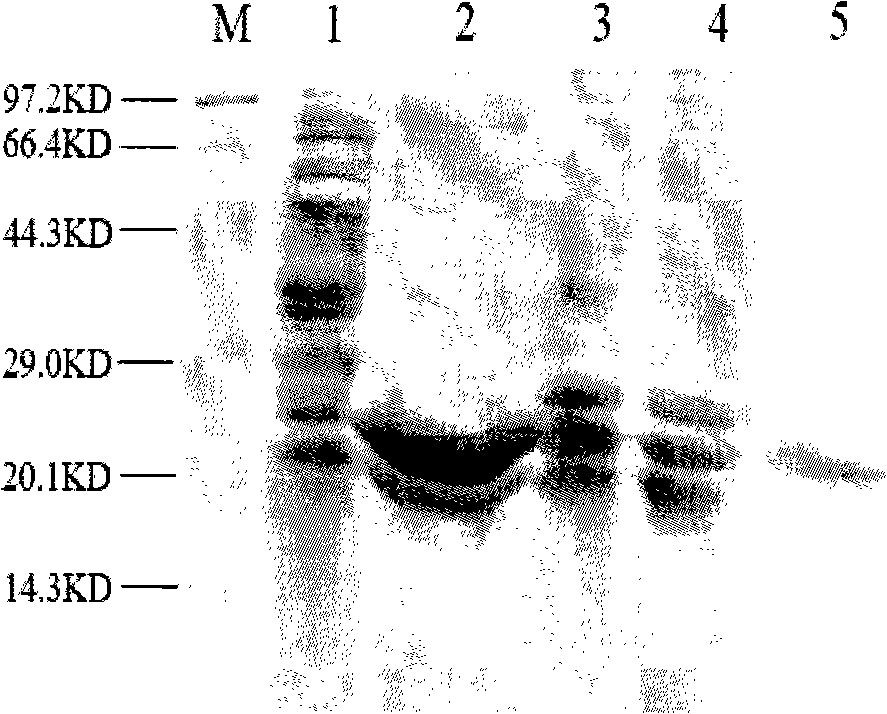
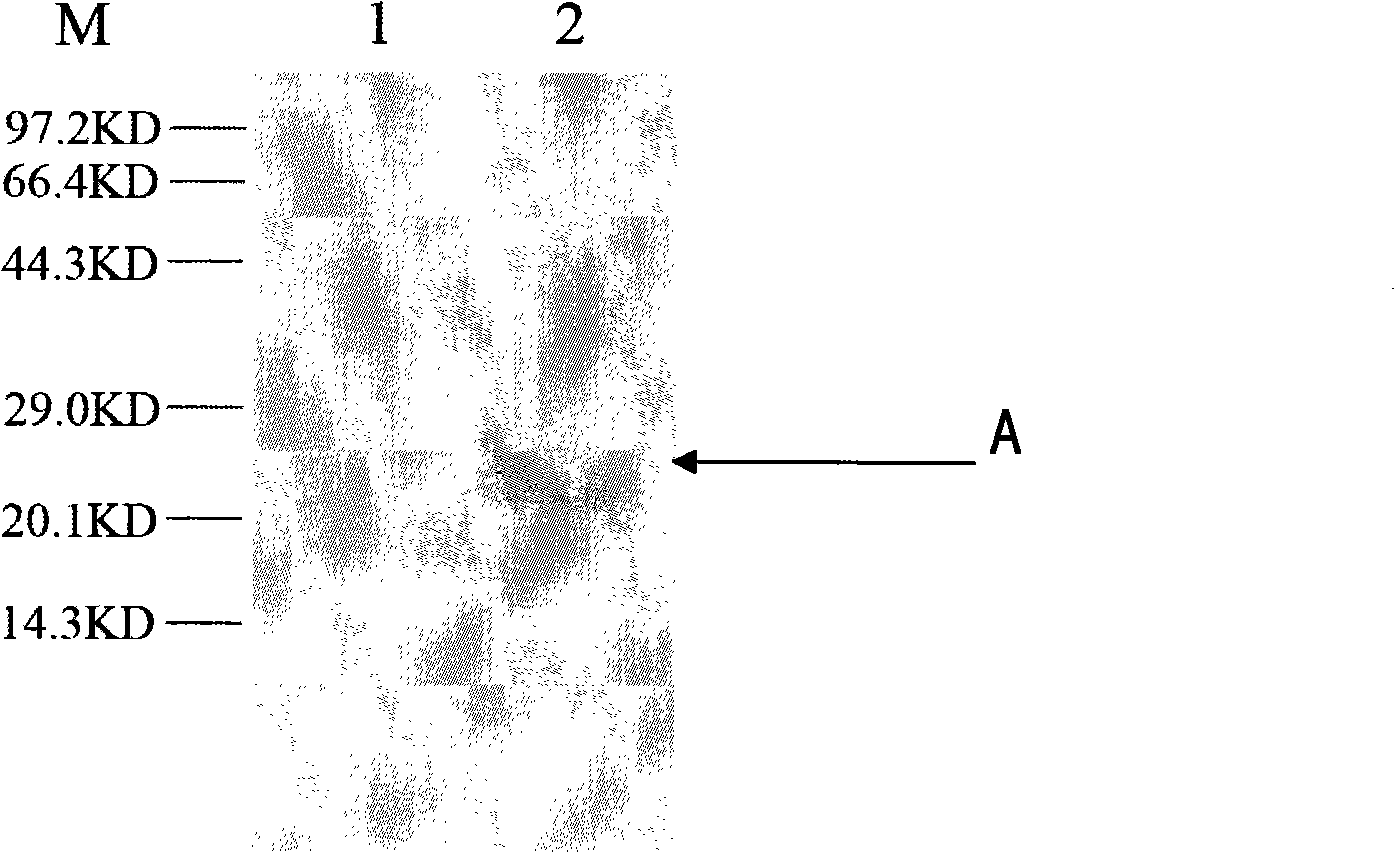
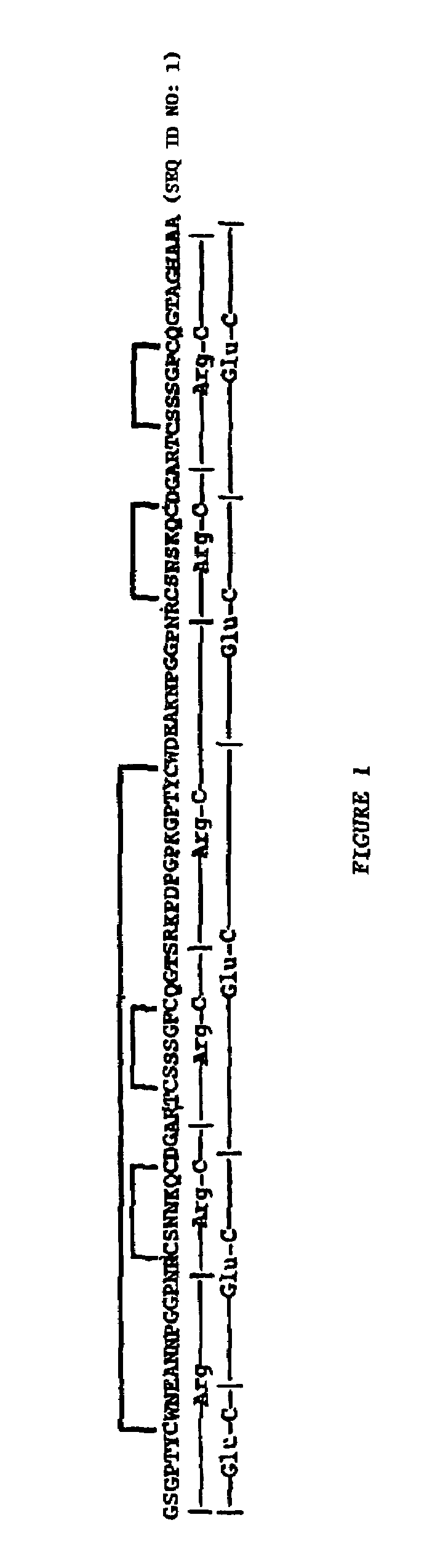
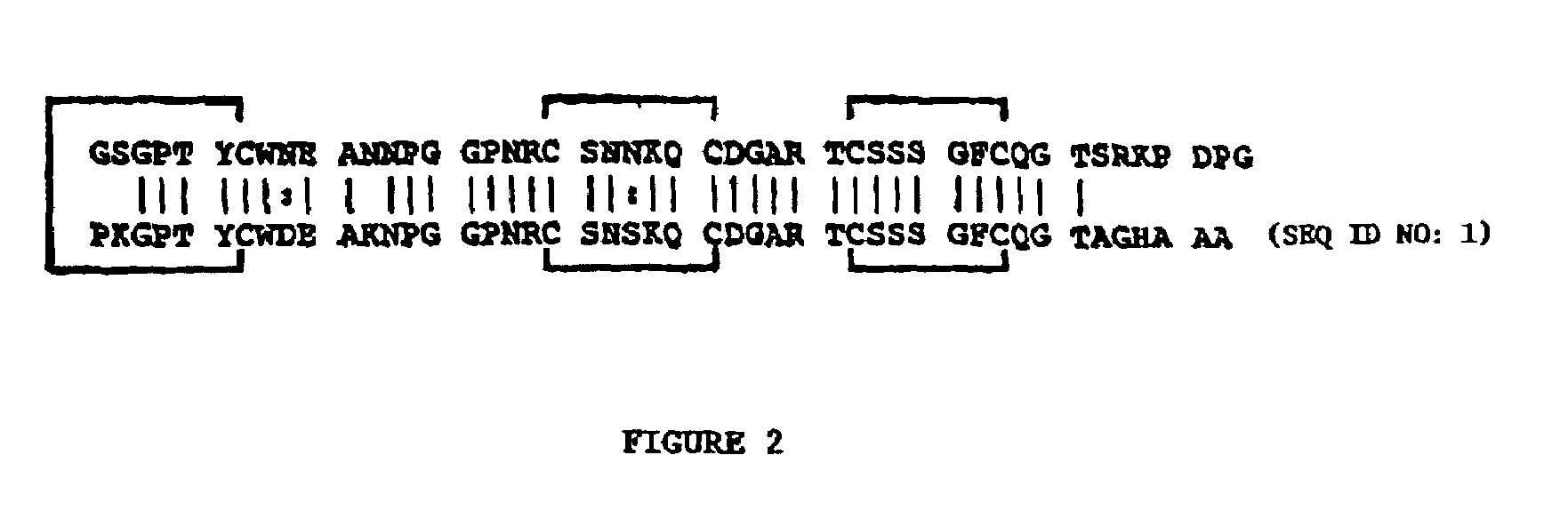
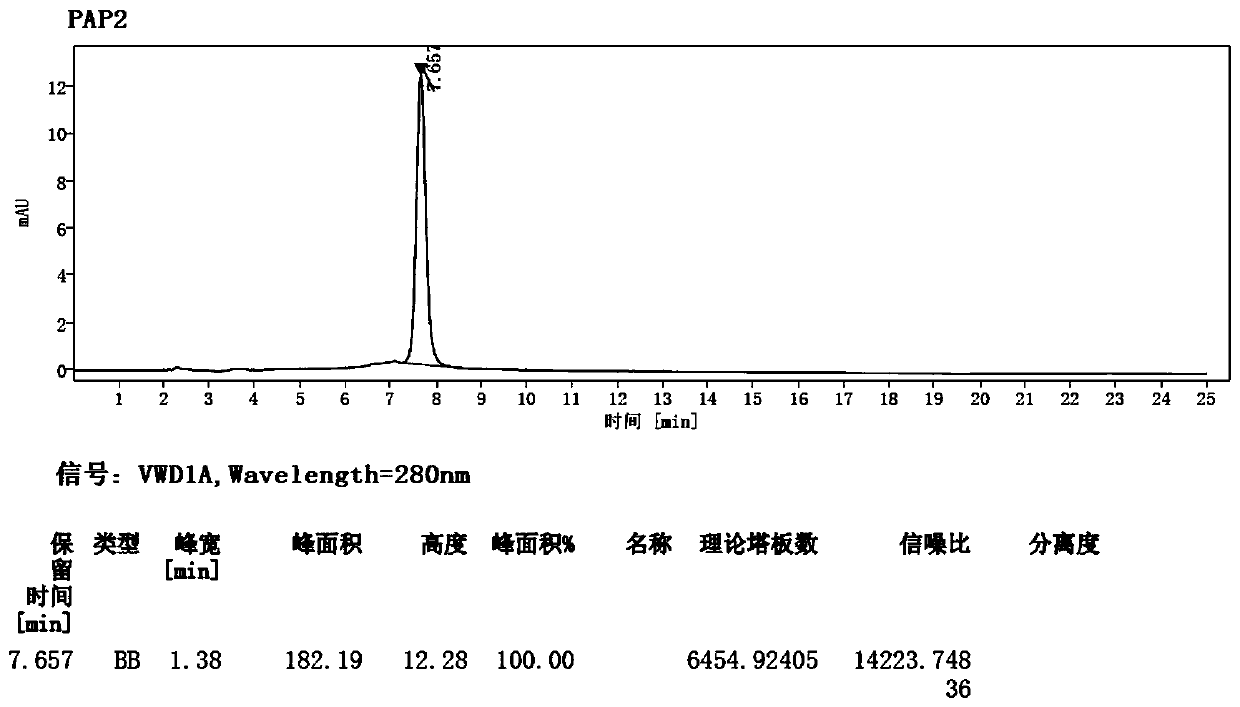
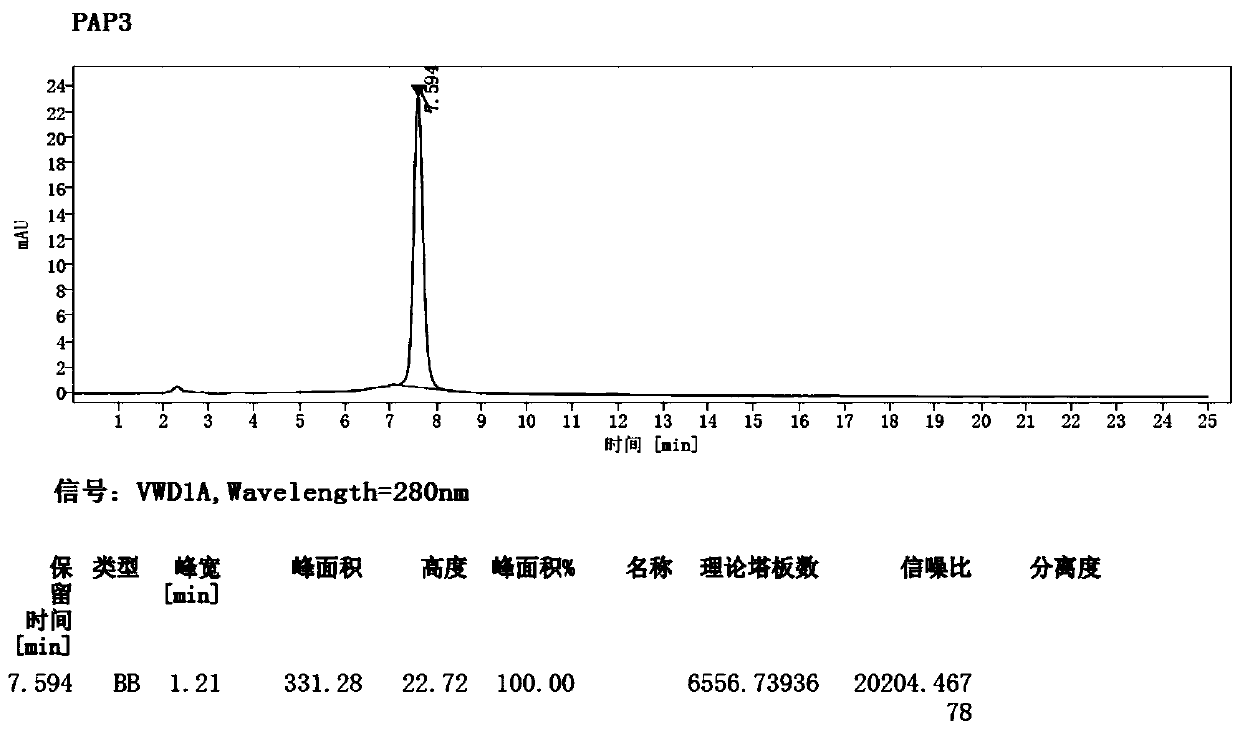
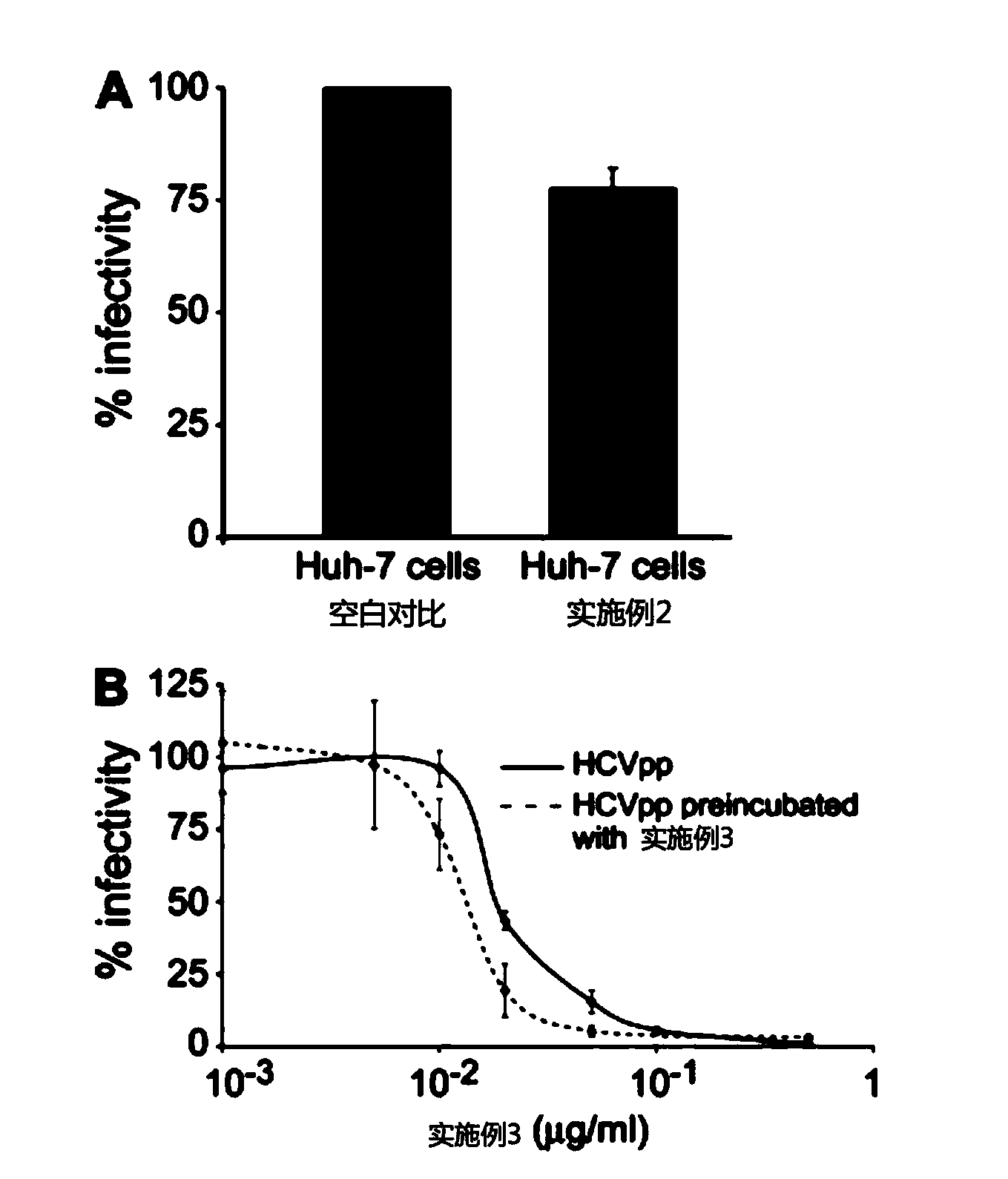
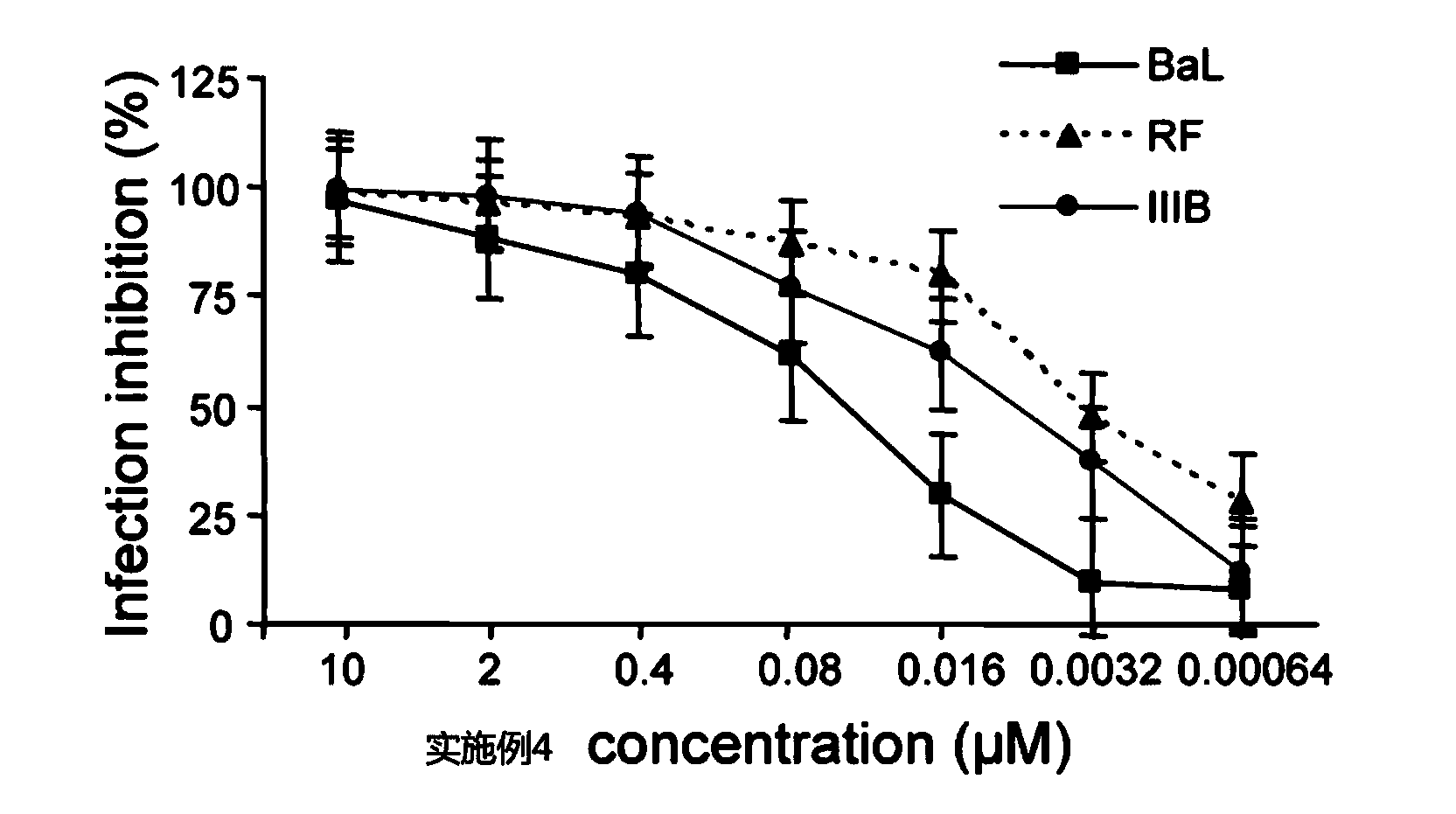
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "ZINC FINGER ANTIVIRAL PROTEIN" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Zinc finger Antiviral Protein (ZAP) is a specific antiviral protein that aids in the destruction of virus particles, specifically the Moloney murine leukemia virus (MLV) and the Sindbis virus (SIN). ZAP prevents the viral mRNA from building up inside the cell.
Method for preparing recombined blue algae antiviral protein and application thereof
InactiveCN101705241APreserve structural featuresSimplify downstream purification routesBacteriaPeptide/protein ingredientsInclusion bodiesAntiviral drug
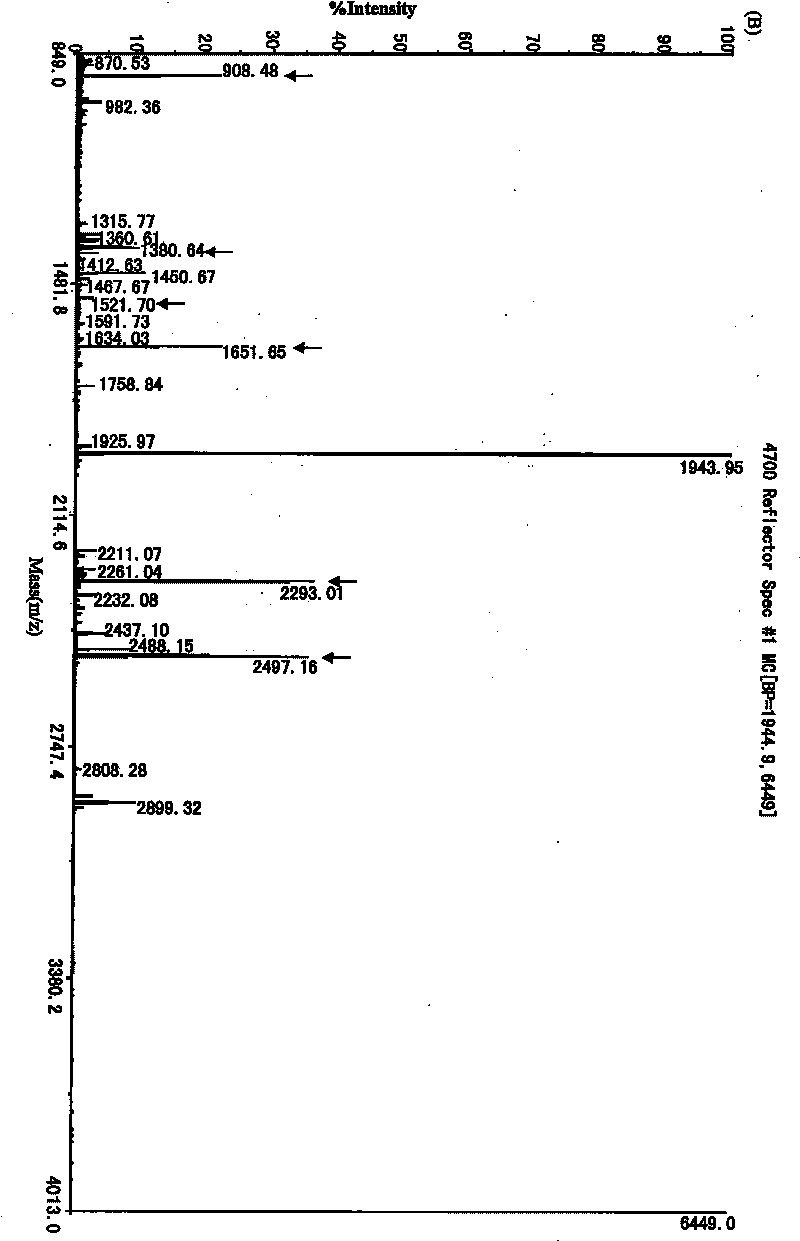
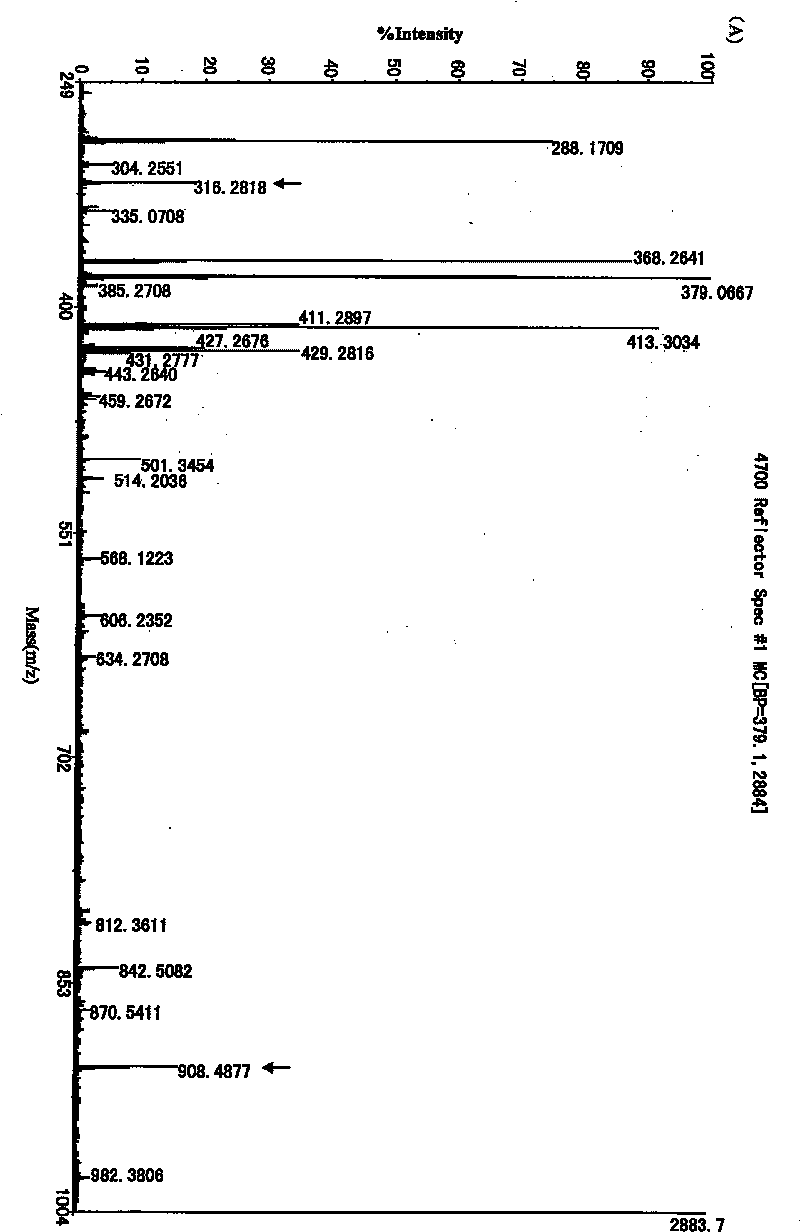
The invention provides an expression vector containing a recombined blue algae antiviral protein N nucleotide sequence, a host cell containing the expression vector and a method for preparing recombined blue algae antiviral protein on the basis to obtain the recombined blue algae antiviral protein and further proves that the expression vector and / or the host cell and / or the recombined blue algae antiviral protein can be applied to prepare an antiviral medicament. The method of the invention overcomes the disadvantages of low yield, an easily formed inclusion body and difficult purification and the like in the prior art. Peptide mass fingerprinting of the recombined CVN protein prepared by the method is totally consistent with theoretical peptide mass fingerprinting; and an antiviral experiment proves that the recombined protein has good antiviral activity.
Owner:JINAN UNIVERSITY
Antiviral protein and uses thereof
InactiveCN101343327ACapable of penetrating membraneLow immunogenicityPeptide/protein ingredientsAntiviralsCytosine deaminaseProtein molecules
The invention relates to anti-viral protein. The anti-viral protein is characterized in that the anti-viral protein is fusion protein and comprises a transmembrane peptide structural domain and a tandem body which is formed through at least two cytosine deaminase structural domains originated from an APOBEC family, a transmembrane peptide structural domain and various cytosine deaminase structural domains. The transmembrane peptide structural domain in the anti-viral protein provided by the invention ensures the anti-viral protein to have the transmembrane capability and to freely shuttle among cells and enters the cells in a non-receptor and non-energy dependent way; the cytosine deaminase structural domains originated from the APOBEC family ensures that the anti-viral protein can effectively inhibit the reproduction of human immunodeficiency virus and / or hepatitis B virus after entering cells; because the non-essential sequences in the APOBEC family protein molecules are removed, not only the antiviral activity of the APOBEC family protein molecules is preserved, but also the immunogenicity of the anti-viral protein is reduced, and the transmembrane efficiency of the anti-viral protein is enhanced.
Owner:SHANTOU UNIV MEDICAL COLLEGE
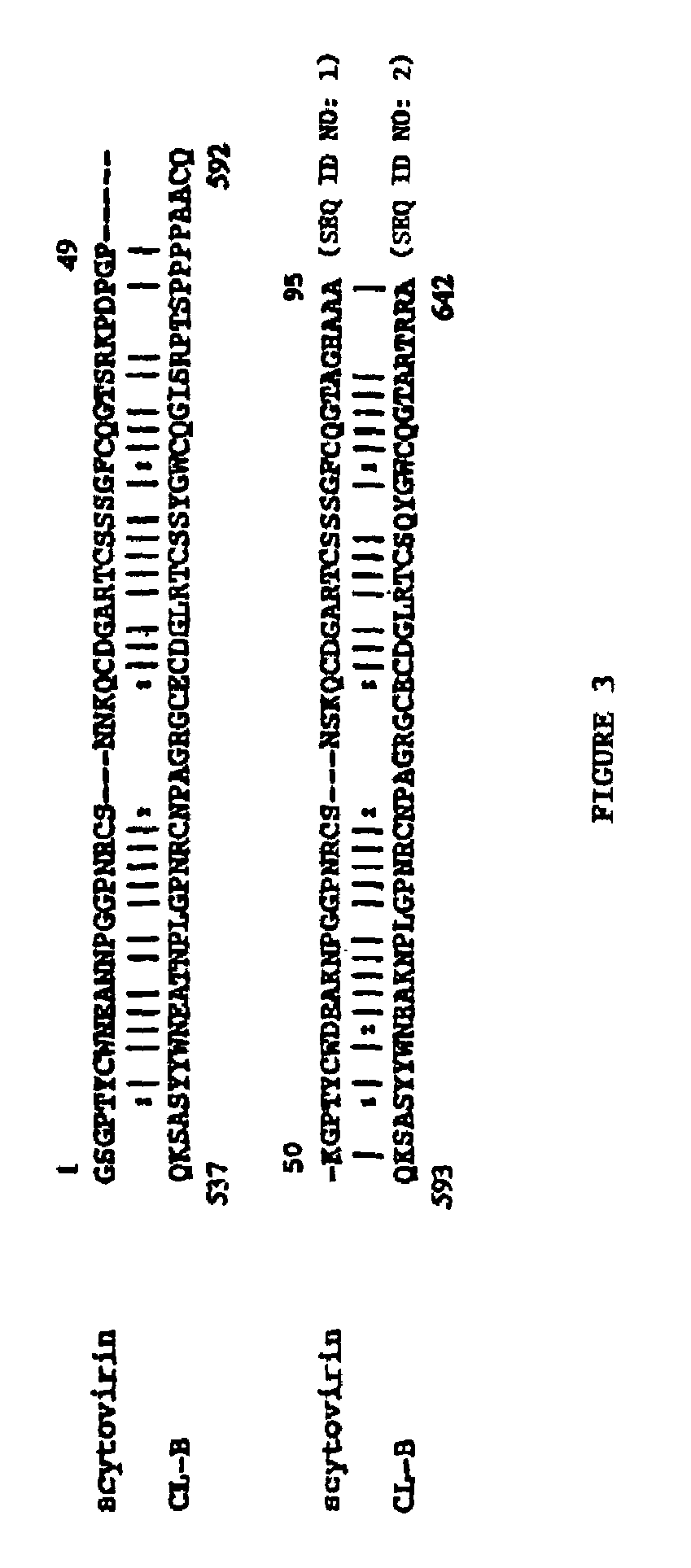
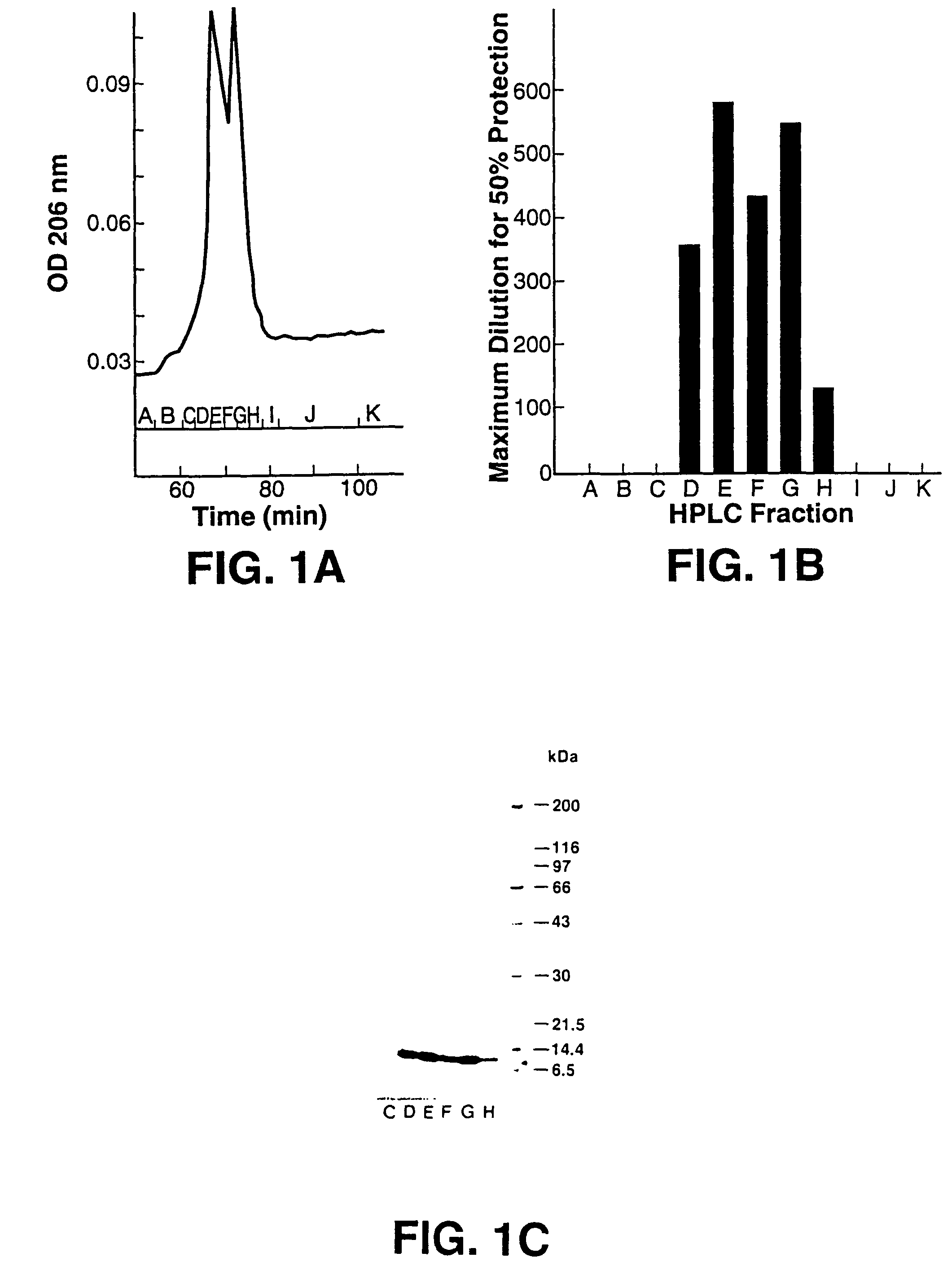
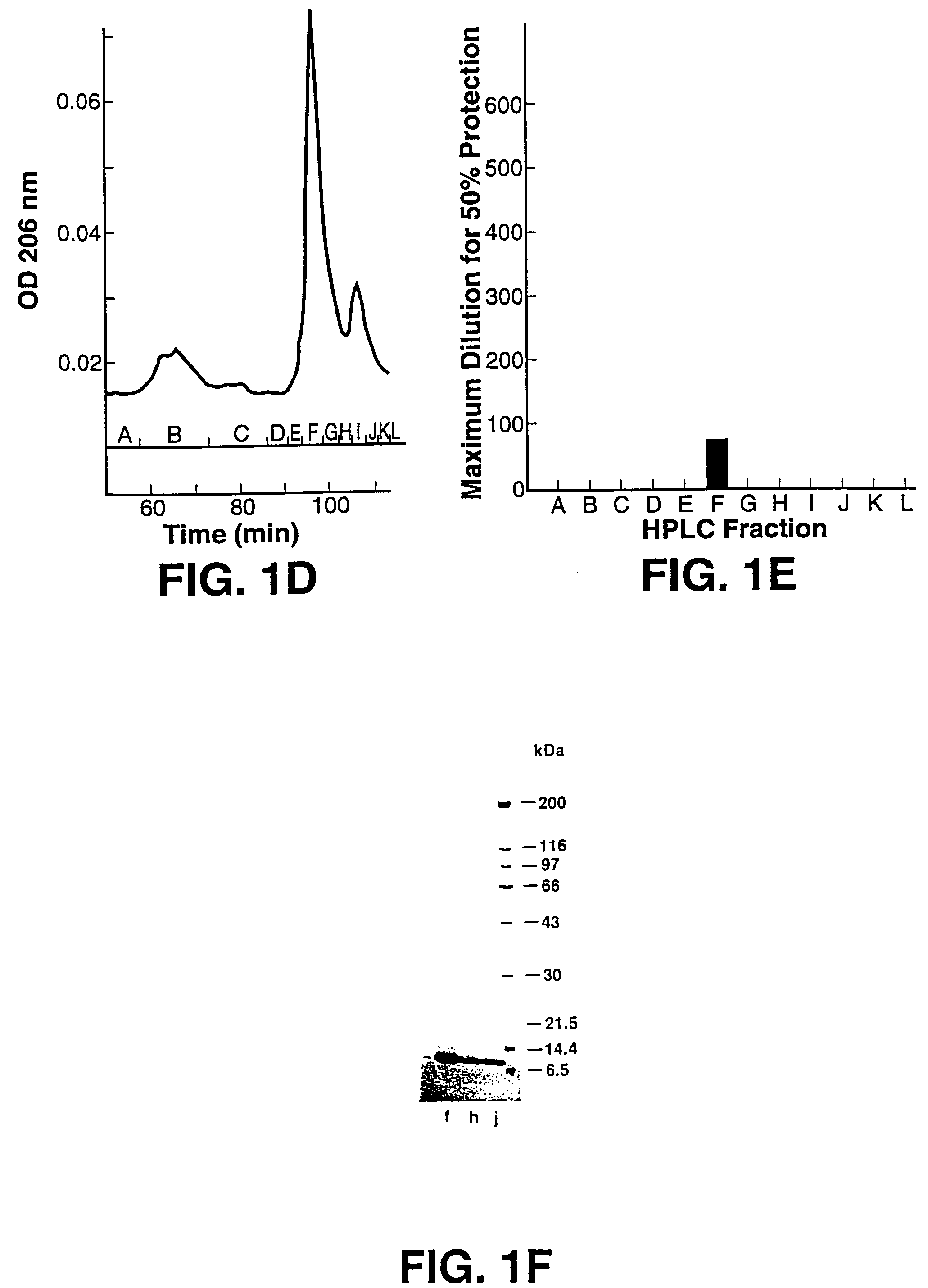
Scytovirins and related conjugates, fusion proteins, nucleic acids, vectors, host cells, compositions, antibodies and methods of using scytovirins
InactiveUS7491798B2Protect against undesired immune responsesExtended half-lifeFungiBacteriaScytovirinZINC FINGER ANTIVIRAL PROTEIN
An isolated or purified antiviral protein of SEQ ID NO: 1, nucleic acids encoding the antiviral protein, cells comprising the nucleic acids, and methods of inhibiting viral infection comprising contacting the virus with the antiviral protein.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Methods of using cyanovirins to inhibit viral infection
InactiveUS7754420B2Protect against undesired immune responsesExtended half-lifeBiocideSaccharide peptide ingredientsZINC FINGER ANTIVIRAL PROTEINAntiviral protein
The present invention provides methods of treating a viral infection of a host using antiviral proteins (collectively referred to as cyanovirins).
Owner:UNITED STATES OF AMERICA
Antiviral protein and preparation method and application thereof
The invention discloses an antiviral protein RC28, the amino acid thereof is shown in SEQ ID NO.1, and the preparation method thereof comprises the following steps: crude separation is carried out to obtain crude extract of RC28, molecular sieve chromatography and ion exchange chromatography are used to further carry out separation and purification to obtain RC28 protein product. The antiviral protein RC28 of the invention has good antiviral activity, and provides an efficient and safe new way for treating virus infection, in particular to enveloped virus infection diseases.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Diagnostic kit for antiviral protein MxA and preparation method of diagnostic kit
The invention discloses a diagnostic kit for antiviral protein MxA and a preparation method of the diagnostic kit. The diagnostic kit and the preparation method are characterized in that the diagnostic kit for the antiviral protein MxA is prepared with a quantum dot technique, a microsphere technique, an intermediate-frequency ultrasonic technique, a suspension technique, a sealing technique and alabeling technique, can be clinically used, is simple to operate, high in precision and sensitivity, long in shelf life and safe to act, application range is wide, detection time is shorter, cost islow, and satisfactory effect is realized.
Owner:安徽金标点生物科技有限公司
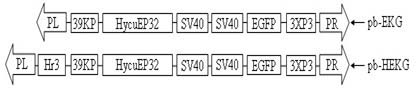
Application of enhancer Hr3 for promoting Hycu-EP32 protein increment expression
ActiveCN102492691AOvercoming Not Expressing Hycu
<i>-</i>
Defects of EP32Does not express Hycu
<i>-</i>
Deficient suppression of EP32Vector-based foreign material introductionAnimal husbandryZINC FINGER ANTIVIRAL PROTEINPromoter
The invention relates to the transgenic technology of bombyx mori, in particular to the application of enhancer Hr3 for promoting Hycu-EP32 protein increment expression. The increment expression vector takes a transposition vector pBac [3 * P3 - EGFPafm] as the base vector; and the transposition vector is sequentially connected with enhancer Hr3, a 39kP promotor, a Hycu-EP32 gene and a termination signal sequence. In the invention, transgenic increment expression exogenous antiviral protein is used for preparing anti-BmNPV bombyx mori, which is the first method of improving the resistance of bombyx mori by adopting transgenic increment expression exogenous resistant protein in diapause bombyx mori; the Hycu-EP32 protein can be expressed in each growth period, so as to overcome the defect that the normal bombyx mori cannot express Hycu-EP32, and provide convenience for inhibiting viral breeding by utilizing Hycu-EP32 protein in each period; and the expressed increment of protein increases with viral increment, so as to reduce impact of expression exogenous protein on normal physiological activities and economic characters of bombyx mori, and obviously improve the resistance of bombyx mori.
Owner:SOUTHWEST UNIVERSITY
Application of IFITM3 (interferon induced transmembrane protein 3) packaging exosome to preparation of dengue virus infection prevention medicine
InactiveCN104587447APeptide/protein ingredientsGenetic material ingredientsZINC FINGER ANTIVIRAL PROTEINInterferon alpha
The invention discloses application of IFITM3 (interferon induced transmembrane protein 3) packaging exosome to preparation of a dengue virus infection prevention medicine. A preparation method of the exosome comprises the following steps: collecting the supernatant of an HUVEC (Human Umbilical Vein Endothelial Cell) culture medium with high-expressed IFITM3, and performing sucrose density gradient ultracentrifugation on the supernatant to prepare the exosome. The action mechanism is that the exosome with high-expressed IFITM3 can strongly inhibit dengue virus from adsorbing and penetrating host cells. The invention builds a new strategy for resisting dengue virus with the IFITM3 packaging exosome. Whether the antiviral protein packaging exosome can replace interferon to be directly applied to research and development of antiviral medicines opens up a new vision and new application of humanized antiviral protein in the field of antiviral prevention and treatment, thus providing new ideas and directions for development of novel antiviral medicines.
Owner:SUN YAT SEN UNIV
Microparticle chemiluminescence detection kit for immunoassay of antiviral protein MxA
InactiveCN109725152AEasy to operateHigh sensitivityChemiluminescene/bioluminescenceBiological testingAntigenMonoclonal antibody
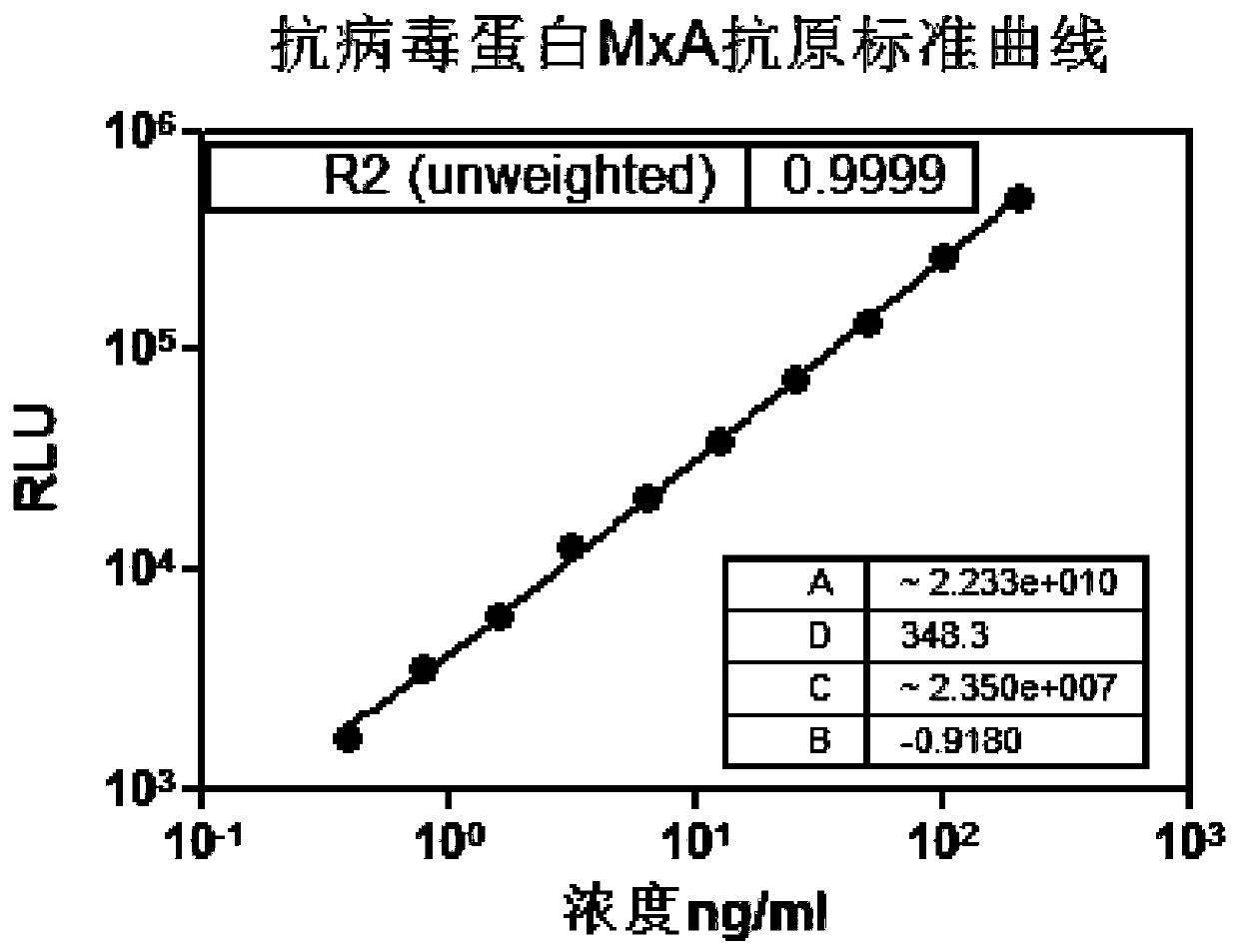
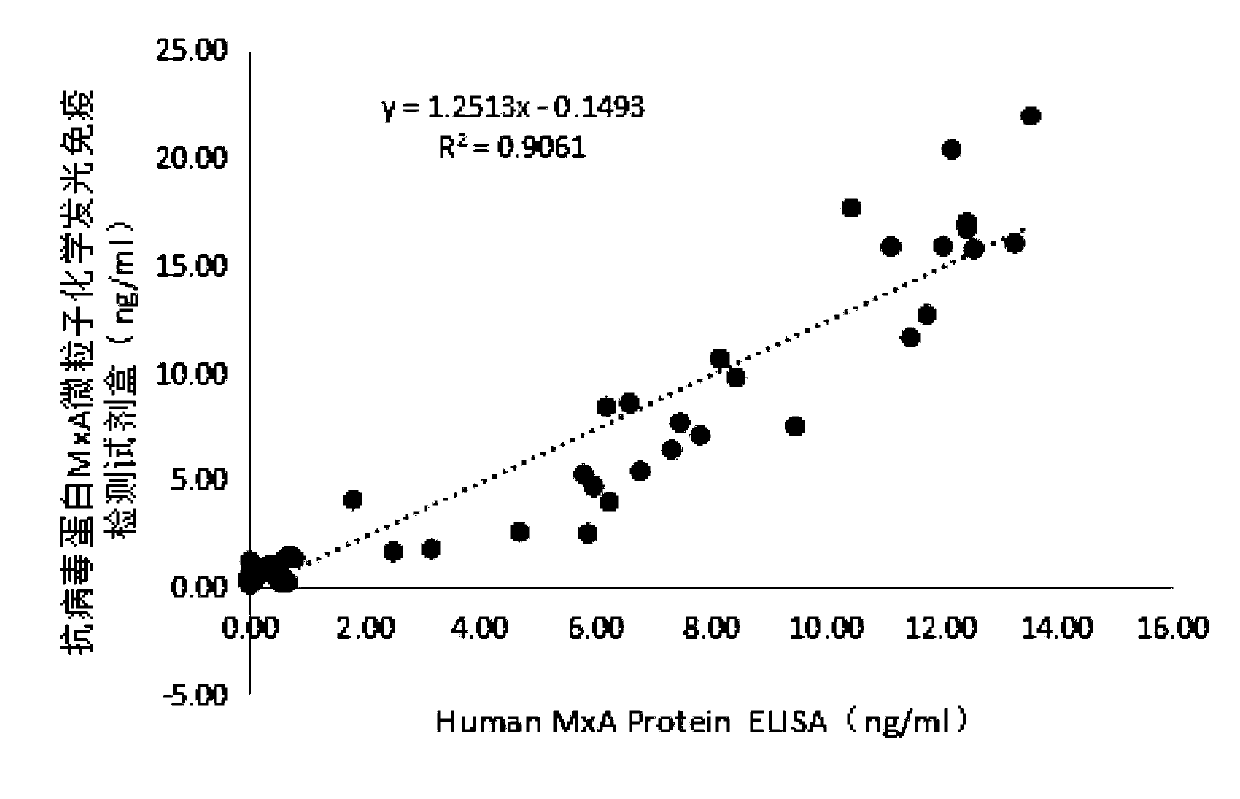
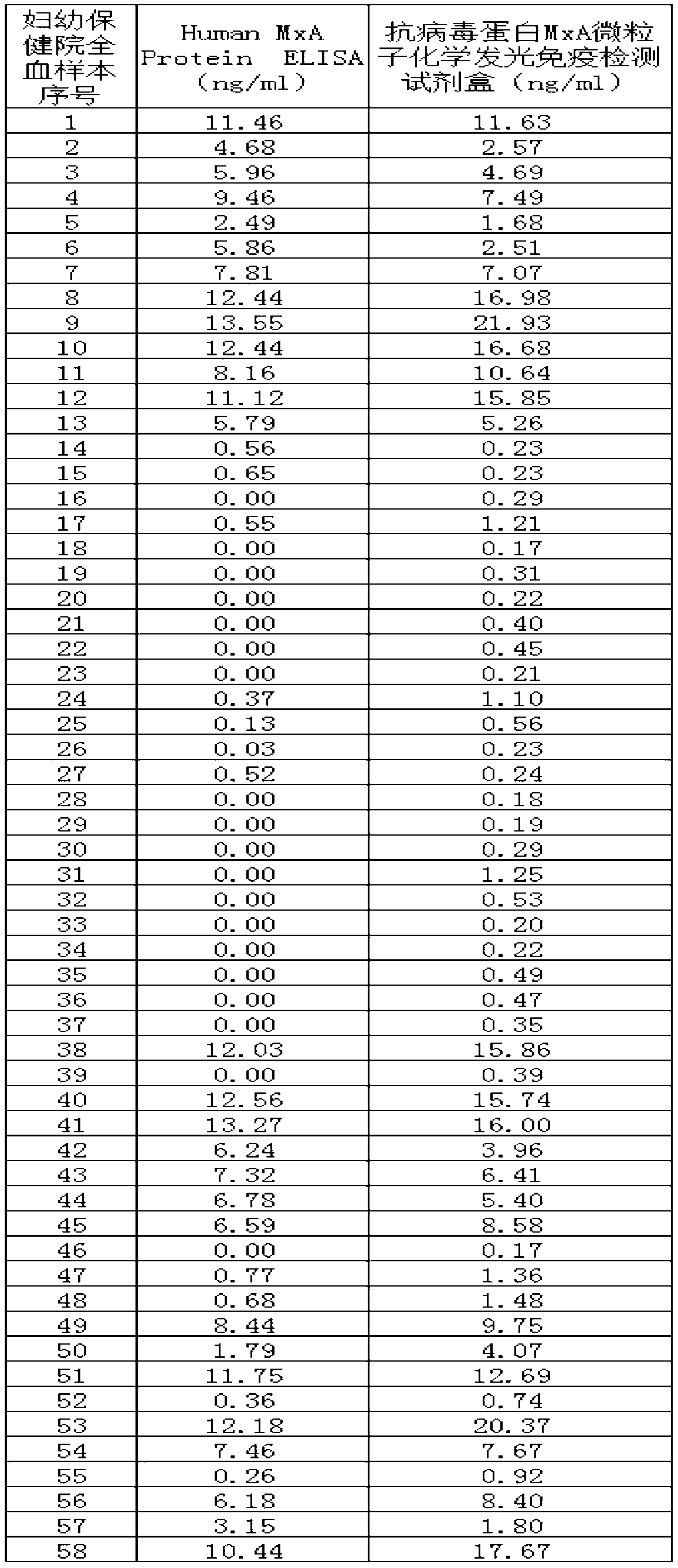
The invention discloses a microparticle chemiluminescence detection kit for immunoassay of antiviral protein MxA, which comprises a coating reaction buffer, a magnetic microparticle solution, a secondantiviral protein MxA monoclonal antibody solution, a first luminescent liquid, a second luminescent liquid, a whole blood lysing solution, different concentrations of antiviral protein MxA antigen standard solution and concentrated washing solution. The invention fills the gap in the production of microparticle chemiluminescence detection reagent for immunoassay of antiviral protein MxA in China, which has the advantages of simple operation, high sensitivity, wide linear range, stable results, good safety, easy automation, and has broad application prospects in clinical testing.
Owner:XIAMEN INNODX BIOTECH CO LTD +1
Application of interferon kappa in preparation of anti-enveloped virus medicines
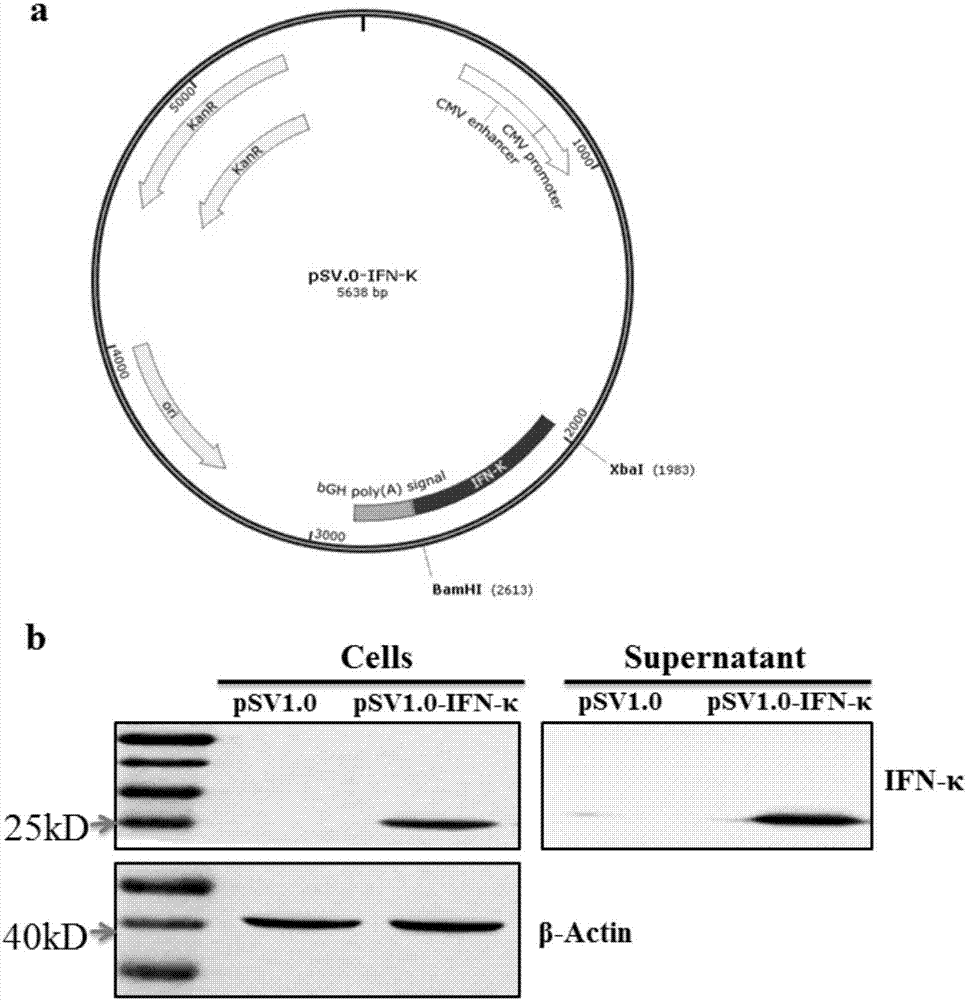
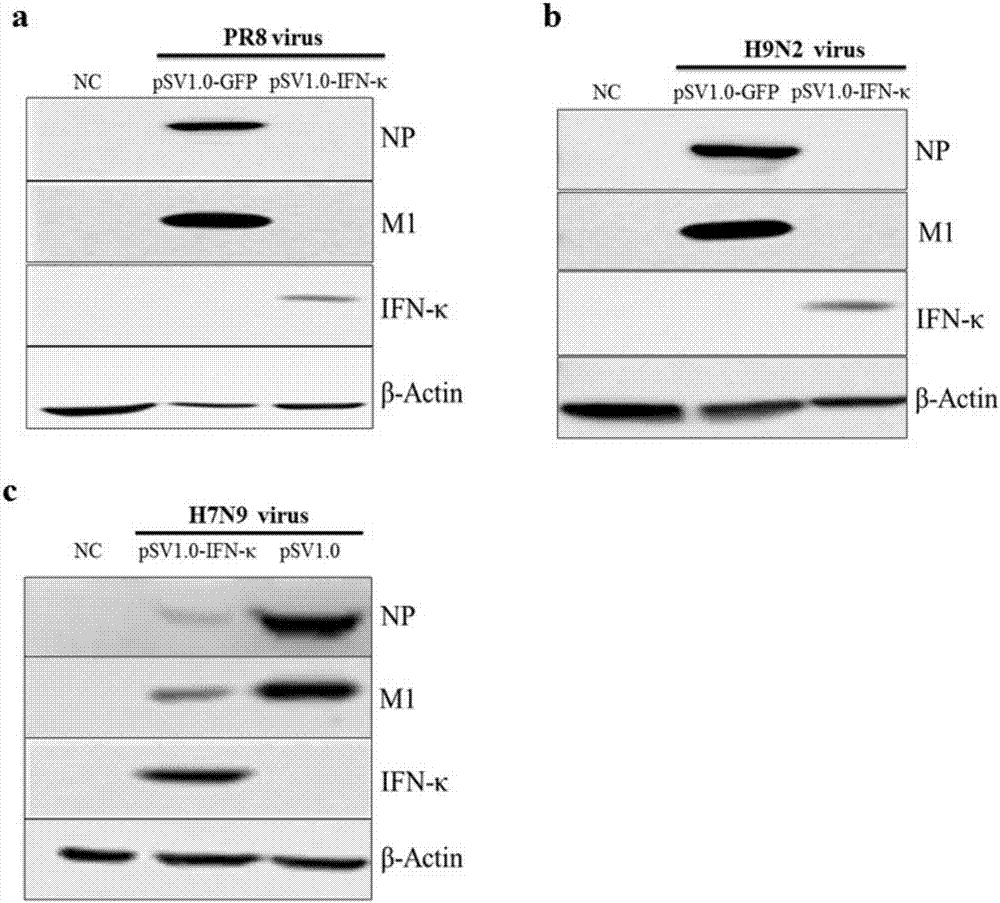
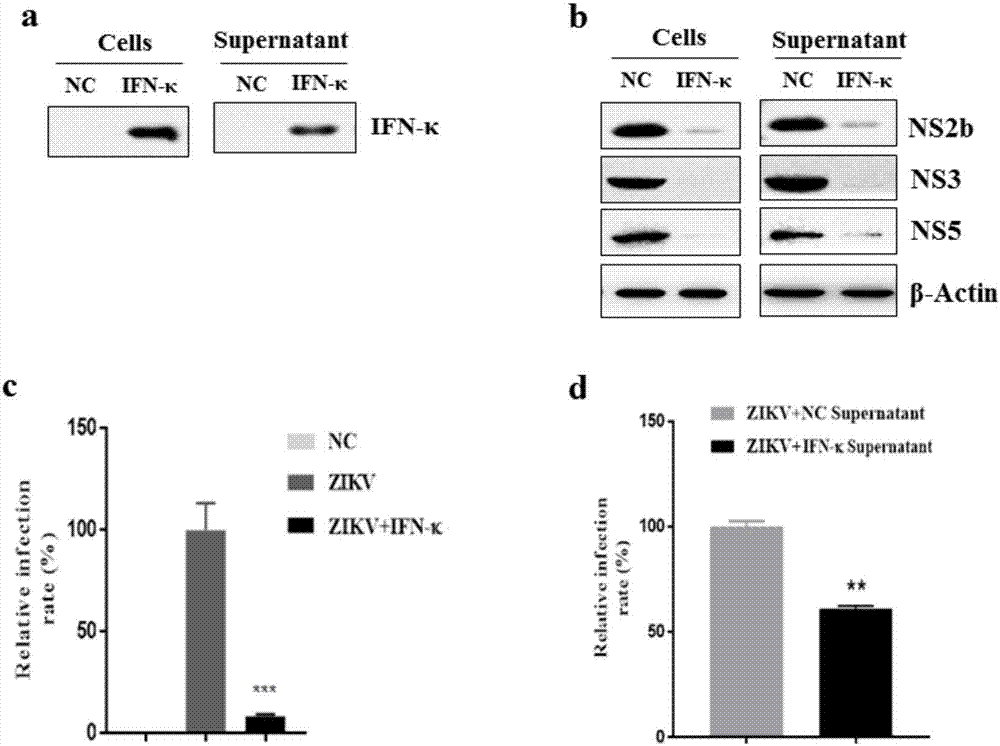
The invention discloses application of an interferon kappa (IFN-kappa) in preparation of anti-enveloped virus medicines, belonging to the field of anti-virus medicines. A nucleotide sequence of a coding gene of the IFN-kappa is as shown in SEQ ID NO:1, and an amino acid sequence is as shown in SEQ ID NO:2; and an enveloped virus comprises but is not limited to flu and ZIKV. The condition that expression of antiviral protein IFITM3 is induced by IFN-kappa is found, so that infection and replication of flu virus and the ZIKV are prohibited. IFN-kappa can effectively prohibit infection and replication of the enveloped-virus in an in-vitro cell model, so as to relieve various diseases caused by massive replication of the flu virus and the ZIKV. The above finding shows that the IFN-kappa can be used for preparing the anti-enveloped virus medicines as well as medicines for treating and / or preventing various diseases caused by the enveloped-virus.
Owner:SHANDONG RUIYING PIONEER PHARMA
Antiviral proteins and peptides, DNA coding sequences therefor, and uses thereof
InactiveUS6987096B1Inhibit growthInhibition of replicationBiocidePeptide/protein ingredientsDna encodingZINC FINGER ANTIVIRAL PROTEIN
The present invention provides antiviral proteins (collectively referred to as cyanovirins), conjugates thereof, DNA sequences encoding such agents, host cells containing such DNA sequences, antibodies directed to such agents, compositions comprising such agents, and methods of obtaining and using such agents.
Owner:UNITED STATES OF AMERICA
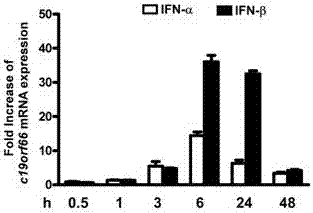
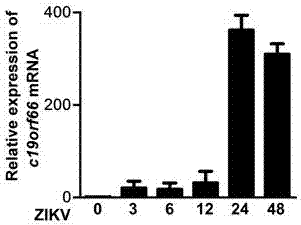
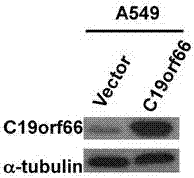
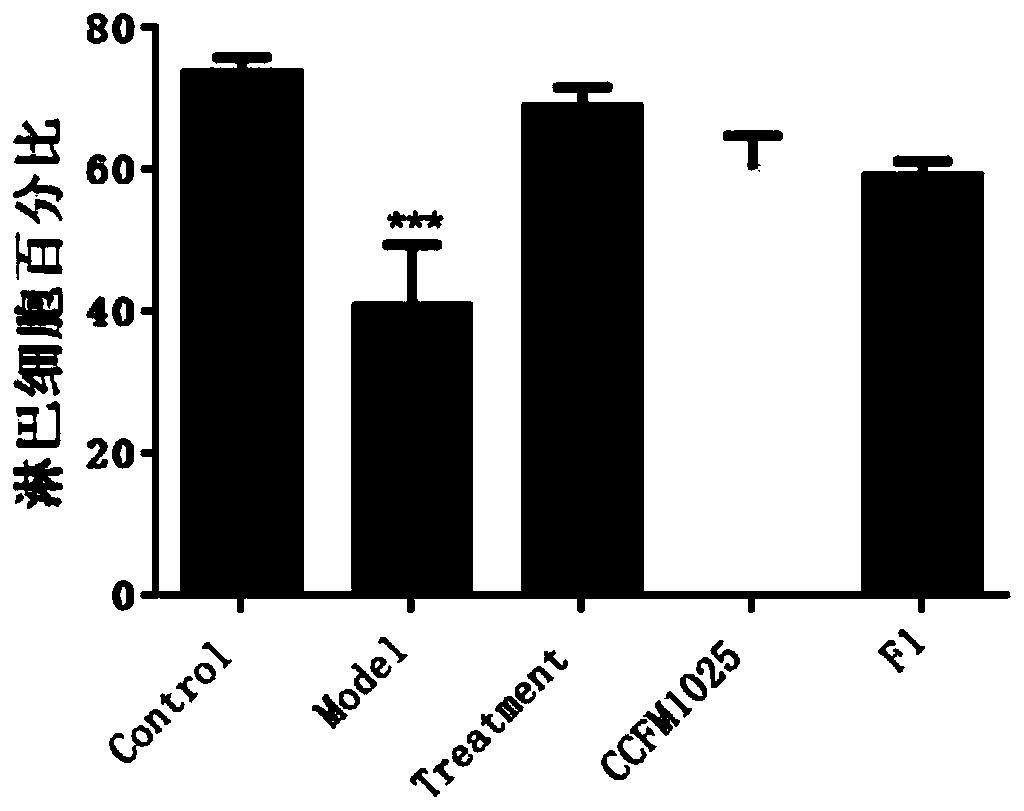
Applications of novel antiviral protein C19orf66 in preparing medicines capable of resisting Zika virus
InactiveCN107050425APrevent proliferationOpen up new areas of clinical applicationPeptide/protein ingredientsAntiviralsZika virusPharmaceutical drug
The invention discloses applications of novel antiviral protein C19orf66 in preparing medicines capable of resisting Zika virus. For the novel antiviral protein C19orf66, through interferon stimulation and Zika virus infection, the increase of the expression level of novel antiviral protein C19orf66 can be effectively stimulated, meanwhile, the C19orf66 with stable and high expression can specifically inhibit the infection of the Zika virus, and moreover, the result also shows that the silent endogenous C19orf66 can promote the infection of the Zika virus. The scheme proves that C19orf66 has the efficient activity in resisting Zika virus infection and proliferation, and thus a novel thought and direction are provided for the development of the medicines capable of resisting the Zika virus.
Owner:SUN YAT SEN UNIV
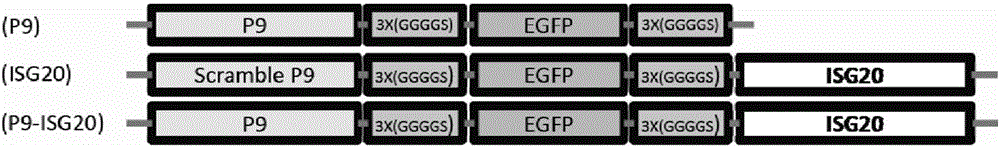
Recombinant antiviral protein as well as preparation method and application thereof
InactiveCN105968211AInhibit synthesisBlock replicationHydrolasesPeptide/protein ingredientsZINC FINGER ANTIVIRAL PROTEINAntiviral protein
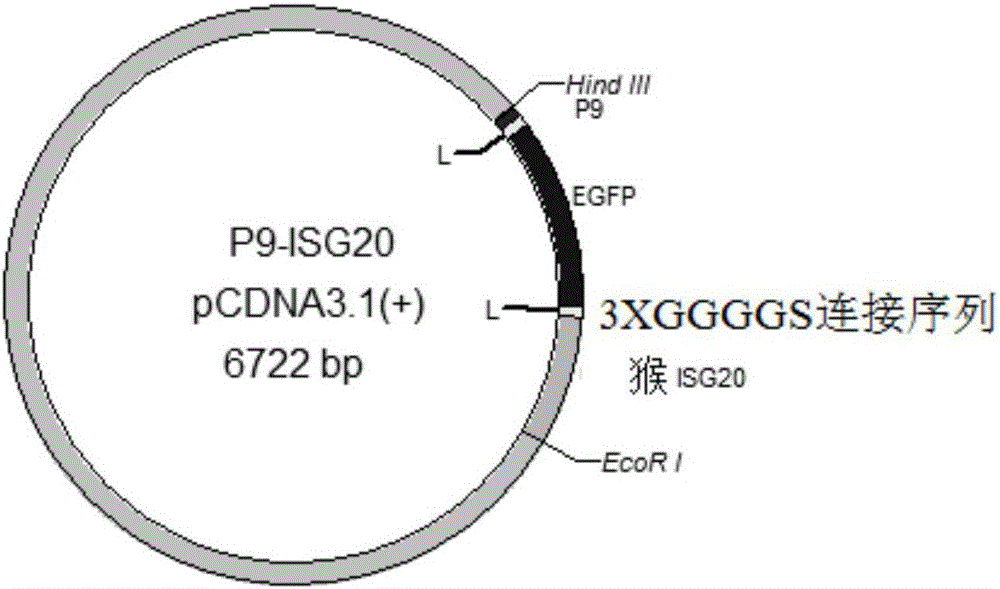
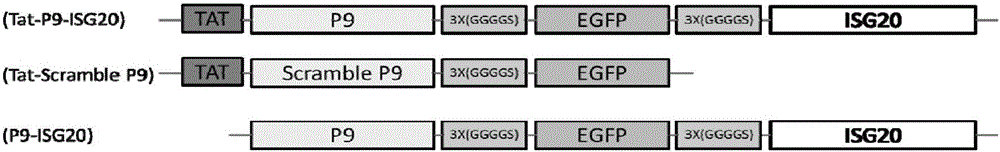
The invention discloses a recombinant antiviral protein, nucleic acid coding the recombinant antiviral protein, a recombinant expression vector comprising the nucleic acid, a transformant comprising the recombinant expression vector, a method for preparing the recombinant antiviral protein and an application of the recombinant antiviral protein to preparation of medicines resistant to porcine reproductive and respiratory syndrome viruses (PRRSV). The recombinant antiviral protein comprises a P9 peptide at a terminal N and an ISG20 protein at a terminal C, wherein the P9 peptide and the ISG20 protein are covalently fused; an amino acid sequence of the P9 peptide is shown in SEQ ID NO.1. The recombinant antiviral protein can effectively inhibit synthesis of ribonucleic acid (RNA) of the PRRSV and block replication of the PRRSV, has very high antiviral ability and high target specificity and also has very low toxicity toward cells.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Primers for obtaining genes of bovine interferon alpha and preparation method for recombinant bovine interferon alpha
The invention discloses primers for obtaining genes of bovine interferon alpha and a preparation method for recombinant bovine interferon alpha. The preparation method comprises the following steps: a, obtaining genes of bovine interferon alpha; b, constructing a cloning vector; c, constructing an expression vector; d, expressing recombinant protein; e, preparing a recombinant bovine interferon alpha crude product; and f, purifying the bovine interferon alpha crude product. Compared with the prior art, the bovine interferon alpha is a cell factor capable of inducing bovine cells to generate various broad-spectrum antiviral proteins and can be used for treating diseases caused by bovine vesicular stomatitis virus, foot and mouth disease virus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and the like.
Owner:ANHUI JIUCHUAN BIOTECH
A shrimp gene PIAV and polypeptides coded thereby
The invention relates to a prawn gene nucleotide sequence, specifically, the invention relates to the cDNA sequence of prawn PLAV polypeptide, wherein the polypeptide protein is an antiviral protein. The invention also relates to polypeptides encoded by the nucleotide sequence, the use of these polynucleotides and polypeptides, and the process for preparing these polynucleotides and polypeptides.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Glycosylation-resistant cyanovirins and related conjugates, compositions, nucleic acids, vectors, host cells, methods of production and methods of using nonglycosylated cyanovirins
InactiveUS7339037B2Avoid virus infectionProtect against undesired immune responsesBiocideFungiZINC FINGER ANTIVIRAL PROTEINAntiviral protein
The invention provides a method of inhibiting prophylactically or therapeutically an influenza viral infection in a host. The method comprises instilling into or onto a host a cell producing an antiviral protein, antiviral peptide, or antiviral conjugate comprising at least nine contiguous amino acids of SEQ ID NO: 2, wherein the at least nine contiguous amino acids are nonglycosylated and have antiviral activity, whereupon the influenza viral infection is inhibited.
Owner:UNITED STATES OF AMERICA
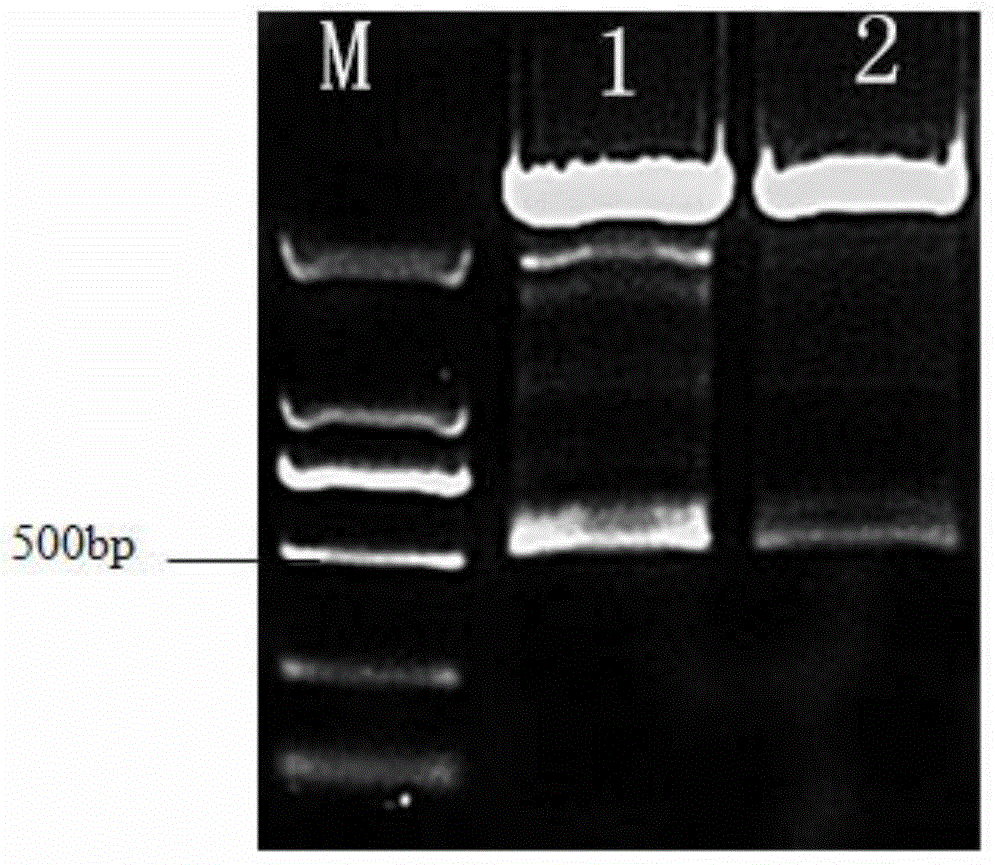
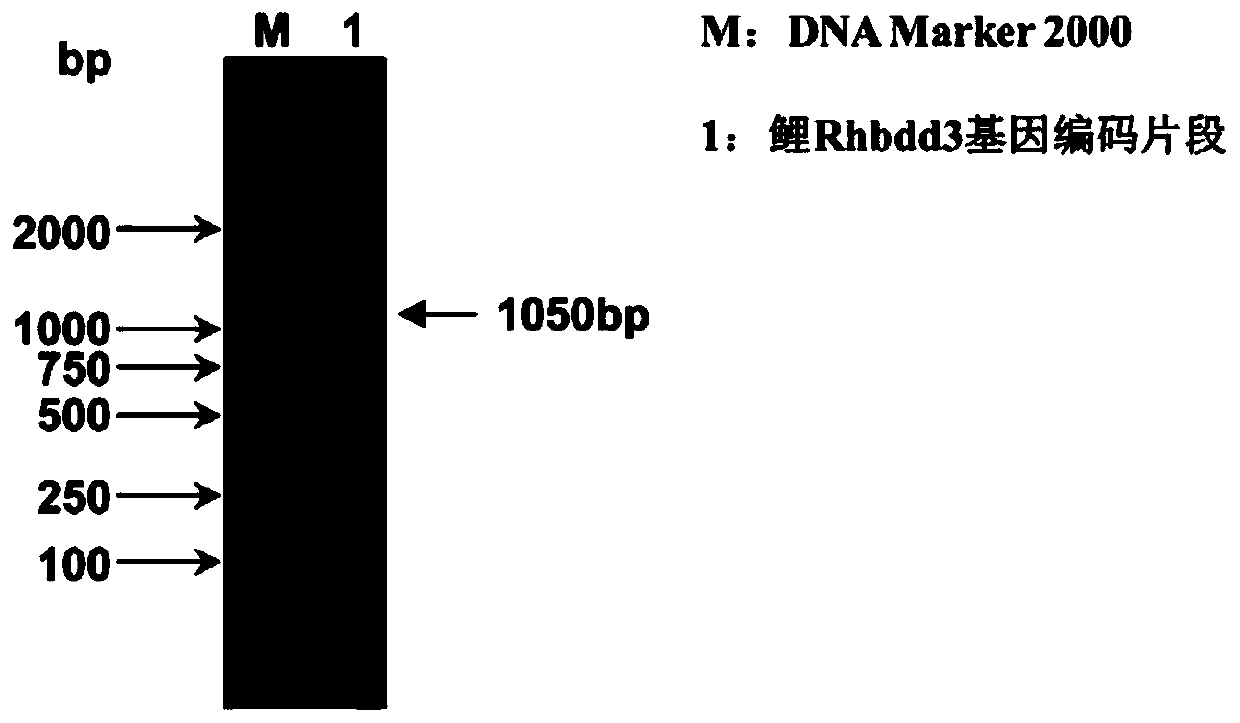
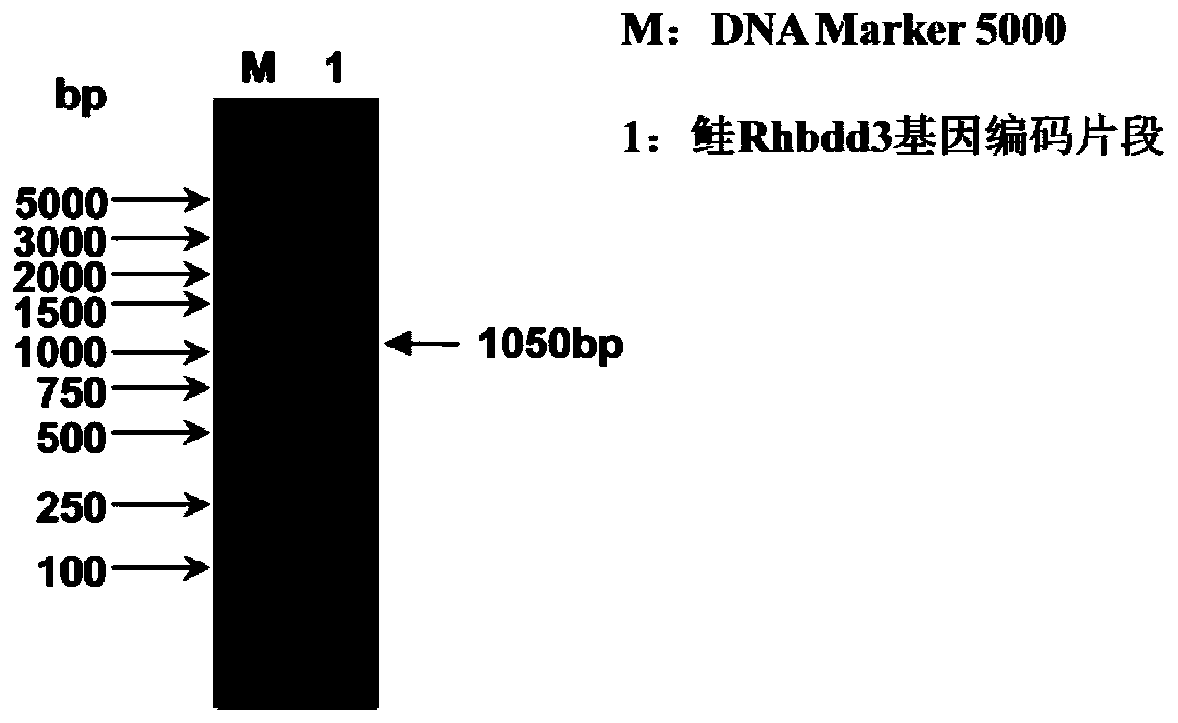
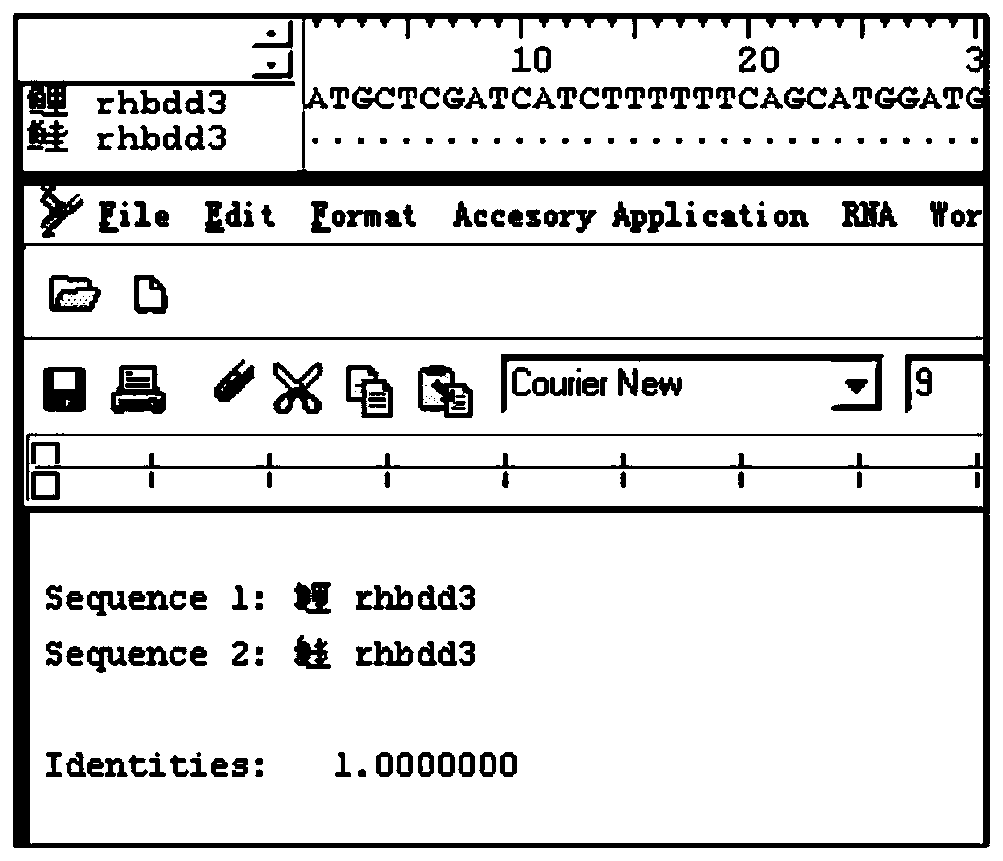
Isolated carp antiviral protein Rhbdd3 and antiviral activity thereof
ActiveCN110295173AInhibition of replicationReduce infectivityPeptide/protein ingredientsAntiviralsAnti virusNucleotide
The invention relates to the technical field of fish genetic engineering, and discloses an isolated carp antiviral protein Rhbdd3 and antiviral activity thereof. The nucleotide sequence of a carp antiviral protein Rhbdd3 encoding gene is shown in 1-1050bp in SEQ ID NO:1, and the amino acid sequence encoded by the carp antiviral protein Rhbdd3 encoding gene is shown in SEQ ID NO:2. The expression of the cloned Rhbdd3 protein not only can inhibit the proliferation of a spring viremia of carp virus (SVCV), but also can inhibit the proliferation of an infectious pancreatic necrosis virus (IPNV). The Rhbdd3 protein plays an important role when cells defend against the infection of the SVCV and IPNV, and does not affect the normal activity of the cells. The gene and the protein provide a new target for preparing drugs resisting the SVCV and the IPNV, and also provide an important technical basis for breeding novel fish anti-virus varieties.
Owner:上海市水产研究所(上海市水产技术推广站)
Use of benzopyridine derivative in preparation of anti-HIV (human immunodeficiency virus) drugs
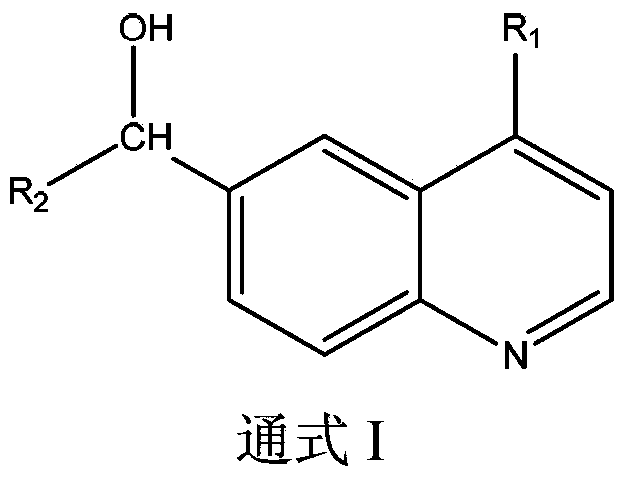
InactiveCN104138375AAntiviralsHeterocyclic compound active ingredientsBenzeneZINC FINGER ANTIVIRAL PROTEIN
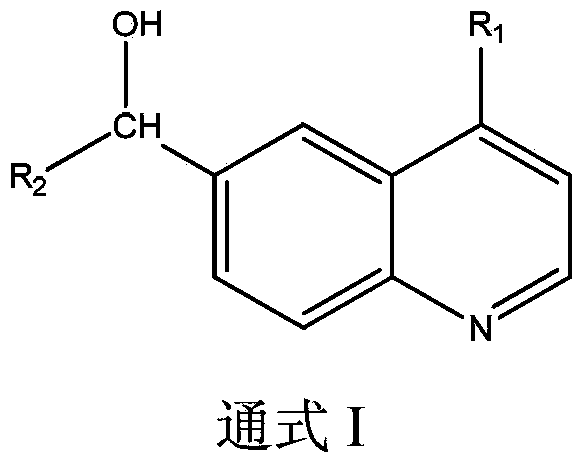
The present invention relates to new use of benzopyridine in preparation of drugs for preventing and / or treatment of acquired immunodeficiency syndrome (AIDS), and particularly relates to use of a compound shown as formula I or pharmaceutically acceptable salts thereof in the preparation of the drugs for preventing and / or treatment of acquired immunodeficiency syndrome (AIDS) and ZAP (zinc finger antiviral protein) inhibitors.
Owner:NANKAI UNIV +1
Detection reagent for antiviral protein MxA and preparation method thereof
PendingCN110988363ASimple compositionSimplify operating proceduresScattering properties measurementsTransmissivity measurementsAssayZINC FINGER ANTIVIRAL PROTEIN
The invention discloses a latex-enhanced immunoturbidimetric assay detection single reagent for antiviral protein MxA and a preparation method thereof. A single reagent is adopted, mixing is not needed, the sensitivity is high, the detection speed is high, various samples such as the whole blood, serum and plasma can be directly measured. Therefore, the reagent can be widely applied to various transmission or scattering analyzers including a common biochemical analyzer, a specific protein analyzer and the like.
Owner:SUZHOU KANGHESHUN MEDICAL TECH
Transgenic plants producing a PAP II protein
Disclosed are recombinant plant cells, plant cell parts, plant parts and transgenic plants containing a DNA molecule comprising a sequence encoding a Pokeweed Antiviral Protein (PAP) II protein. PAP II proteins include full length, wild-type PAP II and substantially nontoxic mutants or analogs including fragments thereof truncated at the C-terminus and other PAP II proteins having an intact catalytic active site amino acid residue E172 but that also have at least one amino acid substitution or deletion, and possess anti-viral and / or anti-fungal activity. DNA molecules comprising sequences encoding the mutants or analogs, as well as the isolated and purified PAP II proteins per se, are also disclosed. Methods of identifying nontoxic PAP II mutants are further disclosed.Transgenic plants that produce a PAP II protein exhibit anti-viral and / or anti-fungal activity. Virtually all flowering plants are included. Seed derived from the transgenic plants are also provided.
Owner:RUTGERS THE STATE UNIV
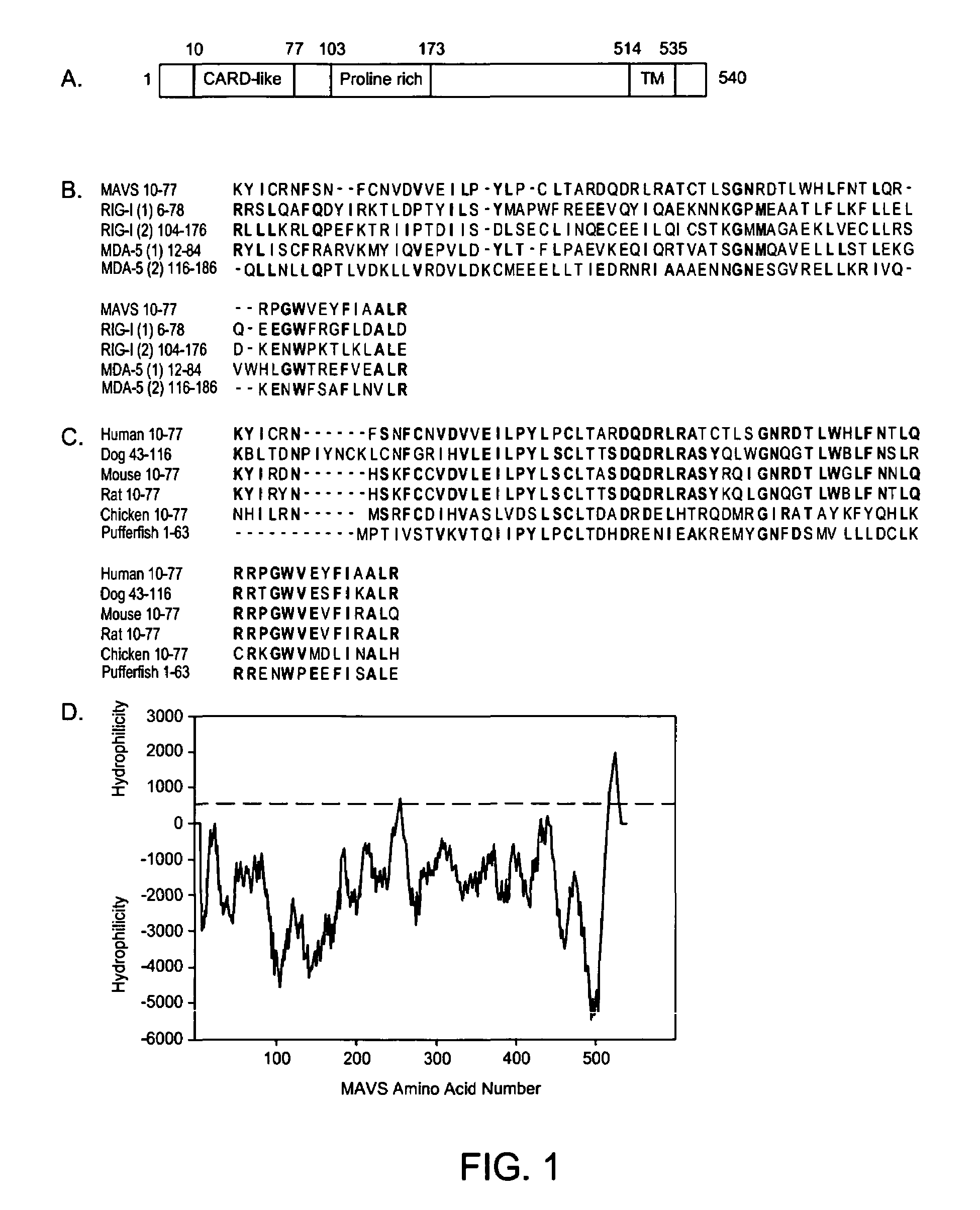
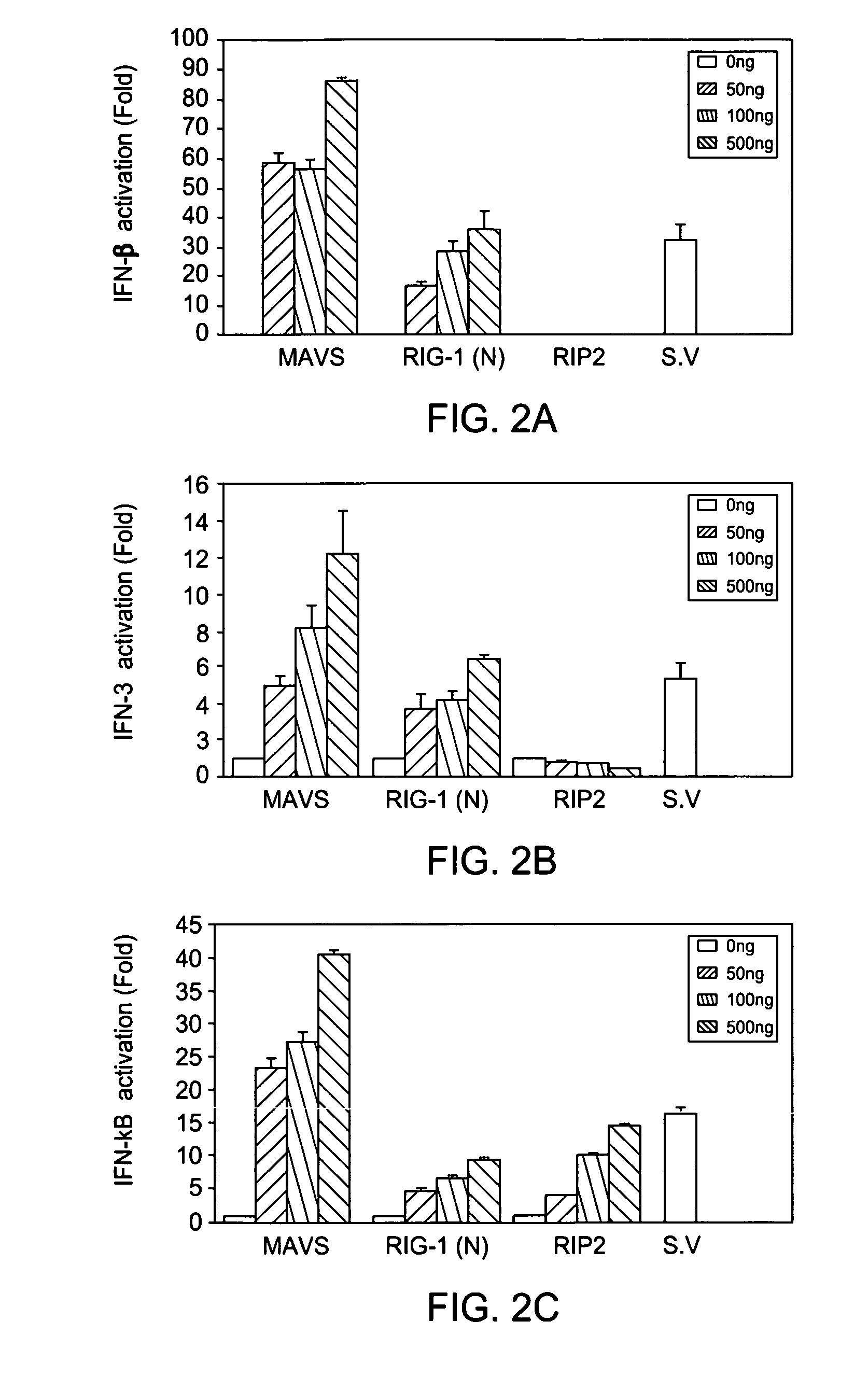
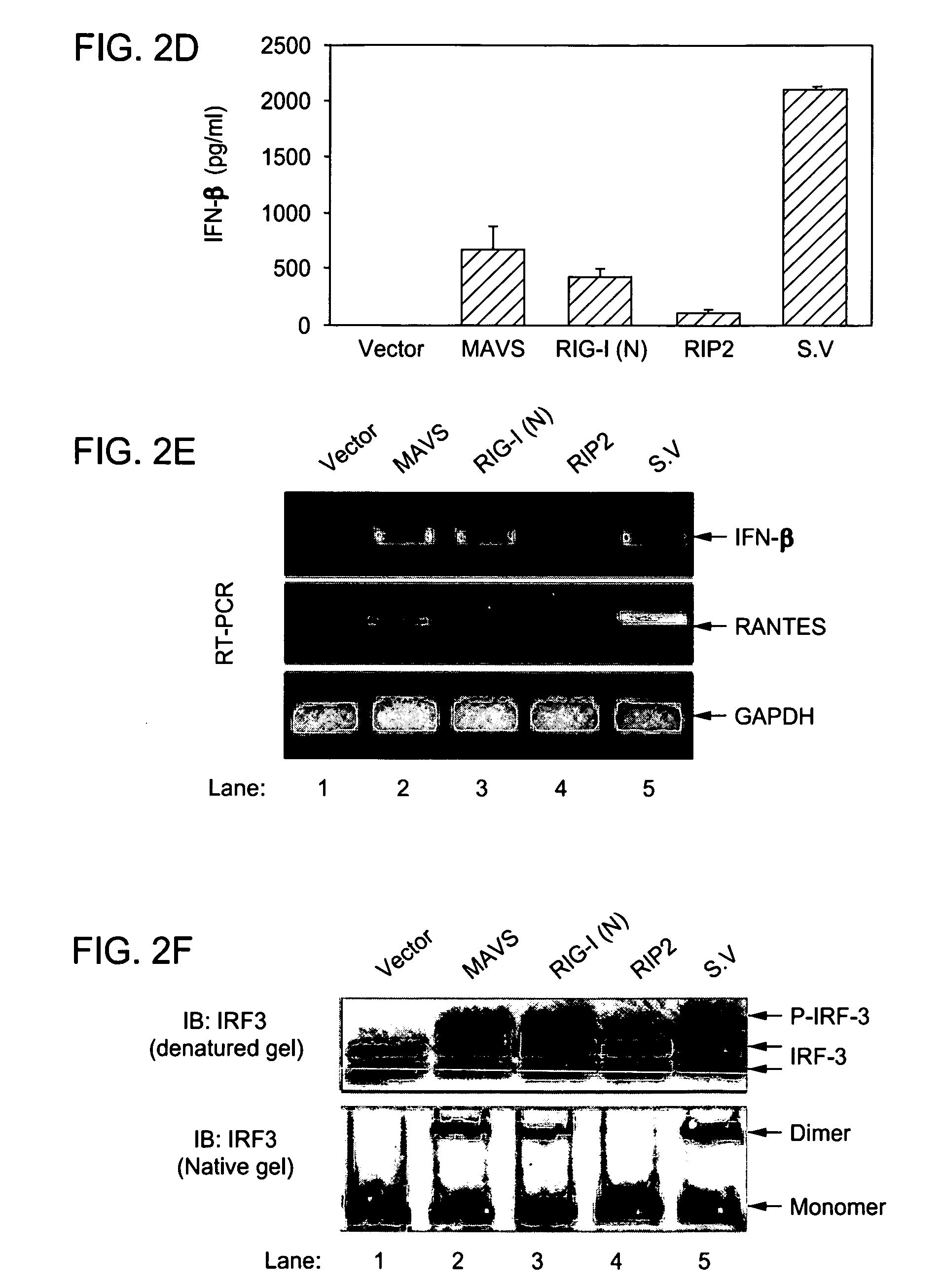
MAVS in the prevention and treatment of viral diseases
InactiveUS7625724B2InhibitionExacerbated the viral replication and killing of the host cellsBacteriaUnicellular algaeViral diseaseZINC FINGER ANTIVIRAL PROTEIN
The present invention includes compositions and methods for the identification, characterization and use of a novel anti-viral protein that includes a mitochondrial anti-viral signaling protein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Isolated fish antiviral protein gene CMPK2 and antiviral activity thereof
The invention belongs to the technical field of genetic engineering of aquatics and particularly relates to isolated fish antiviral protein gene CMPK2 and antiviral activity thereof. An antiviral natural immunoprotein gene CMPK2 is isolated from Pimephales promelas; a nucleotide sequence of this gene is shown as the sequence table SEQ ID NO: 1; an encoded protein sequence of this gene is shown asSEQ ID NO: 2. The expression of the protein gene CMPK2 cloned herein may affect proliferation of SVCV (spring viremia of carp virus); CMPK2 protein plays an important role in the life cycle of SVCV. The gene or protein herein provides a new target for the research on the preparation of anti-SVCV drugs.
Owner:HUAZHONG AGRI UNIV
A probiotic mixed preparation with anti-influenza ability and its application
ActiveCN109576185BHas anti-influenza effectGood for weight lossBacteriaMicroorganism based processesZINC FINGER ANTIVIRAL PROTEINBlood Indices
The invention discloses a probiotic mixed preparation with anti-influenza ability and an application thereof, belonging to the technical field of microbes and the technical field of medicine. This probiotic mixed preparation has the effect of anti-influenza, which is specifically reflected in: (1) significantly improving the degree of weight loss of influenza mice; (2) significantly improving the blood indicators of influenza mice; (3) significantly improving the inflammation of respiratory tract infection in influenza mice (4) Significantly reduce the viral load in the lungs of influenza mice (that is, significantly inhibit the replication and proliferation of influenza virus in influenza mice); (5) significantly enhance the expression of antiviral protein MxA in the lungs of influenza mice, Therefore, the probiotic mixed preparation has great application prospects in the preparation of products for preventing and / or treating atopic dermatitis and even preventing and / or treating influenza.
Owner:JIANGNAN UNIV +1
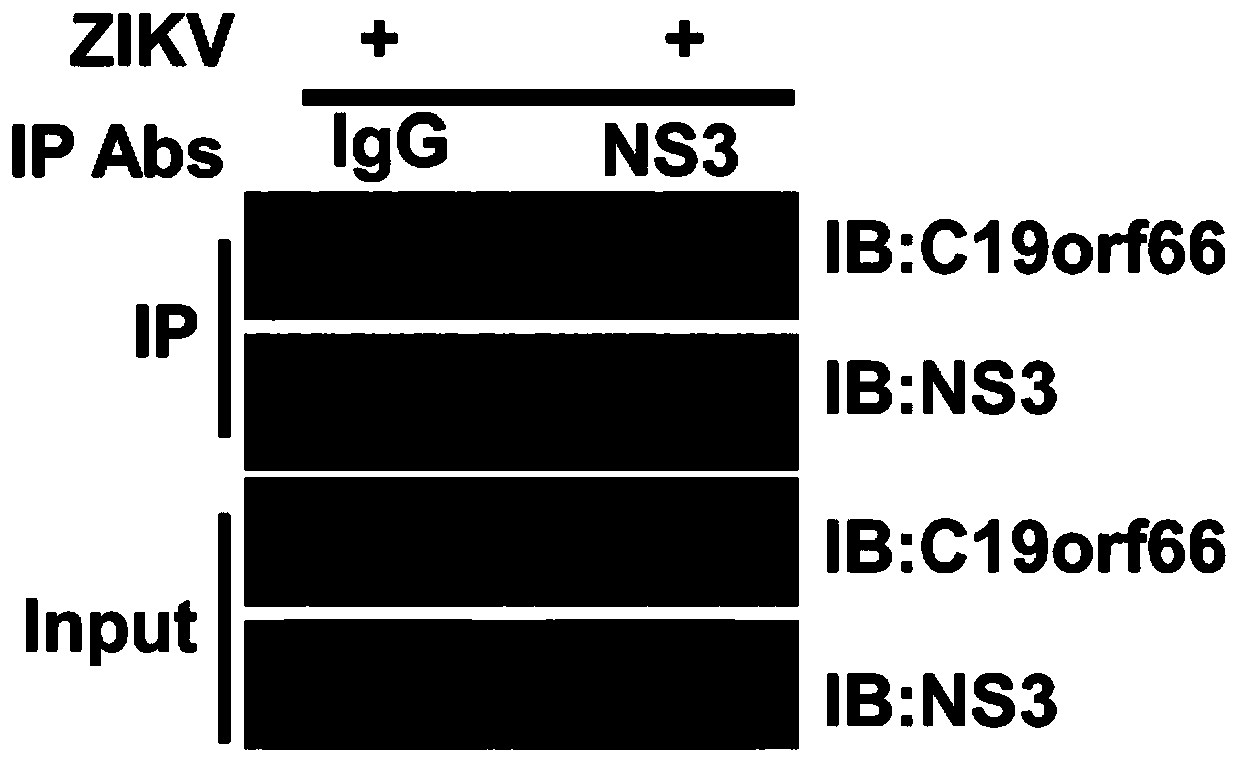
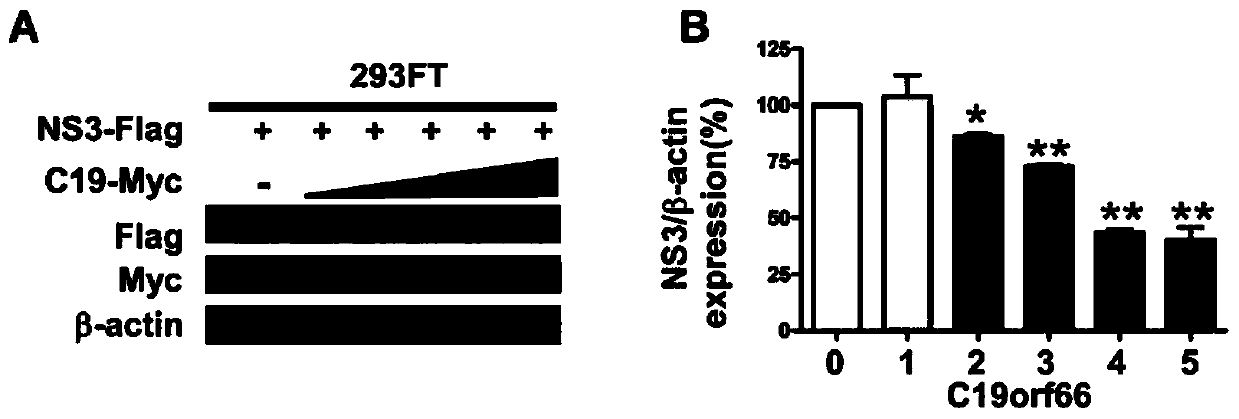
Application of novel antiviral protein C19orf66 in targeted Zika virus non-structural protein NS3 antiviral drugs
ActiveCN111000981AFacilitate pathway degradationOpen up new areas of clinical applicationPeptide/protein ingredientsAntiviralsLysosomeStructural protein
The invention discloses an application of a novel antiviral protein C19orf66 as a Zika virus non-structural protein NS3 inhibitor in antiviral drugs. The novel antiviral protein C19orf66 provided by the invention has a new application of efficiently targeting Zika virus non-structural protein NS3 and promoting lysosome pathway degradation of NS3 so as to inhibit proliferation of Zika virus in a host. The invention develops a new clinical application field of the humanized antiviral protein in the field of antiviral prevention and treatment, thereby providing a new thought and direction for thedevelopment of drugs targeting Zika virus non-structural protein NS3, providing a scientific basis for the development of anti-Zika-virus drugs, and providing a new thought for clinical antiviral treatment.
Owner:SUN YAT SEN UNIV
Method for extracting and purifying pokeweed antiviral protein from pokeweed
PendingCN110016470AAddressing Purity IssuesSolve the problem of standardsGlycosylasesPokeweed antiviral proteinFreeze-drying
The invention belongs to the field of medicines, and relates to a method for extracting and purifying pokeweed antiviral protein from pokeweed. The method comprises the following steps: extracting total protein from pokeweed leaves, performing precipitation with ammonium sulfate salt to obtain pokeweed antiviral crude protein, dissolving the pokeweed antiviral crude protein in a Tris-HCl buffer solution, performing further purification by adopting anion exchange chromatography and cation exchange chromatography, performing elution to obtain pokeweed antiviral protein solution, and performing ultrafiltration concentration and vacuum freeze drying to obtain the pokeweed antiviral protein with the purity of more than or equal to 99%. The method performs salt precipitation crude purification,and performs a process path of further purification by adopting anion exchange chromatography and cation exchange chromatography so as to obtain the pokeweed antiviral protein with the purity of morethan or equal to 99%, so that the problems of low purity of the extracted pokeweed antiviral protein in the prior art and lack of a high-purity pokeweed antiviral protein reference substance and a standard substance are solved, and the pokeweed antiviral protein has good clinical application prospect.
Owner:海南森瑞谱生命科学药业股份有限公司
Anti-h5n1 influenza activity of the antiviral protein cyanovirin
The invention is directed to a method of inhibiting prophylactically or therapeutically an H5N1 viral infection in a host, which method comprises administering to the host an anti-viral effective amount of an antiviral protein comprising the amino acid sequence of SEQ ID NO: 1 or a nucleic acid encoding the antiviral protein, as well as antiviral portions, variants, and conjugates thereof.
Owner:UNITED STATES OF AMERICA
Viral vaccine for the treatment of viral disease
Disclosed is a method for treating virosis, wherein viruses causing virosis can be killed by multiple antiviral proteins produced by interferon evoked cells generated by virus type vaccine stimulation organism, thus curing the virosis. Different dosage, administration mode and and time can be employed for the patients.
Owner:公殿力
New anti-human papilloma virus (HPV) and endometritis preparation
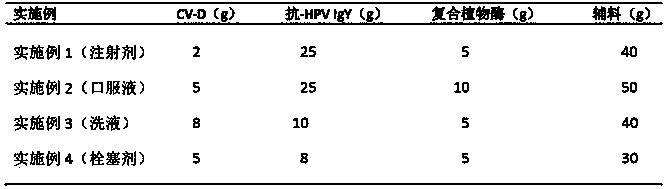
InactiveCN104056268AInhibit bindingAvoid accessAntibacterial agentsPeptide/protein ingredientsSide effectViral disease
The invention provides a new anti-human papilloma virus (HPV) and endometritis preparation comprising the following components by weight: 0.0001-10 parts of plant antiviral protein-D, 0.0001-50 parts of anti-HPV IgY, 1-15 parts of composite plant enzyme and 1-80 parts of accessories, the new preparation has the anti-human papilloma virus (HPV) and endometritis elimination effects, has the effects of resistance to infection of a variety of viral diseases and bacterial infection, and has no toxic and side effect.
Owner:安能(广州)科学技术有限公司
Expression separation and purification method for Chinese Phytolacca acinosa cDNA mutant and TAT gene recombinant in prokaryon and application therefor
InactiveCN1952146AImprove biological activityBacteriaPeptide/protein ingredientsPokeweed antiviral proteinToxin protein
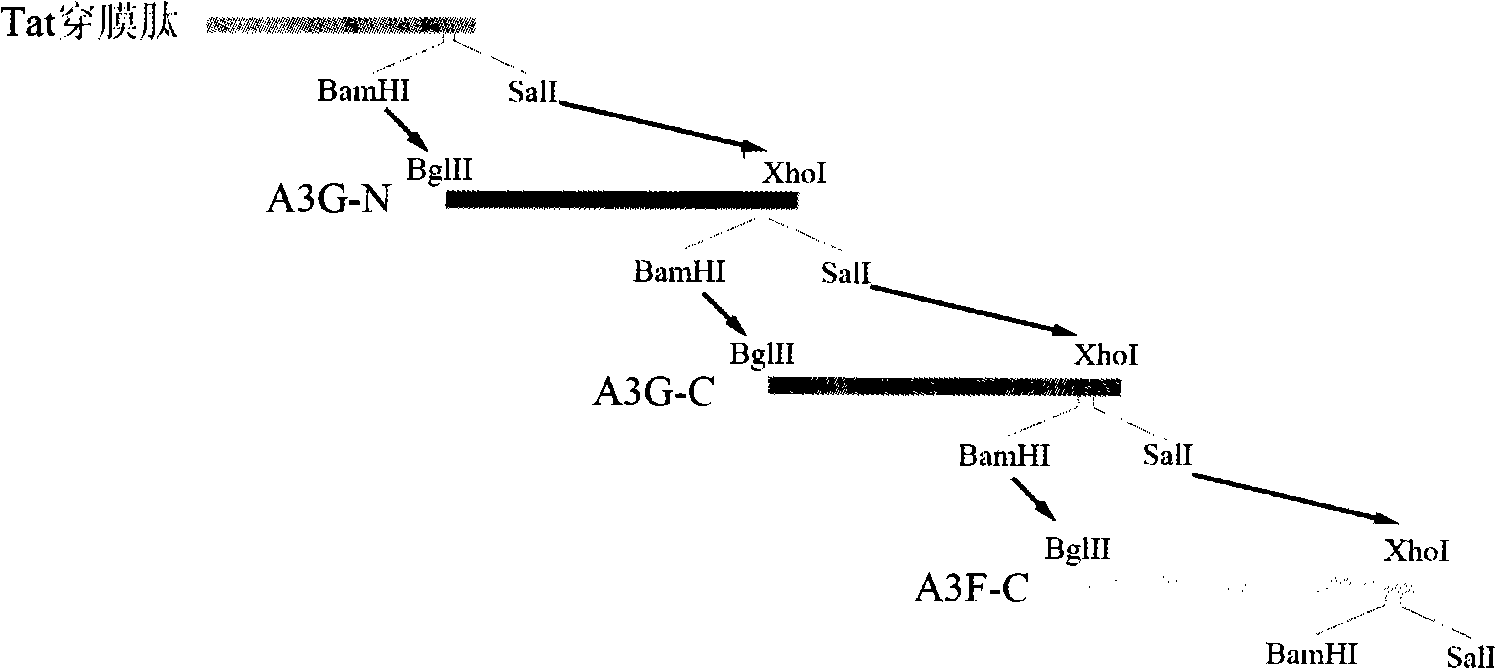
The invention concerns a Ribosome inactibating protein (RIPS) Chinese mainland protein separated from plants which plays a role in anti-AIDS through catalyzing rRNA depurination of prokaryotic and eukaryotic ribosome with highly conserved stem-loop structure at the surface. The invention involves a Tat protein, the basic amino acid enrichment domain of which is protein transduction domain (PTD) and able to carry many exogenous protein directly into cells as protein orientation of cytokines or toxin. The invention involves a fusion protein of Tat and Chinese antiviral protein mutant. Performing deletion mutation to CAP using Quick-mutagenesis site-directed mutagenesis technology: 22 amino acid deletion in N-terminal, 29 amino acid deletion in C-terminal and site-specific mutagenesis: 151KI152 / 151AA152, 191FN192 / 191AA192. The recombinant protein of the invention has biological activity of anti-HIV-1 in vitro. It is found that CAP deletion and site-specific mutagenesis in fusion gene can significantly reduce its ability to combine with rRNA (cytotoxicity), however, without influence to its ability of combination of HIV RNA (anti-HIV activity), has more clinical application.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
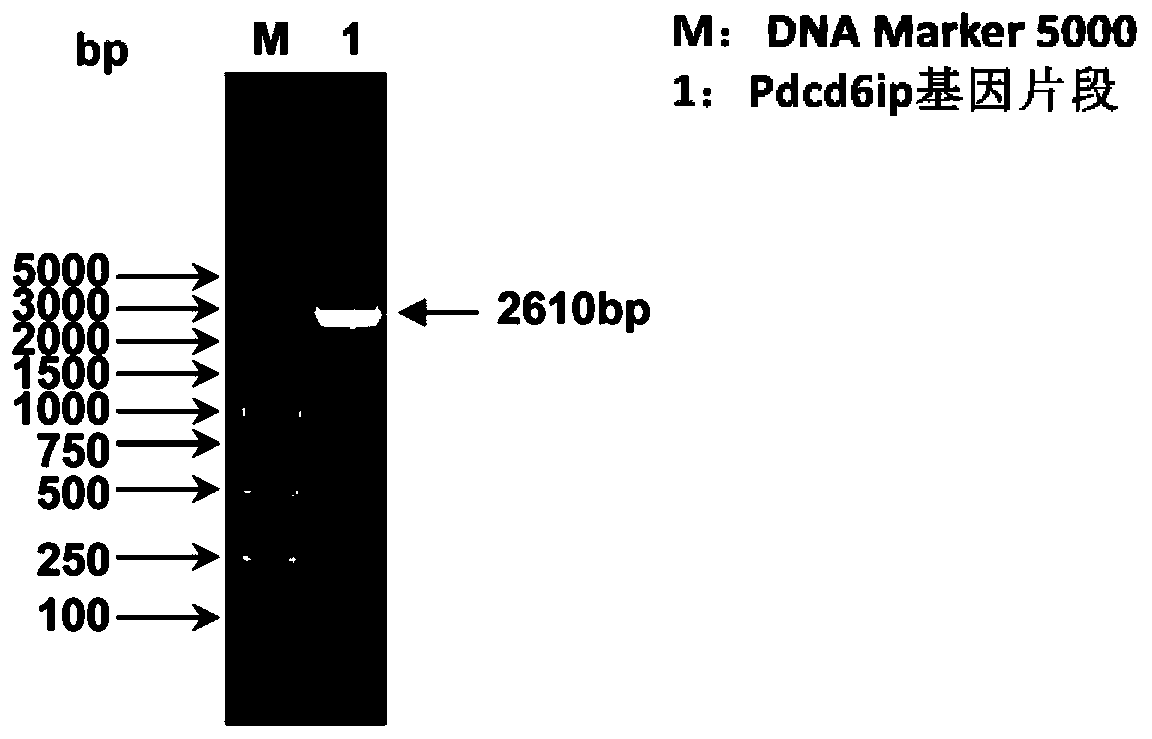
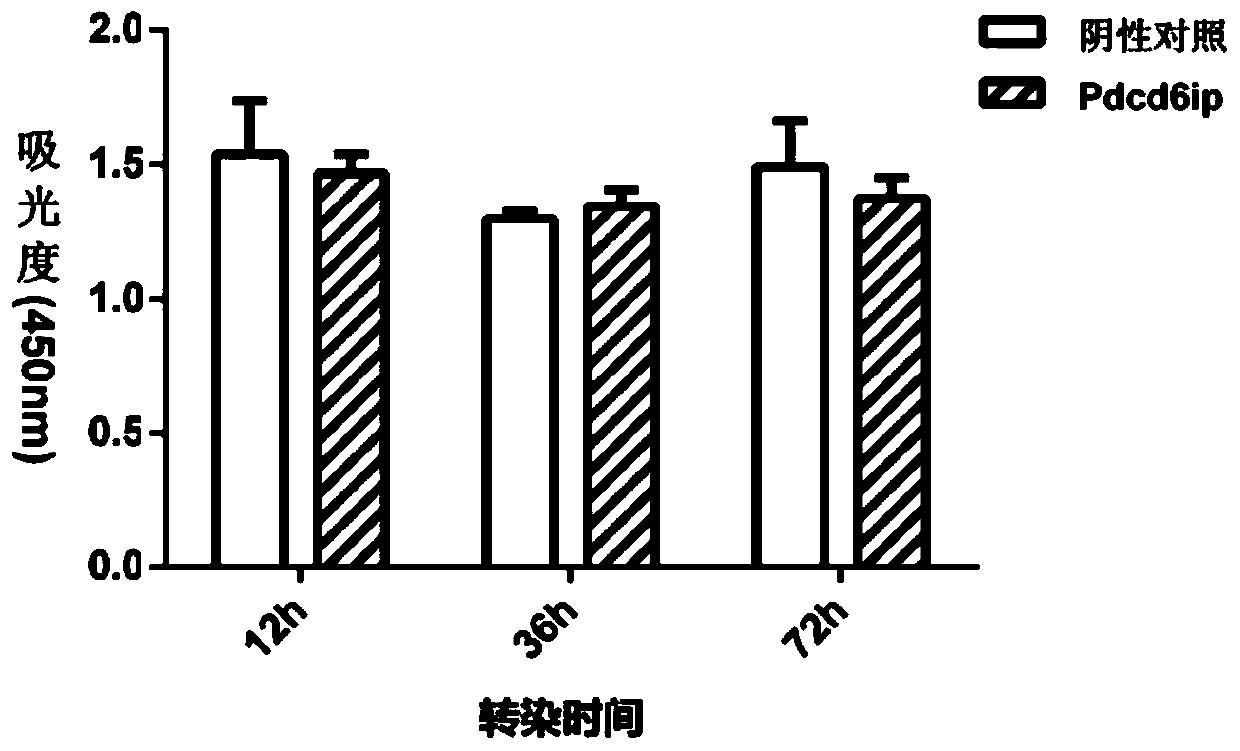
Separated carp antiviral protein Pdcd6ip and antiviral activity
ActiveCN110092823AAffect proliferationInhibition of replicationPeptide/protein ingredientsAntiviralsNucleotideCarp
The invention relates to the technical field of freshwater fish genetic engineering, and discloses a separated carp antiviral protein Pdcd6ip and an antiviral activity. The nucleotide sequence of a carp antiviral protein Pdcd6ip gene is a sequence shown in 1st-2610th bp in SEQ ID NO:1, and an encoded amino acid sequence is a sequence shown in SEQ ID NO:2. Tests indicate that the expression of thecloned Pdcd6ip protein can affect the proliferation of spring viremia of carp virus (SVCV), the Pdcd6ip protein plays an important role in the process of cells resisting to SVCV infection, and at thesame time, the effect on the cell activity of ordinary cells is low. The gene or protein provides a new target for preparing anti-SVCV drugs.
Owner:上海市水产研究所(上海市水产技术推广站)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com