A probiotic mixed preparation with anti-influenza ability and its application
A probiotic and anti-influenza technology, applied in the field of microorganisms, can solve problems such as obvious side effects and achieve great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0065] Example 1-1: Screening and identification of mucosal lactobacilli
[0066] 1. Screening
[0067] Take human feces as the sample, after pretreatment, store the sample in about 20% glycerol in a -80°C refrigerator, take it out and thaw it, mix the sample and add 0.5mL sample to 4.5mL to contain 0.05% cysteine 0.9% normal saline for gradient dilution, select the appropriate gradient dilution and spread it on the MRS plate added with 0.05% cysteine, incubate at 37°C for 48h, pick typical colonies and streak them on the MRS plate for purification, pick A single colony was taken and transferred to liquid MRS medium (containing 0.05% cysteine) for enrichment and preserved in 30% glycerol to obtain strain CCFM1025 and strain F1.
[0068] 2. Identification
[0069] The genomes of CCFM1025 and F1 were extracted, the 16S rDNA of CCFM1025 and F1 were amplified and sequenced (Shanghai Sangon Bioengineering Co., Ltd.), and the sequences were compared in GenBank. The results showed ...
Embodiment 1-2
[0070] Embodiment 1-2: the cultivation of mucosal lactobacillus
[0071] Lactobacillus mucosae CCFM1025 was inserted into MRS solid medium (containing 0.05% cysteine) and cultured at 37°C for 48 hours, and its colonies were observed under a microscope, and the colonies were found to be Round and rough, transparent, and its bacteria are short rod-shaped.
[0072] Lactobacillus mucosae (Lactobacillus mucosae) CCFM1025 was inserted into MRS medium (containing 0.05% cysteine) and cultured at 37°C for 48 hours, and the growth curve was made. It is a heterofermentation that produces acid and gas from glucose.
[0073] Lactobacillus mucosae (Lactobacillus mucosae) CCFM1025 was inserted into MRS medium (containing 0.05% cysteine) and cultured at 10, 15, 20, 25, 30, 35, 40, 45, and 50°C for 48 hours to observe its Growth status: It grows well at 20-35°C, and it can still grow at 45°C, but it hardly grows at 15°C or below 15°C or 50°C.
[0074] Lactobacillus mucosae (Lactobacillus mu...
Embodiment 1-3
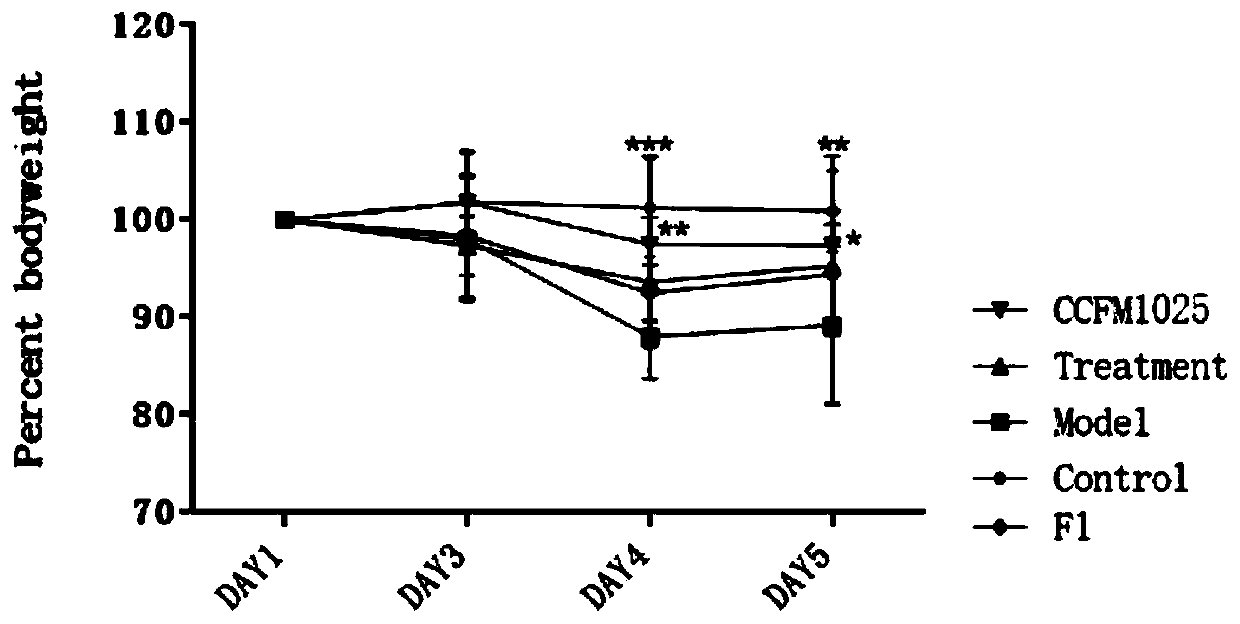
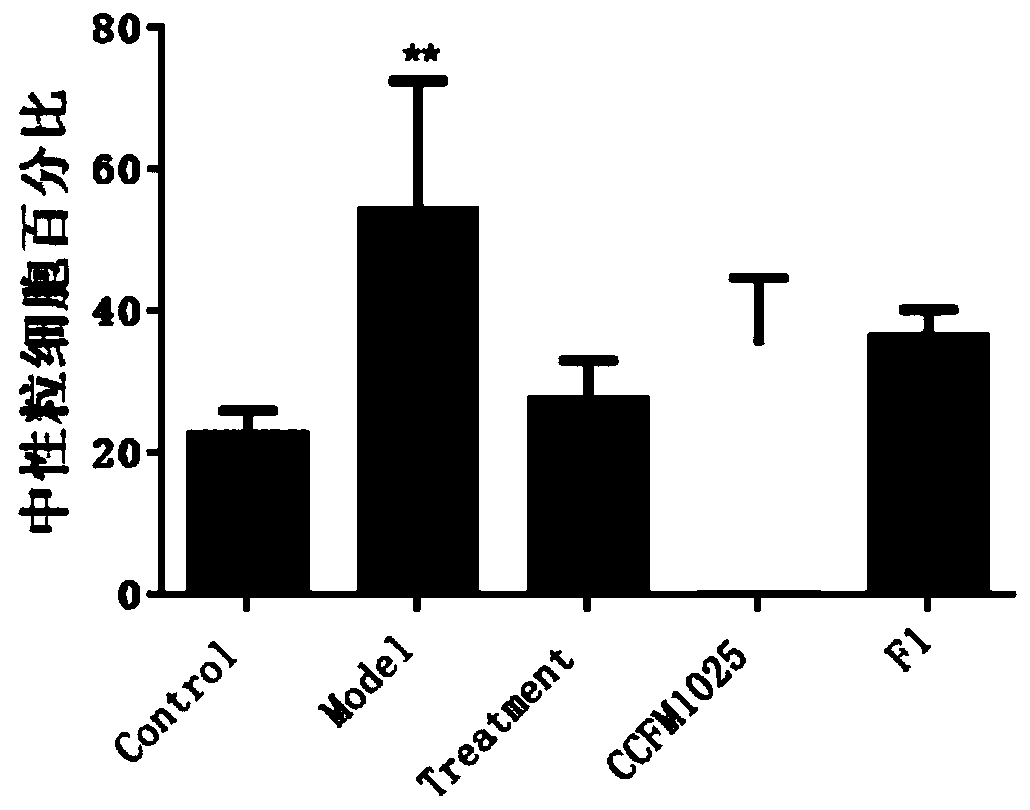
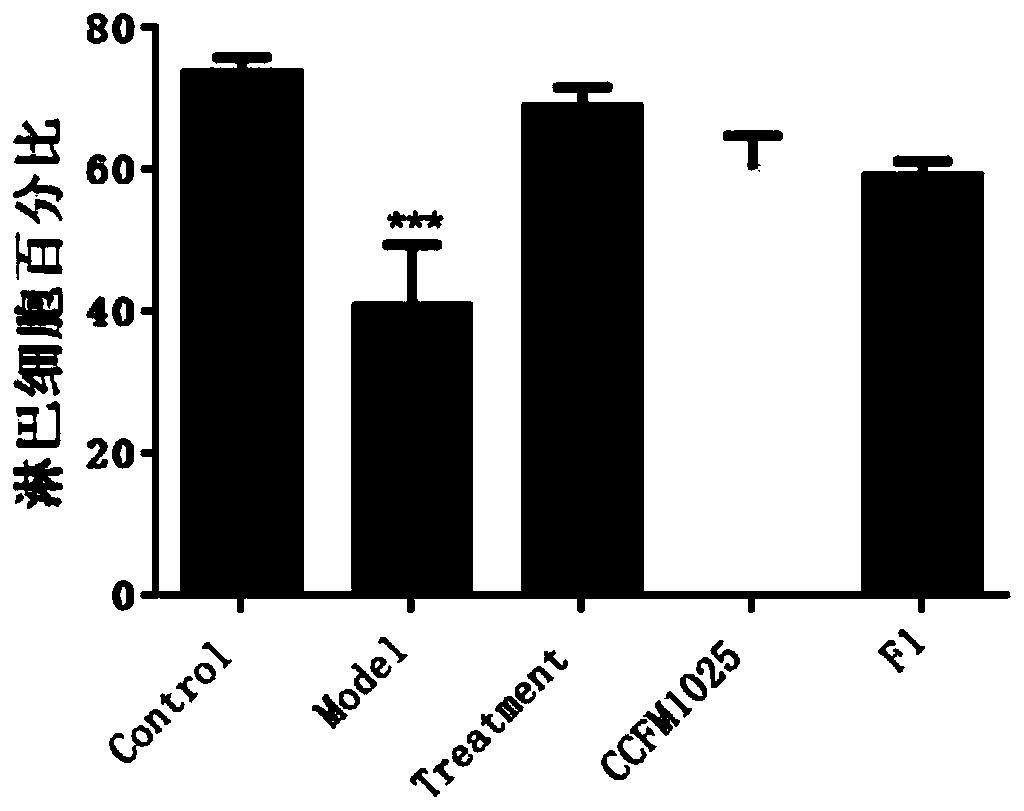
[0075] Example 1-3: Effects of mucosal lactobacilli on the body weight of influenza mice
[0076] Take 40 healthy ICR female mice with a body weight of 20-24g, and divide them into 5 groups randomly, and the 5 groups are respectively named: blank control group (Control), influenza model group (Model), and ribavirin drug treatment group (Treatment). , Lactobacillus mucosal intervention group (CCFM1025) administered intragastrically with Lactobacillus CCFM1025, Lactobacillus mucosal intervention group (F1) administered intragastrically with Lactobacillus F1, 8 rats in each group.
[0077]Two weeks before the experiment, the Lactobacillus Lactobacillus intervention group (CCFM1025) was intragastrically administered 10 times a day. 9 Lactobacillus mucous membranes CCFM1025 bacterial suspension dilution of CFU bacteria amount, Lactobacillus mucous membranes F1 intervention group (F1) gavaged the same amount of Lactobacillus mucous membranes F1 bacterial suspension dilution, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com