Immunoprotection by oral administration of recombinant lactococcus lactis mini-capsules
A technology of lactic acid bacteria and immune response, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problem of expensive and troublesome human and farm animals, impossible to use wild birds, and influenza vaccines that cannot be used for H5N1 Variety and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Materials and methods
[0064] Recombinant Lactococcus lactis vector
[0065] Three different antigen expression plasmids were constructed and named pNZ8150-HA, pN8110-HA and pNZ8110-pgsA-HA1. Plasmid pNZ8148 was purchased from the Netherlands (NIZO). The plasmid was transformed into Lactococcus lactis NZ9000 by electroporation. Highly expressed clones were selected and cultured at 30°C in M17 medium with 0.5% (wt / vol) glucose. Chloramphenicol was used at a concentration of 10 micrograms per milliliter.
[0066] Western blot analysis and immunofluorescence microscopy
[0067] Add nisin A to a final concentration of 10 ng / ml to induce antigen expression in all recombinant L. lactis. Continue to grow for 3 hours. Harvest L1, L2, L4 Lactococcus lactis cells, wash 3 times with 500 μl of sterile phosphate buffered saline (PBS) and resuspend. Aliquots of samples were mixed with 6x loading buffer and boiled for 10 minutes. The extract was subjected to sodium dode...
Embodiment 2
[0085] Recombinant Lactococcus lactis vector
[0086] Construction of recombinant Lactococcus lactis vector expressing H5N1 influenza HA antigen
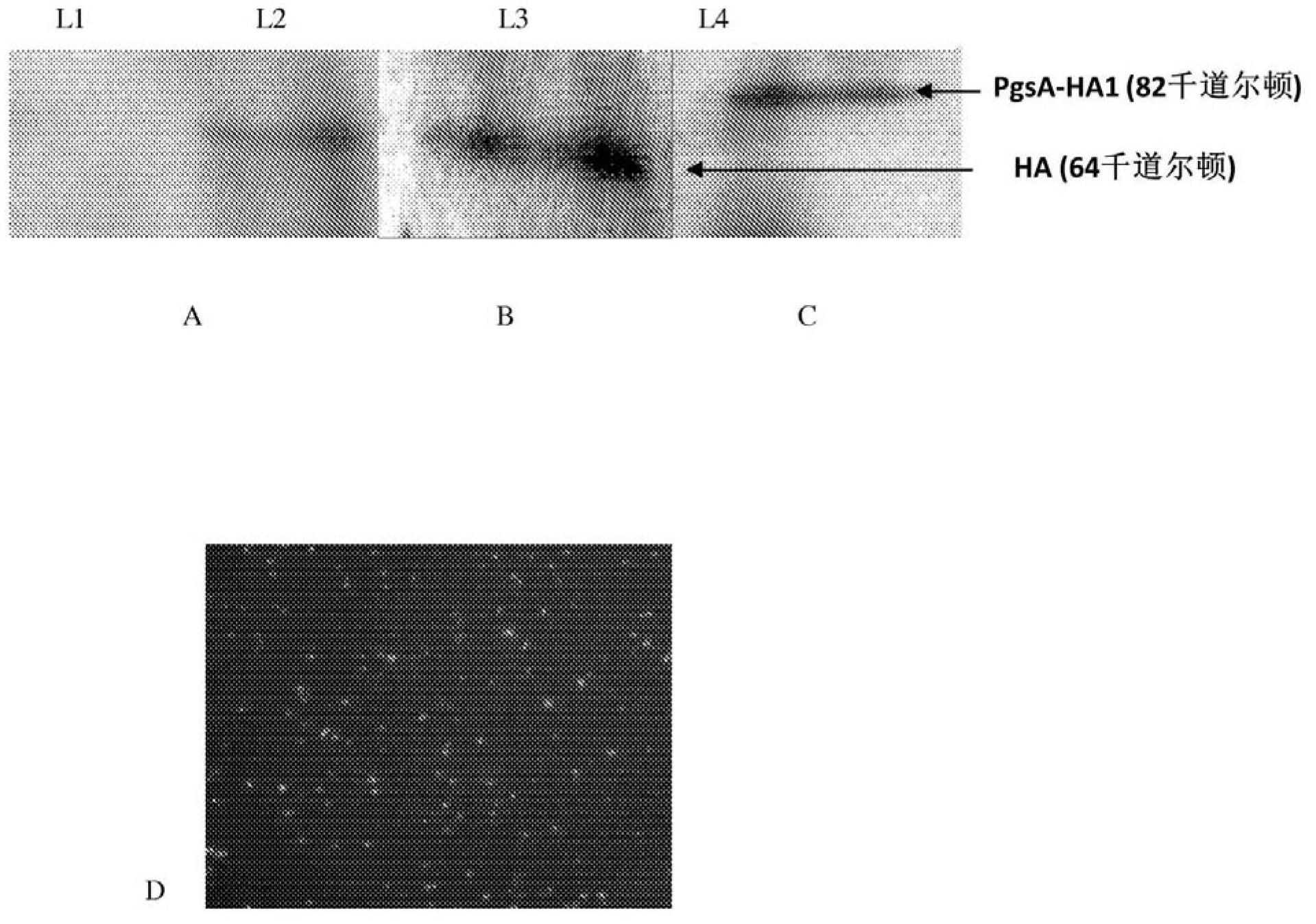
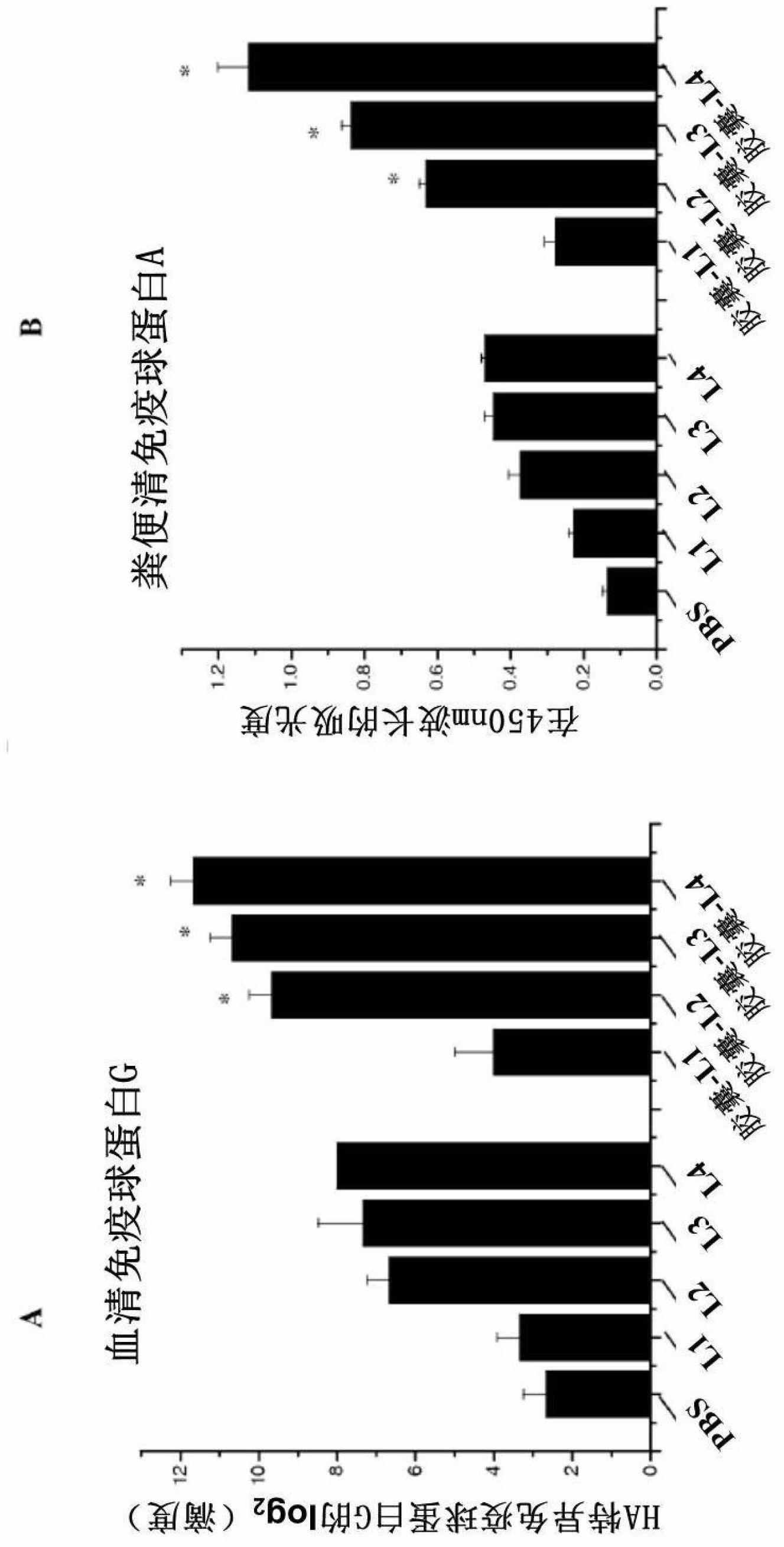
[0087] Four different L. lactis vectors were constructed and named L1, L2, L3 and L4. Table 1 lists the description of each vector and the specific HA expression plasmids within each vector. Immunoblot analysis showed that L1 as a control vector did not express any HA ( figure 1 A), while the HA protein (64 kD) expressed by L2 was mainly found in the cell lysate ( figure 1A). Since a usp45 signal sequence was cloned before the HA gene of L3, most of the HA expressed by L3 was found in the cell culture supernatant ( figure 1 B). L4 carries a plasmid encoding a fusion HA1 protein (38 kD) and a PgsA surface display motif (44 kD); the resulting protein has a molecular mass of 82 kD ( figure 1 C). Furthermore, immunofluorescence staining confirmed the cell wall anchor distribution of HA1 protein (L4) ( figure 1 D).
[0088] ...
Embodiment 3
[0106] further improvement
[0107] Standard in vitro or in vivo titration experiments can be performed to adjust or reduce the dose of bacteria used in the present invention, using the various biological responses described above or animal survival as experimental readouts.
[0108] Similar titration experiments can also be used to determine the effect of coated capsule size on the induction of immune responses. The above-mentioned in vitro or in vivo experiments can be used to investigate the effects of capsules of various sizes on the induction of cellular immune responses, mucosal immune responses and / or protective immune responses.
[0109] Likewise, similar titration experiments can be used to determine the effect of using different coating materials or mucoadhesive polymers. The above-mentioned in vitro or in vivo experiments can be used to investigate the effects of capsules coated with different materials or bacteria formulated with different adhesive polymers on t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com