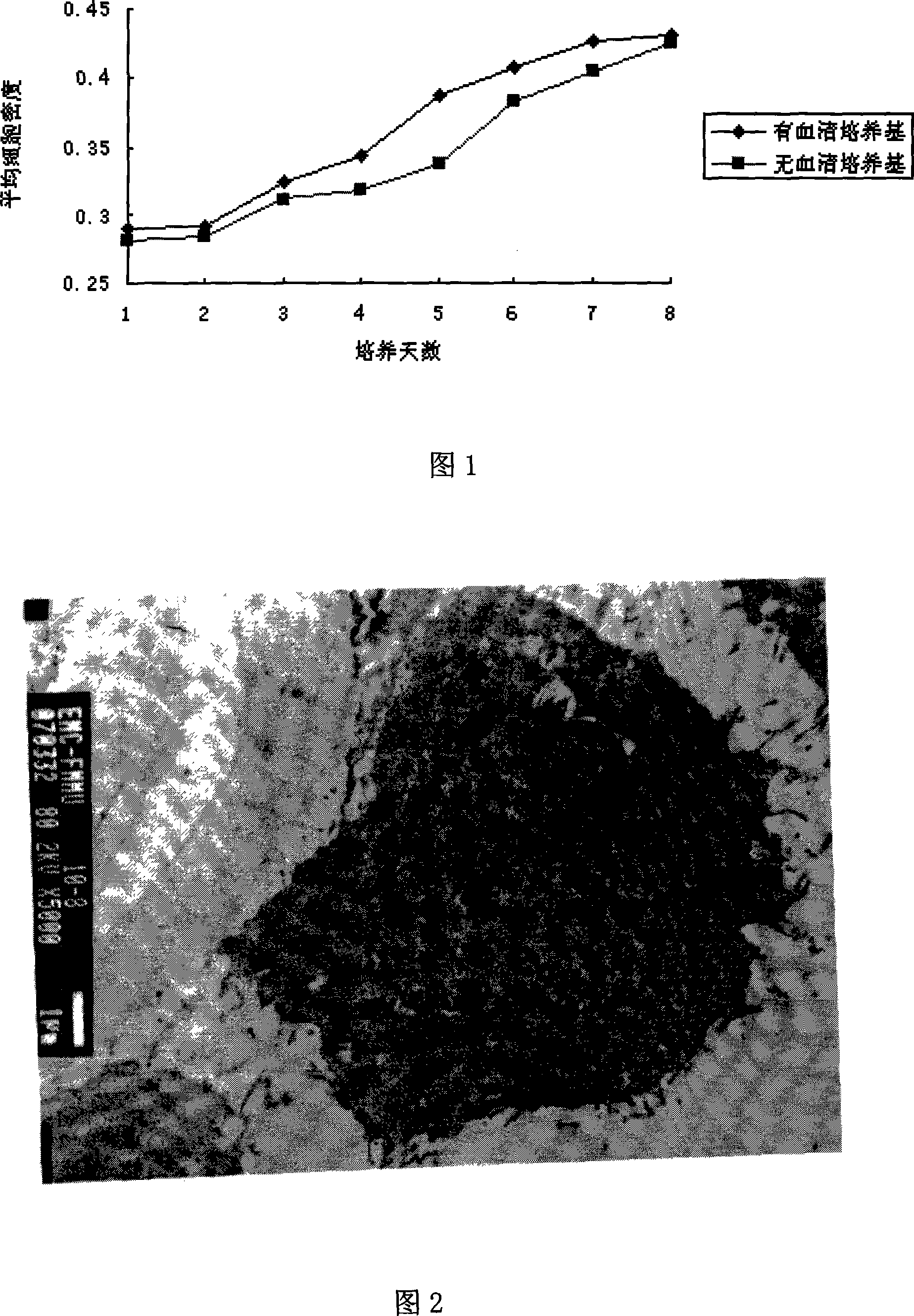
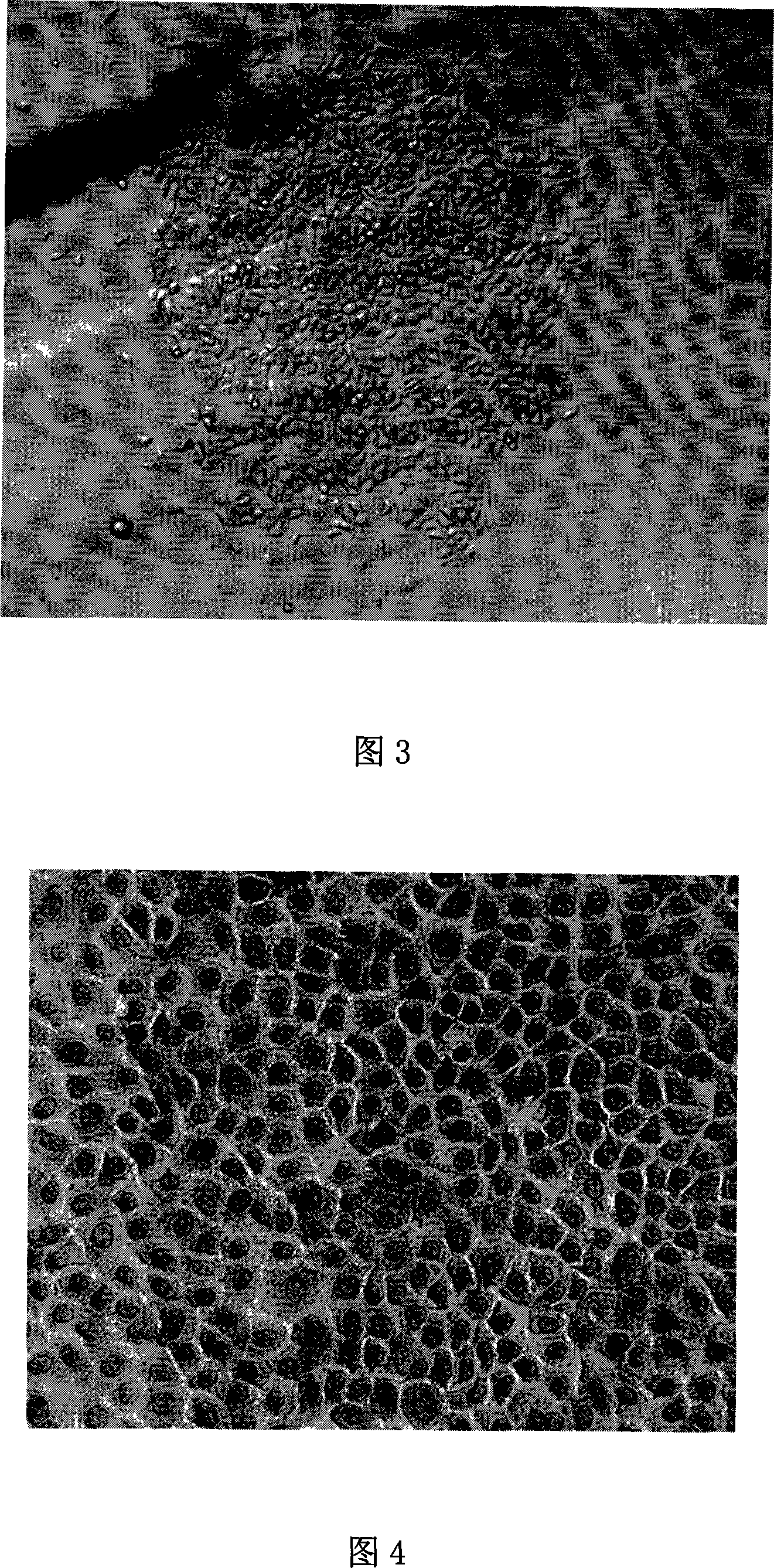
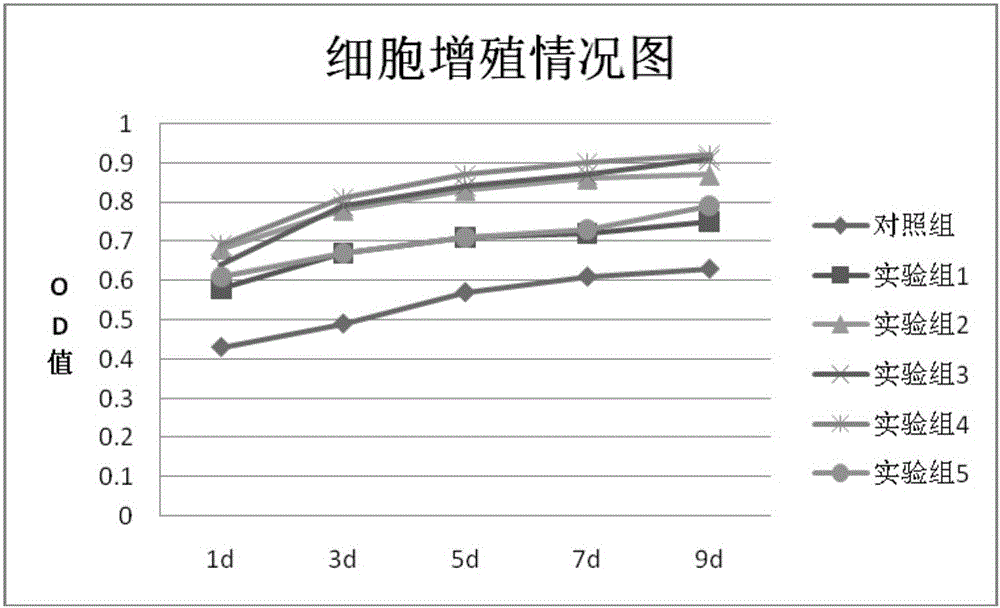
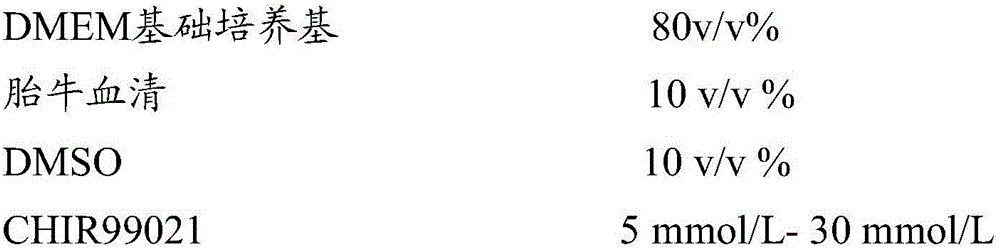Patents
Literature
81 results about "Limbal stem cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
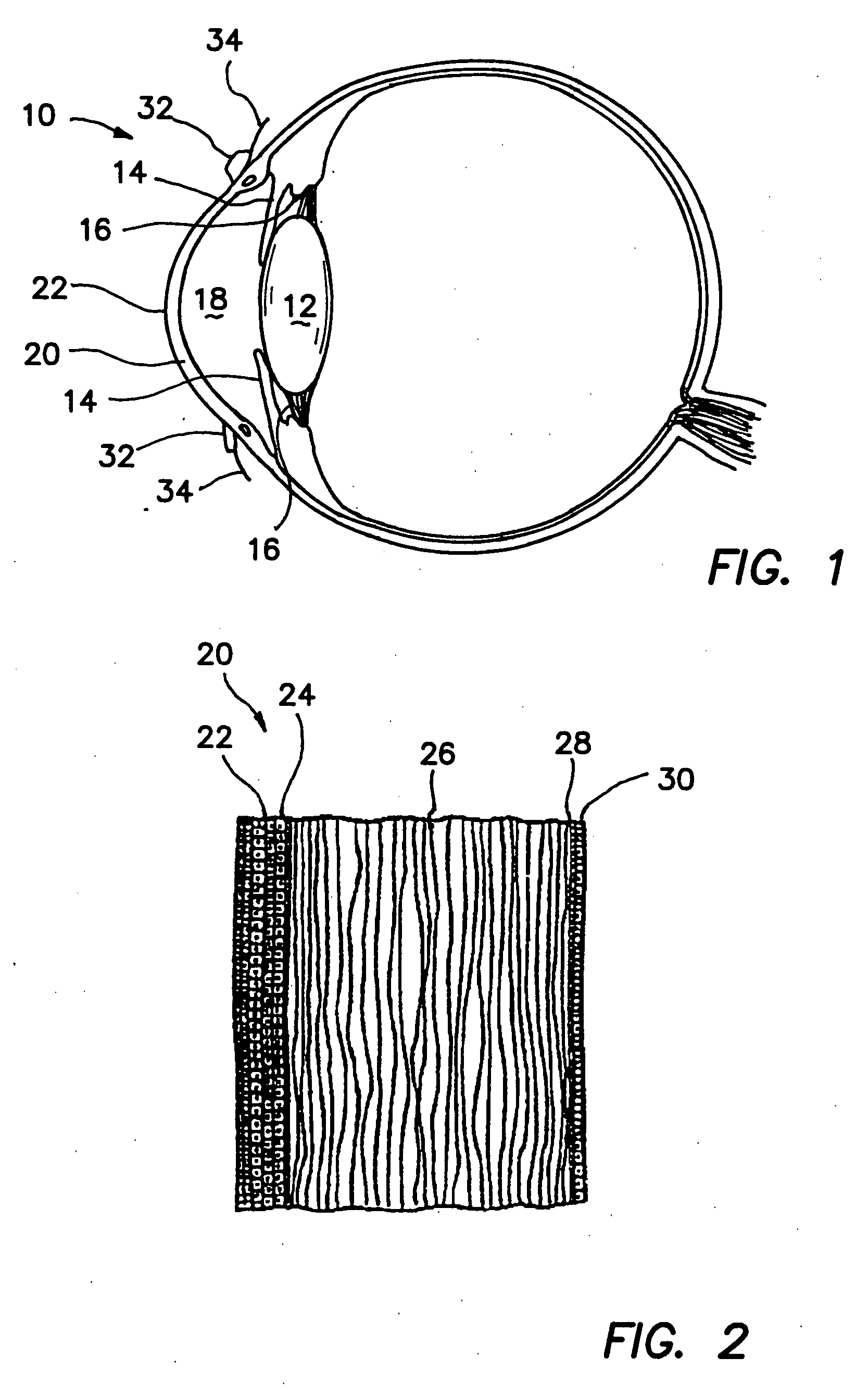
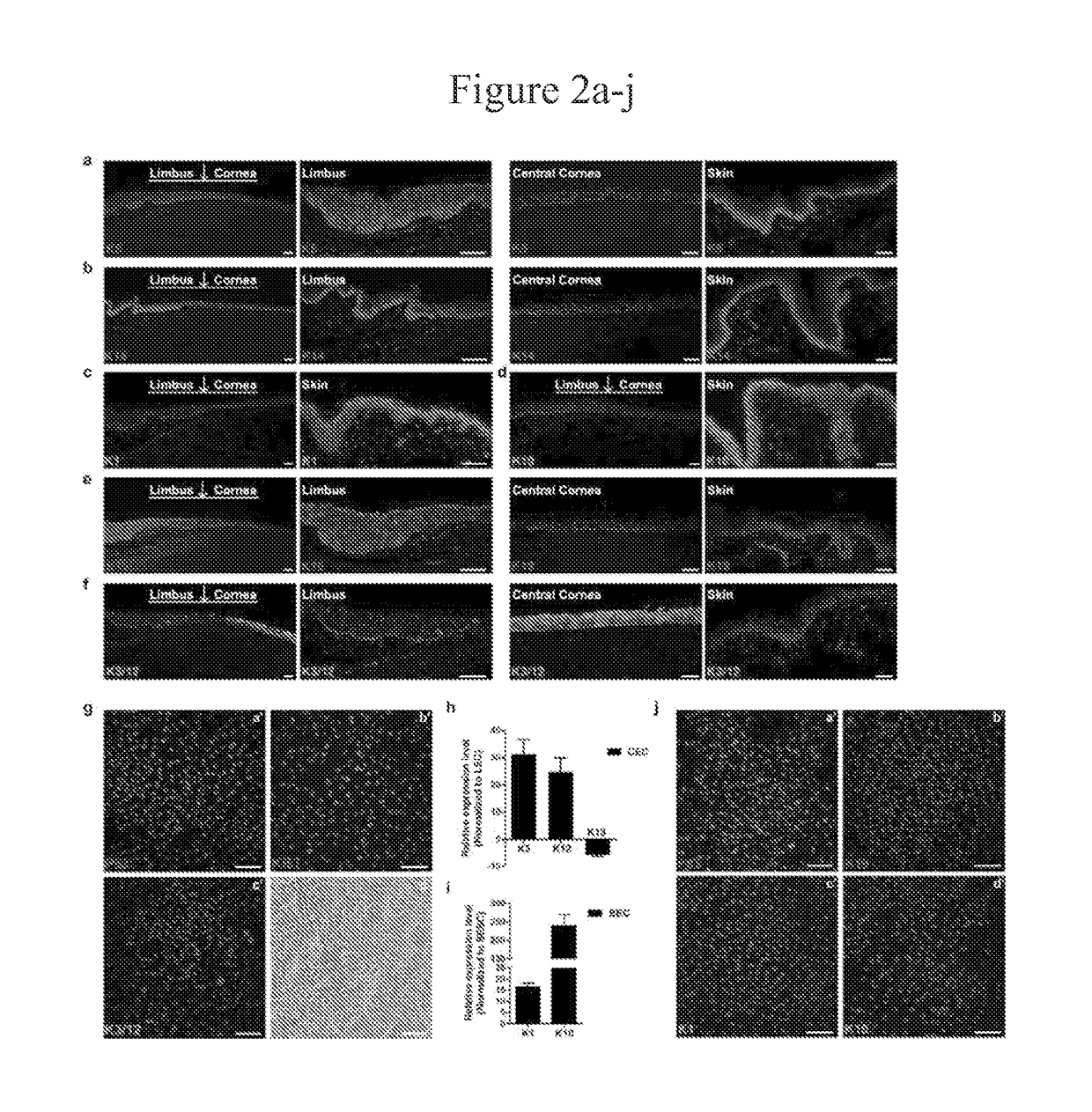
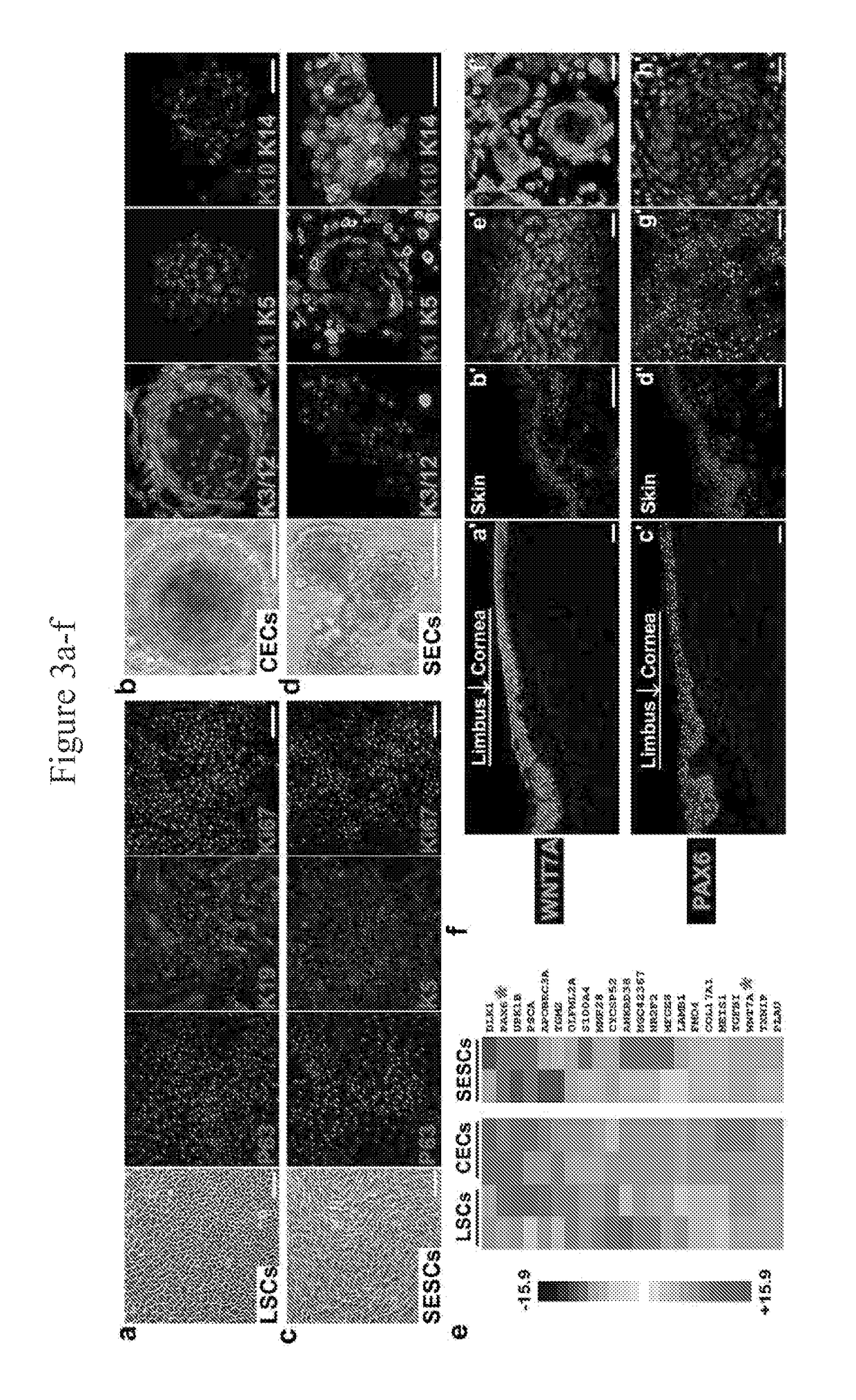
Limbal stem cells, also known as corneal epithelial stem cells, are stem cells located in the basal epithelial layer of the corneal limbus. They form the border between the cornea and the sclera. Characteristics of limbal stem cells include a slow turnover rate, high proliferative potential, clonogenicity, expression of stem cell markers, as well as the ability to regenerate the entire corneal epithelium. Limbal stem cell proliferation has the role of maintaining the cornea; for example, by replacing cells that are lost via tears. Additionally, these cells also prevent the conjunctival epithelial cells from migrating onto the surface of the cornea.
Devices and methods for improving vision
InactiveUS20050080484A1Improve eyesightCorrect and improve visionEye implantsEye surgeryEpitheliumLens plate
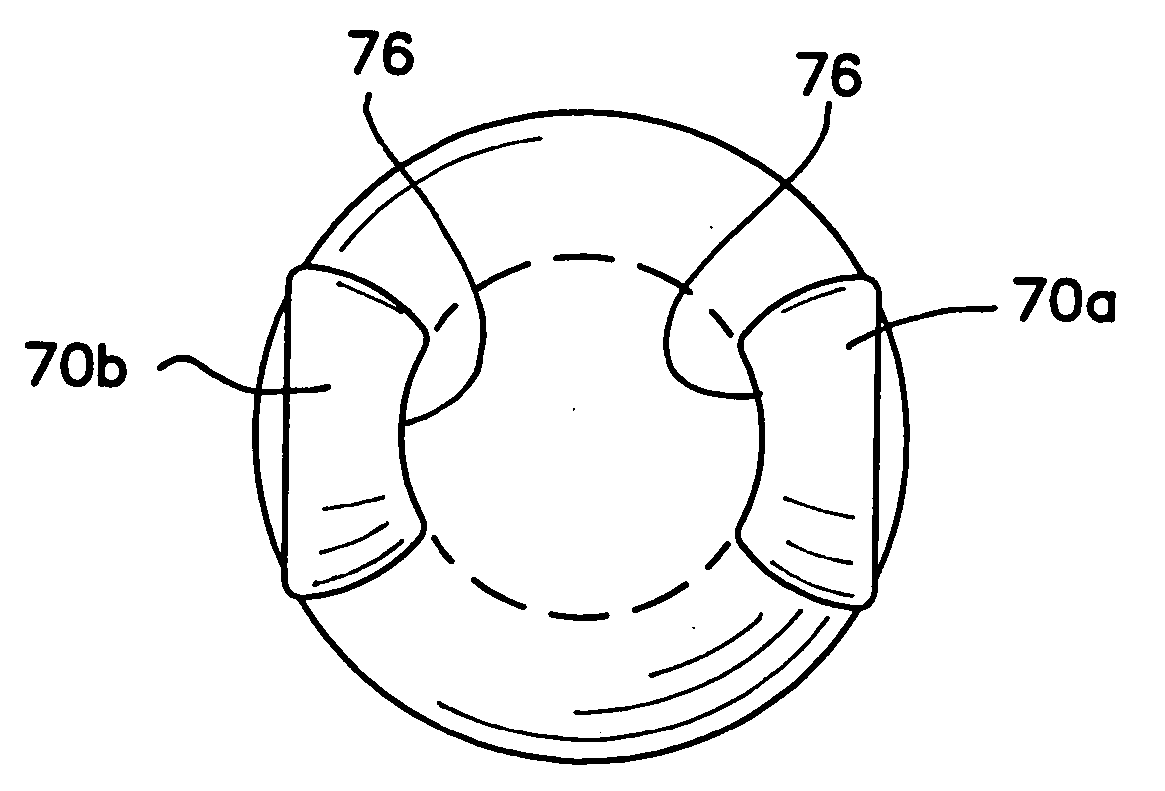
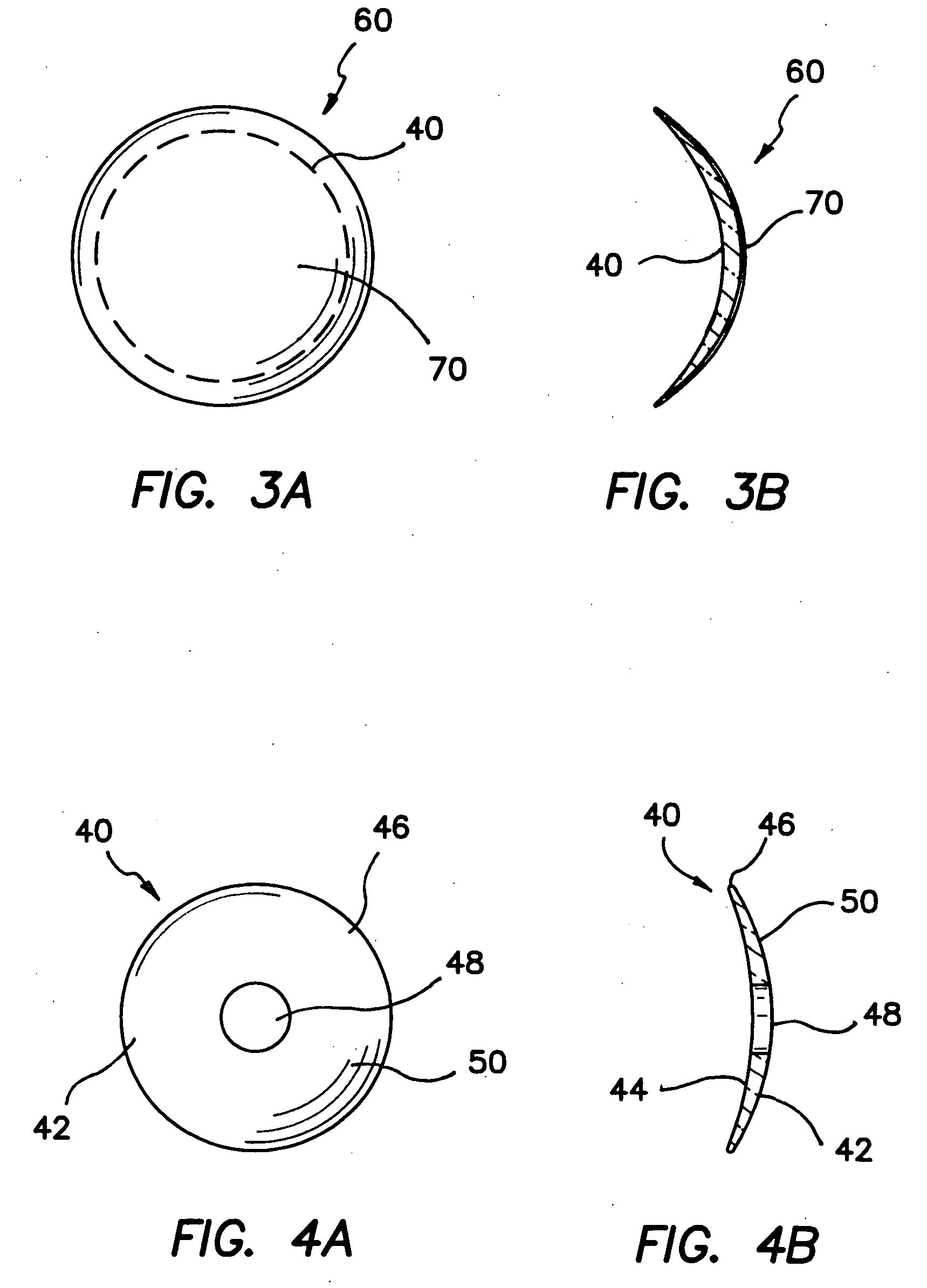
A corneal appliance that is placed over an eye has a lens body and epithelial cells secured over the lens body. The epithelial cells of the appliance may be derived from cultured cells, including stem cells, such as limbal stem cells, or epithelial cell lines, or may include at least a portion of the epithelium of the eye on which the appliance is placed. The corneal appliance may have a cellular attachment element between the lens body and the epithelial cells to facilitate attachment of the epithelial cells over the lens body. The corneal appliance is intended to be used on a deepithelialized eye, which may be an eye that has had the epithelium fully or partially removed. The corneal appliance may be used to improve vision. Methods of producing the corneal appliance and of improving vision are also disclosed.
Owner:FORSIGHT LABS
Tissue system with undifferentiated stem cells derived from corneal limbus
InactiveUS20050186672A1Senses disorderCulture processSurface markerStage-Specific Embryonic Antigens
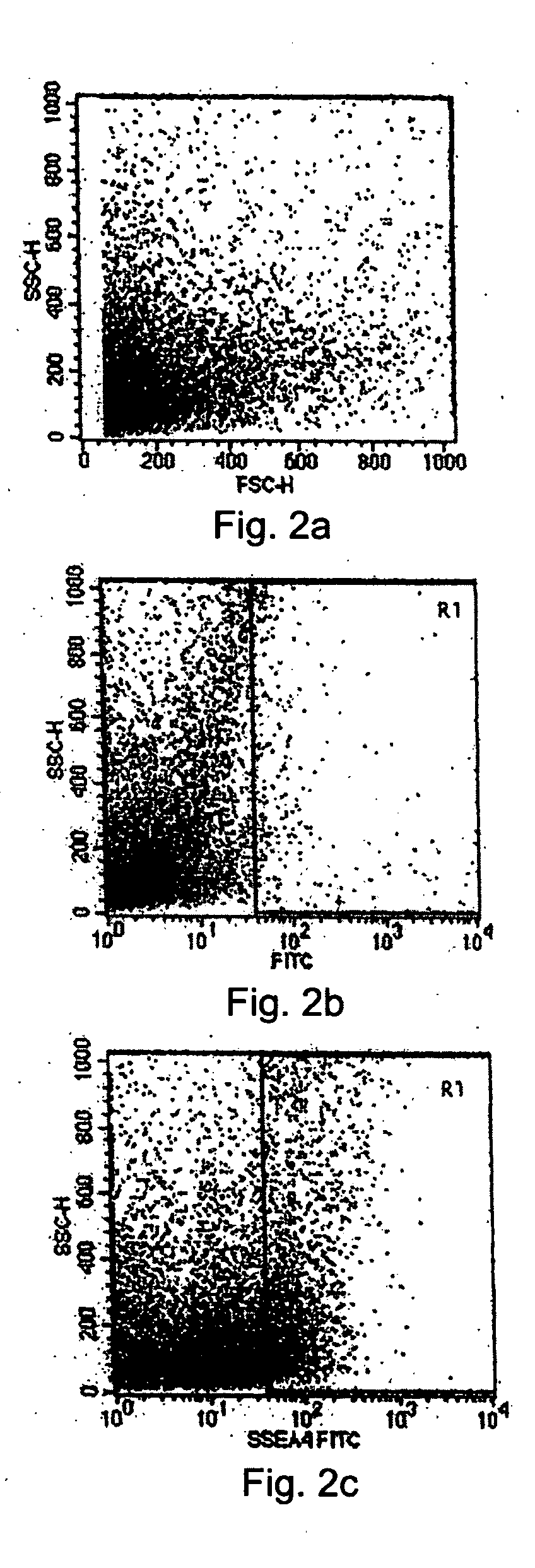
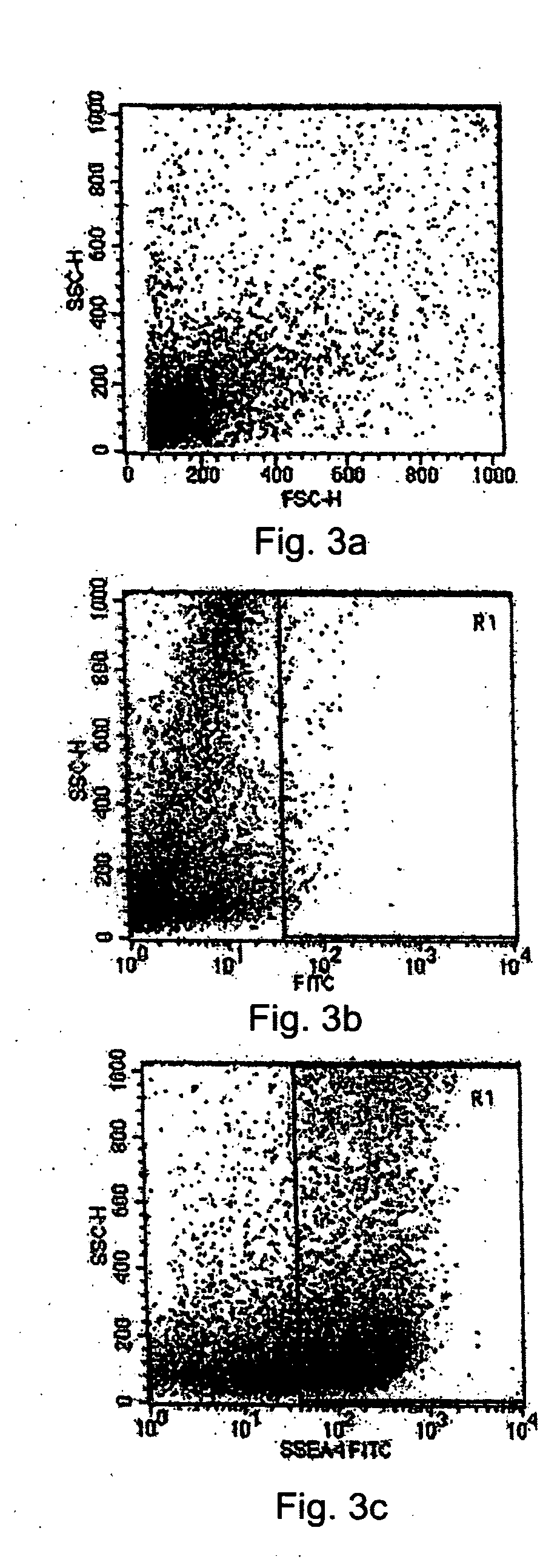
The present disclosure describes a tissue system with self-regenerating limbal stem cells, wherein the limbal stem cells are primarily undifferentiated stem cells (USCs). The tissue system is derived from isolated corneal limbal tissue, and is suitable for restoring ocular surface impairments, particularly those that result from limbal stem cell deficiencies. The tissue system is generated by selectively augmenting the tissue system for USCs, for example by selecting and sorting cells that express stem cell-specific surface markers, such as stage specific embryonic antigen marker 4 (SSEA-4). After isolation, the USCs are cultured on a tissue base in the presence of enriched medium to generate the tissue system, which is suitable for transplantation, implantation, or grafting.
Owner:RELIANCE LIFE SCI PVT
Human corneal limbal stem cell tissue engineering product nourished by fibroblast and preparing process thereof
InactiveCN1635115APromote differentiationTight adhesionArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsDiseaseTrophoblast
A human corneal limbal stem cell-amnion tissue engineering complex using human corneal limbal fibroblasts as the trophoblast is characterized in that: it is close to the human corneal epithelium form in histology form, and is analogous to the human corneal epithelium in immunology phenotype, its ultrastructure shows that the connection between the cells grows mature and the connection between the epithelia and the amnion carrier is a close conglutination. The culture method includes the following steps: (1) preparing the amnion for culturing the human corneal limbal stem cell in vitro; (2) preparing the human corneal limbal fibroblast trophoblast; (3) preparing the human corneal epithelia suspension; (4) culturing the human corneal limbal stem cell-amnion complex. The human corneal limbal stem cell-amnion complex has analogous phenotype to the human corneal epithelium, and provides a safe and effective biology tissue engineering product to treat corneal limbal stem cell defective eye diseases.
Owner:天津医科大学眼科中心
Method for preparing corneal epithelium form epidermis stem cell through fabrication of tissue engineering, and application
InactiveCN1563366AWeakened immune memoryRich sourcesEye implantsTissue cultureSerum free mediaCulture mediums
Owner:陕西九州生物医药科技集团有限公司
Limbus corneae stem cell serum-free culture medium
InactiveCN101121926AMeet the nutritional needs of long-term expansion cultureArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsBiotechnologyPenicillin
The invention discloses a limbal stem cell serum-free medium, consisting of the following raw materials (calculated by weight percent): DMEM / F12 60ml-90ml, BSA 0.5g-4g, EGF 1Mug-4Mug, insulin 0.1mg-1mg, hydrogen 20ug-100ug, cholera toxin 1ug-10ug, transferrin 0.1mg-1mg, selenious acid 0.1ug-1ug, GCLCM 10ml-40ml, penicillin 5*103IU-2*104IU and streptomycin 5*103IU-2*104IU. The invention adopts the method that the culture medium is added with fibroblast conditioned medium, and the nutritive factors are supplemented with the hormone and cytokine combination, fibroblast conditioned medium and BSA. As a result, the nutritive needs for long-time in-vitro expansion and cultivation of the limbal stem cell serum-free medium are satisfied, and the medium is serum-free and suitable for commercial production.
Owner:NORTHWEST A & F UNIV
Preparation method and application thereof of acellular conjunctiva matrix
InactiveCN101590292AEasy to prepareGood biocompatibilityEye implantsDead animal preservationTreatment effectBiocompatibility Testing
The invention relates to a preparation method and application thereof of an acellular conjunctiva matrix used as a tissue engineering corneal scaffold material. The method comprises the following steps: removing cell components in a bulbar conjunctiva first; preparing the acellular conjunctiva matrix; and taking the acellular conjunctiva matrix as a tissue engineering corneal scaffold. A rabbit corneal epithelium or an endothelial cell can form a good cell single layer on the scaffold, can construct a tissue engineering corneal epithelium or endothelium, and can successfully transplant the tissue engineering corneal epithelium or endothelium onto rabbit animal model eyes. The degradation time of the built tissue engineering cornea is longer, rabbit eyes have no obvious immune reject reaction, and the therapeutic effects on the lack of corneal limbus stem cells or the decompensation of the corneal endothelial cell function are obvious. The method and the application thereof have the advantages of simple preparation method, good biocompatibility, low antigenicity, easy growth and proliferation of seed cells, slow degradation, extensive bulbar conjunctiva sources and wide application and development prospects; and the transparency can be maintained all the time in the training process and after transplantation.
Owner:SHANDONG EYE INST
Cultural method for inducing human embryonic stem cell to directionally differentiate into corneal limbal stem cell
ActiveCN102952779AGood in vitro differentiation and proliferation abilitySuppress generationArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsImmunofluorescenceMicroscopic observation
The invention discloses a cultural method for inducing a human embryonic stem cell to directionally differentiate into a corneal limbal stem cell, which comprises the following steps that firstly, a DMEM (Dulbecco's Modified Eagle Medium) / F12 conditioned medium is adopted to culture a human primary corneal limbal stem cell to prepare a corneal limbal stem cell conditioned medium, and then the corneal limbal stem cell conditioned medium is utilized and combined with IV type collagen culture in vitro to induce the human embryonic stem cell to directionally differentiate into the corneal limbal stem cell. According to the corneal limbal stem cell obtained by utilizing the cultural method, through light microscope observation in vitro, electron microscope observation, real-time quantitative polymerase chain reaction, immunofluorescence, flow cytometry, cloning efficiency determination and the like, the induced cell has a similar shape and phenotype with a normal corneal limbal stem cell, has good differentiation and proliferation capacity in vitro, can be transferred in vitro for more than four generations and can be used as a seed cell for preparing a corneal graft.
Owner:SHANDONG UNIV
3D model constructed in vitro by means of serum-free culture medium as well as construction method of 3D model
ActiveCN107326003AEnsure nutritional needsPromote growthEpidermal cells/skin cellsCulture processBiological materialsLiquid surfaces
The invention discloses a 3D model constructed in vitro by means of a serum-free culture medium as well as a construction method of the 3D model and relates to the technical field of biological materials in tissue engineering. Due to selection variety of seed cells and good in-vitro construction repeatability, industrial preparation of the 3D model can be realized. The model is an in-vitro model formed by primary corneal epithelial cells subjected to tissue isolation, limbal stem cells, immortalized corneal epithelial cells, primary oral epithelial cells or oral mucosa squamous cancer cell lines TR146 subjected to in-vitro amplification and stratified through the serum-free culture medium with an undersurface-gas-liquid surface staged culture method.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Preparation method for hydroxyethyl chitosan in-situ hydrogel
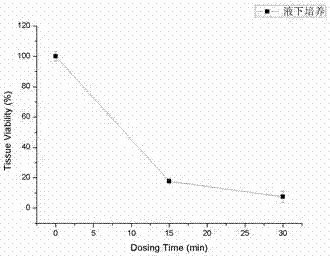
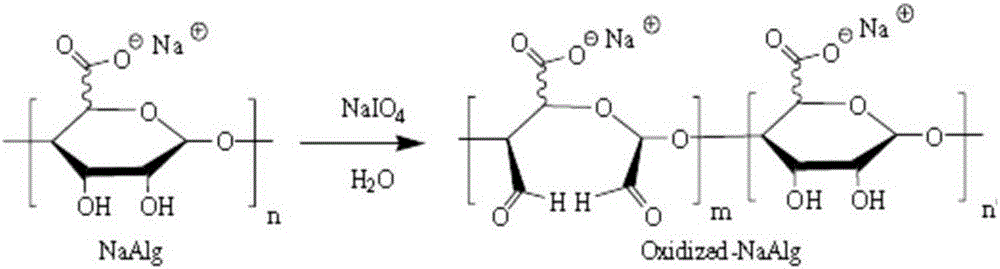
The invention belongs to the technical field of biomedicines and particularly relates to a preparation method for hydroxyethyl chitosan in-situ hydrogel. The preparation method comprises the following steps of firstly, dissolving sodium periodate powder into deionized water to obtain an aqueous solution of the sodium periodate; secondly, stirring an aqueous solution of sodium alginate and the aqueous solution of the sodium periodate to form a mixed solution; after the mixed solution is enabled to perform an oxidizing reaction, adding ethylene glycol to end the reaction; carrying out suction filtration on the mixed solution, carrying out dehydration precipitation on the mixed solution with absolute ethyl alcohol, and then carrying out low-temperature vacuum drying to obtain an oxidized sodium alginate crude product; dissolving the oxidized sodium alginate crude product into an aqueous solution of oxidized sodium alginate crude product with distilled water; carrying out complete dialysis on the aqueous solution of oxidized sodium alginate crude product, centrifuging, taking supernate for freeze drying to obtain oxidized sodium alginate; finally, mixing hydroxyethyl chitosan with the oxidized sodium alginate, carrying out cross-linking reaction on the mixed solution by a double-linkage syringe, and then pushing the reacted product into a test tube to obtain the hydroxyethyl chitosan in-situ hydrogel. The hydroxyethyl chitosan in-situ hydrogel is used for quickly coating and transplanting. The preparation method is simple in preparation process, safe and reliable in principle and low in preparation cost; the hydroxyethyl chitosan in-situ hydrogel has the advantages of good quality, safety and sanitation in use, and environment friendliness in a treatment environment.
Owner:QINGDAO UNIV
Compositions derived from stem cell released molecules & methods for formulation thereof
InactiveUS20140205563A1Ensuring structureEnsure stabilityBiocideCosmetic preparationsFibroblastic cellCytokine
Compositions for use in treatment of a variety of tissue diseases include stem cells and stem cell released molecules (SRM's) suspended in an aqueous solution with a cellulosic material or other thickening agent. The stem cells and SRM's can be derived from one or more distinct cell lines. The SRM's can further include one or more mucins, cytokines, or growth factors. Exemplary formulations include stem cells and SRMs derived from epithelial stem cells, corneal limbal stem cells, and fibroblasts. Other compositions and methods for formulation thereof are described.
Owner:BIOREGENERATIVE SCI
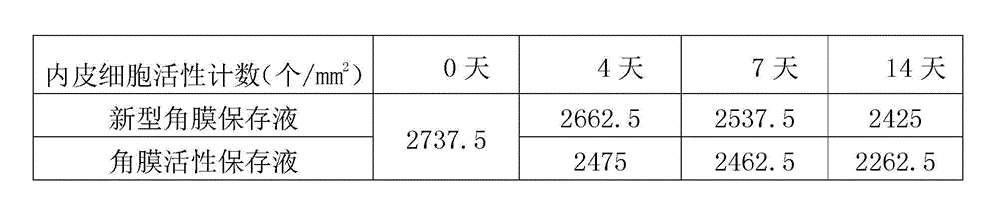
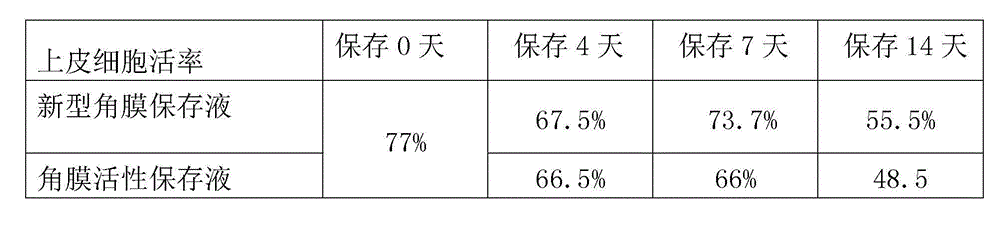
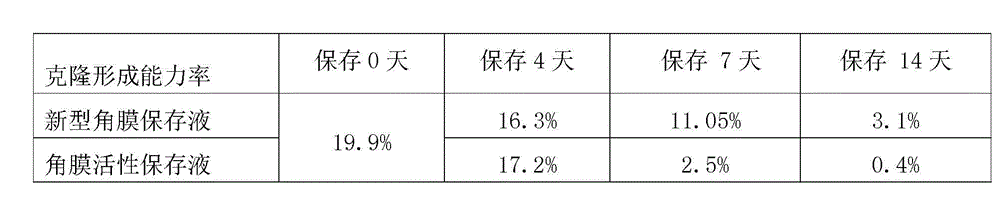
Cornea metaphase preservation solution, and preparing and using methods thereof
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
Separation method of limbal stem cells
InactiveCN105219704AAvoid damageHigh yieldArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsNeutral proteaseStem cell culture
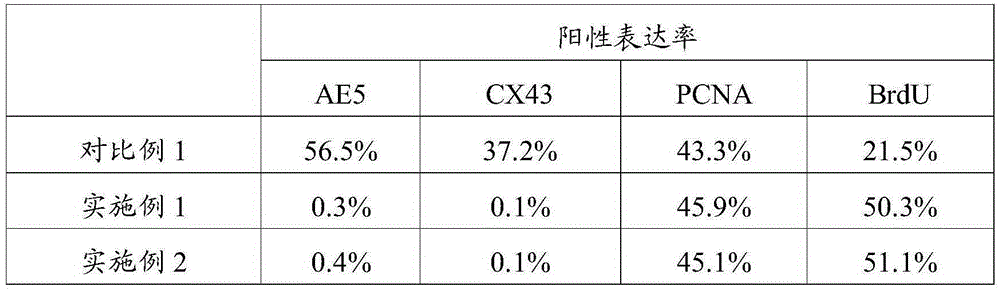
The invention relates to the field of stem cell culture and in particular relates to a separation method of limbal stem cells. The separation method has the beneficial effects that limbal tissues are subjected to enzymolysis under the combined action of a compound enzyme by combining neutral protease with collagenase IV, thus ensuring obtainment of the cells through enzymolysis in a short time, reducing damages to the cells, increasing the cell yield and improving the purity of the obtained cells; the method is simple and convenient to operate, dispenses with high-end instruments and equipment and causes smaller damages to the cells; experiments show that by adopting the method provided by the invention to separate the limbal stem cells, every 1cm<3> of limbal tissues can obtain 8.4*10<5>-9.0*10<5> limbal stem cells, the positive rate of an antibody CX43 in the cells is 0, the positive rate of AE5 is 0.1%, the positive rate of BrdU is 70.1%, and the positive rate of PCNA is 82.1%; the separation method has obviously better effects than the prior art.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Composite membrane based on gelatin and amino acid, and method for cultivating limbal stem cells on membrane
ActiveCN107118552ASolid shapeGood biocompatibilitySkeletal/connective tissue cellsCell culture active agentsPolyethylene glycolAmino acid
The invention discloses a composite membrane based on gelatin and amino acid, and a method for cultivating limbal stem cells (LSCs) on the membrane. A preparation method of the composite membrane comprises the steps of preparing methacrylic anhydride gelatin (Gelma) by utilizing the gelatin, methacrylic anhydride, Dulbecco phosphate buffered saline (DPBS) and distilled water under a controlled condition, preparing poly(ethylene glycol) diacrylate (PEGDA) by utilizing polyethylene glycol (PEG), benzene, triethylamine, acryloyl chloride and glacial ether under a controlled condition, obtaining an unsaturated polymer (U-PEA) by utilizing polymerization of amino acid, diol and diacid under a controlled condition or modification of methacrylic anhydride, and finally preparing the composite membrane by utilizing a reaction product by the action of a photoinitiator. The composite membrane can serve as a matrix layer to support growth of the LSCs on the membrane. The method is very simple and convenient to operate, simple in condition and good in repeatability; a prepared product is reliable in performance; and industrialization is very easy to realize.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Method for preparing amniotic compound corneal limbus stem cell membrane
ActiveCN102166374AAvoid breakingConvenient for clinical operationArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsEpitheliumFeeder Layer
The invention relates to a method for preparing an amniotic compound corneal limbus stem cell membrane, which comprises the following steps of: firstly, preparing a sterile amniotic membrane with epithelium removed, placing the epithelial surface of the amniotic membrane downwards on an end face of a sleeve made of a poly propylene material, and covering an embedded culture transwell on the amniotic membrane of the sleeve to obtain an amniotic-membrane-embedded culture mold; placing the culture mold in holes in a 6-hole plate on which a mouse embryonic fibroblast cell feeder layer is spread; and finally, preparing a corneal limbus stem cell suspension by using a digestion method, inoculating the suspension on the epithelial surface of the amniotic membrane, inoculating 2.0-3.0*10<5> corneal limbus stem cells for each culture mold, and culturing the corneal limbus stem cells for 10 days to obtain the amniotic compound corneal limbus stem cell membrane. Compared with the traditional amniotic membrane spreading method and the latex ring amniotic membrane fixing method, the amniotic compound corneal limbus stem cell membrane prepared with the method has flat amniotic membrane culturing surface and uniform corneal limbus stem cell stratified layer and especially has the advantage of convenience for clinic application and difficulty in rapture of the amniotic membrane.
Owner:SHANDONG EYE INST
Simple limbal stem cell separating and in-vitro culture kit and method
PendingCN104862271AHigh yieldAvoid damageArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsEmbryonic Stem Cell LineBiology
The invention relates to a simple limbal stem cell separating and in-vitro culturing kit and method. The simple limbal stem cell separating and in-vitro culturing kit comprises tissue washing liquid, cold digestive juice, hot digestive juice and culturing liquid. The simple limbal stem cell separating and in-vitro culturing method includes the following steps: limbus tissue extracting, digesting and in-vitro culturing. By means of the simple limbal stem cell separating and in-vitro culturing kit and method, limbal stem cell separating and in-vitro culturing are carried out, operation is simple, the stem cell yield is high, damage to obtained stem cells is small, the appreciation capacity is high, and the in-vitro culturing success rate is high, and the reproducibility is good.
Owner:SHANXI MEDICAL UNIV
Corneal limbal stem cell cryopreservation liquid and cryopreservation method
ActiveCN105850980ADead animal preservationArtificially induced pluripotent cellsCentrifugationCryopreservation
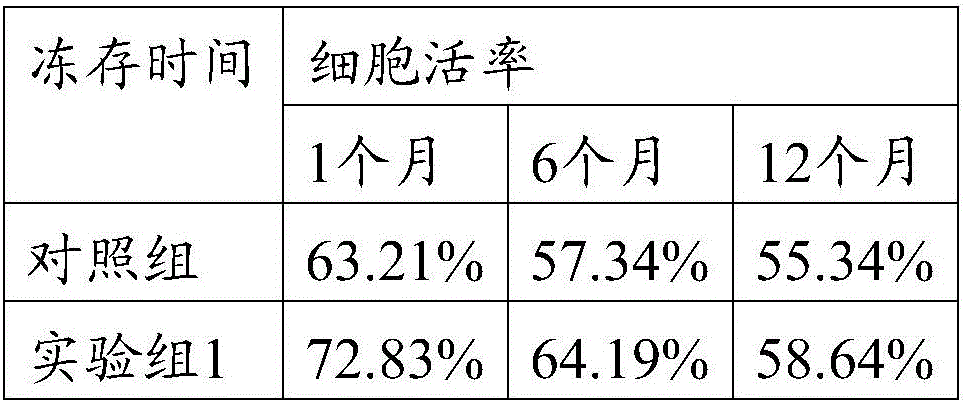
The invention relates to the field of stem cells and discloses corneal limbal stem cell cryopreservation liquid and cryopreservation method. The corneal limbal stem cell cryopreservation liquid comprises CHIR99021. Compared with conventional corneal limbal stem cell cryopreservation liquid, after cryopreserved with the corneal limbal stem cell cryopreservation liquid, stem cells recover, cell viability is obviously higher than that of cells cryopreserved with other types of conventional cryopreservation liquid, the cell viability capability is also superior to that of cells cryopreserved with conventional cryopreservation liquid, and the corneal limbal stem cell cryopreservation liquid can be used for long-time preservation and application of the corneal limbal stem cells. The corneal limbal stem cell cryopreservation method comprises the steps of conducting digestion and centrifugation on the corneal limbal stem cells, then removing supernatant, adding the corneal limbal stem cell cryopreservation liquid, and sub-packing the corneal limbal stem cells in cryopreservation tubes for cryopreservation. Compared with conventional cryopreservation methods, after the cells cryopreserved through the corneal limbal stem cell cryopreservation method recover, cell viability is obviously higher than that of cells cryopreserved through other types of conventional cryopreservation methods, and the cell viability capability is also superior to that of cells cryopreserved thorugh conventional cryopreservation methods.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Cultured mammalian limbal stem cells, methods for generating the same, and uses thereof
The invention provides an isolated limbal stem or progenitor cell (LSC) population or LSC-like population comprising a chemically synthesized, recombinant or isolated nucleic acid encoding PAX6 mtegrated into a chromosome, or alternatively, not integrated remaining as an extrachromosomal genetic material, wherein the isolated LSC population is substantially free of non-LSC cells or wherein the LSC-like population is substantially free of non- LSC-like cells, or wherein the isolated LSC or LSC-like population is substantially free of non-LSC and non-LSC-like cells and uses thereof.
Owner:RGT UNIV OF CALIFORNIA
Corneal limbal stem cell culture medium, and culture method
ActiveCN106916787AMaintain a poorly differentiated statePromote growthCell dissociation methodsCulture processCholera toxinBiology
The invention discloses a corneal limbal stem cell culture medium, and a culture method thereof. The corneal limbal stem cell culture medium comprises 5ml of 100Xdouble-antibody, 10 to 20ng / ml of human recombined EGF, 5 to 10<mu>g / ml of insulin, 1 to 5*10<9>M<3-> iodothyronine, 0.2 to 1<mu>g / ml of hydrocortisone, 10 to 20ng / ml of cholera toxin, 10 to 15% of fetal calf serum, and the balance DMEM and / or DMEM / F12. According to the culture method, I-type collagen is taken as a substrate substance of growth of corneal limbal stem cells to coat materials such as culture plates so as to increase the purity and stability of corneal limbal stem cells. The corneal limbal stem cell culture medium is used for separating corneal limbal stem cells, P63, Pax6 antibody positive rate ranges from 96 to 100%. The corneal limbal stem cell culture method is free of trophoderm and carrier, and a stable cell resource is provided for study on corneal limbal stem cell specificity mechanism and transplantation therapy.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Preservation method for corneal limbus tissue
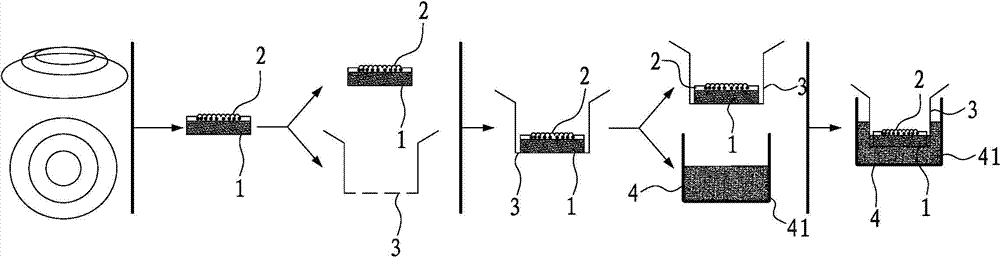
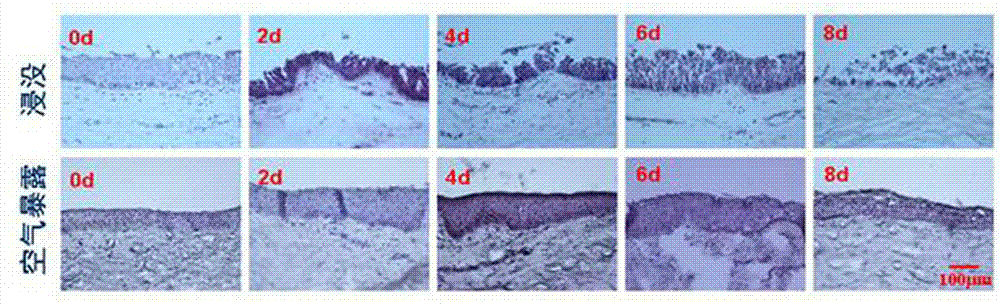
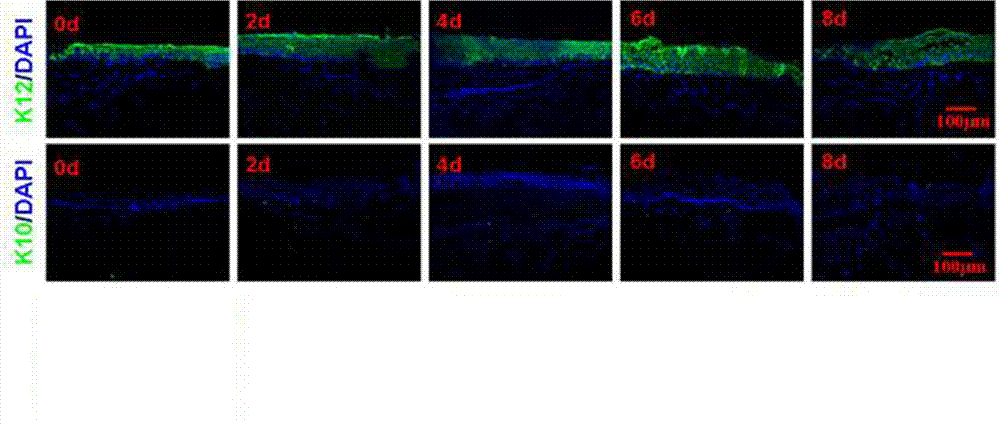
ActiveCN102726370AComplete structureAfter the structure, the present invention preserves the corneal limbal tissue intactDead animal preservationAir exposureCell culture media
The invention discloses a preservation method for corneal limbus tissue. The method comprises a step of disinfecting fresh corneal limbus tissue and a step of air exposure preservation of the disinfected fresh corneal limbus tissue. The step of air exposure preservation is as follows: the fresh corneal limbus tissue is placed on a permeable covering film, preservation liquid or a cell culture medium capable of providing nutrients to the corneal limbus tissue is arranged below the covering film, epitheliums of cornea directly contact with air, the temperature of a culture environment in the step is 0 to 37 DEG C, and oxygen concentration is 0 to 20%. Compared to immersion preservation in the prior art, the preservation method provided in the invention has the following advantages: the structure of the preserved corneal limbus tissue is more integral; obvious apoptosis of the epitheliums of cornea does not occur; stem cells of corneal limbus can maintain high activity; and process flow is simple and reliable, is easy to implement, costs little and has other efficacy.
Owner:XIAMEN EYE CENTER OF XIAMEN UNIVERSITY CO LTD
Novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency
InactiveCN105688282ACurvature maintenanceQuick fitEye implantsTissue regenerationDepyrogenationBiocompatibility
The invention relates to a novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency. The novel biological artificial cornea is prepared with a method in the steps as follows: 1, obtaining of a cornea raw material; 2, preparation of a cornea scaffold; 3, deep modification of the cornea scaffold; 4, deep inactivation of the cornea for removal of pyrogen; 5, irradiation sterilization of the cornea; 6, cultivation and implantation of corneal endothelial cells. Deep and light lamellar biological artificial corneas with different thicknesses and a full-thickness biological artificial cornea can be established. After being implanted, the novel biological artificial cornea can be quickly healed, becomes transparent quickly and keeps good refraction, the eyesight can be recovered and the eyeball is beautified. The obtained biological artificial cornea has good mechanical performance and biocompatibility, a full-thickness cornea graft is formed after endothelial cells are implanted on a full-thickness cornea stroma scaffold and can be rebuilt in vivo, perform in-vivo induction to promote growth of corneal limbal stem cells and growth of corneal epithelial cells and become transparent quickly on the basis, the treatment efficiency and effects are improved, and an application of lamellar keratoplasty and an application of penetrating keratoplasty are both considered.
Owner:广州宏畅生物科技有限公司
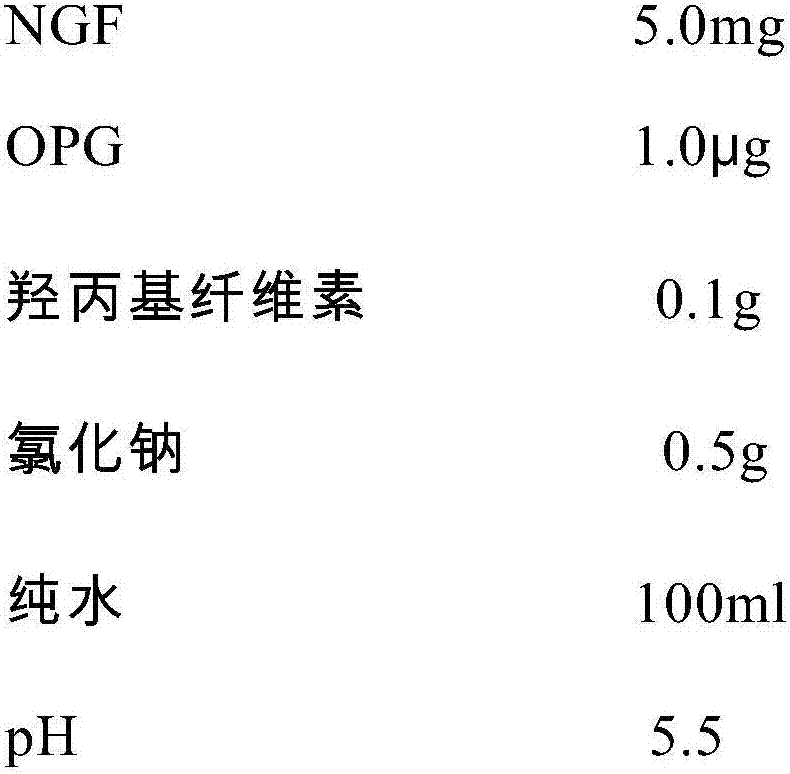
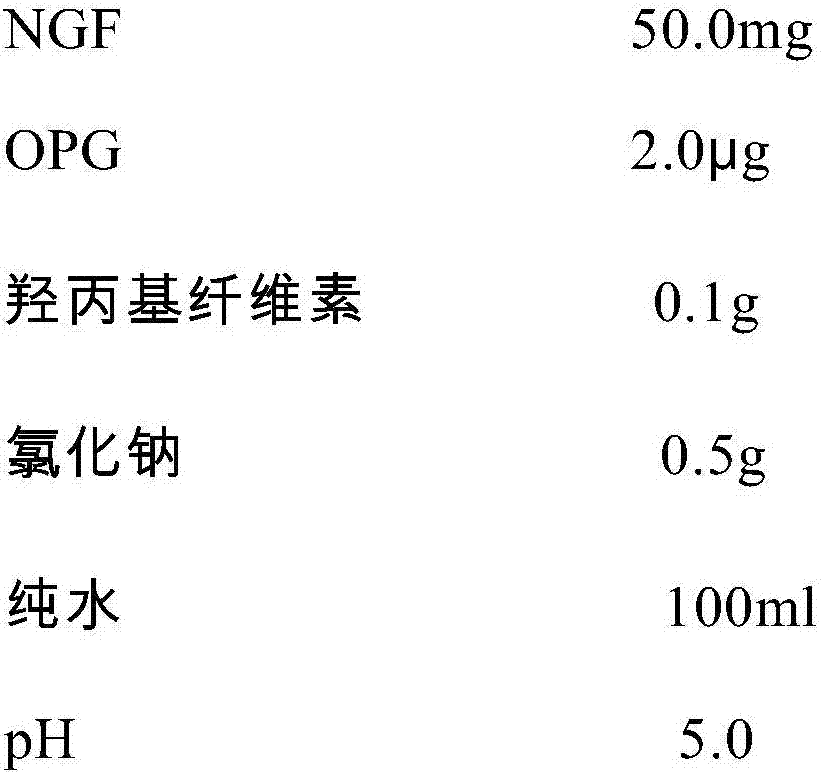
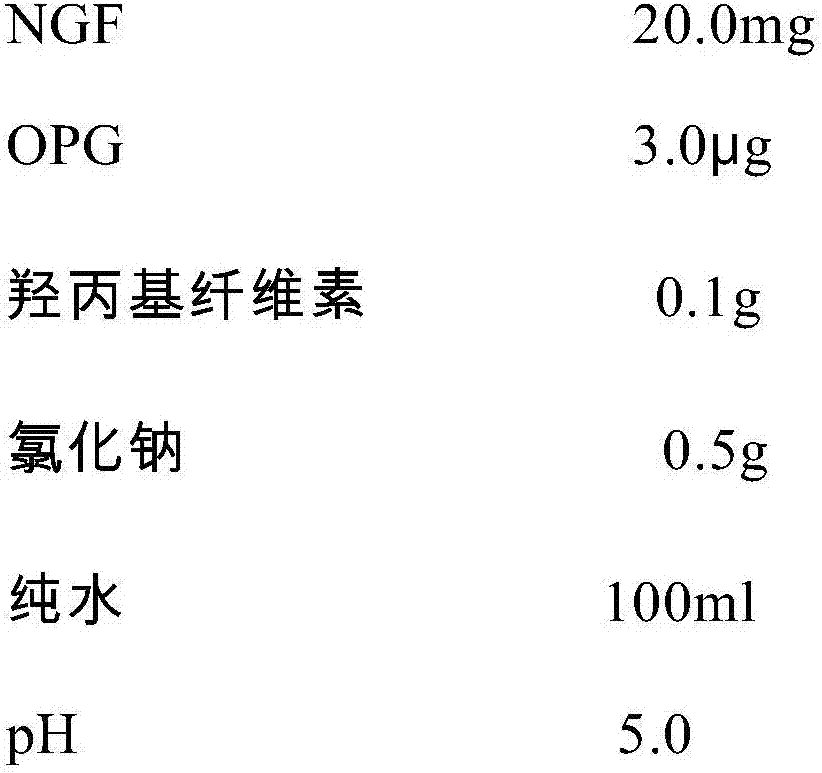
An NGF-containing pharmaceutical composition used for treating corneal epithelium injuries
ActiveCN106943590APromote proliferationPromotes clonogenicitySenses disorderPeptide/protein ingredientsDiseaseDiabetes mellitus
An NGF-containing pharmaceutical composition used for treating corneal epithelium injuries is disclosed. The composition includes an NGF (preferably a human-derived NGF). The composition (in a form of eye drops preferably) can promote proliferation and clonality capabilities of corneal limbal stem cells, and can effectively promote corneal epithelium injury healing including healing of continuous corneal epithelium injuries, diabetic keratopathy treatment, nervous keratitis treatment and treatment of corneal limbal stem cell deficiency.
Owner:PEKING UNIV THIRD HOSPITAL
Method for configurating feeder-layer-free corneal eipithelium for frost self-corneal limbus stem cell
Owner:西北农业科技大学
Limbal stem cell primary culturing method
ActiveCN109439628AKeep natural propertiesNo risk of diseaseCulture processNervous system cellsMitomycin CFrozen storage
Owner:OCEAN UNIV OF CHINA
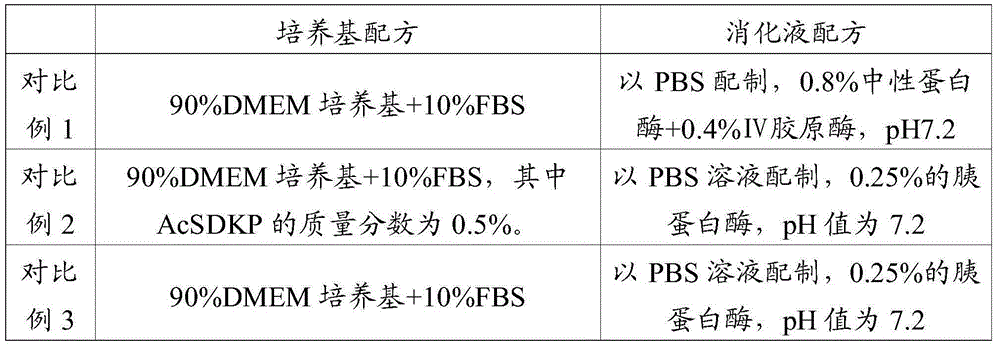
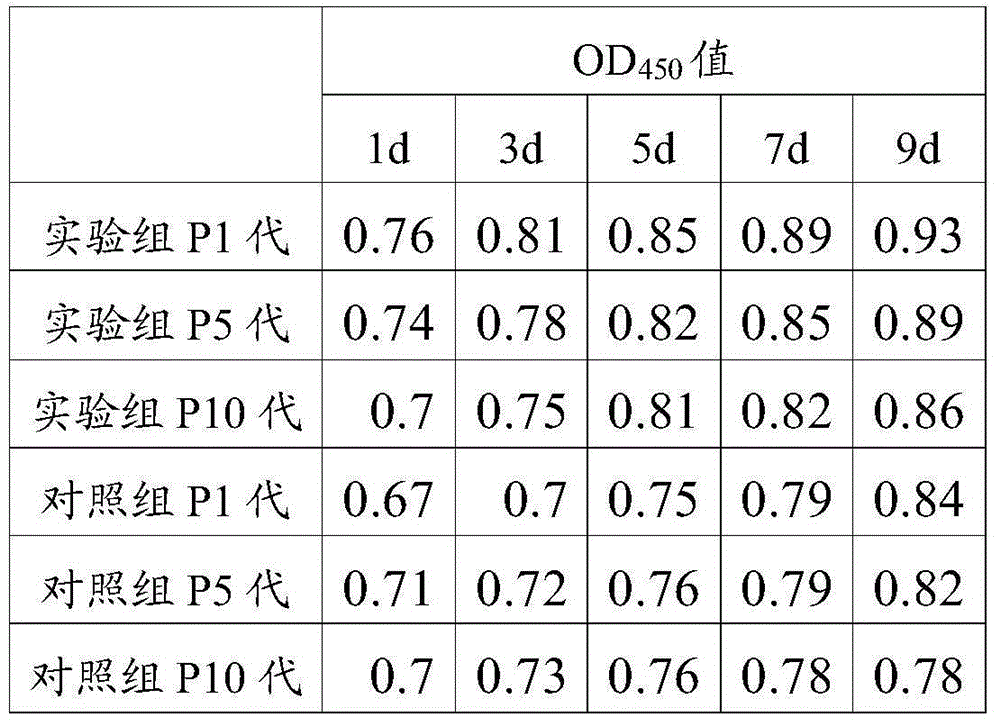
Application of AcSDKP in limbal stem cell separation culture and method for culturing limbal stem cell
InactiveCN105238743APromote growthGrowth inhibitionArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsFibroblastStem cell culture
The invention relates to the field of stem cell culture, and in particular relates to application of AcSDKP in limbal stem cell separation culture and a method for culturing limbal stem cells. According to the culture medium provided by the invention, fetal calf serum is adopted to promote growth of limbal stem cells, and AcSDKP is adopted to inhibit growth of fibroblast. Therefore, by adopting the culture medium provided by the invention, limbal stem cells can be selectively separated, the purity of the limbal stem cells obtained through separation culture is improved, the number of the limbal stem cells obtained through separation culture is increased, and as growth of fibroblast is inhibited, the limbal stem cells are slightly affected by fibroblast secreta, and are in good dryness state. The experiment shows that when limbal stem cells are separated by using the culture medium provided by the invention, the positive rate of an antibody CX43 in cells is 0.1%, the positive rate of AE5 is 0.3%, the positive rate of BrdU is 50.3%, and the positive rate of PCNA is 45.9%. The effect is remarkably superior to that of the prior art.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
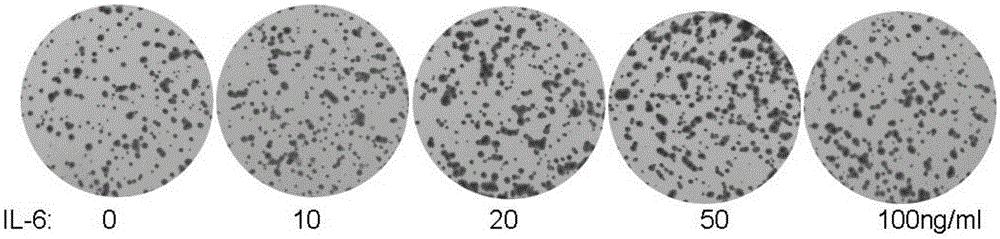
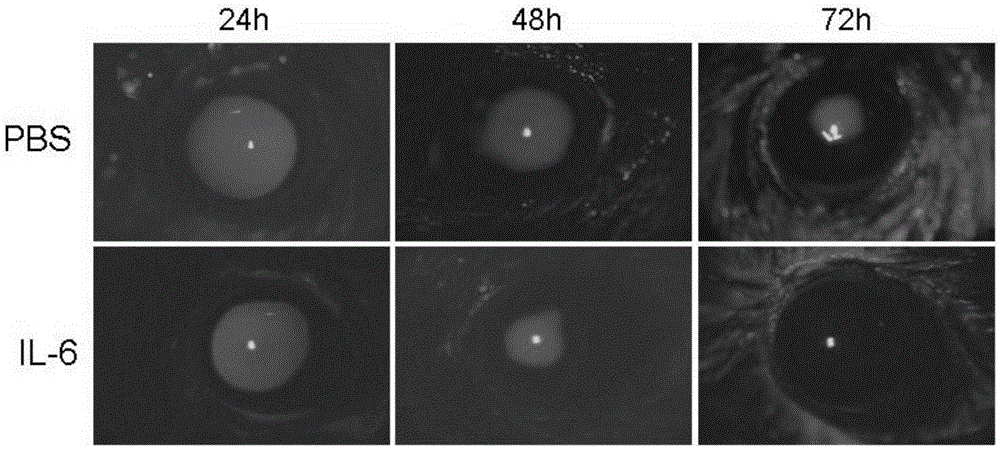
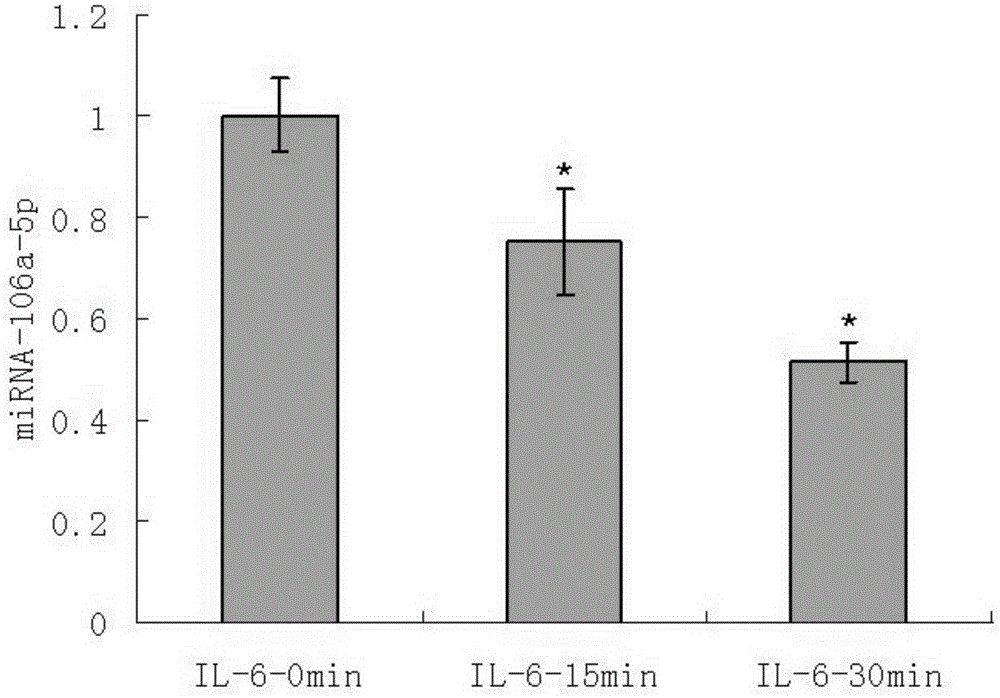
Application of IL-6 to repairing of corneal epithelial injuries
InactiveCN105396125APromote proliferationPromotes clonogenicitySenses disorderPeptide/protein ingredientsDiseaseInterleukin 6
The invention aims at providing an application of interleukin-6 to repairing of corneal epithelial injuries, namely, the application of the interleukin-6 to cultivating of ocular-surface corneal epithelial stem cells, treating of persistent corneal epithelial defects, treating of diabetic keratopathy, treating of neuropathic keratitis and treating of limbal-stem-cell deficiency diseases. By means of the application of the IL-6 to repairing of corneal epithelial injuries, the multiplication capacity and the clone formation capacity of corneal limbal stem cells can be promoted, repairing of injuries to corneal epithelium of normal mice can be further promoted, and the corneal limbal stem cells have the higher multiplication capacity and the higher clone formation capacity. The IL-6 can be used for cultivating ocular-surface corneal epithelial stem cells, treating persistent corneal epithelial defects, treating diabetic keratopathy, treating neuropathic keratitis and treating limbal-stem-cell deficiency diseases.
Owner:SHANDONG EYE INST
Cultured mammalian limbal stem cells, methods for generating the same, and uses thereof
InactiveUS20170233698A1Stimulate proliferationPromote regenerationSenses disorderEpidermal cells/skin cellsGenetic MaterialsProgenitor cell
The invention provides an isolated limbal stem or progenitor cell (LSC) population or LSC-like population comprising a chemically synthesized, recombinant or isolated nucleic acid encoding PAX6 integrated into a chromosome, or alternatively, not integrated remaining as an extrachromosomal genetic material, wherein the isolated LSC population is substantially free of non-LSC cells or wherein the LSC-like population is substantially free of non-LSC-like cells, or wherein the isolated LSC or LSC-like population is substantially free of non-LSC and non-LSC-like cells and uses thereof.
Owner:YOUHEALTH BIOTECH LTD
Limbus corneae stem cell serum-free medium and culture method thereof
ActiveCN109749997APromote growthHigh purityCulture processNervous system cellsLipid formationSodium metasilicate
The invention discloses a limbus corneae stem cell serum-free medium and a culture method thereof. The serum-free medium comprises a basic culture medium and additives, wherein the additives include human recombinant EGF, insulin, 3,3',5-triiodo-L-thyronine, hydrocortisone, forskolin, manganese sulfate monohydrate, sodium selenite, sodium metasilicate, ammonium metavanadate, nickel chloride hexahydrate, stannous chloride dihydrate, ethanol amine, phosphorylethanolamine, ammonium molybdate tetrahydrate, 4-hydroxyethylpiperazine ethane sulfonic acid, vitamin C, bovine serum albumin, lipid concentrate and serum substitution. The serum-free medium contains no fetal calf serum or any animal source ingredient, can supply sufficient nutrients and good environment needed by cell growth proliferation and can effectively replace the effect of serum, cells grow well, the cell purity and stability are improved, rapid and stable cell sources are provided for limbus corneae stem cell specificity mechanism research and transplantation therapy, and the clinic application prospect is wide.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
In vitro culture method of limbal stem cell stability
InactiveCN109321527ASufficient stability is maintainedImprove securityCulture processNervous system cellsFiltrationBiology
The invention relates to an in vitro culture method of limbal stem cell stability. The method is characterized in that after treated by a gentamicin solution, the limbal tissue is cut into small blocks, and then placed in a tissue digestion solution for digestion and filtration, and tissue cell precipitate is collected, and then the precipitate is resuspended by a primary culture solution of limbal stem cells and is inoculated to a cell culture plate pretreated with a limbal stem cell coating solution for primary culture, after a cell cloned sphere appears, the cell cloned sphere is digested with the tissue digestion solution, then centrifugation is performed, the cell precipitate is collected, and the cells are resuspended by a subculture solution of the limbal stem cells, then are inoculated to the pretreated cell culture plate for subculture to obtain stable limbal stem cells. In order to obtain more stable limbal stem cells, multi-generation expansion culture can be carried out after the subculture, and a large number of stem cells with good stability and high safety can be obtained so as to meet the requirement of clinical application.
Owner:OCEAN UNIV OF CHINA
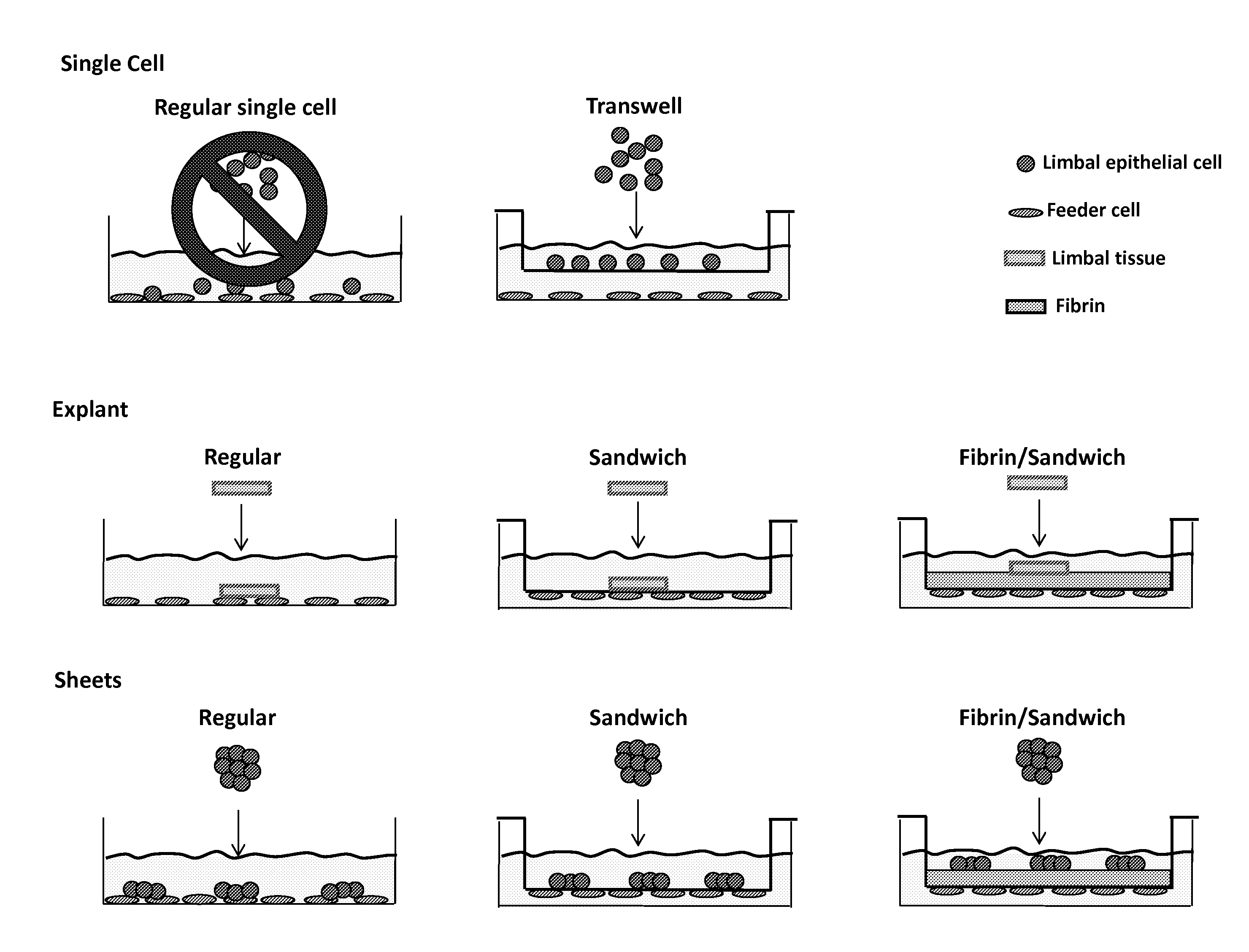
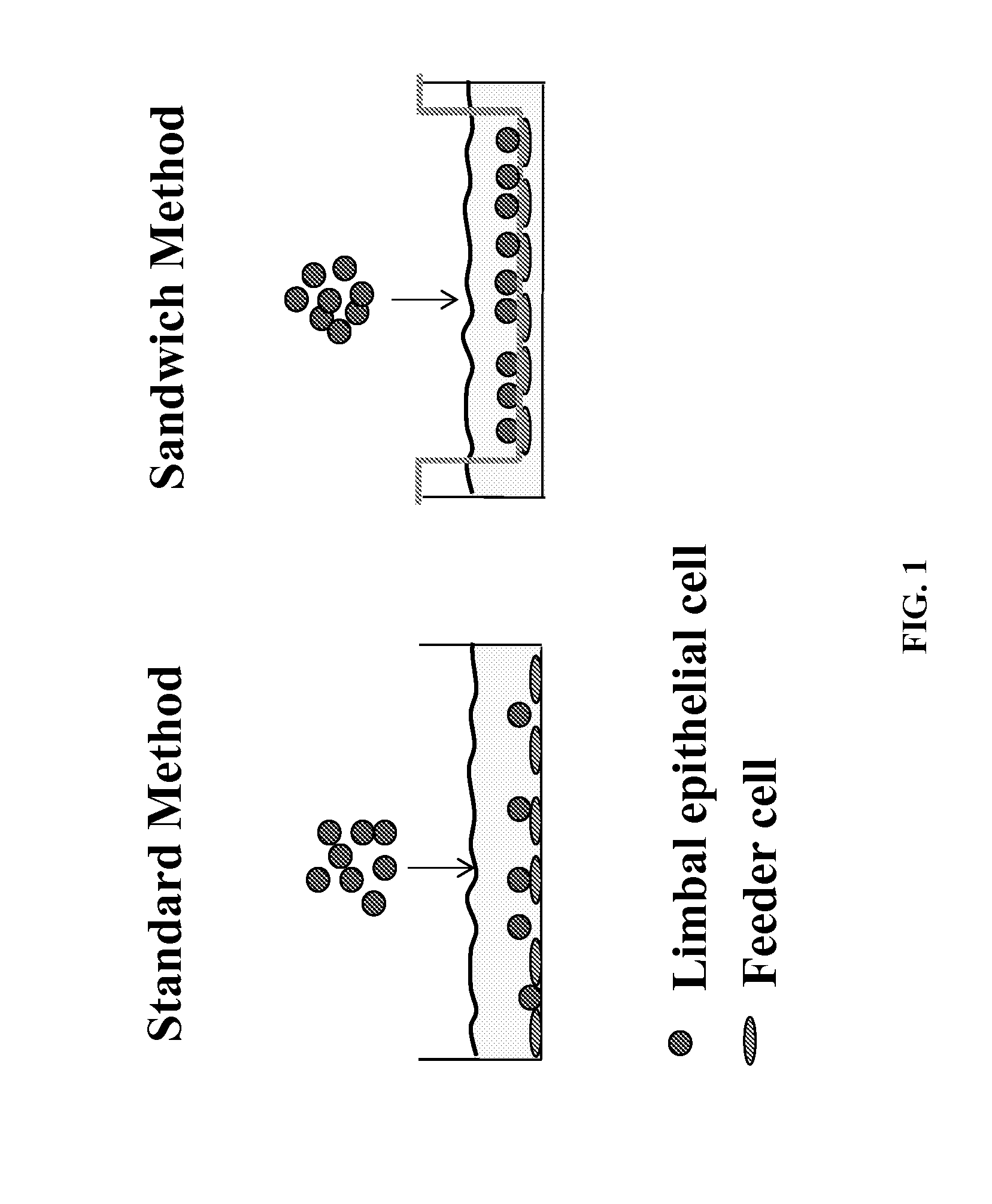
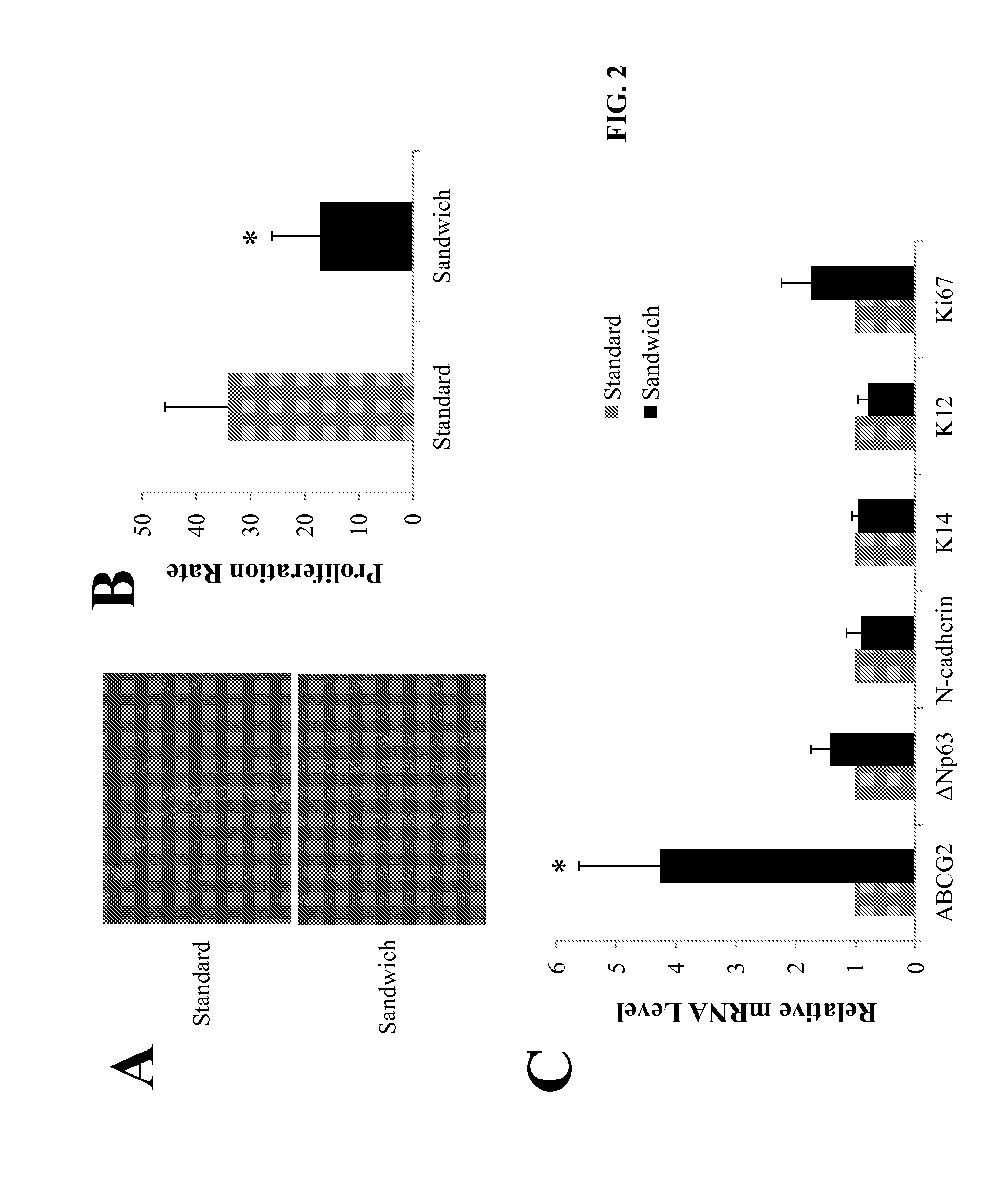
Novel methods to regenerate human limbal stem cells
InactiveUS20150175965A1Facilitated DiffusionBioreactor/fermenter combinationsBiological substance pretreatmentsProgenitorPorous membrane
The invention disclosed herein provides systems and methods designed to facilitate human limbal stem / progenitor cell culture including a novel 3-dimensional (3D) sandwich method / system in which human limbal stem / progenitor cells and feeder cells are separately cultured on opposite sides of a porous membrane.
Owner:RGT UNIV OF CALIFORNIA
Method for culturing human limbal stem cell graft
InactiveCN102586175AConvenient for clinical operationWide range of clinical applicationsArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsFreeze-dryingLimbal epithelium
The invention belongs to the field of tissue engineering and relates to a method for culturing a human limbus stem cell graft by taking a biological amnion as a carrier. According to the method disclosed by the invention, the biological amnion is used as the carrier for culturing a limbus stem cell, wherein the biological amnion is a freeze-dried human amnion which is irradiated and sterilized by colbalt-60; the safety of the biological amnion is higher than that of denuded amniotic membrane; and the biological amnion is easy to preserve and convenient to acquire and is widely used clinically. The method disclosed by the invention has the advantages that an acceptor can be prevented from being infected for a long term; the preparation process is simple; the pollution in the material obtaining and treating process can be reduced; limbus epithelial cells can be cultured more safely and conveniently; and the clinical application of the human limbus stem cell graft is promoted.
Owner:上海交通大学附属第一人民医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com