Recombination antigen composition, vaccine and carrier and method for preparing antigen composition
A composition and antigen technology, applied in the field of immunization, can solve the problems such as the huge effect of antigen vaccines, the difficulty in producing protective effects, and the difficulty in producing immune responses for vaccines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation of candidate vaccines requires effective antigens, and the use of effective antigens to immunize the body can stimulate the body to generate a systemic immune response, and then prevent the body from infection.
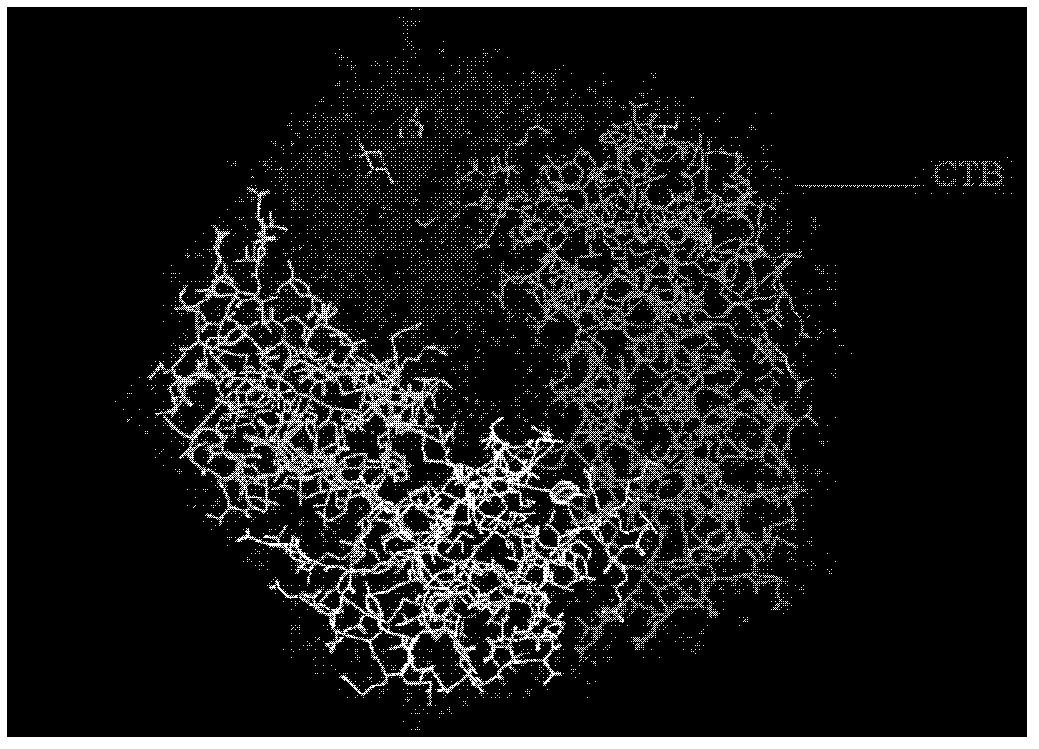
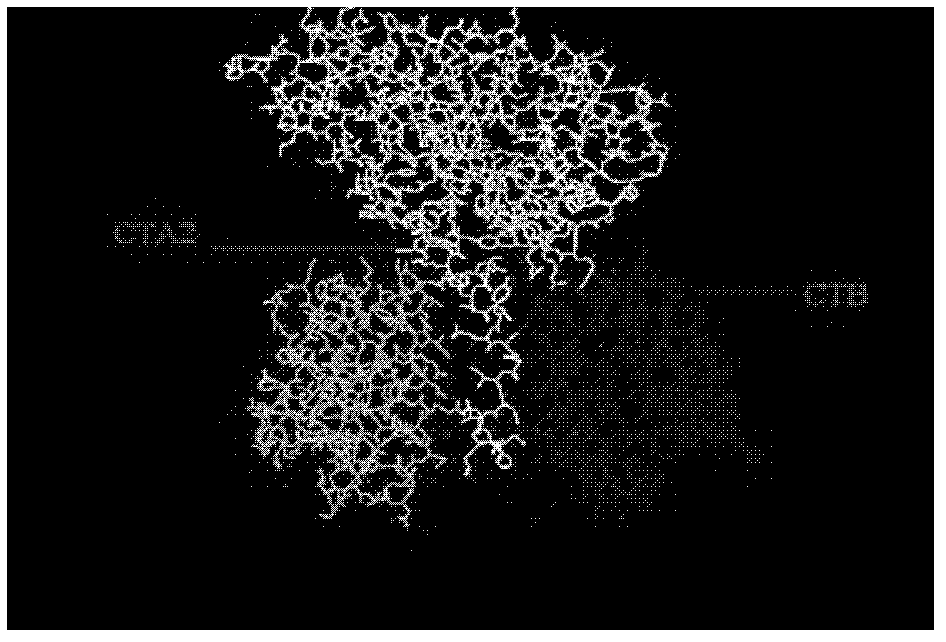
[0030] In the preparation of the antigen used in the vaccine, the present invention adopts the CT-A2-CT-B system to increase the volume of the antigen and utilizes the immune adjuvant function of CT to enhance the immunogenicity of the antigen. Antigens that can use this system include, but are not limited to: Helicobacter pylori-related antigens, typhoid antigens, influenza HA antigens, and the like.
[0031] For these antigens, fusion proteins of corresponding antigens and CT-A2 can be prepared by methods known in the art, and then used together with CT-B protein to achieve the purpose of enhancing immunogenicity.
[0032] Taking the preparation of Helicobacter pylori-related vaccines as an example, the inventors preferentially selected Helico...
Embodiment 1
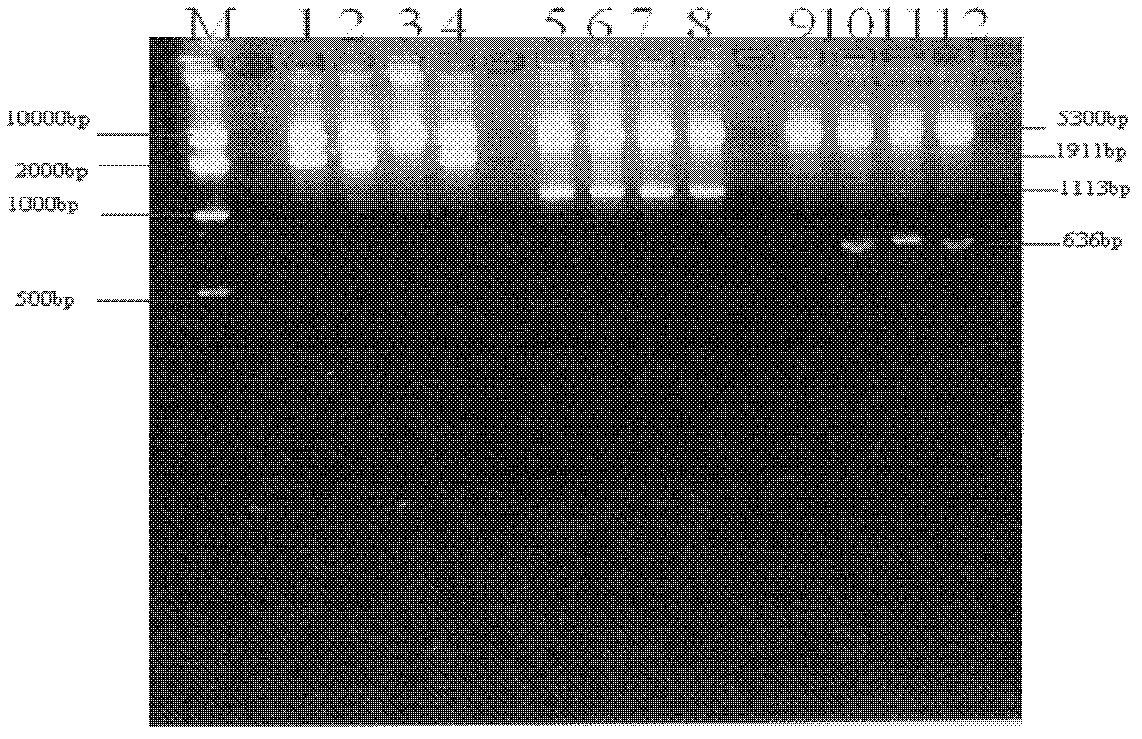
[0038] The preparation of embodiment 1UreB-CTA2 / 5CTB
[0039] 1.1 Construction of recombinant engineering bacteria PET-28a-UreB-CTA2 / BL21-DE3
[0040] 1.1.1 Construction of fusion gene UreB-CTA2:
[0041] The amino acid sequence of UreB comes from Helicobacter pylori strain MEL-HP27, the amino acid sequence of CTA2 comes from Vibrio cholerae O395 strain, and the UreB-CTA2 gene is codon-optimized and synthesized.
[0042] The UreB-CTA2 gene sequence (SEQ ID NO.: 1) is:
[0043] ATGAAAAAAAATCTCTAGGAAAGAGTACGTAAGCATGTACGGTCCGACCAC 50
[0044] CGGTGACAAAGTTCGTCTGGGTGACACCGACCTGATCGCTGAAGTTGAAC 100
[0045]ACGACTACACCATCTACGGTGAAGAACTGAAATTCGGTGGTGGTAAAACC 150
[0046] CTGCGTGAAGGTATGTCTCAGTCTAACAACCCGTCTAAAGAAGAACTGGA 200
[0047] CCTGATCATCACCAACGCTCTGATCGTTGACTACACCGGTATCTACAAAAG 250
[0048] CTGACATCGGTATCAAAGACGGTAAAATCGCTGGTATCGGTAAAAGGTGGT 300
[0049] AACAAAGACATGCAGGACGGTGTTAAAAACAACCTGTCTGTTGGTCCGGC 350
[0050] TACCGAAGCTCTGGCTGGTGAAGGTCTGATCGTTACCGCTGGTGGTATCG 4...
Embodiment 2
[0110] The preparation method of embodiment 2CagA-CTA2 / 5CTB
[0111] CagA-CTA2 / 5CTB was prepared by the same steps as in Example 1, except that the CagA gene was used instead of the UreB gene.
[0112] The corresponding gene sequence (SEQ ID NO.: 2) is:
[0113] ATGAAACTGTTCGGTAACTCTAACAACAACAACAACGGTCTGAAAAACGA 50
[0114] ACCGATCTACGCTCAGGTTAACAAAAAAAAAGCTGGTCAGGCTACCTCTC 100
[0115] CGGAAGAACCGATCTACGCTCAGGTTGCTAAAAAAGTTCTGCTAAAATC 150
[0116] GACCAGCTGAACGAAGCTACCTCTGCTATCAACCGTAAAATCGACCGTAT 200
[0117] CAACAAAATCGCTTCTGCTGGTAAAGGTGTTGGTGGTTTCTCTGGTGCTG 250
[0118] GTCGTTCTGCTTCTCCGGAACCGATCTACGCTACCATCGACTTCGACGAA 300
[0119] GCTAACCAGGCTGGTTTCCCGCTGCGTCGTTCTACCGCTGTTAACGACCT 350
[0120] GTCTAAAGTTGGTCTGTCTCGTGAACAGGAACTGACCCGTCGTATCGGTG 400
[0121] ACCTCAAACCAGGCTGTAAGCGAAGCTAAAACCGGTCACTTCGACAAACTG 450
[0122] GAACAGAAAATGGACGAACTGAAAGACTCTACCAAAAAAAACGCTCTGAA 500
[0123] ACTGTGGGCTGAATCTACCAAACAGGTTCCGACCGGTCTGCAGGCTAAAC 550
[0124] TGGACAACTACGCTA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com