Applications of dihydromyricetin in preparation of anti-influenza virus drugs
A technology against influenza virus, influenza virus, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
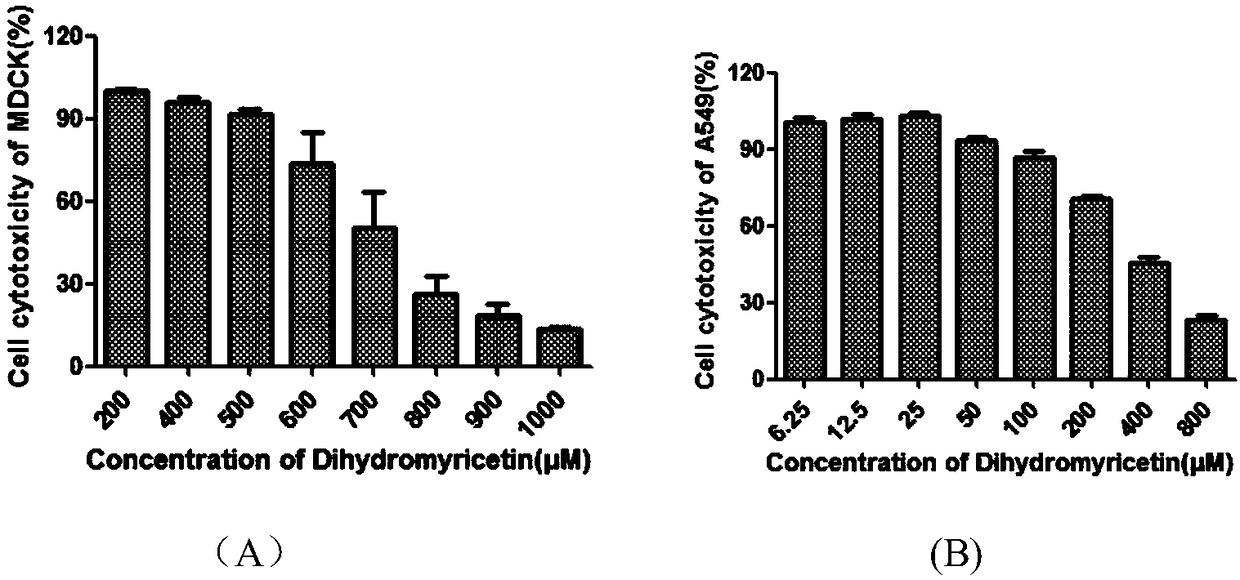
[0030] Cytotoxicity detection of embodiment 1 dihydromyricetin
[0031] The cytotoxicity of dihydromyricetin was detected by MTT method, so as to determine the concentration of the drug. The specific method is as follows:
[0032] MDCK or A549 cells by 1×10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 Cultured in a constant temperature cell incubator to a monolayer, dihydromyricetin was diluted with serum-free DMEM or 1640, and then added to a 96-well plate, 200 μl per well, and continued to culture for 48 hours. Discard the culture supernatant, add 100 μL of 1640 or DMEM medium containing 0.5 mg / ml MTT to each well, and incubate at 37°C for 4 hours. The absorbance at 570 nm was detected with a multifunctional microplate reader (Genios Pro, Tecan, US). The survival rate of the cells was used as the index of the toxicity of dihydromyricetin to MDCK or A549 cells.
[0033] Cell viability (%)=E / N×100
[0034] E is the absorbance of the drug group, and N is the absorbanc...
Embodiment 2 2
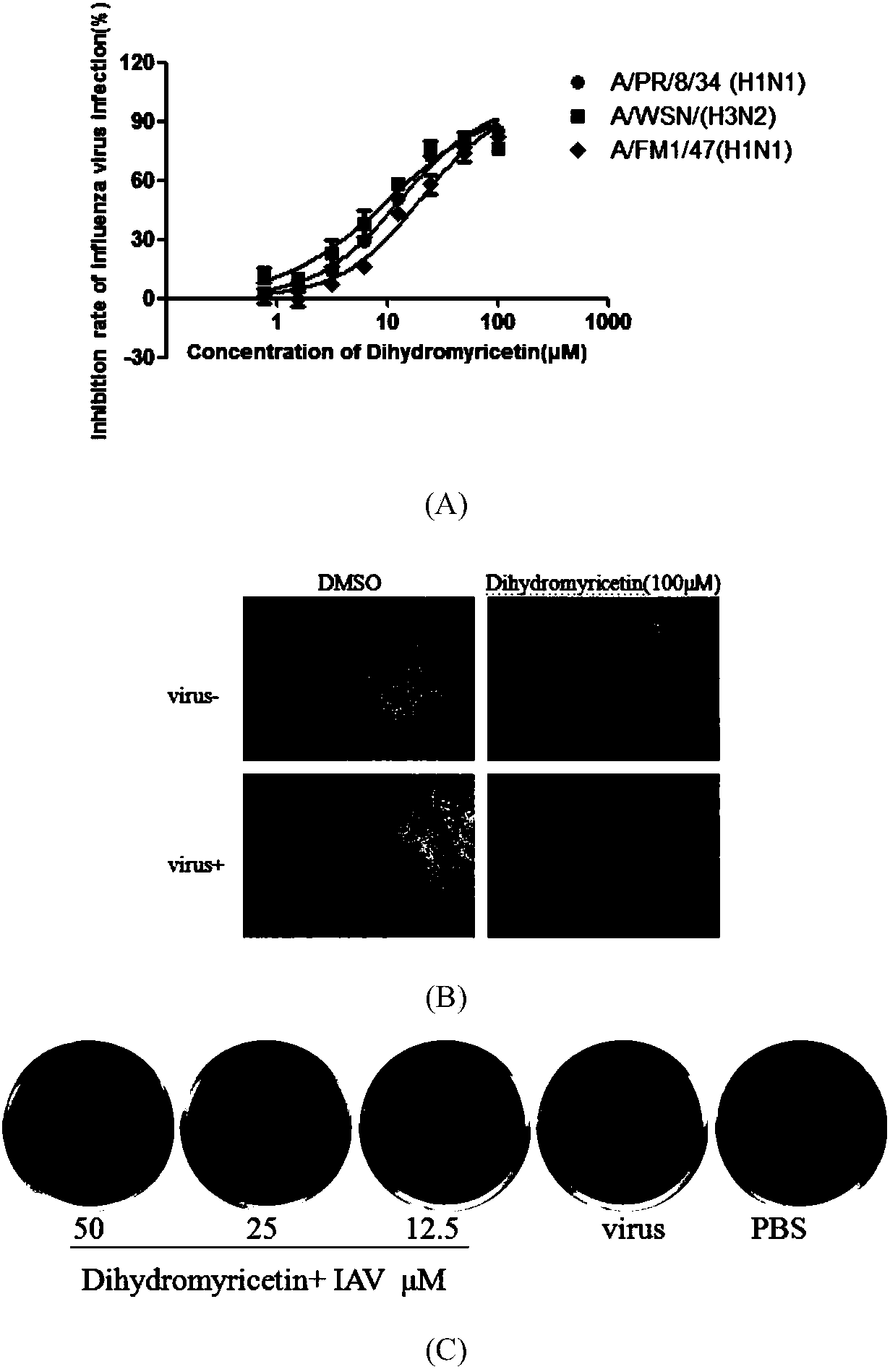
[0037] Example 2 In vitro anti-influenza virus activity detection of dihydromyricetin
[0038] In the in vitro antiviral experiment of the present invention, various subtypes of influenza A viruses are involved, including H1N1 and H3N2, and the specific methods are as follows:
[0039] MDCK cells by 2 x 10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 cultured to a monolayer in a constant temperature cell incubator. Use 100TCID 50 100 μl per well of PR8-infected cells, incubated at 37°C for 1 h, discarded the virus solution, added dihydromyricetin diluted in DMEM (containing 1 μg / ml TPCK), 200 μl per well, and continued to incubate for 48 h. Combined with MTT method and plaque experiment, it reflects that the antiviral activity of dihydromyricetin is determined by the protective effect of dihydromyricetin on cells, including the observation of dihydromyricetin inhibition of virus-induced cell virus phenomenon (CPE) and the detection of cell Survival rate, and further ca...
Embodiment 3
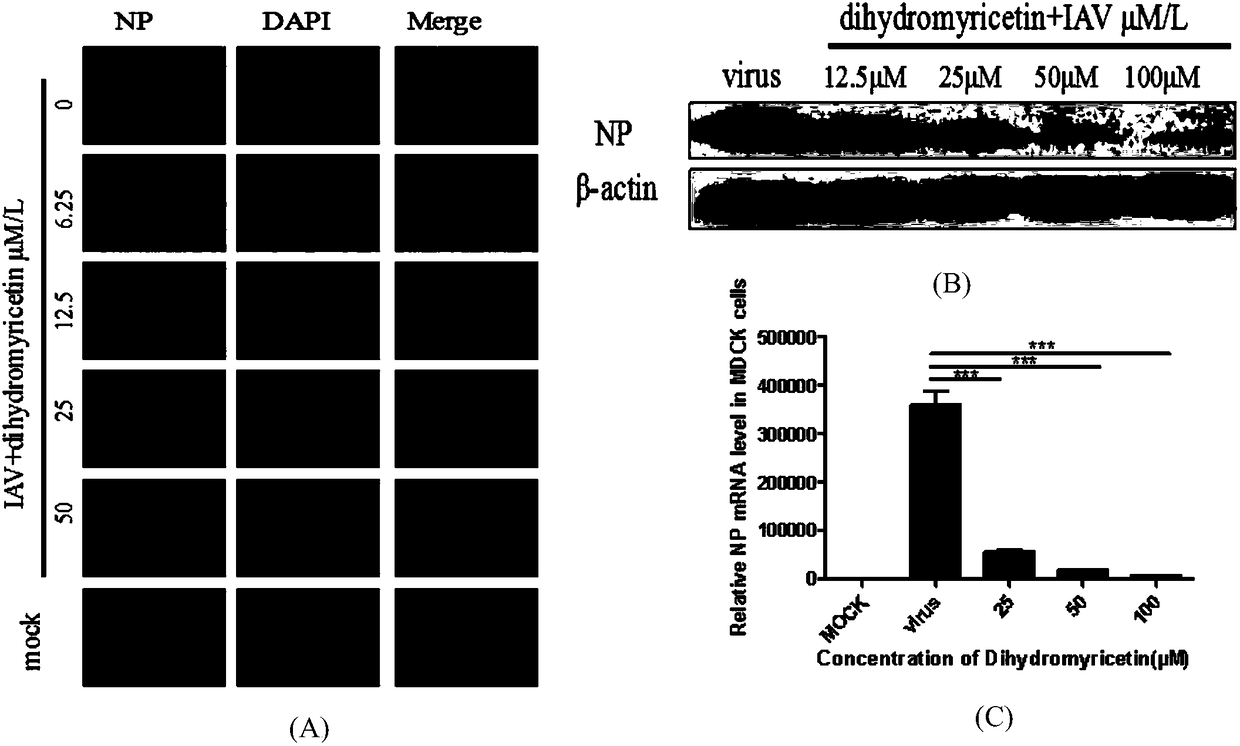
[0044] Example 3 Dihydromyricetin inhibits the replication of influenza A virus
[0045] In order to evaluate the inhibitory effect of dihydromyricetin on influenza virus replication, the present invention uses three methods of indirect immunofluorescence, Q-PCR and Western blotting to detect the effect of dihydromyricetin on virus replication from the expression levels of genes and proteins respectively. influences. The specific method is as follows:
[0046] Indirect immunofluorescence method: MDCK cells were divided into 5×10 4 / well was seeded in a 48-well plate at 37°C, 5% CO 2 cultured to a monolayer in a constant temperature cell incubator. Use 100TCID 50 Infect the cells with PR8, 1ml per well, incubate at 37°C for 1h, discard the virus solution, add dihydromyricetin diluted in DMEM (containing 1μg / ml TPCK), 500μl per well, and continue to incubate for 24h. Afterwards, fix with 4% paraformaldehyde for 20 minutes, stain the NP protein and cell nucleus, and randomly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com