Patents
Literature
245 results about "Influenza C Virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
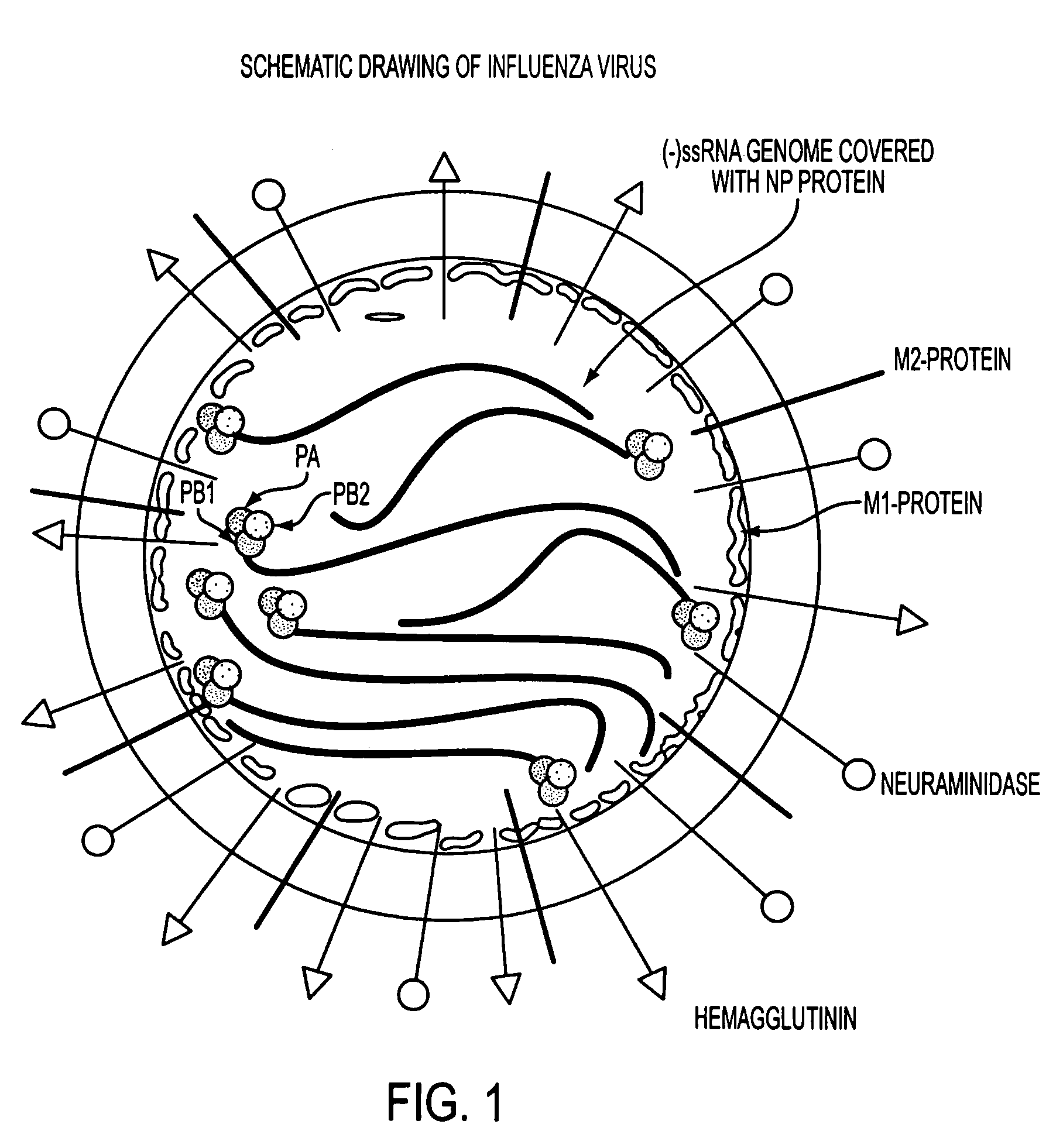
A negative sense single-stranded RNA virus of the orthomyxovirus family that can cause a rare form of influenza. Its genome consists of 7 RNA segments that encode 9 proteins.
Site-directed mutated influenza virus, and live vaccine, preparation method and application thereof
InactiveCN106929482ASsRNA viruses negative-senseViral antigen ingredientsGenomeAttenuated Live Vaccine
The invention relates to an influenza virus subjected to site-directed mutation or an influenza virus with simultaneous mutation of multiple sites. The influenza virus can be derived from human and other animals. The invention further relates to a method of the site-directed mutated influenza virus. The method includes introducing UAG into an influenza virus genome according to a reverse inheritance technology and introducing unnatural amino acid into an influenza virus gene in a site-directed way according to a gene codon extension technology. The invention further relates to application of the site-directed mutated influenza virus or the influenza virus with multiple mutation site combinations, such as serving as attenuated live vaccines and copying controllable safe influenza virus models.
Owner:PEKING UNIV
Avian influenza virus live attenuated vaccine and uses thereof
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
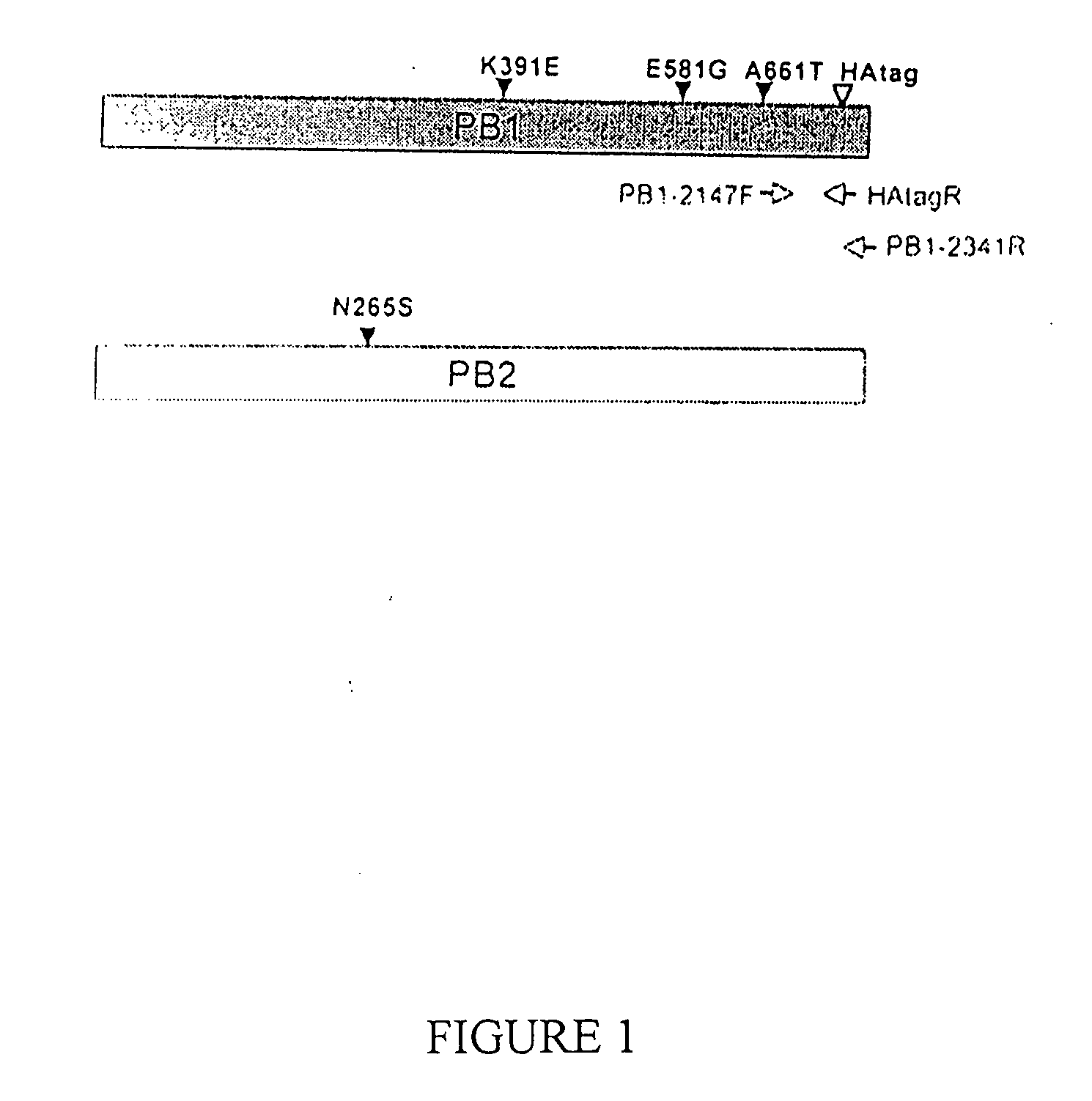
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Influenza viruses and uses thereof
ActiveUS20120244183A1Reduced ability to reassortImprove securitySsRNA viruses negative-senseSugar derivativesDiseaseOpen reading frame
Described herein are chimeric influenza virus gene segments and nucleic acid sequences encoding such chimeric influenza virus gene segments. A chimeric influenza virus gene segment described herein comprises packaging signals found in the non-coding and coding regions of one type of influenza virus gene segment and an open reading frame of a different type of influenza virus gene segment or fragment thereof. Also described herein are recombinant influenza viruses comprising two or more chimeric influenza virus gene segments and the use of such viruses in the prevention and / or treatment of influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
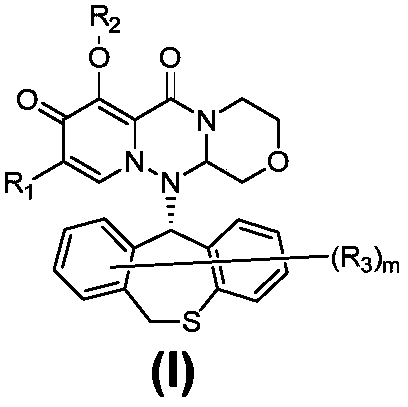
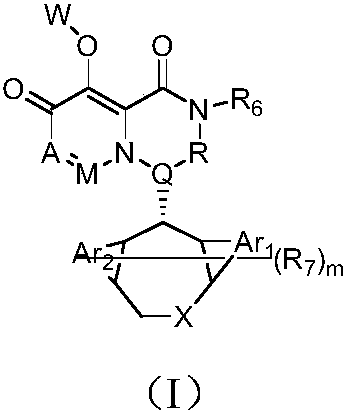
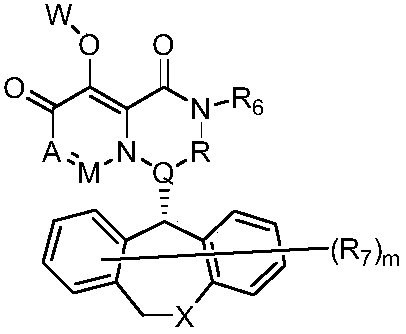
Polycyclic pyridone compound as well as pharmaceutical composition and application thereof
InactiveCN109503625AOrganic active ingredientsGroup 5/15 element organic compoundsPolycyclic compoundMedicine
The invention provides a polycyclic pyridone compound as well as pharmaceutical composition and an application thereof. Specifically, the polycyclic pyridone compound is a compound shown as the formula (I) or pharmaceutically acceptable salt, solvate or hydrate of the compound. The compound can be used for preparing drugs for preventing or treating infectious diseases of mammals, and is particularly used for preparing drugs for preventing, treating, relieving and / or treating orthomyxovirus infection such as influenza A virus, influenza B virus and influenza C virus.
Owner:赵蕾 +3
Materials and methods for treating viral infections
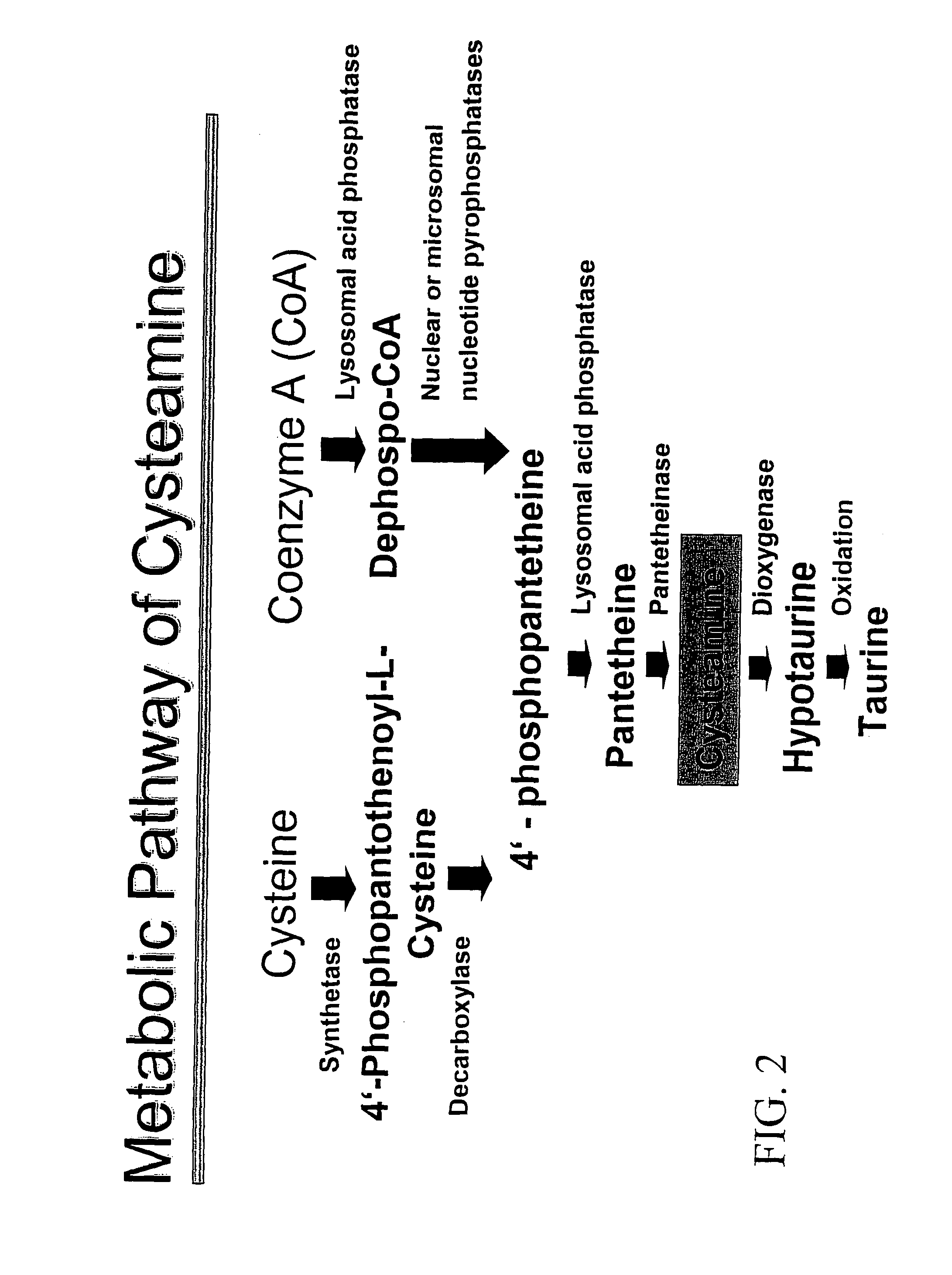
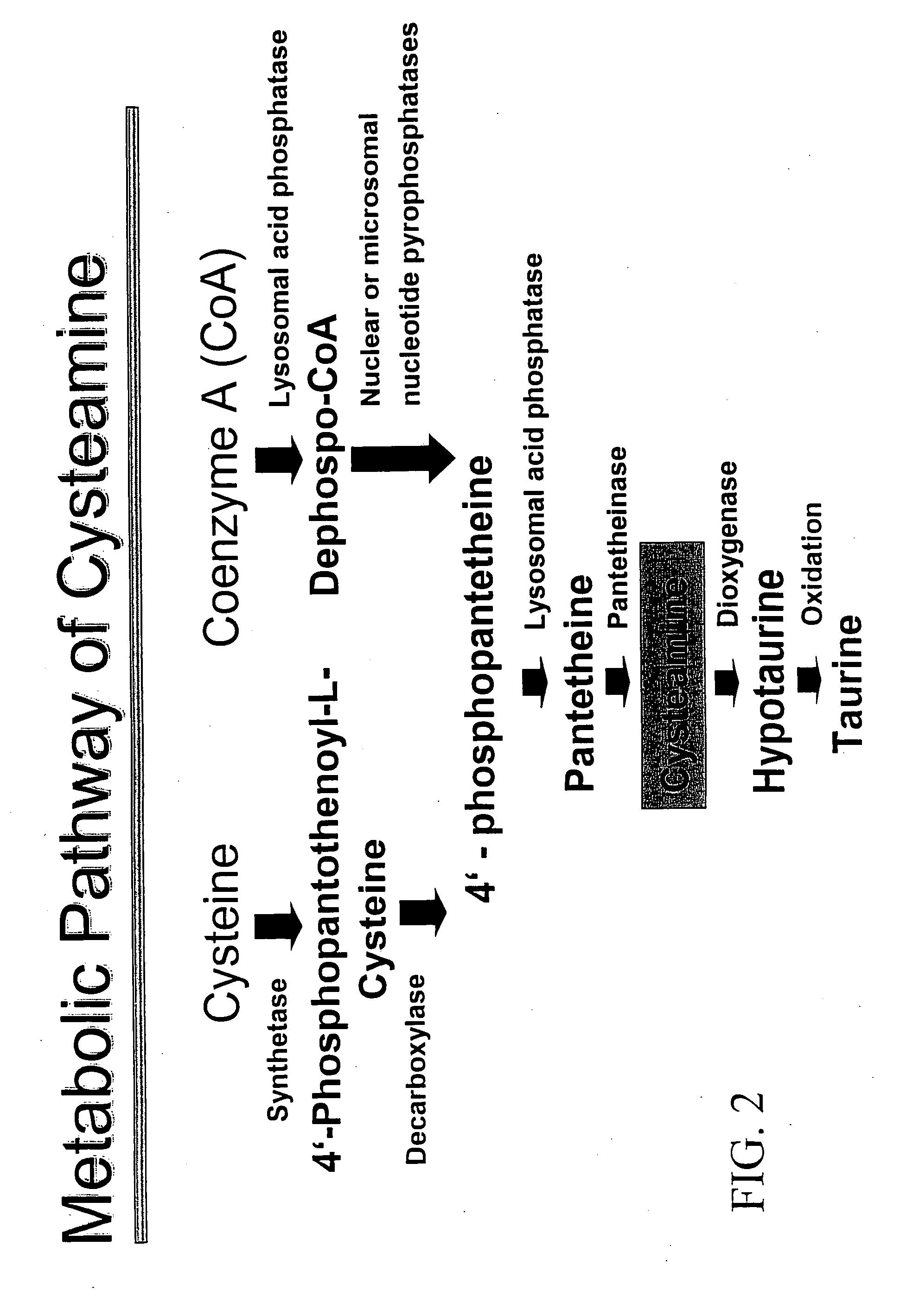
InactiveUS8415398B2Avoid infectionPrevent and delay developmentBiocideAntiviralsCysteamineAvian influenza virus
The subject invention provides materials and methods for treating various health conditions, including the prevention and / or treatment of a viral infection. In a preferred embodiment, a cysteamine compound is administered to a subject to treat an influenza virus infection. More preferably, a cysteamine compound is administered to a subject to treat influenza A, influenza B, influenza C virus infections, including avian influenza virus subtypes (such as H5N1 avian influenza virus).
Owner:OMEGA BIOPHARMA H K
Reagents and kits for detection of influenza virus and the like
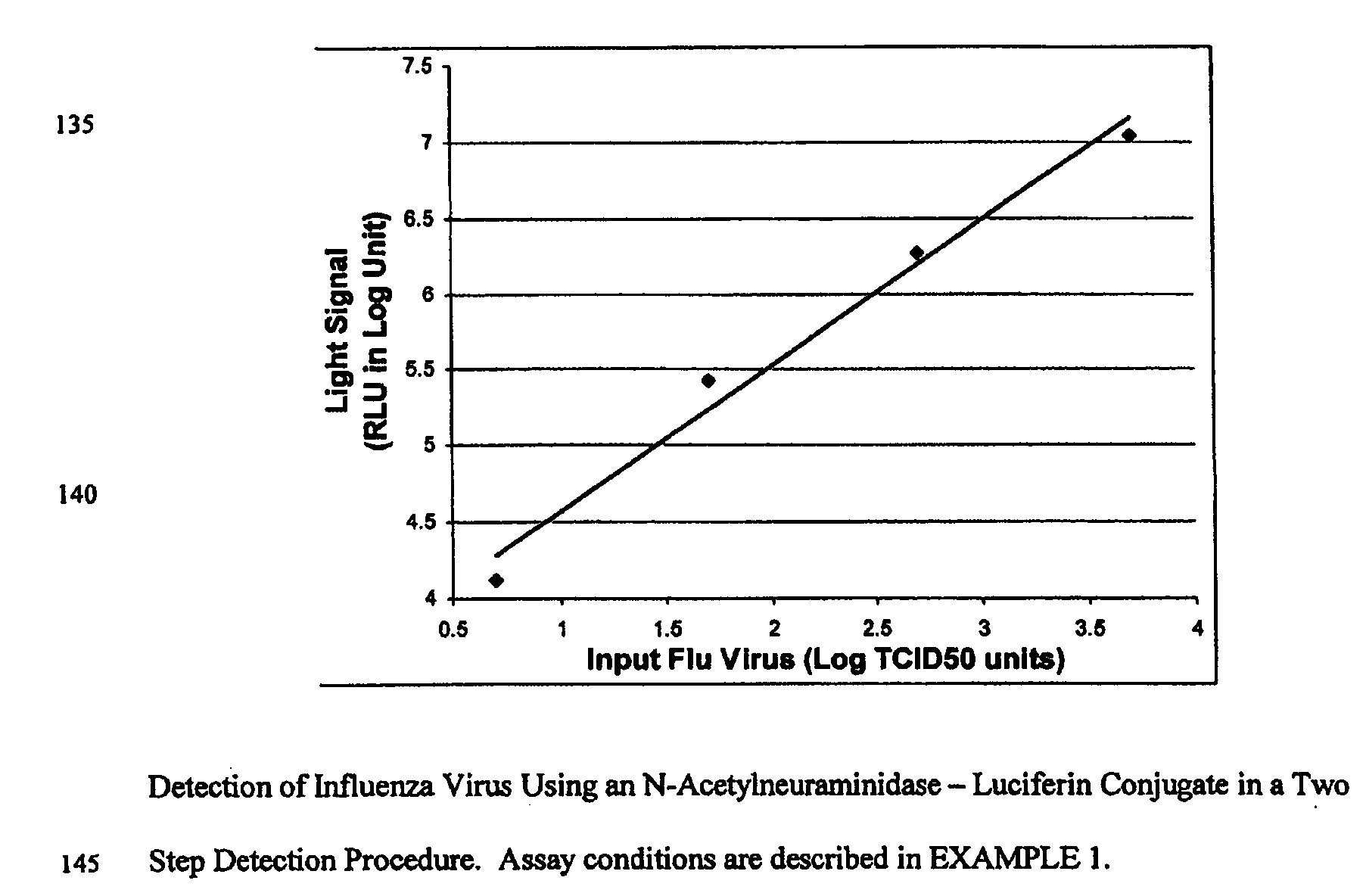
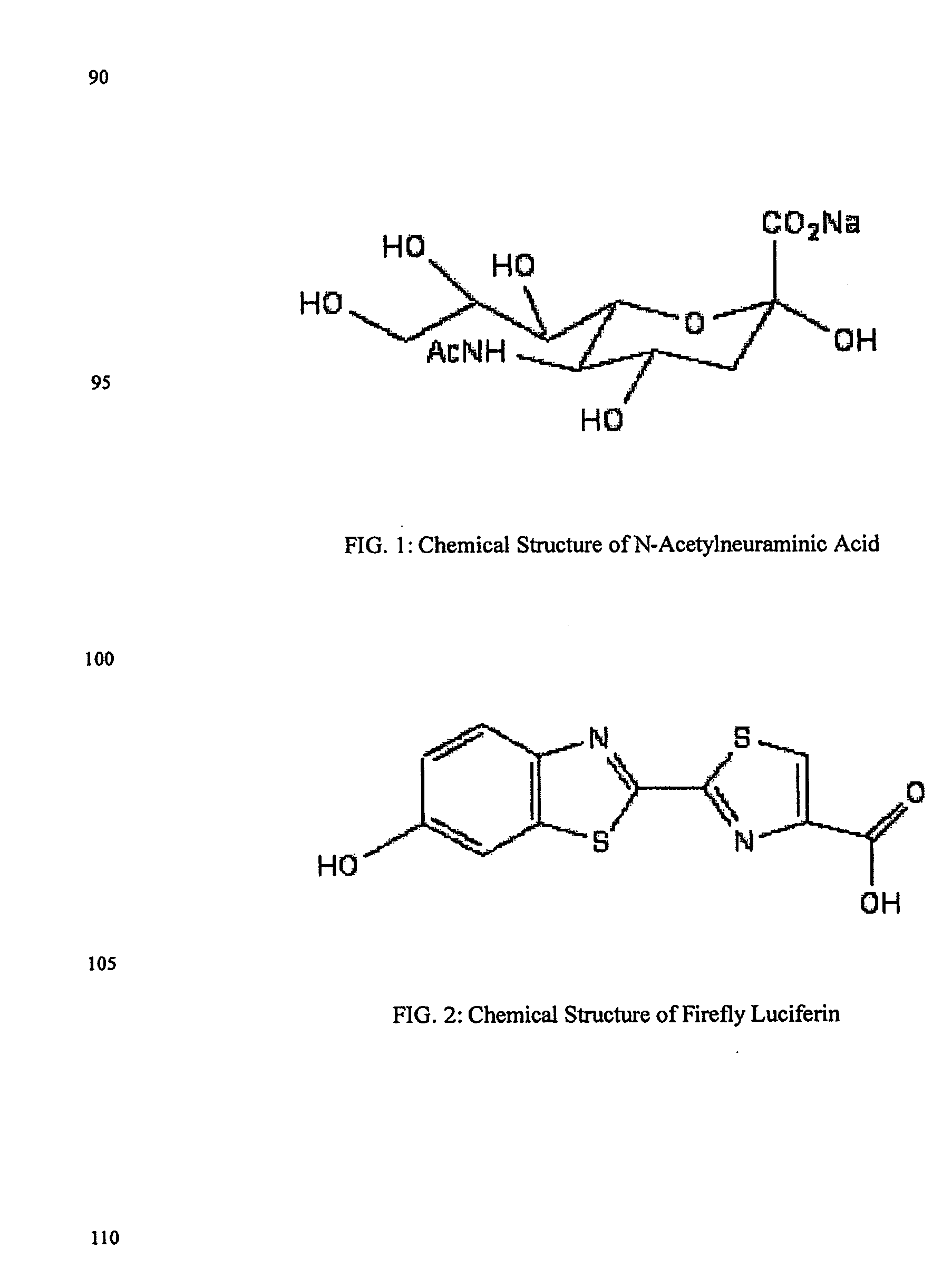
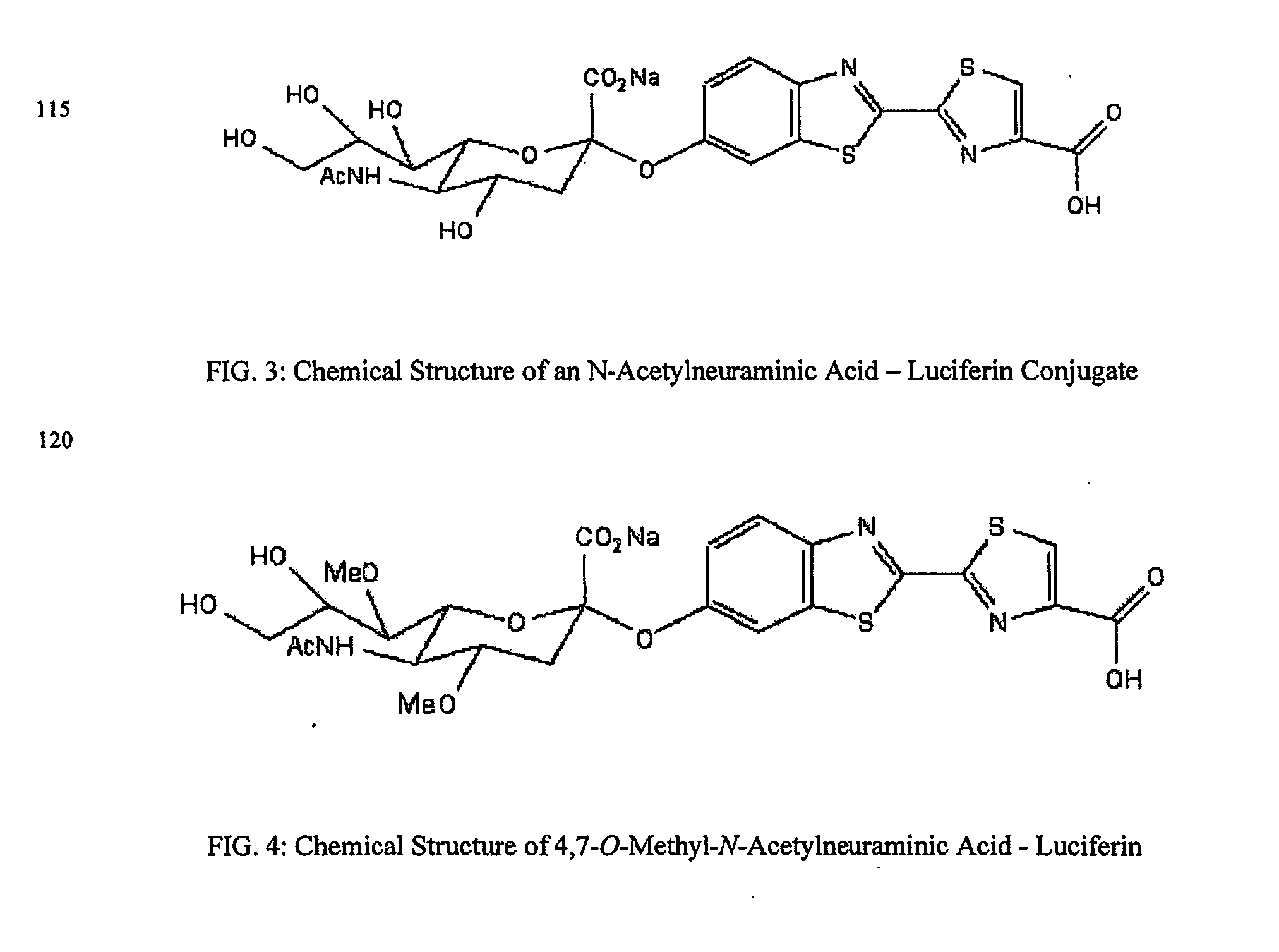
ActiveUS20080286758A1Simple and rapid and specific and sensitive detectionHigh detection sensitivityOrganic chemistryMicrobiological testing/measurementNeuraminidaseFluorescein
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX BIOLOGICAL TECH SUZHOU CO LTD
Novel vaccines against multiple subtypes of influenza virus
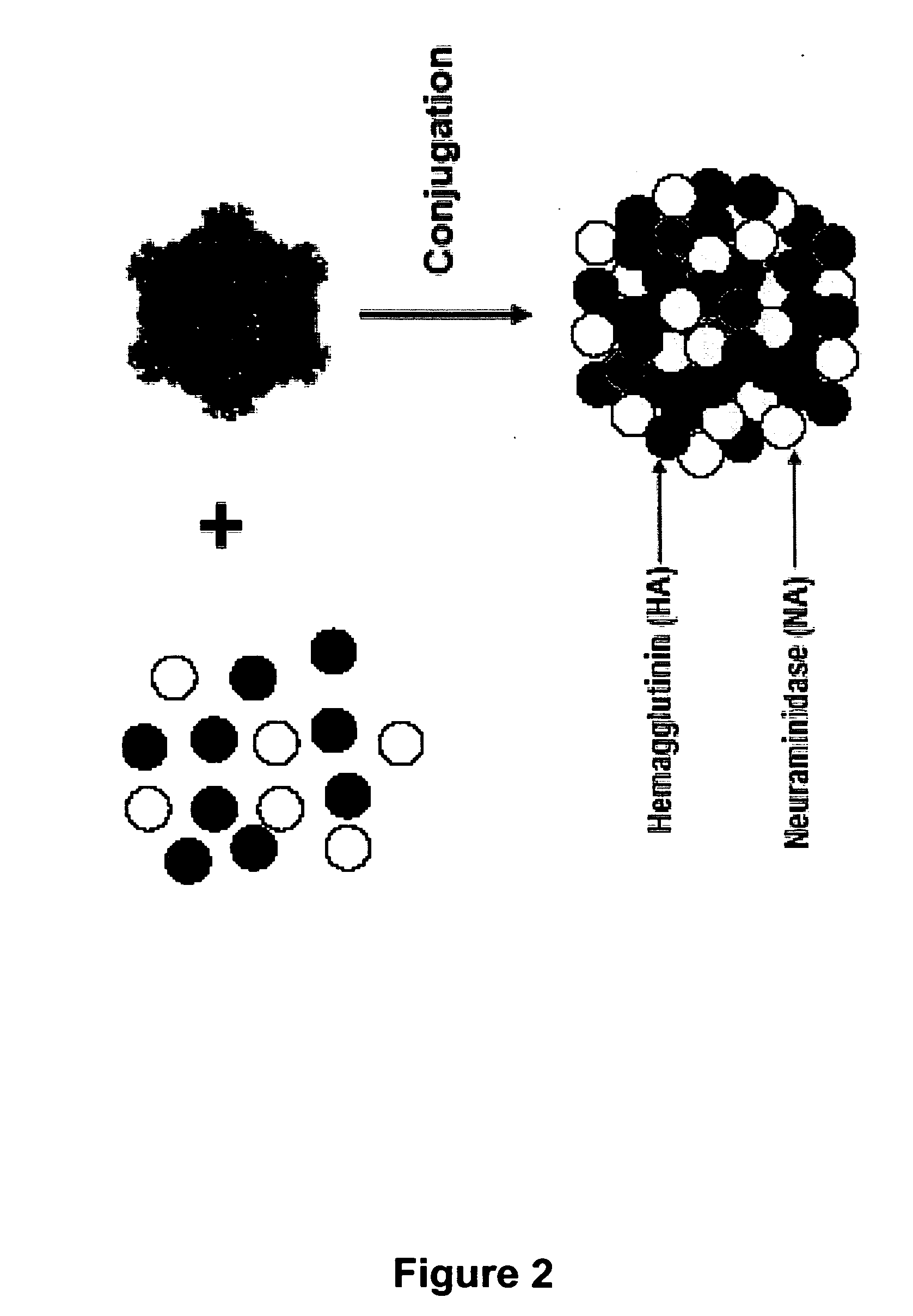
ActiveUS20090169505A1Elicit immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininMammal
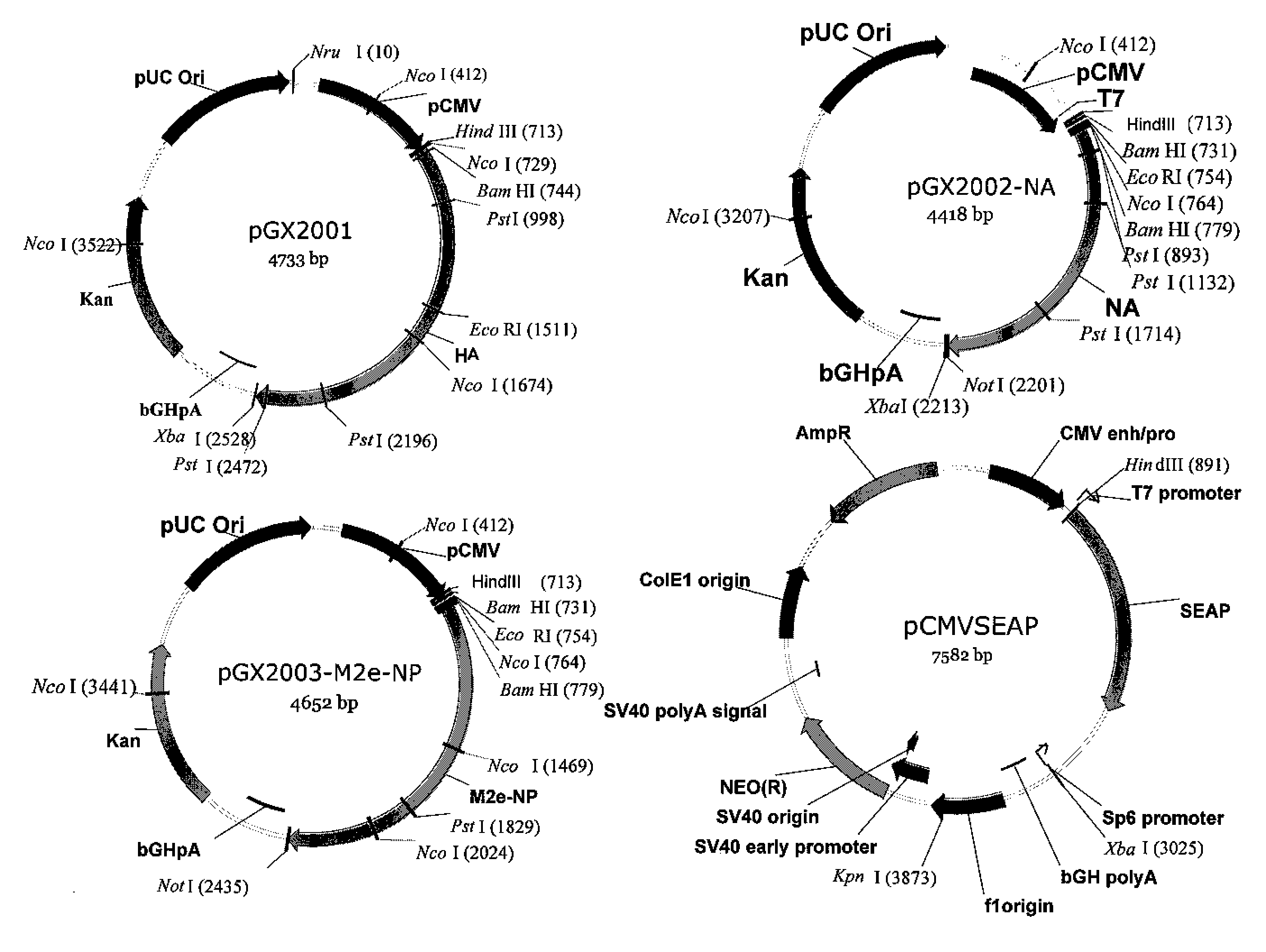
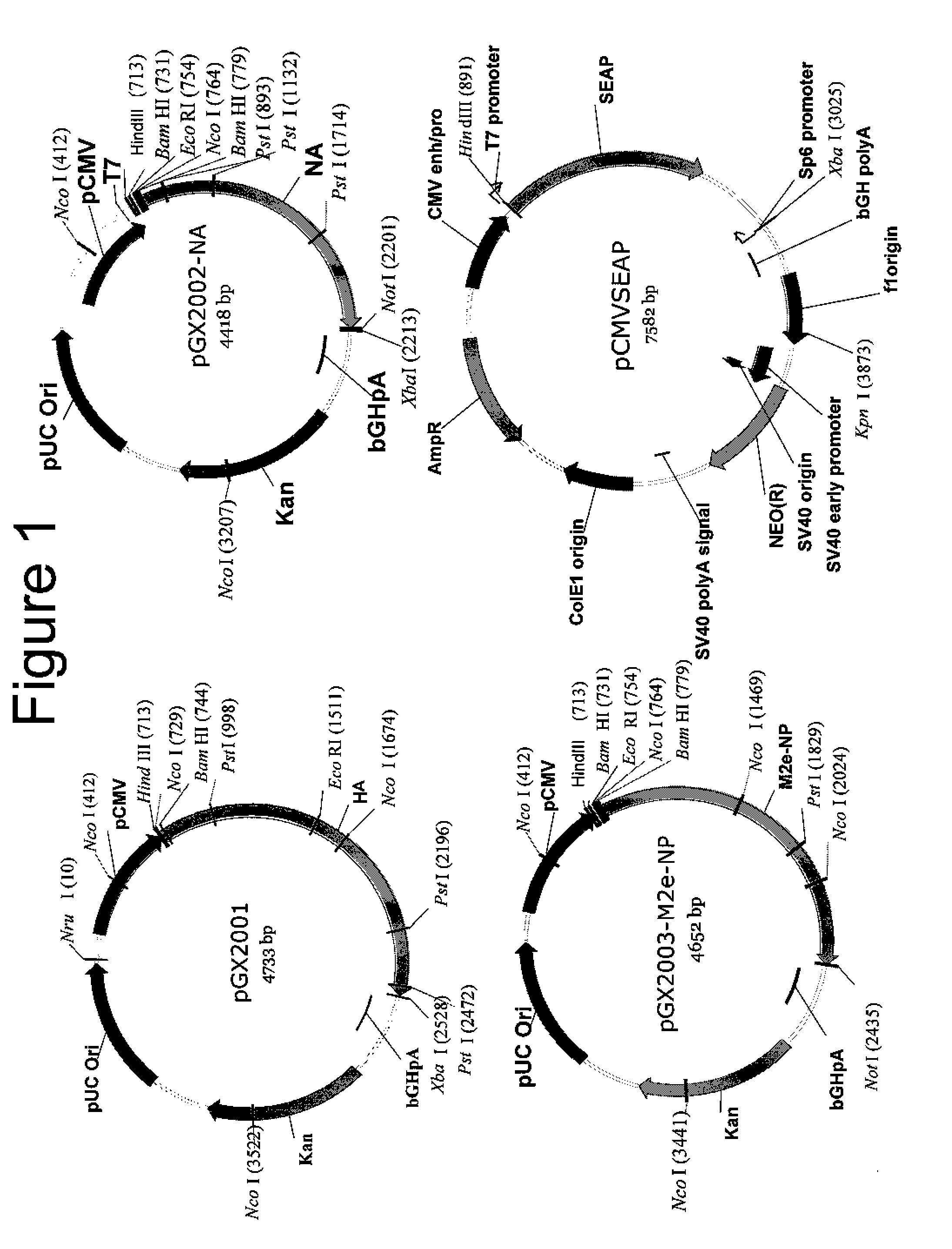
An aspect of the present invention is directed towards DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of influenza virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient. The DNA plasmid is capable of expressing a consensus influenza antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal, wherein the consensus influenza antigen comprises consensus hemagglutinin (HA), neuraminidase (NA), matrix protein, nucleoprotein, M2 ectodomain-nucleo-protein (M2e-NP), or a combination thereof. Preferably the consensus influenza antigen comprises HA, NA, M2e-NP, or a combination thereof. The DNA plasmid comprises a promoter operably linked to a coding sequence that encodes the consensus influenza antigen. Additionally, an aspect of the present invention includes methods of eliciting an immune response against a plurality of influenza virus subtypes in a mammal using the DNA plasmid vaccines provided.
Owner:VGX PHARMA +1
Bi-Functional Polymer-Attached Inhibitors of Influenza Virus
InactiveUS20090081249A1Inhibiting and preventing development of resistanceAntiviralsCarrier-bound antigen/hapten ingredientsPolyethylene glycolDextran
Antimicrobial compositions containing two or more antiviral agents coupled to a polymer and methods of making and using the compositions, are described herein. In one embodiment, two or more antiviral agents are covalently coupled to the polymer. Suitable antiviral agents include, but are not limited to, sialic acid, zanamivir, oseltamivir, amantadine, rimantadine, and combinations thereof. The polymer is preferably a water-soluble, biocompatible polymer. Suitable polymers include, but are not limited to, poly(isobutylene-alt-maleic anhydride) (PIBMA), poly(aspartic acid), poly(l-glutamic acid), polylysine, poly(acrylic acid), plyaginic acid, chitosan, carboxymethyl cellulose, carboxymethyl dextran, polyethyleneimine, and blends and copolymers thereof. In another embodiment, the compositions contain a physical mixture of polymer containing one antiviral agent and polymer containing a second antiviral agent. The compositions can be formulated for enteral or parenteral administration. Suitable oral / intranasal dosage forms include, but are not limited to, tablets, capsules, solutions, suspensions, emulsions, syrups, and lozenges. Suitable dosage forms for parenteral administration include, but are not limited to, solutions, suspensions, and emulsions. The compositions described herein are effective at treating a variety of infections, including viral infections such as influenza, while inhibiting or preventing the development of microbial resistance.
Owner:MASSACHUSETTS INST OF TECH
Inactivated Influenza Virus Compositions
InactiveUS20090297558A1InactivatesMaintain activitySsRNA viruses negative-senseBacterial antigen ingredientsVirus influenzaInfluenza a
The invention provides compositions of inactivated influenza virus that can be used as vaccines and immunological compositions useful for inhibiting, preventing and treating influenza.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Compositions enriched in phenolic compounds and methods for producing the same
Provided are processes for the preparation of compositions enriched in phenolic compounds from a crude plant extract. One process includes a novel column purification step using a polymer resin that releasably adsorbs the phenolic compounds but does not retain polar non-phenolic compounds, wherein the resin comprises aromatic rings substituted with one or more electron-withdrawing groups. This invention also includes compositions enriched in phenolic compounds. This invention encompasses methods of using the phenolic-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease. The compositions are also useful as meat additives to inhibit food-borne pathogens.
Owner:PHENOLICS LLC
Defective Influenza Virus Particles
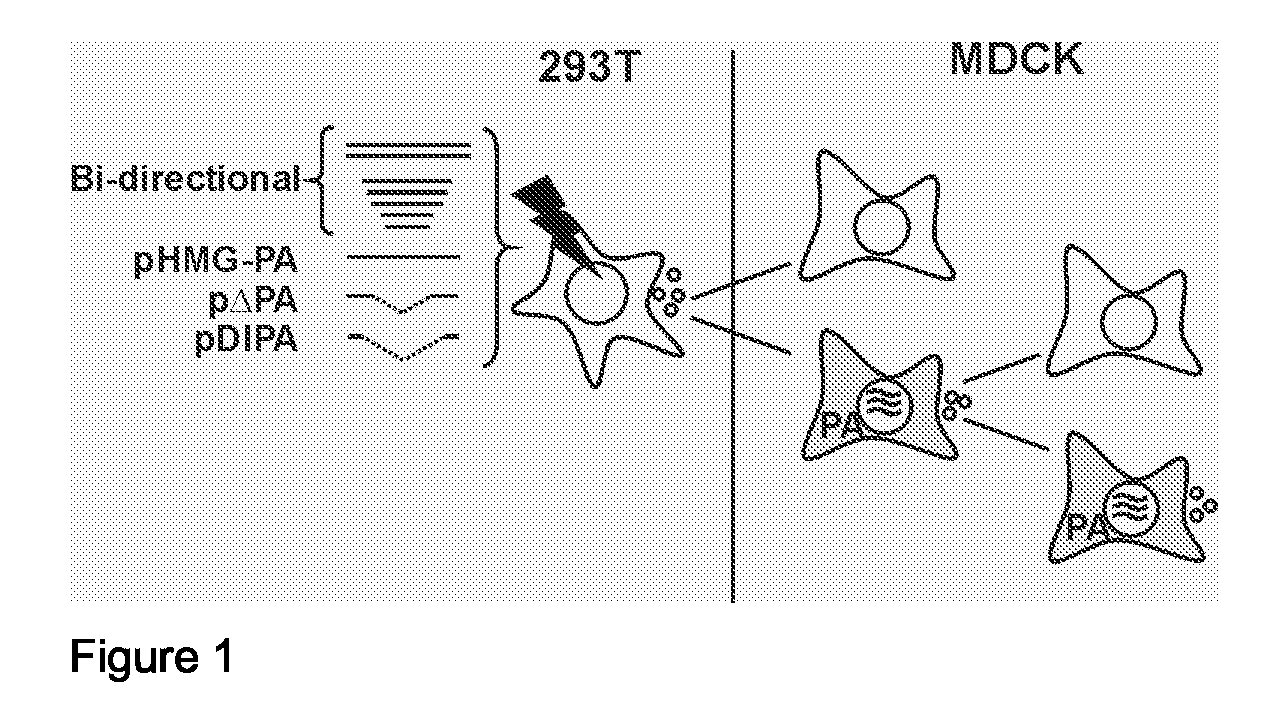
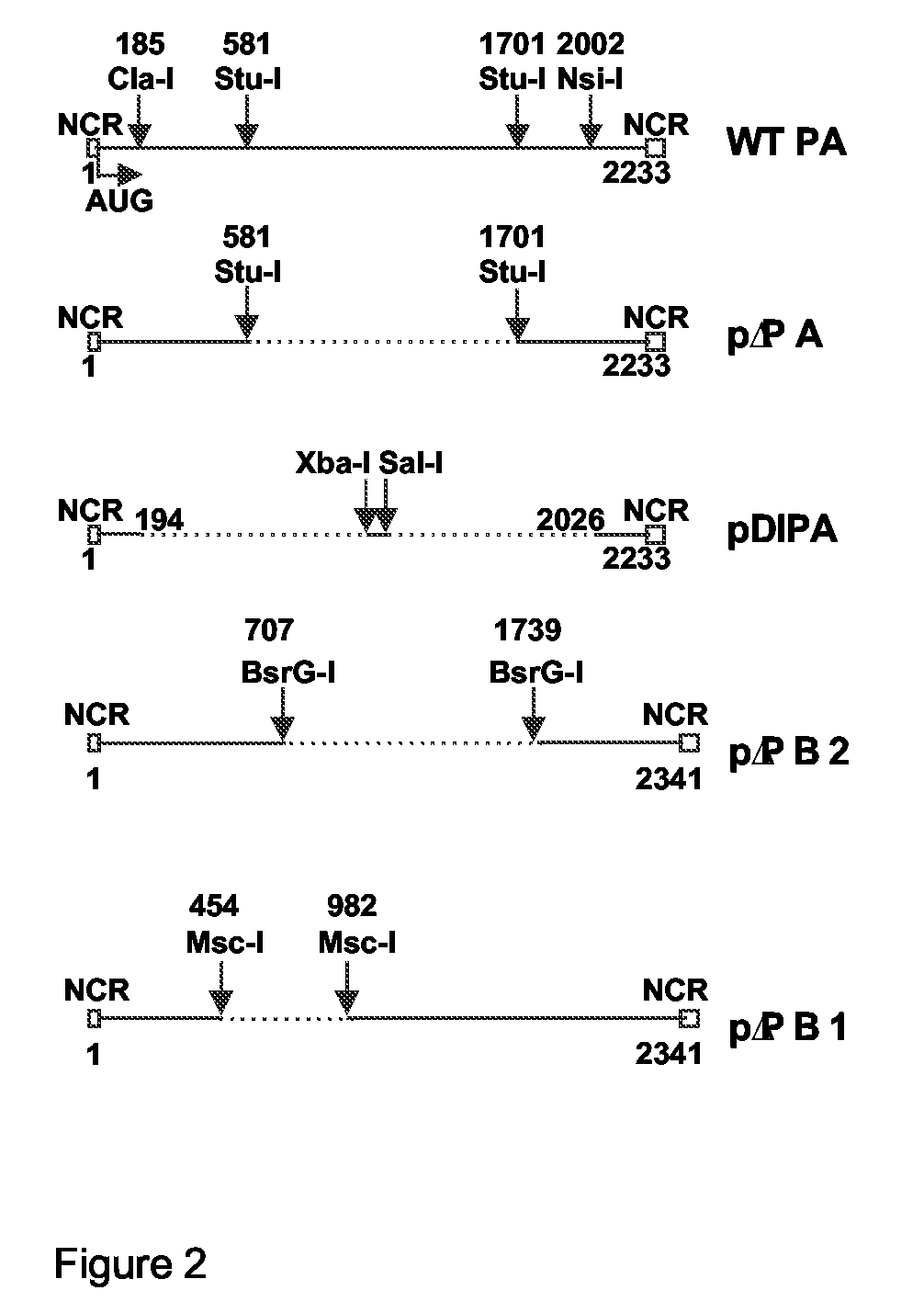
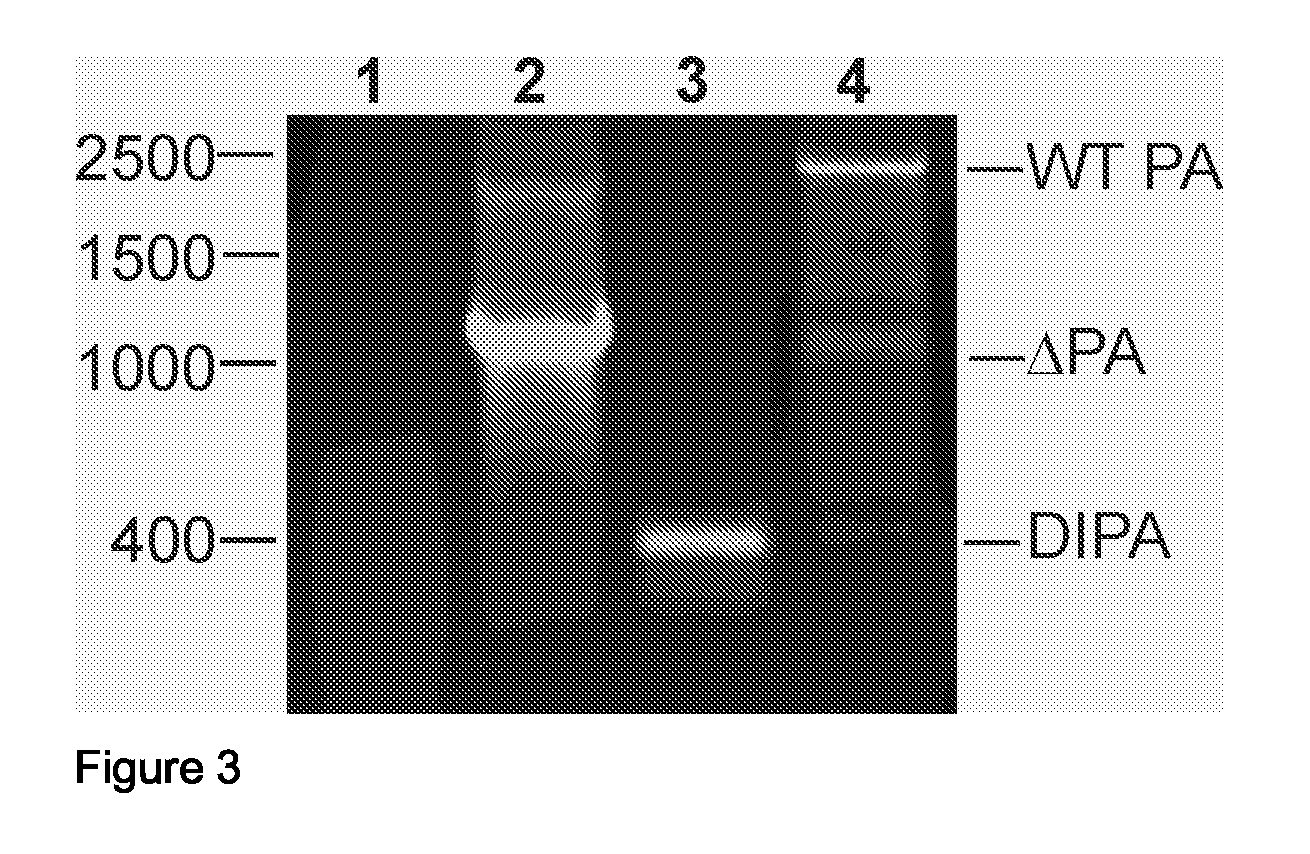
InactiveUS20080292658A1Reduce riskAvoid reinfectionSsRNA viruses negative-senseAnimal cellsFlu immunisationPolymerase L
The invention relates to the field of influenza virus and the vaccination against flu. The invention provides a conditionally defective influenza virus particle having seven different influenza nucleic acid segments. The invention also provides a conditionally defective influenza virus particle lacking an influenza nucleic acid segment selected from the group of segments essentially encoding acidic polymerase (PA), the basic polymerase 1 (PB1) and the basic polymerase 2 (PB2). In particular, the invention provides defective influenza virus particles having seven different influenza nucleic acid segments and lacking an influenza nucleic acid segment essentially encoding acidic polymerase. Furthermore, the invention provides use of a composition comprising a defective influenza virus particle according to the invention for the production of a pharmaceutical composition directed at generating immunological protection against infection of a subject with an influenza virus, and provides a method for generating immunological protection against infection of a subject with an influenza virus comprising providing a subject in need thereof with a composition comprising such defective influenza virus particle.
Owner:ABBOTT BIOLOGICALS
Materials and methods for treating viral infections
InactiveUS20070135525A1Avoid infectionPrevent and delay developmentBiocideAntiviralsCysteamineAvian influenza virus
The subject invention provides materials and methods for treating various health conditions, including the prevention and / or treatment of a viral infection. In a preferred embodiment, a cysteamine compound is administered to a subject to treat an influenza virus infection. More preferably, a cysteamine compound is administered to a subject to treat influenza A, influenza B, influenza C virus infections, including avian influenza virus subtypes (such as H5N1 avian influenza virus).
Owner:OMEGA BIOPHARMA H K
Rescue of influenza virus
The invention relates to the field of influenza vaccine production. Influenza vaccines have been produced in embryonated hens' eggs for over 50 years, but recently there have been considerable efforts to develop cell culture systems for vaccine production. The invention provides a nucleic acid comprising an influenza gene segment and a bacteriophage polymerase promotor or a complementary strand of said nucleic acid, and a cell comprising such a nucleic acid capable of producing desired influenza virus. Furthermore, the invention provides a composition comprising a cell or material derived from a cell according to the invention and a virus or material derived from a viral particle according to the invention.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC +1
Peptide vaccine for influenza virus
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
Method for Replicating Influenza Virus in Culture
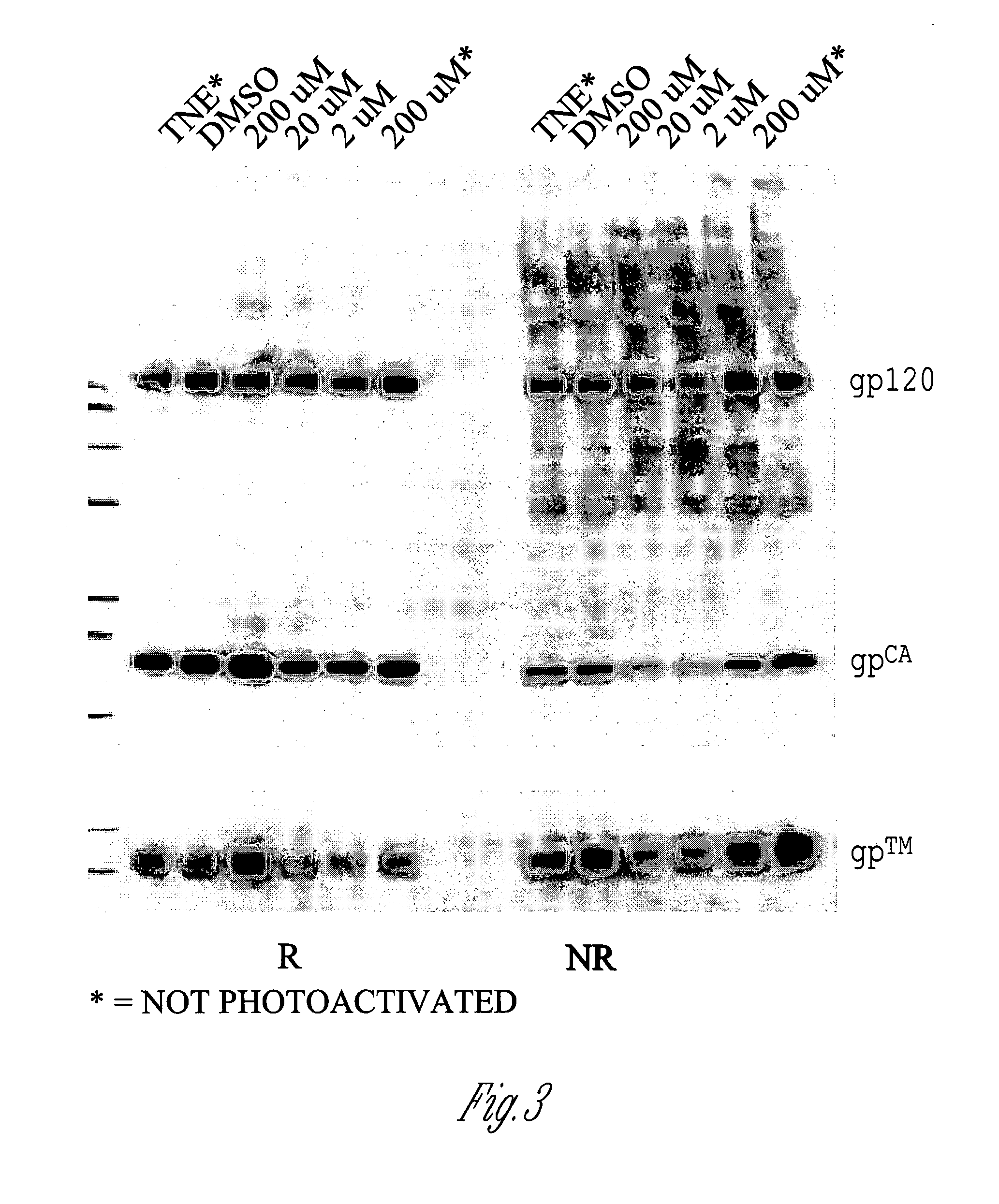
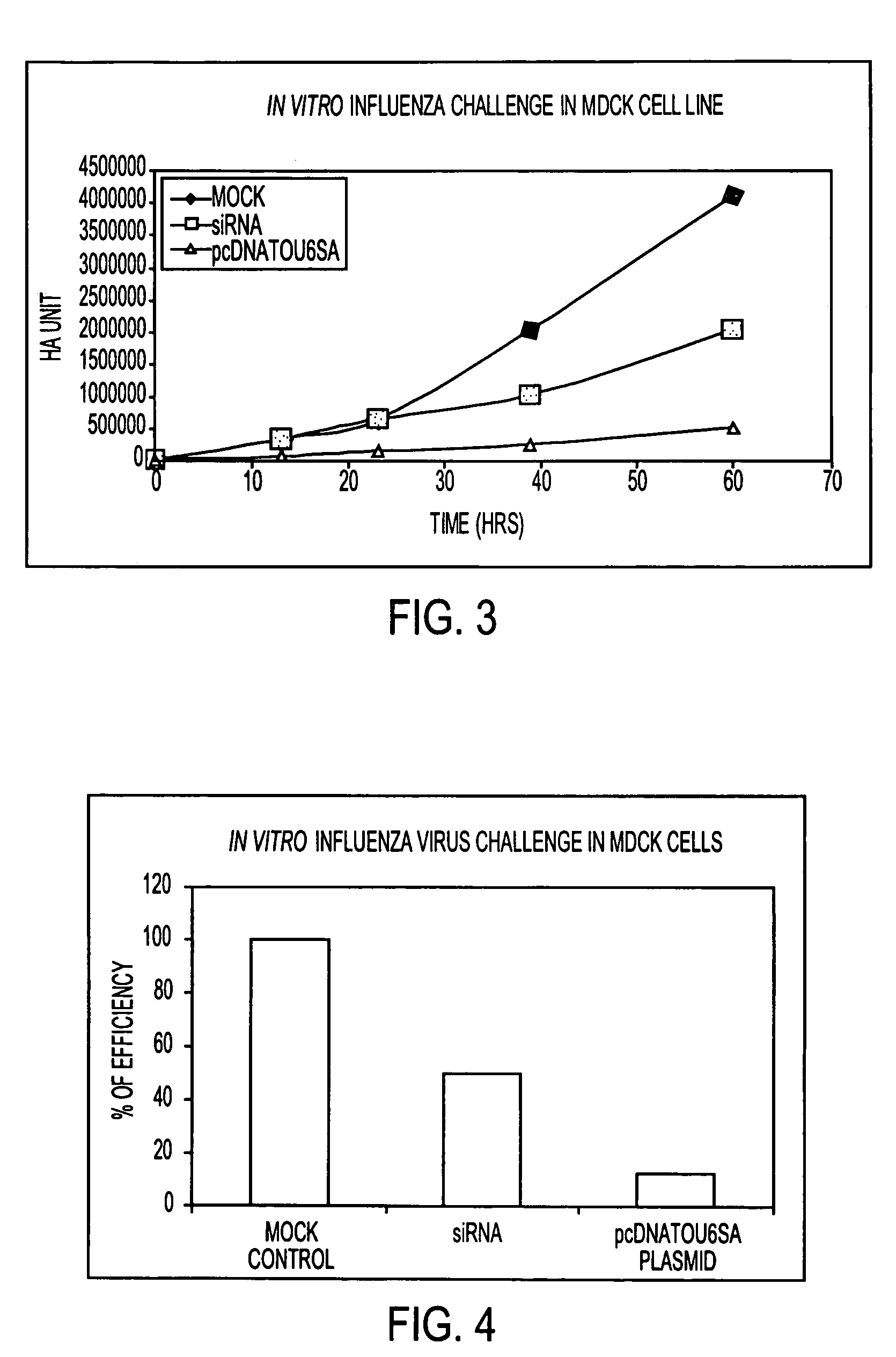
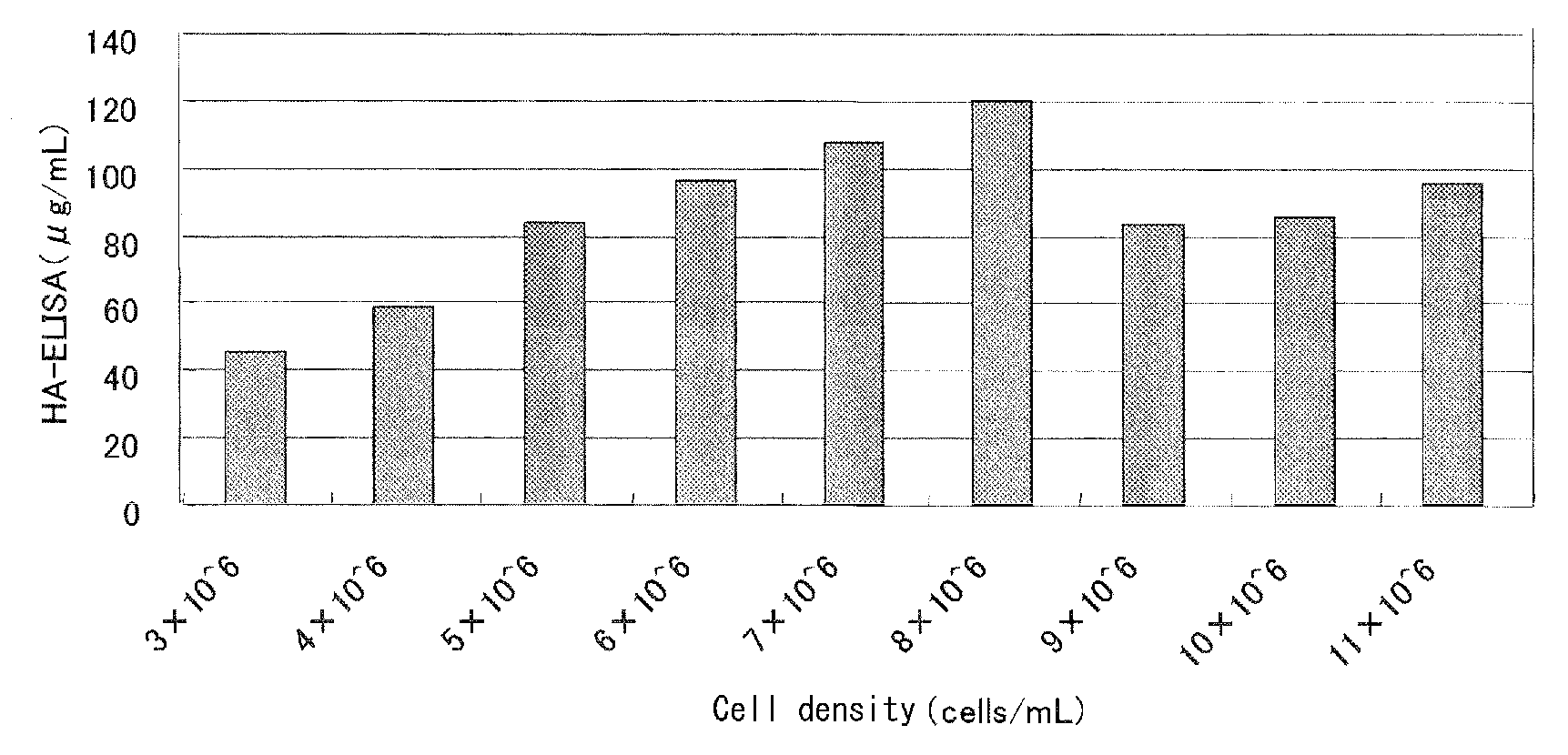
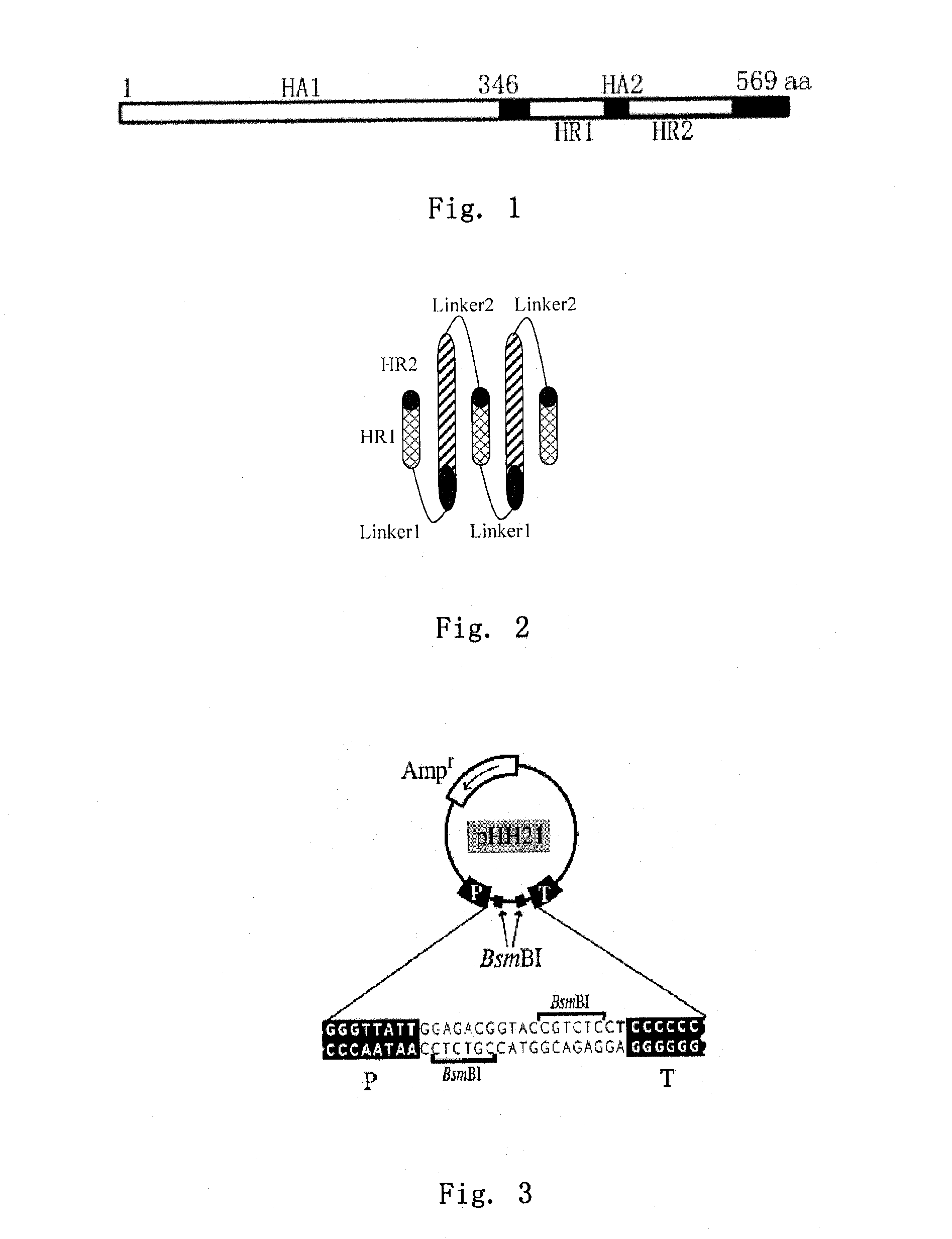
InactiveUS20080187546A1Save critical timeShorten the timeSsRNA viruses negative-senseAntiviralsTissue cultureInfluenza C Virus
The invention is related to a method for selecting an influenza virus for growth on tissue culture cells to produce a tissue-culture adapted viral isolate. The invention also includes vaccines produced from the isolate.
Owner:INTERVET INC
Efficient method for producing compositions enriched in total phenols
This invention provides a process for the preparation of compositions enriched in total phenols from a crude plant extract. The process includes a novel column purification step using a brominated polystyrene resin. This invention also includes compositions enriched in total phenols. The enriched compositions are characterized as containing monomeric, oligomeric and polymeric phenols and having HPLC chromatograms substantially as set forth in FIGS. 10-13. This invention encompasses methods of using the total phenol-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease.
Owner:PHENOLICS LLC
Influenza viruses and uses thereof
ActiveUS8828406B2Reduced ability to reassortImprove securitySsRNA viruses negative-senseSugar derivativesOpen reading frameNucleic acid sequencing
Described herein are chimeric influenza virus gene segments and nucleic acid sequences encoding such chimeric influenza virus gene segments. A chimeric influenza virus gene segment described herein comprises packaging signals found in the non-coding and coding regions of one type of influenza virus gene segment and an open reading frame of a different type of influenza virus gene segment or fragment thereof. Also described herein are recombinant influenza viruses comprising two or more chimeric influenza virus gene segments and the use of such viruses in the prevention and / or treatment of influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
Potent inhibition of influenza virus by specifically designed short interfering RNA
Owner:CALIFORNIA POLYTECHNIC STATE UNIVERSITY
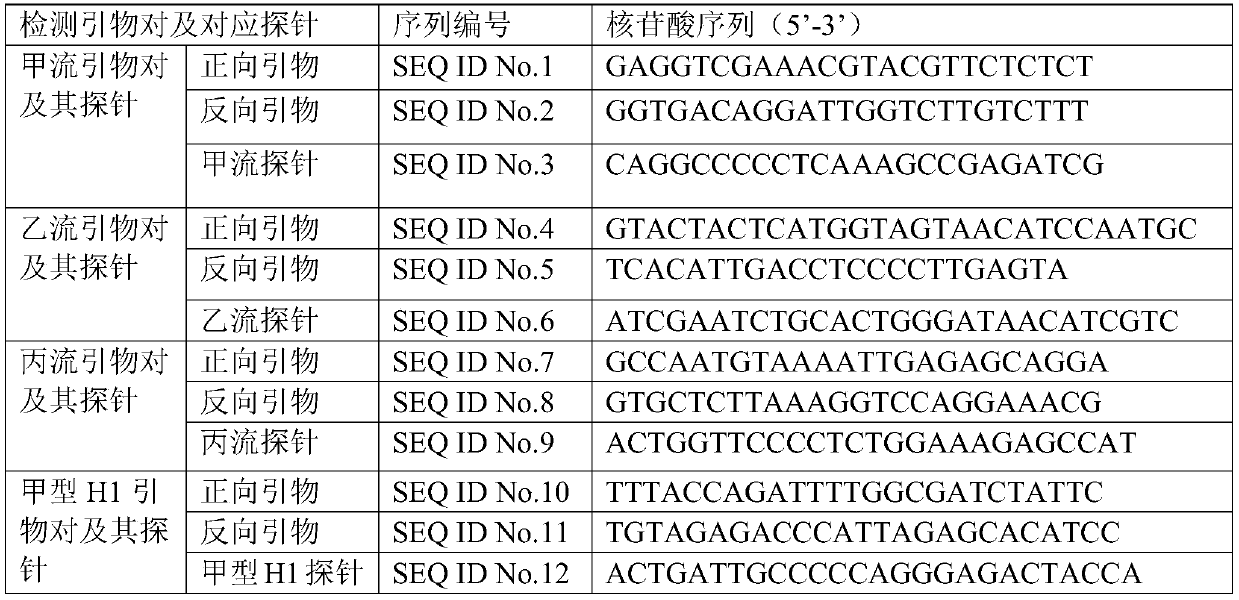
Nucleic acid composition, detection kit for influenza virus and micro-fluidic chip
PendingCN110283940ABioreactor/fermenter combinationsBiological substance pretreatmentsHighly pathogenicInfluenza a
The invention relates to nucleic acid composition, a detection kit for an influenza virus and a micro-fluidic chip. The nucleic acid composition comprises detection primer pairs, wherein the detection primer pairs comprise at least two of the following primer pairs: an influenza A primer pair, an influenza B primer pair, an influenza C primer pair, an influenza A H1 primer pair, an influenza A H3 primer pair, an influenza A H5 primer pair, an influenza A H7 primer pair, an influenza A H9 primer pair, an influenza A N9 primer pair and a high-pathogenicity influenza A H7N9 primer pair. According to the nucleic acid composition, interference between the primers and probes can be avoided, at least two influenza viruses are detected simultaneously once, and detection sensitivity can reach 100 copies / mL.
Owner:深圳市呈晖医疗科技有限公司
Recombinant flu vaccines
InactiveUS20090117144A1Sufficient immunogenicityReduce loadSsRNA viruses negative-senseSsRNA viruses positive-senseInfluenza virus vaccinePlant virus
The present invention provides compositions for use as vaccines against the influenza virus, and rapid methods of producing such compositions. The composition include i) at least one peptide derived from an influenza virus, wherein the peptide is fused to a capsid protein derived from a plant virus forming a recombinant capsid fusion peptide and ii) at least one isolated antigenic protein or protein fragment derived from a human or avian influenza virus. The isolated antigenic protein or protein fragment derived from the human or avian influenza virus can be conjugated to the surface of the recombinant capsid fusion peptide.
Owner:PFENEX
Method for producing influenza viruses in large scale by using bioreactor
The invention provides a method for producing influenza viruses in large scale by using a bioreactor, which comprises the following steps of: (1) inoculating passage cell suspension into a vector tank, and setting the parameters of the bioreactor to perform a cell adsorption and culture process, wherein a serum culture medium is used in the cell adsorption and culture process; (2) when cells are grown to proper density, rinsing the cells to remove serum components, inoculating influenza virus liquid into the vector tank, and setting the parameters of the bioreactor to perform a virus adsorption and propagation process, wherein a serum-free culture medium containing TPCK (tosyl-L-phenylalanine chloromethyl ketone) pancreatin is used in the virus adsorption and propagation process; and (3) harvesting the virus liquid. The method has the advantage that: the high-titer virus liquid can be obtained by adopting the tide type bioreactor and using the passage cells for propagating the influenza viruses. The method overcomes the defects of the traditional transfer bottle culture and other bioreactors, and can continuously produce high-titer viruses in large scale.
Owner:PU LIKE BIO ENG
Avian influenza virus live attenuated vaccine and uses thereof
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Method for propagating influenza virus
InactiveUS20090181446A1Increase productionReduce the amount of solutionSsRNA viruses negative-senseArtificial cell constructsTrypsin inhibitorCanis lupus familiaris
A method for producing influenza virus on large scale is provided. A method for propagating influenza virus which comprises, after removing or decreasing a trypsin inhibitor secreted into culture of MDCK cells (cell line derived from dog kidney) by washing with a culture medium or a buffer, inoculating influenza virus into said cells and culturing said influenza virus-inoculated cells in a culture medium supplemented with trypsin.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Pyridine derivative, composition thereof and application of pyridine derivative and composition as anti-influenza virus drug
ActiveCN110041327AImproved pharmacokinetic propertiesHigh activityGroup 4/14 element organic compoundsAntiviralsDiseaseSolvent
The invention belongs to the antiviral field of medical chemistry and relates to a novel pyridine derivative shown in a formula (I), a stereisomer, pharmaceutical salt, a solvent compound or a crystalof the pyridine derivative and an application of the pyridine derivative and the stereisomer, pharmaceutical salt, the solvent compound or the crystal in preparing drugs for preventing or treating viral infection diseases such as influenza A and / or influenza B, particularly in preparing a cap dependent endonuclease inhibitor in preventing or treating influenza A and / or influenza B viral infectiondiseases. The compound has remarkable activity for inhibiting influenza endonuclease and influenza DNA, can be independently used or can be combined with a neuraminidase inhibitor, nucleoside medicines, a PB2 inhibitor, a PB1 inhibitor, an M2 inhibitor or other anti-influenza drugs, the influenza infection time is notably shortened, the death rate is remarkably reduced, and the pyridine derivative has very good clinical application prospects.
Owner:JIANGXI CAISHI PHARMA TECH CO LTD
Production method for influenza virus vaccines
InactiveCN101818131ASolve yourselfSolve pollutionAntiviralsViruses/bacteriophagesEquine influenza vaccineBioreactor
The invention discloses a production method for vaccines of avian influenza virus and other influenza virus such as swine influenza, dog influenza and equine influenza, which comprises the following steps of: (1) transfer of culture and cultivation of vaccine-made cells; (2) reproduction of vaccine-made virus seeds; (3) microcarrier suspension culture of MDCK cells in a bioreactor; (4) reproduction of vaccine-made virus liquid; and (5) harvest of the virus liquid. The production method has the advantages of reducing the production cost greatly and improving the yield and quality of the vaccines obviously, along with short production period, no restriction to raw material supply, small occupied area, easy and quick expansion of production scale, light environmental pollution, easy processing, high automaticity, low employment and easy realization of balanced and stable quality.
Owner:成都史纪生物制药有限公司
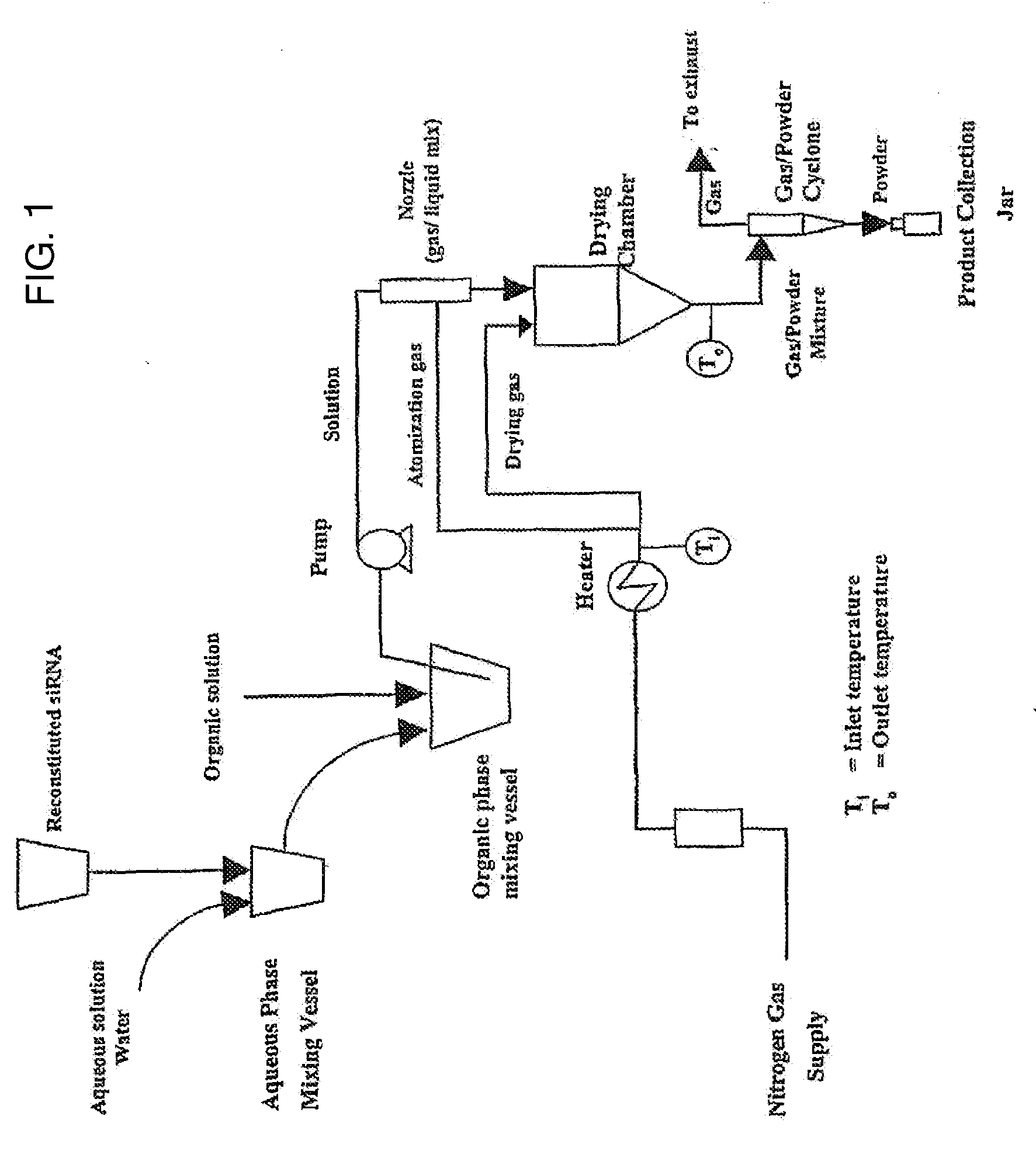
Dry powder compositions for RNA influenza therapeutics
InactiveUS20110077284A1Reduce deliveryIncrease the amount of waterSsRNA viruses negative-sensePowder deliveryInhalationDouble stranded
Owner:MARINA BIOTECH INC
Method and medicament for inhibiting the infection of influenza virus
InactiveUS20100249021A1Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaMedicine use
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and kit
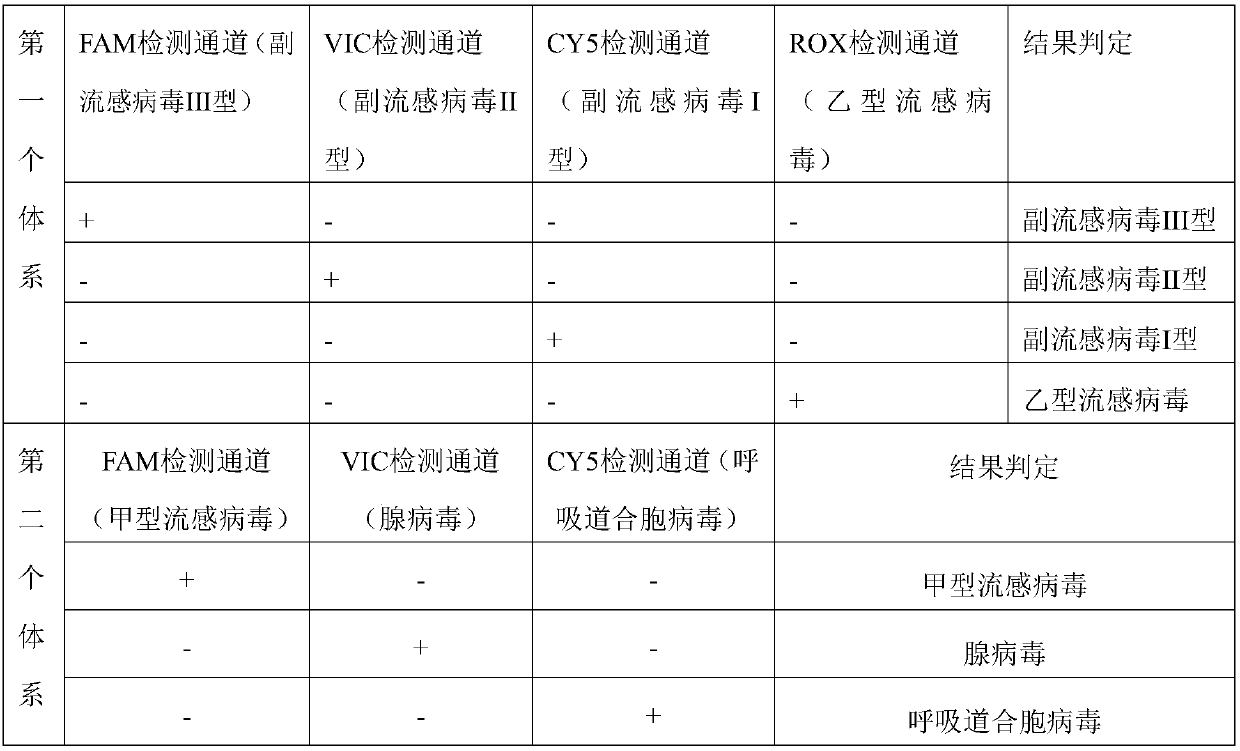
ActiveCN107937613AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceParainfluenza virus
The present disclosure relates to a real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and a kit. Seven influenza viruses are influenza A virus, influenza B virus, parainfluenza virus type I, parainfluenza virus type II, parainfluenza virus type III, respiratory syncytial virus and adenovirus. The present disclosure also provides a kit for multiplex fluorescence quantitative PCR detection of the seven influenza viruses, and the kit includes the primer probe set. The kit significantly improves the sensitivity, specificity, and simplicity of detection of the common respiratory system pathogens.
Owner:北京卓诚惠生生物科技股份有限公司
Method for detecting influenza virus by liquid phase chip
ActiveCN101717830AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescenceMicrosphere
The invention discloses a method for detecting influenza virus by a liquid phase chip. The method is used for carrying out homology analysis on all Non Structural nucleotide sequences of A type influenza virus and B type influenza virus as well as H1, H2, H3, H5, H7 and H9 of the A type influenza virus and hypotypes of N1 and N2, and primers and specific probes are designed aiming at the corresponding gene segments; an improved nest-PCR method is adopted for PCR amplification by using nested primers with different lengths; amination treatment is carried out the 5' end of the specific probe, and the specific probe is coupled with a fluorescent coded microballoon; incubation hybridization is carried out on the PCR amplification product and the mixed microballoon coupled with the specific probe; and finally Luminex 100 is used for detecting. The detection method has higher specificity and sensitivity as well as rapid detection speed, and can be used for indentifying and monitoring the influenza viruses.
Owner:SHANDONG ACV BIOTECH CO LTD
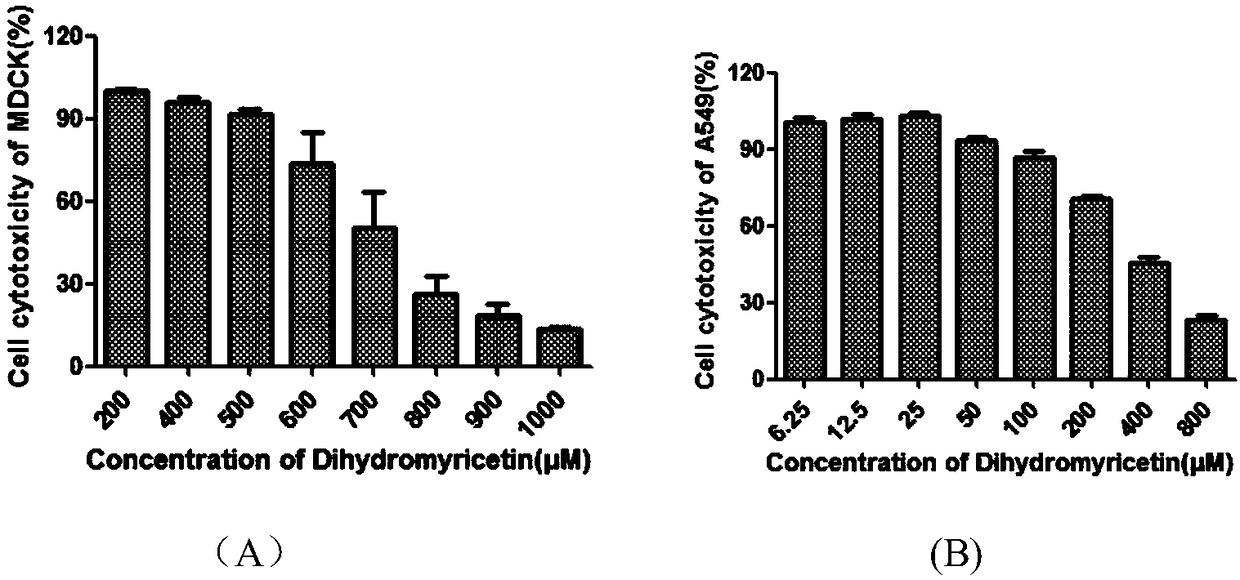
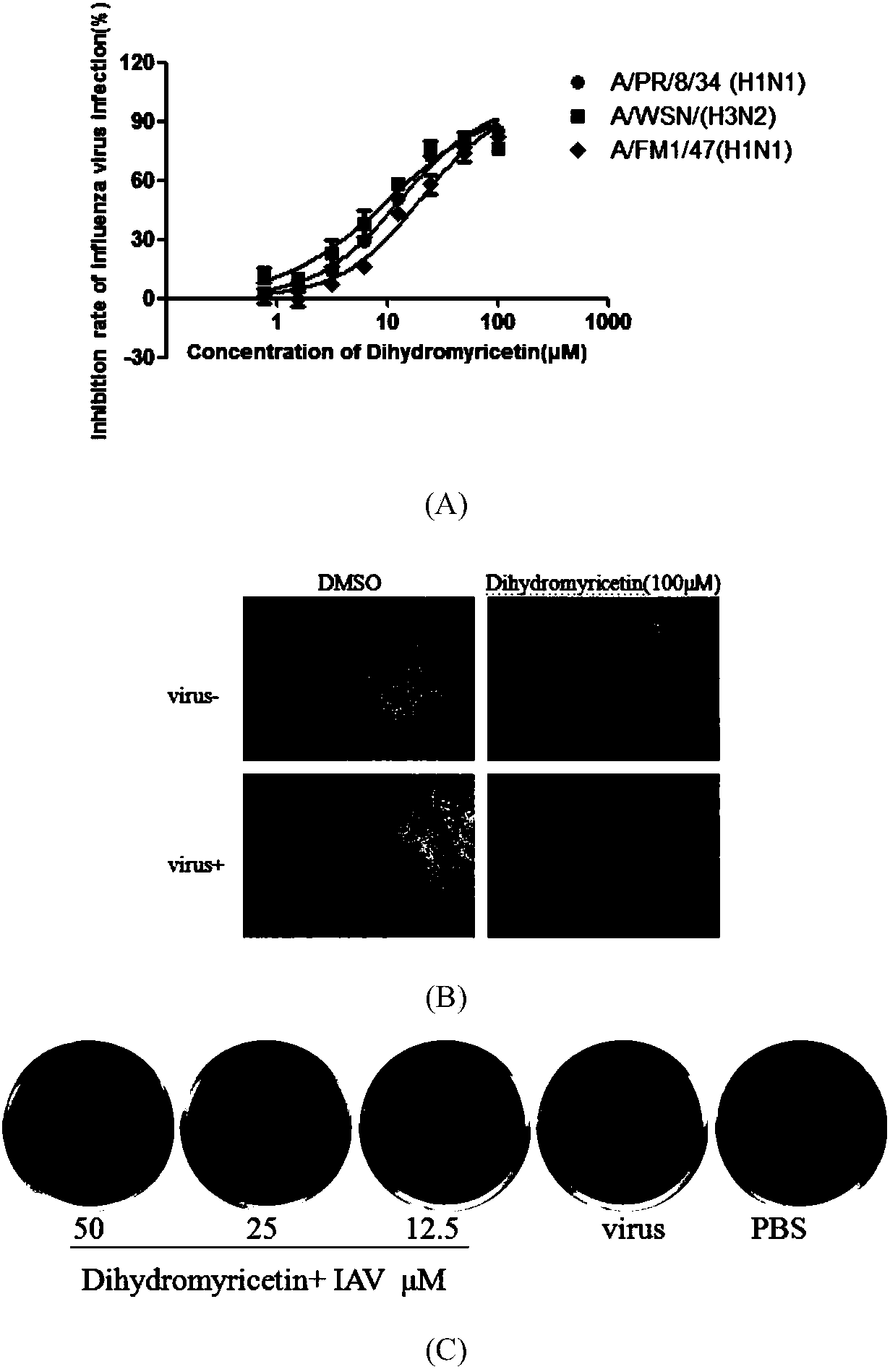
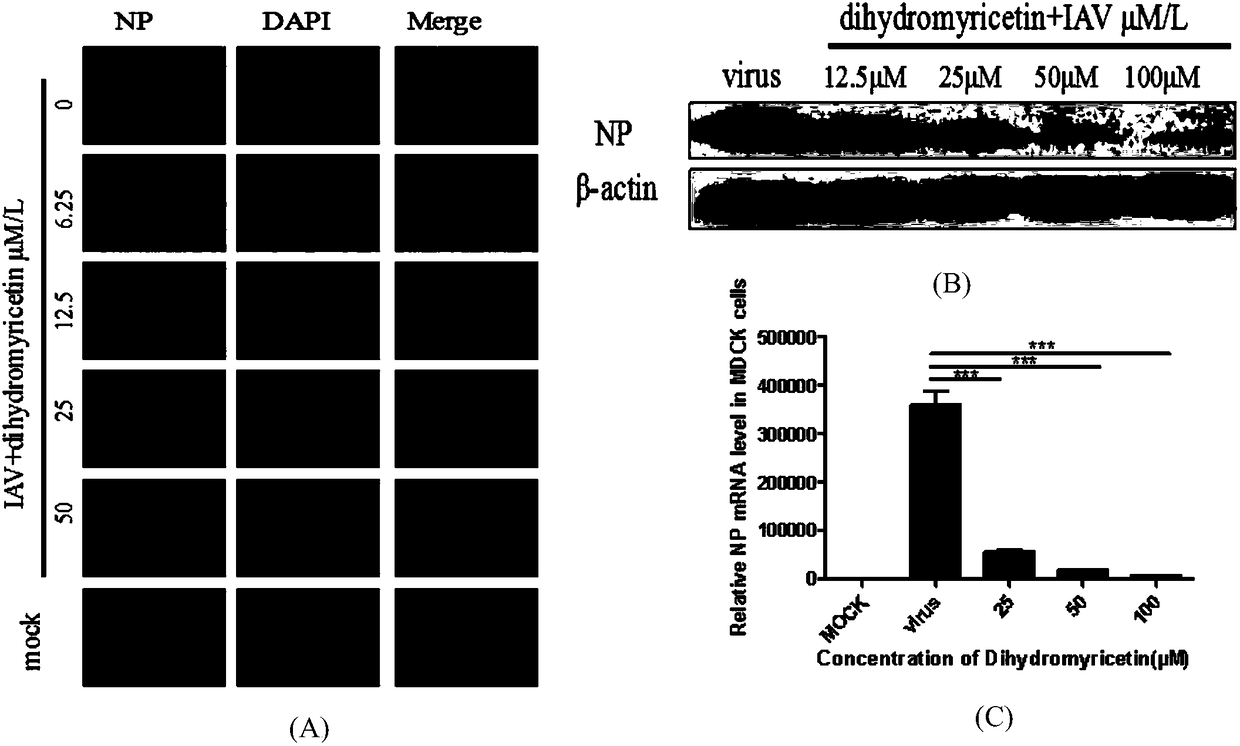
Applications of dihydromyricetin in preparation of anti-influenza virus drugs
InactiveCN108354923ALow toxicitySafe and efficient developmentOrganic active ingredientsAntiviralsPhenylalanineHistidine
The invention discloses applications of dihydromyricetin in preparation of anti-influenza virus drugs, wherein influenza virus is influenza A virus, preferably H1N1, H3N2, H5N1, H7N1, H7N2, H7N3, H7N7, H7N9, H9N2 or H10N8. According to the present invention, the anti-influenza A virus action mechanism of the dihydromyricetin is that the activity of the ribonucleoprotein complex vRNP is inhibited by binding the dihydromyricetin to the PB2cap protein so as to prepare the drugs for prevention and treatment of influenza, wherein the binding loci of the dihydromyricetin and the PB2cap protein are site-432 histidine (His432), site-357 histidine (His357), site-404 phenylalanine (Phe404), site-323 Phenylalanine (Phe323), site-361 glutamic acid (Glu361), site-376 lysine (Lys376), site-363 phenylalanine (Phe363), and site-429 asparagine (Asn429).
Owner:SOUTHERN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com