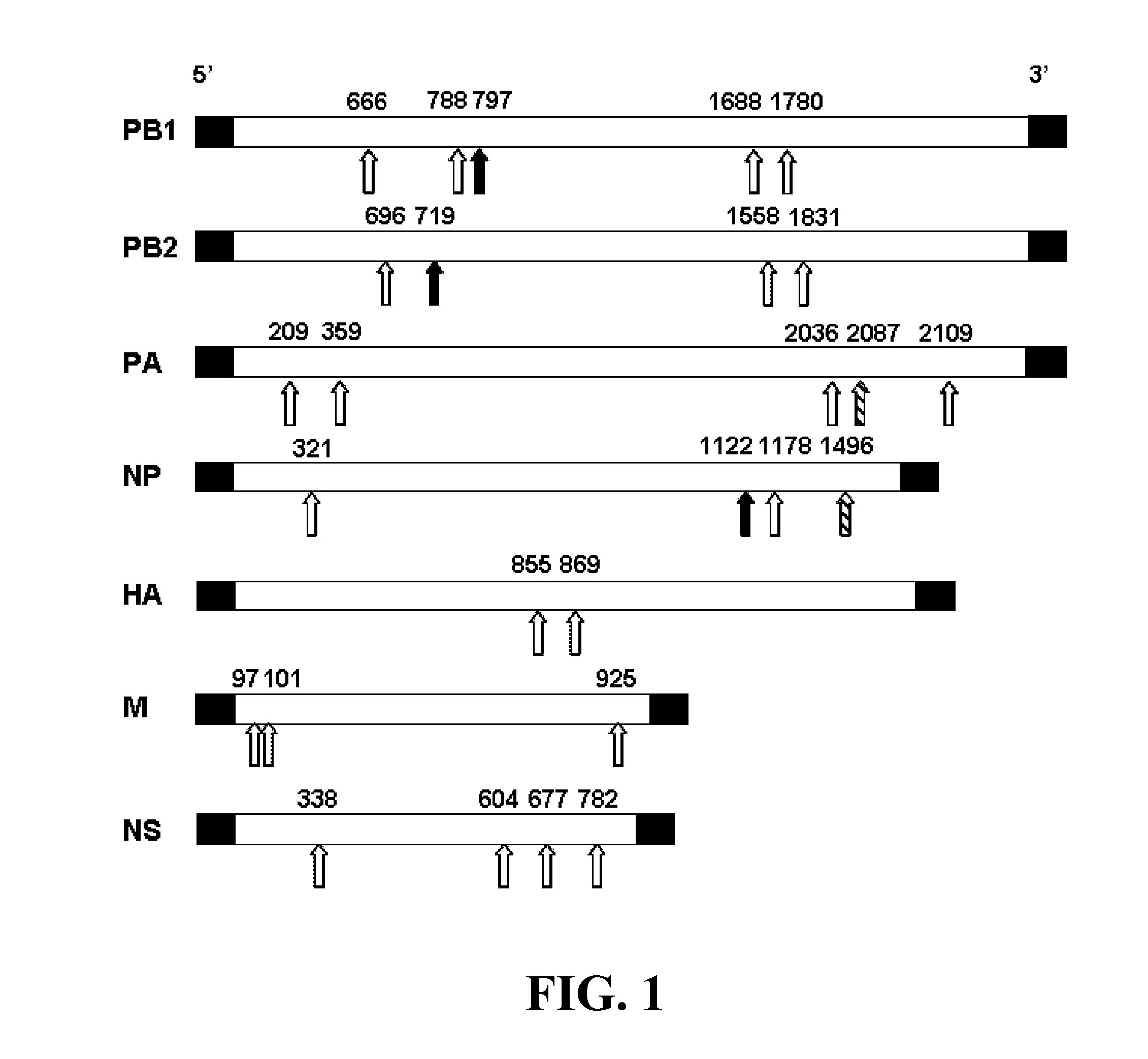
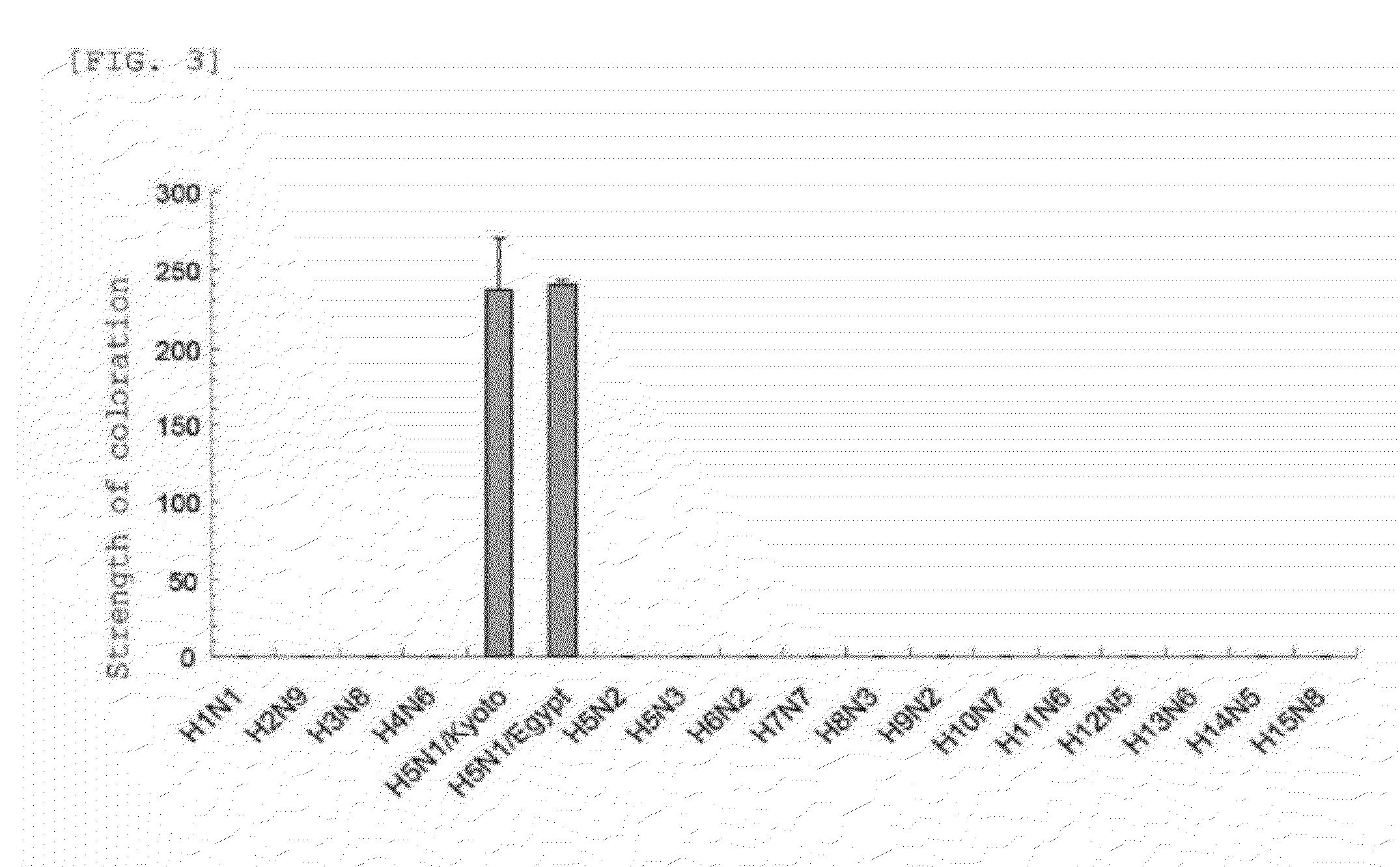
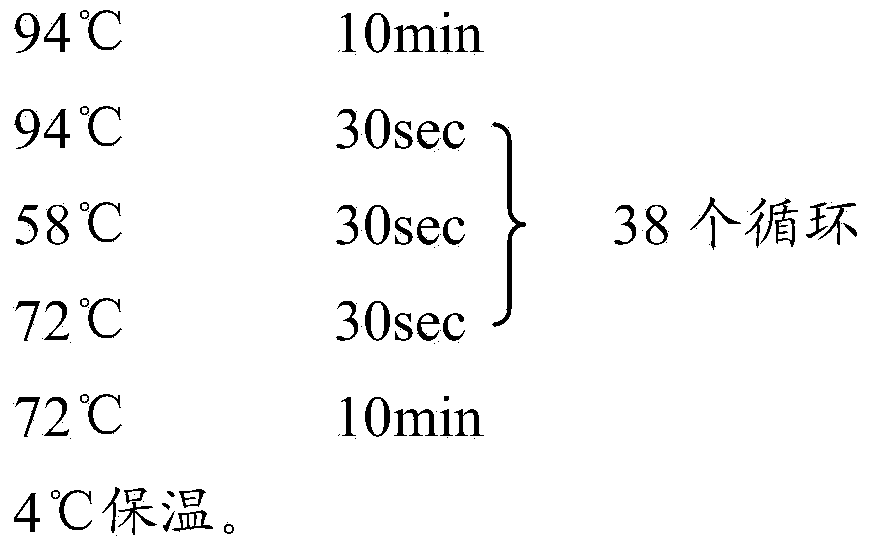Patents
Literature
74 results about "H5N1 virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
H5N1 is an avian influenza virus subtype. The H5N1 flu is what is commonly meant when talking of "bird flu" or "avian influenza". It is a viral disease that causes illness in many species including humans and is a pandemic threat.
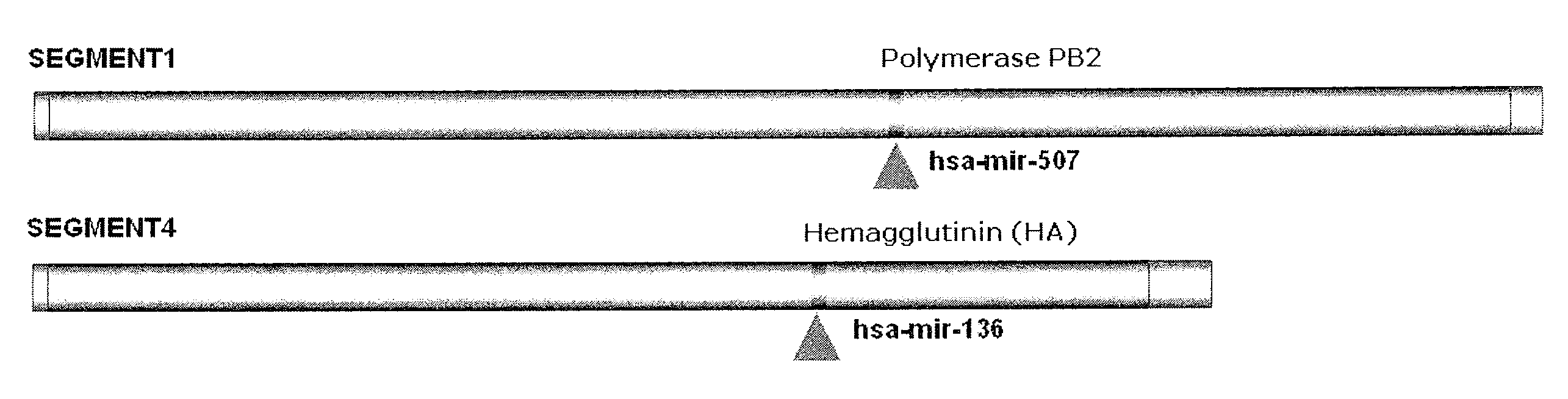
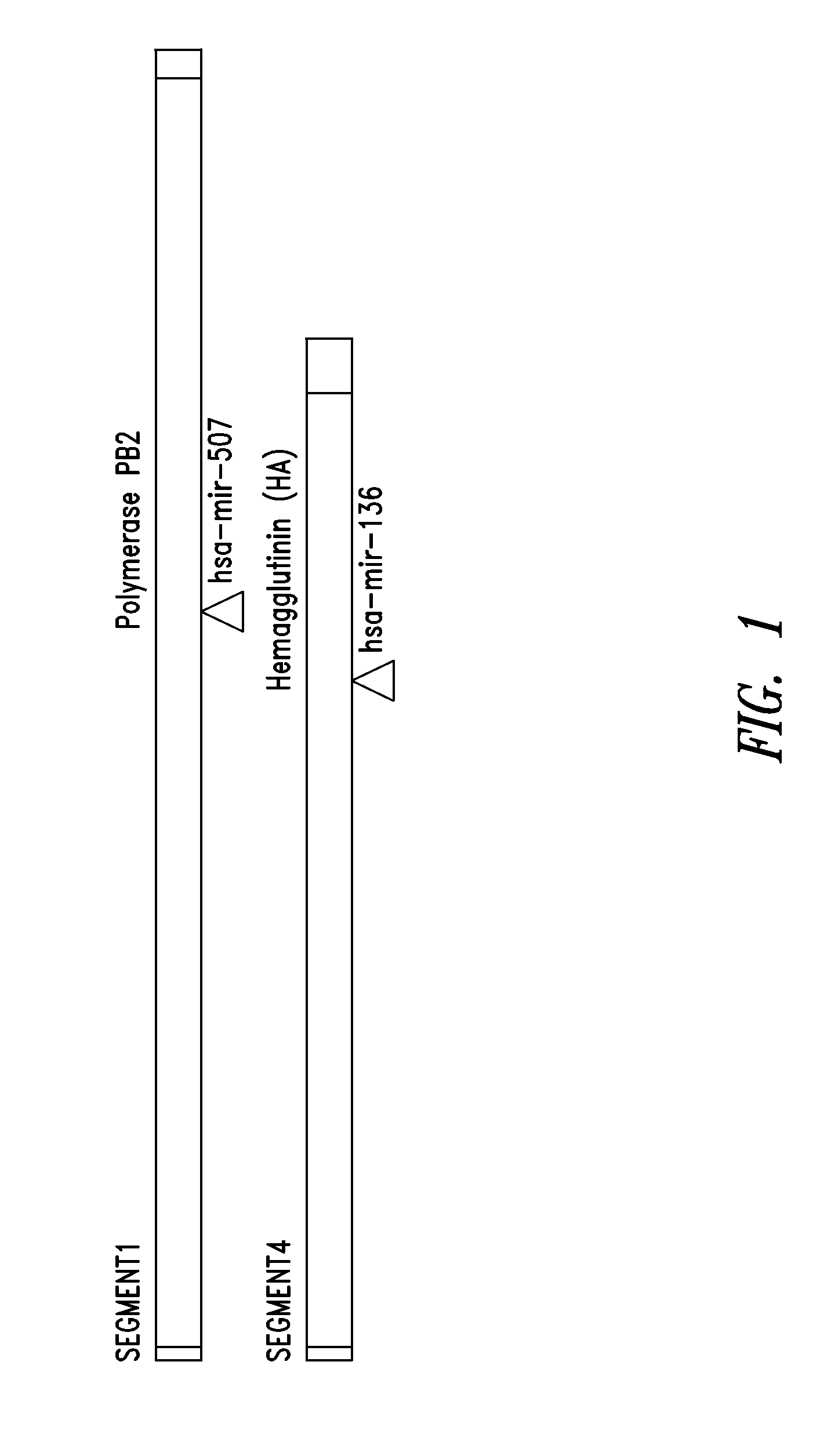
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
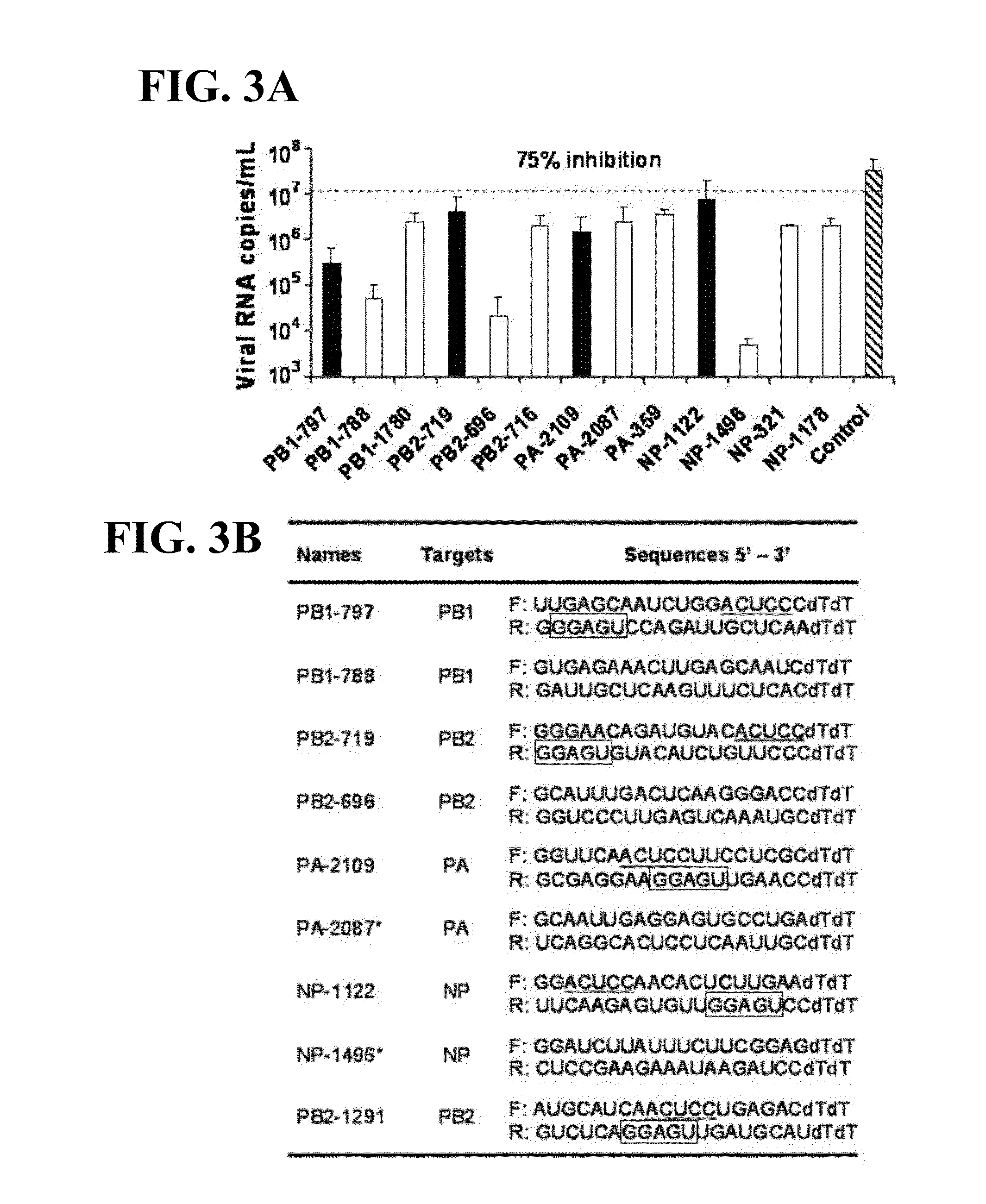
siRNA COMPOSITIONS AND METHODS FOR POTENTLY INHIBITING VIRAL INFECTION
ActiveUS20100166661A1Improve the protective effectLess antiviral effectOrganic active ingredientsSugar derivativesRegimenH5N1 virus
No antiviral regimen has been consistently successful in treating H5N1 virus infection. We demonstrate that a group of highly effective siRNAs targeting different H5N1 viral genes shares a unique motif, GGAGU / ACUCC. We further demonstrate that the effectiveness of siRNAs containing this motif is not sequence specific. The results suggested that the structure of the unique motif is critical in determining the potency of siRNA-mediated protective effects against viral infection and this potent in vivo protection is associated with early productions of β-defensin and IL-6 induced by the motif. Provided are methods and prophylactic and therapeutic agents useful against other viral infections in addition to the H5N1 influenza virus.
Owner:KWUN TONG KOWLOON
Kit for detecting highly pathogenic avian influenza virus subtype h5n1
InactiveUS20120028246A1Reduce pathogenicityMicrobiological testing/measurementBiological material analysisH5N1 virusWater soluble
Disclosed by the invention are an immunoassay kit and an immunoassay method for detecting highly pathogenic avian influenza virus subtype H5N1 rapidly, conveniently and specifically. Also disclosed are an immunochromatographic detection kit and an immunochromatographic detection method for detecting the virus subtype H5N1 rapidly, conveniently and specifically. It is found that a monoclonal antibody 4G6 produced by using the virus subtype H5N1 as an immunogen does not react with the subtype H5N2 virus or a subtype H5N3 virus and reacts only with a subtype H5N1 virus specifically. It is also found that only an avian influenza virus subtype H5N1 can be detected specifically by an immunoassay utilizing the monoclonal antibody 4G6. It is further found that the sensitivity of the detection of immunochromatography can be increased by adding a nonionic surface and a water-soluble vinyl polymer having a polar group containing an oxygen atom and a nitrogen atom to a developing solution to be used in the immunochromatography.
Owner:OSAKA UNIV +1
Oral vaccines produced and administered using edible micro-organisms including lactic acid bacterial strains
InactiveUS20130004547A1Enhance immune responseStrong mucosal immune responseSsRNA viruses negative-senseBacterial antigen ingredientsStaphylococcus lactisH5N1 virus
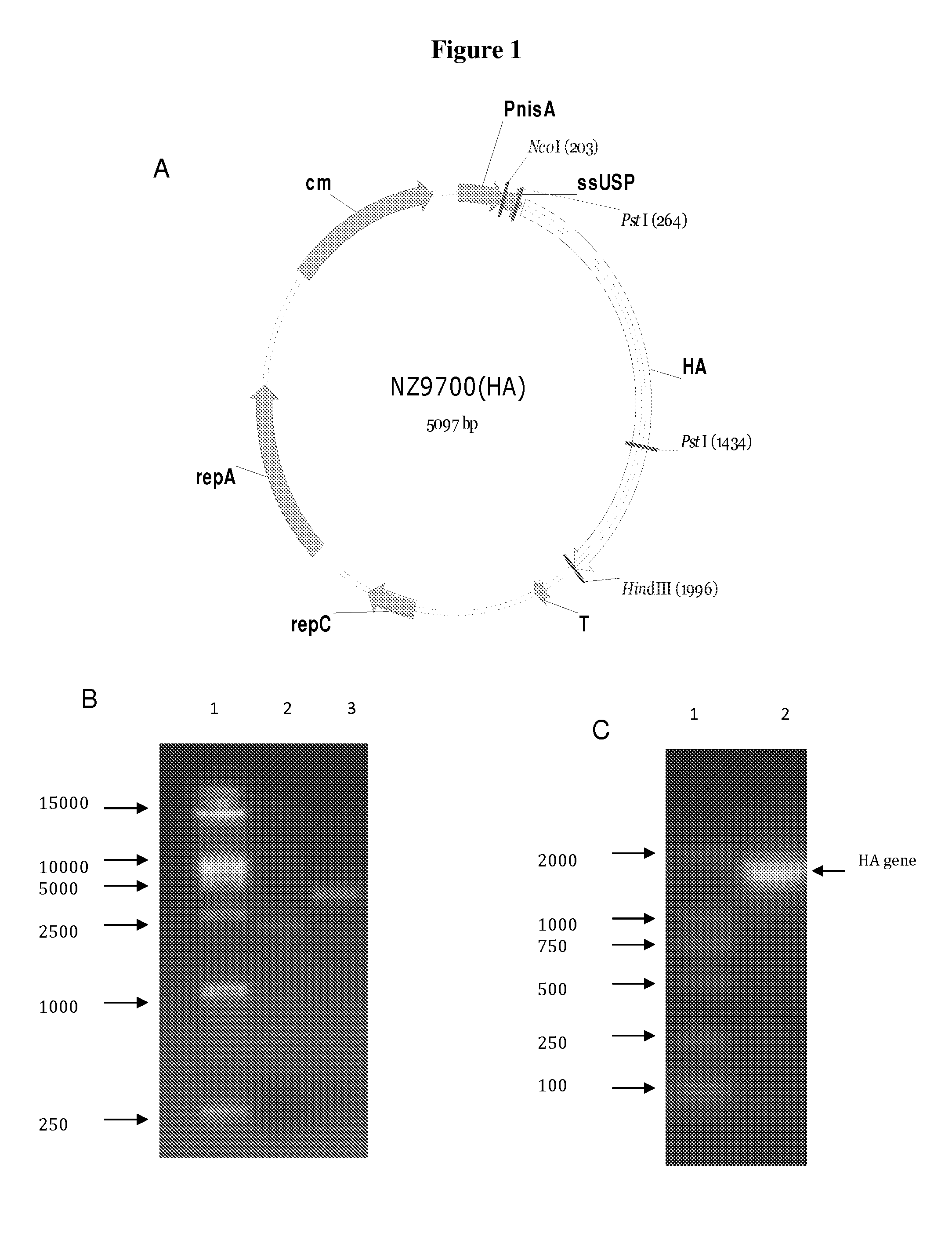
The invention provides for anti-pathogen vaccines produced in recombinant bacteria and / or transgenic plants and administered through standard vaccine introduction methods or oral administration. In one embodiment, the vaccine is administered through the consumption of the edible plant as food, or the bacteria administered orally. The present invention also provides a method of using genetically modified microorganisms, such as lactic acid bacteria, including Lactococcus lactis strains, as oral vaccines. In one embodiment, Lactococcus lactis expressing the avian influenza HA gene can be used as an oral vaccine for protection against H5N1 virus infection. In another embodiment, said Lactococcus lactis is administered as an oral vaccine in conjunction with an adjuvant such as cholera toxin B.
Owner:VAXGENE
Compositions For Treating Respiratory Viral Infections and Their Use
ActiveUS20080279920A1Promote divisionHigh degreeSsRNA viruses negative-senseOrganic active ingredientsH5N1 virusNucleotide
The invention provides siRNA compositions that interfere with viral replication in respiratory viral infections, including respiratory syncytial virus and avian influenza A, including the H5N1 strain. The invention further provides uses of the siRNA compositions to inhibit expression of viral genes in respiratory virus-infected cells, and to uses in the treatment of respiratory virus infections in a subject. Generally the invention provides polynucleotide that includes a first nucleotide sequence of 15 to 30 bases that targets the genome of a respiratory syncytial virus or an influenza A virus, a complement thereof, a double stranded polynucleotide or a hairpin polynucleotide. Additionally the invention provides vectors, cells and pharmaceutical compositions containing siRNA sequences.
Owner:SIRNAOMICS INC
Immunoprotection by oral administration of recombinant lactococcus lactis mini-capsules
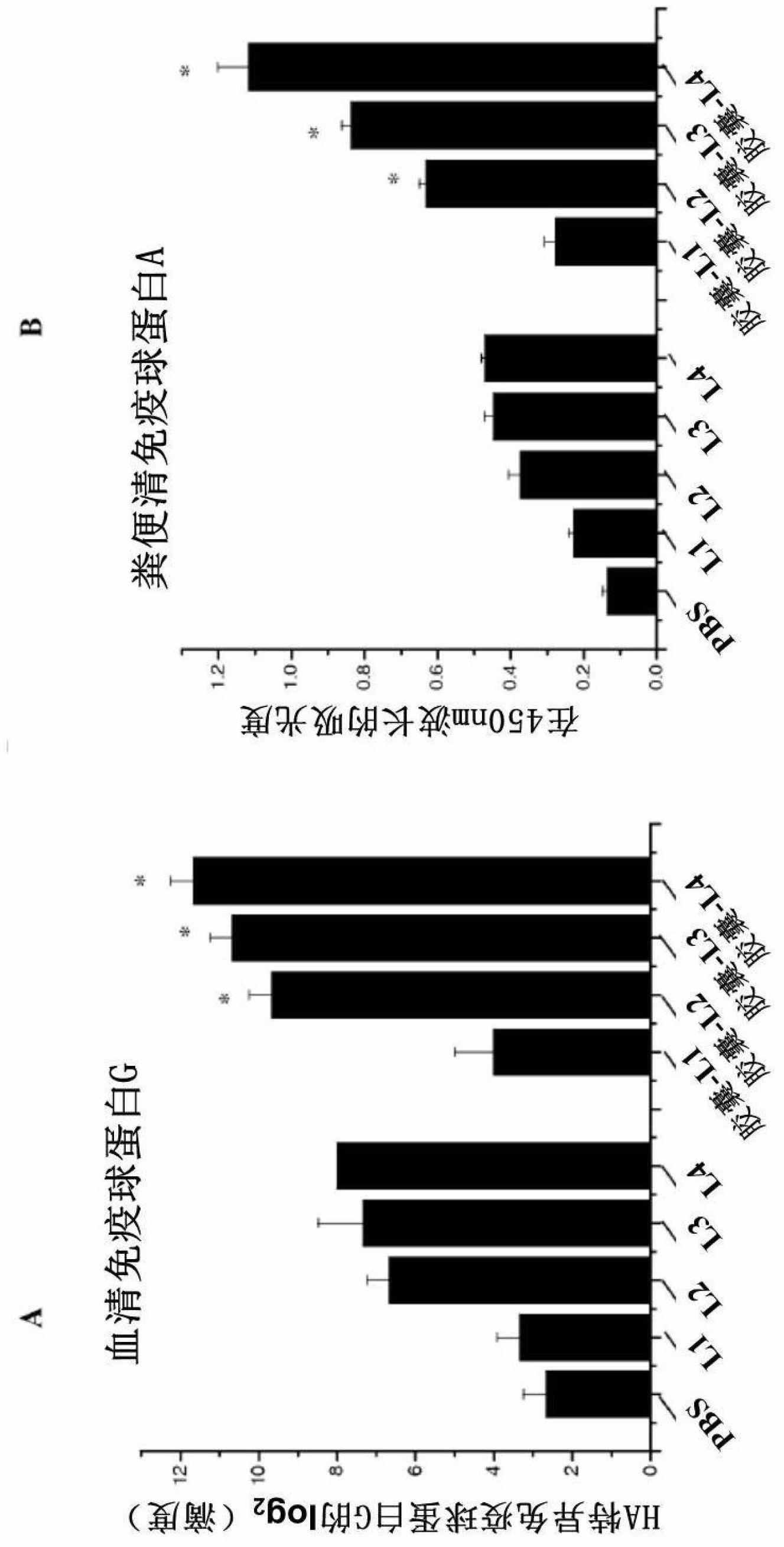
In one embodiment, the present invention provides for an edible mini-capsule form of live, non-persisting, recombinant Lactococcus lactis (L.lactis) vaccine against a pathogen such as the highly virulent influenza H5N1 strain. Enteric coated capsule of the present invention induced high levels of hemagglutinin-specific serum IgG and fecal IgA antibody production after oral administration in mice and chickens, and rendered complete protection against a lethal challenge of H5N1 virus in mice. The present invention thus demonstrates a broadly applicable platform technology for producing and administering edible vaccines against bacterial and viral infections.
Owner:VAXGENE
siRNA Compositions and Methods for Potently Inhibiting Viral Infection
No antiviral regimen has been consistently successful in treating H5N1 virus infection. We demonstrate that a group of highly effective siRNAs targeting different H5N1 viral genes shares a unique motif, GGAGU / ACUCC. We further demonstrate that the effectiveness of siRNAs containing this motif is not sequence specific. The results suggested that the structure of the unique motif is critical in determining the potency of siRNA-mediated protective effects against viral infection and this potent in vivo protection is associated with early productions of β-defensin and IL-6 induced by the motif. Provided are methods and prophylactic and therapeutic agents useful against other viral infections in addition to the H5N1 influenza virus.
Owner:KWUN TONG KOWLOON
CTL epitope polypeptides of bird flu H5N1 virus and applications thereof
The invention discloses a CTL epitope polypeptide of bird flu H5N1 virus and applications thereof. The CTL epitope polypeptide of bird flu H5N1 virus is shown as (a) or (b); (a) the polypeptide consists of amino acid sequences in the sequence I out of a sequence list; and (b) the polypeptide derives from the sequence 1 by the replacing and / or missing and / or adding of one or more amino acid residues of the amino acid sequence in the sequence 1 and is correlate to the CTL epitope of the bird flu H5N1 virus. The invention also protects the DNA that codes the polypeptide. The epitope polypeptide can be used for preparing the bacterin for the bird flu H5N1 virus and can be also used for preparing the kit for detecting the specific T cell of the H5N1 virus. The CTL epitope polypeptides and the applications thereof have important values.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Humanized antibody for resisting avian influenza H5N1 hemagglutinin antigen, and preparation method and application thereof
ActiveCN103739707ALow immunogenicityBroad general hemagglutination inhibitory activityVirus peptidesImmunoglobulins against virusesHemagglutininEpitope
The invention discloses a humanized antibody for resisting avian influenza H5N1 hemagglutinin (HA) antigen. A heavy chain variable region of the humanized antibody has an amino acid sequence shown in SEQ ID No.1; a light chain variable region of the humanized antibody has an amino acid sequence shown in SEQ ID No.2. In addition, the invention also discloses a preparation method and application of the humanized antibody. The humanized antibody is extensive and widespread in coagulation inhibition activity for avian influenza H5N1 virus, high in affinity and neutralization activity for various subtypes of the HA antigen of the avian influenza H5N1, and capable of recognizing a special conserved region of one H5HA; the humanized antibody is high in neutralization activity and capable of reducing immunogenicity; a recognized epitope is highly conserved in the H5 type influenza virus; the humanized antibody is extensive in application prospect in prevention and treatment and vaccine design of the avian influenza H5N1 in the future.
Owner:SHANGHAI INST OF IMMUNOLOGY +1
Dextro-gossypol amino acid sodium salt derivative for blocking invasion of H5N1 avian influenza virus, and preparation method and application thereof
InactiveCN102775320AHigh activityLow toxicityOrganic compound preparationSulfonic acids salts preparationH5N1 virusNitrogen gas
The invention discloses a dextro-gossypol amino acid sodium salt derivative for blocking the invasion of an H5N1 avian influenza virus, and a preparation method and application thereof. The preparation method comprises the following steps: dissolving dextro-gossypol in an organic solvent to prepare a dextro-gossypol organic solution; dissolving amino acid and NaOH in an organic solvent to obtain an amino acid sodium organic solvent; mixing the dextro-gossypol organic solution and the amino acid sodium organic solvent to obtain a reaction liquid, and controlling the pH value of the reaction liquid to be 7.0-7.5; and enabling the reaction liquid to react in reflux conditions under the protection of nitrogen, then distilling out the organic solvent, and recrystallizing the obtained solids with acetone to obtain the dextro-gossypol amino acid sodium salt derivative. The dextro-gossypol amino acid sodium salt derivative has high activity of inhibiting the H5N1 avian influenza virus, and the activity of the derivative is higher than that of amantadine; and the dextro-gossypol amino acid sodium salt derivative acts on the entry phase of H5N1 virus infected cells. Thus, the dextro-gossypol amino acid sodium salt derivative can be used as an effective constituent in medicaments for preventing or treating H5N1 avian influenza.
Owner:WUHAN UNIV
MASA (multi-analyte suspension array) detection kit for influenza virus H5N1 subtype and H7N9 subtype
ActiveCN104388599AStrong specificityHigh sensitivityMicrobiological testing/measurementHemagglutininMulti analyte
The invention relates to the field of biological medicines, particularly to an MASA (multi-analyte suspension array) detection kit for influenza virus H5N1 and H7N9 subtypes, and provides a primer pair and a probe for detecting influenza virus surface hemagglutinin antigen H5 and H7 subtypes, a primer pair and a probe for detecting influenza virus neuraminidase antigen N1 and N9 subtypes as well as a kit comprising the primer pairs and the probes and a usage method of the kit. The kit is good in sensitivity, accuracy and specificity and simple, convenient and quick to use, and detection of to-be-detected samples can be finished within four hours. Tests prove that no false positive result exists when the kit is used for detecting negative samples; the detection rate of 100% is realized when the kit is used for detecting samples containing H7N9 subtype viruses and H5N1 subtype viruses only; the H7N9 and H5N1 subtype viruses contained in samples can be completely detected when the kit is used for detecting the samples containing both the H5N1 subtype viruses and the H7N9 subtype viruses. Besides, the kit can be used for effectively detecting to-be-detected samples containing more than 10 copies.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
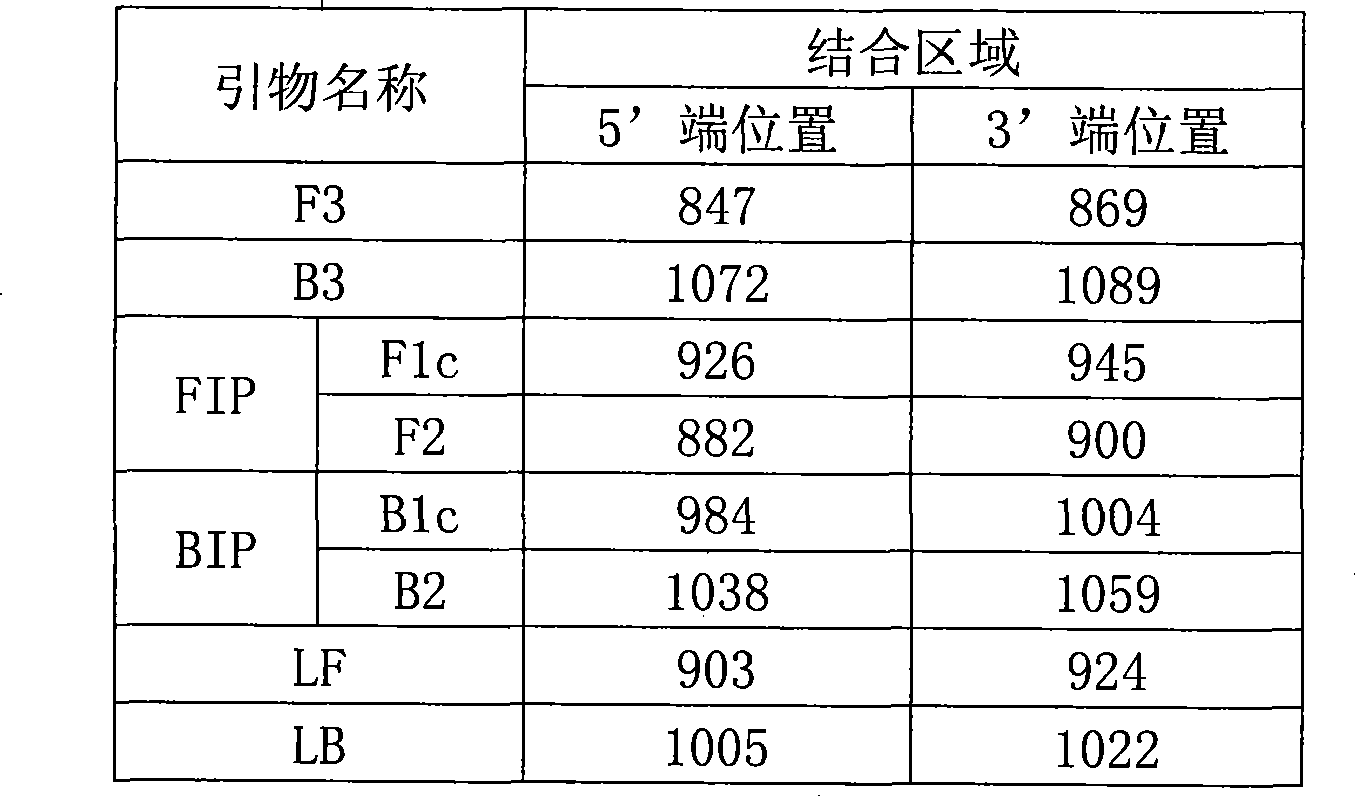
Subtype H5 avian influenza virus detection kit and use thereof
The invention discloses an H5 subtype bird flu virus detection reagent kit and application thereof. The reagent kit provided by the invention comprises three pairs of primers, namely an inner side primer pair, an outer side primer pair and an annular primer pair combined with bird flu H5N1 virus HA genes. The reagent kit also comprises a loop-mediated isothermal amplification reagent, positive contrast, negative contrast and a fluorescent chromogenic reagent; and the positive contrast is an H5 subtype bird flu virus RNA. The invention also provides a method for detecting whether the H5 subtype bird flu virus is carried in animals by applying the reagent kit. The H5 subtype bird flu virus detection reagent kit has high detection sensitivity, can detect a target RNA with 6 to 10 copies, is simple and convenient to operate and is particularly suitable for pathogenic detection carried out in the grass-roots field and the detection of the H5 subtype bird flu virus possibly polluting animal food.
Owner:CHINA AGRI UNIV
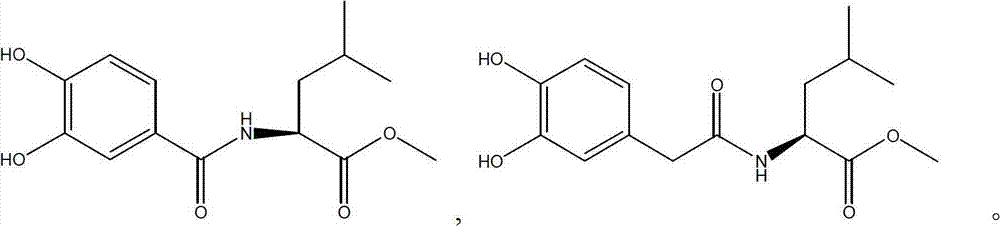
L-leucine derivatives blocking entrance of H5N1 avian influenza virus and preparation method for same
ActiveCN102924318ALow toxicityHigh activityOrganic active ingredientsOrganic compound preparationBenzoic acidH5N1 virus
The invention discloses L-leucine derivatives blocking the entrance of H5N1 avian influenza virus, which are 3,4-dyhydroxyl benzoyl leucine methyl ester and 3,4-dyhydroxyl phenylacetyl leucine methyl ester. The 3,4-dyhydroxyl benzoyl leucine methyl ester is prepared by condensing 3,4-dyhydroxyl benzoic acid with L-leucine methyl ester; the 3,4-dyhydroxyl phenylacetyl leucine methyl ester is prepared by taking 3,4-dyhydroxyl phenylacetic acid as a starting material, treating the 3,4-dyhydroxyl phenylacetic acid by virtue of dimethyl sulphate and thionyl chloride sequentially, then condensing the 3,4-dyhydroxyl phenylacetic acid with L-leucine methyl ester, and then performing demethylation reaction. The compounds, namely, the 3,4-dyhydroxyl benzoyl leucine methyl ester and the 3,4-dyhydroxyl phenylacetyl leucine methyl ester, disclosed by the invention, have a strong inhibitory activity on Influenza strain A / Vietnam / 1194 / 2004 avian influenza virus, and have a mechanism of acting on the entrance phase of H5N1 virus infected cells; and the compounds are a new inhibitor resisting the entrance of avian influenza virus, and can be used as a medicine for preventing or treating H5N1 avian influenza.
Owner:WUHAN UNIV
Influenza h5 vaccines
InactiveUS20140199337A1SsRNA viruses negative-senseViral antigen ingredientsH5N1 virusInfluenza vaccine
The present invention is based on the surprising finding that H5 protein of clade 1 H5N1 induces, in particular by a single-shot vaccination, a cross-clade protective immune response to influenza viruses with H5N1 HA. In one aspect, the invention is thus directed to H5 protein of clade 1 H5N1 virus for use in a method of treating or preventing infections with H5N1 virus of a different clade, namely of a clade different from clade 1 or from any clade with the exception of clade 1, respectively.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Oral vaccines produced and administered using edible micro-organism
The anti-pathogen vaccine of the present invention is produced in recombinant bacteria and / or transgenic plants and then administered through standard vaccine introduction method or through the oral administration. A DNA sequence encoding for the expression of an antigen of a pathogen is isolated and ligated to a promoter which can regulate the production of the surface antigen in a bacterial or transgenic plant. Preferably, a foreign gene is expressed in a portion of the plant or bacteria, and all or part of the antigen expressing plant or bacteria used for vaccine administration. In a preferred procedure, the vaccine is administered through the consumption of the edible plant as food, or the bacteria administered orally. The present invention also provides a method of using genetically modified microorganisms generally recognized to be edible and / or harmless to animals or humans when ingested, such as lactic acid bacteria, including Lactococcus lactis strains, as oral vaccines. In one embodiment, Lactococcus lactis expressing the avian influenza HA gene can be used as an oral vaccine for protection against H5N1 virus infection.
Owner:VAXGENE
Post-exposure therapy of influenza A infections
InactiveUS8569255B2Less likely to drug resistanceEffective and robustSsRNA viruses negative-senseOrganic active ingredientsH5N1 virusLiposome
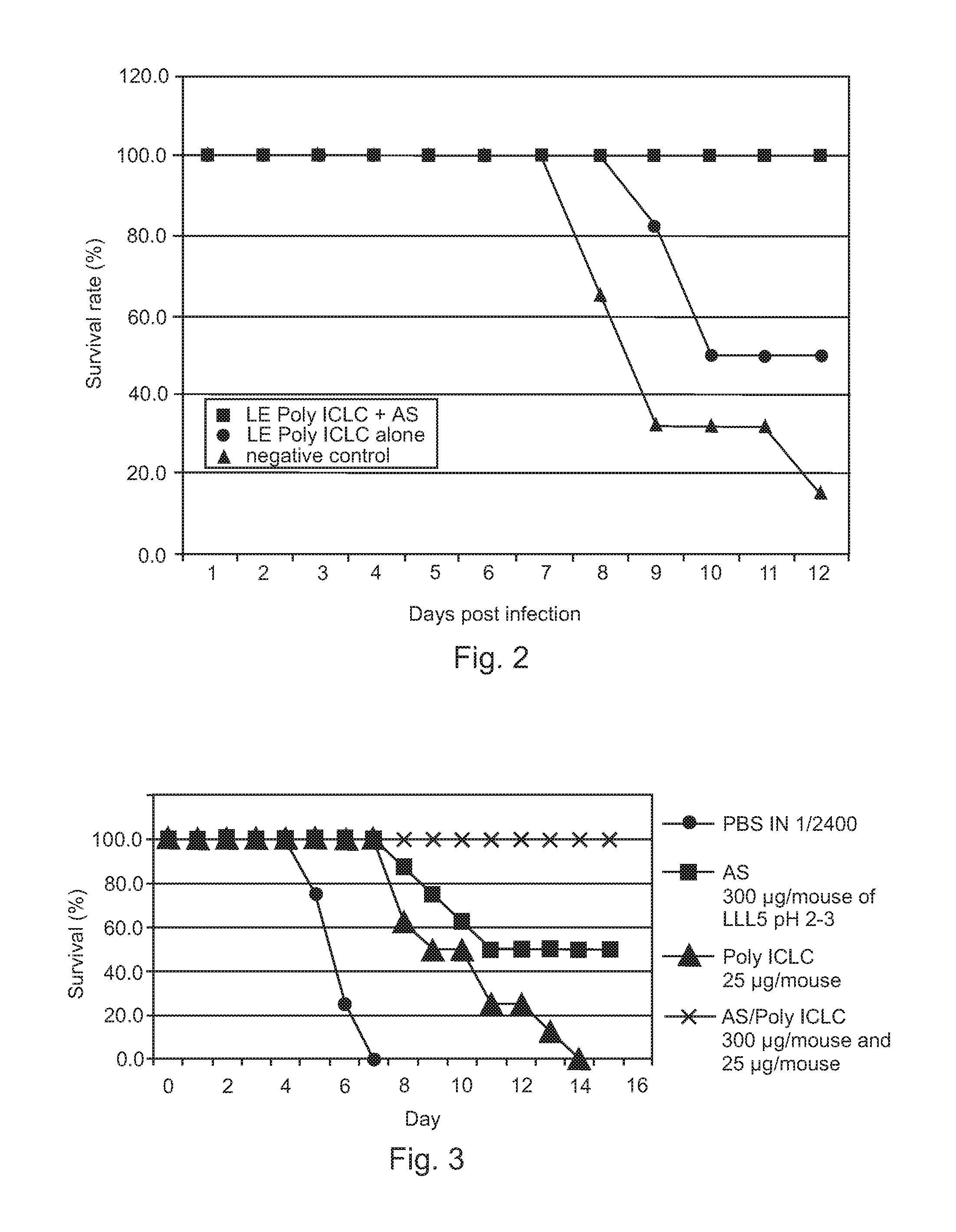
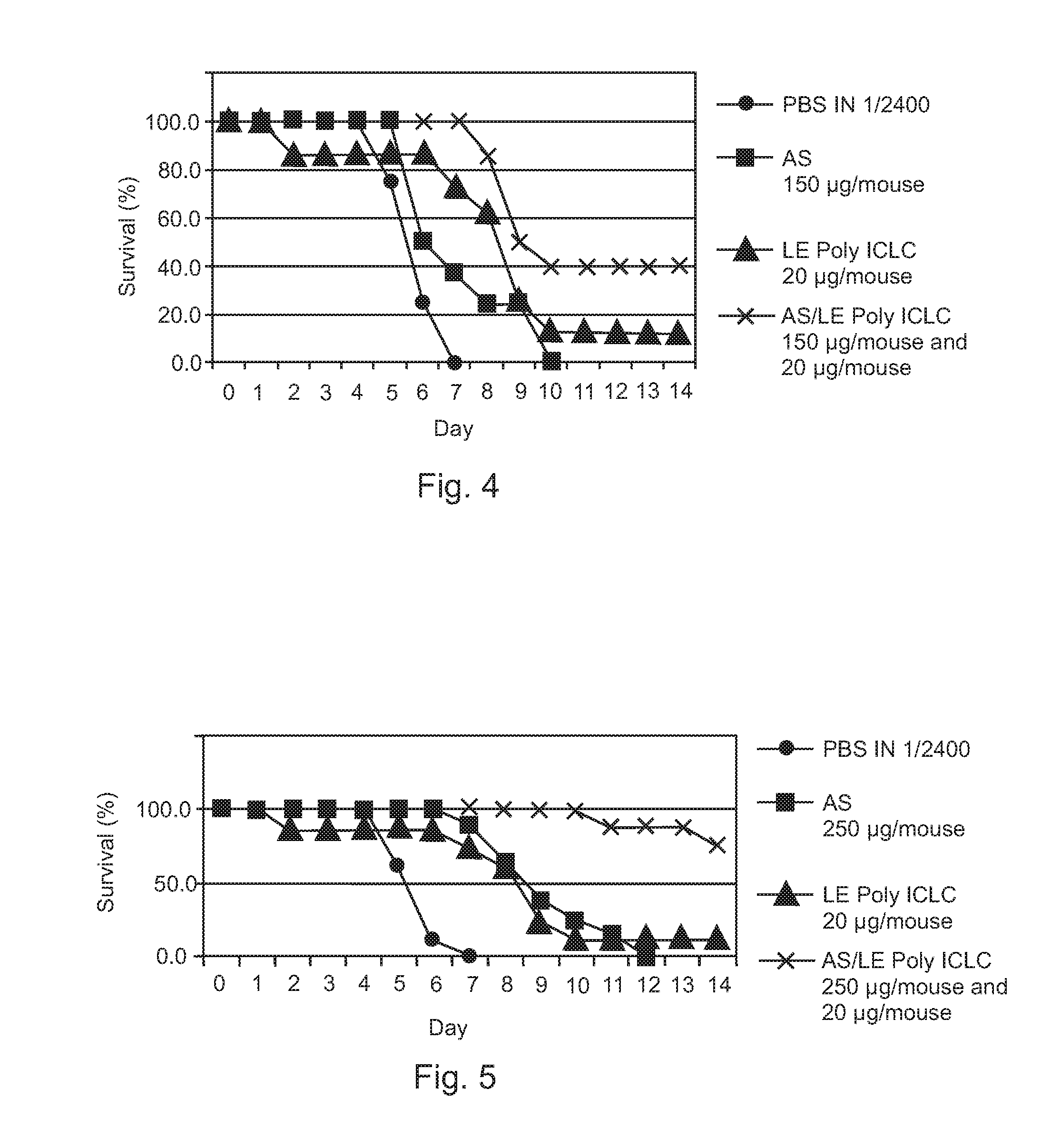
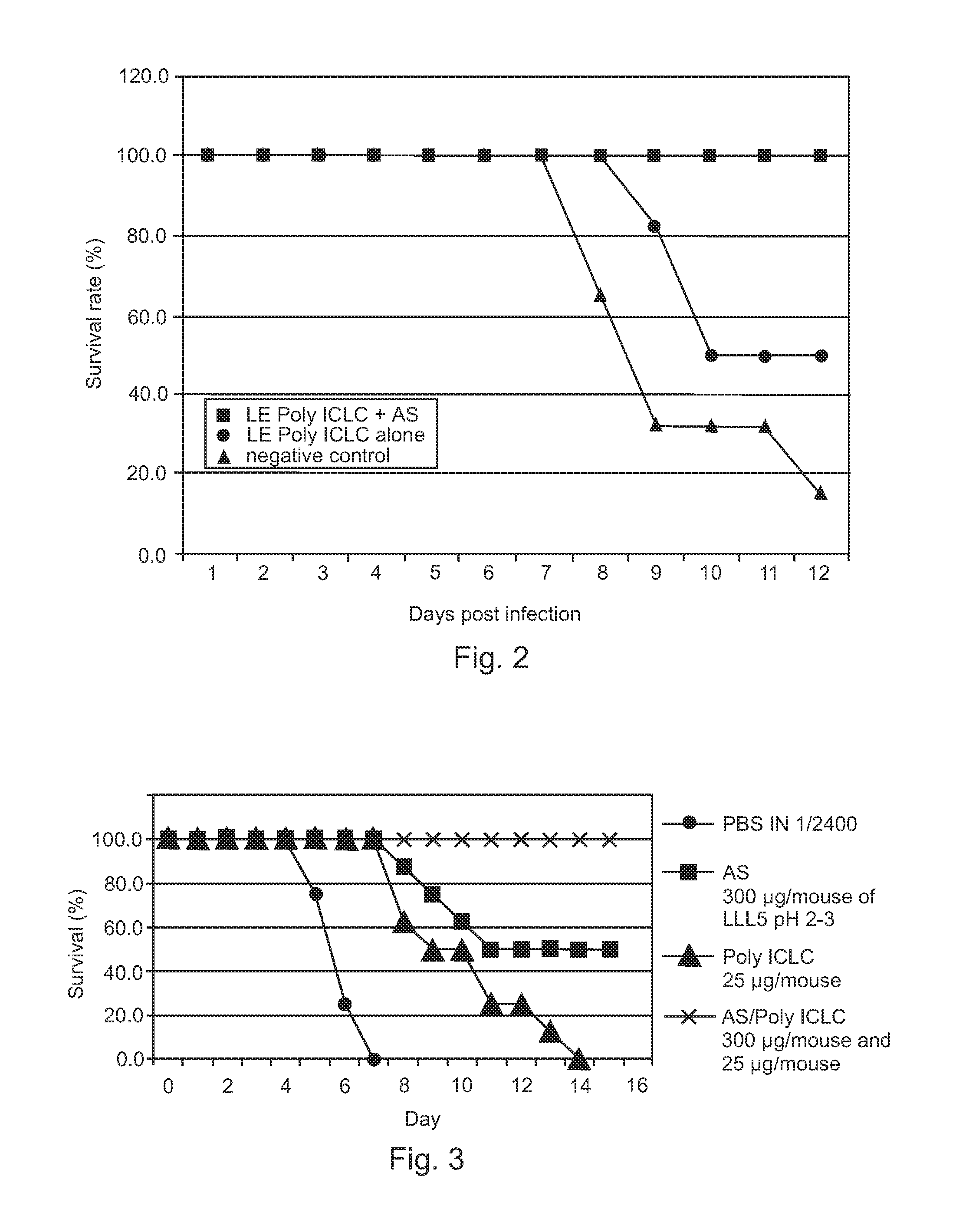
Poly ICLC or liposome-encapsulated Poly ICLC (LE Poly ICLC) in combination with antisense oligonucleotides (AS) act synergistically in post-exposure prophylaxis or therapy of influenza infections, especially H5N1 virus infections.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
CTL epitope polypeptide of avian influenza H5N1 virus and application thereof
The invention discloses a CTL epitope polypeptide of an avian influenza H5N1 virus and application thereof. The CTL epitope polypeptide of the avian influenza H5N1 virus is as shown by (a) or (b), wherein (a) is a polypeptide consisting of amino acid sequences shown by a sequence 1 in a sequence table; and (b) is a polypeptide derived from the sequence 1, is obtained by substituting and / or deleting and / or adding one or more amino acid residues for the amino acid sequence of the sequence 1 and is correlative with a CTL epitope of the avian influenza H5N1 virus. The DNA for coding the polypeptide is protected in the invention. The epitope polypeptide can be applied to preparing vaccines aiming at the avian influenza H5N1 virus and also can be applied to preparing a kit for testing specific T cells of the avian influenza H5N1 virus. The CTL epitope polypeptide of the avian influenza H5N1 virus has great values.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Isothermal amplification detection kit for bird flu H5N1 virus and detecting method thereof
InactiveCN101864498AComparableGood repeatabilityMicrobiological testing/measurementSingle samplePositive control
The invention relates to an isothermal amplification detection kit for bird flu H5N1 virus and a detecting method thereof. The kit comprises RNA extract, bird flu H5N1 virus nucleic acid isothermal amplification reaction liquid, bird flu H5N1 virus positive control and negative control. The detection kit has the advantages of high specificity, high sensitivity, high reaction speed, suitability for sample detection of both high flux and low flux and no need of complicated instrument in the whole reacting process, wherein only 2 hours are required from treating a single sample to finishing detection.
Owner:上海国际旅行卫生保健中心 +1
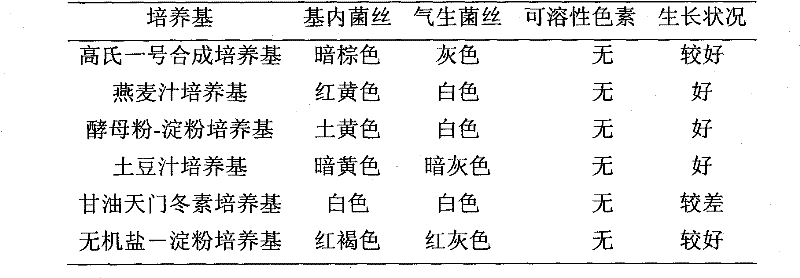
Antiviral actinomyces HA10206 and application thereof
InactiveCN102399717AEnhanced inhibitory effectGood inhibitory effectBacteriaBacteria material medical ingredientsMicroorganismH5N1 virus
The invention discloses an actinomyces HA10206 strain for generating substances with antiviral activity, and the preservation number is CGMCC No.4861, and the invention belongs to the field of microbiological technology. The present invention has the advantages that: the actinomyces HA10206 strain is a new species of streptomyces, and the fermentation liquor of the product has obvious inhibition effects on H1N1 and H2N2, H3N2, H5N1 viruses of the same series, and certain inhibition effects on HIV and HBV, thereby the actinomyces HA10206 has good medicament development prospects.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
Application of cyclic dipeptide C7 in Phellinus igniarius in resisting avian influenza H5N1 virus
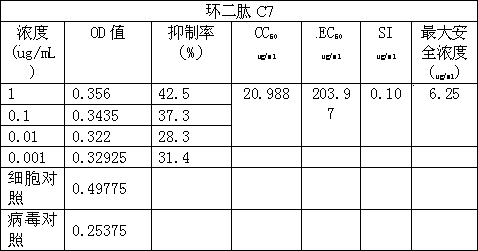
The invention discloses a cyclic dipeptide C7 with anti-avian influenza activity separated from Phellinus igniarius (LexFr) Quel (phellinus linteus (BerketCurt) Teng, Phellinus baumii, Phellinus hartigii (AlleschetSchnabl) Imaz) fermentation liquid and sporophores. The invention discloses new activity of cyclic dipeptide. The cyclic dipeptide is proved to have an anti-avian influenza action. Compared with the application of common compounds, the method detects the new activity of the cyclic dipeptide, and the cyclic dipeptide has the anti-avian influenza action.
Owner:QINGDAO AGRI UNIV
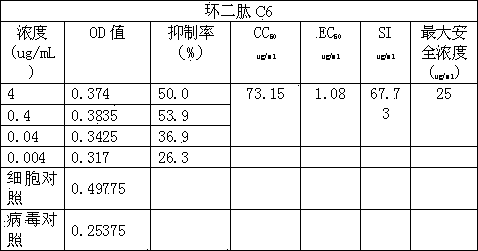
Application of cyclic dipeptide C6 in Phellinus igniarius in resisting avian influenza H5N1 virus
The invention discloses a cyclic dipeptide C6 with anti-avian influenza activity separated from Phellinus igniarius (LexFr) Quel (phellinus linteus (BerketCurt) Teng, Phellinus baumii, Phellinus hartigii (AlleschetSchnabl) Imaz) fermentation liquid and sporophores. The invention discloses new activity of cyclic dipeptide. The cyclic dipeptide is proved to have an anti-avian influenza action. Compared with the application of common compounds, the method detects the new activity of the cyclic dipeptide, and the cyclic dipeptide has the anti-avian influenza action.
Owner:QINGDAO AGRI UNIV
Highly pathogenic avian influenza virus protein vaccine derived from transgenic plant, and preparing method thereof
InactiveUS20120219580A1Quick and easy mannerEfficient expressionSsRNA viruses negative-senseVaccinesHemagglutininH5N1 virus
The present invention relates to a method for producing transgenic plants, which involves producing hemagglutinin proteins of the H5N1 virus using a plant transformation recombinant vector, wherein said vector can express hemagglutinin proteins of the H5N1 virus, the highly pathogenic avian influenza virus, and transport proteins expressed in plants to the endoplasmic reticulum and enable the retention of the proteins in the endoplasmic reticulum to enable glycosylation required for antigen activity. The present invention also relates to a method for the mass production of hemagglutinin proteins of the antigenic H5N1 virus from transgenic plants or a vaccine composition for the avian influenza virus comprising the transgenic plants or the hemagglutinin proteins produced from the plants. The transgenic plants can be used as edible vaccines, antigenic hemagglutinin proteins produced can be used as protein vaccines for the H5N1 avian influenza virus, or as diagnostic reagents for avian influenza virus infection.
Owner:HELIX
Compositions for treating respiratory viral infections and their use
ActiveUS8691781B2Promote divisionHigh degreeSsRNA viruses negative-senseBiocideH5N1 virusRespiratory viral infection
The invention provides siRNA compositions that interfere with viral replication in respiratory viral infections, including respiratory syncytial virus and avian influenza A, including the H5N1 strain. The invention further provides uses of the siRNA compositions to inhibit expression of viral genes in respiratory virus-infected cells, and to uses in the treatment of respiratory virus infections in a subject. Generally the invention provides polynucleotide that includes a first nucleotide sequence of 15 to 30 bases that targets the genome of a respiratory syncytial virus or an influenza A virus, a complement thereof, a double stranded polynucleotide or a hairpin polynucleotide. Additionally the invention provides vectors, cells and pharmaceutical compositions containing siRNA sequences.
Owner:SIRNAOMICS INC
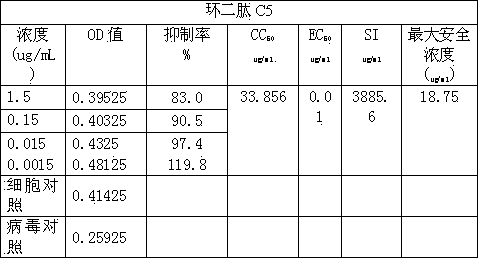
Application of cyclic dipeptide C5 in phellinus igniarius in resisting avian influenza H5N1 virus
The invention discloses cyclic dipeptide C5 with anti-avian influenza activity, and the cyclic dipeptide C5 is separated from fermention broth and fruiting body of phellinus igniarius (L ex Fr) Quel (phellinus linteus (Berk et Curt) Teng, Phellinus baumii and Phellinus hartigii (Allesch et Schnabl)Imaz). A lead compound is provided to research and development of anti-avian influenza medicines, and the application has an important meaning to development and utilization of medical fungus resources.
Owner:QINGDAO AGRI UNIV
Anti-h5n1 influenza activity of the antiviral protein cyanovirin
The invention is directed to a method of inhibiting prophylactically or therapeutically an H5N1 viral infection in a host, which method comprises administering to the host an anti-viral effective amount of an antiviral protein comprising the amino acid sequence of SEQ ID NO: 1 or a nucleic acid encoding the antiviral protein, as well as antiviral portions, variants, and conjugates thereof.
Owner:UNITED STATES OF AMERICA
Post-exposure therapy of influenza a infections
InactiveUS20120195960A1Excellent survival prospectGood initiativeSsRNA viruses negative-senseOrganic active ingredientsH5N1 virusLiposome
Poly ICLC or liposome-encapsulated Poly ICLC (LE Poly ICLC) in combination with antisense oligonucleotides (AS) act synergistically in post-exposure prophylaxis or therapy of influenza infections, especially H5N1 virus infections.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Marine actinomycete with antiviral activity and application of marine actinomycete with antiviral activity
InactiveCN105349448AEnhanced inhibitory effectBacteriaMicroorganism based processesH5N1 virusBacteroides dorei
The invention belongs to the technical field of microorganisms and discloses marine actinomycete capable of generating antiviral active substances. The marine actinomycete refers to Streptomyces HA12284 and is preserved in the China General Microbiological Culture Collection Center on July 16, 2015, and a preservation number is CGMCC No.11079. Fermentation liquor of the Streptomyces HA12284 has an evident inhibition effect on H1N1, H2N2, H3N2 and H5N1 viruses of the same series and has a certain inhibition effect on HBV (hepatitis B virus) and HIV (human immunodeficiency virus), and the marine actinomycete has a bright prospect of medicinal development.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
Influenza h5 vaccines
ActiveUS20170348413A1SsRNA viruses negative-senseViral antigen ingredientsH5N1 virusFlu immunization
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preparation method of recombinant baculovirus vaccine for preventing and treating avian influenza H5N1
The invention relates to a preparation method of a recombinant baculovirus vaccine for preventing and treating avian influenza H5N1, in particular to an H5N1 virus vaccine strain for expressing avian influenza virus hemagglutinin HA gene and a preparation method thereof. The preparation method concretely comprises the following steps of 1) building pS-ITRs-HA plasmids; 2) converting the pS-ITRs-HA plasmids into E .coli DH10 Bac; 3) transfecting Sf9 insect cells by recombinant baculovirus shuttle vectors; 4) amplifying the recombinant baculovirus. When the recombinant H5N1 virus vaccine strain obtained through preparation is used for performing immunodetection on blood serum of immune chicken; the level of IgG antibodies in the blood serum of the immune chicken can reach 0.966 to the highest degree; the concentrations of IL-2, IL-4 and IFN-gamma can respectively reach 89 .67ng / L, 80 .21ng / L and 64 .24ng / L to the highest degree. The results show that the recombinant baculovirus vaccine can effectively irritate the chicken bodies to simultaneously generate the body fluid immunity and the cell immunity; the dual immunity protection effect is provided for the chicken group; the invention lays the theoretical foundation for the research and development of the avian influenza virus based on the baculovirus.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com