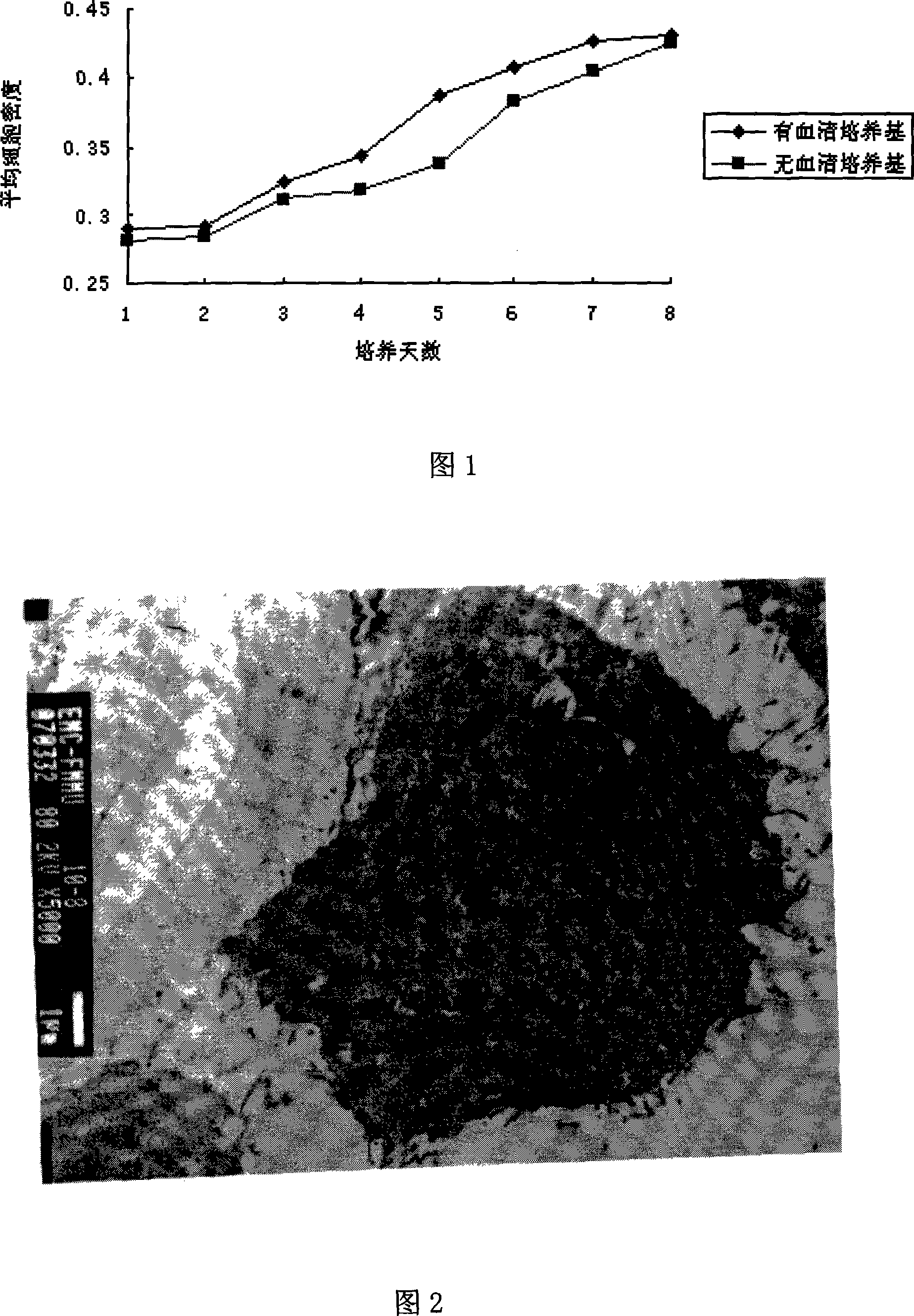
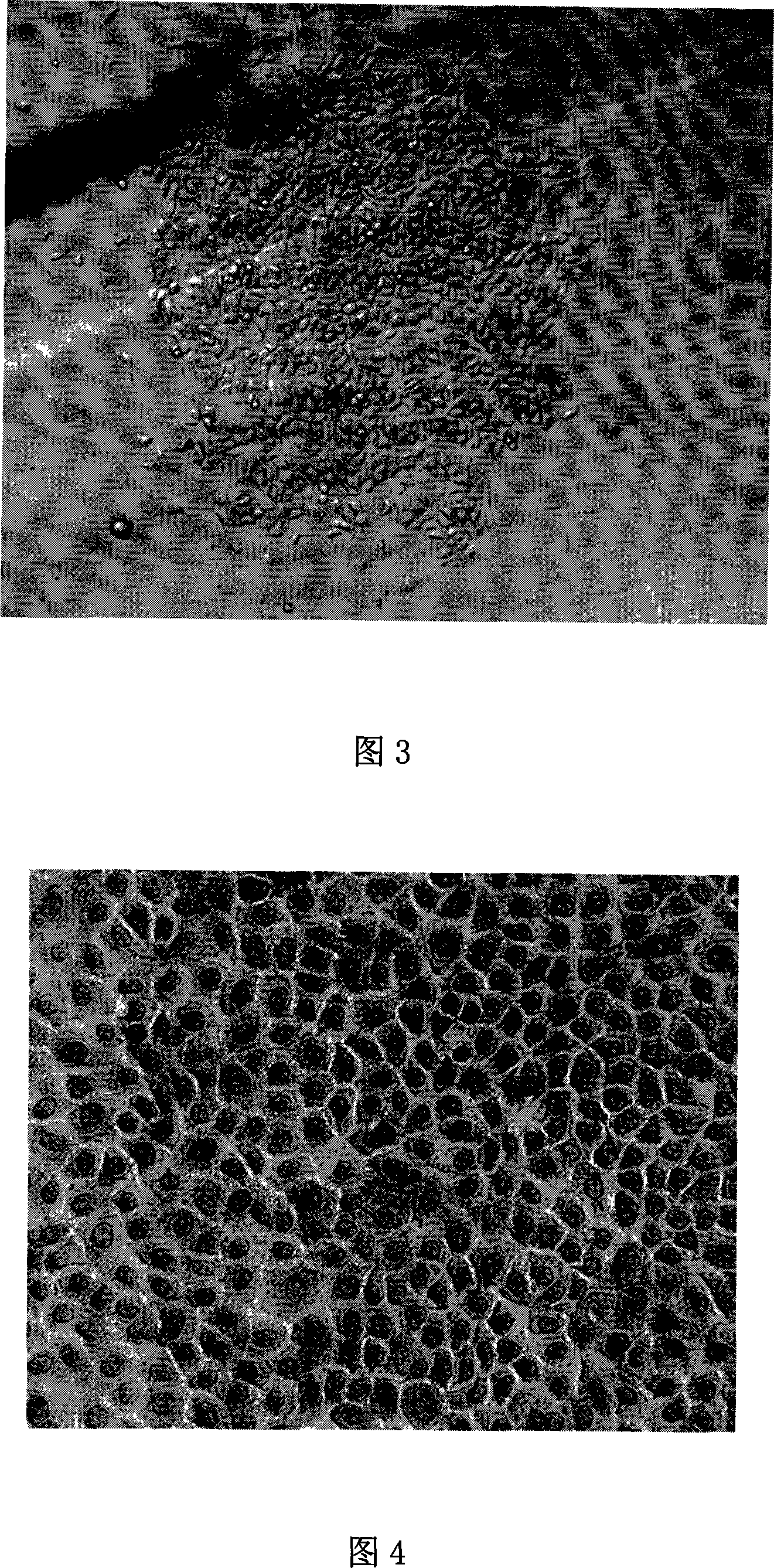
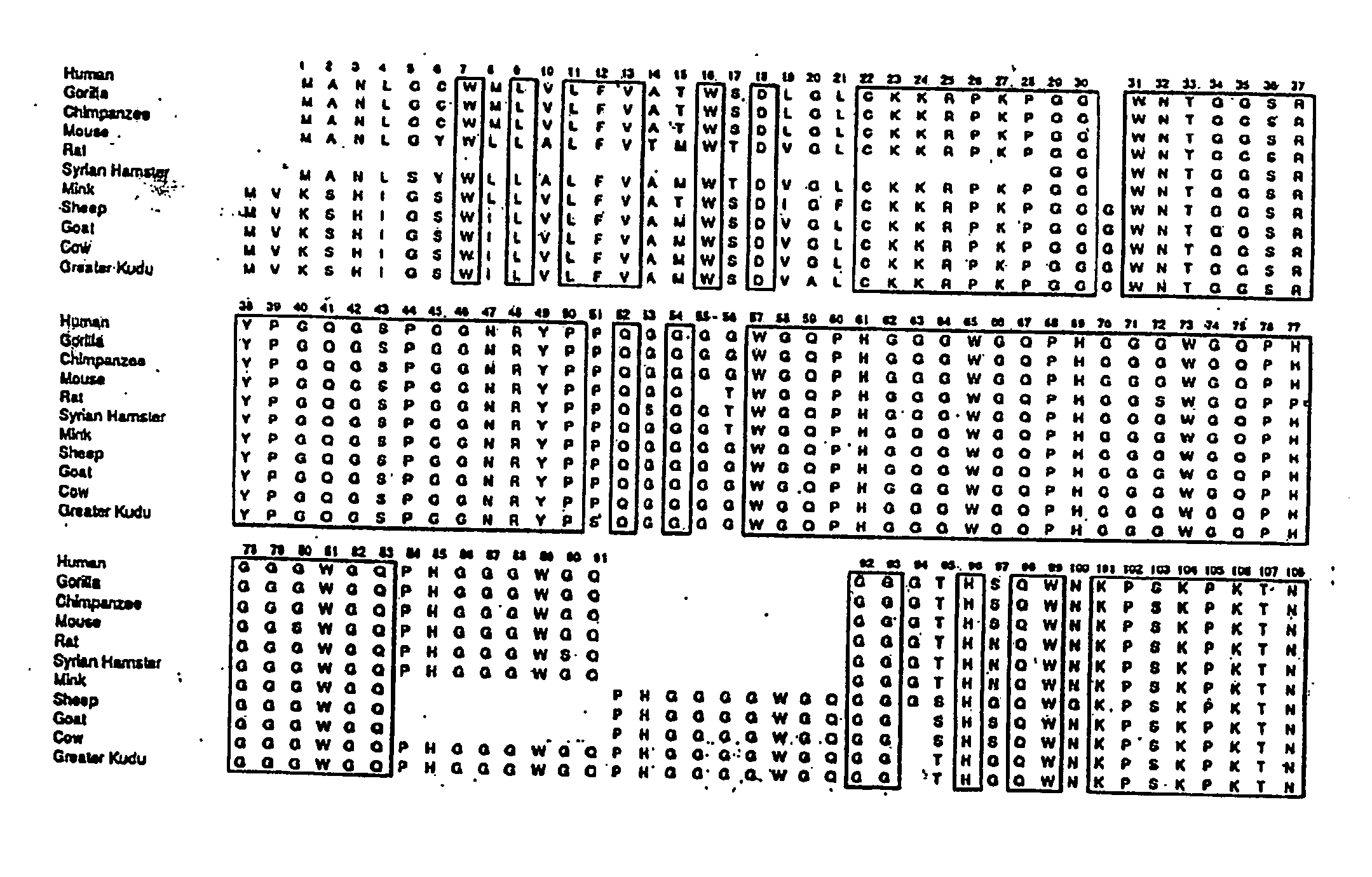
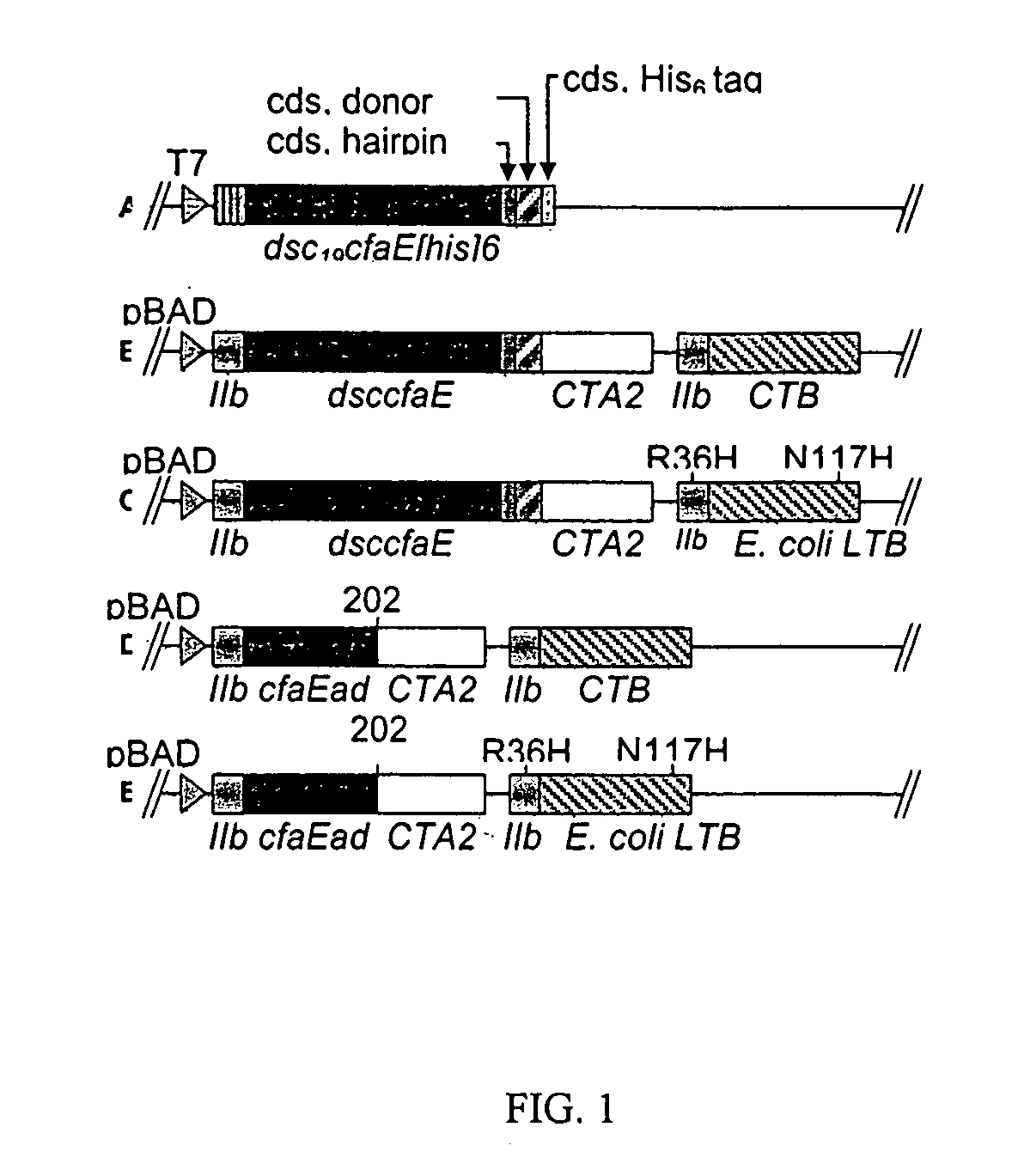
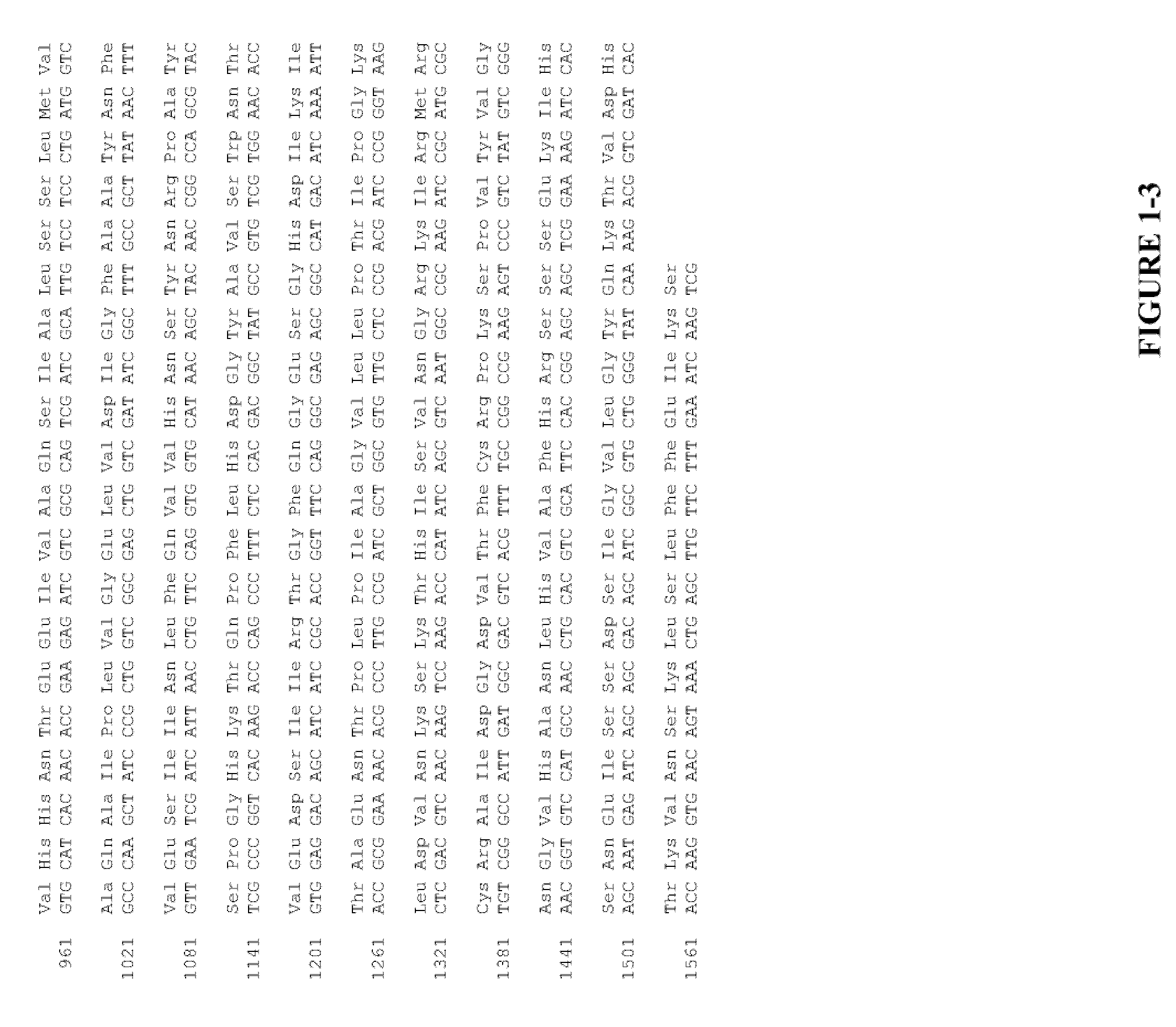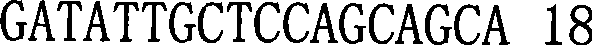Patents
Literature
126 results about "Cholera toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cholera toxin (also known as choleragen and sometimes abbreviated to CTX, Ctx or CT) is AB5 multimeric protein complex secreted by the bacterium Vibrio cholerae. CTX is responsible for the massive, watery diarrhea characteristic of cholera infection. It is a member of the Heat-labile enterotoxin family.
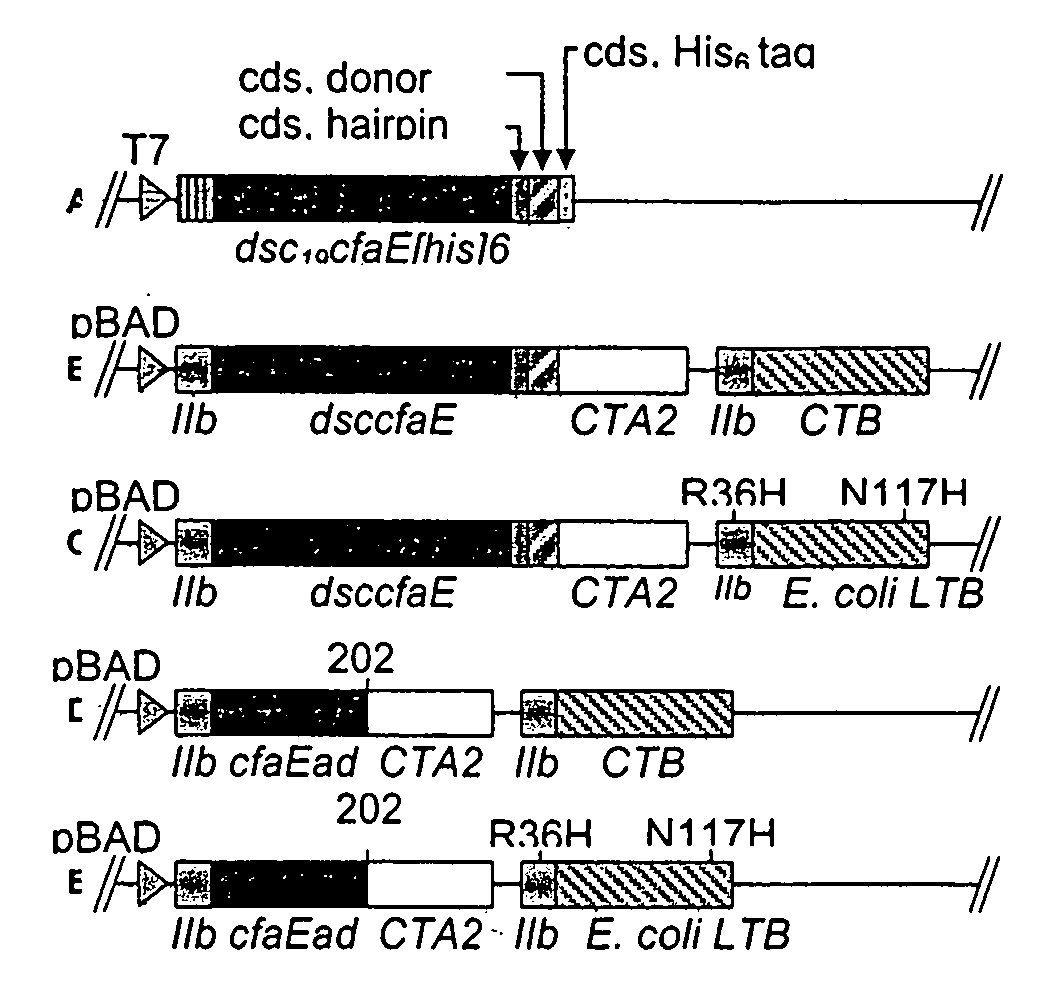
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7285281B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAdjuvantCancer cell
Owner:UNIV OF COLORADO FOUND +1
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
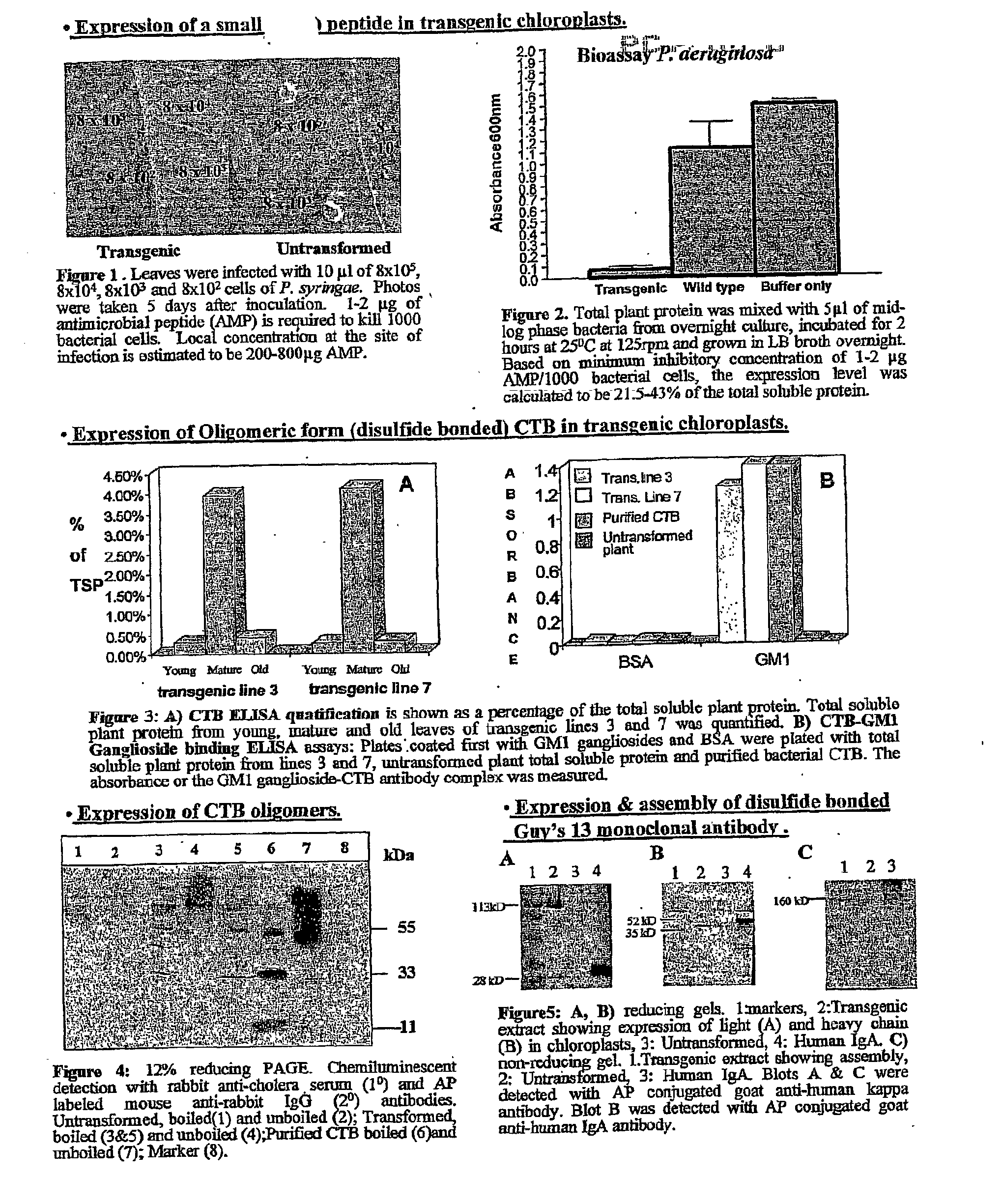
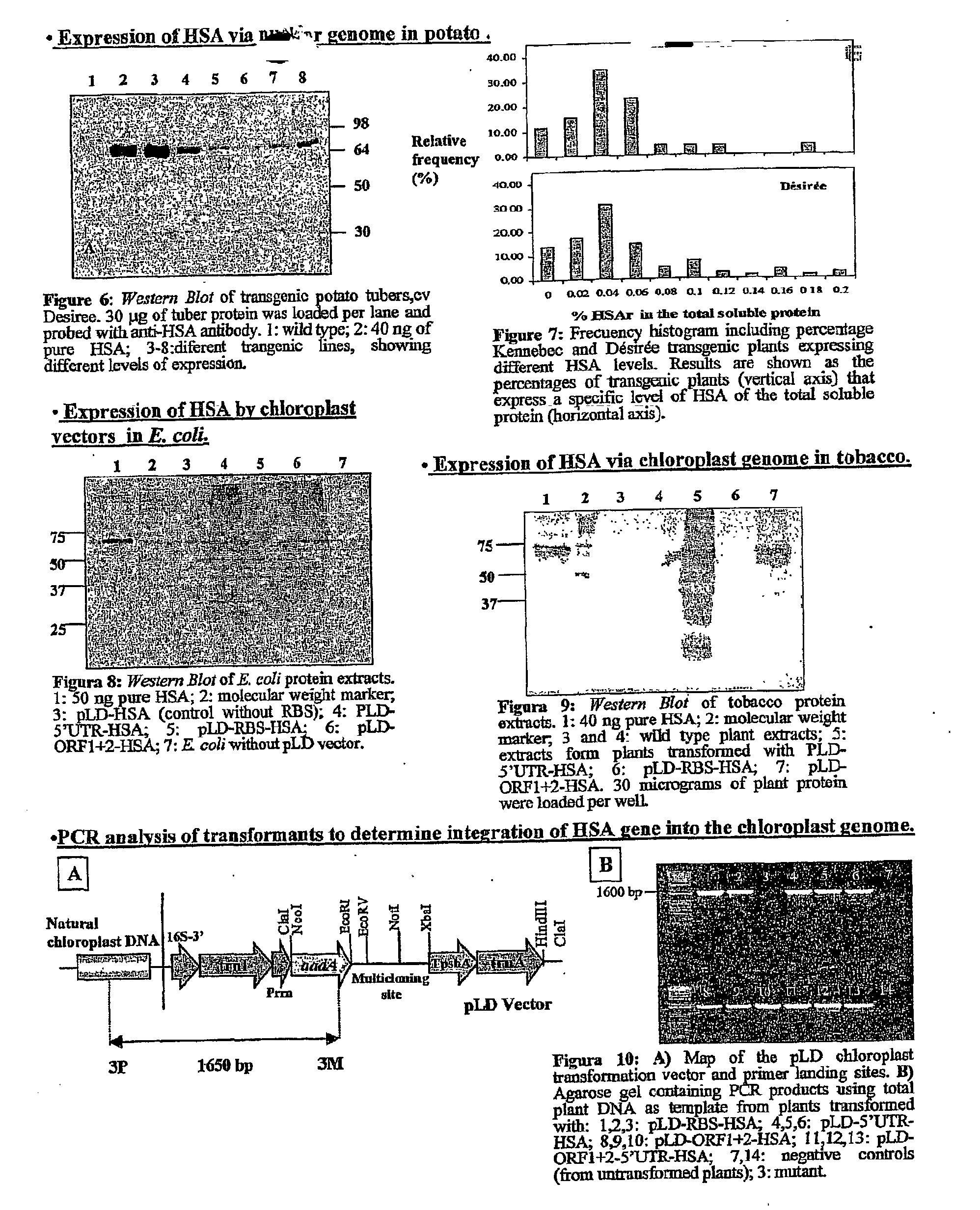
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Primary tumor cell culture medium, culture method and application
ActiveCN108624561ASolve the problem of primary culture immortalizationEnable Personalized TreatmentMicrobiological testing/measurementSkeletal/connective tissue cellsAbnormal tissue growthCell culture media
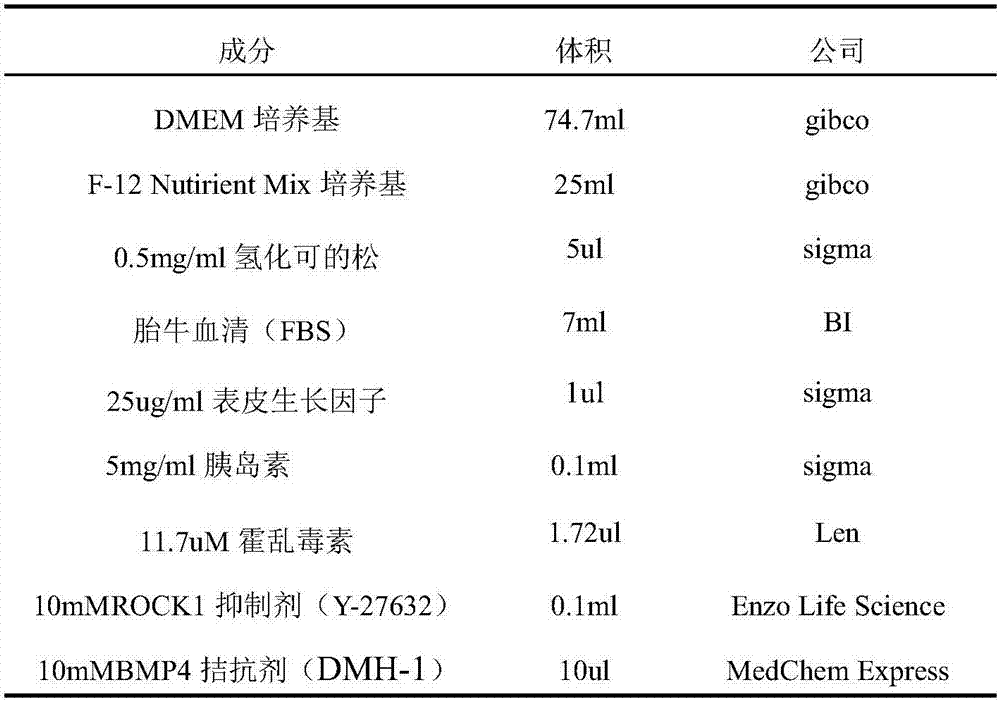
The invention belongs to the technical field of medicine, and specifically relates to a primary tumor cell culture medium, culture method and application. The primary tumor cell culture method provided by the invention comprises the steps as follows: preparing a primary cell culture medium which comprises the following components: hydrocortisone, EGF, Insulin, a ROCK inhibitor, but not contains cholera toxin, and is an improvement of the existing primary cell culture medium; culturing tumor tissue epithelial cells on laid-out trophoblast cells by using the primary cell culture medium, and enabling the tumor tissue epithelial cells to proliferate rapidly under the combined action of growth factors secreted by the trophoblast cells and nutrient factors contained in the culture medium; and digesting and sub-culturing the tumor tissue epithelial cells when the tumor tissue epithelial cells grow to a cell density of about 80% to 90%. A convenient primary cell culture method is used to obtain immortalized cells possessing the biological characteristics of a patient's own tumor, and the problem of immortalization of the primary culture of tumor cells is solved, thereby realizing personalized treatment for the patient.
Owner:FUDAN UNIV
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
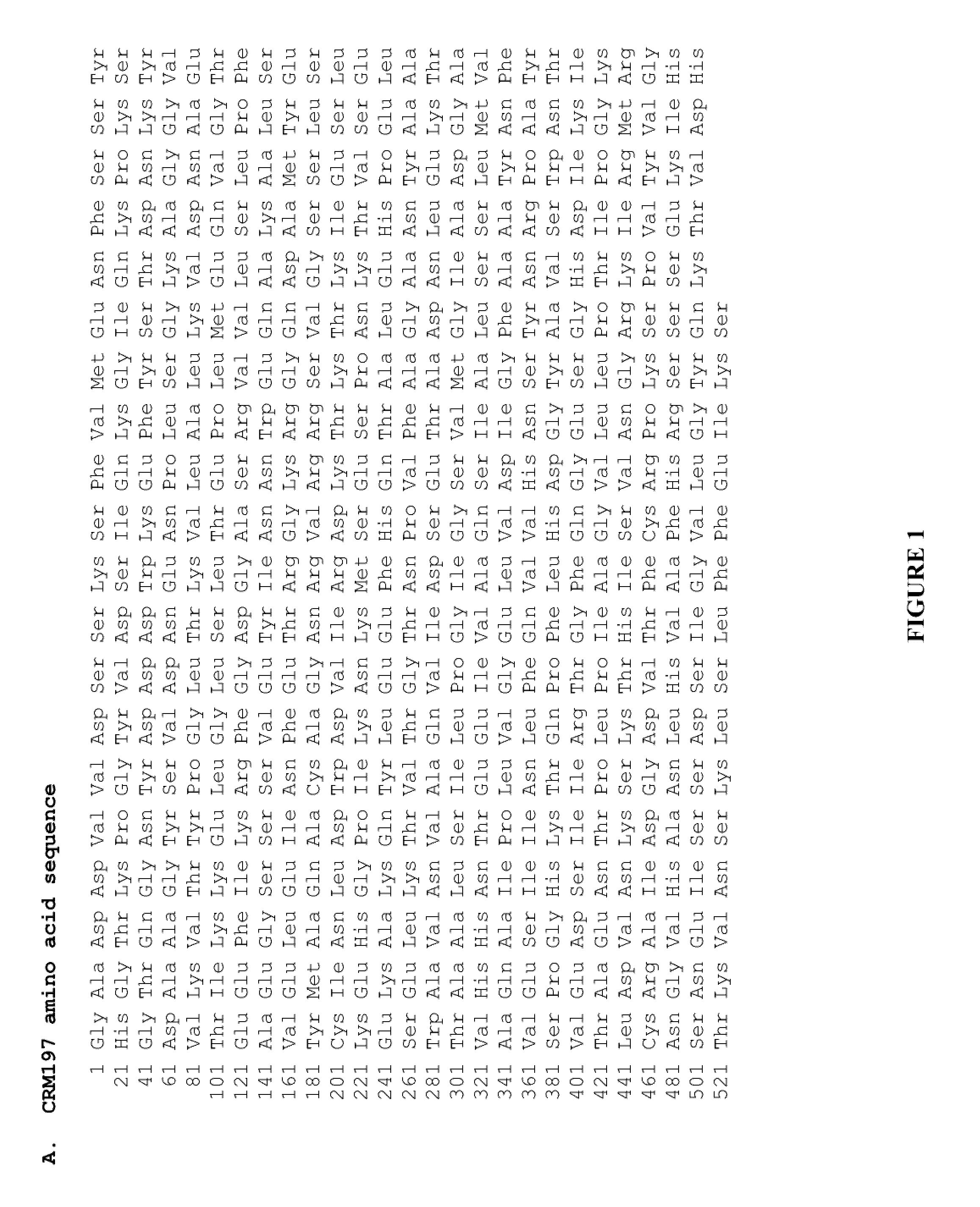
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
Non-viable keratinocyte cell composition or lysate for promoting wound healing
Cultures of keratinocyte cells are provided which are free from nonautologous fibroblasts and organ extracts, and which have a high speed of cell amplification for a minimum seeding density. The cultures can be cryopreserved in a buffered isotonic medium containing serum and a cryoprotectant. The cultures are produced by a process that does not involve the use of a feeder layer and organ extracts. A culture medium which can be used contains Medium 199, serum, epidermal growth factor, cholera toxin and / or hydrocortisone, and optionally insulin. A substance for wound healing and for cosmetic applications is derived from cultured human keratinocytes. A non-viable total keratinocyte lysate for use in promoting wound healing is produced by growing keratinocyte cells on a support, detaching the cells from the support, and lysing the detached cells to obtain the lysate which may be frozen and lyophilized. The cells may be grown without using a support to produce the lysate, or to produce a non-viable keratinocyte cell culture lyophilisate or spray dried non-viable keratinocyte cell composition for use in healing wounds.
Owner:CELLTRAN LTD
Culture medium for normal epithelial cell of human or mammal, culture methods, normal epithelial cell and application of normal epithelial cell
InactiveCN104694460ANormal differentiation physiological functionMicrobiological testing/measurementArtificial cell constructsMatrigelMammal
The invention relates to a culture medium for a normal epithelial cell of a human or mammal, primary separation culture, subculture, 3D gas-liquid culture and 3D matrigel culture methods, the normal epithelial cell generated by using the culture medium and the culture methods and application of the normal epithelial cell to a toxicological evaluation system. The culture medium is prepared by mixing DMEM and Ham's F-12NUTRIENT MIX according to the volume ratio of 3:1 and also adding 4-6% of fetal calf serum, 1-3nM triiodothyronine, 0.4-0.65% of insulin-transferrin-selenium reagent, 4-6mu g / ml transferrin, 9-11ng / mL epidermal growth factors, 0.3-0.5mu g / mL hydrocortisone, 0.5-1.5nM cholera toxin, 0.4-0.6mu g / mL amphoterrible B, 35-45mu g / mL gentamicin, 45-55nM calpeptin, 35-45ng / ml recombinant human IL-1RA and 3mu g / ml recombinant human R-Spondin-1. The culture medium disclosed by the invention can be used for carrying out separation culture or subculture on the normal epithelial cell of the human or the mammal and any other various tissue source, rapidly proliferating the normal epithelial cell in vitro and establishing a cell line; and the normal epithelial cell is a normal diploid cell and is applied to the toxicological evaluation system of the human or the mammal.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
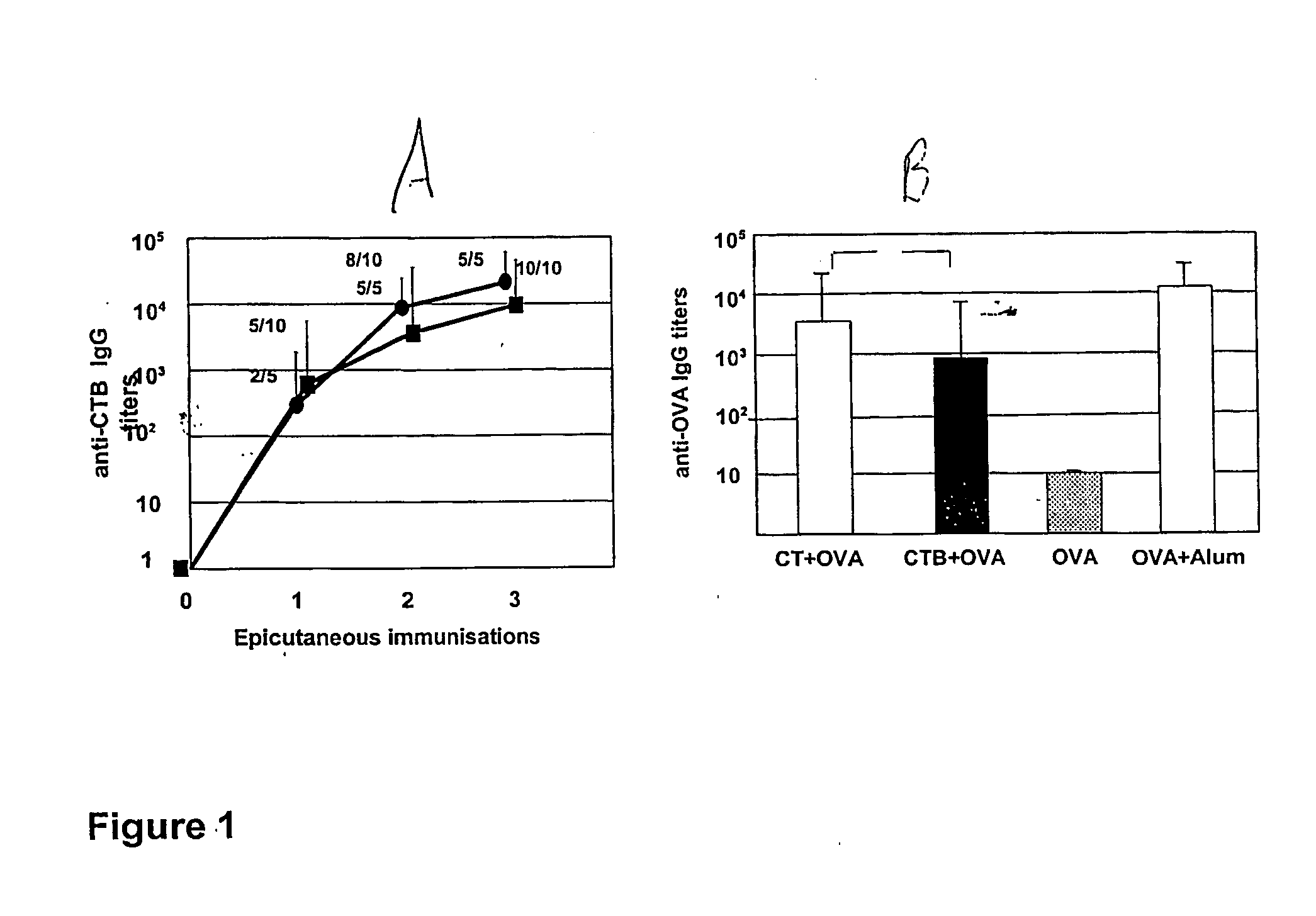
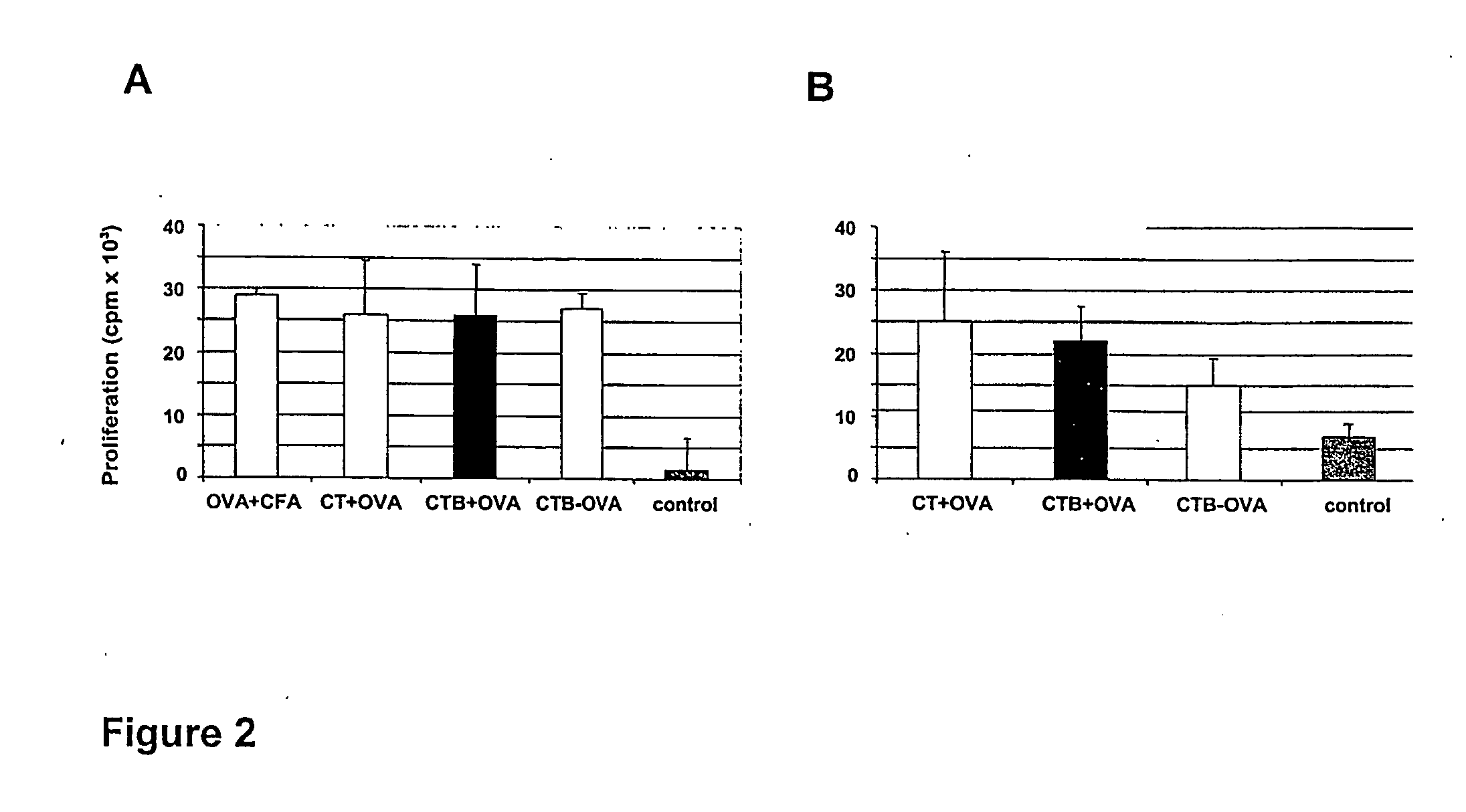
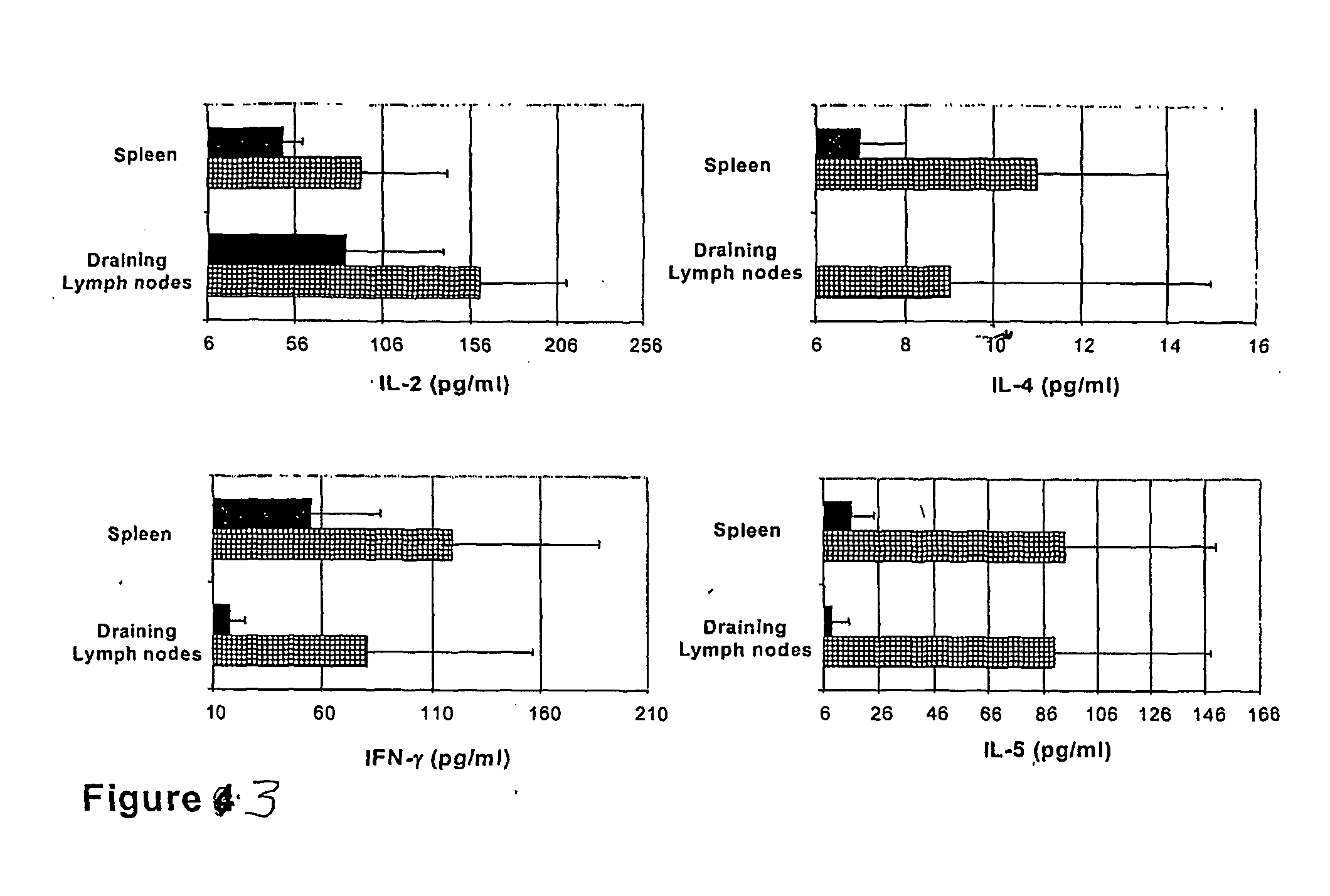
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
New composition and methods for treatment of autoimmune and allergic diseases
ActiveUS20130022634A1High expressionIncrease productionSenses disorderNervous disorderDiseaseArginine
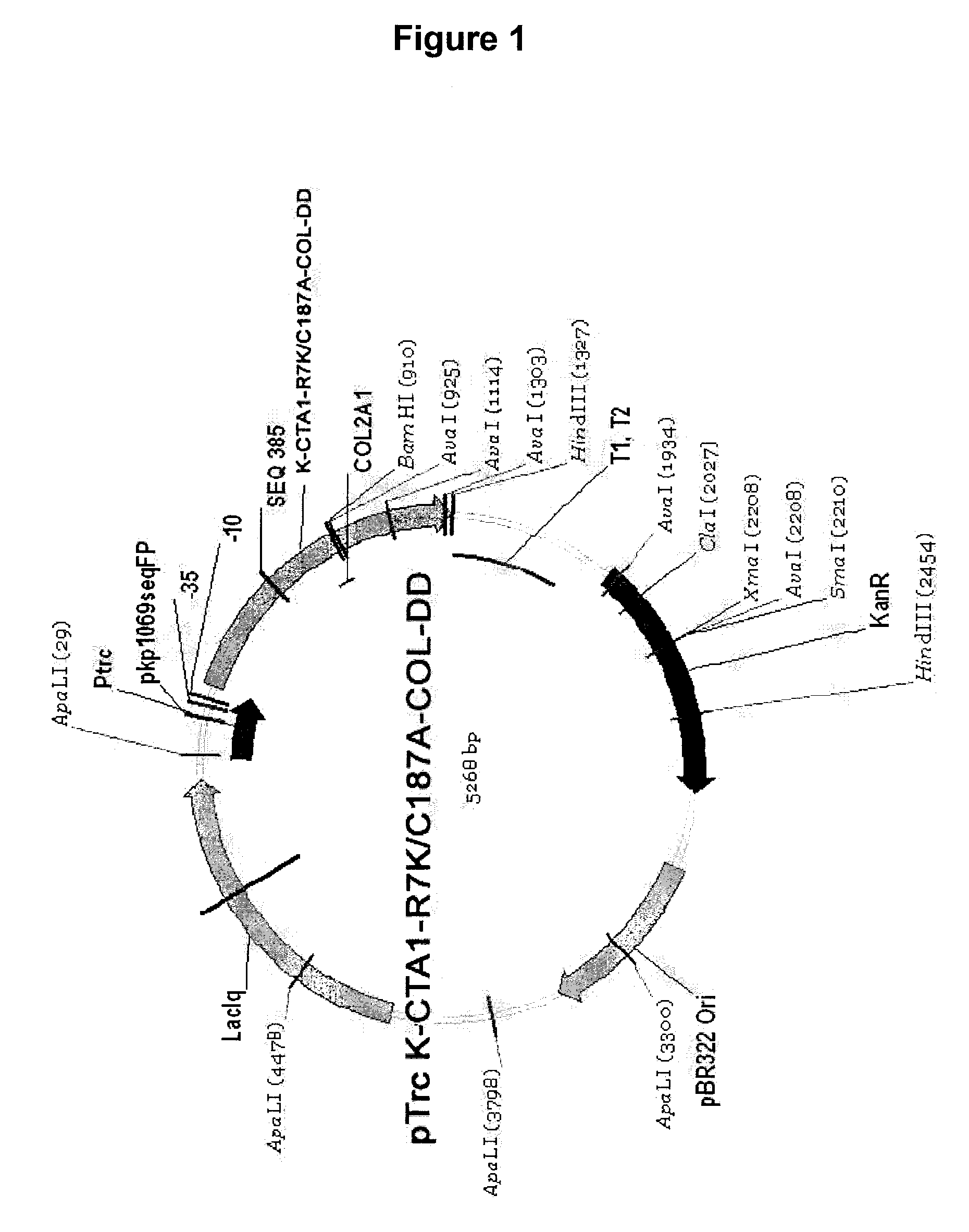
The present invention provides improved methods and compositions for treating and preventing autoimmune and allergic diseases. More specifically, the invention relates to new immunomodulating complexes that are fusion proteins comprising a mutant subunit of the A1-subunit of the cholera toxin (CTA1), a peptide capable of binding to a specific cellular receptor, and one or more epitopes associated with an autoimmune or allergic disease. In the mutant CTA1 subunit, the amino acids corresponding to the amino acid 7, arginine, and amino acid 187, cysteine, in the native CTA1 have been replaced.
Owner:TOLERANZIA
Cholera toxin virulence gene detection kit and detection method thereof
InactiveCN102094090AHigh sensitivityReduce testing costsMicrobiological testing/measurementPositive controlVibrio cholerae
The invention relates to a cholera toxin virulence gene detection kit and a detection method thereof. The kit of the invention contains three pairs of primers which are designed by using vibrio cholera ctxA gene as target gene on the basis of the loop-mediated isothermal amplification technology, namely inner primers FIP / BIP, outer primers F3 / B3 and ring primers LF / LB and also contains Bst DNA polymerases, reaction solution, sample pretreatment solution, coloring liquid, stabilizing solution and positive control. The method for detecting the cholera toxin virulence gene comprises the following steps: extracting bacterial DNA, performing the loop-mediated isothermal amplification of the cholera toxin virulence gene and coloring for detection. The kit of the invention has high amplificationefficiency, specificity and sensitivity, low omission ratio and obvious coloring effect and is suitable for the rapid detection of toxigenic vibrio cholera.
Owner:EAST CHINA NORMAL UNIV +1
Limbus corneae stem cell serum-free culture medium
InactiveCN101121926AMeet the nutritional needs of long-term expansion cultureArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsBiotechnologyPenicillin
The invention discloses a limbal stem cell serum-free medium, consisting of the following raw materials (calculated by weight percent): DMEM / F12 60ml-90ml, BSA 0.5g-4g, EGF 1Mug-4Mug, insulin 0.1mg-1mg, hydrogen 20ug-100ug, cholera toxin 1ug-10ug, transferrin 0.1mg-1mg, selenious acid 0.1ug-1ug, GCLCM 10ml-40ml, penicillin 5*103IU-2*104IU and streptomycin 5*103IU-2*104IU. The invention adopts the method that the culture medium is added with fibroblast conditioned medium, and the nutritive factors are supplemented with the hormone and cytokine combination, fibroblast conditioned medium and BSA. As a result, the nutritive needs for long-time in-vitro expansion and cultivation of the limbal stem cell serum-free medium are satisfied, and the medium is serum-free and suitable for commercial production.
Owner:NORTHWEST A & F UNIV
Mucosal immunization to prevent prion infection
Vaccines against prion disease eliciting a humoral immune response when administered mucosally are described. The vaccines comprise a prion protein, a prion protein fragment, or a non-amyloidogenic prion protein homolog and an adjuvant suitable for inducing a humoral immune response after mucosal administration. Suitable adjuvants include cholera toxin subunit B, heat-labile enterotoxin and aluminum hydroxide. Alternatively, the vaccine comprises a vector encoding a prion protein, fragment, or homolog in an attenuated Salmonella host. The vaccines can be used to prevent or treat prion disease in humans and other mammals.
Owner:NEW YORK UNIV
Urease epitope fusion peptide liposome bacterin for preventing the helicobacter pylori infecting
InactiveCN101062015AEffective in inducing an immune responsePromote wound healingAntibacterial agentsBacterial antigen ingredientsPylorusAdjuvant
The invention discloses an urea enzyme epitope fuse peptiolipid plastid vaccine to against pylorus bolt bacteria infection, which is characterized by the following: choosing fuse peptide of pylorus bolt bacteria urea enzyme B subunit and stick film adjuvant cholera morbus toxin B subunit as immunogen; coating the immunogen with liposome; producing the liposome vaccine. This liposome vaccine can evoke organism to generate special immune response and inhibit planting of pylorus bolt bacteria in stomach.
Owner:CHINA PHARM UNIV
Recombination antigen composition, vaccine and carrier and method for preparing antigen composition
The invention provides a recombination antigen composition, a vaccine and a carrier and a method for preparing the antigen composition. A recombination helicobacter pylori antigen which comprises a helicobacter pylori antigen, the fusion proteins of a cholera toxin CT-A2 subunit and cholera toxin CT-B proteins is optimized. The compatibility of the CT-A2 and the CT-B proteins is used, a chimeric structure of CT-A2-5CT-B is formed, and accordingly an antigen with higher titer is formed through the helicobacter pylori antigen and the fusion proteins of the cholera toxin CT-A2 subunit. Meanwhile, the effect that the CT-B enhances immunization is used, and the effect that the antigen immunogenicity of the recombination antigen composition is enhanced is achieved. In addition, the chimeric protein constituted by the recombination antigen stimulates a mucous membrane to generate secreting type IgA immunization.
Owner:SHANGHAI UNITED CELL BIOTECH
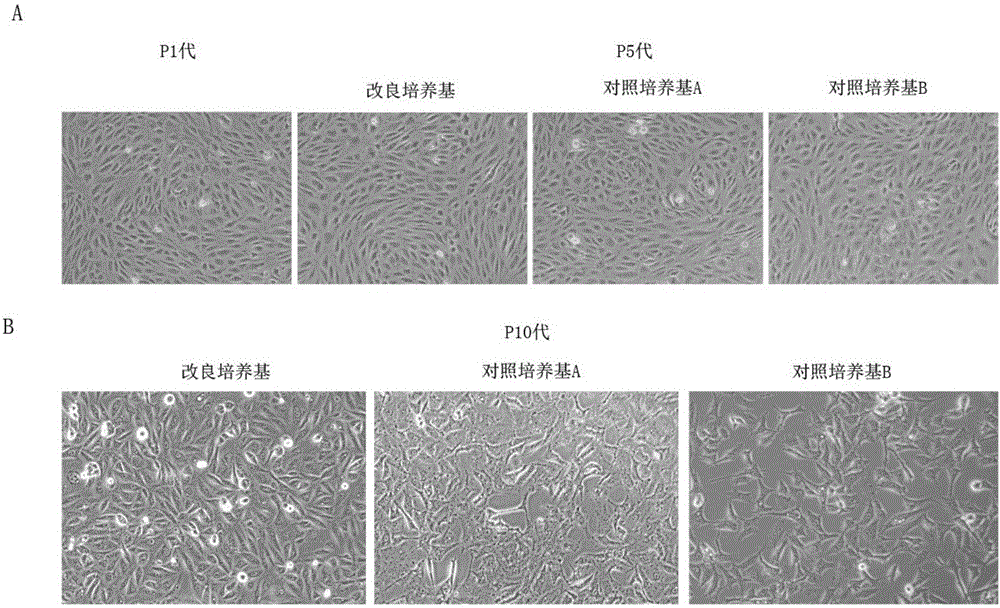
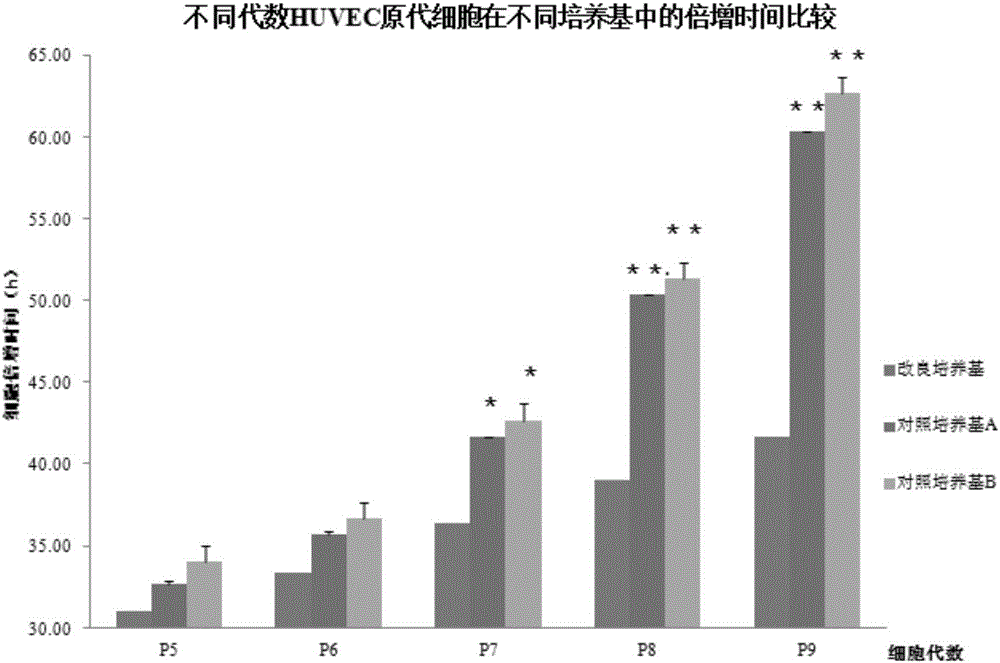
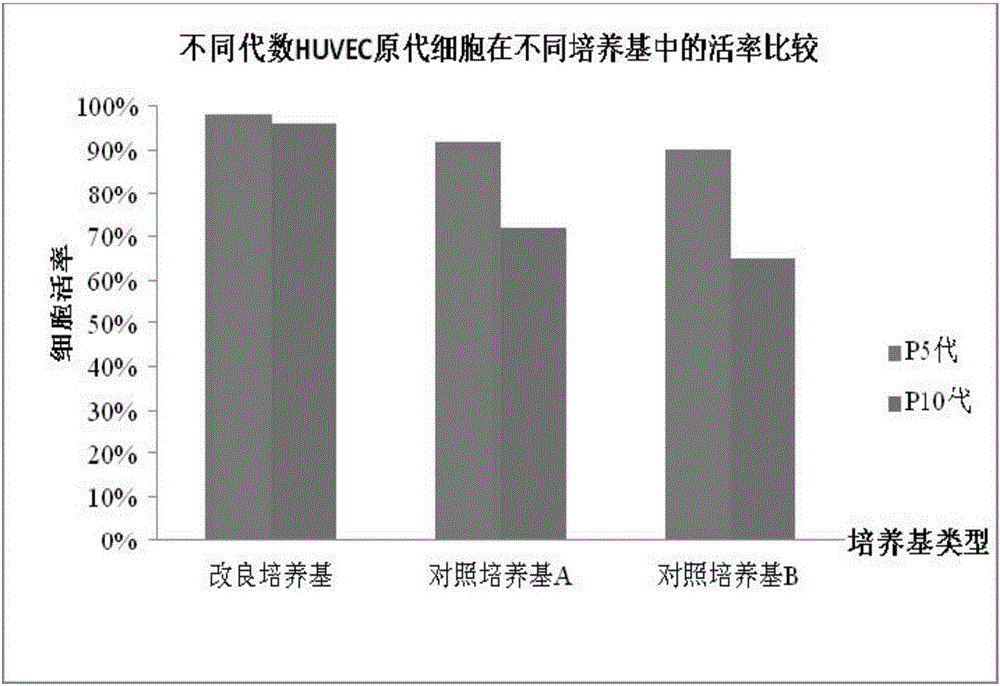
Cell culture medium and application thereof in culturing primary human tumor cells
The invention discloses a cell culture medium. The raw material formula of the cell culture medium comprises 33.33ml of DMEM / High Glucose (DMEM high-glucose culture medium), 66.67ml of HAM'S / F-12 culture medium, 3-5mg / mL of plant-origin recombinant human serum albumin, 100U / ml of penicillin, 100 micrograms / milliliter of streptomycin, 2.5 micrograms / milliliter of amphotericin B, 0.2-0.5 micrograms / milliliter of hydrocortisone, 3-6 micrograms / milliliter of insulin, 6-10ng / ml of cholera toxin B, 9-12ng / ml of human epidermal growth factor EGF, 22-25 micrograms / milliliter of adenine, and 7-9 micromoles / liter of Y-27632. The cell culture medium can be used for culturing primary human tumor cells or tumor stem cells. The cell culture medium is improved; when the cell culture medium is applied to culturing the primary human tumor cells or the tumor stem cells, the cell culture medium is capable of better accelerating the growth of the primary tumor cells and obtaining the human tumor stem cells of a higher ratio.
Owner:山东大学附属千佛山医院
Immunogenic detoxified mutants of cholera toxin
InactiveUS20050136076A1Improved stability characteristicsImprove stabilityVirusesBacteriaEscherichia coliVibrio cholerae
Owner:NOVARTIS AG
Human vascular endothelial cell culture solution and culture method
ActiveCN105969720APromote proliferationShorten split timeCell dissociation methodsCulture processInsulin-like growth factorVitamin C
The invention provides a human vascular endothelial cell culture solution. The human vascular endothelial cell culture solution is prepared from a basic culture medium, fetal calf serum, human recombined epidermal growth factors, human recombined fibroblast growth factors, vascular endothelial growth factors, insulin-like growth factors, vitamin C, cortisol, cholera toxin, aminoethanol and ethanolamine phosphate. Compared with a traditional vascular endothelial cell culture medium, the human vascular endothelial cell culture solution has the advantages that the culture solution can be suitable for multiple kinds of vascular endothelial cells, cell proliferation is effectively promoted, the division time is effectively shortened, the characteristics of the vascular endothelial cells are maintained, the passage number of the in-vitro human vascular endothelial cells is greatly increased, and the survival time of the in-vitro human vascular endothelial cells is greatly prolonged; meanwhile, all the ingredients of the cell culture solution can be obtained in a general laboratory, preparation is convenient, a special finished product culture medium is not needed, therefore, the cost for culturing the endothelial cells is greatly reduced, and the human vascular endothelial cell culture solution is suitable for application and popularization.
Owner:上海瑞鹿生物技术有限公司
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia Coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
Vibrio cholerae typing and virulence gene detection kit and detection method
ActiveCN101967516ADetection helpsStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceVirulent characteristicsEnzyme system
The invention relates to a vibrio cholerae typing and virulence gene detection kit and a vibrio cholerae typing and virulence gene detection method. The kit comprises DNA extracting solution, PCR reaction solution, a Taq enzyme system, a positive quality control product and a negative quality control product, wherein the PCR reaction solution consists of PCR reaction buffer, four pairs of forward and reverse primers for the specificity of vibrio cholerae and four probes for the specificity of the vibrio cholerae; the primers and probes are designed according to the specificity conservative areas of nucleic acid sequences of a hemolysin gene, an O-antigen gene of O139 type vibrio cholerae, the O-antigen gene of O1 type vibrio cholerae and the virulence gene of cholera toxin (CTX). The detection kit and the detection method ensure a reliable and stable result, are easy and fast to operate and can realize the typing of the vibrio cholerae and fast judgment about whether the vibrio cholerae has a virulence gene or not, thereby facilitating the tracing and detection of the public health-related events caused by the vibrio cholerae and facilitating the routine monitoring of the vibrio cholerae.
Owner:DAAN GENE CO LTD +1
Food grade lactic acid bacteria active carrier Group A rotavirus vaccine and preparation method thereof
InactiveCN103656633AWill not spreadNo horizontal transferViral antigen ingredientsDigestive systemEscherichia coliSerotype
The invention discloses food grade lactic acid bacteria active carrier Group A rotavirus vaccine and a preparation method thereof. The food grade lactic acid bacteria active carrier Group A rotavirus vaccine is characterized in that VP6 antigen protein from Group A virus, common serotype VP7 antigen protein (P serotype) and VP4 antigen protein (G serotype-expressed separately in the form of VP5* and VP8* protein subunit), vaccine adjuvant escherichia coli thermal unstable toxin B (LTB) and cholera toxin subunit B (CTB) are expressed and secreted by thyA gene deletion lactic acid bacteria cell or shown by the cell wall. Expressions of antigen protein and vaccine adjuvant protein are controlled by inducible or constitutive promoter, protein expression cassette is integrated onto the chromosome of the expression host lactic acid bacteria strain, and external antibiotics resistance gene introduced in gene manipulation is removed. The lactic acid bacteria active carrier rotavirus vaccine has the advantages of having wide serotype covering range, being easy to produce in large scale, and being safe and convenient to use without a refrigerator and a needle tubing.
Owner:刘占良 +2
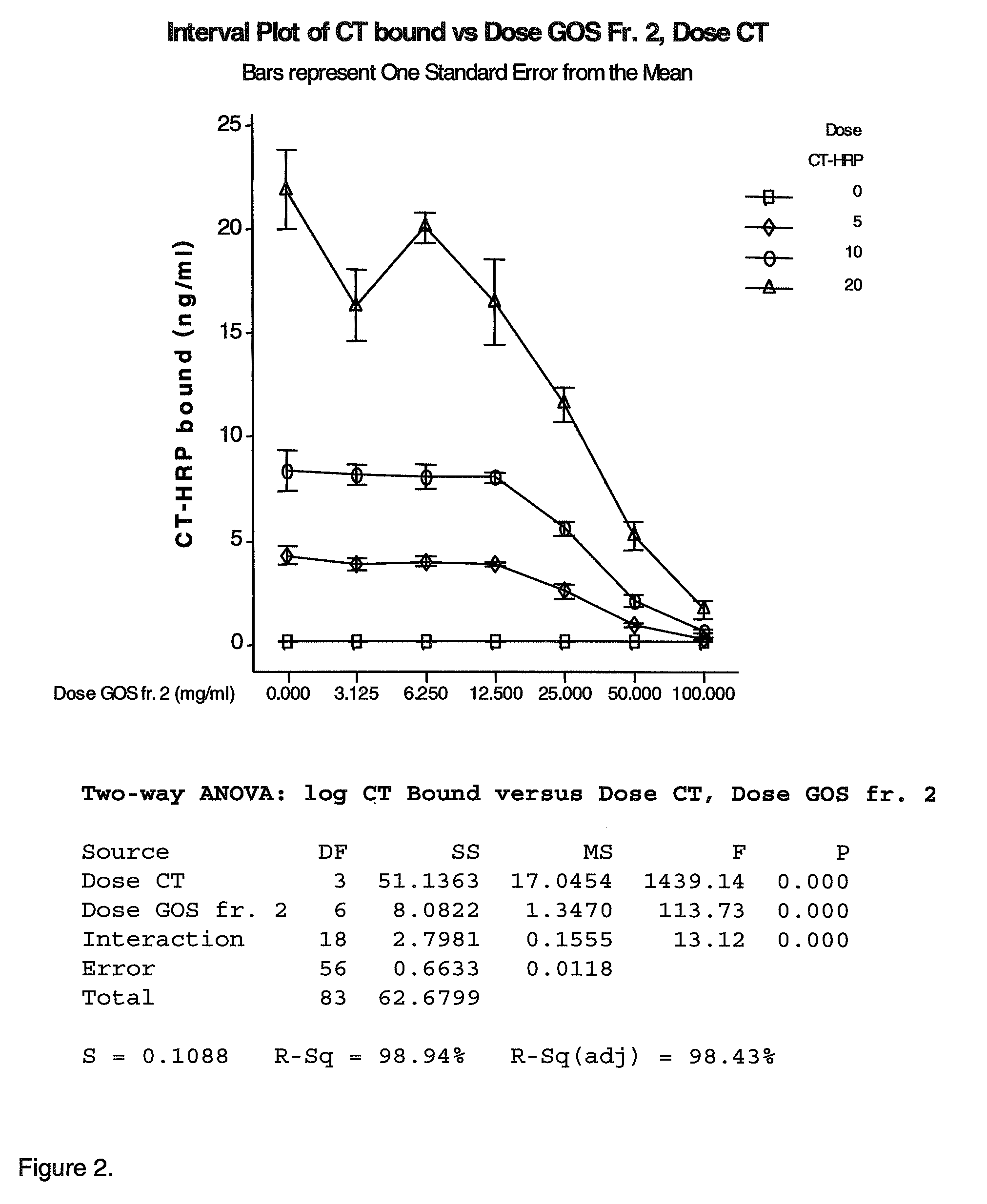
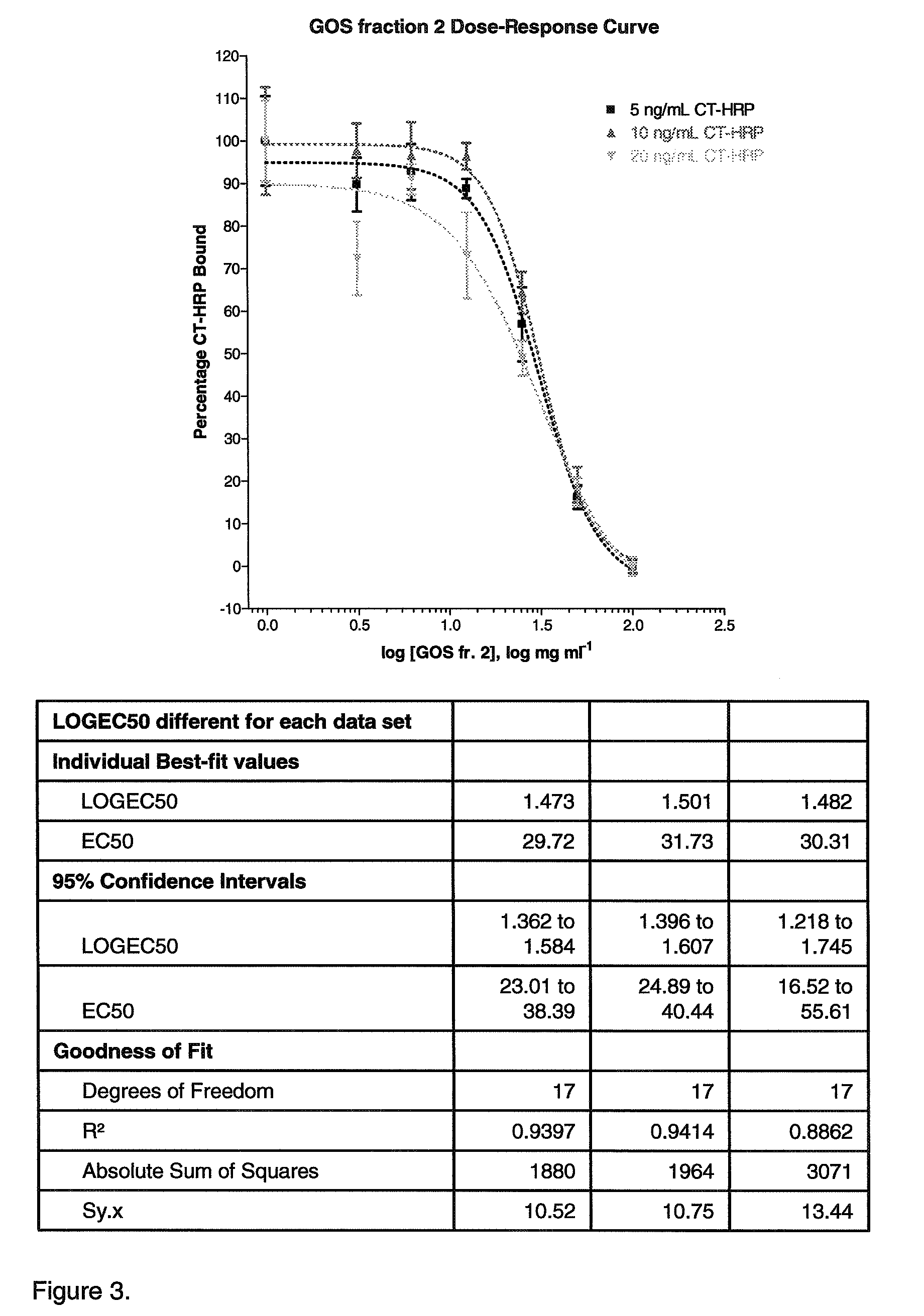
Inhibition of cholera toxins by galatooligosaccharides (GOS)
InactiveUS8202842B2Effective in prophylacticEffective inhibitorBiocideSugar derivativesDiseaseMedicine
The invention relates to nutritional and pharmaceutical compositions comprising non-digestible galactooligosaccharides (GOS) and uses thereof. In particular, it relates to the use of GOS species in preventing or treating disease caused by bacterial toxins. Provided is the use of GOS having a polymerization degree of 5 or higher, preferably 6 or higher, for the manufacture of a nutritional or pharmaceutical composition for the treatment or prevention of an acute or chronic disease associated with or caused by the adhesion and / or uptake of a cholera toxin family member. Also provided is a method for providing a GOS fraction capable of inhibiting cholera toxin (Ctx) binding to GM1 and fractions obtainable thereby.
Owner:FRIESLAND BRANDS BV
Saccharide derivatives
Disclosed are novel saccharide derivatives which inhibit binding of toxins, such as heat-labile enterotoxin or cholera toxin, to their receptors either in vitro or in vivo. Additionally, disclosed are compounds which inhibit binding of enterovirulent organisms (e.g., bacteria, virus, fungi, and the like), such as Vibrio cholerae and enterotoxigenic strains of Escherichia coli, to their cell surface receptors.
Owner:SYNSORB BIOTECH INC
Culture medium for keeping primary airway epithelial cells in physiological state in vivo
InactiveCN102505005AStrong growthEasy to prepareArtificial cell constructsVertebrate cellsPhysiologyBovine serum albumin
The invention relates to preservation of human local living parts and in particular relates to a culture medium for keeping primary airway epithelial cells in the physiological state in vivo during in vitro culture. The invention is characterized in that the culture medium contains the following components (per liter), 12 g of DMEM / F12 medium, 30 mg of penicillin G sodium salt, 50 mg of streptomycin, 10 mg of insulin, 5 mg of transferring, 25 Mug of epidermal growth factors, 100 Mug of cholera toxin, 108.741 Mug of hydrocortisone, 1 g of bovine pituitary extract, 3 g of bovine serum albumin, 1.5*10<-2> Mug of retinoic acid, 50 mg of gentamycin, 0.5 mg of amphotericin B, 50 mL of fetal calf serum and the balance of water. The primary airway epithelial cells cultured by the culture medium have the ciliary beating function and can maintain the physiological state in vivo.
Owner:SOUTHERN MEDICAL UNIVERSITY
Method for rapidly separating and culturing human bronchial epithelial cells, and optimized medium
ActiveCN107974429AEasy to separateEpidermal cells/skin cellsArtificial cell constructsCuticleFeeder Layer
Owner:GENERAL HOSPITAL OF NINGXIA MEDICAL UNIV
Oral vaccines produced and administered using edible micro-organisms including lactic acid bacterial strains
InactiveUS20130004547A1Enhance immune responseStrong mucosal immune responseSsRNA viruses negative-senseBacterial antigen ingredientsStaphylococcus lactisH5N1 virus
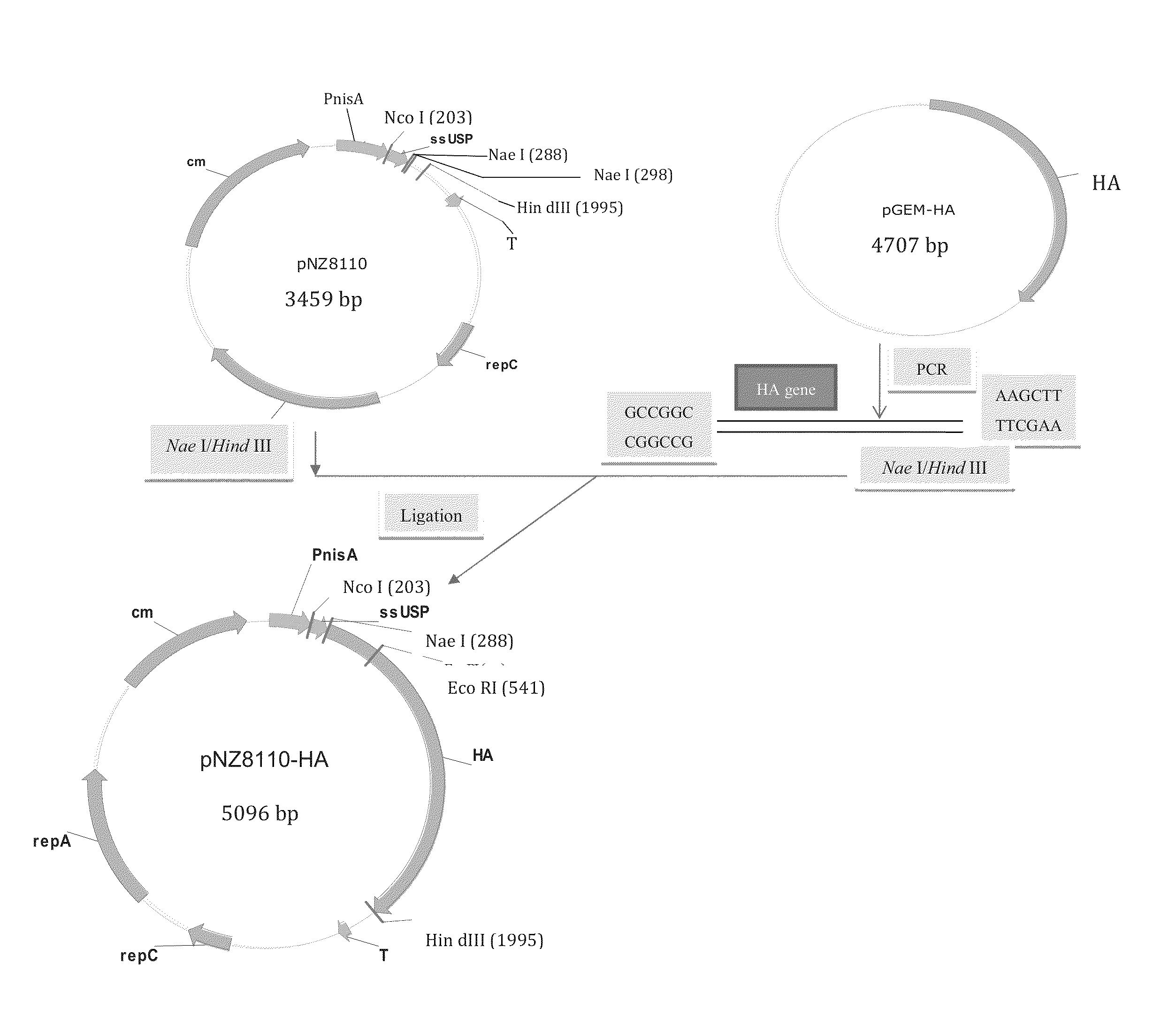
The invention provides for anti-pathogen vaccines produced in recombinant bacteria and / or transgenic plants and administered through standard vaccine introduction methods or oral administration. In one embodiment, the vaccine is administered through the consumption of the edible plant as food, or the bacteria administered orally. The present invention also provides a method of using genetically modified microorganisms, such as lactic acid bacteria, including Lactococcus lactis strains, as oral vaccines. In one embodiment, Lactococcus lactis expressing the avian influenza HA gene can be used as an oral vaccine for protection against H5N1 virus infection. In another embodiment, said Lactococcus lactis is administered as an oral vaccine in conjunction with an adjuvant such as cholera toxin B.
Owner:VAXGENE
Cholera Toxin Chimera and its Use as a Staph Vaccine
The present invention relates to chimeric protein vaccines and methods of use thereof in the treatment of Staphylococcus aureus. One embodiment of the present invention provides a method of generating an immune response in a mammal, that includes administering to the mammal, a composition having a chimeric protein having at least one of: a portion of a cholera toxin, a portion of a heat-labile toxin, and a portion of a shiga toxin; and an antigen having at least one of an antigenic material from S. aureus and an antigenic material from a S. aureus-specific polypeptide.
Owner:BOISE STATE UNIVERSITY
Culture medium for tissue engineering skin convey and formulating method thereof
InactiveCN101486995AEffective maintenance of structureEffective maintenance functionArtificial cell constructsVertebrate cellsBiotechnologyPenicillin
The invention discloses a culture medium that is used in transportation of tissue engineered skin. A basic culture fluid is added with additional hydrocortisone, epidermal growth factors, isoproterenol, insulin, transferrin, triiodothyronine, adenine, vitamin C, cholera toxin, L-serine, L-novain, palmic acid, linoleic acid, arachidonic acid, bovine pituitary extract, bovine serum albumin, penicillin, streptomycin and fungizone B. The obtained culture medium has strong stability and long shelf life of about 12 months, completely meet the demands of large-range transportation of the tissue engineered skin products, can effectively maintain the structure and functions of the tissue engineered skin in the transportation process, becomes solid after being cooled, and consequently has convenient carry in the transportation process.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Toxigenous commabacillus cholera vibrio ring mediated isothermal amplification rapid detection method
InactiveCN101386885AGuaranteed reliabilityStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesBetaineLoop-mediated isothermal amplification
The invention relates to a loop-mediated isothermal amplification method for rapid detection of cholera toxin vibrio cholera. A reagent comprises a loop-mediated isothermal amplification reaction liquid, Bst DNA polymerase, and a chromogenic reagent, wherein the reaction liquid contains a reaction buffer liquid, dNTP, magnesium sulfate, an upstream inner primer 5-TGAATCCACGGCTCTTCCCT-TGGTTATGGATTGGCAGG-3, a downstream inner primer 5-GGTTGTGGGAATGCTCCAAG-ACTTTGGGTTTTTTCATCGC-3, an upstream outer primer 5- GATATTGCTCCAGCAGCA-3, a downstream outer primer 5-CGTCAAGGAATTTTACACCTAG-3, and betaine. The method for detecting the cholera toxin vibrio cholera comprises the steps of the extraction of bacterial DNA, the loop-mediated isothermal amplification of the cholera toxin vibrio cholera, and chromogenic detection. The method has the advantages of rapidness, strong specificity, high sensitivity, and low cost.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Drug-delivery carrier protein based on cholera toxin CT structure as well as in-vitro construction method and application of drug-delivery carrier protein
InactiveCN105504065AImprove purityIn vitro assembly with less interferencePolypeptide with localisation/targeting motifPeptide/protein ingredientsCarrier proteinCholera toxin
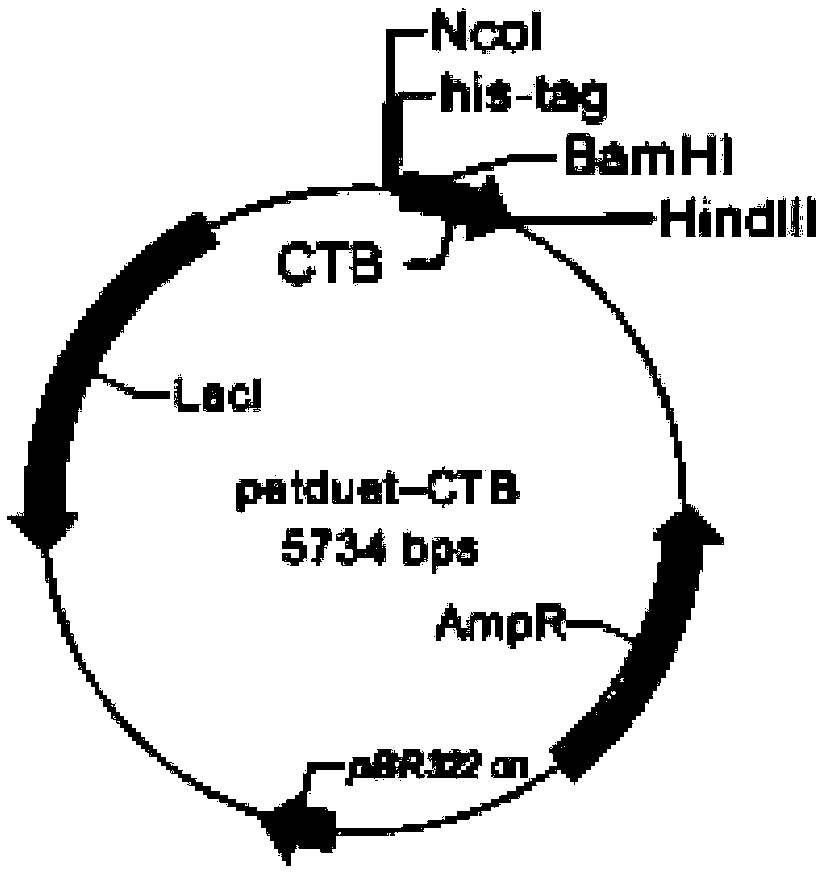
The invention relates to a drug-delivery carrier protein based on a cholera toxin CT structure and an in-vitro construction method of the drug-delivery carrier protein. The drug-delivery carrier protein is a chimeric protein CTB5 / EGFP-CTA2-TAT. The construction method comprises the following steps: constructing carriers petduet-CTB and pet22b-EGFP-CTA2-TAT; introducing host bacteria, carrying purification or renaturation, and assembling an AB5-structured chimeric protein in vitro. The drug-delivery carrier protein prepared by virtue of the construction method is high in purity, is relatively stable and is strong in repeatability; furthermore, no burden is produced during cell expression, and the protein raw material can be rapidly obtained; the drug-delivery carrier protein has high combining activity with GM1 as well as relatively efficient transmembrane capacity.
Owner:INT HEALTHCARE INNOVATION INST JIANGMEN
Process for the isolation of a nontoxinogenic vibrio cholerae strain and a process for preparing cholera vaccine from said vibrio cholerae strain
A process for the isolation of nontoxinogenic V. cholerae strain and a process for preparing a cholera vaccine from said V. cholerae strain, said process comprising (a) isolating V. cholerae from the stool of a patient suffering from cholera by spreading the stool on a selector medium specific for V. cholerae, (b) separating the non-toxinogenic V. cholerae strain from the population of the V. cholerae strains isolated in step (a), and (c) incorporating immunogenic cholera toxin (ctx) B subunit gene into the chromosome of the strain by conventional methods to produce the vaccine.
Owner:COUNCIL OF SCI & IND RES +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com