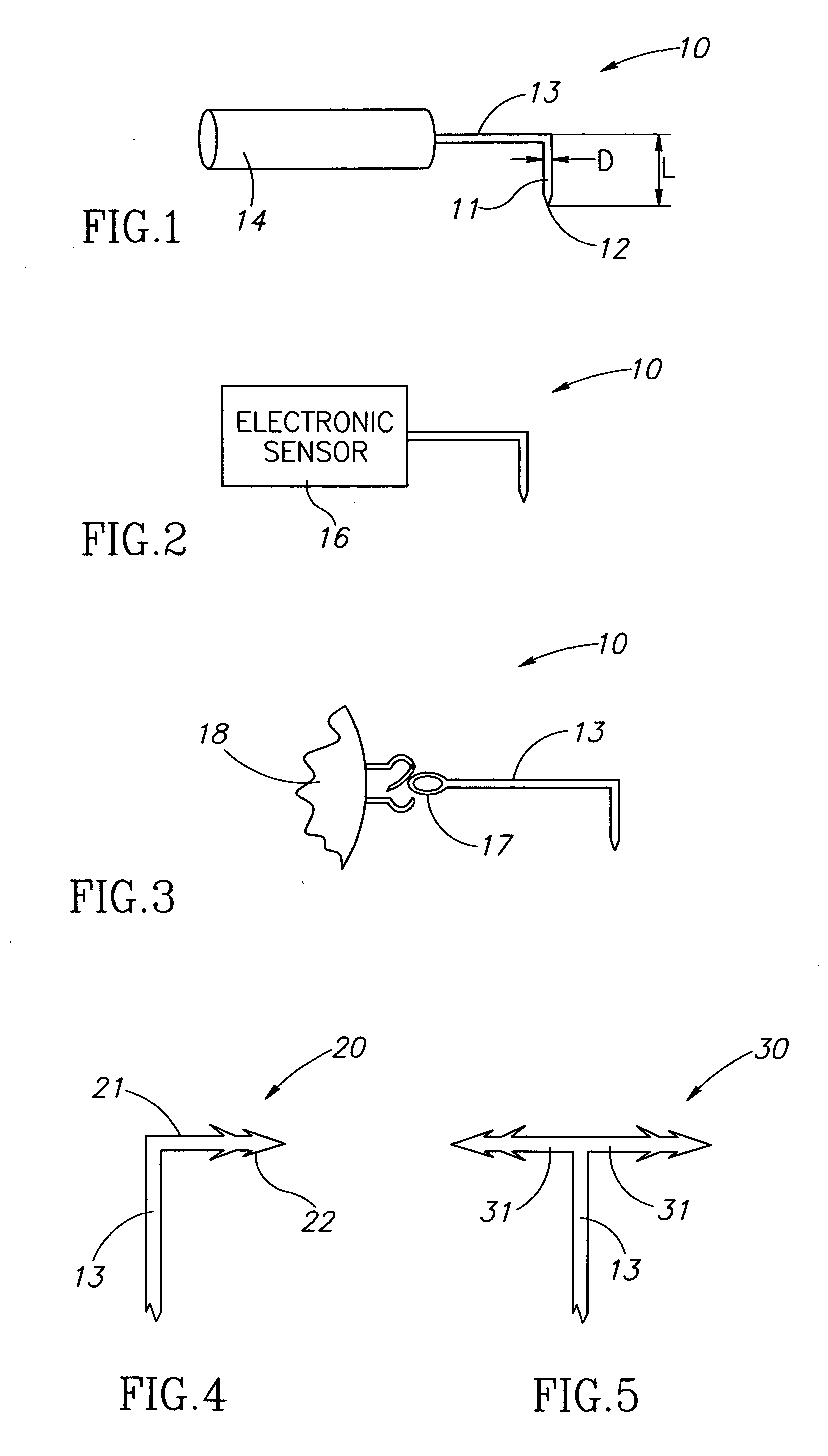
Eye wall anchored fixtures
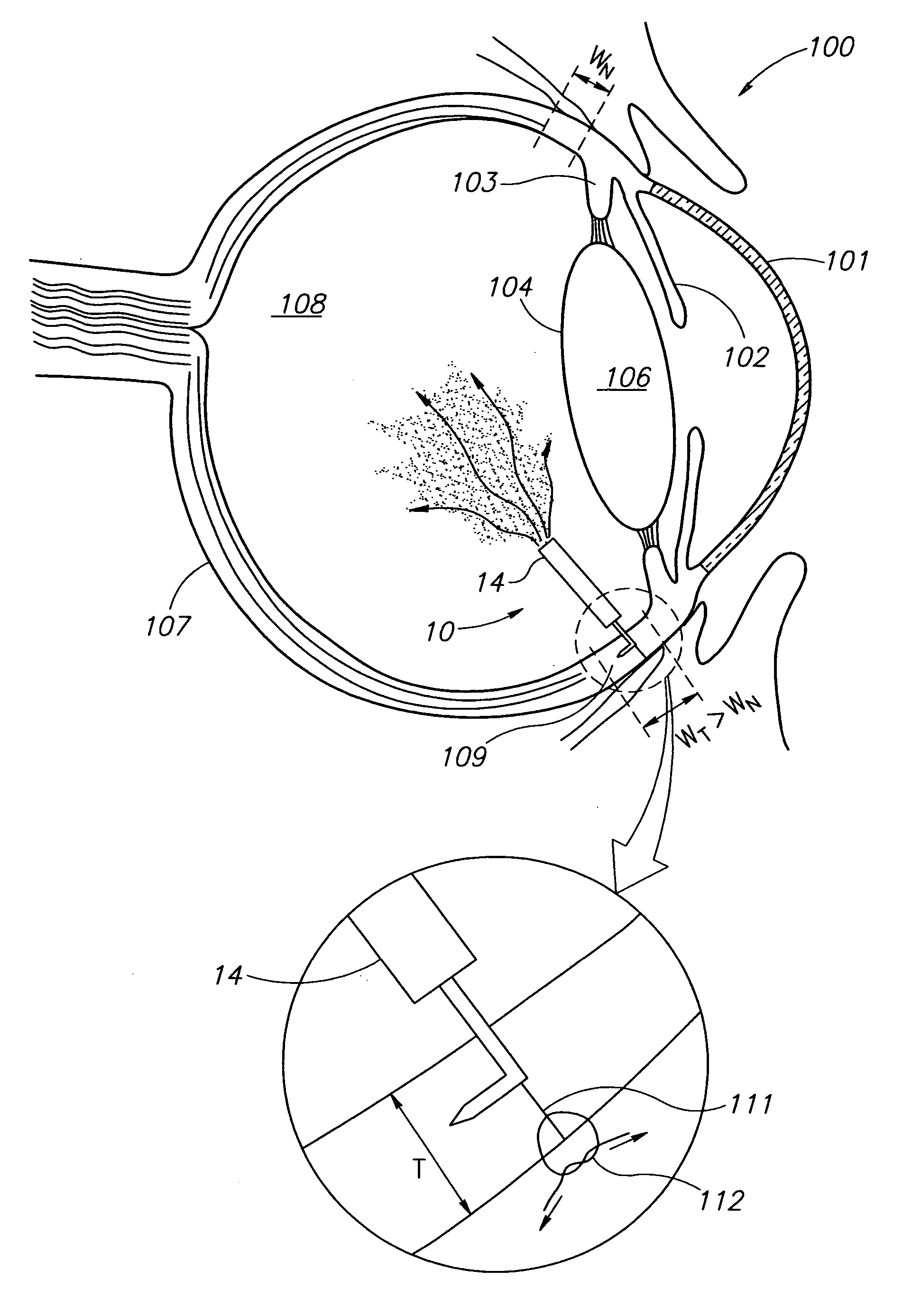
Eye wall anchored fixtures each including at least one elongated anchor member for driven lengthwise insertion into an eye's eye wall in a transverse direction to its thickness for supporting an intraocular device in the eye's vitreous cavity. Fixtures are preferably anchored in circumferential incisions in a human adult eye's pars plana preferably 3.5 mm posterior to its corneal limbus, and perpendicular to a circumferential incision. The fixtures are either generally L-shaped with a single elongated anchor member designed for withdrawal from an eye wall or self-anchoring thereinto, or generally T-shaped with a pair of oppositely directed self-anchoring elongated anchor members for sealing a throughgoing incision. Intraocular devices can be designed for intraocular drug delivery, for acquiring intraocular physiological measurements, and the like.
Owner:FORSIGHT VISION5 INC
System and Device for Correcting Hyperopia and Presbyopia
InactiveUS20080177383A1Reduce sliding frictionCorrect farsightednessEye implantsHydrophilic coatingFar-sightedness
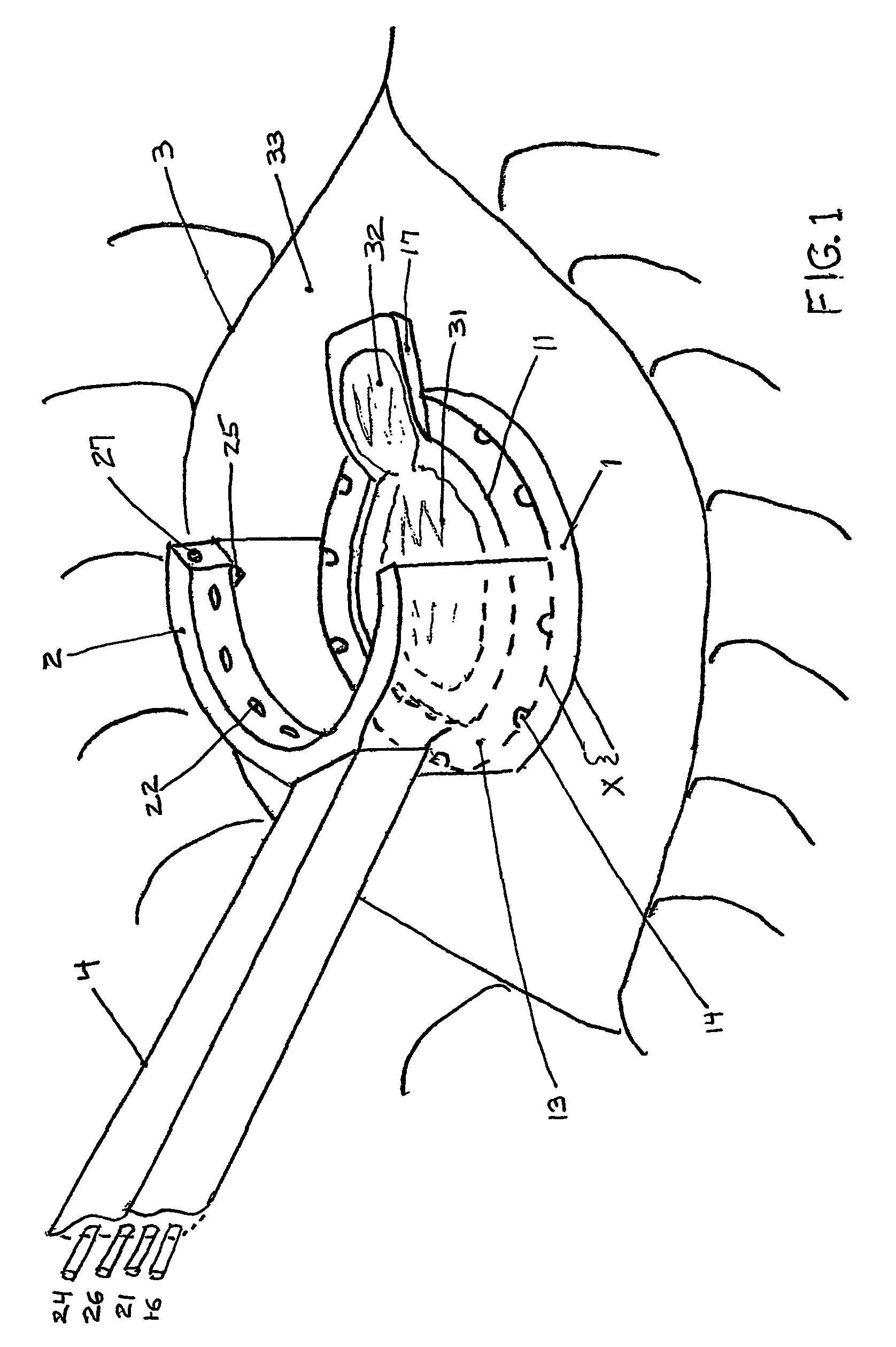
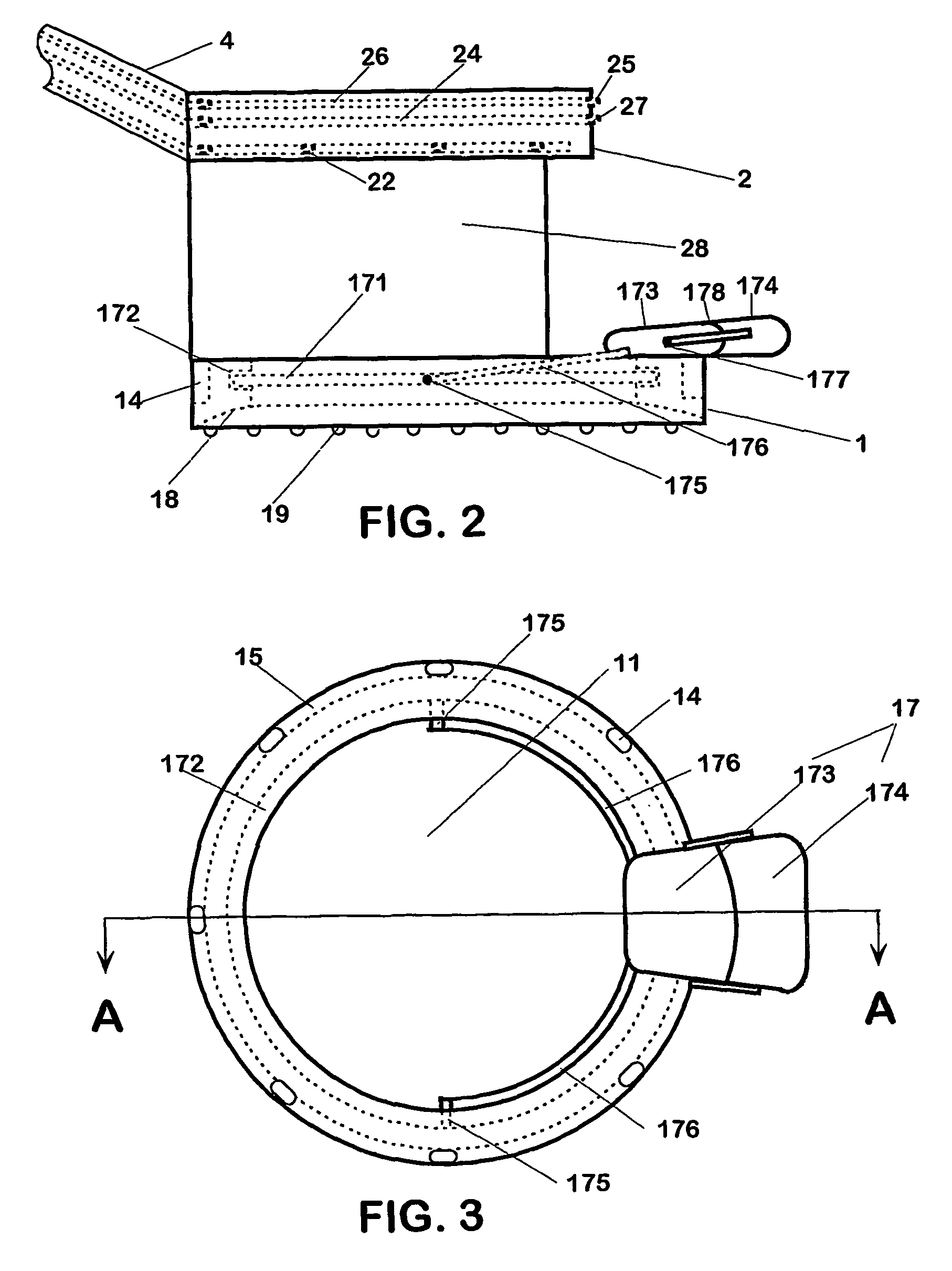
The present invention includes an ophthalmic device and system of mounting for correcting hyperopia and presbyopia. The present invention includes a limbus ring mountable in an encircling relation to a central optic zone of a cornea on a limbus annulus surrounding the cornea. In the limbus ring defines a substantially annular toroid defining a first average diameter that is selectable and has a hydrophilic coating disposed thereon. The inner radius of the limbus ring is selectable such that, upon mounting on the limbus annulus, the limbus ring causes the limbus annulus to contract thereby causing the curvature of the cornea and the eye length to increase. The mounting system of the present invention is adapted to receive a limbus ring and further adapted to selectively place the limbus ring on a limbus annulus.
Owner:SHANINPOOR MOHSEN +2
Strabismus diagnosis device based on mobile platform
InactiveCN102961117AConvenient strabismus detectionEasy to operateEye diagnosticsTablet computerOutput device
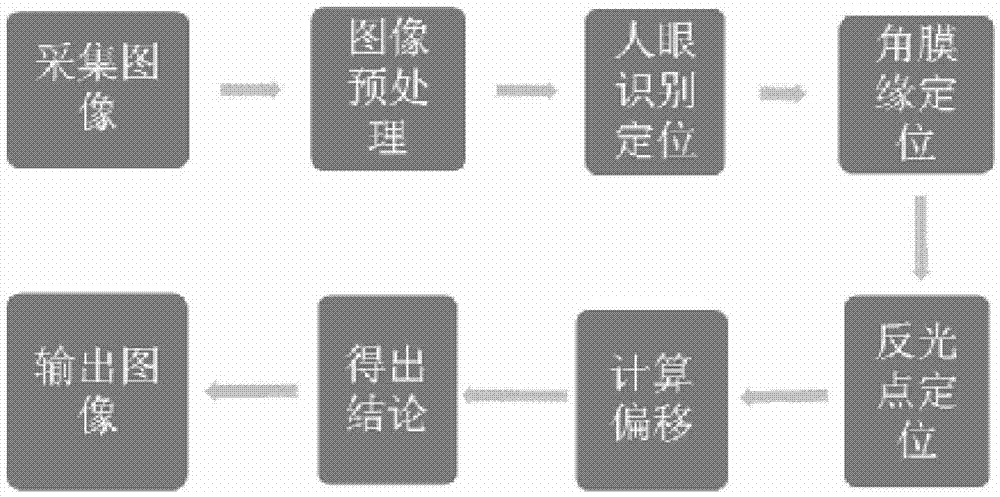
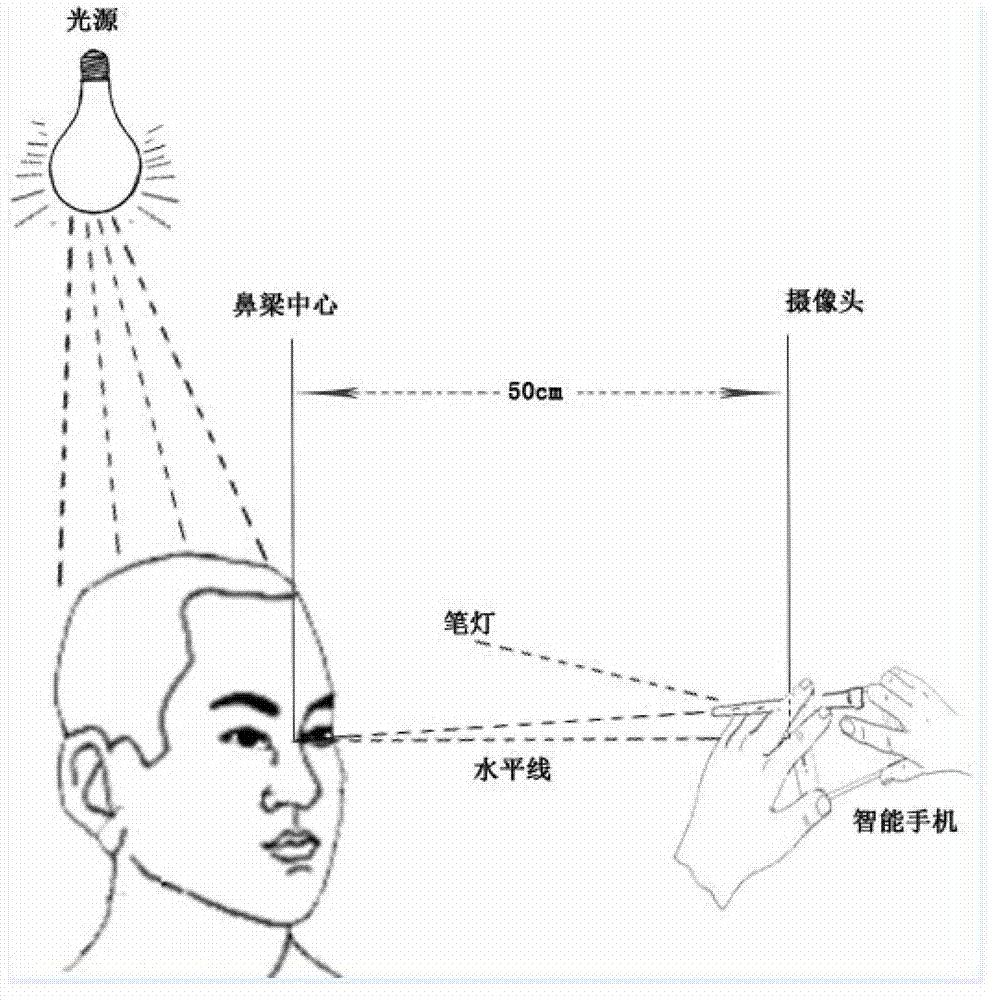
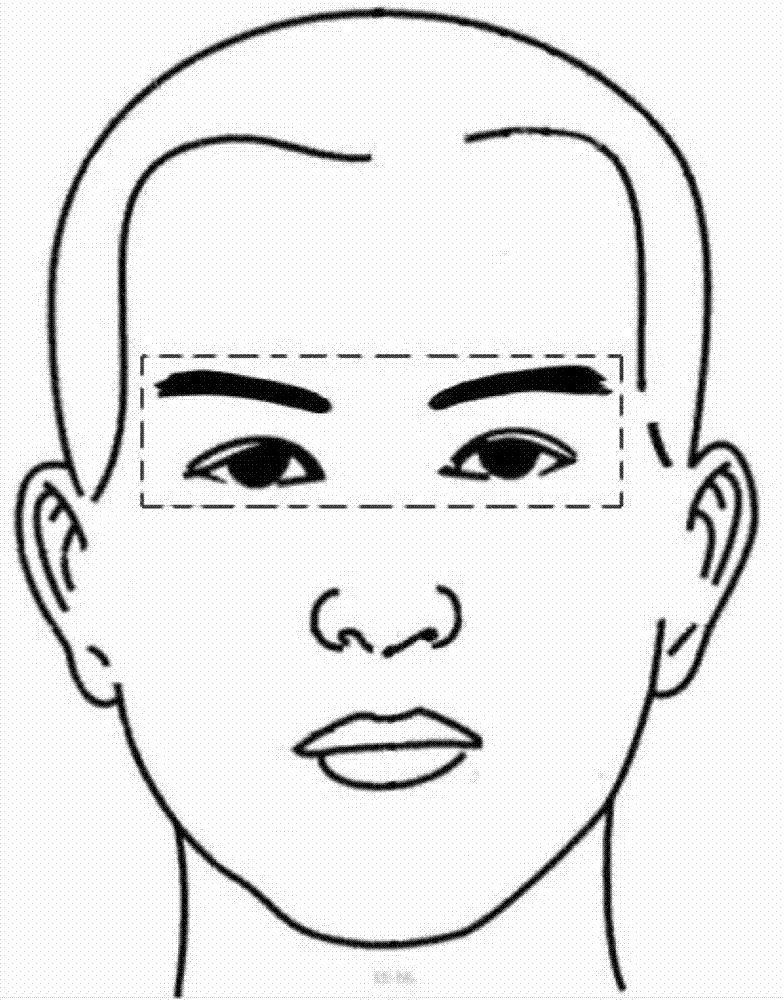
The invention relates to a mobile medical device, in particular to a strabismus diagnosis device based on a mobile platform. The strabismus diagnosis device based on the mobile platform comprises an image input device, an image pretreatment device, an image smoothing and gray level conversion processing unit, an ocular region positioning device, a corneal limbus positioning device, a reflection point positioning device, an offset calculation device and an image output device, wherein the image input device is used for obtaining an ocular region image, and the image pretreatment device also comprises an image resolution regulation image. On the basis of the mobile platform, the strabismus diagnosis device based on the mobile platform can be installed on the intelligent mobile equipment, such as a smart phone and a tablet personal computer, the strabismus detection can be conveniently carried out, and the operation can be conveniently carried out.
Owner:WENZHOU MEDICAL UNIV +2
Human corneal limbal stem cell tissue engineering product nourished by fibroblast and preparing process thereof
InactiveCN1635115APromote differentiationTight adhesionArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsDiseaseTrophoblast
A human corneal limbal stem cell-amnion tissue engineering complex using human corneal limbal fibroblasts as the trophoblast is characterized in that: it is close to the human corneal epithelium form in histology form, and is analogous to the human corneal epithelium in immunology phenotype, its ultrastructure shows that the connection between the cells grows mature and the connection between the epithelia and the amnion carrier is a close conglutination. The culture method includes the following steps: (1) preparing the amnion for culturing the human corneal limbal stem cell in vitro; (2) preparing the human corneal limbal fibroblast trophoblast; (3) preparing the human corneal epithelia suspension; (4) culturing the human corneal limbal stem cell-amnion complex. The human corneal limbal stem cell-amnion complex has analogous phenotype to the human corneal epithelium, and provides a safe and effective biology tissue engineering product to treat corneal limbal stem cell defective eye diseases.
Owner:天津医科大学眼科中心
Preparation method and application thereof of acellular conjunctiva matrix
InactiveCN101590292AEasy to prepareGood biocompatibilityEye implantsDead animal preservationTreatment effectBiocompatibility Testing
The invention relates to a preparation method and application thereof of an acellular conjunctiva matrix used as a tissue engineering corneal scaffold material. The method comprises the following steps: removing cell components in a bulbar conjunctiva first; preparing the acellular conjunctiva matrix; and taking the acellular conjunctiva matrix as a tissue engineering corneal scaffold. A rabbit corneal epithelium or an endothelial cell can form a good cell single layer on the scaffold, can construct a tissue engineering corneal epithelium or endothelium, and can successfully transplant the tissue engineering corneal epithelium or endothelium onto rabbit animal model eyes. The degradation time of the built tissue engineering cornea is longer, rabbit eyes have no obvious immune reject reaction, and the therapeutic effects on the lack of corneal limbus stem cells or the decompensation of the corneal endothelial cell function are obvious. The method and the application thereof have the advantages of simple preparation method, good biocompatibility, low antigenicity, easy growth and proliferation of seed cells, slow degradation, extensive bulbar conjunctiva sources and wide application and development prospects; and the transparency can be maintained all the time in the training process and after transplantation.
Owner:SHANDONG EYE INST
Eye wall anchored fixtures
Eye wall anchored fixtures each including at least one elongated anchor member for driven lengthwise insertion into an eye's eye wall in a transverse direction to its thickness for supporting an intraocular device in the eye's vitreous cavity. Fixtures are preferably anchored in circumferential incisions in a human adult eye's pars plana preferably 3.5 mm posterior to its corneal limbus, and perpendicular to a circumferential incision. The fixtures are either generally L-shaped with a single elongated anchor member designed for withdrawal from an eye wall or self-anchoring thereinto, or generally T-shaped with a pair of oppositely directed self-anchoring elongated anchor members for sealing a throughgoing incision. Intraocular devices can be designed for intraocular drug delivery, for acquiring intraocular physiological measurements, and the like.
Owner:FORSIGHT VISION5 INC
Ophthalmic compositions
ActiveUS20180008538A1High expressionSenses disorderAmide active ingredientsUveitisMedium-chain triglyceride
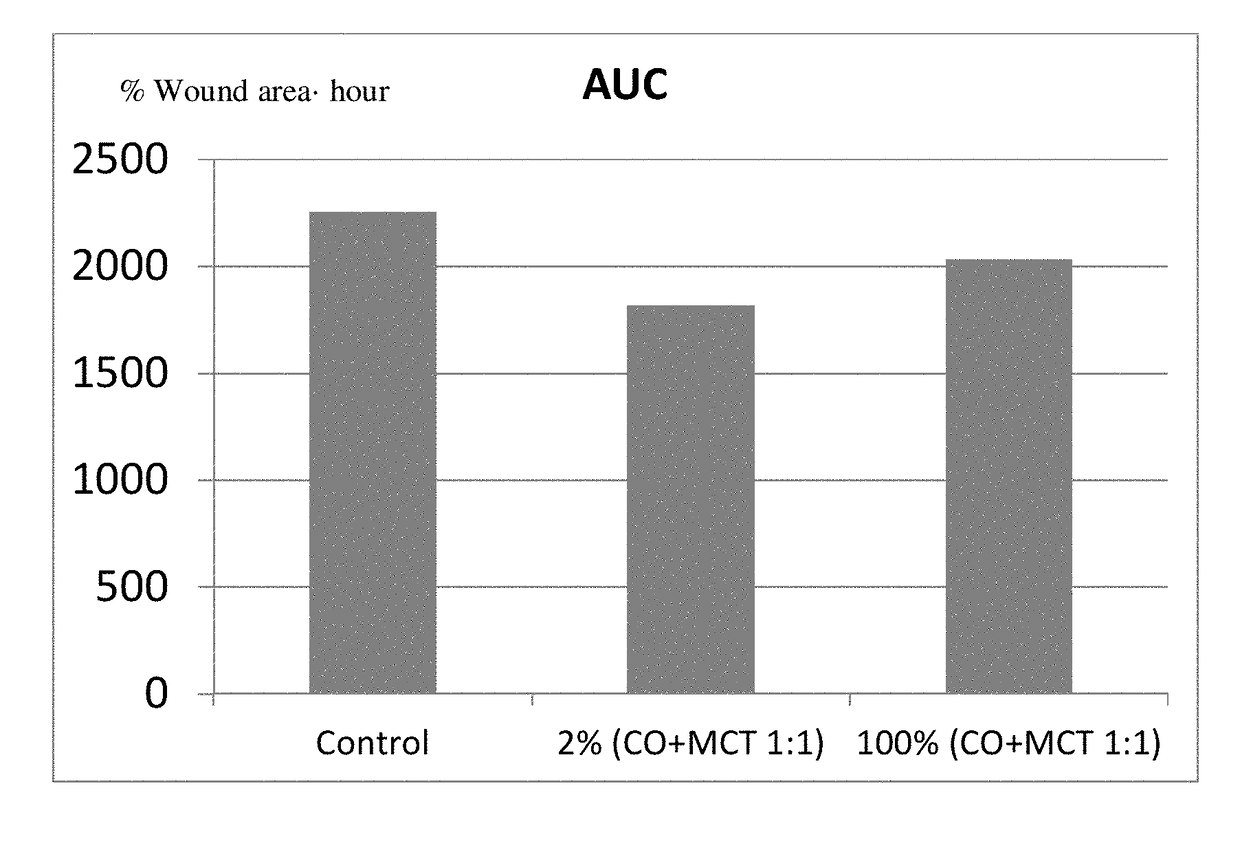
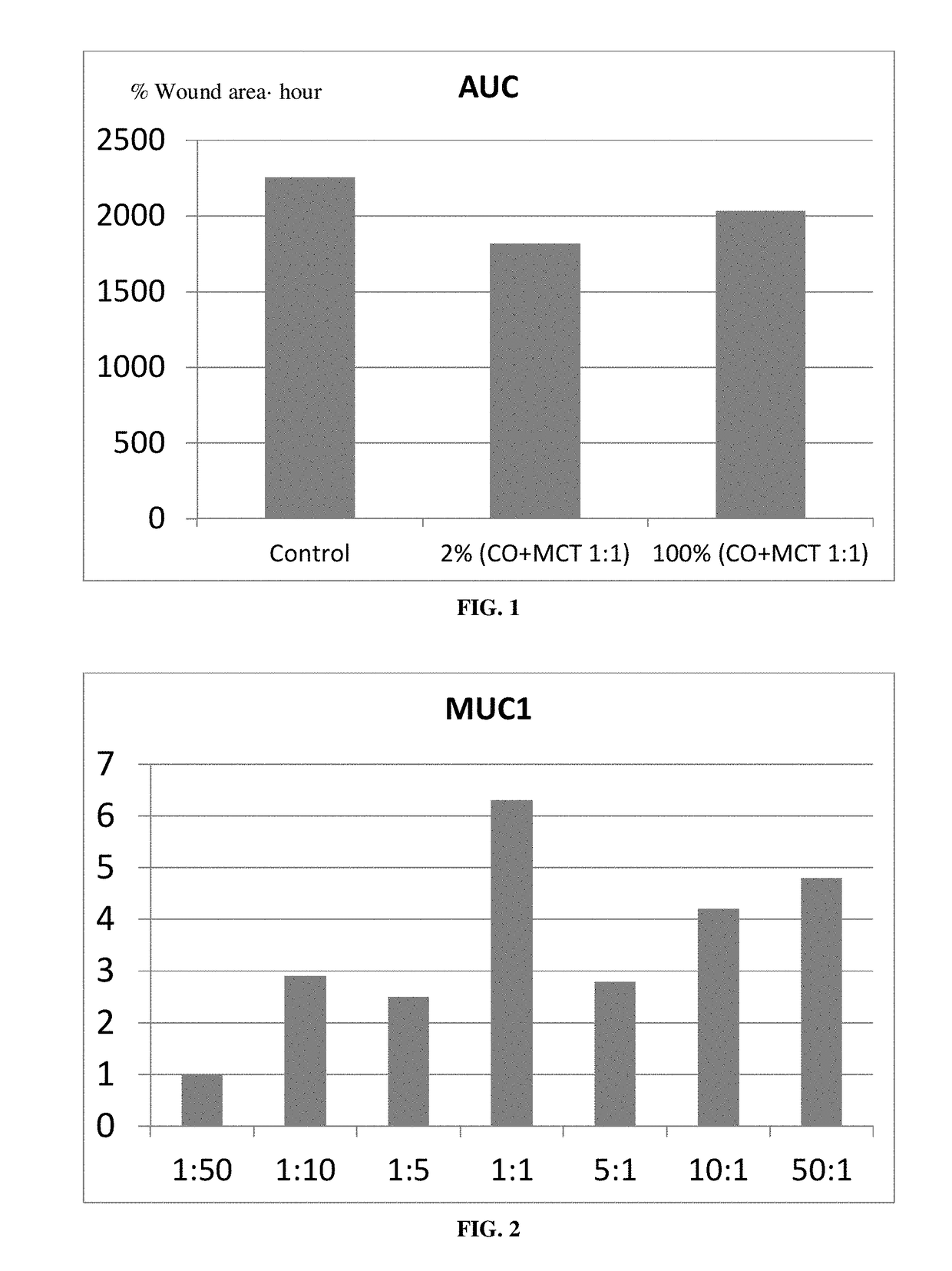
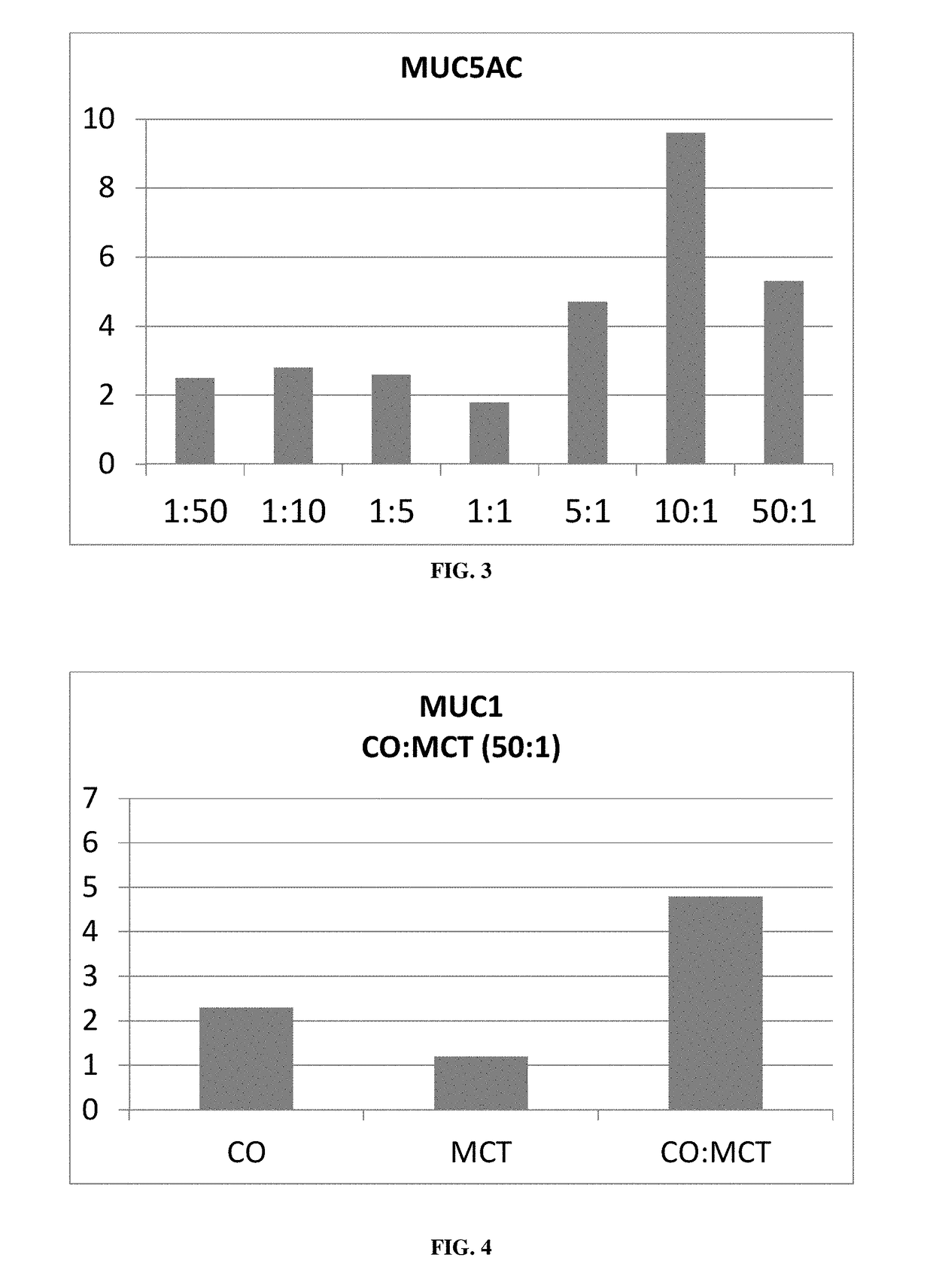
The present invention relates to a sterile ophthalmic composition comprising castor oil and a medium chain triglyceride, to its use in medicine, in particular for the treatment and / or prevention of an ocular disease selected from the group consisting of dry eye, conjunctivitis, dermatitis, blepharitis, entropion, floppy eyelid syndrome, thyroid ophthalmopathy, pterygium, conjunctivochalasis, epithelial damage induced by preservatives, epithelial or anterior chamber damage induced by ocular surgery, limbal cell deficiency, corneal ulcers induced by physical or chemical agents, keratitis, episcleritis and uveitis.
Owner:LAB SALVAT
Method for preparing decellularized lamellar cornea matrix sheet
InactiveCN104645415AThe production process is simple and reliableShorten the timeProsthesisAnterior corneaEthylene Oxide Sterilization
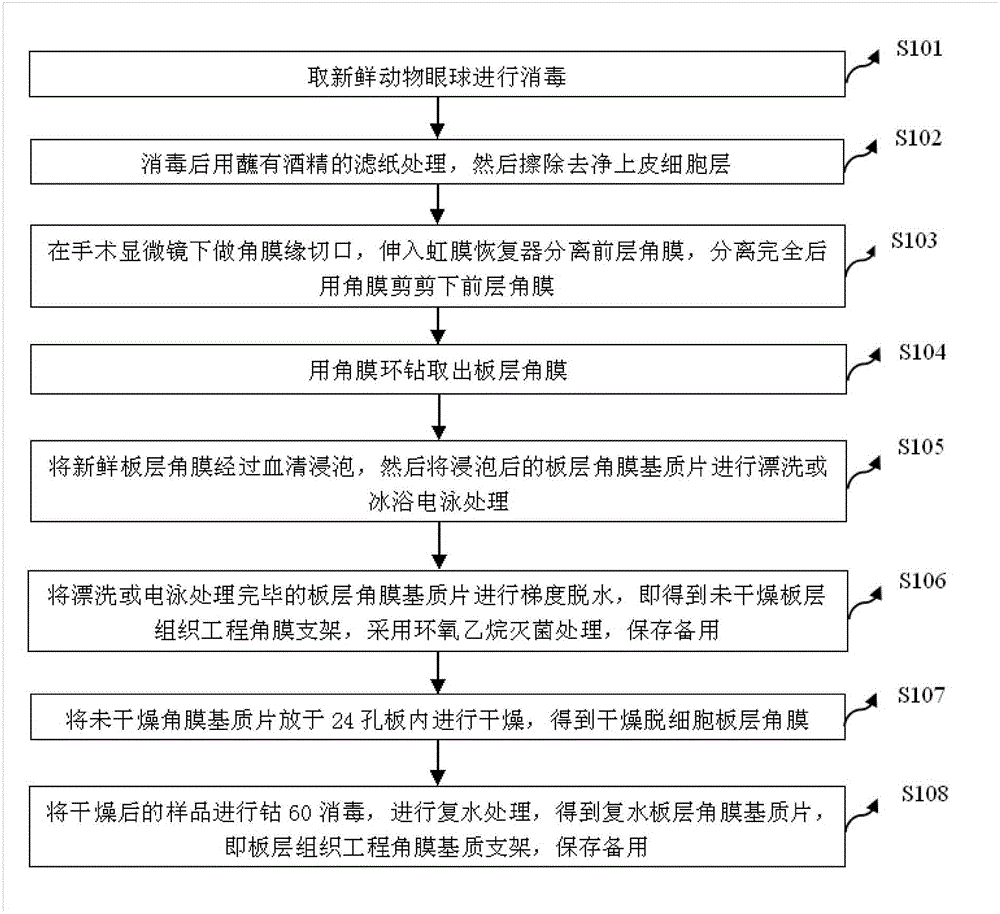
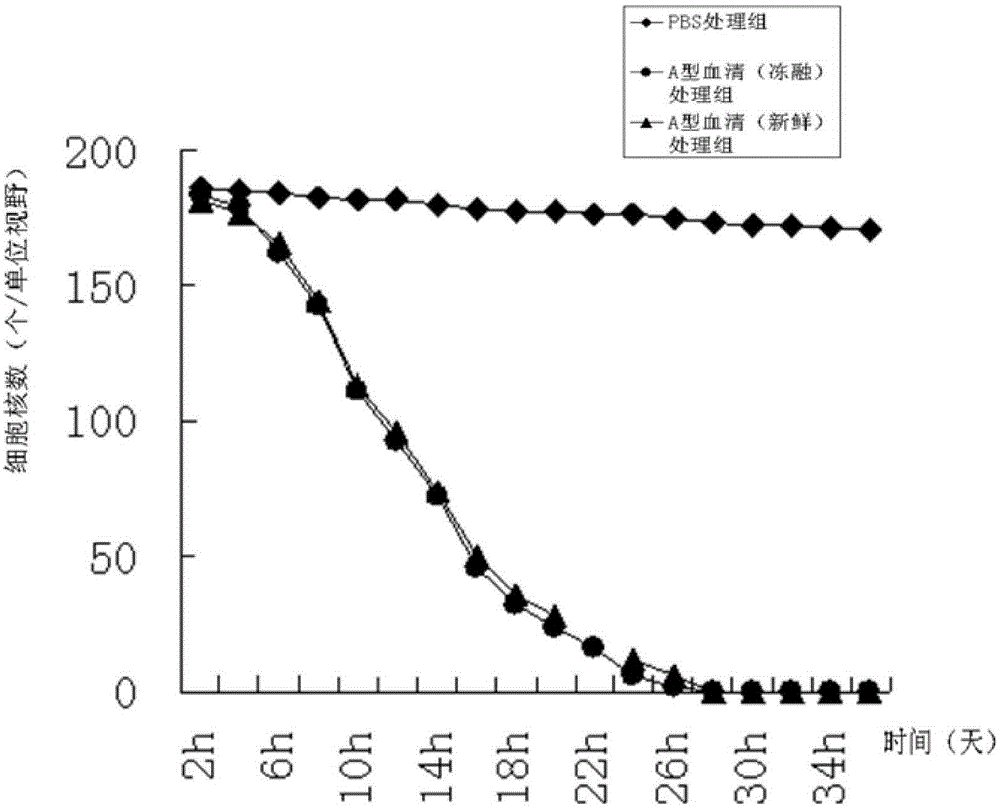
The invention discloses a method for preparing a decellularized lamellar cornea matrix sheet. The method comprises the steps of disinfecting a fresh animal eyeball; treating with filter paper dipped with alcohol after disinfecting, and then erasing an epithelial cell layer; incising the corneal limbus under an operation microscope, stretching into an iris restorer to separate the anterior cornea, and then shearing the anterior cornea with corneal scissors; drilling the lamellar cornea with a corneal annulus; soaking the fresh lamellar cornea into serum, and then rinsing or performing ice-bath electrophoresis treatment; performing gradient dehydration after the treatment to obtain a non-dried lamellar tissue engineering corneal frame, sterilizing with ethylene oxide, and preserving for later use; drying the non-dried cornea matrix sheet in a 24-pore plate to obtain dried decellularized lamellar cornea; and sterilizing the dried sample with cobalt 60, and performing rehydration treatment to obtain a rehydrated lamellar cornea matrix sheet. The obtained cornea matrix sheet is high in transparency, low in structural destroy, good in biocompatibility, close to fresh cornea in performance and thorough in decellularization.
Owner:南昌大学第一附属医院 +1
Multi-function surgical instrument for facilitating ophthalmic laser surgery
Owner:LAHAYE LEON C
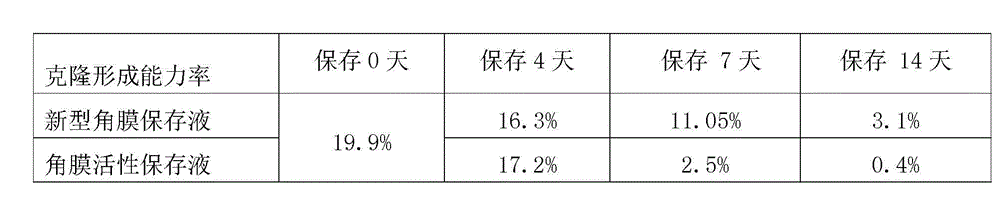
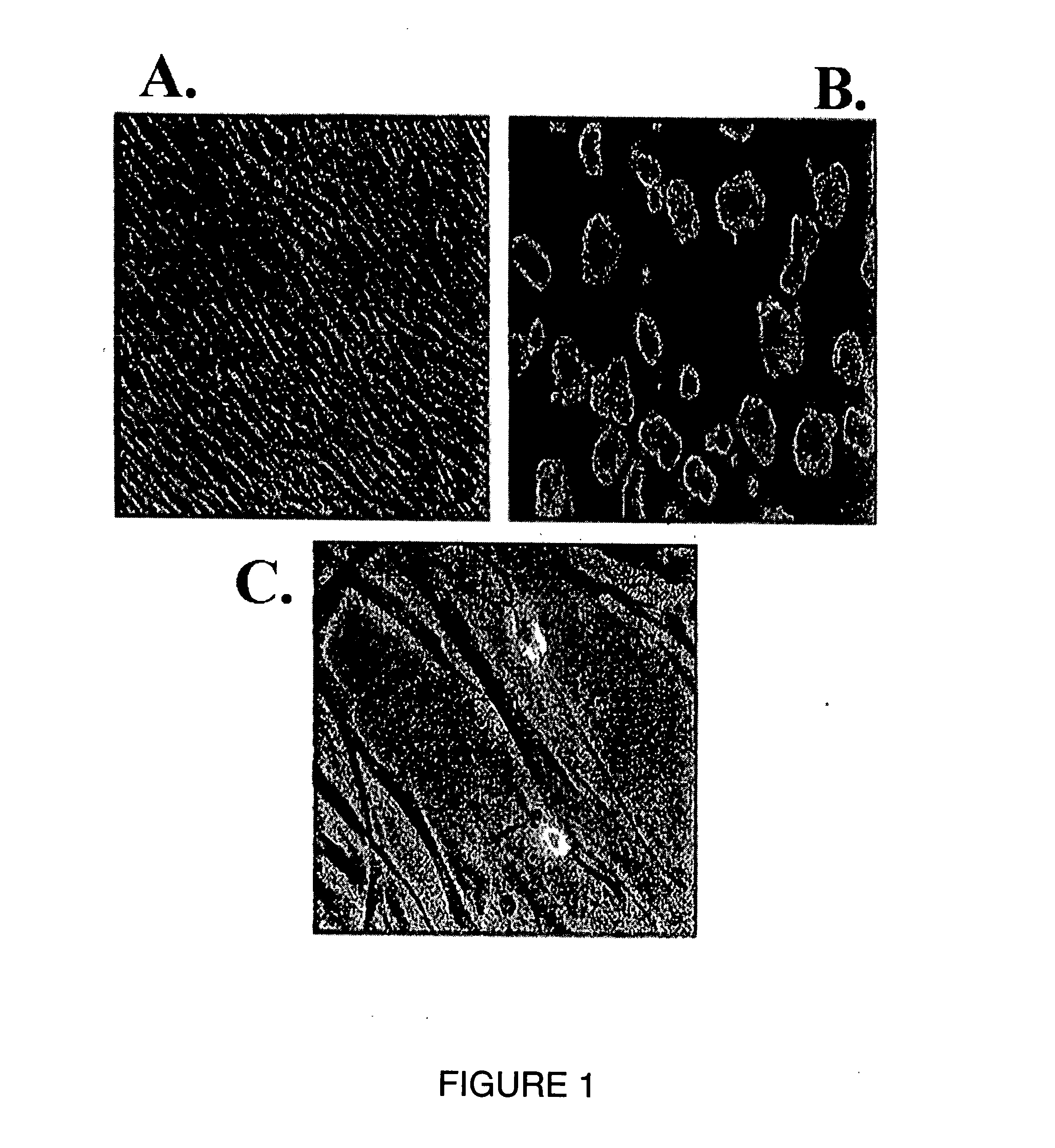
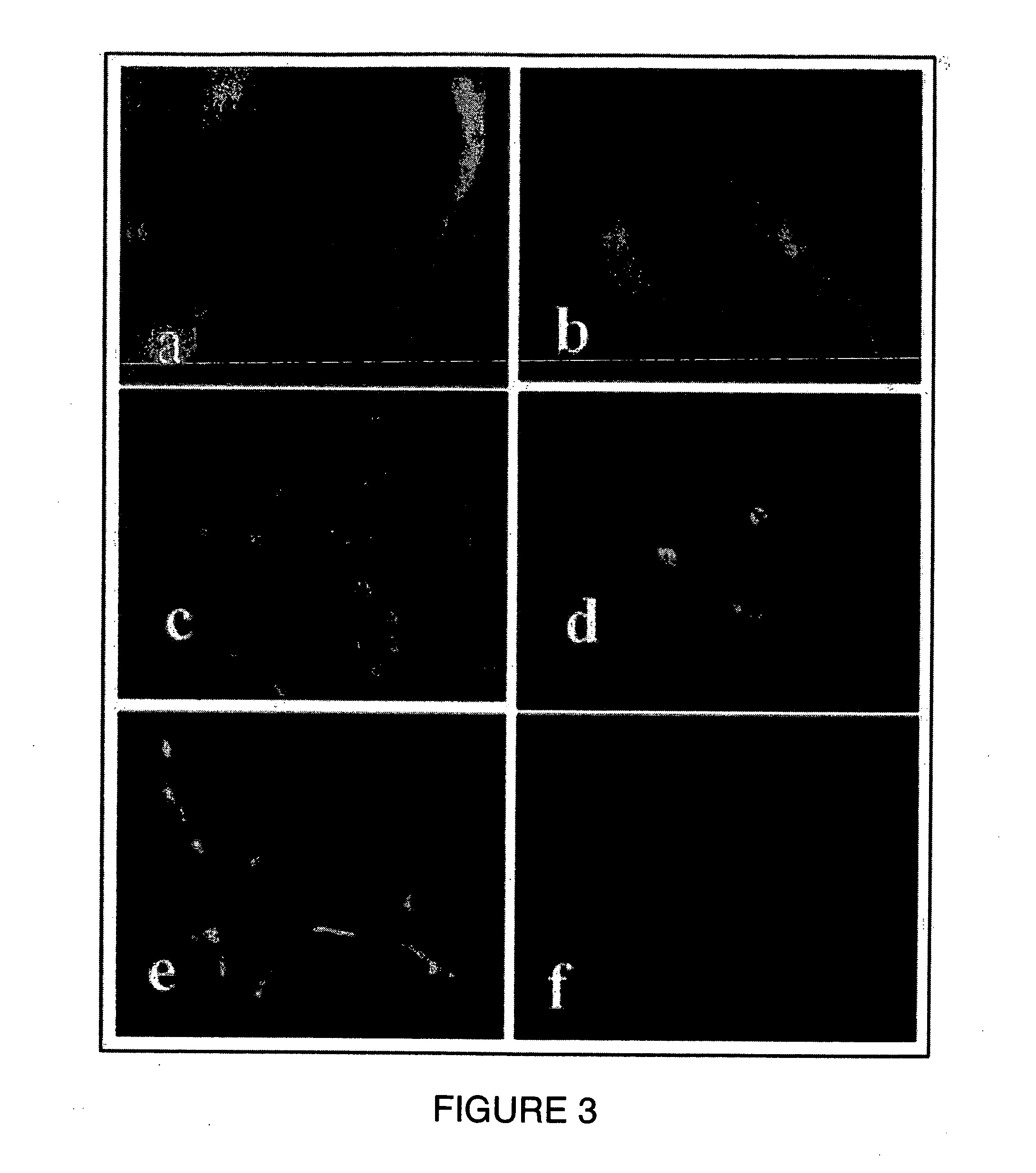
Cornea metaphase preservation solution, and preparing and using methods thereof
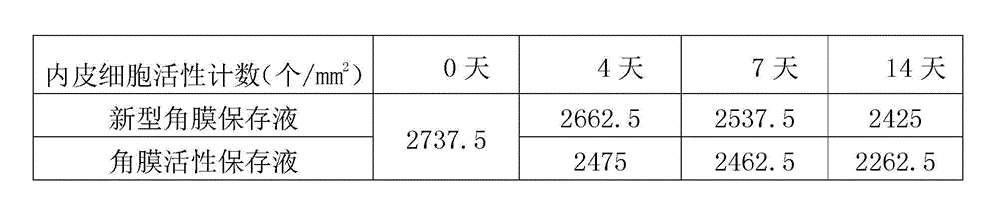
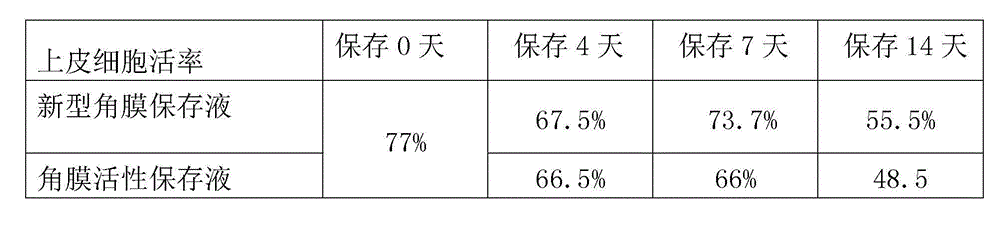
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
Dopaminergic neurons derived from corneal limbus, methods of isolation and uses thereof
The present disclosure describes the generation of neural cells and neurons from mammalian pluripotent embryonic-like stem cells (ELSCs) isolated from corneal limbal tissue, a non-embryonic tissue. Specifically, the present disclosure describes the generation of neuroprogenitor cells and differentiated dopaminergic neurons from ELSCs. The disclosed methods demonstrate the potential of ELSCs as a therapeutic tool, and may provide new therapeutic alternatives for various diseases, conditions, and injuries. Neuroprogenitor cells generated from ELSCs isolated from corneo-limbal tissue were transplanted into a rat model of Parkinson's disease, and were able to alleviate motor abnormalities in the rats for at least six months.
Owner:RELIANCE LIFE SCI PVT
Method for preparing amniotic compound corneal limbus stem cell membrane
ActiveCN102166374AAvoid breakingConvenient for clinical operationArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsEpitheliumFeeder Layer
The invention relates to a method for preparing an amniotic compound corneal limbus stem cell membrane, which comprises the following steps of: firstly, preparing a sterile amniotic membrane with epithelium removed, placing the epithelial surface of the amniotic membrane downwards on an end face of a sleeve made of a poly propylene material, and covering an embedded culture transwell on the amniotic membrane of the sleeve to obtain an amniotic-membrane-embedded culture mold; placing the culture mold in holes in a 6-hole plate on which a mouse embryonic fibroblast cell feeder layer is spread; and finally, preparing a corneal limbus stem cell suspension by using a digestion method, inoculating the suspension on the epithelial surface of the amniotic membrane, inoculating 2.0-3.0*10<5> corneal limbus stem cells for each culture mold, and culturing the corneal limbus stem cells for 10 days to obtain the amniotic compound corneal limbus stem cell membrane. Compared with the traditional amniotic membrane spreading method and the latex ring amniotic membrane fixing method, the amniotic compound corneal limbus stem cell membrane prepared with the method has flat amniotic membrane culturing surface and uniform corneal limbus stem cell stratified layer and especially has the advantage of convenience for clinic application and difficulty in rapture of the amniotic membrane.
Owner:SHANDONG EYE INST
Decellularized corneal limbus protective liquid and preparation method of decellularized corneal limbus
ActiveCN106614519AMaintain structural propertiesMaintain mechanical propertiesDead animal preservationTissue regenerationBiocompatibility TestingTobramycin
The invention relates to the field of bioengineering medical materials, and discloses decellularized corneal limbus protective liquid and a preparation method of decellularized corneal limbus. The component of the decellularized corneal limbus protective liquid is a PBS buffer solution containing hyaluronic acid of 5 to 12 g / L, chondroitin sulfate of 5 to 20 g / L, low molecular dextran of 3 to 10 g / L and tobramycin of 2.5 to 5 mg / L. A fresh pig corneoscleral limbal matrix containing lamina elastica anterior is sealed in the decellularized corneal limbus protective liquid and cells are crushed through high hydrostatic pressure treatment; the corneal limbus of the crushed cells are put into the decellularized corneal limbus protective liquid added with a detergent and nuclease to perform oscillation decellularized treatment; and the decellularized corneal limbus is put into the decellularized corneal limbus protective liquid to be rinsed to finish preparation. The decellularized corneal limbus obtained by the method is complete in decellularization, minimally reduces damage to the corneal limbus structure, has high biocompatibility and is favorable for autologous cell proliferation and crawling.
Owner:健诺维(成都)生物科技有限公司
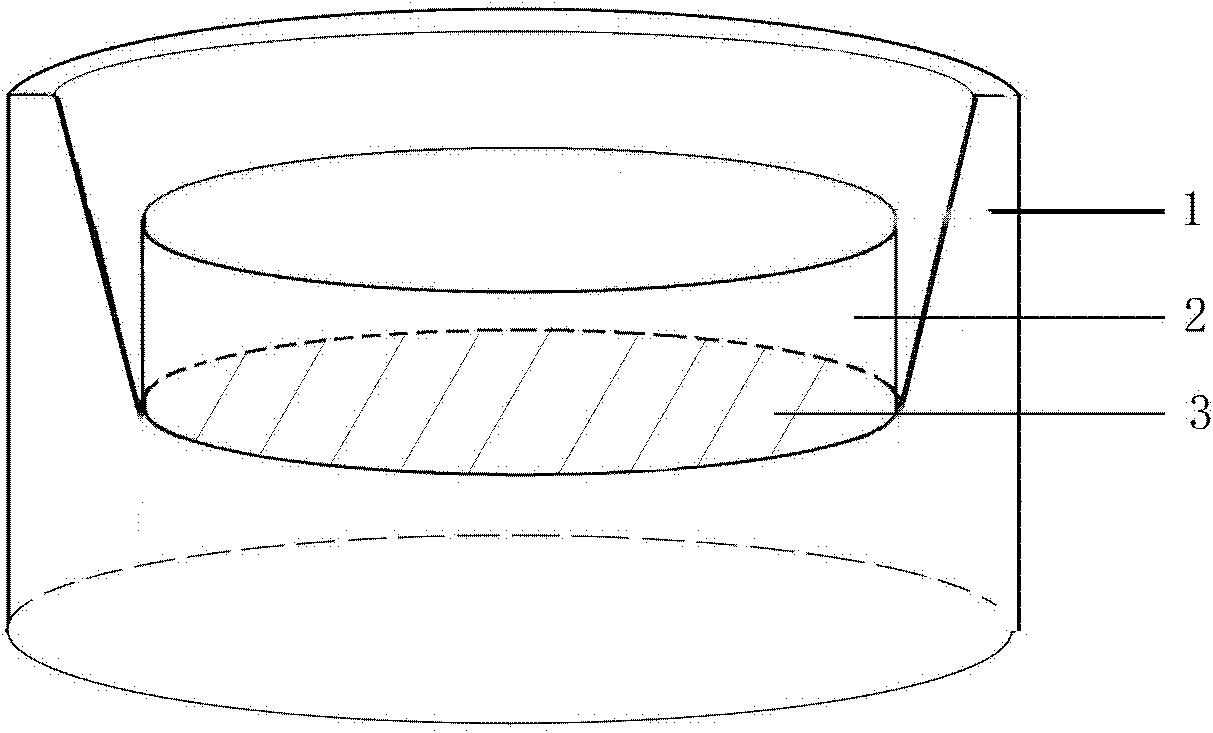
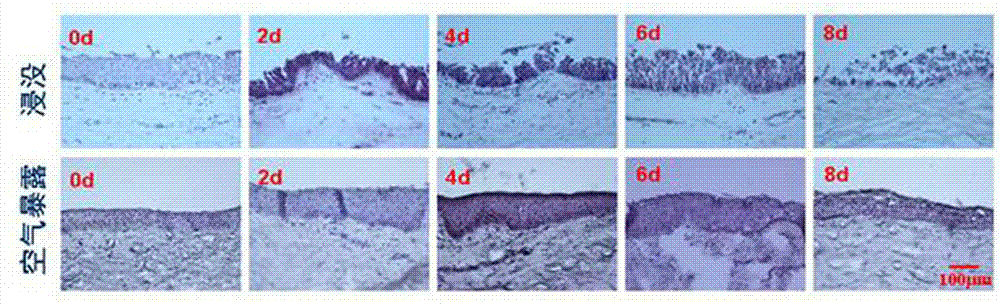
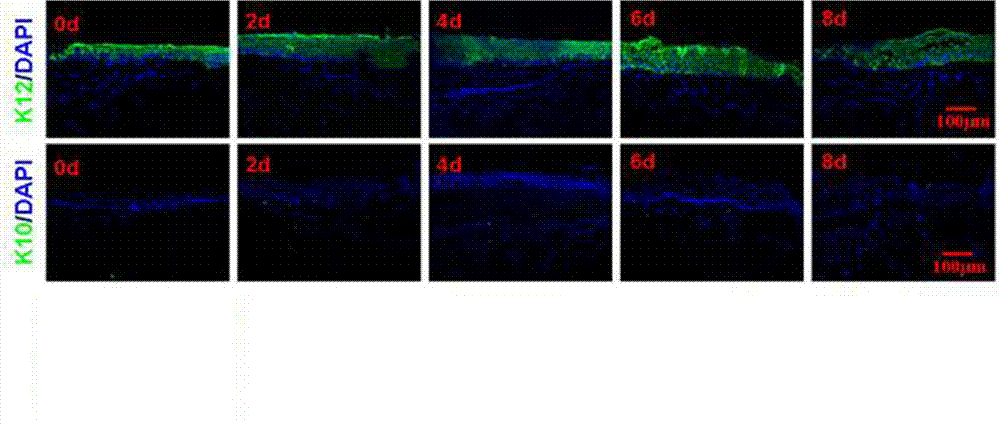
Preservation method for corneal limbus tissue
ActiveCN102726370AComplete structureAfter the structure, the present invention preserves the corneal limbal tissue intactDead animal preservationAir exposureCell culture media
The invention discloses a preservation method for corneal limbus tissue. The method comprises a step of disinfecting fresh corneal limbus tissue and a step of air exposure preservation of the disinfected fresh corneal limbus tissue. The step of air exposure preservation is as follows: the fresh corneal limbus tissue is placed on a permeable covering film, preservation liquid or a cell culture medium capable of providing nutrients to the corneal limbus tissue is arranged below the covering film, epitheliums of cornea directly contact with air, the temperature of a culture environment in the step is 0 to 37 DEG C, and oxygen concentration is 0 to 20%. Compared to immersion preservation in the prior art, the preservation method provided in the invention has the following advantages: the structure of the preserved corneal limbus tissue is more integral; obvious apoptosis of the epitheliums of cornea does not occur; stem cells of corneal limbus can maintain high activity; and process flow is simple and reliable, is easy to implement, costs little and has other efficacy.
Owner:XIAMEN EYE CENTER OF XIAMEN UNIVERSITY CO LTD
One piece flat device of for the drainage of aqueous humor from the eye
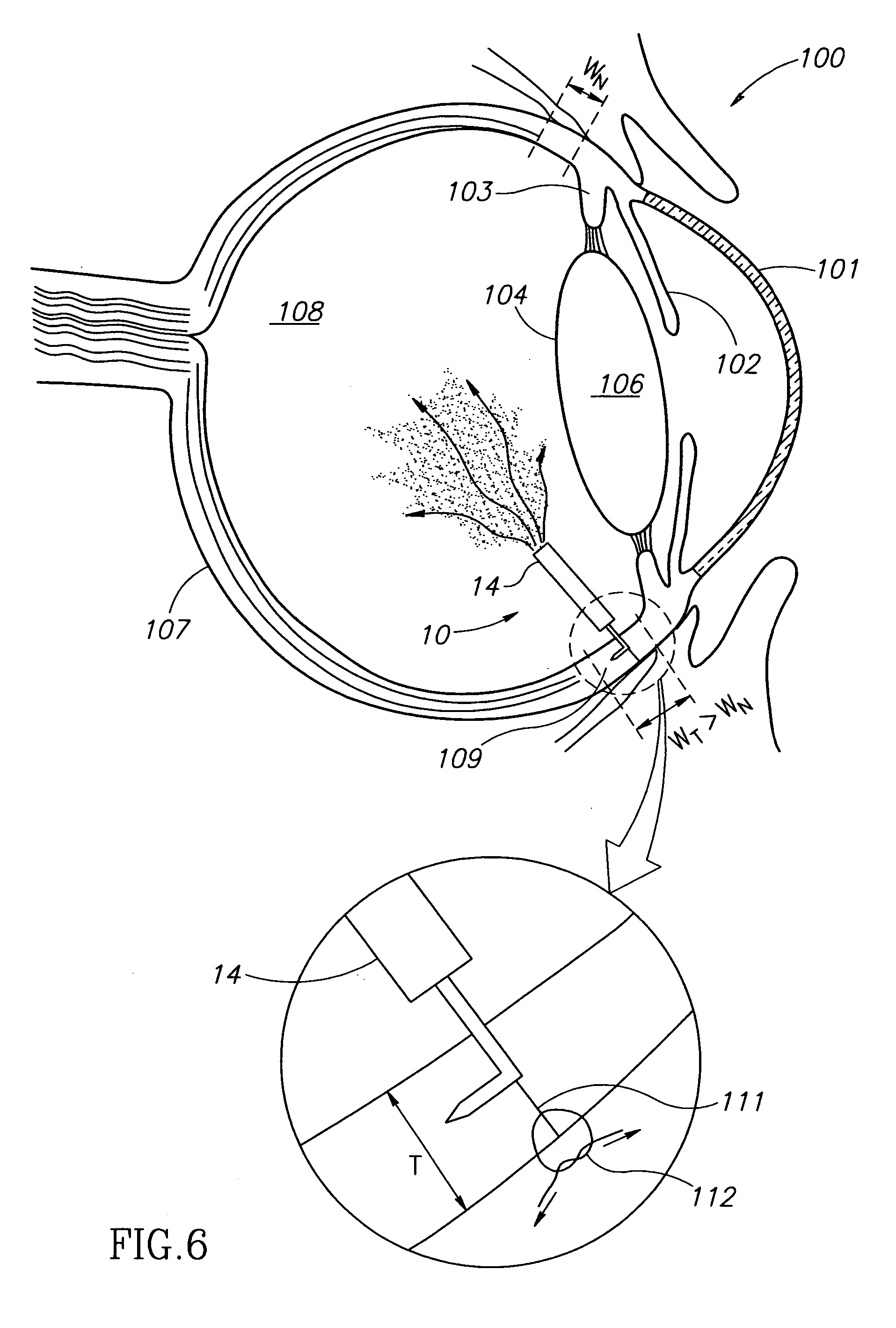
ActiveUS10758412B2Promote resultsReduce pressureLaser surgeryEye implantsSclerocorneal LimbusNeovascular glaucoma
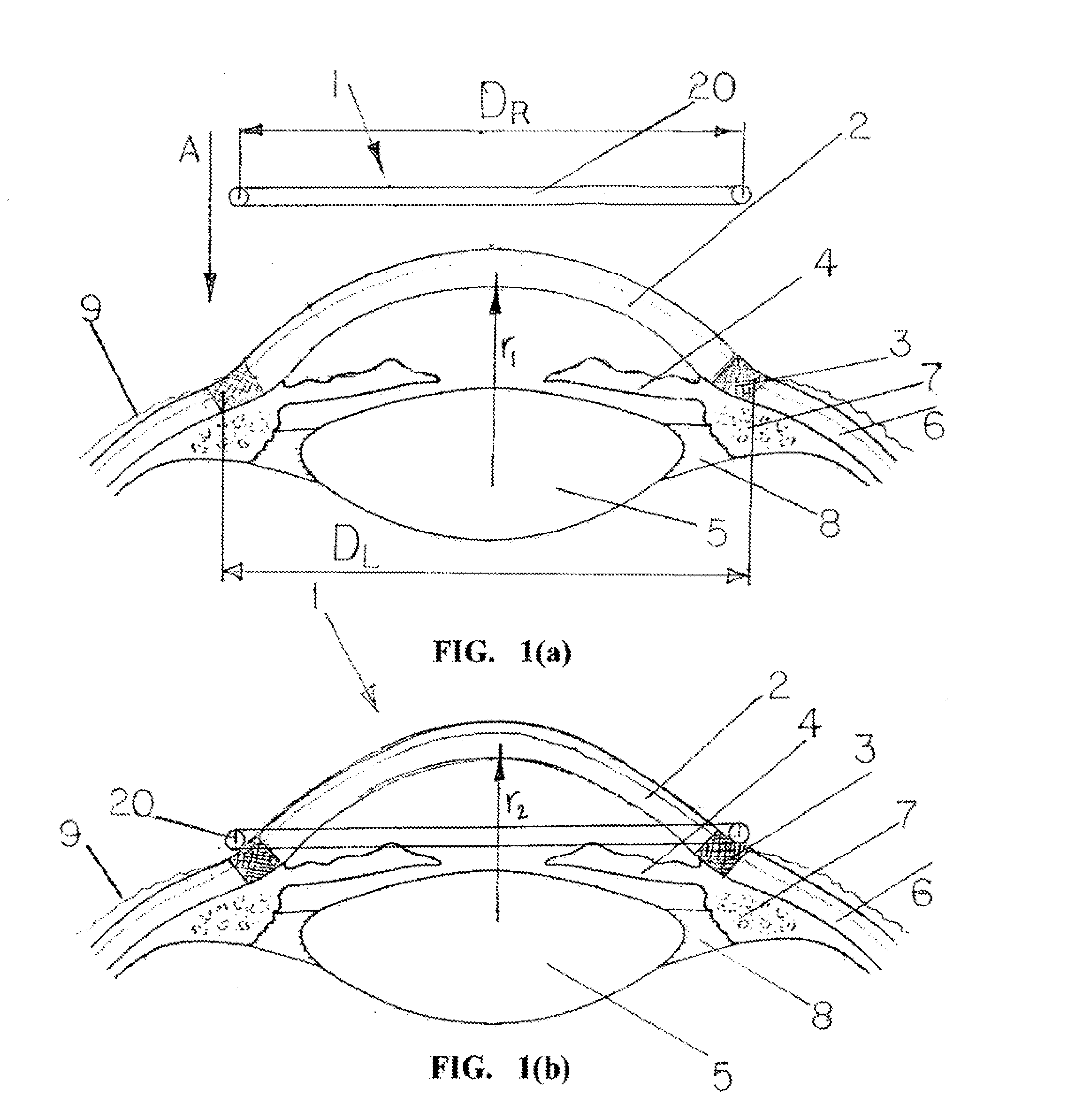
Oriented contact lens with brightly colored sclera
Oriented contact lenses have patterns in which the portion that substantially lies over the sclera when worn on-eye is brightly colored or tinted; the colored or tinted contact lenses can have colored or tinted central portions that enhance the iris when worn on-eye and limbal rings that enhance the limbus.
Owner:JOHNSON & JOHNSON VISION CARE INC
Method for configurating feeder-layer-free corneal eipithelium for frost self-corneal limbus stem cell
Owner:西北农业科技大学
Ophthalmic operation ultrasonic scalpel
The invention discloses an ophthalmic operation ultrasonic scalpel which comprises a handle and a tool bit. The handle is provided with a shell, a power source and an ultrasonic generating device arearranged inside the shell, and the head end of the shell is provided with the output end of the ultrasonic generating device; the tool bit is provided with a tool bit body, the tool bit body is sequentially provided with a connecting part, a limiting installing part and a blade part from bottom to top, the connecting part is used for being detachably connected with the output end of the ultrasonicgenerating device, the limiting installing part is used for installing a limiting mechanism to ensure the cutting depth, and the blade part is used for cutting. The purpose that a blade can instantlycut corneas downwards is achieved, and the scalpel can be used for keratoplasty and corneal limbus dissociation dissection.
Owner:沈阳何氏眼科医院有限公司
Ophthalmic incisional procedure instrument and method
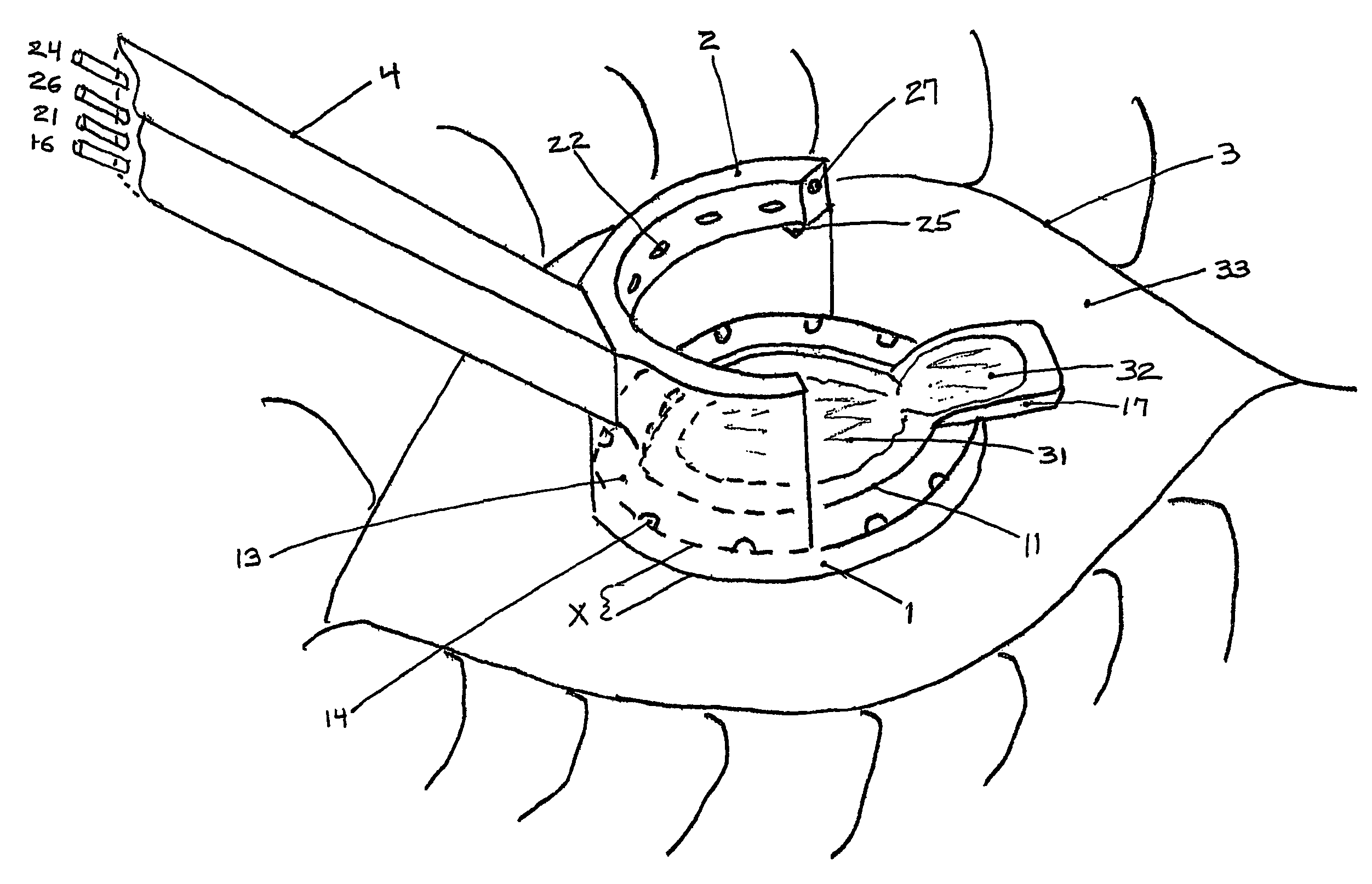
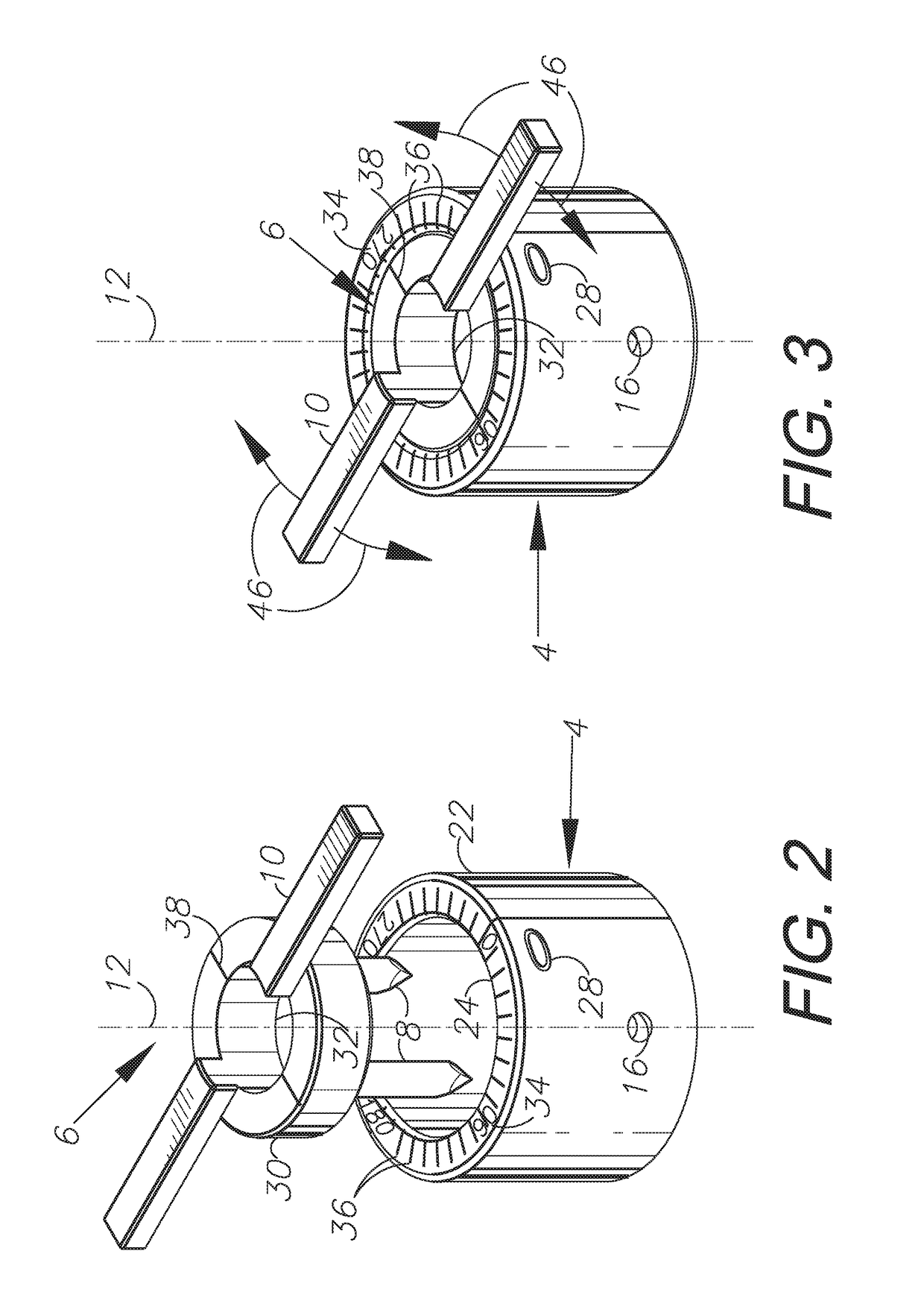
ActiveUS10779990B2Efficient and accurateEye surgerySurgical pincettesSurgical incisionCorneal limbus
An incisional instrument and method of use for creating accurate, reproducible surgical incisions. An exemplary embodiment includes an incisional instrument configured for attachment to a patient's eye and for use performing arcuate limbal relaxing incisions (LRIs). The incisional instrument is made up of two coaxial, interconnecting pieces: a docking piece and a cutting piece. The docking piece includes a suction mechanism and is configured for being secured to a patient's eye just outside the corneal limbus. The cutting piece is configured to fit flush within the docking piece and includes cutting blades and one or more handles for rotating the cutting piece relative to the docking piece. When assembled, the cutting blades extend beyond the proximal end of the docking piece by a length equal to the desired depth of LRIs to be cut. The incisional instrument further includes measurement markings for properly positioning and measuring incisions.
Owner:EYEMDENG LLC
Ophthalmic incisional procedure instrument and method
ActiveUS20180235810A1Efficient and accurateEye surgerySurgical pincettesSurgical incisionCorneal limbus
An incisional instrument and method of use for creating accurate, reproducible surgical incisions. An exemplary embodiment includes an incisional instrument configured for attachment to a patient's eye and for use performing arcuate limbal relaxing incisions (LRIs). The incisional instrument is made up of two coaxial, interconnecting pieces: a docking piece and a cutting piece. The docking piece includes a suction mechanism and is configured for being secured to a patient's eye just outside the corneal limbus. The cutting piece is configured to fit flush within the docking piece and includes cutting blades and one or more handles for rotating the cutting piece relative to the docking piece. When assembled, the cutting blades extend beyond the proximal end of the docking piece by a length equal to the desired depth of LRIs to be cut. The incisional instrument further includes measurement markings for properly positioning and measuring incisions.
Owner:EYEMDENG LLC
Recombinant vector, fibroin film modified by transgenic marrow stromal cells and application thereof
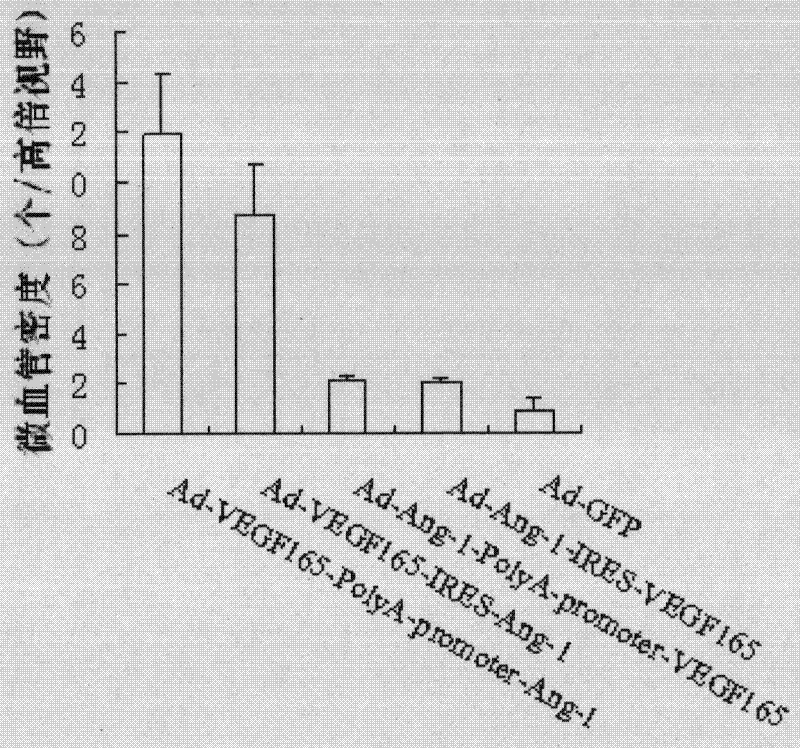
The invention relates to the biology field, and concretely discloses a recombinant plasmid pAdTrack-CMV-VEGF165-PolyA-promoter-Ang of VEGF165 and Ang-1 as well as a recombinant plasmid pAdTrack-CMV-VEGF165-IRES- Ang-1 of VEGF165 and Ang-1, wherein the preservation numbers are CCTCC No: M 2010221, CCTCC NO: M 2010220. The invention also discloses a homologous recombination of the plasmid and adenovirus and a recombinant adenovirus incased and generated from a QBI-293A cell. The invention also provides a fibroin film modified by transgenic marrow stromal cells. Rabbit corneal limbus injected with the recombination adenovirus provided in the invention is capable of successfully inducing the formation of corneal neovascularization; after the fibroin film modified by transgenic cells is implanted in a rabbit cornea layer, the neovascularization can be grown in a cornea of a fibroin film implanted area and the density of blood vessels is obviously increased, therefore, VEGF165, Ang-1, CD34 can be successfully expressed in a rabbit cornea layer. Wound repairing experiments of rats prove that the fibroin film modified by transgenic marrow stromal cells of the invention is capable of promoting the blood vessel generation of the wound organization and repairing wound, so that the recombinant adenovirus and the fibroin film modified by transgenic marrow stromal cells of the invention have medical application value.
Owner:SUZHOU UNIV
Pluripotent embryonic-like stem cells derived from corneal limbus, methods of isolation and uses thereof
The present disclosure describes mammalian pluripotent embryonic-like stem cells (ELSCs) isolated from corneal limbal tissue, a non-embryonic tissue. The ELSCs of the present disclosure are capable of proliferating in an in vitro culture, maintain the potential to differentiate into cells of endoderm, mesoderm, and ectoderm lineage in culture, and are capable of forming embryoid-like bodies when placed in suspension culture. Thus, these cells possess multi-lineage differentiation potential and self-renewing capability. ELSCs may be a promising therapeutic tool, and may provide new therapeutic alternatives for various diseases, conditions, and injuries.
Owner:RELIANCE LIFE SCI PVT
Serum-free cultured corneal limbus stromal stem cells and method for in-vitro inducing balling and induced differentiation
ActiveCN110564688AGood repeatabilityEasy to purifyDrug screeningCulture processCorneal neoplasmBiology
The invention discloses serum-free cultured corneal limbus stromal stem cells and a method for inducing differentiation and stem cell balling of the corneal limbus stromal stem cells. The invention further provides a culture medium combination for inducing corneal limbus stromal stem cells to be pelletized or differentiated into corneal limbus stromal cells in vitro. The serum-free culture mediumcombination used in the invention can provide sufficient nutrition and a good environment required by cell growth and proliferation, can stably carry out in-vitro amplification culture on corneal limbus stromal stem cells, and can ensure that the dryness and specificity of the corneal limbus stromal stem cells are still maintained after amplification culture. Meanwhile, a system for inducing and differentiating into corneal limbus stromal cells is successfully constructed, can be used for experimental research of the corneal limbus stromal stem cells, cell treatment of corneal lesions and transplantation of corneal injuries, and the serum-free cultured corneal limbus stromal stem cells has wide scientific benefits and social and economic benefits.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
RNCEC (rabbit normal corneal epithelial cells) and application thereof
InactiveCN107058213ANormal responsivenessStable proliferative stateMicrobiological testing/measurementEpidermal cells/skin cellsDispaseDigestion
The invention discloses RNCEC (rabbit normal corneal epithelial cells) and an application thereof. The cell is named as RNCEC / HL-032, and the collection number is CCTCC NO: C201677. A primary isolation culture method of the cell is as follows: a normal corneal limbus tissue sample of a New Zealand rabbit is digested, then dispase and DNase I are added after digestion, cells are filtered and collected in a centrifugal manner and then resuspended with an HL culture medium for inoculated culture. The subculturing method of RNCEC comprises steps as follows: the cells are digested with pancreatin-EDTA when proliferating to have the abundance of 70%-90% and then neutralized with a DMEM (dulbecco's modified eagle medium); the cells are collected in a centrifugal manner and resuspended with the HL culture medium for inoculated culture. A 2D and 3D culturing system established for the cell can be used for animal substitutive experiments of corneal drugs and used for detecting pharmacology and toxicology of drugs and establishing a new experimental animal substitution model for corneal drug research.
Owner:SHENZHEN EYE HOSPITAL
Techniques for identifying blepharoptosis from an image
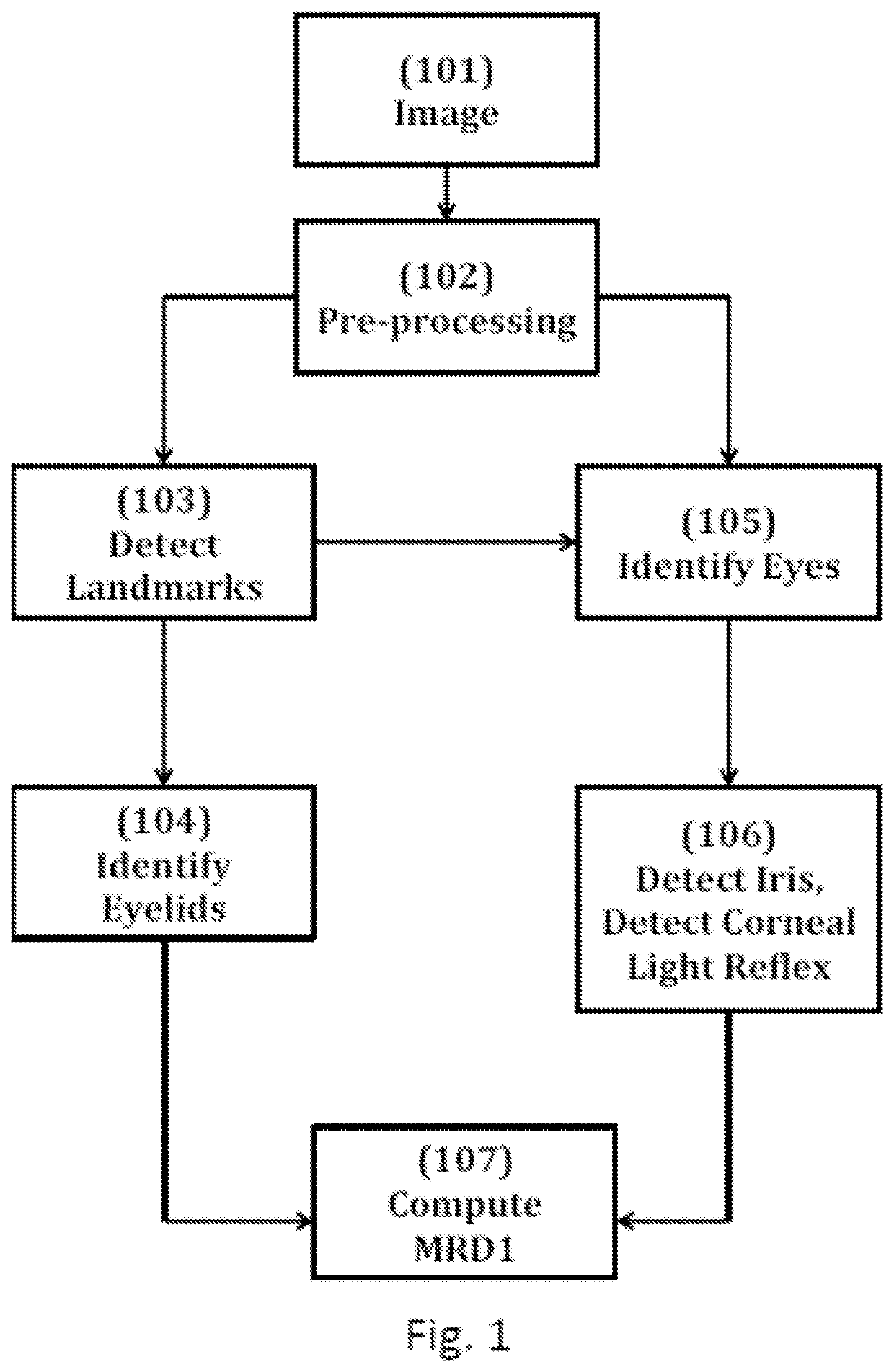
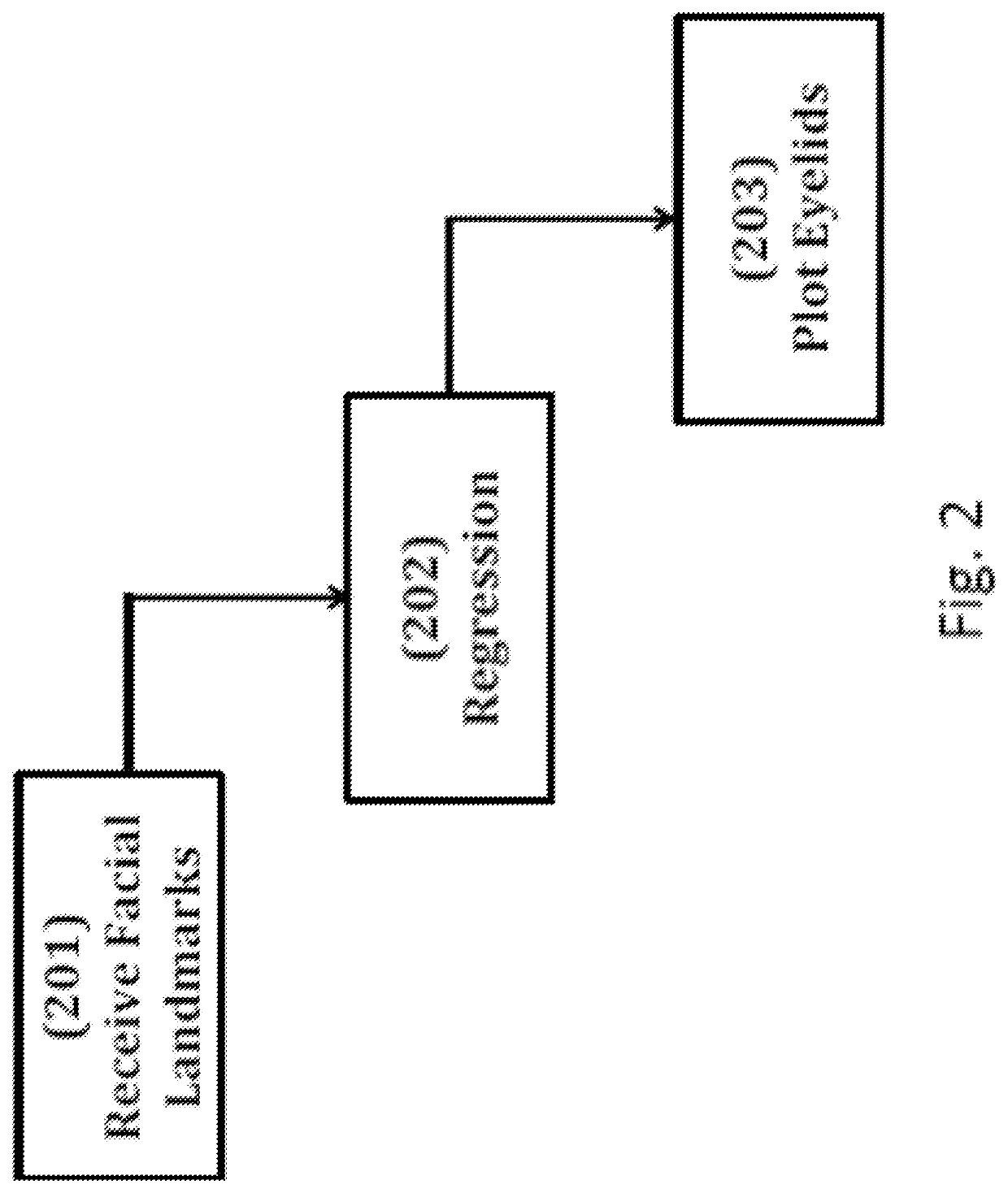
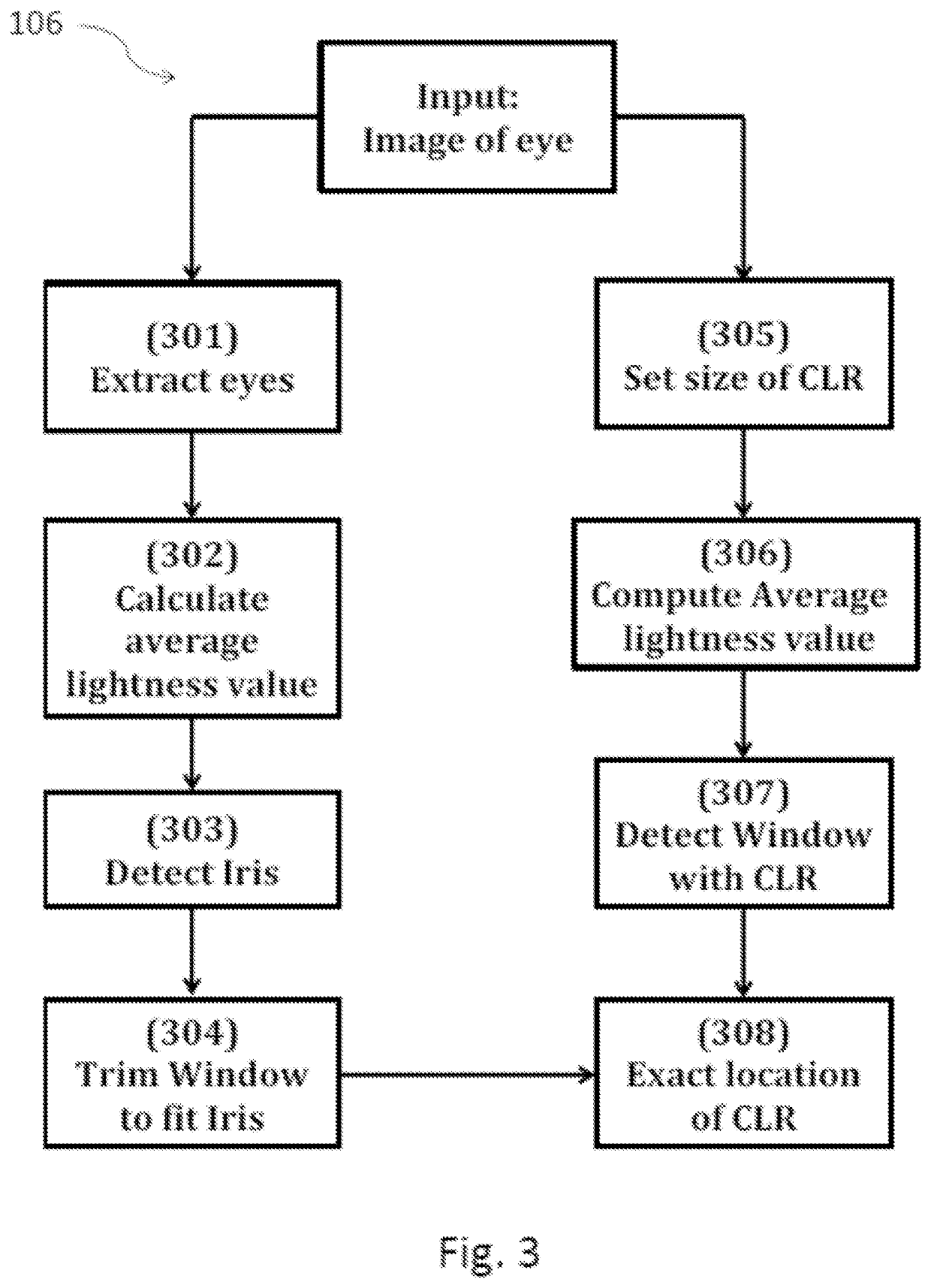
ActiveUS10580133B2High resolutionCancel noiseImage enhancementImage analysisPurkinje imagesLight reflex
Techniques for estimating blepharoptosis from an image of a face are disclosed. The image is captured by a camera. Histogram of Oriented Gradients and Linear Classifiers are used to predict the location of the face and eyes. An Ensemble of Regression Trees is used to detect points on each eyelid and the canthi. The location of the iris is ascertained by thresholding HLS values of individual pixels and applying a sliding window algorithm. Using regression, the eyelid shape curve is approximated. The eyelid shape curve function is used to measure the MRD1 value and distance below the limbus after detecting the Purkinje image / corneal light reflex. Finally, the intensity of blepharoptosis is estimated by categorizing the measured MRD1 and distance-from-Limbus values.
Owner:KRISHNA VISWESH +1
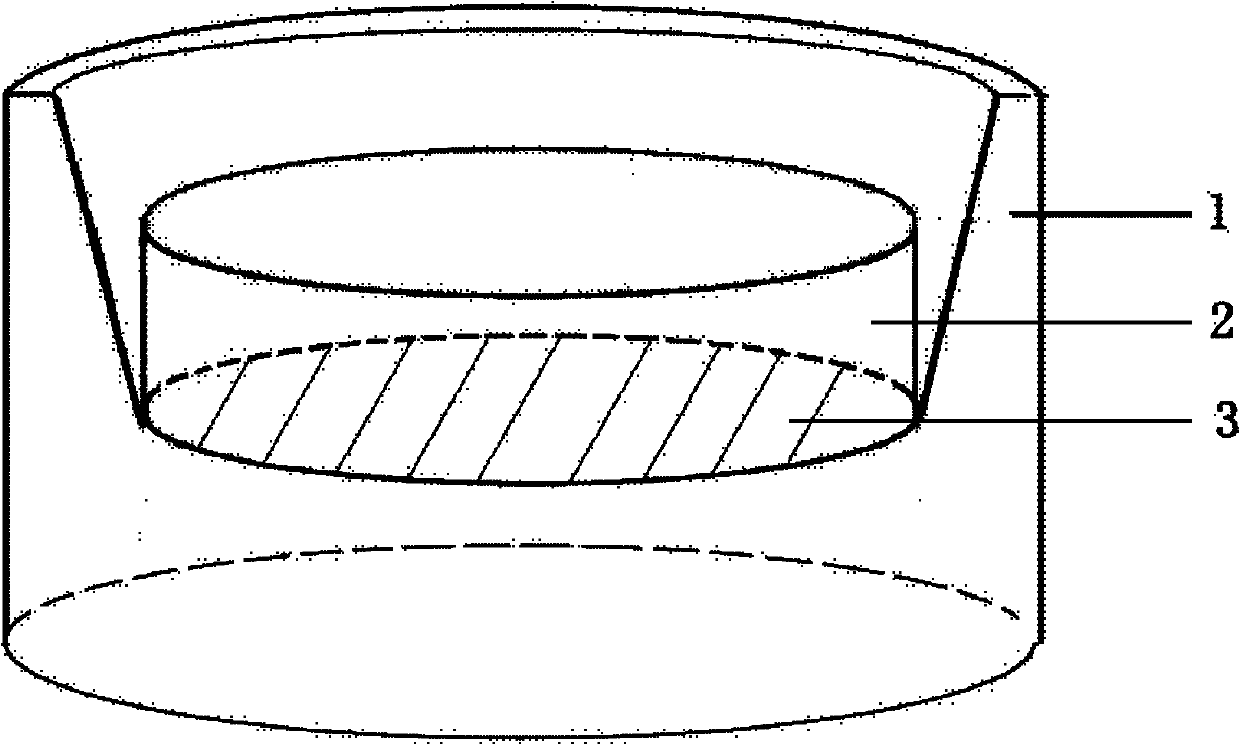
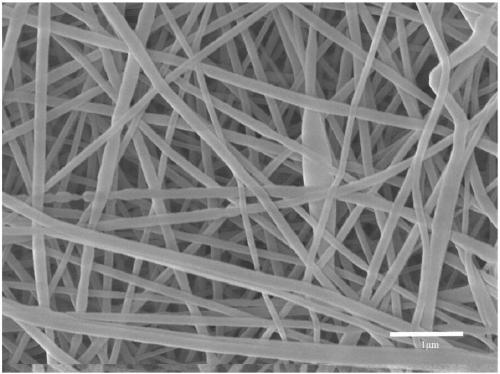
Method for constructing tissue engineering corneal limbus based on electrospinning mixed fiber membranes
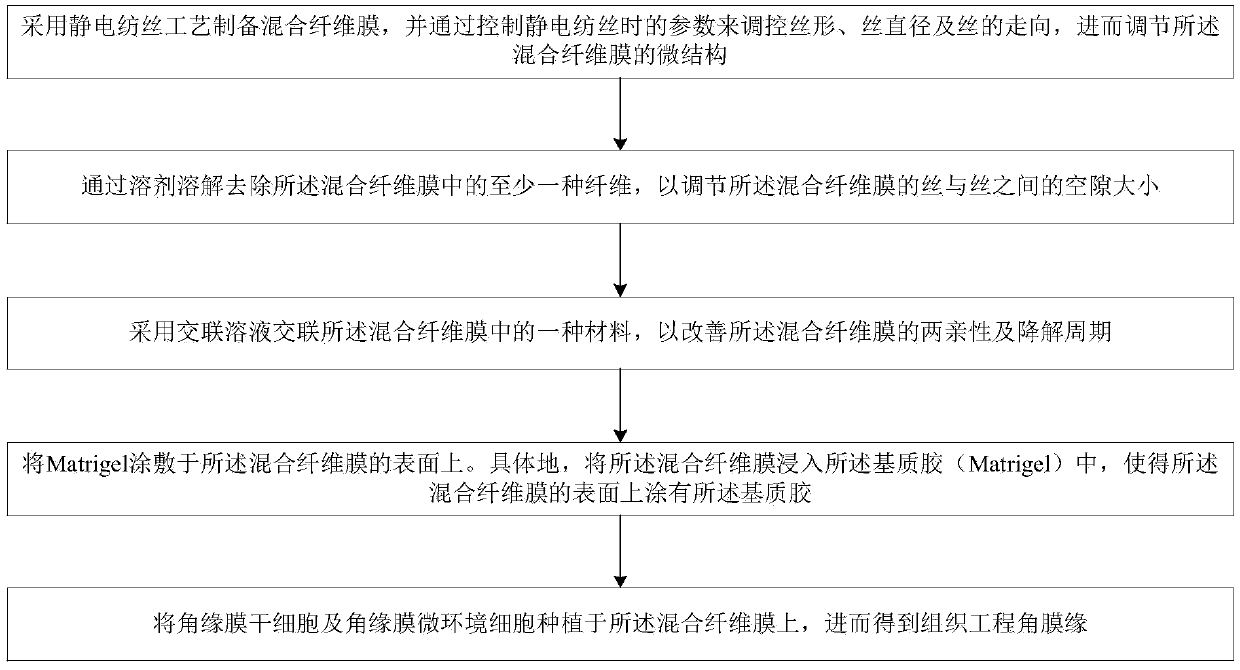
InactiveCN109554340APromote growthEasy to controlNervous system cellsCell culture supports/coatingMicro nanoFiber
The present invention belongs to the technical field of tissue engineering and discloses a method for constructing tissue engineering corneal limbus based on electrospinning mixed fiber membranes. Themethod comprises the following steps: (1) mixed fiber membranes are prepared by using an electrospinning technology, and by controlling parameters of electrospinning to regulate and control spinningshapes, spinning diameters and spinning assembly methods, the fiber membranes with a micro-nano structure conductive for cell growth are obtained; (2) amphiphilicity and degradation cycles of the mixed fiber membranes are adjusted by controlling crosslinking degrees of mixed spinning; and (3) Matrigel is wrapped onto surfaces of fiber filaments, corneal limbal stem cells and corneal limbal microenvironment cells are planted in gaps, and the tissue engineering corneal limbus is further cultured and obtained. The method has strong scientific and technological innovation, and very strong medicalprospects, and solves a limitation that corneas are currently provided only by living body.
Owner:HUAZHONG UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com